Abstract
Background
Aortic dissection (AoD) is associated with high morbidity and mortality. However, the burden of AoD mortality is not well characterized, and contemporary data and mortality trends in different demographic and geographic subgroups have not been described.
Methods and Results
Trends in AoD mortality were assessed using a cross‐sectional analysis of the Centers for Disease Control and Prevention Wide‐Ranging Online Data for Epidemiologic Research database. Crude and age‐adjusted mortality rates (AAMR) per 1 million people with associated annual percent changes were determined. Joinpoint regression was used to assess trends in the overall sample and different demographic (sex, race and ethnicity, age) and geographic subgroups. Between 1999 and 2019, a total of 86 855 AoD deaths occurred within the United States. In the overall population, AAMR was 21.1 per 1 million in 1999 and 21.3 in 2019. After an initial decline in mortality, AAMR increased from 2012 to 2019, with an associated annual change of 2.5% (95% CI, 1.8–3.3). Men, older adults (aged ≥85 years), and non‐Hispanic Black or African American individuals had higher mortality rates than women, younger individuals, and other racial and ethnic individuals, respectively. Despite lower AAMRs throughout the study period, women experienced greater increases in AAMR from 2012 to 2019 compared with men. Similarly, non‐Hispanic Black or African American individuals had a pronounced increase in AAMR from 2012 to 2019.
Conclusions
Despite an initial decline in AoD mortality, the mortality rate has been increasing from 2012 to 2019, with pronounced increases among women and non‐Hispanic Black or African American individuals.
Keywords: aortic dissection, epidemiology, mortality
Subject Categories: Epidemiology, Race and Ethnicity, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- AAMR
age‐adjusted mortality rates
- AoD
aortic dissection
- APC
annual percentage change
- CMR
crude mortality rates
- IRAD
International Registry of Acute Aortic Dissection
- WONDER
Wide‐Ranging Online Data for Epidemiologic Research
Clinical Perspective
What Is New?
Overall, aortic dissection mortality increased from 2012 to 2019 in the United States.
Women experienced greater increases in mortality compared with men from 2012 to 2019.
Non‐Hispanic Black or African American individuals had the highest mortality compared with other racial and ethnic groups.
What Are the Clinical Implications?
Further studies are needed to understand the underlying mechanism of the recent increase in aortic dissection mortality.
More strenuous efforts are needed to reduce the sex and racial disparities in aortic dissection mortality.
Aortic dissection (AoD) is associated with significant mortality and morbidity in the United States. 1 , 2 Challenges associated with the diagnosis and management of AoD result in high rates of mortality. Data from the IRAD (International Registry of Acute Aortic Dissection) indicate that acute Stanford type A AoD carries a 22% in‐hospital mortality, whereas patients with type B dissection have a reported 13% in‐hospital mortality, with increased mortality among patients who were medically managed compared with surgically managed patients. 3
Despite the known morbidity of AoD, few studies have reported epidemiological population‐based estimates of AoD mortality using contemporary data. In a study using inpatient Medicare data, AoD‐related hospitalization rates remained stable from 2000 to 2011, with improvements in short‐term mortality. 1 However, there remains a paucity of data on population‐based estimates of AoD mortality over the past decade. In addition, prior studies using administrative databases are limited by inpatient and regional data that may not be representative of nationwide trends. 2 , 4 , 5 , 6 For example, data from specialty centers or registry analyses may not include deaths occurring outside of the hospital setting. There are also limited data on comparative mortality trends among demographic and regional populations within the United States. Therefore, we used a nationwide database of death certificates to describe contemporary trends in AoD mortality stratified by demographic and regional characteristics.
METHODS
Data Source
The CDC WONDER (Centers for Disease Control and Prevention Wide‐Ranging Online Data for Epidemiologic Research) was used to analyze deaths occurring within the United States related to AoD. 7 The Multiple Cause‐of‐Death Public Use Record database death certificates were studied to determine AoD as an underlying or contributing cause of death on nationwide death certificates. This database has been previously used in several other studies to analyze nationwide trends in mortality of cardiovascular diseases. 8 , 9 , 10 AoD deaths were identified using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) I71.0 code in individuals aged ≥25 years. This study was exempt from local institutional review board approval because the CDC WONDER database includes deidentified publicly available data.
Data Extraction
AoD deaths and population sizes were extracted from 1999 to 2019. Demographic (sex, race and ethnicity, and age) and geographic (urban–rural and state) data were also extracted as identified on the death certificates. Racial and ethnic demographic characteristics were defined as non‐Hispanic White, non‐Hispanic Black or African American, Hispanic or Latino, non‐Hispanic American Indian or Alaskan Native, and non‐Hispanic Asian or Pacific Islander individuals. These racial and ethnic categories were previously used within analyses from the CDC WONDER database and rely on reported data on death certificates. 7 , 11 Non‐Hispanic American Indian/Alaskan Native and non‐Hispanic Asian/Pacific Islander individuals were analyzed together in an Other group because of the limited numbers of cases occurring in each individual group. Age categories were defined as 25 to 39, 40 to 54, 55 to 69, 70 to 84, and ≥85 years. The National Center for Health Statistics Urban‐Rural Classification Scheme was used to classify the study population into urban (large metropolitan area [population ≥1 million], medium/small metropolitan area [population 50 000–999 999]), and rural (population <50 000) counties per the 2013 US Census classification. 12 Location of death was also extracted and included medical facilities (outpatient, emergency room, inpatient, death on arrival, or status unknown), home, hospice, and nursing home/long‐term care facility.
