Abstract
Significance.
Combining 0.01% atropine with soft multifocal contact lenses (SMCL) failed to demonstrate better myopia control than SMCL alone.
Purpose.
The Bifocal & Atropine in Myopia (BAM) study investigated whether combining 0.01% atropine and SMCL with +2.50-D add power leads to greater slowing of myopia progression and axial elongation than SMCL alone.
Methods.
BAM participants wore SMCL with +2.50-D add power daily and administered 0.01% atropine eye drops nightly (n = 46). The BAM subjects (Bifocal + Atropine) were age-matched to 46 participants in the Bifocal Lenses In Nearsighted Kids (BLINK) Study who wore SMCL with +2.50-D add (Bifocal) and 46 BLINK participants who wore single vision contact lenses (Single Vision). The primary outcome was the 3-year change in spherical equivalent refractive error determined by cycloplegic autorefraction, and the 3-year change in axial elongation was also evaluated.
Results.
Of the total 138 subjects, the mean age was 10.1 ± 1.2 years and the mean spherical equivalent was −2.28 ± 0.89 D. The 3-year adjusted mean myopia progression was −0.52 D for Bifocal + Atropine, −0.55 D for Bifocal, and −1.09 D for Single Vision. The difference in myopia progression was 0.03 D (95% CI, −0.14 to 0.21) for Bifocal + Atropine vs Bifocal and 0.57 D (95% CI, 0.38-0.77) for Bifocal + Atropine vs Single Vision. The 3-year adjusted axial elongation was 0.31 mm for Bifocal + Atropine, 0.39 mm for Bifocal, and 0.68 mm for Single Vision. The difference in axial elongation was −0.08 mm (95% CI, −0. 16 to 0.002) for Bifocal + Atropine vs Bifocal and −0.37 mm (95% CI, −0.46 to −0.28) for Bifocal + Atropine vs Single Vision.
Conclusions.
Adding 0.01% atropine to SMCL with +2.50-D add power failed to demonstrate better myopia control than SMCL alone.
Among the currently available myopia control strategies, orthokeratology,1–4 soft multifocal contact lenses,5–10 spectacles,11, 12 and low concentration atropine13–15 are considered to be the most effective methods for slowing myopia progression and eye growth in children in the United States. Soft multifocal contact lenses have been shown to slow both myopia progression and axial elongation.16 A multicenter randomized clinical trial found that the dual-focus MiSight contact lens, the first treatment approved by the FDA with an indication to slow myopia progression, reduced the development of myopia by 0.67 D (95% CI, 0.49 — 0.84) and axial elongation by 0.28 mm (95% CI, −0.36 to 0.20) over 3 years compared to single vision contact lens wearers.10 Similarly, the Bifocal Lenses In Nearsighted Kids (BLINK) Study group recently reported that soft multifocal contact lenses with high add power slowed myopia progression by 0.46 D (95% CI, 0.29 — 0.63) and axial elongation by 0.23 mm (95% CI, 0.17 — 0.30) over 3 years compared to single vision contact lenses.9 Low-concentration atropine is another safe and effective myopia control strategy, although the reduction of axial elongation is not always correlated with slowing of myopia progression.13–15
Combination therapy is a common practice in the medical field to optimize treatment efficacy while minimizing adverse effects. Such examples include cancer care,17, 18 diabetes treatment,19 and glaucoma management,20 among many others. Studies have suggested that combining an optical treatment with a pharmacologic intervention may provide better myopia control than monotherapy.21–26 Recent studies that investigated the combination treatment of 0.01% atropine and orthokeratology found that axial elongation was significantly slower among participants randomly assigned to the combination treatment compared to those who were assigned to orthokeratology alone.24 25 Moreover, a retrospective study found that adding 0.01% atropine to orthokeratology treatment provided additional slowing of axial elongation in children who experienced fast myopia progression.23 One study provided potentially contradictory information. That study compared the axial elongation of participant subgroups based on the concentration of atropine (0.025% or 0.125%) administered and the baseline myopia of the participant (less myopic than −6 D or equal to or more myopic than −6 D). Combination treatment resulted in slower axial elongation in three of the four subgroups, but the high myopes who were administered 0.025% atropine with orthokeratology actually grew an average of 0.18 D more than the participants who wore orthokeratology alone.26 Another study found that adding atropine to orthokeratology did not slow the 3-year axial elongation compared to orthokeratology alone after the participants had used orthokeratology mono-therapy for a year.27
To date, no study has reported the myopia control effects of combining low-concentration atropine and soft multifocal contact lenses. The Bifocal & Atropine in Myopia (BAM) Study investigated whether a combination treatment of 0.01% atropine and soft multifocal contact lenses with high add power lead to slower myopia progression and axial elongation than soft multifocal contact lenses alone.
METHODS
The Biomedical Institutional Review Board at The Ohio State University and the University of Houston approved the study protocols. The study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written parental permission was obtained from a parent or guardian, and written assent was obtained from the participant. The study was registered with ClinicalTrials.gov (NCT03312257).
Study Design and Setting
Details of the BAM Study design were reported previously.28 Briefly, the BAM Study was an ancillary study of the BLINK Study,9, 29 a double masked, randomized clinical trial sponsored by the National Eye Institute. The participant eligibility criteria and protocol for the outcome measures were consistent between the BAM Study and the BLINK Study. The BLINK Study enrolled 294 myopic children aged 7 to 11 years at The Ohio State University College of Optometry and The University of Houston College of Optometry between October 17, 2014 and June 20, 2016. The BAM Study enrolled 49 participants at the Ohio State University between August 2, 2016 and April 4, 2017. The BAM Study participants were all treated with +2.50 D add soft multifocal contact lenses (Biofinity “D”) and nightly administration of 0.01% atropine. The BLINK Study participants were randomly assigned to wear single vision soft contact lenses, soft multifocal contact lenses with a +1.50-D add, or soft multifocal contact lenses with a +2.50-D add. The BAM Study participants were age-matched (see detail of matching below) to a subset of BLINK Study participants who wore soft multifocal contact lenses with +2.50-D add power and another subset of participants who wore single vision contact lenses. All participants underwent annual examinations to measure cycloplegic autorefraction and axial length.
