Abstract
Background
Studies of brain functional connectivity (FC) typically involve massive univariate tests, performing statistical analysis on each individual connection. In this study, we apply a novel whole-matrix regression approach referred to as covariate assisted principal regression to identify resting-state FC brain networks associated with attention-deficit/hyperactivity disorder (ADHD) and response control.
Methods
Participants included 8- to 12-year-old children with ADHD (n = 115; 29 girls) and typically developing control children (n = 102; 35 girls) who completed a resting-state functional magnetic resonance imaging scan and a Go/NoGo task. We modeled three sets of covariates to identify resting-state networks associated with an ADHD diagnosis, sex, and response inhibition (commission errors) and variability (ex-Gaussian parameter tau).
Results
The first network includes FC between striatal-cognitive control (CC) network subregions and thalamic-default mode network (DMN) subregions and is positively related to age. The second consists of FC between CC-visual-somatomotor regions and between CC-DMN subregions and is positively associated with response variability in boys with ADHD. The third consists of FC within the DMN and between DMN-CC-visual regions and differs between boys with and without ADHD. The fourth consists of FC between visual-somatomotor regions and between visual-DMN regions and differs between girls and boys with ADHD and is associated with response inhibition and variability in boys with ADHD. Unique networks were also identified in each of the three models, suggesting some specificity to the covariates of interest.
Conclusions
These findings demonstrate the utility of our novel covariance regression approach to studying functional brain networks relevant for development, behavior, and psychopathology.
Keywords: ADHD, Children, Covariance regression, Functional connectivity, Response control, Sex differences
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder that affects approximately 5% to 10% of children and adolescents (1), characterized by developmentally inappropriate symptoms of inattention, hyperactivity, and impulsivity that impact academic, family, and social functioning (2). These behavioral symptoms are thought to arise from cognitive, motor, and motivational deficits and associated atypical brain structure and function. In particular, impaired response control, including poor inhibition and increased trial-to-trial variability, is often observed in ADHD and central to theoretical models of ADHD (3,4). Recent studies have revealed differential impairments in response control in girls and boys with ADHD, with boys with ADHD showing poorer response inhibition than typically developing (TD) boys and girls with ADHD, whereas girls and boys with ADHD show greater response variability compared with TD children (5). In addition, there is some evidence of ADHD-related sex differences in brain structure and function, with atypical structure and functional connectivity (FC) of frontosubcortical regions among girls with ADHD, whereas boys with ADHD tend to show atypical structure and function of motor and premotor regions (6, 7, 8).
Within the ADHD literature, there is an increased focus on elucidating the neurobiological basis for ADHD and impaired response control to ultimately inform prevention and intervention efforts. Neuroimaging methods have increasingly been applied to characterize brain-behavior relationships in individuals with ADHD. In particular, resting-state functional magnetic resonance imaging (rs-fMRI) is widely being used to examine functional networks that operate differently in children with ADHD and relate to neurocognitive deficits associated with the disorder. The extant literature has shown that the neurobiological basis for ADHD likely involves dysfunctional interactions of, or FC between, brain networks rather than atypical structure or function of isolated brain regions. Default mode network (DMN) hyperconnectivity with other networks among individuals with ADHD is one of the most consistent findings in the literature (10, 11, 12, 13, 9). Some studies have also reported that DMN hyperconnectivity with other networks relates to cognitive deficits in individuals with ADHD (10,14,15). However, the ADHD rs-FC literature extends beyond DMN connectivity to include findings of increased within-network connectivity in motor (16, 17, 18) and visual regions (13,17,19,20) and atypical FC between frontal-subcortical regions (6,21, 22, 23). Thus, a whole-brain approach is warranted to better characterize differences in functional network organization in ADHD. Moving beyond diagnostic group comparisons, characterization of how individual differences in functional networks are associated with behavior has the potential to inform the heterogeneity of neurocognitive deficits and symptom presentation in ADHD.
This study applies a novel whole-matrix regression approach developed by Zhao et al. (24) and applied to data collected from healthy young adults ages 20 to 35 years in the Human Connectome Project (24,25), referred to as covariate assisted principal (CAP). The CAP method offers some advantages over popular network-level FC approaches, including graph theory (26), pattern recognition (27), common reducing subspace model (28), utilization of network-based statistics (29), and a connectome-based analytic protocol for prediction (30). One advantage of the CAP method is that it enables identification of covariate-related networks rather than studying predefined networks. The CAP approach offers higher flexibility in modeling the association, where the covariate can be continuous and the interaction between variables can be included in the model. The CAP method also circumvents the issue of multiplicity (i.e., performing statistical analysis on each individual connection), such that given a set of p brain voxels/regions, statistical inference needs to account for at least p(p − 1)/2 hypothesis tests, one for each element of the connectivity matrix. The CAP method aims to identify a common linear projection of p time courses across subjects such that variations in FC defined by the projection can be explained by the covariates of interest. It is a mesoscale approach in the sense that, with an appropriate threshold, the projection defines a brain subnetwork. However, this approach suffers from the curse of dimensionality, in that the dimension of the data cannot be greater than the number of fMRI volumes. Therefore, it cannot be applied to voxel-level fMRI data. Thus, an integrative approach was proposed to analyze whole-brain voxel-level data, revealing individual and group variations in FC (25) by starting with a dimension reduction step, such as group independent component analysis (ICA) (31), followed by CAP regression on the ICs. Projecting back to the voxel space, it yields a reconstructed brain map that is associated with the covariates.
