Abstract
Rationale & Objective
There is conflicting evidence regarding the type of β-blockers to use in dialysis patients. This systematic review seeks to determine whether highly dialyzable β-blockers are associated with higher rates of cardiovascular events and mortality in hemodialysis patients than poorly dialyzable β-blockers.
Study Design
A systematic review of the existing literature was conducted. A meta-analysis was performed using data from the selected studies.
Setting & Study Populations
Participants were from the United States, Canada, and Taiwan. The mean ages of participants ranged from 55.9-75.7 years.
Selection Criteria for Studies
We searched the Ovid MEDLINE database from 1990 to September 2020. Studies without adult hemodialysis participants and without comparisons of at least 2 β-blockers of different dialyzability were excluded.
Data Extraction
Baseline and adjusted outcome data were extracted from each study.
Analytical Approach
Random-effects models were used to calculate pooled risk ratios using fully adjusted models from individual studies.
Results
Four cohort studies were included. Pooling fully adjusted models, highly dialyzable β-blockers did not influence mortality (HR, 0.94; 95% CI, 0.81-1.08; I2 = 0.84) compared with poorly dialyzable β-blockers but were associated with a reduction in cardiovascular events (HR, 0.88; 95% CI, 0.83-0.93). There was significant heterogeneity between studies (I2 = 0.35). Only 1 study reported on adverse events. Intradialytic hypotension was more common in those on carvedilol (a poorly dialyzable β-blocker) compared with those on metoprolol (a highly dialyzable β-blocker; adjusted incidence rate ratio, 1.10; 95% CI, 1.09-1.11).
Limitations
No randomized controlled trials were identified. Each study used different analytic methods and different definitions for outcomes. Classifications of β-blockers varied. Only 1 study reported on adverse events.
Conclusions
Pooled data suggest highly dialyzable β-blockers are associated with similar mortality events and fewer cardiovascular events compared with poorly dialyzable β-blockers.
Index Words: Acebutolol, adrenergic beta-antagonists, atenolol, β-blockers, beta blockers, bisoprolol, carvedilol, hemodialysis, labetalol, metoprolol, propranolol, renal dialysis
Plain-Language Summary.
Numerous studies exist seeking to address the controversy surrounding which β-blockers are best for patients undergoing hemodialysis. To better delineate the evidence on β-blocker dialyzability in hemodialysis patients, we undertook a systematic review of this past literature. Poorly dialyzable β-blockers (carvedilol, labetalol, betaxolol, and propranolol) were compared with highly dialyzable β-blockers (metoprolol, atenolol, bisoprolol, and acebutolol). For most studies in this review, highly dialyzable β-blockers were associated with lower cardiovascular events and all-cause mortality than poorly dialyzable β-blockers. Not surprisingly, not all of the literature was concordant in their results. These findings demonstrate that more research is needed to determine which class of β-blockers may be most beneficial to hemodialysis patients.
Editorial, •••
β-Blockers are a mainstay for the prevention of adverse cardiovascular outcomes in populations at risk. In the general population, β-blockers have been demonstrated to improve outcomes among patients with a history of cardiovascular disease.1
Dialysis patients are at a particularly high risk of experiencing adverse cardiovascular events. Cardiovascular diseases are responsible for half of all deaths among patients on maintenance dialysis.2
Beta blockers are the only class of drugs that have consistently been shown to reduce cardiovascular events in dialysis patients. Randomized controlled trials among patients receiving dialysis have demonstrated that β-blockers decrease left ventricular hypertrophy in patients with nonischemic dilated cardiomyopathy and decrease cardiovascular events in dialysis patients with left ventricular hypertrophy and hypertension.3 As such, guidelines recommend the use of β-blockers by patients undergoing dialysis.4 Data from Medicare Part D indicate that the most common type of cardiovascular medication among patients receiving dialysis is β-blockers, with approximately 64% of enrolled dialysis patients receiving β-blocker prescriptions in 2007.5
Although most dialysis patients are taking β-blockers, not all medications that belong to this drug class are alike. Individual β-blockers have different pharmacologic and pharmacokinetic properties (Table 1), each of which may influence the effectiveness of the particular β-blocker prescribed to a patient.2,6, 7, 8 An especially relevant property for hemodialysis patients is the degree of β-blocker dialyzability. Dialyzability refers to the ability of a medication to be removed from the circulation during dialysis. Highly dialyzable β-blockers are efficiently removed during dialysis, whereas poorly dialyzable β-blockers are not.
Table 1.
Summary of Pharmacokinetic and Pharmacological Properties of Commonly Prescribed β-Blocker Medications
| β-Blocker | Dialyzability from Literature | Dialyzability in This Review | Cardioselectivity |
|---|---|---|---|
| Acebutolol | Dialyzable8,10,21 | Highly dialyzable | Cardioselective21 |
| Atenolol | Dialyzable6,8,10,14,15 | Highly dialyzable | Cardioselective2,6,14,20 |
| Betaxolol | Not dialyzable8 | Poorly dialyzable | Cardioselective14 |
| Bisoprolol | Not dialyzable10; dialyzable8,14,15 | Highly dialyzable | Cardioselective2,14,20 |
| Carvedilol | Not dialyzable6, 7, 8,14,15 | Poorly dialyzable | Nonselective2,6,14,20,a |
| Labetalol | Not dialyzable6,14 | Poorly dialyzable | Nonselective2,6,14,20,a |
| Metoprolol | Dialyzable6, 7, 8,10,14,15 | Highly dialyzable | Cardioselective2,6,14,20 |
| Propanolol | Not dialyzable8,10,14 | Poorly dialyzable | Nonselective2,14,20 |
Note: Cardioselective means that the β-blocker interacts with only the β-1 receptors. Nonselective means that the β-blocker interacts with both β-1 and β-2 receptors.