The BAM Study had two specific aims (1) to test whether the combined treatment of 0.01% atropine and soft multifocal contact lens wear produces slower myopia progression and axial elongation compared to soft multifocal contact lenses alone over 3 years, and (2) to test whether early changes in choroidal thickness can be used as predictors of long-term myopia progression / axial elongation. This article only addresses the first aim.
Matching
BAM Study participants were matched by age category (7-9 years versus 10-11 years) to participants in the BLINK Study who wore soft multifocal contact lenses with +2.50 D add power and another group of BLINK Study participants who wore single vision contact lenses. There were 98 potential control participants in each group from which the final 49 in each group were randomly selected. The epidemiologist (LAJ) was provided with only the age of the BAM Study participants without knowing the myopia progression or axial elongation at the time of matching. A list of the BLINK Study participants was compiled and matches from the +2.50 D add and the single vision groups were selected for each of the BAM Study participants based on the age group using a random list generator.
Eligibility Criteria
The age range of the eligible participants was 7 to 11 years (inclusive). The requirements of refractive error (by cycloplegic autorefraction) included: spherical component (minus cylinder) myopia of −0.75 to −5.00 diopter (D), inclusive, in each eye; no more than 1.00 D of astigmatism in each eye; and no more than 2.00 D of anisometropia (difference in the spherical component between the eyes). The requirements of best-corrected visual acuities at distance were: +0.1 logMAR (20/25) or better in each eye with spectacle correction and +0.1 logMAR (20/25) or better binocularly while wearing +2.50-D add power soft multifocal contact lenses. Participants could not wear gas-permeable, soft multifocal, or orthokeratology contact lenses, nor could they have undergone myopia control for more than 1 month prior to enrollment. They also could not be chronically using medications that may affect immunity (such as oral or ophthalmic corticosteroids).29 In addition, BAM Study participants needed to pass a 2-week run-in period to ensure adequate compliance with atropine use.28 The run-in period was described in detail previously.28 Briefly, participants were required to self-report on a calendar that they wore contact lenses an average of at least 5 days per week, and the change in weight of the atropine bottle had to indicate similar compliance.
Interventions
All participants in the BAM Study wore Biofinity Multifocal “D” contact lenses with a +2.50-D add power (CooperVision, Victor, NY) daily and were administered 0.01% atropine ophthalmic solution nightly.28 The BLINK Study participants wore either single vision soft contact lenses or soft multifocal contact lenses with a +2.50-D add power daily without using atropine eye drops. Contact lenses, solutions, contact lens cases, and 0.01% atropine ophthalmic solution were provided free of charge for all participants. Participants were told to wear their contact lenses as often as they would like during the day (no overnight wear), and a pair of free or reduced-cost spectacles was also provided for each participant.
The atropine ophthalmic solution was produced at a compounding pharmacy in Columbus, OH. The compounding was performed in a sterile manner according to the United States Pharmacopeia (USP) guidelines by dissolving atropine sulfate USP monohydrate powder in sterile water to a final concentration of 0.01% (for every 0.1 g of atropine, 1000 ml of sterile water was added), and Benzalkonium Chloride was added as a preservative. The atropine solution expired within 120 days from the date of compounding, and the participants received a freshly compounded bottle every 3 months. Twenty-five random samples of atropine eye drops across various lots and expiration dates (including longer than 120 days from the date of compounding) were sent to the Pharmacoanalytical Shared Resource (PhASR) at The Ohio State University Comprehensive Cancer Center for assay validation. The chemist was masked and atropine samples were analyzed and quantitated via Liquid Chromatography - Tandem Mass Spectrometry. All the atropine samples were confirmed to be at a concentration of 0.01%.
Outcomes
The primary outcome was the 3-year change in spherical equivalent refractive error determined by cycloplegic autorefraction using the Grand Seiko WAM-5500 Binocular Autorefractor/Keratometer (AIT Industries, Bensenville, IL). Cycloplegia was achieved by instilling one drop of 0.5% proparacaine followed by two drops of 1.0% tropicamide, five minutes apart. Ten cycloplegic readings were measured 25 minutes after instilling the second drop of tropicamide, and then averaged using the method described by Thibos.30 The participants fixated 6/9 (20/30) size letters on a near point card viewed through a +4.00 D Badal lens at the participants’ far point.
Axial length for each eye was measured under cycloplegia using the Lenstar LS 900 (Haag-Streit USA, Mason, OH). The subject was instructed to fixate an internal, red LED (Light-Emitting Diode) light with the eye being measured, and the contralateral eye was covered with an eye patch. The measurements were repeated until five reliable readings were acquired without a poor-quality warning indicator.
The visual acuity at distance was measured using Bailey-Lovie logMAR charts for high and low contrast visual acuity at 4 m. The visual acuity at near was measured using the Logarithmic Visual Acuity Chart 2000 “New Early Treatment Diabetic Retinopathy Study” (ETDRS) near visual acuity chart at 40 cm. The luminance of all visual acuity charts were calibrated to be between 75 to 120 cd/m2 using the Sekonic L-508 Zoom Master (Sekonic, Tokyo, Japan) light meter. Participants read the first letter of each line until reading one incorrectly, then began two lines above and read every letter of each line until at least three letters were missed on the same line. The number of letters read correctly was recorded and converted to logMAR visual acuity.
