Abstract
Background
Care coordination (CC) interventions involve systematic strategies to address fragmentation and enhance continuity of care. However, it remains unclear whether CC can sufficiently address patient needs and improve outcomes.
Methods
We searched MEDLINE, CINAHL, Embase, Cochrane Database of Systematic Reviews, AHRQ Evidence-based Practice Center, and VA Evidence Synthesis Program, from inception to September 2019. Two individuals reviewed eligibility and rated quality using modified AMSTAR 2. Eligible systematic reviews (SR) examined diverse CC interventions for community-dwelling adults with ambulatory care sensitive conditions and/or at higher risk for acute care. From eligible SR and relevant included primary studies, we abstracted the following: study and intervention characteristics; target population(s); effects on hospitalizations, emergency department (ED) visits, and/or patient experience; setting characteristics; and tools and approaches used. We also conducted semi-structured interviews with individuals who implemented CC interventions.
Results
Of 2324 unique citations, 16 SR were eligible; 14 examined case management or transitional care interventions; and 2 evaluated intensive primary care models. Two SR highlighted selection for specific risk factors as important for effectiveness; one of these also indicated high intensity (e.g., more patient contacts) and/or multidisciplinary plans were key. Most SR found inconsistent effects on reducing hospitalizations or ED visits; few reported on patient experience. Effective interventions were implemented in multiple settings, including rural community hospitals, academic medical centers (in urban settings), and public hospitals serving largely poor, uninsured populations. Primary studies reported variable approaches to improve patient-provider communication, including health coaching and role-playing. SR, primary studies, and key informant interviews did not identify tools for measuring patient trust or care team integration. Sustainability of CC interventions varied and some were adapted over time.
Discussion
CC interventions have inconsistent effects on reducing hospitalizations and ED visits. Future work should address how they should be adapted to different healthcare settings and which tools or approaches are most helpful for implementation.
Trial Registration
PROSPERO #CRD42020156359
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-07158-w.
KEY WORDS: case management, care management, transitional care, implementation tools
INTRODUCTION
Complexity of healthcare services and care fragmentation contribute to adverse health outcomes and poor patient experiences.1–4 To help address fragmentation of health services, the Center for Medicare and Medicaid Innovation (CMMI) has undertaken several demonstration projects evaluating new payment models to incentivize integration of services across the continuum of care.5, 6 Over the past 20 years, there has also been substantial interest in care coordination interventions to address fragmentation, particularly aimed at reducing utilization of acute care services (i.e., hospital admissions and emergency department [ED] visits).7, 8 Care coordination models usually involve systematic strategies to improve continuity and bridge transitions of care.7, 9, 10 These often take the form of care or case management, whereby a designated person or team helps patients manage their medical care and navigate interactions with healthcare system(s). These interventions are frequently targeted at populations thought to be at higher risk for acute care utilization, determined using variable criteria.7, 8 While a variety of care coordination models have been studied across diverse settings, it remains unclear whether these interventions can sufficiently address gaps in care and improve patient outcomes. Additionally, for those considering implementation of care coordination models, there is a need to better understand the evidence for tools and approaches used to assist with and monitor implementation progress.
In support of ongoing efforts to standardize and integrate care coordination services across Veterans Health Administration (VHA) facilities,11 the VA Evidence Synthesis Program (ESP) was asked to review evidence on implementation and outcomes of care coordination models for community-dwelling adults at high risk for acute care use.12 This multi-staged evidence report included a systematic review of reviews,13 examination of primary research studies included by eligible reviews, and key informant interviews. We first present qualitative summaries of results from eligible systematic reviews on key characteristics and effectiveness of care coordination interventions. Then, we describe results from primary research studies of effective interventions (i.e., those able to reduce hospitalizations and/or ED visits) regarding settings, and tools and approaches to assess patient trust and care team integration and to improve patient-provider communications. Finally, we provide results from key informant interviews to address remaining gaps in the published literature, particularly with regard to tools and approaches used by various interventions. We also discuss gaps and limitations in the evidence base, and offer recommendations for future research and policy.
METHODS
Conceptual Model and Scope
Collaboratively with our stakeholders (VA Offices of Nursing Services, and Care Management and Social Work) and expert advisory panel, we selected the framework for Care Coordination in Chronic and Complex Disease Management10(Table 1) to guide scope refinement and protocol development. We examined multiple resources and other existing models for care coordination (e.g., AHRQ Care Coordination Atlas7 and Integrated Team Effectiveness Model14). We chose this particular framework because it delineates specific, potentially measurable concepts within larger domains that largely follow the classic structure-process-outcome model for quality of health care.15 Additionally, this framework delineated relevant concepts within and between healthcare teams; our stakeholders indicated that communications and mechanisms between teams were key issues for VHA programs. We further adapted the selected framework by: (1) specifying that within coordination mechanisms, team roles include who contacted patients (and in what manner); and (2) reorganizing outcomes into those relevant for patients (e.g., patient experience, quality of life, and survival), healthcare teams (e.g., work satisfaction and burnout), and health systems (e.g., acute care utilization) (Table 1). While healthcare utilization may be measured at the patient level (e.g., number of admissions per person), we considered such outcomes to be oriented towards priorities of healthcare systems rather than patients.
Table 1.
Adapted Framework for Care Coordination in Chronic and Complex Disease Management
| Context and Setting | Coordination Mechanisms | Emergent Integrating Conditions | Coordinating Actions | Outcomes | |
|---|---|---|---|---|---|
| Within teams |
• Team composition • Experience and history • Power distribution • Resources |
• Plans, rules, and tools • Objects, representations, artifacts, and information systems • Roles (e.g., who contacts patients and how) • Routines • Proximity |
• Accountability • Predictability • Common understanding • Trust |
• Situation monitoring • Communication • Back-up behavior |
• Patients (e.g., patient experience, quality of life, survival) • Health care teams (e.g., job satisfaction) • Health systems (e.g., acute care utilization, costs) |
| Between teams |
• Multiteam system composition • Linkages between teams • Alignment of organizational cultures/ climates • Governance and payment structure |
• Boundary spanning • Information exchange • Collective problem-solving and decision-making • Negotiation • Mutual adjustment |
Original framework by Weaver et al. (2018)10
Applying this framework, and in accordance with stakeholder priorities, we defined effective interventions as those that reduced hospitalizations and/or ED visits. We sought information on key characteristics of effective interventions, particularly with regard to elements depicted in Context and Setting, and Coordinating Mechanisms. Examples of such characteristics include multidisciplinary teams (vs. primarily single case manager) and home visits (vs. telephone contacts and/or outpatient visits). We also searched for evidence on tools and approaches that were used to assess Emergent Integrating Conditions (e.g., trust within teams) and Coordinating Actions (e.g., within team communication); our stakeholders identified that these tools were as important for monitoring implementation progress of VHA programs before other outcomes may be available. Finally, to better understand the applicability of results, we sought information on characteristics of healthcare systems and communities where effective interventions had been implemented.
