Abstract
Context
Chronic obstructive lung disease (COPD) is a progressive lung disease characterized by persistent airflow limitation. An increasing amount of evidence suggests an effect of dietary quality on the risk of COPD in the general population and pulmonary function decline in patients with COPD.
Objective
The association of dietary intake and nutrient status with COPD risk and onset, as well as pulmonary function decline (change in forced expiratory volume in 1 second, forced vital capacity, or the ratio of the former to the latter) in patients with COPD was investigated in this systematic review.
Data Sources
The PubMed database was searched by combining terms of pulmonary function or COPD with diet, nutrient status, or nutritional supplementation.
Data Extraction
Original studies and systematic reviews and meta-analyses were included. Articles obtained were independently screened for relevance on the bases of title and abstract by 2 researchers. Eventually, 89 articles were included in the analysis.
Results
The unhealthy Western-style diet is associated with an increased risk of COPD and an accelerated decline of pulmonary function. Intake of fruit, vegetables, dietary fibers, vitamins C and E, polyphenols, and β-carotene were individually associated with lower COPD risk, whereas consumption of processed meat was associated with higher COPD risk. Data on the effect of dietary quality on pulmonary function decline in patients with COPD are limited and inconsistent. Strong evidence for beneficial effects on pulmonary function decline was found only for vitamin D supplementation.
Conclusion
Considering the increasing burden of COPD, more attention should be given to dietary quality as a modifiable factor in disease development and progression in patients with COPD.
Systematic Review Registration
PROSPERO registration no. CRD42021240183.
Keywords: COPD, nutrition, progression, pulmonary function, risk
INTRODUCTION
Chronic obstructive pulmonary disease (COPD), cardiovascular disease, and diabetes are the most common chronic diseases worldwide.1,2 A poor diet is a modifiable risk factor for cardiovascular disease and diabetes.3 However, the association between diet and COPD onset and decline in pulmonary function is less established. COPD is characterized by persistent airflow limitation and respiratory symptoms,4 resulting from thickening of the airway walls causing increased mucus production (chronic bronchitis) or destruction of the alveolar walls (emphysema).5,6 Prominent respiratory symptoms include dyspnea, cough, and sputum production. Some patients with COPD have frequent exacerbations, defined as acute events that include pulmonary infections and/or respiratory failure that result in worsening of the respiratory symptoms, which may require hospitalization and often aggravate the decline of pulmonary function.7 Smoking tobacco and air pollution are risk factors for the onset and progression of COPD. There is also increasing evidence for poor diet and malnutrition as additional risk factors.8,9
In a Dutch study of patients with COPD, researchers found that, overall, patients consumed a typical Western diet characterized by low intake of vitamins, fruits, vegetables, and fish and high intakes of saturated fat, cured meat, and refined grains.10 In COPD management, nutrition and metabolism have been topics of scientific research for decades, but that research primarily has been focused on advanced disease, caloric and protein requirements, and extrapulmonary disease progression. Airway and systemic inflammation appear to be associated with disease progression.11,12 Along with inflammation, oxidative stress in lung tissue is enhanced while the antioxidant capacity is substantially depleted due to overproduction of reactive oxygen species as a result of tobacco smoking, air pollution, and acute exacerbations.13 Healthy dietary patterns and specific nutrient intake can attenuate pulmonary and related systemic inflammation as well as oxidative stress associated with COPD.14
Hence, dietary intake may be beneficial for reducing the risk of COPD onset and for decelerating the rate of pulmonary function decline in patients with COPD.15 However, a clear overview of the existing evidence for this notion is lacking. Therefore, our objective for this systematic review was to compile and evaluate available evidence regarding the association between dietary intake and plasma or serum nutrient status on the one hand, with the risk and onset of COPD, as well as the progression of pulmonary function decline in COPD on the other.
