Abstract
The application of autologous fat grafting in reconstructive surgery is commonly used to improve functional form. This review aims to provide an overview of the scientific evidence on the biology of adipose tissue, the role of adipose-derived stem cells, and the indications of adipose tissue grafting in peripheral nerve surgery. Adipose tissue is easily accessible through the lower abdomen and inner thighs. Non-vascularized adipose tissue grafting does not support oxidative and ischemic stress, resulting in variable survival of adipocytes within the first 24 hours. Enrichment of adipose tissue with a stromal vascular fraction is purported to increase the number of adipose-derived stem cells and is postulated to augment the long-term stability of adipose tissue grafts. Basic science nerve research suggests an increase in nerve regeneration and nerve revascularization, and a decrease in nerve fibrosis after the addition of adipose-derived stem cells or adipose tissue. In clinical studies, the use of autologous lipofilling is mostly applied to secondary carpal tunnel release revisions with promising results. Since the use of adipose-derived stem cells in peripheral nerve reconstruction is relatively new, more studies are needed to explore safety and long-term effects on peripheral nerve regeneration. The Food and Drug Administration stipulates that adipose-derived stem cell transplantation should be minimally manipulated, enzyme-free, and used in the same surgical procedure, e.g. adipose tissue grafts that contain native adipose-derived stem cells or stromal vascular fraction. Future research may be shifted towards the use of tissue-engineered adipose tissue to create a supportive microenvironment for autologous graft survival. Shelf-ready alternatives could be enhanced with adipose-derived stem cells or growth factors and eliminate the need for adipose tissue harvest.
Key Words: adipose tissue, adipose-derived stem cells, angiogenesis, autologous fat grafting, nerve injury, nerve regeneration, paracrine environment, peripheral nerve reconstruction, stem cell secretome, tissue engineering
Introduction
Adipose tissue harvesting and grafting have taken a greater role in clinical practice over the past few decades. Adipose tissue grafting techniques have rapidly evolved while advancing reconstructive options to restore functional and aesthetic form in reconstructive surgery. Lipofilling is often used to improve volume or contour, but is associated with the unpredictable resorption of the adipose tissue. This reduction in adipose volume is partly attributed to the insufficient vascularization of the transplanted tissue and may be overcome by pedicled adipofascial flaps (Nguyen et al., 1990). Perforator-based adipofascial flaps are particularly useful in small-to-medium-sized soft-tissue defects that are unsuitable for skin grafting alone (Ozakpinar et al., 2013). In peripheral nerve surgery, the use of platelet-rich plasma (PRP) in the sites of nerve injury may promote tissue healing and relief of neuropathic pain (El Khoury et al., 2017).
Adipose tissue has many advantages; it is easy to harvest, inexpensive, biocompatible, has potential for integration, and is regarded as an abundant source of multipotent stem cells (di Summa et al., 2011). The stromal vascular fraction (SVF) of subcutaneous adipose tissue contains adipose-derived stem cells (ASC). These cells share similarities with mesenchymal stromal cells isolated from the bone marrow and differentiate into multiple cell lineages such as adipocytes, chondrocytes, osteoblasts, and myocytes (Bunnell et al., 2008). ASCs secrete growth factors, which provide the potential of cell-based therapy and tissue engineering for reconstructive and nerve surgery (Bacakova et al., 2018; Travnickova and Bacakova, 2018). Although its clinical applications are still developing, scientific pre-clinical studies have demonstrated promising results. In this review, the scientific evidence on the role of ASCs within adipose tissue will be explored. Moreover, the application of adipose tissue and ASCs in nerve surgery and other applications will be detailed, and their indications in animal models and clinical studies will be described.
Search Strategy and Selection Criteria
Literature search was performed using PubMed, MEDLINE, Cochrane, Web of Science, and Google Scholar databases using the following combination of keywords: “adipose-derived stem cells” OR “cell-based therapy” OR “platelet-rich plasma” OR “adipose-tissue grafting” OR “stromal vascular fraction” AND “nerve regeneration” OR “nerve graft” OR “nerve transplantation” OR “nerve reconstruction” until July 2021. The results were further screened by title and abstract to only present studies in peripheral nerve injuries in both animals and humans. Other inclusion criteria were articles (i) written in English, (ii) published in the last 35 years, and (iii) that had available abstracts. Articles describing the use of ASCs in aesthetic surgery were excluded.
Grafting of Adipose Tissue
The first report of adipose tissue surgery dates back to 1893 when Neuber transferred multiple small fat grafts to fill a facial scar depression (Neuber, 1893). Adipose tissue grafting is now commonly used in soft-tissue reconstruction in conditions such as irradiation wounds, aesthetic surgery, breast reconstruction, burns, and trauma defects (Pu et al., 2015a). Although it has low immunogenicity to the host and is easily obtainable, it is currently limited by the unpredictability of resorption (Pu et al., 2015b; Zhou et al., 2016). While many previously believed that grafted adipocytes survive in the recipient site (Smahel, 1986; Billings and May, 1989; Cortese et al., 2000), newer studies by Eto et al. (2012) suggest that a large proportion of adipocytes in a non-vascularized fat graft are unlikely to survive even when the graft is placed in a good recipient bed. The non-vascularized nature of the graft does not support the oxidative and ischemic stress, resulting in necrosis of some of the adipocytes within the first 24 hours (Eto et al., 2011, 2012; Mashiko and Yoshimura, 2015). In the following days, survival is based on plasmatic imbibition and inosculation, and extensive infiltration of inflammatory cells occurs. Angiogenesis occurs and small graft vessels join local host vessels. If no revascularization occurs, fat degeneration and necrosis with scarring and oil cyst formation ensue with varying degrees of macrophage-mediated replacement of fat (Kato et al., 2014). Phagocytosis of adipocytes by macrophages and its resorption may take weeks to months, therefore the grafted adipose tissue maintains its original size for at least the first four weeks (Mashiko and Yoshimura, 2015). Often the inflammation decreases and the fat graft stabilizes (Kato et al., 2014). Whether or not these cells undergo replacement in the following months is currently a topic of debate (Eto et al., 2012; Kato et al., 2014; Mashiko and Yoshimura, 2015) since many studies have determined adipocyte viability solely by their morphology (Carpaneda and Ribeiro, 1993). Because of the large size of the adipocyte, being 50 to 150 μm, histologic sections (3 to 10 μm) cannot capture a single adipocyte including all its nuclei in one section to evaluate cell viability (Eto et al., 2012). Therefore, the determination of adipose tissue viability by standard histology is limited. The survival of adipose tissue is believed to be volume-dependent. The greater the volume is injected, the higher chances of necrosis, secondary to the decreased plasmatic diffusion of the surrounding host tissue. This has led to current research techniques that focus on purifying adipose tissue or maximizing the number of viable adipocytes prior to grafting (Carpaneda and Ribeiro, 1993; Boschert et al., 2002; Crawford et al., 2010).
Isolation and Harvesting of Adipose-Derived Stem Cells
Adipose graft survival may be influenced by various factors, such as age, donor harvest site, harvesting and processing technique, body mass index, and other variables (Geissler et al., 2014). ASCs demonstrate reduced angiogenic differentiation, proliferation, migration, viability, and an altered and inflammatory transcriptome in obese or overweight lineages compared to lean (Vyas et al., 2019). Donor sites to harvest high concentrations of ASCs and SVF are commonly the lower abdomen and inner thighs. These areas provide an ease of surgical access which is associated with minimal morbidity (Padoin et al., 2008; Li et al., 2013; Tsekouras et al., 2017). There are many adipose harvest techniques which include vacuum suction, syringe suction, or surgical excision (Tan et al., 2016; Fontes et al., 2018). Common to all techniques is the goal of minimizing adipocyte traumatic damage while increasing the survival of adipose tissue. The choice of technique is dependent on the volume required. Syringe aspiration is the preferred technique to maintain the viability and to maximize the cellular yield of ASCs, compared to conventional liposuction. This method can be applied when small volumes of fat, less than 100 mL, are required. When large volumes are needed, low negative-pressure lipoaspiration is preferred over conventional streamlined liposuction devices, to minimize trauma and improve the yield of ASCs. When collecting, the use of larger cannulas may reduce cellular rupture and preserve the native tissue architecture (Campbell et al., 1987). Moreover, wet and dry aspiration can be performed. Wet aspiration uses a tumescent solution containing lidocaine (0.01–0.04%, to maintain adipocyte viability) and a variable concentration of epinephrine injected at the donor site in a ratio of 1:1 or greater (Keck et al., 2010). This causes hydrodissection and enlarges the target fat layer, which facilitates the ease of aspiration with decreased pain, ecchymosis, and lower shear stress resulting in improved graft survival. Dry aspiration directly aspirates fat without the injection of any solution and is associated with more blood loss, ecchymosis, and postoperative pain (Illouz et al., 1989; Kakagia and Pallua, 2014; Kasem et al., 2015).
