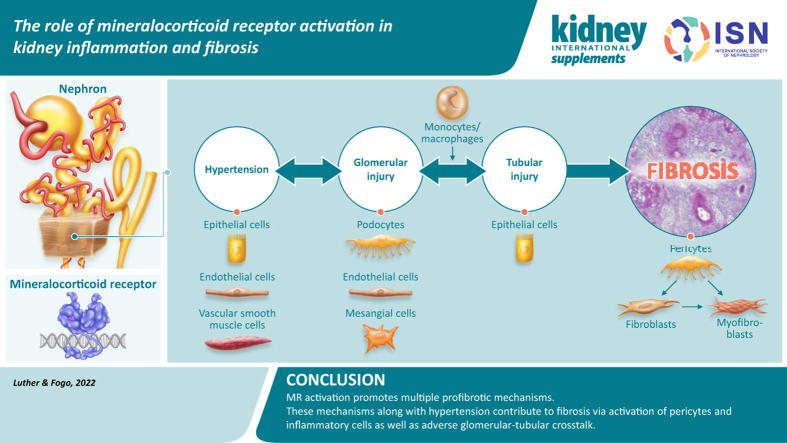
Abstract
Chronic kidney disease is characterized by progressive scarring that results in loss of normal tissue in the kidney and eventually end-stage kidney disease. Interstitial fibrosis and tubular atrophy have been most closely correlated with decline in renal function. Potential mechanisms include profibrotic changes in tubules, influx of profibrotic rather than healing reparative macrophages, and an increase in activated myofibroblasts. Aldosterone activates the mineralocorticoid receptor in the collecting duct to increase sodium reabsorption, resulting in increased blood pressure. Aldosterone also promotes inflammation and fibrosis in the kidney by activating the mineralocorticoid receptor in other cellular compartments, including podocytes, mesangial cells, epithelial cells, and myeloid cells. Aldosterone also may act indirectly by stimulating factors in epithelial tissues that contribute to inflammatory macrophage polarization, myofibroblast differentiation, and progressive fibrosis. This review discusses the potential mechanisms by which aldosterone and mineralocorticoid receptor activation promotes inflammation and fibrosis via nonclassical pathways in the kidney.
Keywords: aldosterone, chronic kidney disease, end-stage kidney disease, fibrosis, inflammation, mineralocorticoid receptor
Graphical abstract
Chronic kidney disease (CKD) is characterized by progressive scarring that results in loss of normal tissue in the kidney and eventually end-stage kidney disease.1 Interstitial fibrosis and tubular atrophy have been correlated most closely with decline in renal function.2 This finding may reflect the fact that interstitial fibrosis and tubular atrophy involves a larger number of nephrons than the small number of glomeruli present in a kidney biopsy sample. Glomerular scarring, resulting in downstream injury to tubules with subsequent atrophy of tubules and interstitial fibrosis, is well recognized. Understanding the elements that drive progressive interstitial fibrosis and tubular atrophy is key to targeting and modulating ongoing progressive kidney disease.2 Potential mechanisms include profibrotic changes in tubular cells, influx of profibrotic rather than healing reparative macrophages, and an increase in activated myofibroblasts.3,4
Injury to proximal tubular cells can result in either healing and return to a normal state or ongoing progression from acute kidney injury (AKI) to CKD. Single-cell RNA sequencing studies have shown that fibroblasts in the kidney cortex after acute injury return to a normal quiescent state, in contrast to the medulla, where these cells maintain an activated profibrotic myofibroblast–like state.5,6 Thus, these proximal tubular cells fail to repair. These cells signal to endothelium, leukocytes, and fibroblasts, via mechanisms including platelet-derived growth factor receptors.4,6 The candidates that form the myofibroblast-like cells include circulating cells, or tubular epithelial cells having undergone so-called epithelial–mesenchymal transition, fibroblasts, and pericytes. Elegant lineage-tracing studies demonstrated that tubules do not enter the interstitium to become myofibroblasts. Circulating cells also do not contribute to kidney myofibroblasts.7 In recent, elegant, single-cell RNA sequencing studies, human kidney specimens and mouse experimental models were investigated in depth.7 To increase resolution and understanding of pathways activated in different cell types, cells were sorted into nonproximal versus proximal tubular cells. Mapping from this approach showed distinct cell populations in patients with hypertension-associated nephrosclerosis. Potential myofibroblast-like cells were studied further by honing in on the cells that highly expressed collagens, glycoproteins, and proteoglycans. These cells appeared to derive from pericytes and fibroblasts and were positive for platelet-derived growth factor receptors alpha and beta.7
