Abstract
For decades, self-monitoring of blood glucose (SMBG) has been considered a cornerstone of adequate diabetes management. Structured SMBG can follow different monitoring patterns, and it results in improved glycemic control, reduced hypoglycemia, and a better quality of life of people with diabetes. The technology, usability, and accuracy of SMBG systems have advanced markedly since their introduction a few decades ago. Current SMBG systems are small and easy to use, require small (capillary) blood sample volumes, and provide measurement results within seconds. In addition, devices are increasingly equipped with features such as connectivity to other devices and/or digital diaries and diabetes management tools. Although measurement quality can come close to or equal that of the glucose monitoring systems used by healthcare professionals, several available SMBG systems still do not meet internationally accepted accuracy standards, such as the International Organization for Standardization 15197 standard. Reports from China, India, and Brazil based on local experience suggest that in addition of the accuracy issues of SMBG systems, other obstacles also need to be overcome to optimize SMBG usage. Nonetheless, adequate usage of SMBG data is of high relevance for the management of people with type 2 diabetes mellitus.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-022-01254-8.
Keywords: Self-monitoring of blood glucose, Type 2 diabetes mellitus, Blood glucose monitoring systems, Accuracy, Diabetes management
Key Summary Points
| Self-monitoring of blood glucose (SMBG) can be a beneficial component of type 2 diabetes therapy. |
| SMBG systems have become more accurate over time and now provide a variety of useful features, such as digital diaries or connectivity. |
| Reports from China, India, and Brazil based on local experience indicate that access to adequate SMBG is often an issue. |
| On the way to achieving optimal diabetes therapy, out-of-pocket costs for the end-user have to be reduced, and self-management education and empowerment have to be improved. |
Current Situation and Outlook
More than half a billion adults (age > 20 years) and a further 1.2 million children and adolescents (age < 20 years) worldwide currently have type 1 diabetes mellitus (T1DM) or type 2 DM (T2DM) [1]. In 2021, 6.7 million people died either as a direct result of diabetes or due to diabetes complications associated with prevailing high blood glucose (BG) levels, including cardiovascular disease, chronic kidney disease, or tuberculosis [1].
Self-monitoring of blood glucose (SMBG) was established a few decades ago as a prerequisite for optimizing anti-diabetic therapy of people with diabetes (PwD) [2], especially for those who are dependent on insulin therapy (T1DM and T2DM) [3] or those using non-insulin glucose-lowering drugs that can induce hypoglycemia. The benefits of structured SMBG in terms of improved glycemic control and quality of life for people with T2DM have been shown repeatedly [4, 5]. However, there are also studies with conflicting data, leading to contrary conclusions, that have shown no or only a short-term benefit (≤ 6 months) of structured BG measurements for people with diabetes not on insulin therapy [6–8]. While these latter studies raised doubts about the effectiveness of structured SMBG, it is important to note that in these studies, the BG values were not used for treatment decisions, and the benefit in non-insulin-dependent people with T2DM was linked to treatment adherence. To optimally benefit from structured SMBG, the obtained BG values therefore have to be actively used by PwD or healthcare professionals (HCPs) for therapeutic decisions or therapy adjustments, such as changes in eating behavior, lifestyle and/or anti-diabetic therapy [9].
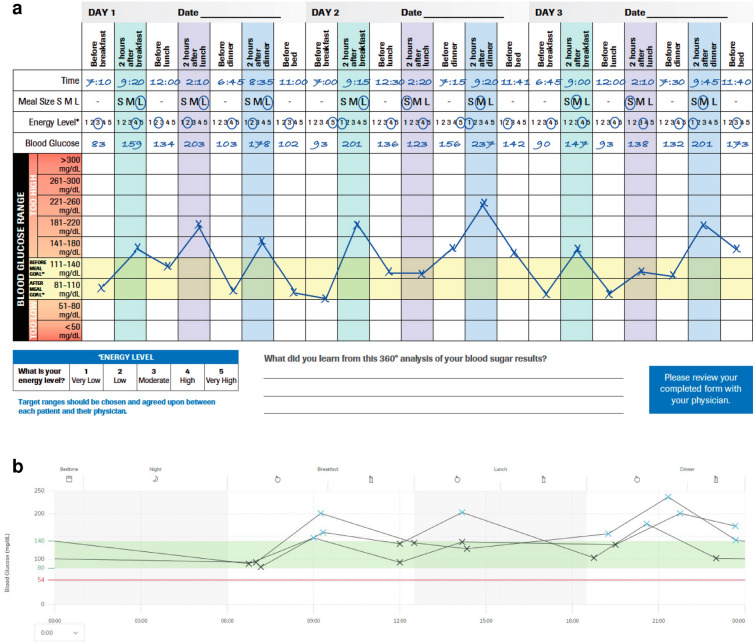
Structured SMBG may be a beneficial strategy if, for example, fasting or postprandial BG values are above a clinically defined glucose target and insulin titration steps are necessary, for which SMBG results are needed. Furthermore, the optimal point in time for the administration of non-insulin glucose-lowering agents, such as metformin or sodium-glucose cotransporter 2 inhibitors (SGLT2 inhibitors), as well as their resulting BG-lowering effect, has to be verified and, if necessary, adjusted by determining BG values. For non-insulin drugs that can induce hypoglycemia as a side effect, such as sulfonylureas, SMBG can be used to detect such glycemic excursions. Structured SMBG is necessary to allow for immediate treatment decisions while the use of glycated hemoglobin (HbA1c) as an estimator of long-term quality of glycemic control is insufficient in that regard [8]. The measurement pattern (frequency and timing of BG measurements) can be adapted based on the individual needs of PwD [10] (Table 1). For example, by measuring BG values before and after meals (pre- and postprandial measurements) and before and after exercise, respectively, PwD can get their individual “biofeedback” (Fig. 1a).
Table 1.
