Abstract
Background and aims
The pathophysiology of sarcopenia in cirrhosis is poorly understood. We aimed to evaluate the histological alterations in the muscle tissue of patients with cirrhosis and sarcopenia, and identify the regulators of muscle homeostasis.
Methods
Computed tomography images at third lumbar vertebral level were used to assess skeletal muscle index (SMI) in 180 patients. Sarcopenia was diagnosed based on the SMI cut-offs from a population of similar ethnicity. Muscle biopsy was obtained from the vastus lateralis in 10 sarcopenic patients with cirrhosis, and the external oblique in five controls (voluntary kidney donors during nephrectomy). Histological changes were assessed by hematoxylin and eosin staining and immunohistochemistry for phospho-FOXO3, phospho-AKT, phospho-mTOR, and apoptosis markers (annexin V and caspase 3). The messenger ribonucleic acid (mRNA) expressions for MSTN, FoxO3, markers of ubiquitin-proteasome pathway (FBXO32, TRIM63), and markers of autophagy (Beclin-1 and LC3) were also quantified.
Results
The prevalence of sarcopenia was 14.4%. Muscle histology in sarcopenics showed atrophic angulated fibers (P = 0.002) compared to controls. Immunohistochemistry showed a significant loss of expression of phospho-mTOR (P = 0.026) and an unaltered phospho-AKT (P = 0.089) in sarcopenic patients. There were no differences in the immunostaining for annexin-V, caspase-3, and phospho-FoxO3 between the two groups. The mRNA expressions of MSTN and Beclin-1 were higher in sarcopenics (P = 0.04 and P = 0.04, respectively). The two groups did not differ in the mRNA levels for TRIM63, FBXO32, and LC3.
Conclusions
Significant muscle atrophy, increase in autophagy, MSTN gene expression, and an impaired mTOR signaling were seen in patients with sarcopenia and cirrhosis.
Keywords: sarcopenia, autophagy, MSTN gene, cirrhosis
Abbreviations: HCC, hepatocellular carcinoma; SMI, skeletal muscle index; EWGSOP, European Working Group on Sarcopenia in Older People; mTOR, mammalian target of rapamycin; Fox-O, forkhead O; 4E-BP1, eukaryotic translation initiation factor 4E binding protein-1; MuRF-1, muscle RING finger 1; CT, computed tomography; HE, hepatic encephalopathy; APASL, Asia Pacific Association for the study of the Liver; RT-PCR, real-time polymerase chain reaction; RNA, ribonucleic acid; qPCR, quantitative polymerase chain reaction; cDNA, complementary deoxyribonucleic acid; BMI, body mass index; mRNA, messenger RNA
Sarcopenia, or reduction in the skeletal muscle mass and/or strength,1 is a relatively unexplored sequela of cirrhosis. Multiple studies have consistently shown that sarcopenia increases mortality in patients with cirrhosis, hepatocellular carcinoma (HCC), and liver transplant recipients.2, 3, 4, 5, 6, 7 The North American consensus defines sarcopenia based on skeletal muscle index (SMI) measured at the level of third lumbar vertebra, whereas the European Working Group on Sarcopenia in Older People (EWGSOP) incorporates both low muscle strength and mass for diagnosing sarcopenia.8,9
Muscle homeostasis is maintained by a balance between protein synthesis and breakdown.10,11 Myostatin, a myokine encoded by the MSTN gene, is released by the muscle cell and is overexpressed in atrophic muscles.12, 13, 14 It impairs myogenesis by inhibiting the Protein kinase B (AKT)-mTOR (mammalian target of rapamycin) pathway and stimulating the forkhead O (Fox-O) dependent protein degradation pathways.15 However, studies in patients with cirrhosis have yielded conflicting results.13,16
AKT-mTOR pathway is the predominant anabolic pathway for muscle protein synthesis. Phosphorylated AKT causes activation of mammalian target of rapamycin complex 1 (mTORC1), which increases the protein synthesis.17, 18, 19Skeletal muscle breakdown occurs predominantly through three major pathways—ubiquitin-proteasome system, caspase-mediated protein cleavage, and the autophagy pathway. The ubiquitin-proteasome and autophagy pathways are interconnected and are under the transcriptional control of transcription factor Fox-O, which regulates various atrophy-related genes including Atrogin-1 (FBXO32, encoding for F-box only protein 32), TRIM63 [encoding for Muscle RING finger 1 (MuRF-1)], and the autophagy-related genes encoding beclin-1, lipidated LC3 and p62.20 Previous studies in cirrhosis have failed to show significant differences in the components of the ubiquitin-proteasome pathway (Atrogin-1, MuRF-1, Proteasome C3, and C5), in comparison to controls.14,16,21,22 In contrast, increased autophagy was the principal contributor to sarcopenia as evidenced by an increased LC3 lipidation and Beclin-1 expression.14,21,22 The significance of the apoptotic pathway in the pathogenesis of sarcopenia in cirrhosis is relatively unexplored, and recent animal studies have shown an increased apoptotic activity shown by an elevated caspase expression.23
These regulatory pathways have been explored in the muscle tissues obtained from patients with cirrhosis, as well as animal models and myotubes.13,14,16,21,22 Although animal models provide an insight into the possible mechanisms, they do not reproduce the changes of sarcopenia. Previous human studies have explored the pathogenesis of muscle depletion in patients with cirrhosis, without clearly defining if they included patients with sarcopenia.14,16,21 In this study, we estimated the prevalence of sarcopenia in cirrhosis, demonstrated the histological changes in the skeletal muscles in sarcopenia, and explored the regulators of muscle protein homeostasis in the muscle biopsies of sarcopenic patients with cirrhosis compared to healthy controls.
