Abstract
Purpose
We evaluated the safety, feasibility, and early treatment outcomes of intraoperative radiotherapy (IORT) using a low-energy X-ray source.
Materials and Methods
Patients with resectable pancreatic cancer were enrolled in this single-institution, prospective, single-arm, phase II trial. Patients underwent surgery and IORT with 10 Gy prescribed at a 5-mm depth from the tumor bed using a 50 kV X-ray source (Intrabeam, Carl Zeiss). Six cycles of adjuvant gemcitabine-based chemotherapy were administered 8–12 weeks after surgery.
Results
A total of 41 patients were included. Thirty-one patients (75.6%) underwent wide R0 resection, while 5 (12.2%) underwent R1 resection and 5 (12.2%) underwent narrow R0 resection (retroperitoneal margin <1 mm). Grade 3 postoperative complications were reported in only one patient (4.9%) who needed additional surgery due to ulcer perforation. At a median follow-up of 9 months, four patients showed local-only recurrence, nine had distant metastases, and two showed both local and distant recurrence. The 1-year local control rate was 76.4%.
Conclusion
Our preliminary report suggests that IORT is well-tolerated and feasible in patients with resectable pancreatic cancer. Further follow-up is needed to confirm the clinical benefits of IORT in terms of local control and overall survival. Trial Registration: Clinical trial registration No. (NCT03273374).
Keywords: Pancreatic neoplasms, radiotherapy, postoperative complications, x-ray therapy, recurrence
INTRODUCTION
Curative surgery followed by adjuvant chemotherapy is the standard treatment for patients with resectable pancreatic cancer. However, despite appropriate treatment, survival rates are low, and local recurrence after curative resection is not uncommon.1 The main reason for the high rate of recurrence is that pancreatic cancer patients often have microscopic residual disease.2 Therefore, postoperative external beam radiotherapy (EBRT) has been proposed as a method to improve local control in resectable pancreatic cancer patients.3
Although previous studies have examined the potential therapeutic benefits of EBRT in an adjuvant setting, its use remains limited.4 The delivery of high-dose radiotherapy (RT) to the pancreas is extremely challenging, as there are several radiosensitive abdominal organs around the pancreas. Recent advanced RT techniques, such as intensity-modulated RT, image-guided RT, magnetic resonance-guided RT, and particle therapy, have shown favorable outcomes in pancreatic cancer patients.5,6 Most of these treatments have been administered to patients with unresectable or borderline resectable pancreatic cancer, and the potential clinical use of these treatments in an adjuvant setting has not yet been established.
The use of intraoperative radiotherapy (IORT) for pancreatic cancer was first reported in Japan in the 1980s for patients with locally advanced pancreatic cancer. During a surgical procedure, IORT can be used to deliver a single fraction of high-dose radiation to the tumor bed after the tumor has been removed. IORT has the potential to improve the efficacy of RT for pancreatic cancer by reducing the radiation dose delivered to the adjacent organs and by allowing radiation dose escalation to the tumor bed, thus improving local control of the disease.
The majority of cases in which IORT has been used for the pancreas has involved the administration of electron beams. However, IORT involving electron beams needs to be delivered in an appropriately shielded room, and transferring patients from the operating room to a shielded radiation room increases the risk of contamination and can undermine patient safety. We have been conducting a phase II study assessing the use of IORT in patients with resectable pancreatic cancer using a 50 kV X-ray source (Intrabeam, Carl Zeiss, Germany) and a study protocol that has been previously described.7 Since Intrabeam employs a miniaturized low-energy X-ray source, it can be used to administer IORT in an operating theater. In this preliminary report, we investigated acute postoperative complications and reviewed the early oncologic outcomes of patients with resectable pancreatic cancer undergoing IORT using a low-energy X-ray source.
MATERIALS AND METHODS
Patient selection
This single-institution prospective phase II study was approved by our Institutional Review Board (protocol number: 3-2015-0102) in 2017 and registered at ClinicalTrials.gov (NCT03273374). Patients diagnosed with pancreatic cancer were recruited between August 2017 and September 2019. The eligibility criteria were as follows: 1) age 20 years or older; 2) histologically or clinically confirmed pancreatic carcinoma; 3) Eastern Cooperative Oncology Group (ECOG) performance status scores of 0–2; 4) resectable disease defined as the absence of distant metastases, absence of direct involvement of the inferior vena cava or aorta, and clear fat planes around the celiac axis, hepatic artery, and superior mesenteric artery; 5) stage I–III disease as per the 7th edition of the American Joint Committee on Cancer (AJCC); 6) good bone marrow function (hemoglobin level >10 g/dL, absolute neutrophil count >1500/mm3, platelet count >100000/mm3); and 7) adequate renal function (serum creatinine level <1.4 mg/dL, blood urea nitrogen level <20 mg/dL). Patients who 1) had previously received RT to the abdominal area; 2) had a tumor bed that could not be adequately covered by the IORT field as defined by a radiation oncologist; 3) had received neoadjuvant chemotherapy; 4) had synchronous distant metastasis; 5) were pregnant or nursing; or 6) had any condition rendering them unsuitable for IORT (at the discretion of a physician) were excluded from this study.