Statistical Analysis
AoD crude and age‐adjusted mortality (AAMR) rates per 1 million people were determined. Crude mortality rates (CMR) were calculated by dividing the number of AoD‐related deaths by the corresponding US population of that year. AAMRs were calculated by standardizing the AoD‐related deaths to the corresponding year 2000 US population, as previously described. 13 The Joinpoint regression program (version 4.9.0.0; National Cancer Institute) was used to describe trends in CMR and AAMR of AoD‐related mortality. 14 Temporal trends in CMR and AAMR were determined by fitting log‐linear regression models. We applied Joinpoint regression to identify inflection points in the temporal trends of CMR and AAMR for AoD from 1999 to 2019 based on published methodological guidelines. 15 For data containing 17 to 21 time points, the guidelines recommend that the analysis identifies a maximum of 3 inflection points across the study period. 15 In this study, 21 years were included; therefore, the Joinpoint regression statistical software was set to determine a maximum of 3 joinpoints where significant temporal variation existed in the trend. Fewer than the maximum allowed number of inflection points could be identified if the magnitude of variation between trends was greatest with fewer inflection points. Therefore, 0 to a maximum of 3 joinpoints were allowed to be identified. The Grid Search method (2, 2, 0), permutation test, and parametric method were used to estimate annual percent change (APC) and 95% confidence intervals (CIs). APC was considered increasing or decreasing if the slope describing the change in mortality was significantly different than 0 using 2‐tailed t testing. Statistical significance was set at P≤0.05.
RESULTS
Overall Population
Between 1999 and 2019, a total of 86 855 deaths related to AoD occurred in the overall study population (Table S1). Of 83 803 deaths with available information on place of death, 81.7% occurred within medical facilities, 2.8% occurred in nursing or long‐term care facilities, 1.5% at hospice, and 13.9% at home (Table S2).
Overall, the AAMR was 21.1 per 1 million in 1999 and 21.3 in 2019 (Table S3). From 1999 to 2012, the overall AAMR of AoD‐related deaths was decreasing, with an associated APC of −1.5% (95% CI, −1.8% to −1.2%). This decreasing AAMR was then followed by increasing AAMR from 2012 to 2019, with an APC of 2.5% (95% CI, 1.8%–3.3%) (Table S3, Figure 1).
Figure 1. Overall and sex‐stratified age‐adjusted aortic dissection mortality rates in the United States, 1999 to 2019.
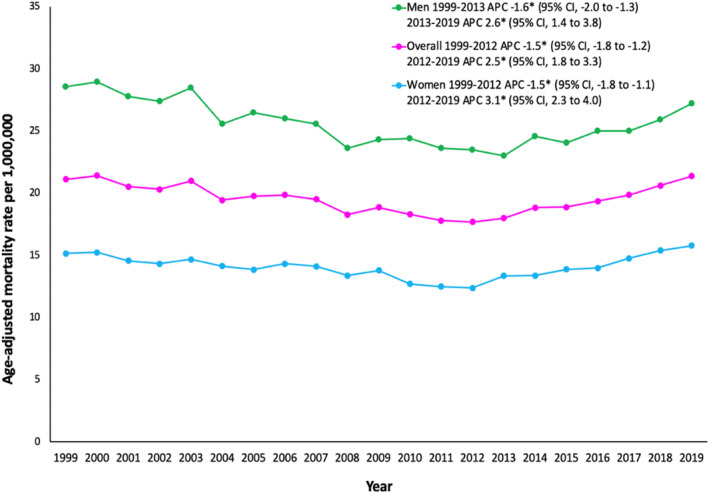
*The annual percent change (APC) is significantly different from 0 at α=0.05.
Demographic Patterns
Sex
In men, the AAMR was 28.5 in 1999 and 27.2 in 2019 (Table S3). The AAMR decreased from 1999 to 2013, which was then followed by increasing AAMR, with an associated APC of 2.6% (95% CI, 1.4%–3.8%) from 2013 to 2019 (Figure 1).
In women, the AAMR was 15.1 in 1999 and 15.7 in 2019 (Table S3). Similar to men, the initial decrease was then followed by increasing AAMR, with an associated APC of 3.1% (95% CI, 2.3%–4.0%) from 2012 to 2019 (Figure 1).
