Abstract
This review synthesizes relations between mindfulness and resting-state fMRI functional connectivity of brain networks. Mindfulness is characterized by present-moment awareness and experiential acceptance, and relies on attention control, self-awareness, and emotion regulation. We integrate studies of functional connectivity and (1) trait mindfulness and (2) mindfulness meditation interventions. Mindfulness is related to functional connectivity in the default mode (DMN), frontoparietal (FPN), and salience (SN) networks. Specifically, mindfulness-mediated functional connectivity changes include (1) increased connectivity between posterior cingulate cortex (DMN) and dorsolateral prefrontal cortex (FPN), which may relate to attention control; (2) decreased connectivity between cuneus and SN, which may relate to self-awareness; (3) increased connectivity between rostral anterior cingulate cortex region and dorsomedial prefrontal cortex (DMN) and decreased connectivity between rostral anterior cingulate cortex region and amygdala region, both of which may relate to emotion regulation; and lastly, (4) increased connectivity between dorsal anterior cingulate cortex (SN) and anterior insula (SN) which may relate to pain relief. While further study of mindfulness is needed, neural signatures of mindfulness are emerging.
Keywords: Mindfulness, Meditation, resting-state fMRI, functional connectivity, default mode network, salience network, frontoparietal network, attention control, emotion regulation, self-awareness, posterior cingulate cortex, anterior cingulate cortex, cuneus, MBSR
1. Introduction to Mindfulness Research
1.1. Defining Mindfulness
Mindfulness is a construct with core features of maintaining present-moment awareness and acceptance of psychological experiences. This term can be used to describe aptitudes, processes, or trainings and originally comes from Buddhist meditation (Van Dam et al., 2018). Mindfulness meditation is indeed rooted in the Buddhist tradition of Bhāvanā, from Pali (the liturgical language of ancient Buddhism): to cultivate and improve core mental faculties (Sugunasiri, 2008). In Pali, the term Sati was adopted and translated as mindfulness in western cultures (Sharf, 2014). Although mindfulness is primarily based within Buddhist contemplative traditions, related forms of meditation have been developed in other religious, spiritual, and philosophical traditions. According to a recent neuroscience-based model, mindfulness meditation incorporates a variety of aspects of self-regulation including attentional control, emotional regulation, and self-related awareness (Tang et al., 2015). Mindfulness meditation has been described as encompassing a variety of different mental practices that span a spectrum ranging from focused attention (FA), which is characterized by practices during which attention is systematically and repeatedly directed towards a specific mental object or proprioceptive experience, to open monitoring (OM), which is defined as meta-awareness of present-moment processes (e.g., thoughts, emotions, bodily sensations) without systematically and repeatedly focusing one’s attention on any specific object of attention (Lutz et al., 2015). Mindfulness meditation practices may be predominantly FA or OM or have characteristics of both FA and OM. Moreover, OM practice may lead to nondual awareness, or dissolution of self, referred to in the Tibetan scripture as Dzogchen or “Great Perfection” (R. Bauer et al., 2019) This form of meditation practice is objectless, as opposed to object-centered FA, and consist of in a state of conscious awareness. Neuroscience research on mindfulness encompasses the study of mindfulness meditation training and of mindful aptitudes or “trait mindfulness”.
1.2. Trait Mindfulness
Trait mindfulness refers to dispositional mindful aptitude, that is, an individual’s inherent levels of personality trait mindfulness. Trait mindfulness exhibits inter-individual variability and is often evaluated by using self-report questionnaires such as the Five Facet Mindfulness Questionnaire (FFMQ; Baer et al., 2008), Freiburg Mindfulness Inventory (FMI; Walach et al., 2006), Mindful Attention and Awareness Scale (MAAS; MacKillop and Anderson, 2007), Kentucky Inventory of Mindfulness Skills (KIMS; Baer et al., 2004), Toronto Mindfulness Scale (TMS; Lau et al., 2006), Cognitive and Affective Mindfulness Scale Revised (CAMS-R; Feldman et al., 2007), Philadelphia Mindfulness Scale (PHLMS; Cardaciotto et al., 2008) and Southampton Mindfulness Questionnaire (SMQ; Chadwick et al., 2008). Some trait mindfulness scales only focus on one aspect of mindfulness. For example, the MAAS focuses on the attentional aspect of mindfulness (MacKillop and Anderson, 2007). Other questionnaires quantify trait mindfulness as a multi-faceted construct. For example, the widely used FFMQ measures trait mindfulness as a variety of capacities that are summarized within five mindfulness-related subscales: observation (containing items such as: “I notice the smells and aromas of things”), description (“I am good at finding words the describe my feelings”), action-awareness (“I find myself doing things without paying attention”; reverse-scored), non-judgmental inner experience (“I disapprove of myself when I have illogical ideas”; reverse-scored), and non-reactivity (“I perceive my feelings and emotions without having to react to them”; Baer et al., 2008).
Reviews and meta-analytic syntheses have summarized relations between trait mindfulness and its relation to psychological and behavioral features (Keng et al., 2011; Mesmer-Magnus et al., 2017; Sala et al., 2019). As reviewed by Keng et al. (2011), trait mindfulness has been shown to correlate with a variety of aspects of psychological health, including increased subjective well-being, reduced psychological symptoms and emotional reactivity, and improved behavior regulation (Keng et al., 2011). According to a quantitative meta-analysis conducted on 270 independent studies (Mesmer-Magnus et al., 2017), trait mindfulness correlates with confidence, mental health, emotional regulation, and life satisfaction; conversely, it correlates negatively with perceived life stress, negative emotions, anxiety, and depression. Within the professional domain, trait mindfulness was found to positively correlate with job satisfaction, performance, and interpersonal relations, while also being related to reduced burnout and work withdrawal (Mesmer-Magnus et al., 2017). Overall, these results suggest a broad spectrum of health-related benefits associated with trait mindfulness and an overall ‘healthier’ lifestyle.
1.3. Mindfulness Training
Mindfulness research additionally includes the study of individuals who have completed mindfulness training programs. In 1979, Dr. Jon Kabat-Zinn introduced the Mindfulness-Based Stress Reduction (MBSR), a mindfulness-based training program that has become one of the most widely applied applications of mindfulness training in clinical contexts (Kabat-Zinn, 2003). This 8-week program targets stress reduction and was originally designed to help chronic pain patients improve aspects of self-regulation related to pain management (Kabat-Zinn, 1982). MBSR includes a variety of techniques for training mindfulness. These techniques include mindful “body-scan” practices that involve systematically focusing one’s attention on, and fostering awareness of, different parts of the body. During body scanning practice, the practitioner may start attending to sensations of the toes and then move dorsally toward the head area, traversing and being aware of various regions throughout the process of attentional scanning. The MBSR practitioner also trains in mindfulness of breathing and other perceptions (including tactile contact regions [e.g., the body against the floor], visual objects, a repeated vocalization or sub-vocalization [sometimes called a mantra]), and mindful movement practices including yoga postures. Since the late 1990’s the MBSR training program, and mindfulness training more broadly, has gained considerable momentum with exponential growth of published academic papers that have investigated primarily mental and physical health-related outcomes of these practices and programs.
Indeed, a major reason for the increasing popularity of mindfulness is growing evidence for its non-pharmacological therapeutic impact on both mental and physical health both in clinical and non-clinical contexts. In this sense, mindfulness practices have been linked to improvements in cognitive processes (Chiesa et al., 2011; Gallant, 2016; Malinowski, 2013), stress-management (Chiesa and Serretti, 2009), social cognition (Campos et al., 2019; Tan et al., 2014), and general well-being (Campanella et al., 2014; Howell et al., 2008; Smith et al., 2015) in healthy populations. Clinically, mindfulness has been shown to reduce the severity of symptoms of a variety of conditions, including anxiety (Hofmann et al., 2010), post-traumatic stress disorder (PTSD; Boyd et al., 2018), attention-deficit/hyperactivity disorder (ADHD; Poissant et al., 2019), eating disorder (Wanden-Berghe et al., 2011), substance use disorder (Priddy et al., 2018), and major depressive disorder (MDD; Hofmann et al., 2010; Piet and Hougaard, 2011).
1.4. Mindfulness and Neuroscience
Neuroscience promises to provide a biologically informed mechanistic model of the health-related effects of mindfulness. The neuroscience of mindfulness has mirrored the broader interest in mindfulness and has grown considerably in recent years. Neuroscientific studies of mindfulness meditation have been published in the fields of cognitive and clinical neuroscience, among many others (Hofmann et al., 2010; Kuyken et al., 2019, 2015; Piet and Hougaard, 2011).
Reviews and meta-analyses concerning the neuroimaging of trait mindfulness and mindfulness training remain sparse and most have focused on activation observed during meditation practice of long-term practitioners or novices following a short-term meditation training program. For instance, Fox and colleagues used an activation likelihood estimation (ALE) approach that included 25 studies in order to identify brain regions whose activity is related to meditation (Fox et al., 2016). The authors argued that dissimilarities between effects of distinct forms of meditation may be greater than their similarities, and, in their meta-analysis, separated PET and functional MRI (fMRI) neuroimaging results according to the type of practice studied: FA, OM, mantra recitation (including transcendental meditation; the repetition of a sound, word, or sentence that is thought to improve concentration), and loving-kindness (meditation practices that cultivate compassion and love for self and others). Predominantly FA meditation techniques were related to increased activation of the left supplementary motor area (SMA; Brodmann Area (BA) 6) and dorsal anterior cingulate cortex (dACC; BA 24), and conversely, deactivation of medial posterior cingulate cortex (PCC; BA 30) and left inferior parietal lobule (IPL; BA 39). OM practices were related to increased activation in the SMA (BA 6), dACC/SMA (BA 32/6), left mid/anterior insular cortex (BA 13), left inferior frontal gyrus (IFG; BA 44/45) and left SMA (BA 6). Conversely, the right pulvinar in the thalamus was associated with deactivation in OM practitioners. However, as emphasized by Fox and colleagues, a key issue pertinent to their meta-analysis was that study designs and meditation practice experience varied vastly across studies, ranging from 4 years of experience to 40 years on average (Fox et al., 2016). This variability may have influenced reported results, which may have rather been related to meditators’ “trait” and/or lifestyle differences.
A subsequent meta-analysis of 21 studies partially addressed this issue by separating fMRI results by level of experience of the practitioners (expert meditators vs. novice participants; Falcone and Jerram, 2018). When contrasting neural activation during mindfulness meditation state vs. a baseline control condition, the ALE approach highlighted increased activation in prefrontal brain regions, rostral ACC (rACC), and insula during a state of mindfulness meditation in both expert and novice mindfulness practitioners. Novice practitioners additionally exhibited increased activity in the insula, whereas expert practitioners exhibited increased activity in the medial frontal gyrus (containing the SMA) and globus pallidus.
Taken together, these results suggest that both FA and OM meditation are associated with distinct patterns of increased activity in regions of the frontal lobe, notably the SMA and rACC; as well as, additionally, the insular region, located deep in the lateral sulcus of the brain (Uddin et al., 2017).
FA meditation is specifically linked to deactivation in the PCC (Falcone and Jerram, 2018; Fox et al., 2016), whereas OM meditation is associated with deactivation in subcortical regions notably the pulvinar and the thalamus (Fox et al., 2016). Additionally, more experience with meditation was linked to increased activation in medial frontal regions, whereas novice practitioners exhibited increased activation in the insula (Falcone and Jerram, 2018). Overall, even though they show distinct patterns, meta-analyses focused on neural correlates of mindfulness remain difficult. Indeed, a large number of articles were excluded (n=53; Fox et al., 2016) because of heterogeneity in data acquisition.
A conceptual model of mindfulness previously proposed by Hölzel et al. suggests that neuroplastic changes in the anterior cingulate cortex, insula, temporo-parietal junction, fronto-limbic network, and the task-negative default mode network (DMN) are associated with enhanced self-regulation mediated by mindfulness, and specifically includes attention regulation, body awareness, emotion regulation and change in perspective on the self (Hölzel et al., 2011). Another model proposed by Vago and Silbersweig has linked mindfulness to putative neurobiological explanations, that is: mindfulness is described as mental training that leads to increased Self-Awareness, Self-Regulation and Self-Transcendence (the “The S-ART model”;(Vago and Silbersweig, 2012). According to the S-ART model mindfulness fosters the development of awareness that transcends self-focus and has prosocial characteristics. This model relies on neurobiological substrates including functional connectivity changes in task-positive networks focused on the self (enactive experiential self; experiential phenomenological self), task-negative network DMN, and cognitive control network, linked to neuroscientific findings, directly or indirectly linked to mindfulness in order to inform future mindfulness research.
In accordance with these previous models from Vago and Silbersweig and Hölzel et al, the current review directly investigates mindfulness-mediated functional connectivity modulation of key cortical regions previously described in the literature, including: anterior and posterior cingulate cortex, insular region (Falcone and Jerram, 2018; Fox et al., 2016; Hölzel et al., 2011), as well as resting-state large-scale brain networks (Hölzel et al., 2011; Vago and Silbersweig, 2012). As previously described in the literature, this review corroborates the important role of those key regions regarding mindfulness. This review builds on Tang et al’s model of pillar concepts of mindfulness improving self-regulation –attentional control, emotional regulation, and self-related awareness - (Tang et al., 2015) in order to define a resting-state functional connectivity (rsFC)-based neurobiological framework of mindfulness. The current review provides a mechanistic explanation and link between mindfulness-related mental aptitudes and modulation of functional connectivity (Box1).
Box 1. Introduction to Resting-state fMRI Functional Connectivity.
As reviewed in the prior section, considerable evidence suggests that mindfulness meditation modulates brain function. Many of these studies focused on neural activation differences between meditative states and non-meditative restful states. While less widely studied in the context of mindfulness meditation, functional interactions among brain regions, sometimes called inter-regional “functional connectivity” or “co-activation”, is garnering increased attention in human neuroscience. Indeed, this burgeoning field is aimed toward characterizing inter-regional synchronized low frequency (<0.1 Hz) spontaneous fluctuations during resting, or non-task, states (Snyder and Raichle, 2012). Resting-state functional connectivity (rsFC) is computed as temporal relations, such as Pearson correlation, between the blood-oxygen level-dependent (BOLD) fMRI timeseries of different brain regions. Stronger temporal relations are generally interpreted as indicating greater functional connectivity, or interaction, between regions (Mohanty et al., 2020).
Functional connectivity fMRI has led to the discovery of sets of highly functionally connected brain regions that are often referred to as resting-state brain networks or systems (Damoiseaux et al., 2006). For instance, sensory and motor regions are organized in specific networks, including visual, auditory, motor, and somatosensory networks (Beckmann et al., 2005). Additional networks are implicated in higher-order processes and include the default mode network (DMN; Raichle et al., 2001), bilateral frontoparietal network (FPN; often called the central executive network [CEN]; Seeley et al., 2007), and salience network (SN; Downar et al., 2002). Resting-state large-scale brain networks are hypothesized to be the result of differential human evolution that has favored cortical expansion of association regions. Those cortices are involved in higher-order top-down processes (including cognitive control). This theory is supported by the visualization of cytoarchitectural properties of those regions and their laminar projections which provide an estimate of underlying cortical circuits (Buckner et al., 2013).
In the context of mindfulness research, rsFC offers new insights. For a long time, neuroscience research considered those “task-negative low frequency fluctuations” as noise or random (Deco et al., 2011). Biswal and colleagues analyzed resting-state fluctuations for the first time in 1995 and discovered sets of highly co-activated neuroanatomical regions when subjects were not engaged in a task (Biswal et al., 1995). The observation of consistently distributed activity during a restful state led to the study of network dynamics independently from a task. Indeed, task-free paradigms offer the advantage of reproducibility across different populations and study designs (Deco et al., 2011; Mulders et al., 2015). This is particularly interesting in the context of mindfulness research. Indeed, above-described mindfulness meditation fMRI activation studies are highly heterogenous in terms of the type of meditation practiced during fMRI acquisition by participants with different experience levels (Falcone and Jerram, 2018; Fox et al., 2016), and rarely relate to changes mediated by trait mindfulness (Lutz et al., 2014). RsFC offers a new understanding of neural correlates of mindfulness while avoiding meditation-style specific activation correlates by observing participants during a restful state. Spontaneous neural oscillations resulting from evolutionary structural adaptations of high specificity (Buckner et al., 2013), differences in rsFC may indicate underlying differences in brain functioning. Indeed, rsFC can additionally be used to visualize abnormalities in co-activation patterns (Fox and Greicius, 2010; Lee et al., 2013). Those dysregulation of brain networks are often linked to neuropsychiatric disorders and have been postulated to underlie neuropsychiatric symptoms (Greicius, 2008; Woodward and Cascio, 2015). There is a burgeoning interest in using functional connectivity methods in human neuroscience to advance neural models of myriad aspects of health and disease (Smitha et al., 2017).
In this context, a growing literature reports the use of resting-state fMRI to investigate functional connectivity, and to map large-scale brain systems, in relation to trait mindfulness and mindfulness meditation training, and the effects of mindfulness training on physical and mental illness.
1.5. Brain Networks and Mindfulness
Several resting-state networks have been most consistently linked to mindfulness-related modulation of their functional connectivity, including both within and across networks. Notably, Uddin and colleagues (Uddin et al., 2019) proposed six large-scale networks referred to with anatomical nomenclature: occipital, pericentral, dorsal frontoparietal, lateral frontoparietal (FPN), midcingulo-insular, and medial frontoparietal networks. Their cognitive domain nomenclature is, respectively, the visual, somatomotor, attention, control, salience (SN) and default mode networks (DMN). Mindfulness has been primarily related to functional changes in the DMN, lateral FPN, and SN.
In this review, we first provide an anatomical description of major network nodes; next, each network is described in relation to cognition and function, followed by a description of each of these networks and their relation to mindfulness; finally, the review discusses interactions among networks and the relations of these interactions to mindfulness.
The DMN (Greicius et al., 2003; Raichle et al., 2001) is comprised primarily of nodes in bilateral medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), precuneus and medial temporal regions (Greicius et al., 2009). The PCC and mPFC are commonly used as a priori-defined seed regions when assessing the DMN. Importantly, a large cortical region involves task-negative functional connectivity to the DMN: posterior medial cortex (PMC). Of note, this region consists of highly functionally and architecturally heterogenous subregions. Specifically, it includes the above-mentioned posterior cingulate cortex (PCC), the retrosplenial cortex and precuneus (Bzdok et al., 2015). It is notable that the PCC, in itself, is a highly parcellated and heterogenous region of association cortex, with distinct cytoarchitectural, functional and structural properties in its ventral and dorsal regions (Bzdok et al., 2015; Leech and Smallwood, 2019; Scheperjans et al., 2008). The DMN is generally deactivated during attention-demanding tasks and activity of the DMN has been associated with self-referential and social processes, retrospective and prospective memory, and mind-wandering (Andrews-Hanna, 2012; Poerio et al., 2017). Abnormalities in DMN activity and functional connectivity have been linked to psychiatric disorders, including major depressive disorder (Hamilton et al., 2015; Sambataro et al., 2014; Wise et al., 2017). Mindfulness meditation training has been shown to down-regulate activity of the DMN (Brewer et al., 2011; Garrison et al., 2015). This may be explained by the fact that DMN-related processes, including mind-wandering and self-reflection, are conceptually opposed to the present-moment awareness component of mindfulness (Brewer et al., 2011).
