Abstract
Background.
Shortcomings of inhaled antibiotic treatments for Pseudomonas aeruginosa infection in patients with cystic fibrosis (CF) include poor drug penetration, inactivation by sputum, poor efficiency due to protective biofilm, and short residence in the lung.
Methods.
Eligible patients with forced expiratory volume in 1 second (FEV1) ≥25% of predicted value at screening and CF with chronic P. aeruginosa infection were randomly assigned to receive 3 treatment cycles (28 days on, 28 days off) of amikacin liposome inhalation suspension (ALIS, 590mg QD) or tobramycin inhalation solution (TIS, 300mg BID). The primary endpoint was noninferiority of ALIS vs TIS in change from baseline to day 168 in FEV1 (per-protocol population). Secondary endpoints included change in respiratory symptoms by Cystic Fibrosis Questionnaire-Revised (CFQ-R).
Results.
The study was conducted February 2012 to September 2013. ALIS was noninferior to TIS (95% CI, −4.95 to 2.34) for relative change in FEV1 (L) from baseline. The mean increases in CFQ-R score from baseline on the Respiratory Symptoms scale suggested clinically meaningful improvement in both arms at the end of treatment in cycle 1 and in ALIS arm at the end of treatment in cycles 2 and 3; however, the changes were not statistically significant between the two treatment arms. Treatment-emergent adverse events (TEAEs) were reported in most patients (ALIS, 84.5%; TIS, 78.8%). Serious TEAEs occurred in 17.6% and 19.9% of patients, respectively; most were hospitalisations for infective pulmonary exacerbation of CF.
Conclusions.
Cyclical dosing of once-daily ALIS was noninferior to cyclical twice-daily TIS in improving lung function.
ClinicalTrials.gov Identifier:
Keywords: Cystic fibrosis, amikacin liposome inhalation suspension, ALIS, Pseudomonas aeruginosa, LAI, liposomal amikacin for inhalation, CFQ-R
1. INTRODUCTION
Cystic fibrosis (CF) is associated with chronic lung infection that may lead to bronchiectasis, respiratory failure, and death [1,2]. In 2012, Pseudomonas aeruginosa infection was reported to occur in approximately 30% of patients with CF aged 6–10 years and 60% of patients aged 18–24 years [3]. While recent studies suggest that rates are declining, P. aeruginosa remains an important pathogen associated with more rapid clinical deterioration than most other CF pathogens, and has proven difficult to eradicate [4-7].
In these patients, the effectiveness of inhaled antibiotics is limited by poor penetration into infection sites, inactivation by sputum, non-penetration of biofilm, emergent resistance, bacterial phenotypic changes, and bacterial retention in mucous plugs [2,5,8-10]. Current approaches for managing chronic P. aeruginosa infection in patients with CF include inhaled tobramycin inhalation solution (TIS) or aztreonam solution, combined with non-antibiotic CF treatments [11]. Inhaled antibiotics can reduce bacterial density in sputum and improve clinical outcomes [12-14]. However, alternative antibiotic treatments are needed, especially for patients with intolerance or non-responsiveness to current inhaled antibiotics [10].
Amikacin liposome inhalation suspension (ALIS)1 is composed of small (≈0.3 μm), charge-neutral, highly biocompatible liposomes encapsulating amikacin. This formulation facilitates drug penetration into sputum and biofilms [15]; when delivered to the lungs, high amikacin concentrations are achieved with an extended half-life enabling once-daily dosing [16]. In a phase 2 study, once-daily ALIS improved lung function, reduced P. aeruginosa sputum density, and improved patient-reported respiratory symptoms over 28 days of treatment [17].
The phase 3 CLEAR-108 study (ClinicalTrials.gov Identifier: NCT01315678) compared the efficacy, safety, and tolerability of 3 treatment cycles of ALIS vs TIS in patients with CF and chronic bronchopulmonary P. aeruginosa infection.
2. METHODS
CLEAR-108 was a randomised, open-label, active-controlled noninferiority study conducted from February 2012 to September 2013 at 70 sites in Europe and Canada. CLEAR-108 was conducted in accordance with the Institutional Review Board/Ethics Committee–approved protocol and the Declaration of Helsinki, Good Clinical Practice, and applicable regulatory requirements. Written informed consent was obtained from study patients (and their guardian, if applicable) before participation.
2.1. Patient eligibility
Eligible patients were aged ≥6 years with CF, confirmed by a positive sweat test (ie, ≥60 mmol/L) or DNA analysis, and chronic P. aeruginosa infection, confirmed by 3 positive cultures within 2 years (≥1 obtained within 6 months) prior to screening, and positive sputum cultures at screening. Key inclusion criteria included forced expiratory volume in 1 second (FEV1) ≥25% of predicted values, oxygen saturation (SaO2) ≥90%, and the ability to expectorate ≥0.4 mL of sputum (see supplementary appendix for complete entry criteria).
Key exclusion criteria included prior exposure to ALIS, major complications of lung disease (eg, atelectasis, pneumothorax, major pleural effusion), haemoptysis ≥60 mL in a 24-hour period, acute pulmonary exacerbation requiring antibiotic treatment, upper respiratory tract infection, clinically significant non-CF pulmonary disease, or use of antipseudomonal antibiotics or initiation of chronic therapy within 4 weeks of day 1.
