Abstract
Drug delivery for osteoarthritis (OA) treatment is a continuous challenge because of their poor bioavailability and rapid clearance in joints. Intra-articular (IA) drug delivery is a common strategy and its therapeutic effects depend mainly on the efficacy of the drug-delivery system used for OA therapy. Different types of IA drug-delivery systems, such as microspheres, nanoparticles, and hydrogels, have been rapidly developed over the past decade to improve their therapeutic effects. With the continuous advancement in OA mechanism research, new drugs targeting specific cell/signaling pathways in OA are rapidly evolving and effective drug delivery is critical for treating OA. In this review, recent advances in various IA drug-delivery systems for OA treatment, OA targeted strategies, and related signaling pathways in OA treatment are summarized and analyzed based on current publications.
Keywords: osteoarthritis, knee, drug delivery, intra-articular
Introduction
Osteoarthritis (OA) is the most common degenerative joint disease that affects approximately more than 300 million people worldwide.1,2 Knee OA(KOA) accounts for approximately 85% of all OA cases worldwide.3 KOA represents a major public health challenge for the coming decades. The disease is ranked as the 10th largest cause of global years lived with disabilities,4 and its prevalence has more than doubled in the last 10 years.5,6 Medication management, hospitalizations, and joint surgery associated with OA treatment cost the healthcare system billions of dollars annually.4,6
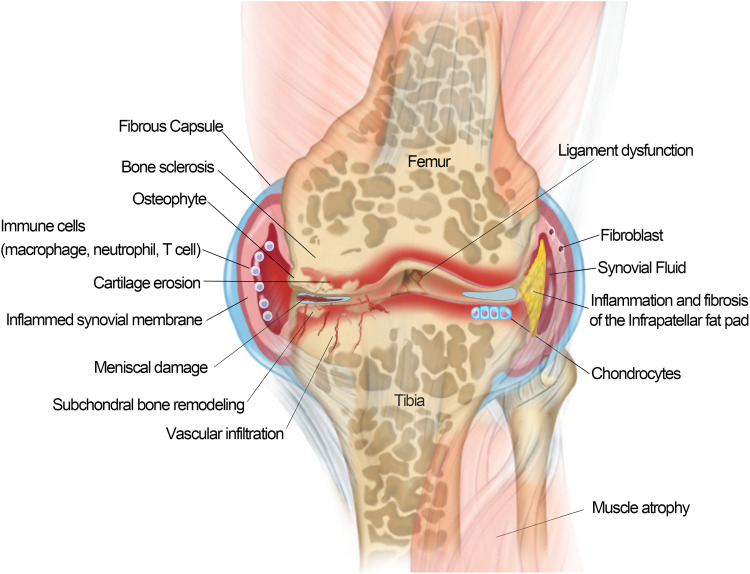
OA is a complex and multifactorial disease that involves various tissues lesion of the joint, including degenerative loss of articular cartilage, osteophyte formation, synovitis, subchondral bone remodeling and sclerosis, inflammation and fibrosis of the infrapatellar fat pad in the joints7 (Figure 1) Traditionally, OA has been defined as an abnormal biomechanic-induced disorder causing extensive changes in joint homeostasis; however, it is not merely a passive degenerative disease of “wear-and-tear” as commonly described, but an active dynamic alteration arising from an imbalance between the repair and destruction of joint tissues with complex inflammatory and metabolic factors involved.8,9 Over the last decade, OA has been increasingly recognized as a heterogeneous disease with the combined effects of aging, obesity, mechanical imbalance, gender, inflammation, metabolic imbalance, and genetic background.10,11 The degradation of the articular cartilage is the central feature of KOA.12 The local unbalanced biomechanical microenvironment and various biological factors in OA induce cartilage homeostasis dysregulation, resulting in collagen- and proteoglycan-rich extracellular matrix (ECM) degradation, articular surface fibrosis and erosion, cell death, matrix calcification, and vascular invasion. The progressive destruction of cartilage stimulated chondrocyte compensatory anabolism hypertrophy. Progressive destruction of cartilage stimulates chondrocytes to increase anabolism in the form of compensatory hypertrophy13 and this process simultaneously produce matrix degradation products and pro-inflammatory mediators to accelerate the development of KOA.14 With the further loss of cartilage and the exposure of subchondral bone, the increasing bone remodeling induces occurrence of osteophytosis, subchondral sclerosis, cyst formation, and abnormalities of bone contour in response to the rapid changes of the mechanical environment.15 Pro-inflammatory factors secreted by hypertrophic chondrocytes stimulate synovial cell proliferation and induce T, B lymphocytes and mast cell infiltration in synovial fluid.16 The triggered synovial tissue releases pro-inflammatory mediators to promote the cascade development of KOA.17 The progressive OA also affects the tissues in and around the joint. As the largest intraarticular adipose tissue, Hoffa’s infrapatellar fat pad (IFP) is a very sensitive tissue containing adipocytes, fibroblasts, leukocytes, macrophages and other immune cells.16,18 The IFP of KOA patients presented an increase in inflammatory infiltration, vascularization, fibrotic changes, and thickness of the interlobular septa.19,20 IFP also in turn to produce pro-inflammatory mediators and induce synovitis.20–23 Meniscal/ligament lesion often leads to subsequent cartilage loss, changes in subchondral bone, lesions of bone marrow and synovitis.24 Meniscal/ligament lesion induced biomechanical imbalance is a risk factor causing OA.25 Conversely, the progressive OA usually caused meniscal/ligament lesions. There appears to be a mutually reinforcing relationship between knee osteoarthritis and meniscal/ligament lesion.16,26 In addition, OA caused dysfunctions of the quadriceps muscle, hamstrings and hip muscles.16,27 The weakness of periarticular muscles of OA has a significant impact on knee biomechanics. However, it is not clear whether muscle weakness is associated with OA onset or OA progression.28,29
Figure 1.
Schematic of an osteoarthritis (OA) joint. OA is a disease of the entire joint in which various tissues in the joint are affected and undergoing progressive lesions, including 1) cartilage degradation and breaking down; 2) bone remodeling and sclerosis; 3) osteophytes formation; 4) synovial hypertrophy/synovitis; 5) meniscal damage; 6) ligament dysfunction; 7) muscle atrophy; and 8) inflammation/fibrosis of the infrapatellar fat pad.
Current KOA treatments include surgery, exercise, weight management, training in self-efficacy and pain-coping skills, and medications.12 There is no cure for OA, even no drugs to stop the progress of the disease.30 Current treatments only rely on symptomatic interventions, especially focus on relieve pain and enhance physical function. Intra-articular (IA) injection is one of the pharmacological treatments recommended for KOA in patients who do not respond to oral or topical analgesics31 Compared with oral administration, IA circumvents systemic exposure and potential adverse side effects32 and strengthens the bioavailability of therapeutic drugs direct access to the joint, which reduces treatment costs. In addition, IA is an attractive strategy for delivering drugs with low oral and local bioavailability. Currently, corticosteroids and hyaluronic acid (HA) are the most common drugs administered by IA injection for pain management and joint lubrication. However, the consensus on IA corticosteroids injections among scientific societies have not yet unified and the efficacy and safety of corticosteroids are debated. The balance between GC benefits and potential safety issues have been highlighted among guidelines. (the 2019 American College of Rheumatology (ACR) guideline, the Osteoarthritis Research Society International (OARSI) guideline, the American Academy of Orthopedic Surgeons (AAOS) guideline)33–35 High molecular weight HA has been used to alleviate the symptoms of OA. HA injection has been shown to provide lubrication, protect the cartilage from mechanical degradation, anti-inflammation, increase proteoglycan and HA synthesis, and reduce nerve impulses and nerve sensitivity associated with OA pain.36
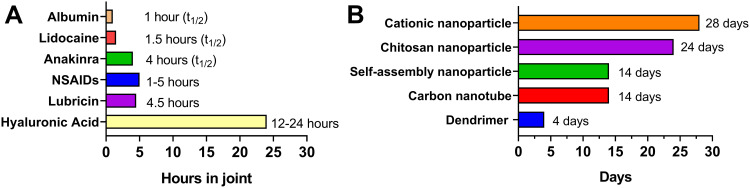
Considering long-term efficacy and safety concerns, current IA drug injection treatments (corticosteroids and HA) are only used for a short-term therapy and this strategy cannot reverse the progression of OA. There is an urgent need to develop new IA treatment strategies for OA. IA injection treatment have been rapidly developing with some promising progress in recent years. Several IA treatment small molecules are investigating in different clinical trial stages, of which some exhibited promising results (Table 1). For example, a single injection of CNTX-4975 (a trans-capsaicin that deactivates TRPV1-expressing nociceptive afferents) provided statistically significant improvements in pain with walking for subjects with moderate to severe knee pain associated with OA.37 However, most drug agents only remain in the joint for a few hours and rapidly clear after IA injection owing to the special physiological environment of the joint (Figure 2A).38–42 Multiple IA injections may result in less compliance and inflammation. Ensuring that drugs are consistently and effectively delivered into joint targets remains a significant challenge.43 Drug-delivery systems (DDSs) have evolved rapidly over the past 20 years, and substantial drug loading formulations have been developed to increase drug duration and provide controlled and/or sustained drug release. Some formulations, such as cationic nanoparticle IA formulations, have increased the drug duration from several hours to nearly one month (Figure 2B).44–49
Table 1.
Clinical Trials of Small Molecules Delivered by IA Injection as Currently Listed on www.clinicaltrials.gov
| Antonia | Antonia | Antonia | Antonia | Antonia | Antonia | Antonia | Antonia |
|---|---|---|---|---|---|---|---|
| Safety and tolerability of 4975 in the treatment of moderate to severe knee pain due to OA. | CNTX-4975 | Relieving pain–targeting the capsaicin receptor (TRPV1) | Centrexion Therapeutics | Phase II | Non randomized, open label | Completed, Dec 2016 | NCT00667654 |
| Fasitibant IA injection in patients with symptomatic OA of the knee | Fasitibant (MEN16132) | Relieving pain–kinin B2 receptor antagonist | Menarini Group | Phase II | Randomized, double blind | Completed, Oct 2015 | NCT02205814 |
| Safety, tolerability and efficacy of IA verapamil in the treatment of joint pain in subjects with OA of the knee | Verapamil | Relieving pain–Wnt/β-catenin inhibitor | Calosyn Pharma, Inc. Health Decisions | Phase II | Randomized, double blind | Terminated, Aug 2014 | NCT01645709 |
| Proof-of-concept study to assess the efficacy, tolerability and safety of a single IA dose of GZ389988 vs placebo in patients with painful OA of the knee | GZ389988 | Relieving pain–tropomyosin receptor-kinase A (TrkA) receptor antagonist | Genzyme, Sanofi | Phase II | Randomized, double blind | Completed, Sep 2017 | NCT02845271 |
| A study evaluating the safety, tolerability and efficacy of SM04690 injected into the target knee joint of moderately to severely symptomatic OA subjects. | SM04690 | Relieving pain–Wnt pathway inhibitor | Samumed LLC | Phase II | Randomized, double blind | Completed, Nov 2017 | NCT02536833 |
| Safety of single doses of SAR113945 and efficacy and safety of a new formulation given into the knee in OA patients | SAR113945 | Inflammation relief–IκB kinase inhibitor (upstream of NF-κB signal transduction cascade) | Sanofi | Phase II | Randomized, double blind | Completed, Oct 2014 | NCT01598415 |
| A multicenter study of rhFGF 18 in patients with knee osteoarthritis not requiring surgery | Sprifermin | Stimulating cartilage anabolism-rhFGF18 | Merck KGaA | Phase I | Randomized, double blind | Completed, Jun 2014 | NCT01033994 |
Abbreviations: API, active pharmaceutical ingredient; rhFGF18, recombinant human fibroblast growth factor 18.
Figure 2.
Representative half-lives or retention times of intra-articular (IA) drug (A)38–42/intra-articular drug (B)44–49 delivery system in a joint.
In this review, we summarized the IA DDSs development over the past five years (Figure 3) and discuss the application prospects of different types of DDSs for OA treatment.
Figure 3.
A statistical chart of the research status of articular injection drug-delivery systems from the past five years.
Methods
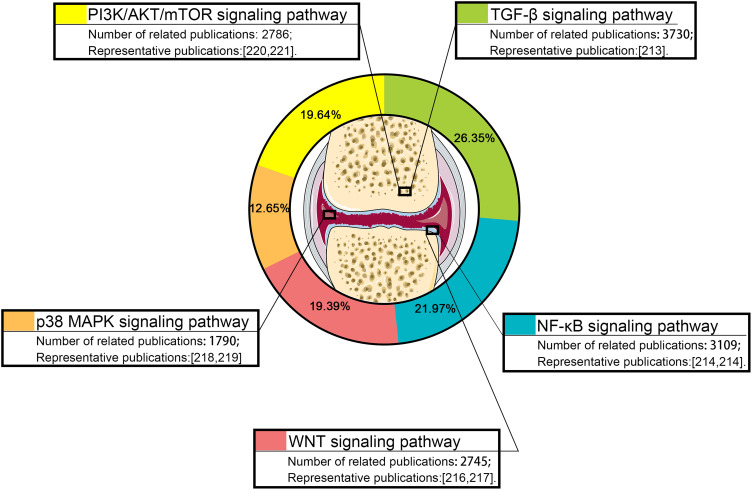
Studies on IA DDSs over the past five years were collected by using the PubMed/Google Scholar/Scopus/Web of Science databases. Search keywords were set as “injection” and “osteoarthritis”. “NoteExpress” software was used to remove the duplicated studies in the collected publications. Then, the publications were categorized into different categories for further analysis (for example: “hydrogel”, “microspheres”, and “nanoparticles”). In addition, the signaling pathway studies related to IA injection for OA treatment over the past five years were also collected by using the PubMed/Google Scholar/Scopus/Web of Science databases, Search keywords were as “Signaling pathway”, “injection” and “osteoarthritis”. Impact factor of the collected publications ≥ 5 were selected and categorized into signaling pathway categories for analysis.
Different Drug-Delivery Systems for Intra-Articular Injection of Knee Joint
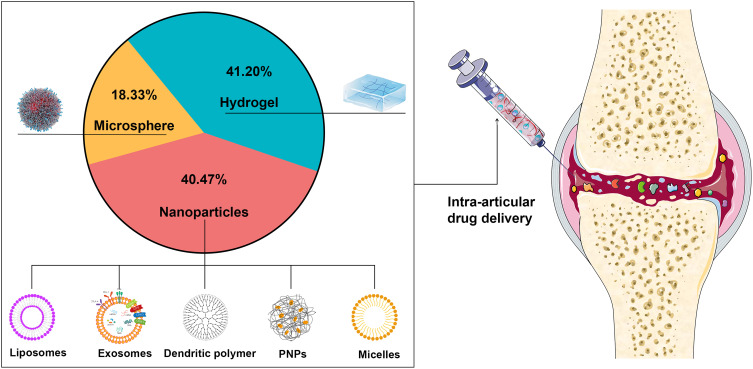
At present, biodegradable and bio-eliminable materials have been widely utilized for IA DDSs with diverse benefits, including, but not limited to, enhancing the stability of encapsulated drugs, decreasing toxicity, reducing adverse effects, improving pharmacokinetics, and targeting specific sites. In this study, the current IA DDSs are divided into three categories according to their material characteristics; that is, nanoparticles, microspheres, and hydrogels. We collected 22,040 publications on IA DDSs from the past five years. Of these publications, hydrogels, nanoparticle carriers, and microspheres accounted for 41.2, 40.47, and 18.33%, respectively, of the IA DDSs used for OA treatment (Figure 3).
Hydrogels
Hydrogels are cross-linked 3D polymer networks with a high water content which expand in water-soluble environments and form polymerization systems with targeted substances such as proteins, nucleic acids, small organic molecules, etc. The high water content of hydrogels (typically 70–99%) is physically similar to tissues, providing excellent biocompatibility and the ability to easily encapsulate hydrophilic drugs. Hydrogel DDSs have been applied in different medicinal fields, including cardiology, oncology, immunology, wound healing, and pain management.50 Hydrogel gelation strategies are selected according to the characteristics of various materials and include Schiff-base cross-linking, enzyme-mediated cross-linking, photocrosslinking, or thermosensitive cross-linking.51 Hydrogels have a long application history as a joint lubricant, and were reported in animals as early as 2002.52
Hydrogels Used as a Drug-Delivery System
Hydrogels are generally composed of macromolecules with long retention times in the knee joint compared with small-molecule materials. Therefore, hydrogels are generally used as a DDS for prolonged drug effects in joints. Chondrogenic factors,53 chondroitin sulfate nanoparticles,54 and oligonucleotides55,56 have been commonly loaded into hydrogel delivery systems for therapeutic studies. Some traditional medicines (such as celecoxib,57 meloxicam,58 dexamethasone,59 and triamcinolone60 loaded in hydrogel delivery systems not only provided improved long-term drug release, but also enhanced anti-inflammatory effect than the original formulations (See Table 2 for details).
Table 2.