METHODS
Data source and search strategy
This systematic review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA; see Supplemental Table S1 in the Supporting Information online).16 A systematic search was conducted of the PubMed database on January 26, 2021, to find relevant articles. The search strategy consisted of terms referring to pulmonary function and COPD, combined with diet, nutrient status, and nutritional supplementation (see Supplemental Appendix 1 in the Supporting Information online for the exact search strategy) and was limited to English written publications of clinical studies; experimental and animal studies were excluded. No time limits were included in the search strategy; PubMed was systematically searched for relevant articles up to January 26, 2021. Observational and interventional studies were included (Table 1). In addition, reference lists of included papers were screened for additional publications.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Inclusion criteria |
|---|---|
| Population | Patients with COPD |
| General population | |
| Intervention/correlate | Dietary intake |
| Nutrient status | |
| Nutritional supplementation | |
| Comparison | Placebo (only applicable for randomized controlled trials) |
| Outcome | Risk of COPD |
| Pulmonary function (FEV1, FVC, or FEV1/FVC) | |
| Incidence of COPD | |
| Study design | Cross-sectional studies |
| Case-control studies | |
| Cohort studies | |
| Randomized controlled trials | |
| Systematic reviews/meta-analysis |
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Study selection and data extraction
Articles identified from the PubMed search were independently screened by 2 researchers (LE.J.v.I. and R.J.H.C.G.B.) for relevance based on title and abstract. Subsequently, relevant full-text articles were assessed for eligibility. Studies were eligible for inclusion if they addressed the association between the risk, onset, or progression of COPD with pulmonary outcome measures (ie, pulmonary function and COPD risk or incidence) on the one hand, and diet, nutrient status, or nutritional supplementation on the other. With respect to nutrient status, only articles reporting nutrient levels in blood were included (papers reporting nutritional status reflecting body composition were excluded). Influence of caloric intake on COPD risk or progression was not examined in this systematic review, because these studies mainly focused on abnormal body composition, not pulmonary function. Original studies as well as systematic reviews/meta-analyses were included. Original studies already summarized in ≥ 1 of the included systematic reviews or meta-analyses (n = 56) were excluded from this review to avoid overrepresentation. Because we included original studies as well as systematic reviews and meta-analyses, the following criteria, adjusted from predefined guidelines and criteria,17–20 were used to draw conclusions from the included studies: A conclusion of “very strong evidence” was drawn if ≥ 1 systematic review or meta-analysis and/or > 5 original studies reported consistent results. Evidence was considered “strong” if > 3 studies reported consistent results. If < 3 studies reported consistent results, evidence was considered “limited.” When contrary results were reported in studies, evidence was considered “inconsistent.” A conclusion of “no evidence” was drawn if no studies were found.
RESULTS
Search results
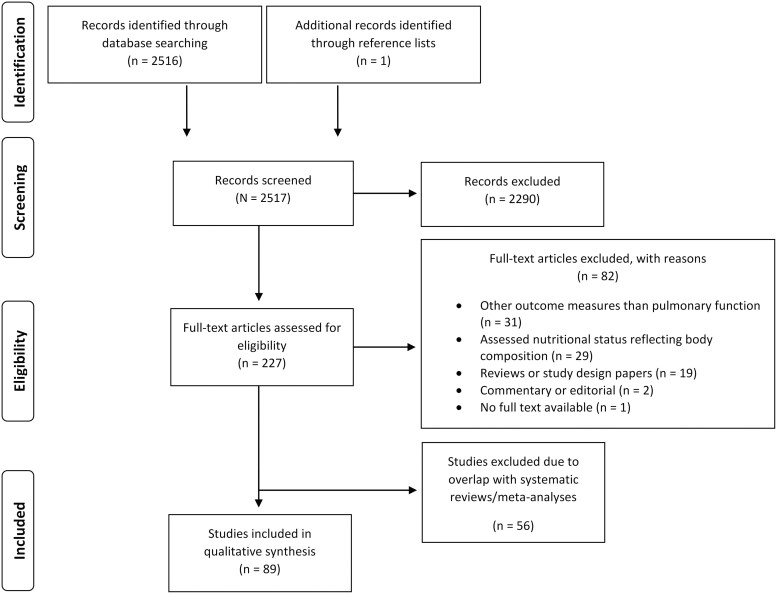
In total, the PubMed search yielded 2517 hits that were assessed for relevance (Figure 1). Subsequently, the full texts of 227 articles were reviewed and, eventually, 89 articles were included on the basis of predefined criteria. A detailed overview of characteristics of the included studies is provided in Table S2 in the Supporting Information online.
Figure 1.
PRISMA flowchart for selection of included studies.
