Abstract
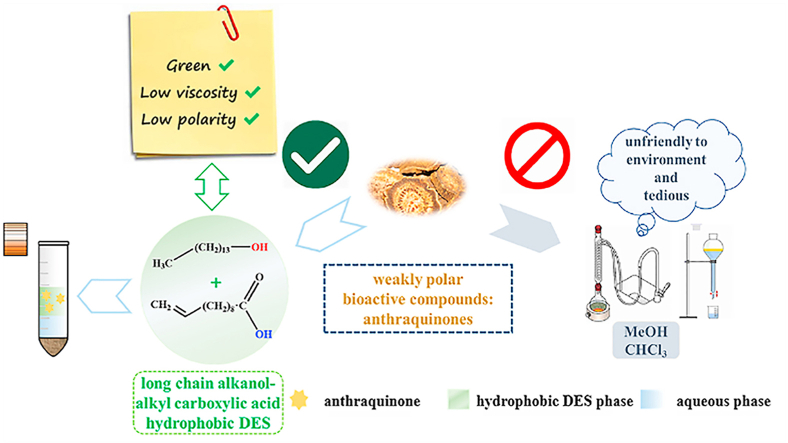
Natural long-chain alkanol and alkyl carboxylic acid were used to prepare novel hydrophobic deep eutectic solvents (HDESs). These HDESs are liquid at room temperature and have low viscosity (<12.26 mPa‧s), low polarity (lower than that of methanol, ChCl-based deep eutectic solvents and other reported HDESs), and low density (<0.928 g/mL). A simple one-pot method based on a novel HDES–water two-phase extraction system was constructed for the extraction of weak-polarity bioactive components, anthraquinones, from Rhei Radix et Rhizoma. This HDES-based new extraction method does not consume hazardous organic solvents and can obtain a total anthraquinone yield of 21.52 mg/g, which is close to that obtained by the Chinese pharmacopoeia method (21.22 mg/g) and considerably higher than those by other reported HDESs-based extraction methods (14.20–20.09 mg/g, p < 0.01). The high extraction yield can be mainly attributed to the severe destruction of the RRR cell walls by the extraction system and the excellent dissolving ability of novel HDESs for anthraquinones.
Keywords: Hydrophobic deep eutectic solvent, Long-chain alkanol, Long-chain alkyl carboxylic acid, One-pot extraction, Anthraquinone
Graphical abstract
Highlights
-
•
Novel long-chain alkanol-alkyl carboxylic acid-based hydrophobic DESs were designed.
-
•
These DESs show the feature of low-viscosity and low-polarity.
-
•
A one-pot extraction method based on DES-water two-phase system was newly established.
-
•
This method can efficiently extract anthraquinones from Rhei Radix et Rhizoma.
-
•
No organic solvents were consumed in this method.
1. Introduction
Bioactive compounds derived from plants have become an essential resource of pharmaceutical industries. Currently, approximately 60% of approved anticancer drugs originate from natural products [1]. The latest research revealed the application potential of the bioactive ingredients such as saikosaponins, cryptotanshinone, and kaempferol in traditional Chinese medicines to prevent and treat SARS-CoV-2 [2,3]. In addition, bioactive components have found applications in food and cosmetics industries as effective ingredients in functional foods and skin care products [4,5]. Thus, acquiring bioactive compounds efficiently is important. Solid–liquid extraction (SLE) is a fundamental technology for extracting and isolating bioactive compounds from medical plants. However, traditional SLE (e.g., reflux, maceration, and Soxhlet extraction) are usually tedious and consume a large amount of hazardous organic solvents such as dichloromethane, chloroform, and acetone, especially when extracting weakly polar compounds [[6], [7], [8]]. In the past decades, green extraction technologies, including supercritical fluid extraction, ultrasound-assisted extraction and microwave-assisted extraction [[9], [10], [11]], have been developed and applied for the extraction of bioactive components. These technologies can reduce, but still cannot avoid, the consumption of hazardous organic solvents [12,13]. The use of green solvents as extractants is a feasible and promising option for the extraction of weakly polar bioactive components.
Deep eutectic solvents (DESs) formed by hydrogen-bond donors (HBDs) and hydrogen-bond acceptors (HBAs) have emerged as attractive green solvents, and they have been extensively applied in the extraction of bioactive ingredients, such as flavonoids, phenolic acids and polysaccharides [[14], [15], [16]]. DESs not only possess some ionic liquid-like excellent characteristics such as low volatility, non-flammability and designability, but also have additional advantages of cheap raw materials and simple synthesis. However, most DESs reported so far have high viscosity and strong polarity [17,18]. The former may reduce the extraction efficiency of bioactive compounds because of low diffusivity, and the latter limits the application of DESs in the extraction of weakly polar compounds [[19], [20], [21]]. Although adding water to DESs can decrease the viscosity of DESs, the DES–water system increases polarity in the case of extracting weakly polar compounds [22]. Undoubtedly, low-viscosity hydrophobic DESs (HDESs) are advantageous for extracting weakly polar compounds.
Since 2015, several HDESs have been reported, mainly including menthol-based neutral HDESs [23,24] and charged HDESs based on long-chain quaternary ammonium salts (LCQASs) [24,25]. Menthol-based neutral HDESs have merits including low toxicity, high biodegradability and low viscosity [23,24]; thus, they have been widely applied to extract bioactive components from various plants [20,26,27]. Charged HDESs based on LCQASs have also been used to extract phytochemicals [[28], [29], [30]]. However, because LCQASs are not easily degraded and some of them, such as methyltrioctylammonium chloride, are toxic [20,31], charged HDESs still pose a threat to human health. By contrast, the high viscosity of charged HDESs, which is attributed to Coulombic forces between charge centers, may lead to their unsatisfactory performance in the extraction of phytochemicals [24]. Clearly, neutral HDESs are superior to charged HDESs; therefore, it is necessary to develop novel low-viscosity neutral HDESs and study their applications in the extraction of weakly polar bioactive compounds from plants.
Rhei Radix et Rhizoma (RRR), which belongs to the Polygonaceae family, is a long-established medicinal herb used in China. Hydrophobic anthraquinones (AQs), including aloe-emodin, rhein, emodin, chrysophanol, and physcion, are the predominant constituents of RRR, and they possess antifungal, antioxidant, anticancer and immune modulatory activities [32]. A well-established method for extracting total AQs from RRR, which is listed in Chinese Pharmacopoeia, comprises heated-reflux extraction with methanol, followed by acid hydrolysis and liquid–liquid extraction with chloroform [33]. This method has complex processes and requires large amounts of toxic organic solvents. Duan et al. [34] evaluated the efficiency of extracting AQs from RRR with hydrophilic DESs based on choline chloride/betaine/l-proline. Unfortunately, the extraction yield was low owing to the low polarity of AQs. Our group applied a new DES–salt aqueous two-phase extraction method to efficiently extract AQs in RRR [35]. However, hexafluoroisopropanol, one of the components of DES involved, is hazardous and expensive. Hence, a cheap, simple, efficient, and green AQs extraction method is urgently needed.
