Summary
Background
Attention-deficit/hyperactivity disorder (ADHD) has been reported to be associated with longer screen time utilization (STU) at the behavioral level. However, whether there are shared neural links between ADHD symptoms and prolonged STU is not clear and has not been explored in a single large-scale dataset.
Methods
Leveraging the genetics, neuroimaging and behavioral data of 11,000+ children aged 9–11 from the Adolescent Brain Cognitive Development cohort, this study investigates the associations between the polygenic risk and trait for ADHD, STU, and white matter microstructure through cross-sectionally and longitudinal analyses.
Findings
Children with higher polygenic risk scores for ADHD tend to have longer STU and more severe ADHD symptoms. Fractional anisotropy (FA) values in several white matter tracts are negatively correlated with both the ADHD polygenic risk score and STU, including the inferior frontal-striatal tract, inferior frontal-occipital fasciculus, superior longitudinal fasciculus and corpus callosum. Most of these tracts are linked to visual-related functions. Longitudinal analyses indicate a directional effect of white matter microstructure on the ADHD scale, and a bi-directional effect between the ADHD scale and STU. Furthermore, reduction of FA in several white matter tracts mediates the association between the ADHD polygenic risk score and STU.
Interpretation
These findings shed new light on the shared neural overlaps between ADHD symptoms and prolonged STU, and provide evidence that the polygenic risk for ADHD is related, via white matter microstructure and the ADHD trait, to STU.
Funding
This study was mainly supported by NSFC and National Key R&D Program of China.
Keywords: Attention-deficit/hyperactivity disorder, Screen time utilization, Polygenic risk for attention-deficit/hyperactivity disorder, Brain tractography, Longitudinal analysis
Research in context.
Evidence before this study
Attention-deficit/hyperactivity disorder (ADHD) is a highly heritable and neurodevelopmental disorder usually starting in childhood. ADHD and its co-occurrent behaviors are frequently reported to be associated with the behavior of screen time utilization (STU), which includes spending time with TV, smart phones and gaming devices. At the genetic level, different types of dopamine receptor or transmission genotypes like DRD2, have been reported to be correlated with ADHD and excessive video game playing. At the neural level, either ADHD or excessive screen-based activities has been found to be related to brain regions like the frontal lobe in their separate imaging studies.
Added value of this study
Previous studies have reported the co-occurrence of ADHD and screen time utilization, while how they are linked is not clear. Our study demonstrates a coherent biological pathway involving genetics, white matter microstructure, and the behaviors of ADHD and STU. As a consequence, this research has important implications for a better science-based understanding of the association between the behaviors of ADHD and STU, at multidimensional levels.
Implications of all the available evidence
This study has uncovered the shared neural links between the ADHD trait and prolonged STU, and how the neural links are associated with the genetics of ADHD. The results demonstrate a biological pathway from the polygenic risk for ADHD, via white matter microstructure (mostly involved in visual-related functions) and the ADHD trait, to STU. This suggests that the reduced structural connectivity involving pathways especially related to visual functions results in less strong executive control of visual functions in individuals with ADHD symptoms, and this increased sensitivity to and distractibility by visual stimuli may lead to increased screen time use.
Alt-text: Unlabelled box
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder usually starting in childhood, with a prevalence of around 5% in school-aged children.1 The disorder is defined by symptoms of inattention and/or hyperactivity/impulsiveness.2 High heritability of ADHD is indicated in genome-wide association studies (GWAS) ranging from 22 to 28%,3,4 while ranging from 72 to 88% in twin studies.5,6 According to a recent GWAS on ADHD, a considerably larger fraction of heritability is indexed by all common variants than only significantly associated common variants.4 This indicates that ADHD has a polygenic architecture, where a large number of common variants with small effects together make a considerable contribution. Therefore, estimating the combined genetic risk of a specific individual with ADHD is of current interest, which provides an opportunity to explore the complex relationship between genetics and ADHD. Typically, this can be achieved by the polygenic risk score (PRS), a secondary genome-wide tool that has been widely used recently and helps to reveal potential associations between the genetic predisposition of brain disorders and other non-genetic factors.7, 8, 9, 10
In the literature, ADHD and its co-occurrent behaviors have been found to be associated with increased screen time utilization (STU).11, 12, 13, 14, 15, 16, 17 STU refers to the time spent with any screens, including smartphones, tablets, television, or computers. With a growing number of children's leisure time spent on screens in recent years,18 concerns have been raised that excessive STU would result in psychological and cognition problems in children.19 A systematic review across 91 studies emphasizes that there is a positive association between the amount of screen time and inattention/hyperactivity problems in school-aged children.12 Other findings also demonstrate that ADHD symptoms and screen based activities, e.g. Internet gaming, may share a bi-directional relationship in preschoolers and adolescents.13,15
Both ADHD and screen-related phenotypes have been reported to correlate with the structure and function of the young, still-developing, brain. For example, in both structural and functional MRI studies, regions of the frontal lobe and anterior cingulate cortex have been found to be associated with ADHD20, 21, 22, 23 and screen-based activities.24,25 Tracts in the corpus callosum, inferior longitudinal fasciculus and inferior fronto-occipital fasciculus, were found to be correlated with ADHD26 and some screen-based activities, e.g. Internet gaming27,28 in diffusion MRI studies. Structural connectivity patterns in frontal-striatal circuitry and dopaminergic systems are reported to be related to ADHD in children.29,30 Among various screen-based activities, video games can influence dopamine release in the striatum that may related to the immediate reward obtained by the game players.15 Overall, these findings indicate a potential neural overlap of ADHD and STU, but it has not been fully tested in a single large-scale dataset that includes their longitudinal relationships.
With the Adolescent Brain Cognitive Development (ABCD) cohort, a large-scale and longitudinal data acquisition project with approximately 11,000 child participants,31 the present investigation aims to analyze the potential relationship between STU, polygenic risks for ADHD, white matter microstructure, and the ADHD trait. The investigation was conducted with the following hypotheses: 1) there are shared neural overlaps between the ADHD trait and prolonged STU; 2) brain structural connectivity or psychopathology mediate the association between polygenic risk for ADHD and STU. To test these hypotheses, several analyses were performed: 1) assessment of the bivariate association between polygenic risks for ADHD, the ADHD trait, STU, and white matter anatomical microstructure in children; 2) examination of the longitudinal association between the ADHD trait and STU; and 3) quantification of the mediation effect between polygenic risks for ADHD and STU through anatomical microstructure and the ADHD trait.
Methods
Participants
The participants for this analysis were from the ABCD cohort,31 an ongoing study of 11,000+ children who completed the baseline and follow-up assessments throughout the United States. The participants (aged 9–11 at baseline) were recruited from sample schools around 21 nationally distributed sites to represent the sociodemographic diversity of the United States.32 The genetic, diffusion tensor imaging (DTI) and behavioral data as well as demographic background of this investigation were obtained from the NIMH Data Archive ABCD Data Release 3.0 (https://nda.nih.gov/study.html?id=901).
Ethics
All procedures of the ABCD study were approved by a central Institutional Review Board (cIRB) at the University of California, San Diego, and in some cases by local IRB in a few research sites (e.g., Washington University in St. Louis).33 Parents provided written informed consent after the procedures had been fully explained, and children provided assent before enrolment in the study.34
Pre-processing of genotype data
Genotyping was acquired through saliva or whole blood samples and was centrally performed by the Rutgers University Cell and DNA Repository using the Affymetrix NIDA SmokeScreen Array.35 The preliminary preprocessed genotype data provided by the ABCD team contains 11,099 unique individuals with 516,598 genetic variants (for more details, see https://nda.nih.gov/study.html?id=901).
