Abstract
Introduction There is limited research on effective treatment of Hyperemesis Gravidarum (HG), the most extreme version of nausea and vomiting during pregnancy (NVP). This paper examines current patterns of use and self-reported effectiveness of cannabis/cannabis-based products (CBP) to treat HG.
Materials/Methods The study employed a 21-question survey to gather information on demographics, antiemetic prescription use, and experience with cannabis/CBPs among individuals who experienced extreme nausea and vomiting or HG during their pregnancy. Age-adjusted unconditional logistic regression was used to compare odds of symptom relief and weight gain between respondents who used prescription antiemetics and those who used cannabis.
Results Of the 550 survey respondents, 84% experienced weight loss during pregnancy; 96% reported using prescription antiemetics and 14% reported cannabis use for HG. Most respondents reported using cannabis/CBPs (71%) because their prescribed antiemetics were self-reported to be ineffective. More than half of cannabis/CBP users reported using products daily or multiple times per day (53%), primarily via smoke inhalation (59%), and mainly either delta-9-tetrahydrocannabinol (THC) only or THC dominant preparations (57%). Eighty-two percent of cannabis/CBP users reported symptom relief, compared to 60% of prescription antiemetic users. Among patients who reported weight loss during pregnancy, 56% of cannabis users reported gaining weight within two weeks of treatment, compared to 25% of prescription antiemetic users.
Conclusions Respondents reported using cannabis primarily because prescribed medications were self-reported to be ineffective. Although the survey approach has inherent limitations so results should be interpreted with caution, in this sample, cannabis was self-reported to be more effective than prescription medications in alleviating HG symptoms and enabling pregnancy weight gain. Therefore, depending on the safety profiles, randomized, double-blinded, placebo-controlled trials of cannabis compared to other antiemetics are warranted to determine whether cannabinoids may provide an effective alternative treatment for HG.
Key words: hyperemesis gravidarum, cannabis, pregnancy
Zusammenfassung
Einleitung Derzeit gibt es nur wenige Studien über effektive Methoden zur Behandlung von Hyperemesis gravidarum (HG), der extremsten Form von Übelkeit und Erbrechen während einer Schwangerschaft. Untersucht werden aktuelle Behandlungsmuster und Selbsteinschätzungen der Wirksamkeit von Cannabis/Cannabidiol-basierten Produkten (CBP) bei der Behandlung von HG.
Material/Methoden Die Studie verwendete einen aus 21 Fragen bestehenden Fragebogen, um Daten zu den demografischen Merkmalen, der Nutzung von verschreibungspflichtigen Antiemetika und Erfahrungen mit dem Konsum von Cannabis/CBP von Frauen zu sammeln, die während ihrer Schwangerschaft unter extremer Übelkeit und Erbrechen oder HG litten. Eine altersangepasste unkonditionale logistische Regression wurde benutzt, um Symptomlinderung und Gewichtszunahme bei Umfrageteilnehmerinnen, die verschreibungspflichtige Antiemetika verwendeten, mit den Erfahrungen von Umfrageteilnehmerinnen, die Cannabisprodukte verwendeten, zu vergleichen.
Ergebnisse Von den insgesamt 550 Umfrageteilnehmerinnen verloren 84% während der Schwangerschaft an Gewicht; 96% berichteten, dass sie verschreibungspflichtige Antiemetika verwendeten, und 14% gaben an, dass sie Cannabis zur Linderung von HG einnahmen. Die meisten dieser Teilnehmerinnen berichteten, dass sie Cannabis/CBP (71%) nutzen, weil sie die verschreibungspflichtigen Antiemetika als ineffektiv einschätzten. Mehr als die Hälfte der Cannabis/CBP-Konsumentinnen gaben an, dass sie die Produkte täglich oder mehrmals täglich (53%) einnahmen, hauptsächlich durch Rauchinhalation (59%), und dass sie meist entweder Delta-9-Tetrahydrocannabinol (THC) oder THC-haltige Präparate (57%) verwendeten. 60% der Nutzerinnen von verschreibungspflichtigen Antiemetika und 82% der Cannabis/CBP-Konsumentinnen berichteten, dass sie eine Linderung ihrer Symptome verspürten. In der Gruppe der Patientinnen, die während der Schwangerschaft an Gewicht verloren, gaben 56% der Cannabis-Konsumentinnen an, dass sie innerhalb von 2 Wochen nach Beginn der Cannabis-Behandlung an Gewicht zunahmen, verglichen mit 25% der Nutzerinnen von verschreibungspflichtigen Antiemetika.
Schlussfolgerungen Die Teilnehmerinnen gaben an, dass sie Cannabis hauptsächlich verwendeten, weil sie die verschriebenen Medikamente als ineffektiv einschätzten. Die Verwendung eines Fragebogens zur Untersuchung einer Fragestellung stößt natürlich an bestimmte Grenzen, und die Ergebnisse sind daher mit Vorsicht zu behandeln. In dieser Studie haben Teilnehmerinnen berichtet, dass Cannabis wirksamer bei der Linderung von HG-Symptomen und zur Förderung der Gewichtszunahme während der Schwangerschaft war als verschreibungspflichtige Medikamente. Je nach Sicherheitsprofil sind daher randomisierte Doppelblindstudien mit Placebokontrolle zum Nutzen von Cannabis und zum Vergleich von Cannabis mit anderen Antiemetika notwendig, um zu beurteilen, ob Cannabinoide eine effektive alternative Behandlungsmethode gegen HG darstellen.
Schlüsselwörter: Hyperemesis gravidarum, Cannabis, Schwangerschaft
Introduction
Approximately 70% of pregnant people experience some form of nausea and vomiting during pregnancy (NVP) 1 . Hyperemesis gravidarum (HG), the extreme version of NVP, is distinguished by the severity of NVP symptoms, presence of complications such as dehydration and metabolic imbalances, and deterioration of the patientʼs quality of life 2 . HG can result in hospitalization, loss of > 15% pre-pregnancy weight, esophageal rupture, postpartum post-traumatic stress disorder (PTSD), Wernickeʼs encephalopathy, and danger to the lives of both mother and fetus 2 . Illustrating the burden of associated disease, a recent survey found rates of suicidal ideation (26%) and pregnancy termination (4.9%) to be independently associated with severity of HG symptoms 3 . HG pregnancies also have increased risk of unfavorable perinatal outcomes such as low birth-weight, preterm birth, neurodevelopmental delay, and autism spectrum disorder 2 . While dietary and lifestyle changes may successfully treat milder NVP, HG patients require further therapy. In addition to fluid supplementation, physicians often prescribe antiemetics that are not completely effective for all patients with NVP/HG. Recent research suggests the placenta and appetite hormone GDF15 plays a role in the disease, but medications treating this novel pathway are still under investigation 4 , 5 .