How, then, do these events in the tubules affect glomeruli? Glomerular scarring causing downstream injury, either by toxic effects of proteinuria or tubular/interstitial ischemia resulting from scarred glomeruli, is well recognized as promoting tubulointerstitial fibrosis.4 More recently, we and others have shown that tubules also can have a deleterious effect to sensitize glomeruli to additional injuries. We developed an experimental model in which tubular injury was followed by podocyte-specific injury. Those mice with preceding recovered AKI with resulting very mild tubulointerstitial fibrosis had marked enhancement of glomerular injury after the second hit, compared with mice without AKI.4 Limiting injury by stabilizing hypoxia-inducible factor ameliorated this adverse crosstalk.8 One possibility is that aldosterone antagonism could similarly decrease adverse tubule–glomerular crosstalk, based on mechanisms of action discussed below.
Possible mediators of this effect include direct effects of interaction via the tubules to parietal epithelial cells, which may migrate to the glomerular tuft, either replacing injured podocytes or becoming profibrotic and contributing to glomerulosclerosis. Because of this cellular migration from one compartment to another, signaling in one compartment can contribute to fibrosis in another, and interventions that target only one aspect of kidney injury (e.g., macrophage infiltration) may not adequately prevent progressive kidney fibrosis.
Potential pathogenic roles of aldosterone and the mineralocorticoid receptor
Mineralocorticoid receptor (MR) antagonists (MRAs) reduce kidney inflammation and fibrosis, and recent studies using tissue-specific MR deletion demonstrate that the beneficial effects are mediated via actions in multiple cell types and that many effects are blood pressure–independent. Nakamura et al.9 detailed the specific actions of the MR in myeloid cells, vascular smooth muscle, podocytes, mesangial cells, and fibroblasts, which may mediate these effects on kidney inflammation and fibrosis. Therefore, MR antagonism may protect against glomerular, tubule, and interstitial injury in part via direct actions in multiple cell types in these tissue compartments.9 Aldosterone and MR activation in one tissue may stimulate inflammatory cytokines or growth factors in this tissue. These cytokines and growth factors may then reach other compartments to promote or alleviate inflammatory or profibrotic pathways.10 Further investigations could determine whether aldosterone contributes to myofibroblast differentiation directly via MR activation or indirectly via activation of platelet-derived growth factor receptor beta by epithelium-derived factors.7,11
Aldosterone stimulates inflammation and fibrosis
