Abstract
T cells genetically engineered to recognize and eliminate tumor cells through synthetic chimeric antigen receptors (CARs) have demonstrated remarkable clinical efficacy against B cell leukemia over the past decade. This therapy is a form of highly personalized medicine that involves genetically modifying a patient’s T cells to recognize and kill cancer cells. With the FDA approval of 5 CAR T cell products, this approach has been validated as a powerful new drug in the therapeutic armamentarium against cancer. Researchers are now studying how to expand this technology beyond its use in conventional polyclonal αβ T cells to address limitations to the current therapy in cancer and applications beyond it. Considering the specific characteristics of immune cell from diverse lineages, several preclinical and clinical studies are under way to assess the advantages of CAR-redirected function in these cells and apply the lessons learned from CAR T cell therapy in cancer to other diseases.
Key words: adoptive cell therapy, cellular immunotherapy, chimeric antigen receptors
Graphical abstract

Chimeric antigen receptors (CARs) expressed in conventional polyclonal αβ T cells have demonstrated remarkable clinical efficacy against B cell leukemia. Current research is exploring ways to expand this technology to alternative immune cells in order to address limitations to current therapy in cancer and applications beyond it.
Introduction
Chimeric antigen receptor (CAR) T cells have achieved remarkable success in the treatment of blood cancers1, 2, 3, 4, 5, 6 and are a fast growing sector of immuno-oncology. This technology involves the genetic modification of autologous αβ T cells to express synthetic receptors against antigen present on tumor cells. These receptors enable modified T cells to recognize and produce an immunogenic response against cancer. To date, five CAR T cell therapies have been approved by the U.S. Food and Drug Administration (FDA) to treat B cell malignancies, four targeted toward CD19 (tisagenlecleucel, axicabtagene ciloleucel, brexucabtagene autoleucel, and lisocabtagene maraleucel) and one directed against B cell maturation agent (BCMA) (idecabtagene vicleucel).4,7, 8, 9, 10 These achievements have sparked new hope for the application of CARs to a wider range of cancers and other diseases. To this end, there are currently more than 500 clinical trials investigating the therapeutic efficacy of CAR T cells.11
Despite some clinical success, many challenges remain that limit the therapeutic applicability and efficacy of CAR T cells against a wider range of diseases. In cancer, obstacles to effective therapy include the reduced durability and tumor homing of CAR T cells, tumor antigen loss, the immunosuppressive tumor microenvironment (TME), and the time and cost associated with manufacturing autologous T cell products.12, 13, 14, 15 Moreover, serious toxicities associated with CAR T cell therapies, such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), have raised major concerns.
Investigators are dedicating tremendous effort worldwide to overcome these issues. A number of clinical and preclinical research are under way to replace the bespoke manufacturing needed with autologous, patient-derived CAR T cells products with donor-derived allogeneic CAR T products. However, the possibility of life-threatening graft-versus-host disease (GvHD) with an allogeneic cell therapy product has motivated researchers to use the CAR molecule in immune cells other than “conventional” polyclonal αβ T cells. These include “nonconventional” T cells, natural killer (NK) cells, and macrophages. In this review we discuss recent developments in CAR-based cellular immunotherapy using immune cell types aside from conventional T cells.
CARs in nonconventional T cells
Natural killer T cells
Type 1 natural killer T (NKT) cells are a small and distinct subset of αβ T cells that are CD1d restricted and express an invariant T cell receptor.16, 17, 18, 19 NKT cells mainly recognize self and foreign lipid antigens.16,20 Invariant natural killer T (iNKT) cells are a major subset of NKT cells and express the unique invariant Vα24Jα18 TCRα chain in humans.21 iNKT cells control the adaptive immune response by coordinating diverse immune cells such as CD4+/CD8+ T cells, NK cells, dendritic cells, and B cells.22, 23, 24, 25, 26
The immune regulatory potential and the rapid cytokine production of iNKT cells suggest that these cells may be harnessed for redirection with CAR. To this end, Poels et al.27 expressed CD38 or B cell maturation antigen-directed CARs in Vα24-iNKT cells and demonstrated effective elimination of multiple myeloma (MM) cells. Additionally, Xu et al.28 demonstrated potent anti-tumor activity by GD2-directed CAR NKT cells against neuroblastoma cells. In addition, autologous GD2-directed CAR NKT cells co-modified to secrete interleukin (IL)-15 demonstrated superior anti-tumor function, cellular persistence, and tumor infiltration compared with GD2-CAR-NKT cells without IL-15 against neuroblastoma. Interim reporting from a phase I clinical trial using these anti-GD2 CAR NKT cells (NCT03294954) demonstrated that the cells underwent in vivo expansion and localization to the tumor in patients.29 Another allogeneic CAR NKT cell (ANCHOR) clinical trial (NCT03774654) is ongoing to study anti-CD19 CAR NKT cells modified to secrete IL-15 in patients with lymphoma.30
Although the CAR NKT cell strategy is in its early stages, the inherent pro-inflammatory characteristics of NKT cells, such as robust cytokine production and rapid cytotoxicity,29 lend these cells great potential in adoptive cell therapy.
Cytokine-induced killer cells
Cytokine-induced killer (CIK) cells are a unique subset of cytotoxic T lymphocytes that demonstrate both T cell and NK cell phenotype and are capable of tumor killing in a major histocompatibility complex (MHC)-unrestricted manner.31, 32, 33 CIK cells are manufactured by ex vivo activation of peripheral blood mononuclear cells (PBMCs) or cord blood cells with interferon (IFN)-γ, anti-CD3 monoclonal antibody, and interleukin-2 for 2–3 weeks. These cells express NK cell markers in addition to T cell markers.34 Increased expression of NKG2D and DAP10 in CIK cells plays a major role in killing tumor cells.35 Importantly, the overproduction of IFN-γ by CIK cells results in reduced GvHD in mice.36,37
Because of the multivariate immunotherapeutic role of CIK cells, incorporation of CAR into this cell type has demonstrated clinical success.38 In a recent phase I and II clinical trial (NCT03389035), allogeneic anti-CD19 CAR-expressing CIK cells (CARCIK-CD19), engineered using the non-viral Sleeping Beauty (SB) transposon, demonstrated complete response (CR) or CR with incomplete blood count recovery (CRi) at day 28 in 6 of 7 patients with B cell acute lymphoblastic leukemia (B-ALL).38,39 Five of 6 patients who achieved CR in this study were minimal residual disease (MRD) negative, and no patients developed GvHD or neurotoxicity at the highest dose tested. Robust expansion of CARCIK-CD19 was observed in the majority of patients, and the CAR-expressing cells were detectable 10 months post-infusion. This research group also developed CD33-targeting CAR CIK cells using the SB transposon system for the treatment of acute myeloid leukemia (AML), which were significantly effective against AML and chemotherapy-resistant AML in a preclinical setting.40 In another study, Merker et al.41 engineered ERBB2-CAR CIK cells to treat high-risk rhabdomyosarcoma. This preclinical study demonstrated that ERBB2-CAR CIK cells eradicated RH30 tumors in mice but failed to clear tumor burden in relapsed disease. Finally, second-generation CAR CIK cells specific to chondroitin sulfate proteoglycan 4 (CSPG4) was developed by Leuci et al.42 to treat soft tissue sarcomas (STS) and demonstrated strong anti-tumor activity in STS spheroid and xenograft mouse models compared with unmodified CIK cells.
Taken together, the promising safety profile in patients in an allogeneic setting, the capacity for MHC-unrestricted cytotoxicity, and a unique NK cell-like phenotype make CAR CIK cells a viable alternative approach to target cancer.
γδ T cells
γδ T cells are a distinct subgroup of T cells based on the variable chains in the T cell receptor (TCR) protein complex.43 Although only 1%–5% of circulating T cells are γδ T cells, these cells are abundant at epithelial surfaces.44 γδ T cells demonstrate functional characteristics of both the innate and adoptive immune systems through expression of cytotoxic receptors such as NKG2D, NKp30, and/or NKp44 and Toll-like receptors (TLRs).45, 46, 47, 48, 49, 50 γδ T cells can kill cancer cells in a major histocompatibility complex-unrestricted manner, a function that distinguishes these cells from αβ T cells.51 Moreover, upon activation, γδ T cells can function as professional antigen-presenting cells to activate CD8 αβ T cells by cross-presenting tumor antigen.44
Given these characteristics, adoptive transfer of CAR-modified γδ T cells can be a viable potential therapeutic option for some cancer patients. A recent study demonstrated that γδ T cells expressing a GD2-targeting second-generation CAR improved the tumor killing and expansion capacity of unmodified γδ T cells, without impairing the cells’ ability for tumor-specific migration.44 Furthermore, a short-term (4 h) in vitro cytotoxicity assay in this study demonstrated that tumor lysis by GD2-specific CAR γδ T cells was equivalent to GD2-specific CAR αβ T cells. A separate study demonstrated that CD19-targeting CAR γδ T cells were effective in clearing disease from the bone marrow in a mice model of leukemia.52 Importantly, this study also showed that unlike conventional CD19-targeting CAR T cells, CAR γδ T cells were also capable of killing leukemia cells in a CD19-independent manner. This approach is noteworthy, as CD19 antigen loss is a major problem for patients who relapse after treatment with conventional CAR T cells.53, 54, 55 Thus, γδ CAR T cells may be able to overcome antigen escape. Additionally, as γδ CAR work in an MHC-independent manner and do not cause GvHD, these cells can be used in allogeneic setting.