Inhaled antibiotics other than study drugs, and other parenteral or oral antibiotics unless clinically indicated, were prohibited. Chronic use of anti-inflammatory therapy, including macrolides, was permitted if the patient was on a stable regimen for at least 28 days preceding day 1 and throughout the trial. Use of prohibited antibiotics triggered interruption of study drug until use of the concomitantly prohibited medication ended. Continuation of a stable bronchodilator regimen (consistent use for ≥28 days preceding day 1 and throughout the trial), was permitted.
2.2. Treatment and follow-up
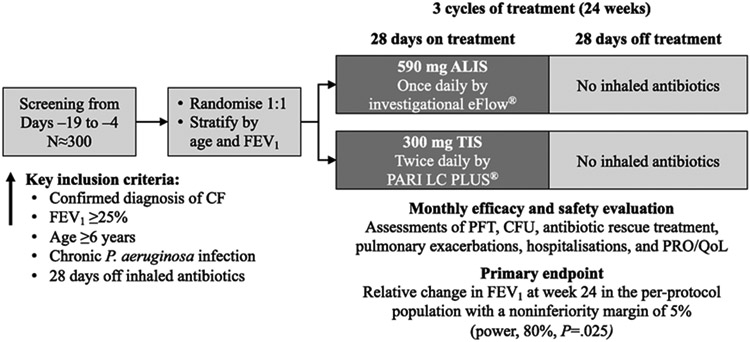
Patients were randomly assigned (1:1) to receive ALIS once daily or TIS twice daily and stratified by age and FEV1% predicted (Figure 1). ALIS was administered by inhalation using an investigational eFlow® nebulizer (PARI Pharma GmbH, Munich, Germany). ALIS was supplied in single-use vials to deliver amikacin 590 mg/8.4 mL. TIS was provided as a single-use, 5-mL ampule of 300 mg tobramycin administered via the PARI LC PLUS® Reusable Nebuliser and dosed at approximately 12-hour intervals but not less than 6 hours apart. One treatment cycle was defined as 28 days on treatment followed by 28 days off treatment; all patients received 3 treatment cycles. Study drug was discontinued at the investigator’s discretion or if a patient withdrew consent, was lost to follow-up, or met predefined criteria for discontinuation.
Figure 1. CLEAR-108 study design.
Patients visited the study site on days 1, 14, 28, 57, 84, 113, 140, and 168; between study visits, patients were followed by telephone contact (days 7, 21, 42, 63, 70, 77, 98, 119, 126, 133, and 154).
ALIS, amikacin liposome inhalation suspension; CF, cystic fibrosis; CFU, colony-forming units; FEV1, forced expiratory volume in 1 second; HRQoL, health-related quality of life; PFT, pulmonary function testing; PRO, patient-reported outcome; TIS, tobramycin inhalation solution.
2.3. Endpoints
The primary endpoint for this 24-week study was relative change from baseline to end of study in FEV1 (L). Secondary endpoints included interim changes from baseline during therapy in FEV1 (L) and FEV1% predicted; change from baseline in Respiratory Symptoms as measured by the Cystic Fibrosis Questionnaire-Revised (CFQ-R); time to first protocol-defined exacerbation (any 4 of 12 signs and symptoms of the Fuchs criteria); change from baseline in log10 colony-forming units (CFU) of P. aeruginosa; and all-cause hospitalisations. After study initiation, the study sponsor amended the statistical analysis plan (for regulatory purposes in the United States) to define time to first protocol-defined pulmonary exacerbation as the primary endpoint. In this manuscript we retain change in FEV1 (L) as the primary endpoint as defined in the study protocol.
The CFQ-R, which comprises 35 items for children, 44 for parents, and 50 for teens/adults on 12 generic and disease-specific scales, is a reliable and validated self-report instrument [18,19]. CFQ-R scales include Respiratory Symptoms, Treatment Burden, Physical Functioning, Vitality, Health Perceptions, Social Functioning, Role Functioning, and School Functioning scales. The minimal clinically important difference for the Respiratory Symptoms scale of the CFQ-R has been defined as a change of ≥4 points on the 100-point scale [20].
Safety endpoints included acute tolerability measured by treatment-emergent adverse events (TEAEs), AEs leading to discontinuation, serious AEs, laboratory abnormalities, audiology, serum creatinine changes, need for concomitant medications, and predose-to-postdose changes in pulmonary function tests, vital signs, and SaO2. Proportions of patients with emergent pathogens and shift of minimum inhibitory concentrations (MICs) for P. aeruginosa were additional endpoints.
Study visits occurred at weeks 2 and 4 and monthly thereafter, with telephone assessments between monthly visits. Patients who discontinued study drug prematurely could remain in the study and were followed for safety evaluation.
