Abstract
Balanced production of immune cells is critical for the maintenance of steady-state immune surveillance, and increased production of myeloid cells is sometimes necessary to eliminate pathogens. Hematopoietic stem and progenitor cell (HSPC) sensing of commensal microbes and invading pathogens has a notable impact on hematopoiesis. In this review, we examine how commensal microbes regulate bone marrow HSPC activity to maintain balanced hematopoiesis in the steady state, and how HSPCs proliferate and differentiate during emergency myelopoiesis in response to infection. HSPCs express a variety of pattern recognition receptors and cytokine receptors that they use to sense the presence of microbes, either directly via detection of microbial components and metabolites, or indirectly by responding to cytokines produced by other host cells. We describe direct and indirect mechanisms of microbial sensing by HSPCs and highlight evidence demonstrating long-term effects of acute and chronic microbial stimuli on HSPCs. We also discuss a possible connection between myeloid-biased hematopoiesis and elevated levels of circulating microbiome-derived components in the context of aging and metabolic stress. Finally, we highlight the prospect of trained immunity-based vaccines that could exploit microbial stimulation of HSPCs.
Keywords: hematopoietic stem and progenitor cells, bone marrow, emergency myelopoiesis, infection, microbiome, pathogens
Graphical Abstract
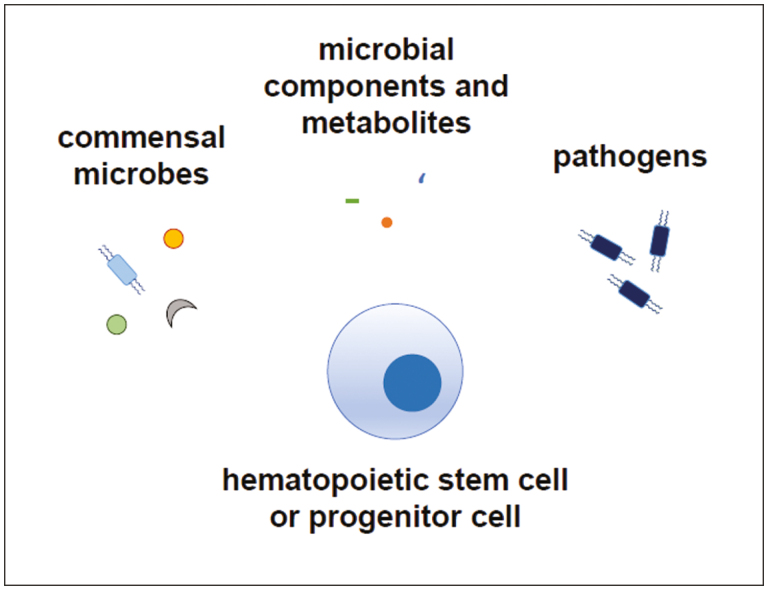
Graphical Abstract.
Hematopoietic stem and progenitor cells can detect the presence of microbes, including commensal and pathogenic organisms, by direct recognition of microbial components or metabolites, and by indirect sensing of cytokines produced in response to microbes by other cells. Microbial detection by hematopoietic stem and progenitor cells supports steady-state hematopoiesis and boosts innate immune cell production in response to infection.
Significance Statement.
Commensal and pathogenic microbes play key roles in the maintenance and differentiation of hematopoietic stem cells in the bone marrow. A healthy microbiome supports balanced hematopoiesis, and the production of innate immune cells can be enhanced during infection to facilitate pathogen clearance and restoration of homeostasis. Dysregulated hematopoiesis, however, can underlie defective immune function, hematopoietic exhaustion, and inflammatory pathology. Defining mechanisms of direct and indirect microbial sensing by hematopoietic stem and progenitor cells could lead to the development of therapeutic strategies to maintain and restore hematopoietic balance, immune health, and organ function.
Introduction
In adults, blood cells of all lineages are produced in the bone marrow via the differentiation of hematopoietic stem and progenitor cells (HSPCs). The most quiescent, self-renewing, and multipotent long-term hematopoietic stem cells (LT-HSCs) give rise to less quiescent short-term HSCs (ST-HSCs), which in turn produce multipotent progenitors (MPPs).1-3 Two fractions of MPP cells, MPP2 and MPP3, are myeloid-biased and thought to give rise to common myeloid progenitors (CMPs), which produce myeloid lineage cells via granulocyte-monocyte progenitors (GMPs) and monocyte-dendritic cell (DC) progenitors (MDPs), and also produce megakaryocyte-erythrocyte progenitors (MEPs). MPP4 cells, on the other hand, are lymphoid-primed and yield lymphoid lineage cells via common lymphoid progenitors (CLPs).
Steady-state hematopoiesis is strictly regulated by cytokines, colony-stimulating factors, and other mediators that induce HSPC differentiation along specific lineages.4,5 Transcription factors and epigenetic changes drive lineage restriction and ultimately lineage commitment and differentiation by promoting expression of lineage-specifying genes and suppressing genes that define other lineages.6-8
In this review, we will discuss how microbial sensing by HSPCs regulates hematopoiesis during homeostasis and in response to infection. We will review evidence that commensal microbes support hematopoiesis and discuss how HSPC proliferation and myeloid-biased differentiation meet the increased demand for myeloid cells to eliminate pathogens. We will consider direct versus indirect mechanisms of microbial detection, and their impact on HSPC maintenance and differentiation, as well as the functional programming of their progeny. Finally, we will discuss the possibility that commensal microbial components that leak into the circulation in the context of microbial dysbiosis and intestinal permeability induce myeloid-biased hematopoiesis during aging and under conditions of metabolic stress such as obesity and type 2 diabetes.