Pupil diameter of the right eye was obtained using the NeurOptics VIP-200 Pupillometer (NeurOptics, Irvine, CA) under both photopic and mesopic lighting conditions. For the photopic condition, the subject stood facing the examiner with his/her back toward the wall-mounted visual acuity chart calibrated to 75 to 120 cd/m2. For the mesopic condition (<1 cd/m2), the subject stood in the same location with all room lights off except an incandescent lamp pointed straight down at the opposite end of the room. The eye cup of the pupillometer was held against the right eye while the measurement was performed. The pupil size was recorded to the nearest 0.1 mm.
Accommodative lag was measured using the Grand Seiko WAM-5500 Binocular Autorefractor/Keratometer (AIT Industries, Bensenville, IL). Subject viewed a 4 X 4 grid of 20/125 Snellen Equivalent letters located at 33 cm through the right eye, and the left eye was occluded. Accommodative lag was defined as the dioptric differences between the accommodative response and stimulus. The participants’ habitual soft contact lens correction was worn during the measurement. Baseline accommodative lag was measured at the 3-week visit after the subjects have started wearing contact lenses and using atropine eye drops.28, 29
Adherence to contact lens wear and atropine was monitored by parental surveys. The parents reported how many days their children usually wore contact lenses and what time they usually inserted and removed contact lenses during weekdays and weekends, respectively. The number of hours that contact lenses were typically worn each week was calculated based on the information, and the average number of hours each day was then calculated by dividing the total hours per week by the number of days the lenses were reported to be worn. Additionally, the parents reported how many days during the week (0 to 5) and weekend (0 to 2) the children typically missed the atropine drops.
Sample Size
Estimates of sample size were computed for each specific aim based on the assumption of an α level of 0.05, 80% power, myopia progression of −1.29 D over three years, and a 50% treatment effect (reducing the myopia progression by 0.65 D for the +2.50-D add power group and by an additional 0.33 D for the combination treatment group). The sample size required was 39 participants for each group, but the ultimate sample size was determined by the second aim related to assessing early changes in choroidal thickness, which required 44 participants per group. Adjusting for 10% loss to follow-up gave a total sample required of 49 participants for each group.
Statistical Analyses
Repeated measures analyses using mixed linear models in STATA (version 15, College Station, TX) were undertaken to model myopia progression (primary outcome) and axial elongation (secondary outcome) to account for the clusters of correlated data due to repeated participant outcome measures.31 Models controlled for the baseline value of the outcome, sex, race, age group (7-9 or 10-11 years), eye (right or left), and pair matching. A significant interaction was retained in the model between treatment group and time (P < .01). Interaction between baseline refractive error and axial elongation was not significant (P = 0.79). Analyses of variance were conducted to compare ages, baseline refractive errors and biometric parameters, visual acuities, pupil sizes, accommodative lag, and contact lenses wear time between the treatment groups. Chi-square tests were used to compare sex and race between the treatment groups. In addition, post hoc analyses were performed to compare the proportion of participants whose myopia progressed −1.00 D or more or eyes grew 0.36 mm or more between the treatment groups (assuming 0.36 mm of eye growth corresponds to −1.00 D of myopia progression).
RESULTS
Of the 49 participants enrolled in the BAM Study, 46 were included in the analyses and all of them completed the 3-year visit. Three participants did not attend any of the subsequent annual visits. Overall, participants attended 138 of the 147 annual visits (93.9%). These 46 BAM Study participants (Bifocal + Atropine) were matched by age category to 46 participants in the BLINK Study who wore soft multifocal contact lenses with +2.50-D add power (bifocal) and another group of 46 BLINK participants who wore single vision contact lenses (single vision).
Of the total 138 participants, 63% were female, the mean (SD) age was 10.1 ± 1.2 years, 54% were aged 10 or 11 years at baseline, and 67% were white. The mean (SD) right eye cycloplegic spherical equivalent at baseline was −2.28 ± 0.89 D. Detailed demographic and ocular characteristics of the BAM participants and matched groups at baseline are shown in Table 1. At baseline, there were no significant differences between the three groups for age, refractive error, biometric parameters (including axial length, anterior chamber depth, lens thickness, and vitreous chamber depth), visual acuities (including high and low contrast distance and high contrast near visual acuity), or pupil sizes (including under photopic and mesopic conditions). However, the participants in the Bifocal + Atropine group had a lower proportion of female participants and more Asian participants than that of the two matched groups (Table 1).
Table 1.
Demographic and ocular characteristics of the BAM participants and matched groups at baseline. Categorical variables are indicated as number (percent) and continuous variables are indicated as mean (standard deviation) unless otherwise noted.
| Bifocal + Atropine (n = 46) | Bifocal (n = 46) | Single Vision (n = 46) | P-value | |
|---|---|---|---|---|
| Age, y | > .99 | |||
| 7-9 | 21 (45.7) | 21 (45.7) | 21 (45.7) | |
| 10-11 | 25 (54.3) | 25 (54.3) | 25 (54.3) | |
| Median (Q1-Q3) | 10.4 (9.4-11.3) | 10.1 (9.3-10.9) | 10.2 (9.3-11.1) | |
| Sex | .001 | |||
| Female | 26 (56.5) | 32 (69.6) | 29 (63.0) | |
| Male | 20 (43.5) | 14 (30.4) | 17 (37.0) | |
| Race | < .01 | |||
| White | 28 (60.9) | 33 (71.7) | 31 (67.4) | |
| > 1 race | 6 (13.0) | 5 (10.9) | 3 (6.5) | |
| Black or African American | 1 (2.2) | 1 (2.2) | 9 (19.6) | |
| Asian | 10 (21.7) | 6 (13.0) | 3 (6.5) | |
| American Indian or Alaska Native | 1 (2.2) | 1 (2.2) | 0 (0) | |
| Right eye refractive error, D | ||||
| Spherical equivalent | −2.31 (1.00) | −2.21 (.80) | −2.31 (.89) | .84 |
| Right eye biometry, mm | ||||
| Axial length | 24.39 (.64) | 24.32 (.75) | 24.54 (.90) | .38 |
| Anterior chamber depth | 3.93 (.21) | 3.96 (.19) | 3.98 (.23) | .56 |
| Lens thickness | 3.32 (.13) | 3.33 (.15) | 3.29 (.13) | .34 |
| Vitreous chamber depth | 17.14 (.65) | 17.02 (.75) | 17.27 (.87) | .31 |
| Visual acuity, logMAR | ||||
| High contrast distance | −0.02 (.01) | −0.01 (.01) | −0.02 (.01) | .76 |
| High contrast near | −0.08 (.01) | −0.05 (.01) | −0.07 (.01) | .24 |
| Low contrast distance | 0.08 (.01) | 0.08 (.01) | 0.09 (.01) | .76 |
| Pupil size, mm | ||||
| Photopic | 5.37 (0.09) | 5.40 (0.10) | 5.37 (0.11) | .96 |
| Mesopic | 6.58 (0.09) | 6.48 (0.10) | 6.40 (0.10) | .41 |
The outcomes from the age-matched subgroups selected from BLINK had outcomes that were essentially identical to the BLINK study as a whole.9 This consistency supports the validity of the inter-study comparisons. Therefore, this article only reports the comparisons between the Bifocal + Atropine group and the BLINK age-matched subgroups (Bifocal and Single Vision groups) and not the comparisons between the two BLINK subgroups.