As accessibility expands, cannabis and cannabis-based products (CBPs) are becoming increasingly popular alternatives to traditional medication 6 , 7 , 8 , 9 . The antiemetic properties of the primary chemical component of the cannabis plant, delta-9-tetrahydrocannabinol (THC) are not novel and have been utilized in treatment of other conditions such as cancer chemotherapy-induced nausea and vomiting 10 . A case series and survey provide preliminary evidence that cannabis may effectively treat pregnancy nausea and vomiting 11 , 12 . However, most studies concerning cannabis use for NVP do not specifically address HG. As self-medicating with cannabis use for NVP becomes more common, so does the importance of prospective research on its use, safety, and efficacy for HG. Furthermore, the development of diverse cannabis product preparations and modes of administration complicates understanding of the drugʼs potential effects; for example, patients can now use cannabinol (CBD) or non-inhalant administrative methods. Using social media platforms to survey people who had an HG-complicated pregnancy, we endeavored to describe recent patterns of cannabis use among people with HG and to estimate the drugʼs self-reported effectiveness in alleviating HG symptoms. We also attempted to clarify relationships between patterns of cannabis use, such as product type and mode of administration, and perceived effectiveness. Finally, we compared reported effectiveness of more traditional treatment methods to cannabis/CBPs.
Methods
Study recruitment and participants
This study utilized a 21-question survey to collect information from 550 respondents who were or had been pregnant (Supporting Information). The survey link was posted on HG-related social media platforms sponsored by the Hyperemesis Education and Research Foundation on Facebook, Instagram, and Twitter, to target individuals who experienced severe NVP or HG. The survey took approximately 5 minutes to complete. The survey was described as an “exploratory treatment survey” to avoid participation bias toward any specific treatment, including cannabis. Responses were collected from February 21 to March 31, 2021. There were no exclusion criteria, all visitors to the sites were allowed to respond to survey questions, completion of the survey was voluntary, and all survey responders were included in the convenience sample reported here. All survey questions were displayed one at a time and asked in the same order, but some questions were only displayed when certain responses involved specific follow-up. For example, follow-up questions on cannabis were not displayed if the respondent answered that they did not use cannabis in their most recent pregnancy.
Survey questions and measures
The survey was anonymous and allowed one response per IP address. This IRB-approved study was exempt because survey responses were anonymous, and no identifiers were obtained by the survey. It collected basic demographic information including country and state/region where the survey was taken and self-reported ethnicity. Additionally, it requested details of respondentsʼ most recent pregnancies. These included information about respondentsʼ age and where they lived during pregnancy (rural, suburban, or urban area). As applicable, it also queried how many weeks pregnant they were when taking the survey, delivery date, and weeks pregnant at delivery.
The survey also asked respondents to report weight loss, weight gain, and antiemetic medication use during their pregnancy. In addition to comparisons between cannabis/CBPs and prescription antiemetics as a whole, a sub-analysis also compared the most commonly reported prescription antiemetic, ondansetron, to cannabis/CBPs. When asking about weight gain after starting prescription antiemetics, the survey requested only self-reported results about the “most effective” medication and the name of this drug. For sub-analyses addressing ondansetron, the population of ondansetron users was identified by searching for mentions of “ondansetron” or “Zofran” in the text self-reporting the most-effective prescription medication.
Finally, the survey asked respondents “do/did you use marijuana/cannabis or marijuana/cannabis-based products for nausea and/or vomiting or HG in your most recent pregnancy?” A subsequent survey question then asked users to describe their productʼs formulation: THC only, CBD only, THC dominant, CBD dominant, equal parts THC and CBD, or an unknown preparation. Cannabis/CBPs users were also asked about their reason for use, timeline of use, frequency of use, and mode of administration. The options for mode of administration were smoking, eating, vaporizing, skin application, dabbing, or drinking. In this study, “cannabis users” refers to respondents who used either cannabis plant material or cannabis-based products (CBP), unless otherwise specified.
Descriptive and statistical analysis
Survey responses were automatically captured by the Alchemer (SurveyGizmo) software. We used Pivet tables in Excel and Google-sheets to manage and tabulate the data and to conduct descriptive analyses. Demographic data were summarized for all respondents as well as for each individual treatment group (cannabis users, ondansetron users, and users of any prescription antiemetic including ondansetron). These characteristics included distributions of residential country/continent during pregnancy, self-identified ethnicity, type of community of residence during most recent pregnancy (urban, suburban, or rural area), weeks pregnant at birth during most recent pregnancy (if applicable), age during most recent pregnancy, whether the respondent was pregnant when the survey was taken, and whether they had experienced weight loss during their most recent pregnancy. Respondents who answered “non-applicable” to any questions regarding these characteristics were excluded from analyses of the corresponding variable.
In further descriptive analyses, we determined frequency of weight gain and symptom relief for the groups of respondents who elected each treatment. Frequencies of NVP relief and weight gain within two weeks of starting treatment were calculated among the cannabis, ondansetron, and any prescription antiemetic user populations. The “any prescription antiemetic” or “all antiemetic” population includes users of all prescription treatments (ondansetron, promethazine, metoclopramide, doxylamine/pyridoxine, and others) besides cannabis. Weight gain was further examined among subgroups who reported weight loss during pregnancy and those who did not.
We used unconditional logistic regression analysis to estimate odds ratio associations of outcomes with the product used. In these analyses, use of cannabis was the reference level to which use of ondansetron alone and use of any prescription antiemetic (including ondansetron) were each compared. Outcome variables in these analyses of self-reported effectiveness were relief from nausea and vomiting and weight gain within two weeks of starting treatment. We first estimated univariate associations, then added potential confounding variables into a multivariate model one-by-one to identify confounders of treatment-outcome associations. After evaluating age (< 30 versus ≥ 30 years of age), ethnicity (white versus non-white), country/continent of residence (United States versus any other), and community of residence (rural versus non-rural), we retained only age in the multivariate analytic model. We conducted all logistic regression analyses using R statistical software (Version 1.4.1106) and reported the results as point and 95% confidence interval estimates of the odds ratio.