Race and Ethnicity
The AAMR for non‐Hispanic White individuals was 20.7 in 1999 and 20.3 in 2019 (Table S4). From 1999 to 2012, White individuals had decreasing AoD‐related mortality (Figure 2). However, from 2012 to 2019, White individuals had increasing AoD‐related mortality, with an associated APC of 2.6% (95% CI, 2.0%–3.2%).
Figure 2. Aortic dissection age‐adjusted mortality rates stratified by race and ethnicity in the United States, 1999 to 2019.
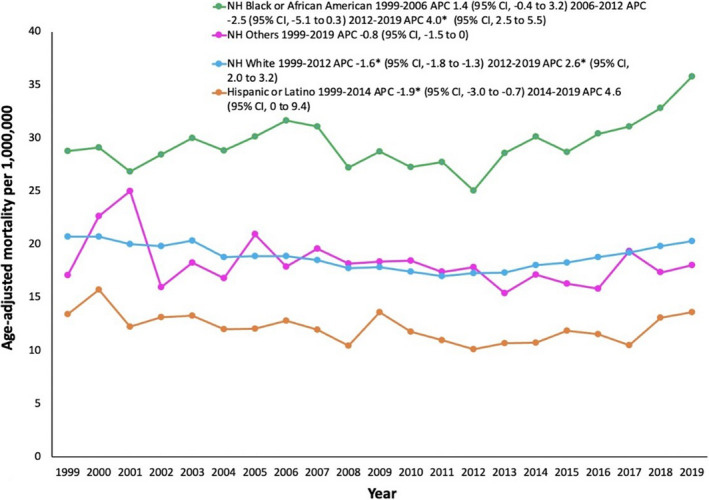
*The annual percent change (APC) is significantly different from 0 at α=0.05. NH indicates non‐Hispanic.
Non‐Hispanic Black or African American individuals had the highest AAMR throughout the study period, which was 28.7 in 1999 and 35.7 in 2019. From 1999 to 2012 there were no significant changes in AoD‐related AAMR for non‐Hispanic Black or African American individuals. However, from 2012 to 2019, AoD‐related AAMR markedly increased at an associated APC of 4.0% (95% CI, 2.5%–5.5%).
Hispanic or Latino individuals had the lowest AAMR throughout the study period, which was 13.4 in 1999 and 13.6 in 2019. From 1999 to 2014, the AoD‐related AAMR decreased; however, from 2014 to 2019, the AoD‐related mortality remained stable without significant changes.
The AAMR for non‐Hispanic Other individuals was 17.1 in 1999 and 18.0 in 2019. There were no significant changes in AAMR for non‐Hispanic Other individuals throughout the study period.
Age
Individuals who were aged ≥85 years had a CMR of 106.8 in 1999 and 135.5 in 2019 (Table S5, Figure 3). From 1999 to 2012, CMR remained stable, followed by increase from 2012 to 2019, with an associated APC of 3.8% (95% CI, 2.4%–5.2%).
Figure 3. Aortic dissection crude mortality rates stratified by age groups in the United States, 1999 to 2019.
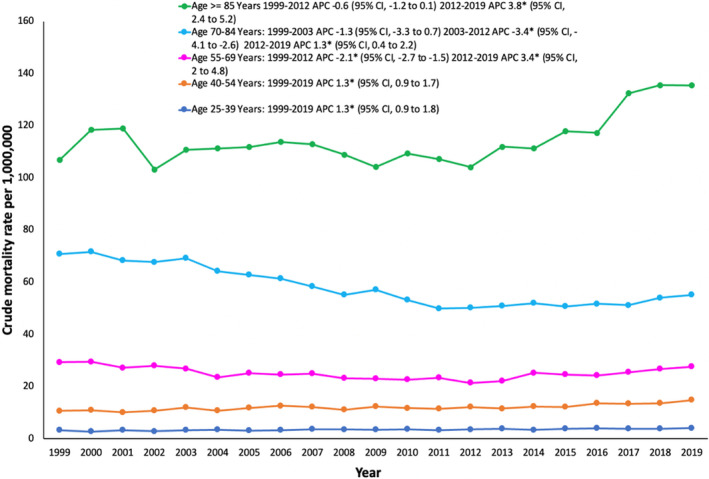
*The annual percent change (APC) is significantly different from 0 at α=0.05.
Individuals aged 70 to 84 years had the second highest CMR of 70.8 to 55.2 from 1999 to 2019. From 2012 to 2019, the CMR increased at an associated APC of 1.3% (95% CI, 0.45%–2.25%).
Individuals aged 55 to 69 years experienced increasing CMR from 2012 to 2019 (APC, 3.4% [95% CI, 2.0%–4.85%]).
Individuals aged 40 to 54 years and 25 to 39 years experienced increasing CMR from 1999 to 2019 (aged 40–54 years: APC, 1.3% [95% CI, 0.9%–1.7%]; aged 25–39 years: APC, 1.3% [95% CI, 0.9%–1.8%]).