Primary Outcomes
For the Bifocal + Atropine group, the mean myopia (mean value of right and left eyes) was −2.28 D at baseline and −2.75 D at 3 years, with a mean unadjusted progression of −0.47 D (95% CI, −0.60 to −0.35). For the Bifocal group, myopia was −2.26 D at baseline and −2.82 D at 3 years, with a mean unadjusted progression of −0.56 D (95% CI, −0.68 to −0.44). For the Single Vision group, myopia was −2.30 D at baseline and −3.37 D at 3 years, with a mean unadjusted progression of −1.08 D (95% CI, −1.23 to −0.92) (Figure 1A).
Figure 1.
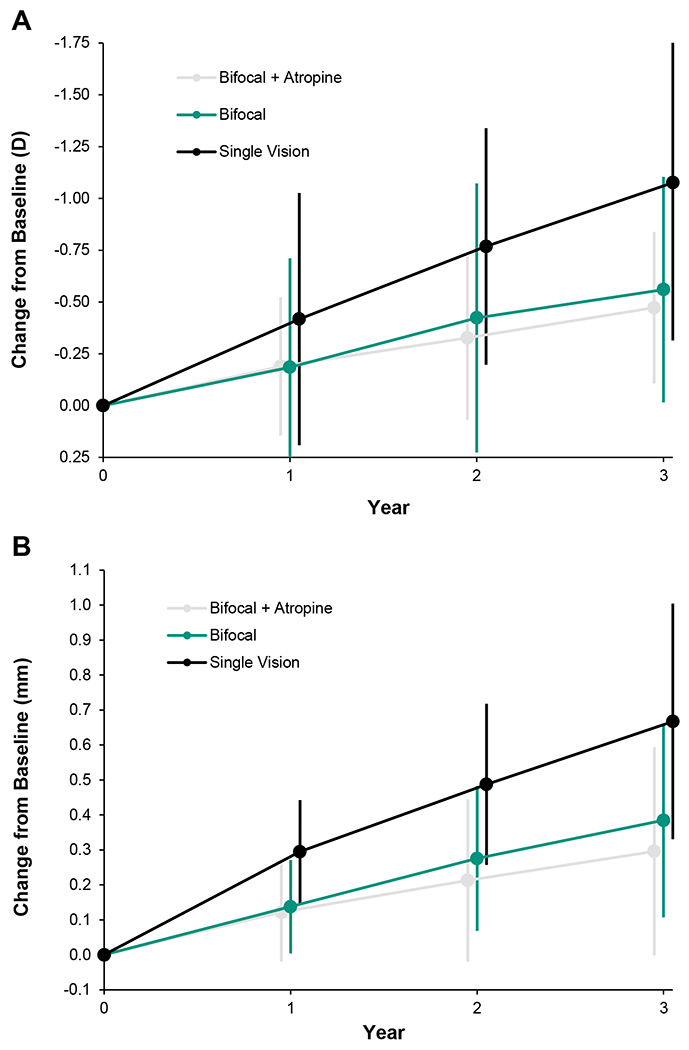
Unadjusted mean (SD) myopia progression (A) and axial elongation (B) of the two eyes from baseline.
A-B reformattedKAZ
The 3-year adjusted (for baseline spherical equivalent, sex, race, age group, eye, and pair matching) mean myopia progression was −0.52 D (95% CI, −0.65 to −0.39) for the Bifocal + Atropine group, −0.55 D (95% CI, −0.66 to −0.44) for the Bifocal group, and −1.09 D (95% CI, −1.24 to −0.95) for the Single Vision group. The difference in progression was 0.03 D (95% CI, −0.14 to 0.21) between the Bifocal + Atropine versus Bifocal group and 0.57 D (95% CI, 0.38 — 0.77) between the Bifocal + Atropine versus Single Vision group (Table 2).
Table 2.