Results
Participant demographics and pregnancy experience
550 individuals completed the survey. There were no partial responses. Basic demographic characteristics are shown in Table 1 . Respondents ranged from 18 to 45 years of age (mean 30 ± 4.6 years). Respondents lived in at least 21 countries during their most recent/current pregnancy, but the majority (68%) resided in the United States. Most respondents identified as white (79%) and lived in the suburbs (56%). Half of the respondents completed the survey about their most recent completed (versus current) pregnancy (49%), of whom the majority delivered their babies between 2019 and 2020 (51%). Of this subsample, most gave birth at term (84%), but there were also reports of preterm delivery between 25 and 36 weeks (13%), and 3% reported that the pregnancy ended between 8 and 24 weeks.
Table 1 Distribution of demographic and clinical features among hyperemesis gravidarum survey respondents. Frequencies represent the demographics of each variable within a treatment category. Sum of nʼs within treatment categories may exceed n of total respondents because treatment categories are not mutually exclusive.
| Variable* | All respondents n (%) |
Cannabis users n (%) |
Ondansetron users n (%) |
Users of any prescription antiemetic n (%) |
|---|---|---|---|---|
| * Sum of nʼs within treatment categories may exceed n of total respondents because treatment categories are not mutually exclusive. † European Union, New Zealand, Norway, South Africa, Africa, Asia, Bermuda, Israel, Kosovo, Kuwait, Malaysia, Mexico, and Turkey (< 5% for each location included in “Other”) ‡ American Indian/Alaska Native, Native Hawaiian or other Pacific Islander, other, prefer not to answer | ||||
| Total (n)* | 550 | 76 | 104 | 527 |
| Country/continent residence during pregnancy | ||||
|
376 (68.4) 51 (9.3) 35 (6.4) 35 (6.4) 53 (9.5) |
64 (84.2) 7 (9.2) 1 (1.3) 2 (2.6) 2 (2.6) |
73 (70.2) 9 (8.7) 8 (7.7) 9 (8.7) 5 (4.8) |
361 (68.5) 47 (8.9) 34 (6.5) 35 (6.6) 50 (9.5) |
| Self-identified ethnicity | ||||
|
433 (79) 37 (7) 24 (4) 21 (4) 35 (6) |
51 (67.1) 10 (13.2) 8 (10.5) 1 (1.3) 6 (3.9) |
89 (85.5) 5 (4.8) 2 (1.9) 1 (1.0) 7 (6.7) |
420 (79.7) 35 (6.6) 21 (4.0) 20 (3.8) 31 (5.9) |
| Type of community of residence during most recent pregnancy | ||||
|
305 (56) 104 (25) 138 (19) 2 (< 1) |
38 (50.0) 17 (22.4) 21 (27.6) 0 (0.0) |
60 (57.7) 23 (22.1) 21 (20.2) 0 (0.0) |
296 (56.2) 132 (25.0) 98 (18.6) 1 (0.2) |
| Pregnancy status when survey was taken | ||||
|
280 (50.9) 270 (49.1) |
42 (55.3) 34 (44.7) |
69 (66.3) 35 (33.7) |
269 (51.0) 258 (49.0) |
| Weeks pregnant at birth during most recent completed pregnancy (if applicable) | ||||
|
226 (83.7) 35 (13.0) 9 (3.3) |
29 (85.3) 5 (14.7) 0 (0.0) |
30 (85.7) 3 (8.6) 2 (5.7) |
214 (82.9) 35 (13.6) 9 (3.5) |
| Age during most recent pregnancy | ||||
|
30.2 ± 4.6 | 28.6 ± 5.1 | 29.7 ± 4.1 | 30.2 ± 4.6 |
| Experienced weight loss during most recent pregnancy | ||||
|
464 (86.9) 70 (13.1) |
71 (93.4) 5 (6.6) |
88 (86.3) 14 (13.7) |
445 (86.9) 67 (13.1) |
Herein we use the term “HG” to include all respondents, although it is possible that some respondents had NVP. Most respondents (84%) reported experiencing weight loss below their pre-pregnancy weight due to nausea and vomiting during their pregnancy ( Fig. 1 ). Almost equal proportions (29%, 28%, and 27%) of respondents reported losing 5%, 10%, and ≥ 15%, respectively, of their pre-pregnancy weight.
Fig. 1.
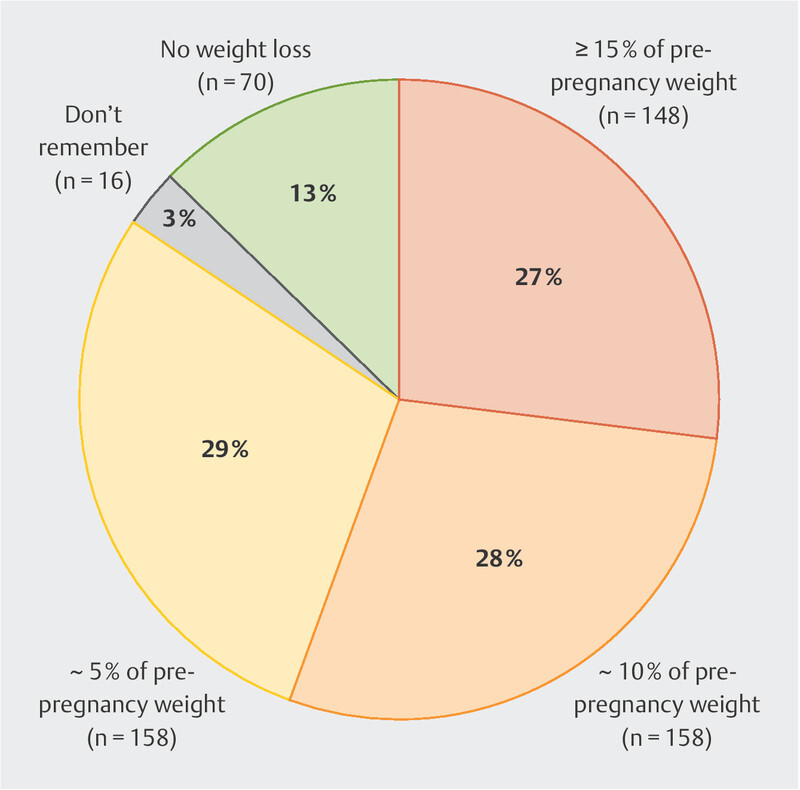
Self-reported weight loss during current or most recent pregnancy. This weight loss may have occurred at any point during the pregnancy.
