Abstract
Chronic kidney disease (CKD) as identified by a reduced glomerular filtration rate (eGFR) is a common comorbidity in patients with heart failure with reduced ejection fraction (HFrEF). The presence of CKD is associated with more severe HF, and CKD itself a strong independent risk factor of poor cardiovascular outcome. Furthermore, the presence of CKD often influences the decision to start, uptitrate or discontinue possible life saving HFrEF therapies. Since pivotal HFrEF randomized clinical trials have historically excluded patients with stage 4 and 5 CKD (eGFR < 30 mL/min/1.73m2), information on the efficacy and tolerability of HFrEF therapies in these patients is limited. However, more recent HFrEF trials with novel classes of drugs, included patients with more severe CKD. In this review on medical therapy in patients with HFrEF and CKD, we show that for both all-cause mortality and/or the combined endpoint of cardiovascular (CV) death or HF hospitalization, most drug classes are safe and effective up to CKD stage 3B (eGFR minimum 30 mL/min/1.73m2). For more severe CKD (stage 4), there is evidence of safety and efficacy of sodium glucose co-transporter 2 inhibitors (SGLT2i), and to a lesser extent angiotensin converting enzyme inhibitors (ACEi), vericiguat, digoxin and omecamtiv mecarbil, although this evidence is restricted to improvement of CV death/HF Hospitalization. Data are lacking on the safety and efficacy for any HFrEF therapies in CKD stage 5 (eGFR < 15 mL/min/1.73m2 or dialysis) for either endpoint. Finally, although an initial decline in eGFR is observed upon initiation of several HFrEF drug classes (ACEi/angiotensin receptor blocker(ARB)/mineralocorticoid receptor antagonist (MRA)/angiotensin receptor blocker neprilysin inhibitor(ARNI)/SGLT2i), renal function often stabilizes over time and the drugs maintain their clinical efficacy. A decline in eGFR in the context of a stable or improving clinical condition should therefore not be cause for concern and should not lead to discontinuation of lifesaving HFrEF therapies.
Keywords: chronic kidney disease, heart failure with reduced ejection fraction, evidence based treatment
Introduction
The management of patients with heart failure with reduced ejection fraction (HFrEF) is increasingly complex. In the current era, patients with HFrEF live longer with heart failure because of an increasing number of evidence based treatments. These patients are also older, frail and suffer from a high number of comorbidities.1 Chronic kidney disease (CKD) has consistently been identified as one of the most prevalent comorbidities, and when present, carries the highest population attributable risk for all-cause mortality and HF hospitalization among all comorbidities in HFrEF.1–3 Furthermore, prevalent CKD (decreased estimated glomerular filtration rate (eGFR)), was one of the key determinants of suboptimal guideline-directed medical therapy (GDMT) utilization in Change the Management of Patients with Heart Failure (CHAMP-HF) registry, as well the most common reason not to uptitrate evidence based therapy in A systems BIOlogy Study to Tailored Treatment in Chronic Heart Failure (BIOSTAT-CHF).4–6 Analysis from TRANSLATE-HF recently showed that with more severe CKD, the use of guideline-directed medical therapies was progressively infrequent. There was only 15 and 5% uptake of three classes of evidence based treatments in patients with eGFR 30–44 or < 30 mL/min/1.73m2, respectively.7
Thus, patients with HFrEF and CKD are at double jeopardy of having worse prognosis yet receiving less evidence based HFrEF treatments, compared with patients without CKD. Adding to the complexity, many evidence-based treatments may influence renal function acutely and chronically, which makes the decision to initiate, titrate, or discontinue therapies a challenge. Renal function can be dynamic and a decline in renal function should not always be cause for concern.8 Earlier reviews already highlighted the increased risk associated with prevalent CKD and the sparse literature of GDMT in patients with severe CKD.9–11 In this review, we will discuss the existing (or lack of) evidence of GDMT in HFrEF in different stages of CKD, with inclusion of most recent (sub)studies, and provide clinical context and guidance on how to optimally monitor and treat HFrEF patients with CKD.
Renal function in HFrEF and modulation by evidence based medical treatments
Heart failure and CKD share common risk factors and comorbidities that have detrimental effects on kidney function. These include factors such as hypertension, atherosclerosis, diabetes mellitus, aging and the use of cardiovascular medication. Both HF and CKD are also risk factors for each other, feeding a vicious circle of perpetual decrease in heart and kidney function.
Therefore even before overt heart or renal failure has developed the kidney has already been exposed to triggers that lead to a worsening in kidney function. In addition to decreasing ability of the kidney to filter blood (i.e., GFR), both CKD and HF can perturb factors such as glomerular barrier function (albuminuria), tubular function (sodium avidity and secretory and absorptive defects) and renal endocrine function (reduced erythropoietin and anemia). Specifically, when important macroalbuminuria or severe anemia is present that is deemed disproportionate to the eGFR, specialist advise should be sought. Overall, these risk factors give the clinician some inference toward the renal reserve of the individual patient, and when present increase the overall risk of adverse events. Renal reserve is the ability of the kidneys to augment function following a challenge or therapeutic maneuver. A kidney with minimal renal reserve will already be maximally compensating and thus unable to augment function.
GFR is heavily influenced by renal hemodynamics, and is the product of the filtration gradient across the glomerular membrane and the glomerular surface area (largely determined by nephron number).12,13 Renal blood flow (RBF) is a primary renal hemodynamic parameter, which is dependent of autoregulation in the kidney by afferent and efferent vasoconstriction and dilation. This renal autoregulation is mediated/influenced by many factors, including the renin-angiotensin system (angiotensin II), adenosine (via the tubuloglomerular feedback (TGF) mechanism), direct and indirect effects of the sympathetic nervous system, inflammation and endothelial dysfunction. These effects are on top of the earlier mentioned clinical risk factors such as diabetes, hypertension and atherosclerosis.14–16 Furthermore, renal venous congestion has direct effects on renal autoregulation which is also influenced by renin angiotensin aldosterone system (RAAS) and sympathetic nervous system (SNS) activation.17 A reduction in arterial pressure (e.g., from reduced cardiac output) and/or increase in renal venous pressure will reduce renal perfusion pressure. Small perturbations in renal perfusion pressure will not influence RBF due to renal autoregulatory mechanisms. However, with more severe perturbations in perfusion pressure, the initial response of the kidney is to maintain GFR through efferent vasoconstriction, resulting increases in the filtration fraction (FF = GFR/renal plasma flow) at the expense of an overall increase in renal vascular resistance and often decreased RBF.18,19 While this classic physiology is well described acutely, it is unclear in contemporary HF populations if this is a relevant chronic physiology. Although albuminuria is a common finding in patients with HFrEF, in many cases this is related to glomerular damage, podocyte dysfunction, and tubular dysfunction rather than glomerular hypertension. However, it should be noted that acute “functional” proteinuria appears to be common with decompensated HF and albuminuria should not be considered evidence of intrinsic CKD until measured in a compensated state. Many of the evidence based medical therapies in HFrEF exert their actions on the basis of this renal pathophysiology in HFrEF. Figure 1 gives an overview of the interaction between HFrEF and CKD, as well as the impact of evidence based treatment on renal function, which will be discussed in detail below.
Figure 1. Overview of the potential mechanisms through which evidence based treatments influence renal function in HFrEF.
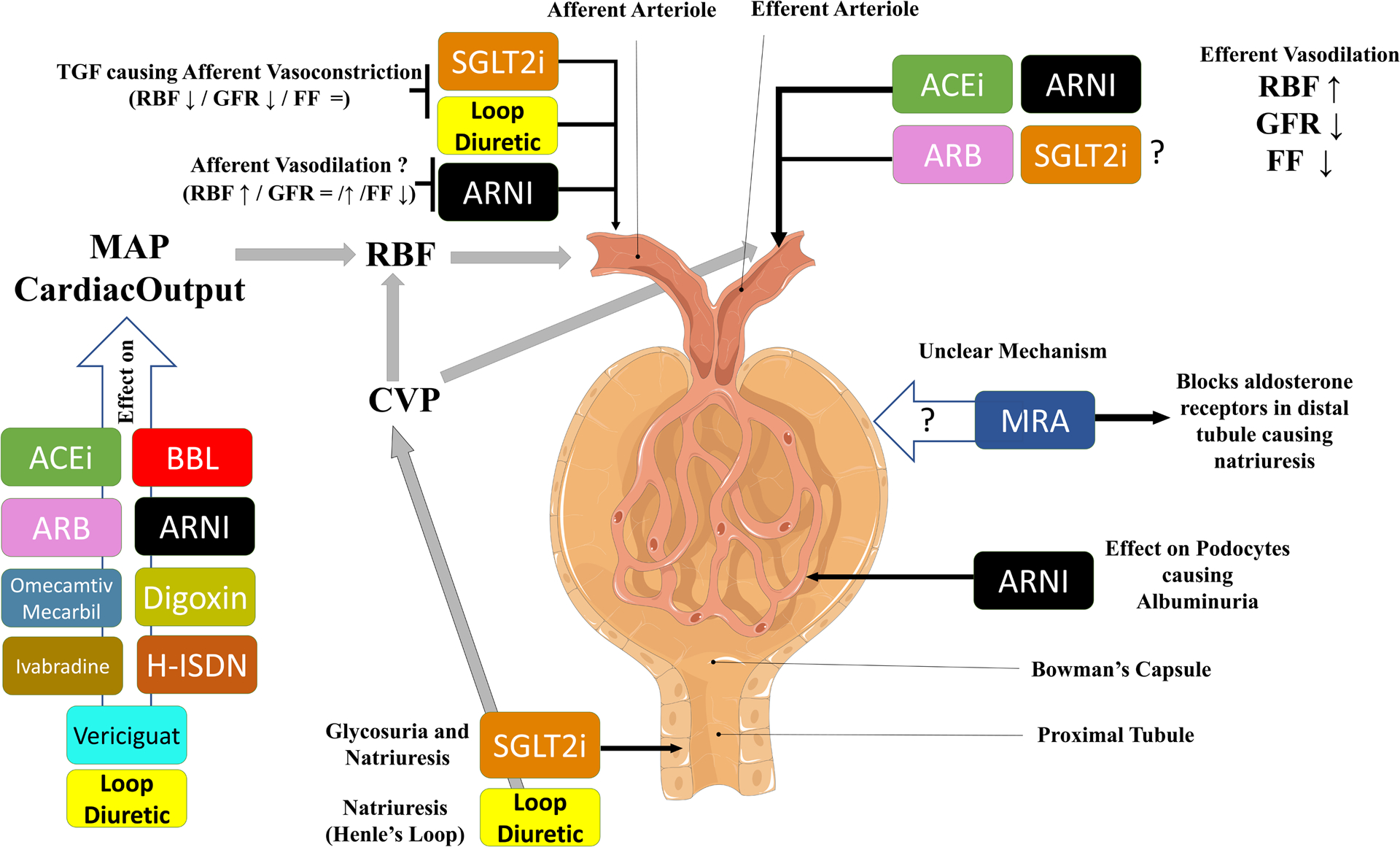
This schematic gives an overview of the potential mechanisms via which evidence based treatments impact renal function in HFrEF. Renin angiotensin system inhibitors (and probably SGLT2i) cause efferent vasodilation, leading to higher RBF, lower GFR and lower FF. It is postulated that SGLT2i have effects on afferent arteriolar tone, causing lower RBF, lower GFR and stable FF. ARNIs may vasodilate the afferent arteriole causing slightly increased RBF and possibly more preserved GFR (as compared with ACEi/ARB alone). ARNIs also influence podocyte function which may be a factor in the modest albuminuria associated with these drugs. It is unclear how MRA influence GFR. Finally, many therapies influence blood pressure, improve contractility and have direct cardiac effects, all of which influence mean arterial pressure and cardiac output/congestion, thereby influencing renal hemodynamics.