Dietary patterns
Generally, 2 dietary patterns are distinguished in nutritional epidemiology. The Western-style diet is characterized by high dietary intake of red and/or processed meats, refined grains, sweets, desserts, and French fries and is generally considered unhealthy.21 The Mediterranean-style diet is characterized mainly by high dietary intake of fruit, vegetables, fish, and whole-grain products and is often considered prudent or healthy. The Healthy Eating Index–2005 is commonly used to assess adherence to dietary guidelines and to score how healthy a diet is.22 A meta-analysis showed that the risk of COPD was higher in the highest category than in the lowest category of the unhealthy dietary pattern and, for the healthy/prudent dietary pattern, the risk of COPD was lower in the highest than in the lowest category.21 In 2 cross-sectional studies focusing on women, impaired pulmonary function, defined as forced vital capacity (FVC) < 80% and/or forced expiratory volume in 1 second (FEV1) < 80% of predicted value and/or the ratio of FEV1 to FVC < 0.7, was positively associated with the Western-style diet, but no association was found with the Mediterranean-style diet.23,24 In a case-control study, men and women with medium or high adherence to a Mediterranean-style diet were, in a dose-response manner, at a significantly lower risk for development of COPD than those with low adherence.25 Furthermore, an overall healthy diet, assessed by the Healthy Eating Index–2005, was positively associated with the ratio of FEV1 to FVC in cross-sectional analyses of the general population.26
Data indicating that healthy dietary patterns also positively modulate progression of pulmonary function decline in patients diagnosed with COPD are scarce. In a longitudinal analysis, researchers found that consumption of foods from a healthy diet (defined as fruit, fish, tea, and dairy products) reflected significant higher levels of FEV1 and FVC in patients with COPD and less decline over a 3-year period.27 Moreover, a positive association was observed between the Mediterranean-style diet and FEV1 and FVC among patients with COPD in a cross-sectional study, indicating that a healthier diet is reflected in higher levels of pulmonary function.28
Thus, there is very strong evidence for an increased risk for development of COPD when an unhealthy Western-style diet is consumed, whereas adherence to a prudent or Mediterranean-style diet decreases that risk. The potential of diet to modulate pulmonary function decline in patients diagnosed with COPD is less well studied because long-term cohort studies in patients with COPD that include dietary data are scarce. Furthermore, to our knowledge, no clinical trials have investigated the effect of a shift toward a healthy dietary pattern.
Processed meat
A meta-analysis including 5 prospective cohort studies involving individuals of the general population29 and a large longitudinal cohort study including middle-aged women30 both showed that consumption of processed meat elevated the risk for development of COPD. These findings correspond with results of a cross-sectional study in the general US population31 that showed a negative association between processed meat intake and FEV1 and FVC. Therefore, it can be concluded that there is very strong evidence that increased consumption of processed meat is negatively associated with pulmonary function in the general population and, hence, an increased risk of COPD. No studies on the effects of processed meat intake on acceleration of pulmonary function impairment in patients with COPD were found; adequate longitudinal studies are required in cohorts of patients with COPD.
Alcohol
The influence of alcohol consumption on the risk of COPD or rate of pulmonary function decline was investigated in 7 cross-sectional23,32–37 and 2 prospective cohort studies38,39 in the general population. It was reported that lifetime alcohol consumption and heavy drinking were predictors of lower FEV1 levels in the general population23,32,33 and that high alcohol consumption (≥350 g/wk) significantly accelerated the decline of FEV1 over 5 years.38 In contrast, no correlation between alcohol consumption and FEV1 and FEV1 percent predicted was observed in 2 other cross-sectional studies at baseline and after 5 years of follow-up.34,35 It was also shown that FEV1 was lower in nondrinkers of alcohol than in light drinkers.37 Strikingly, low to moderate levels of alcohol drinking, especially of beer and wine, was associated with a lower risk and fewer new cases of COPD over 20 years of follow-up,36,39 in contrast to former heavy drinkers, who had significantly increased odds of development of pulmonary obstruction.36
Thus, evidence on alcohol consumption relative to COPD is inconsistent. Heavy drinking is associated with pulmonary function decline, whereas mild to moderate consumption may have protective effects in the general population. For the effect of alcohol consumption on pulmonary function decline in patients already diagnosed with COPD, we found no available studies; therefore, adequate longitudinal cohort studies are required.