The ABCD samples contain many siblings and diverse ethnicity, which might bias the statistical results due to the cryptic relatedness and population stratification.7,36, 37, 38 Therefore, careful pre-processing procedures were adopted on the genotype data to increase the credibility of PRS prediction and subsequent analysis. First, 5807 European samples were selected according to their genetic ancestry factors (genetic_af_european > 0.95). Second, the quality control was performed by PLINK v1.9039 with the following steps: (1) removal of SNPs with minor allele frequency < 5%; (2) removal of SNPs with missing samples > 20%; (3) removal of samples with missing genotypes > 20%; (4) removal of SNPs deviating from Hardy–Weinberg equilibrium (p < 10−6); (5) removal of samples deviating ±3 sd from the samples' heterozygosity rate mean; (6) removal of cryptic relatedness by randomly excluding individuals in a pair of samples with proportion identity by descent PI_HAT > 0.4. Third, missing data imputation was performed using the Michigan Imputation Server,40 with the 1000 Genomes Project EUR (Phase 3, hg19) reference panel and Eagle v2.4 phasing. Fourth, the post imputation QC was conducted to remove those imputed SNPs: 1) with no RS IDs; 2) with imputation quality scores (rsq) < 0.9; 3) with minor allele frequency < 5%; 4) with missingness > 20%; 5) deviating from Hardy–Weinberg equilibrium (p < 10−6). Finally, 2,805,958 genetic variants and 4,673 samples remained for further analysis. The first ten ancestry principles components were calculated by GCTA v1.92.41
PRS calculation
The polygenic risk score is a weighted count of genetic risk alleles from a set of SNPs in a genotype dataset, with weights introduced from another independent and the same ancestry-based GWAS results. We downloaded GWAS summary statistics of the ADHD study4 from the Psychiatric Genomics Consortium (https://www.med.unc.edu/pgc/download-results/adhd/). Only the GWAS results of European ancestry were considered in this study to match the ABCD European samples described above, as the PRS prediction depends on the similarity between the original study population and the external target population.37 Then, we calculated the polygenic scores by PRSice-2,42 with an SNP clumping threshold r2 of 0.1 and a clumping window of 250 kb to remove SNPs in linkage disequilibrium. The PRSs calculated using SNPs at 10 p-value thresholds for their GWAS significance were included: 5 × 10−8, 5 × 10−5, 0.0001, 0.0005, 0.001, 0.005, 0.01, 0.05, 0.1, 0.5. The PRS capturing the largest average phenotypic variance R2 for the measurements focused in this study was used in the following analysis, as R2 is a frequently used indicator to evaluate the performance of PRS models.43,44 In addition, normalization (mean = 0, sd = 1) was performed for all PRSs in order to fairly compare their effects on different phenotypes.
Diffusion tensor imaging
The DTI data were obtained by the ABCD Imaging Acquisition Workgroup on three 3-Tesla scanner platforms, including Phillips, General Electric 750, and Siemens Prisma. The high angular resolution diffusion images were scanned with the following parameters: matrix size 140 × 140, 81 slices, FOV 240 × 240, resolution 1.7 × 1.7 × 1.7 mm, TR 4100ms, flip angle 77–90°, diffusion directions 91, b-values 500/1000/2000/3000.45 The microstructural measures of fractional anisotropy (FA), mean diffusivity, longitudinal diffusivity, and transverse diffusivity were calculated by conventional DTI methods.46, 47, 48 The white matter fiber tracts were segmented using Atlas Track,49 where visualization of each individual fiber tract is shown in Figure S1 and their corresponding connected brain regions are shown in Table S1. Only the measure of FA value in each region of interest (ROI) was considered in this investigation, including 42 metrics. The DTI data were downloaded from the ABCD cohort and preprocessed by the ABCD team,45,50 with 11,736 samples available for baseline, and 5665 samples for 2-year follow-up.
The quality control procedure of the ABCD-preprocessed imaging data was performed by following the recommended image inclusion criteria of the ABCD 3.0. Specifically, we excluded samples with the following criteria: (1) with problematic MR findings (mrif_score = 3 or mrif_score = 4); (2) with dMRI series not passing rawQC (iqc_dmri_ok_ser = 0); (3) with dMRI total number of repetitions for all OK scans less than 103 (iqc_dmri_ok_nreps < 103); (4) with dMRI B0 Unwarp unavailable (apqc_dmri_bounwarp_flag ∼= 1); (5) dMRI Manual Post-Processing QC failed (dmri_postqc_qc = 0) (6) with dMRI registration to T1w larger than 17 (apqc_dmri_regt1_rigid > 17); (7) dMRI Maximum dorsal cutoff score larger than 47 (apqc_dmri_fov_cutoff_dorsal > 47); (8) dMRI Maximum ventral cutoff score larger than 54 (apqc_dmri_fov_cutoff_ventral > 54). Finally, 9459 samples remained at baseline, and 4499 samples were available at 2-year follow-up.
Screen time utilization and ADHD assessments
Screen time utilization was assessed by the total amount of self-reported time using various electronic devices on both typical weekdays and weekend days (abcd_ssmty01), such as spending time on Facebook or watching movies. We calculated a weighted sum score to represent a daily STU as: × hours of STU in a typical weekday + × hours of STU in a typical weekend day. The data were available for baseline (11067 samples) and 1-year follow-up (11236 samples).
To evaluate the children's level of ADHD symptoms, the ADHD CBCL DSM5 Scale from the Parent Report Child behavior checklist (CBCL, abcd_cbcls01) was used.51,52 The data were available for baseline (11067 samples), 1-year follow-up (11235 samples), and 2-year follow-up (6571 samples).
Statistical analysis
We fitted a linear regression model to investigate the relationship between the polygenic risk score for ADHD (PRSADHD), neural measures and behavioral assessments using R software (v3.6.3). For behavioral assessments, we mainly focused on STU and the ADHD scale in this study. For multiple comparison purposes, false discovery rate (FDR)53 was adopted across all association analyses: (1) correction of p-values was performed to evaluate the association between PRSADHD and neural measures, where children's age (month/12), sex, batch, total intracranial volume, the first 10 principal components, household income, parental education, site, body mass index and puberty were regressed out as covariates to reduce model error. These are potential influential factors regarding anthropometry, instrumental measurement or environment and are always regressed out;54,55 (2) correction of p-values was performed to investigate the association between behavioral assessments and neural measures. As no genetic factors were considered here, there was no need to limit to samples of European ancestry. We retained only one child per family according to the family ID, and considered children's age (month/12), sex, batch, total intracranial volume, genetic ancestry proportion factors (genetic_af_african, genetic_af_european, genetic_af_east_asian, and genetic_af_american), household income, parental education, site, body mass index and puberty as covariates. Such a procedure enabled the utilization of all otherwise rejected samples, thereby leading to more robust results. The association analyses were performed on the ABCD baseline data.
A standard three-variable mediation analysis can be performed with the R package Mediation (version 4.5.0)56 to explore whether a potential mediator M of interest can explain the association between an independent variable X and outcome variable Y. To investigate the extent to which the relationship between PRSADHD (X) and STU (Y) is mediated by the brain or the ADHD scale, two mediation models were established to explore the indirect effect between X and Y: (1) a brain morphometry measure (M); (2) the ADHD scale (M). A bootstrap strategy with 10,000 resampling iterations was used to estimate the bias-corrected significance of the mediation. The mediation analyses were performed on the ABCD baseline data.
According to the longitudinal panel data in the ABCD cohort, it was possible to explore the longitudinal relationships in the following aspects: (1) the ADHD scale and STU whose data are both accessible at baseline and 1-year follow-up; (2) the ADHD scale and DTI measures whose data are both accessible at baseline and 2-year follow-up. We conducted a traditional cross-lagged panel model (CLPM)57 with the R package lavaan (version 0.6-7),58 where maximum likelihood estimation was carried out to fit the structural equation model. Specifically, the CLPM model can be described as
| (1) |
| (2) |
where and are the first phenotype at time and , and are the second phenotype at time and , is the covariate, , , , , , are the coefficients, and and are the error terms of the model.
Lastly, a serial mediation analysis was performed by using model 6 in processR (https://github.com/cardiomoon/processR, version 0.2.6), an R package implementation of the PRSCESS Macro,59 through which we assessed the indirect effect of PRSADHD on STU through both the ADHD scale and brain measures. The model investigated the path of PRSADHD (X) – a brain morphometry measure (M1) – the ADHD scale (M2) – STU (Y), and was performed on the ABCD baseline data. Specifically, the serial mediation model can be described as
| (3) |
| (4) |
| (5) |
where is the covariate, , , , , , , , , and are the coefficients, and , and are the error terms of the model. The serial mediation effect is then defined as . A bootstrap strategy with 10,000 resampling iterations was used to estimate the bias-corrected significance of the serial mediation. For all the longitudinal and mediation analyses, the same covariates were regressed out in the same manner as the linear association.
Role of funders
No entity other than the authors listed had roles in the study design, data collection, results interpretation, report writing, decision of manuscript submission or any aspect pertinent to the study for publication.
Results
Study design
As shown in Figure 1, the associations between PRSADHD, brain microstructure, the ADHD trait and STU were explored based on the integrative analysis of genotype, GWAS summary statistics, brain imaging, and behavioral data. Table 1 summarizes the demographic characteristics of all the 11,063 ABCD participants included in this study. When it involves PRSADHD, we limited analyses to the 4673 samples with European ancestry, the demographic characteristics of which were summarized in Table S2. Other models without the involvement of PRSADHD were performed with all available participants to maximize the sample size. We selected the PRSADHD at threshold 0.01 (this will not be specified below for short) for our main analyses, as it reached the largest average R2 among all the thresholds for the measurements focused in this study (Table S3).