Treatment scheme
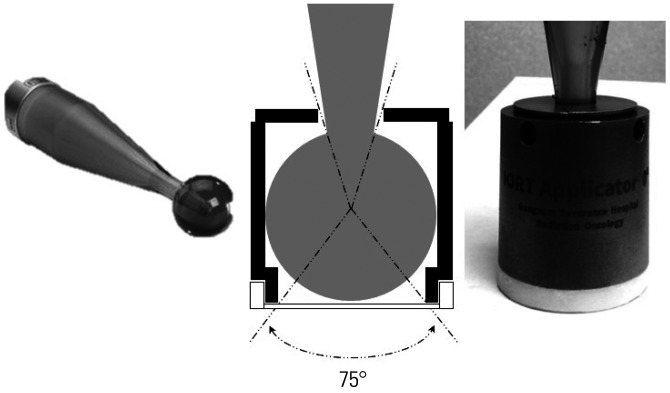
Patients who fulfilled the inclusion criteria and provided written informed consent were assigned to the protocol. Details on the treatment protocol have been described in a previously published study.7 The patients were subjected to curative resection, either pylorus-preserving pancreaticoduodenectomy (PPPD), distal pancreatectomy, or total pancreatectomy. A mobile 50-kV X-ray source was used for IORT. The target volume included the tumor bed, the celiac and superior mesenteric arteries, the mesenteric root, and the portal vein; any areas deemed at risk by the surgeon and radiation oncologist were included as well. A spherical applicator with a diameter of 3.5 cm was used. An additional shielding device was attached to the spherical applicator, leaving only the bottom surface unshielded from which the X-ray beam was delivered to the tumor bed (Fig. 1). The percentage depth dose curve of the shielded applicator is shown in Supplementary Fig. 1 (only online).
Fig. 1. Shielding device of the spherical applicator. Only the bottom surface of the applicator is covered with plastic, while all other parts are shielded by steel use stainless steel.
The target volume was irradiated with a single dose of 10 Gy, prescribed at a 5-mm depth into the tumor bed, resulting in a surface dose of approximately 16 Gy, referring to previous literature.8,9,10 Eight to 12 weeks following surgery, the patients received six cycles of adjuvant gemcitabine-based chemotherapy every 4 weeks. Each chemotherapy cycle consisted of three weekly gemcitabine doses.
Follow-up and analysis
Acute postoperative complications were the primary endpoint of this study; any toxicity occurring within 3 months of surgery was considered an acute toxicity. Delayed gastric emptying was defined and graded according to the International Study Group of Pancreatic Surgery consensus,11 while postoperative pancreatic fistula was defined and graded according to the International Study Group on Pancreatic Fistula consensus.12 Other acute postoperative complications were evaluated using the Clavien-Dindo classification.
Early oncologic outcomes were also investigated. We defined local failure as any failure around the superior mesenteric artery and celiac trunk, including the tumor bed, remnant pancreas, and regional nodes.13 Failures other than local failure were considered as distant failures. Patient survival was determined from the day of surgery.
Kaplan-Meier survival analysis was conducted to evaluate local and distant control. Univariate and multivariate analyses of factors related to local and distant control and overall survival (OS) were conducted using the Cox proportional hazards model. Variables with p<0.1 in univariate analysis were included in the multivariate analysis. P-values<0.05 were considered statistically significant.