Fruit and vegetables
The association between fruit and vegetable intake and the risk of COPD was quantitatively evaluated in a meta-analysis including 8 studies that involved 5787 patients with COPD among 244 154 participants from the general population.40 A significantly lower risk of COPD was observed related to higher fruit and/or vegetable intake. Furthermore, in a cross-sectional analysis, fruit intake was associated with higher FEV1 and lower COPD risk, whereas no such associations were found for vegetable intake.41 A cross-sectional study showed that both fruit and vegetable consumption were positively associated with FEV1 and FVC in the general population,31 and a longitudinal study in middle-aged adults showed that especially higher intake of tomato was associated with an attenuated decline in FVC over 10 years but not in FEV1.42
In several studies, patients with COPD had a significantly lower intake of fruit and vegetables than did healthy individuals.43–45 Also, in a randomized controlled trial (RCT), a dietary shift to a diet rich in fruit and vegetables was associated with higher FEV1 percent predicted in patients with COPD compared with a diet free of choice.46
Overall, there is very strong evidence that dietary fruit and vegetable consumption is associated with a lower risk of COPD in the general population. However, studies on the effect of fruit and vegetable intake on pulmonary function decline in patients diagnosed with COPD are still limited.
Polyphenols
A positive association between polyphenol intake and FEV1 and FVC was reported in a cross-sectional analysis of a general population.47 Total intake of catechins, flavonols, and flavones specifically was positively associated with FEV1.48 Moreover, greater anthocyanin intake significantly attenuated the annual decline in FEV1 and FVC in a longitudinal analysis of men in the United States.49 Furthermore, more frequently drinking green tea, which is rich in polyphenols, was associated with a decreased prevalence of COPD in a cross-sectional analysis of a general population50 and decreased incidence in older adults in a prospective cohort study after 4.5 years of follow-up.51
Likewise, intake of the dietary polyphenol resveratrol was positively associated with FVC in a cross-sectional analysis, but no significant longitudinal change was observed.52 Remarkably, only intake of white wine, which contains much lower levels of resveratrol than red wine, was associated with higher FEV1 levels and lower risk of airway obstruction, whereas no association was found with red wine intake. Dietary total antioxidant capacity was positively associated with FEV1 and FVC in a cross-sectional study of a general population, as well as in premenopausal women or women who never smoked.31,53 In patients with COPD, total antioxidant capacity in blood appeared to be lower than in healthy control subjects.45 A RCT including stable patients with COPD showed that supplementation with antioxidant polyphenol-rich pomegranate juice for 5 weeks had no beneficial effects on any pulmonary function parameter.54
In summary, very strong evidence is available showing positive effects of polyphenols on the risk of COPD. To date, however, limited evidence is available on the effect of polyphenol intake on pulmonary function impairment in patients diagnosed with COPD. Thus, there is a need for RCTs with long-term follow-up to evaluate the effect of polyphenol supplementation on pulmonary function decline.
Vitamin D
Higher dietary intakes of vitamin D were associated with higher FEV1 and lower prevalence of COPD in a cross-sectional analysis of a general population.55 Increased odds of COPD development were reported in a cross-sectional analysis in smokers with a serum 25(OH)D concentration <50 nmol/L compared with smokers with a serum concentration ≥50 nmol/L.56 Vitamin D deficiency was associated with decreased FEV1 and FVC in the Korean population,57 and in another study, researchers reported this association only in men without medical conditions, and not in women.58 Furthermore, 6 cross-sectional59–64 studies reported a positive association between serum 25(OH)D concentrations and FEV1 in a general population, healthy individuals, and adolescents, but no longitudinal change in FEV1 was observed in healthy individuals.63 In addition, in prospective analyses, lower 25(OH)D concentrations were associated with a faster decline rate of FEV1 over 20 years and higher risk of COPD development.64 Although, together, the results of these studies indicate there is very strong evidence of a positive association between 25(OH)D concentrations and FEV1, these positive associations were not confirmed in 5 other cross-sectional studies.55,65–68 In addition, in a prospective cohort study, no association between 25(OH)D concentrations and change in FEV1 over 5 years was observed.69