Materials and methods
Study setting and patients
The study was conducted at a tertiary care center in North India from July 2018 to December 2019. Consecutive patients with cirrhosis between 18 and 60 years of age, irrespective of the etiology, being prospectively followed-up in the liver clinic were included. Cirrhosis was diagnosed based on the clinical features (history and examination, history of decompensation of underlying chronic liver disease), laboratory parameters, and imaging [ultrasonography or computed tomography (CT) abdomen].24 Patients with overt hepatic encephalopathy (HE), gastrointestinal bleed, sepsis, acute-on-chronic liver failure,25 and hospital admission in the preceding month before enrollment were excluded. Additional exclusion criteria included HCC at presentation, non-hepatic malignancy, chronic obstructive pulmonary disease, congestive heart failure, chronic kidney disease, neuromuscular disorders, and those unwilling for participation. The study protocol was approved by the institutional ethics committee (IECPG-320/18.07.2018) and was conducted in accordance with the 1964 Helsinki declaration and its later amendments.
Assessment of sarcopenia
All patients underwent a triple-phase CT abdomen at enrollment to rule out HCC. The CT images were acquired using a 256-slice dual-energy scanner (Somatom definition flash, Siemens, Erlangen, Germany). A transverse CT image in the venous phase at the midbody of the third lumbar (L3) vertebral level was identified. The images were analyzed using an image analyzer software, 3D slicer software version 4.10.2 (http://www.slicer.org), an image computing platform used for quantitative imaging.26 Skeletal muscle was identified by a predefined CT density of −29 to +150 Hounsfield Unit (HU). Skeletal muscle area included the sum of areas of paraspinal, psoas, transversus abdominis, internal oblique, external oblique, and rectus abdominis muscle at the L3 vertebral level. It was then normalized to the height and expressed as SMI in cm2/m2. Skeletal muscle index was used to define sarcopenia, and cut-offs of 36.5 cm2/m2 and 30.2 cm2/m2 for male and female, respectively, were chosen based on previous cut-offs obtained from a healthy population of similar ethnicity.27 Weight was corrected for the presence of ascites and pedal edema, and dry weight was estimated by subtracting the added weight due to fluid retention.28
Muscle biopsy in patients with sarcopenia and healthy controls
Muscle biopsy was performed in patients with sarcopenia within 1 month of enrolment, excluding patients with abnormal coagulation parameters (international normalized ratio >1.5) or thrombocytopenia (<50,000 per mm3). Biopsy was done after overnight fasting using an automatic biopsy gun (BARD MAX-CORE, Tempe, Arizona, US) 16G, 9 cm long with 2 cm throw, from the right vastus lateralis muscle 4–6 inches above the patella and lateral to the outer border of femur. Two cores of muscle tissue were obtained. One core was preserved in RNAlater (Sigma Aldrich, St Louis, US) at −20 °C, whereas the other was fixed in 10% buffered neutral formalin, processed and paraffin-embedded. Local pressure was applied for 5 min at the biopsy site after the procedure. A pressure dressing was done, which was removed at discharge, after an in-hospital observation period of at least 6 h. All patients were followed up daily telephonically for 1 week to follow-up for bleeding, fever, pain, and/or swelling at the procedure site.
Healthy controls included voluntary kidney donors undergoing nephrectomy and not cirrhotics without sarcopenia. Biopsies were taken after obtaining informed consent. Two muscle biopsy specimens measuring about 1 cm in length and 1 cm in width were obtained from the external oblique muscle after skin incision and subcutaneous fat dissection, during the procedure of nephrectomy. This muscle was chosen because both vastus lateralis and external oblique were mixed muscles. No patient developed any complication due to the biopsy procedure.
Muscle histology and immunohistochemistry
Multiple sections (4 micron thick) of muscle tissue were cut and stained with hematoxylin and eosin. Immunohistochemistry (IHC) was performed on the tissue sections using primary antibodies directed against pFOXO3, pAKT, p-mTOR, Annexin V, and Caspase 3 on the Ventana Benchmark XT using UltraView detection system and DAB chromogen. Appropriate positive control tissue sections were run with each batch. For negative controls, the primary antibody was omitted during the staining. Supplementary Table 1 shows the details of all the antibodies used. Presence of nuclear/cytoplasmic staining of any intensity in >25% of muscle fibers was considered as positive expression.29
Assessment of gene expression by real-time polymerase chain reaction (RT-PCR)
The protocols for ribonucleic acid (RNA) extraction and quantitative polymerase chain reaction (qPCR) from other tissues have been established in our laboratory and described earlier.30 Total RNA was extracted from the muscle biopsy using QIAzol reagent (Qiagen, Venlo, Netherland, EU). RNA quantification was done using a spectrophotometer measuring the absorbance at 260 and 280 nm. Total RNA (1 μg) was converted to complementary deoxyribonucleic acid (cDNA) by reverse transcriptase reaction using Revert Aid M-MuLV reverse transcriptase (MBI Fermentas, Vilnius, Lithuania). Real-time PCR reactions were performed and quantified by Maxima SYBR Green (Thermo Scientific, Waltham, MA, USA) using CFX96 Touch™ RT-PCR detection system (BioRad, Hercules, CA, USA), using the β-actin gene as an internal control for normalization. The messenger RNA (mRNA) expression for MSTN, FBXO32 (encoding Atrogin-1), TRIM63 (encoding MURF-1), BECN1 (encoding Beclin-1), FoxO-3, and LC3 was analyzed. The details of primers are given in supplementary Table 2.