RESULTS
Patients and disease characteristics
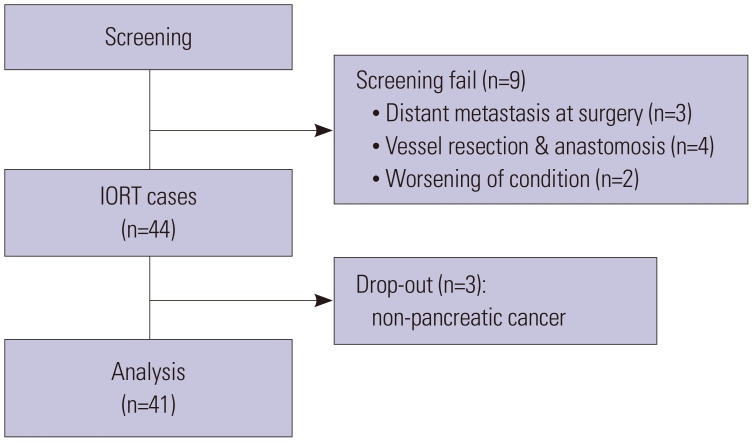
Between November 2017 and August 2019, a total of 53 patients were screened for eligibility. However, nine patients did not fulfill the inclusion criteria: three patients showed peritoneal seeding or liver metastasis during surgery, four underwent superior mesenteric vein or portal vein resection and reconstruction, and two patients’ condition deteriorated during surgery. Thus, a total of 44 patients was initially enrolled in the study. After excluding three patients whose final pathology revealed neoplasms that did not originate from the pancreas, a total of 41 patients was finally included for analysis (Fig. 2).
Fig. 2. Patients selection for this analysis. IORT, intraoperative radiotherapy.
The patient and disease characteristics of the 41 patients included in our analysis are shown in Table 1. The median age of the patients was 66 years (range, 42–84 years), and the cohort consisted of 56.1% male patients and 43.9% female patients. The majority of tumors were located in the pancreatic head or the uncinate process (63.4%). The median serum concentration of carbohydrate antigen 19-9 (CA19-9) was 86 U/mL, and preoperative assessment showed that the median tumor size was 3.0 cm (range, 1.0–8.0 cm). Fifteen (36.6%) patients were pathologically confirmed to have pancreatic cancer before surgery. PPPD, distal pancreatectomy, and total pancreatectomy were performed in 26, 13, and 2 patients, respectively.
Table 1. Patient and Disease Characteristics (n=41).
| Variables | Value | |
|---|---|---|
| Age (yr) | 66 (42–84) | |
| <70 | 23 (56.1) | |
| ≥70 | 18 (43.9) | |
| Sex | ||
| Male | 23 (56.1) | |
| Female | 18 (43.9) | |
| Location | ||
| Head/uncinated process | 26 (63.4) | |
| Body/tail | 15 (36.6) | |
| CEA (ng/mL) | 3.2 (0.8–144.8) | |
| CA19-9 (U/mL) | 86 (0.08–15698.3) | |
| Tumor size (clinical, cm) | 3.0 (1.0–8.0) | |
| Pathologic confirm before surgery | ||
| No | 26 (63.4) | |
| Yes | 15 (36.6) | |
| Types of surgery | ||
| PPPD | 26 (63.4) | |
| Distal pancreatectomy | 13 (31.7) | |
| Total pancreatectomy | 2 (4.9) | |
| Pathological T stage | ||
| T1 | 1 (2.4) | |
| T2 | 23 (56.1) | |
| T3 | 17 (41.5) | |
| Pathological N stage | ||
| N0 | 15 (36.6) | |
| N1 | 16 (39.0) | |
| N2 | 10 (24.4) | |
| AJCC stage (8th) | ||
| I | 9 (22.0) | |
| II | 22 (53.7) | |
| III | 10 (24.4) | |
| Histology | ||
| Adenocarcinoma | 39 (95.1) | |
| Others | 2 (4.9) | |
| LVI | ||
| No | 22 (53.7) | |
| Yes | 19 (46.3) | |
| PNI | ||
| No | 6 (14.6) | |
| Yes | 35 (85.4) | |
| Margin status | ||
| Negative | 36 (87.8) | |
| Positive | 5 (12.2) | |
| Degree of resection | ||
| Wide R0 | 31 (75.6) | |
| Narrow R0 | 5 (12.2) | |
| R1 | 5 (12.2) | |
CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; PPPD, pylorus-preserving pancreaticoduodenectomy; AJCC, American Joint Committee on Cancer; LVI, lymphovascular invasion; PNI, perineural invasion.
Data are presented as median (range) or n (%).
For our analysis, we used the tumor staging system devised by the AJCC 8th edition. More than 40% of patients had tumors larger than 4 cm (T3), and 63.4% of patients had regional lymph node metastasis. Most patients were diagnosed with adenocarcinoma; however, two patients had acinic cell carcinoma of the pancreas. Lymphovascular invasion and perineural invasion were observed in 46.3% and 85.4% of patients, respectively. Resection margins were positive in five patients. Regarding the degree of resection, we defined resections with a retroperitoneal margin of <1 mm as “narrow R0 resections”; five patients underwent R1 resection and five underwent narrow R0 resection.