This study applies this novel integrated CAP analysis to examine how a diagnosis of ADHD, relevant demographic variables, and behavioral measures of response control predict functional interactions between brain networks. Specifically, we applied this novel, high-dimensional statistical method to study interactions between sex and an ADHD diagnosis on FC in children with and without ADHD and in relation to cognitive deficits associated with ADHD. We hypothesized that boys with ADHD would show greater response disinhibition whereas girls and boys with ADHD would show greater response variability than TD children. Given the varied findings across the ADHD neuroimaging literature, we chose to apply an exploratory, data-driven, whole-brain approach to identifying resting-state networks that are related to ADHD, sex, age, and response control.
Methods and Materials
Participants
Participants included 217 8- to 12-year-old children who either had a diagnosis of ADHD (n = 115; 29 girls) or were TD control children (n = 102; 35 girls).1 Participants were recruited through local schools, community-wide advertisement, volunteer organizations, medical institutions, and word of mouth. This study was approved by the local institutional review board. After providing a complete study description to the participants, oral informed consent was obtained from a parent/guardian followed by an initial phone screening. Children with a history of intellectual disability, learning disability, seizures, traumatic brain injury, or other neurologic illnesses were excluded. Participants were determined to be eligible for inclusion in either the ADHD group or the TD group based on review of standardized rating scales and diagnostic interview (see the Supplement for details of the diagnostic procedure). Eligible participants and their parents attended two laboratory sessions. At the initial visit, written informed consent and assent were obtained from the parent/guardian and the child, and intellectual ability was assessed. Children taking psychotropic medications other than stimulants were excluded from participation, and children taking stimulants were asked to withhold medication the day before and day of testing. Basic demographic information is provided in Table 1.
Table 1.
Behavioral Go/NoGo Task Performance and Relevant Demographics for the ADHD and TD Groups Overall and Within Sex
| Demographics | TD |
ADHD |
Group Comparisons, p Value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADHD vs. TD |
Boys vs. Girls |
|||||||||
| Girls | Boys | All | Girls | Boys | All | Girls | Boys | All | ADHD | |
| n | 35 | 67 | 102 | 29 | 86 | 115 | ||||
| Age, Years | 10.26 (0.97) | 10.53 (1.30) | 10.44 (1.20) | 10.09 (1.37) | 10.21 (1.45) | 10.18 (1.43) | .617 | .141 | .155 | .695 |
| GAI | 114 (10.28) | 117 (12.85) | 116 (12.07) | 110 (12.57) | 111 (14.40) | 111 (13.92) | .215 | .010 | .006 | .589 |
| Tau | 4.44 (0.42) | 4.35 (0.46) | 4.38 (0.45) | 4.69 (0.39) | 4.76 (0.57) | 4.74 (0.53) | .004 | <.001 | <.001 | .471 |
| CR | 0.36 (0.18) | 0.39 (0.18) | 0.38 (0.18) | 0.36 (0.20) | 0.51 (0.20) | 0.47 (0.21) | .988 | <.001 | .001 | .001 |
Values are presented as n or mean (SD). Wechsler Intelligence Scale for Children GAI was used.
ADHD, attention-deficit/hyperactivity disorder; CR, commission error rate; GAI, General Ability Index; TD, typically developing.
Behavioral Go/NoGo Data
Participants completed a Go/NoGo (GNG) task programmed in Presentation (Neurobehavioral Systems), from which we have previously published findings from a subset of this sample (5,6,8,32, 33, 34, 35, 36, 37). Participants were seated in front of a computer monitor with a keyboard and were instructed to respond to a single green spaceship for Go trials (80% of 240 trials) and to withhold their response with a red spaceship for NoGo trials (20%) presented for 300 ms, with an interstimulus interval of 2000 ms during which a fixation cross appeared. Response inhibition was quantified as commission error rate (CR), calculated as the proportion of NoGo trials on which participants incorrectly responded. Response variability was quantified using the ex-Gaussian indicator tau, representing infrequent, slow responses contributing to the exponential component of the reaction time distribution (38).
rs-fMRI Data
All children completed a mock scan to acclimate to the scanning environment. The mock was completed on a separate day in a simulated scan environment and lasted 30 to 45 minutes. Trained research assistants observed participants during the mock scan and provided real-time feedback to promote compliance with the scan procedures. Once the child successfully completed the mock scan, rs-fMRI was acquired on a 3.0T Philips scanner (see the Supplement for acquisition and preprocessing details and prior publications from a subset of this sample examining FC without relating this to GNG behavioral performance) (6,39,40). We decomposed the rs-fMRI data into temporally coherent networks using the Group ICA of fMRI Toolbox (http://icatb.sourceforget.net) (31,41). We chose ICA (42) rather than seed-based approaches because of its effectiveness at separating signal from noise (43), its increased sensitivity to detecting individual differences (44), and its ability to identify resting-state networks (RSNs) without defining a seed region by effectively clustering voxels with similar time courses. An additional benefit of this data-driven grouping of functionally related voxels is that it reduces the size of the covariance matrix used in the CAP model and thereby allows for whole-brain analysis. Following group ICA with backward reconstruction (see the Supplement), we excluded noise components (n = 27) (see Figure S1) and identified relevant RSNs (n = 38) by comparing the spatial distribution of each of the group-level, aggregate ICs to a publicly available set of unthresholded IC t-statistic maps that have been classified as RSNs by a group of experts and organized into seven large functional groups: visual, auditory, somatomotor, DM, cognitive control (CC), subcortical, and cerebellar networks (45).