Abbreviations: ACEi: Angiotensin Converting Enzyme Inhibitor, ARB: Angiotensin II Receptor Blocker, ARNI: Angiotensin Blocker Neprilysin Inhibitor, BBL: Beta Blocker, CVP, central venous pressure; FF, filtration fraction; GFR, glomerular filtration rate; H-ISDN: Hydralazine-Isosorbidedinitrate, HFrEF, heart failure with reduced ejection fraction; MAP, mean arterial pressure; MRA: Mineralocorticoid Receptor Antagonist, RBF, renal blood flow; SGLT2i: Sodium-glucose co-transporter-2 inhibitor; TGF, tubuloglomerular feedback.
Study characteristics of landmark clinical trials – baseline eGFR, treatment effect and renal exclusion criteria
We reviewed the original reports and (retrospective) analysis of landmark randomized controlled trials (RCTs) on GDMT in HFrEF and specifically searched for published differential treatment effects based on baseline CKD stages and extracted key efficacy and safety information from these publications (Supplementary File). The paucity of data from landmark clinical trials on patients with HFrEF and severe CKD is evident when trial eligibility criteria pertaining to eGFR and renal function are summarized (Supplementary Figure 1). While most clinical trials excluded patients with more severe stages of CKD (4 and 5), recent studies included patients up to a baseline eGFR of 15–20 mL/min/1.73m2. Furthermore, although some trials set a very low eGFR threshold for inclusion, the mean eGFR of patients included in the study was much higher, suggesting that only a minority of included patients had severe CKD. Crude event rates stratified for baseline eGFR or CKD stages were also scarcely reported. Supplementary figure 2 and 3 show the association between baseline eGFR (either overall population or in CKD stages) and the absolute risk reduction (ARR) at the end of the study. Overall, there was no apparent association between baseline eGFR and ARR indicating that a consistent benefit to these medications appears to occur across the spectrum of renal function seen in these trials.
Established Evidence Based HFrEF treatment – ACEi/ARB, MRA and Beta-Blockers
Efficacy of ACEi/ARB in HFrEF patients with CKD
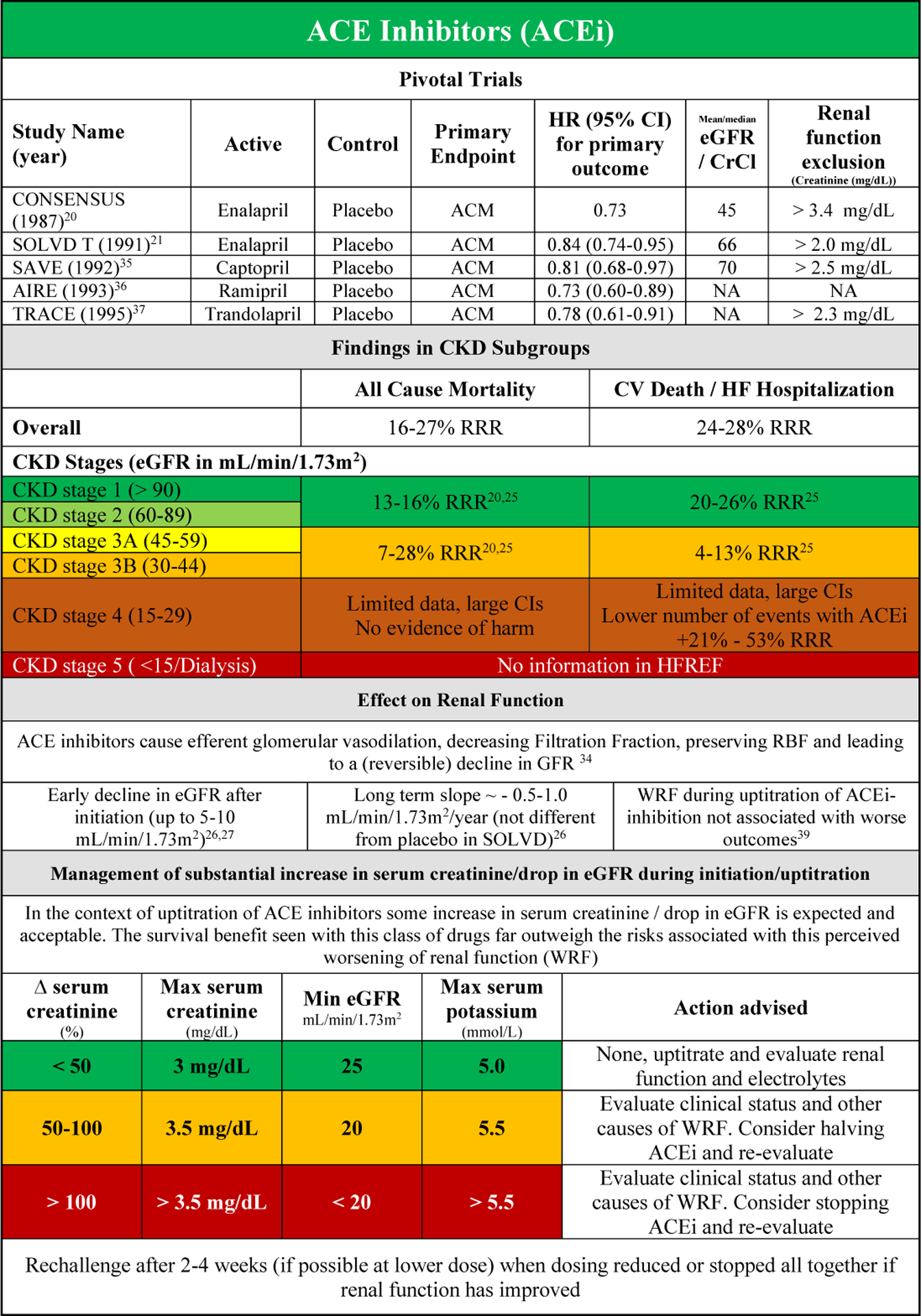
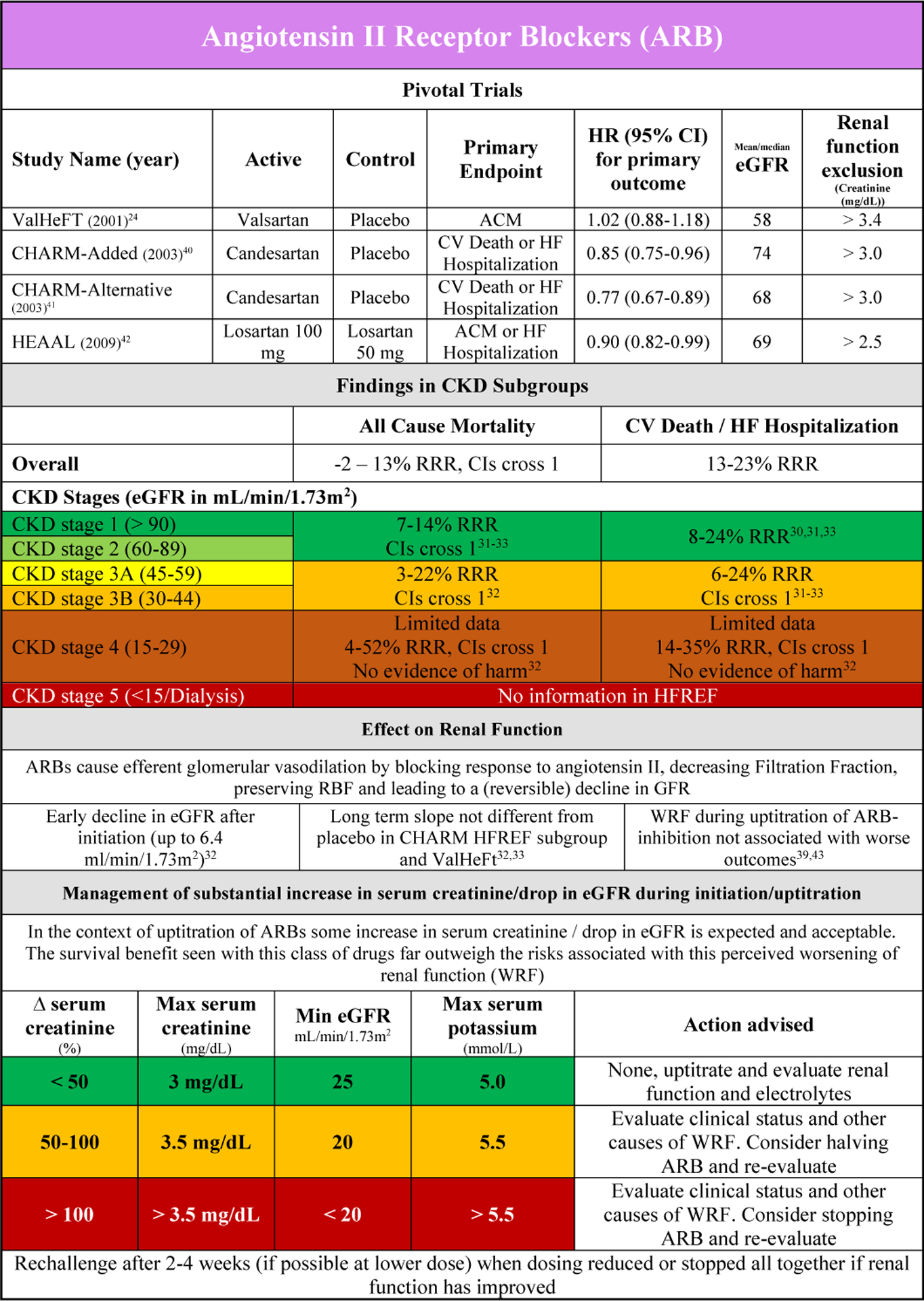
Pivotal trials with ACEi (and or angiotensin receptor blocker (ARB)) in patients with HFrEF typically excluded patients with high serum creatinine at baseline (Supplementary Table 1). ACEis significantly reduce the risk of all-cause mortality and the combined endpoint of CV death or HF hospitalization in HFrEF, while ARBs were more effective in reducing the latter.20–24 In the studies that published interaction and/or subgroups analyses, there was no evidence of effect modification by baseline renal function. However the number of patients in CKD stage 4 was very small and patients in CKD stage 5 were excluded.25–28
In patients with CKD stage 1 to 2 there was clear benefit compared with placebo for ACEi and ARB for both component endpoints.25,28 For CKD stage 3, the evidence for ACEi in reducing CV events was more convincing as compared with ARB treatment, where the confidence intervals were large. (Supplementary Figure 4 A/B). Although the number of patients included in CKD stage 4 for ACEi was small in the SOLVD studies (4–9% of the total population) there was no evidence of harm. However there was also no clear beneficial effect in these patients.21,27,29 Data regarding ARBs in patients with CKD stage 4 are limited as well, however although confidence intervals were large data might suggest a trend towards benefit in reducing CV death and/or HF hospitalization.30 No information on ARB therapy is available in patients with CKD stage 5.
Safety of ACEi/ARB in HFrEF patients with CKD
The safety and adverse events of ACEi/ARB therapy in the subgroup of patients with CKD were often not reported in the (post-hoc analyses of) randomized clinical trials. In SOLVD, the drop in blood pressure (−7 mmHg), increase in serum potassium (+0.2 mEq/L) and increase in serum creatinine (+0.04 mg/dL)(as compared with placebo) were similar in the CKD versus the no CKD group.25,29 In the HEAAL study, a higher dose of losartan was associated with more frequent worsening of renal function (WRF) and hyperkalemia in patients with higher serum creatinine at baseline. However, WRF during initiation was not associated with worse outcomes in the overall study population.31
Renal effects of ACEi/ARB in HFrEF and interaction with outcome
ACEi and ARBs have a heterogenous number of CV effects in HFrEF, including a reduction in blood pressure resulting in afterload reduction, reverse remodeling, but also a decrease in GFR.26,32,33
Early experiments with renin angiotensin system inhibitors (RAASi) have shown that in patients with HFrEF, the inhibition of angiotensin II counteracts the efferent autoregulation, resulting in an increase in RBF, but a drop in FF and as a consequence lower GFR.34 This is the reason ACEi and ARB (can) cause a drop in GFR after initiation, with a mean drop in eGFR of about 6.4 mL/min/1.73m2 in the uptitration phase (Figure 2A and 2B).20,21,25–27,34–43 A modest drop in eGFR (Figure 2A and 2B) should not be worrisome if the clinical status of the patient does not deteriorate, a phenomenon often called pseudo-WRF.8,44
Figure 2. Clinical Summary Figures of class I guideline recommended medical therapies.