Participant prescription antiemetic use
The majority of respondents (96%) reported they were prescribed and tried antiemetic medications, while the remaining respondents either did not take their prescription (1%) or were not prescribed medication (3%). Non-white respondents were less likely to receive antiemetic prescriptions than white respondents (OR: 0.29, 95% CI: 0.11 – 0.79, p = 0.0127): 93% of non-white respondents were prescribed antiemetic medication compared to 98% of white respondents. Among those who reported taking more than one medication, ondansetron (19%) was the most frequently used, although promethazine (4%), metoclopramide (3%), doxylamine/pyridoxine (2%), and others were also reported.
Participant cannabis or cannabis based product use
Seventy-six (14%) respondents reported using cannabis during their pregnancy. Details of their cannabis use are summarized in Fig. 2 . Although 28% of cannabis users reported starting cannabis prior to pregnancy, the majority (71%) reported initiating cannabis use prenatally: 44% reported beginning during their first trimester and 27% during the second trimester. Among respondents who used cannabis during pregnancy, 12% stopped using cannabis in the first trimester, 25% stopped during the second trimester, 16% stopped during the third trimester, and 19% did not stop until after giving birth or at all. Most cannabis users reported using the drug daily or multiple times per day (53%), but many reported using it only a couple of times (21%). With respect to primary mode of administration, 59% reported smoking, 19% reported oral ingestion, 9% reported drinking, 5% reported vaporizing, 5% reported skin application, and 3% reported dabbing.
Fig. 2.
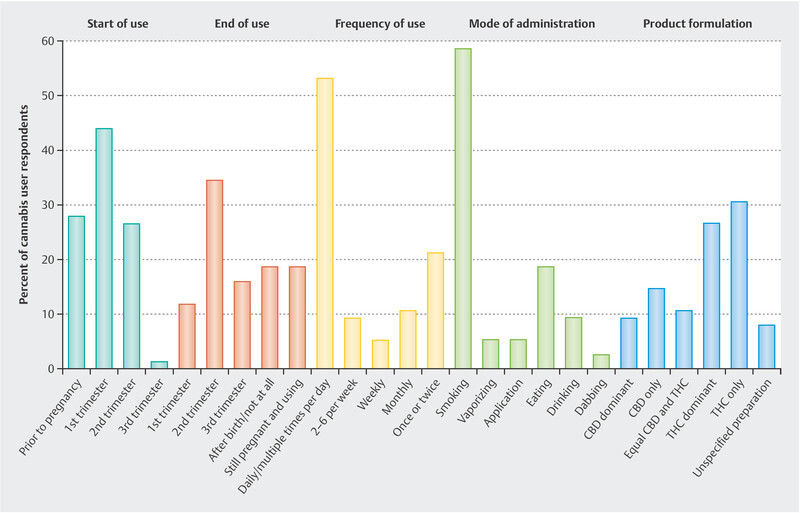
A summary of cannabis use trends among survey respondents. Cannabis users reported when they started using the drug, when they stopped, how often they used it, their mode of administration, and the product formulation.
Self-reported hyperemesis gravidarum symptom relief following treatment
Among those who reported NVP symptom relief, the majority used cannabis daily or multiple times per day (61%) through smoke inhalation (68%). This pattern also persisted among those who experienced weight gain within two weeks of treatment: 69% used cannabis daily/multiple times per day and 64% smoked it. The majority used products that were either THC only or THC dominant (57%). Fewer reported using only CBD or CBD dominant products (24%), products with equal amounts of THC and CBD (11%), or products of unspecified formulation (8%). While users of THC dominant and CBD dominant products reported similar frequencies of weight gain after beginning treatment, those who used THC dominant products were more than 9 times more likely to report NVP symptom relief than those who used CBD dominant products (OR: 9.1, 95% CI: 2.13 – 48.6).
NVP symptom relief was reported by 82% of respondents who self-treated with cannabis, 60% of all antiemetic users (including those who used ondansetron), and 77% of those who indicated ondansetron use specifically. Only 56% of those who used prescription antiemetics other than ondansetron (which included promethazine, metoclopramide, doxylamine/pyridoxine, and others) reported NVP symptom relief. Fig. 3 a compares the self-reported effectiveness of cannabis, ondansetron, and all antiemetic medications (including ondansetron) at treating NVP.
Fig. 3.
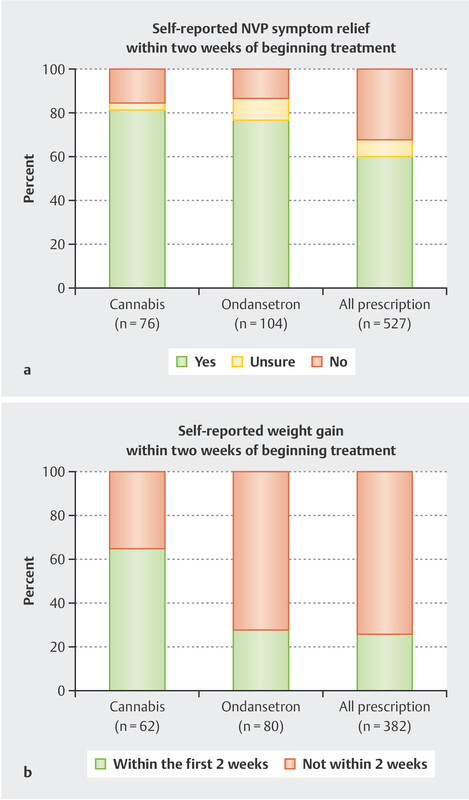
a Self-reported effectiveness of cannabis (n = 76), ondansetron (n = 104), and all prescription antiemetics (n = 527) to provide nausea and vomiting of pregnancy (NVP) symptom relief and b gain weight within two weeks of starting treatment. Weight gain was reported among respondents who reported weight loss. Responses that were unsure about weight loss during pregnancy or weight gain following treatment were excluded. Some individuals used more than one treatment, so nʼs total to greater than 550.
Weight gain within two weeks of starting treatment was reported by 56% of cannabis users, 30% of ondansetron users, 25% of all antiemetic users (including ondansetron), and 23% of those who used prescription antiemetics other than ondansetron. Among the subgroup who experienced weight loss during pregnancy, 57% of cannabis users, 26% of ondansetron users, and 23% of all antiemetic users self-reported gaining weight within two weeks of starting their treatment ( Fig. 3 b ). Table 2 summarizes the frequency of NVP symptom relief and weight gain within two weeks of beginning cannabis/cannabis-based product, ondansetron, or any prescription antiemetic use.