Perioperative conditions and postoperative complications
The duration of the operation depended on the type of surgery. Distal pancreatectomy had a relatively shorter duration than the other surgeries (Table 2). The average IORT time was 35 minutes and 29 seconds. Eighteen patients, all of whom had undergone PPPD, needed to stay in the intensive care unit for 2 days. In these patients, the median Acute Physiology, Age, Chronic Health Evaluation II (APACHE-II) score was 12 (range, 9–18), which has a predicted hospital mortality rate of 13.4% (range, 6.2–33.2%).
Table 2. Details on Perioperative Conditions.
| Variables | Value | |
|---|---|---|
| Postop complications | 12 (29.3) | |
| ICU stays | 18 (43.9) | |
| APACHE-II score | 12 (9–18) | |
| Predicted hospital mortality, % | 13.4 (6.2–33.2) | |
| Hospital stays after surgery, days | 10 (7–36) | |
| Operating time, min | ||
| PPPD | 409 (249–536) | |
| Distal pancreatectomy | 244 (161–309) | |
| Total pancreatectomy | 449 (449–570) | |
PPPD, pylorus-preserving pancreaticoduodenectomy; ICU, intensive care unit; APACHE-II, Acute Physiology, Age, Chronic Health Evaluation II.
Data are presented as median (range) or n (%).
Ten patients (24.4%) experienced postoperative complications, the details of which are listed in Table 3. The most common complication was delayed gastric emptying, experienced by five patients (13.2%); four of these cases were classified as grade B. Other postoperative complications included postoperative pancreatic fistula, chyle leakage, and duodenal ulcer perforation; most of the postoperative complications were tolerable with conservative management. However, one patient required drainage for postoperative pancreatic fistula of grade B, and one patient needed additional surgery due to duodenal ulcer perforation (G3b). These two patients received PPPD, and they were of old age, 75 and 84 years old, respectively.
Table 3. Postoperative Complications (n=41).
| Checklist | Grade | n (%) |
|---|---|---|
| Delayed Gastric emptying* | A | 1 (2.4) |
| B | 4 (9.8) | |
| Postoperative pancreatic fistula† | A | 1 (2.4) |
| B | 1 (2.4) | |
| Chyle leakage | 2 | 2 (4.9) |
| Duodenal ulcer perforation | 3b | 1 (2.4) |
Other acute postoperative complications were evaluated using Clavien-Dindo classification.
*Delayed gastric emptying was graded according to the International Study Group of Pancreatic Surgery consensus definition; †We use the consensus of International Study Group on Pancreatic Fistula (ISGPF) for the definition and grading of postoperative pancreatic fistula.
Two patients did not receive adjuvant gemcitabine chemotherapy at our institution (4.8%): one patient was in a poor condition due to early liver metastases, and the other required reconstructive surgery due to ulcer perforation and received chemotherapy at another hospital. Two patients started receiving adjuvant chemotherapy 13 weeks after the surgery due to their general conditions, while the remaining patients started receiving adjuvant chemotherapy between 8 and 12 weeks after the surgery as per the treatment protocol.
Treatment outcomes
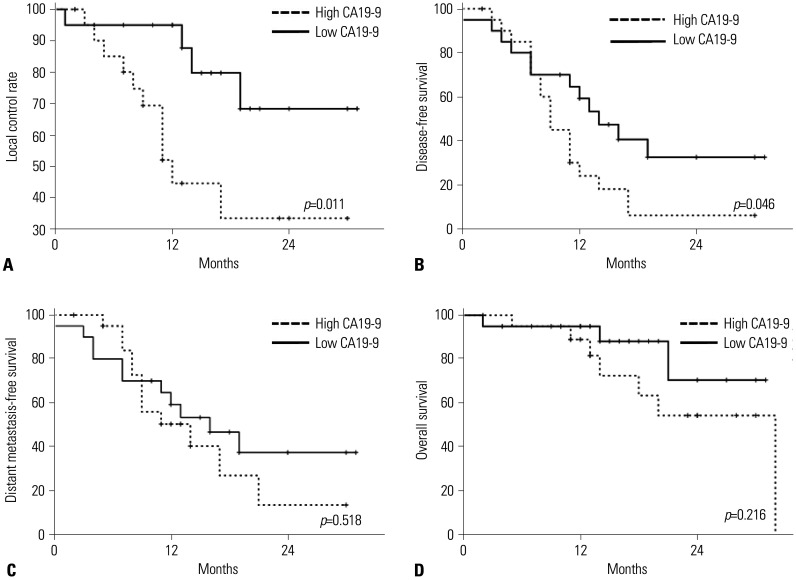
The median follow-up duration was 9 months (range, 1–21 months). Twenty (47.6%) patients had a follow-up duration shorter than 9 months, and 14 (33.3%) had a follow-up duration shorter than 6 months. Five patients died less than a year after treatment, resulting in a 1-year OS rate of 94.1%. The patterns of the first recurrence were as follows: four patients, local-only failure (9.8%); nine, distant-only failure (22.0%); and two, both local and distant failure (4.8%). The 1-year local control and distant control rates were 76.4% and 55.7%, respectively. The survival analysis according to the initial CA19-9 level (with a median CA19-9 of 86 U/mL, high CA19-9 ≥86 U/mL vs. low CA19-9 <86 U/mL) were shown in Fig. 3. Patients with low CA19-9 level showed significantly better local and distant control. Patients characteristics (Supplementary Table 1, only online) and patterns of failure (Supplementary Table 2, only online) according to the CA19-9 level was described in supplementary data.
