Abstract
Purpose of review
This review primarily examines the evidence for areas of consensus and on-going uncertainty or controversy about diet and physical exercise approaches for in the post-CoVID. We propose an ideal dietary and physical activity approach that the patient with obesity should follow after CoVID-19 infection in order to reduce the clinical conditions associated with post-CoVID syndrome.
Recent findings
The CoVID-19 disease pandemic, caused by the severe acute respiratory syndrome coronavirus-2, has spread all over the globe, infecting hundreds of millions of individuals and causing millions of death. It is also known to be is associated with several medical and psychological complications, especially in patients with obesity and weight-related disorders who in general pose a significant global public health problem, and in specific affected individuals are on a greater risk of developing poorer CoVID-19 clinical outcomes and experience a higher rate of mortality. Little is still known about the best nutritional approach to be adopted in this disease especially in the patients post-CoVID syndrome.
Summary
To the best of our knowledge, no specific nutritional recommendations exist to manage in the patients post-CoVID syndrome. We report a presentation of nutritional therapeutic approach based on a ketogenic diet protocol followed by a transition to the Mediterranean diet in patients post-infection by CoVID, combined to a physical activity program to address conditions associated with post-CoVID syndrome.
Keywords: Obesity, Post-CoVID syndrome, Long-CoVID, Ketogenic diet, VLCKD, Mediterranean diet, Muscle mass, Gut, Nutritionist
Background
Now that almost 2 years have elapsed since the first cases of Coronavirus disease 2019 (CoVID-19) were diagnosed in Wuhan (China), it is clear that this disease is much more than a simple viral interstitial pneumonia [1]. It has been, in fact, clearly demonstrated that severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) may damage other organs and tissues besides the lung, including the heart [2], the liver and the gut [3, 4], and the central nervous system [5], either by directly infecting them or by promoting and indirect cytokine-mediated damage. In addition, the clinical manifestations of CoVID-19 do not seem to necessarily stop with the end of acute SARS-CoV-2 infection but may last much longer as a “post CoVID” or “long-CoVID” syndrome, which affects multiple organs and apparatuses and whose pathogenesis is still uncertain but may involve both the persistence of the virus in a latent form and virus-induced triggering of autoimmune responses [6].
A growing body of evidence shows that skeletal muscles are affected during both the acute phase of CoVID-19 and the post-CoVID syndrome and that CoVID-19 related muscle damage requires attention both for prevention and treatment because of its potential detrimental health consequences [7•].
This review primarily examines the evidence for areas of consensus and on-going uncertainty or controversy about diet and physical exercise approaches for in the post-CoVID-19. We come up with a presentation of nutritional therapeutic approach based on a ketogenic diet protocol followed by a transition to Mediterranean diet in patients after CoVID-19 infection (post-CoVID syndrome), combined to a physical activity program for young and older adults to address both short- and long-term conditions associated with CoVID-19 infection and severity.
Main Text
Skeletal Muscle Involvement in Acute CoVID-19 and in the Post-CoVID Syndrome
The severity of muscle involvement in CoVID-19 may vary from mild to extremely severe forms [7•]. Myalgia is one of the most common symptoms of the disease and it often occurs at its onset. It was observed already in the first large case series from China [8–11] and, according to a meta-analysis of 61 studies on a total of 59,254 patients, its prevalence is about 36% [12]. High plasma concentrations of creatine phosphokinase (CPK) levels are also frequently observed although with a lower prevalence than myalgia. In the ALBACOVID registry, which includes 841 Spanish CoVID-19 patients, higher than normal CPK levels were observed in 9.2% of patients at admission [13] whereas it occurred in 13.7% of cases in a large series of 1099 patients from 552 hospitals in China [8]. Importantly, concentrations of CPK levels have a prognostic meaning since very high concentrations of this enzyme are usually associated with an unfavorable course of the disease [14]. Muscle weakness often accompanies myalgia and, in a few cases, a clear diagnosis of myositis was established by magnetic resonance imaging (MRI) examination, which showed diffuse muscle edema [15–18]. The loss of muscle strength may be so severe to cause acute flaccid quadriplegia or contribute in determining ventilatory failure and make difficult the weaning from mechanical ventilation [16, 19]. Severe muscle weakness is usually associated with a significant loss of muscle mass and, therefore, in most cases patients with marked functional impairments are clearly sarcopenic or at high risk to develop this condition [20, 21]. Remarkably, sarcopenia is a predictor of difficult ventilator weaning, length of hospitalization, and in-hospital mortality in patients admitted to intensive care unit (ICU) for severe CoVID-19 [22]. In rare patients, CoVID-19 is complicated by a massive destruction of muscle tissue and several cases of rhabdomyolysis complicating acute CoVID-19 have been reported [23–28]. Quite often muscle symptoms continue in patients recovering from CoVID-19 and sometimes new muscle manifestations such as “autoimmune myositis” appear in these subjects [29]. Muscle weakness occurred in 63% of the subjects in a large series of 1733 patients recovering from CoVID-19 after being discharged from a large hospital in Wuhan [30]. A systematic review of the studies published from January 1, 2020 to March 11, 2021 showed that fatigue scored second after dyspnea as the most frequent symptom persisting for at least 60 days after CoVID-19 diagnosis or at least 30 days after hospital discharge with a median frequency of 39.8% whereas myalgia was far less frequent being observed in about 8% of the patients [31]. Muscle weakness is a frequent finding in patients with the post-CoVID syndrome: a decrease of 69% and 54% has been observed respectively in the strength of the biceps brachii and of the quadriceps femoris in patients recovering from CoVID-19 [32]. Muscle weakness causes a significant impairment in daily life activity, also including personal care, with a consequent decrease in the quality of life of patients recovering from CoVID-19 [33]. In rare cases of post-CoVID, a massive muscle damage may occur and there have been reports of delayed onset acute necrotizing myositis appearing months after hospital discharge for severe acute CoVID-19 [34].
Mechanism of Skeletal Muscle Damage in CoVID-19
Muscle damage in CoVID-19 has likely a multifactorial origin [35]. The first potential culprit is the direct invasion of myocytes by SARS-CoV-2, which has been proposed to take place in infected patients but not yet directly proved. Skeletal muscles express angiotensin-converting enzyme (ACE)-2, the SARS-CoV-2 receptor protein, but only at very low levels, although its expression increases in muscle diseases [36–40]. The analysis of autoptic muscle samples from 43 patients who died of severe CoVID-19 showed marked inflammatory changes with a strong expression of major histocompatibility complex (MHC) class I and, at later stages, of MHC class II antigens in sarcolemma; while SARS-CoV2 was detected by reverse-transcription polymerase chain reaction (RT-PCR) in muscles, no evidence of a direct invasion of muscle cells was obtained, strongly arguing against the hypothesis of a direct viral damage of muscles [41]. Likewise, in a second autoptic series on 35 patients who died of CoVID-19 histological analysis showed muscle inflammatory or degenerative changes but no evidence of a direct SARS-CoV-2 muscle infection [42].
Even in the absence of a direct cytopathic effect of SARS-CoV-2 on myocytes, skeletal fibers may be damaged indirectly through the action of pro-inflammatory cytokines released either locally or systemically in response to the viral infection of other cell types. By interacting with their plasma-membrane receptors, which are expressed in muscle cells, interleukin (IL)-1, IL-6, tumor necrosis factor alpha (TNF-α), and interferons promote muscle cell apoptosis and increase ubiquitin/proteasome-dependent protein degradation by activating p38 and NFκB-dependent pathways [43–45]. Interestingly, the immunocytochemistry evidence of type I interferonopathy has been reported in a patient with proved CoVID-19 but no direct viral infection of muscle cells [46].
Recent evidence shows that the severity of the inflammatory status triggered by the SARS-CoV-2 infection positively correlates with alterations in the gut microbiota [47]. Metagenomic studies showed that in patients with CoVID-19 there is a decrease of fecal commensal symbionts accompanied by an increase in the opportunistic pathogens such as Coprobacillus, Clostridium ramosum, and Clostridium hathewayi, which is proportional to the severity of the disease [48]. Several factors may contribute to alter the fecal microbiota in CoVID-19, including the direct damage of gut mucosa by SARS-CoV-2 infection, antibiotic treatment for concomitant infections, the use of glucocorticoids (GCs) or other immunosuppressant drugs, and the use of enteral or parenteral nutrition. It has been suggested that alterations in the gut microbiota might worsen systemic inflammation in CoVID-19 since some of the bacterial species which are depleted in this disease, including B. adolescentis, F. prausnitzii, E. rectale, R. (Blautia) obeum, and D. formicigenerans, exert a negative modulatory role on immune responses [47]. It is also worth mentioning that dysfunction in the liver–gut microbiome axis have a role in the genesis of autoimmunity and, therefore, that they could also theoretically be involved in the genesis of autoimmune complications of CoVID-19 [49, 50••]. It remains to be established to what extent (if any) gut dysbiosis does contribute to CoVID-19-related muscle damage.
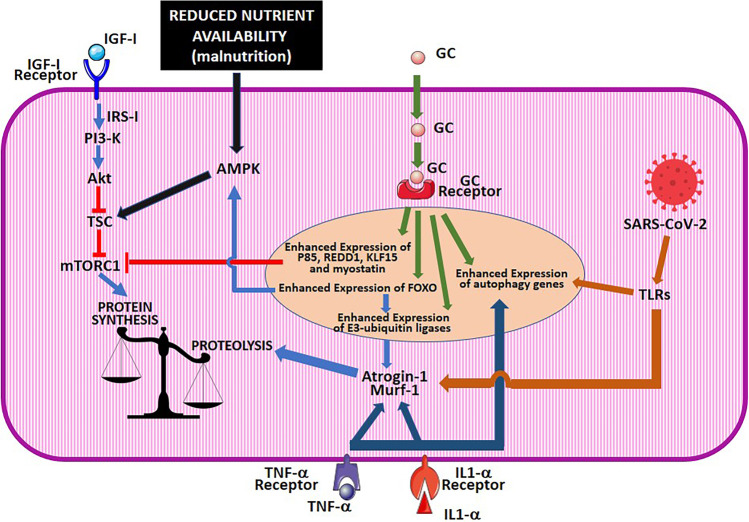
Drug-induced muscle toxicity is an additional important factor, which may contribute to skeletal muscle damage in CoVID-19 [51]. Several myotoxic drugs including azithromycin, darunavir/cobicistat, hydroxychloroquine, lopinavir-ritonavir, and ribavirin have been used to treat CoVID-19 patients at the beginning of the pandemic, but are not recommended anymore in current treatment guidelines. Among the drugs that are in use nowadays for SARS-CoV-2 infection (Table 1), GCs are those with the highest myotoxic potential [52]. These drugs and in particular dexamethasone are very frequently used in CoVID-19 patients since the National Institutes of Health CoVID-19 treatment guidelines recommend their use, alone or in combination with other drugs, in patients with severe disease, National Institute of Allergy and Infectious Disease Ordinal Scale (NIAID-OS) 5 or higher. GC myotoxicity is known since decades and much has been learned on its molecular mechanism [53]. GCs cause a decrease in the strength and size of skeletal muscles by inducing the atrophy of fast twitch type II muscle whereas type I myofibrils are spared [54]. Muscle damage may be caused not only by exogenous GC given as drugs, but also by endogenous glucocorticoid hormones, whose release is enhanced in severe diseases, starvation, metabolic acidosis, and severe insulinopenia; these conditions may be complicated by muscle atrophy which ameliorates in response to GC-receptor blockade [54, 55]. GCs induce muscle atrophy by disturbing at several levels the complex regulatory network that finely tunes the balance between protein synthesis and degradation in myocytes with the final effect of reducing the former and enhancing the latter [54]. In skeletal myocytes, proteins are degraded either by the proteasomal or by the autophagic routes. In myocytes, proteasomal degradation is critically dependent on the levels of two E3 ligases, atrogin-1 and muscle RING-finger protein-1 (MuRF1), whose gene expression is, therefore, strictly regulated [56, 57], whereas the autophagic machinery is mainly controlled through the transcriptional regulation of genes encoding key effector proteins of this pathway including LC-3 and Bnip3 [57, 58]. Transcription factors of the forkhead box O (FOXO) family, mainly FoxO1 and 3, have been identified as master regulators of both the autophagic and proteasomal pathways since they induce the expression of both the genes encoding the E3-ligases and of those encoding autophagy effector proteins [57, 59]. These transcription factors also finely adjust energy metabolism by interacting both with the signaling proteins Akt and the mechanistic target of rapamycin complex (mTORC)-1, which promote cell anabolism, and with 5′-adenosine monophosphate-activated protein kinase (AMPK) that induces cell catabolism [60]. Importantly, the interplay among these regulatory proteins is crucial also for physiological adjustments in response to nutrient deprivation or to specific nutritional programs. Akt, which is activated upon insulin-like growth factor (IGF)-1 binding to its receptors, phosphorylates and inhibits FOXO by preventing its nuclear translocation [61, 62]. Conversely, FOXO activates AMPK, which, in turn, inhibits mTORC1 and activates Akt. Akt and mechanistic target of rapamycin (mTOR) regulate each other in a closed negative feedback loop since Akt activates mTORC1 by phosphorylating and inhibiting tuberous sclerosis complex 2 protein (TSC2), whereas mTORC1 activates S6K, which phosphorylate and inhibit Akt. GCs may act on this cell metabolism regulatory system at different levels [61, 62]. They reduce Akt activity by several mechanisms including the decrease in the expression and the activity of insulin receptor substrate (IRS)-1, an adapter protein with a crucial role in transducing IGF-1 binding to its receptors into the activation of the PI3/Akt/mTOR cascade, and the increase in the expression of the regulatory p85a subunit of PI3K. Besides decreasing mTOR activation indirectly through their effects on IRS-1/PI3-K, GCs also directly reduce mTOR activity by increasing the gene expression of REDD1, KLF15, and myostatin [61, 62]. Because of the GC-induced inhibition of the IGF-1/PI3-K/mTOR signaling pathway, the balance between protein synthesis and degradation is altered in favor of the latter and this effect is further amplified by the promotion of proteolysis, which is exerted by GCs by direct and indirect mechanisms. In fact, GCs induce the transcription of FOXO-3 [63], which, in turn, as mentioned above, increases the transcription of atrogin-1 and MuRF1. In addition, GCs increase the expression of KLF15, which cooperates with FOXO to increase the gene expression of atrogin-1 and MuRF1, further contributing to unbalance muscle metabolism towards proteolysis. A direct role of GC receptors in promoting the expression of MuRF1 has also been demonstrated [64]. Among the other drugs that are recommended by the National Institutes of Health guidelines for CoVID-19, myotoxicity, usually limited to myalgias and CPK increase, has been reported for baricitinib [65] and tocilizumab [66], but not for remdesivir or anti-SARS-CoV-2 antibodies (Fig. 1).