2.4. Statistical methods
A noninferiority margin of 5% was chosen to show that the mean 24-week relative change in FEV1 for ALIS was ≤5% lower vs that for TIS. Sample size was estimated based on prior studies showing that relative change for ALIS would be 2% higher vs that for the standard. Therefore, 129 patients were needed per treatment arm to achieve 80% power with a 1-sided significance level of 0.025. Thus, approximately 150 patients were to be enrolled per treatment arm.
Demographics were described using summary statistics. The primary efficacy analysis, a noninferiority test comparing ALIS with TIS, was performed on the per-protocol (PP) population, defined as patients who completed ≥80% of study drug doses without missing >3 consecutive doses in any cycle. Modified intention-to-treat (mITT) and safety populations were defined as all randomised patients who received ≥1 dose of study drug. All secondary efficacy analyses were completed using the mITT population.
Primary endpoint sensitivity analyses were performed on the PP population using a repeated measures linear mixed model and analysis of covariance (ANCOVA), replacing missing values with the worst-observation-carried-forward (WOCF), including baseline, the least favourable group mean or zero (LFGMZ), and the last-observation-carried-forward (LOCF). An ANCOVA model was also used in the primary analysis with factors for treatment and randomisation strata. Additional analyses repeated employing an ANCOVA model, under the assumption of missing at random, with effects for treatment, randomisation, and consistency of bronchodilator use (ie, whether the bronchodilator regimen was changed on or after day 1) using a lower bound of the 95% CI of ≥ −5%.
The Respiratory Symptoms scale of the CFQ-R was a prespecified secondary endpoint, analysed with a random-effects model; additional CFQ-R domains were analysed posthoc. CFQ-R was analysed using an ANCOVA model with effects for treatment, baseline CFQ-R score, and randomisation strata. Missing data were excluded. We used WOCF, LOCF, and mixed-effects models for repeated measures as the sensitivity analyses. A statistical hierarchy for the secondary endpoints was not prespecified; therefore, all effect sizes and P values presented for secondary endpoints cannot be used to infer statistical significance.
3. RESULTS
3.1. Patients
Overall, 302 patients were randomly assigned to study treatments; 152 patients were assigned to ALIS 590 mg once daily and 150 patients to TIS 300 mg twice daily (Figure S1). Among these, 28 patients (9.3%) did not complete the study (ALIS, 18 (11.8%); TIS, 10 (6.7%)). Four patients in each arm never received study drug and were not included in the safety population. A total of 274 patients completed the study, and 266 completed per protocol (Figure S1). The most common reasons for discontinuation of the study drug were protocol-defined safety criteria or AEs (ALIS, 7.2%; TIS, 2.0%) and other reasons (ALIS, 7.2%; TIS, 4.0%) (Table S1).
Demographics and baseline characteristics were comparable between the 2 arms (Table 1); 59.2% of patients were >18 years of age; 52.7% were male. Baseline FEV1% predicted values of 25%–50%, >50%–75%, and >75% were reported in 86 (29.3%), 109 (37.1%), and 99 (33.7%) patients, respectively. Most patients reported prior use of inhaled antibiotics (ALIS arm, 86.5%; TIS arm, 87.7%). Similar proportions of patients in each arm received study drug for 3 treatment cycles (ALIS arm, 90.5%; TIS arm, 93.2%).
Table 1.
Demographics and baseline characteristics (mITT population).
| Variable | ALIS 590 mg Once Daily N=148 |
TIS 300 mg Twice Daily N=146 |
Total N=294 |
|---|---|---|---|
| Race/ethnicity, n (%) | |||
| White (not of Hispanic origin) | 139 (93.9) | 141 (96.6) | 280 (95.2) |
| Hispanic | 5 (3.4) | 3 (2.1) | 8 (2.7) |
| African | 1 (0.7) | 0 | 1 (0.3) |
| Asian | 0 | 0 | 0 |
| Other | 3 (2.0) | 1 (0.7) | 4 (1.4) |
| Not reported | 0 | 1 (0.7) | 1 (0.3) |
| Sex, n (%) | |||
| Male | 79 (53.4) | 76 (52.1) | 155 (52.7) |
| Female | 69 (46.6) | 70 (47.9) | 139 (47.3) |
| Age | |||
| Mean (SD) | 22.8 (10.2) | 22.0 (10.0) | 22.4 (10.1) |
| 6–12 years, n (%) | 27 (18.2) | 26 (17.8) | 53 (18.0) |
| 13–18 years, n (%) | 34 (23.0) | 33 (22.6) | 67 (22.8) |
| >18 years, n (%) | 87 (58.8) | 87 (59.6) | 174 (59.2) |
| Height, mean (SD), cm | 162.3 (15.0) | 162.2 (15.6) | 162.3 (15.2) |
| Weight, mean (SD), kg | 54.5 (17.2) | 53.1 (15.9) | 53.8 (16.5) |
| BMI, mean (SD), kg/m2 | 20.1 (4.0) | 19.7 (3.7) | 19.9 (3.9) |
| CF genotype, n (%) | |||
| ΔF508 homozygous | 72 (48.6) | 70 (47.9) | 142 (48.3) |
| ΔF508 heterozygous | 40 (27.0) | 43 (29.5) | 83 (28.2) |
| Other | 21 (14.2) | 25 (17.1) | 46 (15.6) |
| FEV1% predicted | |||
| n | 148 | 144 | 292 |
| Mean (SD) | 64.5 (21.5) | 61.9 (22.0) | 63.2 (21.7) |
| FEV1% predicted stratification at screening for randomization | |||
| 25%–50%, n (%) | 42 (28.4) | 44 (30.1) | 86 (29.3) |
| >50%–75%, n (%) | 54 (36.5) | 55 (37.7) | 109 (37.1) |
| >75%, n (%) | 52 (35.1) | 47 (32.2) | 99 (33.7) |
| FEV1 (L) | |||
| n | 148 | 144a | 292 |
| Mean (SD) | 2.142 (0.861) | 2.037 (0.846) | 2.090 (0.854) |
| Use of bronchodilator | 113 (76.4) | 111 (76.0) | 224 (76.2) |
| Use of azithromycin | 76 (51.4) | 73 (50.0) | 149 (50.7) |
Baseline FEV1 data were not available for 2 patients.