Regulation of Hematopoiesis by Commensal Gut Microbes
Induction of severe neutropenia in humans following prolonged antibiotic treatment suggested an association between commensal microbes and hematopoiesis.9 The observation that antibiotic treatment also impairs the function of mouse neutrophils not only in the peritoneal cavity but also in the bone marrow, along with the demonstration that commensal bacteria-derived peptidoglycan is detectable in the circulation and bone marrow,10 raised the possibility of microbiome support of neutrophil differentiation. Subsequent studies showing hematopoietic abnormalities in germ-free (GF) and antibiotic-treated mice further revealed that the gut microbiota plays an important role in steady-state hematopoiesis by communicating with the bone marrow (Fig. 1).11-17 For example, GF mice have defective myelopoiesis, as evidenced by decreased numbers of myeloid progenitors, monocytes, and neutrophils in the bone marrow, and this impairs their resistance to Listeria monocytogenes infection.15 Similarly, decreased HSPC numbers, suppressed MPP cell cycle activity, and defective hematopoietic reconstitution after bone marrow transplantation has been reported in antibiotic-treated mice.11,13,14,18,19
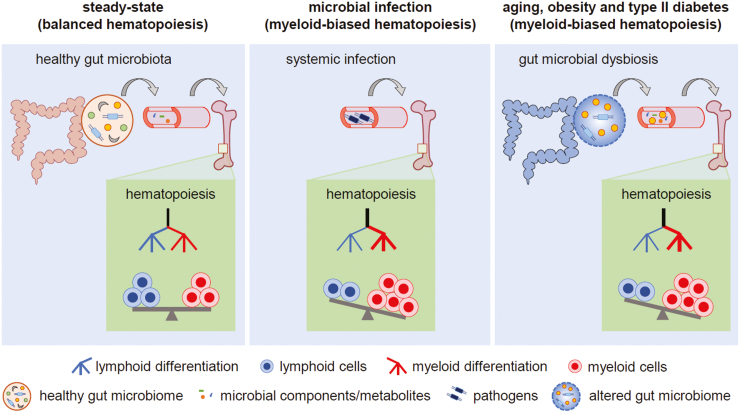
Figure 1.
Regulation of hematopoiesis by microbes. Left panel: Microbial components and metabolites derived from commensal microbes in the healthy gut maintain balanced hematopoiesis in the steady state. Middle panel: During infection, invading pathogens and their components induce myeloid-biased hematopoiesis (emergency myelopoiesis). Right panel: Myeloid-biased hematopoiesis during aging and under stress conditions such as obesity and type II diabetes may be a consequence of commensal microbial dysbiosis and intestinal permeability, which results in elevated levels of microbes and microbiome-derived components in the circulation.
Recolonization of the gut with a complex microbiota, oral administration of heat-killed bacteria, and serum transfer from microbiome-intact specific pathogen-free (SPF) mice have been shown to rescue hematopoiesis defects in GF and antibiotic-treated mice,11,14,15 and a range of mechanisms including nutritional support, microbial metabolites, and microbial cell wall components have been implicated in microbiome-mediated maintenance of hematopoiesis in the steady-state and recovery after HSPC depletion. For instance, supplementation of the drinking water with sucrose rescues antibiotic-induced defects in lymphocyte recovery after bone marrow transplantation, indicating that impaired nutrient absorption may underlie some of the hematopoiesis defects in antibiotic-treated mice.18 Moreover, systemic administration of sodium propionate to mimic short-chain fatty acid metabolism on a high fiber diet increases DC progenitor numbers in the bone marrow of microbiome-intact mice.20 In addition, a role for detection of microbial components by Toll-like receptors (TLRs) in microbiome support of myelopoiesis is evidenced by the demonstration that recolonization and serum transfer from SPF mice are ineffective in GF mice deficient in the TLR signaling adaptors MyD88 and TRIF.11
Infection-Induced Emergency Myelopoiesis
Depletion of myeloid cells from the bone marrow immediately after infection is believed to “pull” HSPCs to differentiate, whereas microbial components and pro-inflammatory cytokines can instruct or “push” HSPCs to undergo emergency myelopoiesis.21 Whole microbes and microbial components induce HSPCs to proliferate and differentiate along the myeloid lineage, resulting in an increased pool of myeloid-committed progenitors and mature myeloid cells (Fig. 1).22-27 For example, polymicrobial sepsis induced by cecal ligation and puncture in mice leads to increased myelopoiesis, as evidenced by elevated numbers of neutrophils and inflammatory (Ly6Chi) monocytes in the circulation and myeloid progenitors (CMPs and GMPs) in the bone marrow.26,27 Similarly, infection of mice with Ehrlichia muris increases the number of splenic granulocytes and myeloid progenitors in the bone marrow.23Candida albicans infection also elevates the production of splenic and bone marrow macrophages by HSPCs.24 Moreover, microbial components such as lipopolysaccharide (LPS) from Gram-negative bacteria, Pam3CSK4 (a synthetic version of bacterial lipopeptide), CpG DNA (mimics bacterial DNA), and β-glucan derived from C. albicans cell walls, as well as the live attenuated mycobacterial Bacillus Calmette-Guerin (BCG) vaccine, have been shown to promote myelopoiesis by inducing HSPC differentiation along the myeloid lineage.19,22,25,28-32 Distinct microbial components have different effects. For instance, LPS selectively induces monocyte and neutrophil differentiation, while CpG DNA promotes the production of monocytes and DCs.32