Activity and connectivity of the lateral FPN has been implicated in cognitive control. The lateral FPN, referred to as FPN in this review, consists of nodes including bilateral dorsolateral prefrontal cortex (dlPFC), premotor cortex (PMC), inferior parietal sulcus (IPS), and IPL, as well as the rostrolateral prefrontal cortex (rlPFC). The dlPFC is commonly used as an a priori-defined seed region in rsFC analyses to quantify effects related to the FPN (Dixon et al., 2018). The FPN is generally thought to be involved in cognitive control including the monitoring and processing of perceptual, interoceptive, and cognitive information (Dixon et al., 2018). More precisely, FPN can be further separated into two relatively functionally distinct subsystems. One subsystem of the FPN involves regions including the dlPFC and PMC and communicates more closely with the DMN. This subsystem is called the FPNA. Another subsystem, called FPNB, includes other regions including the IPS and communicates more with the attention network. The FPNA subsystem is thought to be involved in internally-focused attention and interoceptive processes whereas the FPNB subsystem is thought to be involved in external attention (Dixon et al., 2018; Vincent et al., 2008). Notably, the rlPFC (BA 10) is hypothesized to play a role in switching between internally and externally-focused attention (Burgess et al., 2007). In the sense, this would be a very valuable attribute as mindfulness often aims at reorienting one’s attention from internal self-focused cognitive processes (e.g., past-oriented and future-oriented thoughts including worry and rumination) to other processes (e.g., bodily sensations). Importantly, rlPFC has additionally been evidenced to integrate several cognitive processes for a behavioral goal (Ramnani and Owen, 2004). Taken those information together, it is hypothesized that the rlPFC is a flexible hub that facilitates adaptive functional connectivity and switching between networks according to ongoing task demands (Cole et al., 2013; Desrochers et al., 2015; Gilbert et al., 2005). As such, the rlPFC is thought to be a core component of cognitive control. Overall, FPN regions have been shown to be involved in sustaining attention through integration of bottom-up perception (Ptak, 2012). Given this role, and taking all of this into account, the FPN is sometimes theorized to facilitate mindful present-moment interactions with the environment (Hasenkamp et al., 2012; Kajimura et al., 2020; Taren et al., 2017; Vago and Zeidan, 2016). Future neuroscience studies of mindfulness should evaluate rlPFC, particularly in seed-based studies. This would explicit rlPFC’s role in mindfulness, especially with regard to attention control.
The activity and connectivity of the SN has been widely implicated in salience processing, that is, the processing of elements that stand out from their environment (Uddin, 2015). The SN is composed of primary nodes of bilateral anterior insular cortex and dACC, which are often used as seeds for seed-based approaches, in addition to other subcortical and limbic structures including the amygdala (Seeley et al., 2007). The anterior insula receives interoceptive and external sensory information from other parts of the brain, and has been shown to function as a detector of behaviorally relevant information (Menon and Uddin, 2010). The dACC has been implicated in response selection and conflict monitoring (Ide et al., 2013; Menon, 2015). It has been proposed that the insular and dACC SN nodes act as a “switch” between rsFC of the DMN, which is activated when individuals are not engaging in a cognitively demanding task, and the FPN, which is activated during cognitively challenging tasks that require attention (Sridharan et al., 2008). Evidence for the switching nature of the SN in relation to the DMN and FPN has been shown using Dynamic Causal Modelling (DCM): an fMRI functional connectivity method that provides directionality of functional interactions (Goulden et al., 2014). This switching role of the SN has been theorized to be involved in mindfulness, that is, by the SN favoring FPN activity over DMN activity as a result of mindfulness (Doll et al., 2015). Indeed, rapid switching is thought to be important in mindfulness to refocus attention to present-moment awareness instead of mind wandering. SN has been observed to be involved in the awareness of mind-wandering (Hasenkamp et al., 2012).
Related to this switching role of SN nodes and mindfulness, Hasenkamp and colleagues proposed a neuroscience-based model that describes mindfulness meditation in terms of a constant cycling between four different states that are supported by specific brain networks: (1) mind-wandering mediated by the DMN; (2) awareness of mind-wandering mediated by the SN; and (3) shifting of attention: and (4) sustained attention both implicating attentional subnetworks (Hasenkamp et al., 2012). This model would imply constant interactions between the three main networks during mindfulness and cycling between focused-attention and mind-wandering. Through functional connectivity analyses, the relationships between these networks regarding mindfulness can be further understood.
To observe mindfulness-mediated connectivity changes between these brain networks, below-described studies use two main approaches: seed-based or Independent Component Analysis (ICA) methods. The seed-based approach is a model-based method. Seed-based functional connectivity computes correlations between the time-courses of an a priori defined region of interest (ROI) called a “seed” to other target regions, which may include all voxels of the entire brain (Biswal et al., 1995). Greater correlations between the seed and target region are thought to indicate stronger functional interactions between these regions. Above-mentioned relevant anatomical nodes of networks are used as seeds. Conversely, the ICA method (Bell and Sejnowski, 1995; Calhoun et al., 2001) is a whole-brain model-free method that provides a more data-driven and holistic approach to quantifying functional connectivity. ICA is a computational approach that decomposes BOLD fMRI signal time courses from the whole brain into spatially and temporally independent components. It is based on the separation of noise from low frequency neural fluctuations (<0.1 Hz) that are thought to characterize functionally communicating regions during the resting-state (Venkataraman et al., 2009). Despite their differences, the seed-based and ICA functional connectivity methods described here often provide complementary information that generally replicates across studies. In this context, seed-based approaches focus on the mindfulness-mediated functional connectivity changes observed in relevant nodes of networks in relation to other anatomical regions. Studies that have implemented ICA to assess mindfulness-related change in functional connectivity describe network features as components. Seed-based and ICA methods help understand mindfulness-mediated changes within and between large-scale brain networks.
The influence of mindfulness meditation on each of the above-described networks has been shown using ICA or seed-based methods and a variety of study designs (Table 1) that will be described in the subsequent sections.
Table 1.
Resting-state fMRI Functional Connectivity Studies of Mindfulness. Study design, mindfulness specificity, rsFC methodology and summary of major findings of each mindfulness and rsFC article. N = number of participants.
| Article | Study Design | Participants | Mindfulness specificity | rsFC specificity | Findings (with effect sizes or z-scores) |
|---|---|---|---|---|---|
|
| |||||
| Trait mindfulness: within non-practitioners | |||||
| Bilevicius et al. | Trait mindfulness and rsFC | meditation-naïve (n = 32) | Trait, MAAS | ICA: DMN, SN and FPN | ↗ trait mindfulness linked to FC: ↗ SN and left insula (r = 0.60) ↘ SN and right cuneus (r = −0.70) ↘ right FPN and left cuneus (r = −0.55) ↘ left FPN and left precuneus (r = −0.61) ↗ right FPN and right MFG (r = 0.64) ↗ DMN and right MFG (r = 0.54), right PHG (r = 0.62), left caudate (r = 0.60) ↘ DMN and left MFG (r = −0.66), left STG (r = −0.47), left insula (r = −0.62) |
| Parkinson et al. | Trait mindfulness and rsFC | meditation-naïve (n = 29) | Trait, FFMQ | ICA: DMN, SN, FPN, ATN (VAN and DAN) | ↗ trait mindfulness (FFMQ total score and subscales) linked to FC: ↘ STG and DMN (r = −0.77; Total score), ATN (r = −0.68; Describing subscale), FPN (r = −0.60; Non-reactivity subscale) ↘ SFG (in dmPFC, in DMN) with FPN (r = −0.60; Total score) ↗ insula and ATN (r = 0.75; Observing subscale) ↗ mid-cingulate gyrus and DMN (r = 0.59; Non-judging subscale) ↗ cuneus and DMN (r = 0.63; Acting), SN (r = 0.78; Total, r = 0.68; Acting and r = 0.65; Non-judging subscales) |
| Trait mindfulness: meditation experts vs naïve | |||||
| Bauer et al. | Trait (naïve vs. experienced) and state (resting vs. meditation) mindfulness | meditation-naïve (n = 17) and practitioners (n = 16) | Trait and state | seed-based from fALFF: mPFC for DMN, bilateral IFG and IPL for FPN |
Trait differences in FC, experts vs naïve: ↘ DMN and left SFG, right MFG, IPL and STG ↘ between DMN and FPNa ↘ between mPFC of DMN and MFG positively correlated to experience (r = 0.87) |
| Froeliger et al. | Trait (naïve vs experienced) and state (resting vs meditation) mindfulness | meditation-naïve (n = 7) and practitioners (n = 7) | Trait and state | Seed-based network analysis: DMN, DAN, FPN, SN nodes |
Trait differences in FC, experts vs naïve: ↗ within DAN: right IPL and left FEF (Cohen’s d between 1.3 and 1.7) ↗ between DAN and DMN, ↗ FPN and SN positively correlated to experience (r > 0.71) |
| Mindfulness Training | |||||
| Kilpatrick et al. | Longitudinal approach: 8-week MBSR vs waiting list controls | meditation-naïve (n = 32) | MBSR | group ICA to investigate ICNs |
Training-mediated FC differences: ↗ within auditory network(Cohen’s d = 0.85) and within visual network (Cohen’s d = 1.04) ↘ between auditory and visual networks (Cohen’s d = 0.90) ↘ rACC and visual network(Cohen’s d = 0.84) ↗ rACC and dmPFC of DMN (Cohen’s d = 0.83) ↘ cuneus of DMN and “SN” (Cohen’s d = 0.92) |
| Doll et al. | Longitudinal approach: 2-week audio recording | meditation-naïve (n = 26) | MBSR-based audio recordings daily for two weeks | ICNs: DMN, FPN and SN |
Training-mediated FC differences: ↘ between insula of SN and DMN (r = −0.14) |
| Kral et al. | Longitudinal approach: 8-week MBSR group vs HEP or waiting-list controls | meditation-naïve (n = 140) | MBSR | seed-based: PCC for DMN and dlPFC for FPN |
Training-mediated FC differences: ↗ between PCC (DMN) and right dlPFC (FPN) compared to active (Cohen’s d = 0.28), and passive control group (Cohen’s d = 0.34), no difference after 5.5 months follow-up |
| Yang et al. | Longitudinal approach: 40 days MBSR-based training | meditation-naïve (n = 13) | MBSR-based | seed-based: pgACC and dACC |
Training-mediated FC differences:pgACC seed: ↘ left PCC/precuneus (z-score = 4.71), left dmPFC (z-score = 4.35), right STG (z-score = 4.34), left middle occipital gyrus (z-score = 4.19) ↗ right ITG (z-score = 4.23), right IFG (z-score = 3.84), right TPJ/IPL (z-score = 3.76) dACC seed: ↘ calcarine sulcus, cuneus (z-score = 4.37), ↗ cerebellum (z-score = 4.56), right IPL (z-score = 4.20), PCC (z-score = 4.13) |
| Kwak et al. | Retreat: 4-day mindfulness retreat vs control relaxation retreat | NA, meditation retreat (n = 44), relaxation retreat (n = 23) | 4-day retreat | seed-based: rACC (= pgACC + sgACC) and dACC |
Training-mediated FC differences: ↗ left rACC and DMN: dmPFC, precuneus, angular gyrus ↗ right dACC and PCC(all z-scores > 2.3) |
| Mindfulness and Illness | |||||
| Lifshitz et al. | Longitudinal approach: 2-week MBCT-based audio recording vs active control relaxation | meditation-naïve, MDD, mindfulness (n = 17), relaxation (n = 20) | MBCT audio recordings | seed-based: dlPFC for FPNs, aINS for SN, PCC for DMN |
Training-mediated FC differences: ↘ within FPN: between dlPFC and fusiform gyrus, dlPFC and right angular gyrusa ↘ rsFC correlated to ↘ in depressive scoresangular gyrus (r = −0.505), right fusiform gyrus (r = −0.675), left fusiform gyrus (r = −0.543) |
| Creswell et al. | Retreat: 3-day mindfulness training vs relaxation training | meditation-naïve, chronic stress, mindfulness (n = 18), relaxation (n = 17) | 3-day retreat | seed-based: PCC |
Training-mediated FC differences: ↗ between PCC and left dlPFC (z-score = 3.44) |
| Taren et al. | Retreat: 3-day mindfulness training vs relaxation training | meditation-naïve, chronic stress, mindfulness (n = 18), relaxation (n = 17) | 3-day retreat | seed-based: amygdala with ACC mask |
Training-mediated FC differences: ↘ between sgACC and amygdala (z-score = 3.61) |
| Su et al. | Longitudinal approach: 6-week MBSR training | meditation-naïve with chronic pain (n = 18), or pain-free control (n = 16) | MBSR | seed-based: aINS |
Training-mediated FC differences: ↗ aINS and dorsal anterior midcingulate cortex (z-score = 3.07) |
| King et al. | Longitudinal approach: 8-week MBET vs active control group | Meditation-naïve, PTSD, MBET (n = 14), PCGT control (n = 9) | MBET | seed-based: PCC and vmPFC for DMN |
Training-mediated FC differences: ↗ PCC of DMN and dlPFC of FPN (z-score > 3.66) |
ACC = anterior cingulate cortex;
aINS = anterior insular cortex;
ATN = attentional network;
b = regression coefficient beta
cACC = caudal anterior cingulate cortex;
dACC = dorsal anterior cingulate cortex;
DAN = dorsal attention network;
DMN = default mode network;
dlPFC = dorsolateral prefrontal cortex;
dmPFC = dorsomedial prefrontal cortex;
fALFF = fractional amplitude of low-frequency fluctuations;
FEF = frontal eye field;
FFMQ = five facet mindfulness questionnaire;
FFMQtot = five facet mindfulness questionnaire total score;
FPN = frontoparietal network;
HEP = health enhancement program;
ICA = independent component analysis;
ICNs = intrinsic connectivity networks;
IFG = inferior frontal gyrus;
IPL = inferior parietal lobe;
ITG = inferior temporal gyrus;
MAAS = mindful attention awareness scale;
MBCT = Mindfulness-Based Cognitive Therapy;
MBET = mindfulness-based exposure therapy
MBSR = mindfulness-based stress reduction program;
MDD = major depressive disorder;
MFG = middle frontal gyrus;
MPFC = medial prefrontal cortex;
PCC = posterior cingulate cortex;
PCGT = present-centered group therapy;
PFC = prefrontal cortex;
pgACC = pregenual anterior cingulate cortex;
PHG = parahippocampal gyrus;
PTSD = post-traumatic stress disorder;
r = Pearson correlation coefficient
rACC = rostral anterior cingulate cortex;
ROI = region of interest;
rsFC = resting-state functional connectivity;
SFG = superior frontal gyrus;
sgACC = subgenual anterior cingulate cortex;
SN = salience network;
STG = superior temporal gyrus;
TPJ = temporoparietal junction;
VAN = ventral attention network;
vlPFC = ventrolateral prefrontal cortex;
vmPFC = ventromedial prefrontal cortex;
Results reported without effect sizes or z-scores.
2. Resting-state fMRI Functional Connectivity and Trait Mindfulness
Mindfulness research includes the study of trait mindfulness in relation to fMRI activation. The study of trait mindfulness includes study designs that are different than studies of mindfulness training. Moreover, studies of mindfulness training and trait mindfulness have reported distinct activation patterns compared (see Section 3. Resting-state fMRI Functional Connectivity and Mindfulness Meditation Training). In this sense, several studies investigated rsFC fluctuations correlated to trait mindfulness (Lutz et al., 2014). More studies have to be performed to clearly distinguish neural signatures of dispositional/trait mindfulness from neural signatures of mindfulness training (see Section 5.1. Heterogeneity in Mindfulness Research). It has been hypothesized that individuals who are more mindful will exhibit functional connectivity patterns that are similar to those who practice mindfulness meditation (Wheeler et al., 2017).
Bilevicius and colleagues correlated ICA-based network maps in meditation-naïve individuals with self-reported trait mindfulness as assessed by the MAAS self-report trait mindfulness scale (Bilevicius et al., 2018). Higher trait mindfulness was correlated with decreased functional connectivity of the SN component and the right cuneus, the right FPN component and the left cuneus, as well as decreased functional connectivity of the left FPN component and bilateral precuneus. Of note, the cuneus and precuneus are nodes of the DMN. The cuneus is linked to visual processing (Beason-Held et al., 1998), and could play a role in internally-directed attention (Benedek et al., 2016; See subsection 11.4 Limitations Related to rsFC Methods for the limitation of this type of reverse inference). Notably, the precuneus is extensively linked to self-referential processing and mind-wandering (Utevsky et al., 2014). In this context, these results suggest that trait mindfulness is correlated with decreased functional connectivity of SN and FPNs with a DMN region thought to be involved in mind-wandering processes. In the FPN, as well as in DMN components, MAAS was positively correlated with functional connectivity in the right middle frontal gyrus (MFG) (Bilevicius et al., 2018). These results are consistent with the focus of the MAAS, that is, attentional aspect of mindfulness. The right MFG has been previously shown to be involved in attention re-orienting from an externally driven exogenous stimulus to an internally focused endogenous stimulus, and is hypothesized to act as a major gateway or “circuit-breaker” linking the Ventral Attention Network (VAN) to the Dorsal frontoparietal Attention Network (DAN; Corbetta et al., 2008; Japee et al., 2015; Uddin et al., 2019). The VAN is a less studied network consisting of the temporoparietal junction (TPJ), aspects of the IPL and superior temporal gyrus (IPL/STG), and aspects of the IFG/MFG (Vossel et al., 2014). Lesion studies have determined a marked laterization of the VAN on the right hemisphere (Bartolomeo and Seidel Malkinson, 2019), The VAN has been shown to be involved in the orientation of attention towards unpredicted external exogenous stimuli (Vossel et al., 2014). Conversely, the DAN includes the frontal eye field (FEF) and a region containing the IPS and superior parietal lobule (IPS/SPL). This network is linked to top-down control of attention activated by endogenous stimuli, a goal-directed type of attention (Spreng et al., 2010). In this sense, the right MFG is involved in reallocating attention to a chosen stimulus, a mental process that is a core component of mindfulness, particularly, in FA practices. In the DMN component, trait mindfulness was negatively correlated with functional connectivity to the left MFG and the left STG (Bilevicius et al., 2018). Although Bilevicus et al. state that the MFG and STG are key nodes of the DMN, these regions are not always assigned to the DMN, but rather sometimes the VAN (Vossel et al., 2014). Functional connectivity of the PCC and ventromedial prefrontal cortex (vmPFC) nodes of the DMN have been shown to correlate negatively with the left MFG region but positively with the right MFG region (Uddin et al., 2008). Reasons causing this asymmetry remain unclear, although they could be due to the above-described lateralization of the VAN, which could thus explain lateralized results in rsFC (Bartolomeo and Seidel Malkinson, 2019). Regarding the DMN, high trait mindfulness scores related to decreased functional connectivity of the DMN component with the left insula, a key component of the SN. High MAAS scores were linked to increased functional connectivity in the SN network with the left insula. Those results suggest that greater trait mindfulness is related to a decoupling between the DMN and SN networks, and, further, that more mindful individuals exhibit increased functional connectivity in the SN.