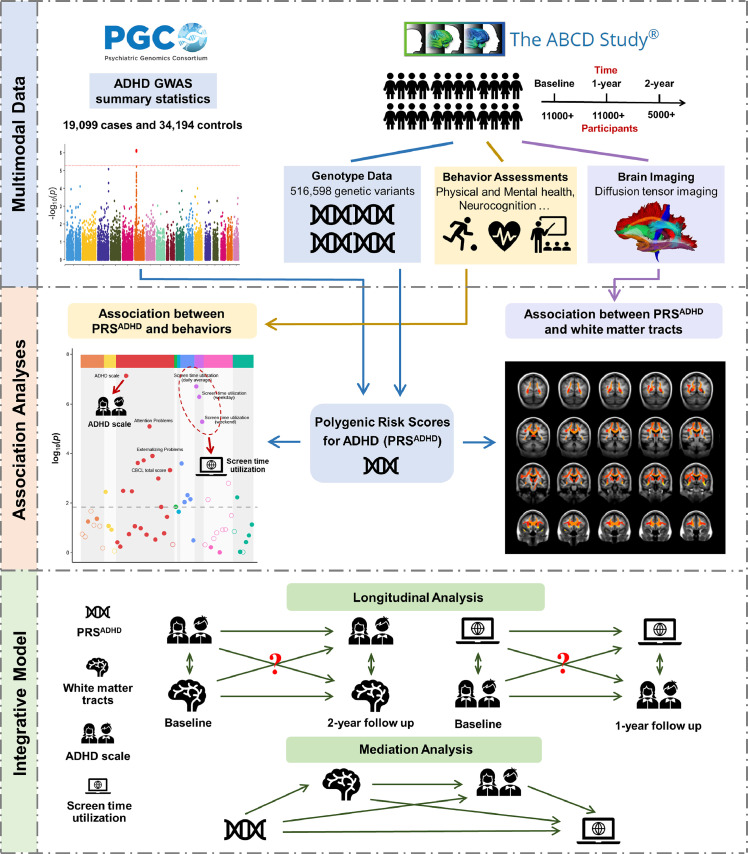
Figure 1.
Schematic diagram of the study design. (1) We collected data from the Psychiatric Genomics Consortium (PGC) and Adolescent Brain Cognitive Development (ABCD) database. The DTI template for white matter fiber tracts we used was from Atlas Track.49 (2) the polygenic risk scores for ADHD (PRSADHD) of 4673 children with European ancestry from the ABCD cohort were obtained based on the summary statistics of an independent ADHD GWAS study.4 Cross-sectionally bivariate associations were performed between PRSADHD, white matter microstructure, and behavioral assessments. The behaviors we mainly focused on in this study are the ADHD scale and screen time utilization (STU). (3) Longitudinal and mediation analyses were performed to explore integrative relationships between PRSADHD, white matter microstructure, the ADHD trait and STU. Potential confounding factors were regressed out in all the analyses (see Methods).
Table 1.
The demographic characteristics of participants from the ABCD analyzed here.
| Basic information | ||||
|---|---|---|---|---|
| Age (month) | Gender (Male/Female) | BMI | Parents income | Parents education |
| 118.96 ± 7.46 | 5847/5216 | 19.07 ± 4.23 | 7.26 ± 2.29 | 16.66 ± 2.68 |
| Puberty | Race (White/Black/Hispanic/Asian/Other) | |||
| 2.08 ± 0.75 | 5914/1694/2118/155/1180 | |||
| Screen time utilization measurement | ||||
| Screen Time Youth Weekday Sum (stq_y_ss_weekday) |
Screen Time Youth Weekend Sum (stq_y_ss_weekday) |
Screen Time Youth daily average (stq_y_ss_ave_daily) |
||
| 3.47 ± 3.1 | 4.64 ± 3.61 | 3.8 ± 3.07 | ||
| Psychiatric problem measurement | ||||
| Anxious/Depressed CBCL Syndrome Scale (cbcl_scr_syn_anxdep) | Withdrawn/Depressed CBCL Syndrome Scale (cbcl_scr_syn_withdep) | Somatic Complaints CBCL Syndrome Scale (cbcl_scr_syn_somatic) | Social Problems CBCL Syndrome Scale (cbcl_scr_syn_social) | Thought Problems CBCL Syndrome Scale (cbcl_scr_syn_thought) |
| 2.52 ± 3.07 | 1.0 ± 1.71 | 1.5 ± 1.95 | 1.62 ± 2.28 | 1.64 ± 2.20 |
| Attention Problems CBCL Syndrome Scale (cbcl_scr_syn_attention) | Rule-Breaking Behavior CBCL Syndrome Scale (cbcl_scr_syn_rulebreak) | Aggressive Behavior CBCL Syndrome Scale (cbcl_scr_syn_aggressive) | Internalizing Problems CBCL Syndrome Scale (cbcl_scr_syn_internal) | Externalizing Problems CBCL Syndrome Scale (cbcl_scr_syn_external) |
| 3 ± 3.51 | 1.2 ± 1.87 | 3.28 ± 4.36 | 5.06 ± 5.53 | 4.48 ± 5.87 |
| Total Problems CBCL Syndrome Scale (cbcl_scr_syn_totprob) |
Depressive Problems CBCL DSM5 Scale (cbcl_scr_dsm5_depress) | Anxiety Disorder CBCL DSM5 Scale (cbcl_scr_dsm5_anxdisord) |
Somatic Problems CBCL DSM5 Scale (cbcl_scr_dsm5_somaticpr) |
ADHD CBCL DSM5 Scale (cbcl_scr_dsm5_adhd) |
| 18.26 ± 17.96 | 1.27 ± 2.01 | 2.06 ± 2.44 | 1.08 ± 1.5 | 2.64 ± 2.98 |
| Oppositional Defiant Problems CBCL DSM5 Scale(cbcl_scr_dsm5_opposit) | Conduct Problems CBCL DSM5 Scale (cbcl_scr_dsm5_conduct) | Sluggish Cognitive Tempo CBCL Scale2007 Scale (cbcl_scr_07_sct) | Obsessive-Compulsive Problems CBCL Scale2007 Scale (cbcl_scr_07_ocd) | Stress Problems CBCL Scale2007 Scale (cbcl_scr_07_stress) |
| 1.77 ± 2.04 | 1.3 ± 2.37 | 0.53 ± 1.01 | 1.35 ± 1.82 | 2.91 ± 3.35 |
Note: The detail information of the measurement can be found on https://nda.nih.gov/data_dictionary.html.
The PRS for ADHD is significantly correlated with screen time utilization and behavioral problems
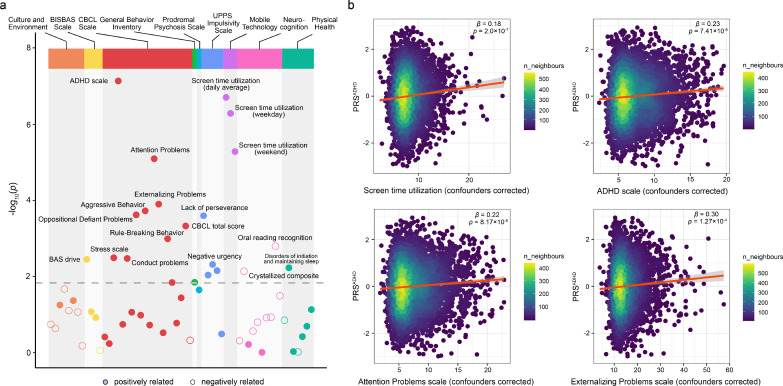
Figure 2 shows the associations found between PRSADHD and behavioral assessments at baseline, including mental and physical health, culture and environment, neurocognition, and mobile technology (see Table S4 for all behavioral assessments used and Table S5 for their associations with PRSADHD). More details about these measures can be found elsewhere.60 The CBCL total score was significantly positively correlated with PRSADHD (β = 0.85, se = 0.242, p = 4.76 × 10−4, t test, R2 = 0.39%, FDR < 0.05). Among the 19 CBCL subscales, three DSM-Oriented subscales (including the ADHD scale, Oppositional Defiant Problems and Conduct Problems), and six empirically derived syndrome subscales (including Attention Problems, Externalizing Problems, Aggressive Behavior, Rule-Breaking Behavior, Stress Scale and Social Problems) were significantly positively correlated with PRSADHD, indicating that a higher PRSADHD is associated with more severe symptoms of those types of behavior. More details of the CBCL subscales can be found in the CBCL manual.51 It is of interest that all of the three mobile technology total scores were significantly positively correlated with PRSADHD (β ranging from 0.18 - 0.2, p < 1 × 10−5, FDR < 0.05), suggesting that adolescents who have higher PRSADHD tend to perform more recreational screen-based activities. For cognitive assessments, the oral reading recognition score defined by the NIH Toolbox61 was found to be significantly negatively correlated with PRSADHD (β = -0.28, se = 0.087, p = 1.62 × 10−3, t test, R2 = 0.35%, FDR < 0.05). In addition, four out of five UPPS impulsivity subscales (i.e. lack of perseverance, negative urgency, positive urgency, lack of planning), and disorders of initiating and maintaining sleep were significantly positively correlated with PRS.