Key Questions (KQ)
For community-dwelling adults with a variety of ambulatory care sensitive conditions and/or at higher risk of having repeat hospitalization or ED visits:
KQ1—What are the key characteristics of care coordination models (of varying types) that aim to reduce hospitalization or ED visits?
KQ2—What is the effect of implementing these care coordination models on hospitalizations, ED visits, and patient experience (e.g., Consumer Assessment of Healthcare Providers and Systems)?
KQ3—What are the characteristics of settings in which effective models have been implemented?
- KQ4—Among effective models, which approaches/tools have been used to:
- Measure patient trust or working alliance?
- Measure team integration?
- Improve communication between patients and providers?
Search Strategy
We first focused on identifying eligible systematic reviews that examined a variety of care coordination models. We searched for English-language systematic reviews, from inception until September 2019, in the following: MEDLINE, CINAHL, Embase, Cochrane Database of Systematic Reviews, AHRQ Evidence-based Practice Center, and VA ESP reports. Search terms included MeSH and free text for care coordination interventions (e.g., care or case management, interdisciplinary care, and intensive primary care) and systematic reviews (Appendix 1 in the supplementary material).
Based on our experience with published evidence on care coordination and information reported by systematic reviews, we anticipated that eligible reviews may not provide sufficient information, particularly regarding characteristics of settings and tools and approaches used (KQ 3 and 4). Therefore, we planned a priori to undertake the following additional steps: (1) examination of primary research studies included by eligible reviews; (2) updated search for relevant randomized controlled trials (RCT) of care coordination interventions in MEDLINE and Embase (from year of most recent eligible review until February 2020) (Appendix 1 in the supplementary material); and (3) key informant interviews with team members who implemented interventions described in primary research studies (see below).
Screening and Selection
Using prespecified criteria (Appendix 2 in the supplementary material), systematic review search results were evaluated and excluded with the consensus of 2 individuals. Eligible populations included community-dwelling adults with a range of ambulatory care sensitive conditions (per AHRQ quality metrics), including conditions like heart failure and chronic lung disease16and/or at higher risk for acute care episodes (as defined by authors of review and/or primary studies). If a review focused exclusively on a single health condition, it was excluded. Eligible interventions covered diverse models, including care or case management and home-based primary care. Eligible reviews included hospitalizations and/or ED visits as outcomes of interest in objectives or results. At full-text review, two individuals separately determined inclusion and resolved conflicts through discussion.
From each eligible review, we also identified all included primary studies and 2 individuals evaluated them for potential relevance to KQ 3–4. As noted above, relevant studies for KQ 3 reported effective care coordination models that reduced acute care visits; if a model was only shown to improve patient experience, it was not considered applicable to KQ 3. In addition to the criteria described above, we also applied the following: conducted in the USA, and RCT or quasi-experimental observational studies (e.g., comparative control cohort or interrupted time series).17 We similarly screened and reviewed results from the additional search of RCT published 2018 to February 2020.
Data Abstraction and Quality Assessment
We assessed quality using criteria adapted from AMSTAR 2,18 rating overall quality as high, medium, or low (Appendix 3 in the supplementary material). We also noted if review authors evaluated for small studies and reporting outcome biases. From all eligible reviews, we abstracted the following: target populations (e.g., frequent utilizers of ED or those with complex chronic conditions); dates of search queries; and number and characteristics of included primary research studies (location, setting, and study design). From high- and medium-quality reviews, we planned to abstract detailed information on the following: characteristics of care coordination models; pooled effects or qualitative summaries of results on hospitalizations, ED visits, and/or patient experience; setting characteristics; and tools and approaches used to measure patient trust or working alliance, assess healthcare team integration, and/or improve patient-provider communications.
From relevant primary studies reporting successful reductions in hospitalizations and/or ED visits, we abstracted the following: effects on main outcomes; participant, intervention, and setting characteristics; and relevant tools and approaches. We also examined associated studies referenced by primary studies for intervention characteristics.
Data Synthesis
Due to heterogeneity of eligible reviews and studies, we undertook qualitative synthesis focusing on key characteristics and effectiveness of care coordination models. If reported, we abstracted strength of evidence determinations by review authors. For primary studies reporting effective interventions, we summarized setting and intervention characteristics; effects on main outcomes; and tools and approaches.
Key Informant Interviews
We conducted semi-structured interviews with researchers and team members who implemented care coordination models, as described in relevant primary studies (regardless of intervention effectiveness). We invited 25 individuals and completed interviews with 11 participants.
The main focus of interviews was to address gaps in published studies regarding intervention tools and approaches (KQ4). We also included questions on intervention uptake and sustainability. A general version of the interview guide is provided in Appendix 4 in the supplementary material; individual guides were adapted using published and online information. Interviews lasted about 30 min and were audio-recorded. We reviewed contemporaneous notes and audio-recordings to develop summaries for each intervention. We then examined all interview summaries to generate key takeaways across major topics.
RESULTS
Overview of Eligible Systematic Reviews
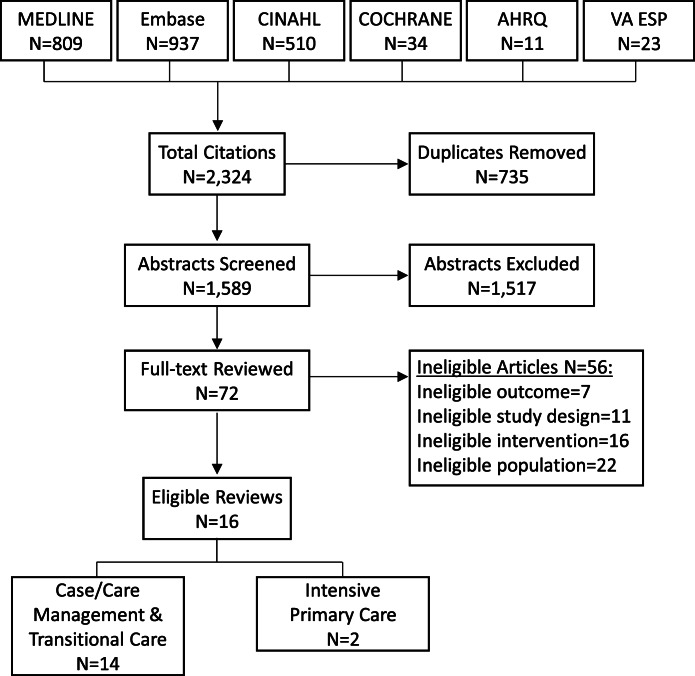
We screened 2,324 unique citations, reviewed 72 full texts, and identified 16 eligible systematic reviews (Figure 1). Fourteen reviews examined case management or transitional care interventions,19–32 and 2 evaluated intensive primary care models (e.g., home-based primary care)33, 34; all reviews included a variety of models within these broad categories. Four reviews included only RCT,19, 25, 26, 31 while others allowed observational studies. Most reviews included studies from different countries, but 3 were limited to US studies.20, 24, 28 Seven reviews focused on patients at higher risk for acute care utilization (i.e., high utilizers),19, 22–24, 27, 28, 30 and one examined interventions for frail adults.31 Six reviews were high quality,23, 26, 27, 29, 30, 34 6 were medium,19, 22, 24, 25, 31, 33 and 4 were low20, 21, 28, 32 (Appendix 3 in the supplementary material). Overall, 8 reviews evaluated selective outcomes reporting bias (7 high and medium quality); of 3 reviews that conducted quantitative meta-analyses, 2 used funnel plots to assess for publication bias (Appendix 3 in the supplementary material). We focused on high- and medium-quality reviews for detailed results, as described below and in Appendix 5 in the supplementary material.