Table 1.
Summary of NIH treatment guidelines for CoVID-19
| Disease severity | Recommended drugs |
|---|---|
| Not requiring hospitalization |
Bamlanivimab plus etesevimab or Casirivimab plus imdevimab or Sotrovimab Do not use dexamethasone or other corticosteroids |
| Discharged from hospital in stable conditions and not requiring supplemental oxygen | Do not use dexamethasone, remdesivir, or baricitinib |
| Discharged from hospital and requiring supplemental oxygen | Insufficient evidence to recommend or not continuing the in-hospital therapy with remdesivir, dexamethasone, and/or baricitinib |
| Discharged from hospital and increasing need for supplemental oxygen | Dexamethasone for the duration of supplemental oxygen |
| Hospitalized and not requiring supplemental oxygen |
Insufficient evidence to recommend or not remdesivir Do not use dexamethasone or other corticosteroids |
| Hospitalized and requiring supplemental oxygen | Remdsivir or Dexamethasone Or Dexamethasone + remdsivir |
| Hospitalized and requiring supplemental oxygen through a high-flow device or non-invasive ventilation | Dexamethasone or Dexamethasone + remdsivir |
| Hospitalized and requiring mechanical ventilation or extracorporeal membrane oxygenation |
Dexamethasone Dexamethasone + tocilizumab (or sarilumab, if tocilizumab unavailable) for patients who are within 24 h of admission to intensive care unit |
Fig. 1.
Molecular mechanism of muscle damage in CoVID-19. The drawing schematically illustrates how, in CoVID-19, muscle damage is produced by different but converging factors, which include malnutrition, cytokine receptor activation, and the activation of glucocorticoid receptors either by exogenous glucocorticoid drugs, given to treat this disease, or by endogenous glucocorticoid hormones, released in response to stress. The figure was prepared using the clip arts freely available under a Creative Commons Attribution 3.0 Unported License at the Servier Medical Art (SMART) website (https://smart.servier.com/). IGF, insulin-like growth factor; GC, glucocorticoids; IRS, insulin receptor substrate; PI3K, phosphoinositide 3-kinase; TSC, tuberous sclerosis complex; mTORC, mechanistic Target of Rapamycim complex; AMPK, 5′-adenosine monophosphate-activated protein kinase; REDD1, regulated in development and DNA damage responses 1; KLF, Krüppel-like factor; FoxO, forkhead box O; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; TLRs, Toll-like receptors; IL, interleukin; TNF-α, tumor necrosis factor alpha
Malnutrition is an important causative factor of sarcopenia and may have a role also in causing muscle damage in CoVID-19 patients. Malnutrition occurs, indeed, in about 50% of hospitalized CoVID-19 patients as a consequence of direct damage of the gut, anorexia caused by systemic inflammation, dysgeusia, and anosmia [67]. By using the Malnutrition Universal Screening Tool a high nutritional risk was detected in 79.6% of the older adults hospitalized for CoVID-19 and was associated with a high risk for sarcopenia as estimated with the Simple Questionnaire to Rapidly Diagnose Sarcopenia [68]. An observational cohort study performed in Spain under the behalf of the NUTRICOVID study research group showed that 83.5% of the patients discharged from the hospital after severe CoVID-19 were malnourished and 86.9% of them were at risk of sarcopenia; nonetheless, specific nutritional intervention had been implemented only in only 38% of these patients [69].
Finally, it is important to mention that among CoVID-19 with severe disease, the hospitalization in ICU may be responsible per se for functional and structural muscle damage [35]. A complex syndrome, known as ICU-acquired weakness (AW), in which polyneuropathy and myopathy cooperate in causing a marked weakness of proximal limb and respiratory muscles occurs in about 30% of critically ill patients during their stay at the ICU [70]. The term “acute sarcopenia” has been proposed to describe the rapid onset of the loss of muscle mass and strength that occur especially in frail subjects during stay in the ICU [71]. The pathogenesis of ICU-AW is presumably multifactorial and might be contributed for by high circulating cytokine levels, high glucocorticoid release from the adrenal glands, bedrest, and malnutrition [35, 71]. Importantly, bedrest may induce per se muscle mass loss and this effect is greatly potentiated by the administration of exogenous GC [72]. The ICU-AW syndrome has a characteristic electrophysiological profile which has been observed also in CoVID-19 patients staying at the ICU, confirming that, at least in some cases, muscle damage in this condition might just represent a consequence of hospitalization in intensive care [73, 74].
Little is known about the mechanism responsible for muscle symptoms in the post-CoVID syndrome. They might reflect the slow recovery of the muscle damage that has been accumulated during the acute phase of the disease, or be the consequence of the persistence of its causative factors; in addition, new pathogenetic mechanisms could be activated during the recovery from the disease with the appearance of forms of muscle damage different from those observed during the acute phase of the disease. By performing nerve conduction studies and quantitative electromyography, Agergaard et al. [75] showed that myopathy changes occur with no nerve fiber impairment in patients complaining of post-CoVID muscular symptoms. It has been suggested that these changes could be dependent on muscle inflammation but, in the absence of muscle biopsy data, this hypothesis remains to be confirmed. However, the evidence that 18F-FDG uptake in skeletal muscles is increased in patients recovering from CoVID-19 [76, 77] has been interpreted as indirect evidence of the accumulation of inflammatory cells in muscle fibers [76]. Coronaviruses are known to establish chronic infections, which may cause the persistent activation of immune responses with the release cytokines, which may cause muscle damage. As a matter of fact, high levels of pro-inflammatory cytokine inflammatory monocytes and activated T cells have been observed in patients recovering from CoVID-19 [78, 79]. In a subset of post-CoVID patients, muscle damage depends on autoimmune mechanisms. Autoimmunity phenomena have been described in patients with CoVID-19 and are considered responsible for complications of this disease including Guillan–Barrè syndrome, systemic vasculitis, and transverse myelitis, which may occur either during the acute phase of the disease or later on during the post-CoVID phase [49]. In a recent literature review, Gracia-Ramos et al. [80] identified 9 cases of patients who developed autoimmune myositis documented by the presence of autoantibodies, 10 days to 3 months after the onset of CoVID-19. Remarkably, in these autoimmune forms, the clinical presentation may be severe as in a patient described by Veyseh et al. [81], who developed a necrotizing myositis with major functional impairment due to the loss of muscle strength in the limbs. The mechanisms that could trigger autoimmune responses during SARS-CoV-2 infection include the sensitization to portions of viral proteins showing sequence analogies with human proteins (molecular mimicry) and the possible activation of T cells through a bystander effect caused virus-induced cytokine release [82, 83]. The alterations in fecal microbiome that we described before often persist beyond the acute phase of the disease and might play a role both in maintaining systemic inflammation and in promoting autoimmunity in the post-CoVID syndrome [47]. Patients who developed ICU-AW during hospitalization are at high risk of showing enduring muscle symptoms which may last for several months after hospital discharge [84] and can be due to the persistence of cardia intestinal metaplasia [85].
Recently, the role of malnutrition in the genesis of post-CoVID muscle symptoms has been highlighted [86]. Gerard et al. [86] showed 36.0% of the patients who were malnourished at the time of hospital discharge after severe CoVID-19 showed persistent malnutrition after 6 months and 14.3% of them also had a significant decrease in muscle strength and a low performance status > 2. Ramos et al. [87] reported that at the time of hospital discharge, almost 100% of patients receiving enteral or parenteral nutrition during hospitalization were at high risk for sarcopenia as assessed with the SARC-F test and that this risk remained high in almost a half of them after 6 months.
In conclusion, muscle damage in CoVID-19 depends mostly on muscle atrophy and inflammation. Since both inflammatory responses and muscle atrophy impinge of transduction pathways which can be modulated by nutrients, dietary intervention may help both preventing and treating muscle complications in CoVID-19 infection and in the post-CoVID syndrome as we will discuss in detail in the next sections.
Ketogenic Diet and CoVID-19
Ketogenic diets (KDs) are high-fat diets, characterized by a marked carbohydrate restriction (< 50 g per day) [88]. Recently, KDs, in particular very low calorie ketogenic diets (VLCKD), were shown to determine a significant weight loss along with improvement of glycemic control in subjects with obesity and type 2 diabetes mellitus (T2DM) [89–91], as well as psychosocial outcomes [92, 93]. It is well known that obesity increases the possibility that infectious diseases lead to serious health consequences; in keeping, it has been shown that obesity and T2DM represent risk factors for an adverse outcome of SARS-CoV-2 infection [94].
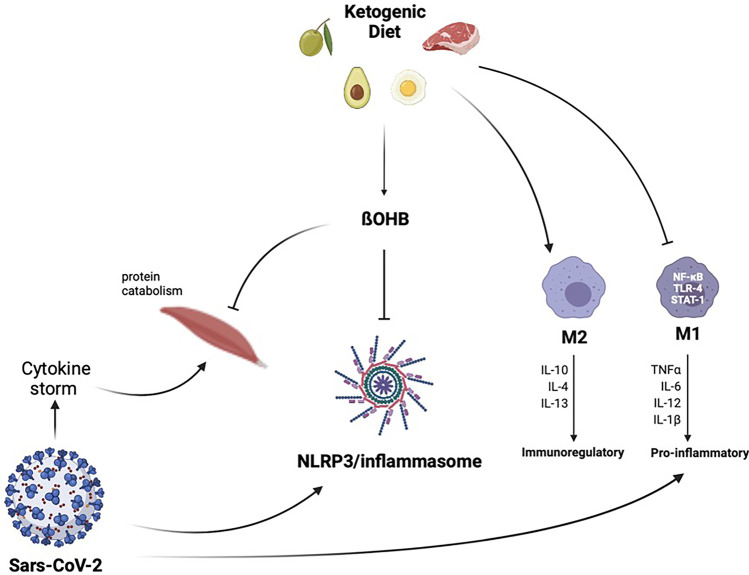
The association between obesity and poor CoVID-19 prognosis may be attributable to the fact that obesity impairs immune response to viral infections, inducing a chronic low-grade inflammatory state and increasing oxidative stress [95]. For this reason, during the pandemic, KD has been considered as a valuable nutritional tool to prevent infection and serious health consequences due to CoVID-19 [96], favoring the improvement of respiratory function [97]. KD displays anti-inflammatory effects through the modulation of immune metabolism. In fact, the cytokine storm is characterized by the overactivation of M1 macrophages [98]. During M1 phenotype activation, there is a metabolic shift from oxidative phosphorylation to aerobic glycolysis [99]. Hence, the scarce glucose availability in patients with CoVID-19 leads to a strong reduction in the development of M1 macrophages and a concomitant trigger for the development of M2 macrophages. Importantly, beta-hydroxybutyrate (β-OHB) inhibits NLRP3/inflammasome activation, through the reduction of K+ efflux from macrophages and the inhibition of the inflammasome assembly [100], as reported in Fig. 2.
Fig. 2.
Contrast effect of the ketogenic diet against SARS-CoV-2 infection. KD leads to a strong reduction in the development of M1 and a concomitant trigger for the development of M2. Moreover, β-OHB inhibits NLRP3/inflammasome activation and exerts anti-catabolic effects on human skeletal muscle. KDs, ketogenic diets; β-OHB, beta-hydroxybutyrate; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2
In a retrospective pilot study, Sukkar and colleagues demonstrated a favorable role of an eucaloric ketogenic diet in patients with CoVID-19, in controlling mortality and intensive care unit admission [101].
Long-CoVID
CoVID-19 pandemic has affected and continues to affect a large number of individuals, with an enormous burden of disease and mortality [31]. As reported by the Italian National Institute of Health in July 2021, although the clinical manifestations of the acute symptomatic phase of the infection are relatively well defined, it has become increasingly evident that the infection can determine a heterogeneous complex of subacute and chronic clinical manifestations that prevent a full return to the previous state of health and well-being [6, 31]. The symptoms attributed to this condition are numerous and heterogeneous: they can affect subjects of any age and with varying severity of the acute phase of the disease.
Long-CoVID represents a clinical condition characterized by the failure of the patient affected by CoVID-19 to return to the state of health prior to the acute infection [102]. The clinical manifestations of long-CoVID are highly variable and, to date, there is no consensus on their features. They can be transient or intermittent and can change their nature over time, or they can be constant [103]. In general, it is possible to distinguish long-CoVID general manifestation such as sarcopenia, malnutrition, muscle weakness, or long-COVID organ-specific [35, 104].
KD as a Post-CoVID Therapy to Increase Skeletal Muscle Mass
The term “sarcopenia” comes from the Greek ‘σαρξ’ (meat) and ‘πενια’ (lost) and was first proposed by Rosenberg in 1988 and indicated only the loss of muscle mass caused by aging [105]. In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) defined sarcopenia as a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with an increased risk of physical disability, deterioration in the quality of life and death [106].
In the updated definition of sarcopenia in 2019, the EWGSOP-2 not only refers to sarcopenia as loss of the muscle mass, but focuses more on the loss of muscle strength and on the reduction of physical performance [107]. Muscle loss related to aging is the result of the reduction in the size and number of muscle fibers, probably determined by multiple factors that depend on physical activity, nutritional intake, oxidative stress, and hormonal changes. Although the specific contribution of each of these factors is unknown, there is increasingly compelling evidence of the prominent role of chronic low-grade inflammation in the development of sarcopenia [108]. Chronic low-grade inflammation that accompanies aging has been defined as inflamm-ageing [109, 110]. It is well established that TNF-α, IL-1α, IL-6, IL-18, C reactive protein (CRP), and fibrinogen are increased during the pro-inflammatory state [111]. Elevated levels of catabolic biomarkers, including CRP, IL-6, and TNF-α, are important predictors of the decline in muscle strength related to aging [112]. The close relationship between inflammation, muscle mass, and muscle strength can be explained on the effects of inflammation on protein synthesis. In the elderly, there is an inverse proportional relationship between IL-6 and skeletal muscle strength and over-expression of IL-6 is associated with muscle atrophy [113]. In the context of the current CoVID-19 pandemic, the importance of inflammatory state is becoming more evident with increased levels of pro-inflammatory cytokines such as IL-6, IL-12, IL-17, IL-18, IL-33, TNF-α, and CRP observed in patients with severe CoVID-19 [114]. Cytokine storm refers to a set of clinical conditions caused by excessive immune reactions and has been recognized as a leading cause of severe CoVID-19 [115]; this directly contributes to skeletal muscle mass damage and sarcopenia, particularly when admission to intensive care is necessary [35].