Outcomes at 3 years for Bifocal + Atropine, Bifocal, and Single Vision (SV) groups.
| Outcome | Bifocal + Atropine | Bifocal | Single Vision (SV) | Mean difference | P-value |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Refractive error, D | |||||
| Baseline mean (SD) | −2.28 (0.98) | −2.26 (0.80) | −2.30 (0.90) | ||
| Year 3, mean (SD) | −2.75 (1.14) | −2.82 (1.09) | −3.37 (1.26) | ||
| 3-y absolute change (95% CI) | −0.47 (−0.60 to −0.35) | −0.56 (−0.68 to −0.44) | −1.08 (−1.23 to −0.92) | ||
| Bifocal + Atropine vs. Bifocal | 0.09 (−0.10 to 0.28) | .36 | |||
| Bifocal + Atropine vs. SV | 0.60 (0.41 to 0.79) | < .01 | |||
| 3-y Adjusted Change (95% CI) | −0.52 (−0.65 to −0.39) | −0.55 (−0.66 to −0.44) | −1.09 (−1.24 to −0.95) | ||
| Bifocal + Atropine vs. Bifocal | 0.03 (−0.14 to 0.21) | .70 | |||
| Bifocal + Atropine vs. SV | 0.57 (0.38 to 0.77) | < .01 | |||
| Secondary Outcomes | |||||
| Axial Length, Mm | |||||
| Baseline Mean (SD) | 24.37 (0.63) | 24.31 (0.75) | 24.50 (0.91) | ||
| Year 3, Mean (SD) | 24.67 (0.70) | 24.70 (0.84) | 25.16 (0.93) | ||
| 3-y Absolute Change (95% CI) | 0.30 (0.23 to 0.36) | 0.38 (0.33 to 0.44) | 0.67 (0.60 to 0.73) | ||
| Bifocal + Atropine vs. Bifocal | −0.09 (−0.18 to −0.0002) | .049 | |||
| Bifocal + Atropine vs. SV | −0.37 (−0.46 to −0.28) | < .01 | |||
| 3-y Adjusted Change (95% CI) | 0.31 (0.25 to 0.37) | 0.39 (0.34 to 0.44) | 0.68 (0.61 to 0.74) | ||
| Bifocal + Atropine vs. Bifocal | −0.08 (−0.16 to 0.002) | .054 | |||
| Bifocal + Atropine vs. SV | −0.37 (−0.46 to −0.28) | < .01 | |||
| VA, Mean (95% CI), logMAR | |||||
| High Contrast Distance | −0.06 (−0.07 to −0.04) | −0.03 (−0.05 to −0.02) | −0.06 (−0.07 to −0.04) | ||
| Bifocal + Atropine vs. Bifocal | −0.02 (−0.05 to −0.004) | .017 | |||
| Bifocal + Atropine vs. SV | 0.00 (−0.02 to 0.02) | > .99 | |||
| High Contrast Near | −0.13 (−0.14 to −0.11) | −0.07 (−0.09 to −0.05) | −0.11 (−0.13 to −0.09) | ||
| Bifocal + Atropine vs. Bifocal | −0.06 (−0.08 to −0.04) | < .01 | |||
| Bifocal + Atropine vs. SV | −0.02 (−0.04 to 0.003) | .10 | |||
| Low Contrast Distance | 0.12 (0.10 to 0.14) | 0.11 (0.09 to 0.13) | 0.07 (0.05 to 0.09) | ||
| Bifocal + Atropine vs. Bifocal | 0.01 (−0.01 to 0.04) | .40 | |||
| Bifocal + Atropine vs. SV | 0.05 (0.03 to 0.08) | < .01 | |||
| Pupil Size, Mean (95% CI), mm | |||||
| Photopic | 5.10 (4.94 to 5.26) | 4.75 (4.59 to 4.90) | 4.87 (4.71 to 5.02) | ||
| Bifocal + Atropine vs. Bifocal | 0.36 (0.13 to 0.58) | < .01 | |||
| Bifocal + Atropine vs. SV | 0.24 (0.01 to 0.46) | .04 | |||
| Mesopic | 6.41 (6.26 to 6.57) | 6.30 (6.15 to 6.45) | 6.23 (6.07 to 6.38) | .25 | |
| Acc. Lag, Mean (95% CI), D | −0.86 (−1.03 to −0.70) | −1.32 (−1.56 to −1.08) | −1.09 (−1.21 to −0.97) | ||
| Bifocal + Atropine vs. Bifocal | 0.46 (0.21 to 0.71) | < .01 | |||
| Bifocal + Atropine vs. SV | 0.23 (−0.02 to 0.48) | .07 | |||
| Post Hoc Outcomes, No. (%) [95% CI] | (n = 46) | (n = 46) | (n = 46) | ||
| Progress >1.00 D | 8 (17.4) | 7 (15.2) | 22 (47.8) | ||
| Bifocal + Atropine vs. Bifocal | 3.3 (−11.6 to 18.1) | .67 | |||
| Bifocal + Atropine vs. SV | −30.4 (−48.6 to −12.3) | .002 | |||
| Eye Growth >0.36 mm | 18 (39.1) | 23 (50.0) | 39 (84.8) | ||
| Bifocal + Atropine vs. Bifocal | −9.8 (−30.0 to 10.4) | .35 | |||
| Bifocal + Atropine vs. SV | −44.6 (−62.3 to −26.9) | < .01 |
SV = single vision; VA = visual acuity; Acc. Lag = accommodative lag; CI = confidence interval; SD = standard deviation; D = diopters
Secondary Outcomes
For the Bifocal + Atropine group, the mean axial length was 24.37 mm at baseline and 24.67 mm at 3 years, and the unadjusted elongation was 0.30 mm (95% CI, 0.23 — 0.36). For the Bifocal group, mean axial length was 24.31 mm at baseline and 24.70 mm at 3 years, and the unadjusted elongation was 0.38 mm (95% CI, 0.33 — 0.44). For the Single Vision group, axial length was 24.50 mm at baseline and 25.16 mm at 3 years, and the unadjusted elongation was 0.67 mm (95% CI, 0.60 — 0.73) (Figure 1B).
The 3-year adjusted axial elongation was 0.31 mm (95% CI, 0.25 — 0.37) for the Bifocal + Atropine group, 0.39 mm (95% CI, 0.34 — 0.44) for the Bifocal group, and 0.68 mm (95% CI, 0.61 — 0.74) for the Single Vision group. The difference in axial elongation was −0.08 mm (95% CI, −0. 16 to +0.002) between the Bifocal + Atropine versus Bifocal group and −0.37 mm (95% CI, −0.46 to −0.28) between the Bifocal + Atropine versus Single Vision group (Table 2).