Structured glucose testing profiles used in the therapy of people with type 2 diabetes mellitus [10]
| Day | Breakfast | Lunch | Dinner | Bedtime | Description | |||
|---|---|---|---|---|---|---|---|---|
| Preprandial | Postprandial | Preprandial | Postprandial | Preprandial | Postprandial | |||
| Staggered profile | ||||||||
| Monday | X | X | Pre- and postprandial BG measurements for 1 meal per day or overnight (e.g. for characterization of occurring glucose values and patterns) | |||||
| Tuesday | X | X | ||||||
| Wednesday | X | X | ||||||
| Thursday | X | |||||||
| Friday | X | |||||||
| Saturday | ||||||||
| Sunday | ||||||||
| Day | Breakfast | Lunch | Dinner | Bedtime | Description | |||
|---|---|---|---|---|---|---|---|---|
| Preprandial | Postprandial | Preprandial | Postprandial | Preprandial | Postprandial | |||
| 7-point profile | ||||||||
| Monday | X | X | X | X | X | X | X | Pre- and postprandial BG measurements for 3 meals per day and bedtime BG on, for example, 3 consecutive days (e.g. for accurate assessment of the glucose profile) |
| Tuesday | X | X | X | X | X | X | X | |
| Wednesday | X | X | X | X | X | X | X | |
| Thursday | ||||||||
| Friday | ||||||||
| Saturday | ||||||||
| Sunday | ||||||||
| Day | Breakfast | Lunch | Dinner | Bedtime | Description | |||
|---|---|---|---|---|---|---|---|---|
| Preprandial | Postprandial | Preprandial | Postprandial | Preprandial | Postprandial | |||
| Paired testing | ||||||||
| Monday | X | X | Pre- and postprandial BG measurements for the same meal (breakfast, lunch, or dinner) on 7 consecutive days (e.g. for evaluation of postprandial glucose excursions or overnight) | |||||
| Tuesday | X | X | ||||||
| Wednesday | X | X | ||||||
| Thursday | X | X | ||||||
| Friday | X | X | ||||||
| Saturday | X | X | ||||||
| Sunday | X | X | ||||||
| Day | Breakfast | Lunch | Dinner | Bedtime | Description | |||
|---|---|---|---|---|---|---|---|---|
| Preprandial | Postprandial | Preprandial | Postprandial | Preprandial | Postprandial | |||
| Modified staggered profile | ||||||||
| Monday | X | X | X | X | Pre- and postprandial BG measurements for 1 meal on 2 days or overnight | |||
| Tuesday | X | X | X | |||||
| Wednesday | ||||||||
| Thursday | ||||||||
| Friday | ||||||||
| Saturday | ||||||||
| Sunday | ||||||||
BG Blood glucose
Fig. 1.
a Example of a structured glucose testing profile (7-point profile) using a system for self-monitoring of blood glucose (SMBG). SMBG results were entered manually into the ACCU-CHEK® 360° View Paper tool (Roche Diabetes Care GmbH, Mannheim, Germany), showing postprandial hyperglycemia. The forms for mmol/L and mg/dL can be downloaded from the Electronic Supplementary Material of this article. b Example of a glucose pattern analysis using an SMBG system. The figure shows the same glucose testing profile as in a, but data of the SMBG system were transferred to the RocheDiabetes Care Platform and visualized. RocheDiabetes, ACCU-CHEK and ACCU-CHEK 360° are trademarks of Roche
In recent years, the interest and focus of industry and academia were very much directed towards continuous glucose monitoring (CGM), i.e., glucose measurements in the interstitial fluid of the subcutaneous tissue [11]. Due to the improved analytical performance of recent generations of CGM systems, SMBG is no longer the only reliable option for glucose monitoring. Nowadays, CGM systems are used by many people with T1DM and also by a number of those with T2DM, at least in some parts of the world [11]. However, while the use of CGM systems has been shown to (further) improve therapy for people with T1DM [12, 13], mixed results have been reported on the use of CGM systems by people with T2DM [14, 15]. It should be kept in mind that SMBG systems tend to be more accurate than CGM systems [16] and that there can be differences between the glucose concentration in interstitial fluid and BG concentrations due to physiological processes [17]. In addition, cost-effectiveness in people with T2DM is still open to discussion [15], and in many countries the costs of SMBG systems are a reason of concern or even prohibitive.
In this article, we briefly described SMBG systems and their state of the art, as well as diabetes management strategies incorporating SMBG devices. We also present the reports of diabetologists from China, India, and Brazil who share their local experience with the treatment of people with T2DM and developments over the last few years.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
SMBG Systems
State of the Art
Since the first SMBG systems were introduced for at-home use of PwD a few decades ago [18], the technology of these systems has markedly advanced. Current SMBG systems are small and easy to use, require very small (capillary) blood samples, and provide the readings within seconds. In addition, their usability and, more importantly, their glucose measurement accuracy have greatly improved. Good SMBG systems nowadays provide a glucose measurement quality that is close to that of systems used by HCPs, such as laboratory analyzers. Such a high measurement accuracy as well as ease of use helps to avoid incorrect treatment decisions, such as insulin dosing errors [19, 20]. Establishing standards and guidance documents for the assessment of measurement accuracy of SMBG and assessments of user performance evaluations for SMBG systems have supported this development considerably [21, 22]. Many SMBG systems that are on the market today meet the accuracy requirements specified in such standards; however, it has been shown repeatedly that numerous systems do not do so when evaluated by independent research sites [23]. In such a study, performed by Klonoff et al. [24], only 33% of the evaluated systems met the accuracy requirements of the International Organization for Standardization (ISO) 15197:2013 standard, whereas in a study by Pleus et al. [25], 77% of the evaluated systems met this standard. It is therefore advisable to consider such evaluations, and not only associated direct costs, before selecting a particular SMBG system for PwD; however, it is also important to verify that the evaluations follow the standards mentioned above or a similar procedure [21, 22, 26].
It is important to acknowledge that in daily practice the measurement accuracy of SMBG systems may be affected by interfering exogenous compounds, such as acetaminophen, ascorbic acid, or L-DOPA, or by endogenous factors (e.g., increased uric acid or triglyceride levels or low or high hematocrit [HCT]). Ambient factors, such as oxygen pressure (depending on the height of the measurement location) or temperature, can also influence the accuracy of SMBG readings [27]. Relevant information on correct use of the device must be provided in the instructions. Quite often the relevance of factors such as HCT is not considered by the treating physician and, subsequently, by the PwD [28]. Low HCT due, for example, to iron deficiency or chronic diseases, as well as high HCT can markedly affect the measurement accuracy of SMBG systems [29, 30]. Many SMBG systems permit only a rather narrow specified HCT range of 30–55% [24], while HCT values are also quite prevalent outside of this range [30]; some SMBG systems may even miss their hematocrit claims [29].