Microbial exposure may alternatively suppress myelopoiesis or decrease HSC fitness. For instance, in contrast to BCG, Mycobacterium tuberculosis (Mtb) infection has been reported to expand HSCs but suppress myelopoiesis in mice by inducing necroptosis of myeloid progenitors via depolarization of mitochondrial membrane potential.33 The effects of acute and chronic microbial exposure may also be different. Chronic LPS treatment induces myeloid-biased hematopoiesis in mice but also compromises the reconstituting ability of bone marrow HSPCs upon serial transplantation.34,35 Chronic inflammatory stress induced by LPS also elevates the cycling rate of LT-HSCs, which compromises their stemness. Similarly, polymicrobial sepsis induces HSPC cell cycle entry but suppresses myelopoiesis during advanced stages of the disease.36-38
In addition to impacting hematopoietic output, microbial sensing by HSPCs can also alter the function of their progeny. For example, macrophages produced by HSPCs exposed to β-glucan have elevated inflammatory cytokine responses to stimulation with Pam3CSK4,29 whereas HSPC exposure to Pam3CSK4 has the opposite effect, impairing the inflammatory responses of macrophage progeny to Pam3CSK4 and LPS stimulation.39 In contrast, antigen-presenting cells derived from HSPCs exposed to either β-glucan or Pam3CSK4 are more efficient at priming Th1 and Th17 cell activation in vitro.40 Mtb and BCG also have opposite effects on macrophage functional programming in mice. BCG induces epigenetic changes in HSPCs consistent with functional priming and increased microbicidal activity (trained immunity), whereas Mtb-exposed HSPCs produce macrophages that are impaired in their ability to control mycobacteria.33
Direct and Indirect Microbial Sensing by HSPCs
Regulation of hematopoiesis by commensal and pathogenic microbes may be mediated via direct sensing of microbial components or metabolites by HSPCs, or via the indirect effects of cytokines and other factors (eg, damage-associated molecular patterns [DAMPs]) produced following microbial detection by other hematopoietic or non-hematopoietic cells in the intestine, vasculature, bone marrow niche, or other tissues (Fig. 2A). For instance, LPS and CpG DNA injection promotes GMP and MDP differentiation, respectively, but it is unclear whether they do so directly or indirectly.32
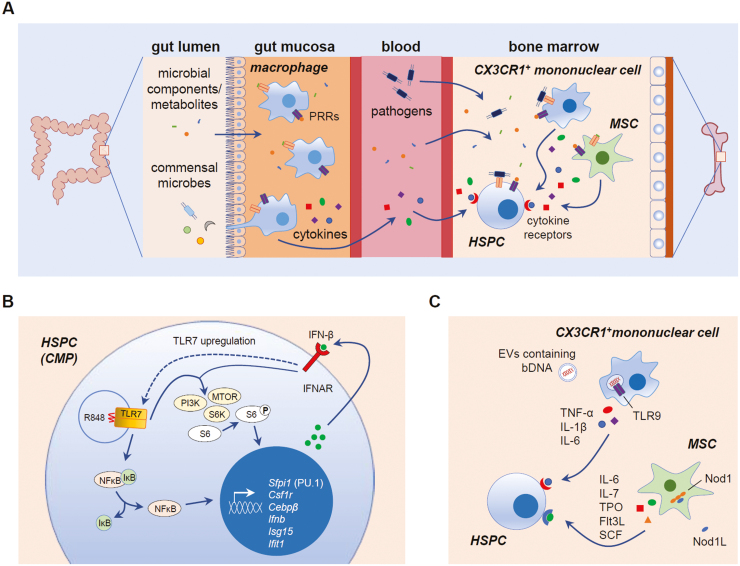
Figure 2.
Mechanisms of direct and indirect microbial sensing by hematopoietic stem and progenitor cells (HSPCs). (A) Direct and indirect mechanisms of microbial sensing regulate HSPC maintenance and differentiation. Microbial components and metabolites derived from the microbiome are present in the circulation, so it is possible that they, as well as invading pathogens and their components, can be sensed directly by bone marrow HSPCs via their pattern recognition receptors (PRRs), including Toll-like receptors (TLRs). HSC niche cells, such as mesenchymal stromal cells (MSCs) and CX3CR1+ mononuclear cells, can also detect microbes using PRRs and release pro-inflammatory cytokines to regulate HSPCs. Circulating cytokines released by mucosal macrophages sensing gut microbiome-derived components and metabolites may also be detected by HSPCs in the bone marrow. DAMPs produced by dying cells or damaged tissues may also be detected by HSPC PRRs. (B) During emergency myelopoiesis induced by the TLR7 agonist R848, direct detection by common myeloid progenitors (CMPs) stimulates NFκB-mediated induction of myeloid lineage genes such as Sfpi1 (PU.1), Csf1r, and Cebpβ. NFκB also induces IFN-β production, and autocrine detection of IFN-β by IFNAR induces TLR7 upregulation and synergistic activation of PI3K-mTOR signaling to promote CMP differentiation into macrophages.46 (C) One mechanism proposed for microbiome-mediated HSPC regulation in the steady-state is detection of commensal bacterial DNA (bDNA) by bone marrow CX3CR1+ mononuclear cells (presumably via endocytic TLR9) following delivery of the bDNA from the gut in extracellular vesicles (EVs).58 Microbiome-derived Nod1 ligands (Nod1L) are also sensed by MSCs.13 Cytokines produced by these cells promote HSPC proliferation and differentiation to maintain steady-state hematopoiesis.