At the final visit, the mean high-contrast distance logMAR visual acuity was significantly different between the three groups (P = .017; Table 2). Specifically, the high-contrast distance logMAR visual acuity for both the Bifocal + Atropine group (−0.06) and the Single Vision group (−0.06) was significantly better than the Bifocal group (−0.03), but the difference was less than 2 letters (i.e., not clinically meaningful). The Bifocal + Atropine group was not significantly different from the Single Vision group for the high-contrast distance logMAR visual acuity. Similarly, the mean high-contrast near logMAR visual acuity was significantly different between the three groups (P < .01). The high-contrast near logMAR visual acuity for both the Bifocal + Atropine group (−0. 13) and the Single Vision group (−0.11) was significantly better than the Bifocal group (−0.07), but the difference was no more than 3 letters. The Bifocal + Atropine group was not significantly different from the Single Vision group for the high-contrast near logMAR visual acuity. The mean low-contrast distance logMAR visual acuity was also significantly different between the three groups (P < .01). The low-contrast distance logMAR visual acuity for the Single Vision group (0.07) was significantly better than both the Bifocal + Atropine group (0.12) and the Bifocal group (0.11), but the difference was again less than 3 letters. The Bifocal + Atropine group was not significantly different from the Bifocal group for the low-contrast distance logMAR visual acuity.
At the baseline, the pupil sizes were not significantly different between the three groups for under either photopic (P = .96) or mesopic conditions (P = .41). At the final visit, the mean photopic pupil size was 5.10 mm for the Bifocal + Atropine group, which was significantly larger than the 4.75 mm for the Bifocal group (P < .01) and the 4.87 mm for the single vision group (P = .04). However, the mean mesopic pupil size was not significantly different between the three groups (P = .25).
At the baseline, the accommodative lag in the Bifocal + Atropine group was −1.44 D (95% CI: −1.60 to −1.27), which was not significantly different from −1.31 D of the Bifocal group (95% CI: −1.52 to −1.11; P = 0.33), but significantly greater than −1.00 D of the Single Vision group (95% CI: −1.18 to −0.82; P < .01). However, at the final visit, the accommodative lag in the Bifocal + Atropine group was −0.86 D (95% CI: −1.03 to −0.70), which was not significantly different from −1.09 D of the Single Vision group (95% CI: −1.21 to −0.97; P = .07), but significantly less than −1.32 D the Bifocal group (95% CI: −1.56 to −1.08; P < .01). The accommodative lag in the Bifocal + Atropine group at the final visit was significantly less than baseline by 0.59 D (95% CI, 0.37 — 0.81; P < .01).
The self-reported contact lenses wear time (hours per day) was significantly longer for the Bifocal + Atropine group (12.7 ± 2.4) compared to the Bifocal (11.8 ± 3.2) and Single Vision (11.9 ± 3.2) groups (both P < .01). The average atropine use in the Bifocal + Atropine group reported by their parents was 6.4 ± 0.2 days/week at year-1 visit, 6.2 ± 0.2 days/week at year-2 visit, and 6.3 ± 0.2 days/week at year-3 visit (P = .91).
The percentage of participants who progressed −1.00 D or more over 3 years was 17.4% (95% CI, 10.9%−26.6%) for the Bifocal + Atropine group, 15.2% (95% CI, 8.4% – 22.9%) for the Bifocal group, and 47.8% (95% CI, 37.8% – 58.0%) for the Single Vision group (Table 2). The percentage was significantly different between the Bifocal + Atropine and Single Vision group (P = .002), but not significantly different between the Bifocal + Atropine and Bifocal group (P = .67). The percentage of participants who had eye growth more than 0.36 mm over 3 years was 39.1% (95% CI, 29.7% – 49.5%) for the Bifocal + Atropine group, 50.0% (95% CI, 38.8%-59.1%) for the Bifocal group, and 84.8% (95% CI, 74.7% – 89.9%) for the Single Vision group. The percentage was significantly different between the Bifocal + Atropine and Single Vision group (P < .01), but not significantly different between the Bifocal + Atropine and Bifocal group (P = .35).
DISCUSSION
In this 3-year non-randomized clinical study, the results indicate that combining 0.01% atropine with soft multifocal contact lens wear failed to demonstrate slower myopia progression or eye growth than using multifocal lenses alone, but both the combination treatment and soft multifocal contact lens treatment slowed myopia progression and eye growth significantly more than single vision contact lenses.
Previous studies have shown that both orthokeratology and soft multifocal contact lenses can slow myopia progression, possibly due to the peripheral myopia defocus that provides inhibiting signal for slowing axial elongation.32–36 It has also been shown that combination treatment of orthokeratology and low dose atropine can be more effective compared to using orthokeratology alone.21,23–25 Two meta-analyses21, 22 showed that combining orthokeratology and low dose atropine slowed axial elongation by about 0.09 mm compared to orthokeratology alone, which was similar to the amount of slowing axial elongation (0.08 mm) by the combination treatment versus using soft multifocal contact lens alone in the BAM Study, although the difference was not statistically different (P = .054). Slowing axial elongation by 0.1 mm corresponds to slowing myopia progression by about 0.25 D, which is not clinically meaningful. Considering the effort of daily administration of atropine, a combination treatment of low dose atropine with optical intervention may not be warranted when the optical intervention by itself is almost as effective in slowing myopia progression. Kinoshita et al. found that the effect of slowing axial elongation through combination treatment of 0.01% atropine and orthokeratology was influenced by the subjects’ initial refractive error.24 In contrast, the BAM Study showed that interaction between baseline refractive error and axial elongation was not significant (P = 0.79), indicating that the initial refractive error did not have an impact on the myopia control effect of combination treatment of 0.01% atropine and soft multifocal contact lenses.