Whereas Bilevicius and colleagues used the MAAS to investigate trait mindfulness, Parkinson et al. used the FFMQ, which subsumes different mindfulness subscales, including Observing, Describing, Acting with Awareness, Non-judging of Inner Experience, and Non-reactivity to Inner Experience (Parkinson et al., 2019). Parkinson et al. correlated FFMQ total and subscale scores with functional connectivity patterns of ICA-derived components: the DMN, SN, bilateral FPN, and ATN (defined as the “attentional network”; encompassing the VAN and the DAN). They observed an overall increased cuneus-SN connectivity related to Total, Acting and Non-judging subscales which is opposite to above-reported decreased cuneus-SN connectivity (Bilevicius et al., 2018). They corroborated the results of Bilevicius and collaborators, that is, increased functional connectivity in the SN component with the left insula correlated with higher total FFMQ scores. They also reported a similar positive correlation linking the Observing subscale of the FFMQ to connectivity between the ATN component and the insula (Parkinson et al., 2019). The Observing subscale measures the attentional component of mindfulness. The insula is a primary hub of the SN, and is thought to support the initiation of appropriate behaviors by integrating salient events and mediating communication between several large-scale networks involved in attentional functions and cognitive control (Menon and Uddin, 2010). Indeed, this formulation is consistent with the results of Parkinson et al. including observed increased functional connectivity between the insula and the ATN related to mindfulness. This increased connectivity reported by Parkinson et al. could facilitate the theorized “switch” role of the insular node of the SN (Goulden et al., 2014; Sridharan et al., 2008), and thus provide a framework that links attentional networks and mindfulness.
Several studies have assessed relations between rsFC and trait mindfulness by comparing experienced meditators to meditation-naïve individuals (Bauer et al., 2019; Froeliger et al., 2012). Instead of using self-report questionnaires to quantify levels of mindfulness, they compared baseline connectivity differences between meditation-naïve subjects and experienced practitioners. Theoretically, this design is supported by the argument that long-term meditation practice leads to changes in brain connectivity and altered trait levels of mindfulness (Luders et al., 2011). That is, this design assumes that experienced mindfulness meditation practitioners exhibit greater trait mindfulness than meditation-naïve individuals (the implications of this assumption are further discussed in subsection 12.4 Limitations of Correlational and Cross-Sectional Studies of “Trait” Mindfulness.)
In the study conducted by Bauer et al., experienced meditators were selected based on extended practice (an average of 1600 hours) of Vipassanā meditation and compared to meditation-naïve controls. This time, instead of correlating questionnaire results to functional connectivity patterns in meditation-naïve individuals, researchers compared functional connectivity correlates of experienced practitioners to meditation-naïve participants. Several findings were consistent across the two different study designs (Bauer et al., 2019; Bilevicius et al., 2018). First, reduced functional connectivity between the DMN seed of mPFC and left SFG (node of the DMN) was confirmed in experienced practitioners compared to meditation-naïve participants (Bauer et al., 2019). Second, relative to meditation-naive individuals, experienced practitioners were characterized by decreased connectivity between the DMN and STG. Similarly, the DMN of experienced practitioners included decreased functional connectivity with the IPL region of FPN (Bauer et al., 2019; Bilevicius et al., 2018). Third, seed-based analysis using a DMN-based mPFC seed revealed reduced functional connectivity with the right MFG in experienced practitioners compared to meditation-naïve individuals (Bauer et al., 2019). This finding contradicts results obtained by assessing trait mindfulness with a self-report questionnaire within the meditation-naïve cohort described above: mindful individuals had increased right MFG-DMN connectivity (Bilevicius et al., 2018). This contradictory finding could stem from distinct experimental paradigms: the former study correlated participants’ mindful aptitudes to rsFC patterns whereas the latter study investigated rsFC differences between long-term meditation practitioners and non-practitioner. Mindful participants and long-term practitioners could have distinct connectivity patterns. However, similarities are superior to dissimilarities and show overall reduced within-DMN connectivity, reduced connectivity between the DMN and the STG, and reduced DMN-FPN connectivity in mindful individuals or experienced practitioners compared to less mindful individuals or meditation-naïve participants.
Froeliger and colleagues enrolled experienced meditators that practiced daily for 5 years on average and compared their functional connectivity patterns to meditation-naïve participants. They focused on the DAN, and found increased functional connectivity in experienced practitioners within the DAN related to IPS and the FEF nodes (Froeliger et al., 2012) as well as the visual area MT. Previous findings in rsFC suggest interactions between visual areas and the DAN (Yeo et al., 2011). Visual areas could play an important role in mindfulness especially linked to a higher DAN connectivity: they may be activated to bring attention to a present sensory stimulus (e.g., a point of visual focus). Froeliger et al. found increased connectivity between the DAN and DMN as well as between the FPN and the SN for more experienced mindfulness meditation practitioners. However, these results should be considered carefully due to the small sample size (n = 7 in each group). Further research should be conducted using this paradigm in addition to larger samples in order to clearly state differences correlated to duration/amount of prior meditation practice.
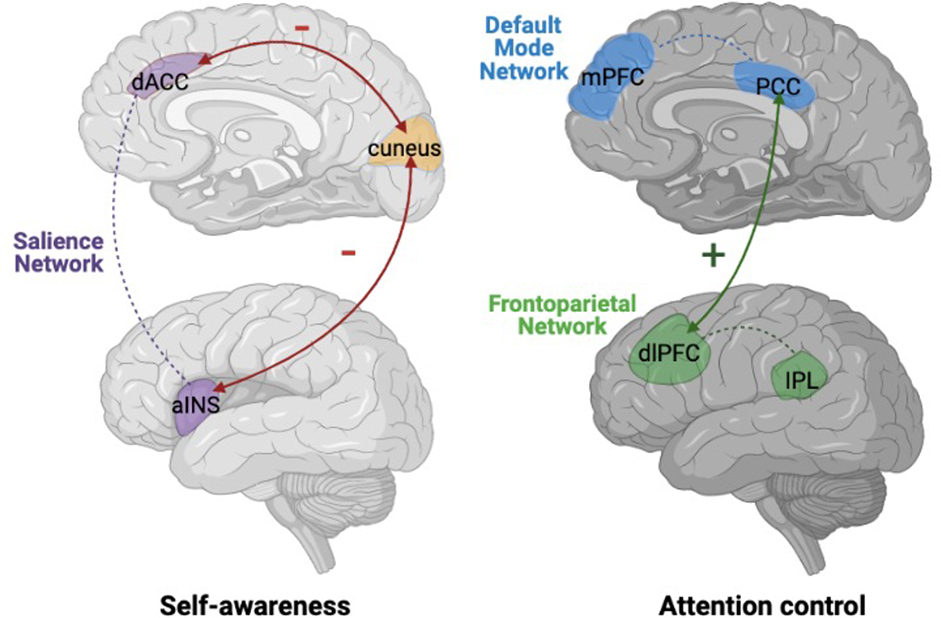
Taken together, these findings from different study designs assessing trait mindfulness in relation to functional connectivity patterns exhibit several patterns. First, decreased functional connectivity between the cuneus and the SN has been related to trait mindfulness (Bilevicius et al., 2018). Additionally, the STG (a node of the VAN) is related to a decoupling with the DMN in individuals with greater trait mindfulness (Bauer et al., 2019; Bilevicius et al., 2018; Parkinson et al., 2019). While the function of the STG and its relations with the DMN remain unclear, some evidence suggests that this region is implicated in visuotemporal attention (Shapiro et al., 2002). Results across studies also suggest that trait mindfulness is related to an overall decoupling of the DMN and FPN (Bauer et al., 2019; Bilevicius et al., 2018; Parkinson et al., 2019). This differential functional connectivity relating to trait mindfulness could be due to a lesser need for mindful individuals to suppress basal DMN activity, which has been related to mind-wandering (Poerio et al., 2017). More mindful individuals may allocate their cognitive processes towards maintaining attention. Furthermore, more mindful individuals exhibited decreased functional connectivity between the SN and the DMN (Bilevicius et al., 2018), and increased connectivity within the SN (Bilevicius et al., 2018). These relations may be linked to the hypothesized switching role of the SN (Goulden et al., 2014). That is, this differential connectivity could prioritize connectivity with the FPN instead of the DMN, which may be mediated by the SN. This hypothesis should be directly tested in future research. Together these findings are starting to indicate evidence for the primacy of awareness of sensation and attention in mindfulness, rather than self-referential processing, and suggest a neural framework for trait mindfulness.
3. Resting-state fMRI Functional Connectivity and Mindfulness Meditation Training
The effects of mindfulness meditation training on functional connectivity have been assessed using longitudinal designs with fMRI collected both before and after mindfulness meditation training. In these studies, mindfulness meditation training programs have included the traditional 8-week MBSR course (Kilpatrick et al., 2011; Kral et al., 2019), MBSR-based trainings such as a self-observation training (Yang et al., 2016), listening to daily recorded audio mindfulness meditation instructions for 2 weeks (Doll et al., 2015), to a few days of intensive mindfulness meditation retreat (Kwak et al., 2019). Participants who completed meditation training were generally compared to active (e.g., relaxation-based training or general health training such as the Health Enhancement Program; Kral et al., 2019) or passive control groups (i.e., waitlist; Kilpatrick et al., 2011; Kral et al., 2019). Some within-subject studies did not include an active or passive control condition, and the control condition only related to functional connectivity patterns of participants before the training (Doll et al., 2015; Yang et al., 2016), for issues raised by this approach, see Subsection 5.6.
Limitations of Studies of Mindfulness Meditation Training.
Kilpatrick and colleagues compared rsFC in a MBSR group (8 weeks of mindfulness meditation training) to a waitlist passive control group (Kilpatrick et al., 2011). They instructed participants to close their eyes and mindfully pay attention to scanner sounds during fMRI acquisition. They found increased functional connectivity within auditory and visual networks and decreased connectivity between them in the active MBSR group compared to controls. The observed effects related to the auditory network, a network not often focused on in other studies of mindfulness, and could have been due to participants having been instructed to “listen to sounds” during acquisition of the fMRI data. In fact, other studies described in this review do not report auditory network functional connectivity changes. This could be explained by the fact that in other paradigms they do not explicitly tell subjects to focus their attention to the surrounding sounds during the scan. The observed effects may thus be related more so to an auditory FA style of meditation rather than OM, as subjects focused their attention on sounds. Moreover, future studies should investigate Increase of functional connectivity within the visual network. It is hypothesized that resting with eyes closed would increase functional connectivity in the retrosplenial cortex, implicated in scene viewing (McAvoy et al., 2008). Another theory is based on the role of attention system on sensory stimuli: inhibiting irrelevant sensory stimuli and enhancing relevant sensory stimuli (Kropotov, 2016). Through mindfulness, there could be increased attentional awareness of sensory stimuli normally suppressed. Overall, results of this particular study are surprising and difficult to interpret as the ICA method used composite networks such as the auditory/salience network and visual/auditory network instead of using typically-described large-scale brain networks.
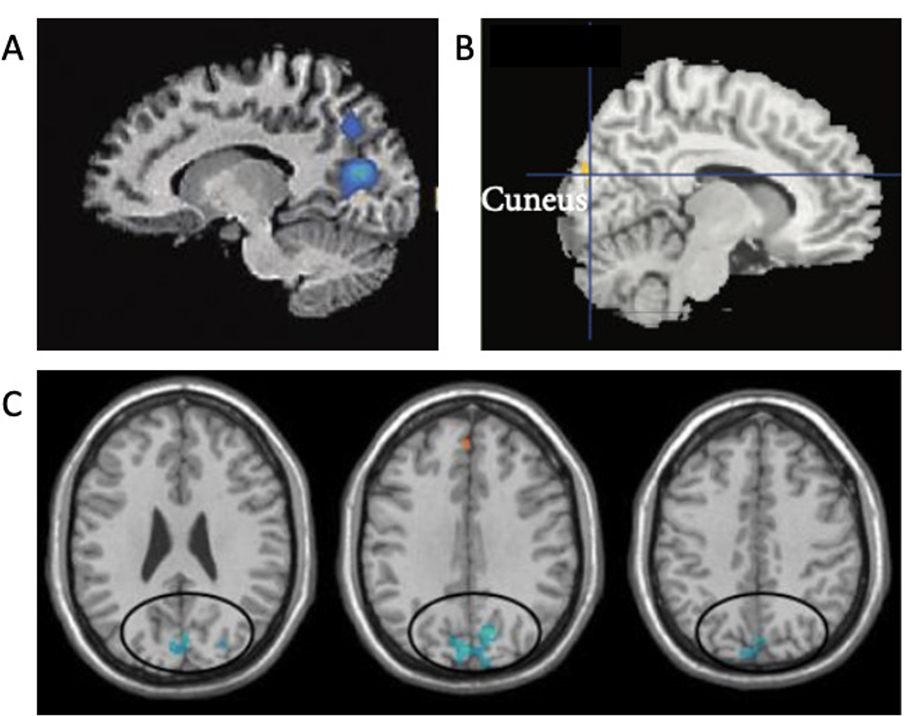
They additionally found increased connectivity between the dorsomedial prefrontal cortex (dmPFC) and pregenual ACC (pgACC), part of the rostral ACC that is encompassing pgACC and the subgenual ACC (sgACC) (Stevens, 2011). Kilpatrick and colleagues interpreted this result as indicating that mindfulness meditators exhibited an increased awareness of attentional and sensory experience, in this case sounds during the fMRI acquisition, rather than engaging in self-referential processing. Kilpatrick et al. also reported decreased functional connectivity in the MBSR training group compared to controls, between a region in the cuneus (part of the posteroventral DMN) and a “composite” network including nodes of the SN, FPN, and auditory network. Decreased functional connectivity of the cuneus with other networks was also identified in the above-described correlational study (Bilevicius et al., 2018).
Doll and colleagues further corroborated this finding by assessing meditation-naïve participants who completed a 2-week audio recording mindfulness meditation training program (Doll et al., 2015). Functional connectivity was compared within the same group before and after training (i.e., there was no passive or active control group). This study focused on functional connectivity of the DMN, SN, and FPN components. Mindfulness meditation training was associated with decreased functional connectivity between the insula region of the SN and the “posteroventral” DMN component. Doll et al. suggest that this result replicates Kilpatrick et al.’s finding of decoupling between the cuneus region—part of the posteroventral DMN—and the insular node of the SN.
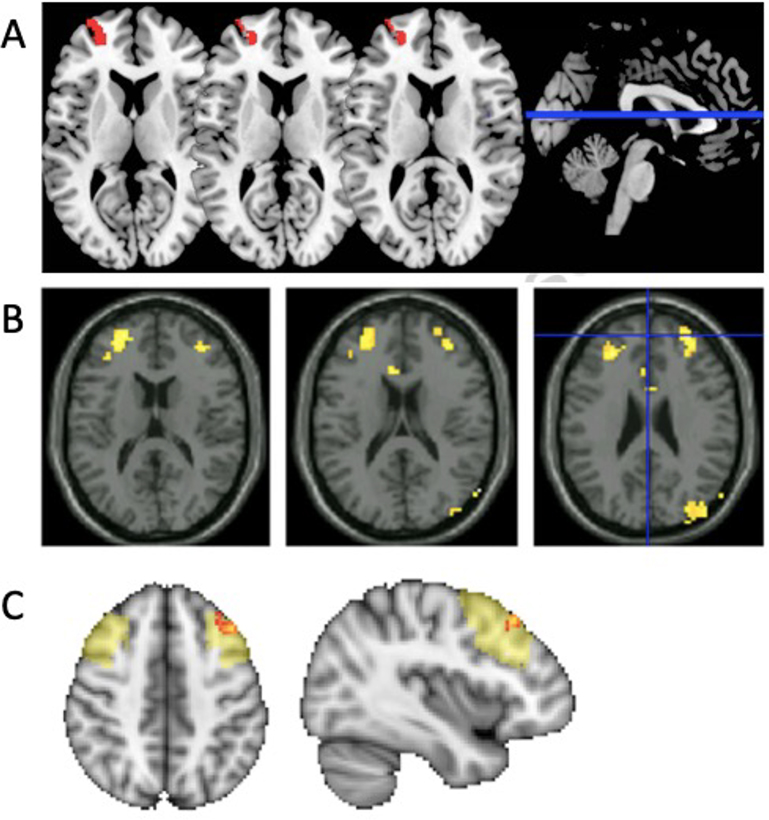
Kral et al. used a seed-based method followed by a whole-brain voxel-wise analysis to compare longitudinal change in functional connectivity between a MBSR group and active Health Enhancement Program (HEP) and passive (waiting) control groups (Kral et al., 2019). Functional connectivity was assessed using a PCC seed and dlPFC target ROI based on a previous study (Creswell et al., 2016). They found that mindfulness training was associated with increased connectivity between the PCC seed and the right and left dlPFC regions. Connectivity between PCC and dlPFC was also linked to decreased mind-wandering as assessed by experience sampling, which was assessed using text-messages sent 6 to 8 times a day that included surveys of the subject’s attention. These functional connectivity effects were not sustained at an approximately 6 months follow-up fMRI assessment. Overall, Kral et al.’s results suggest that mindfulness training is associated with an increased coupling between the PCC node of the DMN and the dlPFC node of the FPNs that is related to decreased mind-wandering.