Classically, aldosterone acts on the MR in principal cells of the cortical collecting duct (CCD), which stimulate sodium reabsorption and potassium excretion via the epithelial sodium channel (Figure 1). This process is essential for maintenance of circulating plasma volume and survival in states of low-sodium diet but becomes maladaptive in the setting of chronic excess sodium intake.9,12 Our current understanding of how aldosterone, even within normal limits, contributes to development of hypertension remains in flux. Hypertension due to primary aldosteronism is clearly detected in only a small minority of patients with the disease, because of low screening rates.13, 14, 15 Even with relatively normal circulating aldosterone concentrations, inappropriate aldosterone signaling in the presence of excess sodium promotes hypertension, which may contribute directly to glomerular injury and kidney fibrosis.13,16
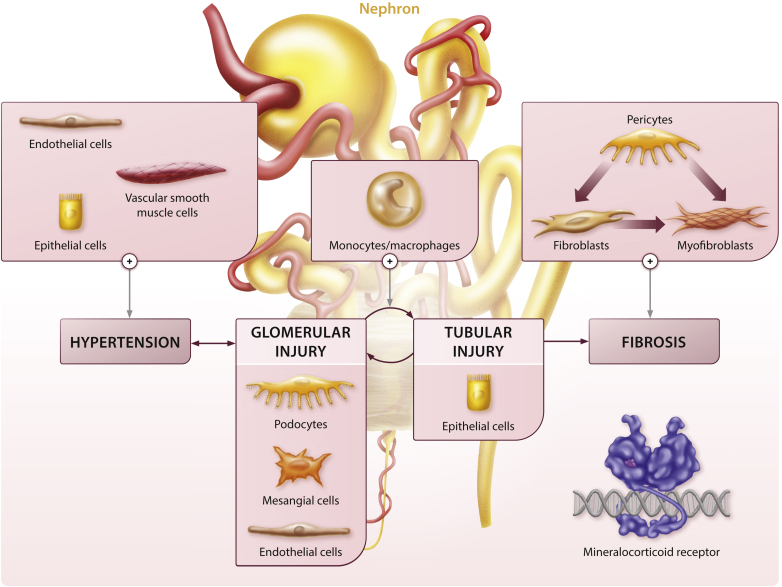
Figure 1.
Potential adverse effects of mineralocorticoid receptor (MR) signaling on inflammation and kidney fibrosis. MR activation in vascular smooth muscle, vascular endothelium, and tubular epithelial cells contributes to glomerular injury via hypertension. In addition, MR activation in mesangial cells and podocytes increases plasminogen activator inhibitor-1 (PAI-1), transforming growth factor-β (TGF-β), nuclear factor (NF)-κB, and interleukin (IL)-6, contributing to glomerular injury. Glomerular injury leads to downstream tubular injury, and conversely, tubular injury can sensitize glomeruli to further injury, thus completing an adverse tubular–glomerular crosstalk cycle. Epithelial cell MR activation contributes to hypertension via increased serum/glucocorticoid regulated kinase 1 (SGK1), epithelial sodium channel (ENaC), and sodium reabsorption, and it promotes inflammation and fibrosis by upregulating PAI-1, NF-κB, and IL-6. Direct MR activation within monocytes/macrophages also promotes M1 macrophage polarization to the M2 phenotype, which enhances fibrosis. Tubular and glomerular factors and direct MR activation may also promote kidney fibrosis via myofibroblast differentiation from fibroblasts and pericytes.
Models of hypertension typically provoke cardiovascular and renal injury via excess endogenous angiotensin II (Ang II) or Ang II administration, which produces hypertension not only via actions on vascular angiotensin type 1 receptors, but also by stimulating aldosterone production.17 A substantial proportion of this injury, including cardiac, kidney, and vascular injury, is attributable to aldosterone and MR activation, which can be prevented via adrenalectomy, aldosterone synthase inhibition, MR antagonism, or a combination of these interventions.18, 19, 20 Aldosterone administration, even in the absence of Ang II, produces glomerular injury, interstitial inflammation, and progressive fibrosis in the kidney,21,22 clearly demonstrating that aldosterone-dependent MR activation contributes significantly to this process. Other steroids, such as cortisol and corticosterone, could activate the MR and contribute to tissue injury, whereas their 11-ketosteroid metabolites (cortisone and 11-dehydrocorticosterone 11β-hydroxysteroid dehydrogenase products) can blunt the effect of mineralocorticoids.23,24 Exogenous glucocorticoids also can contribute to MR-mediated injury, but steroid-induced complications, such as diabetes and hypertension, contribute as well. Studies using specific aldosterone synthase inhibitors or aldosterone synthase–deficient mice demonstrate a specific role for aldosterone in inflammation and fibrosis.18, 19, 20 For example, aldosterone synthase inhibition prevents Ang II–induced kidney injury and inflammation, as