Abstract
Alanine-scanning mutagenesis was applied to the aminoglycoside 6′-N-acetyltransferase type Ib conserved motif B, and the effects of the substitutions were analyzed by measuring the MICs of kanamycin (KAN) and its semisynthetic derivative, amikacin (AMK). Several substitutions resulted in no major change in MICs. E167A and F171A resulted in derivatives that lost the ability to confer resistance to KAN and AMK. P155A, P157A, N159A, L160A, I163A, K168A, and G170A conferred intermediate levels of resistance. Y166A resulted in an enzyme derivative with a modified specificity; it conferred a high level of resistance to KAN but lost the ability to confer resistance to AMK. Although not as pronounced, the resistance profiles conferred by substitutions N159A and G170A were related to that conferred by Y166A. These phenotypes, taken together with previous results indicating that mutant F171L could not catalyze acetylation of AMK when the assays were carried out at 42°C (D. Panaite and M. Tolmasky, Plasmid 39:123–133, 1998), suggest that some motif B amino acids play a direct or indirect role in acceptor substrate specificity. MICs of AMK and KAN for cells harboring the substitution C165A were high, suggesting that the active form of the enzyme may not be a dimer formed through a disulfide bond. Furthermore, this result indicated that the acetylation reaction occurs through a direct mechanism rather than a ping-pong mechanism that includes a transient transfer of the acetyl group to a cysteine residue. Deletion of fragments at the C terminus demonstrated that up to 10 amino acids could be deleted without a loss of activity.
Bacterial resistance to aminoglycosides in the clinical setting is often due to modifying enzymes (16, 31). N-Acetyltransferases, an important group of enzymes that can modify several aminoglycosides (8), belong to the GCN5-related N-acetyltransferase (GNAT) superfamily (24). The GNATs are a superfamily of enzymes that catalyze the transfer of an acetyl group from acetyl coenzyme A (acetyl-CoA) to a primary amine in a wide variety of acceptor molecules. Analysis of primary sequences of GNATs and the determination of three-dimensional structures of 11 of them showed that they share structural features and common domains (11, 24). Four motifs (motifs A to D) were identified, of which A and B are the most conserved (24, 35) (Fig. 1a). Motif A is similar in those acetyltransferases for which the three-dimensional structures have been resolved (11). It includes the β4 strand and the α3 helix, which are the essence of the acetyl-CoA binding site (11). On the other hand, the structure of motif B is not that homogeneous. Its three-dimensional conformation differs in the aminoglycoside 6′-N-acetyltransferase type Ii [AAC(6′)-Ii] and AAC(3)-Ia, the only aminoglycoside acetyltransferases with known structures (44, 48). Furthermore, these two conformations are different from that of motif B in some histone and serotonin acetyltransferases (11, 44, 48). Evidence has been presented that amino acids in motif B play a role in acetyl-CoA binding; but the structural diversity of this motif, together with evidence from mutagenesis experiments and structural data, may also reflect a role in binding or recognition of the acceptor substrate (5, 11, 26, 44, 48). We performed alanine-scanning mutagenesis to further characterize this region in AAC(6′)-Ib, an enzyme that has been often associated with transposition elements and gene cassettes and that is often responsible for resistance to aminoglycosides exhibited by several clinical bacterial isolates (3, 15, 37–40, 45). Alanine-scanning mutagenesis provides a means to perform a systematic analysis of a protein region. It consists of the replacement of each amino acid by an alanine residue. Replacement by this amino acid removes the side chain beyond the β carbon without altering the conformation of the main chain or imposing extreme electrostatic or steric effects (43). Alanine is the most abundant amino acid and is present in exposed as well as buried locations (43). Our results showed that removal of the side chains of amino acids E167 and F171 led to the complete loss of enzymatic activity, while for several others it led to a partial loss of activity. In addition, the phenotypes of some mutants suggest that amino acids within motif B may participate directly or indirectly in recognition of the acceptor substrate. Furthermore, deletion mutagenesis experiments showed that the last 10 amino acids of the protein are dispensable.
FIG. 1.