A) ACEi
B) ARB
C) MRA
D) Beta-Blocker
E) ARNI
F) SGLT2i
Each figure panel provides evidence of the pivotal scientific background of the drug class overall, for different endpoints and stratified for CKD stages. It summarizes the known (and unknown) effect of each drug class on renal function and how to approach a patient with either worsening renal function and/or hyperkalemia.
Abbreviations: ACM: All Cause Mortality, CI: Confidence Interval, CKD: Chronic Kidney Disease, CrCl: Creatinine Clearance, CV: Cardiovascular, eGFR: estimated Glomerular Filtration Rate, HF: Heart Failure, HR: Hazard Ratio, RRR: Relative Risk Reduction, WRF: Worsening Renal Function. For study acronyms see Supplementary Table 1.
Subgroup and interaction analyses from the large randomized clinical trials have shown that although there is some increased risk associated with this WRF (even with ACEi/ARB therapy), the beneficial effect of these agents is maintained or is even greater than patients who experience no drop in eGFR.39,43
Practical Consideration on the use of ACEi/ARB in HFrEF patients with CKD
Figures 2A and B provide an overview of data on ACEi and ARB, their effect on renal function and associated outcome, as well as a guide on the use of these drugs in patients with CKD or WRF. Monitoring of serum creatinine and serum potassium is warranted in the initiation phase of treatment. If the increase in serum creatinine or potassium is excessive or more than anticipated (Figure 2A/B), this requires further investigation. After possible (temporary) downtitration or even discontinuation, a rechallenge should be considered when renal function (and/or potassium) has recovered after 2 to 4 weeks.
Efficacy of Mineralocorticoid Receptor Antagonist in HFrEF patients with CKD
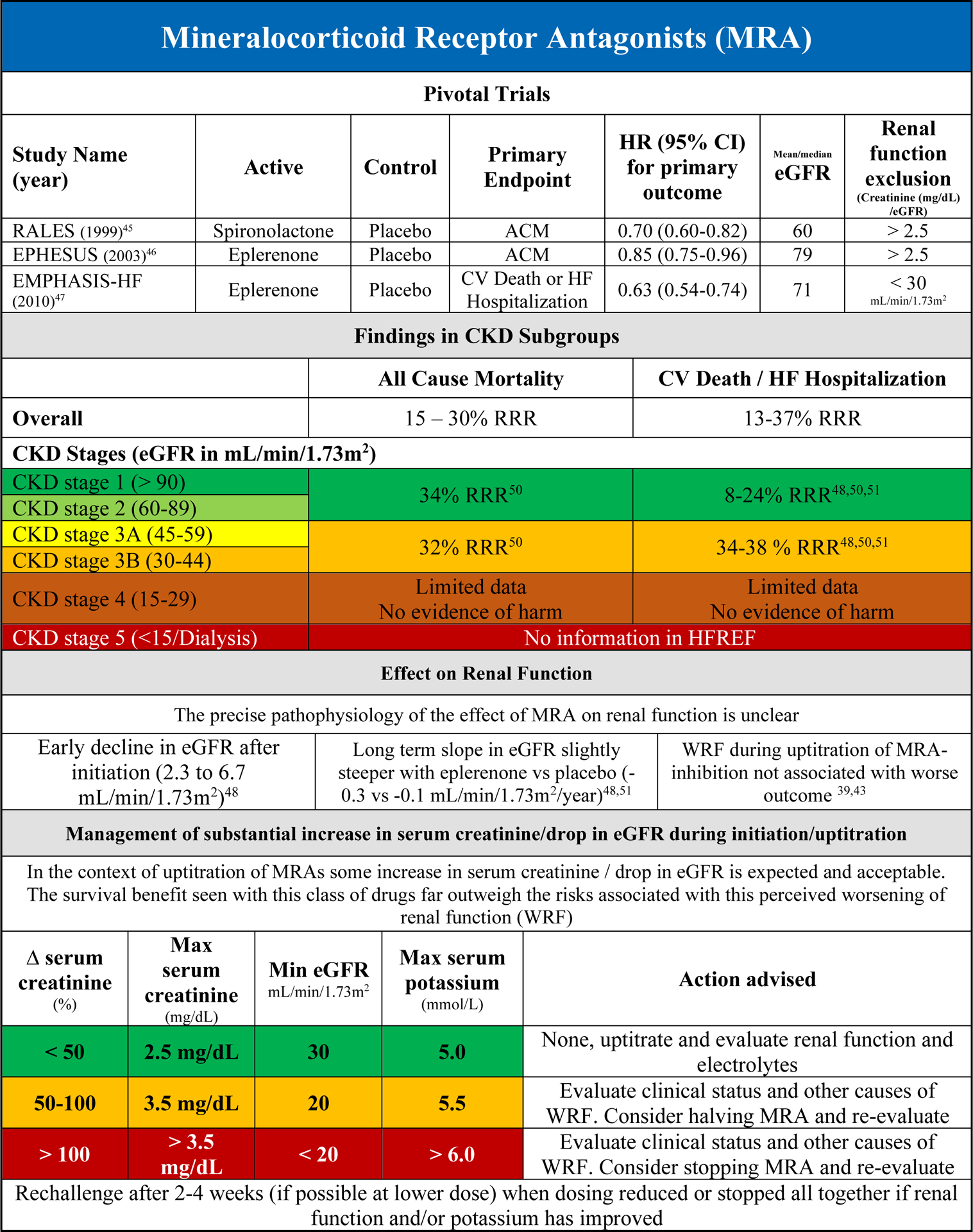
In the two large RCTs with mineralocorticoid receptor antagonist (MRA) in HFrEF (and one in post myocardial left ventricular dysfunction), patients with severe CKD (serum creatinine > 2.5 mg/dL or eGFR < 30 mL/min/1.73m2) were excluded.45–47 Overall, MRAs reduce the risk of all-cause mortality and the combined endpoint of CV death and/or HF hospitalization (Supplementary Figure 4A/B). This effect was found to be irrespective of baseline renal function within the studies, with no evidence of treatment/eGFR interaction.45,47 Although there is very limited data in patients with CKD stage 4, there is no clear evidence of harm in this subgroup of patients.48 However, in patients with severely reduced eGFR at baseline or during follow up, the risk of significant hyperkalemia increases substantially. Despite this, in the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) trial, the beneficial effects of eplerenone were maintained even in the setting of incident hyperkalemia.49 Guidelines recommend the use of MRAs in HFrEF patients with eGFR > 30 mL/min/1.73m2 to avoid the risk of significant hyperkalemia.
Safety of MRA in HFrEF patients with CKD
The safety of MRA in different subgroups, including CKD, was evaluated in an analysis from EMPHASIS-HF.49 Patients with CKD at baseline had increased incidence of hyperkalemia (>5.5 mmol/l) with eplerenone when compared with placebo (70 (16.6) vs 43 (9.3), P=0.002). In the Randomized Aldactone Evaluation Study (RALES), hyperkalemia occurred more frequently in CKD patients, and particularly more frequently in patients with CKD receiving spironolactone when compared with placebo (25.6 vs 8.5%, P<0.001).50. However, the total number of adverse events leading to treatment discontinuation in patients with CKD was significantly lower with MRA treatment compared with placebo. Other adverse events were not reported stratified for CKD presence.
Renal effects of MRA in HFrEF and interaction with outcome
On average, MRA therapy induces a small but significant decline in eGFR during initiation (2.3 to 6.7 mL/min/1.73m2), although the long term decrease in eGFR is similar to placebo.48,51 Even in the setting of a more substantial decrease in eGFR or increase in serum creatinine (WRF), the beneficial effect of MRA therapy remained in both the RALES and EMPHASIS-HF, despite an increasing risk of hyperkalemia.48,50 The mechanism underlying the drop in eGFR with MRA therapy is not entirely clear, but it likely represents acute intra-renal hemodynamic changes much like with ACE-inhibitor initiation.
MRA – Recent insights from CKD populations and future directions
Although there is some data on patients with moderate to severe CKD without heart failure, the overall information is limited. A meta-analysis of MRA therapy in patients on dialysis revealed an association with improved CV outcomes, but a small randomized controlled trial in dialysis patients did not show any benefit.52,53 More recently, finerenone was found to be superior to placebo in The Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) trial in patients with CKD and type 2 Diabetes, reducing a combined renal endpoint by 18%.54 However, patients with eGFR less than 25 mL/min/1.73m2 (Class 4 and 5 CKD) were excluded from FIDELIO-DKD, and only 7% of patients had a history of HF at baseline. Just recently, the results from FIGARO-DKD were announced, where finerenone improved CV endpoints in CKD patients with type 2 diabetes. There is an ongoing trial with finerenone in HF (Finerenone in HF Patients, FINEARTS-HF). In addition, therapies to counteract the increase in serum potassium that is observed with MRA therapy in many patients (and limits uptitration) are currently being investigated in HFrEF (Patiromer in the DIAMOND study, Sodium Zirconium Cyclosilicate in the OPRA-HF (NCT 04789239) and REALIZE-K (NCT04676646) studies.)
Practical Consideration on the use of MRA in HFrEF patients with CKD
Figure 2C summarizes clinical evidence and clinical guidance on the use of MRA in HFrEF patients in different CKD subgroups. MRA treatment should be considered in any HFrEF patient with CKD stages 1–3B. Beyond CKD stage 3B, the scientific evidence is scarce. Initiation and/or uptitration of MRA therapy, if undertaken in patients with slightly lower GFR should include close monitoring of renal function and potassium levels. The focus of monitoring during initiation/uptitration of MRA therapy should be on change in potassium levels (Figure 2C). It is important to realize that the incidence of WRF and hyperkalemia may not evolve simultaneously. WRF can occur without hyperkalemia, while also vice versa is possible. Solitary (severe) hyperkalemia should prompt further endocrine evaluation and thorough review of concomitant medical therapies and diet.