A retrospective study was conducted by Schiaffino et al. to establish how reduced muscle mass was predictive of an adverse clinical outcome in patients with CoVID-19 [116]. A significant association was observed between reduced muscle mass and the onset of complications from CoVID-19. Muscle deficiency was a strong independent predictor of both intensive care unit admission and death.
In recent years, it has become evident that KDs have a favorable impact on lean body mass preservation [117]. As reported in the pilot double-blind study conducted by Merra and colleagues, VLCKD was highly effective in terms of body weight reduction without inducing loss of lean body mass, thereby preventing the risk of sarcopenia [118]. Importantly, β-OHB has also been shown to exert anti-catabolic effects on human skeletal muscle [119], as reported in Fig. 2. Therefore, KD represents a viable approach in post-CoVID patients to preserve skeletal muscle mass.
CoVID-19, Gut Microbiota, and Ketogenic Diet: A Possible Marriage?
CoVID-19 has shown a wide range of severities worldwide, also driven by high individual variation attributable to both advanced age and medical conditions, including hypertension, T2DM, and obesity [120]. Gut microbiome dysbiosis is an important factor that can facilitate the induction and progression of metabolic diseases such as T2DM and obesity. Dysbiosis leads to a “leaky gut” [120]. Moreover, gut dysbiosis, by altering the barrier functions of the intestine and thereby allowing access to toxins in the bloodstream, aggravates the systemic inflammatory response, impairs the immune system, and increases the susceptibility of patients to serious infective diseases such as SARS-CoV-2 [121]. In particular, Akkermansia muciniphila and Faecalibacterium prausnitzii are highly abundant human gut microbes in healthy individuals, and reduced levels are associated with inflammation and alterations of metabolic processes involved in the development of T2DM and obesity [122].
It has been hypothesized that SARS-CoV-2, through its effects on gut microbiota, could display different degrees of CoVID-19 severity [48]. Notably, several studies suggested a close link between gut microbiota dysbiosis and CoVID-19 severity. In a pilot study conducted by Zuo and colleagues, a significant gut microbiome alterations in patients with CoVID-19 has been reported [48]. Patients with CoVID-19 showed significant alterations in fecal microbiomes compared with controls, characterized by enrichment of opportunistic pathogens and depletion of beneficial commensals. The baseline abundance of Coprobacillus, Clostridium ramosum, and Clostridium hathewayi correlated with CoVID-19 severity; there was an inverse correlation between abundance of Faecalibacterium prausnitzii and disease severity. Moreover, they found that abundance of several Bacteroides spp., which down-regulate the expression of ACE2 in the murine gut, was inversely correlated with SARS-CoV-2 load in patient fecal samples [48].
KD is known to determine an alteration of the gut microbiota but current studies have led to conflicting results this is probably due to the different macronutrient composition of KD [123]. Faecalibacterium prausnitzii, an anaerobic bacterium belonging to the Firmicutes phylum, displays beneficial anti-inflammatory effects on the intestinal mucosa and is believed to have a positive influence on colon health [124]. Faecalibacterium prausnitzii seems to be less affected by a decrease in carbohydrates in the diet.
In recent years, many studies have focused on the impact of VLCKDs on gut microbiota, especially on Akkermansia muciniphila, a mucin-degrading bacterium residing in the mucus layer and producing short-chain fatty acids (SCFAs) [124]. The presence of this bacterium is inversely correlated with body weight in rodents and humans. In particular, the role of VLCKD on gut microbiota in relation to their anti-seizure effects on mice has been explored. In the present study, they found that mice exhibited significant changes in gut bacterial taxonomy: particularly, Akkermansia muciniphilia was significantly increased in mice that were fed with ketogenic diets. Importantly, VLCKD has a relevant impact also on human microbiota. In a review by Rondanelli et al., it was reported that a VLCKD induces an increase of Akkermansia muciniphilia [124]. Further studies are needed to investigate the impact of KD on gut microbiota of patients with CoVID-19; however, several evidence suggest that KD displays favorable effects, resulting in an increase of Akkermansia muciniphila and Faecalibacterium prausnitzii, which reduce inflammation and improve gut permeability [124].
Mediterranean Diet and CoVID-19
The term Mediterranean diet (MD) has been first introduced by Ancel Keys, in the middle of the last century, through the Seven Country Study, that showed a lower mortality rate from coronary heart disease in Mediterranean countries, that researchers attributed to the diet adopted in that region [125], known to be rich in monounsaturated and polyunsaturated fats as well as fibers, and from the other side it is poor in saturated fats, the main dietary factor responsible for the increase in the levels of total cholesterol and low-density lipoprotein (LDL)-cholesterol considered to risk factors for cardiovascular diseases [126, 127].
Nowadays, the MD pattern is characterized by the high consumption of plant foods (i.e., cereals, fruits, vegetables, legumes, nuts, seeds, and olives), the moderate consumption of dairy products (i.e., mainly cheeses and low-fat yogurt), eggs no more than four per week and fish, the low intake of sweets and red meat, the moderate intake of alcohol (i.e., mostly red wine during meals), and a main source of fat represented by extra virgin olive oil [128]. Several studies demonstrated the beneficial impact of the MD on health, which confirmed the cardiovascular benefits of this dietary pattern [129, 130] and also showed its ability to reduce the risk of several chronic diseases such T2MD, metabolic syndrome, and certain forms of cancer.
The potential mechanisms behind the pro-health effects of the MD seems to be in its anti-inflammatory and immune-modulating properties [131–133] that derive from the synergy between the various nutrients and foods that interact between each other enhancing their beneficial effects [134]. In this direction, one of the main protagonists is extra virgin olive oil, rich in antioxidant, anti-inflammatory, and immune-modulating substances, such as polyphenols [135].
Moreover, the MD is also characterized by a high intake of fibers that seems to act on the intestinal microbiota by modulating its composition and activity as well as the production of metabolites that regulate the immune function and inflammatory pathways [136]. In particular, a high-fiber diet leads to an increase in the number of intestinal bacterial species responsible for the production of SCFAs such as acetate, propionate, and butyrate [137–139], important for the proper functioning of the immune system and in the prevention of inflammatory diseases [139]. For instance, butyrate can reduce the production of pro-inflammatory molecules such as TNF-α, IL-1β, and nitric oxide, reduce the activity of NFκB, inhibit the production of IL-12, and increase the production of IL-10 by monocytes [140, 141].
In addition, the MD also ensures a high intake of omega-3 polyunsaturated fatty acids from fish (i.e., mackerel, herring, and sardines) and vegetable (i.e. green plants, soybean and canola oils, nuts and seeds) sources and an adequate omega-6/omega-3 ratio [142] by promoting a better inflammatory profile compared to other Western pattern diets in which their intake of omega-6 fatty acids is greater and induces a higher production of pro-inflammatory cytokines and pro-coagulant factors that increase the risk of chronic diseases such as T2MD and atherosclerosis [143]. In fact, the dietary omega-3 fatty acids have a variety of anti-inflammatory and immune-modulating effect and seem to be able to reduce the inflammatory process [144].
Not only due to the anti-inflammatory effects, the beneficial impact on health of the MD seems to be due to its ability to modulate the immune system, through different components, i.e., omega-3 polyunsaturated fatty acids and polyphenols [145–147]. First, omega-3 polyunsaturated fatty acids can in fact modulate the function of T cells either directly, for example by inhibiting the differentiation of T-helper (Th)-1 and Th17, or indirectly by inhibiting the function of antigen-presenting cells like monocytes/macrophages and dendritic cells [147]. In second place, it seems that they are also able to modulate the functions of B cells by promoting their activation and production of cytokines and antibodies [147, 148]. Finally, the intake of polyphenols appears to be associated with a change in the count and differentiation of immune cells [149]. They, for example, down-regulate macrophage activity thereby reducing the production of TNFα, IL-1β and IL-6 expression [150].
The CoVID-19 disease pandemic, caused by the SARS-CoV-2 has spread all over the globe, and known to be is associated with several medical and psychological complications [151••]. Moreover, several studies have shown that obesity and obesity-correlated diseases, i.e., T2MD and cardio-metabolic disorders, may lead to poor CoVID-19 outcomes [152], especially in those with sarcopenic obesity where this condition seems to represent a real problem in CoVID-19 [153, 154].
Little is still known about the nutritional approach to be adopted before (i.e., prevention), during and after CoVID-19 infection in the short and long term. However, in this context, and since the MD is one of the healthiest dietary patterns, it can be considered a valid diet especially [155].
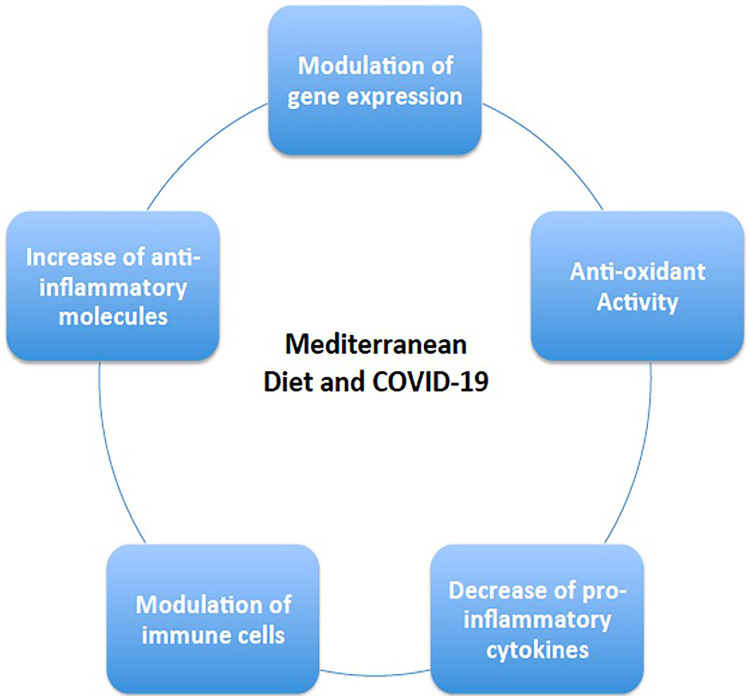
From a preventative point of view, the adherence to the MD included in a context of a healthy lifestyle associated to a regular moderate physical activity that can in general overcome undernutrition-malnutrition and reinforce the immune system and help to fight any potential infection [156]. In fact, as demonstrated by a large Italian study, the “CoVID-19 pandemic” has led to an important modification of food habits and dietary behaviors during the CoVID-19 lockdown with 35.8% of the studied individuals consumed less healthy foods, while 48.6% reported weight gain [157]. Specifically, not to forget, as mentioned above, individuals affected by obesity and correlated morbidities (cardio-metabolic disease) are considered more vulnerable for poorer clinical outcome [158]. Therefore, weight-management programs based on the MD prior to a potential CoVID-19 infection are of utmost importance in these people, which can provide an indispensable tool if not for weight loss but at least to avoid any additional weight gain that may lead to clinical deterioration of obesity and its correlated comorbidities, that may put this population on higher risk of disease severity (i.e., CoVID-19) [159]. More interestingly, the MD showed to be also a valid therapeutic nutritional approach during infection period since it has been demonstrated that a higher adherence to the MD was associated with a decreased CoVID-19 severity and mortality [155], which seems to be due to its high antioxidant, anti-inflammatory, and immune-modulatory properties (Fig. 3). For this reason, it is considered as a promising and relatively easy tool that can attenuate the severity of CoVID-19 infection as well as mortality [160].
Fig. 3.
Potential mechanisms through which the Mediterranean diet contrast SARS-CoV-2 infection
The Potential Dietary Therapy in Patients Post-infection by CoVID
Multiphase Ketogenic Protocol with Meal Replacement
KDs are high-fat, low-carbohydrate diets and have been used primarily to treat epilepsy in children [161]. Interestingly, their use has been adapted to different pathological conditions (severe obesity, metabolic diseases, migraine, cancer, etc.) by changing the macronutrient composition and energy content. In this context, the importance of KDs in the treatment of obesity and its associated comorbidities (T2DM, dyslipidemia, insulin resistance, inflammation) has recently been highlighted [89, 162]. There are several KDs whose common purpose is to induce ketosis. These include the classic ketogenic diet, the Atkins diet, the high-fat ketogenic diet, the very-low-carbohydrate diet, and the VLCKD [88]. VLCKD, which is characterized by a significant restriction of carbohydrate and total energy intake per day with a relative increase in fat and protein, is a highly effective dietary strategy for patients who need rapid weight loss in the short term, such as individuals with moderate to severe obesity and associated cardiovascular risk factors, resulting in significant improvement in insulin resistance, glucose, and blood pressure control [89, 162]. Weight loss achieved with VLCKD is mostly secondary to loss of fat mass, while lean mass and adequate nutritional status are maintained [163]. VLCKD involves several phases and can be formulated using meal replacements or conventional foods [164]. In any case, KDs require strict medical monitoring and therapeutic compliance, along with adequate micronutrient and vitamin supplementation. For this reason, contraindications to their use should be carefully considered [89] (Table 2).
Table 2.