Both human and murine HSPCs express a variety of pattern recognition receptors (PRRs) including TLRs, nucleotide oligomerization domain (Nod)-like receptors (NLRs), and Dectin-1, and in vitro studies have shown that HSPCs can directly respond to microbes and microbial components via these receptors.41-43 For instance, mouse HSCs, MPPs, CMPs, GMPs, and CLPs express TLRs including TLR2, TLR4 (as well as its accessory receptors MD2 and CD14) and TLR9, and purified HSCs, MPPs, and myeloid progenitors differentiate into macrophages in response to Pam3CSK4 (TLR2/TLR1 agonist), LPS (TLR4 agonist) and CpG DNA (TLR9 agonist).31,41,44 Heat-inactivated C. albicans yeast and hyphae also induce the differentiation of HSCs and myeloid progenitors into mature myeloid cells in vitro via TLR2-mediated recognition.44,45 Mechanistically, MyD88-mediated signaling is essential for myeloid cell production by HSPCs in response to LPS, Pam3CSK4, and C. albicans.44 Similarly, R848 (TLR7 agonist) induces CMPs to produce macrophages by upregulating the expression of myeloid transcription factors such as Sfpi1, and Cebpβ via activation of NF-κB, PI3K, and mammalian target of rapamycin (mTOR) signaling pathways46 (Fig. 2B). Exposure of mouse HSPCs to Pam3CSK4 also augments M-CSF-induced macrophage production in vitro.39 Furthermore, microbial exposure reprograms lymphoid-committed progenitors to produce myeloid cells.41,47 For example, mouse CLPs produce DCs instead of B cells upon in vitro challenge with LPS, and Pam3CSK4 also induces DC production by CLPs.41 Similarly, CLPs from HSV-1-infected or CpG DNA-treated mice are biased to DC production in lymphoid enrichment cultures.47
Human HSPCs also constitutively possess a variety of TLRs.48 For example, cord blood HSPCs express TLR9 and are activated by CpG DNA to produce IL-8 via activation of MAPK/AP-1 signaling.48 In contrast, human bone marrow HSPCs do not express TLR9 but do have high levels of TLR4 and TLR7/8, as well as low levels of TLR1 and TLR10.49 Moreover, they produce inflammatory cytokines and differentiate into monocytes and DCs upon R848 (TLR7/8 agonist) exposure. Most TLR agonists induce differentiation of human HSPCs along the myeloid lineage while compromising B-cell production.50 The type of myeloid cells produced by TLR-stimulated HSPCs may depend on the microbial stimulus.43 For example, R848 and loxoribine (TLR7 agonists) preferentially induce DC production by human HSPCs, whereas Pam3CSK4 induces monocyte differentiation.51
HSPCs also express other PRRs. For example, Dectin-1, a phagocytic receptor that detects fungal β-glucans, is expressed by mouse myeloid progenitors, and inactivated C. albicans yeast can induce macrophage and monocyte-derived DC (moDC) production by mouse HSPCs via direct interaction with Dectin-1/TLR2.52 Human HSPCs also express the NLR family member NOD2, and the NOD2 ligand muramyl dipeptide induces DC production by these cells.53
As noted above, PRR stimuli including LPS, CpG DNA, Pam3CSK4, β-glucan, R848, inactivated C. albicans, and microbiome-derived components have also been shown to induce myeloid differentiation in vivo.11,19,22,25,28,29,31,32,46 In contrast to exposure in vitro, it is difficult to determine whether these in vivo effects are mediated by direct HSPC recognition of microbes or indirect microbial effects on other cells. Myelopoiesis induced by microbiome-derived components, for instance, is dependent on MyD88 signaling,11 but this may reflect TLR signaling by mucosal epithelial or immune cells or even TLR-independent responses mediated by IL-1R or IL-18R signaling, rather than direct detection of microbial components by HSPCs in the bone marrow, especially in the context of an intact intestinal barrier. Adoptive transfer experiments using TLR-deficient mice can be informative to define the role of direct stimulation of HSPC TLRs. For example, WT HSPCs transferred into TLR2 and TLR4 KO recipient mice produce macrophages in response to injection of Pam3CSK4 and LPS, respectively, revealing direct recognition of TLR agonists by HSPCs in vivo.31 Similarly, adoptively transferred HSPCs from WT but not TLR2 KO (CD45.2) mice differentiate into macrophages in the spleen and bone marrow of congenic WT (CD45.1) recipient mice following C. albicans challenge, revealing direct recognition of C. albicans by HSPCs via TLR2.24 Moreover, adoptively transferred WT HSPCs produce macrophages in Dectin-1 KO recipient mice in response to β-glucan and C. albicans yeast, further demonstrating direct HSPC recognition of β-glucan via Dectin-1.29 These data demonstrate the relevance of direct recognition of microbes and microbial components by HSPCs via PRRs in the induction of emergency myelopoiesis.