Summary of Hydrogel Drug Delivery System
| Types of Hydrogel | Drug | Carrier Effect | In vivo/vitro | Experimental Subject | Treatment Effect | Ref |
|---|---|---|---|---|---|---|
| CTL | - | Lubrication | In vitro | Macrophages; Chondrocytes | Hydrogels showed a good biocompatibility in vitro and the ability of not stimulating the activity of macrophages in terms of cytokines release. In addition, the beneficial effect of the CTL-boric acid hydrogel was related also to its ability of acting as a scavenger system for ROS; After Fenton reaction, NaOCl and lysozyme treatment, CTL hydrogels has better anti-degradation ability than HA hydrogels. | [185] |
| PEG | BMP2 and sVEGFR1 | Slow release | In vivo | Mice | Stimulating differentiation of skeletal stem cells into cartilage. | [53] |
| Alginate / Poly (vinyl alcohol) | Chondroitin sulfate and nanohydroxyapatite | Slow release; Targeting | In vivo | Rabbits | Promoting the formation of new matrix in cartilage defects, and the new matrix contain matrix, chondrocytes and osteoblasts with the characteristics of hyaline cartilage. | [54] |
| Fibrin/HA | Anti-miR-221 | Slow release | In vitro & vivo | HMSCs; Mice | Fibrin /HA strongly retained functional antimiR-221 over 14 days of in vitro culture and miR-221 knockdown in situ within 7 days; Promoting endogenous cells to repair calf metacarpal cartilage implanted in mice. |
[55] |
| Fibrin/HA | LNA-gapmers | Slow release | In vitro | Chondrocytes | The sustained released profile up to 14 days; Gapmers loaded in hydrogels were able to transfect both co-embedded chondrocytes and chondrocytes in a neighboring gapmer-free hydrogel; Efficient knockdown of ADAMTS5 was shown up to 14 days in both cell populations. |
[56] |
| PCLA-PEG-PCLA | Celecoxib | Slow release Thermal response |
In vivo | Horses | Elevated levels of celecoxib were observed in the joint for up to 30 days; The sustained and controlled intra-articular release in both inflamed and healthy joints together with very low systemic exposure; Celecoxib formulations had a mild, transient effect on inflammatory and structural synovial fluid biomarkers but these returned to baseline within one week of administration; The hydrogels showed a significant inhibition in peak white blood cells concentration at 8 hours after LPS induction. |
[57] |
| CMC-MC-P | Meloxicam | Slow release | In vitro | Chondrocytes | 100% of meloxicam was released from the hydrogels containing the meloxicam solution within 20 days, but it was released slowly from the hydrogels containing nanoparticles in 37 days; Chondrocytes metabolic activity was increased on the 6th and 10th days for all hydrogels. |
[58] |
| Chitosan | Dexamethasone | Slow release Thermal response |
In vivo | Mice | The cumulative release profiles of dexamethasone from hydrogels at 37 °C revealed a rapid release in the first 24 h and a sustained slow release for 7 days; In vivo studies showed that the drug-loaded hydrogels exerted a cartilage protective effect by reducing the expression of MMP-9, MMP-13, and ADAMTS-5, attenuated bone destruction in DMM-induced OA in mice, and improved synovitis and OA progression progress. |
[59] |
| GEL-MAN | Triamcinolone | Slow release | In vivo | Mice | In vitro, hydrogels can be continuously released for 24h under H2O2 environment; In vivo experiments showed that the hydrogels group had a higher OARSI score, less inflammatory response, and less cartilage matrix degradation than triamcinolone acetonide alone. |
[60] |
| Polyglucosamine/glucosamine carbonate | ADMSCs | Trestle | In vivo | Human Patients | The initial WOMAC score of 58.6 ± 11.0 in the study group was reduced by 88% at 6 months (7.1 ± 9.2) and 95% at 24 months (2.9 ± 5.9); Separate biopsies performed at 12 months post-op in the study group also revealed type 2 collagen and hyaline-like cartilage in the regenerated tissue. |
[62] |
| CS/SF/ESM | - | Filling material | In vitro | Chondrocytes | The hydrogels supported better adhesion, growth and differentiation of chondrocytes under standard culture conditions. | [63] |
| HA-TG | Polydactyly chondrocytes of children under the age of 2 | Trestle | In vitro & vivo | Mice, Chondrocytes | Polydactyly chondrocytes have a steady proliferative rate and re-differentiate in 3D pellet culture after up to five passages; Polydactyly chondrocytes produce cartilage-like matrix in a hyaluronan-based hydrogel. |
[64] |
| ACM-BMHP | Rabbit MSC | Trestle | In vitro & vivo | Rabbit, rabbit MSC | Stimulating rabbit MSC proliferation, attachment and chondrogenic differentiation; Upregulation of cartilage-associated aggrecan, Sox9 and type II collagen; At 3 and 6 months after operation, the articular cartilage defect was completely covered by cartilage-like tissue, and the surface was smooth, similar to the surrounding natural cartilage. |
[65] |
| Thermosensitive chitosan-gelatin | Glutathione | Slow release | In vitro | Cisd2 +/+, -/- miPSCs-derived chondrocyte-like cells | The cumulative release percentage within 48 hours is 96.8± 4.6%; Post-treatment of glutathione-loaded hydrogels effectively decreased the oxidative stress-induced damage in Cisd2−/− chondrocytes via increasing catalase activity, down-regulation of inflammation, and decreasing apoptosis. |
[72] |
| PCLA/PEG | Celecoxib | Slow release | In vitro & vivo | Rats | In vitro, release of celecoxib started after a ~10-day lag phase followed by a sustained release of ~90 days; In vivo (subcutaneous injection in rats) experiments showed an initial celecoxib release of ~30% during the first 3 days followed by a sustained release of celecoxib for 4–8 weeks and where no cartilage or bone changes were observed following injection into the knee joints of healthy rats. |
[74] |
| PA-PGE-PA & PAF-PEG-PAF | - | Filling material | In vitro & vivo | Rabbits | Neo-cartilage at 12 weeks post-implantation generated by PAF-PEG-PAF hydrogels carrying BMMSCs possessed higher levels of GAGs and Col II, and lower levels of Col I than that of the PA-PEG-PA and control groups; PAF-PEG-PAF not only promoted proliferation of BMMSCs in vitro, but also facilitated hyaline-like cartilage regeneration with reduced fibrous tissue formation in vivo. |
[76] |
| Glycol chitosan/ hyaluronic | - | - | In vitro & vivo | Chondrocytes, mice | The mitochondrial activity of chondrocytes proliferated in the presence of HGC and AcHA was higher than that of chondrocytes in the control without hydrogel in the medium; On day 3 after implantation, inflammation in the AcHA/HGC thermosensitive hydrogels was slightly worse than that in the HGC gels, and on day 7, the AcHA/HGC thermosensitive hydrogels significantly decreased the inflammatory response, which almost disappeared by day 30. |
[73] |
| CMC/P (NiPAM-co-AA) | - | Filling material | In vitro | Human mesenchymal stem/stromal cells | Cells migration mediated the formation of cells aggregates in the thermosensitive hydrogels and led to a cells dense hollow shell structure; Compared with the control group, the cartilage-related genes (SOX9, Aggrecan, and Col 2A1) induced by hydrogels chondrogenesis in the 4th week were significantly higher than those in the control group. There was still significant difference between SOX9 and Col2A1 6 weeks after chondrogenesis. At the 8th week of chondrogenic induction, the expression of Aggrecan was higher than that of the control group, and there was no significant difference in other genes; Sulfated glycosaminoglycan and COL2A1 were found in the new cartilage induced by chondrogenesis, and the hydrogels group was more obvious than the control group. The structure of multi-empty cells mass formed in the hydrogels group was more compact than that in the control group. |
[77,78] |
| HA/PEG-p (HPMAm-lac) | - | Slow release | In vitro & vivo | Mice | Hydrogels are able to inhibition the inflammatory process in a mouse model of OA through the controlled and sustained release of HA over a time period that goes from 30 to 70 days in vitro; For OA caused by injection of collagenase, the hydrogels demonstrated the ability to restore, to some extent, bone remineralization, proteoglycan production, levels of Sox‐9 and Runx‐2. Furthermore, the downregulation of proinflammatory mediators, such as TNF‐α, NFκB, and RANKL and proinflammatory cytokines was observed. |
[79] |
| Poloxamers | Glucosamine | Slow release | In vitro & vivo | Rabbits | In the in vitro release experiment, the release rate of glucosamine from hydrogel was significantly slower than that of control group under different PH conditions; After intra-articular injection of hydrogel, the degree of swelling and inflammatory factors were significantly decreased in the hydrogel group compared with those in the OA model group; Histological results showed that the Gels group had a good repair effect on damaged cartilage. |
[80] |
| PLEL | Platelet lysate | Slow release | In vitro & vivo | Human Chondrocytes; Rats | Platelet lysate that passes through the hydrogel can be released for up to 35 days in vitro. The complex helped chondrocytes against inflammatory responses and excess catabolism under IL-1β stimulation as well as improved its redifferentiation ability in vitro and RNA sequencing analysis indicated that PL’s protective effects might be associated with modulating hyaluronan synthase 1 (HAS-1) expression; Ameliorating early cartilage degeneration and promoted cartilage repair in the late stage of osteoarthritis. |
[81,82] |
| sEVs load circRNA3503 | Slow release | In vitro & vivo | Chondrocytes; Rats | sEVs that passes through the hydrogel can be released for up to 35 days in vitro; The therapeutic agent enhanced the regenerative/renewal abilities of chondrocytes to neutralize cartilage defects caused by OA progression, and circRNA3503 (1) enhanced ECM synthesis in cartilage (2) suppressed chondrocyte apoptosis caused by OA-induced ER stress, and (3) suppressed ECM degradation in cartilage caused by OA-induced ER stress. |
||
| Amphiphilic poly (organophophazene) | TCA | Slow release | In vitro & vivo | Rats | The in vitro release study showed sustained TCA release for six weeks; The hydrogel was completely degraded in rats for 42 days; Compared with the direct injection of triamcinolone acetonide solution group, the hydrogel group had significant anti-OA effect and could effectively prevent the loss of cartilage tissue. |
[84] |
| HA-VS/SH-2-PEG | - | Viscoelasticity | In vitro & vivo | Rabbits; Chondrocytes | Through the experiments of implanting cells in the hydrogels and injecting the hydrogels into the joints of healthy animals, it is proved that the hydrogels had good biocompatibility; The rabbit OA joint injected with viscoelastic hydrogels had better gross morphology and Mankin score. |
[184] |
| GG/PVA | - | Viscoelasticity | In vitro | NIH3T3 mouse fibroblasts; Human chondrocytes |
No cytotoxicity | [186] |
| PNIPAM; HA | Diclofenac sodium | Slow and controlled release | In vitro & vivo | Chondrocytes; Rats | Diclofenac sodium was released continuously for up to 9 days; Hydrogels particles protected the cells against the H2O2-induced chondrocytes degeneration and prevented the H2O2-caused cartilage marker protein decrease; Hydrogels particles prevented the decreased expression of collagen II and aggrecan. In addition, the concentration of TNF-α, COMP, CTX-II, and MMP-1 were decreased; Histological analysis showed that OA rats treated with hydrogel particles had improved synovial hyperplasia and amount of synovial fluid, and cartilage tissue surface was less damaged and smoother. |
[85] |
Abbreviations: CTL, lactose-modified chitosan; sVEGFR1, soluble VEGFR1, a VEGF receptor antagonist; PAMAM, poly- (amidoamine); GelMA, photo-crosslinked methacrylate gelatin hydrogel; DMA-MPC, self-adhesive polymer; ADSCs, adipose-derived stem cells; DEX-TA, amine-terminated dextran–tyramine conjugates; PA, poly(l-alanine); PEG, poly(ethylene glycol); PLA, poly(DL-lactic acid); PLGA, poly (lactic-co-glycolic acid); PNIPAm, poly(N-isopropylacrylamide); HA, hyaluronic acid; CS, chitosan; LNA-gapmers, locked nucleic acid modified antisense oligonucleotides; ADAMTS, A Disintegrin and Metallo Proteinase with Thrombospondin Motifs; ADMSCs, adipose derived mesenchymal stem cells; SF, silk fibroin; ESM, egg shell membrane; TG, transglutaminase; ACM, acellular cartilage matrix; BMHP, bone marrow homing peptide; NIPAM, N-isopropylacrylamide; AA, acrylic acid; CMC, carboxymethyl cellulose; p(HPMAm-lac), poly(N-(2-hydroxypropyl)methacrylamide lactate); PLEL, poly(d,l-lactide)-poly(ethylene glycol)-poly(d,l-lactide); PL, platelet lysate; HA-VS/SH-2-PEG, vinyl sulfone-modified HA crosslinked by dithiol-terminated poly(ethylene glycol); GG, gellan gum; PVA, polyvinyl alcohol; CMC-MC-P, carboxymethyl chitosan -methylcellulose–pluronic; GEL-MAN, it is a hydrogel formed by benzoxaborole-containing polymers; BMMSCs, bone marrow mesenchymal stem cells; PAF, poly(L-alanine-co-L-phenylalanine); TCA, triamcinolone acetonide.
Hydrogels Used as Cell and Tissue Engineering Scaffolds
Currently, many tissue or cell transplantation techniques have been used for OA cartilage regeneration. However, the cumbersome in vitro cell manipulations and potential cancer risks limit their application.61 In the last few decades, cell and pro-growth factors combined with hydrogel scaffolds applied in tissue engineering has attracted significant attention. As typical representatives, natural hydrogels with high performances are ideal biomaterial scaffolds for cartilage repair because of their preferable reconstructions of cell growth, proliferation, differentiation, and new tissue formation. Hydrogels such as polyglucosamine,62 chitosan,63 hyaluronic acid,64 and acellular cartilage matrix65 showed promising applications in the field of cellular implantation for the treatment of OA.
Functional Hydrogels
Hydrogels made of natural polymer materials have their limited properties (including, rheological, stability, mechanical strength, et al.); therefore, modified hydrogels with different functional groups are rapidly evolving because of the modifications improve the properties of natural polymer. In addition, these modifications create different kinds of smart hydrogels, which could automatically sense and respond to changes in the external environment (include temperature sensitive, pH sensitive, photosensitive, magnetic sensitive and temperature/pH dual sensitivity hydrogels).66–71
Temperature-sensitive hydrogels have always been favored because they are easier to load with drugs or cells and possess more convenient in situ formation properties than other hydrogels.66,67 Thermosensitive hydrogels, such as chitosan with β-glycerol phosphate,72 glycol chitosan,73 PEG-grafted polyalanine or poly(lactide-co-glycolide),74–76 poly(N-isopropylacrylamide-co-acrylic acid) (p(NIPAAm-AA)),77,78 poly(ethyleneglycol)-(p(HPMAm-lac)-PEG),79 poloxamers,80 poly(d,l-lactide)-poly(ethylene glycol)-poly(d,l-lactide) (PLEL),81,82 hyaluronic acid-chitosan-poly(N-isopropylacrylamide),83 and amphiphilic poly(organophosphazene),84 have been increasingly applied in the treatment of OA. A previous study demonstrated that temperature-sensitive PNIPAM (poly(N-isopropylacrylamide)) hydrogels85 could regulate drug release according to the joint temperature. This design offers a novel concept for the hydrogel treatment of OA.
Nanoparticles
Nanoparticles (NPs) are also frequently used in OA treatment studies. NPs are submicron particles with dimensions ranging from 1 to 300 nm, which is approximately 1000 times smaller than that of chondrocytes. NPs as carriers can incorporate drugs on the surface or matrix to protect them from enzymatic degradation, improve their penetration across the cartilage matrix, and regulate drug pharmacokinetics, which is beneficial for improving the efficacy and reducing the toxicity of therapeutic compounds.86 By adjusting the physicochemical properties or decorating with moieties, NPs can be modified with functional groups to target the components and/or cells in the cartilage.87 This section reviews the current developments and novel applications of OA-related NP-based DDSs, including liposomes, micelles, dendrimers, polymeric nanoparticles (PNPs), and exosomes.51,88–92
Liposomes
Liposomes are spherical vesicles composed of phospholipids and steroids,93 and are both hydrophilic and lipophilic.94 Liposomes have been proven to prolong drug efficacy, improve bioavailability, and provide tissue/cell targeting.95–98 Some liposome formulations are already commercially available for treating OA. For example, Lipotalon® (Merckle, Germany) is the first used liposome formulation in Germany to clinically treat OA by IA delivery,43 containing dexamethasone palmitate as active ingredient, preferentially taken up by synovial macrophages after IA injection and transformed to its active form by intracellular esterases, thus preventing the side effects of free dexamethasone (synovitis and tissue irritation).99,100
However, the aqueous environment of the synovial fluid may lead to rapid drug release. These shortcomings have limited the application of liposomes.
1). Prolonged duration of drug efficacy
Liposomes can prolong drug retention because the drugs are encapsulated within the phospholipid bilayers and are more difficult to remove than small-molecule drugs. The efficacy of many drugs has reportedly improved through liposome formulations. For example, the liposome formulations for rapamycin,101 fish oil protein,102 diclofenac sodium,103 and d-glucosamine sulfate104 have been reported to enhance the inhibition of pro-inflammatory factors or promote cartilage-related genes expression (See Table 3 for details).
Table 3.