ALIS, amikacin liposome inhalation suspension; BMI, body mass index; CF, cystic fibrosis; FEV1, forced expiratory volume in 1 second; mITT, modified intention-to-treat; TIS, tobramycin inhalation solution.
3.2. Efficacy
In the PP population, the least squares (LS) mean FEV1 (L) improved from baseline to day 168 similarly in the ALIS and TIS arms (1.56% and 2.87%, respectively, P=.48). The difference in LS mean between the 2 arms (ALIS minus TIS) adjusted for treatment and randomisation strata at day 168 was −1.31% (95% CI −4.95 to 2.34) (Figure 2), indicating noninferiority of improvement in lung function with ALIS vs TIS (ie, lower bound of the 95% CI was ≥ −5%, as per the prespecified analysis plan). In the mITT population, the relative change from baseline in FEV1 (L) at the end of treatment was also comparable in the ALIS and TIS arms in cycle 1 (day 28; LS mean, 4.33% and 7.32%, respectively; P=.08), cycle 2 (day 84; 4.39% and 6.65%, respectively; P=.18), and cycle 3 (day 140; 4.07% and 5.11%, respectively; P=.59) (Figure S2). Relative change in FEV1 from baseline in the mITT population was generally consistent across subgroups regardless of sex, ethnicity, or age. For FEV1% predicted, comparable improvements were observed between the ALIS and TIS arms in the mITT population at the midpoint of treatment cycle 1 when the peak effect was observed (day 14; LS mean, 5.09% and 7.42%, respectively; P=.15) and at the end of treatment cycle 1 (day 28; 4.14% and 7.00%, respectively; P=.09), cycle 2 (day 84; 3.37% and 5.48%, respectively; P=.20), and cycle 3 (day 140; 2.31% and 3.23%, respectively; P=.63).
Figure 2. Primary endpoint of relative change from baseline to day 168 in FEV1 (per-protocol population).
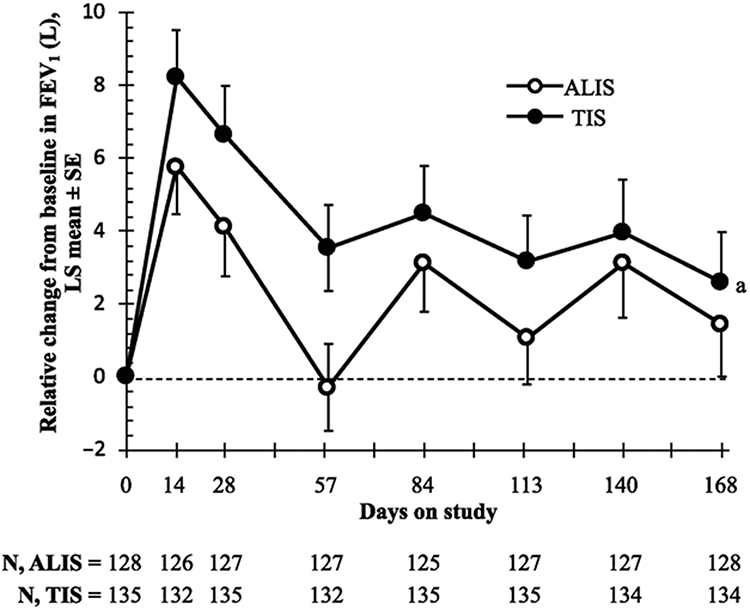
a LS mean difference (ALIS–TIS) adjusted for treatment and randomisation strata at day 168 was −1.31% (95% CI −4.95 to 2.34; P = .48). The lower bound of the 95% CI was > −5%, indicating noninferiority of ALIS to TIS.
ALIS, amikacin liposome inhalation suspension; FEV1, forced expiratory volume in 1 second; LS, least squares; TIS, tobramycin inhalation solution.
Results from the sensitivity analyses for the primary endpoint using a repeated measures linear mixed model and an ANCOVA model that replaced missing values with the WOCF, the LFGMZ, or the LOCF were similar to that of the primary efficacy analysis in both PP and mITT populations (Figure S3). Similarly, the results from the ANCOVA model controlling for consistency of bronchodilator use was consistent with the primary analysis (Figure S3). Overall, these analyses confirmed that both treatment groups had improvements in FEV1 from baseline and that the lower bounds of the 95% CIs were ≥ −5%, indicating noninferiority of ALIS vs TIS.