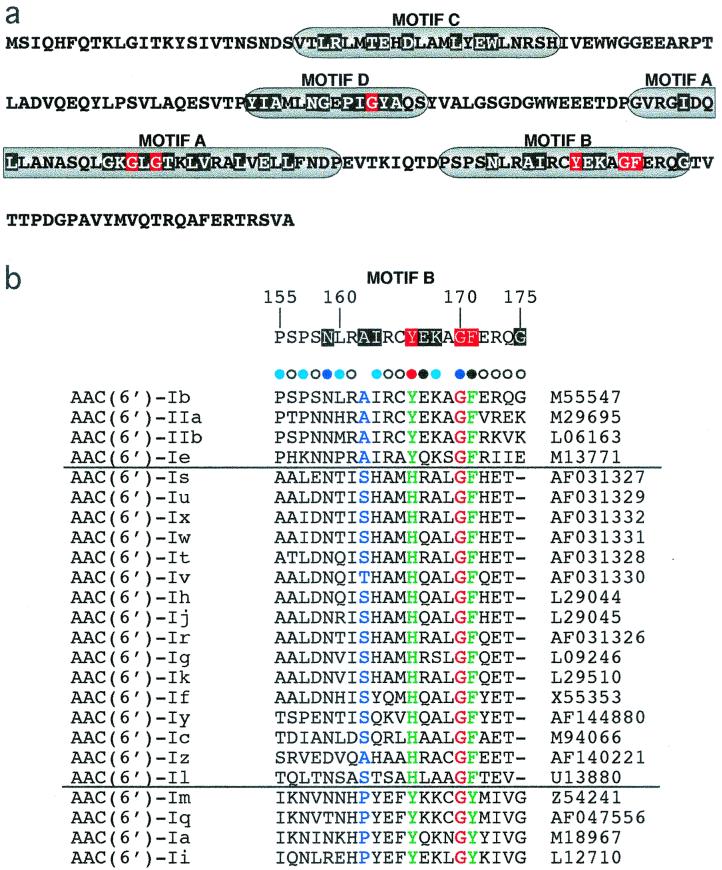
(a) Conserved regions in the amino acid sequence of AAC(6′)-Ib. According to the color scheme defined by Neuwald and Landsman (24), moderately and highly conserved residue positions are highlighted in black and red, respectively. (b) Alignment of the motif B amino acid sequences of AAC(6′) type I and II enzymes generated by using the CLUSTAL W program(36). The horizontal lines separate the members of the three subclasses (13, 31). The sequences are from AAC(6′)-Ia (33), AAC(6′)-Ib (25), AAC(6′)-Ic (32), AAC(6′)-Id (30), AAC(6′)-Ie (12), AAC(6′)-If (34), AAC(6′)-Ig (18), AAC(6′)-Ih (19), AAC(6′)-Ii (6), AAC(6′)-Ij (19), AAC(6′)-Ik (28), AAC(6′)-Il (2), AAC(6′)-Im (13), AAC(6′)-Iq (4), AAC(6′)-Ir (29), AAC(6′)-Is (29), AAC(6′)-It (29), AAC(6′)-Iu (29), AAC(6′)-Iv (29), AAC(6′)-Iw (29), AAC(6′)-Ix (29), AAC(6′)-Iy (22), AAC(6′)-Iz (20). The dashes indicate gaps introduced to optimize similarity. G170 (red) is identical in all proteins; green and blue amino acids denote conservative substitutions with different degrees of similarities. The degrees of conservation of the AAC(6′)-Ib motif B amino acids compared to those of different proteins from the GNAT superfamily are shown at the top of panel b. The phenotypes of alanine substitutions are shown at the top of the amino acids by colored bullets. Black bullets indicate substitutions that abolished resistance to AMK and KAN (group S in Table 1), light blue (group I2) and dark blue (group I1) bullets indicate intermediate MICs, and white bullets indicate substitutions that did not seriously affect resistance (group R in Table 1). The red bullet denotes that there was a change in substrate specificity.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Escherichia coli XL1-Blue [recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac (F′ proAB lacIq ZΔM15 Tn10); Stratagene] was used as the host for wild-type and mutagenized plasmids. Plasmid pJHCMW1, originally from Klebsiella pneumoniae JHCK1 (45), was used for the mutagenesis experiments. Plasmid vector pZerO1 (Invitrogen) was used to generate the deletion derivatives.
General DNA procedures.
Plasmid DNA was prepared by the alkaline lysis method described before (7) or with a plasmid mini kit (Qiagen Inc.). Alanine substitutions were generated by site-directed mutagenesis with the QuikChange mutagenesis kit according to the recommendations of the supplier (Stratagene). Deletions were generated by amplification of the aac(6′)-Ib template gene from pJHCMW1 by using GCCTCGTGATACGCCTATTTTT as the 5′-end primer and GTTTAACGTTTGACATGAGGGC, TTACTCGAATGCCTGGCGTGTTTG, TTATGCCTGGCGTGTTTGAACCAT, TTAGCGTGTTTGAACCATGTACAC, TTATGTTTGAACCATGTACACGGC, and TTATTGAACCATGTACACGGCTG as 3′-terminus primers for the wild-type protein (201 amino acids) or the deletions that included 195, 193, 191, 190, and 189 amino acids, respectively. The amplified DNA fragments were cloned by using pZerO1 (Invitrogen) as the vector. Nucleotide sequencing was performed at the California State University Northridge Sequencing Facility.
General methods.