Figure 2.
Associations between PRSADHD and behavioral assessments. (a) The Manhattan plot of the association between PRSADHD and all the behavioral assessments shown in Table S4. A point above the grey dotted line denotes that this assessment is significantly correlated with PRSADHD (FDR < 0.05). (b) The scatter plots colored by density show the top four non-redundant assessments which are significantly correlated with PRSADHD as shown in (a), including the ADHD scale, daily average screen time utilization, Attention Problems scale and Externalizing Problems scale. ‘n_neighbours’ means ‘number of dots’ around each dot. All p-values were calculated by t test from linear regression analyses.
The top four non-redundant assessments that were significantly correlated with PRSADHD include the ADHD scale (β = 0.23, se = 0.042, p = 7.41 × 10−8, t test, R2 = 0.72%), daily average screen time utilization (also denoted as STU for brevity hereinafter) (β = 0.18, se = 0.035, p = 2 × 10−7, t test, R2 = 1.0%), Attention Problems scale (β = 0.22, se = 0.049, p = 8.17 × 10−6, t test, R2 = 0.52%), and Externalizing Problems scale (β = 0.3, se = 0.078, p = 1.27 × 10−4, t test, R2 = 0.46%) (see Figure 2b). The findings are consistent across different PRS thresholds (see Figure S2), indicating a robust association between PRSADHD and the four behavioral measures.
Lower FA of white matter tracts is associated with higher PRS for ADHD, and longer STU
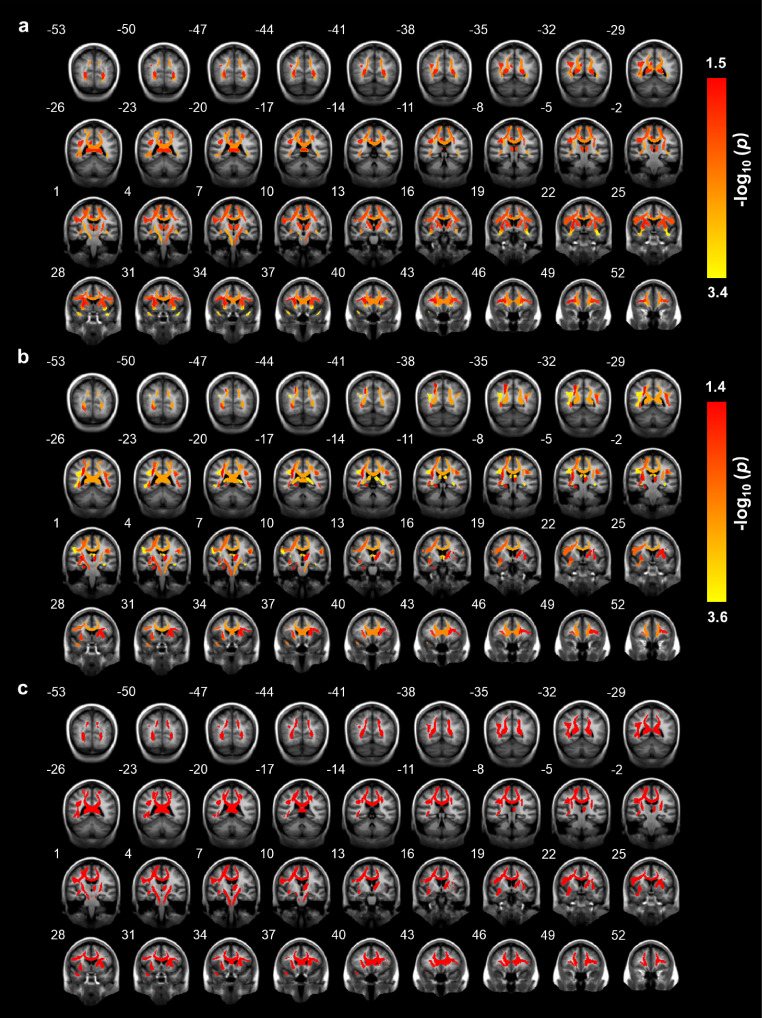
In this section, we described the associations between brain microstructure and PRSADHD as well as behavioral assessments. For global measures, the mean FA value of all fibers tracts was found to be significantly correlated with PRSADHD (standardized β = -3.46, p = 5.48 × 10−4, t test, R2 = 0.33%, FDR < 0.05), which also holds for both left and right tracts (p < 1 × 10−3, FDR < 0.05; Table S6). STU was significantly negatively correlated with the mean FA value of all tracts (standardized β = -3.23, p = 1.25 × 10−3, t test, R2 = 0.78%, FDR < 0.05), and the same for both the left and right hemisphere tracts (p < 5 × 10−3, FDR < 0.05; Table S7).
For regional measures, higher PRS was related to lower FA values of the uncinate fasciculus, corticospinal tract, inferior frontal-occipital fasciculus (IFO), (temporal/parietal) superior longitudinal fasciculus (SLF), corpus callosum (CC), inferior frontal superior frontal tract, anterior thalamic radiations, and inferior frontal-striatal tract (IFS) (all with FDR < 0.05; Figure 3a and Table S6). At the same time, longer STU was found correlated with lower FA values in (parietal/temporal) SLF, fornix, CC, uncinate fasciculus, IFO, corticospinal tract, forceps minor, IFS and superior corticostriate (all with FDR < 0.05; Figure 3b and Table S7).
Figure 3.
The brain regions correlated with PRSADHD or STU. The brain tracts analyzed with DTI significantly (FDR < 0.05) correlated with (a) PRSADHD; (b) STU; (c) both PRSADHD and STU. The number above each brain anatomical section is the MNI coordinate for the selected plane. All p-values were calculated by t test from linear regression analyses.
Table 2 and Figure 3c show the brain areas correlated with both PRSADHD and STU, and Table S8 and Figure S3 show the brain areas correlated with the ADHD scale. Moderate spatial correlation of β maps was observed between the ADHD scale and STU (r = 0.57, p = 1.06 × 10−4, Pearson correlation). The association analysis between the ADHD scale and white matter tracts was repeated while including head motion (iqc_dmri_1_mean_motion) as a covariate (Table S9). The pattern of brain spatial β maps for the ADHD scale was consistent with and without correcting for head motion (r = 0.936, p < 5 × 10−12, Pearson correlation), and lower FA values of the uncinate fasciculus, IFO, parietal SLF and superior corticostriate, can still be found associated with higher ADHD scale (FDR < 0.05). These results support the first hypothesis that prolonged STU and the ADHD trait share a positive neural overlap.
Table 2.