More patients receiving ALIS (63.5%) vs TIS (51.4%) (P=.02) experienced pulmonary exacerbations as defined by the Fuchs criteria. Patients in the ALIS arm had a reduced time to first pulmonary exacerbation (mITT population; hazard ratio 1.51; 95% CI 1.07 to 2.13; P=.03). All-cause hospitalisation was observed in 16.2% (24/148) of patients in the ALIS arm vs 19.9% (29/146) in the TIS arm.
In the mITT population, CFQ-R LS mean scores generally improved in both ALIS and TIS arms during the treatment in cycle 1 (day 14) and at the end of treatment periods (days 28, 84, and 140); except for a few instances, the differences between the 2 arms on various CFQ-R domains during the study were not statistically significant (Table S2). The scores on CFQ-R Respiratory Symptoms scale improved similarly for both ALIS and TIS arms (Figure S4). Mean increases on the CFQ-R Respiratory Symptoms scale–adjusted change from baseline suggested clinically meaningful improvement (≥4 points) for both treatment arms during cycle 1 (LS mean at day 14: ALIS, 4.43; TIS, 4.35; P=.96) and at the end of the on-treatment period of cycle 1 (day 28: ALIS, 5.23; TIS, 5.85; P=.72) and for the ALIS arm at the end of the on-treatment period of cycle 2 (LS mean at day 84: ALIS, 4.25; TIS, 3.22; P=.56) and cycle 3 (LS mean at day 140: ALIS, 4.49; TIS, 2.13; P=.15) (Table S2; Figure S4). In a posthoc analysis, mean improvement in the adjusted change from baseline on the CFQ-R treatment burden score at the end of the cycle 2 on-treatment period (day 84) was 1.69 in the ALIS arm and −2.59 in the TIS arm (P=.03; mITT population). A similar trend was observed at the end of the on-treatment period in cycle 3 (LS mean at day 140: ALIS, 0.739; TIS, −2.826; P=.06). Treatment arms differed regarding reports of CFQ-R Treatment Burden from on- vs off-cycle assessments (estimated difference, 2.53; SE, 0.97; P<.01), with the TIS arm reporting a significant worsening of treatment burden while on cycle (estimate = −2.60, SE = 0.68; P<.01) (Table S3).
Mean reductions in P. aeruginosa sputum density at the end of treatment in cycle 1 (day 28) and cycle 3 (day 140) were, respectively, 1.2 log10 CFU/g and 1.5 log10 CFU/g in the ALIS arm and 1.7 log10 CFU/g and 1.6 log10 CFU/g in the TIS arm, indicating that the microbiologic effect was maintained across the study (Figure 3). Mean P. aeruginosa sputum densities were below baseline level at day 168 (end of off-treatment period in cycle 3) in both the ALIS and TIS arms. The minimum inhibitory concentrations of amikacin and tobramycin required to inhibit the growth of 50% (MIC50) of P. aeruginosa isolates did not increase substantially over treatment cycles.
Figure 3. Change from baseline in sputum density of Pseudomonas aeruginosa (mITT population).
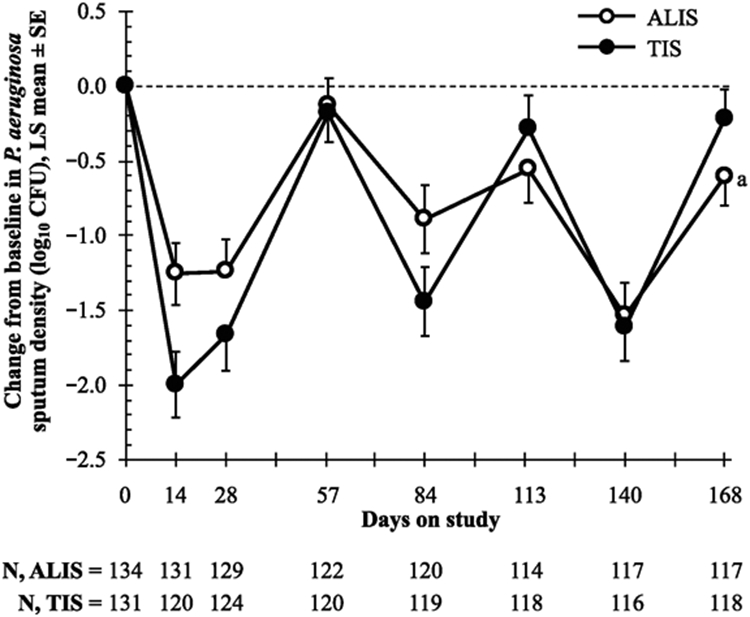
a LS mean difference (ALIS–TIS) adjusted for treatment and randomisation strata at day 168, P=.13. ALIS, amikacin liposome inhalation suspension; CFU, colony-forming units; LS, least squares; mITT, modified intention-to-treat; TIS, tobramycin inhalation solution.