The β-blocker also interacts with the α receptors.
In the nondialysis population, a sudden discontinuation of β-blocker therapy has been linked to tachycardia, hypertension, and adverse cardiovascular events.9 In dialysis patients, efficient removal of highly dialyzable β-blockers from the circulation during dialysis can lead to a rapid decline in the β-blocker concentration.10 This may potentially cause adverse effects equal to or greater than those experienced when simply stopping β-blocker therapy. In comparison, poorly dialyzable β-blockers, which are not removed to a significant degree during hemodialysis, are likely to continue exerting effects during dialysis.7 Because dialysis is a time when patients are undergoing rapid changes in hemodynamics, those taking poorly dialyzable β-blockers may be at a greater risk of both intradialytic and postdialysis hypotension, which can lead to falls and cardiovascular events.
Given the uncertainty regarding how the dialyzability of β-blockers may influence outcomes among dialysis patients, we conducted a systematic review to determine whether the dialyzability of β-blockers influences outcomes in hemodialysis patients. We hypothesized that among dialysis patients, highly dialyzable β-blockers are associated with an increased risk of cardiovascular events and mortality compared with poorly dialyzable β-blockers, with no increase in adverse events (ie, intradialytic hypotension).
Methods
Search Strategy and β-Blocker Article Selection
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.11 We conducted a search in the Ovid MEDLINE database for results published in English from January 1990 to September 2020. The search string was developed to identify randomized controlled trials, prospective and retrospective cohort studies, case report studies, systematic reviews, and meta-analyses. The search strategy used the Medical Subject Heading terms for renal dialysis and adrenergic β-antagonists, as well as those for several individual β-blocker medications. Details of the search string are provided in Item S1.
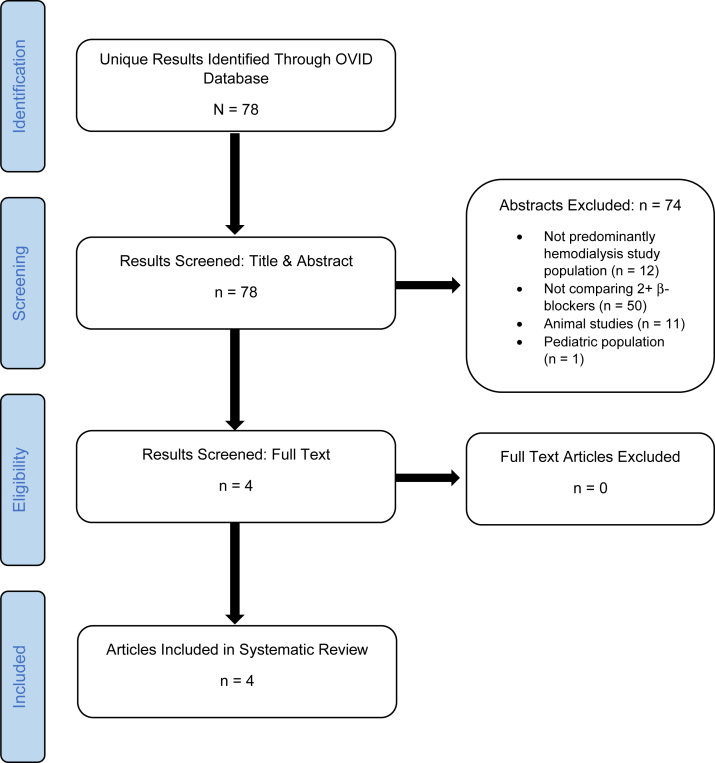
The predetermined inclusion and exclusion criteria were used to screen the results of the search. Two reviewers (OT & WV, EI & AT, and AZ & MM) independently performed an initial screening of each abstract. Any differences in the reviewers’ judgments to include or exclude an abstract for further screening were resolved by team discussion. The results that passed the initial abstract screening then underwent a full-text screening by 2 reviewers (OT & EI and AZ & AT). Differences in judgment were resolved through team discussion. Articles that remained after the full-text review were selected for inclusion in the systematic review (Fig 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. After developing a search string and running it through the Ovid MEDLINE database, 78 unique results were identified. The initial abstract screening resulted in the exclusion of 74 articles, leaving 4 articles for further review. Following a full-text review, 4 articles were determined to meet the eligibility requirements for inclusion in our systematic review.
Bias Assessment
The included articles were evaluated for study quality using the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool.12,13 This tool is designed to assess the risk of bias present in nonrandomized studies that compare the effects of 2 or more interventions. The ROBINS-I tool covers 7 domains: 2 before the intervention, 1 at the intervention, and 4 after the intervention. The rating tool considers biases that may arise from confounding variables, participant selection, classification of interventions, deviations from the intended interventions, missing data, outcome measurements, and selection of the reported results (Table 2). Based on a reviewer’s responses to a set of signaling questions (Table S1), each domain may be assigned a low, moderate, serious, or critical risk of bias. An overall risk of bias is then determined by considering a bias assignment across the set of domains. The overall risk of bias assigned to an article can be no lower than the highest bias judgment given to an individual domain for that article. Each article was evaluated separately by 2 reviewers (OT & AZ, EI & AT, and WV & MM), and differences were resolved by discussion.