ASCs are isolated from whole fat or lipoaspirated fat and undergo a number of processing steps, including sedimentation, filtration, washing, centrifugation (e.g., with a force of 1200 × g for 3 minutes), and collagenase digestion to obtain the SVF (Zuk et al., 2002). After centrifugation, three layers are separated: the top layer contains lipids and debris from adipocyte rupture; the second consists of adipose tissue; the third layer is the aqueous layer containing blood and wetting solutions, and the SVF pellet is formed (Coleman, 2006; Condé-Green et al., 2010; Wilson et al., 2011; Tuin et al., 2016; Figure 1). SVF comprised 10% ASCs and yields a heterogeneous population of cells with distinct surface marker phenotypes (Shukla et al., 2020). The most commonly reported positive markers of ASCs are CD90, CD44, CD29, CD105, CD13, CD73, CD166, CD10, CD49e, and CD59, while the most commonly found negative markers are CD31, CD45, CD14, CD11b, CD34, CD19, CD56, and CD146 (Mildmay-White and Khan, 2017). A sub classification of markers includes the exhibition of a CD90+, CD31–, and CD45– cell surface marker profile (Gronthos et al., 2001; Johal et al., 2015). An overview of markers to identify mesenchymal stem cells (MSC) according to their tissue of origin is presented in Table 1 (Uder et al., 2018). ASCs have many homeostatic functions (Figure 2). ASCs regulate the extracellular matrix (ECM) to support tissue structure, provide the potential for angiogenesis, and produce cytokines that play a role in regeneration (Cherubino et al., 2011).
Figure 1.
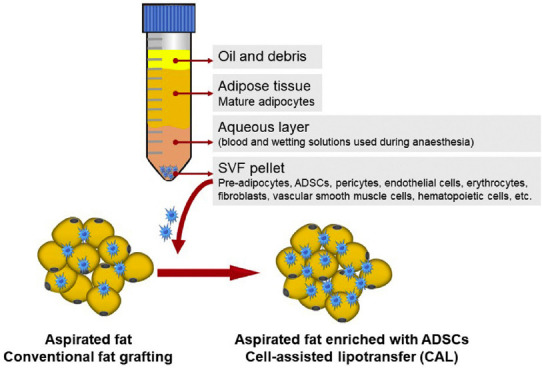
Enrichment of lipoaspirated adipose tissue by cell-assisted lipotransfer (CAL).
After centrifugation of the lipoaspirated fat, three layers are separated; the first contains oil and debris; the second contains adipose tissue; the third is the aqueous layer containing blood and wetting solutions used during anesthesia, and the stromal vascular fraction (SVF) pellet is formed. The SVF pallet is added to the lipoaspirated fat to enhance the yield of adipose-derived stem cells (ADSCs). Copyrighted, used, and reprinted with permission of Fontes and colleagues; all rights reserved (Fontes et al., 2018).
Table 1.
Summary of mesenchymal stem cell (MSC) sources and cell surface markers
| Origin of MSCs | Expressed in MSCs | Not expressed in MSCs |
|---|---|---|
| Adipose tissue | CD90, CD44, CD29, CD105, CD13, CD73, CD166, CD10, CD49e, CD59 | CD31, CD45, CD14, CD11b, CD34, CD19, CD56, CD146 |
| Bone marrow | CD90, CD105, CD73 | CD45, CD14, CD34 |
| Dental pulp | CD90, CD44, CD29, CD105 | CD45, CD14, CD34 |
| Peripheral blood | CD90, CD44, CD105 | CD45, CD133 |
| Skin | CD90, CD44, CD105, CD73, CD166 | CD45, CD34 |
| Differences between species | ||
| CD29, CD40, CD44 | Expressed across all species. | |
| CD45 | Not expressed, across all species. | |
| CD19 | Only not expressed in humans. | |
| CD49e, CD59 | Only expressed in humans. | |
| CD90 | Expressed in humans, pigs and rats. Less expressed in horses and sheep. Not expressed in mice. | |
| CD166 | Only expressed in humans, rats and sheep. | |
An overview of cell surface markers in different tissues is presented. A summary of differences between species is also given, comparing humans to horses, sheep, pigs, rats, and mice (Uder et al., 2018).
Figure 2.
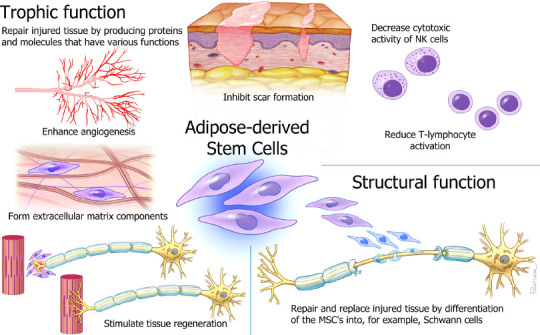
Schematic overview of the homeostatic roles of adipose-derived mesenchymal stem cells (ASC).
ASCs repair injured tissue by producing proteins and molecules that have various functions. They regulate the extracellular matrix to support tissue structure and integrity, support normal blood vessel function and enhance angiogenesis, inhibit scar formation, regulate immune homeostasis in primary and secondary lymphoid organs and in tissues, and decrease the cytotoxic activity of natural killer (NK) cells and reduce T-lymphocyte cells. Moreover, ASCs can stimulate tissue regeneration and repair and replace injured tissue after peripheral nerve injuries. MSC: Mesenchymal stem cells. Modified from (Mathot et al., 2019), used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Fat processing is required since the lipoaspirate also contains collagen fibers, blood, and debris, which can cause inflammation at the recipient site. Injection of these non-fat components can cause degradation of the grafted fat and an erroneous impression of the volume of correction (Mojallal and Foyatier, 2004). Centrifugation speeds higher than 1200 × g may damage the structural integrity of the adipose tissue, resulting in increased necrosis and apoptosis of cells, increased fluid and oil portions, and decreased injectable tissue volume. Damaged cells may also result in decreased adipogenic differentiation capacity and tubule formation, indicating a diminished function of cells (Simonacci et al., 2017). Small-gauge needles, adapted to the recipient site, are argued to reduce trauma and consequently reduce the risks of bleeding and poor graft oxygen diffusion (Kakagia and Pallua, 2014). Fat injection in multiple small aliquots is preferred over single sessions using larger volumes to increase plasmatic diffusion and revascularization (Simonacci et al., 2017). Due to limitations associated with isolation and expansion procedures of purified ASCs, there is a preference to enrich autologous fat grafts with SVF. Cell-assisted lipotransfer (CAL), which uses autologous fat grafting enriched with lipoaspirated SVF, has been proven to augment the eventual volume and long-term stability of fat grafts. Applying CAL results in higher survival rates and reduction of repeat procedures compared to non-enriched fat grafting (Yoshimura et al., 2008a, b). CAL only benefits fat survival rates in small volumes of fat grafting, which are less than 100 mL (Laloze et al., 2018).
Platelet-Rich Plasma
PRP is a concentrate of platelet proteins and contains many growth factors, including epidermal growth factor, insulin-like growth factor-1, platelet-derived growth factor, transforming growth factor-β 1 and 2, thrombospondin and vascular endothelial growth factor. PRP increases the expression of type I collagen to accelerate wound healing (Cho et al., 2012). PRP is blood-derived and is typically obtained by centrifugation of whole blood to obtain five to seven times the concentration of autologous platelets suspended in a small volume of plasma. Centrifugation separates the platelets from red blood cells and leukocytes and allows the collection of the buffy coat (Cervelli et al., 2009; Conese et al., 2020). PRP is suggested to be a promising approach to enhance the applications of ASCs as it promotes the proliferation of endothelial cells and angiogenesis (Liao et al., 2014). In vitro and in vivo studies have proven that PRP and ASCs act synergistically when combined, resulting in better volume fat retention than when used independently. In a rat sciatic crush injury model, PRP was found to play a role in enhancing nerve regeneration (Emel et al., 2011). Moreover, PRP promotes axon growth in spinal cord tissues through mechanisms associated with insulin-like growth factor-1 and vascular endothelial growth factor (Takeuchi et al., 2012).
To date, no clear consensus exists on the beneficial effects of PRP when used independently compared to ASCs only, or ASCs and PRP combined (Vyas et al., 2020b). Optimal platelet concentration and preparation methods must be determined so that the results of studies can be compared. Despite the potential beneficial effects of PRP in reconstructive applications with minimal side effects, the use of PRP has been constrained due to the limited availability of clinical studies (Reddy et al., 2018). Future studies are needed to evaluate preparation and fat reimplantation techniques in larger clinical trials.