Figure 1.
Search and selection for eligible systematic reviews. AHRQ EPC, Agency for Healthcare Research and Quality Evidence-based Practice Centers; VA ESP, Department of Veterans Affairs Evidence Synthesis Program.
Primary research studies included by all eligible reviews were examined for relevance (see METHODS). Among 272 unique primary studies, we identified 16 RCT35–50 and 9 observational studies51–59 that evaluated relevant interventions conducted in the USA. Most studies were included by 1–2 eligible reviews, but 7 studies36, 38, 42, 48, 52, 57 were included by 3 or more reviews (Appendix 6 in the supplementary material). The updated search for RCT (published in 2018 or later) identified 1048 unique citations. We reviewed 21 full texts, finding 2 more relevant RCT; both reported interventions were not effective for reducing hospitalizations and/or ED visits.60, 61 Overall, 20% of relevant RCT (4 of 20)38, 43, 46, 49 showed reductions in hospitalizations and/or ED visits. In contrast, 78% of relevant observational studies (7 of 9)51, 52–55, 57, 58 reported effectiveness for these outcomes.
KQ1: What Are the Key Characteristics of Care Coordination Models?
All reviews summarized different components included by interventions, with variation in team composition and components (e.g., multidisciplinary care plan). Communication with patients was mainly in person (in a clinical setting or at home) or via telephone. Four reviews specifically addressed key characteristics for care coordination models23, 29, 31, 34; all included primary studies conducted in and outside of the USA (e.g., Canada, European countries, and Australia). Among these, Hudon et al.23 used qualitative comparative analysis to examine characteristics of effective case management models, finding that careful participant selection was necessary but not sufficient; additional required elements were high intensity (defined using caseload, frequency, and types of patient contacts) or a multidisciplinary care plan. Smith et al.29 also conducted a qualitative synthesis, reporting that interventions targeting specific risk factors were more likely to be effective. In contrast, Van der Elst et al.31 conducted quantitative subgroup analyses by intervention duration and different approaches to address frailty, finding no significant differences in effect. Finally, Totten et al.34 examined home-based primary care interventions and, using qualitative synthesis, found no specific pattern of components were associated with effectiveness. Additionally, 2 reviews19, 33 sought to determine key components but were unable to draw conclusions; challenges included lack of published information describing components and implementation fidelity.
KQ2: What Is the Effect of Implementing Care Coordination Models?
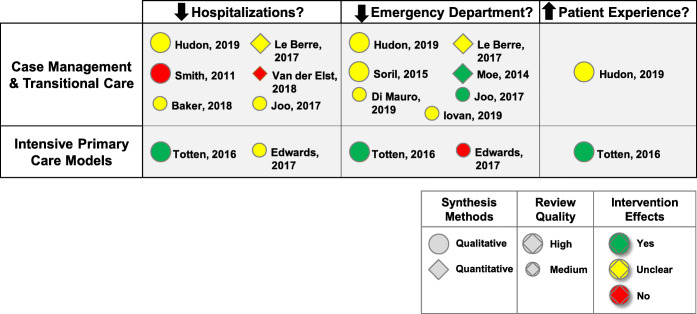
Effects of care coordination interventions on hospitalizations, ED visits, and patient experience are summarized in Figure 2 and described in detail in Appendix 5 in the supplementary material. Of 12 high- and medium-quality reviews, most used qualitative synthesis, and most found unclear or mixed effects across the different outcomes (Figure 2).
Figure 2.
Summary results from eligible high- and medium-quality systematic reviews—effects of care coordination interventions on hospitalizations, emergency department visits, and patient experience.
Case Management and Transitional Care Interventions
Of 10 reviews examining case management or transitional care interventions, 6 evaluated effectiveness for reducing hospitalizations, with 4 reporting mixed or unclear results19, 23, 25, 26 and 2 finding lack of effectiveness29, 31(Figure 2 and Appendix 5 in the supplementary material). Populations addressed by these reviews included high utilizers,19, 23 those with chronic disease or multimorbidity,25, 26, 29 and frail older adults.31 Le Berre et al.26 evaluated transitional care interventions using quantitative meta-analyses; incorporating data from 11 to 35 RCT across different follow-up periods, there was no effect at 1 month (risk difference [RD] −0.03 [−0.05, 0]) but some reduction at 3–18 months (RD range −0.05 to−0.11) (Appendix 5 in the supplementary material). Van der Elst et al.31 conducted quantitative meta-analyses to evaluate diverse case management interventions for frail community-dwelling older adults; pooled results from 5 RCT showed no reduction in hospitalizations (odds ratio [OR] 1.13, 95% confidence interval [CI] 0.95, 1.35) (Appendix 5 in the supplementary material).
Seven reviews examined effects on ED visits, with only 2 indicating that care coordination reduced ED visits.25, 27 Joo et al.25 focused on adults with chronic disease and described 6 studies that showed reductions in ED visits. Moe et al.27 addressed results for high utilizers and reported that the median rate ratio (of care coordination vs. control) was 0.63, with an interquartile range of 0.41–0.71. Le Berre et al.26 also examined care coordination for high utilizers, and reported pooled meta-analyses showing a reduction at 3 months (RD −0.08 [−0.15, −0.01]) but no differences at 1, 6, and 12 months. All 4 remaining reviews22–24, 30 evaluated results for high utilizers, used qualitative synthesis and reported unclear or mixed effects on ED visits (Figure 2 and Appendix 5 in the supplementary material).
One review addressed effects on patient experience and, using qualitative synthesis, found inconsistent results.23
Intensive Primary Care Interventions
Two reviews evaluated intensive primary care interventions, and both used qualitative synthesis. Totten et al.34 focused on home-based primary care for adults with chronic illness and/or disabilities, reporting reduced hospitalizations (moderate strength of evidence) and ED visits (low strength of evidence), along with higher patient and caregiver satisfaction (low strength of evidence) (Figure 2 and Appendix 5 in the supplementary material). In contrast, Edwards et al.33 addressed several different models of intensive primary care for those at high risk for hospital admission and/or death, finding inconsistent results across studies.
KQ3: What Are the Characteristics of Settings in Which Effective Models Have Been Implemented?
Few reviews addressed specific setting characteristics, outside of countries of studies. Soril et al.30 examined case management models and reported that 15 of 16 included studies were single site, usually in an urban setting. Totten et al.34 sought to address organizational settings for home-based primary care but were unable to find published information.