Statistical analysis
Baseline characteristics of patients and controls were presented as number (%) or based on the normalcy of distribution, as mean ± SD/median (interquartile range) as appropriate. Categorical variables were compared using the chi-square or Fischer exact test. Continuous variables were compared using the Student's t-test or Mann–Whitney test, as appropriate. For all statistical tests, a p-value <0.05 was considered to be statistically significant.
The data were entered using Microsoft Excel 2011 and was analyzed using SPSS version 21.0. All authors had full access to the study data and reviewed and approved the final manuscript.
Results
Baseline characteristics of patients with and without sarcopenia
Two hundred seventy-six consecutive patients were assessed for eligibility and 180 patients (143 males and 37 females) were included (Supplementary Figure 1). Alcohol (32.7%) was the commonest etiology of cirrhosis followed by viral hepatitis (28.3%) and cryptogenic (16.7%). Of 180 patients, 26 (14.4%) had sarcopenia. The prevalence of sarcopenia was 21 of 143 (14.7%) and 5 of 37 (13.5%) in males and females, respectively. The Child–Turcotte–Pugh score was significantly higher in the sarcopenic subgroup (P = 0.02). Patients with sarcopenia had a lower body weight [46.6 (40.9–58) vs 60.8 (52.2–70.6) kg, P < 0.001) and body mass index (BMI) [17.0 (15.7–20.4) vs 22.7 (19.9–25.5) kg/m2, P < 0.001], in comparison to patients without sarcopenia. The baseline characteristics are summarized in Table 1.
Table 1.
Demographic and Biochemical Parameters in the Sarcopenic and the Non-sarcopenic Patients with Cirrhosis.
| No sarcopenia (n = 154) | Sarcopenia (n = 26) | P-Value | ||
|---|---|---|---|---|
| Age (years) | 43.2 ± 10.1 | 41 ± 8.5 | 0.28 | |
| Sex (Male) (%) | 122/154 (79.2) | 21/26 (80.8) | 0.85 | |
| Height (cm) | 163.7 ± 8.8 | 164.6 ± 7.8 | 0.65 | |
| Weight (kg) | 60.8 (52.2–70.6) | 46.6 (40.9–58) | <0.001 | |
| BMI (kg/m2) | 22.7 (19.9–25.5) | 17.0 (15.7–20.4) | <0.001 | |
| Etiology (%) | Alcohol | 47/154 (30.5%) | 12/26 (46.2%) | 0.11 |
| Non-alcohol | 107/154 (69.5%) | 14/26 (53.8%) | ||
| Ascites (%) | Absent | 99/154 (64.3%) | 13/26 (50%) | 0.03 |
| Mild/Moderate | 44/154 (28.6%) | 7/26 (26.9%) | ||
| Gross | 11/154 (7.1%) | 6/26 (23.1%) | ||
| Past history of overt HE | 24/154 (15.6%) | 4/26 (15.4%) | 0.99 | |
| Diabetes (%) | 25/154 (16.2%) | 3/26 (11.5%) | 0.54 | |
| Hemoglobin (g/dL) | 11.2 ± 2.3 | 10.1 ± 3.0 | 0.03 | |
| Bilirubin (mg/dL) | 1.5 (0.87–2.6) | 1.9 (1.05–2.5) | 0.22 | |
| AST (U/L) | 50 (38.5–76.5) | 44.5 (33–82) | 0.25 | |
| ALT (U/L) | 35 (25.5–56.5) | 29 (19.5–41.7) | 0.045 | |
| ALP (U/L) | 291 (211–408.5) | 240.5 (190.5–377.2) | 0.61 | |
| Protein (g/dL) | 7.3 ± 0.67 | 7.2 ± 0.8 | 0.57 | |
| Albumin (g/dL) | 3.6 ± 0.8 | 3.4 ± 0.8 | 0.13 | |
| Creatinine (mg/dL) | 0.81 ± 0.20 | 0.79 ± 0.22 | 0.67 | |
| INR | 1.5 ± 0.4 | 1.6 ± 0.5 | 0.15 | |
| Child–Pugh score | 6.9 ± 1.9 | 7.8 ± 2.4 | 0.02 | |
| Child A | 86/154 (55.8%) | 8/26 (30.8%) | 0.03 | |
| Child B | 50/154 (32.5%) | 11/26 (42.3%) | ||
| Child C | 18/154 (11.7%) | 7/26 (26.9%) | ||
| MELD | 12.7 ± 4.4 | 13.9 ± 4.8 | 0.20 | |
All values are expressed as mean ± SD, median (IQR), or percentage, as appropriate.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BMI, body mass index; HE, hepatic encephalopathy; INR, international normalized ratio; MELD, model for end-stage liver disease; IQR, interquartile range; SD, standard deviation. Bold values indicate P < 0.05.