Motion Correction
A significant challenge in the ADHD neuroimaging literature is accounting for artifacts introduced by motion during the scan, which have been shown to impact FC metrics and usually differ between groups with and without psychopathology. Among our sample, mean framewise displacement (FD) [a measure of head motion between consecutive fMRI volumes calculated from the realignment estimates (46)] was greater among girls and boys with ADHD compared with same-sex TD children (girls: p = .006; boys: p = .005), and mean FD was positively correlated with parent-reported ADHD symptoms among girls (r = 0.283, p = .026) and boys (r = 0.269, p < .001) across diagnostic groups. We chose to not match the groups on mean FD for the primary analyses or covary FD, given that variance associated with ADHD symptomatology and cognitive deficits may not be independent from variance associated with motion and that head motion and ADHD may have similar genetic loadings (47). To address potential confounds of motion in this sample, we applied several steps. First, 154 (90 ADHD) participants were excluded for between-volume translational movements >3 mm or rotational movements >3°. Second, a growing body of literature has demonstrated the effectiveness of ICA-based strategies for identifying and removing motion-related variation in fMRI data (13,14). We utilized spatial ICA to isolate spatiotemporally structured signals of noninterest, including motion-related sources from functional network sources. Finally, we regressed the six rigid-body realignment motion parameters from IC time courses associated with components identified as representing signal before estimating between-component FC (45). In secondary analyses, we applied the CAP regression on FD-matched samples, which yielded similar results (Figure S2).
CAP Regression on RSN Components
Details of the CAP regression on the signal components identified by group ICA described above are provided in the Supplement (24). The CAP method identifies a linear projection of the covariance matrices such that between-subject variability in FC is most strongly associated with the covariates of interest. Assuming that the IC time courses are standardized to have identical variance, the CAP regression models the association between FC and the covariates. This association depends on both the sign of the model coefficient and the sign of the loading products. For a positive coefficient estimate, FC between two ICs with the same loading sign (a positive product) is positively associated with the corresponding covariate, while FC for two ICs with the opposite signs (a negative product) is negatively associated with the covariate.
Analysis
We modeled three sets of covariates, and the entire sample was included in all analyses. First, we tested for effects of an ADHD diagnosis, sex, and their interaction, as well as age and intellectual reasoning ability (General Ability Index [GAI]) in the CAP regression model. In subsequent models, we examined associations with behavioral measures of response control, GNG tau (log-transformed), and CR. These models included ADHD diagnosis, sex, the behavioral measure of interest, and all possible interactions among these three variables.
Results
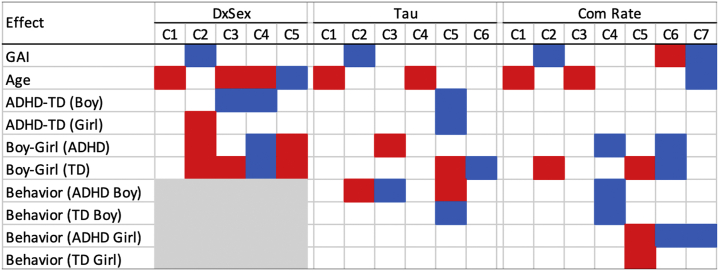
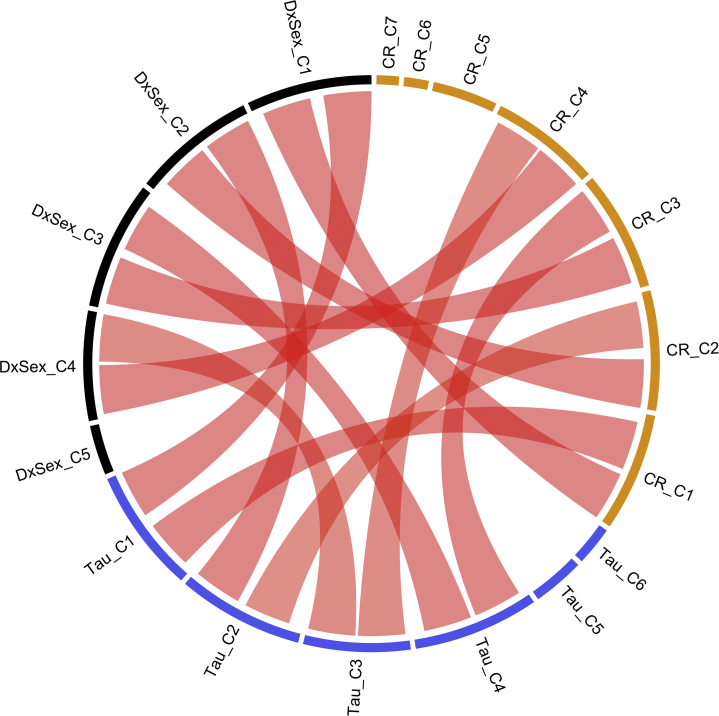
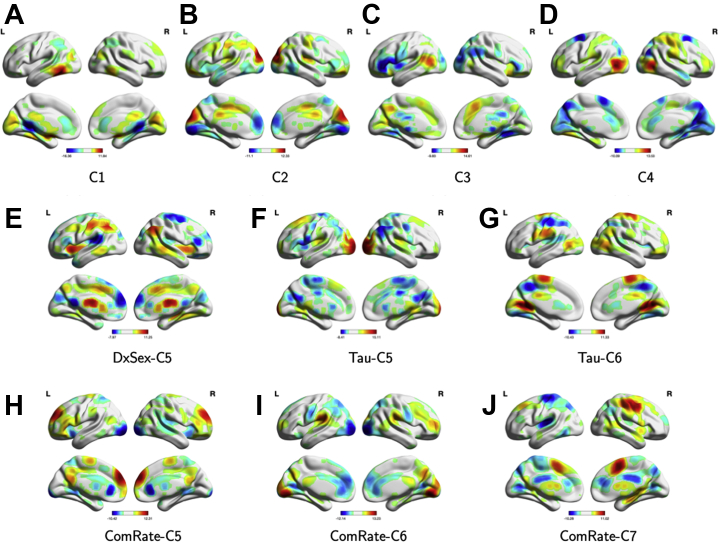
The CAP method identified five brain subnetworks (groups of RSN ICs, or mega-component) in the diagnosis-by-sex (DxSex) model, six in the GNG tau model, and seven in the GNG CR model. We summarize the significance of the coefficient effect in each mega-component in Figure 1. Figure S3 summarizes the RSN ICs clustered within the functional groups described above that contribute to each component as river plots. Among all of these mega-components, four subnetworks were identified in all three models. Figure 2 compares the similarity between the mega-components identified by the three models; a connection between two mega-components suggests a high similarity. Figure 3 illustrates the loadings of RSNs contributing to each mega-component as reconstructed brain maps (Supplemental File S1 is an interactive tool to view 3D images of the 10 mega-components to accompany Figure 3A–J). Finally, Figure S4 shows the percentage of variation explained by the identified components for each subject in each of the models.