Pre-Clinical Evidence for the Use of Adipose-Derived Stem Cells
Paracrine properties of adipose-derived stem cells
Detailed in vivo monitoring provides us insights into the fate of transplanted cells, including their distribution, differentiation, and longevity over time. Various techniques are available to label cells by direct or indirect methods (Srivastava and Bulte, 2014). Luciferase-based non-invasive bioluminescence imaging is an indirect method, which allows real-time in vivo monitoring of location and proliferation of luciferase-expressing MSCs. Using this technique, it was found that labeled MSCs could be detected for up to 29 days when seeded on nerve allografts (Rbia et al., 2019). When influenced by surrounding tissues, ASCs have the ability to enhance the expression of various growth factors (e.g., angiogenic and neurotrophic growth factors) (Rehman et al., 2004; Zhao et al., 2011; Fan et al., 2014; Mathot et al., 2020; Yi et al., 2020). The stem cell secretome, i.e., the paracrine factors secreted by stem cells and utilized for inter-cell communication, is activated under stressful conditions, such as hypoxia. This mechanism of action has been proven by pre-incubation of stem cells under stressful conditions and is called the “paracrine hypothesis” (Tompkins et al., 2018). The survival of grafted ASCs is promoted in acute ischemic and inflammatory environments by differentiation into e.g. endothelial cells and vascular smooth muscle cells (Nagata et al., 2016), which is also explained by the paracrine effects. The paracrine effect also explains the long-term efficacy of MSCs, which extends beyond the survival time of these cells. Many studies have highlighted the role of acute localized tissue inflammation, which leads to hypoxia and triggers the promotion of angiogenesis (Koliaraki et al., 2020). There is a complex interplay between immune cells, ASCs and the paracrine environment. As ASCs are greatly influenced by their paracrine environment, the paracrine secretome varies in different tissues and is dependent on the specific need for regeneration in that area. While acute inflammation is beneficial for angiogenesis, chronic or unresolved inflammation results in the failure of tissue repair mechanisms (Figure 3). This leads to further activation of ASCs and the increased production of extracellular matrix and fibrosis. Although this process is reversible in some cases, its exact mechanism remains unclear (Koliaraki et al., 2020).
Figure 3.
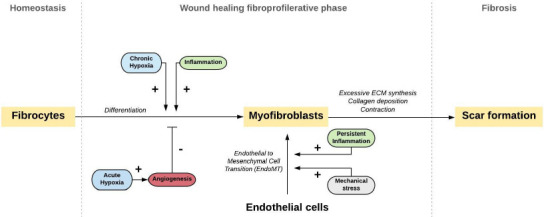
Mechanisms that drive fibrocyte and endothelial cell activation towards myofibroblasts.
Activated myofibroblasts are important mediators of wound healing. During tissue injury, fibroblasts can differentiate into myofibroblasts. This process is mainly driven by hypoxia and could be decreased by angiogenesis. In specific conditions, it is hypothesized that myofibroblasts can also arise from endothelial cells via endothelial to mesenchymal transition (EndoMT). Myofibroblasts follow several different cell fates and can participate in tissue remodeling beyond repair, leading to scar formation. Scar formation, or fibrosis, is characterized by excessive extracellular matrix (ECM) synthesis, collagen deposition, and contraction. Adipose-derived stem cells are found to increase angiogenesis and decrease collagen, which prevents fibrosis. Copyrighted, used, and reprinted with permission of Saffari et al. (2021b); all rights reserved.
Peripheral nerve injuries and reconstruction
Traumatic injuries to peripheral nerves are commonly present after hand trauma and require the use of a nerve graft when tension-free neurorrhaphy is not possible. The use of nerve autografts is considered the gold standard to bridge these large defects. Regeneration is often suboptimal with incomplete target reinnervation, which could be attributed to axonal degeneration and fibrotic scar formation (Ngeow, 2010).
Conduits
Di Summa and colleagues evaluated the delivery of ASCs to fibrin conduits prepared from fibrin glue (Tisseel®) to reconstruct 10-mm sciatic nerve defects in rats (Di Summa et al., 2018). Twelve weeks post-operatively, the histological analysis found lower levels of collagen infiltration in the distal nerve stump in the conduits seeded with the ASCs group when compared to the untreated nerve conduit group. This same pattern was also found for the myelinated area of the middle and distal nerve areas, which was greatly improved by the addition of ASCs. Carriel et al. (2013) also confirmed these findings when ASCs were delivered to nerve conduits. Moreover, the extracellular matrix was also more abundant and better organized around regenerated nerve tissues with ASC conduits than those without (Carriel et al., 2013). Immunohistochemical staining against growth-associated protein-43 and neurofilament can be used to evaluate the proportion of axons in different stages of regeneration to indicate the degree of peripheral nerve regeneration. Conduits filled with MSC-containing hydrogel are found to increase these markers in a rat sciatic nerve defect model (Carriel et al., 2017). The delivery of ASC enhanced outcomes of nerve conduits is found to be comparable to autograft reconstruction (Di Summa et al., 2018). Interestingly, the increase in axon regeneration was also found when SVF was added to conduits (Suganuma et al., 2013). When directly comparing SVF to ASCs, it was found that both treatment groups significantly improved histologic and physiologic parameters compared to empty conduit controls (Shimizu et al., 2018). It is suggested that treatment with SVF is as effective as the delivery of ASCs to enhance nerve regeneration. However, SVF is more translatable to clinical practice (Shimizu et al., 2018).
Nerve autografts
When nerve allografts are augmented with ASCs in a rat sciatic nerve defect model, this results in the increase of neuronal survival, axonal regeneration, and myelination (Masgutov et al., 2018). Saller and colleagues found similar findings when wrapping 20 mm autografts with ASC-loaded hydrogels in rats (Saller et al., 2018).
Nerve allografts
In a different rat sciatic nerve defect model, the delivery of stem cells to nerve allograft as well as wrapping nerve allograft in a pedicled adipofascial flap resulted in increased revascularization of the nerve (Mathot et al., 2020). When combining stem cell delivery with an adipofascial flap, this resulted in a synergic effect and enhanced revascularization (Saffari et al., 2020). Evidence suggests that ASCs have the best potential when used as additives in peripheral nerve regeneration (Hundepool et al., 2014). Delivery of independent ASCs or surgical angiogenesis (i.e., wrapping a pedicled adipofascial flap around the nerve) to nerve allograft also leads to improved nerve regeneration (Mathot, 2021; Mathot et al., 2021; Saffari et al., 2021b). The ASCs in the pedicled adipofascial flap exert various growth factors and modulate the local microenvironment of the nerve to diminish fibrosis (Saffari et al., 2021b). It is suggested that a correlation exists between remyelination and anti-fibrotic activity (Di Summa et al., 2018), which is the result of the paracrine secretome of ASCs (Suganuma et al., 2013).
ASCs could be delivered to the nerve by a number of methods, including intravenous injection, intramuscular injection, intra-neural injection, or dynamic seeding (Saffari et al., 2021a). Each method is associated with its own advantages and limitations. Dynamic seeding combines cells and nerve segments in a tube, which will be placed on a rotator for 12–24 hours. This non-invasive process allows cells to adhere to the outer surface of the nerve (Rbia et al., 2018). Intra-neural microinjection delivers a high quantity of cells directly to the site of the inner and middle nerve zones, however, results in an unpredictable cell distribution and reduction of stem cell viability after needle passage due to pressure build-up (Saffari et al., 2021a). The incorporation of stem cells in a gel is non-invasive and potentially more clinically translatable; however, evaluation on the efficacy and survival of cells using this method has been ongoing in pre-clinical research. A schematic overview of novel experimental techniques to add stem cells to the nerve is provided in Figure 4. Controversy remains on the optimal method of delivery, but in general, slow injection results in lower shear stress and greater fat graft viability (Zhang et al., 2020; Saffari et al., 2021a).
Figure 4.