Geographical Patterns
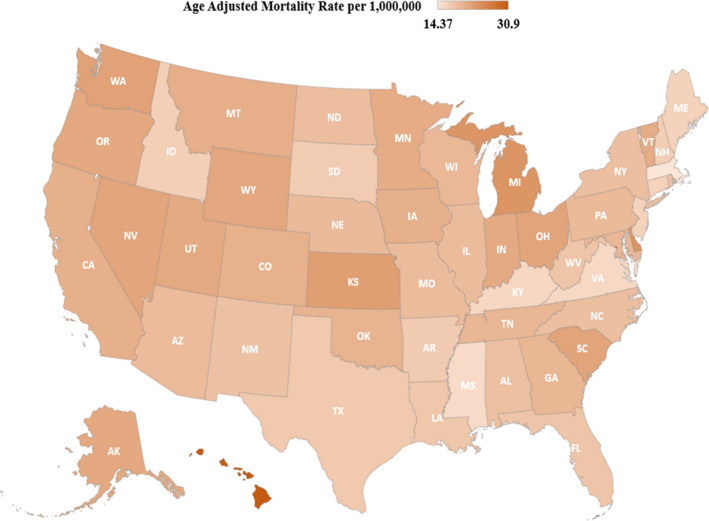
Rural, small/medium metropolitan, and large metropolitan counties had similar trends in AAMR throughout the study period, with initial decreasing AAMR from 1999 to 2012/2013, followed by increasing AAMR from 2012 to 2019 (Figure 4, Table S6). The AAMR varied from 14.3 in Massachusetts to 30.9 in the District of Columbia (Figure 5, Table S7). States in the ≥90th percentile of AoD‐related AAMR included Washington, Kansas, Delaware, Michigan, Hawaii, and the District of Columbia. States in the ≤10th percentile of AoD‐related AAMR included Connecticut, New Jersey, Kentucky, Virginia, Mississippi, and Massachusetts (Table S7).
Figure 4. Aortic dissection age‐adjusted mortality rates stratified by urban–rural classification in the United States, 1999 to 2019.
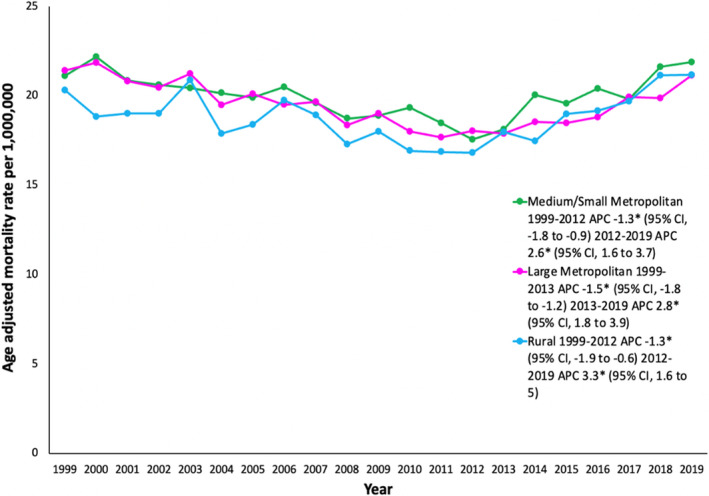
*The annual percent change (APC) is significantly different from 0 at α=0.05.
Figure 5. State‐level aortic dissection age‐adjusted mortality rates per 1 million people in the United States, 1999 to 2019.
DISCUSSION
In this nationwide study, we report several important findings about AoD mortality using data from 1999 to 2019 within the United States. First, after an initial decline from 1999 to 2012, AoD mortality has been increasing from 2012 and 2019, including among most demographic groups within this study. Second, men had higher AAMR throughout the study; however, women experienced a larger relative increase in AAMR compared with men from 2012 onward. Third, non‐Hispanic Black or African American individuals had the highest AAMRs compared with other racial and ethnic groups. In addition, non‐Hispanic Black or African American individuals did not experience declines in AAMR from 1999 to 2012 and had markedly rising AAMR from 2012 to 2019. Fourth, individuals aged ≥85 years had the highest CMR, with markedly rising CMR from 2012 to 2019.
Despite the known morbidity of AoD, the current literature on population‐based AoD mortality is limited. In a study using inpatient Medicare data from 2000 to 2011, the hospitalization rate for AoD remained stable, with significantly decreasing 30‐day mortality, indicating an improvement in outcomes during this limited time period. 1 In addition, other existing studies on AoD mortality are limited by regional populations, inpatient databases, or registries from high‐volume tertiary centers. 2 , 4 , 16 For example, county level data from Olmsted County, Minnesota indicated stable incidence of AoD, with unchanged short‐ and long‐term mortality from 1995 to 2015. 16 Similarly, data from an all‐payer nationwide database indicated rising incidence of AoD hospitalizations and emergency department visits from the early 2000s to 2017, with decreasing in‐hospital mortality rates. 2 , 17 However, to our knowledge, there are no data on nationwide population‐based AoD mortality rates beyond 2012 further stratified by important demographic and regional populations.