Figure 1.
Significant effect of the components estimated from the covariate assisted principal model. The red color denotes a positive effect and blue for negative. DxSex signifies the behavior-free model; Tau signifies the model including Go/NoGo tau as one of the predictors; Com Rate signifies the model including Go/NoGo commission error rate as one of the predictors. ADHD, attention-deficit/hyperactivity disorder; C, component; GAI, General Ability Index; TD, typically developing.
Figure 2.
Chord diagram compares the similarity between the components identified from the three models. DxSex signifies the behavior-free model; Tau signifies the model including Go/NoGo tau as one of the predictors; CR signifies the model including Go/NoGo commission error rate (CR) as one of the predictors. A red connection indicates that the two components are highly similar. C, component.
Figure 3.
Reconstructed brain maps of the components identified by the covariate assisted principal method. DxSex signifies the behavior-free model; Tau signifies the model including Go/NoGo tau as one of the predictors; ComRate signifies the model including Go/NoGo commission error rate as one of the predictors. (A–D) Common components identified by all three models. (E) Unique component identified by the behavior-free model. (F, G) Unique components identified by the model with Go/NoGo tau as one of the predictors. (H–J) Unique components identified by the model with Go/NoGo commission error rate as one of the predictors. C, component; L, left; R, right.
First Common Mega-component (DxSex-C1/Tau-C1/CR-C1)
Across the three models, the identified FC mega-component consisted of subregions of the CC (inferior temporal gyrus [ITG]) and subcortical (striatal) networks with positive loadings, as well as subregions of the DM (posterior cingulate cortex [PCC]) and subcortical (thalamus) networks with negative loadings (Figure 3A). This mega-component was positively related to age, regardless of diagnostic group, in each of the models (all p values < .001), such that as age increases, FC between regions with the same loading signs increases (ITG-striatal FC and PCC-thalamus FC), while FC between regions with opposite loading signs decreases (e.g., PCC-striatum).
Second Common Mega-component (DxSex-C2/Tau-C2/CR-C2)
The second component identified across all three models consisted of subregions of the CC (left central executive), visual (lateral occipital cortex), and sensorimotor (anterior cingulate cortex [ACC]/Brodmann area 6 [BA 6]) networks contributing positively, as well as other subregions of the CC (insula, ITG) and DM (PCC) networks contributing negatively (Figure 3B). This component was negatively associated with GAI (DxSex C2, p = .014; tau C2, p = .039; CR C2, p = .042) and positively associated with tau in boys with ADHD (p = .001), such that lower GAI and greater tau is associated with greater FC between regions with the same loading sign and lower FC between regions with opposite loading signs. Girls with ADHD showed greater FC within this mega-component than TD girls (DxSex C2, p = .022) and lower FC than boys with ADHD (DxSex C2, p < .001). However, this diagnostic difference was not observed when controlling for tau and CR (Figure 1).
Third Common Mega-component (DxSex-C3/Tau-C4/CR-C3)
The third component identified across all three models consisted of a DMN subregion (precuneus) with positive loadings, as well as subregions of the CC (inferior frontal gyrus, insula), DM (PCC), and visual (lateral occipital cortex) networks with negative loadings (Figure 3C). Again, this component was positively associated with age across all three models (all p values < .001). In addition, boys with ADHD showed higher FC within this mega-component than TD boys (p = .009), controlling for age and GAI. However, the diagnostic effect on FC in boys is not significant when controlling for GNG task performance (tau and CR).
Fourth Common Mega-component (DxSex-C4/Tau-C3/CR-C4)
The fourth component identified across all three models consisted of subregions of sensorimotor (right lateralized somatomotor) and visual (lateral occipital cortex) networks with positive loadings, as well as subregions of the DM (ACC, superior parietal lobe, supplementary motor area [SMA]) and visual (anterior primary visual; BA 17) networks with negative loadings (Figure 3D). This component differed among girls and boys with ADHD (DxSex C4 p < .001; tau C3 p = .046; CR C4 p = .045), even when controlling for GNG performance, such that boys with ADHD showed stronger FC within this network with and without CR as a covariate. In contrast, girls with ADHD show stronger FC within this network when tau is included in the model, suggesting network topology differences among girls and boys with ADHD with equivalent response variability. Furthermore, this component was negatively related to tau (p < .001) and CR (p = .015) in boys with ADHD, but not among girls with ADHD.