Summary of Nanoparticles Drug Loading System
| Material (Carrier Type) | Drug | Carrier Effect | In vivo/vitro | Experimental Subject | Treatment Effect | Ref |
|---|---|---|---|---|---|---|
| Liposomes | ||||||
| DSPC/cholesterol/OCT | Rapamycin | Slow release | In vitro & vivo | Chondrocytes; HOACs; Guinea pigs | The release from rapamycin-loaded liposomes was around 85% after 72-hour incubation; Rapamycin-loaded liposomes largely up-regulated aggrecan and collagen II mRNA in human OA chondrocytes; Results on OARSI score showed that intra-articular injection of 5 μM liposomes-rapamycin with LIPUS displayed the greatest anti-OA effects; Immunohistochemistry revealed that liposomes-rapamycin with or without LIPUS predominantly reduced MMP-13 in vivo. |
[101] |
| DPPC | Fish oil protein | Slow release | In vitro & vivo | HIG-82 cells; Rats | Maximum fish oil protein released was 68.98 ± 7.09% within 24 h; The serum and synovial interleukins levels were restored after the treatment with fish oil protein-loaded liposomes; In vitro data also showed that treatment with fish oil protein-loaded liposome leads to apoptosis of the HIG-82 arthritic cells. |
[102] |
| DPPC /Cholesterol | Dex; Diclofenac | Slow release | In vitro & vivo | Chondrocytes; Mice | The continuous release time of liposomes is 7 days; Inhibiting neutrophil elastase and inflammation in vivo; Reducing arthritic inflammation and leukocytes infiltration. |
[103] |
| DSPC | D-glucosamine sulphate | Slow release | In vitro | Mouse chondrocyte | Liposomes prolong D-glucosamine sulphate release for 14 days; The liposomes accelerated the viability and proliferation of primary mouse chondrocytes while also providing the anti-inflammatory and cartilage protective potential for tumor necrosis factor (TNF-α) induced chondrocytes degeneration through the downregulation of pro-inflammatory cytokines, pain related gene and catabolic proteases, as well as the up-regulation of anabolic components. |
[104] |
| Soybean phosphatidylcholine /Cholesterol | Curcumin | Increasing drug stability and improving drug bioavailability | In vitro | Mouse osteoblast-like cells and macrophages | With interleukin (IL)-1β stimulation, curcumin-loaded liposomes successfully down regulated the expression of inflammatory markers on osteoblasts, and showed a high osteoprotegerin (OPG)/receptor activator of nuclear factor κB ligand (RANKL) ratio to prevent osteoclastogenesis. | [106] |
| Phosphatidyl choline/ Cholesterol | Adenosine or CGS21680 | Targeting A2A receptor | In vivo | Mice | Differential expression analysis of mRNA from chondrocytes harvested from knees of rats with OA treated with liposomal A2AR agonist revealed downregulation of genes associated with matrix degradation and upregulation of genes associated with cell proliferation as compared to liposomes alone. | [108,109] |
| Lipofectamine TM 2000 kit | miR-15a | Targeting SMAD2 | In vitro | Human normal chondrocytes | Inhibiting the proliferation and promoting apoptosis of knee arthritis chondrocytes. | [110] |
| Lipo2000 | microRNA-143-3p | Targeting BMPR2 | In vitro | BMSCs | MiR-143-3p could regulate the differentiation process by targeting BMPR2 in BMSCs. | [111] |
| DSPE-PEG-maleimide/ HAP-1 peptide | Prednisone / immunosuppressive peptide CP | Targeting Synovial; Targeting Inflammation |
In vitro & vivo | Synovial fibroblast like and endothelial cells; Rats | Targeted liposomes specifically bound to rabbit FLS and human FLS and showed a 7–10 folds increase in vivo localization in affected joints compared to unaffected joints.; The tissue sections from liposomes treated rats showed very little inflammatory cell infiltrate with normal bone contour. |
[113] |
| DOPC/DOPE/cholesterol/ART-2 | Dex | Targeting inflammation | In vitro & vivo | HUVEC; Rats | ART-2-targeted liposomes-Dex was more effective in suppressing arthritis in rats than untargeted liposomes-DEX or free DEX. | [114] |
| EPC/PEG/cholesterol | Dex | Targeting inflammation | In vivo | Mice | The results indicated that liposomes with 100 nm diameter, a slight negative charge, and 10% incorporation of 5 kDa PEG had better in vivo circulation time and inflamed joint targeting than did other liposomes; Pharmacodynamic studies demonstrated that Dex liposomes could significantly improve the antiarthritic efficacy of Dex in a CIA mouse model of RA. |
[115] |
| Lecithin/ pyrophosphorylated cholesterol; cholesterol | Salvianic acid A | Targeting bone | In vivo | Mice | Locally administered SAA-BTL was found to significantly improve fracture callus formation and micro-architecture with accelerated mineralization rate in callus when compared to the dose equivalent SAA, non-targeting SAA liposome (SAA-NTL) or no treatment on a prednisone-induced delayed fracture union mouse model. | [116] |
| DOPC/ DSPE-PEG2000/ DSPE-PEG2000- maleimide/ type II collagen | - | Targeting cartilage | In vivo | Mice | - | [117] |
| Micelles | ||||||
| DSPE-PEG2000/sPLA | sPLA inhibitor | Targeting lesion tissue | In vitro & vivo | Mice; Cartilage explants | sPLA2i-NPs were able to penetrate into the deep zone of the articular cartilage and exhibit high cartilage accumulation; SPLA2i-NPs prevented cartilage degeneration in OA cartilage explants and reduced joint damage in surgery-induced mouse OA model. In addition, sPLA2i-NPs blocked joint damage in a single load-induced mouse posttraumatic OA model. |
[120] |
| PCL-PEI/PCL-PEG | p65 siRNA and Dex | Targeting NF-κB signaling | In vitro & vivo | Raw264.7; HUVECs; Mice |
This novel hybrid micelles to co-deliver Dex and siRNA targeting p65 could potently suppresses nuclear translocation of p65 and secretion of pro-inflammatory cytokines by activated macrophages and also triggered the re-polarization of macrophages from the pro-inflammatory M1 type to the anti-inflammatory M2 type; The hybrid micelles to accumulate selectively in inflamed joints of the arthritic mice for as long as 24 h. |
[121] |
| PEPS | Celastrol | ROS-responsive | In vitro & vivo | Raw264.7; Mice | Celastrol-loaded micelles may inhibit the re-polarization of macrophages toward the pro-inflammatory M1 phenotype via regulating the NF-κB and Notch1 pathways, which resulted in significantly decreased secretion of multiple pro-inflammatory cytokines to suppress the RA progression and effectively alleviated the major RA-associated symptoms including articular scores, ankle thickness, synovial inflammation, bone erosion and cartilage degradation. | [122] |
| FA/PSA/Cholesterol | Dex | Synovial inflammation targeting | In vitro & vivo | Raw264.7; Mice; Rats | Micelles could also enhance the intracellular uptake of Dex and the suppression of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in vitro and in vivo; FA modification significantly improved the anti-inflammatory efficacy of micelles; Arthritis mice showed reduced paw thickness and clinical arthritis index using micelle treatment. |
[124] |
| PLGA-SeSe-mPEG | Dex and CDMP-1 | ROS-responsive | In vitro | BMSCs; Raw264.7 | The drug-loaded micelles effectively inhibited proliferation of activated macrophages, induced macrophage apoptosis with an anti-inflammatory effect, and caused the BMSCs to differentiate into chondrocytes. | [123] |
| LMWH-TOS | Methotrexate | Targeting inflammatory sites | In vitro & vivo | HUVECs; Mice | The hydrophilic fragment low molecular weight heparin (LMWH) acts as a shield which block the transvascular movement of neutrophils through inhibiting the adhesion cascade by binding to P-selectin on inflamed endothelium; Hydrophobic fragment d-α-tocopherol succinate reduced matrix metalloproteinase-9, which was secreted by neutrophils and degrades the main components of articular cartilage; In collagen-induced arthritis (CIA) mouse model, LT NPs showed significant targeting effect, and exhibited prominent therapeutic efficacy after enveloping the first-line anti-RA drug methotrexate. |
[125] |
| mPEG-PPF | Ibuprofen | pH-responsive | In vitro | HIG-82; | Ibuprofen release was observed to increase with increasing acidic conditions and could be controlled by varying the amount of crosslinker used; Micelles exerted anti-inflammatory effects by significantly decreasing monosodium urate crystal-induced prostaglandin E2 levels in rabbit synoviocytes cultures in vitro. |
[126] |
| Poly (β-amino ester) | Curcumin | Cartilage targeting and PH controlled release | In vitro & vivo | RAW264.7 cells; Mice | The polymer combined with curcumin can form its own micelles and released curcumin under acidic conditions; Micelles drastically protected the articular structures from arthritis through the suppression of tumor necrosis factor-alpha (TNF-α) and interleukin 1β (IL-1β). |
[127] |
| PCL-PEOz-NH2/ MR-Cy5.5/collagen type II | Psoralidin | Enzyme targeting and PH targeting. | In vitro & vivo | Chondrocytes; Mice | Anti-inflammatory effect of micelles on IL-1β-induced chondrocytes via the MAPK, NF-κB, and PI3K/Akt signaling pathways; The incorporation of collagen II peptides and the use of acid responsive polymer PAMAM promoted micelles targeting and retention in the joints of OA. |
[130] |
| Dendritic polymer | ||||||
| PAMAM; PEG | KGN | Improve drug bioavailability | In vitro & vivo | BMSCs; Rats | The combination with polymer could improve the effect of osteogenic induction of KGN; The fluorescein labeled PEG-PAMAM was capable to persist in the joint cavity for a prolonged time of both healthy and osteoarthritis (OA) rats. |
[138] |
| PAMAM; PEG | IGF-1 | Improve tissue binding, penetration and residence time. | In vivo | Rats | When conjugated to insulin like growth factor 1 (IGF-1), the dendrimer penetrated bovine cartilage of human thickness within 2 days and enhanced therapeutic IGF-1 joint residence time in rat knees by 10-fold, for up to 30 days; In the surgical model of osteoarthritis in rats, a single injection of dendrimer-IGF-1 saved cartilage and bone more effectively than free IGF-1. Dendrimer-IGF-1 reduced the width of cartilage degeneration by 60% and the burden of volume osteophyte by 80%. |
[49] |
| CAP-PEG-PAMAM | - | Cartilage targeting | In vitro & vivo | Chondrocytes; Rats | The conjugate was likely internalized by chondrocytes via clathrin and caveolin co-mediated endocytosis, and delivered to lysosomes; Fluorescence labeling showed that nanocarriers could be stored in rats for a very long time. |
[139] |
| Chondroitin sulphate/ PAMAM | Abs | Cartilage targeting | In vitro | ATDC 5; THP-1; Human T lymphocyte cells | Dendrimer nanoparticles did not affect the metabolic activity and proliferation of ATDC5 and THP-1 cells, showed good cytocompatibility and blood compatibility, and had good tumor necrosis factor-α capture ability. | [140] |
| ABP/PAMAM | - | Inflammation targeting | In vitro & vivo | Osteoclasts; Mice | Intravenous injection of dendritic macromolecules inhibited the development of inflammatory arthritis in mice, characterized by normal synovium, decreased levels of inflammatory cytokines and no cartilage destruction and bone erosion. The dendrimer ABP also showed anti-osteoclast activity in mouse and human cells by inhibiting c-FMS. | [141] |
| PNPs | ||||||
| PLGA | Oxaceprol | Slow release | In vitro | - | The in vitro drug release from these nanoparticles showed a sustained release of oxaceprol over 30 days. | [149] |
| PLGA | Diacerein | Slow release | In vitro & vivo | Synoviocytes; Rats | The in vitro studies revealed that DIA/PLGA NPs dose-dependently suppressed mRNA levels of pro-inflammatory cytokines and enzymes; In vivo studies have showed that intra-articular injection of DIA-PLGA nanoparticles could significantly reduce the mRNA level of the above pro-inflammatory factors, increase the mRNA level of anti-inflammatory cytokines (IL-4 and IL-10), and effectively prevent cartilage degeneration. |
[150] |
| PLA | KGN | Improve drug bioavailability; Slow release | In vitro & vivo | Synoviocytes; Mice | Polymer microparticles showed an extended drug release of 62% over 3 months; In vitro, these particles did not change the mitochondrial activity of cultured human osteoarthritis synovial cells. In vivo, KGN- nanocrystals showed higher biological activity than KGN solution in the mouse model of mechanical osteoarthritis. |
[151] |
| PLA/PVA/CS-Hcl | Etoricoxib | Slow release | In vitro | - | Enhanced ALP activity and increased calcium ion deposition and binding | [153] |
| MSNs/pSBMA | - | Lubrication; Slow release | In vitro | - | MSNs@ pSBMA was remarkably improved, with a reduction of 80% in friction coefficient compared with MSNs. | [155] |
| SNF | Celecoxib/ curcumin | Slow release | In vitro | Chondrocytes | Nanoparticles could achieve the controlled release of drugs by changing the drug loading, greatly improve the cytotoxicity of the two drugs, and play an anti-inflammatory effect. | [154] |
| PN | KGN | Slow release | In vitro & vivo | Chondrocytes; Rats | PN-KGN had no cytotoxicity and pro-inflammatory effect on chondrocytes and IA injection of PN-KGN also showed less cartilage degeneration and a significant decrease in OARSI score. | [152] |
| Hollow dextran/ Poly (N-isopropyl acrylamide) | KAFAK peptides. | Thermal response | In vivo | - | The KAFAK-loaded hollow dextran/PNIPAM nanoparticles effectively delivered therapeutic peptides in cartilage explants to suppress inflammation. | [156] |
| PNIPAM-PMPC | Diclofenac sodium | Thermo-Sensitive; Lubrication | In vitro | Chondrocytes | Due to the hydration and lubrication mechanism of zwitterionic head group, the lubrication performance of PNIPAM-PMPC nanospheres had been greatly improved under different experimental conditions, and PNIPAM-PMPC nanospheres could effectively embed anti-inflammatory drugs of DS and achieve temperature-sensitive release of drugs. In addition, in vitro experiments further showed that PNIPAM-PMPC nanospheres were biocompatible and protected chondrocytes from cytokine-induced degeneration. | [157] |
| HA/pNiPAM | - | Thermo-Sensitive; Slow release |
In vitro & vivo | Human synovial fibroblasts; Mice | Nanoparticles were biocompatible, providing a longer residence time at the injection site, protecting cartilage, reducing pro-inflammatory cytokines and maintaining callus thickness. | [158] |
| Chitosan oligosaccharide/ pluronic F127 | KGN/Diclofenac sodium | Thermo-Sensitive/Slow release | In vitro & vivo | Chondrocytes; Macrophage-like cells; BMSCs | In order to achieve dual drug release, KGN was covalently cross-linked to the outer layer of the nanospheres, while DCF was loaded into the core of the nanospheres, showing the immediate release of DCF and the continuous release of KGN, which were independently controlled by temperature changes; The hypothermic nanospheres effectively inhibited the inflammation of chondrocytes and macrophage-like cells induced by endotoxin and induced mesenchymal stem cells to differentiate into cartilage. The nanospheres inhibited the progress in the treatment of osteoarthritis in rats, which was further enhanced by cold therapy. Nanospheres also reduced the expression of cyclooxygenase-2 in serum and synovium of treated rats, and further decreased after cold treatment. |
[159] |
| PLGA | Rhein | pH-responsive | In vitro | THP-1 | Nanoparticles released rhein more effectively in synovial fluid environment (SFE) with low pH value, significantly affected inflammatory cytokines TNF- α and IL-1 β and reduced their release in THP-1 cells stimulated by LPS. It was also found that reactive oxygen species (ROS), a mediator, led to cartilage collapse. | [160] |
| PCFMN/collagen II-binding peptide | FMN | Cartilage targeting | In vitro & vivo | Chondrocytes; Rats | The in vitro test using IL-1β stimulated chondrocytes indicated that PCFMN was biocompatible and upregulated anabolic genes while simultaneously downregulated catabolic genes of the articular cartilage; PCFMN could effectively delay the progression of osteoarthritis and showed a longer joint placement time and better anti-inflammatory effect than FMN, suggesting that the cartilage targeted nanosheets have a certain therapeutic effect on osteoarthritis. |
[163] |
| XG/PSBMA/collagen II-binding peptide | - | Cartilage targeting; Lubrication |
In vitro | - | The nanoparticles possess antioxidation verified by DPPH assay and exhibits synergistically enhanced ROS (OH, O2− and H2O2) scavenging. | [165] |
| PEG-SWCNTs | - | Cartilage targeting | In vitro & vivo | Chondrocytes; Mice | PEG-SWCNTs were capable to persist in the joint cavity for a prolonged time, entered the cartilage matrix, and delivered gene inhibitors into chondrocytes of both healthy and OA mice. | [44] |
| PLGA-PS | - | Cartilage targeting | In vitro | Synoviocytes; Chondrocyte | PLGA NPs surface-modified with a quaternary ammonium cation had the greatest retention within cartilage explants. | [167] |
| PEG/PLGA/WYRGRL | MK-8722 | Cartilage targeting | In vitro & vivo | Chondrocytes; Cartilage tissues; Mice | The novel delivery system binds very specifically to cartilage tissue in vitro and ex vivo because of WYRGRL; When injected into the knee joints of the mice with collagenase‐induced OA, the drug‐loaded nanoparticles can effectively reduce cartilage damage and alleviate the disease severity. |
[164,166] |
| DS | TA | Macrophage targeting | In vitro & vivo | RAW 264.7; Mice | DS-TA nanoparticles with the excellent targeting specificity to scavenger receptor class A; DS-TA nanoparticles could effectively reduce the activity of activated macrophages and the expression of proinflammatory cytokines. Intra-articular injection of DS-TA nanoparticles could effectively reduce the structural damage of articular cartilage. In addition, DS-TA nanoparticles reduced the expression of pro-inflammatory cytokines, including IL-1β, IL-6 and tumor necrosis factor-α in cartilage. |
[168] |
| PEG-4MAL/HAP-1/ WYR | - | Synovial targeting; Cartilage targeting |
In vitro & vivo | Rats | The drug could be released in the carrier for 16 days, near to zero-order release; The microgel display was retained in the joint space for at least 3 weeks. |
[169] |
| O-HTCC | SOD | Slow release | In vitro & vivo | Chondrocytes; Rats | O-HTCC-SOD was nontoxic to chondrocytes and had more long-acting and intracellular protection effects on chondrocytes against MIA-induced oxidative damage; O-HTCC-conjugated SOD significantly prolonged half-life and residence in rat joint cavity, and improved bioavailability compared with unmodified SOD; Intra-articular injection of O-HTCC-SOD significantly attenuated mechanical allodynia in MIA-induced osteoarthritis rats, dramatically suppressed gross morphological and histological lesions of articular cartilage, and greatly enhanced in vivo antioxidant capacity and anti-inflammatory effect. |
[170] |
| PPNP | Dex | ROS-responsive | In vitro & vivo | RAW264.7; Mice | The drug could efficiently inhibit the ROS and nitric oxide production in lipopolysaccharide-activated RAW264.7 macrophages and modulate macrophages M2 polarization at a much lower concentration than free drug dexamethasone; The monosodium iodoacetate-induced OA mice treated with this drug was very similar with the normal mice with the evaluation of body weight and scores including clinical arthritis scores, claw circumference, and kinematics scores. |
[171] |
| PAMAM/ C11 peptide/ CH6 aptamer | - | Bone targeting | In vitro & vivo | Osteoblastic; Rats | Nano-carrier could successfully accumulate in the targeted cells, mineralized areas and tissues. | [172] |
| Exosomes | ||||||
| SMSCs | CircRNA3503 | Improve drug stability | In vitro | Chondrocytes | Alleviating inflammation-induced apoptosis and the imbalance between ECM synthesis and ECM degradation; Promoting chondrocyte renewal to alleviate the progressive loss of chondrocytes. |
[82] |
| Dendritic cells | MicroRNA-140 | Cartilage targeting | In vitro & vivo | Chondrocytes; Rats | By fusing CAP with lysosomal membrane glycoprotein 2b protein on the surface of the exocrine body, the CAP- exosome could specifically enter and transport the goods to chondrocytes; CAP-exosomes also delivered miR-140 to deep cartilage regions through the dense mesochondrium, inhibit cartilage-degrading proteases, and alleviated OA progression in a rat model. |
[176] |
| SF-MSCs | KGN | Increase the effective concentration of the drug in the cell. | In vitro & vivo | Chondrocytes; Rats | The MSC-binding peptide E7 was fused with the extracellular membrane protein Lamp2b to obtain the exosome with SF-MSC targeting ability. The KGN carried by E7-Exo could effectively enter SF-MSCs and induce cartilage differentiation more effectively than KGN alone or KGN transported without E7; E7-Exo-KGN could effectively enter SF-MSCs and induce a higher degree of cartilage differentiation. The combined use of SF-MSCs and E7-Exo/KGN through intra-articular injection in the knee joint also showed a more significant therapeutic effect in rat OA model. |
[177] |
Abbreviations: LIPUS, low-intensity pulsed ultrasound; HOACs, human chondrocytes – osteoarthritis; HUVEC, human umbilical vein endothelial cell; sPLA, secretory phospholipase A2 enzyme; HUVECs, human umbilical vein endothelial cells; Raw264.7, murine macrophages; HIG-82, rabbit synovial cells; KGN, kartogenin; ATDC 5, chondrogenic ATDC 5 cell line; THP-1, human monocytic cell line; c-FMS, cell-cat McDonough strain sarcoma virus oncogene homology; FMN, formononetin; PCFMN, formononetin-poly(ethylene glycol); SWCNTs, single-walled carbon nanotubes; PEG, poly(ethylene glycol); DSPC, 1,2-dioctadecanoyl-sn-glycero-3-phosphocholine; OCT, octadecylamine; DPPC, dipalmitoyl phosphatidylcholine; BMPR2, bone morphogenetic protein 2; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3- 140 phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]; DSPE-PEG2000-maleimide, 1,2-distearoyl-sn-glycero-3-142 phosphoethanolamine-N-[maleimide (polyethylene glycol) 2000]; PCL, polycaprolactone; PEI, polyethylenimine; PEPS, poly(ethylene glycol)-block-poly(propylene sulphide); FA, folic acid; PSA, polysialic acid; PLGA-SeSe-mPEG, the coupling of poly (lactic-co-glycolic acid), methoxy polyethylene glycol and Se; CDMP-1, cartilage-derivedmor-phogeneticprotein-1; LMWH-TOS, the coupling of low molecular weight heparin and d-α-tocopheryl succinate; mPEG-PPF, amphiphilic methoxy polyethylene glycol-polypropylene fumarate; CAP, chondrocyte affinity peptide; Abs, anti-TNF α antibodies; ABP, azabisphosphonate; MSNs, mesoporous silica; pSBMA, photopolymerization of 3-[dimethyl-[2-(2-methylprop-2-enoyloxy) ethyl] azaniumyl] propane-1-sulfonate polymer; SNF, silk fibroin nanoparticles; PN, polyurethane nanoparticles; PNIPAM-PMPC, poly[N-isopropylacrylamide-2-methacryloyloxyethyl phosphorylcholine]; MK-8722, an activator of 5’-adenosine monophosphate-activated protein kinase (AMPK); XG, xanthan gum; SWCNTs, single-walled carbon nanotubes; PS, polystyrene; DS, dextran sulfate; PEG-4MAL, 4-arm-poly(ethylene glycol)-maleimide; HAP-1, SFHQFARATLAS sequence peptide; WYR, peptide WYRGRL; SOD, superoxide dismutase; O-HTCC, O-(2-hydroxyl) propyl-3- trimethyl ammonium chitosan chloride; sPL, super-activated platelet lysate; Dex, dexamethasone; TA, triamcinolone acetonide; PPNP, polyphenol–poloxamer assembled nanoparticle; CAP, chondrocyte-affinity peptide; SF-MSCs, synovial fluid-derived mesenchymal stem cells; PAMAM, poly- (amidoamine); HCL, hydrochloride; CS, chitosan.
2). Improved bioavailability
The phospholipid bilayer structure of liposomes has a high affinity for the cell membrane and good stability, and the uptake of drugs can be improved by liposome formulations. In recent years, natural molecules, such as curcumin,105,106 lornoxicam,107 and rapamycin,101 with poor bioavailability were also loaded into liposomes for OA treatment. The results showed superior therapeutic effects and better bioavailability than the original forms.
3). Liposome tissue/cell targeting
Modified liposomes with functional groups for targeting are generally based on two strategies.