However, microbes also instruct HSPC differentiation indirectly via inflammatory cytokines released by mature immune cells and non-hematopoietic cells during homeostasis and infection, including by altering the hematopoietic niche, which regulates HSPC homing, differentiation, and proliferation.54,55 Bone marrow mesenchymal stromal cells (MSCs), for example, express a variety of PRRs and produce inflammatory cytokines upon exposure to TLR agonists.56,57 Cytokines such as IL-6, IL-7, Flt3L, TPO, and SCF are secreted by MSCs in response to microbiota-derived Nod1 ligands, which have been shown to maintain steady-state hematopoiesis13 (Fig. 2C). Similarly, the expression of TLR4 by non-hematopoietic cells is indispensable for LPS-induced granulopoiesis in mice.28 Furthermore, MSCs from GF mice show dysregulated cytokine production and increased proliferation in cultures, an effect that is normalized upon colonization of the mice with the microbiota of SPF mice.55 Single-cell RNA-sequencing also revealed that altered expression of metabolic pathway, HIF-1/inflammatory signaling, and neurodegenerative pathway genes is associated with the abnormal function MSCs in GF mice. Microbiota-derived bacterial DNA can also induce TLR-mediated production of inflammatory cytokines such as TNF-α, IL-1β, and IL-6 by bone marrow CX3CR1+ mononuclear cells, which in turn promotes progenitor differentiation and myelopoiesis58 (Fig. 2C).
A variety of cytokines and growth factors regulate hematopoiesis in the steady-state and during emergency myelopoiesis, including interleukins (eg, IL-1β, IL-3, IL-6), type I and II interferons, granulocyte and/or monocyte colony-stimulating factors (G-CSF, M-CSF, GM-CSF), stem cell factor (SCF), and Fms-like tyrosine kinase 3 ligand (FLT3L). Some act broadly to promote HSPC survival and lineage restriction, whereas others specifically induce lineage commitment.4,5 For instance, IL-3 is essential for emergency myelopoiesis during polymicrobial sepsis in mice,27 and IL-1β induces proliferation and differentiation of mouse LT-HSCs into myeloid cells by programming their gene expression network toward myeloid differentiation.59,60 JAK/STAT signaling, which is critical for myelopoiesis induced by inflammatory cytokines, is also important for CpG DNA-induced myelopoiesis in vivo, which suggests that CpG acts indirectly via cytokines to promote myelopoiesis.19,61 However, the inflammatory cytokines responsible for CpG DNA-induced myelopoiesis are not known. TNF-α has been shown to increase the expression of PU.1, the master regulator of myeloid differentiation, in mouse HSCs following LPS stimulation, demonstrating its role in LPS-induced myelopoiesis.62 Granulopoiesis induced by infection of mice with Ehrlichia muris is mediated via IFN-γ stimulation of HSPCs,23 and IFN-α induces quiescent HSCs to enter the cell cycle following Poly I:C treatment.63 Autocrine and/or paracrine effects of cytokines produced by HSPCs themselves may also play a role. Indeed, single-cell proteomics revealed that ST-HSCs and MPPs produce a variety of cytokines via NF-κB signaling in response to LPS and Pam3CSK4.64
HSPCs likely sense multiple stimuli simultaneously or sequentially, and type I and type II IFN signaling pathways have been shown to collaborate with TLRs in activating HSPCs during emergency myelopoiesis.22,33,46,63,65 For instance, R848 and IFN-β synergistically induce macrophage production by mouse CMPs, and CMP differentiation to macrophages induced by R848, LPS, and CpG DNA is inhibited in the absence of IFNAR (the IFNα/β receptor)46 (Fig. 2B). HSPC PRRs may also detect DAMPs released by other cells in response to cell death or tissue damage induced by pathogens.
Long-Term Effects of Microbial Sensing by HSPCs
In addition to transiently inducing emergency myelopoiesis, acute exposure to microbes can have longer-lasting consequences, such as metabolic changes and epigenetic modifications in HSPCs that contribute to trained immunity or other forms of innate immune memory.22,25,66,67 Previously activated HSPCs may respond differently to secondary microbial challenge with the same or different microbes or microbial products (homologous or heterologous stimuli), including in terms of gene expression, differentiation, and the functional programming of their progeny. For example, HSPCs from β-glucan-treated mice remain myeloid-biased for at least 28 days, and LT-HSCs from β-glucan-treated donor mice transplanted into naive recipient mice 28 days after β-glucan treatment produce proportionately more myeloid cells and fewer B cells than LT-HSCs from control donors.25 Remarkably, mice that received serially transplanted LT-HSCs from LPS-treated donors are better protected against Pseudomonas aeruginosa infection than those receiving transplants from control PBS-treated donors due to c/EBPβ-driven epigenetic changes that maintain increased myeloid output.67 Functional effects on the progeny of exposed HSPCs may also persist. For example, 3 months after BCG vaccination in humans, bone marrow HSPCs possess transcriptomic alterations consistent with primed innate immune cell function (trained immunity).68
Concluding Remarks and Future Directions
Regulation of hematopoiesis is critical for the balanced production of blood cells in the steady state. An appropriate response of the hematopoietic system to infection is equally important for the increased production of myeloid cells to combat pathogens. Therefore, a thorough understanding of how HSPCs respond to microbial stimuli is important. As we have discussed, HSPCs can respond to microbes both directly via PRR-mediated recognition and indirectly via cytokines and other factors secreted by hematopoietic and non-hematopoietic cells in the steady state and during infection. The current literature suggests that signaling from commensal microbes to HSPCs is essential for steady-state hematopoiesis and that myeloid-biased hematopoiesis is promoted during infection, although it is unclear to what degree the underlying mechanisms of microbial-promoted myeloid differentiation overlap in these contexts. For instance, the indirect effects of microbial components such as TLR agonists in a healthy gut with an intact intestinal barrier may differ considerably from the effects of the same microbial components in the circulation during infection.