Unique Component in the DxSex Model (DxSex-C5)
The DxSex-C5 component was only present in the DxSex model, consisting of subregions of the CC (left central executive network, insula), DM (dorsolateral prefrontal cortex [dlPFC]), sensorimotor (caudate, ACC/BA 6), visual (temporal occipital fusiform cortex), and subcortical (caudate) networks with positive loadings, and subregions of the sensorimotor (SMA), visual (visual cortex), DM (PCC), and auditory (opercular cortex) networks with negative loadings (Figure 3E). Results suggest that this component is negatively related to age (p < .001) and positively related to sex in both ADHD and TD groups, with higher FC in boys (p values < .001).
Unique Components in the GNG Tau Model (Tau-C5-C6)
Two unique components were identified in the GNG tau model (Figures 3F, G). The first component (tau C5) consists of subregions of the DM (PC, SMA), visual (secondary visual area; BA 18), and auditory (opercular cortex) networks with positive loadings and subregions of the CC (medial superior frontal cortex), DM (dlPFC, superior parietal lobe), sensorimotor (ACC/BA 6, primary somatosensory, dorsal somatomotor), and subcortical (caudate) networks with negative loadings. Within this component, FC is reduced among girls and boys with ADHD (controlling for age, GAI, and tau) relative to TD girls and boys (p = .023, p < .001, respectively). In addition, among boys, FC is correlated with tau, but in the opposite direction for those with ADHD (positive, p = .002) compared with TD (negative, p < .001). The last component (tau C6) consists of subregions of the DM (dlPFC, PCC), sensorimotor (SMA, dorsal somatomotor), and visual (lateral occipital cortex, anterior primary visual/BA 17) networks with positive loadings and subregions of the CC (supramarginal gyrus), DM (superior parietal lobe), and sensorimotor (right lateralized somatomotor) networks with negative loadings, with a sex difference in TD children (p = .021). FC between regions in the same loading sign is significantly lower in TD boys than TD girls, while FC between regions in the opposite loading signs is higher.
Unique Components in the GNG Commission Error Rate Model (CR-C5-C7)
The CAP approach identified three unique components in the GNG CR model (Figure 3H–J). The first component (C5) consisted of subregions of the CC (inferior frontal gyrus, dlPFC), DM (PCC, precuneus), and sensorimotor (ACC/BA 6) networks with positive loadings and subregions of the visual (secondary visual area; BA 18), and dorsal attention networks with negative loadings. This component was positively associated with CR among girls with and without ADHD (p = .004). The second component (C6) consisted of subregions of the CC (dlPFC), DM (superior parietal lobe), and visual (posterior primary visual area; BA 17) networks with positive loadings and subregions of the DM (PCC, ACC), visual (secondary visual area; BA 18), and subcortical (striatal) networks with negative loadings. This component was positively related to GAI (p ≤ .001) and negatively related to CR in girls with ADHD (p = .014). In addition, FC within this network was lower in boys with ADHD than girls with ADHD (p = .028) and in TD boys compared with TD girls (p = .003). The third component (C7) consisted of subregions of the DM (SMA, PCC) and sensorimotor (right lateralized somatomotor) networks with positive loadings and subregions of the DM (dlPFC, ACC, superior parietal lobe), sensorimotor (left lateralized somatomotor), and auditory (opercular cortex) networks with negative loadings. This component was negatively related to GAI (p = .001), age (p = .016), and CR (p = .010) in girls with ADHD.
Discussion
In this study, we present a novel statistical approach to characterize relationships between functional brain organization and cognitive function among a large cohort of children with ADHD and TD children. Our approach identifies higher-order networks through simultaneous modeling of multiple lower-order networks, which boosts statistical power compared with more traditional pairwise regression approaches by substantially reducing the number of tests performed. Our results reveal complex, widespread FC patterns associated with ADHD-related sex differences involving regions of the DM, CC, somatomotor, subcortical, and visual networks. The presence and direction of these ADHD-related sex differences in FC changed when we included response control measures. These novel findings are discussed in greater detail below and placed in context of the limited existing literature examining ADHD-related sex differences.
The first network was positively related to age such that older children showed greater ITG-striatal FC and PCC-thalamic FC than younger children, whereas PCC-striatal and ITG-thalamic FC decreased with age. To our knowledge, changes in frontosubcortical connectivity across the 8- to 12-year-old age range have not been the focus of previous reports, with prior studies spanning middle childhood through early adulthood (ages 8–44 years). Our results suggest increasing subcortico-cortical FC with age in middle childhood, which differs from previous findings in a larger age range and different regions (48,49). Our finding of age-related changes in thalamic-cortical FC for frontal and temporal regions is consistent with a prior study of 8- to 32-year-olds (50), providing further support for distinct age-related changes in thalamic-cortical FC for frontal and temporal regions.
The second network identified across models consists of competing contributions from FC among CC-visual-somatomotor regions with FC between CC and DMN regions and was negatively associated with intellectual reasoning ability (GAI). ADHD-related sex differences were observed, such that girls with ADHD displayed greater FC than did TD girls and lower FC than did boys with ADHD, and FC within this component was positively associated with response variability only in boys with ADHD. Collectively, these findings are among the first to identify RSNs related to response variability in children with ADHD and differentially affected in girls and boys with ADHD. One previous study to our knowledge reported that atypical frontosubcortical FC of ICA components was greatest among girls with ADHD (6). These findings expand on the previous results by applying a whole-brain analytic approach to reveal more widespread alterations.