To better understand setting characteristics, we also examined 11 relevant primary studies reporting effective interventions in the USA. We categorized these interventions as transitional care (n=5),37, 43, 51, 53, 54 outpatient care or case management (led by nurse or social worker) (n=4),46, 48, 57, 58 or other intensive primary care models (n=2)52, 55(Table 2). Settings were diverse, including rural community hospitals, academic medical centers (in urban settings), and public hospitals serving largely poor and uninsured populations. Detailed intervention characteristics and effects are also presented in Table 2. There was no clear connection between differences in settings, types of intervention, and various patient populations.
Table 2.
Primary Research Studies—Characteristics, Settings, and Results of Effective Care Coordination Models
|
Author, year; study design*; N(I,C) |
Intervention name; eligibility criteria | Setting characteristics | Description of patient contacts | Intervention effects | |
|---|---|---|---|---|---|
| Hospitalizations | ED visits | ||||
| Transitional care interventions | |||||
|
Capp, 201751; cohort; I=406 C=3396 |
Bridges to care; adults with ≥ 2 ED visits and/or hospitalizations in past 180 days | Large urban academic medical center, Colorado | First home visit by community health worker within 24–72 h, second visit by PCP within 1 week of ED or hospital discharge; 8 visits over 60 days (community health worker, nurse, primary care provider, and/or behavioral health provider) depending on patient needs. |
Average no. of admissions per person, 180 days before enrollment: I=1.04, C=1.15 180 days after 60-day intervention: I=0.75, C=1.02 Difference of differences = −0.16, P<0.1 |
Average no. of visits per person, 180 days before enrollment: I=5.12, C=4.93 180 days after 60-day intervention: I=2.79, C=3.60 Difference of differences = −1.01, P=<0.01 |
|
Hamar, 201654; cohort; I=560 C=3340 |
Care transition solution; adults admitted with ≥ 1 condition (COPD, heart failure, myocardial infarction, pneumonia) | 14 community hospitals in north Texas | Initial visit in hospital with nurse before discharge, then 4 calls over 4 weeks |
Proportion with ≥ 1 readmission at 30 days: AOR=0.56 (0.41–0.77) At 6 months: AOR=0.47 (0.35–0.65) |
NR |
|
Gardner, 201453; cohort; I=21 C=21 |
Care transitions intervention; adults participating in Medicare fee-for-service, admitted to hospital | 6 community hospitals, Rhode Island | Initial visit in hospital by nurse, home visit “shortly after discharge,” 2–3 phone calls during 30-day post-discharge period |
Propensity score–matched no. of readmissions at 6 months: I=0.65, C=0.93 P=0.01 |
Propensity score–matched no. of visits at 6 months: I=0.44, C=0.50 P=0.55 |
|
Coleman, 200638; RCT; I=379 C=371 |
Care transitions intervention; older adults (≥65) admitted with ≥ 1 condition (stroke, heart failure, diabetes mellitus, etc.) | Community health system, Colorado | Nurse met patients in hospital before discharge, home visit within 48–72 h of discharge, then 3 more times during the 28-day post-discharge period. |
Proportion with ≥ 1 readmission at 30 days: I=0.08, C=0.12 (AOR 0.59 [0.35, 1.00], P=0.048) At 90 days: I=0.17, C=0.23 (AOR 0.64 [0.42, 0.99], P=0.04) At 180 days: I=0.26, C=0.31 (AOR 0.80 [0.54, 1.19], P=0.28) |
NR |
|
Naylor, 199943; RCT; I=177 C=186 |
Transitional care model; older adults (≥65) admitted with ≥ 1 condition (heart failure, respiratory infection, orthopedic procedure, etc.) | 2 urban hospitals affiliated with the University of Pennsylvania | Initial nurse visit within 48 h of admission, visits at least every 48 h during admission, home visits after discharge (first within 48 h, second 7–10 days post-discharge, additional visits based on patients’ needs), weekly nurse-initiated phone contact |
Proportion with ≥ 1 readmission at 24 weeks: I=0.20, C=0.37 P<0.01 |
NR |
| Outpatient care or case management | |||||
|
Shah, 201157; cohort; I=98 C=160 |
Care management program; adults aged 18–64, <200% federal poverty level, uninsured, “met frequent user criteria” | Public safety-net hospital and clinics in Kern County, CA | Care managers (social worker or medical office assistant) met with patients at least monthly in the home and/or clinic, for variable lengths of time (care manager decided when patient graduated program) | Adjusted ratio of no. of admissions per year (I:C) was 0.81, P=0.38 | Adjusted ratio of no. of visits per year (I:C) was 0.67, P<0.001 |
|
Peikes, 200946; RCT; Mercy Medical Center (1 of 15 sites) I=669, C=467 |
Medicare coordinated care demonstration; adults participating in Medicare fee-for-service and with ≥ 1 condition (heart failure, COPD, etc.) | Mercy Medical Center—rural community hospital, Iowa | Nurse completed in-person evaluation within 2 weeks of enrollment, contacted patient at least monthly, 69% were in-person (either at home or during clinic visit) |
Average no. of admissions per person per year: I= 1.15, C=0.98 P=0.02 |
NR |
|
Shumway, 200848; RCT; I=167, C=85 |
Comprehensive case management; adults with ≥ 5 ED visits in past 12 months and had “psychosocial problems that could be addressed with case management” | Urban public hospital in San Francisco, CA | Social workers completed assessments, individual and group supportive therapy, assistance to a variety of community resources, and “assertive community outreach” (frequency and schedule of patient contacts NR) | Effect size NR, P=0.08 for treatment effect in adjusted model for visits over 2 years | Effect size NR, P<0.01 for treatment effect in adjusted model for visits over 2 years |
|
Sommers, 200058; Cohort I=280 C=263 |
Senior care connections; adults ≥65 with difficulty in ≥1 instrumental activity of daily living and 2 ≥ chronic conditions | Primary care clinics in San Francisco Bay area, CA | Initial home visit with case manager (nurse or social worker), treatment plan drafted by care team (nurse, social worker, primary care provider), patients contacts via phone, home visits, small group sessions, or office/hospital visits at least once every 6 weeks |
Number of admissions per person per year at baseline: I=0.35, C=0.06 during year 1: I=0.38, C= 0.34 during year 2: I=0.36, C=0.52 P=0.03 |
Proportion with ≥1 visit at baseline: I=0.09, C=0.06 during year 1: I=0.20, C=0.17 during year 2: I=0.21, C=0.17 P=0.77 |
| Other intensive primary care models | |||||
|
Crane, 201252; cohort; I=34 C=36 |
Drop-in group medical appointments; uninsured, family income ≤ 200% federal poverty level, ≥ 6 ED visits in past year | Rural community hospital, North Carolina | Twice weekly groups sessions, short individual visit right after; direct phone access to nurse care manager; team included nurse, primary care, and behavioral health providers | NR |
Median no. of visits per month during 1 year before: I=0.58, C=0.58 during 1 year after: I=0.23, C=0.42 Difference in differences: 0.23, P=0.005 |
|
Meret-Hanke, 201155; cohort; I=3889 C=3103 |
Program for all-inclusive care for the elderly; adults >65, with functional limitations or dementia, income <300% supplemental security income | National US program | Interdisciplinary care teams provided care management, clinical monitoring, and updated care plan in response to changes in enrollee’s health and functional status |
Propensity score-matched any hospitalization at 6 months: AOR 0.35, P<0.01 At 2 years: AOR 0.16, P<0.01 |
NR |
AOR, adjusted odds ratio; C, control group; COPD, chronic obstructive pulmonary disease; ED, emergency department; I, intervention group; RCT, randomized controlled trial
*Study designs were either RCT or observational cohorts with comparative controls
KQ4: What Are Tools and Approaches Used by Effective Models?