assessed by albuminuria, monocyte/macrophage infiltration, and interstitial type IV collagen deposition.18 More recently, endogenous nonsteroidal ligands have been shown to activate the MR and contribute to podocyte injury. The Rac1-MR pathway is activated by hyperglycemia and salt loading, independent of aldosterone, and contributes to podocyte injury.25,26 Activation of the MR in many tissues characteristically increases multiple pathways, which either contribute to inflammation and fibrosis locally (e.g., plasminogen activator inhibitor-1 [PAI-1], transforming growth factor [TGF]-β, interleukin [IL]-6, monocyte chemoattractant protein-1 [MCP-1]) or signal to other cells, such as myeloid cells. These myeloid cells can further augment this injurious cascade by releasing profibrotic molecules or promoting chemotaxis of other inflammatory cells.27, 28, 29
PAI-1 and TGF-β as potential mediators of aldosterone-induced fibrosis
One well-characterized mediator of fibrosis is PAI-1, which directly inhibits tissue plasminogen activator and urokinase. Aldosterone stimulates PAI-1 expression via MR activation in multiple tissues, including in the heart, kidney, and aorta.28 Increased PAI-1 expression contributes to progressive kidney injury by inhibiting plasmin activation, which results in decreased matrix metalloproteinase activation and increased extracellular matrix accumulation and fibrosis.28,29 PAI-1 may also affect fibrosis via vitronectin-binding mechanisms.30 In the kidney, aldosterone stimulates PAI-1 expression via the MR in podocytes, macrophages, mesangial cells, and tubular epithelial cells.29,31 Interventions that prevent kidney injury by MR blockade are typically accompanied by decreased PAI-1 expression, and genetic deletion or pharmacologic inhibition of PAI-1 prevents cardiac and interstitial fibrosis.29 Tubule-specific deletion of PAI-1 also protects against tubulointerstitial fibrosis in the unilateral ureteral obstruction model.32 Although PAI-1 is traditionally viewed as an antifibrinolytic factor, it may also affect the release of other growth factors, such as fibroblast growth factor 23, although its clinical significance remains unclear.33 TGF-β1 promotes fibrosis by stimulating cell transformation to fibroblasts, acting synergistically with aldosterone to increase PAI-1 expression and decrease matrix metalloproteinases.31 The effects of TGF-β1 on kidney fibrosis are complex, such that simply eliminating TGF-β1 signaling with a global or proximal tubule TGF-β1–type 2 receptor knockout worsens interstitial fibrosis.34, 35, 36 This effect may be caused by resulting increased inflammation. Similarly, a T cell–specific TGF-β1–type 2 receptor knockout produced increased interstitial fibrosis at baseline, but additional treatment with aldosterone did not worsen this profibrotic effect.37 These findings suggest that additional strategies to not only block these key profibrotic mediators but also decrease adverse inflammatory infiltrates could be beneficial in preventing or ameliorating scarring.
Despite the success with pharmacologic PAI-1 inhibitors, clinical benefits have proven difficult to achieve because of issues with selectivity and toxicity.30 Likewise, inhibition of TGF-β1 has proven difficult because of its diverse actions in multiple tissues.1 MRAs provide an attractive option for preventing the pathologic effects of aldosterone on PAI-1 and TGF-β1, and beneficial effects of MRAs are likely mediated in part by these actions.