3.3. Safety
Most patients (ALIS, 84.5%; TIS, 78.8%) experienced ≥1 TEAE, mostly mild or moderate in intensity (Table 2). In the ALIS arm, TEAE incidence decreased from 52.0% during the on-treatment period of cycle 1 to 39.6% during the on-treatment period of cycle 3. In the TIS arm, similar proportions of patients experienced TEAEs across all cycles, ranging from 34.6% to 37.4%. TEAEs occurring in ≥10.0% of patients in the ALIS arm included infective pulmonary exacerbation of CF (55.4%), nasopharyngitis (16.2%), haemoptysis (16.2%), cough (12.2%), dysphonia (12.2%), and upper respiratory tract infection (10.1%). With TIS, the most common TEAEs were infective pulmonary exacerbation of CF (48.6%) and nasopharyngitis (22.6%). TEAEs considered related to study drug with the most notable differences in frequency between the ALIS and TIS arms, respectively, included dysphonia (10.8% vs 2.7%), cough (8.8% vs 2.1%), haemoptysis (7.4% vs 3.4%), and infective exacerbation of CF (6.8% vs 2.1%). Concomitant therapies for pulmonary exacerbations were used by 49.7% of patients; antibiotics used by ≥10% of patients were fluoroquinolones, third-generation cephalosporins, other aminoglycosides, and carbapenems.
Table 2.
Summary of TEAEs.
| Variable, n (%) | ALIS 590 mg Once Daily N=148 |
TIS 300 mg Twice Daily N=146 |
Total N=294 |
|---|---|---|---|
| Patients with TEAEs | 125 (84.5) | 115 (78.8) | 240 (81.6) |
| Patients with TEAEs by relationship to study druga | |||
| Related | 57 (38.5) | 21 (14.4) | 78 (26.5) |
| Not related | 68 (45.9) | 94 (64.4) | 162 (55.1) |
| Patients with TEAEs by maximum severity | |||
| Mild | 52 (35.1) | 50 (34.2) | 102 (34.7) |
| Moderate | 62 (41.9) | 59 (40.4) | 121 (41.2) |
| Severe | 11 (7.4) | 5 (3.4) | 16 (5.4) |
| Life-threatening or disabling | 0 | 1 (0.7) | 1 (0.3) |
| Patients with treatment-emergent SAEs | 26 (17.6) | 29 (19.9) | 55 (18.7) |
| Patients with treatment-emergent SAEs by strongest relationship to study drug | |||
| Related | 1 (0.7) | 1 (0.7) | 2 (0.7) |
| Not related | 25 (16.9) | 28 (19.2) | 53 (18.0) |
| Patients with AEs leading to study drug discontinuation | 15 (10.1) | 7 (4.8) | 22 (7.5) |
Assessed by the investigator. Related events are defined as events with relationships to study procedure of probably, possibly, or unknown. Events not related are defined as events with relationships to study procedures of unlikely or not related.
AE, adverse event; ALIS, amikacin liposome inhalation suspension; SAE, serious adverse event; TEAEs, treatment-emergent adverse events; TIS, tobramycin inhaled solution.
Sixteen patients (ALIS, 7.4%; TIS, 3.4%) experienced ≥1 severe event; only one event (severe cough) in the ALIS arm was considered related to study drug. Similar proportions of patients experienced serious AEs in the ALIS (17.6%) and TIS (19.9%) treatment arms; the most serious were hospitalisations for the treatment of infective pulmonary exacerbation of CF. One patient in each arm experienced a serious TEAE considered related to study drug; no deaths were reported.
TEAEs leading to study drug discontinuation were reported in 10.1% and 4.8% of patients in the ALIS and TIS arms, respectively; events were predominantly respiratory. TEAEs requiring antibiotic therapy were reported in 50.0% and 44.5% of patients with pulmonary exacerbations in the ALIS and TIS arms, respectively, and in 23.0% and 28.8% of patients with TEAEs other than pulmonary exacerbations.
No appreciable changes were seen in acute tolerability in vital signs, body weight, SaO2, and pulmonary function as measured by predose-to-postdose changes over a single day. One moderate and five mild AEs of hearing loss were reported in 6 patients receiving ALIS (age, 31–45 years). One adult male patient with a mild AE had moderate 25 decibel (dB) unilateral loss at the highest frequency and one adult male patient with a moderate AE had moderate hearing loss (range 5–30 dB) at multiple frequencies bilaterally. Both patients were aged >35 years. The other 4 patients with mild hearing loss generally had a mixed pattern of loss and improvements. No hearing loss was reported in the TIS arm. No clinically meaningful changes in laboratory values were observed.
4. DISCUSSION
In this open-label, randomised, phase III trial, cyclical dosing of once-daily ALIS or twice-daily TIS in patients with CF and chronic P. aeruginosa infection resulted in improvement in FEV1 (L) from baseline to day 168; the improvement with ALIS was noninferior to TIS, as determined by the prespecified criterion of the lower bound of 95% CI for ALIS to be ≤5% lower than for TIS. The primary analysis of relative change in FEV1 at day 168 (PP population) was observed consistently regardless of sex, ethnicity, or age. Relative changes in each pulmonary function test in the mITT population were also similar in ALIS and TIS arms at the end of all 3 on-treatment cycles, suggesting that the effects of ALIS and TIS were maintained over time. Sensitivity analyses confirmed these results.