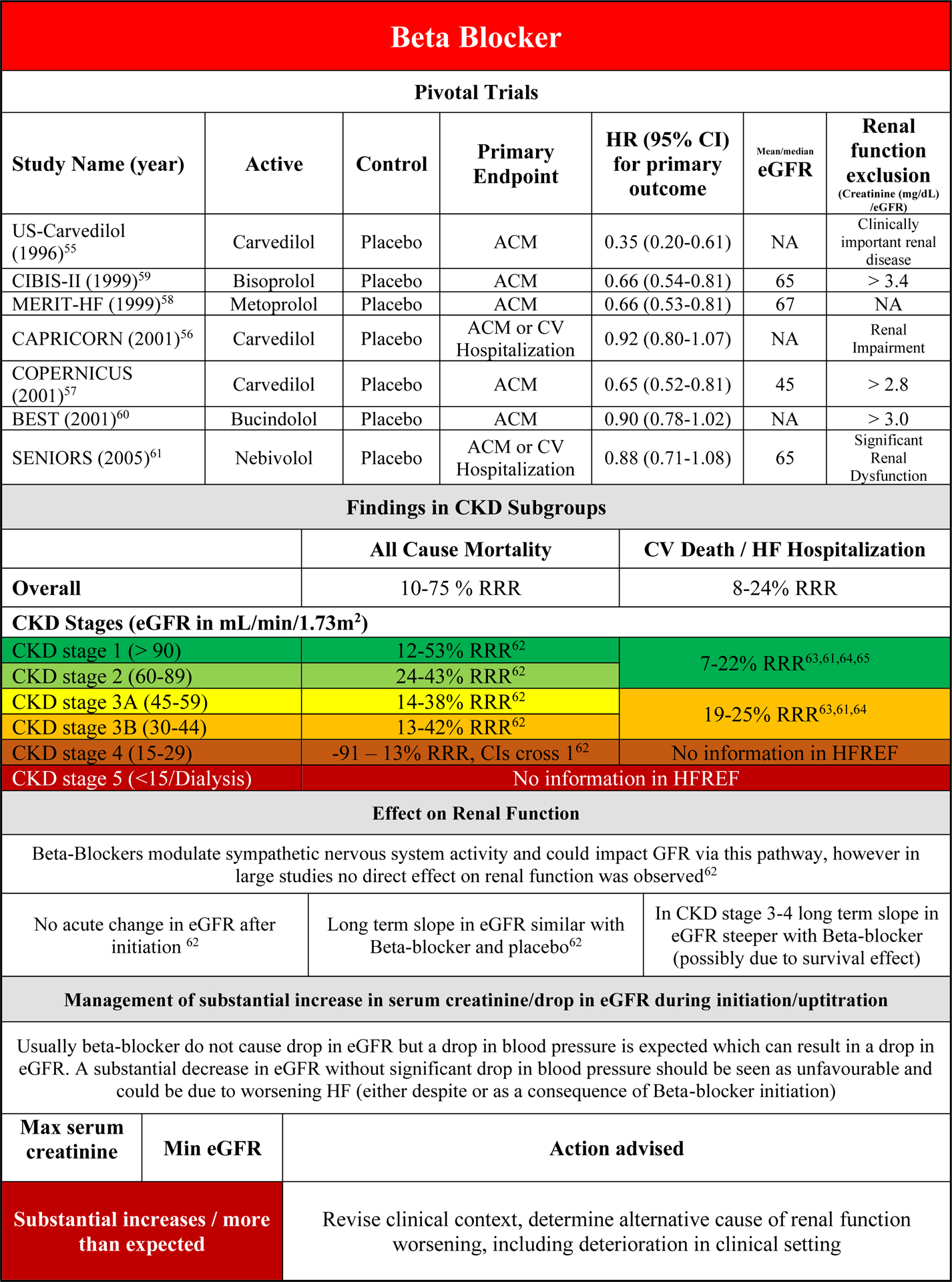
Efficacy of Beta-Blockers in HFrEF patients with CKD
Beta-blocker therapy has consistently shown to be an effective therapy in reducing the risk of all-cause mortality and combined endpoints of all-cause/CV Death or HF hospitalization in HFrEF patients. Exclusion criteria for renal function in the pivotal trials were typically less stringent compared with the RAASi studies (Supplementary Figure 1, Figure 2D).55–66 Data on renal subgroup analyses come from individual substudies or subgroup analyses from the pivotal trials. More robust evidence is published by an individual patient data meta-analysis that included all these trials and evaluated the effect of β-blocker therapy in CKD subgroups.62–64 In that analysis beta-blocker reduced the risk of all-cause mortality up to CKD stage 3B. In contrast in CKD stage 4, there was also no clear benefit of beta-blocker therapy (Supplementary Figure 5A), but also no evidence of harm. There was evidence of a significant interaction between beta-blocker treatment and baseline eGFR on the effect on all-cause mortality. This finding illustrates the uncertainty surrounding beta-blocker therapy as life saving drug in these patients as the current evidence suggest no benefit for all-cause mortality in CKD stage 4 patients with beta-blocker therapy. There may be other important indications to prescribe beta-blockers in these patients, such as rate control in patients with atrial fibrillation and management of ventricular tachycardias.
For the combined end point of cardiovascular death and HF hospitalization, there are only data from the individual studies, showing consistent beneficial effects of β-blocker therapy up to CKD stage 3B, but no evidence in CKD stage 4 (Figure S5B).63–66 No data currently exist for Beta-blocker therapy in HFrEF patients with CKD stage 5 for either endpoint.
Safety of Beta-Blockers in HFrEF patients with CKD
In the individual patient data meta-analysis on beta-blocker therapy in HFrEF, discontinuation rates because of adverse events were higher in patients with more severe CKD, but there was no difference between beta-blocker or placebo.62 Discontinuation rates for renal impairment were similar for placebo and beta-blocker therapy across the entire spectrum of CKD classes.
Renal effects of Beta-Blockers in HFrEF and interaction with outcome
There are no published renal hemodynamic studies on beta-blocker therapy in HFrEF, but it could be hypothesized that by either improving cardiac function, direct renal or neurohormonal effects, beta-blockers may have a beneficial effect on renal function over time. In the individual patient data meta-analysis there was no difference in the change in eGFR over time with Beta-blocker versus placebo.62 However, beta-blockers do lower blood pressure, which can lead to a small decrease in eGFR. If during uptitration of beta-blocker therapy WRF develops without a significant drop in blood pressure, this should always be cause for concern, as this is unexpected and should be interpreted as true WRF until an alternative reason has been found. If WRF developed during uptitration, this was associated with a substantial increase in mortality.62
Practical Consideration on the use of Beta-Blockers in HFrEF patients with CKD
Figure 2D provides an overview of the effect of beta-blocker on clinical and renal endpoints in the context of CKD. Although the improvement in clinical outcome at more severe CKD stages is uncertain, beta-blockers are safe in patients with low eGFR. They can be continued up to severe CKD stages for management of other potential adverse events such as arrythmias.
Other conventional HFrEF therapies – Digoxin, Ivabradine, Hydralazine-Isosorbide Dintrate
In the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT) study, ivabradine treatment was associated with a lower risk of the combined endpoint of CV death and/or HF hospitalization, especially in patients with baseline heart rate ≥ 70 bpm.67 No effect on all-cause mortality was observed (Supplementary Figure 5A). There was no formal renal function cut-off as exclusion criteria besides “severe renal disease”. The beneficial effect of ivabradine on the combined endpoint was similar in patients with eGFR above and below 60 mL/min/1.73m2 (Supplementary Figure 5B). As expected from the mechanism of action, ivabradine had no effect on eGFR or serum creatinine over time.68
In the Digitalis Investigation Group (DIG) study with digoxin, patients were included up to a serum creatinine level of 3.0 mg/dL, which roughly corresponds to an eGFR of around 20 mL/min/1.73m2.69 There were 218 patients with CKD stage 4 included in the study, and the effect of digoxin on HF related death and/or HF rehospitalization was similar in all CKD stages (Supplementary Figure 5B).70 There was no effect on all-cause mortality in the overall study, although there was some evidence of benefit of digoxin in patients with serum creatinine at baseline > 2.0 mg/dL (30% relative risk reduction, P for interaction digoxin x baseline serum creatinine = 0.06) (Supplementary Figure 5A).70 The risk of digoxin toxicity increases with higher CKD stages, which should be a reason to closely monitor digoxin levels and renal function in these patients. Assessment of digoxin levels should be considered early after initiation when at stable doses, after each dose change, and as part of routine follow up in patients with CKD stage 3–4. Digoxin levels should be drawn at sufficient time after initiation/change to allow steady state (8–10 days). The effect of digoxin on renal function is still unclear. With its positive effects on myocardial contractility, it could be hypothesized that digoxin may improve renal perfusion and function, which was retrospectively confirmed in an analysis from DIG.71 Only one small, short term study on intravenous digoxin evaluated (invasive) renal function and found no significant alterations.72
Hydralazine-Isosorbide dinitrate (H-ISDN) was first studied in the Vasodilator-Heart Failure Trials (V-HeFT I and II) but unfortunately the information regarding renal function from these early investigations is scarce.73 Although H-ISDN also carries a class I recommendation, this is specific to the subgroup of African-American patients with HFrEF. For other patients, H-ISDN may be used when RAASis are not tolerated (class II recommendation). In the African-American Heart Failure Trial (A-HeFT) patients with severe renal disease were excluded and H-ISDN was effective in reducing all-cause mortality and/or HF rehospitalization as well as quality of life.74 This effect was regardless of baseline CKD (defined as eGFR above/below 60 mL/min/1.73m2). The effects of H-ISDN on renal function have not been reported.
Other Conventional HFrEF therapies – Future directions
There are new, ongoing studies in modern HFrEF populations with either digoxin (Digoxin Evaluation in Chronic Heart Failure: Investigational Study In Outpatients in the Netherlands, DECISION, NCT NCT03783429) or hydralazine (the DANish randomized, double-blind, placebo controlled trial in patients with chronic HEART failure, DANHEART, NCT03514108), but these studies will exclude CKD class 4 and 5 HF patients. Another study with digitoxin (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure, DIGIT-HF EudraCT [European Union Drug Regulating Clinical Trials Database] 2013-005326-38)75 will only exclude patients with ‘severe renal disease” since digitoxin does not accumulate in patients with renal impairment. This study will therefore provide additional data on the safety and efficacy on glycosides in patients with HFrEF, including those with marked CKD.
Loop Diuretics
There is no randomized, placebo controlled clinical trial on loop diuretic therapy in patients with HFrEF. Loop diuretics are however one of the most used drug classes in chronic HFrEF, but their effect on clinical outcome is unknown. The consensus is that loop diuretics should be used to alleviate congestion in symptomatic patients, and the dose should be downtitrated to the lowest dose that will keep the patient in a euvolemic state. In general, higher stages of CKD will require modestly higher doses of loop diuretics to achieve similar decongestion or euvolemia because tubular delivery of diuretic decreases as GFR falls.76 There is large debate on whether loop diuretics may cause WRF, but in the context of an improvement in clinical status, any deterioration in serum creatinine should be seen as pseudo-WRF. If anything, loop diuretics can cause hypokalemia if decongestion is successful. However, if diuretic response is poor and true WRF does develop, hyperkalemia is possible. For practical reasons, patients with (short term) alterations in loop diuretic dose, including initiation should be monitored closely with respect to renal function and electrolytes.