Induced pluripotent stem cell-derived T cells
Many different strategies are under investigation for the development of “off-the-shelf” CAR T cell therapy. The tedious manufacturing process and high cost of autologous CAR T cells may exclude some patients from receiving this therapy. The use of induced pluripotent stem cells (iPSCs) as a starting source for CAR T cells is an attractive approach. Production and expansion of reprogrammed iPSC from antigen-specific cytotoxic T cell clones have demonstrated anti-tumor function in preclinical models.56, 57, 58, 59 Other approaches produce T lymphocytes from iPSCs in feeder cell or serum-free culture conditions.60, 61, 62 For example, iPSC clones can be generated by retroviral transduction of peripheral blood T lymphocytes with reprogramming factors KLF4, SOX2, OCT-4, and C-MYC.59 These transduced cells are then seeded onto mouse embryonic fibroblast (MEF) feeder cells in T cell media. T-iPSCs are generated after further culturing in embryonic stem cell (ESC) medium for 22–25 days. The resulting T-iPSCs were then lentivirally transduced to express a CD19-directed second-generation CAR (called 19-28zT-iPSC) and expanded on Delta like 1-expressing OP9 (OP9-DL1) feeder cells. The 19-28zT-iPSCs showed activation of T cell markers such as CD25 and CD69, secretion of cytokine markers such as IL-2, tumor necrosis factor (TNF)-α and interferon-γ, and reactivity against CD19 antigen, indicating successful generation of iPSC-derived CAR T cellular therapy. 19-28zT-iPSC lysed tumor cells in vitro and demonstrated anti-tumor function in vivo.
Despite the successful generation of CAR T iPSCs from PBMCs, the use of feeder cells at multiple stages has raised safety concerns for contamination in the final product. Thus, a feeder-free iPSC differentiation system can be a promising approach for cellular immunotherapies. A recent study by Iriguchi et al.60 outlined a feeder-free iPSC system to generate induced T (iT) cells. This approach involves inducing hematopoietic progenitor cells (HPCs) to form embryoid bodies, differentiation of HPCs into CD4+CD8αβ+ cells, differentiation of CD4+CD8αβ+ cells to CD8αβ+ single-positive cells, and finally expansion of CD8αβ+ single-positive cells. The feeder-free iPSC T cells are modified to express anti-CD19 CAR (iCART). These cells express low levels of CD45RA and CCR7 but increased levels of CD45RO and CD62L. iCART cells had no expression of PD1 and TIM3 exhaustion markers but did expressed LAG3 and exerted improved anti-tumor activity in vitro and in vivo against NALM-6 tumor-bearing mice compared with unmodified iT cells.
Regulatory T cells
Given the promising results in targeting hematological malignancies using CARs, efforts have been made to use CARs to redirect T cells for other purposes. Regulatory T cells (Tregs) are a subset of CD4+ T cells characterized by expression of the transcription factor FOXP3 and the immunophenotype CD25+, CD127low. Tregs promote immune tolerance through suppression of multiple immune effectors, including cytotoxic T cells. Adoptive transfer of Tregs for the treatment of multiple medical conditions is an active area of investigation.63,64 Several groups have explored the use of CARs to target Tregs to specific immune cell populations in preclinical models of graft-versus-host disease, organ transplantation, and multiple autoimmune diseases.
CARs targeted to major histocompatibility complex class I molecules have been shown by multiple groups to suppress the activity of alloreactive cytotoxic T cells without eliminating MHC I-expressing cells.65, 66, 67 Tregs expressing CARs on the basis of antibodies against the common MHC allele HLA-A2 (frequently referred to as A2-CAR) were shown to promote immune tolerance in multiple preclinical models of GvHD and tissue transplantation.65, 66, 67, 68, 69, 70 Forced overexpression of FOXP3 to maintain the Treg phenotype in A2-CAR Tregs has also been tested in preclinical GvHD systems.71 Furthermore, co-expressing IL-10 by A2-CAR Tregs enhanced the suppressive capacity of these cells.72 In a more flexible and modular approach, CAR Tregs were designed to bind monoclonal antibodies to MHC I molecules, and this system promoted allograft tolerance in multiple preclinical tissue transplantation settings.73 Immune cells besides MHC I-expressing T cells can also be targeted by CAR Tregs; for example, expression of CD19-targeted CARs in Tregs suppressed differentiation and IgG antibody production by CD19+ B cells, thereby reducing GvHD74 in one model system.
In addition to GvHD and tissue transplantation, the use of CAR Tregs has also been explored in autoimmune diseases. Some of the earliest work in the field demonstrated that CAR Tregs are able to reduce tissue inflammation in multiple preclinical models of autoimmune colitis75, 76, 77 and that this reduction in inflammation reduced subsequent colorectal tumor burden.77 Various CAR Treg approaches have also demonstrated activity in preclinical models of hemophilia A (HA), a clotting disorder characterized by deficiency of clotting factor VIII (FVIII), which is often associated with formation of antibodies that inhibit therapeutic recombinant FVIII. CAR Tregs targeting FVIII suppressed both B and T cell responses to therapeutic FVIII in HA models,78 similar to what was seen using a transgenic T cell receptor.79 T cells modified to express both FOXP3 and a CAR targeted to FVIII and sorted for a Treg immunophenotype inhibited proliferation of FVIII-specific effector T cells and improved clotting activity in a murine model of HA.80 Tregs expressing a CAR incorporating an FVIII antigen (termed a B cell-targeting antibody receptor [BAR]) were shown to inhibit B cells selectively, reducing levels of FVIII-specific antibodies.81,82 Preclinical activity of CAR Tregs has been showing in other autoimmune diseases, including reactive airway disease/asthma,83 rheumatoid arthritis,84 vitiligo,85 and multiple sclerosis.86 Tregs expressing CARs targeting insulin and pancreatic endocrine antigens have been designed to address type 1 diabetes. However, although these efforts have demonstrated proof of concept, they were ultimately unable to prevent diabetes in preclinical models.87,88
Importantly, several of these studies have demonstrated that the CAR molecule provides antigen-specific localization and activation of the CAR Tregs, thus providing an advantage of increased specificity compared with unmodified polyclonal Tregs. Additionally, compared with cytotoxic T cells, Tregs can suppress lymphocytes targeted to other antigens in the local microenvironment (often referred to as “bystander suppression”), without damaging normal cells that express the target antigen. This creates a localized zone of reduced inflammation and immune tolerance even when multiple or unknown antigens are targeted by endogenous effector cells and antibodies.
From a translational perspective, human CAR Tregs have been produced,89,90 and methods to generate CAR Tregs have been described.91,92 Multiple groups have explored the optimal costimulatory domain for CAR Tregs. These efforts have generally suggested that, in contrast to some observations with antineoplastic cytotoxic CAR T cells, CD28-based co-stimulation is superior to other costimulatory systems (e.g., 4-1BB) for CAR Treg activity.93,94 Similar to efforts in conventional CAR αβ T cells (reviewed by Rafiq et al.95), technologies to develop modular CAR Tregs for increased utility have been demonstrated.73,96
CAR NK cells
Natural killer cells are granular lymphocytes that are CD3− and express surface markers CD56+ and NKp46+. Although considered to be a part of innate immunity,97,98 NK cells are functionally parallel to CD8+ cytotoxic T cells and kill target cells through similar cytotoxic mechanisms. However, these cells lack a somatically rearranged and unique antigen-specific TCR.99, 100, 101 NK cells are able to recognize and kill infected cells, allogeneic cells, and tumor cells without prior sensitization in a non-HLA (human leukocyte antigen)-restricted manner. NK cells are able to exhibit potent anti-tumor immunity through multiple mechanisms.102 Activated NK cells are capable of eliminating tumor cells through direct cell cytotoxicity with perforin and granzymes and/or production of pro-inflammatory cytokines.103 In addition, cytokines and chemokines such as IFN-γ and granulocyte macrophage colony-stimulating factor (GM-CSF) produced by NK cells can modulate the immune responses of other cells.104,105
Because of several limitations with autologous αβ T cell therapy, NK cells represent a promising cell type for the application of CAR technology. First, allogeneic NK cells do not need complete HLA matching and cause little or no graft-versus-host disease compared with allogeneic T cells. This opens the possibility to produce “off-the-shelf” allogeneic cell therapy products that can be prepared in advance and are readily available for multiple patients on demand.106, 107, 108 In fact, preclinical data in mouse models suggest that NK cells may prevent GvHD in some cases by inhibiting activated alloreactive T cells while retaining beneficial graft-versus-tumor effects.108 On the contrary, CAR T cells can cause GvHD even when HLA matched, as minor mismatches may still be reactive.108 Additionally, the levels of cytokines release by NK cell infusions are moderate109 compared with the levels seen in life-threatening cases of CRS caused by CAR T cells.106 NK cells have a limited lifespan in circulation and do not produce memory cells except against some viruses. Thus, a primary NK cell-based CAR approach may not need to be modified to include a suicide gene as a safety switch to reduce the CRS and other harmful side effects of long-lasting CAR T cells such as on-target, off-tumor toxicities.14,110,111 However, suicide switches may still be necessary in CAR NK cells that are further modified to enhance in vivo expansion (133). A further potential advantage of using CAR NK cells is that NK cells can be derived from umbilical cord blood (UCB) or cell lines such as NK-92, both of which have the potential for use as “off-the-shelf” therapeutic products.106
NK cell source
PBMCs, UCB, CD34+ hematopoietic progenitor cells, NK cell lines (such as NK-92), and induced pluripotent stem cells can be used to manufacture clinical-grade NK cells. Because of the unlimited proliferative capability of NK-92 cells in vitro and consistent phenotypic and functional features in the presence of interleukin-2, these cells are a major source of CAR-NK cells.112, 113, 114, 115 NK-92 cells provide distinct advantages for use in manufacturing “off-the-shelf” CAR-NK products for clinical use. These include shorter manufacturing time and lower cost. NK-92 cells were initially derived from a non-Hodgkin lymphoma patient, and thus being a tumor-derived cell line, have inherent drawbacks such as the potential risk for tumorigenicity.116 NK-92 cells lack expression of FcγRIII and thus cannot mediate antibody-dependent cell-mediated cytotoxicity. Furthermore, these cells require lethal irradiation before clinical use, which diminishes in vivo expansion potential. These characteristics prevent NK-92 cells from being an ideal cell source for most CAR-NK cell therapy approaches.116,117
Another important source of primary NK cells is human PBMCs, which have been used in numerous clinical trials (see Table 1). Several methods have been used to easily isolate NK cells from healthy donors. The most commonly used method is depletion of CD3 cells followed by selection of CD56 cells that are cultured with IL-2. Good Manufacturing Practices (GMP)-grade NK cells can also be prepared using lymphokine-activated killer (LAK) cell culture media with IL-15 for 28 days.118, 119, 120 Activated PBMC-derived CAR NK cells expressing a wide range of activating receptors and can be administered without irradiation, allowing in vivo expansion. PBMC-derived NK (PB NK) cells, up to 90% of which are CD56dimCD16+, typically exhibit a mature phenotype, with increased cytotoxicity and reduced proliferative capacity.121
Table 1.