Table 2 Intervention and self-reported improvement in nausea and vomiting of pregnancy and weight gain following use of cannabis/cannabis-based products, ondansetron, or any prescription antiemetic including ondansetron. Sum of nʼs within treatment categories may exceed n of total respondents because treatment categories are not mutually exclusive. Responses that selected “not applicable” were excluded from analysis, and those that selected “do not remember” or “do not know” were categorized as “unsure”.
| Intervention | Cannabis users n (%) |
Ondansetron users n (%) |
Users of any prescription antiemetic n (%) |
|---|---|---|---|
| * Totals exceed 100% because some respondents used more than one type of treatment. † Individuals who indicated that they “do not remember” or “do not know”. ‡ Excludes those who did not answer the question or answered “not applicable”. § In the case of any prescription antiemetic, users were asked to report weight gain after beginning their most effective antiemetic drug. | |||
| Number of participants (x/550)* | 76 (14) | 104 (19) | 527 (96) |
| Did you find that the intervention helped treat your nausea/vomiting symptoms in your most recent pregnancy? | |||
|
62 (81.6) 12 (15.8) 2 (2.6) 76 |
80 (76.9) 14 (13.5) 10 (9.6) 104 |
316 (60) 170 (32.3) 41 (7.8) 527 |
| Did you gain weight within the first 2 weeks of starting the intervention? § | |||
|
42 (56) 23 (30.7) 10 (13.3) 75 |
30 (29.7) 64 (63.4) 7 (6.9) 101 |
120 (24.7) 313 (64.5) 52 (10.7) 485 |
| Among those who reported weight loss: did you gain weight within the first 2 weeks of starting the intervention? § | |||
|
40 (57.1) 22 (31.4) 8 (11.4) 70 |
22 (25.6) 58 (67.4) 6 (7.0) 86 |
97 (23.2) 285 (68.2) 36 (8.6) 418 |
| Among those who did not report weight loss: did you gain weight within the first 2 weeks of starting the intervention? § | |||
|
2 (40.0) 1 (20.0) 2 (40.0) 5 |
7 (50.0) 5 (35.7) 2 (14.3) 14 |
20 (28.6) 23 (32.9) 27 (38.6) 70 |
Table 3 reports associations between use of ondansetron or any prescription antiemetic versus cannabis and each of NVP symptom relief and weight gain. While ondansetron users were slightly more likely to report NVP symptom relief than cannabis users (OR: 1.17, 95% CI: 0.49 – 2.76), this result did not achieve statistical significance. Furthermore, users of any prescription antiemetic were only a third as likely to experience NVP symptom relief than cannabis users (OR: 0.36, 95% CI: 0.17 – 0.65). Compared to cannabis users, both those who used ondansetron (OR: 0.26, 95% CI: 0.13 – 0.51) and those who used any prescription antiemetic (OR: 0.21, 95% CI: 0.12 – 0.36) reported lower odds of weight gain within two weeks of starting treatment. Furthermore, compared to cannabis users, prescription antiemetic users (ondansetron included) who lost weight (OR: 0.19, 95% CI: 0.10 – 0.33) were less likely to report weight gain within two weeks of starting treatment than those who did not report weight loss (OR: 0.42, 95% CI: 0.02 – 4.98). Additionally, those who used ondansetron were less likely than those who used cannabis to gain weight, whether they had (OR: 0.21, 95% CI: 0.10 – 0.44) or had not (OR: 0.63, 95% 0.02 – 9.16) lost weight during the pregnancy.
Table 3 Association of use of ondansetron or any prescription antiemetic versus use of cannabis with relief of symptoms and subsequent weight gain, among all respondents and those who did or did not lose weight during the pregnancy, odds ratio* (95% confidence interval). Odds ratios are adjusted for age.
| Agent Used | All participants (n = 550) | Those who lost weight † (n = 464) | Those who did not lose weight † (n = 70) |
|---|---|---|---|
| * Adjusted for age. † 16 participants did not provide information about weight loss. | |||
| Relief of symptoms of nausea and vomiting of pregnancy | |||
|
1.0 (ref) 1.17 (0.49 – 2.76) 0.36 (0.17 – 0.65) |
1.0 (ref) 1.30 (0.51 – 3.32) 0.32 (0.15 – 0.60) |
1.0 (ref) 0.57 (0.01 – 11.92) 0.71 (0.03 – 5.25) |
| Weight gain within two weeks of starting treatment | |||
|
1.0 (ref) 0.26 (0.13 – 0.51) 0.21 (0.12 – 0.36) |
1.0 (ref) 0.21 (0.10 – 0.44) 0.19 (0.10 – 0.33) |
1.0 (ref) 0.63 (0.02 – 9.16) 0.42 (0.02 – 4.98) |
Reason for cannabis or cannabis based product use
When asked “Why did you use marijuana/cannabis or marijuana/cannabis-based products for nausea/vomiting in your most recent pregnancy,” the majority of cannabis users (71%) indicated that they tried it because prescription medications were self-reported to be ineffective. Table 4 shows representative quotes that reflect prolonged self-reported ineffectiveness of prescription antiemetics. Other reasons for use included health professionals recommending cannabis use for NVP (8%), personal decisions not discussed with providers (7%), or hearing about its use during pregnancy from others (7%). Finally, some cannabis users reported that they did not use prescription medication either by choice (4%) or because they were not prescribed any (4%).
Table 4 Patientsʼ representative descriptions of treatment outcomes after use of antiemetic medication they found most effective for hyperemesis gravidarum.
| * 28 pounds |
| “I havenʼt gained any weight yet, even on medication for 8 weeks.” |
| “Even with the prescribed meds, I was never able to gain any weight.” |
| “I weighed 2 stone less at the end of my pregnancy.”* |
| “Delivered weighing almost 30 lbs less than my pre pregnancy weight.” |
| “Iʼve been on 3 medicines for over 10 weeks and still continue losing weight.” |
| “It helped. But marginally. As in a drop in bucket. Did not help weight gain at all. Made it one step from complete agony.” |
| “Zofran alone was ineffective and diclegis alone was ineffective, but the two combined [were] effective… By effective, I mean that I am now throwing up an average of three times a day and can keep some food down. I have been on medication for 15 weeks of my pregnancy and have not gained weight and am still losing weight.” |
Discussion
A minority of respondents in this survey reported using cannabis for HG; however, those who used cannabis or CBPs reported more frequent relief from HG symptoms compared to those who used prescription antiemetics. Furthermore, those cannabis users were more likely to report weight gain within two weeks of treatment than those who used ondansetron, reportedly the most effective prescription antiemetic for survey respondents. Among those who reported NVP symptom relief or weight gain within two weeks of initiating cannabis use, most consumed the drug daily or multiple times per day by smoke inhalation. While users of THC dominant and CBD dominant products reported similar frequencies of weight gain after beginning treatment, those who used THC dominant products were more likely to report NVP symptom relief than those who used CBD dominant products.