Novel HFrEF treatment – Angiotensin Receptor Blocker Neprilysin Inhibitor, Sodium Glucose Co-Transporter 2 inhibitors, vericiguat, omecamtiv mecarbil
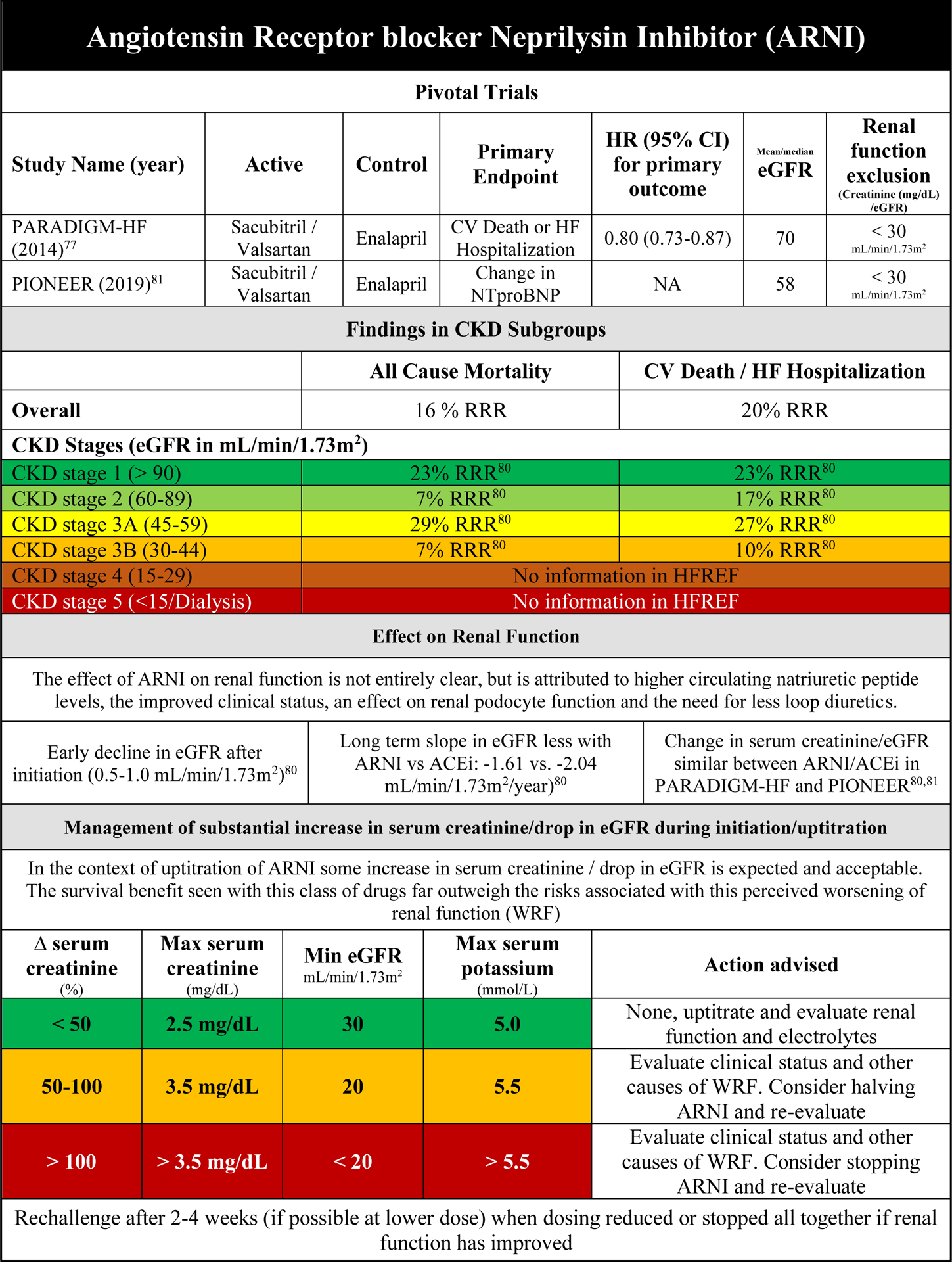
Efficacy of ARNI in HFrEF patients with CKD
Although the pivotal Prospective Comparison of ARNI with ACE inhibition to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial was published in 2014, the uptake of ARNI as replacement for ACEi/ARB or as first line therapy in HFrEF has been slow.77 One reason is that HF guidelines have been conservative recommending ARNI as first line therapy (instead of ACEi). With the most recent update of the ACC/AHA Expert Consensus Decision Pathway for optimized HF treatment as well as other international HF society guidelines, this class has the same level of recommendation as compared with ACEi as first line therapy.78,79 Since there is only one large (endpoint driven) randomized clinical trial in HFrEF, the only available evidence in CKD comes from PARADIGM-HF alone. In a subgroup analysis, sacubitril/valsartan reduced the primary endpoint of CV death and/or HF hospitalization as well as all-cause mortality compared with enalapril up to CKD stage 3B (Supplementary Figure 6A/B).80 As patients with both CKD stage 4 and 5 were excluded, no information exists on the effectiveness of sacubitril/valsartan compared with enalapril in these patients.
Safety of ARNI in HFrEF patients with CKD
In the predefined renal analysis from PARADIGM-HF the renal composite endpoint was numerically but not significantly reduced with sacubitril-valsartan as compared with enalapril in patients with CKD.80 In the same analysis, discontinuation of study drug for renal reasons was significantly less frequent in patients with CKD randomized to sacubitril-valsartan as compared with enalapril. In the comParIson Of sacubitril/valsartan versus Enalapril on Effect on nt-pRo-bnp in patients stabilized from an acute Heart Failure episode (PIONEER-HF) trial in hospitalized HF patients, development of WRF was consistent with sacubitril/valsartan vs enalapril in patients with CKD at baseline. Efficacy was consistent across all high-risk subgroups, including CKD vs no CKD.81 These findings underline the renal safety of the use of ARNI in HFrEF patients with CKD as compared with ACEi therapy.
Renal effects of ARNI in HFrEF and interaction with outcome
Compared with enalapril, sacubitril/valsartan slows the decline in eGFR over time, although the magnitude of the difference is modest. Like other RAAS inhibitors, ARNIs cause a small decline in eGFR during initiation which is reversible after cessation. The cause for this (pseudo) WRF with ARNI is not entirely understood, but may be related to the ARB associated effects of valsartan in the combination drug, as well as additional effects from neprilysin inhibition.80 The latter is also responsible for podocyte alterations that may be the reason for a small increase in urinary albumin excretion that is observed with sacubitril-valsartan. When WRF develops during initiation of ARNI, therapy should be continued (and uptitrated) unless the increase in serum creatinine (and potassium) is large (Figure 2E).77,80,81 Even then, short temporary discontinuation should be sufficient, and if possible, a rechallenge should be considered.
Practical Consideration on the use of ARNI in HFrEF patients with CKD
Figure 2E provides an overview of the effect of ARNI on clinical and renal endpoints in the context of CKD. The suggested response to changes in serum creatinine and potassium for ARNI is similar to that for ACEi/ARB.
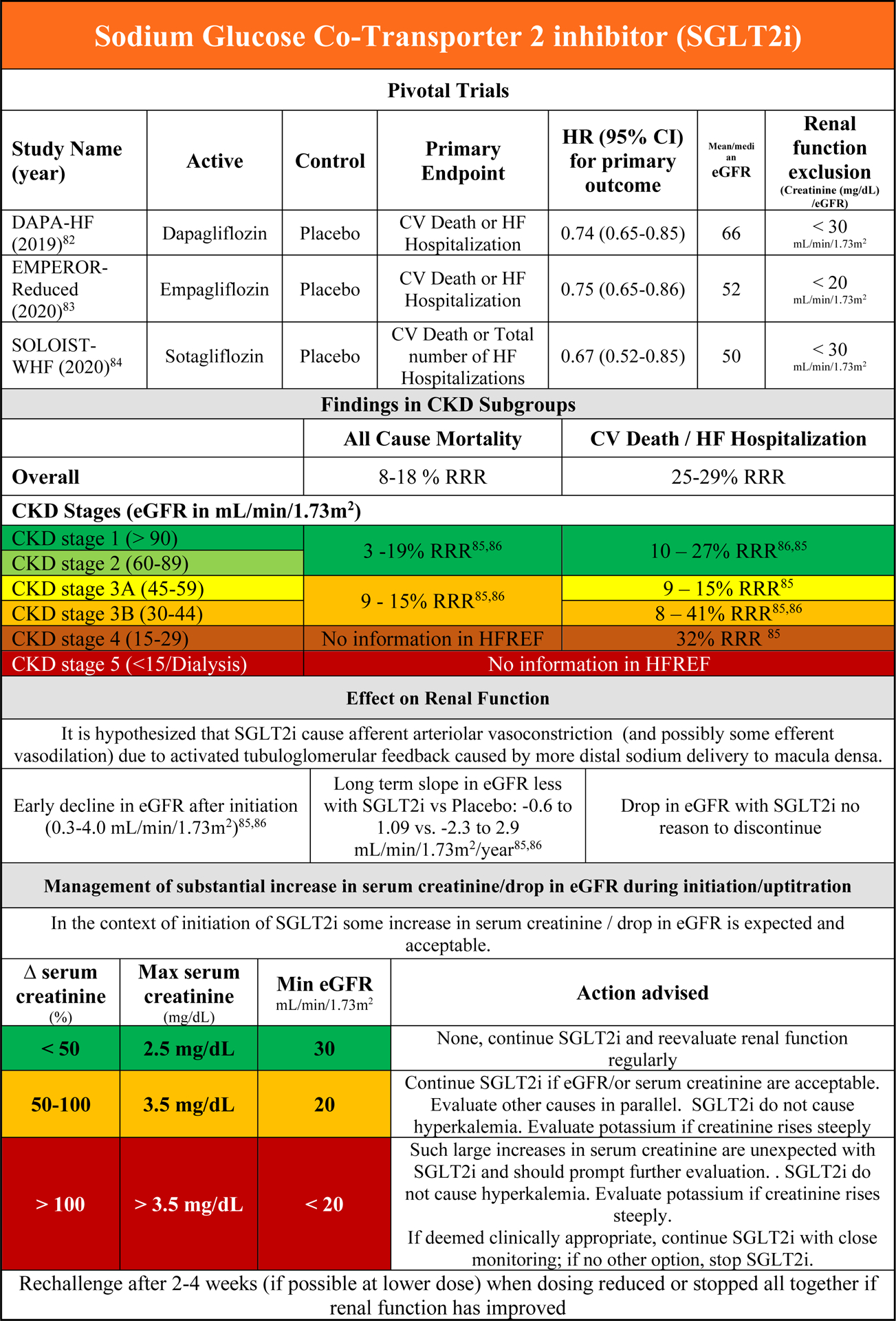
Efficacy of SGLT2i in HFrEF patients with CKD
SGLT2 inhibitors are a new class of drugs in HFrEF that, among many things, reduce renal tubular glucose reabsorption and subsequently increase glucosuria and natriuresis (at least initially). They have been shown to be a safe and effective treatment option that improved clinical outcomes in three large randomized clinical trials in patients with HFrEF (The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure, DAPA-HF; the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and Reduced Ejection Fraction, EMPEROR-Reduced and The effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure, SOLOIST-WHF).82–84 The latter study was conducted with a SGLT1/2 inhibitor primarily among patients recently hospitalized for HF and included patients with preserved ejection fraction. In EMPEROR-Reduced, the lower limit of eGFR for trial inclusion was 20 mL/min/1.73m2, while this was 30 mL/min/1.73m2, for the other studies. Across all trials, there was a clear benefit of SGLT2i up to CKD stage 3B, without evidence of a CKD x treatment interaction (Supplementary Figure 6A/B).83–86 For CKD stage 4, the only published data on the combined endpoint of CV death and/or HF hospitalization comes from EMPEROR-Reduced, where empagliflozin significantly reduced the risk of this endpoint compared with placebo (Supplementary Figure 6B).85 There are no data on SGLT2i therapy in HFrEF patients with CKD stage 5.
Safety of SGLT2i in HFrEF patients with CKD
Detailed information on the safety of SGLT2i in the subgroup of HFrEF patients with CKD have been published.85,86 SGLT2i reduce the risk of study specific combined renal endpoints in patients with or without CKD at baseline. In DAPA-HF, serious renal events were reduced with SGLT2i compared with placebo, while serious adverse events were less frequent with dapagliflozin compared with placebo in patients with CKD.86 Similar findings were published from EMPEROR-HF, showing the (renal) safety of SGLT2i in HFrEF patients with CKD.85
Renal effects of SGLT2i in HFrEF and interaction with outcome
First, it has to be acknowledged that SGLT2i were investigated on top of conventional HFrEF therapies, including ACEi/ARB, MRA and ARNIs. The renal hemodynamics would have already been influenced by these compounds as described above in the section, Renal Effects of ACEi/ARB. Following initiation, SGLT2i caused an early significant drop in eGFR, which on average was 4 mL/min/1.73m2; however over a longer period of time, the decline in eGFR with SGLT2i was slower compared with placebo.83,85,86 Importantly, in EMPEROR-Reduced, the slope of eGFR was included as a predefined, key secondary endpoint in the hierarchical testing strategy and this was significantly improved with empagliflozin compared with placebo.83 Large increases in serum creatinine (and drop in eGFR) are rare. Smaller (mechanistic) studies have demonstrated a decrease in measured GFR (not estimated) after initiation of SGLT2i.87 However, no data exist on the effect on renal hemodynamics (e.g. RBF) in HFrEF patients. It is hypothesized, at least acutely, that SGLT2i cause afferent arteriolar vasoconstriction due to activated TGF caused by more distal sodium/chloride delivery to macula densa. However, in a mechanistic study in patients with diabetes type 2 (without HF), efferent vasodilation rather than afferent vasoconstriction seemed responsible for a drop in GFR.88 Data in patients with HF are lacking. It is important to realize that the drop in GFR with SGLT2i is reversible and should be interpreted in the context of the clinical course of the patient. We are awaiting analyses from EMPEROR-HF and DAPA-HF on the importance of the initial drop in eGFR after initiation of SGLT2i. In most instances, the drop probably represents pseudo-WRF and the SGLT2i should be continued given their beneficial effects on clinical outcomes and preservation of renal function in the long term. However, given the lack of evidence to date, some caution with the continuation (or [temporary] stopping) of SGLT2is when WRF occurs is warranted (Figure 2F).82–86
SGLT2i – Recent insights from CKD populations and future directions
The pivotal SGLT2i studies in populations outside of (primary) HF mostly excluded patients with class 4 and 5 CKD, although the SCORED trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) with sotagliflozin was less restrictive. Even then, only 9% of patients had class 4 CKD at baseline. 89–92 Additional data come from dedicated CKD studies with SGLT2i, including the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial and the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial although even in these studies patients with eGFR < 25 (DAPA-CKD) or 30 (CREDENCE) mL/min/1.73m2 were excluded.93,94 In these trials including patients with and without diabetes, 10% to 15% of patients had HF at baseline, and SGLT2i by dapagliflozin or canagliflozin decreased the risk of renal events (and renal death) and significantly reduced cardiovascular events. Data from these trials in dedicated CKD populations underscore the renal safety and efficacy of these drugs in patients with reduced eGFR. Of note, these trials did not specifically recruit patients with HF.