Medical contraindications to VLCKD of ADI (Associazione Italiana di Dietetica e Nutrizione Clinica) and SIE (Società Italiana di Endocrinologia)
| Contraindications | |
|---|---|
| ADI | SIE |
|
Pregnancy and lactation History of mental disorders and behavioral problems, abuse of alcohol and other substances Hepatic or renal failure Type 1 diabetes |
Type 1 diabetes mellitus Latent autoimmune diabetes in adults β-Cell failure in type 2 diabetes mellitus Use of sodium/glucose cotransporter 2 (SGLT2) inhibitors (risk for euglycemic diabetic ketoacidosis) Pregnancy and breastfeeding Kidney failure and moderate-to-severe chronic kidney disease Liver failure Hearth failure (NYHA III–IV) Respiratory failure Unstable angina, recent stroke, or myocardial infarction (or myocardial infarction (or myocardial infarction (< 12 months) Cardiac arrhythmias Eating disorders and other severe mental illnesses, alcohol and substance abuse Active/severe infections Frail elderly patients 48 h prior to elective surgery or invasive procedures and perioperative period Rare disorders: porphyria, carnitine deficiency, carnitine palmitoyltransferase deficiency, carnitine-acylcarnitine translocase deficiency, mitochondrial fatty acid β-oxidation disorders, pyruvate carboxylase deficiency |
Efficacy of a Multiphase Dietetic Protocol with Meal Replacements
As described in the European Guidelines for Obesity Management in Adults with VLCKD of the European Association for the Study of Obesity (EASO), the protocol includes high-biological-value protein (from milk, peas, whey, and soy), artificial meals, and natural foods [164]. The active phase of VLCKD can be initiated by using meal replacements or conventional foods (meat, fish, and eggs). However, recent data show that the most significant results in terms of safety and efficacy are obtained when using a multi-phase protocol with meal replacement [118, 165–167]. In a prospective pilot study comparing the efficacy and safety of a 45-day VLCKD with whey or vegetable proteins (with meal replacements) or animal proteins (with conventional foods), metabolic parameters, body composition, and gut microbiota composition have been assessed in a population of patients with obesity (body mass index, BMI: 35.9 ± 4.1 kg/m2) and insulin resistance [165]. A significant reduction in initial body weight was observed in both the whey protein and vegetable protein groups. A reduction in body weight was also observed in the animal protein group, although it did not reach statistical significance. Of note, in the animal protein group, urea nitrogen and uric acid increased, while the estimated glomerular filtration rate decreased significantly compared to baseline values [165]. There was also a significant decrease in Firmicutes and an increase in Bacteroidetes, improving the Firmicutes/Bacteroidetes ratio. In particular, whey protein and vegetable protein were more effective than the dietary intervention with animal protein in reducing the proportion of Firmicutes. The authors concluded that VLCKDs based on whey or vegetable protein determined a safer metabolic profile and resulted in a healthier microbiota composition than those containing animal protein [165]. In a multicenter, prospective, uncontrolled study carried out in 33 women with overweight or obesity (BMI: 30.9 ± 2.7 kg/m2) who began a VLCKD meal replacement nutrition program divided into four phases, the VLCKD program resulted in significant decreases in body weight, BMI, and waist circumference [166]. In addition, homeostasis model assessment of insulin resistance was significantly reduced after phase 2 and remained unchanged thereafter. After phase 1, systolic blood pressure decreased and remained unchanged until the end of the program. Finally, total and LDL cholesterol and triglycerides were significantly reduced by VLCKD, while high-density lipoprotein (HDL) cholesterol increased significantly [166]. In a double-blind study, 18 adult participants with overweight and obesity (BMI ≥ 25 kg/m2) were randomized to amino acid–supplemented VLCKD or very low calorie diet [118]. After 3 weeks, significant weight loss was noted in both groups, accompanied by a reduction in fat mass. Interestingly, the VLCKD group showed a reduction in waist circumference and preservation of fat free mass, whereas the very low calorie diet group showed no change in waist circumference and a significant decrease in fat free mass [118]. Finally, the multi-phase protocol approach with meal replacement also seems to provide good safety and tolerance [167]. In a prospective study of 106 subjects with obesity (BMI 34.98 ± 5.43 kg/m2) who followed a multi-phase VLCKD protocol with meal replacement, side effects were assessed during the active phase. At the end of this phase, no serious side effects occurred in the population. Those that did occur were clinically mild and did not lead to discontinuation of the diet protocol, as they were easily treated by healthcare professionals or often resolved spontaneously [167]. Therefore, to ensure the efficacy and safety of a VLCKD based on the emerging scientific evidence, it seems more appropriate to set the first active or ketogenic phase through the use of meal replacements [165].
VLCKD Protocol in Patients Post-infection by CoVID
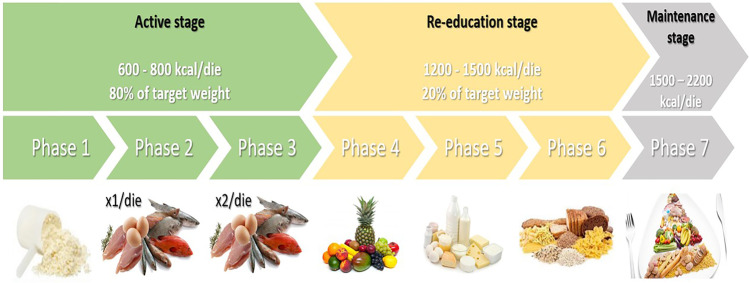
The VLCKD protocol provides < 800 kcal/day with a carbohydrate restriction of 30–50 g/day (≃13% of total energy intake), an increase of 30–40 g/day (≃44%) of fats, and approximately 0.8–1.2 g/day of proteins per kilogram of body weight (≃43%) [89, 168, 169]. VLCKD is based on high biological value protein preparations from non-animal and/or animal protein sources such as peas, eggs, soy, and whey protein [89]. Each artificial meal typically contains 18 g of protein, 4 g of carbohydrate, and 3 g of fat (mainly high oleic vegetable oils) and provides approximately 100–150 kcal. It is also considered a low-fat protocol, mainly from extra virgin olive oil (about 20 g/day). This protocol requires proper guidance from a team of qualified specialists and requires careful monitoring. It is divided into three phases: active (or ketogenic), re-education, and maintenance/transition to a Mediterranean diet [89, 169] (Fig. 4).
Fig. 4.
Overview of VLCKD’s three main phases and their sub-phases
Assessment of anthropometric measurements (BMI, weight, waist and hip circumference), body composition, and hydration status (by bioelectrical impedance analysis) is recommended at baseline, during the active phase, and at the end of the VLCKD program. To investigate the efficacy of VLCKD on the metabolic parameters, glucose, insulin, total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides, these should be determined at the beginning and at the end of the VLCKD program.
Active or Ketogenic Stage
This stage is generally characterized by a very-low-calorie diet containing < 800 kcal/day, of which less than 30 g/day comes from low-carbohydrate sources such as vegetables, protein of high biological value (0.8–1.2 kg/ideal body weight to maintain lean mass and meet the body’s minimum daily requirements), and fats from extra virgin olive oil (10 g/day) [89, 169, 170]. According to international recommendations, the active phase could last between 8 and 12 weeks, but it is important to measure the percentage of weight loss (at least 80% of the desirable weight loss based on the ideal weight calculation) [169]. Three to five days must be considered to reach the active phase and adequate patient education about the protocol must be considered to achieve compliance. The literature also reports that the active phase can be achieved when half of the daily protein is supplemented with a synthetic amino acid supplement containing whey protein (13.42/bag), carbohydrate (0.03/bag), fat (0.15/pouch), isoleucine (0.31/pouch), ornithine-α-ketoglutarate (0.25/pouch), L-citrulline (0.25/pouch), taurine (0.25/pouch), L-tryptophan (0.05/pouch), and potassium citrate (0.45/pouch) for a total of 64 kcal (268 kJ) dissolved in water. This drink is taken with breakfast and lunch or dinner [118]. Because fruit consumption was not allowed at the beginning of the program and the amount of vegetables was limited, patients were instructed to take a multivitamin and multisaline supplement formulated to maintain physiological acid–base balance (vitamins and minerals such as K, B complex, C, E, Na, Mg, Ca, and omega-3 fatty acids) [89, 169, 170]. It was also recommended that, if possible, sodium chloride intake should be adequate to prevent a drop in blood pressure and not to drink less than 1.5–2 L of water per day. Schematically, the active or ketogenic stage is divided into three further different phases:
Phase 1: five meals a day with high biological quality protein supplements, accompanied by low glycemic index vegetables.
Phase 2: one portion of protein is replaced with animal proteins, either at lunch or dinner.
Phase 3: low-fat protein from animal sources for both lunch and dinner.
Re-education Stage
After the active phase, transition to a low-calorie diet should be considered and, in the meantime, participation in a nutritional re-education program to maintain weight loss in the long term. In addition, it represents an important phase of reintroduction (800–1500 kcal/day depending on the specific requirements of the individual) of different food groups that have been excluded until that point (phases 4, 5, and 6, respectively) [89, 169, 170]. The carbohydrates are gradually reintroduced in the following order:
Phase 4: fruit and dairy products.
Phase 5: legumes.
Phase 6: bread, pasta, and cereals.
Transition to the MD in Patients Post-infection by CoVID
This phase is crucial for the introduction of carbohydrates into a balanced diet to achieve a long-term effect. Once the reintroduction of food is complete, there is a maintenance phase that includes a series of balanced dietary patterns with various carbohydrates, proteins, and fats [89, 169, 170]. The main scope of this phase is to promote long-term weight loss and a healthy lifestyle. To this purpose, the transition to a Mediterranean-style dietary pattern is strongly recommended. Not least, it is also important to reintroduce adequate regular physical activity [89, 169, 170].
Phase 7: 2000 kcal, balanced diet and regular physical activity.
MD is a dietary pattern characterized by many bioactive compounds with anti-inflammatory and antioxidant activities (monounsaturated and omega-3 fatty acids, and vitamins, minerals, and phytochemicals, respectively) [133]. Therefore, it might be recommended as a medical prescription for the long-term follow-up of patients after CoVID-19 infection. Individual energy requirement (ER) should be assessed to provide a tailored nutritional therapy also in the post-recovery period. As in standard clinical practice, actual ER should be determined considering patients’ characteristics (mainly age and gender), BMI and body composition (free fat mass and fat mass), as well as food intake and physical activity level [171, 172]. The assessment of anthropometric measures, body composition, and physical activity should be scheduled periodically to adjust individual ER and endorse a well-balanced nutritional plan in the long term. As for dietary composition, most of MD energy intake is provided by carbohydrate (55–60%), nearly 30% from fat, and the remaining part by protein (15%) [173]. More in detail, the recommended dietary allowance (RDA) for carbohydrate is 130 g/day, and it is in line with the MD [174]. According to the MD, carbohydrate is provided by low-glycemic index foods (i.e., wholegrain-based products and legumes) while sugar intake is less than 10% by limiting the consumption of sweets and sugar-sweetened beverages. This avoids postprandial hyperglycemia, which associates with increased oxidative stress and consequent inflammation [175, 176]. In addition, the MD fulfills nutritional recommendations for dietary fiber intake (25–30 g/day) favoring the consumption of fiber-rich foods (i.e., wholegrain, legumes, fruit, vegetables, and nuts) [173]. Notably, soluble and high fermentable fibers contained in these foods (i.e., β-glucan and arabinoxylans from wholegrain, pectins from fruit, vegetables, and legumes, and lignans from nuts) have a prebiotic effect on some butyrate-producing bacteria (i.e., Roseburia, Faecalibacterium prausnitzii, Prevotella) which have shown to reduce inflammation and inflammatory diseases by virtue of SCFAs production [177–179]. Fat is mainly represented by monounsaturated fatty acids (19%), followed by saturated fatty acids (9%) and polyunsaturated fatty acids (5%), and cholesterol is 300 mg/day [180]. This nutritional profile is driven by the consumption of extra-virgin olive oil (monounsaturated fatty acids), low-fat dairies (saturated fatty acids), and fish and nuts (polyunsaturated fatty acids, especially omega-3 fatty acids), and it has been associated with reduced inflammation and a better global health [181–183].
As for protein, the MD complies with RDA for the maintenance of muscle mass (0.8 g protein/kg body weight) [174]. According to the MD, plant protein (i.e., legumes and soy-derived products) should be preferred, while animal protein (fish, lean cuts of meat, eggs, and dairies) should be used as alternative options during the week [133]. Vitamins and some minerals play a pivotal role in modulating immunity as well as in other biological functions. Several studies have shown that hospitalized patients for CoVID-19 presented micronutrient deficiency, particularly in vitamin D (76%) [184].
The MD can provide relevant amounts of vitamins and minerals. Indeed, a cross-sectional study in 6647 individuals showed that higher variety in fruit and/or vegetable consumption was associated with a reduced risk of micronutrient deficiencies, both vitamins and minerals [185]. In addition, increased fruit variety can contribute to the achievement of RDA for vitamin C (90 and 75 mg/day for men and women, respectively) and vitamin A (900 and 700 μg retinol activity equivalents/day for men and women, respectively) in two large cohort studies (n = 9769 and n = 20,069 participants) [186, 187].
Besides, international nutritional recommendations suggested the importance of vitamin D intake, particularly in patients with lower exposure to sunlight (i.e., long-term confinement or hospitalization) [184]. Notably, a cross-sectional study in 402 healthy individuals showed that set aside insufficient exposure to sunlight and other factors (i.e., overweight/obesity, sunscreen use, and seasonality), low adherence to the MD was an independent risk factor for vitamin D deficiency. Conversely, a higher intake of fish and olive oil was positively associated with increased plasma vitamin D concentrations [188]. Therefore, dietary sources such as oily fish, sun light-exposed mushrooms, eggs, and milk can contribute to achieving the RDA for vitamin D (10 µg/day).
As for phytochemicals, no RDA exists. Nevertheless, these bioactive compounds (polyphenols, phytosterols, and carotenoids) are contained in fruits, vegetables, nuts, wholegrain, legumes, and red wine. Therefore, they can play a major role in the antioxidant and anti-inflammatory effects of the MD [189]. It is worth mentioning that the unique nutritional profile of the MD is based on a specific frequency of consumption during the week [190] (Table 3):
Daily consumption of plant-based foods (fruits, vegetables, wholegrain, legumes, and nuts) and extra-virgin olive oil as the primary source of fat.
Moderate amount of animal protein and fat, with fish and low-fat dairies as the preferred sources, respectively.
Limited intake of sweets and processed foods.
Table 3.