MICs were determined by the E-test method (46) with commercial strips (AB Biodisks) by the procedures recommended by the supplier. The numbers given are the median values from three independent tests. Reproducibility was very high when the same commercial source of culture medium (Difco) was used, and many consecutive tests yielded identical results. Amino acid sequence analysis was performed with the CLUSTAL W program (Pôle Bio-Informatique Lyonnais server [http://pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_clustalw.html]) (36).
Nucleotide sequence accession numbers.
The nucleotide sequences of the mutations have been deposited in the GenBank sequence library and assigned accession numbers AF360375 to AF360393.
RESULTS AND DISCUSSION
Mutagenesis analyses have contributed to the characterization of several GNAT enzymes (5, 14, 26, 42). In particular, alanine-scanning mutagenesis has been used to identify specific side chains that are part of active sites (47), that participate in protein binding of different compounds (50), that play a role in protein-protein interaction (43, 49), or that contribute to protein folding and stability (23, 27). Replacement by alanine, which results in the elimination of the side chain beyond the β carbon, does not alter the conformation of the main chain or impose extreme electrostatic or steric effects (43). Therefore, the replacements permit one to determine if the side chains of the amino acids contribute to the structure and/or the function of the enzyme. A reduction in levels of resistance might also be due to reduction of the stability of a mutant derivative. Amino acids in motif B were replaced by alanine, and the resulting derivatives were analyzed by determination of the MICs of kanamycin (KAN) and amikacin (AMK). The MICs, which were determined by using E-test strips (46), are shown in Table 1.
TABLE 1.
Susceptibility to AMK and KAN of E. coli XL1-Blue strains
| Substitutiona | MIC (μg/ml)b
|
Susceptibility phenotypec | |
|---|---|---|---|
| AMK | KAN | ||
| None | 0.25 | 0.25 | |
| pJHCMW1 (wild type) | 32 | 192 | |
| P155A | 12 | 48 | I2 |
| S156A | 32 | 192 | R |
| P157A | 6 | 24 | I2 |
| S158A | 96 | 256 | R |
| N159A | 0.75 | 32 | I1 |
| L160A | 16 | 48 | I2 |
| R161A | 24 | 128 | R |
| I163A | 2 | 24 | I2 |
| R164A | 12 | 128 | R |
| C165A | 16 | 64 | R |
| Y166A | 1.5 | 256 | Specificity |
| E167A | 2 | 4 | S |
| K168A | 6 | 16 | I2 |
| G170A | 6 | 96 | I1 |
| F171A | 1 | 1.5 | S |
| E172A | 32 | 96 | R |
| R173A | 16 | 128 | R |
| Q174A | 32 | 128 | R |
| G175A | 32 | 128 | R |
All mutations were performed with pJHCMW1, and the plasmid was introduced by transformation into E. coli XL1-Blue.
MICs were determined by the E-test.
For the purpose of the comparisons in this paper, the susceptibility phenotypes of the mutants were divided into the following four groups: R, resistant (MICs not more than threefold lower than that for the wild type); I, intermediate levels of resistance; S, susceptible (the MICs of both antibiotics were lower than 6 μg/ml; and Specificity, the MIC of one antibiotic (AMK) was at the susceptible level and the MIC of the other (KAM) was at the resistance level. The I group was subdivided into two groups on the basis of the resistance profile conferred by these derivatives: I1, resistance related to that of Y166A (Specificity) but not as pronounced; I2, the MICs of both antibiotics between those for the R and S groups.
Although most substitutions had some effect on the MICs, amino acids S156, S158, R161, R164, C165, E172, R173, Q174, and G175 could be replaced by an alanine residue and still confer levels of resistance to both antibiotics no more than threefold lower than that for the wild type, suggesting that their side chains do not play essential roles in the enzymatic activity (group R in Table 1). The MICs of AMK and KAN for the strain with the S158 substitution were higher than those for the wild type, indicating that the substitution results in a more efficient enzyme derivative. The hydroxyl group of S158 may be mildly detrimental for the acetylating activity in AAC(6′)-Ib. The amino acid at this position does not show relevant conservation among AAC(6′) enzymes or GNATs (Fig. 1b; see also reference 24). Interestingly, AAC(6′)-Ic and some other GNATs have an alanine residue at this location (Fig. 1b). It would be interesting to test the effect of alanine substitution at this position in other AAC(6′) enzymes as well as the effect of substitutions by other amino acids in AAC(6′)-Ib. The levels of resistance shown by E. coli harboring the substitution derivatives mentioned above varied, which suggests that some of the replaced amino acid side chains may play nonessential roles in the structure or the function of the enzyme.
Substitutions that completely abolish resistance.