Accuracy Evaluations
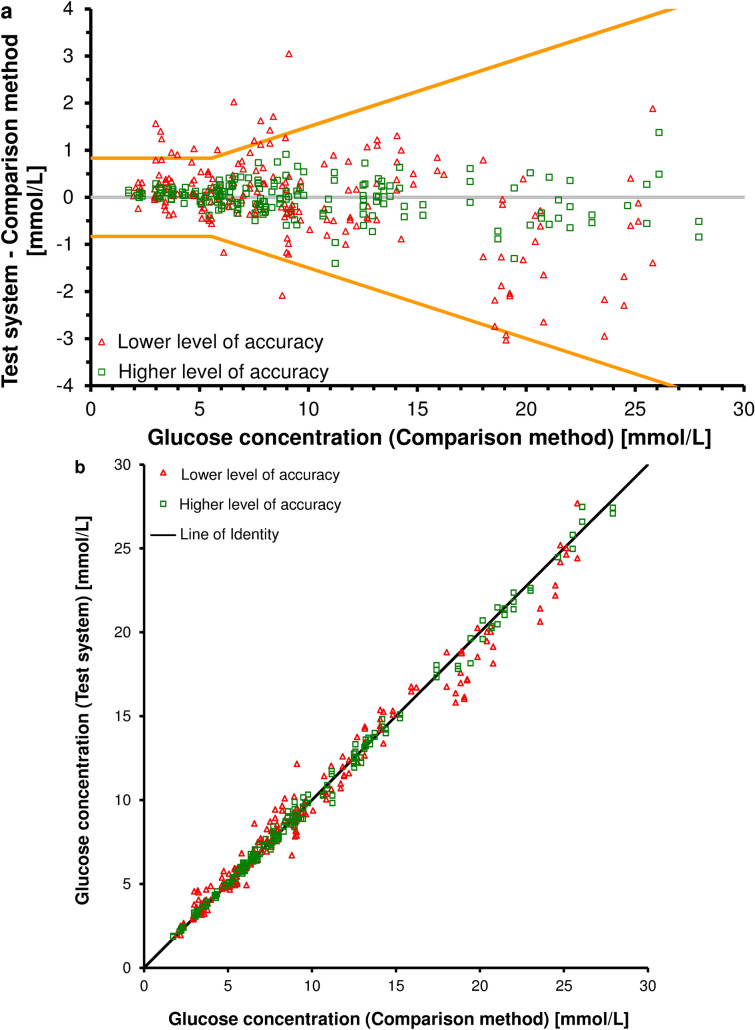
The accuracy of glucose measurements by different SMBG systems can be visualized graphically, such as, for example, in plots that display the difference between the measurement results obtained with a given SMBG system and those obtained with a laboratory system (difference plot) (Fig. 2a) or a direct comparison (scatter plot) (Fig. 2b). Such graphs allow a quick assessment of the analytical performance of different SMBG systems. In addition, these SMBG systems can be assessed in terms of whether and to what extent they actually meet the accuracy requirements set down, for example, in the ISO 15197 standard, in different glucose ranges [22]. Measurement values obtained with accurate SMBG systems are closer to the line indicating zero difference (Fig. 2a) or closer to the line of identity (Fig. 2b), respectively. However, difference plots have certain advantages in comparison to scatter plots [31]; for example, scatter plots show only a correlation between two variables, not their differences. The values obtained with different SMBG systems can be close to the line of identity and have a high correlation coefficient (r = ~ 1), but still have a relevant systematic difference (bias) that is not recognizable with this graphical presentation (Fig. 2b). In contrast, the performance of SMBG systems, especially at low glucose concentrations, can be better assessed with a difference plot (Fig. 2a). Additionally, a bias between the mean differences of SMBG systems can be evaluated and an agreement interval, i.e. a specific difference interval to the comparison method results in which 95% of measurements fall, can be calculated.
Fig. 2.
a Difference plot showing methods comparisons for two systems for SMBG with higher (green squares) and lower (red triangles) levels of accuracy. The orange lines reflect the accuracy requirements of the current ISO 15197:2015 standard [21], i.e., 95% of measurements need to fall within up to ± 0.8 mmol/L (± 15 mg/dL) or ± 15% from the comparison method (for values < 5.6 mmol/L [< 100 mg/dL] or ≥ 5.6 mmol/L [≥ 100 mg/dL]). b Scatter plot of SMBG systems with higher (green squares) and lower (red triangles) levels of accuracy
Diabetes Management and the Role of SMBG
Optimization of diabetes management requires people with T2DM to understand the relationship between lifestyle, food intake, therapeutic interventions, and current BG values [3], all factors that are typically included in diabetes self-management education. PwD experience a clear benefit by putting BG values into such a context and by adjusting their treatment to their glucose patterns. In order to gain valuable information for therapy optimization, PwD do not only have to be educated adequately and their skills re-evaluated on a regular basis, but SMBG measurements and the associated diabetes management have to be carried out frequently, consistently, and in a structured manner [32]. To simplify the cumbersome requirements of diabetes management, modern SMBG systems usually offer several options for data download and analysis.
Many of the current SMBG systems can be connected (wired and/or wirelessly; e.g., via Bluetooth) to the Cloud, a computer, or a smartphone, as well as other diabetes management devices or tools (e.g., apps). This connectivity allows data exchange, so that the BG data stored in a given SMBG system can be downloaded and/or exchanged between different devices. Subsequently, the data can be analyzed with specialized software tools or digital mobile health apps. The results of this analysis can be used for diabetes management decisions. Specialized software or apps can, for example, remind users of scheduled BG measurements or medication intake, keep a digital diary, mark high or low BG values, support the calculation of bolus insulin doses, or link BG values to the insulin doses delivered (Table 2). Reminders for scheduled BG measurements or medication intake can facilitate treatment adherence, which increases the benefit of SMBG in people with T2DM and subsequently counteracts existing clinical inertia [4, 5, 33, 34]. An easy-to-use digital diary can support people with T2DM in discussions on their diabetes therapy with their HCPs. For example, certain clinical events that do not coincide with daily lifestyle or diet, or even illness, can be recorded this way, thus enabling a comprehensive retrospective assessment. For those people with T2DM who use insulin, having the SMBG system connected to smart insulin pens allows a more complete picture of their therapeutic decisions in relation to the BG values measured with SMBG systems. In addition, bolus calculators integrated into medical products or software/apps can reduce wrong bolus calculations by the user and subsequently the risk of hypoglycemic events [35].
Table 2.
