Abstract
Lactobacillus plantarum PS128 has been reported as a psychobiotic to improve mental health through the gut–brain axis in experimental animal models. To explore its mechanism of action in the gut, this study aimed to analyze the effects of L. plantarum PS128 ingestion on naïve and loperamide (Lop)-induced constipation mice. We found that, in the two mouse models, the weight, number, and water content of feces in the L. plantarum PS128 group were higher than those in the vehicle control group. Histological observation revealed that L. plantarum PS128 increased the level of colonic mucins including the major mucin MUC2. In addition, the charcoal meal test showed that L. plantarum PS128 significantly increased the small intestine transit in naïve mice, but not in the Lop-treated mice. Since intestinal serotonin has been found to modulate motility, we further analyzed the expression of genes related to serotonin signal transduction in the small intestine of naïve mice. The results showed that L. plantarum PS128 significantly altered the expression levels of Tph1, Chga, Slc6a4, and Htr4, but did not affect the expression levels of Tph2, Htr3a, and Maoa. Furthermore, immunohistochemistry revealed that L. plantarum PS128 significantly increased the number of serotonin-containing intestinal cells in mice. Taken together, our results suggest that L. plantarum PS128 could promote intestinal motility, mucin production, and serotonin signal transduction, leading to a laxative effect in mice.
Keywords: Lactobacillus plantarum, PS128; Psychobiotic, Gut–brain axis, Serotonin signaling
Introduction
Probiotics are defined as live microorganisms which when administered in adequate amounts confer a health benefit on the host [1]. In the market, probiotics have been extensively available as food products (cheese, yogurt, fermented milk, meat, and vegetables) or food supplements (capsules, tablets, and powders). Numerous studies have demonstrated the diverse beneficial effects of probiotics in the maintenance of gastrointestinal (GI) homeostasis [2, 3], regulation of immune responses [4], and attenuation of metabolic dysfunction [5].
The gut–brain axis facilitates bidirectional communication between the GI tract and the brain or between the enteric nervous system (ENS) and central nervous system (CNS), which involves the neural, immune, and endocrine pathways [6]. A special class of probiotics, termed “psychobiotics,” can improve the CNS-related functions and behaviors of the host through the gut–brain axis [7]. Moreover, psychobiotics have been demonstrated to improve the neurodegenerative and neurodevelopmental disorders, including autism spectrum disorder (ASD) and Parkinson’s disease (PD) [8]. Though, psychobiotic effects are considered strain-specific, a given strain might exert several health-promoting effects in many cases [9].
Lactobacillus plantarum PS128 is a novel psychobiotic that alleviates depression- and anxiety-like behaviors [10, 11], visceral hypersensitivity [12], and neurobehavioral aspects of movement disorders [13, 14] in experimental animals. Clinically, L. plantarum PS128 appears to ameliorate the opposition/defiance behaviors in children with ASD [15], enhance exercise performance in triathletes [16], and improve self-perceived stress and salivary cortisol levels in highly stressed information technology specialists [17]. These studies suggest that L. plantarum PS128 affects CNS-related functions through the gut–brain axis. However, the effect of L. plantarum PS128 on GI function, including motility and secretion, remains largely unknown.
In this study, we aimed to evaluate the effect of L. plantarum PS128 on fecal parameters, intestinal motility, and intestinal secretion in two mouse models, including naïve mice and loperamide (Lop)-induced constipation model mice. Since intestinal serotonin (5-hydroxytryptamine; 5-HT) is a well-known neurotransmitter that regulates GI motility [18], we further studied the effect of L. plantarum PS128 on the expression of genes related to serotonin signaling in the intestine.
Materials and Methods
Preparation of Lactobacillus plantarum PS128 Culture
Lactobacillus plantarum (recently re-classified as Lactiplantibacillus plantarum [19]) PS128 was prepared using the method described by Liao et al. [14]. In brief, L. plantarum PS128 was cultured at 37 °C in the de Man, Rogosa and Sharpe broth (Difco Corp., MD, USA) for 18 h. The bacterial culture was harvested by centrifugation at 6000 × g for 10 min. Before oral administration, the bacterial pellet was resuspended in sterile phosphate-buffered saline (PBS) to attain a final concentration of approximately 1010 colony-forming units (CFUs)/mL.
Animals
Eight-week-old adult male ICR mice were purchased from the National Laboratory Animal Center, Taipei, Taiwan. All mice were maintained on a 12-h light/dark cycle in a humidity-controlled (55–65%) and temperature-controlled (22 ± 2 °C) environment with standardized laboratory chow and tap water ad libitum at the National Yang Ming Chiao Tung University Laboratory Animal Center. The use of animals and the procedures for animal handling and treatments were approved by the Institutional Animal Use and Care Committee (IACUC 1,060,606) at the National Yang Ming Chiao Tung University in Taiwan.
Experimental Design
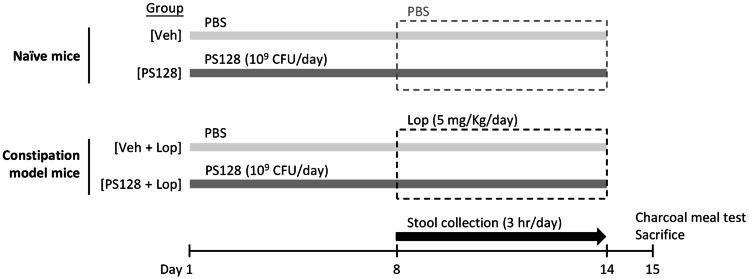
As shown in Fig. 1, mice were divided into four groups (n = 10 per group). The PS128 group received daily oral gavage of PS128 of 109 CFU for 14 consecutive days while vehicle control (Veh) group received PBS (0.2 mL per day). The PS128 + Lop group received daily oral gavage of PS128 of 109 CFU for 14 consecutive days, and orally administered loperamide hydrochloride (5 mg/kg body weight; Sigma-Aldrich, St. Louis, MO, USA) on day 8 to 14 of the experiment [20]. In addition, the Veh + Lop group received daily oral gavage of 0.2 mL PBS for 14 consecutive days and the same treatment of Lop. To analyze fecal parameters, the wet weight and number of feces were measured for 3 h per day on day 8 to 14 of the experiment. Subsequently, the collected feces were dried at 70 °C for 18 h and weighed to determine the percentage of water content, which was calculated using the following formula: Fecal water content (%) = [(wet mass − dry mass)/wet mass] × 100. On day 15 of the experiment, the small intestinal transit rate of mice was determined by a charcoal meal test, and the mice were sacrificed for subsequent analysis.