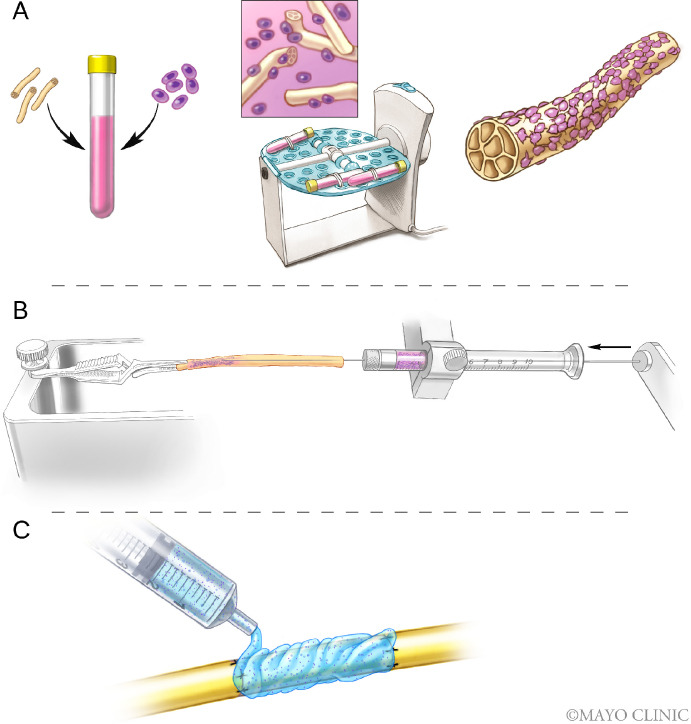
Experimental stem cell delivery methods to the nerve.
Different methods to deliver stem cells to the nerve are presented in this schematic overview. These techniques include the delivery of cells by dynamic seeding (A), intraneural injection of cells (B), and suspension of cells in a gel to be delivered to the nerve (C). Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Other applications of adipose-derived stem cells
ASCs have been previously shown to differentiate into different cell types including cells of the mesenchymal lineage. Their use is promising in full-thickness scar improvement, scar reduction, and wound healing (Cherubino et al., 2011). In animal models, scars injected with ASCs resulted in a reduction of surface area and improvements of color compared to control (Yun et al., 2012; Spiekman et al., 2017). ASCs promote fibroblast proliferation by direct cell-to-cell contact and secretory-induced paracrine activation of growth factors, resulting in acceleration of re-epithelization of wounds (Kim et al., 2007). Post-radiation-induced dermatitis is a clinically relevant problem and suggested to be the result of epidermal thickening and irregular deposition of collagen in the dermis. Previous studies have shown that treatment with lipofilling decreases SMAD3 protein levels, resulting in the reduction of radiation-induced dermatitis (Sultan et al., 2011; Garza et al., 2014). SMAD3 is a pro-fibrotic protein and plays a role in the transforming growth factor-β pathway. Besides the mesodermal lineages (i.e., osteoblast, chondrocyte, and adipocyte lineage) it is hypothesized that adipose-derived MSCs can also differentiate into other cell types such as endothelial cells or neurons. Although the differentiation of these cells into endothelial cells has not robustly been demonstrated yet, we cannot exclude this theory (Laloze et al., 2021). Myofibroblasts in fibrotic diseases are hypothesized to be originating from several sources, including from phenotypic differentiation of fibrocytes and the transition of endothelial cells (EndoMT pathway), often regulated by transforming growth factor-β (Wang et al., 2016; Huang and Ogawa, 2020). The differentiation of epithelial cells to myofibroblasts is associated with mechanical stress on the wound and inflammation. A schematic overview of the EndoMT pathway theory is presented in Figure 3.
ASCs may also expedite wound healing, particularly in difficult healing wounds (Lee et al., 2011; Lam et al., 2013; Uysal et al., 2014; Zonari et al., 2015). Moreover, ASCs are found to decrease fibrotic areas by decreasing elastin deposition (Castiglione et al., 2013). Gene expression profiles of pro-fibrotic markers were found to decrease, while vascular endothelial growth factor, a pro-angiogenic factor, increased (Uysal et al., 2014). ASCs have also been applied to irregularly contoured burn wounds and are found to enhance revascularization, accelerate wound closure, and reduce scar formation (Dong et al., 2020). Thus, treatment of wounds or scars with ASCs in animal models has been shown to reduce scar tissue and enhance wound healing by promoting angiogenesis.
Clinical Applications
Many clinical studies corroborate with the Eto’s pre-clinical evidence (Eto et al., 2012) and also demonstrate that patients receiving higher volumes of injected fat maintained greater total volume retention (Choi et al., 2013). However, this is challenged by the study of Small and colleagues (Small et al., 2014), which suggests that the volume of the injected fat did not play a significant role in the retention of volume after reconstructive breast surgery (Small et al., 2014). The enrichment of adipose tissue with lipoaspirated SVF is proposed to increase the number of ASCs and is believed to augment the long-term stability of adipose tissue grafts (Tan et al., 2016). Clinical application of non-vascularized adipose tissue grafting in peripheral nerve surgery includes the treatment of recurrent or persistent symptoms after primary carpal tunnel release, which can be caused by the incomplete release and abundant scarring (Tung and Mackinnon, 2007; Jones et al., 2012). Tissue interposition flaps, such as the radial artery fascial flap, perforator-based radial forearm fascial flap, thenar or hypothenar fat flap, have been opted to cover nerve fibers in case of scarring disorders, but require technically demanding procedures (Krześniak and Noszczyk, 2015). Krześniak and colleagues performed secondary carpal tunnel release revisions using an open approach combined with autologous lipofilling into the scarred transverse carpal ligament and adjacent subdermal tissue, antebrachial facia, and surrounding subdermal areas. Thickened epineurium was released, and lipofilling was injected under and on top of the nerve, but not into the nerve fibers (Krześniak and Noszczyk, 2015). This minimally invasive and rapid addition has been proven to reduce pathologic fibrosis and decrease a tendency towards excessive collagen production. These findings were corroborated after treatment of 2nd to 5th recurrent carpal tunnel syndrome by extensive neurolysis followed by perineural lipografting, with a longer follow-up time (mean of 30 months) (Gostelie et al., 2020). Moreover, it may stimulate the regeneration and elasticity of the skin and adjacent tissues, and significantly reduce recurrent symptoms of carpal tunnel syndrome (Coleman, 2006; Mojallal et al., 2009; Khouri et al., 2013; Krześniak and Noszczyk, 2015). Thus, depositing adipose tissue directly around the nerve is hypothesized to stimulate nerve fiber regeneration (Krześniak and Noszczyk, 2015).
While pre-clinical studies seem promising, the application of ASCs in clinical practice was initially challenged due to the possibility of ASCs contributing to accelerated tumor growth and recurrence in reconstructive surgery (Wei et al., 2015). Adipose tissue grafting has been often used following oncological mastectomy and combined with autologous flap reconstruction of the breast to create symmetry (Shukla et al., 2020). Various in vitro studies have suggested that adipocytes and ASCs can affect the breast cancer microenvironment and promote breast cancer growth and metastasis, which proposes concerns regarding the use of ASC enriched fat grafting in reconstructive surgery after breast cancer. Nevertheless, in clinical practice, the safety has been closely studied and it has been proven that fat grafting does not increase the local tumor recurrence risk in breast cancer patients (Vyas et al., 2020a). Furthermore, the use of enzymatic dissociation of adipose tissue has not yet been approved by the Food and Drug Administration in clinical practice. To overcome this limitation, ASCs that can be used are required to be minimally manipulated, enzyme-free, and used in the same surgical procedure, e.g. adipose tissue grafts that contain native ASCs or SVF (Dehdashtian et al., 2020; Zhang et al., 2020). The Celution System could be used to harvest adipose tissue and extract ASCs within 1.5 hours using a Celase® processing enzyme reagent. This system is currently being investigated in clinical trials (Fraser et al., 2014). Large clinical cohorts with longer follow-ups are needed to affirm the safety of the application of CAL in patients with malignancy and until consensus has been reached, close follow-up is necessary (Fang et al., 2021). Since the use of ASCs in peripheral nerve reconstruction is relatively new, more studies are needed to explore safety and long-term effects on peripheral nerve regeneration.
Future of Adipose Tissue Grafting
Improved understanding of variables contributing to adipose graft survival will optimize grafting procedures, making them safer and more effective. Tissue engineering relies on regenerative cells or growth factors that are delivered using an appropriate scaffold for grafting and support (Kessler and Grande, 2008). ASCs combined with an ECM that includes various growth factors could be used for a variety of implications to sustain volume. Wang and colleagues used decellularized human adipose tissue ECM from incised fat tissues and combined this with human ASCs to create a graft construct. This construct was subcutaneously injected and compared to the injection of fresh fat grafts containing ASCs in a nude rat model. The graft constructs were found to be well vascularized and without rejection or inflammation. Although the graft construct did not show improved vascularity compared to fresh fat injection, it may serve as a platform to integrate cells and multiple growth factors as the next step for adipose tissue engineering (Wang et al., 2013). Future studies may also focus on the optimization of the recipient site to assist tissue engraftment and long-term survival of adipose tissue (Heit et al., 2012).