The consequences of such exposure may also be distinct. For instance, exposure to commensal microbial components in the context of an intact intestinal barrier, accompanied by the production of protective microbial metabolites may be beneficial to promote a healthy balance of HSC maintenance and immune cell differentiation, whereas commensal microbial components in the circulation as a consequence of microbial dysbiosis and intestinal permeability may disrupt HSC quiescence and induce imbalanced hematopoiesis. Peptidoglycan and DNA derived from commensal bacteria are present in the circulation of healthy young mice,10,58 but microbial dysbiosis and increased intestinal permeability together elevate levels of circulating microbial components, leading to low-grade chronic inflammation during aging, obesity, and type 2 diabetes.54,69-73 Myeloid-biased hematopoiesis, as suggested by increased numbers of myeloid progenitors in the bone marrow and mature myeloid cells in the circulation, has been demonstrated during aging, obesity, and type 2 diabetes,59,74-80 and there may therefore be a direct connection between elevated commensal microbe-derived components and myeloid-biased hematopoiesis under such stress conditions (Fig. 1). Indeed, chow diet-fed mice have increased MPP and myeloid progenitor numbers after fecal transplantation from high fat diet-fed obese mice, which implicates the altered gut microbiome in obesity-associated increased myelopoiesis.54 However, it is not yet clear how bone marrow HSPCs respond to the altered gut microbiome during such stress. Moreover, TNF-α has been implicated in the loss of intestinal barrier integrity73 and in increased myelopoiesis in old mice,79 supporting a role for microbial dysbiosis in myeloid-biased hematopoiesis during aging. Targeting PRR-mediated signaling in HSPCs and niche-associated cells may therefore have therapeutic potential.
Moreover, the concept of microbe-induced training of innate immune cells has recently given rise to the prospect of trained immunity-based vaccines, which might induce long-term immunity against a broad spectrum of pathogens via effects on myeloid cells and their progenitors.81 For example, the elevated responses of monocytes from BCG-vaccinated mice and humans to heterologous microbial challenges ex vivo supports the potential success of trained immunity-based vaccines. Additional studies probing how microbial components impact HSPCs and their progeny are therefore important to inform this area of research.
Funding
This work was supported by National Institutes of Health R01 AI134987 and funds from the Board of Governors Regenerative Medicine Institute at Cedars-Sinai Medical Center (to H.S.G.).
Conflict of Interest
The authors indicated no financial relationships.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Haas S, Trumpp A, Milsom MD.. Causes and consequences of hematopoietic stem cell heterogeneity. Cell Stem Cell 2018;22(5):627-638. [DOI] [PubMed] [Google Scholar]
- 2. Iwasaki H, Akashi K.. Hematopoietic developmental pathways: on cellular basis. Oncogene 2007;26(47):6687-6696. [DOI] [PubMed] [Google Scholar]
- 3. Trzebanski S, Jung S.. Plasticity of monocyte development and monocyte fates. Immunol Lett 2020;227:66-78. [DOI] [PubMed] [Google Scholar]
- 4. Hayashi Y, Sezaki M, Takizawa H.. Development of the hematopoietic system: role of inflammatory factors. Wiley Interdiscip Rev Dev Biol 2019;8(4):e341. [DOI] [PubMed] [Google Scholar]
- 5. Robb L. Cytokine receptors and hematopoietic differentiation. Oncogene 2007;26(47):6715-6723. [DOI] [PubMed] [Google Scholar]
- 6. Kucinski I, Wilson NK, Hannah R, et al. . Interactions between lineage-associated transcription factors govern haematopoietic progenitor states. EMBO J 2020;39(24):e104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenbauer F, Tenen DG.. Transcription factors in myeloid development: balancing differentiation with transformation. Nat Rev Immunol 2007;7(2):105-117. [DOI] [PubMed] [Google Scholar]
- 8. Antoniani C, Romano O, Miccio A.. Concise review: epigenetic regulation of hematopoiesis: biological insights and therapeutic applications. Stem Cells Transl Med 2017;6(12):2106-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neftel KA, Hauser SP, Muller MR.. Inhibition of granulopoiesis in vivo and in vitro by beta-lactam antibiotics. J Infect Dis 1985;152(1):90-98. [DOI] [PubMed] [Google Scholar]
- 10. Clarke TB, Davis KM, Lysenko ES, et al. . Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 2010;16(2):228-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balmer ML, Schurch CM, Saito Y, et al. . Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J Immunol 2014;193(10):5273-5283. [DOI] [PubMed] [Google Scholar]
- 12. Han H, Yan H, King KY.. Broad-spectrum antibiotics deplete bone marrow regulatory T cells. Cells 2021;10(2):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwamura C, Bouladoux N, Belkaid Y, et al. . Sensing of the microbiota by NOD1 in mesenchymal stromal cells regulates murine hematopoiesis. Blood 2017;129(2):171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Josefsdottir KS, Baldridge MT, Kadmon CS, et al. . Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood 2017;129(6):729-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khosravi A, Yanez A, Price JG, et al. . Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 2014;15(3):374-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suva LJ. A link between the gut and bone: bone health impacted by changes in gut microbiota. Am J Pathol 2019;189(2):229-230. [DOI] [PubMed] [Google Scholar]
- 17. Collins A, Mitchell CA, Passegue E.. Inflammatory signaling regulates hematopoietic stem and progenitor cell development and homeostasis. J Exp Med 2021;218(7):e20201545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Staffas A, Burgos da Silva M, Slingerland AE, et al. . Nutritional support from the intestinal microbiota improves hematopoietic reconstitution after bone marrow transplantation in mice. Cell Host Microbe 2018;23(4):447-457 e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weaver LK, Minichino D, Biswas C, et al. . Microbiota-dependent signals are required to sustain TLR-mediated immune responses. JCI Insight 2019;4(1):e124370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trompette A, Gollwitzer ES, Yadava K, et al. . Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014;20(2):159-166. [DOI] [PubMed] [Google Scholar]