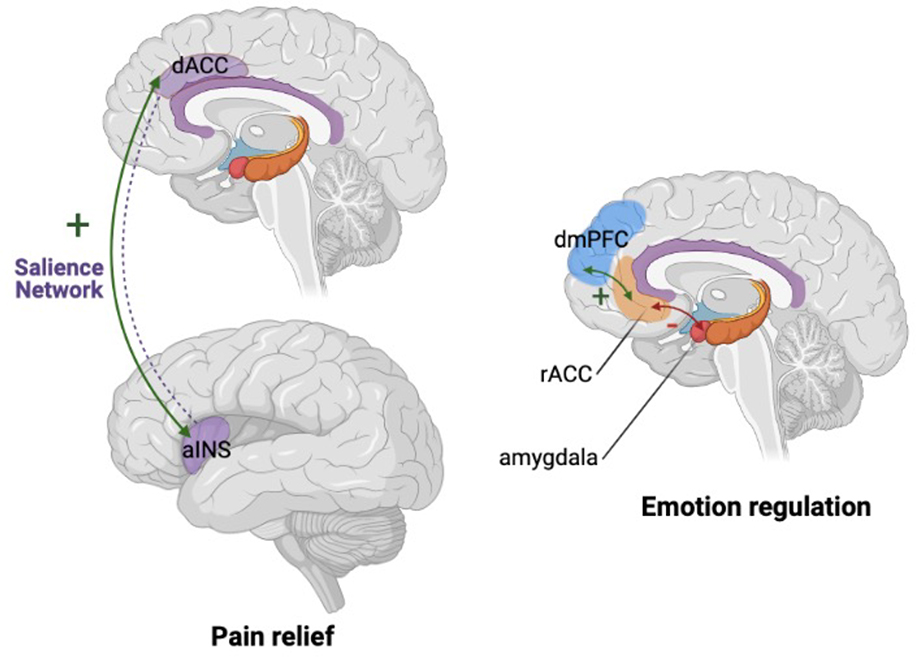
Two studies investigated mindfulness meditation-related rsFC differences of ACC-based network seed regions (Kwak et al., 2019; Yang et al., 2016). One of these studies investigated a 40-day MBSR-based training program (Yang et al., 2016) without a control group, the other included a 4-day intensive mindfulness meditation retreat intervention (Kwak et al., 2019), and compared individuals in this program to those in a relaxation retreat control condition. After intervention, both studies found increased functional connectivity between the dACC and PCC. As the dACC is implicated in control of attentional processes (Benedict et al., 2002; Tian et al., 2006; Weissman et al., 2005) and the PCC in internally directed cognition (Leech and Sharp, 2014), these findings may be related to control of attention and self-reflection. Yang and colleagues additionally found that mindfulness training was associated with increased functional connectivity between the dACC and cerebellum and right IPL, and decreased functional connectivity between the dACC and the calcarine sulcus and cuneus. The latter result corroborates Bilevicius and colleagues’ findings that were reported when comparing meditation-naïve individuals’ trait mindfulness to connectivity patterns (Bilevicius et al., 2018). For the rACC results included increased functional connectivity with the IPL region (including the angular gyrus; Yang et al., 2016) after mindfulness meditation training. Conversely, mindfulness meditation-related functional connectivity between the rACC and dmPFC and the precuneus were inconsistent, with both reports of decreases (Yang et al., 2016) and increases (Kwak et al., 2019) in functional connectivity. Yang and collaborators interpreted the reduced pgACC-DMN connectivity after mindfulness training to the ones found when comparing healthy controls after receiving antidepressant medication (Scheidegger et al., 2012). Because MDD patients exhibit hyperconnectivity of the DMN, especially in relation to the pgACC region (Horn et al., 2010; Sheline et al., 2010), Yang et al. hypothesized that reduced connectivity between pgACC of the rACC and the DMN could be a mechanism for the “antidepressant” effect of mindfulness. Kwak and collaborators interpreted changes in rACC and dmPFC mindfulness training-mediated functional connectivity to a better understanding of the self, arguing that mindfulness meditation strengthens resilience. Indeed, resilience scores increased with mindfulness in the study conducted by Kwak et al. (Kwak et al., 2019). In sum, these two studies show similar effects of mindfulness meditation training on functional connectivity of dACC and rostral ACC regions (Kwak et al., 2019; Yang et al., 2016).
Overall, mindfulness meditation training studies, although varying considerably in terms of paradigms, mindfulness training types and controls groups—or their lack of control groups, exhibited several consistencies. Specifically, these studies highlighted decoupling between key SN nodes and the posterior DMN, in particular the cuneus (Doll et al., 2015; Kilpatrick et al., 2011; Yang et al., 2016). This result mirrors findings from a correlational study between trait mindfulness and functional connectivity patterns (Bilevicius et al., 2018). Furthermore, mindfulness meditation training is associated with increased functional connectivity between PCC DMN and dlPFC FPN regions (Kral et al., 2019) and the dACC node of the SN (Yang et al., 2016). PCC-dlPFC coupling seems, at first glance, contradictory to a generally reported FPN-DMN decoupling associated with mindfulness (Shen et al., 2020) but could be explained by more detailed examination of specific PCC subregions (see section 3.2. Trends in the Literature). Above-described studies state that mindfulness, through emotion regulation practices, could play an important role in alleviating symptoms of psychiatric disorders. The potential neuroprotective effects suggested by Kwak in relation to psychiatric conditions (i.e., major depression) is particularly important for understanding mechanisms in which mindfulness meditation may help to support the alleviation of symptoms of clinical conditions. In this context, mindfulness meditation training has been increasingly implemented in clinical contexts. Several studies have examined mindfulness effects on functional connectivity modulations in clinical contexts; we describe these studies next.
4. Resting-state Functional Connectivity, Mindfulness Meditation, and Illness
The activity of large-scale brain networks that are modulated by mindfulness, in particular the DMN, FPN, and SN (Falcone and Jerram, 2018; Fox et al., 2016, 2014), has been shown to exhibit functional abnormalities in several psychiatric disorders. Next, we briefly introduce several clinical conditions, and then describe functional connectivity modulations as a result of mindfulness training in these clinical populations. It is of note that modulation by mindfulness training of resting-state networks could have different effects on those clinical populations than on healthy populations. Indeed, psychiatric disorders have been associated with modulation of large-scale brain networks (Kaiser et al., 2015; Menon, 2011). These studies include comparisons of mindfulness training in clinical groups to active control groups (Creswell et al., 2016; King et al., 2016; Lifshitz et al., 2019; Taren et al., 2015) and to undiagnosed individuals (Su et al., 2016). These studies suggest that mindfulness meditation modulates corticolimbic systems, which may underlie health-related benefits of mindfulness and relate to emotion regulation.
Major Depressive Disorder.
Neuroimaging meta-analysis suggests that several large-scale neural networks are consistently impaired in MDD (Kaiser et al., 2015). Notably, MDD is correlated to altered and dysfunctional functional connectivity in the PCC, with weakened communication with the FPN and increased communication with the SN (R. Yang et al., 2016), concomitant to insular functional connectivity dysfunctions (Manoliu et al., 2013) overall decreased PCC/caudate nucleus coupling (Bluhm et al., 2009), as well as decreased interhemispheric coupling (Guo et al., 2013). While a growing number of studies have investigated therapeutic effects of mindfulness meditation for MDD (e.g., meta-analyzed in Goldberg et al., 2016), only one has investigated the effects of mindfulness meditation on rsFC in MDD. Specifically, Lifshitz and colleagues used a seed-based approach to compare MDD patients who completed a 2-week mindfulness training program to an active control group of patients who completed a relaxation-based training program (Lifshitz et al., 2019). Relative to the active control intervention, participants assigned to mindfulness training exhibited reduced depressive symptoms and improved mindful aptitudes (quantified using the FFMQ). Moreover, mindfulness training was associated with decreased functional connectivity of the FPN, specifically between bilateral DLFPC seeds and bilateral fusiform and right angular gyri. These regions of the DAN, FPN, and visual networks are involved in top-down processing of sensory input. Increased activations in the right angular gyrus, part of the IPL node of the FPN, has been linked to attention orienting and maintaining (Dixon et al., 2018). This region could be involved in a more mindful self-focus (Freton et al., 2014), shifting away from negative ruminations occurring in MDD. Those ruminations could be the result of a dysregulated functioning of the DMN in MDD with increased functional connectivity between the sgACC and the DMN (Hamilton et al., 2015). This dysregulation is hypothesized to result in negative thought processes centered on the self, prioritized over being in the present moment (Freton et al., 2014). More generally, the results from Lifshitz et al. suggest that mindfulness meditation decouples top-down control regions from brain areas involved in sensory, affective, and attentional processes (Lifshitz et al., 2019).
Chronic stress is a risk factor for MDD (Hammen, 2018; Yang et al., 2015) and causes increased inflammation that can lead to a reduction of neurogenesis (Schoenfeld and Gould, 2012), an increase in neurotoxicity (Lupien et al., 2018), and increased risk of cardiovascular disease and overall mortality (Kopp and Réthelyi, 2004). It is of note that chronic stress could lead to functional connectivity changes compared to controls. Interleukin-6 (IL-6), is a major inflammation and stress biomarker that is increased in chronically stressed populations. IL-6 levels in unemployed, job-seeking individuals, prone to high stress levels, were assessed in a study design involving a 3-day mindfulness meditation intervention or a control relaxation-based intervention (Creswell et al., 2016). Creswell et al. compared between-group functional connectivity differences using a PCC DMN-based seed. Compared to the control condition, the mindfulness meditation group exhibited increased rsFC between the PCC and the left dlPFC node of the FPN. These results corroborate findings from Kral and colleagues who employed a mindfulness training design on healthy participants compared to an active control group (Kral et al., 2019). Results from a 4-month follow-up assessment indicated that participants in the mindfulness meditation training group had relatively decreased levels of IL-6 (a pro-inflammatory cytokine used as a chronic stress biomarker) compared to the active control group. Despite the fact that they did not practice mindfulness meditation leading to the follow-up assessment, they had long-lasting stress-reducing effects. Interestingly, mindfulness meditation-trained participant had sustained levels of IL-6 whereas active control group participant had increased IL-6 levels at 4-months follow-up. Participants who followed mindfulness training, compared to active control participants, had increased functional connectivity between the PCC and the left dlPFC node of the FPN and relatively decreased levels of IL-6 at 4-month follow-up. These results suggest that mindfulness meditation training may prevent complications associated with chronic stress that may be meditated by modulation of large-scale functional connectivity and limiting increases in levels of IL-6. Future studies should directly test this hypothesis.
In secondary analyses from the same chronically-stressed population, Taren and colleagues tested the effects of mindfulness meditation on the functional connectivity of an amygdala seed and sgACC target (Taren et al., 2015). The amygdala is generally implicated in physiological stress responses (LeDoux, 1994) and the sgACC is an important component of the limbic system that modulates emotional processing (Scharnowski et al., 2020). Dysregulated sgACC function is often observed in mood disorders, notably MDD (Ge et al., 2020; Ho et al., 2014). Results from Taren et al. included a functional decoupling of these regions in a mindfulness meditation training compared to the active control group. These results may help to provide further information for a neuroscientific account of reduced physiological stress response (Creswell et al., 2016). Furthermore, the results of Taren et al. complement the studies of undiagnosed community populations that revealed mindfulness training-mediated increase of functional connectivity between the rACC (encompassing the sgACC) and the dmPFC region, described above (Kilpatrick et al., 2011; Kwak et al., 2019). That is, coupling of the sgACC may shift from the amygdala to the anterior DMN regions. Given the role of these regions in emotion processing (LeDoux, 1994), the observed changes in functional coupling may provide a neural signature of improved emotion regulation. Reduced connectivity between the amygdala and ACC was also correlated with reduced concentration of the chronic stress biomarker IL-6 (Taren et al., 2015).
Post-Traumatic Stress Disorder.
Mindfulness meditation training has also been used to alleviate symptoms of Post-Traumatic Stress Disorder (PTSD). PTSD is a debilitating condition that is characterized by intrusion and persistence of traumatic memories, as well as avoidance symptoms and negative alterations in cognition and mood, caused by the direct or indirect exposure to a major stressful event (Friedman et al., 2011). PTSD is associated with the remodulation of large-scale brain networks. Notably, PTSD is evidenced to be related to within-DMN hypoconnectivity, decreased connectivity between the DMN and affective systems, as well as increased connectivity between DMN and the somatomotor network (Bao et al., 2021). There are interindividual differences in vulnerability and susceptibility to the development of PTSD after a trauma (Bomyea et al., 2012). Military veterans exposed to war-zone trauma are an at-risk population for developing PTSD (Friedman et al., 1994). A study by King et al. investigated modulation of functional connectivity by mindfulness-based exposure therapy (MBET) compared to an active control condition of present-centered group therapy (PCGT; King et al., 2016). MBET is a group intervention that incorporates PTSD education, mindfulness training, and in vivo exposure (King et al., 2016). PTSD symptom improvement, specifically related to avoidant and hyperarousal symptoms, were not specific to the MBET group. Compared to the control group, the MBET group exhibited increased connectivity between PCC seed (used for the DMN) and dlPFC seed (FPN). King et al. speculate that the observed modulation of rsFC could mediate improved attentional control and meta-awareness. Of note, these results are based on a small sample (N = 14 for MBET and N = 9 for PCGT) and thus await replications from larger cohorts. The proposed mechanism underlying health-related benefits is similar to that proposed by other investigators of other conditions. For example, findings from Creswell and collaborators’ study (Creswell et al., 2016) also suggest that increased functional coupling of the PCC node of the DMN and dlPFC node of the FPN is linked to reduced psychological symptoms in individuals with chronic stress.
Chronic Pain.
MBSR was originally intended as an intervention for patients suffering from chronic pain (Kabat-Zinn, 2003). This condition is characterized by long-lasting and continuous pain that is believed to be caused by dysregulation of corticolimbic circuitry involving regions including the PFC, ACC, amygdala and nucleus accumbens (Yang and Chang, 2019). Greater mPFC and nucleus accumbens connectivity has been evidence to predict transition from acute to chronic pain (Baliki et al., 2012). In light of this conceptualization, Su et al. compared rsFC with the a priori defined seed region of the anterior insular cortex (aINS) in pain afflicted and healthy participants following MBSR (Su et al., 2016). Compared to the healthy cohort, participants with chronic pain exhibited increased functional connectivity between aINS and dACC after mindfulness meditation training (Su et al., 2016). The dACC region is involved in attentional control (Bush, 2011) and pain cognition and processing has been shown to be modified by attention monitoring (Kabat-Zinn, 1982). This may help explain mindfulness-related benefits for chronic pain, as attention monitoring is a major component of mindfulness meditation (Lutz et al., 2008).
To summarize this section, several studies probed modulation of functional connectivity by mindfulness meditation training in populations suffering from specific health conditions. Several relatively consistent trends emerged. First, each study found decreased symptoms as a result of mindfulness meditation training as assessed by questionnaires or physiological biomarkers (IL-6 for chronic stress; Creswell et al., 2016; Taren et al., 2015). These studies generally found increased functional coupling between PCC nodes of the DMN and dlPFC nodes of the FPN (Creswell et al., 2016; King et al., 2016). Of note, this pattern was also observed in mindfulness-trained and undiagnosed populations discussed (Kral et al., 2019). Modulation of corticolimbic systems by mindfulness meditation training may further decouple the sgACC and the amygdala as observed in a chronically stressed population (Taren et al., 2015). Disruption of amygdala and ventrolateral prefrontal cortex (vlPFC) circuitry has been extensively highlighted in populations with symptoms of anxiety and depression, especially in younger participants (Fowler et al., 2017; Greenberg et al., 2017; Guyer et al., 2008). As chronic stress is linked to MDD (Dieleman et al., 2015; Mcewen, 2004; Tafet and Bernardini, 2003; Vyas et al., 2004), the modulation of disordered frontolimbic systems through mindful emotion regulation is a promising approach to non-pharmacologically treat mood and anxiety disorders, and related conditions. Emotion regulation is a core component of mindfulness meditation training (Tang et al., 2015). Emotional distancing cultivated by mindfulness meditation seems to be an effective mechanism of adaptive coping strategy for processing emotions with negative valence (Grecucci et al., 2015; Guendelman et al., 2017; Jones, 2018; Ortner et al., 2007). In this sense, mindfulness meditation can in this way be understood as a step toward equanimity, that is, a dispositional tendency of evenness of mind towards all experiences, regardless of their emotional valence (Desbordes et al., 2015).
5. Discussion, Limitations and Future Directions
5.1. Heterogeneity in Mindfulness Research
The mindfulness rsFC literature exhibits both consistent and inconsistent findings. This may be explained, in part, by considerable heterogeneity in study design and samples, as well as methodological difficulties. Indeed, methodological issues arise from a number of issues including the inconsistent and broad meaning applied for the term “mindfulness”, and—among others—trait mindfulness, state mindfulness, mindfulness meditation training; the lack of adequate control groups in mindfulness training; and the difficulty in choosing adequate neural targets in analyses (Caspi and Burleson, 2005; Davidson, 2010; Davidson and Kaszniak, 2015).
Researchers have used a variety of paradigms to study effects of mindfulness meditation on rsFC. Studies have included investigation of trait mindfulness in meditation-naïve individuals using MAAS and FFMQ questionnaires (Bilevicius et al., 2018; Parkinson et al., 2019), trait mindfulness differences between experienced mindfulness meditation practitioners and non-practitioners (Bauer et al., 2019; Froeliger et al., 2012), and pre-to-post mindfulness meditation training (Creswell et al., 2016; Doll et al., 2015; Kilpatrick et al., 2011; King et al., 2016; Kral et al., 2019; Kwak et al., 2019; Lifshitz et al., 2019; Su et al., 2016; Taren et al., 2017; Yang et al., 2016). Specific training programs have varied, and included MBSR (Kilpatrick et al., 2011; Kral et al., 2019; Su et al., 2016), variants of MBSR (Yang et al., 2016), study-specific audio recordings of mindfulness meditation training (Doll et al., 2015; Lifshitz et al., 2019), mindfulness meditation retreat-based training (Creswell et al., 2016; Kwak et al., 2019; Taren et al., 2017), as well as MBET training (King et al., 2016). Studies also varied with regard to the duration of treatment: from the common 8-week MBSR program (Kilpatrick et al., 2011; Kral et al., 2019; Su et al., 2016), to 2-week audio recordings (Doll et al., 2015; Lifshitz et al., 2019), and 3-day intensive retreats (Creswell et al., 2016; Kwak et al., 2019; Taren et al., 2017). Studies additionally differed with respect to design, including: correlational (Bilevicius et al., 2018; Parkinson et al., 2019), cross-sectional (Bauer et al., 2019; Froeliger et al., 2012) and controlled longitudinal studies in undiagnosed (Doll et al., 2015; Kilpatrick et al., 2011; Kral et al., 2019; Kwak et al., 2019; Yang et al., 2016) and disordered populations (Creswell et al., 2016; King et al., 2016; Lifshitz et al., 2019; Su et al., 2016; Taren et al., 2017).
The heterogeneity of these studies is in part due to the ambiguous definition of the construct of mindfulness in the neuroscientific literature (Van Dam et al., 2018), indeed, modern science more broadly has had difficulty precisely defining the concept of mindfulness (Keng et al., 2011). In the context of this review, we have attempted to address, in part, this ambiguity by organizing studies according to two different concepts of mindfulness, that is, trait mindfulness and mindfulness meditation training, which have both revealed consistent and unique neural features as well as important limitations.