Practical Consideration on the use of SGLT2i in HFrEF patients with CKD
Figure 2F provides an overview of the effect of SGLT2i on clinical and renal endpoints in the context of CKD. SGLT2i inhibitors are the only class of HFrEF drugs that were systemically investigated in more severe CKD classes and were shown to slow the decline in eGFR over time. Therefore these drugs can be used safely (appropriate laboratory monitoring) in patients with class 4 CKD. As no uptitration is necessary given the fixed dose and SGLT2is do not increase serum potassium, hyperkalemia is less of a concern with SGLT2i.
Efficacy of Vericiguat in HFrEF patients with CKD
The only phase 3 trial to evaluate the clinical benefit of Vericiguat in the HFrEF population was the VerICguaT Global Study in Subjects with HFrEF (VICTORIA) trial.95 In this study the lower limit of eGFR allowed by protocol was 15 mL/min/1.73m2 and it was intended that 15% of patients should have an eGFR between 15 and 30 mL/min/1.73m2. For the endpoint of all-cause mortality, no eGFR subgroup or treatment interaction analysis was published (Supplementary Figure 6A). Vericiguat did not reduce CV death in the overall trial population and in the subgroup of patients with CKD stage 3 and 4. For the combined endpoint of CV death and/or HF hospitalization (the primary outcome of VICTORIA), there was no evidence of treatment x eGFR interaction, and there was also no evidence of harm (Supplementary Figure 6B). Patients with CKD stage 5 were not included.
Safety of Vericiguat in HFrEF patients with CKD
In patients with CKD stage 4 included in VICTORIA, vericiguat showed no excess of adverse events as compared with placebo.96 Across all CKD stage, a higher proportion of patients discontinued vericiguat versus placebo due to WRF after initiation of therapy, but this difference was not significant.
Renal effects of Vericiguat in HFrEF and interaction with outcome
From a physiological perspective, by reducing oxidative stress, increasing cyclic GMP and by improving clinical heart failure status, it could be argued that vericiguat should improve or preserve GFR in HF patients. However, in the VICTORIA study, the decrease in eGFR in the first 16 weeks after the start of vericiguat treatment was larger than placebo, but this difference was not significant after 48 weeks of treatment.
Practical Consideration on the use of Vericiguat in HFrEF patients with CKD
Vericiguat, when adopted in international guidelines, can be used in HFrEF patients with severe CKD stage 4, as VICTORIA included patients eGFR 15 ml/min/1.73m2or greater. As with any evidence based treatment in heart failure, regular monitoring of vitals, serum creatinine and potassium is warranted, but significant WRF should not be expected with vericiguat.
Efficacy of Omecamtiv Mecarbil in HFrEF patients with CKD
The Global Approach to Lowering Adverse Cardiac Outcomes through Improving Contractility in Heart Failure (GALACTIC-HF) trial is the only large randomized controlled trial to assess the effectiveness of omecamtiv mecarbil on clinical endpoints in HFrEF.97 With regard to baseline renal function patients with eGFR < 20 mL/min/1.73m2 were excluded. No information was provided on the basis of baseline eGFR for the end point of all-cause mortality, and omecamtiv mecarbil treatment did not reduce the risk of death in the entire study, also suggesting no benefit in subgroups of CKD stages (Figure S6A). There was a small reduction in the primary outcome of CV death and/or HF hospitalization with treatment, and no significant interaction between eGFR and treatment effect on the primary outcome. The effect in patients with CKD stage 3A/3B even smaller and the confidence intervals crossed 1 (Supplementary Figure 6B). Patients with CKD stage 5 were not included.
Safety of omecamtiv mecarbil in HFrEF patients with CKD
There are no data on the renal safety of omecamtiv mecarbil specifically in patients with CKD.
Renal effects of omecamtiv mecarbil in HFrEF and interaction with outcome
There are very limited data on the effect of omecamtiv mecarbil on renal function in HFrEF patients. In GALACTIC-HF the change in serum creatinine after 24 and 48 weeks of treatment was similar with omecamtiv mecarbil and placebo. In the Acute Treatment with Omecamtiv Mercabil to Increase Contractility in Acute Heart Failure (ATOMIC-AHF) study on intravenous omecamtiv mecarbil, the renal safety was similar to placebo as well as the rate of WRF development.98
Practical Consideration on the use of omecamtiv mecarbil in HFrEF patients with CKD
Omecamtiv mecarbil, upon approval for the treatment of HFrEF and adoption in international guidelines, can be used in HFrEF patients with severe CKD stage 4, as GALACTIC-HF included patients with eGFR 20 ml/min/1.73m2or greater. Omecamtiv mecarbil administration does require drug level monitoring to achieve therapeutic plasma drug concentration and monitoring of vitals and serum electrolytes as standard care in HFrEF patients should be considered. However, there was no effect on serum creatinine (or potassium) with omecamtiv mecarbil, there should be no concern for WRF and hyperkalemia.
Interpretation of results from randomized clinical trials in HFrEF patients with CKD
The implementation of guideline-directed medical treatments and translation of findings from clinical trials to clinical practice are two of the most challenging aspects of improving HFrEF care. There are many reasons physicians are hesitant to either start or continue lifesaving therapies in HFrEF patients, but one of the most important reasons is progressive renal impairment, concomitant hyperkalemia and/or symptomatic hypotension. If the eligibility criteria of pivotal randomized clinical trials are strictly followed, many high-risk patients, including those with severe CKD, would not be eligible for lifesaving therapies. Furthermore, the renal safety of these drug classes was monitored closely in the landmark trials (supplementary table 1), which also employed rigorous downtitration or discontinuation rules when certain thresholds of renal function (eGFR/serum creatinine) or hyperkalemia were crossed. As such, the extrapolation of the findings from large clinical trials into clinical practice is challenging. It is however important to employ a rigorous follow up of laboratory assessment when starting or changing GDMT, especially those that impact GFR and potassium. As a rule of thumb, evaluating serum creatinine and electrolytes should be considered at the start, after each up/downtitration step, after maximum dose has been reached, and with each change in dose thereafter or with clinical deterioration. Standard follow up in patients with CKD should be considered every 4–6 months, depending on clinical stability. Supplementary Table 2 provides an overview of suggested clinical actions when (pseudo)WRF occurs with class I HFrEF therapies.
Because the HF clinician is increasingly faced with an aging, frail and multimorbid HFrEF population with more comorbidities and more severe CKD, trials must aim to be less restrictive in eligibility criteria so that trial results are easier to generalize to clinical setting. The impetus for this change will likely need to be driven by regulators rather than pharmaceutical companies.
Although data are limited, there is consistent evidence of efficacy and safety of most evidence based medical treatments for HFrEF up to at least CKD stage 3 (eGFR 30 mL/min/1.73m2) provided adequate monitoring is present. Furthermore, there is especially robust evidence in CKD among the new classes of drugs (SGLT2i, ARNI) where renal protection may even be possible. The treatment efficacy and safety varies among these pharmacotherapies (Figure 3).
Figure 3. Conceptual overview of evidence based treatments in HFrEF according to baseline CKD status.
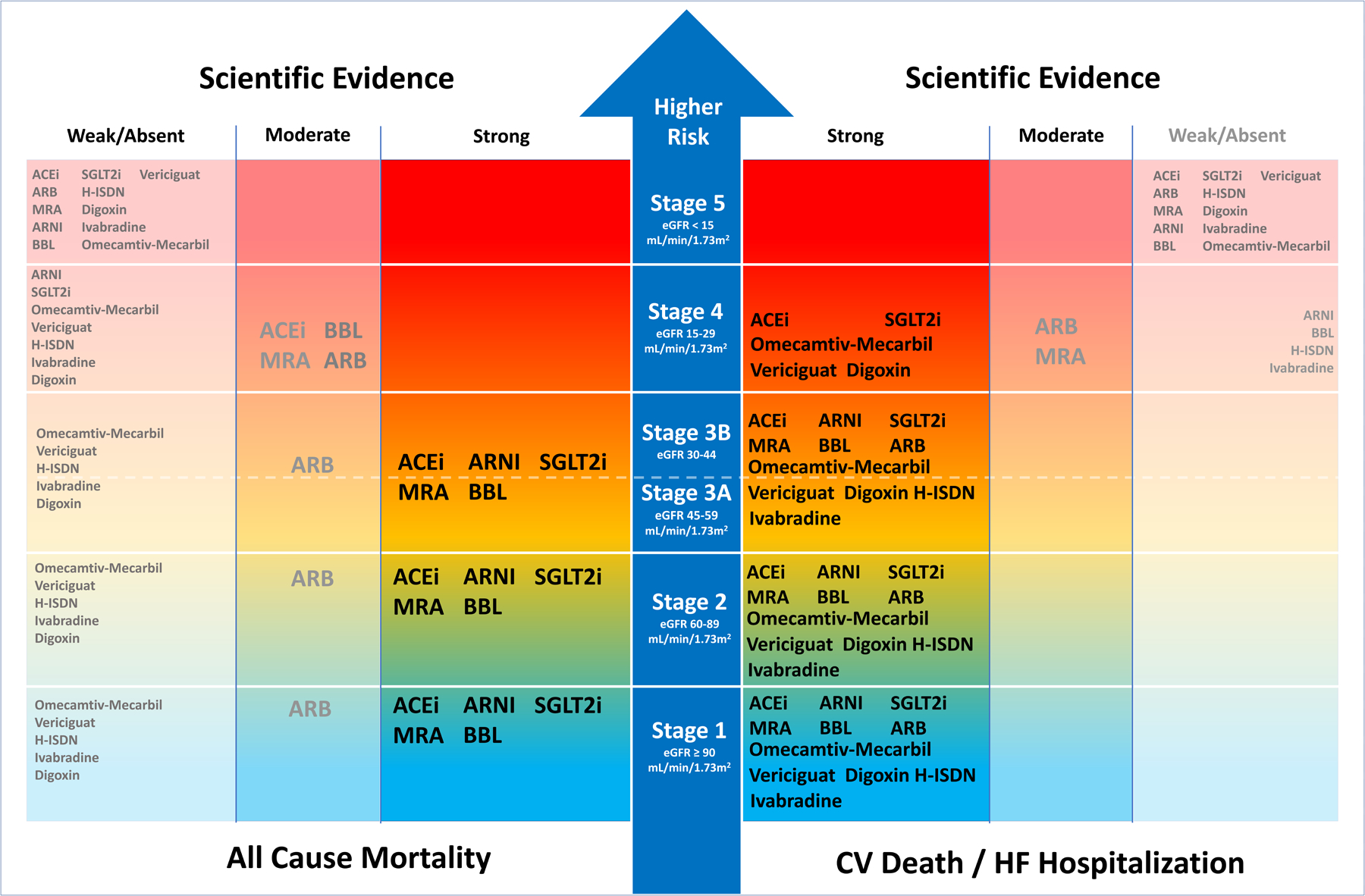
With more severe CKD stages, prognosis worsens, and scientific evidence becomes scarce. There is more evidence for CKD stage 1–4 for preventing CV Death/HF Hospitalization with evidence based treatments as compared with preventing all-cause mortality. Among treatments there is some evidence for efficacy of SGLT2i, omecamtiv-mecarbil, ACEi, digoxin and vericiguat in CKD stage 4. Overall the renal safety profile in all classes of CKD with essentially all treatments is good, if the clinical status is taken into account and renal function and potassium are checked regularly. Loop Diuretics are not depicted in the absence of large randomized placebo controlled trials.