Fig. 3. Kaplan-Meir survival analysis according to the initial CA19-9 level. (A) Local control rate. (B) Disease-free survival. (C) Distant metastasis-free survival. (D) Overall survival. CA19-9, carbohydrate antigen 19-9.
The prognostic factors for local and distant failure and OS are listed in Table 4. Pathologic N2 stage was significantly associated with local failure in multivariate analysis [hazard ratio (HR) 6.51; 95% confidence interval (CI) 1.23–34.46; p=0.027]. Lymphovascular invasion was identified as a prognostic factor for distant metastasis in the multivariate analysis (HR, 3.89; 95% CI, 1.28–11.82; p=0.016). Only LVI was significantly associated with OS in univariate analysis; therefore, multivariate analysis for OS was not conducted.
Table 4. Prognostic Factors for Local, Distal Failure, and Overall Survival.
| Local failure | Distant failure | Overall survival | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | ||||||||||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age | ≥70 (vs. <70) | 0.84 | 0.30–2.35 | 0.732 | 1.08 | 0.29–4.05 | 0.903 | ||||||||||||
| Location | Others (vs. head) | 1.32 | 0.46–3.75 | 0.607 | 2.54 | 0.67–9.60 | 0.169 | ||||||||||||
| Initial CA19-9 | ≥86 (vs. <86) | 1.03 | 1.26–12.85 | 0.019 | 4.12 | 1–17.04 | 0.050 | 2.05 | 0.97–4.332 | 0.060 | 2.17 | 0.275 | |||||||
| pT | T3 (vs. T1, 2) | 2.85 | 1.01–8.03 | 0.048 | 2.51 | 0.75–8.44 | 0.135 | 7.10 | 2.75–18.36 | <0.001 | 1.79 | 0.80–4.00 | 0.159 | 1.06 | 0.28–3.98 | 0.929 | |||
| pN | N2 (vs. N0, 1) | 4.06 | 1.13–14.65 | 0.032 | 6.51 | 1.23–34.46 | 0.027 | 3.35 | 1.47–7.65 | 0.004 | 2.40 | 0.93–6.23 | 0.071 | 70.62 | 0.30–1653.98 | 0.126 | |||
| LVI | Positive (vs. negative) | 3.60 | 1.22–100.64 | 0.020 | 1.64 | 0.46–5.33 | 0.445 | 7.10 | 2.75–18.36 | <0.001 | 3.89 | 1.28–11.82 | 0.016 | 7.31 | 1.47–36.25 | 0.015 | |||
| PNI | Positive (vs. negative) | 28.84 | 0.12–705.75 | 0.230 | 32.88 | 0.87–1442.20 | 0.059 | 26.54 | 0.01–588.60 | 0.401 | |||||||||
| Degree of resection | R1 or narrow R0 (vs. wide R0) | 2.71 | 0.79–9.30 | 0.112 | 2.55 | 1.07–6.09 | 0.035 | 1.37 | 0.51–0.37 | 0.538 | 2.86 | 0.54–15.17 | 0.218 | ||||||
HR, hazard ratio; CI, confidence interval; CA19-9, carbohydrate antigen 19-9; LVI, lymphovascular invasion; PNI, perineural invasion.
DISCUSSION
IORT using a low-energy 50-kV X-ray source was well-tolerated in pancreatic cancer patients. Even though our study included a large number of patients older than 70 years who underwent high-risk surgery, the mortality rate was 0%, and most postoperative complications were classified as grade 2 or less. These results are concordant with those of previous studies on IORT using electron beams that reported that IORT did not increase perioperative morbidity.14,15
Most pancreatic cancer patients undergo surgical resection as a part of disease management. However, one-third of the patients experience at least one postoperative complication, and complications of grade 3 or higher occur in up to 20% of patients.16 Although recent large-scale studies have demonstrated that postoperative mortality rates are less than 6%, pancreatectomy is one a high-risk surgery that often results in poor patient outcomes.17,18 In particular, postoperative complications may result in omission of or delay in adjuvant treatment.19 A previous study also demonstrated that complications of grade 3 or higher after pancreatectomy have a substantial impact on long-term survival.20 Therefore, establishment of treatment strategies that improve local control without increasing postoperative complications is of high priority. Although adjuvant chemotherapy was delayed in two patients in this study, the majority of patients (90.5%) started receiving chemotherapy between 8 and 12 weeks after surgery.