In this study using contemporary data, we report a concerning increase in AoD mortality rates from 2012 to 2019. Despite an initial decline in AoD mortality from 1999 to 2012, the AoD mortality rate has almost uniformly increased among all demographic and regional groups through 2019. There are several plausible explanations of this finding. First, the prevalence of AoD risk factors such as uncontrolled hypertension, obesity, smoking, diabetes, and older age has been increasing within the United States. 18 In a study using prospective data from the Japan‐Specific Health Checkups and UK Biobank, hypertension was associated with a high risk of AoD in a dose‐dependent relationship. 19 The prevalence of hypertension has decreased from 47.0% in 1999 to 2000 to 41.7% in 2013 to 2014 and then increased to 45.4% in 2017 to 2018. 20 Notably, the prevalence of uncontrolled hypertension has also increased after 2014, plausibly contributing to the rising AoD deaths seen within this study. 21 This could be related to the impact of findings from trials such as The Action to Control Cardiovascular Risk in Diabetes trial (ACCORD) and The Secondary Prevention of Small Subcortical Strokes trial (SPS3‐BP) that showed no apparent benefit from intensive blood pressure control. 22 , 23 Other less‐significant risk factors including obesity, smoking, and diabetes have followed similar trends in the United States, possibly supporting the results of our study. 24 , 25 , 26 Therefore, there may be an increasing incidence of AoD resulting in increased AoD deaths after 2012. Second, although improvements in AoD management have occurred, AoD syndromes are associated with high rates of mortality. For example, despite an increase in surgical intervention and chest computed tomography use in the management and diagnosis of AoD, in‐hospital mortality rates for Stanford type A and type B AoD have improved but have remained stagnant for type B AoDs. 3 Importantly, these studies only report in‐hospital mortality of acute AoD requiring repair, which does not capture long‐term mortality of AoD. For example, in patients with type A or type B acute aortic syndromes who survive the acute dissection, around two‐thirds of patients experienced at least 1 aortic or nonaortic adverse event over long‐term follow‐up period, including death. 27 Finally, our analysis also includes deaths occurring in nonmedical facility settings, which comprised ~18% of total deaths throughout the study period, a considerable portion of the total study population.
Considering the increasing AoD deaths found within this study, efforts to control medical comorbidities associated with AoD and operative intervention shown to decrease in‐hospital mortality may be required to improve these epidemiological trends. For example, within the IRAD registry, type A AoD treated with surgical intervention had lower in‐hospital mortality than those with medical treatment alone. 3 In addition, in‐hospital mortality for patients undergoing surgery has decreased from 1996 to 2016 for type A AoD (17.5% versus 12.2%, respectively) within the IRAD registry, and the use of endovascular aortic repair has resulted in a significant decrease in operative mortality for type B AoD (18.6% versus 7.4%, respectively). 28 , 29 Therefore, expeditious referrals for surgical procedures are necessary to improve outcomes for patients with AoD. However, it is also important to note that some patients may not be candidates for aortic intervention because of AoD complications such as hemispheric stroke, cardiogenic shock/visceral malperfusion, or cardiopulmonary arrest >15 minutes, which are associated with worse operative outcomes. 30 , 31 In addition, close surveillance imaging for patients at high‐risk of AoD or enlarged thoracic aortas, such as those with a family history of AoD, bicuspid aortic valve, women, and others, may be needed. 32 , 33
Men had a higher AoD mortality throughout the study period. In the IRAD and population estimates from Olmstead County, Minnesota, AoD was more frequent in men compared with women. 16 , 34 This correlates with previous studies conducted on sex differences and hypertension prevalence within the United States. Men have higher prevalence of hypertension across the majority of age groups compared with women in the United States. 35 Men are more likely to smoke cigarettes than women, with about 15.3% of men versus 12.7% of women using cigarettes. 36 Therefore, the higher rates of hypertension, smoking, and other risk factors among men compared with women within the United States may contribute to the noted sex differences in AoD mortality rates in our study.