Fig. 1.
The experimental design. Laxative effects of L. plantarum PS128 were evaluated in naïve (upper panel) and constipation model mice (lower panel). Male ICR mice (8-week-old) were orally administered 0.2 mL of PBS, as the vehicle control (Veh) group, or 109 CFU of L. plantarum PS128 per day for 14 consecutive days. To induce constipation, mice were orally administered loperamide hydrochloride (Lop. HCl; 5 mg/kg body weight) 1 h after the administration of PBS or PS128 on experimental days 8 to 14. Thirty minutes after the administration of PBS or Lop. HCl, the number and weight of stool from each mouse were measured for 3 h. On experimental day 15, mice were subjected to the charcoal meal test and sacrificed (n = 10 per group)
Charcoal Meal Test
To measure the small intestinal transit rate in mice, a charcoal meal test was performed as previously described with minor modifications [21]. After fasting for 16 h with water ad libitum, mice were orally administered 0.2 mL of PBS or L. plantarum PS128 suspensions. After 1 h, mice were administered PBS or Lop. HCl by oral gavage followed by administration of charcoal meal (5% charcoal and 10% gum arabica) after 30 min. Fifteen minutes later, mice were sacrificed by cervical dislocation and the small intestine was removed from the stomach to the caecum to measure the distance traveled by the charcoal meal and total length of the intestine. The small intestine transit rate was calculated using the following formula: small intestine transit rate (%) = (distance traveled by the charcoal meal/total length of the intestine) × 100.
qRT-PCR Analysis
The total RNA in the ileum tissue was extracted, converted to cDNA, and subjected to quantitative reverse-transcription-polymerase chain reaction (qRT-PCR) analysis as previously described [22]. In brief, cDNA samples from each group were subjected to triplicate real-time PCR experiments with specific primers (Table 1) and KAPA SYBR FAST ABI PRISM Kit (KAPA Biosystems, Woburn, MA, USA) using the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The targets included genes encoding tryptophan hydroxylase 1 and 2 (Tph1 and Tph2), chromogranin A (Chga), the serotonin transporter solute carrier family 6 member 4 (Slc6a4), 5-hydroxytryptamine receptor 3A and 5-hydroxytryptamine receptor 4 (Htr3a and Htr4), and monoamine oxidase A (Maoa). The target threshold cycle (Ct) was subtracted from the Ct for glyceraldehyde-3-phosphate dehydrogenase (Gapdh) to calculate ΔCt, and a relative quantification analysis was performed using the 2−ΔΔCT method.
Table 1.
Primers used in the present study
| Gene | Primer sequence (5′–3′) | Reference |
|---|---|---|
| Tph1 | F: TTTCGAGTCTTTCACTGCACT | [57] |
| R: CTAGGAGTTCATGGCAGGT | ||
| Tph2 | F: GAGTTGCTCCACGCTTTGC | [58] |
| R: ACACTCAGTCTACATCCATCCC | ||
| Chga | F: CCCACTGCAGCATCCAGTT | [59] |
| R: AGTCCGACTGACCATCATCTTTC | ||
| Slc6a4 | F: TATCCAATGGGTACTCCGCAG | [60] |
| R: CCGTTCCCCTTGGTGAATCT | ||
| Htr3a | F: TGACCGCCTGTAGCCTTGAC | [61] |
| R: TCCCACTCGCCCTGATTTATG | ||
| Htr4 | F: AGTTCCAACGAGGGTTTCAGG | [62] |
| R: CAGCAGGTTGCCCAAGATG | ||
| Maoa | F: GGAGAAGCCCAGTATCACAGG | [63] |
| R: GAACCAAGACATTAATTTTGTATTCTGAC | ||
| Gapdh | F: CAATGTGTCCGTCGTGGATCT | [64] |
| R: GTCCTCAGTGTAGCCCAAGATG |
F forward primer, R reverse primer
Histological Analysis
The paraffin-embedded distal colon and ileum tissue blocks were sectioned into 5-μm-thick slices and mounted on poly(lysine)-coated slides. After deparaffinization and rehydration, tissue sections were further subjected to alcian blue staining, and the expression levels of MUC2 and 5-HT were determined by immunohistochemical analysis. For alcian blue staining analysis, distal colon sections were rinsed with 3% acetic acid for 3 min and then incubated with 1% alcian blue solution (pH 2.5) for 15 min. After running tap water for 5 min, the sections were subjected to neutral red staining for 1 min. Slides were mounted and visualized under a microscope.
For immunohistochemical analysis, the expression levels of MUC2 and 5-HT in the distal colon and ileum were detected. The paraffin sections were deparaffinized, blocked with 3% hydrogen peroxide for 10 min, and subjected to antigen retrieval with microwaves in a 0.01 M citrate buffer for 15 min. The slides were then washed twice with PBS and incubated with MUC2 (1:100; ab76774, Abcam, UK) or 5-HT (1:1000; #20,080, Acris, Herford, Germany) antibodies. This was followed by incubation with the polymer conjugated peroxidase for 30 min using a polymer detection system (Zymed Laboratories, San Francisco, CA, USA). Finally, the color was developed using 3, 3-diaminobenzidine (Sigma, St. Louis, MO, USA). The slides were counterstained with Gill’s hematoxylin (Sigma-Aldrich, St. Louis, MO, USA), then dehydrated, and mounted prior to microscopic reading.
Images were observed and photographed using a microscope equipped with a digital image system. Quantitative analyses were performed using ImageJ software, for alcian blue- and MUC2- positive area, or by counting 5-HT-positive cells under five different fields to calculate the mean ± standard error of means (SEM) per filter.