MR activation stimulates inflammatory pathways in the glomerulus and epithelium
Aldosterone administration stimulates PAI-1, TGF-β1, nuclear factor (NF)-κB, and IL-6 in glomeruli and in cultured mesangial cells via MR activation.12,31 In addition to sodium transporters, aldosterone stimulates proinflammatory mediators in CCD epithelial cells, such as the nuclear transcription factor NF-κB, and these responses are blocked by spironolactone.12 Other downstream inflammatory markers, including IL-6, MCP-1, and PAI-1, were similarly increased.12 These cytokines may promote fibrosis by recruiting inflammatory cells or promoting M1 macrophage polarization.3,29
MR activation promotes macrophage polarization and kidney inflammation
Macrophages may be classically activated in vitro to an M1 macrophage phenotype by interferon-γ or lipopolysaccharide, which promotes ongoing inflammation.3 Alternative activation to an M2 macrophage phenotype by IL-4, IL-13, and IL-33 can promote resolution of inflammation.3 Macrophage infiltration and polarization in AKI is characterized by an early proinflammatory M1 phenotype during a period of tubular injury and apoptosis, transitioning to an alternatively activated M2 phenotype during the reparative tubular injury phase.38,39 Secreted factors from kidney epithelial cells influence macrophage polarization independently of the typical IL-4Rα–signal transducer and activator of transcription 6 (STAT6) signaling pathway, in part via the granulocyte-macrophage colony-stimulating factor and activation of signal transducer and activator of transcription 5 (STAT5).38 Polarization to M2 macrophages is hypothesized to reduce the severity of acute injury and the risk of progressive kidney disease, although persistence of M2 macrophages in the setting of incomplete recovery may be maladaptive and contribute to ongoing fibrosis.40 A better understanding of these secreted factors, inflammatory cell responses, and progression of fibrosis is essential for developing treatments to target these pathways. Ongoing clinical studies that affect macrophage polarization by altering IL-6, IL-10, TGF-β, and connective tissue growth factor (a downstream mediator of TGF-β actions) pathways will help define whether this approach can prevent CKD progression.1,27,38
Targeting the MR may also reduce progressive kidney fibrosis and CKD by changing the balance of inflammatory cells, including M1/M2 macrophages, either directly in macrophages or via altering epithelial cell crosstalk.41 Myeloid cell–specific MR is essential for M1 polarization in vitro, and myeloid-specific MR knockout reduces cardiac interstitial and perivascular fibrosis.42 Similarly, myeloid-specific MR knockout increased M2 polarization and reduced kidney fibrosis in an ischemic AKI model.9,27 Administration of aldosterone plus a high-sodium diet for 3 weeks increased protein expression of M1 phenotype markers (inducible nitric oxide synthase and interferon-γ) in rat kidneys, and this effect was prevented by spironolactone.10 Treatment with clodronate reduced M1, but not M2, kidney macrophage markers, and partially reduced tubulointerstitial fibrosis.10 Spironolactone and other MRAs reduce inflammatory cytokine production in animal models and humans, which may provide a portion of the antifibrotic effects in the kidney.10 In a genetic model of Ang II excess, aldosterone synthase inhibition or adrenalectomy reduced kidney injury and macrophage/monocyte cell infiltration to the same extent as losartan treatment, demonstrating the essential role of aldosterone in macrophage infiltration in kidney injury.18 The nonsteroidal MRA finerenone also reduces glomerular MCP-1 and PAI-1 gene expression and macrophage (F4/80+ cells) infiltration in a db/db mouse diabetic model exacerbated by uninephrectomy and a high-sodium diet.26
Clinical benefits of MR antagonism on progressive kidney disease
Although MR antagonism with spironolactone is known to effectively treat heart failure, resistant hypertension, and proteinuria,43, 44, 45 no randomized trials had demonstrated a benefit on hard kidney outcomes, such as risk of progression to dialysis, until recent studies with the nonsteroidal MRA finerenone. Consistent with results during treatment with spironolactone, the initial finerenone phase 2b Mineralocorticoid Receptor Antagonist Tolerability Study in Diabetic Nephropathy (ARTS-DN) study demonstrated a dose-related decrease in albuminuria in participants with diabetic nephropathy who were already on an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.46 The follow-up Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) study demonstrated for the first time that an MRA decreases the risk of diabetic CKD progression, as determined by kidney failure, at least 40% decreased estimated glomerular filtration rate, or death from renal causes (hazard ratio 0.82; 95% confidence interval 0.73–0.93; P = 0.001), with an incidence of 17.8% with finerenone versus 21.1% with placebo over a median follow-up of 2.6 years.47 Finerenone carries a modest risk of hyperkalemia, compared to that found in historical studies of other MRAs. Of note, these studies were designed and conducted prior to the demonstration of sodium–glucose co-transporter-2 (SGLT-2) inhibitor effectiveness for diabetic nephropathy, and the kidney benefits of this combination are undetermined. Secondary analyses of the Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients with Chronic Heart Failure (DAPA-HF) in the subset of 3370 participants also taking an MRA demonstrates additional benefit of this combination on heart failure outcomes.48,49 The additive benefits of these 2 interventions on top of renin–angiotensin system inhibition are of note. If similar results are confirmed in CKD, this could suggest that complementary mechanisms account for the benefits of these multipronged interventions.