The white matter tracts (quantified as FA values) significantly correlated with both PRSADHD and STU. ‘std_β’ denotes the standardized coefficient in the regression analysis.
| DTI measures | Connection |
PRSADHD |
STU |
||
|---|---|---|---|---|---|
| std_β | p | std_β | p | ||
| Global measures | |||||
| all tract fibers | -3.459 | 5.48E-04 | -3.228 | 1.25E-03 | |
| L tract fibers | -3.440 | 5.88E-04 | -3.083 | 2.06E-03 | |
| R tract fibers | -3.389 | 7.08E-04 | -3.311 | 9.34E-04 | |
| R tract fibers without corpus callosum | -3.290 | 1.01E-03 | -3.226 | 1.26E-03 | |
| L tract fibers without corpus callosum | -3.177 | 1.50E-03 | -2.745 | 6.07E-03 | |
| Regional measures | |||||
| R uncinate fasciculus | inferior frontal lobe & anterior temporal lobe | -3.264 | 1.11E-03 | -2.835 | 4.60E-03 |
| R corticospinal tract or pyramidal tract | motor cortex & spinal cord | -3.141 | 1.70E-03 | -2.591 | 9.59E-03 |
| R temporal superior longitudinal fasciculus | temporal lobe & frontal lobe | -3.052 | 2.29E-03 | -3.541 | 4.01E-04 |
| corpus callosum | left cortex & right cortex | -2.965 | 3.05E-03 | -3.139 | 1.70E-03 |
| R inferior fronto-occipital fasciculus | occipital lobe & frontal lobe | -2.862 | 4.24E-03 | -2.431 | 1.51E-02 |
| R superior longitudinal fasciculus | temporal and parietal lobes & frontal lobe | -2.693 | 7.12E-03 | -3.661 | 2.53E-04 |
| L corticospinal tract or pyramidal tract | motor cortex & spinal cord | -2.634 | 8.46E-03 | -2.999 | 2.72E-03 |
| R inferior frontal superior frontal tract | inferior frontal cortex & superior frontal cortex | -2.624 | 8.74E-03 | -2.762 | 5.75E-03 |
| L anterior thalamic radiations | thalamus & frontal lobe | -2.534 | 1.13E-02 | -2.153 | 3.14E-02 |
| L inferior frontal-striatal tract | inferior frontal cortex & striatum | -2.473 | 1.34E-02 | -2.177 | 2.95E-02 |
| R parietal superior longitudinal fasciculus | parietal lobe & frontal lobe | -2.435 | 1.49E-02 | -3.598 | 3.23E-04 |
The longitudinal association between STU, the ADHD scale and white matter tracts
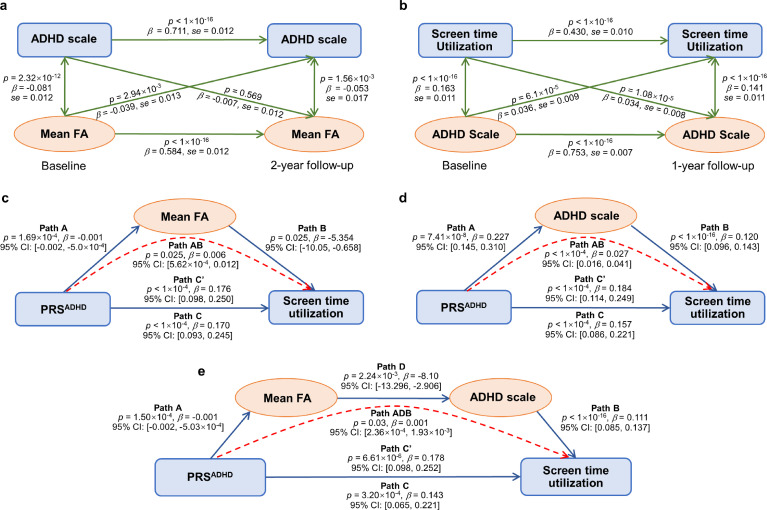
A CLPM model was used to investigate whether the strong association in the cross-sectional data analysis is still evident longitudinally between the ADHD scale and its associated white matter tracts (shown in Figure S3 and Table S8). Note that the mean FA value of white matter tracts at baseline was significantly negativity correlated with the ADHD scale at 2-year follow-up (β = -0.039, se = 0.013, p = 2.94 × 10−3, z test, FDR < 0.05; Figure 4a) while the reverse was not found (β = -0.007, se = 0.012, p = 0.569, z test).
Figure 4.
The longitudinal and mediation analyses. (a) Longitudinal analysis between the ADHD scale and the mean FA of the significant fiber tracts shown in Fig. S3 at baseline and 2-year follow-up. (b) Longitudinal analysis between the STU and the ADHD scale at baseline and 1-year follow-up. The p-values of longitudinal analyses were calculated by z test from the CLPM model. (c, d) Mediation analysis between PRSADHD and STU through (c) mean FA value of regional findings shown in Fig. 3c; (d) the ADHD scale. The p-values of mediation effect were calculated by bootstrap test with 10000 resampling iterations. (e) Serial mediation analysis between PRSADHD and STU through mean FA value of white matter tracts shown in Fig. 3c and the ADHD scale (sequentially). The p-values of the serial mediation effect were calculated by bootstrap test with 10,000 resampling iterations. Path C shows the association between PRSADHD and STU when mediators are not taken into account. Path C’ shows the association between PRSADHD and STU when mediators are taken into account. The mediation relationship is labelled with a red dotted line in (c–e).
A longitudinal analysis was also performed between STU and the ADHD scale. It was found that the ADHD scale at baseline was significantly correlated with STU at 1-year follow-up (β = 0.036, se = 0.009, p = 6.1 × 10−5, z test, FDR < 0.05; Figure 4b), and the reverse was also observed. A similar pattern of results held for the Attention Problems scale and Externalizing Problems scale (Figure S4a, b), the two traits significantly correlated with PRSADHD (Figure 2) and well known to be of high relevance to ADHD, indicating the robustness of our results.
The mean FA of white matter tracts and the ADHD scale mediate the relationship between PRS for ADHD and STU
With the longitudinal analyses, which showed a directional effect of brain white matter microstructure on the ADHD scale, as well as a bi-directional effect of the ADHD scale on STU, we performed mediation analyses to test the second hypothesis that brain structural connectivity or the ADHD trait can mediate the association between PRSADHD and STU. It was found that the FA value of white matter tracts shown in Figure 3c and Table 2 significantly mediated the relationship between PRSADHD and STU (path AB: accounting for 3.17% of the total effect, β = 0.006, 95% CI: [5.62 × 10−4, 0.012], p = 0.025, bootstrap test; Figure 4c). The ADHD scale was also a significant mediator between PRSADHD and STU (path AB: accounting for 14.78% of the total effect, β = 0.027, 95% CI: [0.016, 0.041], p < 1 × 10−4, bootstrap test; Figure 4d).
Based on the associations outlined above, we hypothesized that there was also an indirect path between PRSADHD and STU through both white matter tracts and the ADHD scale (sequentially). As predicted, there was a significant indirect path between PRSADHD and STU through mean FA of white matter tracts and the ADHD scale (path ADB: accounting for 0.53% of the total effect, β = 0.001, 95% CI: [2.36 × 10−4, 1.93 × 10−3], p = 0.03, bootstrap test; Figure 4e). The second hypothesis was thus supported by these mediation analyses. Similar results were also found across neighboring PRS thresholds (Figure S5a–f), except that the indirect path between PRSADHD at threshold 0.05 and STU through both white matter tracts and the ADHD scale did not quite reach but was very close to the significant threshold of p < 0.05 (p = 0.056, Figure S5f).
Discussion
Leveraging cross-sectional and longitudinal data from a large-scale dataset, it was found that individuals with a higher polygenic risk for ADHD tend to prolong their screen activities and have more severe ADHD symptoms (Figure 2). Longitudinal analyses indicate a directional effect of white matter microstructure on the ADHD scale, and a bi-directional effect between the ADHD scale and STU (Figure 4a, b). Furthermore, the mediation analysis demonstrated that some white matter tracts (shown in Figure 3c and Table 2), and the ADHD scale, substantially mediate the association between PRSADHD and STU (Figure 4c–e). Our study has several strengths: (1) the use of longitudinal data with a large number of participants leads to robust findings; (2) the utilization of PRS serves as a good representation of the ADHD polygenic architecture; (3) the feasibility to uncover relationships between genetic risk for ADHD and the ADHD trait with STU. Collectively, this investigation lends a multidimensional perspective to the association between the behaviors of ADHD and STU through multimodal data.
The relationships between ADHD and STU illuminated here can be described at several levels. At the behavioral level, STU had a significant bi-directional long-term correlation with the ADHD scale. The finding is reasonable given that children with ADHD tend to have reward oversensitivity and are intolerant of delay in rewards,62,63 while screen-based activities like video gaming can provide immediate feedback of reward.15 Given the reduced self-control in children with ADHD, these individuals may become lost in screen-based activities to satisfy their yearning for reward. At the genetic level, we found a stronger genetic risk for ADHD was associated with longer STU, and ADHD symptoms. In the literature, either ADHD or excessive STU has been found to be related to dopamine.64,65 For example, a study found that DRD2 TaqlA1, a dopamine D2 receptor gene, was associated with excessive video game playing in a behavioral experiment.64 On the other hand, different types of dopamine receptor or transmission genotypes like DAT1 and DRD2, have also been reported to be correlated with ADHD.65,66 Moreover, due to the high heritability of ADHD, the offspring of adults with ADHD are at increased risk of ADHD. At the same time, ADHD impairs the parenting ability of adults,67,68 resulting in inadequate protection against their children's exposure to digital media, which might lead to the co-occurrence of ADHD and long STU. Our results suggest that the genetic background of ADHD might contribute to prolonged STU, but more research is needed to test this hypothesis in the future.