As mentioned, including response variability in the model eliminated the observed diagnostic effect in girls with ADHD. Intrasubject response variability is among the most ubiquitous findings in the ADHD neuropsychological literature (51), although the neural correlates of this behavioral characteristic are not well defined. There is a lack of research examining RSNs in relation to response variability, with studies instead focusing on hemodynamic response variability during an fMRI task (52) or associations with task-related activation (53). Increased response variability in ADHD is not task specific (54), suggesting that identifying RSNs related to this variability may be more informative than task-specific patterns of brain activation. Our findings suggest that greater FC between executive/motor control and occipital regions and between subregions of the DMN with the insula and ITG relate to increased response variability among boys with ADHD only. Previous studies reporting that abnormal between-network connectivity is associated with ADHD symptom severity suggest that different network topology phenotypes underlie the neurobiological heterogeneity of ADHD subtypes. A similar argument could be made when examining functional network topology in relation to cognitive task performance given the established heterogeneity in cognitive deficits among individuals diagnosed with ADHD. FC among these regions did not differ among boys with ADHD compared with TD boys and was not associated with response variability in TD boys, suggesting that this may be specific to heterogeneity in this cognitive process in boys with ADHD rather than a more general neural correlate of intrasubject variability.
The third network we identified consists of competing contributions from FC within DMN regions with FC among DMN, CC, and visual regions, and boys with ADHD showed higher FC within a DMN subregion (precuneus) with positive loadings, as well as subregions of the CC (inferior frontal gyrus, insula), DM (PCC) and visual (lateral occipital cortex) networks with negative loadings, compared with TD boys. This pattern is consistent with previous findings of hyperconnectivity both within the DMN and between the DMN and other networks, particularly among boys with ADHD who are primarily, if not exclusively, examined in prior studies. Finally, this diagnostic effect in boys is not observed when controlling for GNG performance, suggesting that the FC differences observed in ADHD may contribute to response control deficits during a GNG task with minimal cognitive demands shown to be greatest among boys with ADHD in this age range (5).
The fourth common network identified across models consists of competing contributions of visual-somatomotor FC and visual-DMN FC, such that boys with ADHD show hyperconnectivity between visual regions with DM and motor networks compared with girls with ADHD. This sex difference in children with ADHD remains after accounting for response inhibition, which was greatest among boys with ADHD. However, the direction of the effect changes when response variability is accounted for in the tau model such that girls with ADHD show increased FC in this network compared with TD boys demonstrating similar response variability. Finally, FC within this network is negatively associated with both tau and CR in boys with ADHD, suggesting a possible sex-specific neural correlate, as discussed above. This pattern of findings suggests that visual-somatomotor and visual-DMN FC is differentially affected in girls and boys with ADHD, with boys generally showing greater anomalies and associations with response control.
The unique components of interest from the tau model (C5) showed a diagnostic difference in girls and boys with ADHD and associations with tau in boys only, while the unique component of interest from the CR model (C5–C7) showed a sex difference in children with ADHD (C6) and associations with CR in girls with ADHD (C5–C7). Our behavioral findings are consistent with prior reports of ADHD-related sex differences in response inhibition, such that girls with ADHD show intact response inhibition compared with TD girls when cognitive demands are minimal, whereas boys with ADHD make significantly more response inhibition errors (5). However, within the group of girls with ADHD, individual differences in response inhibition are important to consider, with some girls with ADHD showing impairments in this cognitive process. The results from the three unique components of the CR model suggest that individual variability among subregions of the CC, DM, sensorimotor, visual, and dorsal attention networks relates to response inhibition among girls with ADHD.
In conclusion, using a novel statistical approach to identify a common linear projection of multiple time courses across subjects such that variations in FC defined by the projection can be explained by the covariates of interest, we found a complex pattern of ADHD-related sex differences that would be difficult to capture using traditional pairwise regression approaches. Important next steps include replicating these findings among a larger sample of girls with and without ADHD, as the smaller sample size of girls is a limitation of this study. Additional limitations include the short scan duration, which also impacted our ability to examine changes in FC over the course of the scan, an important direction for future research. These findings add to the growing literature suggesting that the neurobiological basis for ADHD may differ among girls and boys (6, 7, 8,55). This is also important in advancing our understanding of ADHD-related sex differences in cognitive functions, which may suggest different etiologic pathways for girls and boys with ADHD that may ultimately inform prevention and intervention approaches. Future studies oversampling girls with ADHD, as we have done, will be important for replicating these findings and establishing the utility of this novel approach to analysis of intrinsic functional network topology in relation to ADHD and associated cognitive functions.
Acknowledgments and Disclosures
This research was supported by the National Institutes of Health (Grant Nos. R01MH078160 and R01MH085328 [to SHM], Grant No. K23MH101322 [to KSR], Grant No. K01MH109766 [to MBN], and Grant No. K23MH107734 [to Karen Seymour]) and by the Intellectual and Developmental Disabilities Research Center at Kennedy Krieger Institute and The Johns Hopkins University (Grant No. NIH P50HD103538).
A previous version of this article was published as a preprint on bioRxiv: https://doi.org/10.1101/2021.02.09.430522.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
These participants were drawn from a larger sample of 690 8- to 12-year-old children who participated in one of several neuroimaging studies of ADHD conducted at our center between 2008 and 2019, including 318 children with ADHD (228 boys) and 372 TD children (258 boys). Analyses focused on a subset of this sample after excluding participants who did not complete the rs-fMRI scan due to noncompliance (n = 23; 16 ADHD), moved excessively during the rs-fMRI scan (n = 154; 90 ADHD), or did not have relevant behavioral data (n = 296; 97 ADHD).
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.06.003.