Current clinical trials of CAR engineered immune cells, excluding conventional αβ T cells
| CAR immune cells | ClinicalTrials.gov identifier | Target | Disease | Starting material | CAR construct | Sponsor | Purpose |
|---|---|---|---|---|---|---|---|
| NK cells | NCT03692767 | CD22 | refractory B cell lymphoma | NA | NA | Allife Medical Science and Technology Co., Ltd. | to investigate the safety and efficacy of anti-CD22 CAR NK |
| NCT03690310 | CD19 | refractory B cell lymphoma | NA | NA | Allife Medical Science and Technology Co., Ltd. | to assess the safety and efficacy of anti-CD19 CAR NK | |
| NCT03692637 | Mesothelin | epithelial ovarian cancer | NA | NA | Allife Medical Science and Technology Co., Ltd. | to investigate the safety and efficacy of anti-mesothelin CAR NK | |
| NCT03692663 | PSMA | Castration-resistant prostate cancer | NA | NA | Allife Medical Science and Technology Co., Ltd. | to evaluate the safety and feasibility of anti-PSMA CAR NK cells | |
| NCT03824964 | CD19/CD22 | refractory B cell lymphoma | NA | NA | Allife Medical Science and Technology Co., Ltd. | to study on the safety and efficacy of anti-CD19/CD22 CAR NK cells | |
| NCT03940833 | BCMA | R/R multiple myeloma | NK-92 cells | NA | Asclepius Technology Company Group (Suzhou) Co., Ltd. | to assess the safety and feasibility followed by BCMA CAR-NK cells | |
| NCT03941457 | ROBO1 | pancreatic cancer | NK-92 cells | NA | Asclepius Technology Company Group (Suzhou) Co., Ltd. | to assess safety profile followed by administration of BiCAR-NK cells | |
| NCT03940820 | ROBO1 | solid tumor | NK-92 cells | NA | Asclepius Technology Company Group (Suzhou) Co., Ltd. | to assess the safety and effectiveness of ROBO1 CAR-NK cells to treat solid tumors | |
| NCT04324996 | viral S protein and host NKG2DL | COVID-19 | UCB cells | NA | Chongqing Public Health Medical Center | to assess the safety, tolerability, and efficacy of NKG2D-ACE2 CAR-NK cells | |
| NCT04245722 | CD19 | B cell lymphoma, CLL | iPS cells | CAR.19-NKG2D-2B4-CD3ζ-IL15RF-hnCD16 | Fate Therapeutics | to assess the incidence, nature and safety of FT596 (anti-CD19 CAR NK) monotherapy and with combination of anti-CD20 antibody | |
| NCT03383978 | HER2 | GBM | NK-92 cells | anti-HER2 NK-92/5.28.z | Johann Wolfgang Goethe University Hospital | to determine safety, tolerability and maximum tolerated dose (MTD) or maximum feasible dose (MFD) followed by NK-92/5.28z. | |
| NCT04847466 | PDL-1 | gastroesophageal junction (GEJ) cancers, advanced HNSCC | NK-92 cells | NA | National Cancer Institute (NCI) | to determine the clinical response rate (CR + PR) with irradiated PD-L1 CAR-NK cells in combination with N-803 plus pembrolizumab | |
| NCT01974479 | CD19 | B-ALL | NK cells | anti-CD19-BB-zeta | National University Health System, Singapore | to determine the minimal disease residual (MRD) monitoring followed by NK cell infusion | |
| NCT02944162 | CD33 | AML | NK-92 cells | anti-CD33,28,BB and zeta | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | to determine safety profile followed by administration of anti-CD33 CAR-NK cells | |
| NCT02742727 | CD7 | lymphoma, leukemia | NK-92 cells | anti-CD7,28,BB and zeta | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | to determine safety profile followed by administration of the anti-CD7 CAR-pNK cells | |
| NCT02839954 | MUC1 | solid tumor | NK-92 cells | anti-MUC1-pNK | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | to determine safety profile followed by administration of the anti-MUC1 CAR-pNK cells | |
| NCT02892695 | CD19 | lymphoma, leukemia | NK-92 cells | anti-CD19,28,BB and zeta | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | to determine the adverse events attributed to the administration of the anti-CD19 CAR-NK cells | |
| NCT04887012 | CD19 | B cell non-Hodgkin lymphoma | NK cells | NA | Second Affiliated Hospital, School of Medicine, Zhejiang University | to study the safety and effectiveness of HLA haploidentical CAR-NK cells | |
| NCT00995137 | CD19 | B-ALL | NK cells | anti-CD19-BB-zeta | St. Jude Children's Research Hospital | to determine the maximum tolerated dose of genetically modified NK cells | |
| NCT03415100 | NKG2DL | metastatic solid tumor | NK cells | NA | The Third Affiliated Hospital of Guangzhou Medical University | to determine the anti-tumor response followed by CAR-NK cell infusions | |
| NCT04796675 | CD19 | ALL CLL NHL |
CB cells | NA | Wuhan Union Hospital, China | to evaluate the primary safety and efficacy of CAR-NK-CD19 | |
| NCT04639739 | CD19 | NHL | NA | NA | Xinqiao Hospital of Chongqing | to assess safety and efficacy of anti-CD19 CAR NK | |
| NCT03656705 | NA | solid tumor | NK cells | NA | Xinxiang medical university | to evaluate the safety and feasibility of CAR-NK cell treatment | |
| NCT03056339 | CD19 | relapsed and refractory B lymphoid malignancies | UCB | CD19-CD28-zeta-2A-iCasp9-IL15 | M.D. Anderson Cancer Center | to assess the optimal dose, toxicity, and efficacy of CD19-CD28-zeta-2A-iCasp9-IL15 | |
| NKT cells | NCT03774654 | CD19 | refractory B cell lymphoma, relapsed adult ALL relapsed CLL, relapsed non-Hodgkin lymphoma |
NKT cells | NA | Baylor College of Medicine | to determine the dose limiting toxicity (DLT) rate |
| NCT04814004 | CD19 | relapsed, refractory, or high-risk B cell malignancies | iNKT | hCD19.IL15.CAR-iNKT | Kai Lin Xu; Jun Nian Zheng and Xuzhou Medical University | to evaluate the safety and feasibility of IL-15-expressing anti-CD19 CAR iNKT (hCD19.IL15.CAR-iNKT) cells | |
| NCT03294954 | GD2 | neuroblastoma | NKT cells | CAR.GD2-CD28-CD3ζ-IL15 | Baylor College of Medicine | to determine the maximum tolerated dose (mTD) | |
| CAR γδ T cells | NCT04702841 | CD7 | T-ALL | γδ T cells | NA | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | to evaluate the efficacy of CAR - γδ T cells |
| NCT04107142 | NKG2DL | colorectal cancer, triple-negative breast cancer, sarcoma, nasopharyngeal carcinoma, prostate cancer, gastric cancer | γδ T cells | NA | CytoMed Therapeutics Pte Ltd | to evaluate the safety of CTM-N2D and the feasibility to produce CTM-N2D for three target dose levels | |
| NCT04735471 | CD20 | B cell malignancies | γδ T cells | NA | Adicet Bio, Inc | to assess the safety and efficacy study of ADI-001 anti-CD20 CAR-engineered allogeneic gamma delta T cells in adults with B cell malignancies, in monotherapy and combination with IL-2 | |
| CAR Tregs | NCT04817774 | HLA-A2 | kidney transplantation | Treg cells | NA | Sangamo Therapeutics | safety and tolerability of TX200-TR101 |
| CAR macrophages | NCT04660929 | HER2 | HER2-positive carcinoma | macrophages | NA | Carisma Therapeutics Inc | assess the safety and tolerability of CT-0508 and to assess the feasibility of manufacturing CT-0508 |
| CAR CIK cells | NCT03389035 | CD19 | B-ALL | CIK cells | CAR.19-CD28-OX40- CD3ζ | Fondazione Matilde Tettamanti Menotti De Marchi Onlus | feasibility and safety of a single dose of transposon-manipulated allogeneic CARCIK-CD19 |
| iPSC-derived CAR T cells | NCT04629729 | CD19 | B cell lymphoma, CLL, B cell ALL | iPS cells | NA | Fate Therapeutics | to assess the incidence, nature, and safety of FT819 (truncated TCR anti-CD19 CAR T) monotherapy and with combination with IL-2 |
NK cells can also be isolated from UCB or the placenta. Although about 1010 NK cells, with about 92.37% viability, can be obtained from a 21 day culture, these numbers are still limited for clinical use. Compared with PBMC-derived NK cells, UCB NK cells have an immature phenotype, with decreased expression of certain adhesion molecules such as CD2, CD11a, CD18, CD62L, CD16, KIRs, perforin, and granzyme B, and higher expression of inhibitory molecules such as NKG2A.122 This ultimately leads to lower tumor cell lysis potential against K562 cells.123
NK cells derived from induced pluripotent stem cells have become an attractive source of CAR NK cells because of the unlimited proliferative capacity of these cells124 with improved anti-tumor activity.125 Compared with differentiated NK cells, iPSCs can be more efficiently engineered to stably express CARs. Li et al. demonstrated that TrypLe-adapted CAR-expressing iPSCs can be cultured for 11 days in APEL culture media containing human stem cell factor (SCF), human vascular endothelial growth factor (VEGF), and recombinant human bone morphogenetic protein 4 (BMP4) differentiated into hematopoietic progenitor cells. CAR NK cells are then allowed to further differentiate with IL-3, IL-15, IL-7, SCF, and FLT3L in culture medium.115,125 This study demonstrated that NK cells derived from iPSCs (NK-CAR-iPSC-NK) significantly inhibited tumor growth and prolonged survival as compared with CAR T cells.