Characteristics of patients undergoing muscle biopsy and healthy controls
Muscle biopsy was done in 10 patients (9 male and 1 female) with sarcopenia. Sixteen patients were unsuitable or unwilling for biopsy (8 patients had thrombocytopenia and/or coagulopathy, 8 patients did not consent for the procedure). Nine patients did not develop any complication, whereas one patient developed mild pain, which improved after 2 days of oral analgesics. All patients were discharged after an in-hospital observation for 6 h. Control muscle biopsy was obtained from five healthy females. The characteristics of the patients undergoing muscle biopsy and healthy controls are summarized in Table 2. Alcohol was the underlying etiology for cirrhosis in 6 of 10 patients. The proportion of patients with Child A, B, and C cirrhosis were 4, 4, and 2, respectively. The sarcopenic group did not differ from the controls with respect to their age, height, weight, and BMI. The mean SMI in the healthy cohort was 35.9 ± 3.6 cm2/m2, which was higher than the selected cut-off for sarcopenia in females (30.2 cm2/m2).
Table 2.
Baseline Characteristics of Patients With Sarcopenia Undergoing Muscle Biopsy and Healthy Controls.
| Cases (n = 10) | Controls (n = 5) | P-Value | |
|---|---|---|---|
| Age (years) | 41.4 ± 7.6 | 38.8 ± 7.5 | 0.54 |
| Sex (n) | |||
|
9/10 | – | |
|
1/10 | 5/5 | |
| Height (cm) | 166.6 ± 9.6 | 158.8 ± 7.8 | 0.12 |
| Weight (kg) | 59.7 (42.6–72.5) | 55 (49.5–66) | 0.85 |
| BMI (kg/m2) | 20.6 ± 4.7 | 22.7 ± 3.4 | 0.38 |
| Hemoglobin (g/dL) | 10.5 (6.9–13.5) | 11.8 (11.0–12.6) | 0.25 |
| Platelet (per mm3) | 96.5 (54.7–133.5) | 237 (152–277.5) | 0.008 |
| Bilirubin (mg/dL) | 2.2 (1.1–3.6) | 0.4 (0.35–0.6) | 0.003 |
| Albumin (g/dL) | 3.4 (3.0–3.9) | 4.6 (4.4–4.9) | 0.001 |
| Creatinine (mg/dL) | 0.8 ± 0.16 | 0.7 ± 0.1 | 0.13 |
| Child Status (n) | |||
|
4/10 | NA | |
|
4/10 | ||
|
2/10 | ||
| Etiology of cirrhosis (n) | |||
|
6/10 | NA | |
|
4/10 | ||
| SMI (cm2/m2) | |||
|
32.8 ± 3.0 | – | |
|
29.1a | 35.9 ± 3.6 | |
All values are expressed as mean ± SD, median (IQR) or percentage, as appropriate.
BMI, body mass index; SMI, skeletal muscle index; NA, not applicable; IQR, interquartile range; SD, standard deviation. Bold values indicate P < 0.05.
Only one cirrhotic female underwent muscle biopsy.
Histology and immunohistochemistry
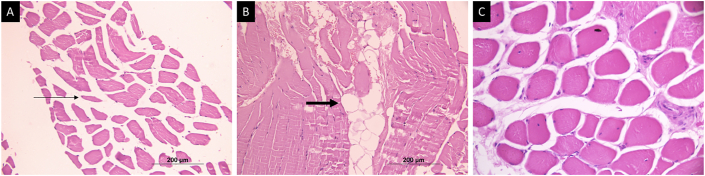
Of the 10 patients with sarcopenia, 9 (90%) showed the presence of muscle atrophy as seen by the presence of many angulated fibers (Figure 1A). None of the patients in the control group showed evidence of muscle atrophy (P = 0.002). Four sarcopenic patients also showed endomysial fat infiltration in the muscle, in contrast to none in the healthy control group. However, this did not reach statistical significance (P = 0.23; Figure 1B).
Figure 1.
Hematoxylin and eosin-stained muscle tissue in patients with sarcopenia. (A) Transverse sections from muscle in sarcopenic patients showing an increase in angulated atrophic fibers (arrows). (B) Fat infiltration (bold arrow) in the muscle. (C) Sections of muscle from a healthy control showing normal fascicular architecture without fat infiltration.