Substitutions at E167 and F171 resulted in derivatives that lost the ability to confer resistance to KAN and AMK (Table 1). With only a few exceptions, in all members of the GNAT superfamily the position equivalent to F171 is occupied by either phenylalanine or tyrosine (24). In the case of the AAC(6′) enzymes, which can be classified into three subclasses (Fig. 1b), members of the major subclass, together with members of the AAC(6′)-Ib subclass, have a phenylalanine residue at this position, while all four members of the AAC(6′)-Iq subclass possess a tyrosine (Fig. 1b). Several mutagenesis studies of this position have been performed with different GNATs, and all show that substitutions have a detrimental effect on the enzymatic activity (5, 14, 17, 26). It has been postulated that in GNATs the phenylalanine or tyrosine residue at this position contacts acetyl-CoA and plays an important role in positioning the acetyl group for the transfer to the acceptor substrate and may play a role in the resolution of the reaction intermediate (11). Our previous results indicated that some substitutions at position F171, although detrimental, still generated an enzyme with a low level of activity (5). Of particular interest was the F171L mutation; the results obtained with the derivative with this mutation indicated that partial activity still exists by replacing the aromatic group of phenylalanine with the aliphatic R group of leucine. In addition, the derivative with the F171L mutation could not acetylate aminoglycoside molecules with substitutions at the C-1 amino group of the deoxystreptamine moiety when the assays were carried out at 42°C (26). All of this information taken together suggests that the phenylalanine or tyrosine side chain at this location plays an important role in the acetylating activities of GNATs. In the case of AAC(6′)-Ib, the phenylalanine side chain is essential for full enzymatic activity, but some substitutions for hydrophobic amino acids do not result in complete loss of activity. Furthermore, the F171 residue in AAC(6′)-Ib may participate in acceptor substrate specificity.
The E167A substitution resulted in a derivative with a highly reduced ability to confer resistance to either AMK or KAN, yet the MICs were consistently higher than those for the derivative with the F171A mutation. These results may indicate that this amino acid plays an important role in the activity of AAC(6′)-Ib but that residual acetylating activity is still possible in the absence of its side chain. The E167 position has a moderate level of conservation, and in the largest subgroup of AAC(6′) enzymes this position is occupied by an alanine residue. In all but one of the GNATs for which the three-dimensional structure was determined, the E167 position equivalent is occupied by an amino acid other than glutamic acid (11). Therefore, there is still not enough information to understand the role played by the amino acid at this position.
The C165A substitution does not abolish resistance.
Since the alanine substitution at the only cysteine residue results in an active enzyme capable of conferring a reasonably high level of resistance to both AMK and KAN, formation of a dimer through a disulfide bond at Cys—Cys does not seem to be necessary for an active enzyme. In addition, this result is in agreement with other lines of biochemical data indicating that acetyltransferases of the GNAT superfamily acetylate the substrates through a direct mechanism rather than through a ping-pong mechanism, in which a covalently bound acetylated enzyme intermediate is formed by the transient transfer of the acetyl group to a cysteine residue, followed by the transfer of this acetyl group to the acceptor substrate (9, 10).
The Y166A substitution modifies the resistance profile.
The Y166A substitution resulted in a derivative that can confer high-level resistance to KAN but that loses the ability to confer resistance to AMK (Table 1). This result suggests that the side chain of this amino acid is directly or indirectly involved in recognition of the acceptor substrate. This position is occupied by a tyrosine residue in most proteins belonging to the GNAT superfamily, but phenylalanine or histidine residues are also found in some of them (24). Within the family of AAC(6′) enzymes, those belonging to two subclasses possess a tyrosine residue, while those belonging to the major subclass contain a histidine residue (Fig. 1b). In the case of a serotonin N-acetyltransferase, Hickman et al. (14) proposed that Y168 [equivalent to Y166 in AAC(6′)-Ib] must play a significant role in catalysis since the Y168F mutation resulted in a dramatic reduction of acetylating activity. Those investigators suggested that the —OH group might play a role in the reprotonation of the thiolate leaving group or in the positioning of the two substrates for catalysis. Histone acetyltransferases show similarities to this serotonin N-acetyltransferase, and a similar role for Y168 has been proposed (1, 21). In the case of AAC(6′)-Ii, Wybenga-Groot et al. (48) suggested the possibility that the side chain of Y147 interacts with acetyl-CoA. However, this does seem not to be the case for the Serratia marcescens aminoglycoside 3-N-acetyltransferase, in which the general crystal structure of motif B is different from those of other GNATs and Y157 is not appropriately positioned for the side chain to be within hydrogen bonding distance of the sulfur atom of acetyl-CoA (44). Furthermore, the data presented by Wolf et al. (44) suggested that amino acids in motif B are involved in forming the “gentamicin-binding slot.” An interesting possibility has been suggested by Trievel et al. (41), indicating that amino acids in motif B together with acetyl-CoA itself play a structural role in acceptor substrate binding. All these results taken together suggest that it is possible that the amino acid at this position may play more than one role in some GNATs or, even when it is conserved, may not play an identical role in all enzymes. We propose that the AAC(6′)-Ib Y166 substitution plays a direct or indirect role related to acceptor substrate recognition.