With respect to the association between vitamin D and pulmonary function decline in patients with COPD, 2 studies reported that dietary intake of vitamin D was lower than recommended.10,70 Authors of a systematic review and meta-analysis in which a total of 21 studies were included, involving 4818 patients with COPD and 7175 control subjects, reported that serum 25(OH)D levels of patients were significantly lower than in the control group.71 Correspondingly, mean serum concentrations of 25(OH)D were significantly lower in patients with COPD than in healthy control subjects,72 and patients with COPD had a higher risk of vitamin D deficiency.73,74 Furthermore, in 3 cross-sectional studies,75–77 researchers demonstrated that vitamin D deficiency was inversely associated with absolute FEV1 or FEV1 percent predicted or FVC in patients with COPD. Also, lower vitamin D levels were associated with lower absolute FEV1 levels in patients with COPD and in healthy control subjects in a cross-sectional analysis, but they were significantly lower in patients with COPD when compared with the control group.78 According to a longitudinal analysis, vitamin D deficiency (< 20 ng/mL) was associated with a greater rate of FEV1 percent predicted decline over 1 year.77 And in 2 cross-sectional studies, no significant correlation was found between serum 25(OH)D levels and absolute FEV1 levels or FEV1-to-FVC ratio.79,80
With respect to the effect of vitamin D supplementation on pulmonary function, a meta-analysis including 8 RCTs involving 687 patients with COPD showed that neither short-term (<6 mo) nor long-term (>6 mo) vitamin D supplementation inhibited FEV1 decline.81 However, an update of this meta-analysis,82 including 19 RCTs involving 2004 patients with COPD, reported a significant positive effect of vitamin D supplementation on FEV1; however, the duration of supplementation was not stated. In another RCT, significant improvements were reported in FEV1 or FVC after vitamin D supplementation in smokers with COPD or vitamin D deficiency only.83
The effect of vitamin D on the risk of COPD and pulmonary function decline has been investigated extensively. However, inconsistent evidence is reported on the relationship between vitamin D intake and serum concentrations and the risk of COPD in the general population. Moreover, despite very strong evidence of low dietary intake and serum concentrations of vitamin D in patients with COPD compared with control subjects, evidence on vitamin D’s association with pulmonary function decline is not consistent. On the other hand, very strong evidence, based on a large meta-analysis, indicates that vitamin D supplementation has beneficial effects on pulmonary function decline in patients with COPD. Potential effects of vitamin D supplementation are stronger in patients deficient in vitamin D.
Other vitamins
Dietary intake and nutrient status of vitamins C and E as well as β-carotene, a precursor of vitamin A, were positively associated with FEV1 in cross-sectional studies,84,85 and higher intake of vitamin C was associated with a reduced risk of COPD in the general population.86 Moreover, in community-dwelling older adults, dietary intake of vitamin C in smokers was associated with a slower rate of FEV1 decline, whereas in former smokers, intake of vitamins C, E, or β-carotene was individually associated with an attenuated decline rate and no associations were found relative to never smokers.87 Also, in another study, higher serum levels of β-carotene protected against FEV1 decline over 8 years in the general population.88 In 2 cross-sectional89,90 studies, a positive association of vitamin E with FEV1 and FVC was observed, and in a prospective cohort study91 with 2 times 2 years of follow-up, a significant decrease in COPD risk was noted with increase in dietary intake of vitamins E and C in the general population. A systematic review of 14 studies showed an association between vitamin intake and the development of COPD. More specifically, intake of vitamins A, C, D, E, and β-carotene was associated with greater pulmonary function in the general population. No clear evidence was found that supplementation with those vitamins by patients with COPD had beneficial effects on FEV1 (range of duration, 4 wk to 5 y).92 Individually, daily supplementation with vitamins A, C, and E was associated with higher FEV1 in a cross-sectional analysis of older adults.68
In patients with COPD, dietary intake of vitamins A, B9, C, and E was lower than recommended,10,93 and plasma concentrations of these vitamins were lower in patients with COPD than in healthy individuals.44 Furthermore, serum vitamin A concentrations were lower in patients with moderate to severe COPD than in healthy smokers in a cross-sectional analysis, and FEV1 and FVC measurements were higher after 30 days of vitamin A supplementation in patients with COPD.94 In addition, lower vitamin K status was observed in patients with COPD compared with smoking and nonsmoking control subjects, although it was not associated with FEV1.95 In 2 RCTs, no effect of vitamin E supplementation was found at 12 weeks and 5 years, respectively, on the rate of annual FEV1 decline in current male smokers and in patients with COPD.96,97
In summary, very strong evidence exists for a positive effect of vitamins C, E, and β-carotene on the risk of COPD in the general population. However, despite lower dietary intake and blood levels of vitamins in patients with COPD than in control subjects, the effect on pulmonary function decline has not yet been longitudinally investigated in these patients.