Table 2.
Bias Assessment Results
| Study | Bias Due to Confounding | Bias in the Selection of Participants in the Study | Bias in the Classification of Interventions | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Data | Bias in the Measurement of Outcome | Bias in the Selection of the Reported Results | Overall Study Bias Rating |
|---|---|---|---|---|---|---|---|---|
| Assimon et al7 | Serious | Low | Low | Low | Low | Low | Low | Serious |
| Shireman et al6 | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious |
| Weir et al10 | Moderate | Serious | Low | Moderate | Low | Moderate | Moderate | Serious |
| Wu et al8 | Moderate | Low | Low | Low | Serious | Low | Moderate | Serious |
Note: Domain-specific and overall bias ratings of the 4 included studies.
β-Blocker Classification
For our analysis, we classified β-blockers as either highly dialyzable or poorly dialyzable, based on the previous literature (Table 1). The highly dialyzable β-blocker group consisted of metoprolol, atenolol, bisoprolol, and acebutolol.6, 7, 8,10,14,15 The comparison group of poorly dialyzable β-blockers consisted of carvedilol, labetalol, betaxolol, and propranolol.6, 7, 8,10,14,15
Three of the studies had comparison groups based on dialyzability, whereas 1 study compared β-blockers based on cardioselectivity.6, 7, 8,10 However, the cardioselective β-blockers included in this study (atenolol and metoprolol) are both highly dialyzable, and the nonselective β-blockers (carvedilol and labetalol) are both poorly dialyzable (Table 1). This allowed us to reclassify the cardioselective and nonselective comparison groups as highly dialyzable and poorly dialyzable, respectively.
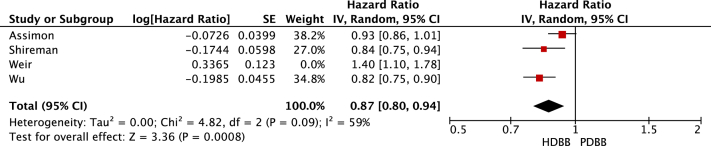
Weir et al10 classified bisoprolol as a “low-dialyzability” β-blocker, whereas Wu et al8 classified it as a “dialyzable” β-blocker. Outside this review, some studies have labeled bisoprolol as “not significantly dialyzable,” whereas others have listed it among the dialyzable β-blockers.10,14 A recent study by Tieu et al15 demonstrated that although bisoprolol (clearance 96 mL/min) is not removed from the body to the same degree as typical highly dialyzable β-blockers such as metoprolol and atenolol (clearances of 114 mL/min and 167 mL/min, respectively), it is still removed far more extensively than the negligibly cleared carvedilol (clearance 24 mL/min). To account for this different classification of bisoprolol, we performed a sensitivity analysis excluding the study by Weir et al.10
Data Extraction and Analysis
Baseline characteristics and outcomes data from each study were compiled by dialyzability group (Table 3; Table S2). For each study, the most adjusted hazard ratio (HR) for outcomes was extracted. Data were extracted for all outcomes reported, with a focus on mortality, cardiovascular outcomes, and adverse events. Table 4 presents the HRs for highly versus poorly dialyzable β-blockers in all 4 studies. The HRs listed in the studies by Weir et al,10 Shireman et al,6 and Wu et al8 are presented as they were reported in their respective studies. The HRs reported by Assimon et al7 compared a minimally dialyzable β-blocker to a highly dialyzable β-blocker. Because this was the inverse of the other studies in our review, we inversed these HRs (and their respective confidence intervals [CIs]) before entering them into our Table 4. Data were synthesized using Review Manager 5.3 (The Cochrane Collaboration) after entering the ln[HR] and the standard error. The standard error was calculated using the following formula:
where the upper and lower limits refer to the CI and x is 3.92 for 95% CI or 5.15 for 99% CI.
Table 3.