Substitutions that confer intermediate levels of resistance.
Some substitutions resulted in derivatives that affected but that did not completely abolish resistance to AMK and/or KAN. We could distinguish two groups that were called I1 and I2 (Table 1). Substitutions in group I1, N159A and G170A, conferred on E. coli a resistance profile related to that conferred by the Y166A derivative, although it was not as pronounced. In these two cases, the percentage of activity lost when AMK was the substrate was considerably higher than the percentage of activity lost when KAN was the substrate. E. coli harboring the N159A derivative showed a low level of resistance to both antibiotics. However, while the MIC of AMK for the strain with the N159A mutation was reduced more than 40-fold with respect to that for the wild type, the MIC of KAN was 32 μg/ml, which is sixfold lower than that for the wild type but still enough to demonstrate that the mutant enzyme is partially active. These MICs suggest that although it is not strictly essential, N159 may play an important role in enzyme function and substrate specificity. N159 is moderately conserved among GNATs and 6′-N-acetyltransferases (Fig. 1b). In the case of G170, a highly conserved residue, its replacement had little effect on resistance to KAN but its replacement resulted in a fivefold reduction in the MIC of AMK, suggesting its probable involvement in substrate specificity. Since glycine allows a wide range of conformations of the main chain, the presence of a very conserved glycine residue at this location in most GNATs may reflect the need for a conformational change at some point in the acetylation process. Replacement by other amino acids will provide more information about the role played by this residue.
The I2 group of mutant derivatives which includes P155A, P157A, L160A, I163, and K168A also showed intermediate levels of resistance to both aminoglycosides. In this group of mutants the percentage of loss of resistance to one of the two antibiotics was no more than twice the percentage of loss of resistance to the other antibiotic. P155 is conserved in all four members of the subclass to which AAC(6′)-Ib belongs but is not present in other acetyltransferases. Further mutagenesis studies may help determine if an important role is played by the side chain of P155. K168 is conserved in two of the subclasses of AAC(6′) enzymes (Fig. 1b) and is moderately conserved among GNATs, while the P157 and L160 residues are not conserved (24). The I163A mutation was placed among those that conferred intermediate levels of resistance, but it showed some differences with the rest of substitutions in group I2. The ratio of the reduction of MIC of AMK with respect to that of KAN was higher, yet it was not enough to be placed within group I1. Amino acid I163 has an intermediate level of conservation among GNATs (24) (Fig. 1b) and is conserved among the four members of the AAC(6′)-Ib subclass but not among members of the other subclasses. Isoleucine is the most common amino acid at this position among the GNAT superfamily, and in others the nonpolar valine or leucine are found at this position (Fig. 1b) (24). However, a large number of enzymes of the superfamily have different kinds of amino acids at this position.
Deletions at the AAC(6′)-Ib C terminus.
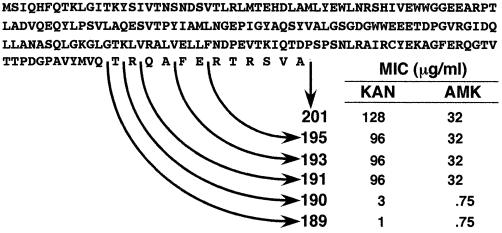
Since motif B is the most C-terminal conserved portion of acetyltransferases, we decided to determine whether a region of the protein located beyond this motif is required for enzymatic activity. For this we generated deletion derivatives and tested them for their ability to confer resistance to KAN and AMK. Figure 2 shows that AAC(6′)-Ib191 and larger derivatives confer resistance, while E. coli strains harboring AAC(6′)-Ib189 or AAC(6′)-Ib190 were susceptible to both antibiotics. These results indicate that amino acid residues located beyond motif B toward the C terminus of the protein are essential for acetylating activity. Information about truncated derivatives of AAC(6′) enzymes is not abundant. However, in the case AAC(3)-Ia, deletion of amino acids from the wild-type 177-amino-acid enzyme to a 168-amino-acid derivative could be made without a loss of resistance to gentamicin (44). More information is available about the N terminus of AAC(6′)-Ib variants; Casin et al. (3) analyzed several variants from Enterobacter cloacae and Citrobacter freundii clinical isolates and found high degrees of variability which led those investigators to conclude that it has flexible structural requirements.
FIG. 2.
C-terminus deletions of AAC(6′)-Ib. The MICs of AMK and KAN for E. coli cells carrying recombinant clones harboring different AAC(6′)-Ib deletion derivatives are shown.
Concluding remarks.