The more pronounced increase in AoD mortality from 2012 to 2019 among women found in this study correlates with recent reports of sex disparities in AoD outcomes. For example, the diagnosis of AoD is more often delayed in women than men, leading to a worse outcome of surgically treated women compared with men, plausibly supporting our reported trends. 34 In addition, thoracic aneurysms in women are more likely to grow at faster rates, and women experience dissections at smaller aortic diameters compared with men, potentially needing indexed aortic diameters for risk stratification. 37 Within the United States, there are discrepancies among the professional society guidelines for abdominal aortic aneurysm (AAA) screening for women compared with men. 38 Moreover, female sex has previously been reported as an independent predictor of mortality among both ruptured and elective AAA repair groups. 38
Non‐Hispanic Black or African American individuals had persistently higher rates of AoD mortality throughout the study, with a marked increase in mortality from 2012 to 2019 compared with remainder of the races and ethnicities. This pronounced population‐estimated AoD mortality in non‐Hispanic Black or African American individuals has not been previously reported. In a study of Maryland hospitals, non‐White race and ethnicity has been reported to be an independent risk factor for AoD admissions. 39 The proportion of non‐Hispanic Black or African American individuals with controlled blood pressure is significantly lower than that of non‐Hispanic White individuals, which may be associated with the pronounced mortality reported in our study. 21 Additionally, the impact of social determinants of health on cardiovascular disease outcomes has been previously described in the United States. Non‐Hispanic Black or African American individuals are disproportionately affected by conditions such as hypertension and have a higher burden of social and economic factors impeding their care. 40 , 41 Individuals who are medically underserved have unequal access to health care resources to control their blood pressure, diabetes, or other potential risk factors for AoD. 42 Additionally, non‐Hispanic Black individuals are more likely to have inadequately controlled hypertension with antihypertensive therapy, possibly increasing mortality rates from AoD. 43
There are several limitations to this study. First, misclassification of AoD mortality may occur upon completion of death certificate data within the database. Second, the CDC WONDER database does not include procedural data on rate or outcomes of AoD repair or social determinants of health information, which may impact the outcomes of demographic subgroups. Therefore, we are unable to determine the exact reasons for reported disparities in AoD‐related mortality within this analysis. Third, our data include deaths for both type A and type B AoD given the lack of specific ICD‐10‐CM diagnostic codes for type A and type B dissection. Fourth, the database lacks important information on underlying comorbidities, risk factors (hypertension, smoking, connective tissue disorders), and imaging data (echocardiography, computed tomography angiography, magnetic resonance angiography). Fifth, given that the database is based on death certificate data only, we cannot ascertain how the diagnosis of AoD was made (autopsy versus imaging versus symptoms), particularly for individuals who died outside of the medical facilities. Finally, residents who may have relocated to another state at the time of death are recorded as deaths occurring in their final location.
In conclusion, despite an initial decline, we report a concerning increase in AoD mortality from 2012 to 2019 in this nationwide population‐based analysis of death certificate data. This increasing mortality is especially pronounced within women, non‐Hispanic Black individuals, and older individuals and requires further investigation into the causative mechanisms of these identified disparities for this highly lethal and morbid condition.
Sources of Funding
The ProMedica Toledo Hospital in Toledo, Ohio funded the open access fee for this study.
Disclosures
None.
Supporting information
Tables S1–S7.
This article was sent to John S. Ikonomidis, MD, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024533
For Sources of Funding and Disclosures, see page 8.
References
- 1. Mody PS, Wang Y, Geirsson A, Kim N, Desai MM, Gupta A, Dodson JA, Krumholz HM. Trends in aortic dissection hospitalizations, interventions, and outcomes among medicare beneficiaries in the united states, 2000–2011. Circ Cardiovasc Qual Outcomes. 2014;7:920–928. doi: 10.1161/CIRCOUTCOMES.114.001140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zimmerman KP, Oderich G, Pochettino A, Hanson KT, Habermann EB, Bower TC, Gloviczki P, DeMartino RR. Improving mortality trends for hospitalization of aortic dissection in the national inpatient sample. J Vasc Surg. 2016;64:e601. doi: 10.1016/j.jvs.2016.03.427 [DOI] [PubMed] [Google Scholar]
- 3. Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, Myrmel T, Larsen M, Harris KM, Greason K. Presentation, diagnosis, and outcomes of acute aortic dissection: 17‐year trends from the international registry of acute aortic dissection. J Am Coll Cardiol. 2015;66:350–358. [DOI] [PubMed] [Google Scholar]