Baseline Characteristics and Outcomes Measured by Study and β-Blocker Dialyzability Cohort
| Characteristics and Outcomes | Highly Dialyzable β-Blocker Cohort |
Poorly Dialyzable β-Blocker Cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Assimon et al7 | Weir et al10 | Shireman et al6 |
Wu et al8 | Assimon et al7 | Weir et al10 | Shireman et al6 |
Wu et al8 | |||
| ACM Model | CVMM Model | ACM Model | CVMM Model | |||||||
| Medications included in cohorts | Metoprolol Succinate, Metoprolol Tartrate | Acebutolol, Atenolol, and Metoprolol tartrate | Atenolol and Metoprolol | Atenolol and Metoprolol | Atenolol, Acebutolol, Metoprolol, and Bisoprolol | Carvedilol | Propranolol and Bisoprolol | Carvedilol and Labetalol | Carvedilol and Labetalol | Betaxolol, Carvedilol, and Propranolol |
| Population/ no. of cases (n = X) | 17,521 | 3,294 | 3,781 | 3,495 (100 %) | 10,446 | 9,533 | 3,294 | 1,157 | 1,042 (100 %) | 10,353 |
| Age, y | 59.5 | 75.6 | 60.4 | 60.1 (15.2%) | 55.9 | 59.8 | 75.7 | 58.3 | 57.6 (16.0%) | 57.2 |
| Females, n (%) | 8,183 | 1,617 | 2,172 | 1,992 (57.0 %) | 2,380 | 4,444 | 1,617 | 625 | 559 (53.7 %) | 513 |
| End of follow-up, d | 365 | 180 | 50% mortality reached at 1,083 d | 50% CVMM reached at 599 d | 730 | 365 | 180 | 50% mortality reached at 894 d | 50% CVMM reached at 481 d | 730 |
| Average follow-up, d | 285 | Median duration of continuous use: 471 | 602 | 276 | Median duration of continuous use: 508 | 544 | ||||
| Outcomes measured | 1-13, 20 | 2, 12, 18 | 1 | 2, 4-7, 12, 14-17 | 2, 12, 19 | 1-13 | 2, 12, 18 | 1 | 2, 4-7, 12, 14-17 | 2, 12, 19 |
Note: Baseline and outcomes data for each of the 4 studies, organized by β-blocker dialyzability cohort. For average follow-up time, the median duration of continuous use in the study by Weir et al10 describes the median number of days participants continuously took their assigned β-blocker. Shireman et al6 provided average follow-up data in terms of when 50% of their study population in each dialyzability group had experienced the outcomes of interest. The following numbers were used to code for the outcomes measured: 1, all-cause mortality; 2, acute myocardial infarction; 3, pericarditis (including cardiac tamponade); 4, atherosclerotic heart disease; 5, cardiomyopathy; 6, cardiac arrhythmia; 7, cardiac arrest (cause unknown); 8, valvular heart disease; 9, pulmonary edema due to exogenous fluid; 10, congestive heart failure; 11, pulmonary embolism and cerebrovascular accident (including intracranial hemorrhage); 12, heart failure; 13, atrial fibrillation; 14, ischemic heart disease; 15, revascularization; 16, cerebrovascular accident; 17, peripheral vascular disease; 18, ventricular arrhythmia; 19, ischemic stroke; and 20, intradialytic hypotension (≥20 mm Hg systolic blood pressure drop plus intradialytic saline administration).
Abbreviations: ACM, all-cause mortality; CVMM, cardiovascular morbidity and mortality.
Table 4.
All-Cause Mortality and Cardiovascular Events Hazard Ratios from Individual Papers, Demonstrating Results from Their Most Fully Adjusted Model
| Study | Model | All-Cause Mortality | Cardiovascular Events | Model Adjusted for |
|---|---|---|---|---|
| Weir et al10 | Adjusted RR | 1.4 (1.1-1.8) | 1.3 (0.9-2.0) | Matched patients from poorly dialyzable cohort to highly dialyzable cohort based on race, sex, and propensity scorea |
| Shireman et al6 | Adjusted HR | 0.84 (0.72-0.97) | 0.86 (0.75-0.99) | Cardioselectivity, start of β-blocker, age, sex, race, BMI category, smoker status, substance use status, employment, inability of ambulate. Inability to transfer, diabetes, congestive heart failure, cerebrovascular accident, peripheral vascular disease, hemoglobin, self-care dialysis |
| Assimon et al7 | Adjusted HR | 0.93 (0.86-0.98) | 0.85 (0.78-0.93) | Patient demographics, comorbid conditions, laboratory data, dialysis treatment parameters, and prescription medication use. Refer to Assimon et al7Table S2 for specifics |
| Wu et al8 | Adjusted HR | 0.82 (0.75-0.88) | 0.89 (0.84-0.93) | Age, sex, comorbid conditions, concomitant medications |
Note: All-cause mortality and cardiovascular events hazard ratios and relative risks for highly dialyzable β-blockers versus poorly dialyzable β-blockers across the 4 studies. The average adjusted ratio was calculated using Review Manager.
Abbreviations: BMI, body mass index; HR, hazard ratio; IRR, incidence rate ratio; RR, risk ratio.
Weir et al10 matched their dialyzability cohorts based on the characteristics above instead of adjusting for the said characteristics.
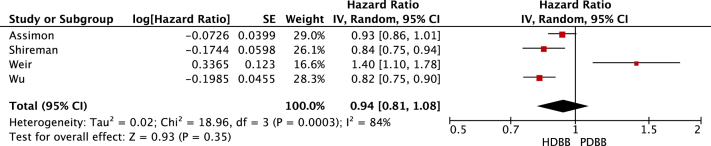
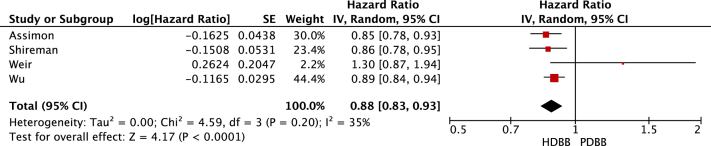
Review Manager calculated the HRs, CIs, and weights for the individual studies. As a result, the CIs generated by Review Manager for the individual studies (Figure 2, Figure 3, Figure 4-5) were slightly different from those listed in Table 4. The statistical heterogeneity of all-cause mortality and cardiovascular mortality was demonstrated by generating HR forest plots (Figure 2, Figure 3, Figure 4-5; Table S3). Data were pooled using a random-effects model, and the heterogeneity (I2) between the studies was calculated using the following formula:
where Q is the Cochran’s heterogeneity statistic and df represent the degrees of freedom.16
Figure 2.