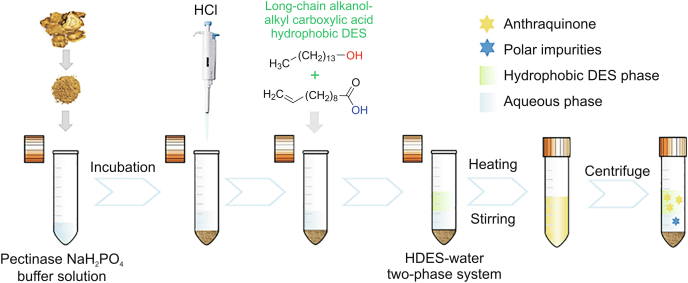
Long-chain alkanol and alkyl carboxylic acid are a family of natural raw materials generated from renewable resources such as fats, oils, plants, or animal waxes [36]. They are cheap and have low toxicity, and long alkyl chains endow them with high hydrophobicity. Accordingly, they are expected to serve as ideal components to prepare cost-effective green HDESs that can easily dissolve and extract low-polarity bioactive components. In this study, a series of novel green neutral HDESs were synthesized using long-chain alkanol as the HBA and long-chain alkyl carboxylic acid as the HBD, and their structures and physicochemical properties were characterized. Subsequently, these novel HDESs were applied to construct a one-pot extraction method based on an HDES–water two-phase system for the extraction of hydrophobic AQs from RRR (the procedure is shown in Fig. 1). The extraction mechanism was also explored.
Fig. 1.
Schematic procedure of long-chain alkanol–alkyl carboxylic acid hydrophobic DESs–water two-phase system for one-pot extraction of weakly polar anthraquinones from Rhei Radix et Rhizoma.
2. Experimental
2.1. Materials
Four RRRs originating from Sichuan Province, Hubei Province, Gansu Province and Qinghai Province of China were purchased from four local drugstores (Wuhan, China). RRR from Sichuan Province was used for optimization experiments. The obtained RRR was first air-dried for one day and cut into small pieces, which were then crushed with a pulverizer. The resulting RRR powder was passed through a 65-mesh sieve, then sealed in a desiccator and stored at room temperature for further use.
Aloe-emodin (≥98.0%), chrysophanol (≥98.0%), emodin (≥99.0%), physcion (≥98.0%), and rhein (≥98.0%) were purchased from Chengdu Must Biotechnology Co., Ltd. (Chengdu, China), and their physicochemical properties are shown in Table S1. 1-Tetradecanol (98.0%), 1-dodecanol (GC, >98.0%), n-decanol (98.0%), decanoic acid (99%), n-octanoic acid (99%), 1,8-dihydroxyanthraquinone (1,8-DHAQ, 97%), and pectinase (30000 U/g) were purchased from Aladdin Chemistry Co. (Shanghai, China). n-Octanol, choline chloride, urea, glycerol, ethylene glycol, and hydrochloric acid were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China). 10-Undecenoic acid (UA, 98.0%) was supplied by Energy Chemical Co. (Shanghai, China). Methanol (CP) was purchased from CINC High Purity Solvents Co., Ltd. (Shanghai, China). Deionized water was obtained from a Milli-Q system (Millipore, Molsheim, France). All the reagents were of analytical grade unless otherwise stated.
2.2. Synthesis and characterization of HDESs
Different kinds of long-chain alkanols (C8, C10, C12, C14) and alkyl carboxylic acids (C8, C10, UA) were used as HBAs and HBDs to synthesize HDESs. Certain amounts of long-chain alkanols and alkyl carboxylic acids were mixed in a glass vial. Then the mixture was heated at 60 °C in a water bath with constant magnetic agitation (500 r/min) until a transparent liquid with freezing temperature (Tf) lower than that of individual components was obtained.
The Tf of HDESs was measured by recording the temperature at which liquid DESs began to solidify (ice bath and thermostatic ethanol bath may be used). The eutectic point of HDESs was determined by plotting their solid–liquid phase diagrams, which were created through measuring the Tf of HDESs formed by different fractions of compositions [37,38]. The 1H nuclear magnetic resonance (NMR) and Fourier transform infrared (FT-IR) spectra were examined by an NMR spectrometer (Bruker DPX-400, Rheinstetten, Germany) and an FT-IR spectrometer (Shimadzu, Tokyo, Japan), respectively. The decomposition temperature of the HDES was measured by thermogravimetric analyzer (TGA, NETZSCH STA449C/3/G, Selb, Germany) with temperature increasing from 25 to 600 °C at a rate of 10 °C/min under nitrogen. The viscosity of HDESs at 25 °C was measured using a rheometer (DISCOVERY HR-2, Milford, MA, USA) with a shear rate of 0.1–1000 s−1. The viscosity-temperature function curve was obtained in the temperature range of 20–85 °C with a shear rate of 100 s−1 at atmospheric pressure. The density of HDESs was determined by drawing 100 μL of the liquid with a micro-syringe at 25 °C, and then weighing the increased mass of syringe (Δm). The density was calculated as ρ = Δm/V. The polarity of the nine HDESs investigated here, other three reported HDESs (i.e., [N8881]Cl–C10 acid, DL-menthol–C12 acid, and C12 acid–C10 acid), conventional hydrophilic DESs (ChCl–urea, ChCl–glycerin, and ChCl–ethanediol), and organic solvents (methanol and n-hexane) were studied using pyrene as the neutral fluorescence probe [39] (see Supplementary data). The hydrophobicity and water stability of the nine HDESs were also studied (see Supplementary data).
2.3. One-pot extraction process of AQs from RRR by HDES–water two-phase system
0.1 g of RRR powder was mixed with 1 mL of NaH2PO4 buffer solution (pH = 4.5) containing 1 mg/mL pectinase, and the mixture was incubated in a thermomixer (MIULAB MTC-100, Hangzhou, China) at a constant temperature (45 °C) and rotation speed of 300 r/min for 120 min [40]. As the enzymatic reaction completed, pectinase was inactivated by heating at 90 °C for 10 min. Then, 385 μL of concentrated HCl was added to make the HCl concentration in the aqueous phase at 10%. Subsequently, C14 alcohol–UA (1:4) HDES was added with a liquid to solid ratio of 12:1 (volume of HDES to mass of RRR, mL/g). The resulting mixture was vortexed vigorously and then placed in a water bath at 67 °C for 20 min under stirring (500 r/min), followed by centrifuging for 10 min at 6,000 r/min. At this moment, the mixture was separated into a liquid–liquid–solid three-phase system, in which AQs were extracted into the top HDES-rich phase and polar impurities were distributed into the middle aqueous phase. The top phase was collected and diluted tenfold with a mobile phase for the quantification analysis of AQs by HPLC-DAD (see Supplementary data).