It has been suggested that motif B may play a role in correctly orienting acetyl-CoA for the catalytic reaction and/or in acceptor substrate-specific recognition or binding in GNATs (11, 41, 44). Our results do not rule out the interaction of amino acids in this motif with either substrate or their participation in the catalytic reaction. They indicate that amino acids F171 and E167 play important roles in AAC(6′)-Ib activity. Furthermore, results from the present study, taken together with the results of our previous work (26), indicate that amino acids N159, Y166, G170, and F171 may participate in acceptor substrate specificity. The deletion mutagenesis experiments showed that the C-terminal portion of the protein up to amino acid 191 is essential for the activity of AAC(6′)-Ib. In addition, the results obtained with mutation C165A indicate that AAC(6′)-Ib catalyzes acetylation of the acceptor substrate through a direct mechanism.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant 1R15AI47115–01. N.W. and K.D. were supported by MSD grant 5R25GM56820–03 from the National Institutes of Health.
REFERENCES
- 1.Angus-Hill M L, Dutnall R N, Tafrov S T, Sternglanz R, Ramakrishnan V. Crystal structure of the histone acetyltransferase Hpa2: a tetrameric member of the Gcn5-related N-acetyltransferase superfamily. J Mol Biol. 1999;294:1311–1325. doi: 10.1006/jmbi.1999.3338. [DOI] [PubMed] [Google Scholar]
- 2.Bunny K, Hall R, Stokes H. New mobile gene cassettes containing an aminoglycoside resistance gene, aacA7, and a chloramphenicol resistance gene, catB3, in an integron in pBWH301. Antimicrob Agents Chemother. 1995;39:686–693. doi: 10.1128/AAC.39.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casin I, Bordon F, Bertin P, Coutrot A, Podglajen I, Brasseur R, Collatz E. Aminoglycoside 6′-N-acetyltransferase variants of the Ib type with altered substrate profile in clinical isolates of Enterobacter cloacae and Citrobacter freundii. Antimicrob Agents Chemother. 1998;42:209–215. doi: 10.1128/aac.42.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centron D, Roy P. Characterization of the 6′-N-acetyltransferase gene aac(6′)-Iq from the integron of a natural multiresistance plasmid. Antimicrob Agents Chemother. 1998;42:1506–1508. doi: 10.1128/aac.42.6.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavideh R, Sholly S, Panaite D, Tolmasky M E. Effects of F171 mutations in the 6′-N-acetyltransferase type Ib [AAC(6′)-Ib] enzyme on susceptibility to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2811–2812. doi: 10.1128/aac.43.11.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa Y, Galimand M, Leclercq R, Duval J, Courvalin P. Characterization of the chromosomal aac(6′)-Ii gene specific for Enterococcus faecium. Antimicrob Agents Chemother. 1993;37:1896–1903. doi: 10.1128/aac.37.9.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosa J, Tolmasky M, Actis L, Falkow S. Plasmids. In: Gerhardt P, editor. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 365–386. [Google Scholar]
- 8.Davies J, Wright G. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 1997;5:234–240. doi: 10.1016/S0966-842X(97)01033-0. [DOI] [PubMed] [Google Scholar]
- 9.De Angelis J, Gastel J, Klein D C, Cole P A. Kinetic analysis of the catalytic mechanism of serotonin N- acetyltransferase (EC 2.3.1.87) J Biol Chem. 1998;273:3045–3050. doi: 10.1074/jbc.273.5.3045. [DOI] [PubMed] [Google Scholar]
- 10.Dutnall R N, Tafrov S T, Sternglanz R, Ramakrishnan V. Structure of the histone acetyltransferase Hat1: a paradigm for the GCN5-related N-acetyltransferase superfamily. Cell. 1998;94:427–438. doi: 10.1016/s0092-8674(00)81584-6. [DOI] [PubMed] [Google Scholar]
- 11.Dyda F, Klein D, Hickman A. GCN5-related N-acetyltransferases: a structural overview. Annu Rev Biophys Biomol Struct. 2000;29:81–103. doi: 10.1146/annurev.biophys.29.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreti J, Gilmore K, Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6′-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986;167:631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannecart-Pokorni E, Depuydt F, De Wit L, Van Bossuyt E, Content J, Vanhoof R. Characterization of the 6′-N-aminoglycoside acetyltransferase gene, aac(6′)-Il, associated with a sulI type integron. Antimicrob Agents Chemother. 1997;41:314–318. doi: 10.1128/aac.41.