NK CAR designs
Considering the distinct properties of NK cells, several novel CAR constructs have been designed especially for signaling within these cells. The novel CARs impart specificity on NK cells and demonstrate improved cytotoxicity and cytokine production.126,127
CAR constructs contain four major structural elements: a target-specific binding domain, a hinge region, a transmembrane domain, and an intracellular signaling domain.14 Manipulating each of these components can significantly change the cellular behaviors and tumor-killing properties of the transduced cell. Thus, CAR NK cell function can greatly depend the CAR design. For instance, Imai et al.128 reported that a second-generation CD19-targeting scFv CAR with 4-1BB costimulatory and CD3ζ activation domain expressed in primary NK-92 cells demonstrated increased activation, interferon production, and markedly enhanced CD19-targeted killing of acute lymphoblastic leukemia (ALL). CD28 costimulatory domains have also been studied in CAR NK cells129 against HER2, EGFR or EGFR variant III, and CS1.130, 131, 132 Although 4-1BB/CD28-containing CARs are engineered for use in T cells, these constructs can exert anti-tumor activities when used in NK cells. Considering the need for NK-specific co-stimulation, domains such as DAP10, DAP12, and 2B4 are under investigation.133, 134, 135 NK cells with 2B4 costimulatory domains were reported to demonstrate enhanced cytotoxicity against CD5+ malignant T-ALL cells with rapid proliferation, increased cytokine production, and decreased apoptosis compared with transduced NK cells bearing the conventional 4-1BB containing CAR.136 In another study, CARs containing the DAP12 signaling domain demonstrated more efficient tumor lysis than receptors with the CD3ζ signaling domain.137 These results suggest that NK cell-specific activation signaling may affect CAR performance.136 An NK CAR construct optimization study was performed by Li et al.125 testing ten different anti-mesothelin CARs in NK-92 cells in cytotoxicity assays. Among the ten CAR structures studied, only the three that contained NKG2D transmembrane and 2B4 costimulatory domains exhibited enhanced expression of CD107a and increased in anti-tumor activity when stimulated by mesothelinhigh targets. The recruitment of DAP10 can be a possible mechanism for improved performance by these NKG2D TM and 2B4 under the condition of mesothelinhigh A1847 stimulation.115
In a recent study, CAR NK (S309-CAR-NK) cells were generated against the highly conserved epitope of spike (S) glycoproteins present on different variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viruses using an S309 scFv domain. This preclinical study demonstrated specific killing of S protein expressing A549 cells representing pseudo-SARS-CoV-2 virus138 and highlighted the potential for the use of CAR NK cells against infectious diseases.
Clinical trials with CAR NK cells
Despite the safety and promising clinical efficacy of unmodified allogeneic NK cells and the potential to address some of the key challenges associated with CAR T cells, the clinical translation of CAR NK cells is still in early stages of development.139
Several of the clinical trials reported thus far demonstrate promising activity against different types of cancer. For instance, early results from the first large-scale trial (NCT03056339) using CAR-NK cells derived from umbilical and cord blood (CB) demonstrated no increase in the levels of inflammatory cytokines and thus no CRS in patients with CD19+ CLL and B cell lymphoma.140 Of the 11 patients who were treated on this trial, 8 (73%) had objective responses, 7 (4 with lymphoma and 3 with CLL) had complete remission, and 1 had remission of the Richter’s transformation component but had persistent CLL. Although this study demonstrated promising preliminary results, most patients had short-term responses, and 5 of 8 patients received post-remission therapy.
As of August 2021, there are 25 studies registered at ClinicalTrials.gov evaluating the safety and efficacy of CAR-NK cells in cancer patients, including two additional completed phase I/II trials (Table 1). Among them, the safety data results from one of the trials (NCT00995137) are not available yet. The other phase I safety trial (NCT02944162) targeting CD33 in relapsed and refractory AML demonstrated no major adverse effect when dosed up to 5 × 109 cells per patient. However, this trial did not demonstrate any obvious clinical efficacy.141
CARs in other immune cells
Multipotent mesenchymal stem cells
Multipotent mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells, are a heterogeneous group of progenitor cells. MSCs were first isolated from the bone marrow stroma in late 1960s and are capable of forming colony-forming unit fibroblast (CFU-fibroblast).142, 143, 144 MSCs have self-renewal capacity and can differentiate into several other cell types, including adipocytes, osteoblasts, and chondrocytes.144, 145, 146, 147 Aside from the immune regulatory role of MSCs, the tumor-promoting phenotype of these cells has also been a focus of research.147, 148, 149, 150, 151, 152
Researchers hypothesize that the crosstalk between the immune suppressing effect of MSCs and tumor cells contributes to tumor growth and progression.151,153 Because of the high tumor-homing capacity of MSCs, including to metastatic lesions and less immunogenic milieu, these cells have become an attractive approach as targeted cellular therapy. A particularly innovative approach to infiltrating solid tumors is the modification of stromal cells themselves, rather than T cells. Multiple groups have investigated the modification of MSCs to express truncated CAR molecules that recognize tumor-associated antigens in order to enhance the innate ability of MSCs to infiltrate solid tumors. This approach was shown to enhance MSC tumor infiltration into glioma xenografts154 and ovarian xenografts155 and facilitated the delivery of the apoptosis-promoting molecule TRAIL to tumor cell in vitro.156
Additionally, Hombach et al.157 engineered MSCs to release IL-7 and IL-12 in the TME to create favorable environment for CAR T cells. The engineered MSCs demonstrated improved activation, proliferation, and cytokine production of carcinoembryonic antigen-targeting CAR T cells. It is well studied that IL-12 and IL-7 not only promote the cytotoxicity of CAR T cells but also modify the tumor microenvironment and enhance T cell infiltration and ultimately exert improved anti-tumor function.158, 159, 160, 161, 162, 163, 164 Thus, secretion of IL-7 and IL-12 by MSCs was an innovative approach to reduce solid cancer progression. Alternatively, Aliperta et al.165 demonstrated that expression of bispecific antibodies (bsAbs) by SCP-1 mesenchymal stromal cells redirected T cells against a CD33-expressing leukemia model.
Macrophages
Tumor-associated macrophages (TAMs) infiltrate solid tumor microenvironments and play role toward immune suppression and tumor progression.166, 167, 168 TAMs can make up to 50% of the total solid tumor mass.169 The ability to infiltrate tumors and the high prevalence of macrophages in tumor microenvironment make macrophages an attractive cell type for CAR expression and have motivated researchers to assess CAR-directed tumor cell phagocytosis by macrophages.
Morrissey et al.170 demonstrated that bone marrow-derived macrophages (BMDMs) engineered to express anti-CD19 CAR with a CD8 transmembrane domain and Megf10 and FcRɣ cytosolic domains (CAR-Ps) triggered antigen-dependent phagocytosis. CAR-P demonstrated enhanced engulfment of different sized antigen-coated beads and significantly reduced Raji cells numbers. Similarly, Klichinsky et al.171 developed CD19-directed CAR macrophages (CAR-Ms) containing a CD3ζ intracellular domain that demonstrated phagocytosis of tumor cells in an antigen-specific manner. Solid tumor targeting anti-mesothelin or anti-HER2 CAR macrophages eradicated antigen-positive cancer cells in vitro and in preclinical mouse models.
Macrophage Toll-like receptor-chimeric antigen receptors (MOTO-CARs) are engineered to add Toll/interleukin-1 receptor (TIR) signaling domain to polarized macrophages toward a pro-inflammatory M1 phenotype.172 A recent conference abstract demonstrated that these cells express high levels of CD14, CD80, and CD206 and low levels of CD163. As M1 macrophages have tumor suppressor activity, MOTO-CARs exhibited target-specific killing of solid tumor. Finally, a recent study of induced pluripotent stem cell-derived, CAR-expressing macrophage cells (CAR-iMac) showed similar enhanced antigen-induced tumor phagocytic properties.173
Apart from targeting cancer, CAR macrophages have been tested against SARS-CoV-2 viruses. A recent study by Fu et al.174 tested a series of CAR macrophages to target various recognition domains of the S protein present in SARS-CoV-2. This study demonstrated that MER tyrosine kinase (MERTK)-expressing CAR macrophages (CARMERTK) are capable of reducing viral load without upregulating of pro-inflammatory cytokines except IFN-γ, IL-6, and IL-8 in an in vitro setting.
Current limitations and next steps
Despite the remarkable success of conventional CAR αβ T cells in certain disease settings, expression of CAR in different immune cells is being explored to address the limitations of current therapy. These include the generation of off-the-shelf/allogeneic products such as NK cells, CIK cells, or γδ T cells to avoid manufacturing failures or using immune cells such as Tregs and macrophages with unique characteristics to address hurdles in cancer and other diseases.
However, similar to CAR T cells, other CAR-expressing cell therapies still face obstacles to clinical efficacy, such as loss of targeted antigen, tumor heterogeneity, and the hostile TME. Therefore, strategies should be considered to maximize the efficacy of CAR-based immune cell therapy in the future. Unlike T cells, many of these alternative cells can mediate cytotoxic activity against cancer cells in both CAR-dependent and CAR-independent manner. Therefore, a CAR can be developed to induce a moderate activating signal. For example, a CAR without costimulatory domains in NK cells could preferentially use the natural killing mechanism of NK cells and weakly use CAR-mediated direct cytotoxicity. One study reported that the use of a 4-1BB costimulatory domain caused excessive activation-induced cell death and reduced numeric expansion of NK cells compared with respective CD28-based constructs,28 indicating the careful consideration should be taken while designing CAR constructs in alternative immune cells. These cells could even be engineered to express a non-signaling CAR that does not induce a direct killing signal but promotes homing and adhesion to targets, allowing cytotoxicity triggered by natural killing mechanisms, with no or minimal on-target, off-tumor effects. In this scenario, a wider range of antigens could be used as targets, including antigens such as HER2, EGFR, and mesothelin that are preferentially expressed by tumor cells but also maintain low levels in some healthy cells.