2.4. Surface morphology characterization of RRR powder
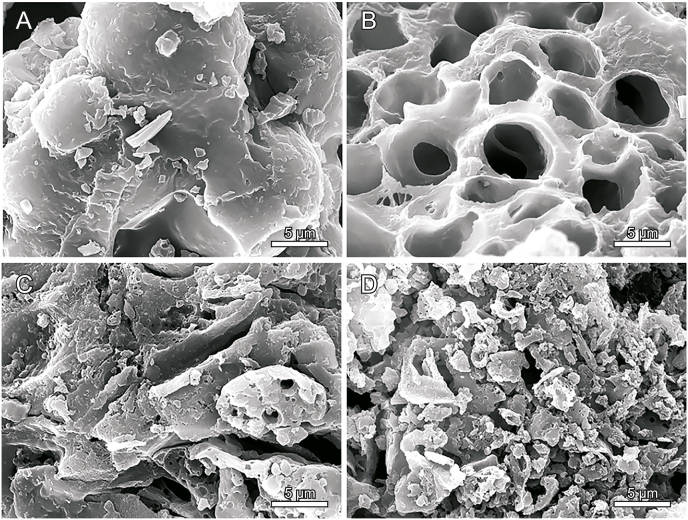
Each RRR sample was treated as follows, respectively: 1) with enzyme only; that is, 0.1 g of RRR powder was incubated with 1 mL of NaH2PO4 buffer solution (pH = 4.5) containing 1 mg/mL pectinase at 45 °C for 120 min; 2) with HDES and HCl; that is, 0.1 g of RRR powder was stirred with the mixture of 1.2 mL of C14 alcohol–UA (1:4) HDES and 1 mL of 10% HCl solution at 67 °C for 20 min; 3) with the whole one-pot extraction process. An untreated RRR sample was set as a control. After freeze-drying, the treated/untreated RRR powder was fixed on a silicon wafer and sputtered with gold. The surface morphology was observed using a scanning electron microscope (SEM; JSM-IT300, Tokyo, Japan), with 1 nm resolution and 5 kV acceleration voltage.
3. Results and discussion
3.1. Synthesis and characterization of HDESs
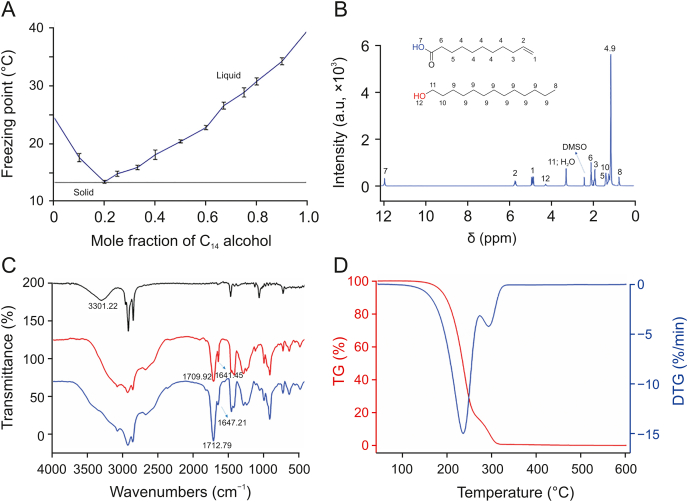
Different long-chain alkanols and alkyl carboxylic acids were mixed with a series of molar ratios to synthesize DESs that are liquid under room temperature. The results (Table S2) showed that the mixtures of C8 alcohol–C8 acid, C10 alcohol–C8 acid/C10 acid/UA, C12 alcohol–C8 acid/C10 acid/UA and C14 alcohol–C8 acid/UA could form HDESs at room temperature. The solid–liquid phase diagrams of the above nine HDESs were studied to obtain the optimal eutectic ratio of stable HDESs. As seen in Fig. 2A (a representative phase diagram of HDESs) and Fig. S1, the freezing points of all HDESs decreased and then increased as the mole fraction of HBA (alkanol) increased. The eutectic ratios and the corresponding freezing points of HDESs are summarized in Table 1. The freezing points of HDESs at the eutectic ratio were all obviously lower than those of their compositions, and the mole fraction of alkanol (or alkyl carboxylic acid) required for reaching the eutectic point gradually decreased as the chain length of alkanol (or alkyl carboxylic acid) increased. The possible reason is the important role of dispersive interactions in HDESs with long alkyl chains [41], which increased with the chain length of a DES component.
Fig. 2.
(A) Solid-liquid phase diagram of C14 alcohol–UA HDES; (B) 1H NMR spectrum (400 MHz, DMSO-d6) of C14 alcohol–UA (1:4) HDES; (C) FT-IR spectra of pure components and C14 alcohol–UA (1:4) HDES, where black line is C14 alcohol, red line is UA and blue line refers to C14 alcohol–UA (1:4) HDES; and (D) red and blue lines represent thermal gravity (TG) and differential thermal gravity (DTG) of C14 alcohol–UA (1:4) HDES, respectively. UA: 10-undecenoic acid.
Table 1.
Compositions and physicochemical properties of long-chain alkanol–alkyl carboxylic acid HDESs and other solvents.
| HDES (HBA–HBD) | Eutectic ratio (HBA:HBD, mol/mol) | Freezing point (oC) |
Viscosity at 25 °C (mPa·s) | Density ± SD (g/mL, n = 6) | Pyrene I1/I3 ± SD (n = 3) | ||
|---|---|---|---|---|---|---|---|
| HBAⅠ | HBDⅠ | DES | |||||
| C14 alcohol–UA | 1:4 | 39.5 | 24.5 | 13.3 ± 0.3 | 8.22 | 0.923 ± 0.027 | 0.883 ± 0.005 |
| C12 alcohol–UA | 1:1 | 24 | 6.5 ± 0.4 | 11.70 | 0.896 ± 0.009 | 0.888 ± 0.001 | |
| C10 alcohol–UA | 2:1 | 6.4 | −13 ± 0.7 | 9.55 | 0.889 ± 0.008 | 0.895 ± 0.004 | |
| C14 alcohol–C8 acid | 1:4 | 39.5 | 16.7 | 6.8 ± 0.4 | 5.89 | 0.928 ± 0.011 | 0.887 ± 0.002 |
| C12 alcohol–C8 acid | 1:2 | 24 | −4.4 ± 0.5 | 8.46 | 0.926 ± 0.008 | 0.893 ± 0.004 | |
| C10 alcohol–C8 acid | 1:1 | 6.4 | −22.3 ± 0.4 | 5.00 | 0.896 ± 0.004 | 0.896 ± 0.002 | |
| C8 alcohol–C8 acid | 3:1 | −16.5 | −34.1 ± 1.3 | 4.03 | 0.896 ± 0.012 | 0.900 ± 0.003 | |
| C12 alcohol–C10 acid | 3:2 | 24 | 31.6 | 9.4 ± 0.6 | 12.26 | 0.890 ± 0.005 | 0.833 ± 0.003 |
| C10 alcohol–C10 acid | 3:1 | 6.4 | −9.7 ± 1.8 | 6.32 | 0.905 ± 0.016 | 0.840 ± 0.002 | |
| ChCl–urea | 1:2 | 303 | 134 | 12 [21] | 214 [43] | 1.1879 [43] | 2.185 ± 0.015 |
| ChCl–glycerin | 1:2 | 17.8 | −40 [21] | 177 [43] | 1.1854 [43] | 1.963 ± 0.002 | |
| ChCl–ethanediol | 1:2 | −12.9 | −66 [21] | 25 [43] | 1.1139 [43] | 1.936 ± 0.015 | |
| ChCl–D-sorbitol | 1:1 | 110–112 | −43.3 [21] | 13736 [43] | 1.2794 [43] | – | |
| ChCl–oxalic acid | 1:1 | 190 | 34 [21] | 89 [43] | 1.2371 [43] | – | |
| ChCl–tartaric acid | 2:1 | 171 | 47 [21] | 66441 [43] | 1.2735 [43] | – | |
| [N8881]Cl–C10 acid | 1:2 | −20 | 31.6 | −0.05 [25] | 783.41 [25] | 0.896 [25] | 1.412 ± 0.026 |
| DL-menthol–C12 acid | 2:1 | 42.5 | 43.2 | 18.39 [23] | 24.42 [23] | 0.896 [23] | 0.968 ± 0.005 |
| C12 acid–C10 acid | 1:2 | 43.2 | 31.6 | 18 [37] | 10.76 [37] | 0.895 [37] | 0.983 ± 0.002 |
| Methanol | – | – | – | – | – | – | 1.437 ± 0.012 |
| n-Hexane | – | – | – | – | – | – | 0.675 ± 0.024 |
ⅠData obtained using SciFinder Scholar from Chemical Abstract Service. [N8881]Cl: methyltrioctylammonium chloride; UA: 10-undecenoic acid.