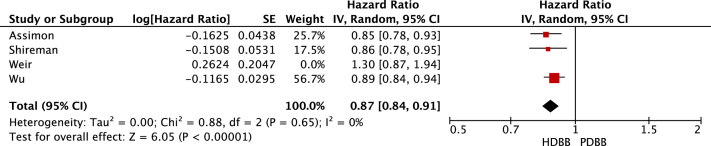
All-cause mortality. Forest plot illustrating the individual and pooled hazard ratios for all-cause mortality for all 4 selected studies. Generated using Review Manager. Abbreviations: CI, confidence interval; HDBB, highly dialyzable β-blocker; IV, inverse variance; PDBB, poorly dialyzable β-blocker; SE, standard error.
Figure 3.
All-cause mortality sensitivity analysis. Forest plot illustrating the individual and pooled hazard ratios for all-cause mortality for 3 of the selected studies. The study by Weir et al10 was excluded from this analysis due to its classification of bisoprolol. Generated using Review Manager. Abbreviations: HDBB, highly dialyzable β-blocker; IV, inverse variance; PDBB, poorly dialyzable β-blocker.
Figure 4.
Cardiovascular events. Forest plot illustrating the individual and pooled hazard ratios for cardiovascular events for all 4 selected studies. Generated using Review Manager. Abbreviations: HDBB, highly dialyzable β-blocker; IV, inverse variance; PDBB, poorly dialyzable β-blocker.
Figure 5.
Cardiovascular events sensitivity analysis. Forest plot illustrating the individual and pooled hazard ratios for cardiovascular events for 3 of the selected studies. The study by Weir et al10 was excluded from this analysis due to its classification of bisoprolol. Generated using Review Manager. Abbreviations: HDBB, highly dialyzable β-blocker; IV, inverse variance; PDBB, poorly dialyzable β-blocker.
Results
Search Strategy and β-Blocker Article Selection
After developing a search string and performing an Ovid MEDLINE search, 78 unique articles were identified. Two reviewers screened each abstract against the inclusion and exclusion criteria (OT & WV, EI & AT, and AZ & MM).
In the initial abstract screening, articles were excluded if they did not involve a comparison of at least 2 β-blockers (n = 50). Articles were also excluded during this stage if the study population was not human (n = 11) or not composed of adult patients (n = 1). Studies that did not include patients on hemodialysis were also excluded (n = 12). Four articles remained for full-text review. All 4 met the inclusion and exclusion criteria to be included in the final meta-analysis (Fig 1).
After the search in September 2020, an article was published that appeared to meet the criteria for inclusion in this systematic review.17 However, the article was determined to involve a subset of the patients of an already-included study. To avoid redundancy in the data, the new article was not included.
β-Blocker Dialyzability Study Summaries
Four studies published between 2015 and 2020 were selected for inclusion into this systematic review (Table 5).
Table 5.
Summary of Studies Included in the Meta-analysis
| Study | Study Design | Comparison Groups | Results |
|---|---|---|---|
| Assimon et al7 | Retrospective cohort | Metoprolol (high dialyzability) vs carvedilol (low dialyzability) | Carvedilol was associated with greater ACM (adjusted HR, 1.08; 95% CI, 1.02-1.16) and cardiovascular mortality (adjusted HR, 1.18; 95% CI, 1.08-1.29) than metoprolol. Intradialytic hypotension was more common in those on poorly dialyzable β-blockers compared with those on highly dialyzable β-blockers (adjusted IRR, 1.10; 95% CI, 1.09-1.11) |
| Shireman et al6 | Retrospective cohort | Atenolol and metoprolol (cardioselective) vs carvedilol and labetalol (noncardioselective) | Cardioselective β-blockers were associated with a lower ACM (AHR, 0.84; 99% CI, 0.72-0.97; P = 0.0026) and CVE (AHR, 0.86; 99% CI, 0.75-0.99; P = 0.0042) than noncardioselective β-blockers |
| Weir et al10 | Retrospective cohort | Acebutolol, atenolol, and metoprolol (high dialyzability) vs bisoprolol and propranolol (low dialyzability) | The high-dialyzability group had 40% higher risk of ACM than the low-dialyzability group (RR, 1.4; 95% CI, 1.1-1.8; P = 0.01) and had a higher risk of CVE than the below-dialyzability group (RR, 1.2; 95% CI, 1.0-1.5; P = 0.03) |
| Wu et al8 | Retrospective cohort | Atenolol, acebutolol, metoprolol (high dialyzability), and bisoprolol vs betaxolol, carvedilol, and propranolol (low dialyzability) | Highly dialyzable β-blockers were associated with lower ACM (HR, 0.82; 95% CI, 0.75-0.88) and a lower risk of CVE (HR, 0.89; 95% CI, 0.84-0.93) than nondialyzable β-blockers |
Abbreviations: ACM, all-cause mortality; AHR, adjusted HR; CI, confidence interval; CVE, cardiovascular event; HR, hazard ratio; IRR, incidence rate ratio; RR, risk ratio.