Studies examining functional connectivity patterns of trait mindfulness show unique neural signatures. Specifically, decoupling between the cuneus region of the DMN and the SN has been shown in individuals with higher trait mindfulness assessed with questionnaires (Bilevicius et al., 2018; Parkinson et al., 2019). In individuals with a higher trait mindfulness assessed with questionnaires (Bilevicius et al., 2018; Parkinson et al., 2019), as well as in meditators compared to non-meditators (Bauer et al., 2019), decoupling between the DMN and the STG region of the VAN has been observed (Bauer et al., 2019; Bilevicius et al., 2018; Parkinson et al., 2019). Additionally, individuals with higher trait mindfulness exhibit an overall decoupling between the DMN and FPNs (Bauer et al., 2019; Bilevicius et al., 2018; Parkinson et al., 2019). As previously described (see section 2. Resting-Sate fMRI Functional Connectivity and Trait Mindfulness), the cuneus region is associated with visual processing (Beason-Held et al., 1998), and could play a role in internally-directed attention (Benedek et al., 2016), while the STG is linked to visuotemporal attention (Shapiro et al., 2002). Those functionally similar roles could indicate a different function of visual and attentional processes in mindful individuals. According to these findings, in individuals with greater trait mindfulness, the DMN network exhibits decreased connectivity with the other networks: VAN, SN, FPNs (Bauer et al., 2019; Bilevicius et al., 2018; Parkinson et al., 2019). This provides evidence for the theory that trait mindfulness distinctly modulates the DMN. This modulation may be related to less self-referential processing and mind-wandering and more cognitive allocation to attentional processes (Andrews-Hanna, 2012; Poerio et al., 2017).
Similarly to trait mindfulness studies, mindfulness training studies also corroborated cuneus-SN decoupling (Doll et al., 2015; Kilpatrick et al., 2011; Yang et al., 2016). Mindfulness training studies reported a distinct neural pattern of increased functional connectivity between the PCC region of the DMN and the dlPFC region of the FPN (Creswell et al., 2016; King et al., 2016; Kral et al., 2019), as well as between the PCC region of the DMN and the dACC region of the SN (Kwak et al., 2019; Yang et al., 2016). Of note, increased DMN PCC to FPN dlPFC connectivity was observed after MBSR training in undiagnosed participants (Kral et al., 2019), retreat-based training in chronically stressed participants (Creswell et al., 2016), and MBCT training in participants diagnosed with PTSD (King et al., 2016). Similarly, increased DMN PCC to dACC SN connectivity was observed after MBSR-based training (Yang et al., 2016) and after an intensive retreat in undiagnosed participants (Kwak et al., 2019). Those highly consistent findings suggest a strong effect unrelated to the type of mindfulness training and the studied population.
In sum, trait mindfulness studies reported unique findings of decreased DMN-STG coupling, and decoupling of DMN and FPNs through the cuneus. Due to fewer number of studies (n=4) than those that studied mindfulness training (n=10), these results are more difficult to interpret and less definitive. Mindfulness training studies reported unique results of increased dlPFC FPN – PCC DMN connectivity, as well as increased PCC DMN – dACC SN connectivity. Both operationalizations of mindfulness described cuneus-SN decoupling.
Increased DMN-FPN connectivity after mindfulness training contradicts findings related to trait mindfulness. This could be due to the DMN regions implicated: cuneus (Bilevicius et al., 2018) as opposed to PCC (Kral et al., 2019), which will be addressed in the next section (see 3.2. Trends in the Literature).
Across studies, even though designs varied, results were more consistent within either trait or training mindfulness studies, than between trait and training mindfulness studies. However, the number of studies presented in this review is not large enough to definitively identify neural correlates of each of trait and training mindfulness. More studies, with larger sample sizes, are warranted. Future research will advance a better understanding of specific neural signatures related to trait mindfulness (e.g., assessed with aptitude questionnaires and in studies of experienced meditators) and specific types of training (e.g., mindfulness retreat instead of listening to audio recordings). This research would ultimately lead to identifying specific features of a particular mindfulness training program that would inform a more definitive and robust neuroscientific understanding of mindfulness.
5.2. Trends in the Literature
Even though the study designs used to study relations between mindfulness and functional connectivity have been heterogenous, several findings from this literature have been relatively consistent across studies. Several effects were observed across different rsFC methods (ICA and seed-based), distinct experimental paradigms (trait mindfulness and mindfulness training) and across different populations (diagnosed and undiagnosed). Those effects were: decreased cuneus-SN connectivity (Fig. 1; Bilevicius et al., 2018; Doll et al., 2015; Kilpatrick et al., 2011; Yang et al., 2016), increased PCC of DMN-dlPFC of FPN connectivity (Fig. 3; Creswell et al., 2016; King et al., 2016; Kral et al., 2019), increased within SN connectivity (Bilevicius et al., 2018; Parkinson et al., 2019; Su et al., 2016), and corticolimbic system modulation including increased dmPFC-rACC connectivity (Kilpatrick et al., 2011; Kwak et al., 2019) and decreased rACC-amygdala connectivity (Taren et al., 2017). Below, we will describe cognitive implications of those changes and their relations to aspects of mindfulness: self-awareness, attention control, and, lastly, particularly important for clinical outcomes: emotion regulation (that encompasses pain relief). This led to the formulation of our proposed theoretical framework (Fig. 2, Fig. 4).
Fig. 1.
Results illustrating decreased cuneus-SN connectivity in non-meditating participants. Studies explored connectivity changes (A) correlated to trait mindfulness using ICA (Bilevicius et al., 2018); (B) after an MBSR-based training using a dACC seed (Yang et al., 2016); and (C) after an MBSR training using ICA (Kilpatrick et al., 2011).
Fig. 3.
Results illustrating increased mindfulness training-related PCC-dlPFC connectivity. Studies used (A) PCC for DMN seed in chronically stressed population after a 3-day retreat (Creswell et al., 2016), (B) PCC for DMN seed in a PTSD-affected population after a MBCT training (King et al., 2016) and (C) PCC for DMN and dlPFC for FPN seeds in undiagnosed participants after an MBSR training, correlated to attention scores (Kral et al., 2019)
Fig. 2.
Visual rendering of the Default Mode Network (DMN; blue), Frontoparietal Network (FPN; green) and Salience Network (SN; violet) and their functional connectivity changes mediated by mindfulness. Studies describe increased rsFC between the PCC (DMN) and dlPFC (FPN) as well as decreased cuneus – SN connectivity. Those changes are hypothesized to be respectively linked to improved self-awareness and improved attention control. Created with BioRender.com. Abbreviations: dACC = dorsal anterior cingulate cortex, mPFC = medial prefrontal cortex, PCC = posterior cingulate cortex, aINS = anterior insula, dlPFC = dorsal prefrontal cortex, IPL = inferior parietal lobule.
Fig. 4.
Summary of findings involving pain relief and limbic system remodulation mediated by mindfulness. Studies describe increased dACC-aINS connectivity by mindfulness, hypothesized to be linked to pain processing regulation, as well as increased sgACC-dmPFC connectivity and decreased sgACC-amygdala connectivity. These changes within the limbic systemic are hypothesized to be linked to emotion regulation. Image created using BioRender.com. Abbreviations: dACC = dorsal anterior cingulate cortex, aINS = anterior insula, dmPFC = dorsomedial prefrontal cortex, rACC = rostral anterior cingulate cortex.
As previously stated, correlational trait mindfulness (Bilevicius et al., 2018) and longitudinal mindfulness training studies (Doll et al., 2015; Kilpatrick et al., 2011; Yang et al., 2016) have consistently highlighted decoupling between the cuneus region of the DMN and the SN (Fig. 1). The cuneus, along with other midline cortical structure, was linked to reduced activation during mindful self-awareness (Lutz et al., 2016). The decoupling of the cuneus with the SN could be related to this previously described decreased activation, which may be in turn related to increasingly mindful, present-moment self-awareness, a primary component of mindfulness practices (Tang et al., 2015) (Fig. 2).
Studies of modulation of the PCC region of the DMN have included this region’s increased functional connectivity with bilateral dlPFC nodes of the FPN in relation to mindfulness training (Fig. 1) in healthy participants (Kral et al., 2019), and also in populations suffering from chronic stress (Creswell et al., 2016) and PTSD (King et al., 2016) (Fig. 3). Given that the PCC is hypothesized to be one of the core regions involved in self-referential processes, autobiographical memory, prospection, and planning (Davey et al., 2016; Maddock et al., 2001), these results related to mindfulness may be considered counterintuitive. However, some studies suggest that the PCC has subregions that exhibit higher specificity, and, furthermore, functional connectivity may not always mirror activity (Lynch et al., 2018). Indeed, cytoarchitectural, structural, functional, and lesion studies leading to parcellation suggest that PCC has highly heterogenous subregions (Bzdok et al., 2015; Leech and Sharp, 2014; Leech and Smallwood, 2019; Scheperjans et al., 2008) and acts as a major hub involving distinct networks (Leech and Smallwood, 2019). Notably, ventral PCC has been shown to communicate with vmPFC, a major node of the DMN, whereas the dorsal subregion of PCC has been related to increased connections to the dlPFC, a core node of the FPN (Bzdok et al., 2015; Fan et al., 2018). Moreover, neuroimaging and lesion studies have found that the dorsal region of PCC is involved in attention regulation (Leech and Sharp, 2014). Indeed, when activated by a task, dorsal PCC tends to exhibit higher functional connectivity with the FPN (Leech et al., 2011). During resting state, the PCC and its subregions are coupled to the DMN (Bzdok et al., 2015).
Taken together, this suggests that the PCC may be involved in modulating DMN-to-FPN interactions. Alternatively, the DMN-specialized subsystem of the FPN may also be involved, specifically the FPNA may modulate dPCC-dlPFC interactions by reorienting and controlling internally-focused attention (Dixon et al., 2018). That is, the DMN-specialized subsystem of the FPN, FPNA, may modulate DMN connectivity through the FPN. This would result in the observed increased DMN-FPN connectivity and explain, in part, a lasting change in attentional processes. Another hypothesis is that dPCC, in complement to rlPFC, acts as a “switch” that modulated FPN regulation of the DMN and DAN. This would be consistent with observed switching for affective (FPN-DMN) and cognitive (FPN-DAN) tasks, respectively (Burgess et al., 2007; Fan et al., 2018). Indeed, higher rsFC changes of DMN and FPN have been associated with higher cognitive flexibility and cognitive performance, overall (Douw et al., 2016). Together, this may explain DMN-FPN increased coupling involving the dPCC, increasing attention control, improving cognitive performance, and thus could help support a mindfulness model informed by dPCC.
In this sense, more precise seed-placement is crucial and further studies should assess how functional connectivity modulation in relation to mindfulness specifically affects the dorsal and ventral subregions of the PCC. Furthermore, future research should be wary of lumping together distinct cortical subregions more generally. With this in mind, here we suggest that overall, growing evidence indicates that increased coupling between PCC, a major node of the DMN, and dlPFC nodes of the FPN, could translate to improved attention control, a primary component of mindfulness (Tang et al., 2015), additionally resulting in improved cognitive flexibility. Future research should explicate subregional interactions increasingly directly.
While cuneus-to-SN decoupling could be linked to self-awareness and PCC-to-dlPFC coupling could relate to attentional control, a final primary component of mindfulness as described by Tang’s model remains: emotion regulation (Tang et al., 2015). Dysregulation of the processing of painful and negative emotions, and pain avoidance, through disordered behavior is related to a variety of psychiatric conditions including MDD and PTSD (Asmundson et al., 1999; Xie et al., 2014). Emotional processing through awareness and acceptance of negative emotions is a core aspect of mindfulness and mindfulness-based trainings and therapies for psychiatric and physical conditions (Hill and Updegraff, 2012). The awareness and acceptance components of mindfulness may also underlie improved pain symptoms.
One neuroanatomical region that has been extensively linked to emotion regulation is the ACC (Etkin et al., 2011; Stevens, 2011). The ACC has separate subdivisions that have roles in distinct mental processes and can be divided into dorsal/caudal ACC, and rostral/ventral ACC (Stevens, 2011). The rACC is further divided into pgACC and sgACC (Stevens, 2011). Extensive cytoarchitectural, lesion and neuroimaging evidence implicates a functional distinction in the ACC wherein the dorsal component, dACC, is related to regulation of cognitive processes, and the rostral component, rACC, in regulating emotional processes (Bush et al., 2000; Stevens, 2011). The dACC, a key node of the SN, is linked to increased connectivity with the SN in mindful individuals (Bilevicius et al., 2018; Parkinson et al., 2019). dACC-SN connectivity is also increased in chronic-pain afflicted individuals after mindfulness training compared to controls (Su et al., 2016). Dorsal ACC additionally has been implicated in pain processing and the anticipation of pain (Vogt, 2005), which suggests a possible mechanism for mindfulness meditation training on altered functional connectivity in chronic pain (Su et al., 2016) and pain relief by mindfulness (Fig. 4).
Apart from the physical and cognitive aspects of pain, the emotional aspect of pain is thought to be processed, in part, in the rACC (Etkin et al., 2011). Relatedly, mindfulness training-mediated modulations of the rACC have shown antidepressant effects (Yang et al., 2016). As aberrant sgACC-DMN hyperconnectivity has been observed in depression (Connolly et al., 2013; Drevets et al., 2008), it has been speculated that decreased connectivity in these regions mediated by mindfulness training may relate to an antidepressant effect (Yang et al., 2016). Additionally, in the pgACC, the modulation of functional connectivity through mindfulness training was related to increased resilience measured through the Resilience Quotient Test (Kwak et al., 2019), a measure of protection against psychiatric conditions. Indeed, regarding mindfulness-mediated rsFC modulations of the affective component of the ACC, the rACC, findings unveil decreased rACC-amygdala connectivity (Taren et al., 2017) and increased rACC-dmPFC connectivity (Kilpatrick et al., 2011; Kwak et al., 2019). As those pathways are evidenced to play major roles in emotion regulation (Fig. 2) (Zotev et al., 2013), dysfunctions are frequently linked to mood disorders. It is indeed notable that cognitive control of emotions mediated by prefrontal cortices coupling to rACC are aberrant in MDD (Disner et al., 2011), and connectivity dysfunctions of rACC and the amygdala component of limbic circuitry are prevalent in mood disorders (Alexander et al., 2020; Connolly et al., 2013; Hakamata et al., 2020; Kamphausen et al., 2012; Scharnowski et al., 2020). All this illustrates that there could a be modulation of corticolimbic circuitry mediated by mindfulness (Fig. 2), resulting in a regulation of emotional processes. However, neuroanatomical specificity in the rACC needs to be further defined as a study highlighted functional decoupling between the dmPFC and the pgACC subregion of the rACC (Yang et al., 2016). Overall, even though further studies should highlight clearer neural signatures of emotion regulation mediated by mindfulness, there seems to be an important role played by mindfulness in neuronal plasticity regarding affect monitoring.
5.3. Underlying brain-behavior relationships
Together these studies suggest that mindfulness is related to the modulation of the functional connectivity of several large-scale brain networks implicated in attention control, self-awareness (Fig. 2), pain processing and emotion regulation (Fig. 4), providing a neural understanding of underlying mechanisms. Notably, even though the neuroscience of mindfulness is in its nascency, several neural patterns emerge that may relate to core psychological features of mindfulness training. The self-awareness component of mindfulness may be related to cuneus/DMN-SN decoupling (Fig. 1, Fig. 2) and the attention regulation component of mindfulness may relate to PCC/DMN and dlPFC/FPN coupling (Fig. 2, Fig. 3). Together, these mindfulness-related effects suggest an overall reconfiguration of DMN connectivity with the SN and FPN. The ACC may play a unique functional connectivity role mediated by mindfulness. Indeed, mindfulness is associated with modulation of dmPFC-rACC-amygdala circuitry with increased rACC-dmPFC connectivity and decreased rACC-amygdala connectivity. These mindfulness-induced changes in the connectivity of the affective region of ACC – the rACC – may relate to improved emotional processing (Fig. 4). Finally, cognitive aspects of emotion processing, and pain processing in particular, involve dACC-SN coupling, or in other words, increased within-SN coupling (Fig. 4). In this sense, mindfulness seems to increase connectivity within the SN, a network implicated in salient event processing, which may help practitioners re-evaluate pain and associated negative affective processing. Overall, these neural signatures could help inform our understanding of therapeutic effects mediated by mindfulness.
5.4. Limitations Related to Resting-State Functional Connectivity Methods
The assessment and synthesis of seed-based rsFC, including in relation to mindfulness, in the context of large-scale resting-state networks is in part limited by investigators’ use of different seed and target regions (please see Table 1 for the class of rsFC methods that each study implemented). While the seed-based method presents several advantages over other functional connectivity methods (including its relative simplicity and efficiency), it may also introduce unwanted variability due to inconsistencies in individual-level seed placements that are based on group-level information (Venkataraman et al., 2009; Wang et al., 2015). Moreover, results are highly dependent on the selection of the a priori defined seed regions and may miss important functional connectivity patterns. For example, mindfulness-related effects on the DMN seem to be dependent on DMN subregions: anterior or posterior DMN (Uddin et al., 2008). This may explain varying DMN-related effects of mindfulness, as a result of seed placements in: vmPFC (Bauer et al., 2019; King et al., 2016) or PCC (Creswell et al., 2016; King et al., 2016; Kral et al., 2019; Lifshitz et al., 2019). Additionally, as reviewed above, different seeds have been used to evaluate the FPN, including: dlPFC (Kral et al., 2019; Lifshitz et al., 2019) or IFG and IPL (Bauer et al., 2019). Furthermore, within a given neuroanatomical region, such as the PCC and the ACC, different seed placement could lead to different results. As previously stated, the PCC and ACC have anatomical subregions that are associated with different functions (Davey et al., 2016; Leech and Sharp, 2014; Maddock et al., 2001; Stevens, 2011). Imprecision in seed placement could lead to different observed effects and even false negatives.
The second most used method in this context, ICA, is based on the computational extraction of main variance-associated components of whole-brain neural signal (Beckmann et al., 2005). Although ICA may facilitate functionally homogeneous interpretation, compared to seed-based approaches, this method is more difficult to understand as it contains a more complex representation of data. Additionally, because of statistical complexity, ICA may complicate between-group comparisons and their translation to clinical contexts (Fox and Raichle, 2007; van den Heuvel and Hulshoff Pol, 2010). Also, because ICA components are defined on a study-by-study basis, this can lead to variance in component structure across studies. For example, some studies of mindfulness have used group-ICA analysis (e.g., Kilpatrick et al., 2011), while others used single-subject analysis (e.g., Parkinson et al., 2019) that have resulted in unique components: a composite attentional component encompassing what other studies have labeled the VAN and the DAN (Parkinson et al., 2019), and a composite auditory/salience component (Kilpatrick et al., 2011). For instance, increased insula-attentional network component connectivity does not reveal which network in particular (VAN or DAN) could be interacting with the insular region (Parkinson et al., 2019). Similarly, it is unclear if increased connectivity within the composite auditory/salience composite network and decreased connectivity of that composite network with the visual network is due to the auditory component or salience component (Kilpatrick et al., 2011). The combining of these different networks into composite networks makes it more difficult to draw direct conclusions between these studies.