There were only two cases of local-only recurrence in our preliminary data, and the patients showed a 1-year local control rate of 76.4%. Ogawa, et al.21 used IORT in a Japanese multicenter retrospective trial; the 2-year local control rate was 83.7%, which is superior to that reported herein. However, in our preliminary data, one-third of the patients had a follow-up duration shorter than 6 months. Thus, the Kaplan-Meier survival analysis results should be interpreted with caution and after careful consideration of the impact of censored data. Further follow-up of our patients might lead to outcomes similar to those in the Japanese report.
Additionally, 30% of patients included in the Japanese study of Ogawa, et al.21 received EBRT as an adjuvant treatment. Other studies on pancreatic IORT also involved preoperative or postoperative EBRT.22,23 Whether additional EBRT could improve oncologic outcomes remains unclear. In some cases, residual tumors could not be covered sufficiently with IORT due to a rapid decrease in the dose of X-ray or electrons. Moreover, the surface of the tumor bed was irregular; thus, there is a possibility that the applicator did not cover the whole tumor bed. We found that high CA19-9 levels, lymph node metastasis, and narrow R0/R1 resection were significantly associated with local recurrence even after IORT. In these cases, the addition of EBRT to the treatment strategy may overcome the limitations of IORT. Moreover, neoadjuvant chemoradiotherapy has been found to improve oncologic outcome in several studies.24,25,26 A further prospective study assessing the potential benefits of adding preoperative or postoperative EBRT should be conducted.
There is no clear evidence regarding the safety of IORT using kV X-rays, and the optimal radiation dose has not been established. In a previous study involving the use of an orthovoltage X-ray beam, only an average of 11.1 Gy was delivered: the study reported that three patients (13% of all patients, n=23) experienced treatment-related complications of grade 3 or higher.9 Our preliminary results indicate that IORT with 10 Gy at a 5-mm depth does not increase postoperative complications. Establishment of an optimal radiation dose in X-ray IORT through dose-escalation studies is essential, as this could improve local control rates.
Additionally, neoadjuvant treatment has been suggested even for resectable tumors to improve disease control.27,28 A recent randomized phase II/III trial showed a significant survival benefit for neoadjuvant gemcitabine-S1 treatment in resectable pancreatic cancer patients.28 A combination of neoadjuvant chemotherapy with IORT might improve local control and OS. A prospective trial assessing the clinical benefit of neoadjuvant chemotherapy followed by IORT for resectable pancreatic cancer will be conducted at our institution in the near future.
Among the patients who agreed to receive IORT, four underwent vessel resections and reconstructions, and IORT was not delivered to these patients at the discretion of the physicians. It has been reported that postoperative RT does not increase morbidity in terms of stability after vessel reconstruction or wound healing.29 However, in most studies, EBRT was administered several weeks after surgery, and currently, there is no report on the safety of RT immediately following vessel reconstruction. Therefore, we decided not to administer IORT to these patients. Additional local treatment may be helpful in such cases since patients requiring vessel resection often have locally advanced disease.30
There are several limitations to this study. First, due to the short-term follow-up duration, the late toxicities of IORT using kV X-rays could not be evaluated. Previous studies have reported late toxicity in only 3% of patients and reported that IORT with doses of approximately 25 Gy are generally well tolerated.21,31 Additionally, due to the short-term follow-up, it is difficult to draw reliable and valid conclusions with regard to the treatment outcomes. Second, since we did not perform a randomized control study comparing patients undergoing IORT and surgery with patients undergoing surgery alone, we could not reach a conclusion on the potential superiority of IORT plus surgery over surgery only in terms of postoperative complications or treatment outcomes. Moreover, since most patients in this study received adjuvant chemotherapy, it is difficult to judge whether IORT has brought about benefits from this study alone. Therefore, in subsequent studies, we plan to compare the results with the further follow-up of the treatment outcomes of patients who received only postoperative adjuvant chemotherapy, which was not included in this study. However, to the best of our knowledge, the current study is the first prospective study to report early outcomes of IORT using kV X-ray for pancreatic cancer, providing a reference for future studies to establish proper protocols of IORT for resectable pancreatic cancer.