The mechanisms by which MR antagonism prevents progressive kidney disease extend beyond blood pressure reduction, and they likely involve decreased profibrotic and inflammatory mediators. These actions involve MR antagonism extending beyond the collecting duct across multiple cell types in the kidney, and actions in one tissue may alter the fibrotic response in an adjacent compartment. Thus, improvement of injury responses in the tubular compartment is postulated to contribute to decreased adverse crosstalk to glomeruli, thus further contributing to decreased scarring. Additional anti-inflammatory effects beyond classic sodium–potassium handling are likely also important elements of efficacy in treating progressive kidney scarring. Better understanding of these mechanisms should guide the development of additional therapies to preserve residual kidney function.26,41
Disclosure
This article is published as part of a supplement sponsored by Bayer AG.
JML serves on the advisory board for Mineralys. ABF declared no competing interests. JML and ABF received no personal funding for this article.
Acknowledgments
Development of this article was funded by an unrestricted educational grant from Bayer AG. The authors acknowledge Nathalie Lawrence and Jo Luscombe, PhD, of Chameleon Communications International, who provided editorial assistance with funding via an unrestricted educational grant from Bayer AG. The authors also acknowledge Alexander Roeder, Ronny Guenther, Katja Marx, and Josephin Schoenrich, of CAST PHARMA, who designed the figure with funding from Bayer AG.
References
- 1.Meng X.M., Nikolic-Paterson D.J., Lan H.Y. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 2.Farris A.B., Colvin R.B. Renal interstitial fibrosis: mechanisms and evaluation. Curr Opin Nephrol Hypertens. 2012;21:289–300. doi: 10.1097/MNH.0b013e3283521cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H., Fessler M.B., Qu P., et al. Macrophage polarization in innate immune responses contributing to pathogenesis of chronic kidney disease. BMC Nephrol. 2020;21:270. doi: 10.1186/s12882-020-01921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim B.J., Yang J.W., Zou J., et al. Tubulointerstitial fibrosis can sensitize the kidney to subsequent glomerular injury. Kidney Int. 2017;92:1395–1403. doi: 10.1016/j.kint.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu S.M., Bonventre J.V. Acute kidney injury and maladaptive tubular repair leading to renal fibrosis. Curr Opin Nephrol Hypertens. 2020;29:310–318. doi: 10.1097/MNH.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirita Y., Wu H., Uchimura K., et al. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci USA. 2020;117:15874–15883. doi: 10.1073/pnas.2005477117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuppe C., Ibrahim M.M., Kranz J., et al. Decoding myofibroblast origins in human kidney fibrosis. Nature. 2021;589:281–286. doi: 10.1038/s41586-020-2941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou J., Yang J., Zhu X., et al. Stabilization of hypoxia-inducible factor ameliorates glomerular injury sensitization after tubulointerstitial injury. Kidney Int. 2021;99:620–631. doi: 10.1016/j.kint.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, Girerd S, Jaisser F, Barrera-Chimal J. Nonepithelial mineralocorticoid receptor activation as a determinant of kidney disease. Kidney Int Suppl. 2022;12:12–18. [DOI] [PMC free article] [PubMed]