Weir et al10 conducted a 1-to-1, propensity-matched, population-based, cohort study using health care databases in Ontario, Canada. The study population included long-term dialysis patients with incident β-blockers usage. The high-dialyzability β-blocker cohort (n = 3,294) included patients taking atenolol, acebutolol, or metoprolol, with a mean age of 74.5 years. The low-dialyzability β-blocker cohort (n = 3,294) included patients taking bisoprolol or propranolol, with a mean age of 74.4 years. Data used in the hemodialysis cohort were collected between April 1, 2002, and March 31, 2011. The outcomes included all-cause mortality and ventricular arrhythmia (a proxy for sudden cardiac death). The authors used propensity matching to reduce differences between the hemodialysis and nondialysis participant cohorts. Conditional logistic regression models were used to generate odds ratios, which were interpreted as risk ratios. In the 180-day follow-up period, dialysis patients who had initiated a high-dialyzability β-blocker had higher risks of death and arrhythmia than those on a low-dialyzability β-blocker (HRs, 1.4 [95% CI, 1.1-1.8] and 1.3 [95% CI, 0.9-2.0], respectively).
Shireman et al6 conducted a cohort study using the US Medicare and Medicaid data and US Renal Data System data collected between 2000-2005. They compared Medicare and Medicaid dually eligible incident hemodialysis and peritoneal dialysis patients who had hypertension and were newly prescribed a cardioselective β-blocker (atenolol or metoprolol) with those who were prescribed a nonselective β-blocker (carvedilol or labetalol). The authors further divided each β-blocker cohort into 2 subgroups: those who experienced the outcome of all-cause mortality and those who experienced cardiovascular morbidity and mortality (defined as inpatient hospitalization for myocardial infarction, ischemic heart disease, revascularization, congestive heart failure, or cerebrovascular accident; or death from myocardial infarction, atherosclerotic heart disease, cardiomyopathy, cardiac arrhythmia, cardiac arrest, or cerebrovascular accident). There was an overlap within each β-blocker cohort, as it was possible for some participants to fall within the all-cause mortality group if they had experienced cardiovascular mortality. In a Cox proportional hazard model that adjusted for potential confounding using covariate adjustment, the use of cardioselective β-blockers was associated with a lower risk of mortality (n = 4,938; HR, 0.84; 99% CI, 0.72-0.97) and a lower risk of cardiovascular events (n = 4,537; HR, 0.86; 99% CI, 0.75-0.99).
Assimon et al7 conducted a cohort study using data extracted from the clinical database of a large US dialysis organization, along with US Renal Data System data, from January 1, 2007, to December 30, 2012. They compared prevalent hemodialysis patients initiating metoprolol (n = 17,521) or carvedilol (n = 9,533). The primary outcome was 1-year all-cause mortality and cardiovascular mortality. The adjusted HRs were calculated by applying inverse probability of treatment weighting to Cox proportional hazard models and Fine and Gray proportional subdistribution hazard models. Carvedilol initiation is associated with greater risks of both all-cause mortality and cardiovascular mortality than metoprolol initiation during the 1 year follow-up period (adjusted HRs, 1.08 [95% CI, 1.02-1.16] and 1.18 [95% CI, 1.08-1.29], respectively). Carvedilol initiation, compared with either metoprolol succinate or metoprolol tartrate initiation, was associated with a higher rate of intradialytic hypotension (adjusted incidence rate ratio, 1.10; 95% CI, 1.09-1.11).
Wu et al8 conducted a cohort study of hemodialysis patients in Taiwan using data from 2004-2011 from the National Health Insurance Research Database. They compared maintenance hemodialysis patients who initiated a dialyzable β-blocker (atenolol, acebutolol, metoprolol, or bisoprolol; n = 10,446) or nondialyzable β-blocker (betaxolol, carvedilol or propranolol; n = 10,353) after the initiation of dialysis. The outcomes included all-cause mortality and major adverse cardiovascular events (acute coronary syndrome, ischemic stroke, heart failure) at 2 years. Using the Cox proportional hazard models, with a multivariable model to control for confounders, the use of dialyzable β-blockers was associated with lower all-cause mortality (HR, 0.82; 95% CI, 0.75-0.88) and lower major adverse cardiovascular events (HR, 0.89; 95% CI, 0.84-0.93) compared to use of nondialyzable β-blockers.
Bias Assessment
Using the ROBINS-I bias rating tool, all 4 studies were graded as having an overall serious risk of bias (Table 2).
Meta-analysis
Pooling of data suggested no difference in the all-cause mortality when comparing highly dialyzable β-blockers with poorly dialyzable β-blockers (HR, 0.94; 95% CI, 0.81-1.08). There was a significant reduction in cardiovascular events in those prescribed highly dialyzable β-blockers compared with those prescribed poorly dialyzable β-blockers (HR, 0.88; 95% CI, 0.83-0.93). A sensitivity analysis limited to studies classifying bisoprolol as highly dialyzable resulted in highly dialyzable β-blockers being associated with a reduced risk of all-cause mortality (HR, 0.87; 95% CI, 0.80-0.94) compared with poorly dialyzable β-blockers. A sensitivity analysis also found highly dialyzable β-blockers to be significantly associated with a reduction in cardiovascular events (HR, 0.87; 95% CI, 0.84-0.91) compared with poorly dialyzable β-blockers. Only 1 study reported on adverse events. Intradialytic hypotension was more common in those on poorly dialyzable β-blockers compared with those on highly dialyzable β-blockers (adjusted incidence rate ratio, 1.10; 95% CI, 1.09-1.11).7
Discussion
A comprehensive literature search from January 1990 to September 2020 identified 4 articles for inclusion. All 4 of these were prospective cohort studies. No randomized controlled trials were identified. Of the 4 studies, 3 demonstrated that highly dialyzable β-blockers are associated with lower cardiovascular events and all-cause mortality compared to poorly dialyzable β-blockers, whereas 1 study suggested that poorly dialyzable β-blockers were associated with lower cardiovascular events and all-cause mortality.6, 7, 8,10 Pooled data suggested no difference between the type of β-blocker and mortality, with highly dialyzable β-blockers being associated with a reduction in cardiovascular mortality compared with poorly dialyzable β-blockers. A sensitivity analysis including only studies classifying bisoprolol as dialyzable suggested a reduction in both all-cause and cardiovascular mortality associated with highly dialyzable β-blockers.