Features of current diabetes management tools (systems for self-monitoring of blood glucose, smart insulin pens, and software/apps)
| Feature | Description |
|---|---|
| Connectivity | Wired and/or wireless transfer of data (e.g., to other diabetes management tools or diabetes management apps) |
| Can grant access to telemedicine/HCP decision support, and parental supervision | |
| Can create additional insights and provide medical value through consented and secure sharing of data, such as via the Cloud, advanced algorithms, and artificial intelligence | |
| Provides an overview of, for example, the time of insulin delivery and the amount of delivered insulin and active insulin through the memory function of a connected smart insulin pen | |
| Digital diary | Quick input of specific incidents or supporting information (e.g., physical activity like sports or gardening or special events like birthday or holiday) |
| Graphical presentation and analysis of data | |
| Display of various parameters and metrics | |
| Pattern recognition | |
| Insulin dose calculator | Calculation and indication of the actual required bolus insulin (after consideration of the carbohydrate-insulin-ratio, activity and the active insulin) |
| Alerts/reminders | Alerts on low/high BG values, as well as missing BG measurements, insulin delivery, or medication intake |
HCP Healthcare professional
The obtained BG data can also be graphically analyzed and presented in reports, in the form of a pattern analysis (Fig. 1b). In the example shown in Fig. 1b, individual SMBG results are plotted along the X-axis on the basis of their timestamp during a 24-h (1-day) period, and consecutive SMBG results are connected by straight lines. Although this analysis only captures patterns obtained from SMBG measurements, and thus the patterns are highly dependent on the frequency and timing of the measurements, it allows clinicians and patients to make better informed therapeutic adjustments [36]. In addition, such reports may show other parameters and metrics, such as mean glucose concentration and the standard deviation (SD), and they support the identification of glucose patterns. Not only are users supported in terms of optimizing their own therapy decisions, these reports also assist HCPs in assessing the success or failure of the diabetes therapy, including factors such as adherence and subsequent possible therapy adjustments. An example of the latter is a change in medication, such as dosage change of anti-diabetic drugs, including insulin, or a complete change in therapy, such as a switch from a pure lifestyle intervention to a pharmacological intervention. SMBG thus supports a closer collaboration between PwD and HCPs and should not be seen as a standalone intervention, but rather as part of a structured feedback loop; for example, an integrated personalized diabetes management shows a considerable improvement in the clinical outcome of insulin-dependent people with T2DM [37]. Last but not least, the opportunity to upload BG data to the Cloud does not only support data sharing and interaction with HCPs; a more in-depth analysis of these data with approaches using advanced algorithms and artificial intelligence will create additional insights of medical value [38].
Local Aspects/Experience
Diabetes mellitus is a chronic disease affecting people around the world. However, new technologies and medications for diabetes therapy are often initially introduced in high-income countries, and PwD in other parts of the world often have to overcome hurdles regarding access to such diagnostic and therapeutic options. This subject is discussed in the following sections.
China
In 2021, the International Diabetes Federation (IDF) reported that 140 million adults in China were living with diabetes, accounting for over one-fourth of the entire world’s population of PwD [1]. Among all countries for which data are available, China has the highest number of adults with diabetes, and it is anticipated that it will remain in this position until at least 2045 [1]. Applying the diagnostic criteria for diabetes suggested by the American Diabetes Association (ADA), the data from two national representative epidemiological surveys in China showed that the prevalence of diabetes regardless of type went up from 10.9% in 2013 to 13.2% in 2020 [39, 40].
A guideline for SMBG was issued by the Chinese Diabetes Society in 2011 and updated 4 years later with the aim to enhance awareness and the standard of practice. SMBG is recognized as an essential component of disease management by helping PwD to better understand their disease, controlling the disease status, and supporting HCPs in their treatment adjustments. Specific monitoring regimen and frequency of SMBG are recommended for subgroups of people with T2DM, including people on insulin and non-insulin glucose-lowering medications [41], applying the principle of structured SMBG monitoring.
It is recommended that the accuracy and precision of SMBG systems be in line with the latest version of the ISO 15197 standard. The National Medical Product Administration agency (China) is working towards adapting the national standard to reflect the same acceptance criteria for accuracy and precision; for example, ≥ 95% of the SMBG system’s results shall fall within ± 0.8 mmol/L (± 15 mg/dL) of the reference measurement results at glucose concentrations < 5.6 mmol/L (< 100 mg/dL), and within ± 15% at glucose concentrations ≥ 5.6 mmol/L (≥ 100 mg/dL), with SD < 0.4 mmol/L (< 7.7 mg/dL) at glucose concentrations < 5.6 mmol/L (< 100 mg/dL) and coefficient of variance (CV) < 7.5% at glucose concentrations ≥ 5.6 mmol/L (≥ 100 mg/dL). In 2020, a draft version of the document was published with the aim to solicit public feedback. The new national standard is anticipated to be in effect in the near future and will match the accuracy requirements of the international ISO standard.
Adherence to SMBG in people with T2DM is poor in China, even in those who are on insulin therapy. A nationwide survey of diabetes education, self-management, and glycemic control in people with T2D using the Summary of Diabetes Self-Care Activities scale showed that the lowest score was for BG testing and the highest scores was for medication, followed by diet [42]. Different cross-sectional studies revealed that only a minority of the participants (< 25%) in these studies performed SMBG more than once per day or at least once a week, and that most (> 70%) did not adhere to the recommendations of the Chinese Diabetes Society or their physician’s instructions regarding frequency and timing of SMBG [43–45]. The reasons for non-compliance to physicians’ recommendations regarding SMBG include [45]: costs of test strips (37.9%); complexity (28.3%); lack of time (24.4%); considered to be unnecessary (15.9%); lack of understanding on how to adjust the insulin dose based on SMBG readings (6.6%).
Thus, the low implementation of structured SMBG is not only due to socio-economic factors, but also due to the lack of knowledge of PwD. For example, a study involving people with T2DM in China showed that their self-efficacy could be improved by increasing their self-management knowledge through training and education [46]. In addition, such an educational approach, in combination with the advanced features of SMBG systems, which have been mentioned in previous sections, may provide a platform for HCP—patient interactions, enabling remote consultation and helping PwD to achieve their glycemic targets in a faster and sustainable way [47].
India
India is home to the second largest number of PwD in the world. According to the tenth edition of the Diabetes Atlas by the IDF, there were an estimated 74 million PwD in India in 2021, and these numbers are set to increase to 92 million by 2030 and 124 million by 2045 [1]. The majority of PwD in India (> 95%) have T2DM; the remaining PwD have various forms of diabetes, including T1DM, gestational diabetes mellitus, fibrocalculous pancreatic diabetes, among other forms [48].
SMBG is recommended by various guidelines in India, including that of the Indian Council of Medical Research [49]. The respective guidelines on T2DM recommend that SMBG is indicated for the following patients/situations: all PwD, to achieve better control of diabetes; all PwD on insulin; those with brittle diabetes; those prone to ketosis/recurrent hypoglycemia; hypoglycemia unawareness; whenever tight control is indicated (e.g., pregnancy, acute illness, complications).