- 4. Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Population‐based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10‐year results from the oxford vascular study. Circulation. 2013;127:2031–2037. doi: 10.1161/CIRCULATIONAHA.112.000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta RH, Bossone E, Evangelista A, O'Gara PT, Smith DE, Cooper JV, Oh JK, Januzzi JL, Hutchison S, Gilon D, et al. Acute type b aortic dissection in elderly patients: clinical features, outcomes, and simple risk stratification rule. The Annals of Thoracic Surgery. 2004;77:1622–1628. doi: 10.1016/j.athoracsur.2003.10.072 [DOI] [PubMed] [Google Scholar]
- 6. Mehta RH, O’Gara PT, Bossone E, Nienaber CA, Myrmel T, Cooper JV, Smith DE, Armstrong WF, Isselbacher EM, Pape LA, et al. Acute type a aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol. 2002;40:685–692. doi: 10.1016/S0735-1097(02)02005-3 [DOI] [PubMed] [Google Scholar]
- 7. Centers for disease control and prevention, national center for health statistics Multiple cause of death 1999‐2019 on cdc wonder online database, released in 2020. Data are from the multiple cause of death files, 1999–2019, as compiled from data provided by the 57 vital statistics jurisdictions through the vital statistics cooperative program. Accessed May 21, 2021. http://wonder.cdc.gov/mcd‐icd10.html
- 8. Aggarwal R, Chiu N, Loccoh EC, Kazi DS, Yeh RW, Wadhera RK. Rural‐urban disparities: Diabetes, hypertension, heart disease, and stroke mortality among black and white adults, 1999–2018. J Am Coll Cardiol. 2021;77:1480–1481. doi: 10.1016/j.jacc.2021.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan MS, Kumar P, Sreenivasan J, Khan SU, Nasir K, Mehra MR, O’Donnell C, Warraich HJ. Rural‐urban differences in mortality from ischemic heart disease, heart failure, and stroke in the united states. Circ Cardiovasc Qual Outcomes. 2021;14:e007341. doi: 10.1161/CIRCOUTCOMES.120.007341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah NS, Lloyd‐Jones DM, O’Flaherty M, Capewell S, Kershaw K, Carnethon M, Khan SS. Trends in cardiometabolic mortality in the united states, 1999–2017. JAMA. 2019;322:780–782. doi: 10.1001/jama.2019.9161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan SU, Kalra A, Kapadia SR, Khan MU, Zia Khan M, Khan MS, Mamas MA, Warraich HJ, Nasir K, Michos ED, et al. Demographic, regional, and state‐level trends of mortality in patients with aortic stenosis in united states, 2008 to 2018. J Am Heart Assoc. 2020;9:e017433. doi: 10.1161/JAHA.120.017433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ingram DD, Franco SJ. 2013 nchs urban‐rural classification scheme for counties. Vital Health Stat 2. 2014;1–73. [PubMed] [Google Scholar]
- 13. Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. National Vital Statistics Reports. 1998;47:1–17. [PubMed] [Google Scholar]
- 14. Methodology National Cancer Institute Division of Cancer Control and Population Sciences. Joinpoint regression program, version 4.7.0.0. Division of Cancer Controls & Population Sciences, National Cancer Institute. Available at: https://surveillance.cancer.gov/joinpoint/. Accessed December 30, 2021.
- 15. National cancer institute . Number of joinpoints. https://surveillance.Cancer.Gov/help/joinpoint/setting‐parameters/method‐and‐parameters‐tab/number‐of‐joinpoints. 2020.
- 16. DeMartino RR, Sen I, Huang Y, Bower TC, Oderich GS, Pochettino A, Greason K, Kalra M, Johnstone J, Shuja F, et al. Population‐based assessment of the incidence of aortic dissection, intramural hematoma, and penetrating ulcer, and its associated mortality from 1995 to 2015. Circ Cardiovasc Qual Outcomes. 2018;11:e004689. doi: 10.1161/CIRCOUTCOMES.118.004689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown J, Usmani B, Arnaoutakis G, Serna‐Gallegos D, Plestis K, Shah S, Navid F, Lewis C, Singh M, Sultan I. 10‐year trends in aortic dissection: mortality and weekend effect within the us nationwide emergency department sample (neds). Heart Surg Forum. 2021;24:E336–E344. doi: 10.1532/hsf.3681 [DOI] [PubMed] [Google Scholar]
- 18. Landenhed M, Engström G, Gottsäter A, Caulfield MP, Hedblad B, Newton‐Cheh C, Melander O, Smith JG. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc. 2015;4:e001513. doi: 10.1161/JAHA.114.001513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hibino M, Otaki Y, Kobeissi E, Pan H, Hibino H, Taddese H, Majeed A, Verma S, Konta T, Yamagata K, et al. Blood pressure, hypertension and the risk of aortic dissection incidence and mortality: Results from the japan‐specific health checkups study, the uk biobank study and a meta‐analysis of cohort studies. Circulation. 2021. Nov 8 [epub ahead of print]. doi: 10.1161/CIRCULATIONAHA.121.056546 [DOI] [PubMed] [Google Scholar]
- 20. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over. United States. 2017–2018. 2020. [PubMed]