The present investigation found that the white matter tracts that were significantly related to both PRSADHD and STU include the IFS, IFO, SLF and CC. Most of our findings are consistent with previous MRI studies on ADHD.26,29,69, 70, 71 The IFS links the inferior frontal cortex to the striatum, and changes of structural connectivity in frontal-striatal circuitry have often been demonstrated to be linked to ADHD in children.72,73 The frontal-striatal circuitry may involve linking inhibitory control and executive function.74,75 In this context, the abnormalities of frontal-striatal circuitry may help explain why children with ADHD find it difficult to tear themselves away from prominent visual sensory stimulation to perform instead more planned activity. With changes in the axonal bundles of frontal-striatal circuitry, problems may arise. The IFO is considered as a key contributor to visual information processing, such as the visual guidance for movement.76 The role of the IFO is mainly supported by its anatomic terminations in the occipital lobe, which contains early visual cortical areas.77 Reduced FA in IFO is a notable finding in previous adolescent and adult ADHD studies.70,71 The SLF is widely known for its connections involving the frontal, temporal, parietal, and occipital cortices, and is involved in visually mediated processes and the maintenance of attention.78,79 Alteration of the SLF has been frequently reported in people with ADHD,80,81 and we also provided evidence for its involvement in STU. Additionally, the CC area indicated in our findings plays a role in integrating visuomotor and cognitive processes between the two hemispheres.82 Clearly, most of these tracts with significant negative correlations with PRSADHD are crucial for visual-related function, except for the IFS which is involved in inhibitory and executive function. It has been shown that in ADHD, there is increased functional connectivity of visual cortical areas, and this was related to the dominant effects of visual stimuli in ADHD.83 Putting these findings together, it is hypothesized that the reduced tractography reported here to be associated with PRSADHD may reduce top-down executive control from prefrontal cortex and related areas on early visual cortical areas.
Few previous studies have examined the linkage between ADHD, white matter microstructure and STU simultaneously. It shows that widespread reductions of FA in white matter tracts at baseline are significantly correlated with more severe ADHD symptoms at two-year follow-up; and the reverse was not found. This provides evidence that early abnormal white matter might be a biomarker for a later diagnosis of ADHD. Concurrently, the longitudinal analysis showed a bi-directional effect between STU and the ADHD scale. Subsequent mediation analysis indicates that PRSADHD is linked to STU through both white matter tracts and the ADHD trait. It is hypothesized that children with high polygenic risk for ADHD tend to have alterations in white matter tracts that are linked to visual-related functions, making individuals with ADHD symptoms sensitive to external visual stimulation with reduced attentional and executive control, and so are easily distracted by screen-related activities. The findings extend a previous study on correlations between PRSADHD, white matter structure and ADHD symptomatology,71 and is informative for future research on biological processes involving ADHD and STU.
However, there are still some issues that need to be addressed in the future. Firstly, we focused on the white matter microstructure in this study because anatomical measures are reported to be more closely related to genetic factors than functional connectivity.84 At the time of the writing of this paper, there is no clear meta-analytic evidence of common functional alterations across individuals with ADHD.85,86 In the future, other types of imaging data like functional MRI may be considered. Secondly, the present analysis does not provide mechanistic insights into how genes may drive brain dysfunction and influence behaviors. Other gene-based methods like transcriptome-wide association studies might replace the PRS used in this study to help understand the molecular mechanisms by which genes affect phenotypes. Thirdly, the STU assessment we used is self-reported, which may skew towards underestimation since the adolescents may pay little attention to the starting or ending time when they use electronic devices. Even if the STU assessment is parent-reported, it may also lead to biased results as parents cannot stay beside their children all the time, and thus the parental reported result maybe based on subjective estimation. Fourthly, the head motion is a noteworthy issue. A previous study reported that the differences in head motion between ADHD and control samples might bias the results of between-group comparison of FA.26 Although the ADHD trait considered here is not a categorical disorder but a continuous scale of ADHD symptoms from samples with healthy backgrounds,50 and consistent patterns of brain spatial β maps for the ADHD scale was observed with and without head motion as a covariate, it is still an open issue that should be considered in future ADHD imaging studies. Finally, due to the lack of some longitudinal measures, we could not test the long-term relation between brain development and STU. However, this could be addressed in the future as the ABCD project is an on-going study.
In summary, leveraging the large-scale data of the ABCD, we found significant associations between the polygenic risk for ADHD, and STU, with white matter microstructure involved in visual function and the ADHD trait as factors involved in the association. These findings may be helpful when designing treatment strategies in future.
Data sharing statement
Genotype, neuroimaging and behavioral data from ABCD dataset can be obtained from https://nda.nih.giv/abcd with the approval of the ABCD consortium. GWAS summary statistics of the ADHD study4 can be directly downloaded from https://www.med.unc.edu/pgc/download-results/adhd/.
Contributors
W.C. and X.M.Z. conceived and designed research; A.Y. performed research; A.Y., and G.D. analyzed data; J.F., J.D., and Y.L. has directly accessed and verified the underlying data reported in the manuscript; E.T.R. supervised the whole project; A.Y. wrote the original draft of the paper; and X.M.Z., W.C., and E.T.R. reviewed and polished the paper. All authors read and approved the final version of the manuscript.
Declaration of interests
The authors declare that they have no conflicts of interest.
Acknowledgments
X.M.Z. was supported by the National Key R&D Program of China (No. 2020YFA0712403), National Natural Science Foundation of China (NSFC) (No. 61932008, No. 61772368), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01). J.F.F. was supported by National Key R&D Program of China (No. 2018YFC1312900 and No. 2019YFA0709502), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), Shanghai Center for Brain Science and Brain-Inspired Technology, and the 111 Project (No. B18015). W.C. was supported by grants from the National Natural Science Foundation of China (No. 82071997) and Shanghai Rising Star Program (No. 21QA1408700).
We deeply appreciate the contributors of the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org). Data of the ABCD Study is held in the NIMH Data Archive (NDA). The ABCD Study is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 years and follow them over 10 years into early adulthood. It is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/scientists/workgroups/. We also deeply appreciate the contributors of Psychiatric Genomics Consortium (PGC, https://www.med.unc.edu/pgc) for making summary statistics publicly and freely available. A full list of funders of the PGC database is available at https://www.med.unc.edu/pgc/about-us/funders/. PGC and ABCD consortium investigators designed and implemented that study and/or provided data but did not participate in analysis for or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of ABCD or PGC consortium investigators.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104039.
Contributor Information
Wei Cheng, Email: wcheng.fdu@gmail.com.
Xing-Ming Zhao, Email: xmzhao@fudan.edu.cn.