Additionally, using advanced gene engineering technologies, immune cells can be developed to co-express other molecules, including cytokines, antibodies, protease, and others, that are able to promote immune cell proliferation, trafficking, and penetration into tumor. Engineered CAR cells that can express molecules to reprogram the tumor microenvironment, switching an immunosuppressive environment to an immuno-activating one, can encourage infiltration of endogenous immune cells to facilitate anti-tumor immune responses independent of the CAR-mediated cytotoxicity.175
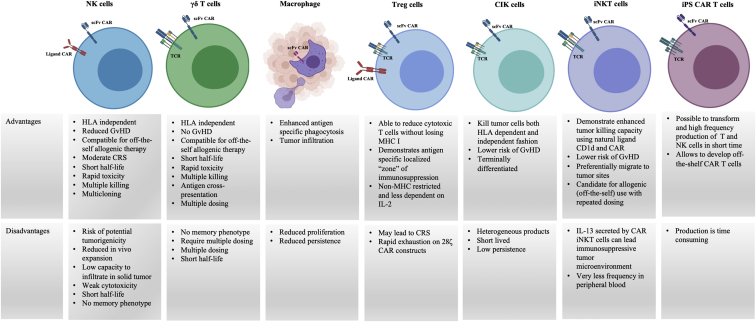
Finally, using these approaches to expand the use of CARs to diseases beyond cancer is promising and highlights the vast potential of this technology. Several preclinical CAR α/β T cell therapy studies are under active investigation to treat diseases such as cardiac fibrosis,176 HIV,177,178 aging,179 and type 1 diabetes.180 The use of other cellular platforms with CAR technology may widen the number of diseases that can be treated using this approach (Figure 1).
Figure 1.
Advantages and disadvantages of CAR engineered immune cells, excluding conventional αβ T cells
NK, natural killer; Treg, regulatory T cell; CIK, cytokine-induced killer; TCR, T cell receptor; HLA, human leukocyte antigen; CRS, cytokine release syndrome; GvHD, graft-versus-host disease. Figure created using BioRender.com
Acknowledgments
C.S.H. is supported by the Mortimer J. Lacher Foundation, the Lymphoma Society, the Druckenmiller Foundation, the Ira Schneider Memorial Foundation, NIH T32 CA009512-29A1, NIH K12 CA184746-05, NIH U01 CA256801-01, and the MSK Technology Development Fund. R.J.B. is supported by NIH K12 CA184746 06, NIH P01 CA190174 05, NIH R35 CA241894 01A1, NIH P01 CA023766 40, NIH P50 CA192937 05, NIH U01 CA256801 01, the Leukemia and Lymphoma Society, the Geoffrey Beene Cancer Research Center, the Center for Experimental Therapeutics, Cycle for Survival, and Center for Experimental Therapeutics Big Bets. S.R. acknowledges funding from Winship Invest$ and the Donaldson Charitable Trust Research Synergy Fund.
Author contributions
A.K.M.N.H, C.S.H., R.J.B., and S.R. planned the manuscript. A.K.M.N.H and C.S.H. wrote and coordinated the draft. A.K.M.N.H. drafted and designed Table 1 and the figures. R.J.B. and S.R. reviewed and revised the manuscript.
Declaration of interests
R.J.B. receives royalties and research funding from BMS/JUNO Therapeutics.
Contributor Information
Renier J. Brentjens, Email: renier.brentjens@roswellpark.org.
Sarwish Rafiq, Email: sarwish.rafiq@emory.edu.
References
- 1.Xin Yu J., Hubbard-Lucey V.M., Tang J. The global pipeline of cell therapies for cancer. Nat. Rev. Drug Discov. 2019;18:821–822. doi: 10.1038/d41573-019-00090-z. [DOI] [PubMed] [Google Scholar]
- 2.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 2016;15:235–247. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 3.Tang J., Pearce L., O'Donnell-Tormey J., Hubbard-Lucey V.M. Trends in the global immuno-oncology landscape. Nat. Rev. Drug Discov. 2018;17:783–784. doi: 10.1038/nrd.2018.167. [DOI] [PubMed] [Google Scholar]
- 4.June C.H., O'Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 5.Guedan S., Ruella M., June C.H. Emerging cellular therapies for cancer. Annu. Rev. Immunol. 2019;37:145–171. doi: 10.1146/annurev-immunol-042718-041407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins P.F., Kassim S.H., Tran T.L., Crystal J.S., Morgan R.A., Feldman S.A., Yang J.C., Dudley M.E., Wunderlich J.R., Sherry R.M., et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin. Cancer Res. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmikangas P., Kinsella N., Chamberlain P. Chimeric antigen receptor T-cells (CAR T-cells) for cancer immunotherapy - moving target for industry? Pharm. Res. 2018;35:152. doi: 10.1007/s11095-018-2436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M., Munoz J., Goy A., Locke F.L., Jacobson C.A., Hill B.T., Timmerman J.M., Holmes H., Jaglowski S., Flinn I.W., et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2020;382:1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramson J.S., Palomba M.L., Gordon L.I., Lunning M.A., Wang M., Arnason J., Mehta A., Purev E., Maloney D.G., Andreadis C., et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 10.First CAR-T Therapy to target BCMA gets FDA nod. Nat. Biotechnol. 2021;39:531. doi: 10.1038/s41587-021-00929-0. [DOI] [PubMed] [Google Scholar]
- 11.Albinger N., Hartman n, J., Ullrich E. Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Ther. 2021 doi: 10.1038/s41434-021-00246-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava S., Riddell S.R. Chimeric antigen receptor T cell therapy: challenges to bench-to-bedside efficacy. J. Immunol. 2018;200:459–468. doi: 10.4049/jimmunol.1701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans A.N., Lin H.K., Hossian A., Rafiq S. Using adoptive cellular therapy for localized protein secretion. Cancer J. 2021;27:159–167. doi: 10.1097/PPO.0000000000000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rafiq S., Hackett C.S., Brentjens R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterner R.C., Sterner R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfrey D.I., Stankovic S., Baxter A.G. Raising the NKT cell family. Nat. Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 17.Berzins S.P., Cochrane A.D., Pellicci D.G., Smyth M.J., Godfrey D.I. Limited correlation between human thymus and blood NKT cell content revealed by an ontogeny study of paired tissue samples. Eur. J. Immunol. 2005;35:1399–1407. doi: 10.1002/eji.200425958. [DOI] [PubMed] [Google Scholar]
- 18.Lee P.T., Putnam A., Benlagha K., Teyton L., Gottlieb P.A., Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J. Clin. Invest. 2002;110:793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montoya C.J., Pollard D., Martinson J., Kumari K., Wasserfall C., Mulder C.B., Rugeles M.T., Atkinson M.A., Landay A.L., Wilson S.B. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology. 2007;122:1–14. doi: 10.1111/j.1365-2567.2007.02647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey D.I., MacDonald H.R., Kronenberg M., Smyth M.J., Van Kaer L. NKT cells: what's in a name? Nat. Rev. Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 21.Rossjohn J., Pellicci D.G., Patel O., Gapin L., Godfrey D.I. Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermans I.F., Silk J.D., Gileadi U., Salio M., Mathew B., Ritter G., Schmidt R., Harris A.L., Old L., Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura H., Ohta A., Sekimoto M., Sato M., Iwakabe K., Nakui M., Yahata T., Meng H., Koda T., Nishimura S., et al. alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199:37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 24.Fujii S., Shimizu K., Smith C., Bonifaz L., Steinman R.M. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doherty D.G., Melo A.M., Moreno-Olivera A., Solomos A.C. Activation and regulation of B cell responses by invariant natural killer T cells. Front Immunol. 2018;9:1360. doi: 10.3389/fimmu.2018.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennan P.J., Brigl M., Brenner M.B. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 27.Poels R., Drent E., Lameris R., Katsarou A., Themeli M., van der Vliet H.J., de Gruijl T.D., van de Donk N., Mutis T. Preclinical evaluation of invariant natural killer T cells modified with CD38 or BCMA chimeric antigen receptors for multiple myeloma. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22031096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X., Huang W., Heczey A., Liu D., Guo L., Wood M., Jin J., Courtney A.N., Liu B., Di Pierro E.J., et al. NKT cells coexpressing a GD2-specific chimeric antigen receptor and IL15 show enhanced in vivo persistence and antitumor activity against neuroblastoma. Clin. Cancer Res. 2019;25:7126–7138. doi: 10.1158/1078-0432.CCR-19-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heczey A., Courtney A.N., Montalbano A., Robinson S., Liu K., Li M., Ghatwai N., Dakhova O., Liu B., Raveh-Sadka T., et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat. Med. 2020;26:1686–1690. doi: 10.1038/s41591-020-1074-2. [DOI] [PubMed] [Google Scholar]
- 30.Allogeneic NKT for Patients with Relapsed or Refractory B-Cell Malignancies (ANCHOR) https://clinicaltrials.gov/ct2/show/results/NCT03774654.
- 31.Pizzitola I., Anjos-Afonso F., Rouault-Pierre K., Lassailly F., Tettamanti S., Spinelli O., Biondi A., Biagi E., Bonnet D. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014;28:1596–1605. doi: 10.1038/leu.2014.62. [DOI] [PubMed] [Google Scholar]
- 32.Hombach A.A., Rappl G., Abken H. Arming cytokine-induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28-OX40 “super-stimulation”. Mol. Ther. 2013;21:2268–2277. doi: 10.1038/mt.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper M.A., Elliott J.M., Keyel P.A., Yang L., Carrero J.A., Yokoyama W.M. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. U S A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt-Wolf I.G., Negrin R.S., Kiem H.P., Blume K.G., Weissman I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verneris M.R., Karimi M., Baker J., Jayaswal A., Negrin R.S. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura R., Baker J., Beilhack A., Zeiser R., Olson J.A., Sega E.I., Karimi M., Negrin R.S. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood. 2008;112:2563–2574. doi: 10.1182/blood-2007-06-092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker J., Verneris M.R., Ito M., Shizuru J.A., Negrin R.S. Expansion of cytolytic CD8(+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97:2923–2931. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 38.Transposon-manipulated allogeneic CARCIK-CD19 cells in pediatric and adult patients with r/r ALL post HSCT. https://ClinicalTrials.gov/show/NCT03389035.