- 21. Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among us adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons‐Morton DG, Basile JN, Corson MA, Probstfield JL. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. New Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Group SS . Blood‐pressure targets in patients with recent lacunar stroke: The sps3 randomised trial. Lancet. 2013;382:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inoue Y, Qin B, Poti J, Sokol R, Gordon‐Larsen P. Epidemiology of obesity in adults: latest trends. Curr Obes Rep. 2018;7:276–288. doi: 10.1007/s13679-018-0317-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer‐Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692 [DOI] [PubMed] [Google Scholar]
- 26. Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: Insights from yesterday, today, and future trends. Popul. Health Manag. 2017;20:6–12. doi: 10.1089/pop.2015.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corsini A, Pacini D, Lovato L, Russo V, Lorenzini M, Foà A, Leone O, Nanni S, Mingardi F, Reggiani LB, et al. Long‐term follow up of patients with acute aortic syndromes: Relevance of both aortic and non‐aortic events. Eur J Vasc Endovasc Surg. 2018;56:200–208. doi: 10.1016/j.ejvs.2018.03.030 [DOI] [PubMed] [Google Scholar]
- 28. Harky A, Chan JSK, Wong CHM, Francis N, Grafton‐Clarke C, Bashir M. Systematic review and meta‐analysis of acute type b thoracic aortic dissection, open, or endovascular repair. J Vasc Surg. 2019;69:1599–1609. doi: 10.1016/j.jvs.2018.08.187 [DOI] [PubMed] [Google Scholar]
- 29. Parikh N, Trimarchi S, Gleason TG, Kamman AV, di Eusanio M, Myrmel T, Korach A, Maniar H, Ota T, Khoynezhad A, et al. Changes in operative strategy for patients enrolled in the international registry of acute aortic dissection interventional cohort program. J Thorac Cardiovasc Surg. 2017;153:S74–S79. doi: 10.1016/j.jtcvs.2016.12.029 [DOI] [PubMed] [Google Scholar]
- 30. Sabe AA, Percy ED, Kaneko T, Plichta RP, Hughes GC. When to consider deferral of surgery in acute type a aortic dissection: a review. Ann Thorac Surg. 2021;111:1754–1762. doi: 10.1016/j.athoracsur.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uehara K, Matsuda H, Matsuo J, Inoue Y, Shijo T, Omura A, Seike Y, Sasaki H, Kobayashi J. Surgical outcomes of acute type a aortic dissection in patients undergoing cardiopulmonary resuscitation. J Thorac Cardiovasc Surg. 2021;161:1173–1180. doi: 10.1016/j.jtcvs.2019.11.135 [DOI] [PubMed] [Google Scholar]
- 32. Vilacosta I, San Román JA, di Bartolomeo R, Eagle K, Estrera AL, Ferrera C, Kaji S, Nienaber CA, Riambau V, Schäfers H‐J, et al. Acute aortic syndrome revisited: Jacc state‐of‐the‐art review. J Am Coll Cardiol. 2021;78:2106–2125. doi: 10.1016/j.jacc.2021.09.022 [DOI] [PubMed] [Google Scholar]
- 33. Wojnarski CM, Svensson LG, Roselli EE, Idrees JJ, Lowry AM, Ehrlinger J, Pettersson GB, Gillinov AM, Johnston DR, Soltesz EG, et al. Aortic dissection in patients with bicuspid aortic valve–associated aneurysms. Ann Thorac Surg. 2015;100:1666–1674. doi: 10.1016/j.athoracsur.2015.04.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, Cooper JV, Januzzi JL, Ince H, Sechtem U, et al. Gender‐related differences in acute aortic dissection. Circulation. 2004;109:3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C [DOI] [PubMed] [Google Scholar]
- 35. Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens. 2018;31:1247–1254. doi: 10.1093/ajh/hpy148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults—united states, 2019. Morb Mortal Wkly Rep. 2020;69:1736. doi: 10.15585/mmwr.mm6946a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho L, Kibbe MR, Bakaeen F, Aggarwal NR, Davis MB, Karmalou T, Lawton JS, Ouzounian M, Preventza O, Russo AM, et al. Cardiac surgery in women in the current era: What are the gaps in care? Circulation. 2021;144:1172–1185. doi: 10.1161/CIRCULATIONAHA.121.056025 [DOI] [PubMed] [Google Scholar]
- 38. Sciria CT, Osorio B, Wang J, Lu DY, Amin N, Vohra A, Yeo I, Feldman DN, Cheung JW, Narula N, et al. Sex‐based disparities in outcomes with abdominal aortic aneurysms. Am J Card. 2021;155:135–148. doi: 10.1016/j.amjcard.2021.06.023 [DOI] [PubMed] [Google Scholar]
- 39. Harris D, Klyushnenkova E, Kalsi R, Garrido D, Bhardwaj A, Rabin J, Toursavadkohi S, Diaz J, Crawford R. Non‐white race is an independent risk factor for hospitalization for aortic dissection. Ethn Dis. 2016;26:363. doi: 10.18865/ed.26.3.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA, Willis M, et al. Cardiovascular health in african americans: a scientific statement from the american heart association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 41. Patel SA, Ali MK, Narayan KV, Mehta NK. County‐level variation in cardiovascular disease mortality in the united states in 2009–2013: comparative assessment of contributing factors. Am J Epidemiol. 2016;184:933–942. doi: 10.1093/aje/kww081 [DOI] [PubMed] [Google Scholar]
- 42. Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and control of hypertension: Jacc health promotion series. J Am Coll Cardiol. 2018;72:1278–1293. doi: 10.1016/j.jacc.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Howard G, Prineas R, Moy C, Cushman M, Kellum M, Temple E, Graham A, Howard V. Racial and geographic differences in awareness, treatment, and control of hypertension: the reasons for geographic and racial differences in stroke study. Stroke. 2006;37:1171–1178. doi: 10.1161/01.STR.0000217222.09978.ce [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7.