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Prism, version 7, La Jolla, CA, USA) and represented as means ± SEM. The changes in the fecal parameters over time between two groups (vehicle control and probiotic groups) were analyzed using two-way analysis of variance (ANOVA), mixed design. For multiple comparisons, one-way ANOVA with Tukey’s post hoc test was used. Statistical significance was set at *P < 0.05.
Results
Increased Fecal Output in Mice Treated with L. plantarum PS128
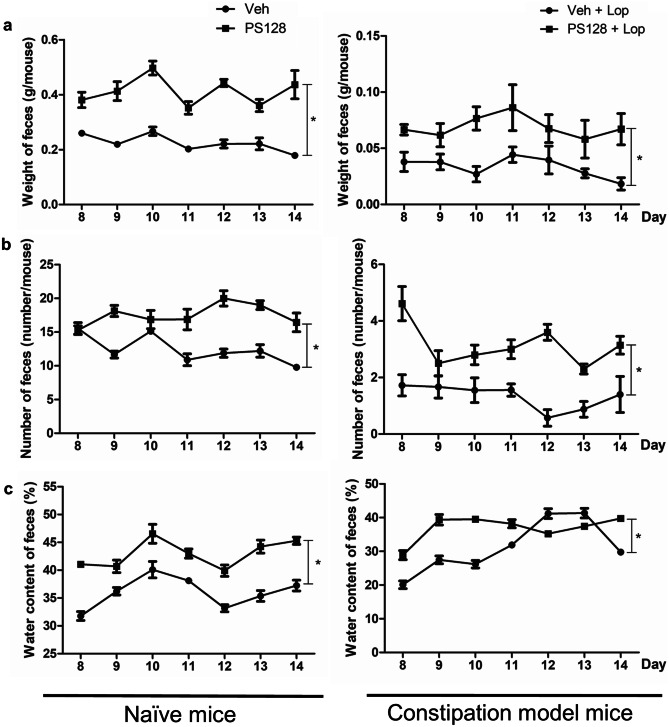
The effects of L. plantarum PS128 on fecal parameters were analyzed in naïve and Lop-treated mice. Compared with the vehicle control (Veh) group, PS128 ingestion resulted in increased fecal weight (Fig. 2a), number (Fig. 2b), and water content (Fig. 2c) in both mouse models, suggesting a laxative effect of L. plantarum PS128.
Fig. 2.
Effects of L. plantarum PS128 on fecal parameters in naïve and Lop-induced constipation model mice. Mice were orally administered PBS or 109 CFU of L. plantarum PS128 for 14 consecutive days. On experimental days 8 to 14, the wet weight (a), number (b), and water content (c) of feces were measured for 3 h. Data were expressed as mean ± SEM. Differences between groups were analyzed using two-way ANOVA, mixed design. *P < 0.05 compared with the indicated groups (n = 10 per group)
Histology of the Distal Colon
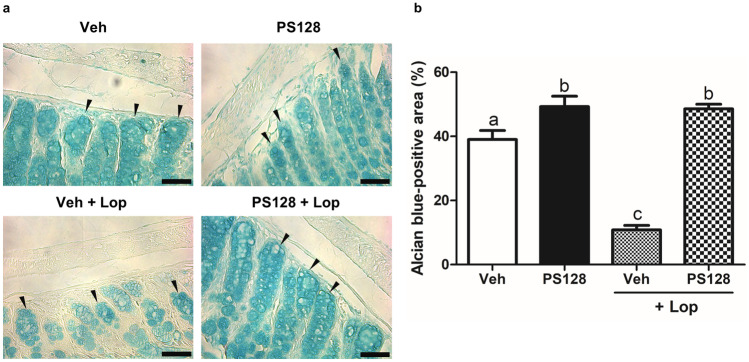
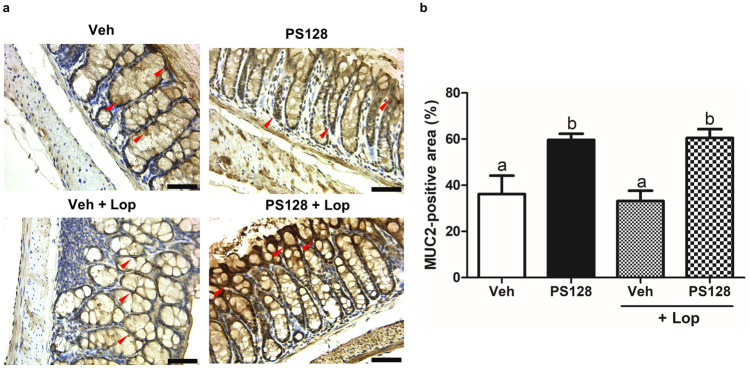
Alcian blue staining was performed to analyze mucin production in the distal colon (Fig. 3a). As shown in Fig. 3b, image quantification of the stained areas showed that, compared with the Veh group, PS128 significantly increased the amount of colonic mucin in naïve mice. In addition, treatment with Lop significantly reduced the amount of colonic mucin, which could be reversed by the ingestion of PS128. Furthermore, immunohistochemical detection and image quantification of MUC2, the major intestinal mucin, showed that PS128 significantly increased the level of MUC2 in both naïve and Lop-treated mice (Fig. 4). However, treatment with Lop did not affect the level of MUC2 in the distal colon.
Fig. 3.
Oral administration of L. plantarum PS128 increased the amount of colonic mucus in mice. a Representative images of Alcian blue stained areas of distal colonic sections (some are indicated by black arrows). Bars, 50 μm. b Quantification using the ImageJ software for each group (n = 6 per group). Data were expressed as mean ± SEM and analyzed by one-way ANOVA with Tukey’s post hoc test, and different superscript letters (a, b, c) differed significantly at P < 0.05
Fig. 4.