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickman A, Namboodiri M, Klein D, Dyda F. The structural basis of ordered substrate binding by serotonin N-acetyltransferase: enzyme complex at 1.8 Å resolution with a bisubstrate analog. Cell. 1999;97:361–369. doi: 10.1016/s0092-8674(00)80745-x. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins J D, Flores A, del Pilar Pla M, Lester S, O'Brien T F. Nosocomial spread of an amikacin resistance gene on both a mobilized, nonconjugative plasmid and a conjugative plasmid. Antimicrob Agents Chemother. 1991;35:1605–1611. doi: 10.1128/aac.35.8.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotra L P, Haddad J, Mobashery S. Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob Agents Chemother. 2000;44:3249–3256. doi: 10.1128/aac.44.12.3249-3256.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo M, Zhou J, Jambeck P, Churchill M, Allis C. Histone acetyltransferase activity of yeast6 Gcn5p is required for the activation of target genes in vivo. Genes Dev. 1998;12:627–639. doi: 10.1101/gad.12.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert T, Gerbaud G, Courvalin P. Characterization of Acinetobacter haemolyticus aac(6′)-Ig gene encoding an aminoglycoside 6′-N-acetyltransferase which modifies amikacin. Antimicrob Agents Chemother. 1993;37:2093–2100. doi: 10.1128/aac.37.10.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert T, Gerbaud G, Courvalin P. Characterization of the chromosomal aac(6′)-Ij gene of Acinetobacter sp. 13 and the aac(6′)-Ih plasmid gene of Acinetobacter baumannii. Antimicrob Agents Chemother. 1994;38:1883–1889. doi: 10.1128/aac.38.9.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert T, Ploy M, Denis F, Courvalin P. Characterization of the chromosomal aac(6′)-Iz gene of Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1999;43:2366–2371. doi: 10.1128/aac.43.10.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y, Fletcher M, Zhou J, Allis D, Wagner G. Solution structure of the catalytic domain of GCN5 histone acetyltransferase bound to coenzyme A. Nature. 1999;400:86–89. doi: 10.1038/21922. [DOI] [PubMed] [Google Scholar]
- 22.Magnet S, Courvalin P, Lambert T. Activation of the cryptic aac(6′)-Iy aminoglycoside resistance gene of Salmonella by a chromosomal deletion generating a transcriptional fusion. J Bacteriol. 1999;181:6650–6655. doi: 10.1128/jb.181.21.6650-6655.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milla M E, Brown B M, Sauer R T. Protein stability effects of a complete set of alanine substitutions in Arc repressor. Nat Struct Biol. 1994;1:518–523. doi: 10.1038/nsb0894-518. [DOI] [PubMed] [Google Scholar]
- 24.Neuwald A, Landsman D. GCN5-related histone N-acetlytransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem Sci. 1997;22:154–155. doi: 10.1016/s0968-0004(97)01034-7. [DOI] [PubMed] [Google Scholar]
- 25.Nobuta K, Tolmasky M E, Crosa L, Crosa J. Sequencing and expression of the 6′-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J Bacteriol. 1988;170:3769–3773. doi: 10.1128/jb.170.8.3769-3773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panaite D M, Tolmasky M E. Characterization of mutants of the 6′-N-acetyltransferase encoded by the multiresistance transposon Tn1331: effect of Phen171-to-Leu171 and Tyr80-to-Cys80 substitutions. Plasmid. 1998;39:123–133. doi: 10.1006/plas.1997.1330. [DOI] [PubMed] [Google Scholar]
- 27.Pons J, Querol E, Planas A. Mutational analysis of the major loop of Bacillus 1,3-1,4-β-d-glucan 4-glucanohydrolases. J Biol Chem. 1997;272:13006–13012. doi: 10.1074/jbc.272.20.13006. [DOI] [PubMed] [Google Scholar]
- 28.Rudant E, Bourlioux P, Courvalin P, Lambert T. Characterization of the aac(6′)-Ik gene of Acinetobacter sp. 6. FEMS Microbiol Lett. 1994;124:49–54. doi: 10.1016/0378-1097(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 29.Rudant E, Bouvet P, Courvalin P, Lambert T. Phylogenetic analysis of proteolytic Acinetobacter strains based on the sequence of genes ancoding aminogylcoside 6′-N-acetyltransferases. Syst Appl Microbiol. 1999;22:59–67. doi: 10.1016/S0723-2020(99)80028-9. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt F, Nucken E, Henschke R. Nucleotide sequence analysis of 2"-aminoglycoside nucleotidyl-transferase ANT (2") from Tn4000: its relationship with AAD(3") and impact on Tn21 evolution. Mol Microbiol. 1988;2:709–711. doi: 10.1111/j.1365-2958.1988.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 31.Shaw K, Rather P, Hare R, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw K, Rather P, Sabatelli F, Mann P, Munayyer H, Mierzwa R, Petrikkos G, Hare R, Miller G, Bennet P, Downey P. Characterization of the chromosomal aac(6′)-Ic gene from Serratia marcescens. Antimicrob Agents Chemother. 