- 39.Magnani C.F., Gaipa G., Lussana F., Belotti D., Gritti G., Napolitano S., Matera G., Cabiati B., Buracchi C., Borleri G., et al. Sleeping Beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J. Clin. Invest. 2020;130:6021–6033. doi: 10.1172/JCI138473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rotiroti M.C., Buracchi C., Arcangeli S., Galimberti S., Valsecchi M.G., Perriello V.M., Rasko T., Alberti G., Magnani C.F., Cappuzzello C., et al. Targeting CD33 in chemoresistant AML patient-derived xenografts by CAR-CIK cells modified with an improved SB transposon system. Mol. Ther. 2020;28:1974–1986. doi: 10.1016/j.ymthe.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merker M., Wagner J., Kreyenberg H., Heim C., Moser L.M., Wels W.S., Bonig H., Ivics Z., Ullrich E., Klingebiel T., et al. ERBB2-CAR-Engineered cytokine-induced killer cells exhibit both CAR-mediated and innate immunity against high-risk rhabdomyosarcoma. Front Immunol. 2020;11:581468. doi: 10.3389/fimmu.2020.581468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leuci V., Donini C., Grignani G., Rotolo R., Mesiano G., Fiorino E., Gammaitoni L., D'Ambrosio L., Merlini A., Landoni E., et al. CSPG4-Specific CAR.CIK lymphocytes as a novel therapy for the treatment of multiple soft-tissue sarcoma histotypes. Clin. Cancer Res. 2020;26:6321–6334. doi: 10.1158/1078-0432.CCR-20-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janeway C.A., Jr. How the immune system works to protect the host from infection: a personal view. Proc. Natl. Acad. Sci. U S A. 2001;98:7461–7468. doi: 10.1073/pnas.131202998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capsomidis A., Benthall G., Van Acker H.H., Fisher J., Kramer A.M., Abeln Z., Majani Y., Gileadi T., Wallace R., Gustafsson K., et al. Chimeric antigen receptor-engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol. Ther. 2018;26:354–365. doi: 10.1016/j.ymthe.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groh V., Rhinehart R., Secrist H., Bauer S., Grabstein K.H., Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl. Acad. Sci. U S A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hudspeth K., Silva-Santos B., Mavilio D. Natural cytotoxicity receptors: broader expression patterns and functions in innate and adaptive immune cells. Front Immunol. 2013;4:69. doi: 10.3389/fimmu.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Correia D.V., Lopes A., Silva-Santos B. Tumor cell recognition by gammadelta T lymphocytes: T-cell receptor vs. NK-cell receptors. Oncoimmunology. 2013;2:e22892. doi: 10.4161/onci.22892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Correia M.P., Stojanovic A., Bauer K., Juraeva D., Tykocinski L.O., Lorenz H.M., Brors B., Cerwenka A. Distinct human circulating NKp30(+)FcepsilonRIgamma(+)CD8(+) T cell population exhibiting high natural killer-like antitumor potential. Proc. Natl. Acad. Sci. U S A. 2018;115:E5980–E5989. doi: 10.1073/pnas.1720564115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rincon-Orozco B., Kunzmann V., Wrobel P., Kabelitz D., Steinle A., Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J. Immunol. 2005;175:2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 50.Hedges J.F., Lubick K.J., Jutila M.A. Gamma delta T cells respond directly to pathogen-associated molecular patterns. J. Immunol. 2005;174:6045–6053. doi: 10.4049/jimmunol.174.10.6045. [DOI] [PubMed] [Google Scholar]
- 51.Vantourout P., Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rozenbaum M., Meir A., Aharony Y., Itzhaki O., Schachter J., Bank I., Jacoby E., Besser M.J. Gamma-Delta CAR-T cells show CAR-directed and independent activity against leukemia. Front Immunol. 2020;11:1347. doi: 10.3389/fimmu.2020.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu H., Sotillo E., Harrington C., Wertheim G., Paessler M., Maude S.L., Rheingold S.R., Grupp S.A., Thomas-Tikhonenko A., Pillai V. Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. Am. J. Hematol. 2017;92:E11–E13. doi: 10.1002/ajh.24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shalabi H., Kraft I.L., Wang H.W., Yuan C.M., Yates B., Delbrook C., Zimbelman J.D., Giller R., Stetler-Stevenson M., Jaffe E.S., et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 2018;103:e215–e218. doi: 10.3324/haematol.2017.183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orlando E.J., Han X., Tribouley C., Wood P.A., Leary R.J., Riester M., Levine J.E., Qayed M., Grupp S.A., Boyer M., et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 2018;24:1504–1506. doi: 10.1038/s41591-018-0146-z. [DOI] [PubMed] [Google Scholar]
- 56.Vizcardo R., Masuda K., Yamada D., Ikawa T., Shimizu K., Fujii S., Koseki H., Kawamoto H. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Minagawa A., Yoshikawa T., Yasukawa M., Hotta A., Kunitomo M., Iriguchi S., Takiguchi M., Kassai Y., Imai E., Yasui Y., et al. Enhancing T cell receptor stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy. Cell Stem Cell. 2018;23:850–858.e854. doi: 10.1016/j.stem.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Maeda T., Nagano S., Ichise H., Kataoka K., Yamada D., Ogawa S., Koseki H., Kitawaki T., Kadowaki N., Takaori-Kondo A., et al. Regeneration of CD8alphabeta T cells from T-cell-derived iPSC imparts potent tumor antigen-specific cytotoxicity. Cancer Res. 2016;76:6839–6850. doi: 10.1158/0008-5472.CAN-16-1149. [DOI] [PubMed] [Google Scholar]
- 59.Themeli M., Kloss C.C., Ciriello G., Fedorov V.D., Perna F., Gonen M., Sadelain M. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol. 2013;31:928–933. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iriguchi S., Yasui Y., Kawai Y., Arima S., Kunitomo M., Sato T., Ueda T., Minagawa A., Mishima Y., Yanagawa N., et al. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat. Commun. 2021;12:430. doi: 10.1038/s41467-020-20658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagano S., Maeda T., Ichise H., Kashima S., Ohtaka M., Nakanishi M., Kitawaki T., Kadowaki N., Takaori-Kondo A., Masuda K., Kawamoto H. High frequency production of T cell-derived iPSC clones capable of generating potent cytotoxic T cells. Mol. Ther. Methods Clin. Dev. 2020;16:126–135. doi: 10.1016/j.omtm.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kennedy M., Awong G., Sturgeon C.M., Ditadi A., LaMotte-Mohs R., Zuniga-Pflucker J.C., Keller G. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Ferreira L.M.R., Muller Y.D., Bluestone J.A., Tang Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov. 2019;18:749–769. doi: 10.1038/s41573-019-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raffin C., Vo L.T., Bluestone J.A. T-reg cell-based therapies: challenges and perspectives. Nat. Rev. Immunol. 2020;20:158–172. doi: 10.1038/s41577-019-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noyan F., Zimmermann K., Hardtke-Wolenski M., Knoefel A., Schulde E., Geffers R., Hust M., Huehn J., Galla M., Morgan M., et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. Am. J. Transpl. 2017;17:917–930. doi: 10.1111/ajt.14175. [DOI] [PubMed] [Google Scholar]
- 66.MacDonald K.G., Hoeppli R.E., Huang Q., Gillies J., Luciani D.S., Orban P.C., Broady R., Levings M.K. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boardman D.A., Philippeos C., Fruhwirth G.O., Ibrahim M.A., Hannen R.F., Cooper D., Marelli-Berg F.M., Watt F.M., Lechler R.I., Maher J., et al. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am. J. Transpl. 2017;17:931–943. doi: 10.1111/ajt.14185. [DOI] [PubMed] [Google Scholar]
- 68.Bezie S., Charreau B., Vimond N., Lasselin J., Gerard N., Nerriere-Daguin V., Bellier-Waast F., Duteille F., Anegon I., Guillonneau C. Human CD8+ Tregs expressing a MHC-specific CAR display enhanced suppression of human skin rejection and GVHD in NSG mice. Blood Adv. 2019;3:3522–3538. doi: 10.1182/bloodadvances.2019000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dawson N.A., Lamarche C., Hoeppli R.E., Bergqvist P., Fung V.C., McIver E., Huang Q., Gillies J., Speck M., Orban P.C., et al. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sicard A., Lamarche C., Speck M., Wong M., Rosado-Sanchez I., Blois M., Glaichenhaus N., Mojibian M., Levings M.K. Donor-specific chimeric antigen receptor Tregs limit rejection in naive but not sensitized allograft recipients. Am. J. Transpl. 2020;20:1562–1573. doi: 10.1111/ajt.15787. [DOI] [PubMed] [Google Scholar]
- 71.Martin A., Daris M., Johnston J.A., Cui J.J. HLA-A∗02:01-directed chimeric antigen receptor/forkhead box P3-engineered CD4+T cells adopt a regulatory phenotype and suppress established graft-versus-host disease. Cytotherapy. 2021;23:131–136. doi: 10.1016/j.jcyt.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 72.Mohseni Y.R., Saleem A., Tung S.L., Dudreuilh C., Lang C., Peng Q., Volpe A., Adigbli G., Cross A., Hester J., et al. Chimeric antigen receptor-modified human regulatory T cells that constitutively express IL-10 maintain their phenotype and are potently suppressive. Eur. J. Immunol. 2021 doi: 10.1002/eji.202048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pierini A., Iliopoulou B.P., Peiris H., Perez-Cruz M., Baker J., Hsu K., Gu X., Zheng P.P., Erkers T., Tang S.W., et al. T cells expressing chimeric antigen receptor promote immune tolerance. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imura Y., Ando M., Kondo T., Ito M., Yoshimura A. CD19-targeted CAR regulatory T cells suppress B cell pathology without GvHD. Jci Insight. 2020;5 doi: 10.1172/jci.insight.136185. ARTN e136185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elinav E., Waks T., Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology. 2008;134:2014–2024. doi: 10.1053/j.gastro.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 76.Elinav E., Adam N., Waks T., Eshhar Z. Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology. 2009;136:1721–1731. doi: 10.1053/j.gastro.2009.01.049. [DOI] [PubMed] [Google Scholar]