1H NMR and FT-IR were adopted to clarify the intermolecular interaction in HDESs. The 1H NMR spectra (Fig. 2B and Fig. S2) indicated that all peaks of HDESs belonged to their initial components and no new other peaks appeared, suggesting the absence of any chemical reaction in the preparation of HDESs. The FT-IR spectra of HDESs (Fig. 2C and Fig. S3) revealed that the O–H stretching vibration peak significantly widened compared to that of alkanol and the carbonyl band shifted to a larger wavenumber (e.g., the carbonyl band shifted from 1709.92 cm−1 to 1712.79 cm−1 in Fig. 2C), which confirmed the existence of hydrogen bonds in long-chain alkanol–alkyl carboxylic acid HDESs [42].
Thermal gravity (TG) and differential thermal gravity (DTG) analysis were performed with the C14 alcohol–UA (1:4) HDES as a representative to explore the thermal stability of HDESs. The results (Fig. 2D) evidenced mass loss of HDES at temperatures higher than 200 °C (TG); the decomposition temperature was determined to be 234.5 °C (DTG), indicating that the C14 alcohol–UA HDES has satisfactory high-temperature tolerance and is appropriate for extracting bioactive compounds, especially when the extraction process requires heating.
As listed in Table 1, the viscosity of long-chain alkanol–alkyl carboxylic acid HDESs at 25 °C ranged 4.03–12.26 mPa‧s, which is lower than that of traditional hydrophilic DESs and some reported hydrophobic DESs, such as ChCl-based hydrophilic DESs (25–66441 mPa‧s) [43], [N8881]Cl–C10 acid hydrophobic DES (783.41 mPa‧s) [25], and DL-menthol–C12 acid hydrophobic DES (24.42 mPa‧s) [23]. The excellent low viscosity of novel HDESs may be due to the absence of coulombic forces (a type of intramolecular resistance in charged DESs such as ChCl-based and LCQASs-based DESs) [24]. Furthermore, the viscosity-temperature function curves (Fig. S4) showed that the viscosity of all HDESs dropped with an increase in temperature, following Arrhenius-like behavior. Furthermore, the trends are in agreement with the Vogel–Fulcher–Tammann (VFT) formula with a fitting constant (R2) more than 0.99 (the detailed VFT formula and corresponding parameters are presented in Table S3). The low viscosity of long-chain alkanol–alkyl carboxylic acid HDESs is conducive to extracting bioactive compounds from the solid matrix owing to the easier mass transfer of targets between the extraction solvent and solid matrix.
Fluorescence probe pyrene was employed to measure the polarity of novel HDESs. As outlined in Table 1, the value of pyrene I1/I3 of HDESs was in the range of 0.833–0.9, which is distinctly smaller than those of methanol (1.437), conventional ChCl-based DESs (1.936–2.185) and other reported HDESs (0.968–1.412), and slightly larger than that of n-hexane (0.675). This may be due to the increase in the molecular symmetry of longer alkyl chain of HDESs, resulting in a reduction in the dipole moment. The above results demonstrate that the novel HDESs are a type of weakly polar solvents and may have a strong affinity for low-polarity compounds.
HDESs were mixed with water at different volume ratios (3:1, 2:1, 1:1, 1:2 and 1:3, V/V) to investigate the hydrophobicity of HDESs. As Fig. S5 shows, all the nine HDESs are immiscible in water at all ratios, confirming that they are a class of hydrophobic solvents. Since the density (0.889–0.928 g/mL) of HDESs (Table 1) is lower than that of water, the HDES phase is located in the top phase, making the HDES–water two-phase system suitable to extract bioactive compounds from solid Chinese herb powder because the target compounds can be easily separated from the solid matrix. To further verify the hydrophobicity and water stability of HDESs, the water content of the DES phase was measured, and the 1H NMR spectrum of the water phase was characterized (for a 1:1 volume ratio of HDESs–water mixtures). As shown in Table S4, after an HDES was fully mixed with water, only 2.136%–4.413 % (m/V) water content was found in the DES phase for all HDESs, considerably lower than that of water–saturated ChCl–acid DESs (9.88%–19.40%, m/V) [44]. Fig. S6 shows no peaks of the components of HDESs, indicating that the HDES content is extremely low in the water phase. These results prove the strong hydrophobicity and excellent water stability of novel HDESs, which can be attributed to the low solubility of alkanol and alkyl carboxylic acid in water caused by the long alkyl chains. The excellent hydrophobicity and water stability of novel HDESs allow them to efficiently extract low-polarity bioactive ingredients from medicinal plants and to be possibly recycled and reused after extraction.
3.2. Establishment of HDES–water two-phase extraction system and single factor optimization
In our pre-experiment, long-chain alkanol–alkyl carboxylic acid HDESs were adopted as the extraction solvent to directly extract AQs from RRR powder, but the extraction yield of AQs was very low because of the poor permeability of HDES toward plant tissue. To improve extraction efficiency, pectinase, which was dissolved in an aqueous medium (NaH2PO4 buffer solution at pH 4.5), was used to damage the cell walls of RRR. The aqueous medium was combined with the HDES to construct the HDES–water two-phase system, which was further used for the one-pot extraction of AQs from RRR. The water phase not only allowed RRR powder to be well wetted and dispersed, facilitating the full contact between the HDES and solid matrix during the extraction process, but also facilitated the removal of hydrophilic impurities from RRR, which was verified by the plentiful impurity peaks in the first five minutes of the chromatogram of the aqueous phase obtained after extracting AQs from the RRR sample (Fig. S7).
To obtain the most effective performance for promoting the release of AQs, the pectinase incubation conditions were optimized (Fig. S8) as follows: 1 mg/mL pectinase, enzymatic time of 120 min, enzymatic pH of 5.0 and enzymatic temperature of 45 °C. In addition, a series of extraction factors, including the type of long-chain alkanol–alkyl carboxylic acid HDESs, extraction method, extraction time and temperature, liquid–solid ratio, and HCl concentration, were optimized by a single factor method and the results are shown in Fig. S9.