Dietary recommendations of Mediterranean diet
| Foodstuff | Amount |
|---|---|
| Daily | |
| Extra-virgin olive oil | At least 2 servings (main meal) |
| Wholegrain-based products | 1–2 servings (main meal) |
| Vegetables | > 2 servings (main meal) |
| Fruits | 1–2 servings (main meal) |
| Nuts | 1–2 servings |
| Low-fat dairies | 2 servings |
| Weekly | |
| Legumes | 2–3 servings |
| Fish | > 2 servings |
| Eggs | 2–4 servings |
| Lean meat (preferring poultry) | 1–2 servings |
| With moderation | |
| Red meat | ≤ 1 serving |
| Processed meat | < 2 servings |
| Sweets | < 2 serving week |
| Red wine | Wine in moderation and respecting social beliefs |
Physical Rehabilitation in Post-CoVID Patients: Proposal of a Physical Treatment Protocol
Physical activity (PA) is a potent modulator of multiple physiologic functions. According to the last WHO guidelines, PA improves all-cause mortality in adults, including cardiovascular disease mortality, hypertension, site-specific cancers, and T2DM. Regular PA influences mental health (reduced symptoms of anxiety and depression), improving cognitive functions and sleep, reducing visceral adiposity, and limiting weight gain [191]. In industrialized countries, a decrement of PA was observed prior to the CoVID-19 pandemic. On November 25, 2020, World Health Organization (WHO) released a recent version of the WHO guidelines on physical activity and sedentary behaviors to increase individual PA levels by 15% within 2030. New guidelines include at least 150–300 min of moderate-intensity aerobic physical activity, or at least 75–150 min of vigorous-intensity aerobic PA, or an equivalent combination of moderate- and vigorous-intensity activity throughout the week, for substantial health benefits, for adults from 14 to 65 years [191]. Adults should also do muscle-strengthening activities at a moderate or greater intensity that involve all major muscle groups two or more days a week, as these provide additional health benefits [191]. Low levels of PA due to at-home confinement were registered during CoVID-19 pandemic, with a decrement of 33.5% in the number of minutes/day of PA and the consequent increase in sedentary behaviors [191]. An international survey conducted on 1047 subjects highlighted that lower levels of PA were associated with an increased number of meals and snacking, contributing to a positive energy intake and fat accumulation [192]. Italy was one of the first countries to introduce severe limitations to citizen mobility, with the scope of avoiding virus diffusion. A high prevalence of sleep disturbances (57.1%) and distress (41.8%), and increased anxiety (32.1%) was observed in the general population, in an Italian study, in the first 4 months of severe restrictions [193].
Stress and sleep disturbances, like lower daily PA, can directly contribute to muscle loss through changes in key metabolic, hormonal, and chemical pathways related to muscle mass homeostasis. Short periods of reduced activity have been shown a rapid muscle mass loss, which is appreciable after 2 days of immobilization, with a 5.5% of muscle volume loss after 7 days of inactivity [194]. In the case of CoVID-19 infection, the length of hospitalization days reported is an average of 11 days of bedrest, a median size from 8 to 12 days, in case of intensive care unit admission, with more prolonged periods for older individuals (> 65 years) [195].
In this scenario, a considerable muscle mass loss can be observed in CoVID-19 patients and, more generally, in subjects in at-home quarantine. A study on 27 subjects highlighted that muscle tissue lost during intensity care unit permanence of 7 days did not normalize even after 6 months of PA intervention, even if a significant improvement was reached in the majority of subjects [196]. The etiology of rapid muscle loss due to inactivity is associated with multiple mechanisms like oxidative stress, mitochondrial dysfunction, insulin resistance, and pro-inflammatory cytokine increase [197–200]. A specific loss of type I and II fibers was observed by muscle biopsy in 30 young men after 7 days of knee immobilization [201]. A paper from Camozzi et al. observed that post-acute CoVID patients undergoing intensive care units suffered from dyspnea and shortness of breath, with deambulatory difficulties and severe disability as revealed by 6-min walking test results [202]. Acute infection can be resolved in 4 weeks; however, in 10 to 20% of patients, infection can evolve in a persistent phase of the long-lasting disease (up to 12 weeks and more), characterized by dyspnea, neurocognitive alterations, fatigue, stress, irritability, and confusion, also called post-CoVID syndrome [203]. Genetic host factors, pre-existing comorbidities, potential virus integration in the host genome [204], neuronal sufferance by hypoxia [205], and cytokine storm by innate immune response hyperactivation are the suggested mechanisms underlying post-CoVID syndrome [206]. The dysregulation of the immune response determines progressive endothelial dysfunction responsible for multiple organ failures. The mechanisms of cardiovascular damage seem to involve the protein ACE2, to which the virus binds to penetrate cells, and other mechanisms, among these the endothelial damage. Cardiovascular sequelae of CoVID-19 include heart failure, cardiomyopathy, acute coronary syndrome, arrhythmias, and venous thromboembolism [207].
Exercise training is primarily a tool for cardiac rehabilitation but can be influenced by respiratory problems of various degrees in CoVID-19 patients. However, it represents a powerful tool capable of inducing significant changes in the cardiovascular system causing the recovery of endothelial dysfunction, useful for containment thromboembolic complications [207]. The major recommendations for post-CoVID rehabilitations regard pulmonary and cardiac interventions. Exercise training must be conducted with some precautions as monitoring temperature before training, starting with a strengthening workout to compensate sarcopenia, before initiating an intensive cardiovascular program [208].
Regarding the illness severity, CoVID-19 patients can be classified into
Asymptomatic infected patients;
Paucisymptomatic or symptomatic patients isolated and treated at home;
Symptomatic patients treated in hospital;
Symptomatic patients undergoing intensive and sub-intensive care unit for ventilatory support.
In relationship to the symptoms during CoVID-19 infection, patients who required oxygen therapy or with acute lymphopenia should perform pulmonary radiological and pulmonary function tests and undergo pulmonary rehabilitation, if necessary, before starting exercise. Patients who experience symptoms like cough, fever, chest pain, shortness of breath, body aches, and severe sore throat should avoid starting moderate PA (> 3 metabolic equivalent tasks, MET) the first 3 weeks after symptoms. Patients with very mild symptoms should avoid prolonged or high-intensity training and practice light activity (< 3 MET) to avoid sedentary behaviors, and asymptomatic patients can continue the previous training before contracting the infection [208]. Exercise is a powerful medicine preventing a large spectrum of diseases and disabilities, but dosage and typologies of exercise should be personalized for each individual, based on his starting conditions, and monitored by wearable devices for a better understanding of adherence to the training programs and physiological parameter modification induced by exercise [208].
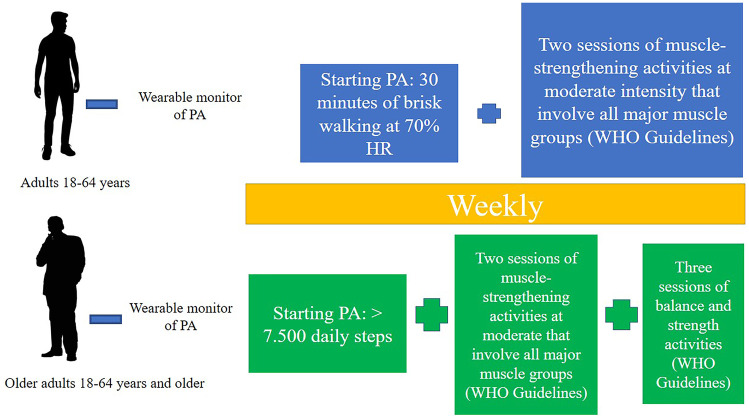
Wearable ambulatory sensors (i.e., smartphones, smart watches, wrist bracelets, patches, necklaces, headbands, eyeglasses) can provide critical monitoring and diagnostic insights to prevent and treat such health problems, while also being small in size and comfortable to use on a long-term basis [208]. These devices can monitor physiologic parameters like temperature, blood pressure, heart rate, the maximum volume of oxygen used to perform exercises (VO2max), blood oxygen saturation, sleep disturbances, and apnea. Many wearable devices have been introduced for remote and long-term cardiac monitoring in patients with heart diseases [209], muscular-skeletal disorders, neurodegenerative disorders, and others [210]. Some devices can also execute electrocardiogram monitoring and arrhythmia detection [211, 212], becoming useful for telemedicine interventions and telehealth programs. A protocol proposal of exercise training after CoVID-19 infection should be constructed considering WHO recommendations contemplating at least 30 min of moderate daily activity. At 70% of heart rate, a starting monitored brisk walking is able to ensure cardiometabolic positive effects. Brisk walking is characterized by simplicity of training and equipment, low levels of injury, and the possibility to conduct an outdoor activity, associated with the advantage of being exposed to sunlight and increasing vitamin D levels [213] (Fig. 5).
Fig. 5.
A proposal of a starting physical treatment protocol for post-CoVID rehabilitation after 3 weeks from symptoms for adults (18–64 years) and older adults (+ 65 years)
Few trials are available in the literature regarding physical activity in post-CoVID infection. A Finnish clinical trial used supervised walking sessions consisting of a warm-up, including walking at self-selected speed and progressive, dynamic balance exercises, and continuous walking for 10–20 min at a target intensity of 13–15 on the Borg scale combined with resistance exercise sessions started with a 10-min warm-up and balance exercises. The subjects targeted in the study were characterized by 159 older adults from 70 to 85 years old. Thereafter, 8–9 resistance exercises targeting the lower body, trunk, and upper body muscles were performed with machines utilizing air pressure technology. The protocol determined an improvement of executive functions in elderly patients after 6 months of exercise training and cognitive training after CoVID-19 infection [214].
Sarcopenia remains one of the more frequent consequences of CoVID-19 infection or quarantine and its etiology, as previously reported, is influenced by aging, pharmacological therapy, and cytokine storm during CoVID-19 acute phase, hormonal perturbations, sedentary behaviors, and poor nutrient quality intake [215]. Multiple approaches combining nutritional and exercise training intervention should be adopted for sarcopenia.
A clinical trial combining nutritional and exercise interventions was conducted on 32 older adults (77.4 ± 2.8 years) for 3 weeks to restore the effects of sarcopenia due to aging. The combined intervention was characterized by 12-week multi-component therapy consisting of 3/day week home-based light, whole-body elastic band resistance exercise, and daily intake of a protein-based five-ingredient supplement or isocaloric/isonitrogenous placebo. Sub-group receiving supplement and exercise training showed greater gains in total lean mass (+ 1.65 kg/ + 3.4%, p < 0.05) [216].
Conclusion
On a general scale, nutritional interventions have attained considerable scientific evidence in diseases’ prevention and treatment. More specifically, little is still known about the best nutritional approach to adopt during CoVID-19 pandemic, especially in the post-infection stage (i.e., short and long term). In fact, to the best of our knowledge, no specific nutritional recommendations exist to manage this condition. However, we speculate that a healthy nutrition has a beneficial role in its holistic concept. This review examines the evidence for areas of consensus and on-going uncertainty or controversy about the best nutritional and exercise-based approaches during the post-CoVID. We come up with a presentation of nutritional therapeutic approach based on a KD protocol followed by a transition to the MD in patients post-infection by CoVID, combined to a physical activity program for young and older adults to address both short- and long-term conditions associated with CoVID-19 infection and severity.
Acknowledgements
BCM—Club delle Terapie Dietetiche in Endocrinologia e Metabolismo. Italian Society of Endocrinology (SIE). Coordinators: Luigi Barrea; Massimiliano Caprio. Secretariat: Giovanna Muscogiuri. http://www.societaitalianadiendocrinologia.it/html/pag/club-home.asp?id=15. Accessed on 21 January 2022.