Abbreviations: ACEi: Angiotensin Converting Enzyme Inhibitor, ARB: Angiotensin II Receptor Blocker, ARNI: Angiotensin Receptor blocker neprilysin Inhibitor, Beta-blocker: Beta-Blocker, CV: Cardiovascular, eGFR: Estimated Glomerular Filtration Rate, H-ISDN: Hydralazine IsosorbideDinitrate, HR: Hazard Ratio, HF: Heart Failure, MRA: Mineralocorticoid Receptor Antagonist, SGLT2i: Sodium glucose co-transporter 2 inhibitor.
It is important to note that several therapies transiently reduce eGFR after initiation, yet remain effective in the prevention of HF events and are associated with stabilization of renal function in the long term. Figures 2A–F give guidance to the clinical use of these drugs if renal function deteriorates and how to interpret changes, resembling the advice given by international HF guidelines and consensus documents.8,78,99 In all situations, it should not be the height or change in serum creatinine that determines changes in prescription of evidence-based treatments, but the change in clinical status of the patient.
Conclusions
CKD plays a crucial role in the pathophysiology and prognosis of HFrEF and is often a perceived limitation in the optimization of evidence based HFrEF therapies. Yet available evidence suggest that most guideline directed medical therapies are effective up to CKD stage 3B, while some drug classes have even shown efficacy in CKD stage 4. Many therapies influence renal function direct or indirectly, as well as associated conditions such as hyperkalemia, warranting close monitoring during initiation. A decrease in eGFR is expected with initiation of RAASi (including ARNI) and SGLT2i, and should not be a reason to discontinue these life saving drugs. Knowledge, correct interpretation, and possible treatment of changes in renal function in relation to evidence-based HFrEF treatments are therefore essential assets for the HF caregiver.
Supplementary Material
Conflict of Interest Disclosures
IEB and JMtM report no disclosures. KD reports speaker fees from Abbott, AstraZeneca, Boehringer Ingelheim. AAV reports consultancy fees and/or research support from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novartis, NovoNordisk, Roche diagnostics. HGCV is funded by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. JMT reports grants or personal fees from 3ive labs, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Astra Zeneca, Novartis, Cardionomic, MagentaMed, Reprieve inc., FIRE1, W.L. Gore, Sanofi, Sequana Medical, Otsuka, Abbott, Merck, Windtree Therapeutics, Lexicon pharmaceuticals, Precardia, BD, Regeneron, Edwards. In addition, JMT has a patent Treatment of diuretic resistance issued to Yale and Corvidia Therapeutics Inc, a patent Methods for measuring renalase issued to Yale, and a patent Treatment of diuretic resistance
Abbreviations list
- ACEi
angiotensin-converting-enzyme inhibitors
- ARB
angiotensin II receptor blockers
- ARNI
angiotensin receptor blocker neprilysin inhibitors
- BBL
betablockers
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- GDMT
guideline-directed medical therapy
- GFR
glomerular filtration rate
- H-ISDN
hydralazine-isosorbide dinitrate
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- MRA
mineralocorticoid receptor antagonists
- RAASi
renin angiotensin aldosterone system inhibitor
- RBF
renal blood flow
- SGLT2i
sodium glucose co-transporter 2 inhibitors
- WRF
worsening renal function
Footnotes
References
- 1.van Deursen VM, Urso R, Laroche C, Damman K, Dahlström U, Tavazzi L, Maggioni AP, Voors AA. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014; 16: 103–111. [DOI] [PubMed] [Google Scholar]
- 2.van Deursen VM, Damman K, van der Meer P, Wijkstra PJ, Luijckx GJ, van Beek A, van Veldhuisen DJ, Voors AA. Co-morbidities in heart failure. Heart Fail Rev. 2014; 19: 163–172. [DOI] [PubMed] [Google Scholar]
- 3.Damman K, Valente MA, Voors AA, O’Connor CM, Van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014; 35: 455–469. [DOI] [PubMed] [Google Scholar]
- 4.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018; 72: 351–366. [DOI] [PubMed] [Google Scholar]
- 5.Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2019; 73: 2365–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J. 2017; 38: 1883–1890. [DOI] [PubMed] [Google Scholar]
- 7.Patel RB, Fonarow GC, Greene SJ, Zhang S, Alhanti B, DeVore AD, Butler J, Heidenreich PA, Huang JC, Kittleson MM, et al. Kidney Function and Outcomes in Patients Hospitalized with Heart Failure. J Am Coll Cardiol. 2021; 78: 330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, Tang WHW, Skouri H, Verbrugge FH, Orso F, et al. Evaluation of kidney function throughout the heart failure trajectory - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020; 22: 584–603. [DOI] [PubMed] [Google Scholar]
- 9.Shlipak MG. Pharmacotherapy for heart failure in patients with renal insufficiency. Ann Intern Med. 2003; 138: 917–924. [DOI] [PubMed] [Google Scholar]
- 10.Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H, McMurray JJ. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol. 2014; 63: 853–871. [DOI] [PubMed] [Google Scholar]
- 11.Hein AM, Scialla JJ, Edmonston D, Cooper LB, DeVore AD, Mentz RJ. Medical Management of Heart Failure With Reduced Ejection Fraction in Patients With Advanced Renal Disease. JACC Heart Fail. 2019; 7: 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damman K, Voors AA, Navis G, Van Veldhuisen DJ, Hillege HL. The cardiorenal syndrome in heart failure. Prog Cardiovasc Dis. 2011; 54: 144–153. [DOI] [PubMed] [Google Scholar]
- 13.Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WH, Mullens W. The kidney in congestive heart failure: ‘are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur J Heart Fail. 2014; 16: 133–142. [DOI] [PubMed] [Google Scholar]
- 14.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015; 36: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005; 26: 11–17. [DOI] [PubMed] [Google Scholar]
- 16.Mullens W, Martens P. Exploiting the Natriuretic Peptide Pathway to Preserve Glomerular Filtration in Heart Failure. JACC Heart Fail. 2018; 6: 499–502. [DOI] [PubMed] [Google Scholar]
- 17.Fiksen-Olsen MJ, Strick DM, Hawley H, Romero JC. Renal effects of angiotensin II inhibition during increases in renal venous pressure. Hypertension. 1992; 19: 137–141. [DOI] [PubMed] [Google Scholar]
- 18.Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs. 1990; 39 Suppl 4: 10–21. [DOI] [PubMed] [Google Scholar]
- 19.Smilde TD, Damman K, van der Harst P, Navis G, Daan Westenbrink B, Voors AA, Boomsma F, van Veldhuisen DJ, Hillege HL. Differential associations between renal function and “modifiable” risk factors in patients with chronic heart failure. Clin Res Cardiol. 2009; 98: 121–129. [DOI] [PubMed] [Google Scholar]
- 20.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987; 316: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 21.SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991; 325: 293–302. [DOI] [PubMed] [Google Scholar]
- 22.SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992; 327: 685–691. [DOI] [PubMed] [Google Scholar]
- 23.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003; 362: 759–766. [DOI] [PubMed] [Google Scholar]
- 24.Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001; 345: 1667–1675. [DOI] [PubMed] [Google Scholar]
- 25.Bowling CB, Sanders PW, Allman RM, Rogers WJ, Patel K, Aban IB, Rich MW, Pitt B, White M, Bakris GC, et al. Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: insights from the SOLVD Treatment trial. Int J Cardiol. 2013; 167: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCallum W, Tighiouart H, Ku E, Salem D, Sarnak MJ. Acute declines in estimated glomerular filtration rate on enalapril and mortality and cardiovascular outcomes in patients with heart failure with reduced ejection fraction. Kidney Int. 2019; 96: 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011; 4: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jose P, Skali H, Anavekar N, Tomson C, Krumholz HM, Rouleau JL, Moye L, Pfeffer MA, Solomon SD. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J Am Soc Nephrol. 2006; 17: 2886–2891. [DOI] [PubMed] [Google Scholar]
- 29.McCallum W, Tighiouart H, Ku E, Salem D, Sarnak MJ. Trends in Kidney Function Outcomes Following RAAS Inhibition in Patients With Heart Failure With Reduced Ejection Fraction. Am J Kidney Dis. 2020; 75: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damman K, Solomon SD, Pfeffer MA, Swedberg K, Yusuf S, Young JB, Damman K, Granger CB, McMurray JV. Worsening Renal Function and Outcome in Heart Failure patients with Reduced and Preserved Ejection Fraction and the Impact of Angiotensin Receptor Blocker Treatment. Eur J Heart Fail. 2016; 18: 1508–1517. [DOI] [PubMed] [Google Scholar]
- 31.Kiernan MS, Gregory D, Sarnak MJ, Rossignol P, Massaro J, Kociol R, Zannad F, Konstam MA. Early and late effects of high- versus low-dose angiotensin receptor blockade on renal function and outcomes in patients with chronic heart failure. JACC Heart Fail. 2015; 3: 214–223. [DOI] [PubMed] [Google Scholar]
- 32.Damman K, Perez AC, Anand IS, Komajda M, McKelvie RS, Zile MR, Massie B, Carson PE, McMurray JJ. Worsening renal function and outcome in heart failure patients with preserved ejection fraction and the impact of angiotensin receptor blocker treatment. J Am Coll Cardiol. 2014; 64: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 33.Lesogor A, Cohn JN, Latini R, Tognoni G, Krum H, Massie B, Zalewski A, Kandra A, Hua TA, Gimpelewicz C. Interaction between baseline and early worsening of renal function and efficacy of renin-angiotensin-aldosterone system blockade in patients with heart failure: insights from the Val-HeFT study. Eur J Heart Fail. 2013; 15: 1236–1244. [DOI] [PubMed] [Google Scholar]
- 34.Levine TB, Olivari MT, Garberg V, Sharkey SW, Cohn JN. Hemodynamic and clinical response to enalapril, a long-acting converting-enzyme inhibitor, in patients with congestive heart failure. Circulation. 1984; 69: 548–553. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992; 327: 669–677. doi: 10.1056/NEJM199209033271001 [DOI] [PubMed] [Google Scholar]
- 36.Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet. 1993; 342: 821–828. [PubMed] [Google Scholar]
- 37.Køber L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995; 333: 1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 38.Tokmakova MP, Skali H, Kenchaiah S, Braunwald E, Rouleau JL, Packer M, Chertow GM, Moyé LA, Pfeffer MA, Solomon SD. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the Survival And Ventricular Enlargement (SAVE) study. Circulation. 2004; 110: 3667–3673. doi: 10.1161/01.CIR.0000149806.01354.BF [DOI] [PubMed] [Google Scholar]
- 39.Beldhuis IE, Streng KW, Ter Maaten JM, Voors AA, van der Meer P, Rossignol P, McMurray JJ, Damman K. Renin-angiotensin system inhibition, worsening renal function, and outcome in heart failure patients with reduced and preserved ejection fraction: a meta-analysis of published study data. Circ Heart Fail. 2017; 10: e003588. doi: 10.1161/CIRCHEARTFAILURE.116.003588 [DOI] [PubMed] [Google Scholar]
- 40.McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003; 362: 767–771. doi: 10.1016/S0140-6736(03)14283-3 [DOI] [PubMed] [Google Scholar]
- 41.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003; 362: 772–776. doi: 10.1016/S0140-6736(03)14284-5 [DOI] [PubMed] [Google Scholar]
- 42.Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GA, Malbecq W, Smith RD, Guptha S, et al. ; HEAAL Investigators. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009; 374: 1840–1848. doi: 10.1016/S0140-6736(09)61913-9 [DOI] [PubMed] [Google Scholar]