Only 1 study reported on adverse events. Patients on carvedilol (a poorly dialyzable β-blocker) had a greater incidence of intradialytic hypotension than those on metoprolol (a highly dialyzable β-blocker). This finding is not surprising given the kinetic differences in various β-blockers during dialysis. Poorly dialyzed β-blockers are not removed during dialysis, and their antihypertensive effect is maintained consistently during dialysis. Dialyzable β-blockers are removed as dialysis progresses and have diminishing blood pressure effects over the course of dialysis.
In 2014, the Hypertension in Hemodialysis Patients Treated with Atenolol or Lisinopril (HDPAL) trial compared 2 highly dialyzable antihypertensive agents: lisinopril (an angiotensin-converting enzyme inhibitor) and atenolol (a β-blocker). Atenolol was associated with fewer adverse cardiovascular events.3 Although these results reflected favorably on highly dialyzable β-blockers, the study only incorporated 1 highly dialyzable β-blocker and did not compare β-blockers of different dialyzabilities. In addition, the trial was conducted with a predominantly Black study population, limiting its generalizability. Although this review considers a much different question than that considered by the HDPAL trial, our findings reinforce this preference for highly dialyzable β-blockers over other agents.
Of note in the HDPAL trial was the dosing regimen. Because of the dialyzable nature of both antihypertensive agents, they were administered to patients thrice weekly after dialysis treatments. The 4 observational studies included in this systematic review did not report on how each β-blocker was prescribed or administered. The timing of administration is something that clinicians may choose to consider when prescribing highly dialyzable β-blockers to hemodialysis patients. Future randomized controlled trials that investigate this question of β-blocker dialyzability in hemodialysis patients should consider standardizing the timing of administration of highly dialyzable β-blockers with respect to dialysis treatments.
Carvedilol has been considered a favored β-blocker among patients with heart failure in both the general population and dialysis settings.14 However, this preference has been based on studies comparing carvedilol with other β-blockers in nondialysis settings and on studies comparing carvedilol with placebo in dialysis.18,19 In contrast, the studies in this review compared different β-blockers in dialysis settings. With respect to all-cause mortality and cardiovascular events, our review did not favor poorly dialyzable β-blockers, such as carvedilol. Carvedilol was also associated with a higher risk of intradialytic hypotension in one of our included studies. Carvedilol may not have shown the same positive effects on patients undergoing hemodialysis because it stays longer during dialysis, leading to intradialytic hypotension induced by its α-1 receptor blocker activity, which offsets the potential cardiovascular benefits of the drug.2,1
All 4 studies reported both all-cause mortality and cardiovascular outcomes. A key limitation was our review’s standardization of outcomes, as each article reported slightly different composites of outcomes. In our attempt to standardize and aggregate the HRs and relative ratios between papers, all cardiovascular outcomes were classified as “events” and aggregated together. Data were pooled from 4 observational studies, each using different cohort designs and different analytic methods. Each study likely had residual confounding, which then carried forward through the pooling of results. Pooled results should be viewed in the context of the underlying observational studies. The included studies were all classified as having a serious risk of bias.
An additional limitation to consider is the inclusion of the study by Shireman et al6 in our systematic review. In the all-cause mortality model from Shireman et al,6 94.7% of the patients on highly dialyzable β-blockers and 95.8% of patients taking poorly dialyzable β-blockers received in-center hemodialysis. In the cardiovascular mortality and morbidity model, 94.8% of patients on highly dialyzable β-blockers and 95.5% of patients taking poorly dialyzable β-blockers received in-center hemodialysis. Those not on in-center hemodialysis were on self-care dialysis (which includes both home hemodialysis and peritoneal dialysis). Although Shireman et al6 did not report exactly how many patients in each β-blocker cohort received peritoneal dialysis, we know based on the available data that the numbers could not have exceeded 5.3%. In our review, we have generalized our findings with reference to patients undergoing hemodialysis. We did not analyze the outcomes of all-cause mortality or cardiovascular events as they relate to peritoneal dialysis.
A strength of our review includes the comprehensive review process and an evaluation for risk of bias for each study. In addition, the included studies were large, represented multiple countries, and included a broadly generalizable hemodialysis population.
In conclusion, pooled results from cohort studies suggest that highly dialyzable β-blockers have no effect on all-cause mortality but may reduce cardiovascular outcomes compared with low-dialyzability β-blockers. However, a sensitivity analysis excluding studies that categorized bisoprolol as poorly dialyzable suggested that the use of highly dialyzable β-blockers reduced both all-cause mortality and cardiovascular mortality compared with the use of poorly dialyzable β-blockers. Only 1 study commented on adverse events and suggested that poorly dialyzable β-blockers are associated with a greater incidence of intradialytic hypotension. Future randomized controlled trials comparing β-blockers of varying dialyzabilities should be undertaken to determine both cardiovascular outcomes and adverse effects, such as intradialytic hypotension.