① Targeting agents loaded in liposome formulation
The targeting agents can be loaded into the liposomes. This strategy typically targets protein receptors or genes, such as CGS21680 (adenosine A2A receptor agonist),108,109 miR-15a (a microRNA that silences SMAD2 gene expression),110 and microRNA-143-3p (targeted regulation of BMPR2 expression).111 The stable lipid bilayer structure of liposomes can help transport sensitive cargo into cells and enhance the effect of the agents.112
② Liposome surface modification with functional targeting groups
Liposome surface modifications with functional targeting groups is another common strategy. The synovium and inflammatory microenvironment can be targeted by modifying with the HAP-1 (ALSQAFRHAFTS; sc-HAP-1) peptide,113 ART-2 (CKPFDRALC) peptide,114 or a specified number of PEG groups.115 Bone-targeted delivery of liposomes can be achieved by modifying with alendronate, pyrophosphate, and oligopeptides.116 Considering that cartilage is rich in type II collagen, liposomes modified with type II collagen antibodies117 reportedly provided cartilage targeting for targeted drug delivery.
Micelles
Micelles are nanoscale materials comprised of amphiphilic polymers that self-assemble in aqueous solvents, and their size range is generally 5–100 nm.118 Micelle formulation requires a critical polymer concentration known as the critical micelle concentration (CMC). The self-assembly process occurs when the amphiphile concentration in the aqueous solution reaches the CMC.119
Micelle formulations are commonly used as a DDS for hydrophobic agents. Wei et al.120 encapsulated the sPLA2 (secretory phospholipase A2) inhibitor into micelles for lesion tissue targeted drug delivery, and Wang et al.121 loaded siRNA in micelles to target p56 of the NF-κB pathway. Oxygen species (ROS)-responsive micelles were developed based on elevated ROS exposure in arthritic joints. For example, the diblock copolymer poly (ethylene glycol)-block-poly (propylene sulfide)122 and PLGA-SeSe-mPEG (poly (lactic-co-glycolic acid-SeSe-poly (ethylene glycol)) were used to modify micelles for ROS-sensitive drug delivery.123 Considering that the folic acid (FA) receptor is highly expressed on arthritic macrophage membranes, FA-modified PSA (polysialic acid)-CC (natural cholesterol) micelles were developed to target macrophages in the synovial inflammatory microenvironment.124 Conjugate polymers of heparin and d-α-tocopheryl succinate (LMWH-TOS) also reportedly target the inflammatory microenvironment.125
The acidic environment and overexpressed matrix metalloproteinases-13 (MMP-13) are typical OA markers, which enable the development of stimulus-responsive drug-delivery systems with high specificity for OA. pH-sensitive amphiphilic methoxy polyethylene glycol-polypropylene fumarate micelles,126 poly (β-amino ester) micelles,127–129 and multiple targeted micelles have been developed for OA drug delivery. For example, Lan et al.130 developed an MMP-13 enzyme and pH-responsive theranostic micelle for OA treatment and a specific collagen type II targeting peptide (WRYGRL) conjugate PPL was used to target the cartilage.
To date, few studies have reported micelle applications for clinical OA treatment,131 which may be related to the common limitations of micelles, including their inability to encapsulate hydrophilic drugs, CMC dependency, and toxicity concerns.
Dendrimers
Dendrimers are highly branched polymers with demonstrated therapeutic potential for drug delivery. Their structure can be divided into three main components: (1) the core or nucleus, (2) inner layers consisting of repetitive molecular units called dendrons, and (3) terminal groups on the surface.132–135 Poly(lysine) (PLL), polypropyleneimine (PPI), polyethylenimine (PEI), poly(arylether), and poly(amidoamine) (PAMAM) are the most popular dendrimers used in oral delivery.136 Dendrimers can steadily incorporate many active compounds and/or ligands that improve their solubility.137 Hu et al.138 conjugated kartogenin (KGN), PAMAM, and PEG to obtain PEG-PAMAM-KGN (PPK) and KGN-PEG-PAMAM (KPP) conjugates. This strategy improved KGN release and enhanced chondrogenic effects in OA joints. In addition, the dendrimer formed by PAMAM is a dense cationic macromolecule that binds anionic cartilage and easy to penetrates anionic cartilage tissues. Geiger et al.49 conjugated insulin-like growth factor 1 and PEG to PAMAM and improved cartilage-targeted drug delivery. Chondrocyte-affinity peptides139 or chondroitin sulfate (CS)-modified PAMAM polymers140 further enhanced polymer cartilage targeting, and azabisphosphonate (ABP)-capped dendrimers selectively targeted monocytes and directed them toward anti-inflammatory activation in the RA joint.141
Dendrimers possess various advantages, such as increased solubility of hydrophobic drugs and tunable physicochemical properties. Currently, there are many pre-clinical studies related to dendrimer applications;135,142–145 however, their application in OA treatment is limited. Disadvantages such as non-entrapment of hydrophilic drugs, cellular toxicity, and rapid drug release (up to 70% of the encapsulated drugs are released within a few hours146) limit their application in OA treatment.
Polymer Nanoparticles (PNPs)
Polymer nanoparticles (PNPs) are solid particles that can be prepared in the range of nanometers to microns.147 There are two structural types: nanocapsules, which consist of a hydrophilic drug reservoir and a polymer shell, and nanospheres, which contain a homogeneous polymer matrix that can load dispersed/intercepted drugs. The release kinetics of both types can be adjusted according to the formulation strategy, chemical composition, and molecular weight of polymers and drugs.148 PNPs have many promising advantages for drug delivery, including:
Improved drug release
Several studies report significantly improved and sustained drug release using PNPs for drug delivery. PLGA,149,150 polylactic acid (PLA),151 polyurethane,152 different polymer combinations,153 silk fibroin,154 and polymer-grafted mesoporous silica155 used to prepare PNPs for drug delivery. These formulations reportedly provide improved and sustained drug-release profiles for celecoxib, curcumin, and KGN by IA drug delivery.
2. Improved drug efficacy by microenvironmental responsive drug delivery (PH/Thermo sensitive)
Microenvironmental-sensitive drug-release strategies have been attractive for PNP drug delivery. pH and temperature sensitive PNPs are rapidly evolving, including hollow dextran/poly (N-isopropyl acrylamide) NPs,156 dual-functional poly[N-isopropylacrylamide-2-methacryloyloxyethyl phosphorylcholine] (PNIPAM-PMPC) nanospheres,157 hyaluronic acid-poly(N-isopropylacrylamide) (HA-pNiPAM) nanospheres,158 and chitosan oligosaccharide-conjugated pluronic F127 grafting carboxyl group nanospheres159 which possess temperature-responsive release properties. Previous studies showed that NH4HCO3 laden poly (lactic-co-glycolic acid) (PLGA) NPs demonstrate pH-responsive drug release.160,161
3. PNPs tissue/cell targeting
In recent years, more and more PNPs targeted drug-delivery systems have been developed for OA therapy. Cartilage targeting is the most popular strategy that can be achieved by modifying the PNPs with cartilage affinity groups.162 Collagen II-binding peptide-modified formononetin (FMN)-poly (ethylene glycol) (PEG) NPs,163,164 xanthan gum-poly (sulfobetaine methacrylate) (XG-PSBMA) NPs,165 WYRGRL (a short cartilage-targeting peptide sequence)-modified PLGA NPs,164,166 polyethylene glycol (PEG) chains (PEG-SWCNTs) modified with single-walled carbon nanotube (SWCNT) NPs,44 and quaternary ammonium cation modified PLGA NPs167 have been developed for cartilage targeting. In some instances, cartilage targeting originates in the basic material of the PNPs.163–166In addition, dextran sulfate used to target macrophages,168 HAP-1 peptide used to target synovial,169 O-HTCC (O-(2-hydroxyl) propyl-3trimethyl ammonium chitosan chloride) and polyphenol–poloxamer used to target ROS,170,171 C11 peptide (the C-terminal region of rh174) used to target bone,172 PNPs have also been developed to target the specific microenvironment of OA joint.
Over the past decade, significant progress has been made in the functional modification of PNPs. However, the side effects, toxicity, and complicated preparation processes of PNPs continue to challenge their application in OA treatment.
Exosomes
Exosomes are extracellular vesicles (loading content including lipids, nucleic acids, and proteins) secreted by cells for cell-to-cell communication.92 The size of exosomes is generally between 30–150 nm.173 Previous studies demonstrated the utility of exosomes as drug-delivery systems. Compared with other nanoparticles, exosomes possess higher stability, biocompatibility, biological barrier permeability, and lower immunogenicity,174 and can overcome some challenges, such as toxicity and a high clearance rate.175 Current studies on exosomes for OA treatment are mainly focused on two aspects: 1) to investigate the diagnostic significance and biological effects of endogenous exosomes in OA patients; 2) to investigate stem cell-derived exosomes for OA treatment.92
On the other hand, in recent years, the utility of exosomes as drug-delivery systems has become increasingly attractive for OA treatment. Tao et al.82 prepared circRNA3503 loaded exosomes from synovial mesenchymal stem cells and further incorporated the exosomes into an injectable thermosensitive hydrogel. This composite gel strategy successfully delivered small-molecule RNAs, improved drug targeting, and prevented OA progression. Liang et al.176 reported chondrocyte-affinity peptides (CAP) and lysosome-associated membrane glycoprotein 2b (Lamp2b) protein-modified exosomes (CAP-exosomes) that can efficiently encapsulate miR-140 and target chondrocytes in vitro. The CAP-exosomes successfully delivered miR-140 to deep cartilage regions and alleviated OA progression, demonstrating a potential organelle-based, cell-free therapy for OA. Xu et al.177 reported KGN-loaded MSC-binding peptide E7 modified exosomes (E7-Exo) that targeted synovial fluid-derived mesenchymal stem cells. The E7-Exo induced a higher MSC cartilage differentiation than KGN alone or KGN-loaded unmodified exosomes.
Exosomes have shown promising potential as drug carriers in OA treatment. However, studies of using exosome as drug-delivery systems are still limited. The greatest technical challenge is the difficulty in obtaining sufficient amounts of exosomes for in vivo studies. Table 3 demonstrated more details of the nanoparticle drug delivery systems mentioned in this review.
Microspheres
Microspheres are skeletal spherical drug-delivery systems formulated by dispersing or dissolving a drug in a polymer, with a particle size of approximately 1–200 μm. Microspheres are commonly used in OA treatment to prolong the retention time of drugs and lubricate joints.178,179
Liang et al.180 reported that mometasone furoate-loaded PLGA microspheres increased the drug retention time in joints by up to 35 days. Stefani et al.181 developed an acellular agarose hydrogel incorporated with dexamethasone-loaded PLGA microspheres for OA treatment. Combining microspheres and hydrogels improved the sustained drug-release properties for OA treatment for up to 99 days. Li et al.182 demonstrated that super-activated platelet lysate (sPL)-loaded PLGA/chitosan/gelatin microspheres improved the sustained drug release, inhibited osteoarthritis, and promoted cartilaginous repairs. Park et al.183 reported the utility of gelatin microspheres that are responsive to proteolytic enzymes typically expressed in arthritic flares, resulting in the on-demand and spatiotemporally controlled release of anti-inflammatory cytokines for cartilage preservation and repair.
On the other hand, IA DDS are not only committed to improving drug effectiveness, but also focus on improving joint lubrication properties. Bio-lubricants have been designed for the treatment of early OA by improving the lubrication performance of synovial joints. Therefore, some people have also improved the lubricating properties of hydrogels by modifying polymer materials. Sulfone-modified HA,184 lactose modified chitosan185 and gellan gum186 could modulate viscoelastic properties of osteoarthritis synovial fluids and improve OA joint lubrication. Han et al.179 reported photocrosslinked methyl methacrylate gelatin (GelMA) hydrogel microspheres incorporated into a self-adhesive polymer (DMA-MPC) to prepare a new composite microsphere (GelMA@DMA-MPC). This biomimetic injectable hydrogel microsphere enhanced lubrication and controlled the drug release for OA treatment. Zhang et al.187 demonstrated that YAP (Yes-associated protein)-selective inhibitor-loaded chitosan microspheres could target subcellular YAP activity and alleviate OA progression. Yang et al.188 reported ball-bearing-inspired polyampholyte-modified microspheres with a super lubricated ability (MGS@DMA-SBMA). The MGS@DMA-SBMA microspheres significantly enhanced joint lubrication and improved the sustained drug release, which are highly desirable for IA OA treatment.
Several microsphere formulations have been commercially available or proved to be promising for OA treatment. A triamcinolone acetonide sustained-release microsphere formulation (Triamcinolone acetonide extended-release (ER), Zilretta®) was approved for OA treatment in the United States.189 In addition, several microsphere formulations, such as fluvastatin,190 celecoxib,191 and etoricoxib,192 are planning to tested in clinical trials.
Generally, different polymers (such as polylactic acid, gelatin, and chitosan) can be developed into microspheres, which can also be modified with different functional groups to help improve drug delivery/targeting.193 Table 4 demonstrated more details of the microsphere drug delivery system mentioned in this review.
Table 4.
Summary of Microspheres Drug Delivery System
| Material | Drug | Carrier Effect | In vivo/vitro | Experimental Subject | Treatment Effect | Ref |
|---|---|---|---|---|---|---|
| PLGA | Mometasone furoate | Slow release | In vitro | - | The drug could be released for 35 days. | [180] |
| PLGA/CS/Gelatin | sPL | Slow release | In vitro & vivo | Chondrocytes; Rats | Continuous release of sPL from microspheres could significantly increase cartilage proliferation, reduce cell necrosis, and increase the expression of type II collagen, ACAN and SOX9 in OA chondrocytes. The microspheres in vivo could also smooth the surface of cartilage. | [182] |
| PLGA | Dexamethasone | Slow release | In vivo | Dogs | Cartilage repairing | [181] |
| GelMA and DMA-MPC | DS | Lubrication; Slow release | In vitro & vivo | Chondrocytes; Rats | The microspheres had the characteristics of lubricity and sustained release so they could significantly up-regulate the expression level of cartilage anabolism genes and down-regulate the expression level of cartilage catabolism protease genes. | [179] |
| CS | YAP-selective inhibitor | Targeting | In vitro & vivo | Chondrocyte; Mice | YAP could maintain the phenotype of chondrocytes and prevent cartilage degeneration in OA by targeting downstream molecular activity of ECM hardness. | [187] |
| Poly (dopamine methacrylamide-ethyl methacrylate methanesulfonate); Methyl methacrylate gelatin | Diclofenac sodium | Lubrication; Slow release | In vitro & vivo | Chondrocytes; Rats | The modified microspheres had the properties of enhancing lubrication, reducing degradation and slow release of drugs. The drug-loaded super-lubricating microspheres with good biocompatibility had a protective effect on chondrocyte degeneration induced by inflammatory factors in vitro and had a therapeutic effect on osteoarthritis in DMM model rats, that is, to reduce the load of osteophyte and cartilage degradation. | [188] |
| Gelatin/Genipin | IL-4; IL-13 | On-demand and spatiotemporally controlled release | In vitro | Chondrocytes | Exposure of the IL-4 and IL-13 loaded microspheres reduced the inflammation of chondrocytes up to 80%. | [183] |
Abbreviations: sPL, super-activated platelet lysate; DS, diclofenac sodium; GelMA, photo-crosslinked methacrylate gelatin hydrogel; DMA-MPC, self-adhesive polymer; ADSCs, adipose-derived stem cells; DEX-TA, amine-terminated dextran–tyramine conjugates; PLGA, poly (lactic-co-glycolic acid); CS, chitosan; YAP, yes-associated protein.
Current Active and Passive Targeting Strategies for IA DDSs
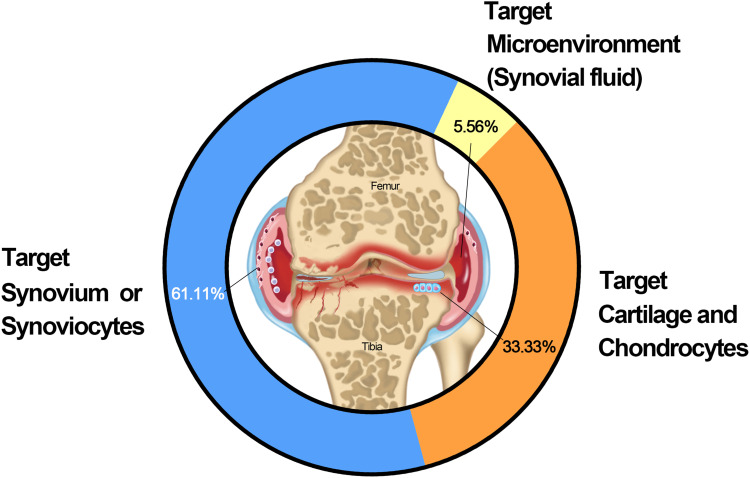
Tissue/cell-specific active and passive targeting technologies have been developed to improve drug retention and efficacy in joints. We collected and analyzed 36 studies with clear target mechanisms. The results demonstrated that targeted synovium/synoviocytes, cartilage and chondrocytes, and joint microenvironments (synovial fluid) accounted for 61.11, 33.33, and 5.56%, respectively, of the IA DDSs used for OA treatment (Figure 4). The details are listed in Table 5.
Figure 4.
Representative research targets of IA DDSs.
Table 5.