Oral administration of L. plantarum PS128 increased the amount of colonic MUC2 in mice. a The distal colons were analyzed for the expression of MUC2 by immunohistochemistry (some are indicated by red arrows). Bars, 50 μm. b Quantification using the ImageJ software for each group (n = 6 per group). Data were expressed as mean ± SEM and analyzed by one-way ANOVA with Tukey’s post hoc test, and different superscript letters (a, b) differed significantly at P < 0.05
L. plantarum PS128 Increases the Small Intestine Transit Time in Naïve Mice
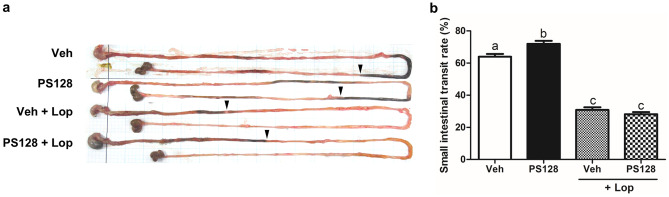
To investigate whether L. plantarum PS128 affects intestinal motility, a charcoal meal test was performed. As shown in Fig. 5a, a representative photograph shows the total length of the small intestine and the distance traveled by the charcoal meal in the intestine. Compared with the Veh group, PS128 significantly increased the small intestinal transit rate in naïve mice (Fig. 5b). In addition, treatment with Lop significantly reduced the small intestinal transit rate. However, this reduction could not be ameliorated by PS128. Since PS128 only increased the intestinal motility in naïve mice, but not in Lop-treated mice, we focused on two groups of naïve mice to further analyze how PS128 affects the intestinal motility.
Fig. 5.
Effect of L. plantarum PS128 on the small intestinal transit rate assessed by the charcoal meal test. a An actual image of the charcoal meal transit site in the small intestine. The stomach (left side), small intestine, and cecum were excised from mice, and their morphology was observed using a digital camera. The black arrows indicate the position of the charcoal meal. b Small intestinal transit rate for each group treated with charcoal meal (n = 6 per group). Data were expressed as mean ± SEM and analyzed by one-way ANOVA with Tukey’s post hoc test, and different superscript letters (a, b, c) differed significantly at P < 0.05
L. plantarum PS128 Modulates the Serotonin Signal Transduction in the Intestine
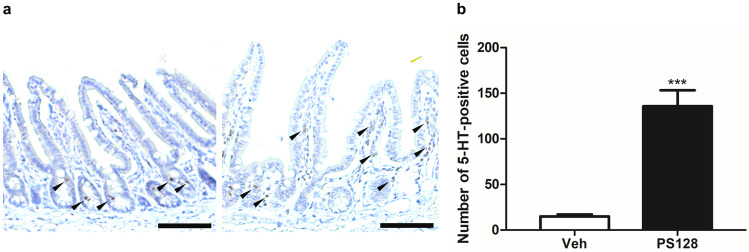
To further investigate the mechanism of action of L. plantarum PS128 on intestinal motility, qRT-PCR was performed to analyze the expression of genes related to serotonin signal transduction (Table 1). As shown in Table 2, compared with the Veh group, PS128 ingestion significantly increased Tph1 expression and decreased the expression levels of Chga, Slc6a4, and Htr4. In addition, no other significant differences in the expression levels of Tph2, Htr3a, and Maoa were observed. Furthermore, we examined 5-HT expression in the ileum sections by immunohistochemical staining (Fig. 6a). Compared with the Veh group, the number of 5-HT-positive cells was significantly increased in the PS128 group (Fig. 6b).
Table 2.
Gene expression of 5-HT related genes in the ileum of naïve ICR mice
| Gene | Vehicle | PS128 |
|---|---|---|
| Tph1 | 1.0 ± 0.40 | 3.2 ± 1.18*** |
| Tph2 | 1.0 ± 0.67 | 0.8 ± 0.87 |
| Chga | 1.0 ± 0.53 | 0.7 ± 0.31* |
| Slc6a4 | 1.0 ± 0.46 | 0.5 ± 0.15*** |
| Htr3a | 1.0 ± 0.61 | 1.0 ± 0.70 |
| Htr4 | 1.0 ± 0.93 | 0.2 ± 0.17*** |
| Maoa | 1.0 ± 0.44 | 1.2 ± 0.29 |
Gene expression levels of Tph1, Tph2, Chga, Slc6a4, Htr3a, Htr4, and Maoa were quantified by real-time PCR relative to the expression of Gapdh. Values were expressed as mean ± SEM and analyzed using an unpaired t test
*P < 0.05; **P < 0.01; ***P < 0.001 versus the vehicle control group
Fig. 6.
Oral administration of L. plantarum PS128 increased the number of 5-HT-positive cells in the ileum of naïve mice. a Representative images showing 5-HT-positive cells in the ileum tissues from mice. The 5-HT-positive cells were indicated by black arrows. Bars, 5 μm. b Numbers of 5-HT-positive cells per area in the ileum tissues of mice (n = 4 per group). Data were expressed as mean ± SEM and analyzed using unpaired t test. ***P < 0.001 compared with the vehicle control groups
Discussion
Emerging evidence has demonstrated that intestinal homeostasis affects CNS-related functions through the gut–brain axis in a bidirectional manner [23]. Patients with psychiatric and neurological disorders, including ASD [24], major depressive disorder [25], PD [26], and Alzheimer’s disease [27], are commonly reported to have GI symptoms and imbalanced gut microbiota. Moreover, psychological stress leads to several GI symptoms and plays a crucial role in the development of irritable bowel syndrome (IBS) [28]. In contrast, dysregulated GI mucus secretion and increased intestinal permeability may lead to systemic inflammation and impairment of the blood–brain barrier (BBB), thus negatively influencing CNS-related functions [29]. Therefore, maintaining intestinal homeostasis and improving gut health may help to alleviate CNS disorders. Probiotic food supplementation can reduce stress-induced GI symptoms in volunteers [30], decrease depression scores, alter brain activity in patients with IBS [31], and improve symptoms associated with ASD and PD [8]. These probiotics that affect CNS-related functions and behaviors are classified as psychobiotics, including L. plantarum PS128, Bifidobacterium longum 1714 [32], B. longum NCC3001 [31], Lactobacillus rhamnosus JB-1 [33], Lactobacillus helveticus R0052, and B. longum R0175 [30]. Although these psychobiotics have been suggested to regulate CNS-related functions through the gut-brain axis, their regulation in the intestine remains largely unknown.