Article Information
Authors’ Full Names and Academic Degrees
Abhinav Tella, MBBS, William Vang, BS, Eustacia Ikeri, BS, Olivia Taylor, BA, Alicia Zhang, BS, Megan Mazanec, BS, Srihari Raju, MD, and Areef Ishani, MD, MS.
Authors’ Contributions
Research idea and study design: AI; data acquisition: EI, AT, OT, AZ, MM, WV, SR; data analysis/interpretation: EI, AT, OT, AZ, MM, WV, SR; statistical analysis: EI, AT, OT, AZ, MM, WV, SR; supervision or mentorship; AI, SR. Each author contributed important intellectual content during manuscript drafting or revision and accepted accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received September 23, 2021. Evaluated by 3 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form February 14, 2022.
Footnotes
Complete author and article information provided before references.
Item S1: Search string.
Table S1: ROBINS-I bias assessment tool questions and reviewer responses for each paper used in this systematic review.
Table S2: Primary outcomes for each article used, their definitions, and the sources of these definitions.
Table S3: I2 values.
Supplementary Material
Item S1, Table S1-S3.
References
- 1.Freemantle N., Cleland J., Young P., Mason J., Harrison J. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318(7200):1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furgeson S.B., Chonchol M. Beta-blockade in chronic dialysis patients. Semin Dial. 2008;21(1):43–48. doi: 10.1111/j.1525-139X.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R., Sinha A.D., Pappas M.K., Abraham T.N., Tegegne G.G. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant. 2014;29(3):672–681. doi: 10.1093/ndt/gft515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Kidney Foundation Inc. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4):16–153. [PubMed] [Google Scholar]
- 5.Frankenfield D.L., Weinhandl E.D., Powers C.A., Howell B.L., Herzog C.A., St Peter W.L. Utilization and costs of cardiovascular disease medications in dialysis patients in Medicare part D. Am J Kidney Dis. 2012;59(5):670–681. doi: 10.1053/j.ajkd.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 6.Shireman T.I., Mahnken J.D., Phadnis M.A., Ellerbeck E.F. Effectiveness comparison of cardio-selective to non-selective β-blockers and their association with mortality and morbidity in end-stage renal disease: a retrospective cohort study. BMC Cardiovasc Disord. 2016;16(1):60. doi: 10.1186/s12872-016-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assimon M.M., Brookhart M.A., Fine J.P., Heiss G., Layton J.B., Flythe J.E. A comparative study of carvedilol versus metoprolol initiation and 1-year mortality among individuals receiving maintenance hemodialysis. Am J Kidney Dis. 2018;72(3):337–348. doi: 10.1053/j.ajkd.2018.02.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu P.H., Lin Y.T., Kuo M.C., et al. Β-blocker dialyzability and the risk of mortality and cardiovascular events in patients undergoing hemodialysis. Nephrol Dial Transplant. 2020;35(11):1959–1965. doi: 10.1093/ndt/gfaa058. [DOI] [PubMed] [Google Scholar]
- 9.Houston M.C., Hodge R. Beta-adrenergic blocker withdrawal syndromes in hypertension and other cardiovascular diseases. Am Heart J. 1988;116(2 Pt 1):515–523. doi: 10.1016/0002-8703(88)90627-8. [DOI] [PubMed] [Google Scholar]
- 10.Weir M.A., Dixon S.N., Fleet J.L., et al. Β-blocker dialyzability and mortality in older patients receiving hemodialysis. J Am Soc Nephrol. 2015;26(4):987–996. doi: 10.1681/ASN.2014040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutton B., Salanti G., Caldwell D.M., et al. The Prisma Extension Statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 12.Sterne J.A.C., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne J.A.C., Higgins J.P.T., Elbers R.G., Reeves B.C., development group for ROBINS-I Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I): detailed guidance. http://www.riskofbias.info
- 14.McQuillan R.F., Chan C.T. The intuitive case for β-blockers in patients with ESRD. Semin Dial. 2012;25(1):15–21. doi: 10.1111/j.1525-139X.2011.01016.x. [DOI] [PubMed] [Google Scholar]
- 15.Tieu A., Velenosi T.J., Kucey A.S., Weir M.A., Urquhart B.L. Β-blocker dialyzability in maintenance hemodialysis patients: a randomized clinical trial. Clin J Am Soc Nephrol. 2018;13(4):604–611. doi: 10.2215/CJN.07470717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu P.H., Lin Y.T., Liu J.S., et al. Comparative effectiveness of bisoprolol and carvedilol among patients receiving maintenance hemodialysis. Clin Kidney J. 2021;14(3):983–990. doi: 10.1093/ckj/sfaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiNicolantonio J.J., Lavie C.J., Fares H., Menezes A.R., O'Keefe J.H. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol) Am J Cardiol. 2013;111(5):765–769. doi: 10.1016/j.amjcard.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 19.Cice G., Ferrara L., D’Andrea A., et al. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41(9):1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 20.Hundemer G.L., Sood M.M., Canney M. Β-blockers in hemodialysis: simple questions, complicated answers. Clin Kidney J. 2021;14(3):731–734. doi: 10.1093/ckj/sfaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roux A., Aubert P., Guedon J., Flouvat B. Pharmacokinetics of acebutolol in patients with all grades of renal failure. Eur J Clin Pharmacol. 1980;17(5):339–348. doi: 10.1007/BF00558446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1, Table S1-S3.