Appendix. Supplementary materials
References
- 1.Polanczyk G., de Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Segal D.L. The corsini encyclopedia of psychology. John Wiley & Sons, Inc.; 2010. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) pp. 1–3. [Google Scholar]
- 3.Lee S.H., Ripke S., Neale B.M., Faraone S.V., Wray N.R. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demontis D., Walters R.K., Martin J., et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson H., Chang Z., D'Onofrio B.M., Lichtenstein P. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol Med. 2014;44(10):2223–2229. doi: 10.1017/S0033291713002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson H., Dilshad R., Lichtenstein P., Barker E.D. Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: genetic effects, family risk and associated psychopathology. J Child Psychol Psychiatry Res. 2011;52(9):954–963. doi: 10.1111/j.1469-7610.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- 7.Li R., Chen Y., Ritchie M.D., Moore J.H. Electronic health records and polygenic risk scores for predicting disease risk. Nat Rev Genet. 2020;21(8):493–502. doi: 10.1038/s41576-020-0224-1. [DOI] [PubMed] [Google Scholar]
- 8.Sudre G., Frederick J., Sharp W., et al. Mapping associations between polygenic risks for childhood neuropsychiatric disorders, symptoms of attention deficit hyperactivity disorder, cognition, and the brain. Mol Psychiatry. 2020;25(10):2482–2492. doi: 10.1038/s41380-019-0350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang S., Yang L., Wang Y., Faraone S.V. Shared polygenic risk for ADHD, executive dysfunction and other psychiatric disorders. Transl Psychiatry. 2020;10(1):1–9. doi: 10.1038/s41398-020-00872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohi K., Ochi R., Noda Y., et al. Polygenic risk scores for major psychiatric and neurodevelopmental disorders contribute to sleep disturbance in childhood: adolescent brain cognitive development (ABCD) study. Transl Psychiatry. 2021;11(1):1–11. doi: 10.1038/s41398-021-01308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lissak G. Adverse physiological and psychological effects of screen time on children and adolescents: Literature review and case study. Environ Res. 2018;164:149–157. doi: 10.1016/j.envres.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Suchert V., Hanewinkel R., Isensee B. Sedentary behavior and indicators of mental health in school-aged children and adolescents: a systematic review. Prev Med. 2015;76:48–57. doi: 10.1016/j.ypmed.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Swing E.L., Gentile D.A., Anderson C.A., Walsh D.A. Television and video game exposure and the development of attention problems. Pediatrics. 2010;126(2):214–221. doi: 10.1542/peds.2009-1508. [DOI] [PubMed] [Google Scholar]
- 14.Nikkelen S.W., Valkenburg P.M., Huizinga M., Bushman B.J. Media use and ADHD-related behaviors in children and adolescents: a meta-analysis. Dev Psychol. 2014;50(9):2228. doi: 10.1037/a0037318. [DOI] [PubMed] [Google Scholar]
- 15.Weiss M.D., Baer S., Allan B.A., Saran K., Schibuk H. The screens culture: impact on ADHD. ADHD Atten Deficit Hyperact Disord. 2011;3(4):327–334. doi: 10.1007/s12402-011-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suchert V., Pedersen A., Hanewinkel R., Isensee B. Relationship between attention-deficit/hyperactivity disorder and sedentary behavior in adolescence: a cross-sectional study. ADHD Atten Deficit Hyperact Disord. 2017;9(4):213–218. doi: 10.1007/s12402-017-0229-6. [DOI] [PubMed] [Google Scholar]
- 17.Vohr B.R., McGowan E.C., Bann C., et al. Association of high screen-time use with school-age cognitive, executive function, and behavior outcomes in extremely preterm children. JAMA Pediatr. 2021;175(10):1025–1034. doi: 10.1001/jamapediatrics.2021.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Twenge J.M., Martin G.N., Spitzberg B.H. Trends in US Adolescents’ media use, 1976–2016: the rise of digital media, the decline of TV, and the (near) demise of print. Psychol Popul Media Cult. 2019;8(4):329. [Google Scholar]
- 19.Johnson S., Lawrence D., Hafekost J., et al. Internet use and electronic gaming by children and adolescents with emotional and behavioural problems in Australia – results from the second child and adolescent survey of mental health and wellbeing. Aust N Z J Psychiatry. 2016;16(1):887–898. doi: 10.1186/s12889-016-3058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoogman M., Bralten J., Hibar D.P., et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry. 2017;4(4):310–319. doi: 10.1016/S2215-0366(17)30049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoogman M., Muetzel R., Guimaraes J.P., et al. Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am J Psychiatry. 2019;176(7):531–542. doi: 10.1176/appi.ajp.2019.18091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukito S., Norman L., Carlisi C., et al. Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol Med. 2020;50(6):894–919. doi: 10.1017/S0033291720000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart H., Radua J., Nakao T., Mataix-Cols D., Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi H., Taki Y., Hashizume H., et al. The impact of television viewing on brain structures: cross-sectional and longitudinal analyses. Cereb Cortex. 2015;25(5):1188–1197. doi: 10.1093/cercor/bht315. [DOI] [PubMed] [Google Scholar]
- 25.Efraim M., Kirwan C.B., Muncy N.M., Tucker L.A., Kwon S., Bailey B.W. Acute after-school screen time in children decreases impulse control and activation toward high-calorie food stimuli in brain regions related to reward and attention. Brain Imaging Behav. 2021;15(1):177–189. doi: 10.1007/s11682-019-00244-y. [DOI] [PubMed] [Google Scholar]
- 26.Aoki Y., Cortese S., Castellanos F.X. Research Review: Diffusion tensor imaging studies of attention-deficit/hyperactivity disorder: meta-analyses and reflections on head motion. J Child Psychol Psychiatry. 2018;59(3):193–202. doi: 10.1111/jcpp.12778. [DOI] [PubMed] [Google Scholar]
- 27.Dong G., Wu L., Wang Z., Wang Y., Du X., Potenza M.N. Diffusion-weighted MRI measures suggest increased white-matter integrity in Internet gaming disorder: evidence from the comparison with recreational Internet game users. Addict Behav. 2018;81:32–38. doi: 10.1016/j.addbeh.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Rahmani F., Sanjari Moghaddam H., Aarabi M.H. Microstructural changes and internet addiction behaviour: a preliminary diffusion MRI study. Addict Behav. 2019;98 doi: 10.1016/j.addbeh.2019.106039. [DOI] [PubMed] [Google Scholar]
- 29.Weyandt L., Swentosky A., Gudmundsdottir B.G. Neuroimaging and ADHD: fMRI, PET, DTI findings, and methodological limitations. Dev Neuropsychol. 2013;38(4):211–225. doi: 10.1080/87565641.2013.783833. [DOI] [PubMed] [Google Scholar]
- 30.Kollins S.H., Adcock R.A. ADHD, altered dopamine neurotransmission, and disrupted reinforcement processes: Implications for smoking and nicotine dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:70–78. doi: 10.1016/j.pnpbp.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jernigan T.L., Brown S.A., Dowling G.J. The adolescent brain cognitive development study. J Res Adolesc. 2018;28(1):154–156. doi: 10.1111/jora.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garavan H., Bartsch H., Conway K., et al. Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auchter A.M., Hernandez Mejia M., Heyser C.J., et al. A description of the ABCD organizational structure and communication framework. Dev Cogn Neurosci. 2018;32:8–15. doi: 10.1016/j.dcn.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark D.B., Fisher C.B., Bookheimer S., et al. Biomedical ethics and clinical oversight in multisite observational neuroimaging studies with children and adolescents: The ABCD experience. Dev Cogn Neurosci. 2018;32:143–154. doi: 10.1016/j.dcn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baurley J.W., Edlund C.K., Pardamean C.I., Conti D.V., Bergen A.W. Smokescreen: a targeted genotyping array for addiction research. BMC Genom. 2016;17(1):1–12. doi: 10.1186/s12864-016-2495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin A.R., Gignoux C.R., Walters R.K., et al. Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet. 2017;100(4):635–649. doi: 10.1016/j.ajhg.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wand H., Lambert S.A., Tamburro C., et al. Improving reporting standards for polygenic scores in risk prediction studies. Nature. 2021;591(7849):211–219. doi: 10.1038/s41586-021-03243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sohail M., Maier R.M., Ganna A., et al. Polygenic adaptation on height is overestimated due to uncorrected stratification in genome-wide association studies. Elife. 2019;8:e39702. doi: 10.7554/eLife.39702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell S., Neale B., Todd-Brown K., et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das S., Forer L., Schonherr S., et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J., Lee S.H., Goddard M.E., Visscher P.M. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi S.W., O’Reilly P.F. PRSice-2: polygenic risk score software for biobank-scale data. Gigascience. 2019;8(7):giz082. doi: 10.1093/gigascience/giz082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amariuta T., Ishigaki K., Sugishita H., et al. Improving the trans-ancestry portability of polygenic risk scores by prioritizing variants in predicted cell-type-specific regulatory elements. Nat Genet. 2020;52(12):1346–1354. doi: 10.1038/s41588-020-00740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Márquez-Luna C., Gazal S., Loh P.R., et al. Incorporating functional priors improves polygenic prediction accuracy in UK Biobank and 23andMe data sets. Nat Commun. 2021;12(1):1–11. doi: 10.1038/s41467-021-25171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casey B.J., Cannonier T., Conley M.I., et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basser P.J., Mattiello J., LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Bihan D., Mangin J.F., Poupon C., et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 48.Pierpaoli C., Jezzard P., Basser P.J., Barnett A., Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 49.Hagler D.J., Ahmadi M.E., Kuperman J., Holland D., Dale A.M. Automated white-matter tractography using a probabilistic diffusion tensor atlas: application to temporal lobe epilepsy. Hum Brain Mapp. 2010;30(5):1535–1547. doi: 10.1002/hbm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hagler D.J., Hatton S., Cornejo M.D., et al. Image processing and analysis methods for the adolescent brain cognitive development study. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Achembach T.M., Rescorla L.A. University of Vermont. University of Vermont Research Center for Children Youth & Families; Burlington, VT: 2001. Manual for the ASEBA school-age forms & profiles. [Google Scholar]