RENUVA® (Musculoskeletal Transplant Foundation, Edison, NJ, USA; https://www.mtfbiologics.org/our-products/detail/renuva) is a commercially available human-derived allograft adipose matrix (AAM) and used as an off-the-shelf alternative for autologous fat transfer to restore adipose volumes in the body. This matrix is processed as such that it preserves the ECM containing collagens and growth factors that are derived from adipose tissue, while not inducing allorejection. It has been adopted in clinical studies and used for a variety of applications (Shahin et al., 2017). Giatsidis and colleagues developed a shelf-ready AAM, which is derived from human cadavers. AAM was subcutaneously injected on the left dorsum of mice to evaluate volume retention over time. It was found that AAM grafts retained volume over time and similarly promoted angiogenesis in surrounding recipient tissues. This matrix could be used to create a vascularized, pro-adipogenic environment to enhance volume sustention of the grafted tissue and promote adipogenesis through an inflammation-mediated process (Giatsidis et al., 2019). In line with the effect of acute inflammation on angiogenesis, this study also reported inflammation to be a major inductive factor for adipogenesis (Lancerotto et al., 2013; Lujan-Hernandez et al., 2016; Giatsidis et al., 2019). In humans, AAM grafts are comparable to pre-clinical evidence and found to remodel perilipin-positive adipocytes after six months, with no severe adverse events reported (Kokai et al., 2020). Future studies are needed to evaluate the use of AAM grafts in peripheral nerve reconstruction. Other future implications may include the use of ASC-derived exosomes. Exosomes are a subtype of extracellular vesicles released from cell types and contain a range of growth factors. A recent study demonstrated that the local administration of exosomes improved nerve regeneration in a reverse sciatic nerve autograft rat model (Ikumi et al., 2021). Exosomes hold promise for peripheral nerve reconstruction, as these vesicles contain fewer membrane-bound proteins and may be available off-the-shelf (Shukla et al., 2020; Saffari et al., 2021a).
Conclusions
Fat grafting is a safe and dynamic procedure used by surgeons for a variety of indications. Current practice for autologous fat grafting for soft tissue reconstruction or augmentation has been limited by variability in long-term graft retention. Research suggests that SVF and ASCs may improve fat graft survival, largely through angiogenic properties and reduction of inflammation and fibrosis. Basic science nerve research suggests an increase in nerve regeneration and nerve revascularization, and a decrease in nerve fibrosis after the addition of ASCs or adipose tissue. In clinical studies, the use of autologous lipofilling is mostly applied to secondary carpal tunnel release revisions with promising results. Since the use of ASCs in peripheral nerve reconstruction is relatively new, more studies are needed to explore safety and long-term effects on peripheral nerve regeneration. Furthermore, the use of enzymatic dissociation of adipose tissue has not yet been approved by the Food and Drug Administration in clinical practice. To overcome this limitation, ASCs can be used that are minimally manipulated, enzyme-free, and used in the same surgical procedure, e.g. adipose tissue grafts that contain native ASCs or SVF. Future research may be shifted towards the use of tissue-engineered adipose tissue to create a supportive microenvironment for autologous graft survival. High-quality clinical trials to demonstrate safety and efficacy are required to further guide the development of protocols for clinical practice.
Acknowledgments:
We would like to thank Fontes and colleagues from Department of Biomechanics, Medical Faculty, University of Porto, Portugal for their permission to reprint Figure 1 in this review. We would also like to thank Jim Postier (Mayo Clinic, MN, USA) for the artwork in Figures 2 and 4.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, Molitor M. Stem cells:their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36:1111–1126. doi: 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Billings E, Jr, May JW., Jr Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83:368–381. doi: 10.1097/00006534-198902000-00033. [DOI] [PubMed] [Google Scholar]
- 3.Boschert MT, Beckert BW, Puckett CL, Concannon MJ. Analysis of lipocyte viability after liposuction. Plast Reconstr Surg. 2002;109:761–765. doi: 10.1097/00006534-200202000-00054. [DOI] [PubMed] [Google Scholar]
- 4.Bunnell BA, Estes BT, Guilak F, Gimble JM. Differentiation of adipose stem cells. Methods Mol Biol. 2008;456:155–171. doi: 10.1007/978-1-59745-245-8_12. [DOI] [PubMed] [Google Scholar]
- 5.Campbell GL, Laudenslager N, Newman J. The effect of mechanical stress on adipocyte morphology and metabolism. The Am J of Cosmetic Surg. 1987;4:89–94. [Google Scholar]
- 6.Carpaneda CA, Ribeiro MT. Study of the histologic alterations and viability of the adipose graft in humans. Aesthetic Plast Surg. 1993;17:43–47. doi: 10.1007/BF00455048. [DOI] [PubMed] [Google Scholar]
- 7.Carriel V, Garzón I, Campos A, Cornelissen M, Alaminos M. Differential expression of GAP-43 and neurofilament during peripheral nerve regeneration through bio-artificial conduits. J Tissue Eng Regen Med. 2017;11:553–563. doi: 10.1002/term.1949. [DOI] [PubMed] [Google Scholar]
- 8.Carriel V, Garrido-Gómez J, Hernández-Cortés P, Garzón I, García-García S, Sáez-Moreno JA, del Carmen Sanchez-Quevedo M, Campos A, Alaminos M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J Neural Eng. 2013;10:026022. doi: 10.1088/1741-2560/10/2/026022. [DOI] [PubMed] [Google Scholar]
- 9.Castiglione F, Hedlund P, Van der Aa F, Bivalacqua TJ, Rigatti P, Van Poppel H, Montorsi F, De Ridder D, Albersen M. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie's disease. Eur Urol. 2013;63:551–560. doi: 10.1016/j.eururo.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervelli V, Gentile P, Scioli MG, Grimaldi M, Casciani CU, Spagnoli LG, Orlandi A. Application of platelet-rich plasma in plastic surgery:clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009;15:625–634. doi: 10.1089/ten.TEC.2008.0518. [DOI] [PubMed] [Google Scholar]
- 11.Cherubino M, Rubin JP, Miljkovic N, Kelmendi-Doko A, Marra KG. Adipose-derived stem cells for wound healing applications. Ann Plast Surg. 2011;66:210–215. doi: 10.1097/SAP.0b013e3181e6d06c. [DOI] [PubMed] [Google Scholar]
- 12.Cho JW, Kim SA, Lee KS. Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med. 2012;29:32–36. doi: 10.3892/ijmm.2011.803. [DOI] [PubMed] [Google Scholar]
- 13.Choi M, Small K, Levovitz C, Lee C, Fadl A, Karp NS. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg. 2013;131:185–191. doi: 10.1097/PRS.0b013e3182789b13. [DOI] [PubMed] [Google Scholar]
- 14.Coleman SR. Structural fat grafting:more than a permanent filler. Plast Reconstr Surg. 2006;118:108S–120S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 15.Condé-Green A, de Amorim NFG, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue:a comparative study. J Plast Reconstr. 2010;63:1375–1381. doi: 10.1016/j.bjps.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Conese M, Annacontini L, Carbone A, Beccia E, Cecchino LR, Parisi D, Di Gioia S, Lembo F, Angiolillo A, Mastrangelo F, Lo Muzio L, Portincasa A. The role of adipose-derived stem cells, dermal regenerative templates, and platelet-rich plasma in tissue engineering-based treatments of chronic skin wounds. Stem Cells Int. 2020;2020:7056261. doi: 10.1155/2020/7056261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortese A, Savastano G, Felicetta L. Free fat transplantation for facial tissue augmentation. J Oral Maxillofac Surg. 2000;58:164–169. doi: 10.1016/s0278-2391(00)90331-8. [DOI] [PubMed] [Google Scholar]
- 18.Crawford JL, Hubbard BA, Colbert SH, Puckett CL. Fine tuning lipoaspirate viability for fat grafting. Plast Reconstr Surg. 2010;126:1342–1348. doi: 10.1097/PRS.0b013e3181ea44a9. [DOI] [PubMed] [Google Scholar]