- 77.Blat D., Zigmond E., Alteber Z., Waks T., Eshhar Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol. Ther. 2014;22:1018–1028. doi: 10.1038/mt.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoon J., Schmidt A., Zhang A.H., Konigs C., Kim Y.C., Scott D.W. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood. 2017;129:238–245. doi: 10.1182/blood-2016-07-727834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim Y.C., Zhang A.H., Su Y., Rieder S.A., Rossi R.J., Ettinger R.A., Pratt K.P., Shevach E.M., Scott D.W. Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood. 2015;125:1107–1115. doi: 10.1182/blood-2014-04-566786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu R.Y., Chen A.C., Lyle M.J., Chen C.Y., Liu C.L., Miao C.H. CD4(+) T cells engineered with FVIII-CAR and murine Foxp3 suppress anti-factor VIII immune responses in hemophilia a mice. Cell Immunol. 2020;358 doi: 10.1016/j.cellimm.2020.104216. ARTN 104216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang A.H., Yoon J., Kim Y.C., Scott D.W. Targeting antigen-specific B cells using antigen-expressing transduced regulatory T cells. J. Immunol. 2018;201:1434–1441. doi: 10.4049/jimmunol.1701800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pohl A.D., Venkatesha S.H., Zhang A.H., Scott D.W. Suppression of FVIII-specific memory B cells by chimeric BAR receptor-engineered natural regulatory T cells. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00693. ARTN 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skuljec J., Chmielewski M., Happle C., Habener A., Busse M., Abken H., Hansen G. Chimeric antigen receptor-redirected regulatory T cells suppress experimental allergic airway inflammation, a model of asthma. Front Immunol. 2017;8:1125. doi: 10.3389/fimmu.2017.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raffin C., Muller Y., Barragan J., Zhou Y., Piccoli L., Lanzavecchia A., et al. Development of citrullinated-vimentin-specific CAR for targeting Tregs to treat autoimmune rheumatoid arthritis. J. Immunol. 2018;200:176.17. [Google Scholar]
- 85.Mukhatayev Z., Dellacecca E.R., Cosgrove C., Shivde R., Jaishankar D., Pontarolo-Maag K., Eby J.M., Henning S.W., Ostapchuk Y.O., Cedercreutz K., et al. Antigen specificity enhances disease control by Tregs in vitiligo. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.581433. ARTN 581433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fransson M., Piras E., Burman J., Nilsson B., Essand M., Lu B., Harris R.A., Magnusson P.U., Brittebo E., Loskog A.S. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J. Neuroinflammation. 2012;9:112. doi: 10.1186/1742-2094-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tenspolde M., Zimmermann K., Weber L.C., Hapke M., Lieber M., Dywicki J., Frenzel A., Hust M., Galla M., Buitrago-Molina L.E., et al. Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. J. Autoimmun. 2019;103:102289. doi: 10.1016/j.jaut.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 88.Radichev I.A., Yoon J., Scott D.W., Griffin K., Savinov A.Y. Towards antigen-specific Tregs for type 1 diabetes: construction and functional assessment of pancreatic endocrine marker, HPi2-based chimeric antigen receptor. Cell Immunol. 2020;358 doi: 10.1016/j.cellimm.2020.104224. ARTN 104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hombach A.A., Kofler D., Rappl G., Abken H. Redirecting human CD4+CD25+ regulatory T cells from the peripheral blood with pre-defined target specificity. Gene Ther. 2009;16:1088–1096. doi: 10.1038/gt.2009.75. [DOI] [PubMed] [Google Scholar]
- 90.Lee J.C., Hayman E., Pegram H.J., Santos E., Heller G., Sadelain M., Brentjens R. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 2011;71:2871–2881. doi: 10.1158/0008-5472.CAN-10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vimond N., Lasselin J., Anegon I., Guillonneau C., Bezie S. Genetic engineering of human and mouse CD4(+) and CD8(+) Tregs using lentiviral vectors encoding chimeric antigen receptors. Mol. Ther-meth Clin. D. 2021;20:69–85. doi: 10.1016/j.omtm.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fritsche E., Volk H.D., Reinke P., Abou-El-Enein M. Toward an optimized process for clinical manufacturing of CAR-treg cell therapy. Trends Biotechnol. 2020;38:1099–1112. doi: 10.1016/j.tibtech.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 93.Dawson N.A.J., Rosado-Sanchez I., Novakovsky G.E., Fung V.C.W., Huang Q., McIver E., Sun G., Gillies J., Speck M., Orban P.C., et al. Functional effects of chimeric antigen receptor co-receptor signaling domains in human regulatory T cells. Sci. Translational Med. 2020;12 doi: 10.1126/scitranslmed.aaz3866. ARTN eaaz3866. [DOI] [PubMed] [Google Scholar]
- 94.Boroughs A.C., Larson R.C., Choi B.D., Bouffard A.A., Riley L.S., Schiferle E., Kulkarni A.S., Cetrulo C.L., Ting D., Blazar B.R., et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight. 2019;5 doi: 10.1172/jci.insight.126194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rafiq S., Hackett C.S., Brentjens R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019 doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koristka S., Kegler A., Bergmann R., Arndt C., Feldmann A., Albert S., Cartellieri M., Ehninger A., Ehninger G., Middeke J.M., et al. Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J. Autoimmun. 2018;90:116–131. doi: 10.1016/j.jaut.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 97.Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N., Mebius R.E., et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 98.Ishizuka I.E., Constantinides M.G., Gudjonson H., Bendelac A. The innate lymphoid cell precursor. Annu. Rev. Immunol. 2016;34:299–316. doi: 10.1146/annurev-immunol-041015-055549. [DOI] [PubMed] [Google Scholar]
- 99.Herberman R.B., Nunn M.E., Holden H.T., Lavrin D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer. 1975;16:230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 100.Wang F., Lau J.K.C., Yu J. The role of natural killer cell in gastrointestinal cancer: killer or helper. Oncogene. 2021;40:717–730. doi: 10.1038/s41388-020-01561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun J.C., Lanier L.L. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat. Rev. Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mehta R.S., Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol. 2018;9:283. doi: 10.3389/fimmu.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Childs R.W., Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat. Rev. Drug Discov. 2015;14:487–498. doi: 10.1038/nrd4506. [DOI] [PubMed] [Google Scholar]
- 104.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 105.Delneste Y., Charbonnier P., Herbault N., Magistrelli G., Caron G., Bonnefoy J.Y., Jeannin P. Interferon-gamma switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101:143–150. doi: 10.1182/blood-2002-04-1164. [DOI] [PubMed] [Google Scholar]
- 106.Hu Y., Tian Z.G., Zhang C. Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol. Sin. 2018;39:167–176. doi: 10.1038/aps.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller J.S., Soignier Y., Panoskaltsis-Mortari A., McNearney S.A., Yun G.H., Fautsch S.K., McKenna D., Le C., Defor T.E., Burns L.J., et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 108.Olson J.A., Leveson-Gower D.B., Gill S., Baker J., Beilhack A., Negrin R.S. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:e28147. doi: 10.4161/onci.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Glienke W., Esser R., Priesner C., Suerth J.D., Schambach A., Wels W.S., Grez M., Kloess S., Arseniev L., Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol. 2015;6:21. doi: 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu S.Y., Fu T., Jiang Y.Z., Shao Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer. 2020;19:120. doi: 10.1186/s12943-020-01238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gong J.H., Maki G., Klingemann H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 113.Klingemann H., Boissel L., Toneguzzo F. Natural killer cells for immunotherapy - advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7:91. doi: 10.3389/fimmu.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suck G., Odendahl M., Nowakowska P., Seidl C., Wels W.S., Klingemann H.G., Tonn T. NK-92: an 'off-the-shelf therapeutic' for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2016;65:485–492. doi: 10.1007/s00262-015-1761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie G., Dong H., Liang Y., Ham J.D., Rizwan R., Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rezvani K., Rouce R., Liu E., Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol. Ther. 2017;25:1769–1781. doi: 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang J., Zheng H., Diao Y. Natural killer cells and current applications of chimeric antigen receptor-modified NK-92 cells in tumor immunotherapy. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schonfeld K., Sahm C., Zhang C., Naundorf S., Brendel C., Odendahl M., Nowakowska P., Bonig H., Kohl U., Kloess S., et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol. Ther. 2015;23:330–338. doi: 10.1038/mt.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Klingemann H. Challenges of cancer therapy with natural killer cells. Cytotherapy. 2015;17:245–249. doi: 10.1016/j.jcyt.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 120.Becker P.S., Suck G., Nowakowska P., Ullrich E., Seifried E., Bader P., Tonn T., Seidl C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016;65:477–484. doi: 10.1007/s00262-016-1792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poli A., Michel T., Theresine M., Andres E., Hentges F., Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sarvaria A., Jawdat D., Madrigal J.A., Saudemont A. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol. 2017;8:329. doi: 10.3389/fimmu.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luevano M., Daryouzeh M., Alnabhan R., Querol S., Khakoo S., Madrigal A., Saudemont A. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum. Immunol. 2012;73:248–257. doi: 10.1016/j.humimm.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 124.Valamehr B., Robinson M., Abujarour R., Rezner B., Vranceanu F., Le T., Medcalf A., Lee T.T., Fitch M., Robbins D., Flynn P. Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Rep. 2014;2:366–381. doi: 10.1016/j.stemcr.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Y., Hermanson D.L., Moriarity B.S., Kaufman D.S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23:181–192.e185. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kotanides H., Sattler R.M., Lebron M.B., Carpenito C., Shen J., Li J., Surguladze D., Haidar J.N., Burns C., Shen L., et al. Characterization of 7A5: a human CD137 (4-1BB) receptor binding monoclonal antibody with differential agonist properties that promotes antitumor immunity. Mol. Cancer Ther. 2020;19:988–998. doi: 10.1158/1535-7163.MCT-19-0893. [DOI] [PubMed] [Google Scholar]