A plausible explanation of why the combination treatment was not better than using soft multifocal contact lens alone is the relatively low concentration of atropine used in the BAM Study. The Low-Concentration Atropine for Myopia Progression (LAMP) Study suggests that low-concentration atropine slowed myopia progression in a dose-dependent manner, with 0.05% being more effective compared to 0.01%.13 The concentration of atropine used in the BAM Study was 0.01%, which might be too weak of a dose for evaluation of combination therapy. Another factor that should be considered is compliance for contact lens wear and atropine use were both evaluated through self-report in this study. It is possible that the participants/parents may have overestimated their compliance.
The BAM Study found that after receiving combination treatment of +2.5-Add soft bifocal contact lenses and 0.01% atropine for 3 years, the Bifocal + Atropine group had slightly better high-contrast distance and near logMAR visual acuity compared to the Bifocal group and worse low-contrast distance logMAR visual acuity compared to the Single Vision group, although none of the differences was clinically meaningful. The BLINK Study reported that children wearing +2.50-D add power contact lenses had worse low-contrast distance visual acuity compared to those wearing single vision contact lenses (similar to the BAM Study, the difference was not clinically meaningful), but the high-contrast distance and near visual acuity was not significantly different between the two groups.9 The LAMP (Low-Concentration Atropine for Myopia Progression) and ATOM2 (Atropine for the Treatment of Myopia 2) Study found that high-contrast visual acuity was not affected by low-concentration atropine (low-contrast visual acuity was not evaluated in these two studies).13,14 Two other studies that used a combination treatment of atropine and orthokeratology also found that low dose atropine did not impact the outcome of high-contrast visual acuity (neither study reported results of low-contrast visual acuity).24,25
The BAM Study found that the pupil dilation under photopic condition was modest (less than 0.36 mm) in the Bifocal + Atropine group compared to the Bifocal group and single vision group (the mesopic pupil size was not significantly different between the three groups), which is consistent with findings in previous studies that involved atropine for myopia control.13,14,25 The LAMP Study reported a dose-dependent response in pupil size when treating myopia using atropine with different concentrations,13 and Tan et al. found that adding atropine to orthokeratology resulted in modest pupil dilation compared to orthokeratology alone.25
Previous studies showed that low-concentration atropine had a minimal impact on accommodation.13,14,25 The BAM Study found that after 3 years of combination treatment with +2.5-Add soft bifocal contact lenses and 0.01% atropine, the amount of accommodative lag in the Bifocal + Atropine group decreased by 0.59 D compared to baseline, which was a surprising finding. It’s possible that the reduction of accommodative lag was a result of participants changing their accommodative behavior toward a preference for the distance zone of the soft multifocal contact lenses.
Limitations
The BAM Study was supported by a K23 Mentored Patient-Oriented Research Career Development Award through the National Eye Institute, which does not allow randomized clinical trials. As a result, a historical control comparison was made between the BAM Study group and the +2.50 Add group and the single vision group from a historical study. Utilizing an age-matching process that was masked to the outcome and conducting identical study protocols reduced the potential for bias, but is not as strong of a study design as a randomized clinical trial. Although including an additional monotherapy treatment group of atropine only would have been interesting, that was not part of the study question to be answered.
CONCLUSIONS
The results of this non-randomized study do not support a combination treatment using 0.01% atropine and soft multifocal contact lenses with high add power to slow myopia progression in children.
ACKNOWLEDGEMENTS
Funding/Support: National Institutes of Health grants K23 EY025273, U10 EY023204, EY023206, EY023208, EY023210, UL1 TR001070, P30 EY007551, and P30 EY005722.
REFERENCES
- 1.Si JK, Tang K, Bi HS, et al. Orthokeratology for Myopia Control: A Meta-Analysis. Optom Vis Sci 2015;92:252–57. [DOI] [PubMed] [Google Scholar]
- 2.Huang J, Wen D, Wang Q, et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-Analysis. Ophthalmology 2016;123:697–708. [DOI] [PubMed] [Google Scholar]
- 3.Kang P. Optical and Pharmacological Strategies of Myopia Control. Clin Exp Optom 2018;101:321–32. [DOI] [PubMed] [Google Scholar]
- 4.Cho P, Cheung SW, Edwards M. The Longitudinal Orthokeratology Research in Children (LORIC) in Hong Kong: A Pilot Study on Refractive Changes and Myopic Control. Curr Eye Res 2005;30:71–80. [DOI] [PubMed] [Google Scholar]
- 5.Walline JJ, Greiner KL, McVey ME, Jones-Jordan LA. Multifocal Contact Lens Myopia Control. Optom Vis Sci 2013;90:1207–14. [DOI] [PubMed] [Google Scholar]
- 6.Sankaridurg P, Holden B, Smith E 3rd, et al. Decrease in Rate of Myopia Progression with a Contact Lens Designed to Reduce Relative Peripheral Hyperopia: One-Year Results. Invest Ophthalmol Vis Sci 2011;52:9362–67. [DOI] [PubMed] [Google Scholar]
- 7.Lam CS, Tang WC, Tse DY, et al. Defocus Incorporated Soft Contact (DISC) Lens Slows Myopia Progression in Hong Kong Chinese Schoolchildren: A 2-Year Randomised Clinical Trial. Br J Ophthalmol 2014;98:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Pomeda A, Perez-Sanchez B, Valls I, et al. Misight Assessment Study Spain (MASS). A 2-Year Randomized Clinical Trial. Graefes Arch Clin Exp Ophthalmol 2018;256:1011–21. [DOI] [PubMed] [Google Scholar]