No review commented on tools and approaches used to measure patient trust or care team integration, or to improve patient-provider communication. Among relevant primary studies, 5 described various approaches to improve patient-provider communications, such as coaching patients, making lists of key concerns, and role-playing.38, 46, 53, 57, 62 In 2 studies, care coordinators also attended outpatient visits with patients and their providers.46, 57 No study described specific measures to assess patient trust or care team integration. We identified an accompanying article for one study38 that described results from qualitative interviews to evaluate patient experiences and relationship with care coordinators.45
Key Informant Interviews
Main takeaways from interviews and selected supporting quotes are presented in Table 3. We did not identify any additional tools or approaches to assess patient trust or care team integration, or to improve patient-provider communications. Additional materials provided by interviewees indicated that sometimes, assessments of patient experience included factors conceptually related to patient trust (e.g., perception that care coordinator was knowledgeable and understood patients’ needs).
Table 3.
Summary of Takeaways from Key Informant Interviews
| What worked well | |
|
1. Multidisciplinary collaboration • Relationships and communication with providers from primary care, specialty care, and a variety of disciplines (e.g., social work) “Patients had issues that crossed medical, behavioral, and social domains, so it was critical to have experts in these areas on the team” • Collaboration approaches varied (e.g., outreach calls, email, and in-person meetings) 2. Dedicated staff for intervention • Important for ensuring continuity of care, timely changes in management, and developing trust with patient and family “It made a difference that [there was a] dedicated person to respond to alerts, contact physicians to facilitate interactions, etc.) …[C]are suggestions…were quickly implemented” “The fact that we had the same nurse following the patient…was viewed by patients and family caregivers as really central…” 3. Knowledge of and relationship with community-based services • Services included transportation, food banks, home-delivered meals, and substance abuse treatment “Establishing a vast network of relationships [with community organizations] was important … to effectively help patients” 4. Staff key qualities and skills • Dedication and compassion, communication and relationship-building skills | |
| Challenges and sustainability | |
|
1. Difficulty of impacting readmissions • Timeframe for metrics is too short, and there are often many challenging factors to address “30 days doesn’t give you sufficient time…especially in elderly patients with many issues… Everything that could be possibly going wrong is going wrong…” 2. Adaptation of interventions • Modified over time to meet changing priorities (e.g., adaptation for different high-risk populations) • Certain components or techniques selected for adoption by sites, while others were not 3. Stakeholder engagement and financial viability • Engagement with senior leadership as key to sustainability “[P]artnership between the architects of these models and the health systems, …strong collaboration…is required to meaningfully move evidence into health systems…” • Difficulty in determining which group (within health system) benefits most and should bear financial responsibility for staffing, etc. |
Overall, several elements were noted to work well: multidisciplinary collaboration; dedicated staff for intervention (e.g., who contacted providers and patients); relationships with community organizations and services; and staff dedication, compassion, and ability to establish relationships with relevant actors (Table 3). There was variation in sustainability of interventions, with some stopping after completion of research studies. Challenges encountered by interventions (and successfully addressed by some) included the following: need to meet readmission metrics; adaptation to changing health system priorities; and achieving buy-in and ownership (e.g., of staffing costs) from key health system stakeholders (Table 3).
For future interventions, some suggested that interventions may work better for patients with less severe conditions and/or modifiable factors; an important challenge with such an approach is that interventions may need to serve a larger number of patients before there are appreciable differences in acute care utilization. One individual stated, “You can allocate a lot of resources to extremely high need patients…or you can allocate resources to a larger population and … have a smaller impact on individual level, but on population level have greater impact…”
DISCUSSION
We conducted a multi-staged evidence review and key informant interviews to evaluate care coordination interventions. Interventions were complex and differed along multiple dimensions, presenting substantial challenges in comparing and summarizing the evidence. Most reviews reported unclear or inconsistent effects in reducing hospitalizations or ED visits. Two reviews drew conclusions about key characteristics; both highlighted the importance of selection criteria focused on clear risk factors and/or needs. One of these further indicated the requirement for a high-intensity model (defined by lower caseload and more patient contacts) or use of multidisciplinary plans.
Among 11 primary studies demonstrating effective interventions, none reported specific tools or approaches for measuring patient trust or healthcare team integration. Approaches used to improve patient-provider communication included coaching and role-playing. In some interventions, care coordinators directly communicated with providers on patients’ behalf, including participation at clinic appointments. Key informants highlighted multiple challenges to implementation and sustainability of interventions, including difficulty with impacting short-term readmission metrics, adapting to changing health system priorities, and stakeholder engagement.
Among multiple factors contributing to healthcare fragmentation in the USA, foremost are complex payment policies and a regulatory environment that present substantial barriers to service integration.63, 64 These factors make it financially challenging for healthcare entities to invest in programs aiming to improve long-term health outcomes. Existing barriers also limit the potential impact of care coordination interventions, which must work to patch ongoing gaps in care and manage misaligned incentives among various providers. CMMI demonstration projects are testing several new payment models to incentivize restructuring of healthcare delivery, including bundled payments to promote integration of care across settings for certain indications,5 and establish patient-centered medical homes in outpatient care.6 Results from these demonstrations may lead to longer-term policy changes that will support greater integration of US healthcare services.
Evidence Gaps and Future Research
Primary research studies using observational designs were more likely to report reductions in hospitalizations and/or ED visits. Observational studies may have residual confounding, be more affected by selective outcomes and publication bias, and are susceptible to false-positive results from regression to the mean (particularly for pre-post cohort designs). Also, there was very limited evidence for intensive primary care models; the 2 eligible reviews on these models included a total of 4 relevant primary studies.40, 50, 55, 56
Additionally, studies did not report standardized tools used to assess patient trust or care team integration. Key informant interviews revealed that some assessments of patient experience included concepts closely related to patient trust, but interviews did not identify any measures of care team integration. Gaps in our knowledge about tools to reliably assess these important aspects of patient coordinator and health team relationships may reflect the general focus of many care coordination studies on impacting acute care utilization; there may have been relatively less attention on verifying successful implementation as demonstrated by improvements in communication and relationships. Lack of standardized and validated tools to assess these aspects may hinder evaluation of existing services and implementation of new programs.