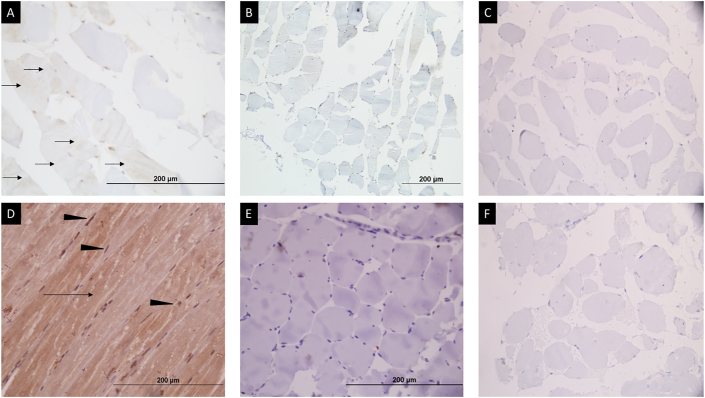
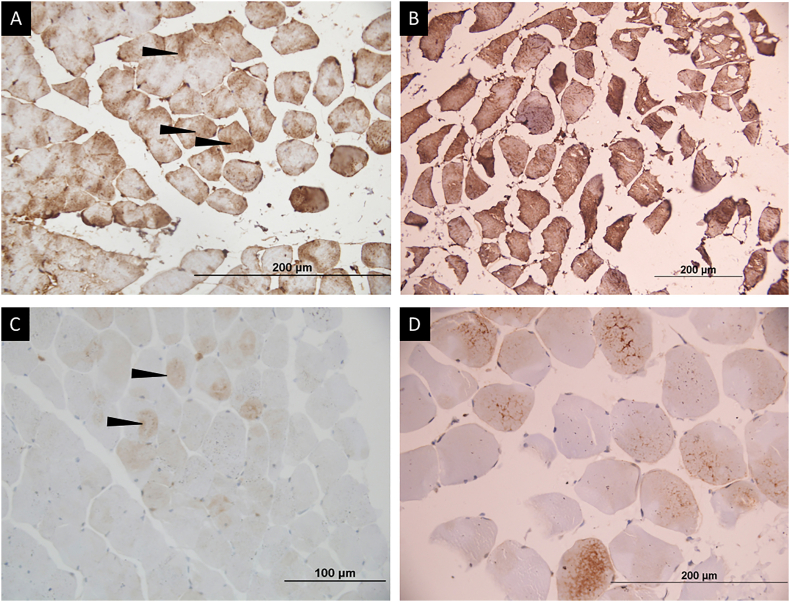
Immunohistochemistry for phospho-mTOR showed a weak cytoplasmic localization in all controls, whereas a loss of phospho-mTOR was seen in 7 of 10 cases (P = 0.026; Figure 2A and B). A moderate to strong intensity cytoplasmic and focal nuclear staining for phospho-AKT was seen in a lesser proportion of sarcopenic patients (2/10, 20%) in comparison to 80% controls; however, it did not reach statistical significance (P = 0.089; Figure 2C and D). Phospho-FoxO3 immunostaining showed a strong intensity nuclear and cytoplasmic positivity in both cases and controls (P = 0.99; Figure 3B and C). The significance of the apoptotic pathway in the regulation of muscle homeostasis was assessed by the IHC for caspase 3 and annexin V. Caspase 3 immunostaining showed cytoplasmic positivity in scattered muscle fibers in 50% cases and 60% controls (P = 0.99), whereas annexin V immunostaining showed cytoplasmic positivity in all cases and controls (Supplementary Figure 2).
Figure 2.
Immunohistochemistry for phospho-mTOR and phospho-AKT in healthy controls and cases (patients with sarcopenia). (A) Weak to moderate intensity brown cytoplasmic staining for phospho-mTOR protein in >25% of muscle fibers (black arrows) in healthy control. (B) Absent brown staining in majority of fibers for phospho-mTOR expression in patients with sarcopenia. (D) Moderate to strong intensity diffuse brown cytoplasmic (black arrow) and nuclear (black arrow head) staining in most muscle fibers for phospho-AKT protein in healthy control. (E) Absent brown staining for phospho-AKT expression in patients with sarcopenia. (C) and (F) represent the negative controls (immunohistochemistry procedure performed without application of primary antibodies) showing complete absence of brown staining in the muscle fibers.
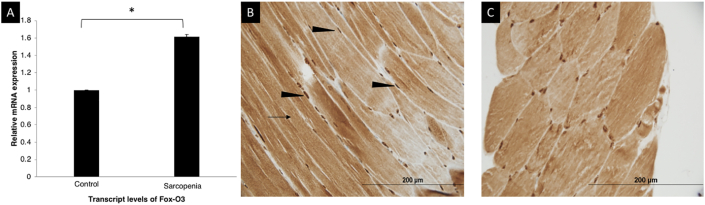
Figure 3.
Skeletal muscle expression of Fox-O3 mRNA and IHC for phospho-FoxO3. (A) Skeletal muscle expression of Fox-O3 mRNA showing a significantly higher expression in sarcopenic patients (n = 10) in comparison to controls (n = 5) (P = 0.009). (B) Immunohistochemistry showing strong intensity nuclear (black arrowhead) and cytoplasmic (arrow) staining for phospho-FOXO3 in healthy controls. (C) Similar immunostaining pattern in patients with sarcopenia. (∗ suggests P < 0.05).
RT-PCR for genes regulating muscle synthesis and breakdown
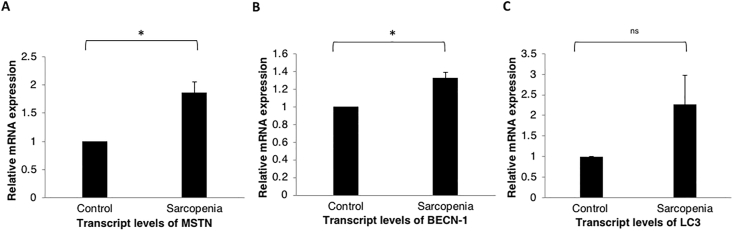
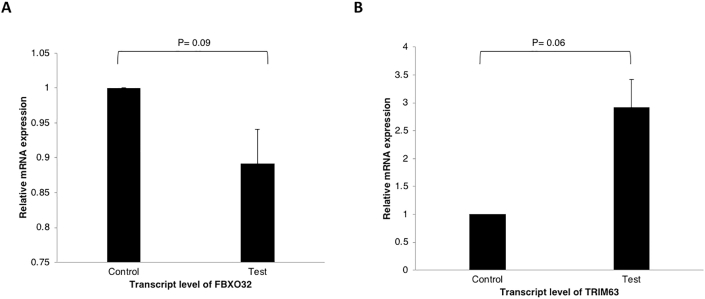
Significantly higher gene expression of MSTN mRNA (1.9 times) was seen in patients with sarcopenia in comparison to controls (P = 0.04; Figure 4A). The expression of the mRNA for FoxO3 was 1.6 times higher in the sarcopenic patients (P = 0.009), in contrast to the results of the IHC for FoxO3 (Figure 3A). Critical components of the ubiquitin-proteasome pathway, MuRF-1 and Atrogin-1 encoded by the TRIM63 and FBXO32 genes, respectively, did not differ between the two groups (P = 0.06 and P = 0.09 for TRIM63 and FBXO32, respectively; Supplementary Figure 3). Increased expression for Beclin-1, a marker of autophagy, was seen in sarcopenic patients (P = 0.04) (Figure 4B). Although the gene expression for LC3 was 2.3 times higher in patients with sarcopenia, it was not statistically significant (P = 0.12; Figure 4).