- 21. King KY, Goodell MA.. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol 2011;11(10):685-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaufmann E, Sanz J, Dunn JL, et al. . BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 2018;172(1-2): 176-190 e119. [DOI] [PubMed] [Google Scholar]
- 23. MacNamara KC, Oduro K, Martin O, et al. . Infection-induced myelopoiesis during intracellular bacterial infection is critically dependent upon IFN-gamma signaling. J Immunol 2011;186(2):1032-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Megias J, Maneu V, Salvador P, et al. . Candida albicans stimulates in vivo differentiation of haematopoietic stem and progenitor cells towards macrophages by a TLR 2 -dependent signalling. Cell Microbiol 2013;15(7): 1143-1153. [DOI] [PubMed] [Google Scholar]
- 25. Mitroulis I, Ruppova K, Wang B, et al. . Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 2018;172(1-2): 147-161 e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen Q, Zhang Q, Shi Y, et al. . Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature 2018;554(7690):123-127. [DOI] [PubMed] [Google Scholar]
- 27. Weber GF, Chousterman BG, He S, et al. . Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 2015;347(6227):1260-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boettcher S, Ziegler P, Schmid MA, et al. . Cutting edge: LPS-induced emergency myelopoiesis depends on TLR 4 -expressing nonhematopoietic cells. J Immunol 2012;188(12): 5824-5828. [DOI] [PubMed] [Google Scholar]
- 29. Bono C, Martinez A, Megias J, et al. . Dectin-1 stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward trained macrophages via an indirect cell-autonomous mechanism. mBio 2020;11(3):e00781-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brook B, Harbeson DJ, Shannon CP, et al. . BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci Transl Med 2020;12(542):eaax4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Megias J, Yanez A, Moriano S, et al. . Direct Toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells 2012;30(7):1486-1495. [DOI] [PubMed] [Google Scholar]
- 32. Yanez A, Coetzee SG, Olsson A, et al. . Granulocyte-monocyte progenitors and monocyte-dendritic cell progenitors independently produce functionally distinct monocytes. Immunity 2017;47(5):890-902 e894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khan N, Downey J, Sanz J, et al. . M. tuberculosis reprograms hematopoietic stem cells to limit myelopoiesis and impair trained immunity. Cell 2020;183(3):752-770 e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Esplin BL, Shimazu T, Welner RS, et al. . Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol 2011;186(9):5367-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao Y, Ling F, Wang HC, et al. . Chronic TLR signaling impairs the long-term repopulating potential of hematopoietic stem cells of wild type but not Id1 deficient mice. PLoS One 2013;8(2):e55552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skirecki T, Kawiak J, Machaj E, et al. . Early severe impairment of hematopoietic stem and progenitor cells from the bone marrow caused by CLP sepsis and endotoxemia in a humanized mice model. Stem Cell Res Ther 2015;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang P, Wang J, Li YH, et al. . Phenotypical changes of hematopoietic stem and progenitor cells in sepsis patients: correlation with immune status? Front Pharmacol 2020;11:640203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang H, Rodriguez S, Wang L, et al. . Sepsis induces hematopoietic stem cell exhaustion and myelosuppression through distinct contributions of TRIF and MYD88. Stem Cell Reports 2016;6(6):940-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yanez A, Hassanzadeh-Kiabi N, Ng MY, et al. . Detection of a TLR2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce. Eur J Immunol 2013;43(8):2114-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martinez A, Bono C, Gozalbo D, et al. . TLR2 and Dectin-1 signaling in mouse hematopoietic stem and progenitor cells impacts the ability of the antigen presenting cells they produce to activate CD4 T cells. Cells 2020;9(5):1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagai Y, Garrett KP, Ohta S, et al. . Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 2006;24(6):801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sioud M. Microbial sensing by haematopoietic stem and progenitor cells: Vigilance against infections and immune education of myeloid cells. Scand J Immunol 2020;92(5):e12957. [DOI] [PubMed] [Google Scholar]
- 43. Yanez A, Goodridge HS, Gozalbo D, et al. . TLRs control hematopoiesis during infection. Eur J Immunol 2013;43(10):2526-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yanez A, Flores A, Murciano C, et al. . Signalling through TLR2/MyD88 induces differentiation of murine bone marrow stem and progenitor cells to functional phagocytes in response to Candida albicans. Cell Microbiol 2010;12(1):114-128. [DOI] [PubMed] [Google Scholar]
- 45. Yanez A, Murciano C, O’Connor JE, et al. . Candida albicans triggers proliferation and differentiation of hematopoietic stem and progenitor cells by a MyD88-dependent signaling. Microbes Infect 2009;11(4):531-535. [DOI] [PubMed] [Google Scholar]
- 46. Buechler MB, Akilesh HM, Hamerman JA.. Cutting edge: direct sensing of TLR7 ligands and type I IFN by the common myeloid progenitor promotes mTOR/PI3K-dependent emergency myelopoiesis. J Immunol 2016;197(7):2577-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Welner RS, Pelayo R, Nagai Y, et al. . Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood 2008;112(9):3753-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim JM, Kim NI, Oh YK, et al. . CpG oligodeoxynucleotides induce IL-8 expression in CD34+ cells via mitogen-activated protein kinase-dependent and NF-kappaB-independent pathways. Int Immunol 2005;17(12):1525-1531. [DOI] [PubMed] [Google Scholar]