Finally, multiple reviews found a lack of information on intervention implementation and fidelity. To improve evaluation and interpretation of effectiveness, future studies should consider application of frameworks and designs that address implementation outcomes (e.g., hybrid effectiveness implementation designs, Consolidated Framework for Implementation Research [CFIR], and Reach, Effectiveness, Adoption, Implementation, and Maintenance [RE-AIM]).65–68 Studies should clearly define the “core” set of key components and the “adaptable periphery” of elements that can be adjusted to accommodate the local context.66
Therefore, we recommend the following:
Evaluate future care coordination interventions using randomized designs
Develop and apply standardized tools to assess patient trust or working alliance, healthcare team integration, and communication between patients and providers
Consider study designs that explicitly consider implementation outcomes in future studies of care coordination models
Define “core” intervention components and describe local adaptations, particularly in multisite studies
Implications for Policy and Practice
It remains unclear whether specific care coordination models should be implemented and how adaptations may improve effectiveness and sustainability. Current VHA initiatives have piloted new tools for evaluating patient needs and matching the level of care coordination services.11 It will be important to evaluate feasibility of wider implementation and downstream effects on service delivery and patient outcomes. Additionally, it will be important to understand differences in utility across large and small medical centers and clinics, and those located in urban and more rural communities. These evaluations may also be informative for non-VHA health systems seeking better tools to match services to patient needs. As local adaptations were noted to be important for uptake and sustainability, it may also be valuable to develop materials and resources to guide adaptations. Such guidance must necessarily depend on further work to better understand the rationale and impacts of various adaptations in different settings.
As noted above, fragmentation of healthcare services in the USA is unlikely to improve without substantial policy changes to better align incentives and remove regulatory hurdles. Our results suggest that high intensity and multidisciplinary care are needed for effective interventions, and these care coordination models are likely to incur higher costs and potentially more regulatory burden in the current environment. Thus, longer-term investments to implement such models, and improve patient outcomes, are likely not feasible until substantial changes in policy have occurred.
Finally, there may be specific patient populations that require care models beyond additional care coordination services (e.g., by a nurse and/or social worker). For example, VHA has implemented a collaborative model of collocated primary care and mental health, to improve access to mental health services.69 Another collaborative (often collocated) model involves longitudinal integration of oncology and palliative care for cancer patients.70 Notably, these care models target specific needs that are prevalent among the relevant populations, and for which there are clearly effective treatments.
Limitations
To address our VA stakeholder priorities, we focused on interventions effective in reducing hospitalizations and/or ED visits; reviews and studies not addressing these outcomes were excluded. While we acknowledge the importance of patient experience, our stakeholders and key informant interviews all indicated that impacting acute care utilization was often the top priority for health system leadership, and particularly important for sustainability. Because interventions occurring outside the USA may be less informative, due to substantial differences in healthcare financing and delivery, we limited relevant primary studies to those conducted in the USA. We completed interviews with less than half of those whom we invited; it is possible there was unpublished information on tools and approaches that we did not identify. Finally, we relied on determinations of overall effectiveness and strength of evidence from high- and medium-quality systematic reviews; thus, methodological concerns with the conduct of these reviews would affect the validity of our results.
CONCLUSIONS
Existing evidence on care coordination interventions indicates inconsistent effects on reducing hospitalizations and/or ED visits for high-risk community-dwelling adults. It remains unclear whether such interventions should be implemented and how they may be adapted to different healthcare settings. Implementation of new care coordination models should be carefully evaluated using randomized designs. Policymakers should also consider whether a larger scale redesign of healthcare services may be necessary to improve continuity and collaboration for certain patient populations.
Supplementary Information
(DOCX 145 kb)
Funding
This work was supported by VA Health Services Research & Development funding for ESP (VA-ESP Project No. 09-009; 2020). The funder had no role in study design, data collection, analysis and interpretation of data, writing of the report, or decision to submit article for publication.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533–6. doi: 10.7326/0003-4819-141-7-200410050-00009. [DOI] [PubMed] [Google Scholar]
- 2.Gadbois EA, Tyler DA, Shield R, McHugh J, Winblad U, Teno JM, et al. Lost in Transition: a Qualitative Study of Patients Discharged from Hospital to Skilled Nursing Facility. J Gen Intern Med. 2019;34(1):102–9. doi: 10.1007/s11606-018-4695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison A, Verhoef M. Understanding coordination of care from the consumer’s perspective in a regional health system. Health Serv Res. 2002;37(4):1031–54. doi: 10.1034/j.1600-0560.2002.64.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery JE, Irish JT, Wilson IB, Chang H, Li AC, Rogers WH, et al. Primary care experiences of medicare beneficiaries, 1998 to 2000. J Gen Intern Med. 2004;19(10):991–8. doi: 10.1111/j.1525-1497.2004.30381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Medicare and Medicaid Services. Bundled Payments for Care Improvement (BPCI) Initiative: General Information. 2021. https://innovation.cms.gov/innovation-models/bundled-payments. Accessed 08/10/21.
- 6.Centers for Medicare and Medicaid Services. Comprehensive Primary Care Plus. 2021. https://innovation.cms.gov/innovation-models/comprehensive-primary-care-plus. Accessed 08/10/21.
- 7.MacDonald K, Schultz E, Albin L, Pineda N, Lonhart J, Sundaram V, et al. Care Coordination Atlas Version 4 (AHRQ Publication No. 14-0037-EF) Agency for Healthcare Research and Quality: Rockville, MD; 2014. [Google Scholar]
- 8.Shojania KG, Wachter RM, Owens DK, Ranji SR, Shetty K, Posley KA, et al. Closing the quality gap: a critical analysis of quality improvement strategies. Care Coordination. 2007;7.