1992;36:1447–1455. doi: 10.1128/aac.36.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenover F, Filpula D, Phillips K, Plorde J. Cloning and sequencing of a gene encoding an aminoglycoside 6′-N-acetyltransferase from an R factor of Citrobacter diversus. J Bacteriol. 1988;170:471–473. doi: 10.1128/jb.170.1.471-473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teran F, Suarez J, Mendoza M. Cloning, sequencing, and uses as a molecular probe of a gene encoding an aminoglycoside 6′-N-acetyltransferase of broad substrate profile. Antimicrob Agents Chemother. 1991;35:714–719. doi: 10.1128/aac.35.4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tercero J, Riles L, Wickner R. Localized mutagenesis and evidence for post-transcriptional regulation of MAK3. J Biol Chem. 1992;267:20270–20276. [PubMed] [Google Scholar]
- 36.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolmasky M E, Chamorro R M, Crosa J H, Marini P M. Transposon-mediated amikacin resistance in Klebsiella pneumoniae. Antimicrob Agents Chemother. 1988;32:1416–1420. doi: 10.1128/aac.32.9.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolmasky M E, Crosa J H. Genetic organization of antibiotic resistance genes (aac(6′)-Ib, aadA, and oxa9) in the multiresistance transposon Tn1331. Plasmid. 1993;29:31–40. doi: 10.1006/plas.1993.1004. [DOI] [PubMed] [Google Scholar]
- 39.Tolmasky M E, Crosa J H. Tn1331, a novel multiresistance transposon encoding resistance to amikacin and ampicillin in Klebsiella pneumoniae. Antimicrob Agents Chemother. 1987;31:1955–1960. doi: 10.1128/aac.31.12.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran Van Nhieu G, Bordon F, Collatz E. Incidence of an aminoglycoside 6′-N-acetyltransferase, ACC(6′)-Ib, in amikacin-resistant clinical isolates of gram-negative bacilli, as determined by DNA-DNA hybridisation and immunoblotting. J Med Microbiol. 1992;36:83–88. doi: 10.1099/00222615-36-2-83. [DOI] [PubMed] [Google Scholar]
- 41.Trievel R, Rojas J, Sterner D, Venkataramani R, Wang L, Zhou J, Allis C, Berger S, Marmorstein R. Crystal structure and mechanism of histone acetylation of the yeast GCN5 transcriptional coactivator. Proc Natl Acad Sci USA. 1999;96:8931–8936. doi: 10.1073/pnas.96.16.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Liu L, Berger S. Critical residues for histone acetylation by GCN5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo. Genes Dev. 1998;12:640–653. doi: 10.1101/gad.12.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells J A. Systematic mutational analyses of protein-protein interfaces. Methods Enzymol. 1991;202:390–411. doi: 10.1016/0076-6879(91)02020-a. [DOI] [PubMed] [Google Scholar]
- 44.Wolf E, Vassilev A, Makino Y, Sali A, Nakatani Y, Burley S. Crystal structure of a GCN5-related N-acetyltranferase: Serratia marcescens aminoglycoside 3-N-acetyltransefrase. Cell. 1998;94:439–449. doi: 10.1016/s0092-8674(00)81585-8. [DOI] [PubMed] [Google Scholar]
- 45.Woloj M, Tolmasky M E, Roberts M C, Crosa J H. Plasmid-encoded amikacin resistance in multiresistant strains of Klebsiella pneumoniae isolated from neonates with meningitis. Antimicrob Agents Chemother. 1986;29:315–319. doi: 10.1128/aac.29.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woods G, Washington J. Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 1327–1341. [Google Scholar]
- 47.Wu D, Hersch L. Identification of an active site arginine in rat choline acetyltransferase by alanine scanning mutagenesis. J Biol Chem. 1995;270:29111–29116. doi: 10.1074/jbc.270.49.29111. [DOI] [PubMed] [Google Scholar]
- 48.Wybenga-Groot L, Draker K, Wright G, Berghuis A. Crystal structure of an aminoglycoside 6′-N-acetyltransferase: defining the GCN5-related N-acetyltransferase superfamily fold. Structure. 1999;7:497–507. doi: 10.1016/s0969-2126(99)80066-5. [DOI] [PubMed] [Google Scholar]
- 49.Yan K, Pearce K, Payne D. A conserved residue at the extreme C-terminus of ftsZ is critical for the FtsA-FtsZ interaction in Staphylococcus aureus. Biochem Biophys Res Commun. 2000;270:387–390. doi: 10.1006/bbrc.2000.2439. [DOI] [PubMed] [Google Scholar]
- 50.Yan M, Chen X, Militello K, Hoffman R, Fernandez B, Baumann C, Gollnick P. Alanine-scanning mutagenesis of Bacillus subtilis trp RNA-binding attenuation protein (TRAP) reveals residues involved in tryptophan binding and RNA binding. J Mol Biol. 1997;270:696–710. doi: 10.1006/jmbi.1997.1149. [DOI] [PubMed] [Google Scholar]