Representative Research Targets of IA DDSs
| Target | Nanomaterial Type | Targeting Modality | Physical Properties | Major Findings | Ref |
|---|---|---|---|---|---|
| Cartilage and chondrocytes | PAMAM dendrimer, PEGylated to control surface charge, with IGF-1 conjugated | Passive (positive charge for cartilage) | “Gen 4” 14 kDa (4.5 nm), 64 NH2 groups per molecule (less cationic) | The more charged “Gen 6” dendrimer transported more slowly into cartilage (6 days vs 2 days for “Gen 4” for full thickness distribution), but to a greater extent (nearly twice as much “Gen 6” compared to “Gen 4” after 6 days). | [49] |
| “Gen 6” 58 kDa (6.7 nm), 256 NH2 groups per molecule (more cationic) | |||||
| Globular protein (Avidin) | Passive (positive charge for cartilage) | 7 nm +20 mV |
Positively charged Avidin had stronger interactions with cartilage than the neutral form of the protein. | [229] | |
| Triblock self-assembly nanoparticle | Passive | 300 nm | Nanoparticles penetrated the full-thickness of cartilage in vivo, shown by fluorescence microscopy. | [45] | |
| Micelle ± CPP | Passive (naked) | 15 nm | CPP modification increased association with chondrocytes. Only smaller micelles could diffuse through the cartilage (larger liposomes were trapped in the superficial zone). | [200] | |
| Active (CPP) | 106 nm | ||||
| Liposome ± CPP | Passive (naked) | 138 nm | |||
| Active (CPP) | 397 nm | ||||
| Peptidic siRNA carrier (CPPsiRNA complex, coated with albumin) | Active (contains a CPP) | 55 nm | After incubating the cartilage for 48 h with the carrier, the carrier was present throughout the cartilage, primarily accumulated intracellularly and aggregated in the superficial zone. Signal was detectable in chondrocyte lacunae after 14–21 days in culture. | [230] | |
| Poly (propylene sulphide) nanoparticle with collagen type II peptide | Active (targeting peptide for collagen II) | 38 nm 96 nm -Peptide: −3mV ± 9 mV +Peptide: +18 mV ± 3.5 mV |
The 38 nm targeted particles were immobilised within the tissue with a 71-fold greater accumulation than scrambled controls at 48 hr. For targeted particles, 38 nm particles had 14.9-fold more accumulation than 96 nm particles in the cartilage matrix. | [196] | |
| Cartilage and chondrocytes | Liposome with an anticollagen II antibody | Active (antibody for collagen II) | 150–250 nm | Antibody-enhanced liposomes qualitatively showed selective binding to OA cartilage. Liposomes without the targeting antibody did not show significant binding to cartilage. | [199] |
| Liposome with an anticollagen II antibody | Active (antibody for collagen II) | 100–300 nm | The amount of nanomaterial increased proportionately with disease severity via fluorescence tracking. | [231] | |
| Hyaluronic acid-coated bovine serum albumin nanoparticles | Active (binding to chondrocyte CD44) | 108.1 nm ± 5.9 nm -21.1 mV ± 3.2 mV |
Hyaluronic acid coating statistically improved chondrocyte uptake of the loaded drug through active transport processes. | [232] | |
| Hyaluronic acid-coated polylactide (PLA) nanoparticles | Active (binding to chondrocyte CD44) | 650 nm ± 40 nm | Hyaluronic acid coating improved uptake into chondrocytes relative to poly vinyl alcohol (passive strategy). | [233] | |
| Globular protein (Avidin) | Passive (positive charge for cartilage) | 7 nm +20 mV |
At 24 h, significantly more Avidin (cationic) than Neutravidin (neutral) in remained various joint tissues. At 7 days, Avidin is mostly cleared from joint tissues. | [195] | |
| DOTAM derivative with collagen type II targeting peptide | Active (targeting peptide for cartilage) | N/A | Targeted molecules had greater retention compared to untargeted controls at 125 h post injection, and increasing the sites of peptide conjugation increased joint retention. | [194] | |
| Synovium or Synoviocytes | Gold nanoparticles (no drug) | Passive | 5 nm–52 nm | Effective tissue permeation was only achieved with the smallest particles (5 nm). Exposure to pro-inflammatory factors did not affect permeation. | [234] |
| Chloroquine loaded solid lipid nanoparticle | Passive | 113.6 nm | TNF-A levels were significantly reduced when chloroquine was loaded into a solid lipid nanoparticle versus free suspension of chloroquine. | [235] | |
| Brucine loaded PLGA nanoparticles in PLGA microparticles | Passive | 12.38 nm | Burst release of brucine was slowed and particles stayed in the articular cavity for significant time. | [236] | |
| Dendritic polyglycerol sulfate (no drug) | Passive | 3 nm | Cells treated with NPs did not show any change in the synthesis of proinflammatory cytokines (TNF-A and IL-6) and exhibited a greater expression of anti-inflammatory cytokines (IL-10). | [237] | |
| Synovium or Synoviocytes | Thiolated glycol chitosan nanoparticles encapsulating polymerised Notch 1 targeting siRNA | Passive | 200 nm | In vitro Notch-1 inhibition of siRNA-NPs in murine macrophage cell was confirmed. siRNA-NPs exhibited higher targeting efficiency in the arthritic joints of CIA mice. | [238] |
| Chitosan-graft-PEI nanoparticles complexed with plasmid enhanced green fluorescent protein | Passive (gene delivery) | 100 nm–300 nm | Nanoparticles were able to carry plasmid DNA inside synoviocytes where the DNA was detected entering the cell nuclei. | [239] | |
| Melittin-Derived Cationic Amphipathic Nanocomplexes combined with siRNA targeting the p65 subunit of NF-κB | Passive (gene delivery) | 55 nm | Administration of p5RHH-p65 siRNA nanocomplexes decreased inflammatory cytokine expression and cellular influx into the joints, protected against bone erosions, and preserved cartilage integrity. | [240] | |
| Multiwall Carbon Nanotubes (no drug) | Passive | 60 nm diameter 10 mm length |
MWCNTs led to formation of granulation tissues within adipose tissues at higher concentrations in vivo. With RAW 264.7 cells, the MWCNTs increased the TNF-A, MCP-1 and RANTES induced inflammatory responses in a dose dependent manner while decrease MIP-1a. With HFLS, they decreased secretion of IL-6 and MCP-1. | [241] | |
| Carbon nanotubes coated with PEG and DEX | Passive | 180 nm (DEX-PEG coated CNT) −18 mV | DEX and PEG coated CNTs were able to decrease inflammatory cytokines (IL-1b, TNF-A and IL-6) and MMP3 at both a gene and protein expression level at a lower concentration than free DEX in vitro with FLS. | [242] | |
| N-trimethyl chitosan-polysilicon acid nanoparticles coated with decoy oligodeoxynucleotides | Passive (gene delivery) | Without methotrexate:159 nm, +23 mV With methotrexate:184 nm, +33 mV |
Nanoparticles decorated with decoy oligodeoxynucleotides specific to transcription factor NFκB resulted in significant decreases in inflammatory mediators (IL-6 and IL-8). |
[243] | |
| Clodronate liposomes | Passive | 120 nm–160 nm | A single IA dose of clodronate liposomes significantly reduced the number of CD68-positive macrophages and the expression of ICAM-1 and VCAM-1 in the synovial lining. | [244] | |
| DEX-HPMA copolymer conjugate | Passive (Stimuli Responsive pH sensitivity) | 73 kDa (2.8 nm) | The conjugate has greater anti-inflammatory effects compared with systemically administered free DEX. This differential effect of the conjugate was related to its selective accumulation, potential macrophage-mediated retention, and pH-sensitive drug release in arthritic joints. | [245] | |
| Synovium or Synoviocytes | Mineralised nanoparticles composed of PEGylated hyaluronic acid as the hydrophilic shell, 5b-cholanic acid as the hydrophobic core, and calcium phosphate as the pH-responsive mineral and loaded with methotrexate | Passive (Stimuli Responsive pH sensitivity) | 218 nm–265 nm | The mineralised nanoparticles revealed pH-dependent demineralisation followed by acceleration of methotrexate release into the cytosol. | [246] |
| PLGA gold/iron/gold half-shell nanoparticles conjugated with RGD and loaded with methotrexate | Passive (Stimuli Responsive - NIR and magnetic) | 135 nm | When combined with consecutive NIR irradiation and external magnetic field application, these nanoparticles provided enhanced therapeutic effects. | [247] | |
| Alginate nanoparticles decorated with tuftsin peptide and loaded with IL-10 plasmid DNA | Active (IL-1 surface receptors) | ~300 nm | Targeted alginate nanoparticles loaded with IL-10 plasmid DNA efficiently repolarized macrophages from an M1 to an M2 state. | [204] | |
| Self-assembling block copolymer with protein tethering moiety (IL-1Ra) | Active (surface receptors) | 270 nm ± 5 nm | IL-1Ra-tethered particles bound to synoviocytes via the IL-1 receptor and significantly increased whole joint retention of IL-1Ra relative to soluble IL-Ra. | [45] | |
| Micelle carriers of camptothecin, surface modified by vasoactive intestinal peptide | Active (surface receptors) | Not reported | Single subcutaneous injections of the micelles led to mitigated inflammation in joint if CIA mice up to 4.5 weeks after induction. | [248] | |
| RGD peptide–exposing long circulating PEG liposomes loaded with dexamethasone and targeted to ɑνβ3 integrins expressed on angiogenic VECs | Active (surface receptors) | 100 nm | In vivo, increased targeting of radiolabeled RGD-PEG–L to areas of LPS-induced inflammation in rats was observed. | [205] | |
| ανβ3-targeted fumagillin nanoparticles | Active (surface receptors) | 250 nm | Synovial tissues from animals treated with targeted fumagillin nanoparticles showed a significant decrease in inflammation and angiogenesis, and preserved proteoglycan integrity. | [206] | |
| Nanocarrier composed of lipids, PEG-PLGA forming a hydrophilic shell, folic acid around the hydrophilic shell as a targeting ligand, and poly(cyclohexane- 1,4-diylacetone dimethylene ketal) (PCADK) and PLGA as a hydrophobic core and loaded with methotrexate | Active (surface receptors) | 133.6–208.5 nm (varied based on composition of components) | Folate targeted particles demonstrated superior uptake in RAW 264.7 cells than untargeted nanoparticles. A smaller paw size and less swelling was observed in the AIA model of rat when injected with the targeted particles compared to the untargeted particles. | [207] | |
| Synovium or Synoviocytes | PLGA nanoparticles coated either with macrophage-derived microvesicle proteins or red blood cell membrane proteins and loaded with tacrolimus | Active (surface receptors) | 130 nm | In vitro binding of the microvesicle coated particles was greater than controls. The microvesicle coated particles also showed greater therapeutic impact in CIA model than controls. | [208] |
| Superparamagnetic iron oxide nanoparticles (no drug) | Passive (Stimuli Responsive -magnetic) | Not Reported | Intra-articular and peri-articular injection of the particles led to uptake in the synovium, with increased local concentration when an extracorporeal magnet was applied. | [249] | |
| Microenvironments (synovial fluid) | Positively surface-charged poly(lactide-co-glycolide) (PLGA)/Eudragit RL nanoparticles (no drug) |
Passive | 170.1 nm | Increasing retention time and sustain release profile in joints after intra-articular injection, by forming micrometer-sized electrostatic aggregates with hyaluronic acid. | [48] |
| Nanoparticles based on Eudragit RL100 or cationically modified dextran. | Passive | ~130nm | Hydrogels formed after the nanoparticles were mixed with synovial fluid | [209] |
Abbreviations: CPP, cell penetrating peptide (nonspecific to chondrocytes); DMM, destabilization of the medial meniscus; IGF-1, insulin-like growth factor 1; PAMAM, polyamidoamine; PEG, polyethylene glycol; AIA, adjuvant-induced arthritis; CAIA, collagen antibody–induced arthritis; CIA, collagen induced arthritis; CFA, complete Freund’s adjuvant; CNT, carbon nanotube; DEX, dexamethasone; FLS, fibroblast-like synoviocytes; HFLS, human fibroblast-like synoviocytes; HPMA, N-(2-hydroxypropyl) methacrylamide; IA, intra-articular; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; MIP-1a, macrophage inflammatory protein 1 alpha; MMP, matrix metalloproteinase; MWCNT, multiwall carbon nanotubes; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NIR, near infrared; PEI, polyethylenimine; PEG, poly (ethylene glycol); PLGA, poly(lactic-co-glycolic acid); RA, rheumatoid arthritis; RANTES, regulated on activation, normal T cell expressed and secreted; RAW 264.7, macrophage- like cell line derived from tumors induced in male BALB/c mice by the Abelson murine leukemia virus; RGD, arginine-glycine-aspartic acid; SLNs, solid lipid nanoparticles; TNF-A, tumor necrosis factor alpha; VCAM- 1, vascular cell adhesion molecule 1; AIA, antigen-induced arthritis; CIA, collagen induced arthritis; HUVEC, human umbilical vein endothelial cells; IL-1Ra, interleukin 1 receptor antagonist; LPS, lipopolysaccharide; MPCM, mouse peritoneal cavity macrophages; RA, rheumatoid arthritis; RAW264.7, macrophage-like cell line derived from tumors induced in male BALB/c mice by the Abelson murine leukemia virus; RGD, arginine-glycine-aspartic acid; VEC, vascular endothelial cells.
Different strategies have been used to target cartilages/chondrocytes, including the passively targeting anionic cartilage ECM/collagen type II.49,194–196 For example, a six-amino acid collagen II-binding peptide (WYRGRL)-conjugated drug-delivery system targets collagen and provides significantly longer joint retention than a normal control peptide.195,196 In addition, drug retention increased with the collagen II-binding peptide content in the drug-delivery system. Antibodies for collagen II197,198 have also been used as ligands in drug-delivery systems to target cartilage.117,199 A drug-delivery system incorporating a cell-penetrating peptide (YGRKKRRQRRR-C) significantly enhanced the drug uptake by chondrocytes by approximately 4-folds compared to the non-targeting formulation.200 A passive, electrostatic-based strategy was also developed for targeting cartilage.49,139,201,202 For example, drug-delivery systems with cationic surface charges (especially dendrimers136,139) significantly improved the joint retention of drugs, as the cartilage ECM accumulated anionic charges because of the dense sulfated proteoglycan networks.49,141,203
The OA targeting strategy not only focuses on the cartilage/chondrocytes, but also on other joint tissues/cells, such as the synovium/synoviocytes, owing to the complicated disease progression in OA. This strategy focused on targeting surface receptors in two major cells: 1) the minor type A synovial macrophages that significantly proliferate and activate under inflammatory conditions; 2) and the dominant type B fibroblast-like cells which produce ECM components of the synovial membrane and synovial fluid. Previous studies have demonstrated tuftsin peptide incorporated nanoparticles that target macrophages in the joint;204 copolymer nanoparticles incorporated with IL-1Ra proteins targeted synoviocytes through the IL-1 receptor and increased drug retention in the joint;45 RGD (Arg-Gly-Asp) peptide-modified liposomes targeted vascular endothelial cells of synovium though RGD peptide binding to αvβ3 integrins,205 which are strongly up-regulated on angiogenic endothelium at the inflammatory sites; αvβ3-targeted fumagillin nanoparticles significantly decreased inflammation and angiogenesis, and preserved proteoglycan integrity in synovial tissues.206
Overexpressed activated macrophages and acidic microenvironments are two significant features of RA joints. Multifunctional FA receptor-targeting and pH-responsive nanocarriers have been developed for RA IA treatment. Folate-incorporated nanoparticles promoted drug uptake of macrophages compared to untargeted nanoparticles,207 and drug-delivery systems incorporated with macrophage-derived micro-vesicles can target macrophages in the synovium or pannus of inflamed tissues.208 Drug deliveries were also designed to target synovial fluid by ionic cross-linking with endogenous/exogenous hyaluronic acid.48,209
Evidently, numerous targeted strategies have been developed to deliver and improve the efficacy of drugs in joints. Active and passive targeting strategies for OA joints have rapidly evolved over the past decades (Figure 4 and Table 5).
Current Signaling Pathways Targeted by IA Drug-Delivery Systems
Many pathways are involved in the pathogenesis of OA, and drug delivery system targeting different pathways have been used in IA drug-delivery systems. Articular drug-delivery systems provide significant support for the precise targeting of key sites in OA.210–221 We searched 14,160 studies on articular drug-delivery systems with clear signaling pathways from the 1st of January 2016 to the 13th of July 2021. The results demonstrated that the current research on articular drug-delivery systems mainly focus on five signaling pathways. TGF-β is the leading signaling pathway accounting for up to 26.35% of the studies (3730), followed by the NF-κB, PI3K, Wnt, and p38 MAPK (1790) signaling pathways which account for 21.97 (3109), 19.64 (2786), 19.39 (2745), and 12.65% (1790), respectively, of the studies (3109) (Figure 5, Table 6). None of the signaling pathways were proven to play the dominant role in OA initiation and progression. The mechanism of action of OA has yet to be fully elucidated.
Figure 5.
Statistics over the past five years on the current research status of IA DDSs target signaling pathways.
Table 6.
Summary of Previous Studies on Signaling Pathway in IA DDSs
| Signaling Pathway | Publication Ratio | Carrier Type | Material or Source | Drug Delivery | Carrier Effect | Treatment Effect | Ref |
|---|---|---|---|---|---|---|---|
| TGF-β signaling pathways | 26.35% | Hydrogels | Collagen-genipin -carbon dot nanoparticles | - | - | Contributing to chondrogenic differentiation | [213] |
| NF-κB signaling pathways | 21.e97% | Nanoparticles | Gd2(CO3)3-PDA-PEG | Hesperetin | Target chondrocytes specifically and release drug smartly. | Inhibiting receptor activator of nuclear factor κB ligand (RANKL)-induced osteoclast formation while promoting osteoblast differentiation. | [214] |
| Hyaluronic acid | - | - | Inhibiting CD44-induced NF-κB activation in chondrocytes. | [215] | |||
| PI3K/AKT/mTOR signaling pathways | 19.64% | Nanoparticles | hBMSCs | Curcumin | Increase the solubility and stability of the drug. | Alleviating IL-1β-induced catabolic effects on OA-CH. | [220] |
| PVP(K30)-K3Fe (CN)6 | Prussian blue | - | Alleviating ROS and apoptosis of chondrocytes by activating the PI3K/Akt/mTOR pathway; Reducing inflammatory cytokines and inhibit ECM degradation by reducing MMPs expression. |
[221] | |||
| Wnt signaling pathways | 19.39% | Hydrogels | Sodium hyaluronate and sodium alginate | Berberine | Provide a drug release environment. | The subchondral bone was partially repaired and the cartilage was protected from degeneration. | [216] |
| Amnion membrane | Adipose-derived stem cells (ADSCs) | Retain ADSCs. | Inhibiting the catabolic responses of IL-1β and inhibiting the Wnt/βcatenin signaling pathway. | [217] | |||
| p38 MAPK signaling pathways | 12.65% | Microspheres | Poly (D, L-lactic acid)-Cyanine 7 | PH-797804 | Slow release. | Significantly reducing inflammation and joint destruction and by inhibiting several biomarkers. | [218] |
| Hydrogels | Hyaluronic acid | Lingzhi and San-Miao-San | Lubricate joints | Down-regulate inflammatory factors. | [219] |
Abbreviations: PDA, polydopamine; PEG, poly (ethylene glycol); OA-CH, OA-patients; PVP, polyvinylpyrrolidone.
Discussion
IA injections play an important role in the treatment of OA owing to their significant advantages. In this review, we summarized the current drug-delivery systems for IA DDSs. We collected and analyzed studies that reported the application of different nanoparticle-based IA DDSs in pre-clinical/clinical trials. Polymeric microspheres were the leading nanoparticles and accounted for 35.17% of IA DDSs. Nanoparticle and hydrogel/gel formulations accounted for 28.57 and 14.29%, respectively, of IA DDSs (Figure 6A). Although OA treatment remains a challenge in clinical practice, there are many IA drug delivery formulations for clinical application are ongoing, and several formulations including ZILRETTA, TIVORBEX, ZORVOLEX, and VIVLODEX have been approved222–225 (Figure 6B, Table 7).
Figure 6.
Recent reported IA DDSs in pre-clinical/clinical trials/approved stages. (A) Proportion of different formulation of recent reported IA DDSs in pre-clinical/clinical trials/approved stages; (B) proportion of recent reported IA DDSs in pre-clinical/clinical trials/approved stages.
Table 7.