- 19.Dehdashtian A, Bratley JV, Svientek SR, Kung TA, Awan TM, Cederna PS, Kemp SW. Autologous fat grafting for nerve regeneration and neuropathic pain:current state from bench-to-bedside. Regen Med. 2020;15:2209–2228. doi: 10.2217/rme-2020-0103. [DOI] [PubMed] [Google Scholar]
- 20.di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278–291. doi: 10.1016/j.neuroscience.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 21.Di Summa PG, Schiraldi L, Cherubino M, Oranges CM, Kalbermatten DF, Raffoul W, Madduri S. Adipose derived stem cells reduce fibrosis and promote nerve regeneration in rats. Anat Rec (Hoboken) 2018;301:1714–1721. doi: 10.1002/ar.23841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Y, Cui M, Qu J, Wang X, Kwon SH, Barrera J, Elvassore N, Gurtner GC. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020;108:56–66. doi: 10.1016/j.actbio.2020.03.040. [DOI] [PubMed] [Google Scholar]
- 23.El Khoury J, Awaida C, Nasr M, Hokayem N. Platelet-rich plasma and fat grafting for the treatment of inferior alveolar nerve neuropathy:the first case report. Oral Maxillofac Surg Cases. 2017;3:107–111. [Google Scholar]
- 24.Emel E, Ergün SS, Kotan D, Gürsoy EB, Parman Y, Zengin A, Nurten A. Effects of insulin-like growth factor-I and platelet-rich plasma on sciatic nerve crush injury in a rat model. J Neurosurg. 2011;114:522–528. doi: 10.3171/2010.9.JNS091928. [DOI] [PubMed] [Google Scholar]
- 25.Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno S, Yoshimura K. The fate of adipocytes after nonvascularized fat grafting:evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;129:1081–1092. doi: 10.1097/PRS.0b013e31824a2b19. [DOI] [PubMed] [Google Scholar]
- 26.Eto H, Suga H, Inoue K, Aoi N, Kato H, Araki J, Doi K, Higashino T, Yoshimura K. Adipose injury-associated factors mitigate hypoxia in ischemic tissues through activation of adipose-derived stem/progenitor/stromal cells and induction of angiogenesis. Am J Pathol. 2011;178:2322–2332. doi: 10.1016/j.ajpath.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan L, Yu Z, Li J, Dang X, Wang K. Schwann-like cells seeded in acellular nerve grafts improve nerve regeneration. BMC Musculoskelet Disord. 2014;15:165. doi: 10.1186/1471-2474-15-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang J, Chen F, Liu D, Gu F, Wang Y. Adipose tissue-derived stem cells in breast reconstruction:a brief review on biology and translation. Stem Cell Res Ther. 2021;12:1–13. doi: 10.1186/s13287-020-01955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontes T, Brandão I, Negrão R, Martins MJ, Monteiro R. Autologous fat grafting:harvesting techniques. Ann Med Surg (Lond) 2018;36:212–218. doi: 10.1016/j.amsu.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraser JK, Hicok KC, Shanahan R, Zhu M, Miller S, Arm DM. The Celution®system:automated processing of adipose-derived regenerative cells in a functionally closed system. Adv Wound Care (New Rochelle) 2014;3:38–45. doi: 10.1089/wound.2012.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garza RM, Paik KJ, Chung MT, Duscher D, Gurtner GC, Longaker MT, Wan DC. Studies in fat grafting:Part III. Fat grafting irradiated tissue--improved skin quality and decreased fat graft retention. Plast Reconstr Surg. 2014;134:249–257. doi: 10.1097/PRS.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geissler PJ, Davis K, Roostaeian J, Unger J, Huang J, Rohrich RJ. Improving fat transfer viability:the role of aging, body mass index, and harvest site. Plast Reconstr Surg. 2014;134:227–232. doi: 10.1097/PRS.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 33.Giatsidis G, Succar J, Haddad A, Lago G, Schaffer C, Wang X, Schilling B, Chnari E, Matsumine H, Orgill DP. Preclinical optimization of a shelf-ready, injectable, human-derived, decellularized allograft adipose matrix. Tissue Eng Part A. 2019;25:271–287. doi: 10.1089/ten.TEA.2018.0052. [DOI] [PubMed] [Google Scholar]
- 34.Gostelie O NG, Paulusma SB, van Dongen JA, Tellier MA, Coert, JH, Jaquet JB. Re-neurolysis and infiltration of autologous lipoaspirate around the median nerve in secondary recurrent carpal tunnel syndrome:a prospective cohort study. SSRN Electronic Journal. 2020 doi:10.2139/ssrn.3522582. [Google Scholar]
- 35.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 36.Heit YI, Lancerotto L, Mesteri I, Ackermann M, Navarrete MF, Nguyen CT, Mukundan S, Jr, Konerding MA, Del Vecchio DA, Orgill DP. External volume expansion increases subcutaneous thickness, cell proliferation, and vascular remodeling in a murine model. Plast Reconstr Surg. 2012;130:541–547. doi: 10.1097/PRS.0b013e31825dc04d. [DOI] [PubMed] [Google Scholar]
- 37.Huang C, Ogawa R. The vascular involvement in soft tissue fibrosis-lessons learned from pathological scarring. Int J Mol Sci. 2020;21:2542. doi: 10.3390/ijms21072542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hundepool CA, Nijhuis TH, Mohseny B, Selles RW, Hovius SE. The effect of stem cells in bridging peripheral nerve defects:a meta-analysis. J Neurosurg. 2014;121:195–209. doi: 10.3171/2014.4.JNS131260. [DOI] [PubMed] [Google Scholar]
- 39.Ikumi A, Gingery A, Toyoshima Y, Zhao C, Moran SL, Livia C, Rolland T, Peterson T, Sabbah MS, Boroumand S, Saffari TM, Behfar A, Shin AY, Amadio PC. Administration of purified exosome product in a rat sciatic serve reverse autograft model. Plast Reconstr Surg. 2021;148:200e–211e. doi: 10.1097/PRS.0000000000008202. [DOI] [PubMed] [Google Scholar]
- 40.Illouz YG, Illouz YG, De Villers YT. Edinburgh: Churchill Livingstone; 1989. Body sculpturing by lipoplasty. [Google Scholar]
- 41.Johal KS, Lees VC, Reid AJ. Adipose-derived stem cells:selecting for translational success. Regen Med. 2015;10:79–96. doi: 10.2217/rme.14.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129:683–692. doi: 10.1097/PRS.0b013e3182402c37. [DOI] [PubMed] [Google Scholar]
- 43.Kakagia D, Pallua N. Autologous fat grafting:in search of the optimal technique. Surg Innov. 2014;21:327–336. doi: 10.1177/1553350613518846. [DOI] [PubMed] [Google Scholar]
- 44.Kasem A, Wazir U, Headon H, Mokbel K. Breast lipofilling:a review of current practice. Arch Plast Surg. 2015;42:126. doi: 10.5999/aps.2015.42.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato H, Mineda K, Eto H, Doi K, Kuno S, Kinoshita K, Kanayama K, Yoshimura K. Degeneration, regeneration, and cicatrization after fat grafting:dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg. 2014;133:303e–313e. doi: 10.1097/PRS.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 46.Keck M, Zeyda M, Gollinger K, Burjak S, Kamolz LP, Frey M, Stulnig TM. Local anesthetics have a major impact on viability of preadipocytes and their differentiation into adipocytes. Plast Reconstr Surg. 2010;126:1500–1505. doi: 10.1097/PRS.0b013e3181ef8beb. [DOI] [PubMed] [Google Scholar]
- 47.Kessler MW, Grande DA. Tissue engineering and cartilage. Organogenesis. 2008;4:28–32. doi: 10.4161/org.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khouri RK, Smit JM, Cardoso E, Pallua N, Lantieri L, Mathijssen IM, Khouri RK, Jr, Rigotti G. Percutaneous aponeurotomy and lipofilling:a regenerative alternative to flap reconstruction? Plast Reconstr Surg. 2013;132:1280–1290. doi: 10.1097/PRS.0b013e3182a4c3a9. [DOI] [PubMed] [Google Scholar]
- 49.Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose-derived stem cells:a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Kokai LE, Sivak WN, Schilling BK, Karunamurthy A, Egro FM, Schusterman MA, Minteer DM, Simon P, D'Amico RA, Rubin JP. Clinical evaluation of an off-the-shelf allogeneic adipose matrix for soft tissue reconstruction. Plast Reconstr Surg Glob Open. 2020;8:e2574. doi: 10.1097/GOX.0000000000002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koliaraki V, Prados A, Armaka M, Kollias G. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol. 2020;21:974–982. doi: 10.1038/s41590-020-0741-2. [DOI] [PubMed] [Google Scholar]