This study also reveals the need for a better understanding of HG and its treatment within the medical community. Although 96% of respondents were prescribed prescription antiemetics, 88% reported weight loss during pregnancy, and less than one fourth reported weight gain within two weeks of starting their most self-reportedly effective medication. Thus, although doctors prescribe medications, these treatments do not help many patients regain weight lost from HG within 14 days of the treatment start date. Given that inadequate weight gain in pregnancy is associated with poor perinatal outcomes 2 , this study highlights the importance of including weight gain as an outcome measure for HG treatment effectiveness. Furthermore, survey results indicate that medical inadequacy motivates some people with HG to try cannabis for symptom relief: most respondents who used cannabis reported initiating use during pregnancy because prescribed antiemetic medications were self-reported to be ineffective.
Despite increased research and understanding of HG, most available prescription treatments do not provide adequate symptom relief or effectively assist weight gain. Cochrane reviews of NVP and HG published in 2015 and 2018 both conclude there is insufficient evidence to establish superiority of any prescription treatment 13 , 14 . The present survey identifies likely consequences of unclear treatment protocols: physicians prescribe a wide variety of antiemetics, but they may only help a fraction of their pregnant patients regain weight lost to HG within 14 days of treatment. The representative statements of respondents show that although people should gain weight during pregnancy, HG patients may give birth weighing up to 30 pounds less than their pre-pregnancy weight, despite using prescription antiemetics.
Our survey indicates that ondansetron may provide self-reported NVP symptom relief and allow for weight gain more than other prescription antiemetics. These results align with a retrospective study on ondansetron treatment of HG which showed that HG pregnancies treated with ondansetron had lower rates of miscarriage and therapeutic termination, and higher rates of live birth compared to those that were not 15 .
Although the study is limited because it is based on self-reporting, this is not the first study to suggest that cannabis or a constituent of cannabis may effectively treat NVP. Westfallʼs 2006 survey on cannabis use during pregnancy showed that people who experienced NVP self-reported that cannabis was effective at treating symptoms and stimulating appetite 12 . Accordingly, NVP symptom relief appears to be a major incentive for prenatal cannabis use 16 , with cannabis used more frequently by those who experienced nausea and vomiting during pregnancy 7 , 8 .
The consequences of HG can be severe for both mother and fetus. It can result in hospitalization, esophageal rupture, postpartum PTSD, Wernickeʼs encephalopathy, or danger to the mother and fetusʼ life 2 . It is also shown to affect fetal growth and neurodevelopment 2 . Based on a 15-year cohort study, Meinich directly linked decreased maternal weight gain to decreased fetal growth 17 . With this in mind, the finding that 88% of respondents experienced weight loss and less than 25% reported weight gain within two weeks of initiating antiemetic use, highlights the importance of improving HG management and treatment.
This study adds to growing literature supporting antiemetic properties of cannabis and cannabinoid compounds while also suggesting their potential to treat HG. There is limited scholarly literature addressing cannabinoids and HG, specifically. A PubMed search using the terms “hyperemesis gravidarum” together with “cannabis”, “cannabinoid”, or “marijuana” yielded only one paper with an HG focus: Koren and Cohenʼs 2020 article showed measured improvement of HG symptoms in four people 11 . Our study provides a larger sample size to support these conclusions and further examines patterns of cannabis use.
Although our survey indicates that cannabis may effectively treat HG based on self-reporting of effectiveness, several results are concerning: most respondents who used cannabis were new rather than long-term users, and most respondents attributed their use to the inadequacy of prescription treatments. Of note, there were 9 pregnancy terminations reported among prescription antiemetic users, but none among cannabis users. The safety of cannabis use during pregnancy is currently unclear 6 , 18 , 19 . However, safety profiles of prescription antiemetics are also not well-studied 20 . Our survey underscores pressing needs to both evaluate effectiveness of traditional treatments with respect to both symptom improvement and weight gain and to explore new ones.
This study also highlights the exigency of research into the safety of cannabis use for HG. Recent studies show an association between prenatal cannabis use and adverse neonatal outcomes such as preterm birth, decreased fetal growth, and death within the first year 6 , 18 , 19 . A 2019 study examining over 600 000 Canadian women found that cannabis users were almost twice as likely to experience preterm birth than non-cannabis users. Interestingly, it also found significantly lower frequencies of maternal obstetric outcomes such as preeclampsia and gestational diabetes, although without addressing specific indications for use 21 . Other studies have found evidence suggesting increased risk of childhood psychopathology following prenatal cannabis exposure 22 . Unfortunately, there are many obstacles to performing substantial and accurate studies on the effects of prenatal cannabis use. As summarized in National Academies of Sciences, Engineering, and Medicineʼs 2017 consensus report on the health effects of cannabis and cannabinoids, self-reported use statistics, missing data on cannabis dosage or potency, small sample sizes, and confounding tobacco and alcohol use all challenge this research 19 . Furthermore, it is unclear whether there is a distinction between the impacts of chronic or recreational cannabis use prior to pregnancy versus cannabis use initiated during pregnancy on fetal development. Most studies that examine the effects of cannabis on fetal development have focused on long-term users or failed to specify extent of use.
Importantly, future research assessing the safety of cannabis and cannabinoid use in pregnant populations must address HG as a potentially confounding variable. Many of the adverse fetal and child outcomes associated with prenatal cannabis use are also associated with HG 22 , 23 , but most extant research addressing these outcomes has not addressed potential influences of HG. For instance, a recent study exploring effects of cannabis use during pregnancy did not include HG as one of the six obstetric complications 24 . It reported increased risks of autism spectrum disorder, learning disorders, and ADHD among children with prenatal cannabis exposure; however, all of these complications are also associated with HG. To rectify this gap in scholarly literature, new studies on cannabis use during pregnancy must assess HG status of their participants. Similarly, conclusions about the safety of cannabis as an antiemetic treatment during pregnancy must distinguish between NVP and HG.