- 127.Lanier L.L. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Imai C., Iwamoto S., Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marofi F., Saleh M.M., Rahman H.S., Suksatan W., Al-Gazally M.E., Abdelbasset W.K., Thangavelu L., Yumashev A.V., Hassanzadeh A., Yazdanifar M., et al. CAR-engineered NK cells; a promising therapeutic option for treatment of hematological malignancies. Stem Cell Res Ther. 2021;12:374. doi: 10.1186/s13287-021-02462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kruschinski A., Moosmann A., Poschke I., Norell H., Chmielewski M., Seliger B., Kiessling R., Blankenstein T., Abken H., Charo J. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc. Natl. Acad. Sci. U S A. 2008;105:17481–17486. doi: 10.1073/pnas.0804788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chu J., Deng Y., Benson D.M., He S., Hughes T., Zhang J., Peng Y., Mao H., Yi L., Ghoshal K., et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28:917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Han J., Chu J., Keung Chan W., Zhang J., Wang Y., Cohen J.B., Victor A., Meisen W.H., Kim S.H., Grandi P., et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci. Rep. 2015;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Billadeau D.D., Upshaw J.L., Schoon R.A., Dick C.J., Leibson P.J. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat. Immunol. 2003;4:557–564. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 134.Lanier L.L., Corliss B.C., Wu J., Leong C., Phillips J.H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 135.Nakajima H., Colonna M. 2B4: an NK cell activating receptor with unique specificity and signal transduction mechanism. Hum. Immunol. 2000;61:39–43. doi: 10.1016/s0198-8859(99)00170-6. [DOI] [PubMed] [Google Scholar]
- 136.Xu Y., Liu Q., Zhong M., Wang Z., Chen Z., Zhang Y., Xing H., Tian Z., Tang K., Liao X., et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J. Hematol. Oncol. 2019;12:49. doi: 10.1186/s13045-019-0732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Topfer K., Cartellieri M., Michen S., Wiedemuth R., Muller N., Lindemann D., Bachmann M., Fussel M., Schackert G., Temme A. DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J. Immunol. 2015;194:3201–3212. doi: 10.4049/jimmunol.1400330. [DOI] [PubMed] [Google Scholar]
- 138.Ma M.T., Badeti S., Chen C.H., Kim J., Choudhary A., Honnen B., Reichman C., Calianese D., Pinter A., Jiang Q., et al. CAR-NK cells effectively target SARS-CoV-2-spike-expressing cell lines in vitro. Front Immunol. 2021;12:652223. doi: 10.3389/fimmu.2021.652223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Romee R., Rosario M., Berrien-Elliott M.M., Wagner J.A., Jewell B.A., Schappe T., Leong J.W., Abdel-Latif S., Schneider S.E., Willey S., et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl Med. 2016;8:357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., Nassif Kerbauy L., Overman B., Thall P., Kaplan M., et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020;382:545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tang X., Yang L., Li Z., Nalin A.P., Dai H., Xu T., Yin J., You F., Zhu M., Shen W., et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 2018;8:1083–1089. [PMC free article] [PubMed] [Google Scholar]
- 142.Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. The development of fibroblast colonies in monolayer cultures of Guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 143.Luria E.A., Panasyuk A.F., Friedenstein A.Y. Fibroblast colony formation from monolayer cultures of blood cells. Transfusion. 1971;11:345–349. doi: 10.1111/j.1537-2995.1971.tb04426.x. [DOI] [PubMed] [Google Scholar]
- 144.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 145.Shi Y., Su J., Roberts A.I., Shou P., Rabson A.B., Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou B.O., Yue R., Murphy M.M., Peyer J.G., Morrison S.J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Quante M., Tu S.P., Tomita H., Gonda T., Wang S.S., Takashi S., Baik G.H., Shibata W., Diprete B., Betz K.S., et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ren G., Zhao X., Wang Y., Zhang X., Chen X., Xu C., Yuan Z.R., Roberts A.I., Zhang L., Zheng B., et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell. 2012;11:812–824. doi: 10.1016/j.stem.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 150.Ramasamy R., Lam E.W., Soeiro I., Tisato V., Bonnet D., Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 151.Djouad F., Plence P., Bony C., Tropel P., Apparailly F., Sany J., Noel D., Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 152.Ame-Thomas P., Maby-El Hajjami H., Monvoisin C., Jean R., Monnier D., Caulet-Maugendre S., Guillaudeux T., Lamy T., Fest T., Tarte K. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109:693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- 153.Karnoub A.E., Dash A.B., Vo A.P., Sullivan A., Brooks M.W., Bell G.W., Richardson A.L., Polyak K., Tubo R., Weinberg R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 154.Balyasnikova I.V., Ferguson S.D., Sengupta S., Han Y., Lesniak M.S. Mesenchymal stem cells modified with a single-chain antibody against EGFRvIII successfully inhibit the growth of human xenograft malignant glioma. PLoS One. 2010;5:e9750. doi: 10.1371/journal.pone.0009750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Komarova S., Roth J., Alvarez R., Curiel D.T., Pereboeva L. Targeting of mesenchymal stem cells to ovarian tumors via an artificial receptor. J. Ovarian Res. 2010;3:12. doi: 10.1186/1757-2215-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Golinelli G., Grisendi G., Prapa M., Bestagno M., Spano C., Rossignoli F., Bambi F., Sardi I., Cellini M., Horwitz E.M., et al. Targeting GD2-positive glioblastoma by chimeric antigen receptor empowered mesenchymal progenitors. Cancer Gene Ther. 2020;27:558–570. doi: 10.1038/s41417-018-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hombach A.A., Geumann U., Gunther C., Hermann F.G., Abken H. IL7-IL12 engineered mesenchymal stem cells (MSCs) improve A CAR T cell attack against colorectal cancer cells. Cells. 2020;9 doi: 10.3390/cells9040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Agliardi G., Liuzzi A.R., Hotblack A., De Feo D., Nunez N., Stowe C.L., Friebel E., Nannini F., Rindlisbacher L., Roberts T.A., et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat. Commun. 2021;12:444. doi: 10.1038/s41467-020-20599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Adachi K., Kano Y., Nagai T., Okuyama N., Sakoda Y., Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018;36:346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 160.Kerkar S.P., Goldszmid R.S., Muranski P., Chinnasamy D., Yu Z., Reger R.N., Leonardi A.J., Morgan R.A., Wang E., Marincola F.M., et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tugues S., Burkhard S.H., Ohs I., Vrohlings M., Nussbaum K., Vom Berg J., Kulig P., Becher B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22:237–246. doi: 10.1038/cdd.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fry T.J., Mackall C.L. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–571. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 163.Bradley L.M., Haynes L., Swain S.L. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005;26:172–176. doi: 10.1016/j.it.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 164.Jiang Q., Li W.Q., Aiello F.B., Mazzucchelli R., Asefa B., Khaled A.R., Durum S.K. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 165.Aliperta R., Cartellieri M., Feldmann A., Arndt C., Koristka S., Michalk I., von Bonin M., Ehninger A., Bachmann J., Ehninger G., et al. Bispecific antibody releasing-mesenchymal stromal cell machinery for retargeting T cells towards acute myeloid leukemia blasts. Blood Cancer J. 2015;5:e348. doi: 10.1038/bcj.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Biswas S.K., Allavena P., Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin. Immunopathol. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 167.Zhang Q.W., Liu L., Gong C.Y., Shi H.S., Zeng Y.H., Wang X.Z., Zhao Y.W., Wei Y.Q. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Medrek C., Ponten F., Jirstrom K., Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Murdoch C., Giannoudis A., Lewis C.E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 170.Morrissey M.A., Williamson A.P., Steinbach A.M., Roberts E.W., Kern N., Headley M.B., Vale R.D. Chimeric antigen receptors that trigger phagocytosis. Elife. 2018;7 doi: 10.7554/eLife.36688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Klichinsky M., Ruella M., Shestova O., Lu X.M., Best A., Zeeman M., Schmierer M., Gabrusiewicz K., Anderson N.R., Petty N.E., et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020;38:947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Velazquez E.J., Lattin J.E., Brindley T.D., Reinstein Z.Z., Chu R., Liu L., Weagel E.G., Townsend M.H., Whitley K.V., Lawrence E.L. Macrophage Toll-like receptor-chimeric antigen receptors (MOTO-CARs) as a novel adoptive cell therapy for the treatment of solid malignancies. Cancer Res. 2018;78 doi: 10.1158/1538-7445.AM2018-2563. [DOI] [Google Scholar]
- 173.Zhang L., Tian L., Dai X., Yu H., Wang J., Lei A., Zhu M., Xu J., Zhao W., Zhu Y., et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. 2020;13:153. doi: 10.1186/s13045-020-00983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fu W., Lei C., Ma Z., Qian K., Li T., Zhao J., Hu S. CAR macrophages for SARS-CoV-2 immunotherapy. Front Immunol. 2021;12:669103. doi: 10.3389/fimmu.2021.669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bailey S.R., Maus M.V. Gene editing for immune cell therapies. Nat. Biotechnol. 2019;37:1425–1434. doi: 10.1038/s41587-019-0137-8. [DOI] [PubMed] [Google Scholar]
- 176.Aghajanian H., Kimura T., Rurik J.G., Hancock A.S., Leibowitz M.S., Li L., Scholler J., Monslow J., Lo A., Han W., et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–433. doi: 10.1038/s41586-019-1546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tebas P., Jadlowsky J.K., Shaw P.A., Tian L., Esparza E., Brennan A.L., Kim S., Naing S.Y., Richardson M.W., Vogel A.N., et al. CCR5-edited CD4+ T cells augment HIV-specific immunity to enable post-rebound control of HIV replication. J. Clin. Invest. 2021;131 doi: 10.1172/JCI144486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maldini C.R., Claiborne D.T., Okawa K., Chen T., Dopkin D.L., Shan X., Power K.A., Trifonova R.T., Krupp K., Phelps M., et al. Dual CD4-based CAR T cells with distinct costimulatory domains mitigate HIV pathogenesis in vivo. Nat. Med. 2020;26:1776–1787. doi: 10.1038/s41591-020-1039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Amor C., Feucht J., Leibold J., Ho Y.J., Zhu C., Alonso-Curbelo D., Mansilla-Soto J., Boyer J.A., Li X., Giavridis T., et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kobayashi S., Thelin M.A., Parrish H.L., Deshpande N.R., Lee M.S., Karimzadeh A., Niewczas M.A., Serwold T., Kuhns M.S. A biomimetic five-module chimeric antigen receptor ((5M)CAR) designed to target and eliminate antigen-specific T cells. Proc. Natl. Acad. Sci. U S A. 2020;117:28950–28959. doi: 10.1073/pnas.2012495117. [DOI] [PMC free article] [PubMed] [Google Scholar]