- 9.Peterson K, Anderson J, Bourne D, Boundy E. Scoping Brief: Care Coordination Theoretical Models and Frameworks: VA ESP Project #09-199. 2018. [PubMed] [Google Scholar]
- 10.Weaver SJ, Che XX, Petersen LA, Hysong SJ. Unpacking Care Coordination Through a Multiteam System Lens: A Conceptual Framework and Systematic Review. Med Care. 2018;56(3):247–59. doi: 10.1097/MLR.0000000000000874. [DOI] [PubMed] [Google Scholar]
- 11.Greenstone CL, Peppiatt J, Cunningham K, Hosenfeld C, Lucatorto M, Rubin M, et al. Standardizing Care Coordination Within the Department of Veterans Affairs. J Gen Intern Med. 2019;34(Suppl 1):4–6. doi: 10.1007/s11606-019-04997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan-Porter W UK, Majeski B, Miake-Lye I, Diem S, Wilt TJ. Evidence review: care coordination models and tools. VA ESP Project #09-009. 2020. [PubMed]
- 13.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemieux-Charles L, McGuire WL. What do we know about health care team effectiveness? A review of the literature. Med Care Res Rev. 2006;63(3):263–300. doi: 10.1177/1077558706287003. [DOI] [PubMed] [Google Scholar]
- 15.Donabedian A. Evaluating the quality of medical care. 1966. Milbank Q. 2005;83(4):691–729. doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AHRQ . Quality Indicators. Guide to Prevention Quality Indicators: Hospital Admission for Ambulatory Care Sensitive Conditions (AHRQ Pub. No. 02-R0203) Agency for Healthcare Research and Quality: Rockville, MD; 2001. [Google Scholar]
- 17.Rockers PC, Rottingen JA, Shemilt I, Tugwell P, Barnighausen T. Inclusion of quasi-experimental studies in systematic reviews of health systems research. Health Policy. 2015;119(4):511–21. doi: 10.1016/j.healthpol.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker JM, Grant RW, Gopalan A. A systematic review of care management interventions targeting multimorbidity and high care utilization. BMC Health Serv Res. 2018;18(1):65. doi: 10.1186/s12913-018-2881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleich SN, Sherrod C, Chiang A, Boyd C, Wolff J, DuGoff E, et al. Systematic Review of Programs Treating High-Need and High-Cost People With Multiple Chronic Diseases or Disabilities in the United States, 2008-2014. Prev Chronic Dis. 2015;12:E197. doi: 10.5888/pcd12.150275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Pourcq K, Meijboom B, Trybou J, Mortier E, Eeckloo K. The role of hospitals in bridging the care continuum: a systematic review of coordination of care and follow-up for adults with chronic conditions. BMC Health Serv Res. 2017;17(1):550. doi: 10.1186/s12913-017-2500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Mauro R, Di Silvio V, Bosco P, Laquintana D, Galazzi A. Case management programs in emergency department to reduce frequent user visits: a systematic review. Acta Biomed. 2019;90(6-S):34–40. doi: 10.23750/abm.v90i6-S.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudon C, Chouinard MC, Pluye P, El Sherif R, Bush PL, Rihoux B, et al. Characteristics of Case Management in Primary Care Associated With Positive Outcomes for Frequent Users of Health Care: A Systematic Review. Ann Fam Med. 2019;17(5):448–58. doi: 10.1370/afm.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iovan S, Lantz PM, Allan K, Abir M. Interventions to Decrease Use in Prehospital and Emergency Care Settings Among Super-Utilizers in the United States: A Systematic Review. Med Care Res Rev. 2020;77(2):99–111. doi: 10.1177/1077558719845722. [DOI] [PubMed] [Google Scholar]
- 25.Joo JY, Liu MF. Case management effectiveness in reducing hospital use: a systematic review. Int Nurs Rev. 2017;64(2):296–308. doi: 10.1111/inr.12335. [DOI] [PubMed] [Google Scholar]
- 26.Le Berre M, Maimon G, Sourial N, Gueriton M, Vedel I. Impact of Transitional Care Services for Chronically Ill Older Patients: A Systematic Evidence Review. J Am Geriatr Soc. 2017;65(7):1597–608. doi: 10.1111/jgs.14828. [DOI] [PubMed] [Google Scholar]
- 27.Moe J, Kirkland SW, Rawe E, Ospina MB, Vandermeer B, Campbell S, et al. Effectiveness of Interventions to Decrease Emergency Department Visits by Adult Frequent Users: A Systematic Review. Acad Emerg Med. 2017;24(1):40–52. doi: 10.1111/acem.13060. [DOI] [PubMed] [Google Scholar]
- 28.Raven MC, Kushel M, Ko MJ, Penko J, Bindman AB. The Effectiveness of Emergency Department Visit Reduction Programs: A Systematic Review. Ann Emerg Med. 2016;68(4):467–83. doi: 10.1016/j.annemergmed.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016;3:CD006560. doi: 10.1002/14651858.CD006560.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soril LJ, Leggett LE, Lorenzetti DL, Noseworthy TW, Clement FM. Reducing frequent visits to the emergency department: a systematic review of interventions. PLoS ONE. 2015;10(4):e0123660. doi: 10.1371/journal.pone.0123660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Elst M, Schoenmakers B, Duppen D, Lambotte D, Fret B, Vaes B, et al. Interventions for frail community-dwelling older adults have no significant effect on adverse outcomes: a systematic review and meta-analysis. BMC geriatr. 2018;18(1):249. doi: 10.1186/s12877-018-0936-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weeks LE, Macdonald M, Martin-Misener R, Helwig M, Bishop A, Iduye DF, et al. The impact of transitional care programs on health services utilization in community-dwelling older adults: a systematic review. JBI Database System Rev Implement Rep. 2018;16(2):345-84. 10.11124/JBISRIR-2017-003486 [DOI] [PubMed]
- 33.Edwards ST, Peterson K, Chan B, Anderson J, Helfand M. Effectiveness of Intensive Primary Care Interventions: A Systematic Review. J Gen Intern Med. 2017;32(12):1377–86. doi: 10.1007/s11606-017-4174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Totten AM, White-Chu EF, Wasson N, Morgan E, Kansagara D, Davis-O’Reilly C, et al.Home-based primary care interventions. Review. Rockville, MD: Agency for Healthcare Research and Quality2016 02 Contract No.: 16. [PubMed]
- 35.Balaban RB, Galbraith AA, Burns ME, Vialle-Valentin CE, Larochelle MR, Ross-Degnan D. A patient navigator intervention to reduce hospital readmissions among high-risk safety-net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–15. doi: 10.1007/s11606-015-3185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boult C, Reider L, Leff B, Frick KD, Boyd CM, Wolff JL, et al. The effect of guided care teams on the use of health services: results from a cluster-randomized controlled trial. Archives of internal medicine. 2011;171(5):460–6. doi: 10.1001/archinternmed.2010.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman EA, Grothaus LC, Sandhu N, Wagner EH. Chronic care clinics: a randomized controlled trial of a new model of primary care for frail older adults. J Am Geriatr Soc. 1999;47(7):775–83. doi: 10.1111/j.1532-5415.1999.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 38.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–8. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 39.Hughes SL, Cummings J, Weaver F, Manheim LM, Conrad KJ, Nash K. A randomized trial of Veterans Administration home care for severely disabled veterans. Med Care. 1990;28(2):135–45. doi: 10.1097/00005650-199002000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Hughes SL, Weaver FM, Giobbie-Hurder A, Manheim L, Henderson W, Kubal JD, et al. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. Jama. 2000;284(22):2877–85. doi: 10.1001/jama.284.22.2877. [DOI] [PubMed] [Google Scholar]