- 49. Sioud M, Floisand Y, Forfang L, et al. . Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. J Mol Biol 2006;364(5):945-954. [DOI] [PubMed] [Google Scholar]
- 50. De Luca K, Frances-Duvert V, Asensio MJ, et al. . The TLR1/2 agonist PAM( 3 )CSK( 4 ) instructs commitment of human hematopoietic stem cells to a myeloid cell fate. Leukemia 2009;23(11):2063-2074. [Google Scholar]
- 51. Sioud M, Floisand Y.. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th 1 -type response. Eur J Immunol 2007;37(10): 2834-2846. [DOI] [PubMed] [Google Scholar]
- 52. Yanez A, Megias J, O’Connor JE, et al. . Candida albicans induces selective development of macrophages and monocyte derived dendritic cells by a TLR2 dependent signalling. PLoS One 2011;6(9):e24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sioud M, Floisand Y.. NOD2/CARD15 on bone marrow CD34+ hematopoietic cells mediates induction of cytokines and cell differentiation. J Leukoc Biol 2009;85(6):939-946. [DOI] [PubMed] [Google Scholar]
- 54. Luo Y, Chen GL, Hannemann N, et al. . Microbiota from obese mice regulate hematopoietic stem cell differentiation by altering the bone niche. Cell Metab 2015;22(5):886-894. [DOI] [PubMed] [Google Scholar]
- 55. Xiao E, He L, Wu Q, et al. . Microbiota regulates bone marrow mesenchymal stem cell lineage differentiation and immunomodulation. Stem Cell Res Ther 2017;8(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Najar M, Krayem M, Meuleman N, et al. . Mesenchymal stromal cells and toll-like receptor priming: a critical review. Immune Netw 2017;17(2):89-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Romieu-Mourez R, Francois M, Boivin MN, et al. . Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol 2009;182(12):7963-7973. [DOI] [PubMed] [Google Scholar]
- 58. Lee S, Kim H, You G, et al. . Bone marrow CX3CR1+ mononuclear cells relay a systemic microbiota signal to control hematopoietic progenitors in mice. Blood 2019;134(16):1312-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nagareddy PR, Kraakman M, Masters SL, et al. . Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab 2014;19(5):821-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pietras EM, Mirantes-Barbeito C, Fong S, et al. . Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol 2016;18(6):607-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Furqan M, Mukhi N, Lee B, et al. . Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res 2013;1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Etzrodt M, Ahmed N, Hoppe PS, et al. . Inflammatory signals directly instruct PU.1 in HSCs via TNF. Blood 2019;133(8):816-819. [DOI] [PubMed] [Google Scholar]
- 63. Essers MA, Offner S, Blanco-Bose WE, et al. . IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 2009;458(7240):904-908. [DOI] [PubMed] [Google Scholar]
- 64. Zhao JL, Ma C, O’Connell RM, et al. . Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell 2014;14(4):445-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baratono SR, Chu N, Richman LP, et al. . Toll-like receptor 9 and interferon-gamma receptor signaling suppress the B-cell fate of uncommitted progenitors in mice. Eur J Immunol 2015;45(5):1313-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen L, Ozato K.. Innate immune memory in hematopoietic stem/progenitor cells: myeloid-biased differentiation and the role of interferon. Front Immunol 2021;12:621333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. de Laval B, Maurizio J, Kandalla PK, et al. . C/EBPbeta-dependent epigenetic memory induces trained immunity in hematopoietic stem cells. Cell Stem Cell 2020;26(5):657-674 e658. [DOI] [PubMed] [Google Scholar]
- 68. Cirovic B, de Bree LCJ, Groh L, et al. . BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 2020;28(2):322-334 e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bosco N, Noti M.. The aging gut microbiome and its impact on host immunity. Genes Immun 2021;22:289-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gurung M, Li Z, You H, et al. . Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020;51:102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ragonnaud E, Biragyn A.. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun Ageing 2021;18(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dabke K, Hendrick G, Devkota S.. The gut microbiome and metabolic syndrome. J Clin Invest 2019;129(10):4050-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thevaranjan N, Puchta A, Schulz C, et al. . Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2018;23(4):570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Barman PK, Urao N, Koh TJ.. Diabetes induces myeloid bias in bone marrow progenitors associated with enhanced wound macrophage accumulation and impaired healing. J Pathol 2019;249(4):435-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guidi N, Sacma M, Standker L, et al. . Osteopontin attenuates aging-associated phenotypes of hematopoietic stem cells. EMBO J 2017;36(7):840-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ho YH, Del Toro R, Rivera-Torres J, et al. . Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell 2019;25(3):407-418 e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Maryanovich M, Zahalka AH, Pierce H, et al. . Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med 2018;24(6):782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nagareddy PR, Murphy AJ, Stirzaker RA, et al. . Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 2013;17(5):695-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Puchta A, Naidoo A, Verschoor CP, et al. . TNF drives monocyte dysfunction with age and results in impaired anti-pneumococcal immunity. PLoS Pathog 2016;12(1):e1005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Singer K, DelProposto J, Morris DL, et al. . Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab 2014;3(6):664-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sanchez-Ramon S, Conejero L, Netea MG, et al. . Trained immunity-based vaccines: a new paradigm for the development of broad-spectrum anti-infectious formulations. Front Immunol 2018;9:2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.