Fatty acids
Fish and omega-3 polyunsaturated fatty acids are common components of the prudent or healthy dietary pattern. Authors of a systematic review summarized evidence on the role of fatty acid intake in the onset of COPD, but results were not consistent.98 In addition, in a prospective cohort study, no association between omega-3 polyunsaturated fatty acid intake and the risk of COPD was observed,99 and 1 cross-sectional study showed no associations with FEV1.41 However, in 2 cross-sectional analyses of current and former smokers, an inverse association between docosahexaenoic acid and the risk of COPD was observed,100,101 and in 2 cross-sectional studies, dietary intake of fish had a protective association with FEV1.68,102 A positive association was also observed between serum docosahexaenoic acid concentration and FEV1 percent predicted and FVC percent predicted in men.103 With respect to saturated fatty acid intake, only 1 cross-sectional study was found and the authors reported no effects on the onset of COPD.104
In terms of pulmonary function decline in COPD, a significant positive association between intake of pentadecanoic acid and FEV1-to-FVC ratio was observed in patients with COPD in a cross-sectional study,105 whereas in another cross-sectional study, no associations between polyunsaturated fatty acid intake and pulmonary function (ie, FEV1 percent predicted and FVC percent predicted) were observed.106
Evidence for the association between dietary intake of fatty acids and the risk and progression of COPD is still very limited and inconsistent and needs to be further elucidated.
Fiber
A reduced risk of COPD in the general population was reported for dietary fiber intake in a systematic review that included 2 longitudinal cohort studies, a case-control study, and a cross-sectional study.98 Moreover, low fiber intake was associated with reduced outcomes of FEV1 and thereby an increased risk of COPD, particularly in current and former smokers in a cross-sectional and prospective cohort study.107,108 In a prospective cohort study, dietary fiber intake was even found to be associated with a long-term 30% reduced risk of COPD development, especially for fruit- and cereal-derived fibers.109 Accordingly, there is very strong evidence indicating that high intake of dietary fibers reduces the risk of COPD, but we found no studies that reported on the effect of dietary fiber intake on pulmonary function decline in patients with COPD.
DISCUSSION
This systematic review highlights the effect of dietary intake, nutrient status, and nutritional supplementation in the general population on the risk for development and onset of COPD and on progression of pulmonary function decline in patients diagnosed with COPD. Generally, patients with COPD often consume a typical Western-style diet, which is poor in dietary quality, and thus they probably eat more processed meat and fewer fruits and vegetables, and less fiber. This review clearly reveals strong evidence that a Western diet, high consumption of processed meat, and low consumption of fruits, vegetables, and fiber are associated with an increased risk of COPD.
Tobacco-smoke exposure and air pollution enhance oxidant burden and chronic pulmonary and systemic inflammation.110 Oxidant burden in the respiratory tract is increased by tobacco exposure and by the release of reactive oxygen species from neutrophils and macrophages, attracting inflammatory cells into the lungs and inducing inflammatory responses by activating transcription factors like nuclear factor-κB.111 Enhanced oxidative stress also results in lung-tissue injury by oxidation of proteins, DNA, and lipids.112 These processes can contribute to loss of pulmonary function and the onset of COPD. Dietary intake of nutrients with antioxidant or anti-inflammatory properties, like vitamins and fiber, theoretically could attenuate pulmonary function decline, which can reduce the risk of COPD in the general population and disease progression in patients with COPD.