Finally, this study identified potential racial disparities in HG treatment because a greater proportion of non-white respondents reported not receiving antiemetic medication(s) than white respondents. Therefore, in addition to studying cannabisʼ effectiveness and safety in a clinical setting, future research should focus on evaluating existing treatment algorithms 2 , 25 and developing standards of care across all populations.
The study had several limitations. The survey was posted on social media sites related to HG and was answered primarily by white respondents living in suburban areas of the United States. This is not a representative sample of pregnant people with severe NVP or HG. This study may reflect response bias because users of these social media sites are more likely to have greater resources (particularly internet access) and more frequent or more severe NVP/HG may motivate their social media site use. Determining the generalizability of the findings reported here requires further research. Additionally, the study has potential for inaccurate recall because while the survey occurred in February/March of 2021, half of the respondents reported on their most recent completed pregnancy and presumably were no longer experiencing NVP or HG at the time. Half of these (one-quarter of all respondents) were recalling pregnancies completed in 2019 or 2020. This leaves another one-quarter of respondents recalling pregnancies completed before 2019 (greater than 2 years before the survey). Additionally, all survey respondents were included in the study, so while the survey was posted only on HG-specific websites, it is possible some respondents had less severe NVP rather than HG. It is not possible to determine whether NVP severity is different between treatment groups in this study because it was not measured. Future research should include a measurement of nausea severity using a validated tool such as the PUQE or HELP questionnaire to determine whether severity confounded the results 26 . That being said, weight loss can be an indicator of more severe symptoms 26 . Accordingly, this study performed a sub-analysis limited to respondents that reported weight loss to identify which treatments lead to self-reported weight gain within two weeks. Similar frequencies of respondents reported “no weight gain” between the sub-analysis and unstratified results within every treatment group: 30.7% vs. 31.4% of cannabis users, 63.4% vs. 67.4% of ondansetron users, and 64.5% vs. 68.2% of any prescription antiemetic user. Thus, results do not appear to be sensitive to this indicator of severity.
There is also a potential for misclassification between HG and Cannabis Hyperemesis Syndrome (CHS). However, most of the respondents reported initiating use during pregnancy to treat symptoms already existing prior to use when other antiemetics were self-reportedly ineffective, and CHS is a condition of long-term users 27 . In addition, it is unlikely that respondents with CHS would report weight gain within two weeks of initiating use, so if anything, the inclusion of respondents with CHS would bias results in the opposite direction.
Also, other treatments such as hospitalization/rehydration or nutritional treatment were not included in the survey and are potential confounders in comparing the outcomes from the antiemetic treatment group. However, since cannabis cannot be used in most inpatient settings, one might predict that patients using prescription treatments would be more likely to simultaneously have used other prescribed interventions than the cannabis group, and one might expect any resulting confounding to be negative, leading to an underestimate of the differences between cannabis and antiemetics. If such confounding did occur then true differences between groups who used cannabis and prescription antiemetics could be even greater than the estimate presented here.
This study relies on the self-reporting of prescription antiemetic and cannabis use. As a result, it may under-represent the population of users. Cannabisʼ complex legal status threatens honest reporting because law enforcement may oppose prenatal cannabis use 9 . That being said, patients may feel more comfortable reporting cannabis use in the anonymous setting of this survey. The survey also uses self-reported weight statistics, which could yield inaccurate results; however, the majority reported on current/recent pregnancies, and allowed respondents to choose “do not remember” when answering questions about weight gain/loss. A systematic review examining the accuracy of self-reported pregnancy-related weight found slight under-reporting of pre-pregnancy weight and slight over-reporting of gestational weight gain but concluded that self-reporting is a practical and cost-effective approach to assessing weight in pregnancy 28 . In addition, this survey focuses on comparing rates of self-reported HG symptom relief between cannabis and non-cannabis users, and there is no reason to believe the validity of self-reported weight gain would be different between these populations.
Respondents may have used antiemetics and cannabis simultaneously. As a result, NVP symptom relief and weight gain during cannabis use may reflect synergistic effects of combined drug use. While this survey did not ask cannabis users if they were taking antiemetics concurrently, only three respondents reported using cannabis and never having been prescribed antiemetics. Regardless, any changes in symptom status after beginning cannabis use are important, even if other drugs were present. A larger study that explicitly distinguishes between cannabis users, antiemetic users, and those who use both is needed to better understand the effectiveness of cannabis.
Conclusions
Many people who suffer from HG experience weight loss during pregnancy, even when using physician-prescribed antiemetics. More respondents reported NVP symptom relief and weight gain with ondansetron than other antiemetics, but many were still unable to gain weight within two weeks of their first dose. Cannabis products may be perceived as a more effective alternative, but more research is required to understand its mechanism and safety. Given that the findings reported herein are based on survey responses in a convenience sample, the study should be replicated in a well-controlled clinical setting. It is important to note that a recent study found a higher risk (5-fold) of having a baby born small for gestational age associated with in utero exposure to HG, than exposure to cannabis, as well as chronic hypertension, pre-gestational diabetes, preeclampsia, autoimmune disease, cocaine use, amphetamine use, and tobacco use 29 . Therefore, in the meantime, providers must weigh unknown risks of recommending cannabis, which may be perceived as having a greater effectiveness profile in this convenience sample, with the well-established risk of adverse maternal and fetal outcomes for refractory HG.
Acknowledgements
This study received funding from the National Institute of Drug Abuse Grant DA047296 and the Semel Charitable Foundation. The results were presented, in part, as a poster at the International Cannabinoid Research Society annual meeting in Jerusalem, Israel, June 21 – 24, 2021. No earlier version of this manuscript has been published on any preprint server.
Footnotes
Conflict of Interest Outside of this work, Dr. Cooper reports grants from National Center for Complementary and Integrative Health, Center for Medical Cannabis Research, and the California Bureau of Cannabis Control. In the past three years, Dr. Cooper has served as a consultant to the following companies: GB Sciences and Beckley Canopy Therapeutics and has served on the scientific advisory board of FSD Pharma. Dr. Fejzo is a consultant for Materna Biosciences, Inc. and a voluntary (unpaid) board member of the Hyperemesis Education and Research Foundation.