This relates to more general inconsistencies in how investigators have defined resting-state networks. Indeed, similar nodes are sometimes inconsistently assigned to different resting-state networks. For example, the VAN and SN have similar roles and are often used interchangeably. They are defined by a comparable function: activation to a salient event (Seeley et al., 2007) or an exogenous stimulus (Vossel et al., 2014), and also using anatomically proximal regions in functional connectivity analyses. That is, the insular cortex is often used to define the SN while its neuroanatomically neighboring region, the IFG/MFG node, (Vossel et al., 2014), is assigned to the VAN (Farrant and Uddin, 2015). However, while the VAN is mainly linked to attentional processes (Corbetta and Shulman, 2002; Vossel et al., 2014), the SN has been associated with processing internally and externally relevant (Seeley, 2019; Seeley et al., 2007). The existence of VAN was initially identified through lesion studies notably in stroke patients with spatial hemineglect (Corbetta and Shulman, 2002; Vossel et al., 2014). Conversely, the salience network was defined through intrinsic functional connectivity observations at resting-state (Seeley et al., 2007). Prior studies have noted that it is unclear if the VAN is an aggregate of networks including the SN or if the VAN is closely adjacent to the SN (Yeo et al., 2011). In sum, further clarification is needed regarding the study of the VAN and SN.
The definition of the DAN and FPN also raises similar ambiguities regarding their distinct cognitive roles and neuroanatomical correlates. For example, the IPS is often included in both the DAN (Farrant and Uddin, 2015) and also in the FPN (Dixon et al., 2018). Additionally, the networks described using this framework are thought to have similar roles involving attentional processes, although the FPN is thought to be more so involved in higher-order cognitive processes—including cognitive control—than the DAN (Corbetta and Shulman, 2011). These anatomical and functional ambiguities are diminished when a given taxonomy refers to those networks instead as dorsal frontoparietal attention network (DAN) and lateral frontoparietal control network (FPN; Uddin et al., 2019), with an FPNB subsystem functionally connected to DAN (Dixon et al., 2018). Overall, similarities between FPN and DAN can be understood when discriminating FPNA from FPNB and further observing the attention control role of FPNB, communicating with the DAN, indeed involved in attention.
The investigator-initiated selection of seed and target regions may introduce bias and limit our understanding. The DAN and VAN have been relatively less studied in the mindfulness literature compared to other resting-state networks. Given the involvement of these networks in attentional processes and the importance of attention in mindfulness, the assessment of these networks may provide additional insight into neural models of mindfulness. To overcome issues related to the similarities of the SN and VAN and the FPN and DAN, increasingly specific and distinct regions could be used as seeds: for instance, TPJ for the VAN and the FEF for the DAN. Taking all of this into account, future research should study the VAN and DAN.
There are considerable ongoing efforts to advance a scientific consensus for the taxonomy of large-scale resting-state brain networks. Notably, Uddin and colleagues’ taxonomy (Uddin et al., 2019) describe six large-scale networks referred to using anatomical/cognitive domain nomenclature: occipital/visual, pericentral/somatomotor, dorsal frontoparietal/attention, lateral frontoparietal/control, midcingulo-insular/salience, and medial frontoparietal/default mode networks. This has been proposed as a viable universal taxonomy of large-scale functional brain networks.
Furthermore, and more broadly, the mindfulness and functional connectivity literature is limited by considerable reverse inference with regard to linking the modulation of functional connectivity as a result of mindfulness meditation to changes in behavioral, attentional, emotional and cognitive processes. Indeed, a certain behavior may be linked to the activation or deactivation of a given neuroanatomical region, however, rsFC changes of that same neuroanatomical region are not always related to a difference in such behavior. Reverse inferences are even made in the present review by hypothesizing links and causality of rsFC modulations by changes in inherent brain functions of relevant neuroanatomical regions (e.g., PCC rsFC modulations explained by the role of PCC in attentional processes). Research should move cautiously especially when prior belief in the involvement of a behavioral or cognitive process is unprecise and the neuroanatomical region’s activation specificity and selectivity is low. However, reverse inferences can suggest new hypotheses and give direction to future experimental testing (Poldrack, 2006). In sum, to move beyond reverse inference, these links need to be subsequently studied directly (Chang et al., 2013; Sprooten et al., 2017).
5.5. Limitations of Correlational and Cross-Sectional Studies of “Trait” Mindfulness
Correlational studies have linked self-reported trait mindfulness to rsFC patterns (Bilevicius et al., 2018; Parkinson et al., 2019). These studies are limited by questions about the validity of using self-report personality-trait questionnaires to quantify meditation-naïve individuals’ and experienced meditators’ mindful aptitudes. Assessment of mindfulness using self-report questionnaires may not adequately address all related facets, and their interactions, of mindfulness (Bergomi et al., 2013). Similarly, Park et al. argue that questionnaires do not provide exhaustive construct coverage, content validity, or inter-testing responsiveness and reliability (Park et al., 2013). Additionally, questionnaires may not provide differential sensitivity of general wellbeing and effects related to mindfulness (Baer et al., 2019). Several systematic reviews and meta-analyses have suggested a need to revise questionnaires (Baer et al., 2019; Bergomi et al., 2013; Park et al., 2013). For example, with regard to the widely used FFMQ, only the non-judging subscale has been shown to exhibit strong validity, while the observing, describing, acting with awareness and non-reactivity subscales have not (Goldberg et al., 2016; Mattes, 2019).
Additionally, it has been reported that individuals vary in their tendency to adopt a mindful attitude across different modalities and contexts (Kiken et al., 2015; Tang, 2017), which may influence state-specific results obtained in functional connectivity studies of trait mindfulness (e.g., Parkinson et al., 2019). Taken together, these limitations should be taken into account when evaluating the utility of assessing reported relations between mindfulness questionnaire measures and functional connectivity.
When studying trait mindfulness by comparing the rsFC of meditation practitioners to meditation-naïve individuals, researchers operate on the assumption that prior meditation practice increases a personality trait (Kiken et al., 2015). This is a construct weakness as it makes “trait” not inherent and time-varying. A possible resolution to this issue may be that trait changes linked to meditation practice may only be observed over long time periods, which may help explain meditation-naïve and long-time meditator comparisons (Bauer et al., 2019; Froeliger et al., 2012).
However, the comparison of experienced meditators to meditation-naïve individuals may be confounded by uncontrolled factors between these groups, for example, differences in: lifestyle and health habits including nutrition, exercise, and spiritual beliefs. Indeed, it has been shown that trait mindfulness positively relates to an overall healthier lifestyle and psychological wellbeing (Keng et al., 2011).
An additional consideration in this literature is the varying levels of expertise of practitioners. That is, experienced meditators’ years of training may relate to different patterns of functional connectivity. Indeed, several studies have reported that mindfulness meditation-related modulation of functional connectivity is related to years of mindfulness practice (Bauer et al., 2019; Froeliger et al., 2012), and a meta-analysis found unique functional connectivity patterns when comparing results of studies of novice and experienced meditation practitioners (Falcone and Jerram, 2018).
In sum, studies of trait mindfulness may be limited by high variability and uncertainty related to measurement approach and construct validity. Questionnaires that assess trait mindfulness should be further tested for validity, and revised accordingly. The time-stability of “trait” mindfulness is uncertain and cross-sectional studies comparing meditator to non-meditating individuals should control confounding factors. Functional connectivity studies using these methods may be increasingly useful if future studies further cluster individuals based on their expertise level of meditation in order to refine findings.
5.6. Limitations of Studies of Mindfulness Meditation Training
Studies of mindfulness meditation training overcome some of the limitation described above by directly assessing effects of mindfulness on functional connectivity in individuals who have completed mindfulness meditation training.
With that said, there are issues raised by the lack of inclusion of adequate baseline controls. In fact, some of these studies are limited by having assessed mindfulness training effects without an active control group (Doll et al., 2015; Yang et al., 2016). Instead, these studies used within-subjects designs that limit inferential confidence. Alternatively, randomized active-controlled studies are considered a gold standard in intervention research (Chalmers et al., 1981). This is because active control groups help to specify intervention-specific effects by eliminating considerable confounding factors (e.g., practice effects). In this way, active-controlled studies will benefit our understanding of mindfulness’ effects on large-scale brain connectivity.
Although some researchers investigated lasting effects of mindfulness meditation training by performing follow-up assessments (Creswell et al., 2016; Kral et al., 2019), studies have largely not correlated practice time with change in functional connectivity. This could have been done by observing rsFC changes at different time points during mindfulness training (e.g., after days, weeks, or months of mindfulness practice). There still are not sufficient findings in the current literature to conclude if the impact of mindfulness training on functional connectivity relates to practice time.
Moreover, mindfulness meditation training includes both FA and OM features of meditation (Lutz et al., 2015). Mindfulness training programs usually provide aspects of both FA and OM (Williams and Kabat-Zinn, 2011), and thus the unique effects of each practice are difficult to differentiate (Britton et al., 2018). In this context, it will be important to characterize differences between FA and OM components of mindfulness meditation on functional connectivity (Kilpatrick et al., 2011). Such differences in mindfulness meditation practices could help explain high variability in results. Future studies may benefit from more explicit descriptions of the type of mindfulness training studied or explicitly dismantling components of mindfulness training (Britton et al., 2018). Similarly, future studies may further our understanding of effects related to similar training modalities, for example: retreat, 8-week courses, or brief interventions. Limitations encountered in mindfulness training paradigms could be countered by further examining practiced mindfulness techniques in active-controlled studies.
6. Summary and Conclusion
In the current review, we have aggregated and synthesized studies of functional connectivity and mindfulness—operationalized as both a trait and form of training—across multiple study designs and rsFC methodologies. These studies suggest that functional connectivity is related to mindfulness, especially with the cingulate cortex playing a major role across multiple modalities. Mindfulness meditation may modulate the DMN with PCC node of DMN and dlPFC node of FPN coupling that may underlie increases in attentional processes (Fig. 2, Fig. 3), and cuneus-SN decoupling that may underlie increases in self-awareness (Fig. 1, Fig. 2). Increased within SN coupling (dACC-anterior insula) mediated by mindfulness might point to improved pain processing leading to pain relief (Fig. 4). Mindfulness may also modulate cortico-limbic circuitry with notably increased dmPFC-rACC connectivity and decreased rACC-amygdala connectivity, which may relate to improved emotion regulation (Fig. 4). In this way, mindfulness meditation is speculated to be linked to, among other effects, antidepressant, neuroprotectant and anxiolytic outcomes. Indeed, its effects ranged from decreased depressive and anxious symptom scores, decreased biological IL-6 stress biomarker quantities, lessening of avoidant and hyperarousal symptoms of PTSD and decreased pain perception for chronic pain patients. Additionally, results were paralleled to increased resilience scores and overall increased psychological well-being. The heterogeneity of study designs and analytic approaches of rsFC and mindfulness may explain inconsistencies, and future research may benefit from (1) increasingly clear conceptual and operational definitions of mindfulness; (2) more precise and consistent seed and target definitions; and (3) consistency of ICA components. Additionally, consensus with the description and designation of rsFC networks may further improve comparisons across studies. Moreover, future mindfulness and brain network research will benefit from recent developments in approaches in this field (e.g., connectomics; Smith et al., 2013). Despite these limitations, preliminary results provide evidence for the utility of functional connectivity for informing neural models of mindfulness which promises to contribute to improved training programs and better wellbeing outcomes in both clinical and non-clinical contexts.
Highlights.
We reviewed mindfulness research including trait mindfulness and mindfulness training
Mindfulness decreases default mode network and frontoparietal network connectivity
Attention control relates to posterior cingulate and - dorsolateral prefrontal cortex coupling
Self-awareness relates to decreased connectivity between cuneus and salience network
Emotional regulation aspect of mindfulness is linked to corticolimbic modulation
Mindfulness decreases psychiatric symptoms
Pain relief relates to coupling between dorsal anterior cingulate and salience network
Scientific consensus is needed on definitions of mindfulness and brain networks
Acknowledgements
DAP was partially supported by grants 2R37MH068376 and 2R01MH095809 from the National Institute of Mental Health. MDS was partially supported by the Phyllis and Jerome Lyle Rappaport Foundation, Ad Astra Chandaria Foundation, BIAL Foundation, Brain and Behavior Research Foundation, and the Center for Depression, Anxiety, and Stress Research at McLean Hospital.
Declaration of Competing Interest
Over the past 3 years, Dr. Pizzagalli has received consulting fees from Albright Stonebridge Group, BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Concert Pharmaceuticals, Engrail Therapeutics, Neurocrine Biosciences, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, Millennium Pharmaceuticals. In addition, he has received stock options from BlackThorn Therapeutics. All views expressed are solely those of the authors. All other authors report no financial relationships with commercial interest. The authors declare that there were no conflicts of interest with respect to the writing of this article.
Abbreviations
- ACC
anterior cingulate cortex
- ADHD
attention-deficit/hyperactivity disorder
- aINS
anterior insula
- ATN
attentional network
- CAMS-R
Cognitive and Affective Mindfulness Scale Revised
- CEN
central executive network
- dACC
dorsal anterior cingulate cortex
- DAN
dorsal attention network
- DCM
dynamic causal modelling
- dlPFC
dorsolateral prefrontal cortex
- DMN
default mode network
- dmPFC
dorsomedial prefrontal cortex
- FA
focused attention
- FEF
frontal eye field
- FFQM
Five Facet Mindfulness Questionnaire
- FMI
Freiburg Mindfulness Inventory
- FPN
bilateral frontoparietal network
- HEP
health enhancement program
- ICA
independent component analysis
- IFG
inferior frontal gyrus
- IL-6
Interleukin-6
- IPL
inferior parietal lobule
- IPS
inferior parietal sulcus
- KIMS
Kentucky Inventory of Mindfulness Skills
- MAAS
Mindful Attention and Awareness Scale
- MBET
mindfulness-based exposure therapy
- MBSR
mindfulness-based stress reduction
- MDD
major depressive disorder
- MFG
middle frontal gyrus
- mPFC
medial prefrontal cortex
- OM
open monitoring
- PCC
posterior cingulate cortex
- PCGT
present-centered group therapy
- PFC
prefrontal cortex
- pgACC
pregenual anterior cingulate cortex
- PHLMS
Philadelphia Mindfulness Scale
- PMC
premotor cortex
- PTSD
post-traumatic stress disorder
- rACC
rostral anterior cingulate cortex
- ROI
region of interest
- rsFC
resting-state functional connectivity
- sgACC
subgenual anterior cingulate cortex
- SMA
supplementary motor area
- SMQ
Southampton Mindfulness Questionnaire
- SMS
State Mindfulness Scale
- SN
salience network
- STG
superior temporal gyrus
- TMS
Toronto Mindfulness Scale
- TPJ
temporoparietal junction
- VAN
ventral attention network
- vlPFC
ventrolateral prefrontal cortex
- vmPFC
ventromedial prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. References
- Alexander L, Wood CM, Gaskin PLR, Sawiak SJ, Fryer TD, Hong YT, McIver L, Clarke HF, Roberts AC, 2020. Over-activation of primate subgenual cingulate cortex enhances the cardiovascular, behavioral and neural responses to threat. Nature Communications 11, 5386. 10.1038/s41467-020-19167-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, 2012. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18, 251–270. 10.1177/1073858411403316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJG, Norton PJ, Norton GR, 1999. Beyond pain: The role of fear and avoidance in chronicity. Clinical Psychology Review 19, 97–119. 10.1016/S0272-7358(98)00034-8 [DOI] [PubMed] [Google Scholar]
- Baer R, Gu J, Cavanagh K, Strauss C, 2019. Differential sensitivity of mindfulness questionnaires to change with treatment: a systematic review and meta-analysis. Psychol Assess 31, 1247–1263. 10.1037/pas0000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Allen KB, 2004. Assessment of mindfulness by self-report: the Kentucky inventory of mindfulness skills. Assessment 11, 191–206. 10.1177/1073191104268029 [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Lykins E, Button D, Krietemeyer J, Sauer S, Walsh E, Duggan D, Williams JMG, 2008. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment 15, 329–342. 10.1177/1073191107313003 [DOI] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV, 2012. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 15, 1117–1119. 10.1038/nn.3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W, Gao Y, Cao L, Li H, Liu J, Liang K, Hu Xinyue, Zhang L, Hu Xinyu, Gong Q, Huang X, 2021. Alterations in large-scale functional networks in adult posttraumatic stress disorder: A systematic review and meta-analysis of resting-state functional connectivity studies. Neuroscience & Biobehavioral Reviews 131. 10.1016/j.neubiorev.2021.10.017 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, Seidel Malkinson T, 2019. Hemispheric lateralization of attention processes in the human brain. Curr Opin Psychol 29, 90–96. 10.1016/j.copsyc.2018.12.023 [DOI] [PubMed] [Google Scholar]
- Bauer CCC, Whitfield-Gabrieli S, Díaz JL, Pasaye EH, Barrios FA, 2019. From State-to-Trait meditation: reconfiguration of central executive and default mode networks. eNeuro 6. 10.1523/ENEURO.0335-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Diplomate Psychology C, 2019. The Experience of Presence In Dzogchen: A Phenomenology. [Google Scholar]
- Beason-Held LL, Purpura KP, Krasuski JS, Maisog JM, Daly EM, Mangot DJ, Desmond RE, Optican LM, Schapiro MB, VanMeter JW, 1998. Cortical regions involved in visual texture perception: a fMRI study. Brain Res Cogn Brain Res 7, 111–118. 10.1016/s0926-6410(98)00015-9 [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM, 2005. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360, 1001–1013. 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ, 1995. An information-maximization approach to blind separation and blind deconvolution. Neural Computation 7, 1129–1159. 10.1162/neco.1995.7.6.1129 [DOI] [PubMed] [Google Scholar]
- Benedek M, Jauk E, Beaty RE, Fink A, Koschutnig K, Neubauer AC, 2016. Brain mechanisms associated with internally directed attention and self-generated thought. Scientific Reports 6, 22959. 10.1038/srep22959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB, Shucard DW, Maria MPS, Shucard JL, Abara JP, Coad ML, Wack D, Sawusch J, Lockwood A, 2002. Covert auditory attention generates activation in the rostral/dorsal anterior cingulate cortex. Journal of Cognitive Neuroscience 14, 637–645. 10.1162/08989290260045765 [DOI] [PubMed] [Google Scholar]