In this study, we used two mouse models, naïve and Lop-induced constipation mice, to investigate the effect of L. plantarum PS128 on intestinal homeostasis. Daily administration of L. plantarum PS128 appeared to increase the fecal output, colonic mucin production, and intestinal motility in naïve mice (Figs. 2, 3, 4, and 5), suggesting that L. plantarum PS128 can be used as a laxative food supplement. A recent clinical study has also shown that daily administration of L. plantarum PS128 for eight weeks significantly improved self-perceived stress and GI symptoms in highly stressed information technology specialists [17], which supported the results of this study. However, in mice with Lop-induced constipation, the laxative effect of L. plantarum PS128 was relatively faint (Fig. 2). Lop has been widely used in animal experiments to reduce intestinal motility and colonic water secretion, thus prolonging the evacuation time of feces [34]. Lop treatment in the Sprague–Dawley rats also resulted in a thinner mucus layer at the fecal surface [35]. In this study, we found that treatment with Lop significantly reduced the level of colonic mucins stained with alcian blue (Fig. 3), which is a central feature of this constipation model [20, 36]. Several studies have also shown that treatment with Lop reduced the expression of colonic MUC2 at mRNA [37, 38] and protein levels [39]. However, the effect of Lop treatment on the expression of MUC2 cannot be observed in this study (Fig. 4). Possible reasons include that, first, alcian blue and MUC2 antibodies detect different targets, which may lead to different results. The staining of alcian blue is restricted mainly to acidic carbohydrates but not the protein core of mucin [40]. Second, although MUC2 is the major colonic mucin, the expression of other mucins, such as MUC3 and MUC6 [41], may affect the results. Third, there are some differences in the response of ICR mice derived from different sources to Lop treatment [36]. It is still unclear how Lop treatment may affect the expression and glycosylation of different colonic mucins.
As shown in Fig. 5, Lop significantly reduced the small intestinal motility; however, this reduction could not be reversed by the administration of L. plantarum PS128. These findings indicate that L. plantarum PS128 may be ineffective in patients with serious or chronic constipation. Chronic constipation is known to have many possible causes, including blockage of the colon or rectum, dysfunction of the ENS, difficulty in moving the pelvic muscle involved in elimination, and imbalance of hormones in the body. We assume that L. plantarum PS128 is only effective for specific subtypes of constipation, and this assumption requires further investigation. On the other hand, pharmacological therapy for constipation includes the use of bulking agents, osmotic agents, stool softeners, stimulant laxatives, lubricants, etc. [42], while non-pharmacological therapy includes increased intake of dietary fibers and water, increased physical activity, and supplementation of probiotics [43, 44]. Fermented milk containing Lactobacillus casei Shirota increases bowel movement frequency and stool consistency in patients with constipation [45, 46]. In addition, daily consumption of L. helveticus for one week alleviates constipation-related symptoms and reduces both fecal pH and intestinal transit time in patients with constipation-predominant IBS [47]. The efficacy of probiotic products is suggested to be both strain-specific and disease-specific [48], which should be proven by further evidence-based research. Clinically, whether L. plantarum PS128 is effective in constipation and PD- and ASD-complicated gut dysfunction remains to be studied.
GI epithelial cells sense luminal signals from the ingested food and microbiota, and then translate and deliver signals to exert local and systematic effects. One of the important signals is 5-HT, which originates from the enterochromaffin (EC) cells of the GI tract, which act as the major source of peripheral 5-HT by secreting 95% of total 5-HT in the body [49]. Although peripheral 5-HT does not cross the BBB [50], gut-derived 5-HT can be carried and released by circulating platelets, remain free in the serum, and interact with the CNS through the ENS, thus affecting various biological phenomena, including gut motility and secretion, bowel inflammation, bone development, and platelet aggregation [51]. In this study, we found that L. plantarum PS128 modulated the expression levels of genes related to serotonin signaling in the intestine (Table 2). TPH1 is a key enzyme for 5-HT biosynthesis and Chromogranin A (ChgA) is colocalized with 5-HT in EC cell storage granules [52, 53]. The upregulation of Tph1 and downregulation of ChgA suggested that there is an increase in the biosynthesis and storage of 5-HT in EC cells. Moreover, immunohistochemical analysis showed that L. plantarum PS128 increased the number of 5-HT-containing cells in the ileum (Fig. 6). These results support the previous finding that L. plantarum PS128 ingestion can increase 5-HT levels in the ileum, colon, and serum of rats [13]. Besides, a previous study has shown that heat-killed L. casei 327 promotes colonic 5-HT biosynthesis and GI motility in mice [54], which is similar to the effects exerted by L. plantarum PS128. However, heat-killed L. plantarum PS128 is ineffective in mice [11]. Thus, we suggest that L. plantarum PS128 regulates intestinal 5-HT biosynthesis and motility through mechanisms different from those of heat-killed L. casei 327, which may include bacterial secretory molecules, heat-labile constituents, and specific metabolic activities. In addition, L. plantarum PS128 downregulates the expression of Slc6a4 and Htr4 in the ileum (Table 2), which suggested that 5-HT reuptake would be reduced and may compromise 5-HT4 receptor signaling. Correlated expressions of Slc6a4 and Htr4 in the mouse colon have been previously reported, which is relevant in the pathogenesis of visceral hypersensitivity by influencing local 5-HT abundance/availability [55]. Moreover, a previous study also reported that L. plantarum PS128 alleviated visceral hypersensitivity induced by subcutaneous injection of 5-hydroxytryptophan (5-HTP), a precursor of 5-HT, in rats [56]. Therefore, modulation of the peripheral serotonin signal transduction may be a crucial action mechanism of L. plantarum PS128, and this requires further investigation.
This study had several limitations. First, the psychobiotic effects of L. plantarum PS128 on the two mouse models were not evaluated; thus, PS128 regulation in the intestine cannot be directly correlated to the host behavior and previous findings in the CNS. Second, although the qRT-PCR results showed that PS128 modulated the expression of 5-HT-related genes in the intestine, the expression level of the protein was not analyzed. Moreover, the mechanism by which PS128 modulates the intestinal serotonin signaling remains largely uncharacterized. Third, the specific role of gut microbiota in this process remains unknown. Future studies of L. plantarum PS128 action mechanisms should focus on both the gut and the brain, and experiments using vagotomy or 5-HT agonists/antagonists should be conducted to obtain a more comprehensive understanding of these mechanisms.