3.3. Optimization of significant extraction conditions by response surface methodology
To evaluate the effects of various factors on the extraction efficiency, seven experimental factors, namely, extraction temperature, extraction time, liquid–solid ratio, HCl concentration, vortex time, centrifugal time, and centrifugal rate, which may affect the extraction yield, were chosen for the Plackett–Burman design (PBD) experiments. Table S5 lists the boundary level values of each factor, which were selected on the basis of the results of the single-factor optimization experiments. The obtained results were visualized with Pareto charts, as shown in Fig. S10. The bar length in the Pareto chart is proportional to the effect of the corresponding factor. Among the seven factors, extraction temperature (P = 0.0001), liquid–solid ratio (P = 0.0005), and concentration of HCl (P = 0.0277) showed significant effects on the extraction yield of AQs. Thus, these three factors were required to be further optimized by response surface methodology (RSM) while maintaining the other four insignificant factors constant, that is, extraction time of 20 min; vortex time of 5 s; centrifugation time of 10 min; and centrifugation rate of 6,000 r/min.
Next, the extraction temperature (A: 30–80 °C), liquid–solid ratio (B: 5–15 mL/g) and concentration of HCl (C: 0.5%–15%) were optimized by Box-Behnken Design (BBD) with the extraction yield of total AQs as the response (R). The experimental design and results of the three-variable and three-level BBD project are listed in Table S6. Through multiple regression analysis, the response and three variables could be matched to the following second-order polynomial model:
| R = 20.59 + 2.93A + 1.13B + 0.70C − 0.28AB + 0.33AC + 0.32BC − 2.92A2 − 1.30B2 − 1.29C2 |
Analysis of variance (ANOVA) was conducted to assess the significance of the model. As Table S7 shows, the high F-value (263.56) and low P-value (<0.0001) indicate that the model is significant and reliable enough to be applied to analyze the experimental data. The P-value of lack-of-fit (0.8019) was considerably larger than 0.05, indicating that the model is not significant relative to the pure error and thus has credible prediction. The determination coefficient (R2), adjusted determination coefficient (adjusted R2), and predicted determination coefficient (predicted R2) were all larger than 0.98, suggesting that the model has very high accuracy, reliability, and predictive capability. In addition, the P-values of AC (0.0283) and BC (0.0324) suggest that the concentration of HCl interacts with extraction temperature and liquid–solid ratio in the extraction of AQs from RRR samples.
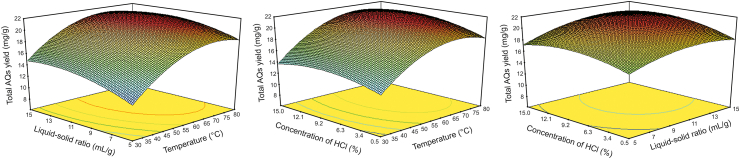
The three-dimensional response surface curves are plotted in Fig. 3 to visualize the interactive effect of every two variables on the response. All curves have an upward convex shape and their maximum values are within the experimental range of variables, demonstrating that the selected experimental ranges of the three variables are reasonable. Furthermore, the varying trends of total AQ yield with the changes in the three variables are similar to that of observed in the single-factor optimization. On the basis of the RSM model, the highest predicted total extraction yield of AQs (21.71 mg/g) was achieved in the following conditions: extraction temperature of 67.6 °C, liquid–solid ratio of 12.14 mL/g and HCl concentration of 10.59%. For ease of operation, the conditions were adjusted slightly to 67 °C, 12 mL/g and 10%, respectively. Under the modified conditions, the experimental value (total AQs yield of 21.52 mg/g) was in great agreement with the predicted value, with an error of 0.87%, thus validating the accuracy and reliability of the fitted model.
Fig. 3.
Response surface plots for total AQs extraction yield using Box-Behnken Design (BBD).
3.4. Analysis of total AQs in real RRR samples from different origins
The proposed method, C14 alcohol–UA DES-based extraction with HPLC-DAD for AQ quantification, was first validated under the optimal extraction conditions (see Supplementary data). Then, the total AQs content of real RRR samples from four different origins was determined by the proposed method, and the results were compared with those measured by the Chinese Pharmacopoeia method (the detailed method is described in Supplementary data). As seen in Table 2, the total AQs contents of these RRR samples were greater than 18.54 mg/g, which is not less than 1.5% (m/m); this complies with the standard regulated by Chinese Pharmacopoeia [33]. Moreover, the total AQ contents determined by the two methods showed no significant difference for all the four RRR samples, with relative error less than 1.4%. Most importantly, the proposed method did not require the consumption of organic solvents, while large amounts of toxic solvents (266.67 mL/g chloroform and 166.67 mL/g methanol) were consumed in the Chinese Pharmacopoeia method. In addition, acid hydrolysis and extraction were performed simultaneously in the proposed method, whereas the pharmacopoeia method was complicated, requiring crude extraction with methanol, followed by HCl hydrolysis and extraction with chloroform. Thus, the proposed method is simple, green, and efficient for the extraction of AQs, and it is an excellent alternative to the Chinese Pharmacopoeia method.
Table 2.
Total AQs analysis of real RRR samples from different areas by this method and Chinese Pharmacopoeia method.
| RRR sample | Extraction method | The amount of extracted AQs ± SD (mg/g, n = 3) |
Organic solvent consumption/1.0 g RRR powder | |||||
|---|---|---|---|---|---|---|---|---|
| Aloe-emodin | Rhein | Emodin | Chrysophanol | Physcion | Total AQs | |||
| Sample 1 | This method | 2.38 ± 0.05 | 4.43 ± 0.05 | 3.76 ± 0.07 | 8.45 ± 0.19 | 2.49 ± 0.08 | 21.52 ± 0.35 | No |
| Pharmacopoeia method | 2.57 ± 0.03 | 4.22 ± 0.34 | 3.57 ± 0.18 | 8.39 ± 0.16 | 2.46 ± 0.06 | 21.22 ± 0.46 | CHCl3: 266.67 mL MeOH: 166.67 mL |
|
| Relative error (%) | −7.4 | 5.0 | 5.3 | 0.7 | 1.2 | 1.4 | ||
| Sample 2 | This method | 1.53 ± 0.10 | 2.57 ± 0.05 | 2.85 ± 0.06 | 9.37 ± 0.22 | 2.20 ± 0.02 | 18.54 ± 0.19 | No |
| Pharmacopoeia method | 1.47 ± 0.01 | 2.62 ± 0.08 | 2.71 ± 0.07 | 9.60 ± 0.25 | 2.23 ± 0.08 | 18.64 ± 0.31 | CHCl3: 266.67 mL MeOH: 166.67 mL |
|
| Relative error (%) | 4.1 | −1.9 | 5.2 | −2.4 | −1.3 | −0.5 | ||
| Sample 3 | This method | 1.91 ± 0.04 | 4.69 ± 0.16 | 3.06 ± 0.02 | 8.00 ± 0.29 | 1.93 ± 0.07 | 19.59 ± 0.53 | No |
| Pharmacopoeia method | 2.08 ± 0.02 | 4.32 ± 0.05 | 3.02 ± 0.04 | 8.39 ± 0.02 | 2.02 ± 0.03 | 19.82 ± 0.10 | CHCl3: 266.67 mL MeOH: 166.67 mL |
|
| Relative error (%) | −8.2 | 8.6 | 1.3 | −4.6 | −4.5 | −1.2 | ||
| Sample 4 | This method | 1.69 ± 0.04 | 4.38 ± 0.04 | 3.60 ± 0.02 | 7.74 ± 0.09 | 1.84 ± 0.01 | 19.25 ± 0.11 | No |
| Pharmacopoeia method | 1.90 ± 0.03 | 3.75 ± 0.06 | 3.59 ± 0.06 | 7.94 ± 0.13 | 1.84 ± 0.03 | 19.02 ± 0.26 | CHCl3: 266.67 mL MeOH: 166.67 mL |
|
| Relative error (%) | −11.1 | 16.8 | 0.3 | −2.5 | 0 | 1.2 | ||
Samples 1, 2, 3, and 4 were from Sichuan, Hubei, Gansu and Qinghai Provinces of China, respectively.