- Bergomi C, Tschacher W, Kupper Z, 2013. The assessment of mindfulness with self-report measures: existing scales and open issues. Mindfulness 4, 191–202. 10.1007/s12671-012-0110-9 [DOI] [Google Scholar]
- Bilevicius E, Smith SD, Kornelsen J, 2018. Resting-state network functional connectivity patterns associated with the mindful attention awareness scale. Brain Connect 8, 40–48. 10.1089/brain.2017.0520 [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS, 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34, 537–541. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Théberge J, Densmore M, Bartha R, Neufeld R, Osuch E, 2009. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci 63, 754–761. 10.1111/j.1440-1819.2009.02030.x [DOI] [PubMed] [Google Scholar]
- Bomyea J, Risbrough V, Lang AJ, 2012. A consideration of select pre-trauma factors as key vulnerabilities in PTSD. Clin Psychol Rev 32, 630–641. 10.1016/j.cpr.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JE, Lanius RA, McKinnon MC, 2018. Mindfulness-based treatments for posttraumatic stress disorder: a review of the treatment literature and neurobiological evidence. J Psychiatry Neurosci 43, 7–25. 10.1503/jpn.170021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang Y-Y, Weber J, Kober H, 2011. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. U.S.A. 108, 20254–20259. 10.1073/pnas.1112029108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton WB, Davis JH, Loucks EB, Peterson B, Cullen BH, Reuter L, Rando A, Rahrig H, Lipsky J, Lindahl JR, 2018. Dismantling mindfulness-based cognitive therapy: creation and validation of 8-week focused attention and open monitoring interventions within a 3-armed randomized controlled trial. Behav Res Ther 101, 92–107. 10.1016/j.brat.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, DiNicola LM, 2019. The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci 20, 593–608. 10.1038/s41583-019-0212-7 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BTT, 2013. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 16, 832–837. 10.1038/nn.3423 [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ, 2007. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci 11, 290–298. 10.1016/j.tics.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Bush G, 2011. Cingulate, frontal and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry 69, 1160–1167. 10.1016/j.biopsych.2011.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI, 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences 4, 215–222. 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Bzdok D, Heeger A, Langner R, Laird AR, Fox PT, Palomero-Gallagher N, Vogt BA, Zilles K, Eickhoff SB, 2015. Subspecialization in the human posterior medial cortex. NeuroImage 106, 55–71. 10.1016/j.neuroimage.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ, 2001. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping 14, 140–151. 10.1002/hbm.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella F, Crescentini C, Urgesi C, Fabbro F, 2014. Mindfulness-oriented meditation improves self-related character scales in healthy individuals. Comprehensive Psychiatry 55, 1269–1278. 10.1016/j.comppsych.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Campos D, Modrego-Alarcón M, López-del-Hoyo Y, González-Panzano M, Van Gordon W, Shonin E, Navarro-Gil M, García-Campayo J, 2019. Exploring the role of meditation and dispositional mindfulness on social cognition domains: a controlled study. Front. Psychol. 10. 10.3389/fpsyg.2019.00809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardaciotto L, Herbert JD, Forman EM, Moitra E, Farrow V, 2008. The assessment of present-moment awareness and acceptance: the philadelphia mindfulness scale. Assessment 15, 204–223. 10.1177/1073191107311467 [DOI] [PubMed] [Google Scholar]
- Caspi O, Burleson KO, 2005. Methodological challenges in meditation research. Adv Mind Body Med 21, 4–11. [PubMed] [Google Scholar]
- Chadwick P, Hember M, Symes J, Peters E, Kuipers E, Dagnan D, 2008. Responding mindfully to unpleasant thoughts and images: Reliability and validity of the Southampton mindfulness questionnaire (SMQ). British Journal of Clinical Psychology 47, 451–455. 10.1348/014466508X314891 [DOI] [PubMed] [Google Scholar]
- Chalmers TC, Smith H, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A, 1981. A method for assessing the quality of a randomized control trial. Controlled Clinical Trials 2, 31–49. 10.1016/0197-2456(81)90056-8 [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG, 2013. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex 23, 739–749. 10.1093/cercor/bhs065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa A, Calati R, Serretti A, 2011. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin Psychol Rev 31, 449–464. 10.1016/j.cpr.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A, 2009. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med 15, 593–600. 10.1089/acm.2008.0495 [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS, 2013. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16, 1348–1355. 10.1038/nn.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, Tapert SF, Banerjee D, Simmons AN, Yang TT, 2013. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry 74, 898–907. 10.1016/j.biopsych.2013.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL, 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, 2002. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience 3, 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Creswell JD, Taren AA, Lindsay EK, Greco CM, Gianaros PJ, Fairgrieve A, Marsland AL, Brown KW, Way BM, Rosen RK, Ferris JL, 2016. Alterations in resting-state functional connectivity link mindfulness meditation with reduced interleukin-6: a randomized controlled trial. Biol. Psychiatry 80, 53–61. 10.1016/j.biopsych.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts S. a. R.B., Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF, 2006. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U.S.A. 103, 13848–13853. 10.1073/pnas.0601417103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Pujol J, Harrison BJ, 2016. Mapping the self in the brain’s default mode network. NeuroImage 132, 390–397. 10.1016/j.neuroimage.2016.02.022 [DOI] [PubMed] [Google Scholar]
- Davidson RJ, 2010. Empirical explorations of mindfulness: conceptual and methodological conundrums. Emotion 10, 8–11. 10.1037/a0018480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Kaszniak AW, 2015. Conceptual and methodological issues in research on mindfulness and meditation. Am Psychol 70, 581–592. 10.1037/a0039512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR, 2011. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci 12, 43–56. 10.1038/nrn2961 [DOI] [PubMed] [Google Scholar]
- Desbordes G, Gard T, Hoge EA, Hölzel BK, Kerr C, Lazar SW, Olendzki A, Vago DR, 2015. Moving beyond mindfulness: defining equanimity as an outcome measure in meditation and contemplative research. mindfulness (N Y) 6, 356–372. 10.1007/s12671-013-0269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers TM, Chatham CH, Badre D, 2015. The necessity of rostrolateral prefrontal cortex for higher-level sequential behavior. Neuron 87, 1357–1368. 10.1016/j.neuron.2015.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman GC, Huizink AC, Tulen JHM, Utens EMWJ, Creemers HE, van der Ende J, Verhulst FC, 2015. Alterations in HPA-axis and autonomic nervous system functioning in childhood anxiety disorders point to a chronic stress hypothesis. Psychoneuroendocrinology, This issue includes a Special Section on Biomarkers in the Military - New Findings from Prospective Studies 51, 135–150. 10.1016/j.psyneuen.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, Beck AT, 2011. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience 12, 467–477. 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Dixon ML, De La Vega A, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, Christoff K, 2018. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci U S A 115, E1598–E1607. 10.1073/pnas.1715766115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll A, Hölzel BK, Boucard CC, Wohlschläger AM, Sorg C, 2015. Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Front Hum Neurosci 9. 10.3389/fnhum.2015.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw L, Wakeman DG, Tanaka N, Liu H, Stufflebeam SM, 2016. State-dependent variability of dynamic functional connectivity between frontoparietal and default networks relates to cognitive flexibility. Neuroscience 339, 12–21. 10.1016/j.neuroscience.2016.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD, 2002. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J. Neurophysiol. 87, 615–620. 10.1152/jn.00636.2001 [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M, 2008. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13, 663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15, 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone G, Jerram M, 2018. Brain activity in mindfulness depends on experience: a meta-analysis of fmri studies. Mindfulness 9, 1319–1329. 10.1007/s12671-018-0884-5 [DOI] [Google Scholar]
- Fan Y, Borchardt-Lohölter V, Düring F, Leutritz A, Dietz M, Herrera-Meléndez A, Bajbouj M, li M, Grimm S, Walter M, 2018. Dorsal and ventral PCC switch network assignment via changes in relative functional connectivity strength to non-canonical networks. Brain Connectivity. 10.1089/brain.2018.0602 [DOI] [PubMed] [Google Scholar]
- Farrant K, Uddin LQ, 2015. Asymmetric development of dorsal and ventral attention networks in the human brain. Dev Cogn Neurosci 12, 165–174. 10.1016/j.dcn.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman G, Hayes A, Kumar S, Greeson J, Laurenceau J-P, 2007. Mindfulness and emotion regulation: the development and initial validation of the cognitive and affective mindfulness scale-revised (CAMS-R). J Psychopathol Behav Assess 29, 177–190. 10.1007/s10862-006-9035-8 [DOI] [Google Scholar]
- Fowler CH, Miernicki ME, Rudolph KD, Telzer EH, 2017. Disrupted amygdala-prefrontal connectivity during emotion regulation links stress-reactive rumination and adolescent depressive symptoms. Developmental Cognitive Neuroscience 27, 99–106. 10.1016/j.dcn.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KCR, Dixon ML, Nijeboer S, Girn M, Floman JL, Lifshitz M, Ellamil M, Sedlmeier P, Christoff K, 2016. Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. arXiv:1603.06342 [q-bio]. [DOI] [PubMed] [Google Scholar]
- Fox KCR, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, Sedlmeier P, Christoff K, 2014. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neuroscience & Biobehavioral Reviews 43, 48–73. 10.1016/j.neubiorev.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M, 2010. Clinical applications of resting state functional connectivity. Front. Syst. Neurosci 4. 10.3389/fnsys.2010.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME, 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience 8, 700–711. 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- Freton M, Lemogne C, Delaveau P, Guionnet S, Wright E, Wiernik E, Bertasi E, Fossati P, 2014. The dark side of self-focus: brain activity during self-focus in low and high brooders. Soc Cogn Affect Neurosci 9, 1808–1813. 10.1093/scan/nst178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ, Resick PA, Bryant RA, Brewin CR, 2011. Considering PTSD for DSM-5. Depress Anxiety 28, 750–769. 10.1002/da.20767 [DOI] [PubMed] [Google Scholar]
- Friedman MJ, Schnurr PP, McDonagh-Coyle A, 1994. Post-traumatic stress disorder in the military veteran. Psychiatr. Clin. North Am. 17, 265–277. [PubMed] [Google Scholar]
- Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen N-K, McClernon FJ, Greeson JM, Sobin P, 2012. Meditation-state functional connectivity (msfc): strengthening of the dorsal attention network and beyond. Evid Based Complement Alternat Med 2012, 680407. 10.1155/2012/680407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant SN, 2016. Mindfulness meditation practice and executive functioning: Breaking down the benefit. Conscious Cogn 40, 116–130. 10.1016/j.concog.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Garrison KA, Zeffiro TA, Scheinost D, Constable RT, Brewer JA, 2015. Meditation leads to reduced default mode network activity beyond an active task. Cogn Affect Behav Neurosci 15, 712–720. 10.3758/s13415-015-0358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Vila-Rodriguez F, 2020. Functional connectivity of the anterior cingulate cortex predicts treatment outcome for rTMS in treatment-resistant depression at 3-month follow-up. Brain Stimulation 13, 206–214. 10.1016/j.brs.2019.10.012 [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Frith CD, Burgess PW, 2005. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. European Journal of Neuroscience 21, 1423–1431. 10.1111/j.1460-9568.2005.03981.x [DOI] [PubMed] [Google Scholar]
- Goldberg SB, Wielgosz J, Dahl C, Schuyler B, MacCoon DS, Rosenkranz M, Lutz A, Sebranek CA, Davidson RJ, 2016. Does the Five Facet Mindfulness Questionnaire measure what we think it does? Construct validity evidence from an active controlled randomized clinical trial. Psychol Assess 28, 1009–1014. 10.1037/pas0000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG, 2014. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage 99, 180–190. 10.1016/j.neuroimage.2014.05.052 [DOI] [PubMed] [Google Scholar]
- Grecucci A, Pappaianni E, Siugzdaite R, Theuninck A, Job R, 2015. Mindful Emotion Regulation: Exploring the Neurocognitive Mechanisms behind Mindfulness. Biomed Res Int 2015. 10.1155/2015/670724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg T, Bertocci MA, Chase HW, Stiffler R, Aslam HA, Graur S, Bebko G, Lockovich JC, Phillips ML, 2017. Mediation by anxiety of the relationship between amygdala activity during emotion processing and poor quality of life in young adults. Translational Psychiatry 7, e1178–e1178. 10.1038/tp.2017.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M, 2008. Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol. 21, 424–430. 10.1097/WCO.0b013e328306f2c5 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V, 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 100, 253–258. 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF, 2009. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19, 72–78. 10.1093/cercor/bhn059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guendelman S, Medeiros S, Rampes H, 2017. Mindfulness and emotion regulation: insights from neurobiological, psychological, and clinical studies. Front Psychol 8. 10.3389/fpsyg.2017.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Dai Y, Jiang M, Zhang J, Yu L, Long L, Chen H, Gao Q, Xiao C, 2013. Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry 41, 24–29. 10.1016/j.pnpbp.2012.11.003 [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JYF, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJR, Leibenluft E, Fox NA, Ernst M, Pine DS, Nelson EE, 2008. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry 65, 1303–1312. 10.1001/archpsyc.65.11.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata Y, Mizukami S, Izawa S, Moriguchi Y, Hori H, Kim Y, Hanakawa T, Inoue Y, Tagaya H, 2020. Basolateral amygdala connectivity with subgenual anterior cingulate cortex represents enhanced fear-related memory encoding in anxious humans. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 5, 301–310. 10.1016/j.bpsc.2019.11.008 [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH, 2015. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry 78, 224–230. 10.1016/j.biopsych.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, 2018. Risk factors for depression: an autobiographical review. Annu Rev Clin Psychol 14, 1–28. 10.1146/annurev-clinpsy-050817-084811 [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW, 2012. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage 59, 750–760. 10.1016/j.neuroimage.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Hill CLM, Updegraff JA, 2012. Mindfulness and its relationship to emotional regulation. Emotion 12, 81–90. 10.1037/a0026355 [DOI] [PubMed] [Google Scholar]
- Ho TC, Yang G, Wu J, Cassey P, Brown SD, Hoang N, Chan M, Connolly CG, Henje-Blom E, Duncan LG, Chesney MA, Paulus MP, Max JE, Patel R, Simmons AN, Yang TT, 2014. Functional connectivity of negative emotional processing in adolescent depression. Journal of Affective Disorders 155, 65–74. 10.1016/j.jad.2013.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D, 2010. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol 78, 169–183. 10.1037/a0018555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Hoge EA, Greve DN, Gard T, Creswell JD, Brown KW, Barrett LF, Schwartz C, Vaitl D, Lazar SW, 2013. Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. Neuroimage Clin 2, 448–458. 10.1016/j.nicl.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U, 2011. How does mindfulness meditation work? proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci 6, 537–559. 10.1177/1745691611419671 [DOI] [PubMed] [Google Scholar]
- Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, Eckert U, Zierhut KC, Schiltz K, He H, Biswal B, Bogerts B, Walter M, 2010. Glutamatergic and resting-state functional connectivity correlates of severity in major depression -the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci 4. 10.3389/fnsys.2010.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AJ, Digdon NL, Buro K, Sheptycki AR, 2008. Relations among mindfulness, well-being, and sleep. Personality and Individual Differences 45, 773–777. 10.1016/j.paid.2008.08.005 [DOI] [Google Scholar]
- Ide JS, Shenoy P, Yu AJ, Li CR, 2013. Bayesian prediction and evaluation in the anterior cingulate cortex. J. Neurosci. 33, 2039–2047. 10.1523/JNEUROSCI.2201-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG, 2015. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci 9, 23. 10.3389/fnsys.2015.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TM, 2018. The effects of mindfulness meditation on emotion regulation, cognition and social skills. ESJ 14, 18. 10.19044/esj.2018.v14n14p18 [DOI] [Google Scholar]
- Kabat-Zinn J, 2003. Mindfulness-based stress reduction (MBSR). Constructivism in the Human Sciences 8, 73–107. [Google Scholar]
- Kabat-Zinn J, 1982. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry 4, 33–47. 10.1016/0163-8343(82)90026-3 [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA, 2015. Large-scale network dysfunction in Major Depressive Disorder: Meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Masuda N, Lau JKL, and Murayama K (2020). Focused attention meditation changes the boundary and configuration of functional networks in the brain. Sci Rep 10, 18426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphausen S, Schröder P, Maier S, Bader K, Feige B, Kaller C, Glauche V, Ohlendorf S, Tebartz van Elst L, Klöppel S, Jacob G, Silbersweig D, Lieb K, Tuescher O, 2012. Medial prefrontal dysfunction and prolonged amygdala response during instructed fear processing in borderline personality disorder. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry 14. 10.3109/15622975.2012.665174 [DOI] [PubMed] [Google Scholar]
- Keng S-L, Smoski MJ, Robins CJ, 2011. Effects of mindfulness on psychological health: A review of empirical studies. Clinical Psychology Review 31, 1041–1056. 10.1016/j.cpr.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiken LG, Garland EL, Bluth K, Palsson OS, Gaylord SA, 2015. From a state to a trait: trajectories of state mindfulness in meditation during intervention predict changes in trait mindfulness. personality and individual differences, Dr. Sybil Eysenck Young Researcher Award 81, 41–46. 10.1016/j.paid.2014.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Suyenobu BY, Smith SR, Bueller JA, Goodman T, Creswell JD, Tillisch K, Mayer EA, Naliboff BD, 2011. Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. NeuroImage 56, 290–298. 10.1016/j.neuroimage.2011.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, Angstadt M, Kessler D, Welsh R, Liberzon I, 2016. Aletered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depress Anxiety 33, 289–299. 10.1002/da.22481 [DOI] [PubMed] [Google Scholar]
- Kong F, Wang X, Song Y, Liu J, 2016. Brain regions involved in dispositional mindfulness during resting state and their relation with well-being. Social Neuroscience 11, 331–343. 10.1080/17470919.2015.1092469 [DOI] [PubMed] [Google Scholar]
- Kopp MS, Réthelyi J, 2004. Where psychology meets physiology: chronic stress and premature mortality—the Central-Eastern European health paradox. Brain Research Bulletin 62, 351–367. 10.1016/j.brainresbull.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Kral TRA, Imhoff-Smith T, Dean DC, Grupe D, Adluru N, Patsenko E, Mumford JA, Goldman R, Rosenkranz MA, Davidson RJ, 2019. Mindfulness-based stress reduction-related changes in posterior cingulate resting brain connectivity. Soc Cogn Affect Neurosci 14, 777–787. 10.1093/scan/nsz050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Lewis G, Watkins E, Brejcha C, Cardy J, Causley A, Cowderoy S, Evans A, Gradinger F, Kaur S, Lanham P, Morant N, Richards J, Shah P, Sutton H, Vicary R, Weaver A, Wilks J, Williams M, Taylor RS, Byford S, 2015. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. The Lancet 386, 63–73. 10.1016/S0140-6736(14)62222-4 [DOI] [PubMed] [Google Scholar]