Further, the guidelines state that the frequency of SMBG should be individualized; for example, SMBG should occur at higher frequency during pregnancy of in other situations where tight glycemic control is indicated. While no specifications are given in the guidelines about the accuracy of SMBG systems, it is implied that the standard international guidelines regarding the accuracy of SMBG systems are followed.
According to a 2008 study carried out by the SMBG International Working Group only 0.2% of PwD in India performed SMBG [50], although this number has markedly increased to 20.8% of PwD in urban India and 14.5% of those in rural India according to more recent data (unpublished) reported in the India Diabetes Study carried out by the Indian Council of Medical Research. However, the frequency of SMBG remains low and grossly inadequate in India and lags behind that of other countries [51]. There are a number of possible explanations for this low use of SMBG in India. Until a couple of decades ago, the cost of SMBG systems was extremely high in India and amounted to about 1 month’s salary of the average Indian. Around 2004, with the introduction of low-cost SMBG systems, there was a surge in the purchase of SMBG systems in India; however, the cost of the test strips still remains high [52]. A lack of reimbursement for SMBG systems and test strips has also been a major deterrent to frequent SMBG testing in India [50, 51]. It is worth noting that 80% of PwD in India pay ‘out of pocket’ for their medications and devices [53]. Thus, the costs associated with SMBG systems (both direct and indirect) may explain partially their low use in India. Another possible explanation is that HCPs do not have the time to go through the laborious sheets of paper produced by PwD when they present their SMBG readings to them. If the HCP does not show sufficient interest in the SMBG records, the individual gets disheartened and often stops performing SMBG altogether [54]. Another reason why SMBG has not taken off in India is that the PwD are not empowered to use the data to make adjustments to their diet, physical activity, and medication, either insulin or oral drugs, based on the SMBG readings obtained [51]. Additional reasons for the low use of SMBG may be the pain of obtaining a drop of capillary blood for SMBG, although lancets are getting better all the time and this should no longer be a deterrent, and a lack of medical insurance for outpatient or domiciliary diabetes care.
The chronic nature of diabetes mellitus means that lifelong BG testing is needed. Initially, each PwD is enthusiastic about checking BG levels. However, motivation generally drops over time and, with the exception of a few motivated people, PwD tend to give up and stop testing. It should be noted that social media propagate fake news consisting of all kinds of myths and untruths about diabetes [55], thereby contribution to PwD not using SMBG devices. For example, messages are frequently sent to the mobile phones of people stating that high BG levels are a normal, physiological reaction and treatment is not needed. Some messages even state that there is no such thing as diabetes and that this disorder is a creation of physicians and the pharmaceutical industry to make money. There is also a large lobby against scientific and modern allopathic systems. Practitioners of other 'alternative' health systems (e.g., Ayurveda, Siddha, Unani, and Homeopathy) claim that they can ‘cure’ or reverse diabetes whereas allopathy only masks the ‘symptoms’ of diabetes [51]. Many people are lured to these practitioners by these false promises and claims, with the result often being deterioration of their diabetes control.
The solution to these problems is to increase awareness of the need for performing SMBG. Even more importantly, people should be empowered about what action should be taken with their SMBG readings. This would help propagate SMBG in developing countries like India.
Brazil
The prevalence of diabetes in Brazil varies greatly according to the region of the country, ranging from 5.2% to 13.5% [56]. Data from 2018 showed a mean diabetes prevalence of 7.1% in males and 8.1% in females [57]. It is important to note, as also shown in other parts of the world, that around 50% of PwD did not previously know they have the disease prior to getting the results from an oral glucose tolerance test [58].
An estimated 15.7 million people were living with diabetes in Brazil in 2021 [1]. In terms of number of PwD, this estimate positions the country in the sixth place globally and in first place in Latin America, with the vast majority of people living with T2DM. This number is expected to grow in the next 20–30 years. The economic impact of direct and indirect costs of diabetes for a developing country is huge, as studies have shown that the total cost of diabetes is around 15 billion Int$/year [59].
Brazil is a continental size country whose population is characterized by great disparities and inequities; consequently, it is expected that major differences exist regarding diabetes control. Indeed, a study involving 5750 PwD with data in the public healthcare system showed a mean duration of T2DM of 11.8 years, with only 26% of PwD having a HbA1c < 7% (53 mmol/mol) [60]. In contrast, 40% of PwD from private clinics in São Paulo had HbA1c < 7% (53 mmol/mol) according to real-world data from 1034 PwD [60].
The Brazilian Diabetes Society recommends SMBG in cases of T1DM, gestational diabetes mellitus, and insulin-treated T2DM. It also recommends SMBG in people with T2DM not on insulin when therapy is modified or in the presence of unstable glycemic control and risk of metabolic decompensation. Sporadic monitoring after meals is also recommended for PwD using medications with prandial action. The Society emphasizes that the indication must be individualized [61].
In Brazil, the National Health System (Sistema Único de Saúde) is responsible for the distribution of medications, meters, test strips, and lancets. Regarding monitoring, a federal law from 2006 sets out that all PwD are entitled to receive these materials free of charge as long as they are taking insulin as part of their treatment, together with enrollment in educational programs [62]. The reason to only distribute SMBG to insulin users was that, according to the regulators, there is no proven cost–benefit relation of SMBG to people with T2DM not using insulin. Regarding the accuracy of meters in Brazil, a federal resolution from 2018 requires that SMBG systems have a maximum of ± 15% variation in > 95% of the glucose tests for approval [63].
In addition to the distribution of materials to people with T2DM not using insulin being restricted, the use of SMBG is limited by the paucity of specialized HCPs to educate and train these people in ways that would ensure that SMBG would improve control. PwD also showed some resistance to performing painful procedures. It should be noted that a national multicenter study showed that acceptance of SMBG varied depending both on the device used (considering time for getting results, amount of blood needed, technology used) and peoples’ characteristics (age, glycemic control) [64]. Another issue to consider is the number of capillary BG measurements needed to improve glycemic control. A regional study showed that the use of a restricted structured scheme of SMBG (fasting, 3 times a week) did not improve control in people with T2DM not treated with insulin, suggesting that a more intensive anti-diabetic approach would be necessary to obtain better results [65].
Some of these challenges could be solved through the establishment of programs on diabetes education. The importance of education was shown to be relevant in a Brazilian study in which training for self-titrating insulin doses was combined with structured SMBG. Improvement of glycemic control was found in poorly controlled people with T2DM on insulin therapy [66]. This strategy may facilitate effective insulin therapy in routine medical practice, compensating for any reluctance on the part of physicians to optimize insulin therapy and thus to improve the achievement of recommended targets of diabetes care.