Abbreviations
- CoVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- CPK
Creatine phosphokinase
- MRI
Magnetic resonance imaging
- ICU
Intensive care unit
- ACE
Angiotensin-converting enzyme
- MHC
Major histocompatibility complex
- RT-PCR
Reverse-transcription polymerase chain reaction
- IL
Interleukin
- TNF-α
Tumor necrosis factor alpha
- GC
Glucocorticoids
- NIAID-OS
National Institute of Allergy and Infectious Disease Ordinal Scale
- MuRF1
Muscle RING-finger protein-1
- FoxO
Forkhead box O
- mTORC
Mechanistic Target of Rapamycim complex
- AMPK
5′-Adenosine monophosphate-activated protein kinase
- IGF
Insulin-like growth factor
- mTOR
Mechanistic target of rapamycin
- TSC2
Tuberous sclerosis complex 2 protein
- IRS
Insulin receptor substrate
- ICU-AW
Intensive care unit-acquired weakness
- KDs
Ketogenic diets
- VLCKD
Very low calorie ketogenic diet
- T2DM
Type 2 diabetes mellitus
- β-OHB
Beta-hydroxybutyrate
- EWGSOP
European Working Group on Sarcopenia in Older People
- CRP
C reactive protein; SCFAs, short-chain fatty acids
- MD
Mediterranean diet
- LDL
Low-density lipoprotein
- Th
T-helper
- EASO
European Association for the Study of Obesity
- BMI
Body mass index
- HDL
High-density lipoprotein
- ER
Energy requirement
- RDA
Recommended dietary allowance
- PA
Physical activity
- WHO
World Health Organization
- MET
Metabolic equivalent tasks
Author contribution
The authors’ responsibilities were as follows: LB was responsible for the concept of this paper; CV, MC, MC, MEG, AE, EC, and LV drafted the manuscript; SS, AM, and GM provided a critical review of the paper. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Metabolism
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Liu YC, Kuo RL, Shih SR. COVID-19: The first documented coronavirus pandemic in history. Biomed J. 2020. [DOI] [PMC free article] [PubMed]
- 2.Dhakal BP, Sweitzer NK, Indik JH, Acharya D, William P. SARS-CoV-2 infection and cardiovascular disease: COVID-19 heart. Heart Lung Circ. 2020. [DOI] [PMC free article] [PubMed]
- 3.Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc. 2020. [DOI] [PMC free article] [PubMed]
- 4.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021. [DOI] [PMC free article] [PubMed]
- 5.Cataldi M, Pignataro G, Taglialatela M. Neurobiology of coronaviruses: Potential relevance for COVID-19. Neurobiol Dis. 2020. [DOI] [PMC free article] [PubMed]
- 6.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nature Med. 2021. [DOI] [PMC free article] [PubMed]
- 7.• Ali AM, Kunugi H. Skeletal muscle damage in covid-19: a call for action. Medicina (Lithuania). 2021;57(4):372. 10.3390/medicina57040372. Muscle loss in symptomatic COVID-19 patients is associated with a poor prognosis of the disease. Particular attention to longer-term consequences of muscle loss in recovering COVID-19 patients is necessary. [DOI] [PMC free article] [PubMed]
- 8.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. New England J Med. 2020. [DOI] [PMC free article] [PubMed]
- 9.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurology. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widyadharma IPE, Sari NNSP, Pradnyaswari KE, Yuwana KT, Adikarya IPGD, Tertia C, et al. Pain as clinical manifestations of COVID-19 infection and its management in the pandemic era: a literature review. Egypt J Neurol Psychiatry Neurosurg. 2020. [DOI] [PMC free article] [PubMed]
- 11.Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan. Chin Clin Microbiol Infect. 2020;26(6):767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do Nascimento IJB, Cacic N, Abdulazeem HM, von Groote TC, Jayarajah U, Weerasekara I, et al. Novel coronavirus infection (Covid-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020. [DOI] [PMC free article] [PubMed]
- 13.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020. [DOI] [PMC free article] [PubMed]
- 14.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020. [DOI] [PMC free article] [PubMed]
- 15.Beydon M, Chevalier K, Al Tabaa O, Hamroun S, Delettre AS, Thomas M, et al. Myositis as a manifestation of SARS-CoV-2. Ann Rheum Dis. 2021. [DOI] [PubMed]
- 16.Islam B, Ahmed M, Islam Z, Begum SM. Severe acute myopathy following SARS-CoV-2 infection: a case report and review of recent literature. Skelet Muscle. 2021. [DOI] [PMC free article] [PubMed]
- 17.Uslu S. Myositis due to COVID-19. Postgrad Med J. 2021. [DOI] [PMC free article] [PubMed]
- 18.Zhang H, Charmchi Z, Seidman RJ, Anziska Y, Velayudhan V, Perk J. COVID-19–associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020. [DOI] [PMC free article] [PubMed]
- 19.Madia F, Merico B, Primiano G, Cutuli SL, De Pascale G, Servidei S. Acute myopathic quadriplegia in patients with COVID-19 in the intensive care unit. Neurology. 2020. [DOI] [PubMed]
- 20.Gobbi M, Bezzoli E, Ismelli F, Trotti G, Cortellezzi S, Meneguzzo F, et al. Skeletal muscle mass, sarcopenia and rehabilitation outcomes in post-acute COVID-19 patients. J Clin Med. 2021. [DOI] [PMC free article] [PubMed]
- 21.Wierdsma NJ, Kruizenga HM, Konings LA, Krebbers D, Jorissen JR, Joosten MHI, et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin Nutr ESPEN. 2021. [DOI] [PMC free article] [PubMed]
- 22.Damanti S, Cristel G, Ramirez GA, Bozzolo EP, Da Prat V, Gobbi A, et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin Nutr. 2021. [DOI] [PMC free article] [PubMed]
- 23.Anwar H, Al Lawati A. Adolescent COVID-19-associated fatal rhabdomyolysis. J Prim Care Community Health. 2020. [DOI] [PMC free article] [PubMed]
- 24.Gefen AM, Palumbo N, Nathan SK, Singer PS, Castellanos-Reyes LJ, Sethna CB. Pediatric COVID-19-associated rhabdomyolysis: a case report. Pediatr Nephrol. 2020. [DOI] [PMC free article] [PubMed]
- 25.Husain R, Corcuera-Solano I, Dayan E, Jacobi AH, Huang M. Rhabdomyolysis as a manifestation of a severe case of COVID-19: a case report. Radiol Case Rep. 2020. [DOI] [PMC free article] [PubMed]
- 26.Reggio C, Paudel A, Specht CS, Donato AA. Necrotising myopathy and concurrent thyroiditis in a patient with COVID-19 infection. BMJ Case Rep. 2021. [DOI] [PMC free article] [PubMed]
- 27.Singh B, Kaur P, Mechineni A, Maroules M. Rhabdomyolysis in COVID-19: report of four cases. Cureus. 2020. [DOI] [PMC free article] [PubMed]
- 28.Taxbro K, Kahlow H, Wulcan H, Fornarve A. Rhabdomyolysis and acute kidney injury in severe COVID-19 infection. BMJ Case Rep. 2020. [DOI] [PMC free article] [PubMed]
- 29.Karaarslan F, Güneri FD, Kardeş S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin Rheum. 2022. [DOI] [PMC free article] [PubMed]
- 30.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021. [DOI] [PMC free article] [PubMed]
- 31.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Network Open. 2021. [DOI] [PMC free article] [PubMed]
- 32.Paneroni M, Simonelli C, Saleri M, Bertacchini L, Venturelli M, Troosters T, et al. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehab. 2021. [DOI] [PubMed]
- 33.Belli S, Balbi B, Prince I, Cattaneo D, Masocco F, Zaccaria S, et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur Respir J. 2020. [DOI] [PMC free article] [PubMed]
- 34.Lokineni S, Mortezavi M. Delayed-onset necrotizing myositis following COVID-19 infection. Eur J Case Rep Intern Med. 2021;8(4):002461. doi: 10.12890/2021_002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piotrowicz K, Gąsowski J, Michel JP, Veronese N. Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res. 2021. [DOI] [PMC free article] [PubMed]
- 36.Echeverría-Rodríguez O, Del Valle-Mondragón L, Hong E. Angiotensin 1–7 improves insulin sensitivity by increasing skeletal muscle glucose uptake in vivo. Peptides. 2014. [DOI] [PubMed]
- 37.Fernandes T, Hashimoto NY, Oliveira EM. Characterization of angiotensin-converting enzymes 1 and 2 in the soleus and plantaris muscles of rats. Braz J Med Biol Res. 2010. [DOI] [PubMed]
- 38.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Letters. 2002. [DOI] [PubMed]
- 39.Riquelme C, Acuña MJ, Torrejón J, Rebolledo D, Cabrera D, Santos RA, et al. ACE2 is augmented in dystrophic skeletal muscle and plays a role in decreasing associated fibrosis. PLoS ONE. 2014. [DOI] [PMC free article] [PubMed]
- 40.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme: cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000. [DOI] [PubMed]
- 41.Aschman T, Schneider J, Greuel S, Meinhardt J, Streit S, Goebel HH, et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurology. 2021. [DOI] [PMC free article] [PubMed]
- 42.Suh J, Mukerji SS, Collens SI, Padera RF, Pinkus GS, Amato AA, et al. Skeletal muscle and peripheral nerve histopathology in COVID-19. Neurology. 2021. [DOI] [PubMed]
- 43.Franch HA, Price SR. Molecular signaling pathways regulating muscle proteolysis during atrophy. Curr Opin Clin Nutr Metab Care. 2005. [DOI] [PubMed]
- 44.Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun. 2021. [DOI] [PMC free article] [PubMed]
- 45.Zhou J, Liu B, Liang C, Li Y, Song YH. Cytokine signaling in skeletal muscle wasting. Trends Endocrinol Metab. 2016. [DOI] [PubMed]
- 46.Manzano GS, Woods JK, Amato AA. Covid-19–associated myopathy caused by type I interferonopathy. New England J Med. 2020. [DOI] [PMC free article] [PubMed]
- 47.Yeoh YK, Zuo T, Lui GCY, Zhang F, Liu Q, Li AYL, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021. [DOI] [PMC free article] [PubMed]
- 48.Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed]
- 49.Ehrenfeld M, Tincani A, Andreoli L, Cattalini M, Greenbaum A, Kanduc D, et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020. [DOI] [PMC free article] [PubMed]
- 50.•• Jabczyk M, Nowak J, Hudzik B, Zubelewicz-Szkodzińska B. Microbiota and its impact on the immune system in COVID-19—a narrative review. Journal of Clinical Medicine. 2021. Due to its impact on the host immune system, the microbiota is of interest for the development of a therapeutic strategy against COVID-19. The severity of COVID-19 is associated with the modulation of microbiota. [DOI] [PMC free article] [PubMed]
- 51.De Giorgio MR, Di Noia S, Morciano C, Conte D. The impact of SARS-CoV-2 on skeletal muscles. Acta Myol. 2020;39(4):307–312. doi: 10.36185/2532-1900-034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Institutes of Health (NIH). Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. 2022. [cited 2022 Jan 21]. Available from: https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 53.Bodine SC, Furlow JD. Glucocorticoids and skeletal muscle. Adv Exp Med Biol. 2015. [DOI] [PubMed]
- 54.Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013. [DOI] [PubMed]
- 55.Braun TP, Marks DL. The regulation of muscle mass by endogenous glucocorticoids. Front Physiol. 2015. [DOI] [PMC free article] [PubMed]
- 56.Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003. [DOI] [PubMed]
- 57.Sandri M. Autophagy in skeletal muscle. FEBS Letters. 2010. [DOI] [PubMed]
- 58.Grumati P, Bonaldo P. Autophagy in skeletal muscle homeostasis and in muscular dystrophies. Cells. 2012. [DOI] [PMC free article] [PubMed]
- 59.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007. [DOI] [PubMed]
- 60.Hay N. Interplay between FOXO, TOR, and Akt. Biochimica et Biophysica Acta - Mol Cell Res. 2011. [DOI] [PMC free article] [PubMed]
- 61.Gilson H, Schakman O, Combaret L, Lause P, Grobet L, Attaix D, et al. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology. 2007. [DOI] [PubMed]
- 62.Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, Takeda SI, et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011. [DOI] [PubMed]
- 63.Lützner N, Kalbacher H, Krones-Herzig A, Rösl F. FOXO3 is a glucocorticoid receptor target and regulates LKB1 and its own expression based on cellular AMP levels via a positive autoregulatory loop. PLoS ONE. 2012. [DOI] [PMC free article] [PubMed]
- 64.Waddell DS, Baehr LM, Van Den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, et al. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Ame J Physiol - Endocrinol Metab. 2008; [DOI] [PMC free article] [PubMed]
- 65.PrOLUMIANT® [Internet]. April 9, 2020. [cited 2022 Jan 21]. p. 46. Available from: http://pi.lilly.com/ca/olumiant-ca-pm.pdf
- 66.ACTEMRA® (tocilizumab) injection, for intravenous or subcutaneous use [Internet]. Revised: 08/2017. [cited 2022 Jan 21]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125276s114lbl.pdf
- 67.Abate SM, Chekole YA, Estifanos MB, Abate KH, Kabthymer RH. Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: a systematic review and meta-analysis. Clin Nutr ESPEN. 2021. [DOI] [PMC free article] [PubMed]
- 68.Riesgo H, Castro A, Del Amo S, San Ceferino MJ, Izaola O, Primo D, et al. Prevalence of risk of malnutrition and risk of sarcopenia in a reference hospital for COVID-19: relationship with mortality. Ann Nutr Metab. 2021; [DOI] [PubMed]
- 69.Cuerda C, Sánchez López I, Gil Martínez C, Merino Viveros M, Velasco C, Cevallos Peñafiel V, et al. Impact of COVID-19 in nutritional and functional status of survivors admitted in intensive care units during the first outbreak. Preliminary results of the NUTRICOVID study. Clin Nutr [Internet]. 2021; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0261561421005264 [DOI] [PMC free article] [PubMed]
- 70.Wang W, Xu C, Ma X, Zhang X, Xie P. Intensive care unit-acquired weakness: a review of recent progress with a look toward the future. Front Med. 2020. [DOI] [PMC free article] [PubMed]
- 71.Welch C, Hassan-Smith ZK, Greig CA, Lord JM, Jackson TA. Acute sarcopenia secondary to hospitalisation – an emerging condition affecting older adults. Aging Dis. 2018. [DOI] [PMC free article] [PubMed]
- 72.Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, et al. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006. [DOI] [PubMed]
- 73.Tankisi H, Ochala J. Myopathy in acute and long-term COVID-19. Clin Neurophysiol [Internet]. 2022;134:141–2. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1388245721007987 [DOI] [PMC free article] [PubMed]