Supporting Information
References
- 1.Einarson T R, Piwko C, Koren G. Quantifying the global rates of nausea and vomiting of pregnancy: a meta analysis. J Popul Ther Clin Pharmacol. 2013;20:e171–e183. [PubMed] [Google Scholar]
- 2.Fejzo M S, Trovik J, Grooten I J. Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat Rev Dis Primers. 2019;5:62. doi: 10.1038/s41572-019-0110-3. [DOI] [PubMed] [Google Scholar]
- 3.Nana M, Tydeman F, Bevan G. Hyperemesis gravidarum is associated with increased rates of termination of pregnancy and suicidal ideation: results from a survey completed by > 5000 participants. Am J Obstet Gynecol. 2021;224:629–631. doi: 10.1016/j.ajog.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Fejzo M S, Arzy D, Tian R. Evidence GDF15 Plays a Role in Familial and Recurrent Hyperemesis Gravidarum. Geburtshilfe Frauenheilkd. 2018;78:866–870. doi: 10.1055/a-0661-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fejzo M S, Fasching P A, Schneider M O. Analysis of GDF15 and IGFBP7 in Hyperemesis Gravidarum Support Causality. Geburtshilfe Frauenheilkd. 2019;79:382–388. doi: 10.1055/a-0830-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metz T D, Stickrath E H. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol. 2015;213:761–778. doi: 10.1016/j.ajog.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Roberson E K, Patrick W K, Hurwitz E L. Marijuana use and maternal experiences of severe nausea during pregnancy in Hawai′i. Hawaii J Med Public Health. 2014;73:283–287. [PMC free article] [PubMed] [Google Scholar]
- 8.Young-Wolff K C, Sarovar V, Tucker L Y. Trends in marijuana use among pregnant women with and without nausea and vomiting in pregnancy, 2009–2016. Drug Alcohol Depend. 2019;197:66–70. doi: 10.1016/j.drugalcdep.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metz T D, Borgelt L M. Marijuana Use in Pregnancy and While Breastfeeding. Obstet Gynecol. 2018;132:1198–1210. doi: 10.1097/AOG.0000000000002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koren G, Cohen R. Medicinal Use of Cannabis in Children and Pregnant Women. Rambam Maimonides Med J. 2020;11:e0005. doi: 10.5041/RMMJ.10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koren G, Cohen R. The use of cannabis for Hyperemesis Gravidarum (HG) J Cannabis Res. 2020;2:4. doi: 10.1186/s42238-020-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westfall R E, Janssen P A, Lucas P. Survey of medicinal cannabis use among childbearing women: patterns of its use in pregnancy and retroactive self-assessment of its efficacy against ʼmorning sicknessʼ. Complement Ther Clin Pract. 2006;12:27–33. doi: 10.1016/j.ctcp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Boelig R C, Barton S J, Saccone G. Interventions for treating hyperemesis gravidarum: a Cochrane systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2018;31:2492–2505. doi: 10.1080/14767058.2017.1342805. [DOI] [PubMed] [Google Scholar]
- 14.Matthews A, Haas D M, OʼMathúna D P.Interventions for nausea and vomiting in early pregnancy Cochrane Database Syst Rev 2015(2015)CD007575 10.1002/14651858.CD007575.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fejzo M S, MacGibbon K W, Mullin P M. Ondansetron in pregnancy and risk of adverse fetal outcomes in the United States. Reprod Toxicol. 2016;62:87–91. doi: 10.1016/j.reprotox.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Skelton K R, Hecht A A, Benjamin-Neelon S E. Womenʼs cannabis use before, during, and after pregnancy in New Hampshire. Prev Med Rep. 2020;20:101262. doi: 10.1016/j.pmedr.2020.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meinich T, Trovik J. Early maternal weight gain as a risk factor for SGA in pregnancies with hyperemesis gravidarum: a 15-year hospital cohort study. BMC Pregnancy Childbirth. 2020;20:255. doi: 10.1186/s12884-020-02947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y, Zhu B, Liang D. The associations between prenatal cannabis use disorder and neonatal outcomes. Addiction. 2021;116:3069–3079. doi: 10.1111/add.15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice ; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda . Washington, DC: National Academies Press; 2017. The Health Effects of Cannabis and Cannabinoids: the current State of Evidence and Recommendations for Research. [PubMed] [Google Scholar]
- 20.Boelig R C, Barton S J, Saccone G. Interventions for treating hyperemesis gravidarum: a Cochrane systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2018;3:2492–2505. doi: 10.1080/14767058.2017.1342805. [DOI] [PubMed] [Google Scholar]
- 21.Corsi D J, Walsh L, Weiss D. Association Between Self-reported Prenatal Cannabis Use and Maternal, Perinatal, and Neonatal Outcomes. JAMA. 2019;322:145–152. doi: 10.1001/jama.2019.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul S E, Hatoum A S, Fine J D. Associations Between Prenatal Cannabis Exposure and Childhood Outcomes: Results From the ABCD Study. JAMA Psychiatry. 2021;78:64–76. doi: 10.1001/jamapsychiatry.2020.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Rolls E T, Du X. Severe nausea and vomiting in pregnancy: psychiatric and cognitive problems and brain structure in children. BMC Med. 2020;18:228. doi: 10.1186/s12916-020-01701-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corsi D J, Donelle J, Sucha E. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat Med. 2020;26:1536–1540. doi: 10.1038/s41591-020-1002-5. [DOI] [PubMed] [Google Scholar]
- 25.MacGibbon K W. Hyperemesis Gravidarum: Strategies to Improve Outcomes. J Infus Nurs. 2020;43:78–96. doi: 10.1097/NAN.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 26.MacGibbon K W, Kim S, Mullin P M. Level Prediction (HELP Score) Identifies Patients with Indicators of Severe Disease: a Validation Study. Geburtshilfe Frauenheilkd. 2021;8:90–98. doi: 10.1055/a-1309-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senderovich H, Patel P, Jimenez Lopez B. A Systematic Review on Cannabis Hyperemesis Syndrome and its Management Options. Med Princ Pract. 2021 doi: 10.1159/000520417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Headen I, Cohen A K, Mujahid M. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev. 2017;18:350–369. doi: 10.1111/obr.12486. [DOI] [PubMed] [Google Scholar]
- 29.Sasso E B, Bolshakova M, Bogumil D. Marijuana use and perinatal outcomes in obstetric patients at a safety net hospital. Eur J Obstet Gynecol Reprod Biol. 2021;266:36–41. doi: 10.1016/j.ejogrb.2021.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