Figure 4.
Skeletal muscle expression of various regulators of muscle synthesis and breakdown. (A) A significantly higher MSTN mRNA expression in patients with sarcopenia (n = 10) in comparison to healthy controls (n = 5) (P = 0.04). (B) BECN1 mRNA expression in patients with sarcopenia (n = 10) versus healthy controls (n = 5) (P = 0.04). (C) No differences in the mRNA expression of LC3 in patients with sarcopenia (n = 10) versus healthy controls (n = 5) (P = 0.12). (ns-not significant, ∗ suggests P < 0.05).
Discussion
In this study, we evaluated 180 patients with cirrhosis to estimate the prevalence of sarcopenia. Sarcopenia was defined based on the depletion of muscle mass, following the North American consensus definition.8 Owing to differences in the body composition of Asians and Caucasians,31 the threshold for defining sarcopenia was based on estimates obtained from a similar ethnic group.27 The SMI cut-offs are lower in the Asian cohort in comparison to the Caucasians; therefore, all patients with sarcopenia classified by the Asian estimates would also satisfy the SMI-based criteria obtained from the Western populations.8,32 The prevalence of sarcopenia was 14.4% in our cohort, which is similar to previous results obtained from a similar ethnic group.27 The prevalence of sarcopenia was, however, lower than the estimates from the Western population due to disparities in the cut-offs.7,33,34
The pathogenesis of sarcopenia in cirrhosis is evolving, and the difficulties in obtaining muscle tissue in patients with cirrhosis who frequently have thrombocytopenia and/or coagulopathy limit human data. Our study showed the feasibility and safety of muscle biopsy, even in patients with decompensated cirrhosis who did not have severe coagulopathy or thrombocytopenia. Previous studies have also shown the feasibility of needle-guided biopsy in patients with cirrhosis.33,35 Hence, selecting suitable patients for the procedure decreases the risks of complications.
This study reported the histological changes of muscle atrophy in sarcopenic patients with cirrhosis. Ninety percent of sarcopenic patients who underwent a muscle biopsy had evidence of muscle atrophy, as seen by the presence of angulated fibers on histology. Previous animal models have shown similar results in mice in whom cirrhosis was induced by bile duct ligation and carbon tetrachloride.36 Forty percent patients also had fat infiltration in the muscle, although not significant. Intramuscular fat deposition, as assessed by the muscle attenuation of the paravertebral muscle on abdominal CT, have been associated with higher mortality in patients with cirrhosis and post-transplant mortality.37,38 Though myosteatosis is diagnosed based on the imaging of the axial musculature, whether such changes are also seen in appendicular muscles need to be further explored. Further studies with a larger sample size are warranted to explore the significance of fat infiltration in appendicular muscles.
Molecular studies and IHC highlight the significance of various pathways in the regulation of muscle mass. The gene expression of MSTN (coding for myostatin mRNA) was significantly higher in patients with sarcopenia. Previous literature on the MSTN gene expression is conflicting.13,16 Elevated serum myostatin causes downstream inhibition of the mTOR pathway, thereby impairing skeletal muscle protein synthesis.39 Immunohistochemistry also showed reduced expression of phospho-mTOR in patients with sarcopenia. Our data are consistent with previous reports of an elevated myostatin mRNA and a downregulated mTOR pathway in cirrhosis.13,14 AKT immunostaining was negative in 80% patients; however, it did not reach a statistical significance, likely due to the small sample size.
Previous studies have shown that muscle breakdown, mediated by the expression of autophagy components and ubiquitin-proteasome markers, is under the transcriptional control of FoxO.20 Consistently, our results showed an increased FoxO3 mRNA expression in sarcopenic patients. Interestingly, IHC did not demonstrate any differences between controls and sarcopenics. Discordance between the mRNA and protein expression has been reported by others,40,41 and can be explained by multiple factors, such as low levels of protein expression, post-translational changes, and technical error. These results need to be further explored in studies with larger sample size and by assessing FoxO3 protein expression using western blot. Our observations also suggest increased muscle autophagy in patients with sarcopenia and are consistent with previous studies.21,22 Previous studies have also shown that autophagy is the predominant driver of sarcopenia in patients with alcohol-related liver disease.22,42 The mRNA expression for Beclin-1 was significantly higher, whereas that of LC3, although elevated, failed to reach statistical significance. Components of the ubiquitin-proteasome pathway, quantified by the mRNA expression of TRIM63 and FBXO32, did not differ between controls and patients with cirrhosis and sarcopenia. Multiple studies involving patients with cirrhosis and mice models have also shown increased skeletal muscle autophagy without significant alterations in the ubiquitin-proteasome pathway.21,22,43
Our study is limited by the small number of patients undergoing muscle biopsy. The lack of frozen section examination of the muscle biopsies precluded study for the predominant type of fibers involved (Type 1 or 2) and changes in enzyme histochemistry. We did not estimate the gene expression of Unc-51 like kinase (ULK1) and therefore, could not show a link between the mTOR and autophagy pathways. We expected that the differences in the mediators of sarcopenia would be most evident in sarcopenic patients when compared to healthy individuals. Assessing these pathways in patients with cirrhosis without sarcopenia can be explored in the future, to show the transition of changes that occur from a healthy individual to patients with cirrhosis and sarcopenia. The number of female patients in the sarcopenic cohort is small, and muscle biopsy could be obtained in only one. It is still unknown if the regulatory pathways for sarcopenia in cirrhosis differ between males and females, and hence we analyzed our results irrespective of the gender, similar to previous human studies.13,21 Control biopsies were obtained from the external oblique muscle rather than the vastus lateralis due to the limitations of obtaining invasive biopsies from healthy individuals. To the best of our knowledge, there is no study that demonstrates differences in regulators of muscle synthesis and breakdown in the external oblique versus vastus lateralis muscle of healthy individuals, and therefore, we choose the former as the control tissue. About 15% patients with and without sarcopenia had past history (beyond 1 month) of overt HE and were on ammonia lowering therapy, hence estimation of serum ammonia was not done for the purpose of this study. In addition, skeletal muscle ammonia estimation was not done because of the difficulties in assessing ammonia in tissues and the limited tissue obtained by biopsy in patients with cirrhosis.