- 74.Tankisi H, Tankisi A, Harbo T, Markvardsen LK, Andersen H, Pedersen TH. Critical illness myopathy as a consequence of Covid-19 infection. Clin Neurophysiol. 2020. [DOI] [PMC free article] [PubMed]
- 75.Agergaard J, Leth S, Pedersen TH, Harbo T, Blicher JU, Karlsson P, et al. Myopathic changes in patients with long-term fatigue after COVID-19. Clin Neurophysiol. 2021. [DOI] [PMC free article] [PubMed]
- 76.Rudroff T, Workman CD, Ponto LLB. 18 F-FDG-PET imaging for post-COVID-19 brain and skeletal muscle alterations. Viruses. 2021. [DOI] [PMC free article] [PubMed]
- 77.Topuz OV, Aksu A, Yilmaz B. Is there any change in the muscle uptake pattern of FDG during or after COVID 19 infection in oncological FDG PET CT patients? J Nucl Med [Internet]. 62 no. Available from: https://jnm.snmjournals.org/content/62/supplement_1/1389
- 78.Ong SWX, Fong SW, Young BE, Chan YH, Lee B, Amrun SN, et al. Persistent symptoms and association with inflammatory cytokine signatures in recovered coronavirus disease 2019 patients. Open Forum Infect Dis. 2021. [DOI] [PMC free article] [PubMed]
- 79.Singh R, Hemati H, Bajpai M, Yadav P, Maheshwari A, Kumar S, et al. Sustained expression of inflammatory monocytes and activated T cells in COVID-19 patients and recovered convalescent plasma donors. Immun Inflamm Dis. 2021. [DOI] [PMC free article] [PubMed]
- 80.Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells [Internet]. 2021;10:3592. Available from: https://www.mdpi.com/2073-4409/10/12/3592 [DOI] [PMC free article] [PubMed]
- 81.Veyseh M, Koyoda S, Ayesha B. COVID-19 IgG-related autoimmune inflammatory necrotizing myositis. BMJ Case Rep. 2021. [DOI] [PMC free article] [PubMed]
- 82.Karami Fath M, Jahangiri A, Ganji M, Sefid F, Payandeh Z, Hashemi ZS, et al. SARS-CoV-2 Proteome harbors peptides which are able to trigger autoimmunity responses: implications for infection, vaccination, and population coverage. Front Immunol. 2021; [DOI] [PMC free article] [PubMed]
- 83.Shah S, Danda D, Kavadichanda C, Das S, Adarsh MB, Negi VS. Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment. Rheumatol Int. 2020. [DOI] [PMC free article] [PubMed]
- 84.Medrinal C, Prieur G, Bonnevie T, Gravier FE, Mayard D, Desmalles E, et al. Muscle weakness, functional capacities and recovery for COVID-19 ICU survivors. BMC Anesthesiology. 2021. [DOI] [PMC free article] [PubMed]
- 85.Bagnato S, Ferraro M, Boccagni C, Battaglia G, D’Agostino T, Prestandrea C, et al. COVID-19 Neuromuscular involvement in post-acute rehabilitation. Brain Sci [Internet]. 2021;11:1611. Available from: https://www.mdpi.com/2076-3425/11/12/1611 [DOI] [PMC free article] [PubMed]
- 86.Gérard M, Mahmutovic M, Malgras A, Michot N, Scheyer N, Jaussaud R, et al. Long-term evolution of malnutrition and loss of muscle strength after covid-19: a major and neglected component of long covid-19. Nutrients. 2021. [DOI] [PMC free article] [PubMed]
- 87.Ramos A, Joaquin C, Ros M, Martin M, Cachero M, Sospedra M, et al. Impact of COVID-19 on nutritional status during the first wave of the pandemic. Clin Nutr. 2021. [DOI] [PMC free article] [PubMed]
- 88.Trimboli P, Castellana M, Bellido D, Casanueva FF. Confusion in the nomenclature of ketogenic diets blurs evidence. Rev Endocr Metab Disord. 2020. [DOI] [PubMed]
- 89.Caprio M, Infante M, Moriconi E, Armani A, Fabbri A, Mantovani G, et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J Endocrinol Invest. 2019. [DOI] [PubMed]
- 90.Goday A, Bellido D, Sajoux I, Crujeiras AB, Burguera B, García-Luna PP, et al. Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr Diabetes. 2016. [DOI] [PMC free article] [PubMed]
- 91.Romano L, Marchetti M, Gualtieri P, Renzo LD, Belcastro M, De Santis GL, et al. Effects of a personalized VLCKD on body composition and resting energy expenditure in the reversal of diabetes to prevent complications. Nutrients. 2019; [DOI] [PMC free article] [PubMed]
- 92.Saslow LR, Kim S, Daubenmier JJ, Moskowitz JT, Phinney SD, Goldman V, et al. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS ONE. 2014. [DOI] [PMC free article] [PubMed]
- 93.El Ghoch M, Calugi S, Grave RD. The effects of low-carbohydrate diets on psychosocial outcomes in obesity/overweight: a systematic review of randomized, controlled studies. Nutrients. 2016. [DOI] [PMC free article] [PubMed]
- 94.Petrakis D, Margină D, Tsarouhas K, Tekos F, Stan M, Nikitovic D, et al. Obesity – a risk factor for increased COVID‑19 prevalence, severity and lethality (Review). Mol Med Rep. 2020. [DOI] [PMC free article] [PubMed]
- 95.Moriconi E, Camajani E, Fabbri A, Lenzi A, Caprio M. Very-low-calorie ketogenic diet as a safe and valuable tool for long-term glycemic management in patients with obesity and type 2 diabetes. Nutrients. 2020. [DOI] [PMC free article] [PubMed]
- 96.Gangitano E, Tozzi R, Gandini O, Watanabe M, Basciani S, Mariani S, et al. Ketogenic diet as a preventive and supportive care for covid-19 patients. Nutrients. 2021. [DOI] [PMC free article] [PubMed]
- 97.Gangitano E, Tozzi R, Mariani S, Lenzi A, Gnessi L, Lubrano C. Ketogenic diet for obese COVID-19 patients: is respiratory disease a contraindication? A narrative review of the literature on ketogenic diet and respiratory function. Front Nutr [Internet]. 2021;8. Available from: https://www.frontiersin.org/articles/10.3389/fnut.2021.771047/full [DOI] [PMC free article] [PubMed]
- 98.Sukkar SG, Bassetti M. Induction of ketosis as a potential therapeutic option to limit hyperglycemia and prevent cytokine storm in COVID-19. Nutrition. 2020. [DOI] [PMC free article] [PubMed]
- 99.Qian Z, Travanty EA, Oko L, Edeen K, Berglund A, Wang J, et al. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol. 2013. [DOI] [PMC free article] [PubMed]
- 100.Paoli A, Gorini S, Caprio M. The dark side of the spoon – glucose, ketones and COVID-19: a possible role for ketogenic diet? J Transl Med. 2020. [DOI] [PMC free article] [PubMed]
- 101.Sukkar SG, Cogorno L, Pisciotta L, Pasta A, Vena A, Gradaschi R, et al. Clinical efficacy of eucaloric ketogenic nutrition in the COVID-19 cytokine storm: a retrospective analysis of mortality and intensive care unit admission. Nutrition. 2021. [DOI] [PMC free article] [PubMed]
- 102.Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med. 2021. [DOI] [PMC free article] [PubMed]
- 103.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA - J Am Med Assoc. 2020. [DOI] [PMC free article] [PubMed]
- 104.Moreno-Pérez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JM, Arenas-Jiménez J, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021. [DOI] [PMC free article] [PubMed]
- 105.Sammarco R, Marra M, Di Guglielmo ML, Naccarato M, Contaldo F, Poggiogalle E, et al. Evaluation of hypocaloric diet with protein supplementation in middle-aged sarcopenic obese women: a pilot study. Obes Facts. 2017. [DOI] [PMC free article] [PubMed]
- 106.Wing JS, Sanderson LM, Brender JD, Perrotta DM, Beauchamp RA. Acute health effects in a community after a release of hydrofluoric acid. Arch Environ Health. 1991. [DOI] [PubMed]
- 107.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019. [DOI] [PMC free article] [PubMed]
- 108.Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Current Opinion in Clinical Nutrition and Metabolic Care. 2012. [DOI] [PubMed]
- 109.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018. [DOI] [PMC free article] [PubMed]
- 110.Cevenini E, Monti D, Franceschi C. Inflamm-ageing. Curr Opin Clin Nutr Metab Care. 2013. [DOI] [PubMed]
- 111.Li CW, Yu K, Shyh-Chang N, Li GX, Jiang LJ, Yu SL, et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia Sarcopenia Muscle. 2019. [DOI] [PMC free article] [PubMed]
- 112.Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017. [DOI] [PubMed]
- 113.Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019. [DOI] [PMC free article] [PubMed]
- 114.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020. [DOI] [PMC free article] [PubMed]
- 115.Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2020. [DOI] [PMC free article] [PubMed]
- 116.Schiaffino S, Albano D, Cozzi A, Messina C, Arioli R, Bnà C, et al. CT-derived chest muscle metrics for outcome prediction in patients with COVID-19. Radiology. 2021. [DOI] [PMC free article] [PubMed]
- 117.Benlloch M, López-Rodríguez MM, Cuerda-Ballester M, Drehmer E, Carrera S, Ceron JJ, et al. Satiating effect of a ketogenic diet and its impact on muscle improvement and oxidation state in multiple sclerosis patients. Nutrients. 2019; [DOI] [PMC free article] [PubMed]
- 118.Merra G, Miranda R, Barrucco S, Gualtieri P, Mazza M, Moriconi E, et al. Very-low-calorie ketogenic diet with aminoacid supplement versus very low restricted-calorie diet for preserving muscle mass during weight loss: a pilot double-blind study. Eur Rev Med Pharmacol Sci. 2016. [PubMed]
- 119.Thomsen HH, Rittig N, Johannsen M, Møller AB, Jørgensen JO, Jessen N, et al. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr. 2018. [DOI] [PubMed]
- 120.Hussain I, Cher GLY, Abid MA, Abid MB. Role of gut microbiome in COVID-19: an insight into pathogenesis and therapeutic potential. Front Immunol. 2021. [DOI] [PMC free article] [PubMed]
- 121.Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol. 2020. [DOI] [PMC free article] [PubMed]
- 122.Verhoog S, Taneri PE, Díaz ZMR, Marques-Vidal P, Troup JP, Bally L, et al. Dietary factors and modulation of bacteria strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: a systematic review. Nutrients. 2019. [DOI] [PMC free article] [PubMed]
- 123.Paoli A, Mancin L, Bianco A, Thomas E, Mota JF, Piccini F. Ketogenic diet and microbiota: friends or enemies? Genes. 2019. [DOI] [PMC free article] [PubMed]
- 124.Rondanelli M, Gasparri C, Peroni G, Faliva MA, Naso M, Perna S, et al. The potential roles of very low calorie, very low calorie ketogenic diets and very low carbohydrate diets on the gut microbiota composition. Front Endocrinol. 2021;12:662591. [DOI] [PMC free article] [PubMed]
- 125.Keys A, Menotti A, Aravanis C, Blackburn H, Djordevič BS, Buzina R, et al. The seven countries study: 2,289 deaths in 15 years. Prevent Med. 1984. [DOI] [PubMed]
- 126.Estruch R, Camafort M. The Mediterranean diet and plasma lipid profile. Rev Esp Cardiol. 2015. [DOI] [PubMed]
- 127.Blackburn H. Invited commentary: 30-year perspective on the seven countries study. Am J Epidemiol. 2017. [DOI] [PubMed]
- 128.Mazzocchi A, Leone L, Agostoni C, Pali-Schöll I. The secrets of the mediterranean diet. Does [only] olive oil matter? Nutrients. 2019. [DOI] [PMC free article] [PubMed]
- 129.Buckland G, González CA, Agudo A, Vilardell M, Berenguer A, Amiano P, et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC cohort study. Am J Epidemiol. 2009. [DOI] [PubMed]
- 130.Vargas G, Azarbal J, Tota-Maharaj R. A comparative review of established diets for prevention of cardiovascular disease and newer dietary strategies. Curr Probl Cardiol. 2021. [DOI] [PubMed]
- 131.Soldati L, Di Renzo L, Jirillo E, Ascierto PA, Marincola FM, De Lorenzo A. The influence of diet on anti-cancer immune responsiveness. J Transl Med. 2018. [DOI] [PMC free article] [PubMed]
- 132.Tosti V, Bertozzi B, Fontana L. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol - Series A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed]
- 133.Barrea L, Muscogiuri G, Frias-Toral E, Laudisio D, Pugliese G, Castellucci B, et al. Nutrition and immune system: from the Mediterranean diet to dietary supplementary through the microbiota. Crit Rev Food Sci Nutr. 2020. [DOI] [PubMed]
- 134.Lăcătușu CM, Grigorescu ED, Floria M, Onofriescu A, Mihai BM. The Mediterranean diet: from an environment-driven food culture to an emerging medical prescription. Int J Environ Res Publ Health. 2019. [DOI] [PMC free article] [PubMed]
- 135.De Santis S, Liso M, Verna G, Curci F, Milani G, Faienza MF, et al. Extra virgin olive oil extracts modulate the inflammatory ability of murine dendritic cells based on their polyphenols pattern: correlation between chemical composition and biological function. Antioxidants. 2021. [DOI] [PMC free article] [PubMed]
- 136.Cronin P, Joyce SA, O’toole PW, O’connor EM. Dietary fibre modulates the gut microbiota. Nutrients. 2021. [DOI] [PMC free article] [PubMed]
- 137.Barrea L, Muscogiuri G, Annunziata G, Laudisio D, Pugliese G, Salzano C, et al. From gut microbiota dysfunction to obesity: could short-chain fatty acids stop this dangerous course? Hormones. 2019;18. [DOI] [PubMed]
- 138.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014. [DOI] [PubMed]
- 139.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014. [DOI] [PubMed]
- 140.Ni YF, Wang J, Yan XL, Tian F, Zhao JB, Wang YJ, et al. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respir Res. 2010. [DOI] [PMC free article] [PubMed]
- 141.Säemann MD, Böhmig GA, Österreicher CH, Burtscher H, Parolini O, Diakos C, et al. Anti‐inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL‐12 and up‐regulation of IL‐10 production. The FASEB Journal. 2000. [DOI] [PubMed]
- 142.Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord-Drug Targets. 2014; [DOI] [PMC free article] [PubMed]
- 143.DiNicolantonio JJ, O’Keefe J. The importance of maintaining a low omega-6/omega-3 ratio for reducing the risk of inflammatory cytokine storms. Mo Med. 2020. [PMC free article] [PubMed]
- 144.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013. [DOI] [PMC free article] [PubMed]
- 145.Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab. 2009. [DOI] [PubMed]
- 146.Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog Lipid Res. 2010. [DOI] [PMC free article] [PubMed]
- 147.Wu D, Lewis ED, Pae M, Meydani SN. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. 2019. [DOI] [PMC free article] [PubMed]
- 148.Gurzell EA, Teague H, Harris M, Clinthorne J, Shaikh SR, Fenton JI. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. J Leukocyte Biol. 2013. [DOI] [PMC free article] [PubMed]
- 149.Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018. [DOI] [PMC free article] [PubMed]