- 10.Martin-Fernandez B., Rubio-Navarro A., Cortegano I., et al. Aldosterone induces renal fibrosis and inflammatory m1-macrophage subtype via mineralocorticoid receptor in rats. PloS One. 2016;11 doi: 10.1371/journal.pone.0145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koszegi S., Molnar A., Lenart L., et al. RAAS inhibitors directly reduce diabetes-induced renal fibrosis via growth factor inhibition. J Physiol. 2019;597:193–209. doi: 10.1113/JP277002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leroy V., De Seigneux S., Agassiz V., et al. Aldosterone activates NF-kappaB in the collecting duct. J Am Soc Nephrol. 2009;20:131–144. doi: 10.1681/ASN.2008020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown J.M., Robinson-Cohen C., Luque-Fernandez M.A., et al. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann Intern Med. 2017;167:630–641. doi: 10.7326/M17-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J.B., Cohen D.L., Herman D.S., et al. Testing for primary aldosteronism and mineralocorticoid receptor antagonist use among U.S. veterans: a retrospective cohort study. Ann Intern Med. 2021;174:289–297. doi: 10.7326/M20-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe G., Gray Z., Krishnan G., et al. Screening rates for primary aldosteronism in resistant hypertension: a cohort study. Hypertension. 2020;75:650–659. doi: 10.1161/HYPERTENSIONAHA.119.14359. [DOI] [PubMed] [Google Scholar]
- 16.Toshiro F. Mineralocorticoid receptors, salt-sensitive hypertension, and metabolic syndrome. Hypertension. 2010;55:813–818. doi: 10.1161/HYPERTENSIONAHA.109.149062. [DOI] [PubMed] [Google Scholar]
- 17.Forrester S.J., Booz G.W., Sigmund C.D., et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiebeler A., Nussberger J., Shagdarsuren E., et al. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation. 2005;111:3087–3094. doi: 10.1161/CIRCULATIONAHA.104.521625. [DOI] [PubMed] [Google Scholar]
- 19.Lea W.B., Kwak E.S., Luther J.M., et al. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int. 2009;75:936–944. doi: 10.1038/ki.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luther J.M., Luo P., Wang Z., et al. Aldosterone deficiency and mineralocorticoid receptor antagonism prevent angiotensin II-induced cardiac, renal, and vascular injury. Kidney Int. 2012;82:643–651. doi: 10.1038/ki.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha R., Stier C.T., Jr., Kifor I., et al. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- 22.Blasi E.R., Rocha R., Rudolph A.E., et al. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 23.Brem A.S., Morris D.J., Gong R. Aldosterone-induced fibrosis in the kidney: questions and controversies. Am J Kidney Dis. 2011;58:471–479. doi: 10.1053/j.ajkd.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brem A.S., Gong R. Therapeutic targeting of aldosterone: a novel approach to the treatment of glomerular disease. Clin Sci (Lond) 2015;128:527–535. doi: 10.1042/CS20140432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata S., Nagase M., Yoshida S., et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–1376. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 26.Hirohama D., Nishimoto M., Ayuzawa N., et al. Activation of Rac1-mineralocorticoid receptor pathway contributes to renal injury in salt-loaded db/db mice. Hypertension. 2021;78:82–93. doi: 10.1161/HYPERTENSIONAHA.121.17263. [DOI] [PubMed] [Google Scholar]
- 27.Barrera-Chimal J., Estrela G.R., Lechner S.M., et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018;93:1344–1355. doi: 10.1016/j.kint.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Brown N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9:459–469. doi: 10.1038/nrneph.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J., Weisberg A., Griffin J.P., et al. Plasminogen activator inhibitor-1 deficiency protects against aldosterone-induced glomerular injury. Kidney Int. 2006;69:1064–1072. doi: 10.1038/sj.ki.5000201. [DOI] [PubMed] [Google Scholar]