Supplementary Material
References
- 1.Polanczyk G.V., Willcutt E.G., Salum G.A., Kieling C., Rohde L.A. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43:434–442. doi: 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 3.Barkley R.A. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Nigg J.T. Response inhibition and disruptive behaviors: Toward a multiprocess conception of etiological heterogeneity for ADHD combined type and conduct disorder early-onset type. Ann N Y Acad Sci. 2003;1008:170–182. doi: 10.1196/annals.1301.018. [DOI] [PubMed] [Google Scholar]
- 5.Seymour K.E., Mostofsky S.H., Rosch K.S. Cognitive load differentially impacts response control in girls and boys with ADHD. J Abnorm Child Psychol. 2016;44:141–154. doi: 10.1007/s10802-015-9976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosch K.S., Mostofsky S.H., Nebel M.B. ADHD-related sex differences in fronto-subcortical intrinsic functional connectivity and associations with delay discounting. J Neurodev Disord. 2018;10:34. doi: 10.1186/s11689-018-9254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirlikov B., Shiels Rosch K., Crocetti D., Denckla M.B., Mahone E.M., Mostofsky S.H. Distinct frontal lobe morphology in girls and boys with ADHD. Neuroimage Clin. 2015;7:222–229. doi: 10.1016/j.nicl.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson L.A., Peterson D.J., Rosch K.S., Crocetti D., Mori S., Mostofsky S.H. Sex-based dissociation of white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:938–946. doi: 10.1016/j.jaac.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoekzema E., Carmona S., Ramos-Quiroga J.A., Richarte Fernández V., Bosch R., Soliva J.C., et al. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum Brain Mapp. 2014;35:1261–1272. doi: 10.1002/hbm.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills B.D., Miranda-Dominguez O., Mills K.L., Earl E., Cordova M., Painter J., et al. ADHD and attentional control: Impaired segregation of task positive and task negative brain networks. Netw Neurosci. 2018;2:200–217. doi: 10.1162/netn_a_00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sripada C., Kessler D., Fang Y., Welsh R.C., Prem Kumar K.P., Angstadt M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2014;35:4693–4705. doi: 10.1002/hbm.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellanos F.X., Margulies D.S., Kelly C., Uddin L.Q., Ghaffari M., Kirsch A., et al. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler D., Angstadt M., Welsh R.C., Sripada C. Modality-spanning deficits in attention-deficit/hyperactivity disorder in functional networks, gray matter, and white matter. J Neurosci. 2014;34:16555–16566. doi: 10.1523/JNEUROSCI.3156-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fassbender C., Zhang H., Buzy W.M., Cortes C.R., Mizuiri D., Beckett L., Schweitzer J.B. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helps S.K., Broyd S.J., James C.J., Karl A., Chen W., Sonuga-Barke E.J.S. Altered spontaneous low frequency brain activity in attention deficit/hyperactivity disorder. Brain Res. 2010;1322:134–143. doi: 10.1016/j.brainres.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 16.Tian L., Jiang T., Liang M., Zang Y., He Y., Sui M., Wang Y. Enhanced resting-state brain activities in ADHD patients: A fMRI study. Brain Dev. 2008;30:342–348. doi: 10.1016/j.braindev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Carmona S., Hoekzema E., Castellanos F.X., García-García D., Lage-Castellanos A., Van Dijk K.R.A., et al. Sensation-to-cognition cortical streams in attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2015;36:2544–2557. doi: 10.1002/hbm.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcos-Vidal L., Martínez-García M., Pretus C., Garcia-Garcia D., Martínez K., Janssen J., et al. Local functional connectivity suggests functional immaturity in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2018;39:2442–2454. doi: 10.1002/hbm.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Q., Zang Y., Sun L., Sui M., Long X., Zou Q., Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: A resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- 20.Wang L., Zhu C., He Y., Zang Y., Cao Q., Zhang H., et al. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30:638–649. doi: 10.1002/hbm.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao X., Cao Q., Long X., Sun L., Sui M., Zhu C., et al. Abnormal resting-state functional connectivity patterns of the putamen in medication-naive children with attention deficit hyperactivity disorder. Brain Res. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Castellanos F.X., Proal E. Large-scale brain systems in ADHD: Beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posner J., Park C., Wang Z. Connecting the dots: A review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev. 2014;24:3–15. doi: 10.1007/s11065-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y., Wang B., Mostofsky S.H., Caffo B.S., Luo X. Covariate Assisted Principal regression for covariance matrix outcomes. Biostatistics. 2021;22:629–645. doi: 10.1093/biostatistics/kxz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y., Caffo B.S., Wang B., Li C.-S.R., Luo X. A whole-brain modeling approach to identify individual and group variations in functional connectivity. Brain Behav. 2021;11 doi: 10.1002/brb3.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farahani F.V., Karwowski W., Lighthall N.R. Application of graph theory for identifying connectivity patterns in human brain networks: A systematic review. Front Neurosci. 2019;13:585. doi: 10.3389/fnins.2019.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joules R., Doyle O.M., Schwarz A.J., O’Daly O.G., Brammer M., Williams S.C., Mehta M.A. Ketamine induces a robust whole-brain connectivity pattern that can be differentially modulated by drugs of different mechanism and clinical profile. Psychopharmacology. 2015;232:4205–4218. doi: 10.1007/s00213-015-3951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W., Zhang X., Li L. Common reducing subspace model and network alternation analysis. Biometrics. 2019;75:1109–1120. doi: 10.1111/biom.13099. [DOI] [PubMed] [Google Scholar]