Taking into account the aim to improve glycemic control of PwD in Brazil, in addition to prevention programs and new pharmacological approaches, better definitions of the importance of SMBG are needed. As one of the main components of diabetes care, national policies on SMBG should be more valued, with national studies evaluating characteristics of PwD, number of measurements, and structured glucose test profiles. As everywhere else in the world, people in Brazil should have the opportunity to be trained on how to use SMBG devices and interpret the glucose values, and to undertake the appropriate action based on these values, as well as having their provision of supplies assured. In the near future, technology and digital health approaches may offer new ways to turn monitoring diabetes into something more inexpensive and easier to use, enabling PwD worldwide to achieve better metabolic control.
Summary of Local Experience
Performance of SMBG is and remains a cornerstone in the therapy of many people with diabetes. As suggested by the reports on local experience from China, India, and Brazil, access to adequate SMBG can be limited by issues such as the high cost of SMBG systems, insufficient self-management education and empowerment, and, at least in some cases, cultural beliefs. Issues such as these must be overcome on the way to optimal diabetes therapy.
Conclusion
SMBG was established as a cornerstone of the therapy for PwD a few decades ago. Its benefit for PwD who are on insulin therapy, including people with T2DM on insulin therapy, is undisputed. In people with T2DM not using insulin, effectiveness is linked to the active use of BG values for therapeutic decisions or therapy adjustments. SMBG systems have markedly evolved over time. Not only have they become more accurate, modern SMBG systems offer several options for data connectivity and interoperability with diabetes management tools and devices, and thus simplify diabetes therapy notably. However, reports of local experiences from three of the ten countries with the highest number of PwD in the world show that not all PwD have access to SMBG or other diagnostic and therapeutic options. Major influences seem to be the high cost of SMBG systems as well as insufficient self-management education and empowerment. Removing these hurdles will not only lead to improved therapy for PwD and their resulting outcomes, but also to a higher quality of life.
Digital platforms that enable data analysis and secure data sharing, and foster education and motivation of PwD, help to streamline the workflow of HCPs and provide information to the payers in healthcare systems. Smart phones are already the most prevalently used communication tool worldwide, and wirelessly connected SMBG systems can also empower PwD to make use of their personal SMBG results. Such developments will play a major role in achieving better glycemic control in PwD in highly and less developed regions of the world.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Medical writing was supported and the Rapid Service Fee was funded by Roche Diabetes Care GmbH, Germany.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Writing—original draft preparation: Sebastian Schauer. Writing—review and editing: Stefan Pleus, Guido Freckmann, Sebastian Schauer, Lutz Heinemann, Ralph Ziegler, Linong Ji, Viswanathan Mohan, Luis Eduardo Calliari, and Rolf Hinzmann. Funding acquisition: Rolf Hinzmann. Supervision: Rolf Hinzmann.
Medical Writing, Editorial or Other Assistance
Editorial assistance was provided by Prof. Dr. Bernhard Kulzer (Research Institute of the Diabetes-Academy Mergentheim, Bad Mergentheim, Germany) who kindly reviewed an earlier draft of this manuscript.
Disclosures
Guido Freckmann is general manager of the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm (IfDT, Ulm, Germany), which carries out clinical studies on the evaluation of BG meters and medical devices for diabetes therapy on its own initiative and on behalf of various companies. Guido Freckmann/IfDT have received grants, speakers’ honoraria or consulting fees from Abbott, Agamatrix, Ascensia, Berlin Chemie, Beurer, Boydsens, CRF Health, Dexcom, i-SENS, LifeScan, Lilly, Metronom Health, Medtronic, Menarini, MySugr, Novo Nordisk, PharmaSens, Roche, Sanofi, Sensile, and Ypsomed. Stefan Pleus and Sebastian Schauer are employees of IfDT. Lutz Heinemann is a consultant for a number of companies that are developing novel diagnostic and therapeutic options for diabetes treatment. He is a shareholder of the Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany. Ralph Ziegler has received speaker’s honoraria and/or served on advisory boards from/of Abbott, Dexcom, Glooko, Lilly, Menarini, MySugr, Novo Nordisk, and Roche Diabetes Care. Linong Ji has nothing to disclose. Viswanathan Mohan has acted as consultant and speaker, received research or educational grants from Abbott, Astra Zeneca, Boehringer Ingelheim, Dr. Reddy’s Laboratories, Johnson & Johnson, LifeScan, Lilly, MSD, Novartis, Novo Nordisk, Roche Diabetes Care India Pvt. Ltd, Sanofi-Aventis, USV Private Limited, and other Indian pharmaceutical companies. Luis Eduardo Calliari has acted as consultant and speaker for Abbott, Lilly, Novo Nordisk, Medtronic, Roche, and Sanofi-Aventis. Rolf Hinzmann is an employee of Roche Diabetes Care.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.International Diabetes Federation. IDF diabetes atlas 10th edition 2021. 2021. https://diabetesatlas.org/data/en/. Accessed 09 Dec 2021. [PubMed]
- 2.Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/nejm199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl_1). 10.2337/dc21-SINT.