Recent IA DDSs Reported in Preclinical and Clinical Trials
| Stage | Name | Formulation Type | Agent | Company |
|---|---|---|---|---|
| Approved | ZILRETTA | Polymeric microsphere | Triamcinolone acetonide | Flexion Therapeutics |
| TIVORBEX | Nanoparticle formulation | Indomethacin | iCeutica Inc. | |
| ZORVOLEX | Nanoparticle formulation | Diclofenac | iCeutica Inc. | |
| VIVLODEX | Nanoparticle formulation | Meloxicam | iCeutica Inc. | |
| Phase 3 | FX005 | Polymeric microsphere | p38 MAPK inhibitor | Flexion Therapeutics |
| FX006 | Polymeric microsphere | Glucocorticoid | Flexion Therapeutics | |
| Phase 2 | SB-061 | Polymeric formulation | Peptide | Symic Bio, Inc. |
| TLC-599 | Liposome formulation | Ropivacaine | Taiwan Liposome Co. | |
| SOLUMATRIX | Nanoparticle formulation | Naproxen | iCeutica Inc. | |
| Phase 1 | FX007 | Polymeric microsphere | TrkA receptor antagonist | Flexion Therapeutics |
| EP-104IAR | Polymeric microsphere | Fluticasone Propionate | Eupraxia Pharmaceuticals Inc. | |
| Pre-clinical | FX301 | Polymeric thermosensitive | Funapide | Flexion Therapeutics |
| OA GEL | Gel formulation | Diclofenac | PolyActiva Pty Ltd | |
| HA-based | Intra-articular formulation | Clodronate | Abiogen Pharma Sp |
Different IA drug delivery systems have their advantages and disadvantages. Hydrogel often used as lubricant as its high water content and rheological properties. The excellent biocompatibility of hydrogel is also attractive for loading live cells and drugs. However, the loose network and high water content of hydrogels makes it difficult to increase drug retention time in joint. Microspheres are widely used for controlled delivery of therapeutics via different routes of administration (mainly subcutaneous and intramuscular) for augmenting effectiveness and reducing toxicity of the incorporated agents to non-targeted cells and tissues. However, the stability of microspheres is a challenge issue and they are not easily to mass-produce. Nanoparticle is a promising drug delivery system as its unique ability to penetrate tissues and easy to modify functional groups. However, some challenge issues still hinder the application of these nanoparticles. For example, 1) the sustained release and retention properties of most nanoparticles are still weak; 2) the drug leakage issue of liposome formulations; 3) the entrapment efficiency issue of hydrophilic drug micelles; 4) drug release too fast issue of dendrimer. Although exosomes have unique advantages in biocompatibility and non-toxicity, it is difficult to address the large quantities production issue, which significant hinder its future application. At present, significant challenges remain regarding to the safety, efficacy duration, and targeting properties to all IA DDSs. There is another important issue that may raise significant concerns: how to sterilize DDs while maintaining their effectiveness? Liposomes and microspheres are more suitable for gamma ray sterilization.226 For unstable nanoparticles, aseptic filtration is recommended.227 High-pressure steam sterilization and ethylene oxide sterilization are not suitable for most drug delivery systems as the properties of DDSs and its loaded drugs would easy to be destroy.227 However, there still are some formulations had been proved that their properties maintained their properties after autoclave.228
A multifunctional drug-delivery system with excellent biosafety and drug-targeting properties is a promising direction of future IA drug delivery system development. Combined different strategies to prepare drug delivery systems for IA injection treatment are increasingly attractive. However, the more complex strategy of the drug delivery system, the more it is required to address synergies effects between different strategies.
Conclusions
Different IA drug delivery systems are developing rapidly with some significant progress. The ideal performance of an IA DDS depends on the nature of the drug delivery platform. Desirable properties of an IA DDS for OA included excellent continuous and controlled drug delivery, lubrication performance, and disease-targeting that could significantly improve the treatment of OA. At last, the most important issue is the benefits of new IA treatments must be carefully weighed against their cost and potential risks.
Funding Statement
This research was funded by grants from the National Natural Science Foundation of China (No. 81703584); The regional joint fund of natural science foundation of Guangdong province (No. 2020B1515120052); Guangdong Province Natural Science Foundation of China (No. 2017A030310614, 2018A030313390, 2016ZC0178, 2021A1515010975); Discipline construction project of Guangdong Medical University (No. 4SG22002G, 4SG21156G); Special Funds for Scientific Technological Innovation of Undergraduates in Guangdong Province (No. pdjh2022a0214); Affiliated Hospital of Guangdong Medical University “Clinical Medicine+” CnTech Co-operation Project (No. CLP2021B012); Guangdong Medical University scientific research fund (No. B2017001); the Fund of Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (No. ZJW-2019-007).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this review.
References
- 1.Boer CG, Hatzikotoulas K, Southam L, et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell. 2021;184(18):4784–4818 e4717. doi: 10.1016/j.cell.2021.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dantas LO, Salvini TF, McAlindon TE. Knee osteoarthritis: key treatments and implications for physical therapy. Braz J Phys Ther. 2021;25(2):135–146. doi: 10.1016/j.bjpt.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DALYs GBD, Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–138. doi: 10.1016/j.rehab.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 7.Jotanovic Z, Mihelic R, Sestan B, Dembic Z. Emerging pathways and promising agents with possible disease modifying effect in osteoarthritis treatment. Curr Drug Targets. 2014;15(6):635–661. doi: 10.2174/1389450115666140306153115 [DOI] [PubMed] [Google Scholar]
- 8.Prieto-Alhambra D, Arden N, Hunter DJ. Osteoarthritis: The Facts. OUP Oxford; 2014. [Google Scholar]
- 9.Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016;59(5–6):333–339. doi: 10.1016/j.rehab.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 10.Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2(1):16072. doi: 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- 11.Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293–311. doi: 10.1016/j.mcna.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 12.Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384(1):51–59. doi: 10.1056/NEJMcp1903768 [DOI] [PubMed] [Google Scholar]
- 13.Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632–644. doi: 10.1038/nrrheum.2016.148 [DOI] [PubMed] [Google Scholar]
- 14.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 15.Zhen G, Cao X. Targeting TGFbeta signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol Sci. 2014;35(5):227–236. doi: 10.1016/j.tips.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Primorac D, Molnar V, Rod E, et al. Knee osteoarthritis: a review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes. 2020;11(8):854. doi: 10.3390/genes11080854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsia AW, Emami AJ, Tarke FD, et al. Osteophytes and fracture calluses share developmental milestones and are diminished by unloading. J Orthop Res. 2018;36(2):699–710. doi: 10.1002/jor.23779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paduszynski W, Jeskiewicz M, Uchanski P, Gackowski S, Radkowski M, Demkow U. Hoffa’s fat pad abnormality in the development of knee osteoarthritis. Adv Exp Med Biol. 2018;1039:95–102. [DOI] [PubMed] [Google Scholar]
- 19.Zeng N, Yan ZP, Chen XY, Ni GX. Infrapatellar fat pad and knee osteoarthritis. Aging Dis. 2020;11(5):1317–1328. doi: 10.14336/AD.2019.1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belluzzi E, Macchi V, Fontanella CG, et al. Infrapatellar fat pad gene expression and protein production in patients with and without osteoarthritis. Int J Mol Sci. 2020;21(17):6016. doi: 10.3390/ijms21176016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belluzzi E, Stocco E, Pozzuoli A, et al. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. Biomed Res Int. 2019;2019:6390182. doi: 10.1155/2019/6390182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macchi V, Stocco E, Stecco C, et al. The infrapatellar fat pad and the synovial membrane: an anatomo-functional unit. J Anat. 2018;233(2):146–154. doi: 10.1111/joa.12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belluzzi E, Olivotto E, Toso G, et al. Conditioned media from human osteoarthritic synovium induces inflammation in a synoviocyte cell line. Connect Tissue Res. 2019;60(2):136–145. doi: 10.1080/03008207.2018.1470167 [DOI] [PubMed] [Google Scholar]
- 24.Roemer FW, Guermazi A, Hunter DJ, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis Cartilage. 2009;17(6):748–753. doi: 10.1016/j.joca.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu CL, Harasymowicz NS, Klimak MA, Collins KH, Guilak F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthritis Cartilage. 2020;28(5):544–554. doi: 10.1016/j.joca.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol. 2012;8(7):412–419. doi: 10.1038/nrrheum.2012.69 [DOI] [PubMed] [Google Scholar]
- 27.Alnahdi AH, Zeni JA, Snyder-Mackler L. Muscle impairments in patients with knee osteoarthritis. Sports Health. 2012;4(4):284–292. doi: 10.1177/1941738112445726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JR, Yoo JJ, Kim HA. Therapeutics in osteoarthritis based on an understanding of its molecular pathogenesis. Int J Mol Sci. 2018;19(3). doi: 10.3390/ijms19030674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos EM, Herzog W, Block JA, Bennell KL. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol. 2011;7(1):57–63. doi: 10.1038/nrrheum.2010.195 [DOI] [PubMed] [Google Scholar]
- 30.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. doi: 10.1186/s13075-017-1229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014;43(6):701–712. doi: 10.1016/j.semarthrit.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 32.Nguyen C, Rannou F. The safety of intra-articular injections for the treatment of knee osteoarthritis: a critical narrative review. Expert Opin Drug Saf. 2017;16(8):897–902. doi: 10.1080/14740338.2017.1344211 [DOI] [PubMed] [Google Scholar]
- 33.Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):577–579. doi: 10.5435/JAAOS-21-09-577 [DOI] [PubMed] [Google Scholar]
- 34.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220–233. doi: 10.1002/art.41142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 36.Altman R, Hackel J, Niazi F, Shaw P, Nicholls M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: a systematic review. Semin Arthritis Rheum. 2018;48(2):168–175. doi: 10.1016/j.semarthrit.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 37.Stevens RM, Ervin J, Nezzer J, et al. Randomized, double-blind, placebo-controlled trial of intraarticular trans-capsaicin for pain associated with osteoarthritis of the knee. Arthritis Rheumatol. 2019;71(9):1524–1533. doi: 10.1002/art.40894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58(2):226–242. doi: 10.1016/j.addr.2006.01.018 [DOI] [PubMed] [Google Scholar]
- 39.Owen SG, Francis HW, Roberts MS. Disappearance kinetics of solutes from synovial fluid after intra-articular injection. Br J Clin Pharmacol. 1994;38(4):349–355. doi: 10.1111/j.1365-2125.1994.tb04365.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schumacher HR Jr. Aspiration and injection therapies for joints. Arthritis Rheum. 2003;49(3):413–420. doi: 10.1002/art.11056 [DOI] [PubMed] [Google Scholar]
- 41.Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61(3):344–352. doi: 10.1002/art.24096 [DOI] [PubMed] [Google Scholar]
- 42.Vugmeyster Y, Wang Q, Xu X, et al. Disposition of human recombinant lubricin in naive rats and in a rat model of post-traumatic arthritis after intra-articular or intravenous administration. AAPS J. 2012;14(1):97–104. doi: 10.1208/s12248-011-9315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014;10(1):11–22. doi: 10.1038/nrrheum.2013.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacchetti C, Liu-Bryan R, Magrini A, Rosato N, Bottini N, Bottini M. Polyethylene-glycol-modified single-walled carbon nanotubes for intra-articular delivery to chondrocytes. ACS Nano. 2014;8(12):12280–12291. doi: 10.1021/nn504537b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitmire RE, Wilson DS, Singh A, Levenston ME, Murthy N, Garcia AJ. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials. 2012;33(30):7665–7675. doi: 10.1016/j.biomaterials.2012.06.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh A, Agarwal R, Diaz-Ruiz CA, et al. Nanoengineered particles for enhanced intra-articular retention and delivery of proteins. Adv Healthc Mater. 2014;3(10):1562–1567, 1525. doi: 10.1002/adhm.201400051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang ML, Ko JY, Kim JE, Im GI. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 2014;35(37):9984–9994. doi: 10.1016/j.biomaterials.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 48.Kim SR, Ho MJ, Lee E, Lee JW, Choi YW, Kang MJ. Cationic PLGA/Eudragit RL nanoparticles for increasing retention time in synovial cavity after intra-articular injection in knee joint. Int J Nanomedicine. 2015;10:5263–5271. doi: 10.2147/IJN.S88363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geiger BC, Wang S, Padera RF Jr., Grodzinsky AJ, Hammond PT. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci Transl Med. 2018;10(469):469. doi: 10.1126/scitranslmed.aat8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12). doi: 10.1038/natrevmats.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahimi M, Charmi G, Matyjaszewski K, Banquy X, Pietrasik J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021;123:31–50. doi: 10.1016/j.actbio.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 52.Barbucci R, Lamponi S, Borzacchiello A, et al. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials. 2002;23(23):4503–4513. doi: 10.1016/S0142-9612(02)00194-1 [DOI] [PubMed] [Google Scholar]
- 53.Murphy MP, Koepke LS, Lopez MT, et al. Articular cartilage regeneration by activated skeletal stem cells. Nat Med. 2020;26(10):1583–1592. doi: 10.1038/s41591-020-1013-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radhakrishnan J, Manigandan A, Chinnaswamy P, Subramanian A, Sethuraman S. Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials. 2018;162:82–98. doi: 10.1016/j.biomaterials.2018.01.056 [DOI] [PubMed] [Google Scholar]
- 55.Lolli A, Sivasubramaniyan K, Vainieri ML, et al. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J Control Release. 2019;309:220–230. doi: 10.1016/j.jconrel.2019.07.040 [DOI] [PubMed] [Google Scholar]
- 56.Garcia JP, Stein J, Cai Y, et al. Fibrin-hyaluronic acid hydrogel-based delivery of antisense oligonucleotides for ADAMTS5 inhibition in co-delivered and resident joint cells in osteoarthritis. J Control Release. 2019;294:247–258. doi: 10.1016/j.jconrel.2018.12.030 [DOI] [PubMed] [Google Scholar]
- 57.Cokelaere SM, Plomp SGM, de Boef E, et al. Sustained intra-articular release of celecoxib in an equine repeated LPS synovitis model. Eur J Pharm Biopharm. 2018;128:327–336. doi: 10.1016/j.ejpb.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 58.Fattahpour S, Shamanian M, Tavakoli N, et al. An injectable carboxymethyl chitosan-methylcellulose-pluronic hydrogel for the encapsulation of meloxicam loaded nanoparticles. Int J Biol Macromol. 2020;151:220–229. doi: 10.1016/j.ijbiomac.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 59.Wang QS, Xu BX, Fan KJ, Fan YS, Teng H, Wang TY. Dexamethasone-loaded thermo-sensitive hydrogel attenuates osteoarthritis by protecting cartilage and providing effective pain relief. Ann Transl Med. 2021;9(14):1120. doi: 10.21037/atm-21-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen K, Li S, Yuan F, Sun P, Zhang Y. GEL-MAN hydrogel loaded with triamcinolone acetonide for the treatment of osteoarthritis. Front Bioeng Biotechnol. 2020;8:872. doi: 10.3389/fbioe.2020.00872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao W, Li M, Yang Y, et al. Advancements and frontiers in the high performance of natural hydrogels for cartilage tissue engineering. Front Chem. 2020;8:53. doi: 10.3389/fchem.2020.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pipino G, Risitano S, Alviano F, et al. Microfractures and hydrogel scaffolds in the treatment of osteochondral knee defects: a clinical and histological evaluation. J Clin Orthop Trauma. 2019;10(1):67–75. doi: 10.1016/j.jcot.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adali T, Kalkan R, Karimizarandi L. The chondrocyte cell proliferation of a chitosan/silk fibroin/egg shell membrane hydrogels. Int J Biol Macromol. 2019;124:541–547. doi: 10.1016/j.ijbiomac.2018.11.226 [DOI] [PubMed] [Google Scholar]
- 64.Cavalli E, Levinson C, Hertl M, et al. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci Rep. 2019;9(1):4275. doi: 10.1038/s41598-019-40575-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu J, Shen X, Sun X, et al. Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranostics. 2018;8(18):5039–5058. doi: 10.7150/thno.26981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moon HJ, Ko du Y, Park MH, Joo MK, Jeong B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem Soc Rev. 2012;41(14):4860–4883. doi: 10.1039/c2cs35078e [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Yu J, Ren K, Zuo J, Ding J, Chen X. Thermosensitive hydrogels as scaffolds for cartilage tissue engineering. Biomacromolecules. 2019;20(4):1478–1492. doi: 10.1021/acs.biomac.9b00043 [DOI] [PubMed] [Google Scholar]
- 68.Sattari S, Dadkhah Tehrani A, Adeli M. pH-Responsive hybrid hydrogels as antibacterial and drug delivery systems. Polymers. 2018;10(6):660. doi: 10.3390/polym10060660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang Q, Chen C, Jiang Y, et al. Engineering an adhesive based on photosensitive polymer hydrogels and silver nanoparticles for wound healing. J Mater Chem B. 2020;8(26):5756–5764. doi: 10.1039/D0TB00726A [DOI] [PubMed] [Google Scholar]
- 70.Liu Z, Liu J, Cui X, Wang X, Zhang L, Tang P. Recent advances on magnetic sensitive hydrogels in tissue engineering. Front Chem. 2020;8:124. doi: 10.3389/fchem.2020.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavanya K, Chandran SV, Balagangadharan K, Selvamurugan N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2020;111:110862. doi: 10.1016/j.msec.2020.110862 [DOI] [PubMed] [Google Scholar]
- 72.Cheng YH, Chavez E, Tsai KL, et al. Effects of thermosensitive chitosan-gelatin based hydrogel containing glutathione on Cisd2-deficient chondrocytes under oxidative stress. Carbohydr Polym. 2017;173:17–27. doi: 10.1016/j.carbpol.2017.05.069 [DOI] [PubMed] [Google Scholar]
- 73.Lee EJ, Kang E, Kang SW, Huh KM. Thermo-irreversible glycol chitosan/hyaluronic acid blend hydrogel for injectable tissue engineering. Carbohydr Polym. 2020;244:116432. doi: 10.1016/j.carbpol.2020.116432 [DOI] [PubMed] [Google Scholar]
- 74.Petit A, Sandker M, Muller B, et al. Release behavior and intra-articular biocompatibility of celecoxib-loaded acetyl-capped PCLA-PEG-PCLA thermogels. Biomaterials. 2014;35(27):7919–7928. doi: 10.1016/j.biomaterials.2014.05.064 [DOI] [PubMed] [Google Scholar]
- 75.Mok SW, Fu SC, Cheuk YC, et al. Intra-articular delivery of quercetin using thermosensitive hydrogel attenuate cartilage degradation in an osteoarthritis rat model. Cartilage. 2020;11(4):490–499. doi: 10.1177/1947603518796550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu H, Cheng Y, Chen J, et al. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018;73:103–111. doi: 10.1016/j.actbio.2018.04.035 [DOI] [PubMed] [Google Scholar]