Conclusions
In this study, we found that daily administration of the psychobiotic strain L. plantarum PS128 could increase the fecal output, colonic mucin production, and small intestinal motility in mice. Moreover, L. plantarum PS128 appeared to promote serotonin signal transduction in the intestine, which might indirectly affect the CNS-related functions and host behaviors through the gut–brain axis.
Acknowledgements
The authors would like to appreciate Ms. Wu-Shun Peng, a research assistant from the Institute of Biochemistry and Molecular Biology, National Yang Ming Chiao Tung University, Taipei, Taiwan, for providing technical assistance in this study.
Author Contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Chin-Lin Huang, Min-Yu Chang, and Shih-Hsuan Cheng. The first draft of the manuscript was written by Chin-Lin Huang, Chien-Chen Wu, and Chih-Ming Chen, and all authors commented on previous versions of the manuscript. The study was supervised by Ching-Ting Lin and Ying-Chieh Tsai. All authors read and approved the final manuscript.
Funding
This study was supported by a grant (RD10601) from the Bened Biomedical R&D Project.
Availability of Data and Material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics Approval
The use of animals and the procedures for animal handling and treatments were approved by the Institutional Animal Use and Care Committee (IACUC 1,060,606) at the National Yang Ming Chiao Tung University in Taiwan.
Conflict of Interest
Chih-Ming Chen, Chien-Chen Wu, Chin-Lin Huang, Min-Yu Chang, and Shih-Hsuan Cheng are employees of Bened Biomedical Co., Ltd. Ying-Chieh Tsai owns stock from Bened Biomedical Co., Ltd. The views presented in this article reflect those of the authors and not necessarily those of the funder. Ching-Ting Lin declares no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chih-Ming Chen and Chien-Chen Wu contributed equally to this study.
Contributor Information
Ching-Ting Lin, Email: gingting@mail.cmu.edu.tw.
Ying-Chieh Tsai, Email: tsaiyc@ym.edu.tw.
References
- 1.FAO, WHO, Guidelines for the evaluation of probiotics in food. Rome: FAO; 2002. [Google Scholar]
- 2.Dimidi E, Christodoulides S, Scott SM, Whelan K. Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv Nutr. 2017;8(3):484–494. doi: 10.3945/an.116.014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin L, Deng L, Wu W, Wang Z, Shao W, Liu J. Systematic review and meta-analysis of the effect of probiotic supplementation on functional constipation in children. Medicine (Baltimore) 2018;97(39):e12174. doi: 10.1097/MD.0000000000012174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, Velez E, Perdigon G. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab. 2019;74(2):115–124. doi: 10.1159/000496426. [DOI] [PubMed] [Google Scholar]
- 5.Green M, Arora K, Prakash S (2020) Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int J Mol Sci 21 (8). 10.3390/ijms21082890 [DOI] [PMC free article] [PubMed]
- 6.Richards P, Thornberry NA, Pinto S (2021) The gut-brain axis: identifying new therapeutic approaches for type 2 diabetes, obesity, and related disorders. Mol Metab:101175. 10.1016/j.molmet.2021.101175 [DOI] [PMC free article] [PubMed]
- 7.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Cheng LH, Liu YW, Wu CC, Wang S, Tsai YC. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J Food Drug Anal. 2019;27(3):632–648. doi: 10.1016/j.jfda.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11 (8):506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed]
- 10.Liu YW, Liu WH, Wu CC, Juan YC, Wu YC, Tsai HP, Wang S, Tsai YC. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naive adult mice. Brain Res. 2016;1631:1–12. doi: 10.1016/j.brainres.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Liu WH, Chuang HL, Huang YT, Wu CC, Chou GT, Wang S, Tsai YC. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav Brain Res. 2016;298(Pt B):202–209. doi: 10.1016/j.bbr.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Liu YW, Wang YP, Yen HF, Liu PY, Tzeng WJ, Tsai CF, Lin HC, Lee FY, Jeng OJ, Lu CL, Tsai YC. Lactobacillus plantarum PS128 ameliorated visceral hypersensitivity in rats through the gut-brain axis. Probiotics Antimicrob Proteins. 2019 doi: 10.1007/s12602-019-09595-w. [DOI] [PubMed] [Google Scholar]
- 13.Liao JF, Cheng YF, Li SW, Lee WT, Hsu CC, Wu CC, Jeng OJ, Wang S, Tsai YC. Lactobacillus plantarum PS128 ameliorates 2,5-dimethoxy-4-iodoamphetamine-induced tic-like behaviors via its influences on the microbiota-gut-brain-axis. Brain Res Bull. 2019;153:59–73. doi: 10.1016/j.brainresbull.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Liao JF, Cheng YF, You ST, Kuo WC, Huang CW, Chiou JJ, Hsu CC, Hsieh-Li HM, Wang S, Tsai YC. Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse models of Parkinson's disease. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 15.Liu YW, Liong MT, Chung YE, Huang HY, Peng WS, Cheng YF, Lin YS, Wu YY, Tsai YC (2019) Effects of Lactobacillus plantarum PS128 on children with autism spectrum disorder in Taiwan: a randomized, double-blind, placebo-controlled trial. Nutrients 11 (4). 10.3390/nu11040820 [DOI] [PMC free article] [PubMed]
- 16.Huang WC, Wei CC, Huang CC, Chen WL, Huang HY (2019) The Beneficial Effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients 11 (2). 10.3390/nu11020353 [DOI] [PMC free article] [PubMed]