3.5. Extraction mechanism
Commonly, DES-based extraction effect for bioactive components depends on the ability of the extraction method to destruct plant powder and the capacity of the solvent to dissolve the components [1,45]. To investigate the destructive effect of the extraction method on the plant tissue, the microscopic structure of RRR powder that was untreated and treated with different methods (with the enzyme alone, with C14 alcohol–UA and HCl, and with C14 alcohol–UA, HCl, and enzyme, i.e., the whole one-pot extraction process) was characterized and the results are shown in Fig. 4. There were many cavities in the surface of RRR particles treated with enzyme alone (Fig. 4B), indicating that pectinase could break the cell surface [46]. The RRR powder treated with C14 alcohol–UA and HCl showed gully-like ruptures and porous pieces (Fig. 4C), suggesting that the HDES and HCl also have abilities to destroy the fiber [45]. Fig. 4D indicates that the proposed extraction method could break the RRR powder into many small pieces and showed stronger capability of destroying the cell walls compared with the previous two treatment methods. The severe damage of the RRR powder tissue structure was due to the combined effect of pectinase, C14 alcohol–UA, and HCl, in which the enzyme plays the role of boring holes, while DES and HCl break the cells apart. The strong ability of this method to destroy RRR could enhance the mass transfer of AQs from the RRR powder into the external solvent, resulting in a high extraction efficiency.
Fig. 4.
SEM images of RRR powder untreated (A), treated with enzyme only (B), treated with C14 alcohol–UA (1:4) HDES and HCl (C), treated through whole extraction process (D). Each image has a magnification of 5000.
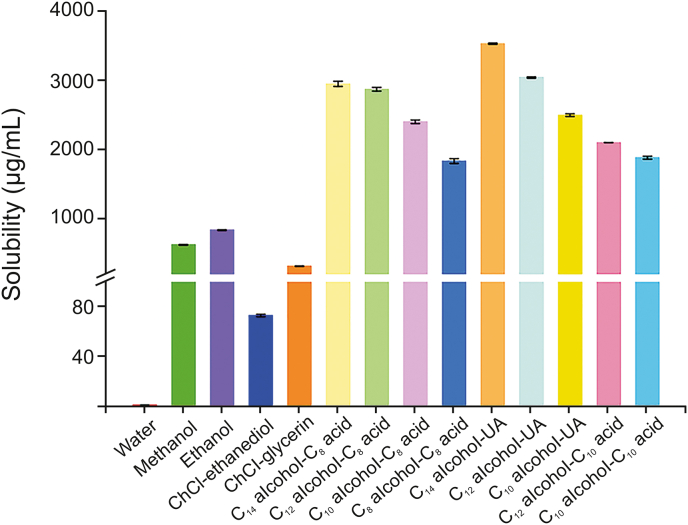
The solubility of AQs in the nine HDESs investigated herein was determined with 1,8-DHAQ as a representative of AQ compounds (see Supplementary data), and it was compared with those of traditional DESs (ChCl–glycerin and ChCl–ethylene glycol), common organic solvents (methanol and ethanol), and water. As seen in Fig. 5, the solubilities of 1,8-DHAQ in the nine HDESs were 1840.2–3539.1 μg/mL, which were 1122–2158 times higher than that in water (1.64 μg/mL), 5.8–48.4 times those in traditional DESs (73.1–319.5 μg/mL) and 2.2–5.6 times those in common organic solvents (628.6–842.3 μg/mL). The superior dissolution ability of HDESs may be due to the fact that the hydrophobic moiety of 1,8-DHAQ is more inclined to be attracted by long alkyl chains of HDESs via van der Waals interactions [47]. In addition, the C14 alcohol–UA HDES showed the best dissolution performance among the nine HDESs, which is in line with the trend of AQs extraction yield in optimizing the type of HDES (Fig. S9A). The excellent dissolving capacity of HDESs allows AQs to easily distribute into the HDES phase with lower polarity in terms of the “like-dissolve-like” principle. To sum up, the strong destructive ability to RRR cells and the superior dissolving capacity to AQs of HDESs provide forceful support for the excellent extraction performance of the proposed method.
Fig. 5.
Solubilities of 1,8-dihydroxyanthraquinone in water, organic solvents (methanol and ethanol), traditional DESs (ChCl–ethanediol and ChCl–glycerin) and long-chain alkanol–alkyl carboxylic acid HDESs (at eutectic ratio) at room temperature.
3.6. Comparison with other HDESs
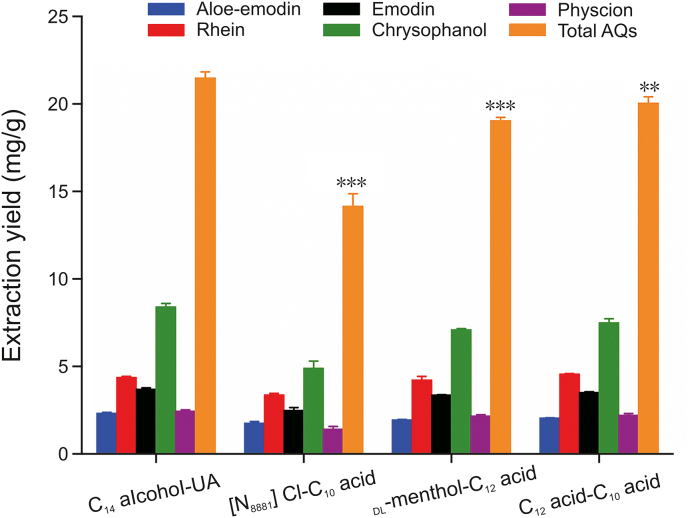
Three reported HDESs, namely, [N8881]Cl–C10 acid (charged HDES) [25], DL-menthol–C12 acid (neutral HDES) [23] and C12 acid–C10 acid (neutral HDES) [37], were selected as extraction media for the comparison of the extraction yield of AQs with the proposed C14 alcohol–UA HDES using the one-pot extraction method described above. Under the optimized extraction conditions, the extraction performance of four HDESs is depicted in Fig. 6, which indicates that C14 alcohol–UA obtained the highest extraction yield for total AQs (P < 0.01). The total AQ yields of the above solvents are in the following order: C14 alcohol–UA (21.52 mg/g) > C12 acid–C10 acid (20.09 mg/g) > DL-menthol–C12 acid (19.08 mg/g) > [N8881]Cl–C10 acid (14.20 mg/g). This indicates that the neutral HDESs have higher extraction efficiency than the charged HDES. The superior extraction yield of C14 alcohol–UA is on account of the lower viscosity (8.22 mPa‧s) and polarity (pyrene I1/I3 0.833) than those of other HDESs (10.76–783.41 mPa·s, pyrene I1/I3 0.968–1.412) (Table 1), leading to easier mass transfer and more efficient interactions of AQs with the solvent. The above results suggest that the designed C14 alcohol–UA HDES is an attractive and promising solvent to extract weakly polar bioactive compounds such as AQs from the plant matrix.