- 52.Achenbach T., Rescorla L.A. Lawrence Erlbaum Associates, Inc.; 2004. The Achenbach system of empirically based assessment (ASEBA) for ages 1.5 to 18 years. The use of psychological testing for treatment planning and outcomes assessment; pp. 179–213. [Google Scholar]
- 53.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 54.Csa Q., Qiang L., Src F., et al. What is the link between attention-deficit/hyperactivity disorder and sleep disturbance? A multimodal examination of longitudinal relationships and brain structure using large-scale population-based cohorts - sciencedirect. Biol Psychiatry. 2020;88(6):459–469. doi: 10.1016/j.biopsych.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng W., Rolls E., Gong W., et al. Sleep duration, brain structure, and psychiatric and cognitive problems in children. Mol Psychiatry. 2021;26(8):3992–4003. doi: 10.1038/s41380-020-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tingley D., Yamamoto T., Hirose K., Keele L., Imai K. Mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1–38. [Google Scholar]
- 57.Hamaker E.L., Kuiper R.M., Grasman R.P. A critique of the cross-lagged panel model. Psychol Methods. 2015;20(1):102–116. doi: 10.1037/a0038889. [DOI] [PubMed] [Google Scholar]
- 58.Rosseel Y. Lavaan: an R package for structural equation modeling and more. Version 0.5–12 (BETA) J Stat Softw. 2012;48(2):1–36. [Google Scholar]
- 59.Hayes A. Introduction to mediation, moderation, and conditional process analysis. J Educ Meas. 2013;51(3):335–337. [Google Scholar]
- 60.Barch D.M., Albaugh M.D., Avenevoli S., et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci. 2018;32:55–66. doi: 10.1016/j.dcn.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horn J.L., Cattell R.B. Refinement and test of the theory of fluid and crystallized general intelligences. Educ Psychol. 1966;57(5):253–270. doi: 10.1037/h0023816. [DOI] [PubMed] [Google Scholar]
- 62.Luman M., van Meel C.S., Oosterlaan J., Geurts H.M. Reward and punishment sensitivity in children with ADHD: validating the sensitivity to punishment and sensitivity to reward questionnaire for children (SPSRQ-C) J Abnorm Child Psychol. 2012;40(1):145–157. doi: 10.1007/s10802-011-9547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marx I., Hacker T., Yu X., Cortese S., Sonuga-Barke E. ADHD and the choice of small immediate over larger delayed rewards: a comparative meta-analysis of performance on simple choice-delay and temporal discounting paradigms. J Atten Disord. 2021;25(2):171–187. doi: 10.1177/1087054718772138. [DOI] [PubMed] [Google Scholar]
- 64.Han D.H., Lee Y.S., Yang K.C., Kim E.Y., Lyoo I.K., Renshaw P.F. Dopamine genes and reward dependence in adolescents with excessive internet video game play. J Addict Med. 2007;1(3):133–138. doi: 10.1097/ADM.0b013e31811f465f. [DOI] [PubMed] [Google Scholar]
- 65.Drtilkova I., Sery O., Theiner P., Uhrova A., Znojil V. Clinical and molecular-genetic markers of ADHD in children. Neuro Endocrinol Lett. 2008;29(3):320–327. [PubMed] [Google Scholar]
- 66.Paclt I., Drtílková I., Kopeckova M., Theiner P., Sery O., Cermakova N. The association between TaqI A polymorphism of ANKK1 (DRD2) gene and ADHD in the Czech boys aged between 6 and 13 years. Neuroendocrinol Lett. 2010;31(1):131–136. [PubMed] [Google Scholar]
- 67.Ni H.C., Gau S.S. Co-occurrence of attention-deficit hyperactivity disorder symptoms with other psychopathology in young adults: parenting style as a moderator. Compr Psychiatry. 2015;57:85–96. doi: 10.1016/j.comppsych.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Johnston C., Mash E.J., Miller N., Ninowski J.E. Parenting in adults with attention-deficit/hyperactivity disorder (ADHD) Clin Psychol Rev. 2012;32(4):215–228. doi: 10.1016/j.cpr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saad J.F., Griffiths K.R., Korgaonkar M.S. A systematic review of imaging studies in the combined and inattentive subtypes of attention deficit hyperactivity disorder. Front Integr Neurosci. 2020;14:31. doi: 10.3389/fnint.2020.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaw P., Sudre G., Wharton A., Weingart D., Sharp W., Sarlls J. White matter microstructure and the variable adult outcome of childhood attention deficit hyperactivity disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2015;40(3):746–754. doi: 10.1038/npp.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Albaugh M.D., Hudziak J.J., Ing A., et al. White matter microstructure is associated with hyperactive/inattentive symptomatology and polygenic risk for attention-deficit/hyperactivity disorder in a population-based sample of adolescents. Neuropsychopharmacology. 2019;44(9):1597–1603. doi: 10.1038/s41386-019-0383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qian A., Wang X., Liu H., et al. Dopamine D4 receptor gene associated with the frontal-striatal-cerebellar loop in children with ADHD: a resting-state fMRI study. Neurosci Bull. 2018;34(3):497–506. doi: 10.1007/s12264-018-0217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yap K.H., Abdul Manan H., Sharip S. Heterogeneity in brain functional changes of cognitive processing in ADHD across age: a systematic review of task-based fMRI studies. Behav Brain Res. 2021;397 doi: 10.1016/j.bbr.2020.112888. [DOI] [PubMed] [Google Scholar]
- 74.Meck W.H., Benson A.M. Dissecting the brain's internal clock: how frontal–striatal circuitry keeps time and shifts attention. J Brain Cognit. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- 75.Seo M., Lee E., Averbeck B.B. Action selection and action value in frontal-striatal circuits. Neuron. 2012;74(5):947–960. doi: 10.1016/j.neuron.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conner A.K., Briggs R.G., Sali G., et al. A connectomic atlas of the human cerebrum-chapter 13: tractographic description of the inferior fronto-occipital fasciculus. Oper Neurosurg. 2018;15(suppl_1):S436–SS43. doi: 10.1093/ons/opy267. (Hagerstown) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rehman A., Al Khalili Y. StatPearls Publishing; FL: 2021. Neuroanatomy, occipital lobe. Treasure island. G, ed. [PubMed] [Google Scholar]
- 78.Ailion A.S., King T.Z., Roberts S.R., et al. Double dissociation of auditory attention span and visual attention in long-term survivors of childhood cerebellar tumor: a deterministic tractography study of the cerebellar-frontal and the superior longitudinal fasciculus pathways. J Int Neuropsychol Soc. 2020;26(10):939–953. doi: 10.1017/S1355617720000417. [DOI] [PubMed] [Google Scholar]
- 79.Kinoshita M., Nakajima R., Shinohara H., et al. Chronic spatial working memory deficit associated with the superior longitudinal fasciculus: a study using voxel-based lesion-symptom mapping and intraoperative direct stimulation in right prefrontal glioma surgery. J Neurosurg. 2016;125(4):1024–1032. doi: 10.3171/2015.10.JNS1591. [DOI] [PubMed] [Google Scholar]
- 80.Sudre G., Choudhuri S., Szekely E., et al. Estimating the heritability of structural and functional brain connectivity in families affected by attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(1):76–84. doi: 10.1001/jamapsychiatry.2016.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chiang H.L., Chen Y.J., Lo Y.C., Tseng W.Y., Gau S.S. Altered white matter tract property related to impaired focused attention, sustained attention, cognitive impulsivity and vigilance in attention-deficit/hyperactivity disorder. J Psychiatry Neurosci. 2015;40(5):325–335. doi: 10.1503/jpn.140106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schulte T., Muller-Oehring E.M. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychol Rev. 2010;20(2):174–190. doi: 10.1007/s11065-010-9130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rolls E.T., Cheng W., Feng J. Brain dynamics: the temporal variability of connectivity, and differences in schizophrenia and ADHD. Transl Psychiatry. 2021;11(1):1–11. doi: 10.1038/s41398-021-01197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arnatkeviciute A., Fulcher B.D., Bellgrove M.A., Fornito A. Where the genome meets the connectome: understanding how genes shape human brain connectivity. Neuroimage. 2021;244 doi: 10.1016/j.neuroimage.2021.118570. [DOI] [PubMed] [Google Scholar]
- 85.Cortese S., Aoki Y.Y., Itahashi T., Castellanos F.X., Eickhoff S.B. Systematic review and meta-analysis: resting-state functional magnetic resonance imaging studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(1):61–75. doi: 10.1016/j.jaac.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 86.Samea F., Soluki S., Nejati V., et al. Brain alterations in children/adolescents with ADHD revisited: a neuroimaging meta-analysis of 96 structural and functional studies. Neurosci Biobehav Rev. 2019;100:1–8. doi: 10.1016/j.neubiorev.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.