- 4.Mannucci E, Antenore A, Giorgino F, Scavini M. Effects of structured versus unstructured self-monitoring of blood glucose on glucose control in patients with non-insulin-treated type 2 diabetes: a meta-analysis of randomized controlled trials. J Diabetes Sci Technol. 2018;12:183–189. doi: 10.1177/1932296817719290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnell O, Alawi H, Battelino T, Ceriello A, Diem P, Felton AM, et al. Self-monitoring of blood glucose in type 2 diabetes: recent studies. J Diabetes Sci Technol. 2013;7:478–488. doi: 10.1177/193229681300700225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;1:Cd005060. doi: 10.1002/14651858.CD005060.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farmer AJ, Perera R, Ward A, et al. Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes. BMJ. 2012;344:e486. doi: 10.1136/bmj.e486. [DOI] [PubMed] [Google Scholar]
- 8.O'Kane MJ, Bunting B, Copeland M, Coates VE. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336:1174–1177. doi: 10.1136/bmj.39534.571644.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34:262–267. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnell O, Alawi H, Battelino T, et al. Addressing schemes of self-monitoring of blood glucose in type 2 diabetes: a European perspective and expert recommendation. Diabetes Technol Ther. 2011;13:959–965. doi: 10.1089/dia.2011.0028. [DOI] [PubMed] [Google Scholar]
- 11.Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. J Lab Med. 2020;44:71–79. doi: 10.1515/labmed-2019-0189. [DOI] [Google Scholar]
- 12.Dicembrini I, Cosentino C, Monami M, Mannucci E, Pala L. Effects of real-time continuous glucose monitoring in type 1 diabetes: a meta-analysis of randomized controlled trials. Acta Diabetol. 2021;58:401–410. doi: 10.1007/s00592-020-01589-3. [DOI] [PubMed] [Google Scholar]
- 13.Slattery D, Choudhary P. Clinical use of continuous glucose monitoring in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:S55–61. doi: 10.1089/dia.2017.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin R, Brown F, James S, Jones J, Ekinci E. Continuous glucose monitoring: A review of the evidence in type 1 and 2 diabetes mellitus. Diabet Med. 2021;38:e14528. doi: 10.1111/dme.14528. [DOI] [PubMed] [Google Scholar]
- 16.Freckmann G, Pleus S, Grady M, Setford S, Levy B. Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices. J Diabetes Sci Technol. 2019;13:575–583. doi: 10.1177/1932296818812062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freckmann G, Nichols JH, Hinzmann R, Klonoff DC, Ju Y, Diem P, et al. Standardization process of continuous glucose monitoring: Traceability and performance. Clin Chim Acta. 2021;515:5–12. doi: 10.1016/j.cca.2020.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Skyler JS, Lasky IA, Skyler DL, Robertson EG, Mintz DH. Home blood glucose monitoring as an aid in diabetes management. Diabetes Care. 1978;1:150–157. doi: 10.2337/diacare.1.3.150. [DOI] [PubMed] [Google Scholar]
- 19.Freckmann G, Jendrike N, Baumstark A, Pleus S, Liebing C, Haug C. User performance evaluation of four blood glucose monitoring systems applying ISO 15197:2013 accuracy criteria and calculation of insulin dosing errors. Diabetes Ther. 2018;9:683–697. doi: 10.1007/s13300-018-0392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breton MD, Kovatchev BP. Impact of blood glucose self-monitoring errors on glucose variability, risk for hypoglycemia, and average glucose control in type 1 diabetes: an in silico study. J Diabetes Sci Technol. 2010;4:562–570. doi: 10.1177/193229681000400309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Organization for Standardization. In vitro diagnostic test systems−requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (ISO 15197:2013). Geneva: International Organization for Standardization; 2015.
- 22.US Food and Drug Administration. Self-monitoring blood glucose test systems for over-the-counter use—guidance for industry and food and drug administration staff. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/self-monitoring-blood-glucose-test-systems-over-counter-use. Accessed 06 Apr 2022.
- 23.King F, Ahn D, Hsiao V, Porco T, Klonoff DC. A review of blood glucose monitor accuracy. Diabetes Technol Ther. 2018;20:843–856. doi: 10.1089/dia.2018.0232. [DOI] [PubMed] [Google Scholar]
- 24.Klonoff DC, Parkes JL, Kovatchev BP, et al. Investigation of the accuracy of 18 marketed blood glucose monitors. Diabetes Care. 2018;41:1681–1688. doi: 10.2337/dc17-1960. [DOI] [PubMed] [Google Scholar]
- 25.Pleus S, Baumstark A, Jendrike N, et al. System accuracy evaluation of 18 CE-marked current-generation blood glucose monitoring systems based on EN ISO 15197:2015. BMJ Open Diabetes Res Care. 2020 doi: 10.1136/bmjdrc-2019-001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabetes Technology Society. Blood glucose monitoring system surveillance program. 2017. https://www.diabetestechnology.org/surveillance.shtml. Accessed 26 Aug 2021.
- 27.Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3:903–913. doi: 10.1177/193229680900300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Z, Lee JH, Louie RF, Kost GJ. Effects of different hematocrit levels on glucose measurements with handheld meters for point-of-care testing. Arch Pathol Lab Med. 2000;124:1135–1140. doi: 10.5858/2000-124-1135-EODHLO. [DOI] [PubMed] [Google Scholar]
- 29.Hattemer A, Wardat S. Evaluation of hematocrit influence on self-monitoring of blood glucose based on ISO 15197:2013: comparison of a novel system with five systems with different hematocrit ranges. J Diabetes Sci Technol. 2018;12:333–340. doi: 10.1177/1932296818757550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinzmann R, Militz D, Zima T, et al. Real-world data from Europe and Africa suggest that accuracy of systems for self-monitoring of blood glucose is frequently impaired by low hematocrit. Diabetes Res Clin Pract. 2021 doi: 10.1016/j.diabres.2021.108860. [DOI] [PubMed] [Google Scholar]
- 31.Giavarina D. Understanding Bland Altman analysis. Biochem Med (Zagreb) 2015;25:141–151. doi: 10.11613/BM.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter R, Dichiacchio T, Barker K. Interventions for self-management of type 2 diabetes: an integrative review. Int J Nurs Sci. 2018;6(1):70-91. 10.1016/j.ijnss.2018.12.002. [DOI] [PMC free article] [PubMed]
- 33.Jimenez G, Lum E, Huang Z, Theng YL, Boehm BO, Car J. Reminders for medication adherence in Type 2 diabetes management apps. J Pharm Pract Res. 2020;50:78–81. doi: 10.1002/jppr.1595. [DOI] [Google Scholar]