In conclusion, this preliminary report demonstrated that IORT is well-tolerated and feasible in patients with resectable pancreatic cancer and does not cause significant postoperative complications. Our results also suggested that IORT using kV X-rays might yield favorable outcomes, concordant with the results of previous studies involving IORT with electron beams. Future prospective randomized trials comparing the current standard of care for pancreatic cancer with or without IORT are required.
ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Republic of Korea (2017R1D1A1B03035047).
This work was also supported by a National Research Foundation of Korea grant funded by the Korean government (No. NRF-2019R1A2C1085958 and NRF-2018R1D1A1B07048234).
This study was supported by the 2020 Research Grant of Gangnam Severance Hospital Research Committee.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Ik Jae Lee, Joon Seong Park, and Jun Won Kim.
- Data curation: Yeona Cho and Hyung Sun Kim.
- Formal analysis: Yeona Cho.
- Methodology: Yeona Cho and Hyung Sun Kim.
- Project administration: Jun Won Kim, Joon Sung Park, and Ik Jae Lee.
- Writing—original draft: Yeona Cho and Jun Won Kim.
- Writing—review & editing: Jun Won Kim.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
The percent depth dose (PDD) of applicator (A) and longitudinal central axis dose distribution of IORT (B). IORT, intraoperative radiotherapy.
Comparison of Patient Characteristics between Low CA19-9 and High CA19-9 Groups
Patterns of Failure according to Initial CA19-9
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Griffin JF, Smalley SR, Jewell W, Paradelo JC, Reymond RD, Hassanein RE, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Rich TA, Evans DB, Curley SA, Ajani JA. Adjuvant radiotherapy and chemotherapy for biliary and pancreatic cancer. Ann Oncol. 1994;5 Suppl 3:75–80. doi: 10.1093/annonc/5.suppl_3.s75. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 5.Boldrini L, Cusumano D, Cellini F, Azario L, Mattiucci GC, Valentini V. Online adaptive magnetic resonance guided radiotherapy for pancreatic cancer: state of the art, pearls and pitfalls. Radiat Oncol. 2019;14:71. doi: 10.1186/s13014-019-1275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reese AS, Lu W, Regine WF. Utilization of intensity-modulated radiation therapy and image-guided radiation therapy in pancreatic cancer: is it beneficial? Semin Radiat Oncol. 2014;24:132–139. doi: 10.1016/j.semradonc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Kim JW, Cho Y, Kim HS, Choi WH, Park JS, Lee IJ. A phase II study of intraoperative radiotherapy using a low-energy x-ray source for resectable pancreatic cancer: a study protocol. BMC Surg. 2019;19:31. doi: 10.1186/s12893-019-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roeder F, Timke C, Saleh-Ebrahimi L, Schneider L, Hackert T, Hartwig W, et al. Clinical phase I/II trial to investigate neoadjuvant intensity-modulated short term radiation therapy (5×5 Gy) and intraoperative radiation therapy (15 Gy) in patients with primarily resectable pancreatic cancer-NEOPANC. BMC Cancer. 2012;12:112. doi: 10.1186/1471-2407-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachireddy P, Tseng D, Horoschak M, Chang DT, Koong AC, Kapp DS, et al. Orthovoltage intraoperative radiation therapy for pancreatic adenocarcinoma. Radiat Oncol. 2010;5:105. doi: 10.1186/1748-717X-5-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfieri S, Morganti AG, Di Giorgio A, Valentini V, Bossola M, Trodella L, et al. Improved survival and local control after intraoperative radiation therapy and postoperative radiotherapy: a multivariate analysis of 46 patients undergoing surgery for pancreatic head cancer. Arch Surg. 2001;136:343–347. doi: 10.1001/archsurg.136.3.343. [DOI] [PubMed] [Google Scholar]
- 11.Malleo G, Crippa S, Butturini G, Salvia R, Partelli S, Rossini R, et al. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: validation of International Study Group of pancreatic surgery classification and analysis of risk factors. HPB (Oxford) 2010;12:610–618. doi: 10.1111/j.1477-2574.2010.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10:511–518. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Krempien R, Roeder F. Intraoperative radiation therapy (IORT) in pancreatic cancer. Radiat Oncol. 2017;12:8. doi: 10.1186/s13014-016-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reni M, Panucci MG, Ferreri AJ, Balzano G, Passoni P, Cattaneo GM, et al. Effect on local control and survival of electron beam intraoperative irradiation for resectable pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2001;50:651–658. doi: 10.1016/s0360-3016(01)01470-5. [DOI] [PubMed] [Google Scholar]