- 52.Krześniak NE, Noszczyk BH. Autologous fat transfer in secondary carpal tunnel release. Plast Reconstr Surg Global Open. 2015;3:e401. doi: 10.1097/GOX.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laloze J, Fiévet L, Desmoulière A. Adipose-derived mesenchymal stromal cells in regenerative medicine:state of play, current clinical trials, and future prospects. Adv Wound Care (New Rochelle) 2021;10:24–48. doi: 10.1089/wound.2020.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laloze J, Varin A, Gilhodes J, Bertheuil N, Grolleau J, Brie J, Usseglio J, Sensebe L, Filleron T, Chaput B. Cell-assisted lipotransfer:friend or foe in fat grafting?Systematic review and meta-analysis. J Tissue Engi Regen Med. 2018;12:e1237–e1250. doi: 10.1002/term.2524. [DOI] [PubMed] [Google Scholar]
- 55.Lam MT, Nauta A, Meyer NP, Wu JC, Longaker MT. Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing. Tissue Eng Part A. 2013;19:738–747. doi: 10.1089/ten.tea.2012.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lancerotto L, Chin MS, Freniere B, Lujan-Hernandez JR, Li Q, Vasquez AV, Bassetto F, Del Vecchio DA, Lalikos JF, Orgill DP. Mechanisms of action of external volume expansion devices. Plast Reconstr Surg. 2013;132:569–578. doi: 10.1097/PRS.0b013e31829ace30. [DOI] [PubMed] [Google Scholar]
- 57.Lee SH, Lee JH, Cho KH. Effects of human adipose-derived stem cells on cutaneous wound healing in nude mice. Ann Dermatol. 2011;23:150–155. doi: 10.5021/ad.2011.23.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li K, Gao J, Zhang Z, Li J, Cha P, Liao Y, Wang G, Lu F. Selection of donor site for fat grafting and cell isolation. Aesthetic Plast Surg. 2013;37:153–158. doi: 10.1007/s00266-012-9991-1. [DOI] [PubMed] [Google Scholar]
- 59.Liao HT, Marra KG, Rubin JP. Application of platelet-rich plasma and platelet-rich fibrin in fat grafting:basic science and literature review. Tissue Eng Part B Rev. 2014;20:267–276. doi: 10.1089/ten.teb.2013.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lujan-Hernandez J, Lancerotto L, Nabzdyk C, Hassan KZ, Giatsidis G, Khouri RK, Jr, Chin MS, Bassetto F, Lalikos JF, Orgill DP. Induction of adipogenesis by external volume expansion. Plast Reconstr Surg. 2016;137:122–131. doi: 10.1097/PRS.0000000000001859. [DOI] [PubMed] [Google Scholar]
- 61.Masgutov R, Masgutova G, Mukhametova L, Garanina E, Arkhipova SS, Zakirova E, Mukhamedshina YO, Margarita Z, Gilazieva Z, Syromiatnikova V. Allogenic adipose derived stem cells transplantation improved sciatic nerve regeneration in rats:autologous nerve graft model. Front Pharmacol. 2018;9:86. doi: 10.3389/fphar.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mashiko T, Yoshimura K. How does fat survive and remodel after grafting? Clin Plast Surg. 2015;42:181–190. doi: 10.1016/j.cps.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Mathot F, Rbia N, Bishop AT, Hovius SER, Shin AY. Adipose derived mesenchymal stem cells seeded onto a decellularized nerve allograft enhances angiogenesis in a rat sciatic nerve defect model. Microsurgery. 2020;40:585–592. doi: 10.1002/micr.30579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathot F, Saffari TM, Rbia N, Nijhuis THJ, Bishop AT, Hovius SER, Shin AY. Functional outcomes of nerve allografts seeded with undifferentiated and differentiated mesenchymal stem cells in a rat sciatic nerve defect model. Plast Reconstr Surg. 2021;148:354–365. doi: 10.1097/PRS.0000000000008191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathot F, Shin AY, van Wijnen AJ. Targeted stimulation of MSCs in peripheral nerve repair. Gene. 2019;710:17–23. doi: 10.1016/j.gene.2019.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mildmay-White A, Khan W. Cell surface markers on adipose-derived stem cells:a systematic review. Curr Stem Cell Res Ther. 2017;12:484–492. doi: 10.2174/1574888X11666160429122133. [DOI] [PubMed] [Google Scholar]
- 67.Mojallal A, Foyatier JL. The effect of different factors on the survival of transplanted adipocytes. Ann Chir Plast Esthet. 2004;49:426–436. doi: 10.1016/j.anplas.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Mojallal A, Lequeux C, Shipkov C, Breton P, Foyatier JL, Braye F, Damour O. Improvement of skin quality after fat grafting:clinical observation and an animal study. Plast Reconstr Surg. 2009;124:765–774. doi: 10.1097/PRS.0b013e3181b17b8f. [DOI] [PubMed] [Google Scholar]
- 69.Nagata H, Ii M, Kohbayashi E, Hoshiga M, Hanafusa T, Asahi M. Cardiac adipose-derived stem cells exhibit high differentiation potential to cardiovascular cells in C57BL/6 Mice. Stem Cells Transl Med. 2016;5:141–151. doi: 10.5966/sctm.2015-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neuber F. Fettransplantation. Chir Kongr Verhandl Dsch Gesellch Chir. 1893;22:66. [Google Scholar]
- 71.Ngeow WC. Scar less:a review of methods of scar reduction at sites of peripheral nerve repair. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:357–366. doi: 10.1016/j.tripleo.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen A, Pasyk KA, Bouvier TN, Hassett CA, Argenta LC. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg. 1990;85:378–386. [PubMed] [Google Scholar]
- 73.Ozakpinar HR, Tellioglu AT, Eryilmaz T, Durgun M, Inozu E, Oktem F. A reliable option for wrist soft tissue defects:adipofascial flaps for immediate and late reconstruction. Int Wound J. 2013;10:661–665. doi: 10.1111/j.1742-481X.2012.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Padoin AV, Braga-Silva J, Martins P, Rezende K, da Rosa Rezende AR, Grechi B, Gehlen D, Machado DC. Sources of processed lipoaspirate cells:influence of donor site on cell concentration. Plast Reconstr Surg. 2008;122:614–618. doi: 10.1097/PRS.0b013e31817d5476. [DOI] [PubMed] [Google Scholar]
- 75.Pu LL, Yoshimura K, Coleman SR. Fat grafting:current concept, clinical application, and regenerative potential, Part 2. Preface. Clin Plast Surg. 2015a;42:xiii–xiv. doi: 10.1016/j.cps.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 76.Pu LL, Yoshimura K, Coleman SR. Fat grafting:current concept, clinical application, and regenerative potential, part 1. Clin Plast Surg. 2015b;42:ix–x. doi: 10.1016/j.cps.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Rbia N, Bulstra LF, Bishop AT, Van Wijnen AJ, Shin AY. A simple dynamic strategy to deliver stem cells to decellularized nerve allografts. Plast Reconstr Surg. 2018;142:402. doi: 10.1097/PRS.0000000000004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rbia N, Bulstra LF, Thaler R, Hovius SE, van Wijnen AJ, Shin AY. In vivo survival of mesenchymal stromal cell–enhanced decellularized nerve grafts for segmental peripheral nerve reconstruction. J Hand Surg. 2019;44:514. doi: 10.1016/j.jhsa.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 79.Reddy SHR, Reddy R, Babu NC, Ashok G. Stem-cell therapy and platelet-rich plasma in regenerative medicines:a review on pros and cons of the technologies. J Oral Maxillofac Pathol. 2018;22:367–374. doi: 10.4103/jomfp.JOMFP_93_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 81.Saffari S, Saffari TM, Ulrich DJO, Hovius SER, Shin AY. The interaction of stem cells and vascularity in peripheral nerve regeneration. Neural Regen Res. 2021a;16:1510–1517. doi: 10.4103/1673-5374.303009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saffari TM, Mathot F, Thaler R, van Wijnen AJ, Bishop AT, Shin AY. Microcomputed analysis of nerve angioarchitecture after combined stem cell delivery and surgical angiogenesis to nerve allograft. J Plast Reconstr Aesthet Surg. 2020;74:1919–1930. doi: 10.1016/j.bjps.2020.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saffari TM, Mathot F, Friedrich PF, Bishop AT, Shin AY. Surgical angiogenesis of decellularized nerve allografts improves early functional recovery in a rat sciatic nerve defect model. Plast Reconstr Surg. 2021b;148:561–570. doi: 10.1097/PRS.0000000000008291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saller MM, Huettl R-E, Mayer JM, Feuchtinger A, Krug C, Holzbach T, Volkmer E. Validation of a novel animal model for sciatic nerve repair with an adipose-derived stem cell loaded fibrin conduit. Neural Regen Res. 2018;13:854–861. doi: 10.4103/1673-5374.232481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shahin TB, Vaishnav KV, Watchman M, Subbian V, Larson E, Chnari E, Armstrong DG. Tissue augmentation with allograft adipose matrix for the diabetic foot in remission. Plast Reconstr Surg Glob Open. 2017;5:e1555. doi: 10.1097/GOX.0000000000001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shimizu M, Matsumine H, Osaki H, Ueta Y, Tsunoda S, Kamei W, Hashimoto K, Niimi Y, Watanabe Y, Miyata M. Adipose-derived stem cells and the stromal vascular fraction in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Wound Repair Regen. 2018;26:446–455. doi: 10.1111/wrr.12665. [DOI] [PubMed] [Google Scholar]