- 41.Lin MP, Blanchfield BB, Kakoza RM, Vaidya V, Price C, Goldner JS, et al. ED-based care coordination reduces costs for frequent ED users. Am J Manag Care. 2017;23(12):762–6. [PubMed] [Google Scholar]
- 42.Linden A, Butterworth S. A comprehensive hospital-based intervention to reduce readmissions for chronically ill patients: a randomized controlled trial. Am J Manag Care. 2014;20(10):783–92. [PubMed] [Google Scholar]
- 43.Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. Jama. 1999;281(7):613–20. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 44.Newcomer R, Maravilla V, Faculjak P, Graves MT. Outcomes of preventive case management among high-risk elderly in three medical groups: a randomized clinical trial. Eval Health Prof. 2004;27(4):323–48. doi: 10.1177/0163278704270011. [DOI] [PubMed] [Google Scholar]
- 45.Parry C, Min S-J, Chugh A, Chalmers S, Coleman EA. Further application of the care transitions intervention: results of a randomized controlled trial conducted in a fee-for-service setting. Home Health Care Serv Q. 2009;28(2-3):84–99. doi: 10.1080/01621420903155924. [DOI] [PubMed] [Google Scholar]
- 46.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. Jama. 2009;301(6):603–18. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 47.Shannon GR, Wilber KH, Allen D. Reductions in costly healthcare service utilization: findings from the Care Advocate Program. J Am Geriatr Soc. 2006;54(7):1102–7. doi: 10.1111/j.1532-5415.2006.00799.x. [DOI] [PubMed] [Google Scholar]
- 48.Shumway M, Boccellari A, O’Brien K, Okin RL. Cost-effectiveness of clinical case management for ED frequent users: results of a randomized trial⋆. Am J Emerg Med. 2008;26(2):155–64. doi: 10.1016/j.ajem.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 49.Sledge WH, Brown KE, Levine JM, Fiellin DA, Chawarski M, White WD, et al. A randomized trial of primary intensive care to reduce hospital admissions in patients with high utilization of inpatient services. Dis Manag. 2006;9(6):328–38. doi: 10.1089/dis.2006.9.328. [DOI] [PubMed] [Google Scholar]
- 50.Zulman DM, Pal Chee C, Ezeji-Okoye SC, Shaw JG, Holmes TH, Kahn JS, et al. Effect of an Intensive Outpatient Program to Augment Primary Care for High-Need Veterans Affairs Patients: A Randomized Clinical Trial. JAMA Intern Med. 2017;177(2):166–75. doi: 10.1001/jamainternmed.2016.8021. [DOI] [PubMed] [Google Scholar]
- 51.Capp R, Misky GJ, Lindrooth RC, Honigman B, Logan H, Hardy R, et al. Coordination program reduced acute care use and increased primary care visits among frequent emergency care users. Health Affairs. 2017;36(10):1705–11. doi: 10.1377/hlthaff.2017.0612. [DOI] [PubMed] [Google Scholar]
- 52.Crane S, Collins L, Hall J, Rochester D, Patch S. Reducing utilization by uninsured frequent users of the emergency department: combining case management and drop-in group medical appointments. J Am Board Fam Med. 2012;25(2):184–91. doi: 10.3122/jabfm.2012.02.110156. [DOI] [PubMed] [Google Scholar]
- 53.Gardner R, Li Q, Baier RR, Butterfield K, Coleman EA, Gravenstein S. Is implementation of the care transitions intervention associated with cost avoidance after hospital discharge? J Gen Intern Med. 2014;29(6):878–84. doi: 10.1007/s11606-014-2814-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamar B, Rula EY, Wells AR, Coberley C, Pope JE, Varga D. Impact of a scalable care transitions program for readmission avoidance. Am J Manag Care. 2016;22(1):28–34. [PubMed] [Google Scholar]
- 55.Meret-Hanke LA. Effects of the program of all-inclusive care for the elderly on hospital use. The Gerontologist. 2011;51(6):774–85. doi: 10.1093/geront/gnr040. [DOI] [PubMed] [Google Scholar]
- 56.Schubert CC, Myers LJ, Allen K, Counsell SR. Implementing Geriatric Resources for Assessment and Care of Elders Team Care in a Veterans Affairs Medical Center: Lessons Learned and Effects Observed. J Am Geriatr Soc. 2016;64(7):1503–9. doi: 10.1111/jgs.14179. [DOI] [PubMed] [Google Scholar]
- 57.Shah R, Chen C, O’Rourke S, Lee M, Mohanty SA, Abraham J. Evaluation of care management for the uninsured. Med Care. 2011;49(2):166–71. doi: 10.1097/MLR.0b013e3182028e81. [DOI] [PubMed] [Google Scholar]
- 58.Sommers LS, Marton KI, Barbaccia JC, Randolph J. Physician, nurse, and social worker collaboration in primary care for chronically ill seniors. Arch Intern Med. 2000;160(12):1825–33. doi: 10.1001/archinte.160.12.1825. [DOI] [PubMed] [Google Scholar]
- 59.Weerahandi H, Basso Lipani M, Kalman J, Sosunov E, Colgan C, Bernstein S, et al. Effects of a Psychosocial Transitional Care Model on Hospitalizations and Cost of Care for High Utilizers. Soc Work Health Care. 2015;54(6):485–98. doi: 10.1080/00981389.2015.1040141. [DOI] [PubMed] [Google Scholar]
- 60.Finkelstein A, Zhou A, Taubman S, Doyle J. Health Care Hotspotting - A Randomized. Controlled Trial. N Engl J Med. 2020;382(2):152–62. doi: 10.1056/NEJMsa1906848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon J, Chang E, Rubenstein LV, Park A, Zulman DM, Stockdale S, et al. Impact of Primary Care Intensive Management on High-Risk Veterans’ Costs and Utilization: A Randomized Quality Improvement Trial. Ann Intern Med. 2018;168(12):846–54. doi: 10.7326/M17-3039. [DOI] [PubMed] [Google Scholar]
- 62.Brown R, Peikes D, Chen A, Ng J, Schore J, Soh C, editors. The evaluation of the Medicare coordinated care demonstration: Findings for the first two years. Second Report to Congress. Mathematica Policy Research, Inc. Princeton, NJ; 2007.
- 63.Wolfe A. Institute of Medicine report: crossing the quality chasm: a new health care system for the 21st century. Policy, Politics, & Nursing Practice. 2001;2(3):233–5. doi: 10.1177/152715440100200312. [DOI] [Google Scholar]
- 64.Elhauge E. The fragmentation of US health care: causes and solutions: Oxford University Press on Demand. 2010. [Google Scholar]
- 65.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rabin BA, McCreight M, Battaglia C, Ayele R, Burke RE, Hess PL, et al. Systematic, Multimethod Assessment of Adaptations Across Four Diverse Health Systems Interventions. Front Public Health. 2018;6:102. doi: 10.3389/fpubh.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wray LO, Szymanski BR, Kearney LK, McCarthy JF. Implementation of primary care-mental health integration services in the Veterans Health Administration: program activity and associations with engagement in specialty mental health services. J Clin Psychol Med Settings. 2012;19(1):105–16. doi: 10.1007/s10880-011-9285-9. [DOI] [PubMed] [Google Scholar]
- 70.Fulton J, Williams J, LeBlanc T, McDuffie J, Porter K, Adam S, et al. Integrated Outpatient Palliative Care in Oncology. VA ESP. 2017. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 145 kb)