- Kuyken W, Warren FC, Taylor RS, Whalley B, Crane C, Bondolfi G, Hayes R, Huijbers M, Ma H, Schweizer S, Segal Z, Speckens A, Teasdale JD, Van Heeringen K, Williams M, Byford S, Byng R, Dalgleish T, 2019. Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse. JAMA Psychiatry 73, 565–574. 10.1001/jamapsychiatry.2016.0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak S, Lee TY, Jung WH, Hur J-W, Bae D, Hwang WJ, Cho KIK, Lim K-O, Kim S-Y, Park HY, Kwon JS, 2019. The immediate and sustained positive effects of meditation on resilience are mediated by changes in the resting brain. front. Hum. Neurosci. 13. 10.3389/fnhum.2019.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau MA, Bishop SR, Segal ZV, Buis T, Anderson ND, Carlson L, Shapiro S, Carmody J, Abbey S, Devins G, 2006. The toronto mindfulness scale: Development and validation. J. Clin. Psychol. 62, 1445–1467. 10.1002/jclp.20326 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, 1994. The amygdala: contributions to fear and stress. Seminars in Neuroscience 6, 231–237. 10.1006/smns.1994.1030 [DOI] [Google Scholar]
- Lee MH, Smyser CD, Shimony JS, 2013. Resting-State fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol 34, 1866–1872. 10.3174/ajnr.A3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ, 2011. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci 31, 3217–3224. 10.1523/JNEUROSCI.5626-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ, 2014. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. 10.1093/brain/awt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Smallwood J, 2019. The posterior cingulate cortex: Insights from structure and function. Handbook of clinical neurology. 10.1016/b978-0-444-64196-0.00005-4 [DOI] [PubMed] [Google Scholar]
- Lifshitz M, Sacchet MD, Huntenburg JM, Thiery T, Fan Y, Gärtner M, Grimm S, Winnebeck E, Fissler M, Schroeter TA, Margulies DS, Barnhofer T, 2019. Mindfulness-based therapy regulates brain connectivity in major depression. Psychother Psychosom 88, 375–377. 10.1159/000501170 [DOI] [PubMed] [Google Scholar]
- Luders E, Clark K, Narr KL, Toga AW, 2011. Enhanced brain connectivity in long-term meditation practitioners. Neuroimage 57, 1308–1316. 10.1016/j.neuroimage.2011.05.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Juster R-P, Raymond C, Marin M-F, 2018. The effects of chronic stress on the human brain: From neurotoxicity, to vulnerability, to opportunity. Front Neuroendocrinol 49, 91–105. 10.1016/j.yfrne.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Lutz A, Jha AP, Dunne JD, Saron CD, 2015. Investigating the Phenomenological Matrix of Mindfulness-related Practices from a Neurocognitive Perspective. Am Psychol 70, 632–658. 10.1037/a0039585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ, 2008. Attention regulation and monitoring in meditation. Trends Cogn Sci 12, 163–169. 10.1016/j.tics.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz J, Brühl AB, Scheerer H, Jäncke L, Herwig U, 2016. Neural correlates of mindful self-awareness in mindfulness meditators and meditation-naïve subjects revisited. Biological Psychology 119, 21–30. 10.1016/j.biopsycho.2016.06.010 [DOI] [PubMed] [Google Scholar]
- Lutz J, Herwig U, Opialla S, Hittmeyer A, Jäncke L, Rufer M, Grosse Holtforth M, Brühl AB, 2014. Mindfulness and emotion regulation—an fMRI study. Soc Cogn Affect Neurosci 9, 776–785. 10.1093/scan/nst043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch LK, Lu K-H, Wen H, Zhang Y, Saykin AJ, Liu Z, 2018. Task-evoked functional connectivity does not explain functional connectivity differences between rest and task conditions. Human Brain Mapping 39, 4939–4948. 10.1002/hbm.24335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Anderson EJ, 2007. Further psychometric validation of the mindful attention awareness scale (MAAS). J Psychopathol Behav Assess 29, 289–293. 10.1007/s10862-007-9045-1 [DOI] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH, 2001. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104, 667–676. 10.1016/S0306-4522(01)00108-7 [DOI] [PubMed] [Google Scholar]
- Malinowski P, 2013. Neural mechanisms of attentional control in mindfulness meditation. Front Neurosci 7. 10.3389/fnins.2013.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Schwerthöffer D, Zimmer C, Förstl H, Bäuml J, Riedl V, Wohlschläger AM, Sorg C, 2013. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci 7, 930. 10.3389/fnhum.2013.00930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes J, 2019. Systematic review and meta-analysis of correlates of FFMQ mindfulness facets. Front. Psychol 10. 10.3389/fpsyg.2019.02684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy M, Larson-Prior L, Nolan TS, Vaishnavi SN, Raichle ME, d’Avossa G, 2008. Resting states affect spontaneous BOLD oscillations in sensory and paralimbic cortex. J Neurophysiol 100, 922–931. 10.1152/jn.90426.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcewen BS, 2004. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences 1032, 1–7. 10.1196/annals.1314.001 [DOI] [PubMed] [Google Scholar]
- Menon V, 2015. Salience network, in: brain mapping. Elsevier, pp. 597–611. 10.1016/B978-0-12-397025-1.00052-X [DOI] [Google Scholar]
- Menon V, 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15, 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ, 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214, 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesmer-Magnus J, Manapragada A, Viswesvaran C, Allen JW, 2017. Trait mindfulness at work: A meta-analysis of the personal and professional correlates of trait mindfulness. Human Performance 30, 79–98. 10.1080/08959285.2017.1307842 [DOI] [Google Scholar]
- Mohanty R, Sethares WA, Nair VA, Prabhakaran V, 2020. Rethinking measures of functional connectivity via feature extraction. Scientific Reports 10, 1298. 10.1038/s41598-020-57915-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I, 2015. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev 56, 330–344. 10.1016/j.neubiorev.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Ortner C, Kilner S, Zelazo P, 2007. Mindfulness meditation and reduced emotional interference on a cognitive task. Motivation and Emotion 31, 271–283. 10.1007/s11031-007-9076-7 [DOI] [Google Scholar]
- Park T, Reilly-Spong M, Gross CR, 2013. Mindfulness: A systematic review of instruments to measure an emergent patientreported outcome (PRO). Qual Life Res 22. 10.1007/s11136-013-0395-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson TD, Kornelsen J, Smith SD, 2019. Trait mindfulness and functional connectivity in cognitive and attentional resting state networks. Front Hum Neurosci 13. 10.3389/fnhum.2019.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piet J, Hougaard E, 2011. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clin Psychol Rev 31, 1032–1040. 10.1016/j.cpr.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Poerio GL, Sormaz M, Wang H-T, Margulies D, Jefferies E, Smallwood J, 2017. The role of the default mode network in component processes underlying the wandering mind. Soc Cogn Affect Neurosci 12, 1047–1062. 10.1093/scan/nsx041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissant H, Mendrek A, Talbot N, Khoury B, Nolan J, 2019. Behavioral and cognitive impacts of mindfulness-based interventions on adults with attention-deficit hyperactivity disorder: a systematic review. Behav Neurol 2019, 5682050. 10.1155/2019/5682050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, 2006. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences 10, 59–63. 10.1016/j.tics.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Priddy SE, Howard MO, Hanley AW, Riquino MR, Friberg-Felsted K, Garland EL, 2018. Mindfulness meditation in the treatment of substance use disorders and preventing future relapse: neurocognitive mechanisms and clinical implications. Subst Abuse Rehabil 9, 103–114. 10.2147/SAR.S145201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R, 2012. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist 18, 502–515. 10.1177/1073858411409051 [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL, 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM, 2004. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci 5, 184–194. 10.1038/nrn1343 [DOI] [PubMed] [Google Scholar]
- Reineberg AE, Gustavson DE, Benca C, Banich MT, Friedman NP, 2018. The relationship between resting state network connectivity and individual differences in executive functions. Front Psychol 9. 10.3389/fpsyg.2018.01600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Rochefort C, Lui PP, Baldwin AS, 2019. Trait mindfulness and health behaviours: A meta-analysis. Health Psychology Review No Pagination Specified-No Pagination Specified. 10.1080/17437199.2019.1650290 [DOI] [PubMed] [Google Scholar]
- Sambataro F, Wolf ND, Pennuto M, Vasic N, Wolf RC, 2014. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychol Med 44, 2041–2051. 10.1017/S0033291713002596 [DOI] [PubMed] [Google Scholar]
- Scharnowski F, Nicholson AA, Pichon S, Rosa MJ, Rey G, Eickhoff SB, Ville DVD, Vuilleumier P, Koush Y, 2020. The role of the subgenual anterior cingulate cortex in dorsomedial prefrontal–amygdala neural circuitry during positive-social emotion regulation. Human Brain Mapping 41, 3100–3118. 10.1002/hbm.25001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, Seifritz E, 2012. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS ONE 7, e44799. 10.1371/journal.pone.0044799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K, 2008. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex 18, 846–867. 10.1093/cercor/bhm116 [DOI] [PubMed] [Google Scholar]
- Schoenfeld T, Gould E, 2012. Stress, stress hormones, and adult neurogenesis. Exp Neurol 233, 12–21. 10.1016/j.expneurol.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, 2019. The salience network: a neural system for perceiving and responding to homeostatic demands. J. Neurosci. 39, 9878–9882. 10.1523/JNEUROSCI.1138-17.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K, Hillstrom AP, Husain M, 2002. Control of visuotemporal attention by inferior parietal and superior temporal cortex. Current Biology 12, 1320–1325. 10.1016/S0960-9822(02)01040-0 [DOI] [PubMed] [Google Scholar]
- Sharf R, 2014. Mindfulness and mindlessness in early chan. Philosophy East and West 64, 933–964. [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA, 2010. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A 107, 11020–11025. 10.1073/pnas.1000446107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y-Q, Zhou H-X, Chen X, Castellanos FX, Yan C-G, 2020. Meditation effect in changing functional integrations across large-scale brain networks: Preliminary evidence from a meta-analysis of seed-based functional connectivity. Journal of Pacific Rim Psychology 14, e10. 10.1017/prp.2020.1 [DOI] [Google Scholar]
- Smith B, Metzker K, Waite R, Gerrity P, 2015. Short-form mindfulness-based stress reduction reduces anxiety and improves health-related quality of life in an inner-city population. Holist Nurs Pract 29, 70–77. 10.1097/HNP.0000000000000075 [DOI] [PubMed] [Google Scholar]
- Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, Nichols TE, Robinson E, Salimi-Khorshidi G, Woolrich MW, Barch DM, Uğurbil K, Van Essen DC, 2013. Functional connectomics from resting-state fMRI. Trends Cogn Sci 17, 666–682. 10.1016/j.tics.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitha K, Akhil Raja K, Arun K, Rajesh P, Thomas B, Kapilamoorthy T, Kesavadas C, 2017. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J 30, 305–317. 10.1177/1971400917697342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AZ, Raichle ME, 2012. A brief history of the resting state: the Washington University perspective. Neuroimage 62, 902–910. 10.1016/j.neuroimage.2012.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL, 2010. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53, 303–317. 10.1016/j.neuroimage.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprooten E, Rasgon A, Goodman M, Carlin A, Leibu E, Lee WH, Frangou S, 2017. Addressing reverse inference in psychiatric neuroimaging: Meta-analyses of task-related brain activation in common mental disorders. Human Brain Mapping 38, 1846–1864. 10.1002/hbm.23486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V, 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U.S.A. 105, 12569–12574. 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens FL, 2011. Anterior Cingulate Cortex: Unique Role in Cognition and Emotion. J Neuropsychiatry Clin Neurosci 6. [DOI] [PubMed] [Google Scholar]
- Su I-W, Wu F-W, Liang K-C, Cheng K-Y, Hsieh S-T, Sun W-Z, Chou T-L, 2016. Pain perception can be modulated by mindfulness training: a resting-state fMRI study. Front. Hum. Neurosci. 10. 10.3389/fnhum.2016.00570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugunasiri SHJ, 2008. Establishing of mindfulness meditation (satipatthana bhavana): the creative interplay of cognition, praxis and affection. [Google Scholar]
- Tafet GE, Bernardini R, 2003. Psychoneuroendocrinological links between chronic stress and depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 27, 893–903. 10.1016/S0278-5846(03)00162-3 [DOI] [PubMed] [Google Scholar]
- Tan LBG, Lo BCY, Macrae CN, 2014. Brief mindfulness meditation improves mental state attribution and empathizing. PLoS ONE 9, e110510. 10.1371/journal.pone.0110510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-Y, 2017. Traits and states in mindfulness meditation, in: tang, y.-y. (ed.), the neuroscience of mindfulness meditation: how the body and mind work together to change our behaviour. Springer International Publishing, Cham, pp. 29–34. 10.1007/978-3-319-46322-3_4 [DOI] [Google Scholar]
- Tang Y-Y, Hölzel BK, Posner MI, 2015. The neuroscience of mindfulness meditation. Nature Reviews Neuroscience 16, 213–225. 10.1038/nrn3916 [DOI] [PubMed] [Google Scholar]
- Taren AA, Gianaros PJ, Greco CM, Lindsay EK, Fairgrieve A, Brown KW, Rosen RK, Ferris JL, Julson E, Marsland AL, Bursley JK, Ramsburg J, Creswell JD, 2015. Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: a randomized controlled trial. Soc Cogn Affect Neurosci 10, 1758–1768. 10.1093/scan/nsv066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taren AA, Gianaros PJ, Greco CM, Lindsay EK, Fairgrieve A, Brown KW, Rosen RK, Ferris JL, Julson E, Marsland AL, Creswell JD, 2017. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med 79, 674–683. 10.1097/PSY.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, Zhuo Y, 2006. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neuroscience Letters 400, 39–43. 10.1016/j.neulet.2006.02.022 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, 2015. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16, 55–61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Clare Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP, 2008. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp 30, 625–637. 10.1002/hbm.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O, 2017. Structure and function of the human insula. J Clin Neurophysiol 34, 300–306. 10.1097/WNP.0000000000000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Yeo BTT, Spreng RN, 2019. Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr 32, 926–942. 10.1007/s10548-019-00744-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA, 2014. Precuneus is a functional core of the default-mode network. J Neurosci 34, 932–940. 10.1523/JNEUROSCI.4227-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago DR, and Silbersweig DA (2012). Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. Front Hum Neurosci 6, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago DR, and Zeidan F (2016). The brain on silent: mind wandering, mindful awareness, and states of mental tranquility. Ann N Y Acad Sci 1373, 96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam NT, van Vugt MK, Vago DR, Schmalzl L, Saron CD, Olendzki A, Meissner T, Lazar SW, Kerr CE, Gorchov J, Fox KCR, Field BA, Britton WB, Brefczynski-Lewis JA, Meyer DE, 2018. Mind the hype: a critical evaluation and prescriptive agenda for research on mindfulness and meditation. Perspect Psychol Sci 13, 36–61. 10.1177/1745691617709589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE, 2010. Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology 20, 519–534. 10.1016/j.euroneuro.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Venkataraman A, Van Dijk KRA, Buckner RL, Golland P, 2009. Exploring functional connectivity in fMRI via clustering. Proc IEEE Int Conf Acoust Speech Signal Process 2009, 441–444. 10.1109/ICASSP.2009.4959615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL, 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100, 3328–3342. 10.1152/jn.90355.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, 2005. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6, 533–544. 10.1038/nrn1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR, 2014. Dorsal and ventral attention systems. Neuroscientist 20, 150–159. 10.1177/1073858413494269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S, 2004. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 128, 667–673. 10.1016/j.neuroscience.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Walach H, Buchheld N, Buttenmüller V, Kleinknecht N, Schmidt S, 2006. Measuring mindfulness—the Freiburg Mindfulness Inventory (FMI). Personality and Individual Differences 40, 1543–1555. 10.1016/j.paid.2005.11.025 [DOI] [Google Scholar]
- Wanden-Berghe RG, Sanz-Valero J, Wanden-Berghe C, 2011. The application of mindfulness to eating disorders treatment: a systematic review. Eat Disord 19, 34–48. 10.1080/10640266.2011.533604 [DOI] [PubMed] [Google Scholar]
- Wang D, Buckner RL, Fox MD, Holt DJ, Holmes AJ, Stoecklein S, Langs G, Pan R, Qian T, Li K, Baker JT, Stufflebeam SM, Wang K, Wang X, Hong B, Liu H, 2015. Parcellating cortical functional networks in individuals. Nat Neurosci 18, 1853–1860. 10.1038/nn.4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG, 2005. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex 15, 229–237. 10.1093/cercor/bhh125 [DOI] [PubMed] [Google Scholar]
- Wheeler MS, Arnkoff DB, Glass CR, 2017. The neuroscience of mindfulness: how mindfulness alters the brain and facilitates emotion regulation. Mindfulness 8, 1471–1487. 10.1007/s12671-017-0742-x [DOI] [Google Scholar]
- Williams JMG, Kabat-Zinn J, 2011. Mindfulness: diverse perspectives on its meaning, origins, and multiple applications at the intersection of science and dharma. Contemporary Buddhism 12, 1–18. 10.1080/14639947.2011.564811 [DOI] [Google Scholar]
- Winnebeck E, Fissler M, Gärtner M, Chadwick P, Barnhofer T, 2017. Brief training in mindfulness meditation reduces symptoms in patients with a chronic or recurrent lifetime history of depression: A randomized controlled study. Behaviour Research and Therapy 99, 124–130. 10.1016/j.brat.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Wise T, Marwood L, Perkins AM, Herane-Vives A, Joules R, Lythgoe DJ, Luh W-M, Williams SCR, Young AH, Cleare AJ, Arnone D, 2017. Instability of default mode network connectivity in major depression: a two-sample confirmation study. Transl Psychiatry 7, e1105. 10.1038/tp.2017.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Cascio CJ, 2015. Resting-state functional connectivity in psychiatric disorders. JAMA Psychiatry 72, 743–744. 10.1001/jamapsychiatry.2015.0484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Li H, Luo X, Fu R, Ying X, Wang N, Yin Q, Zou Y, Cui Y, Wang X, Shi C, 2014. Anhedonia and pain avoidance in the suicidal mind: behavioral evidence for motivational manifestations of suicidal ideation in patients with major depressive disorder. J Clin Psychol 70, 681–692. 10.1002/jclp.22055 [DOI] [PubMed] [Google Scholar]
- Yang C-C, Barrós-Loscertales A, Pinazo D, Ventura-Campos N, Borchardt V, Bustamante J-C, Rodríguez-Pujadas A, Fuentes-Claramonte P, Balaguer R, Ávila C, Walter M, 2016. State and training effects of mindfulness meditation on brain networks reflect neuronal mechanisms of its antidepressant effect. Neural Plasticity 2016, 1–14. 10.1155/2016/9504642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhao Y, Wang Y, Liu L, Zhang X, Li B, Cui R, 2015. The effects of psychological stress on depression. Curr Neuropharmacol 13, 494–504. 10.2174/1570159X1304150831150507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Gao C, Wu X, Yang J, Li S, Cheng H, 2016. Decreased functional connectivity to posterior cingulate cortex in major depressive disorder. Psychiatry Research: Neuroimaging 255, 15–23. 10.1016/j.pscychresns.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Yang S, Chang MC, 2019. Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci 20. 10.3390/ijms20133130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V, Phillips R, Young KD, Drevets WC, Bodurka J, 2013. Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLOS ONE 8, e79184. 10.1371/journal.pone.0079184 [DOI] [PMC free article] [PubMed] [Google Scholar]