- 87.Shukla L, Yuan Y, Shayan R, Greening DW, Karnezis T. Fat therapeutics:the clinical capacity of adipose-derived stem cells and exosomes for human disease and tissue regeneration. Front Pharmacol. 2020;11:158. doi: 10.3389/fphar.2020.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E. Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg (Lond) 2017;20:49–60. doi: 10.1016/j.amsu.2017.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smahel J. Adipose tissue in plastic surgery. Ann Plast Surg. 1986;16:444–453. [PubMed] [Google Scholar]
- 90.Small K, Choi M, Petruolo O, Lee C, Karp N. Is there an ideal donor site of fat for secondary breast reconstruction? Aesthet Surg J. 2014;34:545–550. doi: 10.1177/1090820X14526751. [DOI] [PubMed] [Google Scholar]
- 91.Spiekman M, van Dongen JA, Willemsen JC, Hoppe DL, van der Lei B, Harmsen MC. The power of fat and its adipose-derived stromal cells:emerging concepts for fibrotic scar treatment. J Tissue Eng Regen Med. 2017;11:3220–3235. doi: 10.1002/term.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Srivastava AK, Bulte JW. Seeing stem cells at work in vivo. Stem Cell Rev Rep. 2014;10:127–144. doi: 10.1007/s12015-013-9468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suganuma S, Tada K, Hayashi K, Takeuchi A, Sugimoto N, Ikeda K, Tsuchiya H. Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration. J Orthop Sci. 2013;18:145–151. doi: 10.1007/s00776-012-0306-9. [DOI] [PubMed] [Google Scholar]
- 94.Sultan SM, Stern CS, Allen RJ, Jr, Thanik VD, Chang CC, Nguyen PD, Canizares O, Szpalski C, Saadeh PB, Warren SM, Coleman SR, Hazen A. Human fat grafting alleviates radiation skin damage in a murine model. Plast Reconstr Surg. 2011;128:363–372. doi: 10.1097/PRS.0b013e31821e6e90. [DOI] [PubMed] [Google Scholar]
- 95.Takeuchi M, Kamei N, Shinomiya R, Sunagawa T, Suzuki O, Kamoda H, Ohtori S, Ochi M. Human platelet-rich plasma promotes axon growth in brain-spinal cord coculture. Neuroreport. 2012;23:712–716. doi: 10.1097/WNR.0b013e3283567196. [DOI] [PubMed] [Google Scholar]
- 96.Tan SS, Ng ZY, Zhan W, Rozen W. Role of adipose-derived stem cells in fat grafting and reconstructive surgery. J Cutan Aesthet Surg. 2016;9:152–156. doi: 10.4103/0974-2077.191672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tompkins BA, Balkan W, Winkler J, Gyongyosi M, Goliasch G, Fernandez-Aviles F, Hare JM. Preclinical studies of stem cell therapy for heart disease. Circ Res. 2018;122:1006–1020. doi: 10.1161/CIRCRESAHA.117.312486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Travnickova M, Bacakova L. Application of adult mesenchymal stem cells in bone and vascular tissue engineering. Physiol Res. 2018;67:831–850. doi: 10.33549/physiolres.933820. [DOI] [PubMed] [Google Scholar]
- 99.Tsekouras A, Mantas D, Tsilimigras DI, Moris D, Kontos M, Zografos GC. Comparison of the viability and yield of adipose-derived stem cells (ASCs) from different donor areas. In Vivo. 2017;31:1229–1234. doi: 10.21873/invivo.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tuin AJ, Domerchie PN, Schepers RH, Willemsen JC, Dijkstra PU, Spijkervet FK, Vissink A, Jansma J. What is the current optimal fat grafting processing technique?A systematic review. J Cranio-Max Surg. 2016;44:45–55. doi: 10.1016/j.jcms.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 101.Tung TH, Mackinnon SE. Secondary carpal tunnel surgery. Plast Reconstr Surg. 2001;107:1830–1843. doi: 10.1007/978-3-540-49008-1_40. [DOI] [PubMed] [Google Scholar]
- 102.Uder C, Brückner S, Winkler S, Tautenhahn HM, Christ B. Mammalian MSC from selected species:Features and applications. Cytometry Part A. 2018;93:32–49. doi: 10.1002/cyto.a.23239. [DOI] [PubMed] [Google Scholar]
- 103.Uysal CA, Tobita M, Hyakusoku H, Mizuno H. The effect of bone-marrow-derived stem cells and adipose-derived stem cells on wound contraction and epithelization. Adv Wound Care (New Rochelle) 2014;3:405–413. doi: 10.1089/wound.2014.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vyas KS, Bole M, Vasconez HC, Banuelos JM, Martinez-Jorge J, Tran N, Lemaine V, Mardini S, Bakri K. Profile of adipose-derived stem cells in obese and lean environments. Aesthetic Plast Surg. 2019;43:1635–1645. doi: 10.1007/s00266-019-01397-3. [DOI] [PubMed] [Google Scholar]
- 105.Vyas KS, DeCoster RC, Burns JC, Rodgers LT, Shrout MA, Mercer JP, Coquillard C, Dugan AJ, Baratta MD, Rinker BD, Vasconez HC. Autologous fat grafting does not increase risk of oncologic recurrence in the reconstructed breast. Ann Plast Surg. 2020a;84:S405–410. doi: 10.1097/SAP.0000000000002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vyas KS, Vasconez HC, Morrison S, Mogni B, Linton S, Hockensmith L, Kabir T, Zielins E, Najor A, Bakri K, Mardini S. Fat graft enrichment strategies:a systematic review. Plast Reconstr Surg. 2020b;145:827–841. doi: 10.1097/PRS.0000000000006557. [DOI] [PubMed] [Google Scholar]
- 107.Wang L, Johnson JA, Zhang Q, Beahm EK. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomater. 2013;9:8921–8931. doi: 10.1016/j.actbio.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang S, Meng XM, Ng YY, Ma FY, Zhou S, Zhang Y, Yang C, Huang XR, Xiao J, Wang YY, Ka SM, Tang YJ, Chung AC, To KF, Nikolic-Paterson DJ, Lan HY. TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget. 2016;7:8809–8822. doi: 10.18632/oncotarget.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei HJ, Zeng R, Lu JH, Lai WF, Chen WH, Liu HY, Chang YT, Deng WP. Adipose-derived stem cells promote tumor initiation and accelerate tumor growth by interleukin-6 production. Oncotarget. 2015;6:7713–7726. doi: 10.18632/oncotarget.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilson A, Butler P, Seifalian A. Adipose-derived stem cells for clinical applications:a review. Cell Prolif. 2011;44:86–98. doi: 10.1111/j.1365-2184.2010.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yi S, Zhang Y, Gu X, Huang L, Zhang K, Qian T, Gu X. Application of stem cells in peripheral nerve regeneration. Burns Trauma. 2020;8:tkaa002. doi: 10.1093/burnst/tkaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation:supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008a;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell-assisted lipotransfer for facial lipoatrophy:efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008b;34:1178–1185. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 114.Yun IS, Jeon YR, Lee WJ, Lee JW, Rah DK, Tark KC, Lew DH. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model:a pilot study. Dermatol Surg. 2012;38:1678–1688. doi: 10.1111/j.1524-4725.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- 115.Zhang J, Liu Y, Chen Y, Yuan L, Liu H, Wang J, Liu Q, Zhang Y. Adipose-derived stem cells:current applications and future directions in the regeneration of multiple tissues. Stem Cells Int. 2020;2020:8810813. doi: 10.1155/2020/8810813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao Z, Wang Y, Peng J, Ren Z, Zhan S, Liu Y, Zhao B, Zhao Q, Zhang L, Guo Q, Xu W, Lu S. Repair of nerve defect with acellular nerve graft supplemented by bone marrow stromal cells in mice. Microsurgery. 2011;31:388–394. doi: 10.1002/micr.20882. [DOI] [PubMed] [Google Scholar]
- 117.Zhou Y, Wang J, Li H, Liang X, Bae J, Huang X, Li Q. Efficacy and safety of cell-assisted lipotransfer:a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:44e–57e. doi: 10.1097/PRS.0000000000001981. [DOI] [PubMed] [Google Scholar]
- 118.Zonari A, Martins TM, Paula AC, Boeloni JN, Novikoff S, Marques AP, Correlo VM, Reis RL, Goes AM. Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomater. 2015;17:170–181. doi: 10.1016/j.actbio.2015.01.043. [DOI] [PubMed] [Google Scholar]
- 119.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]