- 17.Wu SI, Wu CC, Tsai PJ, Cheng LH, Hsu CC, Shan IK, Chan PY, Lin TW, Ko CJ, Chen WL, Tsai YC. Psychobiotic supplementation of PS128TM improves stress, anxiety, and insomnia in highly stressed information technology specialists: a pilot study. Front Nutr. 2021 doi: 10.3389/fnut.2021.614105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Colle A, Israelyan N, Gross Margolis K. Novel aspects of enteric serotonergic signaling in health and brain-gut disease. Am J Physiol Gastrointest Liver Physiol. 2020;318(1):G130–G143. doi: 10.1152/ajpgi.00173.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J, Wittouck S, Salvetti E, Franz C, Harris HMB, Mattarelli P, O'Toole PW, Pot B, Vandamme P, Walter J, Watanabe K, Wuyts S, Felis GE, Ganzle MG, Lebeer S. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 20.Zhou M, Jia P, Chen J, Xiu A, Zhao Y, Zhan Y, Chen P, Zhang J. Laxative effects of Salecan on normal and two models of experimental constipated mice. BMC Gastroenterol. 2013;13:52. doi: 10.1186/1471-230X-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Zhou X, Chen C, Deng Q, Huang Q, Yang J, Yang N, Huang F. Laxative effects of partially defatted flaxseed meal on normal and experimental constipated mice. BMC Complement Altern Med. 2012;12:14. doi: 10.1186/1472-6882-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin A, Shih CT, Huang CL, Wu CC, Lin CT, Tsai YC (2019) Hypnotic effects of Lactobacillus fermentum PS150(TM) on pentobarbital-induced sleep in mice. Nutrients 11 (10). 10.3390/nu11102409 [DOI] [PMC free article] [PubMed]
- 23.Zhu X, Han Y, Du J, Liu R, Jin K, Yi W. Microbiota-gut-brain axis and the central nervous system. Oncotarget. 2017;8(32):53829–53838. doi: 10.18632/oncotarget.17754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasilewska J, Klukowski M. Gastrointestinal symptoms and autism spectrum disorder: links and risks - a possible new overlap syndrome. Pediatric Health Med Ther. 2015;6:153–166. doi: 10.2147/PHMT.S85717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanada K, Nakajima S, Kurokawa S, Barcelo-Soler A, Ikuse D, Hirata A, Yoshizawa A, Tomizawa Y, Salas-Valero M, Noda Y, Mimura M, Iwanami A, Kishimoto T. Gut microbiota and major depressive disorder: a systematic review and meta-analysis. J Affect Disord. 2020;266:1–13. doi: 10.1016/j.jad.2020.01.102. [DOI] [PubMed] [Google Scholar]
- 26.Scheperjans F, Derkinderen P, Borghammer P. The gut and Parkinson's disease: hype or hope? J Parkinsons Dis. 2018;8(s1):S31–S39. doi: 10.3233/JPD-181477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chok KC, Ng KY, Koh RY, Chye SM. Role of the gut microbiome in Alzheimer's disease. Rev Neurosci. 2021 doi: 10.1515/revneuro-2020-0122. [DOI] [PubMed] [Google Scholar]
- 28.Qin HY, Cheng CW, Tang XD, Bian ZX. Impact of psychological stress on irritable bowel syndrome. World J Gastroenterol. 2014;20(39):14126–14131. doi: 10.3748/wjg.v20.i39.14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herath M, Hosie S, Bornstein JC, Franks AE, Hill-Yardin EL. The role of the gastrointestinal mucus system in intestinal homeostasis: implications for neurological disorders. Front Cell Infect Microbiol. 2020;10:248. doi: 10.3389/fcimb.2020.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diop L, Guillou S, Durand H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial. Nutr Res. 2008;28(1):1–5. doi: 10.1016/j.nutres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O, Welsh C, Rieder A, Traynor J, Gregory C, De Palma G, Pigrau M, Ford AC, Macri J, Berger B, Bergonzelli G, Surette MG, Collins SM, Moayyedi P, Bercik P (2017) Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153 (2):448–459 e448. 10.1053/j.gastro.2017.05.003 [DOI] [PubMed]
- 32.Allen AP, Hutch W, Borre YE, Kennedy PJ, Temko A, Boylan G, Murphy E, Cryan JF, Dinan TG, Clarke G. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016;6(11):e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Steinhausen K, Bharwani A, Mian MF, Neufeld KAM, Forsythe P (2020) Increased persistence of avoidance behaviour and social deficits with L. rhamnosus JB-1 or selective serotonin reuptake inhibitor treatment following social defeat. Sci Rep 10 (1):13485. 10.1038/s41598-020-69968-y [DOI] [PMC free article] [PubMed]
- 34.Yin J, Liang Y, Wang D, Yan Z, Yin H, Wu D, Su Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int J Mol Med. 2018;41(2):649–658. doi: 10.3892/ijmm.2017.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Decreased colonic mucus in rats with loperamide-induced constipation. Comp Biochem Physiol A: Mol Integr Physiol. 2000;126(2):203–212. doi: 10.1016/s1095-6433(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 36.Kim JE, Yun WB, Sung JE, Lee HA, Choi JY, Choi YS, Jung YS, Kim KS, Hwang DY. Characterization the response of Korl:ICR mice to loperamide induced constipation. Lab Anim Res. 2016;32(4):231–240. doi: 10.5625/lar.2016.32.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JE, Yun WB, Lee ML, Choi JY, Park JJ, Kim HR, Song BR, Hong JT, Song HK, Hwang DY. Synergic laxative effects of an herbal mixture of Liriope platyphylla, Glycyrrhiza uralensis, and Cinnamomum cassia in loperamide-induced constipation of Sprague Dawley rats. J Med Food. 2019;22(3):294–304. doi: 10.1089/jmf.2018.4234. [DOI] [PubMed] [Google Scholar]
- 38.Kim JE, Park JW, Kang MJ, Choi HJ, Bae SJ, Choi YS, Lee YJ, Lee HS, Hong JT, Hwang DY (2019) Anti-inflammatory response and muscarinic cholinergic regulation during the laxative effect of Asparagus cochinchinensis in loperamide-induced constipation of SD rats. Int J Mol Sci 20 (4). 10.3390/ijms20040946 [DOI] [PMC free article] [PubMed]
- 39.Chen Z, Lin S, Jiang Y, Liu L, Jiang J, Chen S, Tong Y, Wang P. Effects of bread yeast cell wall beta-glucans on mice with loperamide-induced constipation. J Med Food. 2019;22(10):1009–1021. doi: 10.1089/jmf.2019.4407. [DOI] [PubMed] [Google Scholar]
- 40.Mowry EASRW. Alcian Blue. J Histotechnol. 1983;6(2):65–69. doi: 10.1179/his.1983.6.2.65. [DOI] [Google Scholar]