Both ALIS and TIS arms demonstrated reductions in P. aeruginosa sputum densities during the on-treatment periods, suggesting that both treatments were effective antimicrobials. However, more patients receiving ALIS than TIS experienced protocol-defined pulmonary exacerbations and a shorter time to first pulmonary exacerbation. The observed differences in pulmonary exacerbations in this relatively short study should be evaluated in future studies assessing the efficacy of ALIS for P. aeruginosa infection in CF over a longer period, especially given the fact the definition of exacerbation includes respiratory symptoms that may be short-term adverse effect from the treatment with inhaled study medication. Both arms also demonstrated improvements in CFQ-R Respiratory Symptoms scores; the improvements were clinically meaningful (increased by ≥4 points) for both arms at the end of the on-treatment period of cycle 1 and for only the ALIS arm at the end of the on-treatment period in cycles 2 and 3.
Most AEs in the ALIS group were respiratory in nature, including local respiratory events and infective exacerbation of CF, and were associated with patients’ underlying diagnoses. The TEAE incidence in the ALIS arm was higher vs TIS arm during the on-treatment period of cycle 1, but it decreased progressively during the study to proportions similar to that in the TIS arm by cycle 3.
One significant limitation is that this study was of relatively short duration for evaluation of a long-term chronic therapy. The requirement for all patients with prior exposure to be tolerant of TIS may have created a potential selection bias in favour of TIS. Another limitation of the study was that the patients were not blinded as they were aware of being on a once or twice daily therapy.
Overall, the results support the hypothesis that ALIS is noninferior to TIS for treatment of chronic P. aeruginosa infection in patients with CF. Improvements in FEV1% predicted and reductions in P. aeruginosa sputum density were similar in the 2 arms, and although acute pulmonary exacerbations were more common in the ALIS arm, some patient-reported outcomes tended to favour ALIS. ALIS administered once daily was comparable to TIS administered twice daily in improving lung function. At study completion, patients reported improvement in their respiratory symptoms and had a lower treatment burden as measured by CFQ-R, with trends in favour of ALIS compared with TIS despite a higher frequency of pulmonary exacerbations reported in the ALIS arm.
Supplementary Material
Acknowledgements
The authors would also like to acknowledge: The ALIS Study Group (ie, principal investigators, sub-investigators, and study coordinators who participated in the study); Arikayce Clinical Program Steering Committee members; Accelsiors CRO & Consultancy Services; Chiltern International Ltd; ICON Central Laboratories; PARI Pharma GmbH; Vitalograph Ltd; Xerimis Inc; Cystic Fibrosis Foundation Therapeutics Development Network; European Cystic Fibrosis Society–Clinical Trials Network; and the patients who contributed their time and faith to participate in the study.
The authors acknowledge Susan Torchio for contributing to data collection for the CLEAR-108 study while employed by Insmed; Rebecca Monroe for contributing to data collection and management of the CLEAR-108 study while employed by Insmed; Marie Bialek, PharmD, and Duke Duguay, PhD, of Connexion Healthcare (Newtown, PA) for medical writing assistance; editorial and creative support were also provided by Connexion Healthcare. Additional editorial support was provided by Richard Boehme, PhD, of Meditech Media (Hamilton, NJ). Insmed Incorporated (Bridgewater, NJ) provided funding to Connexion Healthcare and Meditech Media for these services.
Funding
The CLEAR-108 study was funded by Insmed Incorporated (Bridgewater, NJ). The sponsor designed the study, analysed the data, and contributed to data interpretation and report preparation.
Footnotes
Competing interests
Diane Bilton: National Institute for Health Research funding support through the Imperial College, Royal Brompton Hospital, Specialist Respiratory Bio-Medical Research Unit.
Tacjana Pressler: Received research contract support from Insmed Incorporated.
Isabelle Fajac: Received research contract support from Insmed Incorporated, has received a research grant from Actelion, and has served on advisory boards for Gilead Sciences and Vertex.
John Paul Clancy: Scientific Advisory Board for Insmed; prior clinical trial support for phase II studies of ALIS in CF for Insmed.
Dorota Sands: Received research contract support from Insmed Incorporated.
Predrag Minic: Received research contract support from Insmed Incorporated.
Marco Cipolli: Received research contract support from Insmed Incorporated.
Ivanka Galeva: Received research contract support from Insmed Incorporated.
Amparo Solé: Has served on the advisory board for Gilead Sciences and Vertex.
Alexandra L. Quittner: Consulting for Aradigm, Vertex, and AbbVie; investigator-initiated studies for the Cystic Fibrosis Foundation.
Keith Liu: Former employee of Insmed Incorporated.
John P. McGinnis II: Former employee of Insmed Incorporated.
Gina Eagle: Former employee of Insmed Incorporated.