- 29.Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: Identifying differences in brain networks. NeuroImage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 30.Shen X., Finn E.S., Scheinost D., Rosenberg M.D., Chun M.M., Papademetris X., Constable R.T. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. 2017;12:506–518. doi: 10.1038/nprot.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wodka E.L., Mahone E.M., Blankner J.G., Larson J.C., Fotedar S., Denckla M.B., Mostofsky S.H. Evidence that response inhibition is a primary deficit in ADHD. J Clin Exp Neuropsychol. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- 33.Shiels Rosch K., Dirlikov B., Mostofsky S.H. Increased intrasubject variability in boys with ADHD across tests of motor and cognitive control. J Abnorm Child Psychol. 2013;41:485–495. doi: 10.1007/s10802-012-9690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosch K.S., Dirlikov B., Mostofsky S.H. Reduced intrasubject variability with reinforcement in boys, but not girls, with ADHD: Associations with prefrontal anatomy. Biol Psychol. 2015;110:12–23. doi: 10.1016/j.biopsycho.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinelli M.K., Mostofsky S.H., Rosch K.S. Investigating the impact of cognitive load and motivation on response control in relation to delay discounting in children with ADHD. J Abnorm Child Psychol. 2017;45:1339–1353. doi: 10.1007/s10802-016-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patros C.H.G., L Sweeney K, Mahone E.M., Mostofsky S.H., Rosch K.S. Greater delay discounting among girls, but not boys, with ADHD correlates with cognitive control. Child Neuropsychol. 2018;24:1026–1046. doi: 10.1080/09297049.2017.1359525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang X., Seymour K.E., Crocetti D., Miller M.I., Mostofsky S.H., Rosch K.S. Response control correlates of anomalous basal ganglia morphology in boys, but not girls, with attention-deficit/hyperactivity disorder. Behav Brain Res. 2019;367:117–127. doi: 10.1016/j.bbr.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leth-Steensen C., Elbaz Z.K., Douglas V.I. Mean response times, variability, and skew in the responding of ADHD children: A response time distributional approach. Acta Psychol. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 39.Shappell H.M., Duffy K.A., Rosch K.S., Pekar J.J., Mostofsky S.H., Lindquist M.A., Cohen J.R. Children with attention-deficit/hyperactivity disorder spend more time in hyperconnected network states and less time in segregated network states as revealed by dynamic connectivity analysis. Neuroimage. 2021;229:117753. doi: 10.1016/j.neuroimage.2021.117753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schirmer M.D., Venkataraman A., Rekik I., Kim M., Mostofsky S.H., Nebel M.B., et al. Neuropsychiatric disease classification using functional connectomics - Results of the connectomics in neuroimaging transfer learning challenge. Med Image Anal. 2021;70:101972. doi: 10.1016/j.media.2021.101972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erhardt E.B., Rachakonda S., Bedrick E.J., Allen E.A., Adali T., Calhoun V.D. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mejia A.F., Nebel M.B., Wang Y., Caffo B.S., Guo Y. Template independent component analysis: Targeted and reliable estimation of subject-level brain networks using big data population priors. J Am Stat Assoc. 2020;115:1151–1177. doi: 10.1080/01621459.2019.1679638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Martino F., Gentile F., Esposito F., Balsi M., Di Salle F., Goebel R., Formisano E. Classification of fMRI independent components using IC-fingerprints and support vector machine classifiers. Neuroimage. 2007;34:177–194. doi: 10.1016/j.neuroimage.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 44.Koch W., Teipel S., Mueller S., Buerger K., Bokde A.L., Hampel H., et al. Effects of aging on default mode network activity in resting state fMRI: Does the method of analysis matter? Neuroimage. 2010;51:280–287. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Allen E.A., Erhardt E.B., Damaraju E., Gruner W., Segall J.M., Silva R.F., et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2013;76:439–441. doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couvy-Duchesne B., Ebejer J.L., Gillespie N.A., Duffy D.L., Hickie I.B., Thompson P.M., et al. Head motion and inattention/hyperactivity share common genetic influences: Implications for fMRI studies of ADHD. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porter J.N., Roy A.K., Benson B., Carlisi C., Collins P.F., Leibenluft E., et al. Age-related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Dev Cogn Neurosci. 2015;11:83–95. doi: 10.1016/j.dcn.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Duijvenvoorde A.C.K., Westhoff B., de Vos F., Wierenga L.M., Crone E.A. A three-wave longitudinal study of subcortical–cortical resting-state connectivity in adolescence: Testing age- and puberty-related changes. Hum Brain Mapp. 2019;40:3769–3783. doi: 10.1002/hbm.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fair D.A., Bathula D., Mills K.L., Dias T.G.C., Blythe M.S., Zhang D., et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kofler M.J., Rapport M.D., Sarver D.E., Raiker J.S., Orban S.A., Friedman L.M., Kolomeyer E.G. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clin Psychol Rev. 2013;33:795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Sørensen L., Eichele T., van Wageningen H., Plessen K.J., Stevens M.C. Amplitude variability over trials in hemodynamic responses in adolescents with ADHD: The role of the anterior default mode network and the non-specific role of the striatum. Neuroimage Clin. 2016;12:397–404. doi: 10.1016/j.nicl.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simmonds D.J., Fotedar S.G., Suskauer S.J., Pekar J.J., Denckla M.B., Mostofsky S.H. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Epstein J.N., Langberg J.M., Rosen P.J., Graham A., Narad M.E., Antonini T.N., et al. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25:427–441. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seymour K.E., Tang X., Crocetti D., Mostofsky S.H., Miller M.I., Rosch K.S. Anomalous subcortical morphology in boys, but not girls, with ADHD compared to typically developing controls and correlates with emotion dysregulation. Psychiatry Res Neuroimaging. 2017;261:20–28. doi: 10.1016/j.pscychresns.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.