- 41.Grondin JA, Kwon YH, Far PM, Haq S, Khan WI. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front Immunol. 2020;11:2054. doi: 10.3389/fimmu.2020.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basilisco G, Coletta M. Chronic constipation: a critical review. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2013;45(11):886–893. doi: 10.1016/j.dld.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Banares F. Nutritional care of the patient with constipation. Best Pract Res Clin Gastroenterol. 2006;20(3):575–587. doi: 10.1016/j.bpg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Prasad VG, Abraham P. Management of chronic constipation in patients with diabetes mellitus. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology. 2017;36(1):11–22. doi: 10.1007/s12664-016-0724-2. [DOI] [PubMed] [Google Scholar]
- 45.Gill HS, Guarner F. Probiotics and human health: a clinical perspective. Postgrad Med J. 2004;80(947):516–526. doi: 10.1136/pgmj.2003.008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen S, Ou Y, Zhao L, Li Y, Qiao Z, Hao Y, Ren F. Differential effects of Lactobacillus casei strain shirota on patients with constipation regarding stool consistency in China. J Neurogastroenterol Motil. 2019;25(1):148–158. doi: 10.5056/jnm17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bahrudin MF, Abdul Rani R, Tamil AM, Mokhtar NM, Raja Ali RA. Effectiveness of sterilized symbiotic drink containing Lactobacillus helveticus comparable to probiotic alone in patients with constipation-predominant irritable bowel syndrome. Dig Dis Sci. 2020;65(2):541–549. doi: 10.1007/s10620-019-05695-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McFarland LV, Evans CT, Goldstein EJC. Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med (Lausanne) 2018;5:124. doi: 10.3389/fmed.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Mann JJ, McBride PA, Brown RP, Linnoila M, Leon AC, DeMeo M, Mieczkowski T, Myers JE, Stanley M. Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients. Arch Gen Psychiatry. 1992;49(6):442–446. doi: 10.1001/archpsyc.1992.01820060022003. [DOI] [PubMed] [Google Scholar]
- 51.Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cubeddu LX, O'Connor DT, Parmer RJ. Plasma chromogranin A: a marker of serotonin release and of emesis associated with cisplatin chemotherapy. J Clin Oncol. 1995;13(3):681–687. doi: 10.1200/JCO.1995.13.3.681. [DOI] [PubMed] [Google Scholar]
- 53.Chen X, Meng S, Li S, Zhang L, Wu L, Zhu H, Zhang Y. Role of 5-hydroxytryptamine and intestinal flora on depressive-like behavior induced by lead exposure in rats. Biomed Res Int. 2021;2021:5516604. doi: 10.1155/2021/5516604. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Hara T, Mihara T, Ishibashi M, Kumagai T, Joh T. Heat-killed Lactobacillus casei subsp. casei 327 promotes colonic serotonin synthesis in mice. Journal of Functional Foods. 2018;47:585–589. doi: 10.1016/j.jff.2018.05.050. [DOI] [Google Scholar]
- 55.Reigstad CS, Linden DR, Szurszewski JH, Sonnenburg JL, Farrugia G, Kashyap PC. Correlated gene expression encoding serotonin (5-HT) receptor 4 and 5-HT transporter in proximal colonic segments of mice across different colonization states and sexes. Neurogastroenterol Motil. 2016;28(9):1443–1448. doi: 10.1111/nmo.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu YW, Wang YP, Yen HF, Liu PY, Tzeng WJ, Tsai CF, Lin HC, Lee FY, Jeng OJ, Lu CL, Tsai YC. Lactobacillus plantarum PS128 ameliorated visceral hypersensitivity in rats through the gut-brain axis. Probiotics Antimicrob Proteins. 2020;12(3):980–993. doi: 10.1007/s12602-019-09595-w. [DOI] [PubMed] [Google Scholar]
- 57.Keating C, Nocchi L, Yu Y, Donovan J, Grundy D. Ageing and gastrointestinal sensory function: altered colonic mechanosensory and chemosensory function in the aged mouse. J Physiol. 2016;594(16):4549–4564. doi: 10.1113/JP271403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu YJ, Meng FT, Wu L, Zhou JN. Serotoninergic and melatoninergic systems are expressed in mouse embryonic fibroblasts NIH3T3 cells. Neuro Endocrinol Lett. 2013;34(3):236–240. [PubMed] [Google Scholar]
- 59.Bell KN, Shroyer NF. Krupple-like factor 5 is required for proper maintenance of adult intestinal crypt cellular proliferation. Dig Dis Sci. 2015;60(1):86–100. doi: 10.1007/s10620-014-3307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sada A, Jacob F, Leung E, Wang S, White BS, Shalloway D, Tumbar T. Defining the cellular lineage hierarchy in the interfollicular epidermis of adult skin. Nat Cell Biol. 2016;18(6):619–631. doi: 10.1038/ncb3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hata T, Asano Y, Yoshihara K, Kimura-Todani T, Miyata N, Zhang XT, Takakura S, Aiba Y, Koga Y, Sudo N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS ONE. 2017;12(7):e0180745. doi: 10.1371/journal.pone.0180745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lund ML, Sorrentino G, Egerod KL, Kroone C, Mortensen B, Knop FK, Reimann F, Gribble FM, Drucker DJ, de Koning EJP, Schoonjans K, Backhed F, Schwartz TW, Petersen N. L-Cell Differentiation Is Induced by Bile Acids Through GPBAR1 and Paracrine GLP-1 and Serotonin Signaling. Diabetes. 2020;69(4):614–623. doi: 10.2337/db19-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee AK, Mojtahed-Jaberi M, Kyriakou T, Astarloa EA, Arno M, Marshall NJ, Brain SD, O'Dell SD. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition. 2010;26(4):411–422. doi: 10.1016/j.nut.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss U, Moller M, Husseini SA, Manderscheid C, Hausler J, Geisslinger G, Niederberger E (2020) Inhibition of HDAC enzymes contributes to differential expression of pro-inflammatory proteins in the TLR-4 signaling cascade. International journal of molecular sciences 21 (23). 10.3390/ijms21238943 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.