- 77.Tan J, Xie S, Wang G, et al. Fabrication and optimization of the thermo-sensitive hydrogel carboxymethyl cellulose/Poly(N-isopropylacrylamide-co-acrylic acid) for U(VI) removal from aqueous solution. Polymers. 2020;12(1):151. doi: 10.3390/polym12010151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Yun S, Du Y, Zannettino ACW, Zhang H. Fabrication of a cartilage patch by fusing hydrogel-derived cell aggregates onto electrospun film. Tissue Eng Part A. 2020;26(15–16):863–871. doi: 10.1089/ten.tea.2019.0318 [DOI] [PubMed] [Google Scholar]
- 79.Agas D, Laus F, Lacava G, et al. Thermosensitive hybrid hyaluronan/p(HPMAm-lac)-PEG hydrogels enhance cartilage regeneration in a mouse model of osteoarthritis. J Cell Physiol. 2019;234(11):20013–20027. doi: 10.1002/jcp.28598 [DOI] [PubMed] [Google Scholar]
- 80.Zhang T, Chen S, Dou H, et al. Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Mater Sci Eng C Mater Biol Appl. 2021;118:111352. doi: 10.1016/j.msec.2020.111352 [DOI] [PubMed] [Google Scholar]
- 81.Tang Q, Lim T, Shen LY, et al. Well-dispersed platelet lysate entrapped nanoparticles incorporate with injectable PDLLA-PEG-PDLLA triblock for preferable cartilage engineering application. Biomaterials. 2021;268:120605. doi: 10.1016/j.biomaterials.2020.120605 [DOI] [PubMed] [Google Scholar]
- 82.Tao SC, Huang JY, Gao Y, et al. Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact Mater. 2021;6(12):4455–4469. doi: 10.1016/j.bioactmat.2021.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen CH, Chen SH, Mao SH, et al. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr Polym. 2017;173:721–731. doi: 10.1016/j.carbpol.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 84.Seo BB, Kwon Y, Kim J, et al. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact Mater. 2022;7:14–25. doi: 10.1016/j.bioactmat.2021.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang L, Liu Y, Shou X, Ni D, Kong T, Zhao Y. Bio-inspired lubricant drug delivery particles for the treatment of osteoarthritis. Nanoscale. 2020;12(32):17093–17102. doi: 10.1039/D0NR04013D [DOI] [PubMed] [Google Scholar]
- 86.Gu W, Wu C, Chen J, Xiao Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int J Nanomedicine. 2013;8:2305–2317. doi: 10.2147/IJN.S44393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li X, Dai B, Guo J, et al. Nanoparticle-cartilage interaction: pathology-based intra-articular drug delivery for osteoarthritis therapy. Nanomicro Lett. 2021;13(1):149. doi: 10.3847/1538-4357/abeb18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maudens P, Jordan O, Allemann E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov Today. 2018;23(10):1761–1775. doi: 10.1016/j.drudis.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 89.Pullan JE, Confeld MI, Osborn JK, Kim J, Sarkar K, Mallik S. Exosomes as drug carriers for cancer therapy. Mol Pharm. 2019;16(5):1789–1798. doi: 10.1021/acs.molpharmaceut.9b00104 [DOI] [PubMed] [Google Scholar]
- 90.Huyan T, Li H, Peng H, et al. Extracellular vesicles - advanced nanocarriers in cancer therapy: progress and achievements. Int J Nanomedicine. 2020;15:6485–6502. doi: 10.2147/IJN.S238099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pourakbari R, Khodadadi M, Aghebati-Maleki A, Aghebati-Maleki L, Yousefi M. The potential of exosomes in the therapy of the cartilage and bone complications; emphasis on osteoarthritis. Life Sci. 2019;236:116861. doi: 10.1016/j.lfs.2019.116861 [DOI] [PubMed] [Google Scholar]
- 92.Ni Z, Zhou S, Li S, et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi: 10.1038/s41413-020-0100-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037 [DOI] [PubMed] [Google Scholar]
- 95.Fonseca-Santos B, Gremiao MP, Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomedicine. 2015;10:4981–5003. doi: 10.2147/IJN.S87148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patil YP, Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids. 2014;177:8–18. doi: 10.1016/j.chemphyslip.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 98.Cipollaro L, Trucillo P, Bragazzi NL, Della PG, Reverchon E, Maffulli N. Liposomes for intra-articular analgesic drug delivery in orthopedics: state-of-art and future perspectives. insights from a systematic mini-review of the literature. Medicina. 2020;56(9). doi: 10.3390/medicina56090423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen YC, Gad SF, Chobisa D, Li Y, Yeo Y. Local drug delivery systems for inflammatory diseases: status quo, challenges, and opportunities. J Control Release. 2021;330:438–460. doi: 10.1016/j.jconrel.2020.12.025 [DOI] [PubMed] [Google Scholar]
- 100.Lin W, Goldberg R, Klein J. Poly-phosphocholination of liposomes leads to highly-extended retention time in mice joints. J Mater Chem B. 2022;10(15):2820–2827. doi: 10.1039/D1TB02346B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen CH, Kuo SM, Tien YC, Shen PC, Kuo YW, Huang HH. Steady augmentation of anti-osteoarthritic actions of rapamycin by liposome-encapsulation in collaboration with low-intensity pulsed ultrasound. Int J Nanomedicine. 2020;15:3771–3790. doi: 10.2147/IJN.S252223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sarkar A, Carvalho E, D’Souza AA, Banerjee R. Liposome-encapsulated fish oil protein-tagged gold nanoparticles for intra-articular therapy in osteoarthritis. Nanomedicine. 2019;14(7):871–887. doi: 10.2217/nnm-2018-0221 [DOI] [PubMed] [Google Scholar]
- 103.Chang MC, Chiang PF, Kuo YJ, Peng CL, Chen KY, Chiang YC. Hyaluronan-loaded liposomal dexamethasone-diclofenac nanoparticles for local osteoarthritis treatment. Int J Mol Sci. 2021;22(2):665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ji X, Yan Y, Sun T, et al. Glucosamine sulphate-loaded distearoyl phosphocholine liposomes for osteoarthritis treatment: combination of sustained drug release and improved lubrication. Biomater Sci. 2019;7(7):2716–2728. doi: 10.1039/C9BM00201D [DOI] [PubMed] [Google Scholar]
- 105.Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22(1):156–165. doi: 10.1177/2156587216636747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yeh CC, Su YH, Lin YJ, et al. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug Des Devel Ther. 2015;9:2285–2300. doi: 10.2147/DDDT.S78277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He K, Huang X, Shan R, et al. Intra-articular injection of lornoxicam and MicroRNA-140 co-loaded cationic liposomes enhanced the therapeutic treatment of experimental osteoarthritis. AAPS PharmSciTech. 2021;23(1):9. doi: 10.1208/s12249-021-02149-w [DOI] [PubMed] [Google Scholar]
- 108.Corciulo C, Castro CM, Coughlin T, et al. Intraarticular injection of liposomal adenosine reduces cartilage damage in established murine and rat models of osteoarthritis. Sci Rep. 2020;10(1):13477. doi: 10.1038/s41598-020-68302-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Friedman B, Corciulo C, Castro CM, Cronstein BN. Adenosine A2A receptor signaling promotes FoxO associated autophagy in chondrocytes. Sci Rep. 2021;11(1):968. doi: 10.1038/s41598-020-80244-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma T, Cheng Y, Tan L. Mechanism of miR-15a regulating the growth and apoptosis of human knee joint chondrocytes by targeting SMAD2. Artif Cells Nanomed Biotechnol. 2019;47(1):3188–3193. doi: 10.1080/21691401.2019.1613420 [DOI] [PubMed] [Google Scholar]
- 111.Tian J, Rui YJ, Xu YJ, Zhang SA. MiR-143-3p regulates early cartilage differentiation of BMSCs and promotes cartilage damage repair through targeting BMPR2. Eur Rev Med Pharmacol Sci. 2018;22(24):8814–8821. doi: 10.26355/eurrev_201812_16649 [DOI] [PubMed] [Google Scholar]
- 112.Rahman M, Beg S, Anwar F, et al. Liposome-based nanomedicine therapeutics for rheumatoid arthritis. Crit Rev Ther Drug Carrier Syst. 2017;34(4):283–316. doi: 10.1615/CritRevTherDrugCarrierSyst.2017016067 [DOI] [PubMed] [Google Scholar]
- 113.Vanniasinghe AS, Manolios N, Schibeci S, et al. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clin Immunol. 2014;151(1):43–54. doi: 10.1016/j.clim.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 114.Meka RR, Venkatesha SH, Acharya B, Moudgil KD. Peptide-targeted liposomal delivery of dexamethasone for arthritis therapy. Nanomedicine. 2019;14(11):1455–1469. doi: 10.2217/nnm-2018-0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ren H, He Y, Liang J, et al. Role of liposome size, surface charge, and PEGylation on rheumatoid arthritis targeting therapy. ACS Appl Mater Interfaces. 2019;11(22):20304–20315. doi: 10.1021/acsami.8b22693 [DOI] [PubMed] [Google Scholar]
- 116.Liu Y, Jia Z, Akhter MP, et al. Bone-targeting liposome formulation of Salvianic acid A accelerates the healing of delayed fracture Union in Mice. Nanomedicine. 2018;14(7):2271–2282. doi: 10.1016/j.nano.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 117.Cho H, Pinkhassik E, David V, Stuart JM, Hasty KA. Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model. Nanomedicine. 2015;11(4):939–946. doi: 10.1016/j.nano.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 118.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–131. doi: 10.1016/S0169-409X(00)00124-1 [DOI] [PubMed] [Google Scholar]
- 119.Jones M, Leroux J. Polymeric micelles - A new generation of colloidal drug carriers. Eur J Pharm Biopharm. 1999;48(2):101–111. doi: 10.1016/S0939-6411(99)00039-9 [DOI] [PubMed] [Google Scholar]
- 120.Wei Y, Yan L, Luo L, et al. Phospholipase A2 inhibitor-loaded micellar nanoparticles attenuate inflammation and mitigate osteoarthritis progression. Sci Adv. 2021;7:15. doi: 10.1126/sciadv.abe6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Q, Jiang H, Li Y, et al. Targeting NF-kB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy. Biomaterials. 2017;122:10–22. doi: 10.1016/j.biomaterials.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 122.An L, Li Z, Shi L, et al. Inflammation-targeted celastrol nanodrug attenuates collagen-induced arthritis through NF-kappaB and notch1 pathways. Nano Lett. 2020;20(10):7728–7736. doi: 10.1021/acs.nanolett.0c03279 [DOI] [PubMed] [Google Scholar]
- 123.Wu X, Li P, Cheng J, et al. ROS-sensitive nanoparticles co-delivering dexamethasone and CDMP-1 for the treatment of osteoarthritis through chondrogenic differentiation induction and inflammation inhibition. Front Bioeng Biotechnol. 2021;9:608150. doi: 10.3389/fbioe.2021.608150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang N, Xu C, Li N, et al. Folate receptor-targeted mixed polysialic acid micelles for combating rheumatoid arthritis: in vitro and in vivo evaluation. Drug Deliv. 2018;25(1):1182–1191. doi: 10.1080/10717544.2018.1472677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li J, Long Y, Guo R, et al. Shield and sword nano-soldiers ameliorate rheumatoid arthritis by multi-stage manipulation of neutrophils. J Control Release. 2021;335:38–48. doi: 10.1016/j.jconrel.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 126.Seetharaman G, Kallar AR, Vijayan VM, Muthu J, Selvam S. Design, preparation and characterization of pH-responsive prodrug micelles with hydrolyzable anhydride linkages for controlled drug delivery. J Colloid Interface Sci. 2017;492:61–72. doi: 10.1016/j.jcis.2016.12.070 [DOI] [PubMed] [Google Scholar]
- 127.Kang C, Jung E, Hyeon H, Seon S, Lee D. Acid-activatable polymeric curcumin nanoparticles as therapeutic agents for osteoarthritis. Nanomedicine. 2020;23:102104. doi: 10.1016/j.nano.2019.102104 [DOI] [PubMed] [Google Scholar]
- 128.Perni S, Prokopovich P. Poly-beta-amino-esters nano-vehicles based drug delivery system for cartilage. Nanomedicine. 2017;13(2):539–548. doi: 10.1016/j.nano.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koo H, Lee H, Lee S, et al. In vivo tumor diagnosis and photodynamic therapy via tumoral pH-responsive polymeric micelles. Chem Commun (Camb). 2010;46(31):5668–5670. doi: 10.1039/c0cc01413c [DOI] [PubMed] [Google Scholar]
- 130.Lan Q, Lu R, Chen H, et al. MMP-13 enzyme and pH responsive theranostic nanoplatform for osteoarthritis. J Nanobiotechnology. 2020;18(1):117. doi: 10.1186/s12951-020-00666-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Biswas S, Kumari P, Lakhani PM, Ghosh B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci. 2016;83:184–202. doi: 10.1016/j.ejps.2015.12.031 [DOI] [PubMed] [Google Scholar]
- 132.Li J, Liang H, Liu J, Wang Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int J Pharm. 2018;546(1–2):215–225. doi: 10.1016/j.ijpharm.2018.05.045 [DOI] [PubMed] [Google Scholar]
- 133.Abedi-Gaballu F, Dehghan G, Ghaffari M, et al. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today. 2018;12:177–190. doi: 10.1016/j.apmt.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kavanaugh TE, Werfel TA, Cho H, Hasty KA, Duvall CL. Particle-based technologies for osteoarthritis detection and therapy. Drug Deliv Transl Res. 2016;6(2):132–147. doi: 10.1007/s13346-015-0234-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dias AP, da Silva Santos S, da Silva JV, et al. Dendrimers in the context of nanomedicine. Int J Pharm. 2020;573:118814. doi: 10.1016/j.ijpharm.2019.118814 [DOI] [PubMed] [Google Scholar]
- 136.Yellepeddi VK, Ghandehari H. Pharmacokinetics of oral therapeutics delivered by dendrimer-based carriers. Expert Opin Drug Deliv. 2019;16(10):1051–1061. doi: 10.1080/17425247.2019.1656607 [DOI] [PubMed] [Google Scholar]
- 137.Lee CC, MacKay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23(12):1517–1526. doi: 10.1038/nbt1171 [DOI] [PubMed] [Google Scholar]
- 138.Hu Q, Ding B, Yan X, et al. Polyethylene glycol modified PAMAM dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomedicine. 2017;13(7):2189–2198. doi: 10.1016/j.nano.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 139.Hu Q, Chen Q, Yan X, Ding B, Chen D, Cheng L. Chondrocyte affinity peptide modified PAMAM conjugate as a nanoplatform for targeting and retention in cartilage. Nanomedicine. 2018;13(7):749–767. doi: 10.2217/nnm-2017-0335 [DOI] [PubMed] [Google Scholar]
- 140.Oliveira IM, Goncalves C, Oliveira EP, et al. PAMAM dendrimers functionalised with an anti-TNF alpha antibody and chondroitin sulphate for treatment of rheumatoid arthritis. Mater Sci Eng C Mater Biol Appl. 2021;121:111845. doi: 10.1016/j.msec.2020.111845 [DOI] [PubMed] [Google Scholar]
- 141.Hayder M, Poupot M, Baron M, et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci Transl Med. 2011;3(81):81ra35. doi: 10.1126/scitranslmed.3002212 [DOI] [PubMed] [Google Scholar]
- 142.Vergallo C, Hafeez MN, Iannotta D, et al. Conventional nanosized drug delivery systems for cancer applications. Adv Exp Med Biol. 2021;1295:3–27. [DOI] [PubMed] [Google Scholar]
- 143.Ortega MA, Guzman Merino A, Fraile-Martinez O, et al. Dendrimers and dendritic materials: from laboratory to medical practice in infectious diseases. Pharmaceutics. 2020;12(9):874. doi: 10.3390/pharmaceutics12090874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Singh P. Dendrimers and their applications in immunoassays and clinical diagnostics. Biotechnol Appl Biochem. 2007;48(Pt 1):1–9. doi: 10.1007/s12010-007-0004-9 [DOI] [PubMed] [Google Scholar]
- 145.Rupp R, Rosenthal SL, Stanberry LR. VivaGel (SPL7013 Gel): a candidate dendrimer–microbicide for the prevention of HIV and HSV infection. Int J Nanomedicine. 2007;2(4):561–566. [PMC free article] [PubMed] [Google Scholar]
- 146.Patri AK, Kukowska-Latallo JF, Baker JR Jr. Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev. 2005;57(15):2203–2214. doi: 10.1016/j.addr.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 147.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70(1–2):1–20. doi: 10.1016/S0168-3659(00)00339-4 [DOI] [PubMed] [Google Scholar]
- 148.Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48(3):416–427. doi: 10.1016/j.ejps.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alarcin E, Demirbag C, Karsli-Ceppioglu S, Kerimoglu O, Bal-Ozturk A. Development and characterization of oxaceprol-loaded poly-lactide-co-glycolide nanoparticles for the treatment of osteoarthritis. Drug Dev Res. 2020;81(4):501–510. doi: 10.1002/ddr.21642 [DOI] [PubMed] [Google Scholar]
- 150.Jung JH, Kim SE, Kim HJ, Park K, Song GG, Choi SJ. A comparative pilot study of oral diacerein and locally treated diacerein-loaded nanoparticles in a model of osteoarthritis. Int J Pharm. 2020;581:119249. doi: 10.1016/j.ijpharm.2020.119249 [DOI] [PubMed] [Google Scholar]
- 151.Maudens P, Seemayer CA, Thauvin C, Gabay C, Jordan O, Allemann E. Nanocrystal-polymer particles: extended delivery carriers for osteoarthritis treatment. Small. 2018;14(8). doi: 10.1002/smll.201703108 [DOI] [PubMed] [Google Scholar]
- 152.Fan W, Li J, Yuan L, et al. Intra-articular injection of kartogenin-conjugated polyurethane nanoparticles attenuates the progression of osteoarthritis. Drug Deliv. 2018;25(1):1004–1012. doi: 10.1080/10717544.2018.1461279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salama AH, Abdelkhalek AA, Elkasabgy NA. Etoricoxib-loaded bio-adhesive hybridized polylactic acid-based nanoparticles as an intra-articular injection for the treatment of osteoarthritis. Int J Pharm. 2020;578:119081. doi: 10.1016/j.ijpharm.2020.119081 [DOI] [PubMed] [Google Scholar]
- 154.Crivelli B, Bari E, Perteghella S, et al. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur J Pharm Biopharm. 2019;137:37–45. doi: 10.1016/j.ejpb.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 155.Wan L, Tan X, Sun T, Sun Y, Luo J, Zhang H. Lubrication and drug release behaviors of mesoporous silica nanoparticles grafted with sulfobetaine-based zwitterionic polymer. Mater Sci Eng C Mater Biol Appl. 2020;112:110886. doi: 10.1016/j.msec.2020.110886 [DOI] [PubMed] [Google Scholar]
- 156.Song Y, Zhang T, Cheng H, et al. Imidazolium-based ionic liquid-assisted preparation of nano-spheres loaded with bio-active peptides to decrease inflammation in an osteoarthritis model: ex vivo evaluations. J Biomed Nanotechnol. 2021;17(5):859–872. doi: 10.1166/jbn.2021.3069 [DOI] [PubMed] [Google Scholar]
- 157.Zhang K, Yang J, Sun Y, et al. Thermo-sensitive dual-functional nanospheres with enhanced lubrication and drug delivery for the treatment of osteoarthritis. Chemistry. 2020;26(46):10564–10574. doi: 10.1002/chem.202001372 [DOI] [PubMed] [Google Scholar]
- 158.Maudens P, Meyer S, Seemayer CA, Jordan O, Allemann E. Self-assembled thermoresponsive nanostructures of hyaluronic acid conjugates for osteoarthritis therapy. Nanoscale. 2018;10(4):1845–1854. doi: 10.1039/C7NR07614B [DOI] [PubMed] [Google Scholar]
- 159.Kang ML, Kim JE, Im GI. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 2016;39:65–78. doi: 10.1016/j.actbio.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 160.Hu B, Gao F, Li C, et al. Rhein laden pH-responsive polymeric nanoparticles for treatment of osteoarthritis. AMB Express. 2020;10(1):158. doi: 10.1186/s13568-020-01095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zerrillo L, Que I, Vepris O, et al. pH-responsive poly(lactide-co-glycolide) nanoparticles containing near-infrared dye for visualization and hyaluronic acid for treatment of osteoarthritis. J Control Release. 2019;309:265–276. doi: 10.1016/j.jconrel.2019.07.031 [DOI] [PubMed] [Google Scholar]
- 162.Faust HJ, Sommerfeld SD, Rathod S, et al. A hyaluronic acid binding peptide-polymer system for treating osteoarthritis. Biomaterials. 2018;183:93–101. doi: 10.1016/j.biomaterials.2018.08.045 [DOI] [PubMed] [Google Scholar]