Fig. 6.
Extraction yields of each AQ and total AQs obtained by C14 alcohol–UA (1:4) HDES and other HDESs ([N8881]Cl–C10 acid, DL-menthol–C12 acid, C12 acid–C10 acid). Comparison between two groups was performed using a two-tailed unpaired Student's t-test, and P < 0.05 was considered statistically significant (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). All experiments were performed at least three times.
4. Conclusions
Novel HDESs based on long-chain alkanol–alkyl carboxylic acid were designed and prepared. These HDESs possess the advantages of being environmentally friendly, low viscosity, and low polarity, and they have promising applications in extracting weakly polar bioactive compounds. A one-pot extraction acid hydrolysis method based on the HDES–water two-phase system was then established to maximize the yield of AQs from RRR samples and simplify the operation procedure. The optimal extraction conditions after being optimized by single-factor experiment and RSM are as follows: C14 alcohol–UA HDES, stirring at 67 °C for 20 min, liquid–solid ratio of 12:1 (mL/g) and HCl concentration of 10%. Under these conditions, up to 21.52 mg/g of total AQs could be extracted without organic solvent consumption. This yield is very close to that by the Chinese Pharmacopoeia method (21.22 mg/g) and considerably higher than those of other reported HDESs-based methods (total AQs yield: 14.20–20.09 mg/g). The excellent extraction performance of our proposed method is attributed to the synergistic damaging effect of pectinase, C14 alcohol–UA and HCl on the RRR cell walls as well as the predominant dissolution ability of long-chain alkanol–alkyl carboxylic acid HDESs for AQs. Our research illustrates that the novel long-chain alkanol–alkyl carboxylic acid HDESs are a family of green and promising hydrophobic media, and they are expected to be combined with multiple processes to achieve sustainable applications for the extraction and purification of low-polarity compounds.
CRediT author statement
Anqi Huang: Conceptualization, Methodology, Software, Data curation, Investigation, Writing - Original draft preparation; Wenwen Deng: Methodology, Software, Writing - Reviewing and Editing; Xiao Li: Investigation, Writing - Reviewing and Editing; Qutong Zheng: Software, Data curation; Xuanxuan Wang: Writing - Reviewing and Editing; Yuxiu Xiao: Conceptualization, Methodology, Supervision, Funding acquisition, Resources, Writing - Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We thank the National Natural Science Foundation of China (Grant Nos.: 81673394 and 82073811), the Fundamental Research Funds for the Central Universities (Grant No.: 2042020kf1010), and the Large-scale Instrument and Equipment Sharing Foundation of Wuhan University (Grant No.: LF20170838).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2021.03.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zainal-Abidin M.H., Hayyan M., Hayyan A., et al. New horizons in the extraction of bioactive compounds using deep eutectic solvents: a review. Anal. Chim. Acta. 2017;979:1–23. doi: 10.1016/j.aca.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Zhang D.-H., Wu K.-L., Zhang X., et al. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020;18:152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha S.K., Shakya A., Prasad S.K., et al. An in-silico evaluation of different saikosaponins for their potency against SARS-CoV-2 using NSP15 and fusion spike glycoprotein as targets. J. Biomol. Struct. Dyn. 2021;39:3244–3255. doi: 10.1080/07391102.2020.1762741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morone J., Alfeus A., Vasconcelos V., et al. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals — a new bioactive approach. Algal Res. 2019;41:101541. [Google Scholar]
- 5.Maqsood S., Adiamo O., Ahmad M., et al. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020;308:125522. doi: 10.1016/j.foodchem.2019.125522. [DOI] [PubMed] [Google Scholar]
- 6.Kukula-Koch W., Koch W., Stasiak N., et al. Quantitative standarization and CPC-based recovery of pharmacologically active components from Polygonum tinctorium Ait. leaf extracts. Ind. Crop. Prod. 2015;69:324–328. [Google Scholar]
- 7.Kubola J., Meeso N., Siriamornpun S. Lycopene and beta carotene concentration in aril oil of gac (Momordica cochinchinensis Spreng) as influenced by aril-drying process and solvents extraction. Food Res. Int. 2013;50:664–669. [Google Scholar]
- 8.Wójciak-Kosior M., Sowa I., Kocjan R., et al. Effect of different extraction techniques on quantification of oleanolic and ursolic acid in Lamii albi flos. Ind. Crop. Prod. 2013;44:373–377. [Google Scholar]
- 9.Goyeneche R., Di Scala K., Ramirez C.L., et al. Recovery of bioactive compounds from beetroot leaves by supercritical CO2 extraction as a promising bioresource. J. Supercrit. Fluids. 2020;155:104658. [Google Scholar]
- 10.Rouhani M. Modeling and optimization of ultrasound-assisted green extraction and rapid HPTLC analysis of stevioside from Stevia Rebaudiana. Ind. Crop. Prod. 2019;132:226–235. [Google Scholar]
- 11.Hou K., Bao M., Wang L., et al. Aqueous enzymatic pretreatment ionic liquid–lithium salt based microwave–assisted extraction of essential oil and procyanidins from pinecones of Pinus koraiensis. J. Clean. Prod. 2019;236:117581. [Google Scholar]
- 12.Tsiaka T., Fotakis C., Lantzouraki D.Z., et al. Expanding the role of sub-exploited DOE-high Energy extraction and metabolomic profiling towards agro-byproduct valorization: the case of carotenoid-rich apricot pulp. Molecules. 2020;25:2702. doi: 10.3390/molecules25112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agnieszka M., Michał S., Robert K. Selection of condition of ultrasound-assisted, three-step extraction of ellagitannins from selected berry fruit of the rosaceae family using the response surface methodology. Food Anal. Method. 2020;13:1650–1665. [Google Scholar]
- 14.Nam M.W., Zhao J., Lee M.S., et al. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from Flos sophorae. Green Chem. 2015;17:1718–1727. [Google Scholar]
- 15.Yoo D.E., Jeong K.M., Han S.Y., et al. Deep eutectic solvent-based valorization of spent coffee grounds. Food Chem. 2018;255:357–364. doi: 10.1016/j.foodchem.2018.02.096. [DOI] [PubMed] [Google Scholar]