Our study provides mechanistic basis of the alterations in various regulatory pathways in patients with sarcopenia of cirrhosis. The study establishes direct translational relevance of previous preclinical results and is probably the first study to demonstrate the histological changes of muscle atrophy in patients with cirrhosis. Elevated gene expression for MSTN and a decreased mTOR expression, in combination with autophagy upregulation, was identified. Further studies, exploring these results in non-sarcopenic patients with cirrhosis in comparison to patients with sarcopenia and healthy controls would help establish the differential significance of each regulatory pathway in the natural course of sarcopenia and help develop treatment strategies.
CRediT authorship contribution statement
Abhinav Anand: Formal analysis. Aruna Nambirajan: Formal analysis, drafting of the manuscript. Vikas Kumar: Formal analysis, drafting of the manuscript. Samagra Agarwal: drafting of the manuscript, acquisition of data, Formal analysis, drafting of the manuscript, acquisition of data. Sanchit Sharma: acquisition of data, drafting of the manuscript. Srikant Mohta: acquisition of data, drafting of the manuscript. Srikanth Gopi: acquisition of data. Kanav Kaushal: acquisition of data. Deepak Gunjan: Supervision, drafting of the manuscript. Namrata Singh: Supervision, drafting of the manuscript. Kumble S. Madhusudhan: acquisition of radiological data. Shyam S. Chauhan: acquisition of data. Mehar C. Sharma: acquisition of data. Virinder K. Bansal: acquisition of data. Anoop Saraya: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, Supervision.
Conflicts of interest
The authors have none to declare.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2021.05.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
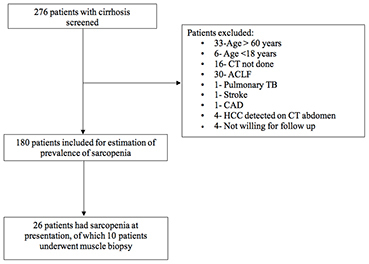
Flow diagram illustrating the number of participants evaluated in the diseased cohort. Two-hundred seventy-six patients were screened, 180 were included and 96 excluded due to reasons as mentioned.
Supplementary Figure 2.
Immunohistochemistry for annexin-V and caspase-3 in healthy controls and patients with sarcopenia. (A) Cytoplasmic staining (arrowhead) for Annexin-V in a healthy control. (B) Similar immunostaining for Annexin-V observed in patients with sarcopenia. (C) Cytoplasmic staining (arrowhead) for Caspase-3 in scattered muscle fibers in a healthy control. (D) Similar immunostaining for Caspase 3 in patients with sarcopenia.
Supplementary Figure 3.
Skeletal muscle expression of regulators of ubiquitin-proteasome pathway. (A) FBXO32 mRNA expression in patients with sarcopenia (n = 10) in comparison to healthy controls (n = 5) (P = 0.09). (B) TRIM63 mRNA expression in patients with sarcopenia (n = 10) versus healthy controls (n = 5) (P = 0.06).