- 16.Kneuertz PJ, Pitt HA, Bilimoria KY, Smiley JP, Cohen ME, Ko CY, et al. Risk of morbidity and mortality following hepato-pancreatobiliary surgery. J Gastrointest Surg. 2012;16:1727–1735. doi: 10.1007/s11605-012-1938-y. [DOI] [PubMed] [Google Scholar]
- 17.McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill JS, McPhee JT, Whalen GF, Sullivan ME, Warshaw AL, Tseng JF. In-hospital mortality after pancreatic resection for chronic pancreatitis: population-based estimates from the nationwide inpatient sample. J Am Coll Surg. 2009;209:468–476. doi: 10.1016/j.jamcollsurg.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372–377. doi: 10.1097/SLA.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 20.Kamphues C, Bova R, Schricke D, Hippler-Benscheidt M, Klauschen F, Stenzinger A, et al. Postoperative complications deteriorate long-term outcome in pancreatic cancer patients. Ann Surg Oncol. 2012;19:856–863. doi: 10.1245/s10434-011-2041-4. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa K, Karasawa K, Ito Y, Ogawa Y, Jingu K, Onishi H, et al. Intraoperative radiotherapy for resected pancreatic cancer: a multi-institutional retrospective analysis of 210 patients. Int J Radiat Oncol Biol Phys. 2010;77:734–742. doi: 10.1016/j.ijrobp.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Kokubo M, Nishimura Y, Shibamoto Y, Sasai K, Kanamori S, Hosotani R, et al. Analysis of the clinical benefit of intraoperative radiotherapy in patients undergoing macroscopically curative resection for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2000;48:1081–1087. doi: 10.1016/s0360-3016(00)00673-8. [DOI] [PubMed] [Google Scholar]
- 23.Valentini V, Morganti AG, Macchia G, Mantini G, Mattiucci GC, Brizi MG, et al. Intraoperative radiation therapy in resected pancreatic carcinoma: long-term analysis. Int J Radiat Oncol Biol Phys. 2008;70:1094–1099. doi: 10.1016/j.ijrobp.2007.07.2346. [DOI] [PubMed] [Google Scholar]
- 24.Nagakawa Y, Sahara Y, Hosokawa Y, Murakami Y, Yamaue H, Satoi S, et al. Clinical impact of neoadjuvant chemotherapy and chemoradiotherapy in borderline resectable pancreatic cancer: analysis of 884 patients at facilities specializing in pancreatic surgery. Ann Surg Oncol. 2019;26:1629–1636. doi: 10.1245/s10434-018-07131-8. [DOI] [PubMed] [Google Scholar]
- 25.Vidri RJ, Vogt AO, Macgillivray DC, Bristol IJ, Fitzgerald TL. Better defining the role of total neoadjuvant radiation: changing paradigms in locally advanced pancreatic cancer. Ann Surg Oncol. 2019;26:3701–3708. doi: 10.1245/s10434-019-07584-5. [DOI] [PubMed] [Google Scholar]
- 26.Hirono S, Kawai M, Okada KI, Miyazawa M, Shimizu A, Kitahata Y, et al. Treatment strategy for borderline resectable pancreatic cancer with radiographic artery involvement. Pancreas. 2016;45:1438–1446. doi: 10.1097/MPA.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 27.Artinyan A, Anaya DA, McKenzie S, Ellenhorn JD, Kim J. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer. 2011;117:2044–2049. doi: 10.1002/cncr.25763. [DOI] [PubMed] [Google Scholar]
- 28.Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05) Jpn J Clin Oncol. 2019;49:190–194. doi: 10.1093/jjco/hyy190. [DOI] [PubMed] [Google Scholar]
- 29.Choi S, Schwartz DL, Farwell DG, Austin-Seymour M, Futran N. Radiation therapy does not impact local complication rates after free flap reconstruction for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:1308–1312. doi: 10.1001/archotol.130.11.1308. [DOI] [PubMed] [Google Scholar]
- 30.Chua TC, Saxena A. Extended pancreaticoduodenectomy with vascular resection for pancreatic cancer: a systematic review. J Gastrointest Surg. 2010;14:1442–1452. doi: 10.1007/s11605-009-1129-7. [DOI] [PubMed] [Google Scholar]
- 31.Sindelar WF, Kinsella TJ. Normal tissue tolerance to intraoperative radiotherapy. Surg Oncol Clin N Am. 2003;12:925–942. doi: 10.1016/s1055-3207(03)00087-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The percent depth dose (PDD) of applicator (A) and longitudinal central axis dose distribution of IORT (B). IORT, intraoperative radiotherapy.
Comparison of Patient Characteristics between Low CA19-9 and High CA19-9 Groups
Patterns of Failure according to Initial CA19-9