- 34.Pantalone KM, Misra-Hebert AD, Hobbs TM, et al. Clinical inertia in type 2 diabetes management: evidence from a large, real-world data set. Diabetes Care. 2018;41:e113–e114. doi: 10.2337/dc18-0116. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt S, Nørgaard K. Bolus calculators. J Diabetes Sci Technol. 2014;8:1035–1041. doi: 10.1177/1932296814532906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkin CG, Davidson JA. Value of self-monitoring blood glucose pattern analysis in improving diabetes outcomes. J Diabetes Sci Technol. 2009;3:500–508. doi: 10.1177/193229680900300314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulzer B, Daenschel W, Daenschel I, et al. Integrated personalized diabetes management improves glycemic control in patients with insulin-treated type 2 diabetes: results of the PDM-ProValue study program. Diabetes Res Clin Pract. 2018;144:200–212. doi: 10.1016/j.diabres.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Contreras I, Vehi J. Artificial intelligence for diabetes management and decision support: literature review. J Med Internet Res. 2018;20:e10775. doi: 10.2196/10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi G, Zhu N, Qiu L, et al. Impact of the 2020 China Diabetes Society Guideline on the prevalence of diabetes mellitus and eligibility for antidiabetic treatment in China. Int J Gen Med. 2021;14:6639–6645. doi: 10.2147/ijgm.s331948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinese Diabetes Society Guideline for clinical use of blood glucose monitoring (2015) Chin J Diabetes Mellitus. 2015;7:603–613. [Google Scholar]
- 42.Guo XH, Yuan L, Lou QQ, et al. A nationwide survey of diabetes education, self-management and glycemic control in patients with type 2 diabetes in China. Chin Med J. 2012;125:4175–4180. [PubMed] [Google Scholar]
- 43.Wang X, Luo J-F, Qi L, Long Q, Guo J, Wang H-H. Adherence to self-monitoring of blood glucose in Chinese patients with type 2 diabetes: current status and influential factors based on electronic questionnaires. Patient Prefer Adher. 2019;13:1269–1282. doi: 10.2147/PPA.S211668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao J, Wang H, Yan J, Shao D, Sun Q, Yin X. Understanding the profiles of blood glucose monitoring among patients with type 2 diabetes mellitus: a cross-sectional study in Shandong. China Patient Prefer Adher. 2021;15:399–409. doi: 10.2147/ppa.s292086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji L, Su Q, Feng B, et al. Glycemic control and self-monitoring of blood glucose in Chinese patients with type 2 diabetes on insulin: baseline results from the COMPASS study. Diabetes Res Clin Pract. 2016;112:82–87. doi: 10.1016/j.diabres.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Yao J, Wang H, Yin X, Yin J, Guo X, Sun Q. The association between self-efficacy and self-management behaviors among Chinese patients with type 2 diabetes. PLoS ONE. 2019;14:e0224869-e. doi: 10.1371/journal.pone.0224869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, He X, Shen Y, et al. Effectiveness of smartphone app-based interactive management on glycemic control in Chinese patients with poorly controlled diabetes: randomized controlled trial. J Med Internet Res. 2019;21:e15401. doi: 10.2196/15401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unnikrishnan R, Anjana RM, Mohan V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. 2016;12:357–370. doi: 10.1038/nrendo.2016.53. [DOI] [PubMed] [Google Scholar]
- 49.Indian Council of Medical Research . ICMR guidelines for management of type 2 diabetes. Delhi: Indian Council of Medical Research; 2018. [Google Scholar]
- 50.SMBG International Working Group Self-monitoring of blood glucose in type 2 diabetes: an inter-country comparison. Diabetes Res Clin Pract. 2008;82:e15–e18. doi: 10.1016/j.diabres.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chittem M, Sridharan SG, Pongener M, Maya S, Epton T. Experiences of barriers to self-monitoring and medication-management among Indian patients with type 2 diabetes, their primary family-members and physicians. Chronic Illn. 2021 doi: 10.1177/17423953211032251. [DOI] [PubMed] [Google Scholar]
- 52.Deshwal S, Barola A, Tiwari P. Costing of ADA-recommended self-monitoring of blood glucose: Early results from a northern Indian city couplet. Value Health. 2016;19:A301–A302. doi: 10.1016/j.jval.2016.03.705. [DOI] [Google Scholar]
- 53.Joshi SR. Self-monitoring of blood glucose in the Asia-Pacific region. Diabetes Technol Ther. 2008;10:S89–S92. doi: 10.1089/dia.2008.0014. [DOI] [Google Scholar]
- 54.Rao PV, Makkar BM, Kumar A, et al. RSSDI consensus on self-monitoring of blood glucose in types 1 and 2 diabetes mellitus in India. Int J Diabetes Dev Ctries. 2018;38:260–279. doi: 10.1007/s13410-018-0677-3. [DOI] [Google Scholar]
- 55.Verma M, Junaid A, Verma K, Das Gupta D. Exploring the role of social media as a support mechanism among persons with diabetes: an online ethnographic study. J Content Community Commun. 2019;10:78–85. doi: 10.31620/JCCC.12.19/09. [DOI] [Google Scholar]
- 56.de Almeida-Pititto B, Dias ML, de Moraes ACF, Ferreira SR, Franco DR, Eliaschewitz FG. Type 2 diabetes in Brazil: epidemiology and management. Diabetes Metab Syndr Obes. 2015;8:17–28. doi: 10.2147/DMSO.S72542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ministério de Saúde da Brasil. Vigitel Brasil 2018. 2021. https://portalarquivos2.saude.gov.br/images/pdf/2019/julho/25/vigitel-brasil-2018.pdf. Accessed 07 Sep 2021.
- 58.Correr CJ, Coura-Vital W, Frade JCQP, et al. Prevalence of people at risk of developing type 2 diabetes mellitus and the involvement of community pharmacies in a national screening campaign: a pioneer action in Brazil. Diabetol Metab Syndr. 2020;12:89. doi: 10.1186/s13098-020-00593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bahia LR, da Rosa MQM, Araujo DV, et al. Economic burden of diabetes in Brazil in 2014. Diabetol Metab Syndr. 2019;11:54. doi: 10.1186/s13098-019-0448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harder L, Andrade TK, Serra FB, Pinho FM, Pinzon A, Orengo JC. Number of type 2 diabetes patients achieving HbA1c goal and Related Factors in Brazil: a real world evidence study. Res Sq. 2021 doi: 10.21203/rs.3.rs-61621/v1. [DOI] [Google Scholar]
- 61.Diretrizes da Sociedade Brasileira de Diabetes 2019. 2022. http://diabetes.org.br. Accessed 31 Jan 2022.
- 62.Ministro de Estado da Saúde. 2007. https://bvsms.saude.gov.br/bvs/saudelegis/gm/2007/prt2583_10_10_2007.html. Accessed 31 Jan 2022.
- 63.Diário Oficial da União (D.O.U.). Resolution RE 3.161 2018. 2022. https://www.in.gov.br/web/guest/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/50727633/imprensanacional. Accessed 31 Jan 2022.
- 64.Hissa MN. Brazilian multicenter study for the evaluation of patients’ satisfaction of blood glucose self-monitoring with BGStar(®) blood glucose meter in insulinized patients with diabetes mellitus type 1 and 2. Diabetol Metab Syndr. 2016;8:66. doi: 10.1186/s13098-016-0180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Viana LV, Leitão CB, Grillo Mde F, et al. Are diabetes management guidelines applicable in ‘real life’? Diabetol Metab Syndr. 2012;4:47. doi: 10.1186/1758-5996-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silva DD, Bosco AA. An educational program for insulin self-adjustment associated with structured self-monitoring of blood glucose significantly improves glycemic control in patients with type 2 diabetes mellitus after 12 weeks: a randomized, controlled pilot study. Diabetol Metab Syndr. 2015;7:2. doi: 10.1186/1758-5996-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.