- 150.González R, Ballester I, López-Posadas R, Suárez MD, Zarzuelo A, Martínez-Augustin O, et al. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011. [DOI] [PubMed]
- 151.•• Shanbehzadeh S, Tavahomi M, Zanjari N, Ebrahimi-Takamjani I, Amiri-arimi S. Physical and mental health complications post-COVID-19: scoping review. J Psychosom Res. 2021. (Several physical and mental health problems have been reported up to 3 months post-COVID-19. A comprehensive assessment and post-COVID-19 rehabilitation is needed to promote quality of life.) [DOI] [PMC free article] [PubMed]
- 152.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020. [DOI] [PMC free article] [PubMed]
- 153.Kara M, Ata AM, Özçakar L. Sarcopenic obesity is the real problem in COVID-19 ! Eur J Intern Med. 2021. [DOI] [PMC free article] [PubMed]
- 154.Khazem S, Itani L, Kreidieh D, El Masri D, Tannir H, Citarella R, et al. Reduced lean body mass and cardiometabolic diseases in adult males with overweight and obesity: a pilot study. Int J Environ Res Publ Health. 2018; [DOI] [PMC free article] [PubMed]
- 155.Angelidi AM, Kokkinos A, Katechaki E, Ros E, Mantzoros CS. Mediterranean diet as a nutritional approach for COVID-19. Metabolism: Clin Exp. 2021. [DOI] [PMC free article] [PubMed]
- 156.El Ghoch M, Valerio A. Let food be the medicine, but not for coronavirus: nutrition and food science, telling myths from facts. J Popul Ther Clin Pharmacol. 2020. [DOI] [PubMed]
- 157.Di Renzo L, Gualtieri P, Pivari F, Soldati L, Attinà A, Cinelli G, et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med [Internet]. 2020;18. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85086297209&doi=10.1186%2Fs12967-020-02399-5&partnerID=40&md5=04cd8d902535d75395c85a604cc728ff [DOI] [PMC free article] [PubMed]
- 158.Valerio A, Nisoli E, Rossi AP, Pellegrini M, Todesco T, El Ghoch M. Obesity and higher risk for severe complications of COVID-19: what to do when the two pandemics meet. J Popul Ther Clin Pharmacol. 2020. [DOI] [PubMed]
- 159.Barazzoni R, Bischoff SC, Busetto L, Cederholm T, Chourdakis M, Cuerda C, et al. Nutritional management of individuals with obesity and COVID-19: ESPEN expert statements and practical guidance. Clin Nutr. 2021. [DOI] [PMC free article] [PubMed]
- 160.Greene MW, Roberts AP, Frugé AD. Negative association between Mediterranean diet adherence and COVID-19 cases and related deaths in Spain and 23 OECD countries: an ecological study. Front Nutr. 2021. [DOI] [PMC free article] [PubMed]
- 161.Wheless JW. History of the ketogenic diet. Epilepsia. 2008. [DOI] [PubMed]
- 162.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013. [DOI] [PMC free article] [PubMed]
- 163.Gomez-Arbelaez D, Crujeiras AB, Castro AI, Martinez-Olmos MA, Canton A, Ordoñez-Mayan L, et al. Resting metabolic rate of obese patients under very low calorie ketogenic diet. Nutr Metab. 2018. [DOI] [PMC free article] [PubMed]
- 164.Muscogiuri G, El Ghoch M, Colao A, Hassapidou M, Yumuk V, Busetto L. European guidelines for obesity management in adults with a very low-calorie ketogenic diet: a systematic review and meta-analysis. Obes Facts. 2021. [DOI] [PMC free article] [PubMed]
- 165.Basciani S, Camajani E, Contini S, Persichetti A, Risi R, Bertoldi L, et al. Very-low-calorie ketogenic diets with whey, vegetable, or animal protein in patients with obesity: a randomized pilot study. J Clin Endocrinol Metab. 2020. [DOI] [PubMed]
- 166.Tragni E, Vigna L, Ruscica M, Macchi C, Casula M, Santelia A, et al. Reduction of cardio-metabolic risk and body weight through a multiphasic very-low calorie ketogenic diet program in women with overweight/obesity: a study in a real-world setting. Nutrients. 2021. [DOI] [PMC free article] [PubMed]
- 167.Barrea L, Verde L, Vetrani C, Marino F, Aprano S, Savastano S, et al. VLCKD: a real time safety study in obesity. J Transl Med [Internet]. 2022;20:23. Available from: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-021-03221-6 [DOI] [PMC free article] [PubMed]
- 168.Castellana M, Conte E, Cignarelli A, Perrini S, Giustina A, Giovanella L, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020. [DOI] [PubMed]
- 169.Moreno B, Crujeiras AB, Bellido D, Sajoux I, Casanueva FF. Obesity treatment by very low-calorie-ketogenic diet at two years: reduction in visceral fat and on the burden of disease. Endocrine. 2016. [DOI] [PubMed]
- 170.Muscogiuri G, Barrea L, Laudisio D, Pugliese G, Salzano C, Savastano S, et al. The management of very low-calorie ketogenic diet in obesity outpatient clinic: a practical guide. J Transl Med. 2019. [DOI] [PMC free article] [PubMed]
- 171.Müller MJ, Enderle J, Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. 2016. [DOI] [PMC free article] [PubMed]
- 172.Westerterp KR. Control of energy expenditure in humans. Eur J Clin Nutr. 2017. [DOI] [PubMed]
- 173.Barrea L, Pugliese G, Laudisio D, Colao A, Savastano S, Muscogiuri G. Mediterranean diet as medical prescription in menopausal women with obesity: a practical guide for nutritionists. Crit Rev Food Sci Nutr [Internet]. 2021;61:1201–11. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85085028190&doi=10.1080%2F10408398.2020.1755220&partnerID=40&md5=9f8bbea1d75c4f65e9b0d2125921453d [DOI] [PubMed]
- 174.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002. [DOI] [PubMed]
- 175.Sun Q, Li J, Gao F. New insights into insulin: the anti-inflammatory effect and its clinical relevance. World J Diabetes. 2014; [DOI] [PMC free article] [PubMed]
- 176.Vetrani C, Costabile G, Di Marino L, Rivellese AA. Nutrition and oxidative stress: a systematic review of human studies. Int J Food Sci Nutr. 2013. [DOI] [PubMed]
- 177.Fu X, Liu Z, Zhu C, Mou H, Kong Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit Rev Food Sci Nutr. 2019. [DOI] [PubMed]
- 178.Salamone D, Rivellese AA, Vetrani C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: the possible role of dietary fibre. Acta Diabetol. 2021. [DOI] [PMC free article] [PubMed]
- 179.Bailey MA, Holscher HD. Microbiome-mediated effects of the Mediterranean diet on inflammation. Adv Nutr. 2018. [DOI] [PMC free article] [PubMed]
- 180.Pugliese G, Barrea L, Laudisio D, Aprano S, Castellucci B, Framondi L, et al. Mediterranean diet as tool to manage obesity in menopause: a narrative review. Nutrition. 2020. [DOI] [PubMed]
- 181.Fernández-Quintela A, Milton-Laskibar I, Trepiana J, Gómez-Zorita S, Kajarabille N, Léniz A, et al. Key aspects in nutritional management of covid-19 patients. J Clin Med. 2020. [DOI] [PMC free article] [PubMed]
- 182.Majumder D, Debnath M, Sharma KN, Shekhawat SS, Prasad GBK, Maiti D, et al. Olive oil consumption can prevent non-communicable diseases and COVID-19: a review. Curr Pharm Biotechnol. 2021. [DOI] [PubMed]
- 183.Cândido FG, Valente FX, Grześkowiak ŁM, Moreira APB, Rocha DMUP, Alfenas R de CG. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: mechanisms and clinical implications on obesity. Int J Food Sci Nutr. 2018. [DOI] [PubMed]
- 184.Cawood AL, Walters ER, Smith TR, Sipaul RH, Stratton RJ. A review of nutrition support guidelines for individuals with or recovering from COVID-19 in the community. Nutrients. 2020. [DOI] [PMC free article] [PubMed]
- 185.López-González L, Becerra-Tomás N, Babio N, Martínez-González MÁ, Díaz-López A, Corella D, et al. Variety in fruits and vegetables, diet quality and lifestyle in an older adult mediterranean population. Clin Nutr. 2021; [DOI] [PubMed]
- 186.Foote JA, Murphy SP, Wilkens LR, Basiotis PP, Carlson A. Dietary variety increases the probability of nutrient adequacy among adults. J Nutr. 2004. [DOI] [PubMed]
- 187.Oude Griep LM, Verschuren WMM, Kromhout D, Ocké MC, Geleijnse JM. Variety in fruit and vegetable consumption and 10-year incidence of CHD and stroke. Publ Health Nutr. 2012. [DOI] [PMC free article] [PubMed]
- 188.Dalamaga M, Muscogiuri G, Paganitsa G, Parvouleskou G, Syriou V, Karagkoynis P, et al. Adherence to the Mediterranean diet is an independent predictor of circulating vitamin D levels in normal weight and non-smoker adults: an observational cross-sectional study. Int J Food Sci Nutr. 2021. [DOI] [PubMed]
- 189.Martucci M, Ostan R, Biondi F, Bellavista E, Fabbri C, Bertarelli C, et al. Mediterranean diet and inflammaging within the hormesis paradigm. Nutr Rev. 2017. [DOI] [PMC free article] [PubMed]
- 190.Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, et al. Mediterranean diet pyramid today. Science and cultural updates: Public Health Nutrition; 2011. [DOI] [PubMed] [Google Scholar]
- 191.DiPietro L, Al-Ansari SS, Biddle SJH, Borodulin K, Bull FC, Buman MP, et al. Advancing the global physical activity agenda: recommendations for future research by the 2020 WHO physical activity and sedentary behavior guidelines development group. Int J Behav Nutr Phys Act. 2020. [DOI] [PMC free article] [PubMed]
- 192.Ammar A, Brach M, Trabelsi K, Chtourou H, Boukhris O, Masmoudi L, et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 international online survey. Nutrients. 2020. [DOI] [PMC free article] [PubMed]
- 193.Casagrande M, Favieri F, Tambelli R, Forte G. The enemy who sealed the world: effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. 2020. [DOI] [PMC free article] [PubMed]
- 194.Kilroe SP, Fulford J, Jackman SR, Van Loon LJC, Wall BT. Temporal muscle-specific disuse atrophy during one week of leg immobilization. Med Sci Sports Exerc. 2020. [DOI] [PubMed]
- 195.Kim L, Whitaker M, O’Halloran A, Kambhampati A, Chai SJ, Reingold A, et al. Hospitalization rates and characteristics of children aged. MMWR Morb Mortal Wkly Rep. 2020. [DOI] [PMC free article] [PubMed]
- 196.Dos Santos C, Hussain SNA, Mathur S, Picard M, Herridge M, Correa J, et al. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay: a pilot study. Am J Respir Crit Care Med. 2016. [DOI] [PubMed]
- 197.Dirks ML, Wall BT, Van De Valk B, Holloway TM, Holloway GP, Chabowski A, et al. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016. [DOI] [PubMed]
- 198.Pišot R, Narici MV, Šimunič B, De Boer M, Seynnes O, Jurdana M, et al. Whole muscle contractile parameters and thickness loss during 35-day bed rest. Eur J Appl Physiol. 2008. [DOI] [PubMed]
- 199.Gram M, Dahl R, Dela F. Physical inactivity and muscle oxidative capacity in humans. Eur J Sport Sci. 2014. [DOI] [PubMed]
- 200.Webster JM, Kempen LJAP, Hardy RS, Langen RCJ. Inflammation and skeletal muscle wasting during cachexia. Front Physiol. 2020. [DOI] [PMC free article] [PubMed]
- 201.Backx EMP, Horstman AMH, Marzuca-Nassr GN, van Kranenburg J, Smeets JS, Fuchs CJ, et al. Leucine supplementation does not attenuate skeletal muscle loss during leg immobilization in healthy, young men. Nutrients. 2018. [DOI] [PMC free article] [PubMed]
- 202.CURCI C, PISANO F, BONACCI E, CAMOZZI DM, CERAVOLO C, BERGONZI R, et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 Rehabilitation Unit and proposal of a treatment protocol. Eur J Phys Rehab Med. 2020. [DOI] [PubMed]
- 203.Baig AM. Deleterious outcomes in Long-Hauler COVID-19: the effects of SARS-CoV-2 on the CNS in chronic COVID syndrome. ACS Chem Neurosci. 2020. [DOI] [PubMed]
- 204.Stefano GB, Ptacek R, Ptackova H, Martin A, Kream RM. Selective neuronal mitochondrial targeting in SARS-CoV-2 infection affects cognitive processes to induce “Brain Fog” and results in behavioral changes that favor viral survival. Med Sci Monit. 2021. [DOI] [PMC free article] [PubMed]
- 205.Camargo-Martínez W, Lozada-Martínez I, Escobar-Collazos A, Navarro-Coronado A, Moscote-Salazar L, Pacheco-Hernández A, et al. Post-COVID 19 neurological syndrome: implications for sequelae’s treatment. J Clin Neurosci. 2021. [DOI] [PMC free article] [PubMed]
- 206.Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. The BMJ. 2020. [DOI] [PubMed]
- 207.Calabrese M, Garofano M, Palumbo R, Di Pietro P, Izzo C, Damato A, et al. Exercise training and cardiac rehabilitation in covid-19 patients with cardiovascular complications: State of art. Life. 2021. [DOI] [PMC free article] [PubMed]
- 208.Barker-Davies RM, O’Sullivan O, Senaratne KPP, Baker P, Cranley M, Dharm-Datta S, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020. [DOI] [PMC free article] [PubMed]
- 209.Sana F, Isselbacher EM, Singh JP, Heist EK, Pathik B, Armoundas AA. Wearable devices for ambulatory cardiac monitoring: JACC state-of-the-art review. J Am Coll Cardiol. 2020. [DOI] [PMC free article] [PubMed]
- 210.Negrini F, De Sire A, Lazzarini SG, Pennestrì F, Sorce S, Arienti C, et al. Reliability of activity monitors for physical activity assessment in patients with musculoskeletal disorders: a systematic review. J Back Musculoskelet Rehab. 2021. [DOI] [PubMed]
- 211.Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014. [DOI] [PMC free article] [PubMed]
- 212.Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med. 2014. [DOI] [PMC free article] [PubMed]
- 213.Nigro E, Polito R, Alfieri A, Mancini A, Imperlini E, Elce A, et al. Molecular mechanisms involved in the positive effects of physical activity on coping with COVID-19. Eur J Appl Physiol. 2020. [DOI] [PMC free article] [PubMed]
- 214.Savikangas T, Törmäkangas T, Tirkkonen A, Alen M, Fielding RA, Kivipelto M, et al. The effects of a physical and cognitive training intervention vs. physical training alone on older adults’ physical activity: a randomized controlled trial with extended follow-up during COVID-19. PLoS ONE. 2021. [DOI] [PMC free article] [PubMed]
- 215.Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, Martínez-Cava A, Franco-López F, Sánchez-Alcaraz Martínez BJ, et al. Post-covid-19 syndrome and the potential benefits of exercise. Int J Environ Rese Publ Health. 2021. [DOI] [PMC free article] [PubMed]
- 216.Nilsson MI, Mikhail A, Lan L, Carlo A Di, Hamilton B, Barnard K, et al. A five-ingredient nutritional supplement and home-based resistance exercise improve lean mass and strength in free-living elderly. Nutrients. 2020. [DOI] [PMC free article] [PubMed]