- 30.Sillen M., Declerck P.J. Targeting PAI-1 in cardiovascular disease: structural insights into PAI-1 functionality and inhibition. Front Cardiovasc Med. 2020;7:622473. doi: 10.3389/fcvm.2020.622473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W., Xu C., Kahng K.W., et al. Aldosterone and TGF-beta1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am J Physiol Renal Physiol. 2008;294:F1287–F1295. doi: 10.1152/ajprenal.00017.2008. [DOI] [PubMed] [Google Scholar]
- 32.Yao L., Wright M.F., Farmer B.C., et al. Fibroblast-specific plasminogen activator inhibitor-1 depletion ameliorates renal interstitial fibrosis after unilateral ureteral obstruction. Nephrol Dial Transplant. 2019;34:2042–2050. doi: 10.1093/ndt/gfz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eren M., Place A.T., Thomas P.M., et al. PAI-1 is a critical regulator of FGF23 homeostasis. Sci Adv. 2017;3 doi: 10.1126/sciadv.1603259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung S., Overstreet J.M., Li Y., et al. TGF-β promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight. 2018;3 doi: 10.1172/jci.insight.123563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorelik L., Flavell R.A. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 36.Nlandu-Khodo S., Neelisetty S., Phillips M., et al. Blocking TGF-beta and beta-catenin epithelial crosstalk exacerbates CKD. J Am Soc Nephrol. 2017;28:3490–3503. doi: 10.1681/ASN.2016121351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreier B., Rabe S., Schneider B., et al. Aldosterone/NaCl-induced renal and cardiac fibrosis is modulated by TGF-beta responsiveness of T cells. Hypertens Res. 2011;34:623–629. doi: 10.1038/hr.2011.16. [DOI] [PubMed] [Google Scholar]
- 38.Huen S.C., Huynh L., Marlier A., et al. GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. J Am Soc Nephrol. 2015;26:1334–1345. doi: 10.1681/ASN.2014060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S., Huen S., Nishio H., et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki K., Terker A.S., Pan Y., et al. Deletion of myeloid interferon regulatory factor 4 (irf4) in mouse model protects against kidney fibrosis after ischemic injury by decreased macrophage recruitment and activation. J Am Soc Nephrol. 2021;32:1037–1052. doi: 10.1681/ASN.2020071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel V., Joharapurkar A., Jain M. Role of mineralocorticoid receptor antagonists in kidney diseases. Drug Dev Res. 2021;82:341–363. doi: 10.1002/ddr.21760. [DOI] [PubMed] [Google Scholar]
- 42.Usher M.G., Duan S.Z., Ivaschenko C.Y., et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams B., MacDonald T.M., Morant S.V., Brown M.J. Pathway-2: spironolactone for resistant hypertension—authors' reply. Lancet. 2016;387:1373–1374. doi: 10.1016/S0140-6736(16)00698-X. [DOI] [PubMed] [Google Scholar]
- 44.Currie G., Taylor A.H., Fujita T., et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 2016;17:127. doi: 10.1186/s12882-016-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 46.Bakris G.L., Agarwal R., Chan J.C., et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884–894. doi: 10.1001/jama.2015.10081. [DOI] [PubMed] [Google Scholar]
- 47.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 48.Rossing P., Persson F., Frimodt-Møller M., Hansen T.W. Linking kidney and cardiovascular complications in diabetes-impact on prognostication and treatment: the 2019 Edwin Bierman Award Lecture. Diabetes. 2021;70:39–50. doi: 10.2337/dbi19-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen L., Kristensen S.L., Bengtsson O., et al. Dapagliflozin in HFrEF patients treated with mineralocorticoid receptor antagonists: an analysis of DAPA-HF. JACC Heart Fail. 2021;9:254–264. doi: 10.1016/j.jchf.2020.11.009. [DOI] [PubMed] [Google Scholar]