- 9.Walline JJ, Walker MK, Mutti DO, et al. Effect of High Add Power, Medium Add Power, or Single-Vision Contact Lenses on Myopia Progression in Children: The BLINK Randomized Clinical Trial. JAMA 2020;324:571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamberlain P, Peixoto-de-Matos SC, Logan NS, et al. A 3-Year Randomized Clinical Trial of Misight Lenses for Myopia Control. Optom Vis Sci 2019;96:556–67. [DOI] [PubMed] [Google Scholar]
- 11.Bao J, Yang A, Huang Y, et al. One-Year Myopia Control Efficacy of Spectacle Lenses with Aspherical Lenslets. Br J Ophthalmol 2021;April 4:pre-print before publication: 10.1136/bjophthalmol-2020-318367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam CS, Tang WC, Lee PH, et al. Myopia Control Effect of Defocus Incorporated Multiple Segments (DIMS) Spectacle Lens in Chinese Children: Results of a 3-Year Follow-up Study. Br J Ophthalmol 2021;March 19:pre-print before publication: 10.1136/bjophthalmol-2020-317664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology 2019;126:113–24. [DOI] [PubMed] [Google Scholar]
- 14.Chia A, Chua WH, Cheung YB, et al. Atropine for the Treatment of Childhood Myopia: Safety and Efficacy of 0.5%, 0.1%, and 0.01% Doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012;119:347–54. [DOI] [PubMed] [Google Scholar]
- 15.Chia A, Chua WH, Wen L, et al. Atropine for the Treatment of Childhood Myopia: Changes after Stopping Atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol 2014;157:451–57 e1. [DOI] [PubMed] [Google Scholar]
- 16.Walline JJ, Lindsley K, Vedula SS, et al. Interventions to Slow Progression of Myopia in Children. Cochrane Database Syst Rev 2020;1:CD004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong J, Yun CO. Emergence of Ad-Mediated Combination Therapy against Cancer: What to Expect? Curr Cancer Drug Targets 2018;18:139–52. [DOI] [PubMed] [Google Scholar]
- 18.Aumeeruddy MZ, Mahomoodally MF. Combating Breast Cancer Using Combination Therapy with 3 Phytochemicals: Piperine, Sulforaphane, and Thymoquinone. Cancer 2019;125:1600–11. [DOI] [PubMed] [Google Scholar]
- 19.Cersosimo E, Johnson EL, Chovanes C, Skolnik N. Initiating Therapy in Patients Newly Diagnosed with Type 2 Diabetes: Combination Therapy Vs a Stepwise Approach. Diabetes Obes Metab 2018;20:497–507. [DOI] [PubMed] [Google Scholar]
- 20.Mehran N, Sinha S, Razeghinejad R. New Glaucoma Medications: Latanoprostene Bunod, Netarsudil, and Fixed Combination Netarsudil-Latanoprost. Eye (Lond) 2020;34:72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao C, Wan S, Zhang Y, Han J. The Efficacy of Atropine Combined with Orthokeratology in Slowing Axial Elongation of Myopia Children: A Meta-Analysis. Eye Contact Lens 2021;47:98–103. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Wang J, Wang N. Combined Orthokeratology with Atropine for Children with Myopia: A Meta-Analysis. Ophthalmic Res 2021;64:723–31. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Huang S, Zhou J, et al. Adjunctive Effect of Orthokeratology and Low Dose Atropine on Axial Elongation in Fast-Progressing Myopic Children-a Preliminary Retrospective Study. Cont Lens Anterior Eye 2019;42:439–42. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita N, Konno Y, Hamada N, et al. Efficacy of Combined Orthokeratology and 0.01% Atropine Solution for Slowing Axial Elongation in Children with Myopia: A 2-Year Randomised Trial. Sci Rep 2020;10:12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan Q, Ng AL, Choy BN, et al. One-Year Results of 0.01% Atropine with Orthokeratology (Aok) Study: A Randomised Clinical Trial. Ophthalmic Physiol Opt 2020;40:557–66. [DOI] [PubMed] [Google Scholar]
- 26.Wan L, Wei CC, Chen CS, et al. The Synergistic Effects of Orthokeratology and Atropine in Slowing the Progression of Myopia. J Clin Med 2018;7:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.1. Chen Z, Zhou J, Xue F, et al. Two-Year Add-on Effect of Using Low Concentration Atropine in Poor Responders of Orthokeratology in Myopic Children. Br J Ophthalmol 2021;March 13:pre-print before publication: 10.1136/bjophthalmol-2020-317980. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Mutti DO, Jones-Jordan L, Walline JJ. Bifocal & Atropine in Myopia Study: Baseline Data and Methods. Optom Vis Sci 2019;96:335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walline JJ, Gaume Giannoni A, Sinnott LT, et al. A Randomized Trial of Soft Multifocal Contact Lenses for Myopia Control: Baseline Data and Methods. Optom Vis Sci 2017;94:856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thibos LN, Wheeler W, Horner D. Power Vectors: An Application of Fourier Analysis to the Description and Statistical Analysis of Refractive Error. Optom Vis Sci 1997;74:367–75. [DOI] [PubMed] [Google Scholar]
- 31.Glynn R, Rosner B. Regression Methods When the Eye Is the Unit of Analysis. Ophthalmic Epidemiol 2012;19:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charman WN, Radhakrishnan H. Peripheral Refraction and the Development of Refractive Error: A Review. Ophthalmic Physiol Opt 2010;30:321–38. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Wildsoet C. The Effect of Two-Zone Concentric Bifocal Spectacle Lenses on Refractive Error Development and Eye Growth in Young Chicks. Invest Ophthalmol Vis Sci 2011;52:1078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith EL 3rd, Hung LF, Huang J. Relative Peripheral Hyperopic Defocus Alters Central Refractive Development in Infant Monkeys. Vision Res 2009;49:2386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith EL 3rd, Kee CS, Ramamirtham R, et al. Peripheral Vision Can Influence Eye Growth and Refractive Development in Infant Monkeys. Invest Ophthalmol Vis Sci 2005;46:3965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tepelus TC, Vazquez D, Seidemann A, et al. Effects of Lenses with Different Power Profiles on Eye Shape in Chickens. Vision Res 2012;54:12–9. [DOI] [PubMed] [Google Scholar]