References
- 1.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krell R.W., Kaul D.R., Martin A.R., et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396–1402. doi: 10.1002/lt.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montano-Loza A.J., Meza-Junco J., Prado C.M.M., et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173. doi: 10.1016/j.cgh.2011.08.028. 173.e1. [DOI] [PubMed] [Google Scholar]
- 4.DiMartini A., Cruz R.J., Dew M.A., et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19:1172–1180. doi: 10.1002/lt.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamaguchi Y., Kaido T., Okumura S., et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl. 2014;20:1413–1419. doi: 10.1002/lt.23970. [DOI] [PubMed] [Google Scholar]
- 6.Durand F., Buyse S., Francoz C., et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151–1157. doi: 10.1016/j.jhep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Carey E.J., Lai J.C., Wang C.W., et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017;23:625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey E.J., Lai J.C., Sonnenday C., et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology. 2019;70:1816–1829. doi: 10.1002/hep.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft A.J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Periyalwar P., Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong S., Wang L., Peng Z., et al. The mechanisms and treatments for sarcopenia: could exosomes be a perspective research strategy in the future? J Cachexia Sarcopenia Muscle. 2020;11:348–365. doi: 10.1002/jcsm.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.H., Jun H.-S. Role of myokines in regulating skeletal muscle mass and function. Front Physiol. 2019;10:42. doi: 10.3389/fphys.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu J., Thapaliya S., Runkana A., et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110:18162–18167. doi: 10.1073/pnas.1317049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsien C., Davuluri G., Singh D., et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han H.Q., Zhou X., Mitch W.E., Goldberg A.L. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol. 2013;45:2333–2347. doi: 10.1016/j.biocel.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Merli M., Giusto M., Molfino A., et al. MuRF-1 and p-GSK3β expression in muscle atrophy of cirrhosis. Liver Int. 2013;33:714–721. doi: 10.1111/liv.12128. [DOI] [PubMed] [Google Scholar]
- 17.Egerman M.A., Glass D.J. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49:59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiaffino S., Dyar K.A., Ciciliot S., Blaauw B., Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 19.Ebadi M., Bhanji R.A., Mazurak V.C., Montano-Loza A.J. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845–859. doi: 10.1007/s00535-019-01605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J., Brault J.J., Schild A., et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metabol. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Qiu J., Tsien C., Thapalaya S., et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:E983–E993. doi: 10.1152/ajpendo.00183.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thapaliya S., Runkana A., McMullen M.R., et al. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10:677–690. doi: 10.4161/auto.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrigo J., Marín T., Aguirre F., et al. N-acetyl cysteine attenuates the sarcopenia and muscle apoptosis induced by chronic liver disease. Curr Mol Med. 2019;20:60–71. doi: 10.2174/1566524019666190917124636. [DOI] [PubMed] [Google Scholar]
- 24.Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 25.Moreau R., Jalan R., Gines P., et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 26.Yip S.S.F., Parmar C., Blezek D., et al. Application of the 3D slicer chest imaging platform segmentation algorithm for large lung nodule delineation. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0178944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamin J., Shasthry V., Kaal C.R., et al. Characterization of body composition and definition of sarcopenia in patients with alcoholic cirrhosis: a computed tomography based study. Liver Int. 2017;37:1668–1674. doi: 10.1111/liv.13509. [DOI] [PubMed] [Google Scholar]
- 28.Tandon P., Low G., Mourtzakis M., et al. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1473–1480. doi: 10.1016/j.cgh.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 29.Kumari K., Sharma M., Kakkar A., et al. Role of mTOR signaling pathway in the pathogenesis of subependymal giant cell astrocytoma – a study of 28 cases. Neurol India. 2016;64:988. doi: 10.4103/0028-3886.190274. [DOI] [PubMed] [Google Scholar]
- 30.Pandey G., Bakhshi S., Thakur B., Jain P., Chauhan S.S. Prognostic significance of cathepsin L expression in pediatric acute myeloid leukemia. Leuk Lymphoma. 2018;59:2175–2187. doi: 10.1080/10428194.2017.1422865. [DOI] [PubMed] [Google Scholar]
- 31.Raji A., Seely E.W., Arky R.A., Simonson D.C. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86:5366–5371. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 32.Prado C.M.M., Lieffers J.R., McCargar L.J., et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 33.Tsien C., Garber A., Narayanan A., et al. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation: post-liver transplant sarcopenia. J Gastroenterol Hepatol. 2014;29:1250–1257. doi: 10.1111/jgh.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anand A., Mohta S., Agarwal S., et al. European Working Group on Sarcopenia in Older People (EWGSOP2) criteria with population-based skeletal muscle index best predicts mortality in Asians with cirrhosis. J Clin Exp Hepatol. 2021 doi: 10.1016/j.jceh.2021.03.015. S0973688321000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maschio G., D'Angelo A., Sirigu F., et al. Muscle biopsy studies in liver cirrhosis. Scand J Gastroenterol. 1971;6:363–368. doi: 10.3109/00365527109181134. [DOI] [PubMed] [Google Scholar]
- 36.Giusto M., Barberi L., Di Sario F., et al. Skeletal muscle myopenia in mice model of bile duct ligation and carbon tetrachloride-induced liver cirrhosis. Phys Rep. 2017;5 doi: 10.14814/phy2.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montano-Loza A.J., Angulo P., Meza-Junco J., et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis: sarcopenic obesity and myosteatosis in cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126–135. doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamaguchi Y., Kaido T., Okumura S., et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 2017;101:565–574. doi: 10.1097/TP.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 39.Nishikawa H., Enomoto H., Ishii A., et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J Cachexia Sarcopenia Muscle. 2017;8:915–925. doi: 10.1002/jcsm.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pascal L.E., True L.D., Campbell D.S., et al. Correlation of mRNA and protein levels: cell type-specific gene expression of cluster designation antigens in the prostate. BMC Genom. 2008;9:246. doi: 10.1186/1471-2164-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinn H.-P., Schneeweiss A., Keller M., et al. Comparison of immunohistochemistry with PCR for assessment of ER, PR, and Ki-67 and prediction of pathological complete response in breast cancer. BMC Canc. 2017;17:124. doi: 10.1186/s12885-017-3111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh S.S., Kumar A., Welch N., et al. Multiomics-identified intervention to restore ethanol-induced dysregulated proteostasis and secondary sarcopenia in alcoholic liver disease. Cell Physiol Biochem. 2021;55:91–116. doi: 10.33594/000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dasarathy S., McCullough A.J., Muc S., et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54:915–921. doi: 10.1016/j.jhep.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.