- 163.Xiong W, Lan Q, Liang X, et al. Cartilage-targeting poly(ethylene glycol) (PEG)-formononetin (FMN) nanodrug for the treatment of osteoarthritis. J Nanobiotechnology. 2021;19(1):197. doi: 10.1186/s12951-021-00945-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ai X, Duan Y, Zhang Q, et al. Cartilage-targeting ultrasmall lipid-polymer hybrid nanoparticles for the prevention of cartilage degradation. Bioeng Transl Med. 2021;6(1):e10187. doi: 10.1002/btm2.10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ren K, Ke X, Chen Z, et al. Zwitterionic polymer modified xanthan gum with collagen II-binding capability for lubrication improvement and ROS scavenging. Carbohydr Polym. 2021;274:118672. doi: 10.1016/j.carbpol.2021.118672 [DOI] [PubMed] [Google Scholar]
- 166.Jiang T, Kan HM, Rajpura K, Carbone EJ, Li Y, Lo KW. Development of targeted nanoscale drug delivery system for osteoarthritic cartilage tissue. J Nanosci Nanotechnol. 2018;18(4):2310–2317. doi: 10.1166/jnn.2018.14311 [DOI] [PubMed] [Google Scholar]
- 167.Brown S, Pistiner J, Adjei IM, Sharma B. Nanoparticle properties for delivery to cartilage: the implications of disease state, synovial fluid, and off-target uptake. Mol Pharm. 2019;16(2):469–479. doi: 10.1021/acs.molpharmaceut.7b00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.She P, Bian S, Cheng Y, et al. Dextran sulfate-triamcinolone acetonide conjugate nanoparticles for targeted treatment of osteoarthritis. Int J Biol Macromol. 2020;158:1082–1089. doi: 10.1016/j.ijbiomac.2020.05.013 [DOI] [PubMed] [Google Scholar]
- 169.Mancipe CLM, Sequeira A, Garcia AJ, Guldberg RE. Articular cartilage- and synoviocyte-binding Poly(ethylene glycol) nanocomposite microgels as intra-articular drug delivery vehicles for the treatment of osteoarthritis. ACS Biomater Sci Eng. 2020;6(9):5084–5095. doi: 10.1021/acsbiomaterials.0c00960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gao X, Ma Y, Zhang G, et al. Targeted elimination of intracellular reactive oxygen species using nanoparticle-like chitosan- superoxide dismutase conjugate for treatment of monoiodoacetate-induced osteoarthritis. Int J Pharm. 2020;590:119947. doi: 10.1016/j.ijpharm.2020.119947 [DOI] [PubMed] [Google Scholar]
- 171.Li X, Wang X, Liu Q, et al. ROS-responsive boronate-stabilized polyphenol-poloxamer 188 assembled dexamethasone nanodrug for macrophage repolarization in osteoarthritis treatment. Adv Healthc Mater. 2021;10(20):e2100883. doi: 10.1002/adhm.202100883 [DOI] [PubMed] [Google Scholar]
- 172.Ren M, Li Y, Zhang H, et al. An oligopeptide/aptamer-conjugated dendrimer-based nanocarrier for dual-targeting delivery to bone. J Mater Chem B. 2021;9(12):2831–2844. doi: 10.1039/D0TB02926B [DOI] [PubMed] [Google Scholar]
- 173.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;3(2):22. doi: 10.1002/0471143030.cb0322s30 [DOI] [PubMed] [Google Scholar]
- 174.Rasheed MH, E B, Helal GK, et al. Exosomes: from garbage bins to promising therapeutic targets. Int J Mol Sci. 2017;18(3):538. doi: 10.3390/ijms18030538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liang Y, Xu X, Li X, et al. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12(33):36938–36947. doi: 10.1021/acsami.0c10458 [DOI] [PubMed] [Google Scholar]
- 177.Xu X, Liang Y, Li X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. doi: 10.1016/j.biomaterials.2020.120539 [DOI] [PubMed] [Google Scholar]
- 178.Edwards SH. Intra-articular drug delivery: the challenge to extend drug residence time within the joint. Vet J. 2011;190(1):15–21. doi: 10.1016/j.tvjl.2010.09.019 [DOI] [PubMed] [Google Scholar]
- 179.Han Y, Yang J, Zhao W, et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact Mater. 2021;6(10):3596–3607. doi: 10.1016/j.bioactmat.2021.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liang Y, Zhang J, Zhao X, et al. Study on the slow-release mometasone furoate injection of PLGA for the treatment of knee arthritis. Curr Drug Deliv. 2021;18(3):357–368. doi: 10.2174/1567201817666200917124759 [DOI] [PubMed] [Google Scholar]
- 181.Stefani RM, Lee AJ, Tan AR, et al. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater. 2020;102:326–340. doi: 10.1016/j.actbio.2019.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li J, Liu N, Huang Z, Wang W, Hou D, Wang W. Intra-articular injection of loaded sPL sustained-release microspheres inhibits osteoarthritis and promotes cartilaginous repairs. J Orthop Surg Res. 2021;16(1):646. doi: 10.1186/s13018-021-02777-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Park E, Hart ML, Rolauffs B, Stegemann JP, TA R. Bioresponsive microspheres for on-demand delivery of anti-inflammatory cytokines for articular cartilage repair. J Biomed Mater Res A. 2020;108(3):722–733. doi: 10.1002/jbm.a.36852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cai Z, Zhang H, Wei Y, Wu M, Fu A. Shear-thinning hyaluronan-based fluid hydrogels to modulate viscoelastic properties of osteoarthritis synovial fluids. Biomater Sci. 2019;7(8):3143–3157. doi: 10.1039/C9BM00298G [DOI] [PubMed] [Google Scholar]
- 185.Scognamiglio F, Travan A, Donati I, Borgogna M, Marsich E. A hydrogel system based on a lactose-modified chitosan for viscosupplementation in osteoarthritis. Carbohydr Polym. 2020;248:116787. doi: 10.1016/j.carbpol.2020.116787 [DOI] [PubMed] [Google Scholar]
- 186.Leone G, Consumi M, Pepi S, et al. Enriched Gellan Gum hydrogel as visco-supplement. Carbohydr Polym. 2020;227:115347. doi: 10.1016/j.carbpol.2019.115347 [DOI] [PubMed] [Google Scholar]
- 187.Zhang X, Cai D, Zhou F, et al. Targeting downstream subcellular YAP activity as a function of matrix stiffness with Verteporfin-encapsulated chitosan microsphere attenuates osteoarthritis. Biomaterials. 2020;232:119724. doi: 10.1016/j.biomaterials.2019.119724 [DOI] [PubMed] [Google Scholar]
- 188.Yang J, Han Y, Lin J, et al. Ball-bearing-inspired polyampholyte-modified microspheres as bio-lubricants attenuate osteoarthritis. Small. 2020;16(44):e2004519. doi: 10.1002/smll.202004519 [DOI] [PubMed] [Google Scholar]
- 189.Paik J, Duggan ST, Keam SJ. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79(4):455–462. doi: 10.1007/s40265-019-01083-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Goto N, Okazaki K, Akasaki Y, et al. Single intra-articular injection of fluvastatin-PLGA microspheres reduces cartilage degradation in rabbits with experimental osteoarthritis. J Orthop Res. 2017;35(11):2465–2475. doi: 10.1002/jor.23562 [DOI] [PubMed] [Google Scholar]
- 191.Janssen M, Timur UT, Woike N, et al. Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J Control Release. 2016;244(Pt A):30–40. doi: 10.1016/j.jconrel.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 192.Arunkumar P, Indulekha S, Vijayalakshmi S, Srivastava R. Synthesis, characterizations, in vitro and in vivo evaluation of Etoricoxib-loaded Poly (Caprolactone) microparticles–a potential intra-articular drug delivery system for the treatment of osteoarthritis. J Biomater Sci Polym Ed. 2016;27(4):303–316. doi: 10.1080/09205063.2015.1125564 [DOI] [PubMed] [Google Scholar]
- 193.Sulaiman SB, Idrus RBH, Hwei NM. Gelatin microsphere for cartilage tissue engineering: current and future strategies. Polymers. 2020;12(10):2404. doi: 10.3390/polym12102404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM. Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints. J Orthop Res. 2014;32(8):1044–1051. doi: 10.1002/jor.22630 [DOI] [PubMed] [Google Scholar]
- 195.Hu HY, Lim NH, Ding-Pfennigdorff D, et al. DOTAM derivatives as active cartilage-targeting drug carriers for the treatment of osteoarthritis. Bioconjug Chem. 2015;26(3):383–388. doi: 10.1021/bc500557s [DOI] [PubMed] [Google Scholar]
- 196.Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7(3):248–254. doi: 10.1038/nmat2116 [DOI] [PubMed] [Google Scholar]
- 197.Noyori K, Koshino T, Takagi T, Okamoto R, Jasin HE. Binding characteristics of antitype II collagen antibody to the surface of diseased human cartilage as a probe for tissue damage. J Rheumatol. 1994;21(2):293–296. [PubMed] [Google Scholar]
- 198.Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148(7):2103–2108. [PubMed] [Google Scholar]
- 199.Cho H, Stuart JM, Magid R, et al. Theranostic immunoliposomes for osteoarthritis. Nanomedicine. 2014;10(3):619–627. doi: 10.1016/j.nano.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Elsaid KA, Ferreira L, Truong T, Liang A, Machan J, D’Souza GG. Pharmaceutical nanocarrier association with chondrocytes and cartilage explants: influence of surface modification and extracellular matrix depletion. Osteoarthritis Cartilage. 2013;21(2):377–384. doi: 10.1016/j.joca.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brown S, Kumar S, Sharma B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019;93:239–257. doi: 10.1016/j.actbio.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Brown SB, Wang L, Jungels RR, Sharma B. Effects of cartilage-targeting moieties on nanoparticle biodistribution in healthy and osteoarthritic joints. Acta Biomater. 2020;101:469–483. doi: 10.1016/j.actbio.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Formica FA, Barreto G, Zenobi-Wong M. Cartilage-targeting dexamethasone prodrugs increase the efficacy of dexamethasone. J Control Release. 2019;295:118–129. doi: 10.1016/j.jconrel.2018.12.025 [DOI] [PubMed] [Google Scholar]
- 204.Jain S, Tran TH, Amiji M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials. 2015;61:162–177. doi: 10.1016/j.biomaterials.2015.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Koning GA, Schiffelers RM, Wauben MH, et al. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate-containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006;54(4):1198–1208. doi: 10.1002/art.21719 [DOI] [PubMed] [Google Scholar]
- 206.Zhou HF, Chan HW, Wickline SA, Lanza GM, Pham CT. Alphavbeta3-targeted nanotherapy suppresses inflammatory arthritis in mice. FASEB J. 2009;23(9):2978–2985. doi: 10.1096/fj.09-129874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao J, Zhao M, Yu C, et al. Multifunctional folate receptor-targeting and pH-responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis. Int J Nanomedicine. 2017;12:6735–6746. doi: 10.2147/IJN.S140992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li R, He Y, Zhu Y, et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19(1):124–134. doi: 10.1021/acs.nanolett.8b03439 [DOI] [PubMed] [Google Scholar]
- 209.Morgen M, Tung D, Boras B, Miller W, Malfait AM, Tortorella M. Nanoparticles for improved local retention after intra-articular injection into the knee joint. Pharm Res. 2013;30(1):257–268. doi: 10.1007/s11095-012-0870-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang Y, Fan X, Xing L, Tian F. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun Signal. 2019;17(1):97. doi: 10.1186/s12964-019-0411-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage. 2020;28(4):400–409. doi: 10.1016/j.joca.2020.02.027 [DOI] [PubMed] [Google Scholar]
- 212.Cherifi C, Monteagudo S, Lories RJ. Promising targets for therapy of osteoarthritis: a review on the Wnt and TGF-beta signalling pathways. Ther Adv Musculoskelet Dis. 2021;13:1759720X211006959. doi: 10.1177/1759720X211006959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lu Z, Liu S, Le Y, et al. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials. 2019;218:119190. doi: 10.1016/j.biomaterials.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 214.Ouyang Z, Tan T, Liu C, et al. Targeted delivery of hesperetin to cartilage attenuates osteoarthritis by bimodal imaging with Gd2(CO3)3@PDA nanoparticles via TLR-2/NF-kappaB/Akt signaling. Biomaterials. 2019;205:50–63. doi: 10.1016/j.biomaterials.2019.03.018 [DOI] [PubMed] [Google Scholar]
- 215.Kang LJ, Yoon J, Rho JG, et al. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials. 2021;275:120967. doi: 10.1016/j.biomaterials.2021.120967 [DOI] [PubMed] [Google Scholar]
- 216.Chen P, Xia C, Mo J, Mei S, Lin X, Fan S. Interpenetrating polymer network scaffold of sodium hyaluronate and sodium alginate combined with berberine for osteochondral defect regeneration. Mater Sci Eng C Mater Biol Appl. 2018;91:190–200. doi: 10.1016/j.msec.2018.05.034 [DOI] [PubMed] [Google Scholar]
- 217.Bhattacharjee M, Ivirico JLE, Kan HM, et al. Preparation and characterization of amnion hydrogel and its synergistic effect with adipose derived stem cells towards IL1beta activated chondrocytes. Sci Rep. 2020;10(1):18751. doi: 10.1038/s41598-020-75921-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Maudens P, Seemayer CA, Pfefferle F, Jordan O, Allemann E. Nanocrystals of a potent p38 MAPK inhibitor embedded in microparticles: therapeutic effects in inflammatory and mechanistic murine models of osteoarthritis. J Control Release. 2018;276:102–112. doi: 10.1016/j.jconrel.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 219.Chu M, Wu P, Hong M, et al. Lingzhi and San-Miao-San with hyaluronic acid gel mitigate cartilage degeneration in anterior cruciate ligament transection induced osteoarthritis. J Orthop Translat. 2021;26:132–140. doi: 10.1016/j.jot.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li S, Stockl S, Lukas C, et al. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1beta-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res Ther. 2021;12(1):252. doi: 10.1186/s13287-021-02317-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zuo D, Tan B, Jia G, Wu D, Yu L, Jia L. A treatment combined prussian blue nanoparticles with low-intensity pulsed ultrasound alleviates cartilage damage in knee osteoarthritis by initiating PI3K/Akt/mTOR pathway. Am J Transl Res. 2021;13(5):3987–4006. [PMC free article] [PubMed] [Google Scholar]
- 222.Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release. 2014;190:15–28. doi: 10.1016/j.jconrel.2014.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Im GI, Kang ML. Kartogenin derivative-containing polymeric micelle, hyaluronic acid hydrogel, method for producing the same, and use thereof. Google Patents; 2019.
- 224.Bodick N, Jackson JD, Joshi U. Fluticasone extended-release formulations and methods of use thereof. Google Patents; 2020.
- 225.Ghandehari H, Oottamasathien S, Jensen MM, Cappello J, Jia W, Prestwich GD. Situ gelling compositions for the treatment or prevention of inflammation and tissue damage. Google Patents; 2019.
- 226.Sakar F, Ozer AY, Erdogan S, et al. Nano drug delivery systems and gamma radiation sterilization. Pharm Dev Technol. 2017;22(6):775–784. doi: 10.3109/10837450.2016.1163393 [DOI] [PubMed] [Google Scholar]
- 227.Zielinska A, Soles BB, Lopes AR, et al. Nanopharmaceuticals for eye administration: sterilization, depyrogenation and clinical applications. Biology. 2020;9(10). doi: 10.3390/biology9100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lucke M, Winter G, Engert J. The effect of steam sterilization on recombinant spider silk particles. Int J Pharm. 2015;481(1–2):125–131. doi: 10.1016/j.ijpharm.2015.01.024 [DOI] [PubMed] [Google Scholar]
- 229.Bajpayee AG, Wong CR, Bawendi MG, Frank EH, Grodzinsky AJ. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials. 2014;35(1):538–549. doi: 10.1016/j.biomaterials.2013.09.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yan H, Duan X, Pan H, et al. Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl Acad Sci U S A. 2016;113(41):E6199–E6208. doi: 10.1073/pnas.1608245113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cho H, Kim BJ, Park SH, Hasty KA, Min BH. Noninvasive visualization of early osteoarthritic cartilage using targeted nanosomes in a destabilization of the medial meniscus mouse model. Int J Nanomedicine. 2018;13:1215–1224. doi: 10.2147/IJN.S149375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen Z, Chen J, Wu L, et al. Hyaluronic acid-coated bovine serum albumin nanoparticles loaded with brucine as selective nanovectors for intra-articular injection. Int J Nanomedicine. 2013;8:3843–3853. doi: 10.2147/IJN.S50721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Laroui H, Grossin L, Leonard M, et al. Hyaluronate-covered nanoparticles for the therapeutic targeting of cartilage. Biomacromolecules. 2007;8(12):3879–3885. doi: 10.1021/bm700836y [DOI] [PubMed] [Google Scholar]
- 234.Labens R, Lascelles BD, Charlton AN, et al. Ex vivo effect of gold nanoparticles on porcine synovial membrane. Tissue Barriers. 2013;1(2):e24314. doi: 10.4161/tisb.24314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bhalekar MR, Upadhaya PG, Madgulkar AR. Fabrication and efficacy evaluation of chloroquine nanoparticles in CFA-induced arthritic rats using TNF-alpha ELISA. Eur J Pharm Sci. 2016;84:1–8. doi: 10.1016/j.ejps.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 236.Chen Z, Liu D, Wang J, et al. Development of nanoparticles-in-microparticles system for improved local retention after intra-articular injection. Drug Deliv. 2014;21(5):342–350. doi: 10.3109/10717544.2013.848495 [DOI] [PubMed] [Google Scholar]
- 237.Schneider T, Welker P, Haag R, et al. Effects of dendritic polyglycerol sulfate on articular chondrocytes. Inflamm Res. 2015;64(11):917–928. doi: 10.1007/s00011-015-0875-0 [DOI] [PubMed] [Google Scholar]
- 238.Kim MJ, Park JS, Lee SJ, et al. Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy. J Control Release. 2015;216:140–148. doi: 10.1016/j.jconrel.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 239.Lu H, Dai Y, Lv L, Zhao H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS One. 2014;9(1):e84703. doi: 10.1371/journal.pone.0084703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhou HF, Yan H, Pan H, et al. Peptide-siRNA nanocomplexes targeting NF-kappaB subunit p65 suppress nascent experimental arthritis. J Clin Invest. 2014;124(10):4363–4374. doi: 10.1172/JCI75673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nomura H, Takanashi S, Tanaka M, et al. Specific biological responses of the synovial membrane to carbon nanotubes. Sci Rep. 2015;5(1):14314. doi: 10.1038/srep14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lee YK, Kim SW, Park JY, Kang WC, Kang YJ, Khang D. Suppression of human arthritis synovial fibroblasts inflammation using dexamethasone-carbon nanotubes via increasing caveolin-dependent endocytosis and recovering mitochondrial membrane potential. Int J Nanomedicine. 2017;12:5761–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wardwell PR, Forstner MB, Bader RA. Investigation of the cytokine response to NF-kappaB decoy oligonucleotide coated polysaccharide based nanoparticles in rheumatoid arthritis in vitro models. Arthritis Res Ther. 2015;17(1):310. doi: 10.1186/s13075-015-0824-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Barrera P, Blom A, van Lent PL, et al. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000;43(9):1951–1959. doi: [DOI] [PubMed] [Google Scholar]
- 245.Wang D, Miller SC, Liu XM, Anderson B, Wang XS, Goldring SR. Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res Ther. 2007;9(1):R2. doi: 10.1186/ar2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Alam MM, Han HS, Sung S, et al. Endogenous inspired biomineral-installed hyaluronan nanoparticles as pH-responsive carrier of methotrexate for rheumatoid arthritis. J Control Release. 2017;252:62–72. doi: 10.1016/j.jconrel.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 247.Kim HJ, Lee SM, Park KH, Mun CH, Park YB, Yoo KH. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials. 2015;61:95–102. doi: 10.1016/j.biomaterials.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 248.Koo OM, Rubinstein I, Onyuksel H. Actively targeted low-dose camptothecin as a safe, long-acting, disease-modifying nanomedicine for rheumatoid arthritis. Pharm Res. 2011;28(4):776–787. doi: 10.1007/s11095-010-0330-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Schulze K, Koch A, Schöpf B, et al. Intraarticular application of superparamagnetic nanoparticles and their uptake by synovial membrane—an experimental study in sheep. J Magn Magn Mater. 2005;293(1):419–432. doi: 10.1016/j.jmmm.2005.02.075 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.