- 43.Clark H, Krum H, Hopper I. Worsening renal function during renin-angiotensin-aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. Eur J Heart Fail. 2014; 16: 41–48. doi: 10.1002/ejhf.13 [DOI] [PubMed] [Google Scholar]
- 44.Damman K, Tang WH, Testani JM, McMurray JJ. Terminology and definition of changes renal function in heart failure. Eur Heart J. 2014; 35: 3413–3416. doi: 10.1093/eurheartj/ehu320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 46.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 47.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011; 364: 11–21.21073363 [Google Scholar]
- 48.Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Messig M, Vincent J, Girerd N, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail. 2014; 7: 51–58. [DOI] [PubMed] [Google Scholar]
- 49.Eschalier R, McMurray JJ, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ, Shi H, Vincent J, Rossignol P, Zannad F, et al. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). J Am Coll Cardiol. 2013; 62: 1585–1593. [DOI] [PubMed] [Google Scholar]
- 50.Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD. Influence of Baseline and Worsening Renal Function on Efficacy of Spironolactone in Patients With Severe Heart Failure: Insights From RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol. 2012; 60: 2082–2089. [DOI] [PubMed] [Google Scholar]
- 51.Rossignol P, Cleland JG, Bhandari S, Tala S, Gustafsson F, Fay R, Lamiral Z, Dobre D, Pitt B, Zannad F. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study. Circulation. 2012; 125: 271–279. [DOI] [PubMed] [Google Scholar]
- 52.Quach K, Lvtvyn L, Baigent C, Bueti J, Garg AX, Hawley C, Haynes R, Manns B, Perkovic V, Rabbat CG, et al. The Safety and Efficacy of Mineralocorticoid Receptor Antagonists in Patients Who Require Dialysis: A Systematic Review and Meta-analysis. Am J Kidney Dis. 2016; 68: 591–598. [DOI] [PubMed] [Google Scholar]
- 53.Charytan DM, Himmelfarb J, Ikizler TA, Raj DS, Hsu JY, Landis JR, Anderson AH, Hung AM, Mehrotra R, Sharma S, et al. Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney Int. 2019; 95: 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020; 383: 2219–2229. [DOI] [PubMed] [Google Scholar]
- 55.Colucci WS, Packer M, Bristow MR, Gilbert EM, Cohn JN, Fowler MB, Krueger SK, Hershberger R, Uretsky BF, Bowers JA, et al. Carvedilol inhibits clinical progression in patients with mild symptoms of heart failure. US Carvedilol Heart Failure Study Group. Circulation. 1996; 94: 2800–2806. [DOI] [PubMed] [Google Scholar]
- 56.CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999; 353: 9–13. [PubMed] [Google Scholar]
- 57.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999; 353: 2001–2007. [PubMed] [Google Scholar]
- 58.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001; 357: 1385–1390. doi: 10.1016/s0140-6736(00)04560-8 [DOI] [PubMed] [Google Scholar]
- 59.Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, et al. ; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. Circulation. 2002; 106: 2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf [DOI] [PubMed] [Google Scholar]
- 60.Beta-Blocker Evaluation of Survival Trial Investigators, Eichhorn EJ, Domanski MJ, Krause-Steinrauf H, Bristow MR, Lavori PW. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001; 344: 1659–1667. doi: 10.1056/NEJM200105313442202 [DOI] [PubMed] [Google Scholar]
- 61.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, et al. ; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005; 26: 215–225. doi: 10.1093/eurheartj/ehi115 [DOI] [PubMed] [Google Scholar]
- 62.Kotecha D, Gill SK, Flather MD, Holmes J, Packer M, Rosano G, Böhm M, McMurray JJV, Wikstrand J, Anker SD, et al. Impact of Renal Impairment on Beta-Blocker Efficacy in Patients With Heart Failure. J Am Coll Cardiol. 2019; 74: 2893–2904. [DOI] [PubMed] [Google Scholar]
- 63.Ghali JK, Wikstrand J, Van Veldhuisen DJ, Fagerberg B, Goldstein S, Hjalmarson A, Johansson P, Kjekshus J, Ohlsson L, Samuelsson O, et al. The influence of renal function on clinical outcome and response to beta-blockade in systolic heart failure: insights from Metoprolol CR/XL Randomized Intervention Trial in Chronic HF (MERIT-HF). J Card Fail. 2009; 15: 310–318. [DOI] [PubMed] [Google Scholar]
- 64.Castagno D, Jhund PS, McMurray JJ, Lewsey JD, Erdmann E, Zannad F, Remme WJ, Lopez-Sendon JL, Lechat P, Follath F, et al. Improved survival with bisoprolol in patients with heart failure and renal impairment: an analysis of the Cardiac Insufficiency Bisoprolol Study II (CIBIS-II) trial. Eur J Heart Fail. 2010; 12: 607–616. doi: 10.1093/eurjhf/hfq038 [DOI] [PubMed] [Google Scholar]
- 65.Wali RK, Iyengar M, Beck GJ, Chartyan DM, Chonchol M, Lukas MA, Cooper C, Himmelfarb J, Weir MR, Berl T, et al. Efficacy and safety of carvedilol in treatment of heart failure with chronic kidney disease: a meta-analysis of randomized trials. Circ Heart Fail. 2011; 4: 18–26. doi: 10.1161/CIRCHEARTFAILURE.109.932558 [DOI] [PubMed] [Google Scholar]
- 66.Cohen-Solal A, Kotecha D, van Veldhuisen DJ, Babalis D, Böhm M, Coats AJ, Roughton M, Poole-Wilson P, Tavazzi L, Flather M; SENIORS Investigators. Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. Eur J Heart Fail. 2009; 11: 872–880. doi: 10.1093/eurjhf/hfp104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010; 376: 875–885. [DOI] [PubMed] [Google Scholar]
- 68.Voors AA, van Veldhuisen DJ, Robertson M, Ford I, Borer JS, Böhm M, Komajda M, Swedberg K, Tavazzi L, SHIFT investigators. The effect of heart rate reduction with ivabradine on renal function in patients with chronic heart failure: an analysis from SHIFT. Eur J Heart Fail. 2014; 16: 426–434. [DOI] [PubMed] [Google Scholar]
- 69.Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997; 336: 525–533. [DOI] [PubMed] [Google Scholar]
- 70.Shlipak MG, Smith GL, Rathore SS, Massie BM, Krumholz HM. Renal function, digoxin therapy, and heart failure outcomes: evidence from the digoxin intervention group trial. J Am Soc Nephrol. 2004; 15: 2195–2203. [DOI] [PubMed] [Google Scholar]
- 71.Testani JM, Brisco MA, Tang WH, Kimmel SE, Tiku-Owens A, Forfia PR, Coca SG. Potential effects of digoxin on long-term renal and clinical outcomes in chronic heart failure. J Card Fail. 2013; 19: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly HG, Wong SY. Effect of Intravenous Digoxin on Blood Pressure, Serum Electrolytes, Renal Hemodynamics and Excretory Function in Normal and Hypertensive Subjects, and Subjects in Congestive Heart Failure. Can Med Assoc J. 1961; 85: 1131–1135. [PMC free article] [PubMed] [Google Scholar]
- 73.Ziesche S, Cobb FR, Cohn JN, Johnson G, Tristani F. Hydralazine and isosorbide dinitrate combination improves exercise tolerance in heart failure. Results from V-HeFT I and V-HeFT II. The V-HeFT VA Cooperative Studies Group. Circulation. 1993; 87: VI56–64. [PubMed] [Google Scholar]
- 74.Taylor AL, Ziesche S, Yancy CW, Carson P, Ferdinand K, Taylor M, Adams K, Olukotun AY, Ofili E, Tam SW, et al. Early and sustained benefit on event-free survival and heart failure hospitalization from fixed-dose combination of isosorbide dinitrate/hydralazine: consistency across subgroups in the African-American Heart Failure Trial. Circulation. 2007; 115: 1747–1753. [DOI] [PubMed] [Google Scholar]
- 75.Bavendiek U, Berliner D, Dávila LA, Schwab J, Maier L, Philipp SA, Rieth A, Westenfeld R, Piorkowski C, Weber K, et al. Rationale and design of the DIGIT-HF trial (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure): a randomized, double-blind, placebo-controlled study. Eur J Heart Fail. 2019; 21: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, et al. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019; 21: 137–155. [DOI] [PubMed] [Google Scholar]
- 77.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 78.Writing Committee, Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021; 77: 772–810. [DOI] [PubMed] [Google Scholar]
- 79.O’Meara E, McDonald M, Chan M, Ducharme A, Ezekowitz JA, Giannetti N, Grzeslo A, Heckman GA, Howlett JG, Koshman SL, et al. CCS/CHFS Heart Failure Guidelines: Clinical Trial Update on Functional Mitral Regurgitation, SGLT2 Inhibitors, ARNI in HFpEF, and Tafamidis in Amyloidosis. Can J Cardiol. 2020; 36: 159–169. [DOI] [PubMed] [Google Scholar]
- 80.Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, et al. Renal Effects and Associated Outcomes During Angiotensin-Neprilysin Inhibition in Heart Failure. JACC Heart Fail. 2018; 6: 489–498. [DOI] [PubMed] [Google Scholar]
- 81.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER-HF Investigators. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med. 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 82.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 83.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 84.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 85.Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, Filippatos G, Hauske SJ, Brueckmann M, Pfarr E, et al. Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function: Insights From EMPEROR-Reduced. Circulation. 2021; 143: 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, Chopra V, de Boer RA, Desai AS, Ge J, et al. Efficacy of Dapagliflozin on Renal Function and Outcomes in Patients With Heart Failure With Reduced Ejection Fraction: Results of DAPA-HF. Circulation. 2021; 143: 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jensen J, Omar M, Kistorp C, Tuxen C, Gustafsson I, Køber L, Gustafsson F, Faber J, Malik ME, Fosbøl EL, et al. Effects of empagliflozin on estimated extracellular volume, estimated plasma volume, and measured glomerular filtration rate in patients with heart failure (Empire HF Renal): a prespecified substudy of a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2021; 9: 106–116. [DOI] [PubMed] [Google Scholar]
- 88.van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, Bozovic A, Danser AHJ, Geurts F, Hoorn EJ, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020; 97: 202–212. [DOI] [PubMed] [Google Scholar]
- 89.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 90.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 91.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 92.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med. 2021; 384: 129–139. [DOI] [PubMed] [Google Scholar]
- 93.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020; 383: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 94.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 95.Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2020; 382: 1883–1893. [DOI] [PubMed] [Google Scholar]
- 96.Voors AA, Mulder H, Reyes E, Cowie MR, Lassus J, Hernandez AF, Ezekowitz JA, Butler J, O’Connor CM, Koglin J, et al. Renal Function and the Effects of Vericiguat in Patients with Worsening Heart Failure with Reduced Ejection Fraction: Insights from VICTORIA (VerICiguaT Global Study in Subjects with HFrEF) Trial. Eur J Heart Fail. 2021; 23: 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias-Mendoza A, Biering-Sørensen T, et al. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N Engl J Med. 2021; 384: 105–116. [DOI] [PubMed] [Google Scholar]
- 98.Teerlink JR, Felker GM, McMurray JJV, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, Cleland JGF, Kim JB, et al. Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure: The ATOMIC-AHF Study. J Am Coll Cardiol. 2016; 67: 1444–1455. [DOI] [PubMed] [Google Scholar]
- 99.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