Renu Gupta: Former employee of Insmed Incorporated.
Michael Konstan: Served on the Arikayce Clinical Program Steering Committee and on advisory boards for Albumedix, Celtaxsys, Corbus, Genentech, Gilead Sciences, Merck, Novartis, Savara, and Vertex.
The Supplementary Appendix contains a complete list of contributing investigators.
Previously referred to as liposomal amikacin for inhalation or LAI.
REFERENCES
- [1].Elborn JS. Cystic fibrosis. Lancet 2016;388:2519–31. 10.1016/S0140-6736(16)00576-6 [DOI] [PubMed] [Google Scholar]
- [2].Mayer-Hamblett N, Rosenfeld M, Gibson RL, Ramsey BW, Kulasekara HD, Retsch-Bogart GZ, et al. Pseudomonas aeruginosa in vitro phenotypes distinguish cystic fibrosis infection stages and outcomes. Am J Respir Crit Care Med 2014;190:289–97. 10.1164/rccm.201404-0681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cystic Fibrosis Foundation. Patient Registry 2012. Annual Data Report, www.cysticfibrosisdata.org/LiteratureRetrieve.aspx?ID=149756; 2012 [accessed 12 December 2018].
- [4].Strausbaugh SD, Davis PB. Cystic fibrosis: A review of epidemiology and pathobiology. Clin Chest Med 2007;28:279–88. 10.1016/j.ccm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- [5].Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003;168:918–51. 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- [6].Crull MR, Ramos KJ, Caldwell E, Mayer-Hamblett N, Aitken ML, Goss CH. Change in Pseudomonas aeruginosa prevalence in cystic fibrosis adults over time. BMC Pulm Med 2016;16:176. 10.1186/s12890-016-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Crull MR, Somayaji R, Ramos KJ, Caldwell E, Mayer-Hamblett N, Aitken ML, et al. Changing rates of chronic Pseudomonas aeruginosa infections in cystic fibrosis: A population-based cohort study. Clin Infect Dis 2018;67:1089–95. 10.1093/cid/ciy215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Quittner AL, Zhang J, Marynchenko M, Chopra PA, Signorovitch J, Yushkina Y, et al. Pulmonary medication adherence and health-care use in cystic fibrosis. Chest 2014;146:142–51. 10.1378/chest.13-1926. [DOI] [PubMed] [Google Scholar]
- [9].Sawicki GS, Ren CL, Konstan MW, Millar SJ, Pasta DJ, Quittner AL. Treatment complexity in cystic fibrosis: Trends over time and associations with site-specific outcomes. J Cyst Fibros 2013;12:461–7. 10.1016/j.jcf.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Høiby N. Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Med 2011;9:32. 10.1186/1741-7015-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mogayzel PJ Jr., Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB. Cystic fibrosis pulmonary guidelines. chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2013;187:680–9. [DOI] [PubMed] [Google Scholar]
- [12].Sheinberg M, Elborn JS. Use of inhaled tobramycin in cystic fibrosis. Adv Ther 2015;32:1–9. 10.1007/s12325-015-0179-3. [DOI] [PubMed] [Google Scholar]
- [13].Stuart Elborn J, Geller DE, Conrad D, Aaron SD, Smyth AR, Fischer R. A phase 3, open-label, randomized trial to evaluate the safety and efficacy of levofloxacin inhalation solution (APT-1026) versus tobramycin inhalation solution in stable cystic fibrosis patients. 2015;14:507–14. 10.1016/j.jcf.2014.12.013. [DOI] [PubMed] [Google Scholar]
- [14].Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med 1999;340:23–30. [DOI] [PubMed] [Google Scholar]
- [15].Meers P, Neville M, Malinin V, Scotto AW, Sardaryan G, Kurumunda R, et al. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother 2008;61:859–68. 10.1093/jac/dkn059. [DOI] [PubMed] [Google Scholar]
- [16].Weers J, Metzheiser B, Taylor G, Warren S, Meers P, Perkins WR. A gamma scintigraphy study to investigate lung deposition and clearance of inhaled amikacin-loaded liposomes in healthy male volunteers. J Aerosol Med Pulm Drug Deliv 2009;22:131–8. 10.1089/jamp.2008.0693. [DOI] [PubMed] [Google Scholar]
- [17].Clancy JP, Dupont L, Konstan MW, Billings J, Fustik S, Goss CH, et al. Phase II studies of nebulised arikace in CF patients with Pseudomonas aeruginosa infection. Thorax 2013;68:818–25. 10.1136/thoraxjnl-2012-202230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of the cystic fibrosis questionnaire in the united states: A health-related quality-of-life measure for cystic fibrosis. Chest 2005;128:2347–54. 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- [19].Quittner AL, Sawicki GS, McMullen A, Rasouliyan L, Pasta DJ, Yegin A, et al. Psychometric evaluation of the cystic fibrosis questionnaire-revised in a national sample. Qual Life Res 2012;21:1267–78. 10.1007/s11136-011-0036-z. [DOI] [PubMed] [Google Scholar]
- [20].Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the cystic fibrosis questionnaire-revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135:1610–8. 10.1378/chest.08-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.