- 16.Liang J., Zeng Y., Wang H., et al. Extraction, purification and antioxidant activity of novel polysaccharides from Dendrobium officinale by deep eutectic solvents. Nat. Prod. Res. 2019;33:3248–3253. doi: 10.1080/14786419.2018.1471480. [DOI] [PubMed] [Google Scholar]
- 17.Benvenutti L., Zielinski A.A.F., Ferreira S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019;90:133–146. [Google Scholar]
- 18.Ruesgas-Ramón M., Figueroa-Espinoza M.C., Durand E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: overview, challenges, and opportunities. J. Agric. Food Chem. 2017;65:3591–3601. doi: 10.1021/acs.jafc.7b01054. [DOI] [PubMed] [Google Scholar]
- 19.Dai Y., van Spronsen J., Witkamp G.-J., et al. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta. 2013;766:61–68. doi: 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Wang L.-T., Yang Q., Cui Q., et al. Recyclable menthol-based deep eutectic solvent micellar system for extracting phytochemicals from Ginkgo biloba leaves. J. Clean. Prod. 2020;244:118648. [Google Scholar]
- 21.Zhang Q., De Oliveira Vigier K., Royer S., et al. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 2012;41:7108–7146. doi: 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- 22.Dai Y., Witkamp G.-J., Verpoorte R., et al. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015;187:14–19. doi: 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro B.D., Florindo C., Iff L.C., et al. Menthol-based eutectic mixtures: hydrophobic low viscosity solvents. ACS Sustain. Chem. Eng. 2015;3:2469–2477. [Google Scholar]
- 24.Florindo C., Branco L.C., Marrucho I.M. Quest for green-solvent design: from hydrophilic to hydrophobic (deep) eutectic solvents. ChemSusChem. 2019;12:1549–1559. doi: 10.1002/cssc.201900147. [DOI] [PubMed] [Google Scholar]
- 25.van Osch D.J.G.P., Zubeir L.F., van den Bruinhorst A., et al. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015;17:4518–4521. [Google Scholar]
- 26.Křížek T., Bursová M., Horsley R., et al. Menthol-based hydrophobic deep eutectic solvents: towards greener and efficient extraction of phytocannabinoids. J. Clean. Prod. 2018;193:391–396. [Google Scholar]
- 27.Silva Y.P.A., Ferreira T.A.P.C., Jiao G., et al. Sustainable approach for lycopene extraction from tomato processing by-product using hydrophobic eutectic solvents. J. Food Sci. Technol. 2019;56:1649–1654. doi: 10.1007/s13197-019-03618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao J., Chen L., Li M., et al. Two-phase systems developed with hydrophilic and hydrophobic deep eutectic solvents for simultaneously extracting various bioactive compounds with different polarities. Green Chem. 2018;20:1879–1886. [Google Scholar]
- 29.Cao J., Yang M., Cao F., et al. Well-designed hydrophobic deep eutectic solvents as green and efficient media for the extraction of artemisinin from Artemisia annua leaves. ACS Sustain. Chem. Eng. 2017;5:3270–3278. [Google Scholar]
- 30.Cao J., Yang M., Cao F., et al. Tailor-made hydrophobic deep eutectic solvents for cleaner extraction of polyprenyl acetates from Ginkgo biloba leaves. J. Clean. Prod. 2017;152:399–405. [Google Scholar]
- 31.Florindo C., Lima F., Branco L.C., et al. Hydrophobic deep eutectic solvents: a circular approach to purify water contaminated with ciprofloxacin. ACS Sustain. Chem. Eng. 2019;7:14739–14746. [Google Scholar]
- 32.Arvindekar A.U., Laddha K.S. An efficient microwave-assisted extraction of anthraquinones from Rheum emodi: optimisation using RSM, UV and HPLC analysis and antioxidant studies. Ind. Crop. Prod. 2016;83:587–595. [Google Scholar]
- 33.Chinese Pharmacopoeia Commission . Vol. 1. China Medical Science Press; Beijing, China: 2020. Pharmacopoeia of the People’s Republic of China, [Google Scholar]
- 34.Duan L., Dou L.-L., Guo L., et al. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng. 2016;4:2405–2411. [Google Scholar]
- 35.Deng W.-W., Zong Y., Xiao Y.-X. Hexafluoroisopropanol-Based Deep Eutectic Solvent/Salt Aqueous Two-Phase Systems for Extraction of Anthraquinones from Rhei Radix et Rhizoma Samples. ACS Sustain. Chem. Eng. 2017;5:4267–4275. [Google Scholar]
- 36.Noweck K., Grafahrend W. Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley & Sons, Inc.; 2006. Fatty alcohols. [Google Scholar]
- 37.Florindo C., Romero L., Rintoul I., et al. From phase change materials to green solvents: hydrophobic low viscous fatty acid-based deep eutectic solvents. ACS Sustain. Chem. Eng. 2018;6:3888–3895. [Google Scholar]
- 38.Gilmore M., McCourt É.N., Connolly F., et al. Hydrophobic deep eutectic solvents incorporating trioctylphosphine oxide: advanced liquid extractants. ACS Sustain. Chem. Eng. 2018;6:17323–17332. [Google Scholar]
- 39.Pandey A., Rai R., Pal M., et al. How polar are choline chloride-based deep eutectic solvents? Phys. Chem. Chem. Phys. 2014;16:1559–1568. doi: 10.1039/c3cp53456a. [DOI] [PubMed] [Google Scholar]
- 40.Fu X.Q., Ma N., Sun W.P., et al. Microwave and enzyme co-assisted aqueous two-phase extraction of polyphenol and lutein from marigold (Tagetes erecta L.) flower. Ind. Crop. Prod. 2018;123:296–302. [Google Scholar]
- 41.Martins M.A.R., Crespo E.A., Pontes P.V.A., et al. Tunable hydrophobic eutectic solvents based on terpenes and monocarboxylic acids. ACS Sustain. Chem. Eng. 2018;6:8836–8846. [Google Scholar]
- 42.Florindo C., Celia-Silva L.G., Martins L.F.G., et al. Supramolecular hydrogel based on a sodium deep eutectic solvent. Chem. Commun. (Camb) 2018;54:7527–7530. doi: 10.1039/c8cc03266a. [DOI] [PubMed] [Google Scholar]
- 43.Zhao B.-Y., Xu P., Yang F.-X., et al. Biocompatible deep eutectic solvents based on choline chloride: characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015;3:2746–2755. [Google Scholar]
- 44.Florindo C., Oliveira F.S., Rebelo L.P.N., et al. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2014;2:2416–2425. [Google Scholar]
- 45.Wan Mahmood W.M.A., Lorwirachsutee A., Theodoropoulos C., Gonzalez-Miquel M. Polyol-based deep eutectic solvents for extraction of natural polyphenolic antioxidants from Chlorella vulgaris. ACS Sustain. Chem. Eng. 2019;7:5018–5026. [Google Scholar]
- 46.Puri M., Sharma D., Barrow C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012;30:37–44. doi: 10.1016/j.tibtech.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Jin W., Yang Q., Huang B., et al. Enhanced solubilization and extraction of hydrophobic bioactive compounds using water/ionic liquid mixtures. Green Chem. 2016;18:3549–3557. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.