In this systematic review, we found consumption of fruit and vegetables is positively associated with COPD risk in the general population. Intake influences the oxidant and anti-oxidant balance, including that in the respiratory tract, because fruit and vegetables have high content of antioxidant substances (eg, vitamins C and E). Vitamin C is involved in the immune defense and potentially protects against environmental oxidative stress.113 Moreover, vitamin C deficiency can cause an impaired immune function, rendering patients more susceptible to acute viral or bacterial infections.113 Furthermore, we found beneficial effects of nutritional fibers, especially cereal fibers and fibers in fruits, on the risk of COPD development. A potential mechanism to explain the inverse association between dietary-fiber intake and COPD risk may be that nutritional fibers modulate the innate immune system and attenuate systemic inflammation via the gut-lung axis.114 A chronically overactive innate immune response can contribute to accelerated loss of pulmonary function.115 The gut microbiome plays a pivotal role in functioning of the host immune system. Bacterial fermentation of dietary fibers in the gut results in the production of short-chain fatty acids, such as butyrate, which can modify the immune response by inhibition of macrophage and neutrophil activation, inducing regulatory T cells, and improving microbial composition.116,117 A previous longitudinal and cross-sectional study demonstrated that high intake of dietary fibers decreased concentrations of pro-inflammatory markers like C-reactive protein and interleukin-6.118,119
In contrast to the beneficial effects of fruit, vegetable, and fiber intake, the studies in this review revealed greater consumption of processed meat is identified as potential risk factor in the onset of COPD. This may be due to the potential pro-inflammatory properties and the high concentrations of nitrates, nitrites, and nitrosamine compounds.120 Nitrites are pro-oxidants and can produce oxidizing reactive nitrogen species such as peroxynitrite, which may amplify inflammatory and oxidative stress processes involved in the lung, leading, for example, to DNA damage, inhibition of mitochondrial respiration, and cell dysfunction.121 Moreover, processed meat contains advanced glycation end products that can increase inflammation and oxidative stress by activating NF-κB after binding to the cell-surface receptor.122,123 Long-term persistence of inflammation and reactive nitrogen species may contribute to progressive loss of pulmonary function; however, the underlying mechanisms of processed meat intake in the pathogenesis of COPD remains unclear and needs to be further elucidated.
Micronutrients were not included in this systematic review and, as far as we know, they have not been implicated in the risk of COPD or pulmonary function decline. However, it has been reported that patients with COPD have lower dietary intake of calcium, phosphorus, and iron compared with that of healthy control subjects,124 and patients with COPD with iron deficiency have less exercise capacity, more frequent exacerbations, and increased COPD severity than do patients with COPD who do not have iron deficiency.125,126 Whether low mineral status also affects lung function and COPD risk remains to be elucidated.
This systematic review shows that the association between dietary intake and the risk of COPD in the general population has been extensively investigated, but no or few studies are available on the association between nutrition and progression of pulmonary function decline in patients with COPD. The available evidence suggests increased nutritional intake of fruit and vegetables or vitamins C, E, and β-carotene; polyphenols; or dietary fibers could very well play a role in the prevention of COPD. Poor nutrition, in addition to tobacco smoking and air pollution, thus is identified as a modifiable COPD risk factor in the general population. Hence, improving quality of dietary intake and adherence to a healthier diet like the Mediterranean diet should be considered as an important lifestyle adaptation with respect to COPD prevention.
CONCLUSION
Considering the increasing burden of COPD worldwide, the scarce or absent data on the effect of dietary intake, nutrient status, and supplementation on pulmonary function decline in patients with COPD highlight the need for large, longitudinal cohort studies and RCTs with long-term follow-up to clarify the role of nutrition in the progression of pulmonary function decline in this disease. Overall, stimulating a shift toward a healthy Mediterranean dietary pattern during lifestyle counseling in patients with COPD may contribute to attenuated decline of pulmonary function, reduced risk of comorbidities, and improved health-related quality of life.
Supplementary Material
Acknowledgements
Author contributions. L.E.J.v.I. and R.J.H.C.G.B. conducted the literature search and assessed the articles independently for eligibility. L.E.J.v.I. wrote the manuscript; R.J.H.C.G.B., H.R.G., and A.M.W.J.S. commented and revised drafts of the manuscript. All authors approved the final version for submission.
Funding. This work was supported by a LSH-TKI Lung Foundation grant (10.2.16.119, 2017: “Food for thought and active lifestyle in COPD”).
Declaration of interest. The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Table S1 Items from the PRIMA checklist included in the systematic review.
Appendix S1 Details of the database search strategy.
Table S2 Results of each cross-sectional, longitudinal cohort study or randomized controlled trial summarized in the systematic review.
Contributor Information
Lieke E J van Iersel, Department of Respiratory Medicine, School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Centre+, Maastricht, The Netherlands.
Rosanne J H C G Beijers, Department of Respiratory Medicine, School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Centre+, Maastricht, The Netherlands.
Harry R Gosker, Department of Respiratory Medicine, School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Centre+, Maastricht, The Netherlands.
Annemie M W J Schols, Department of Respiratory Medicine, School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Centre+, Maastricht, The Netherlands.
References
- 1. Hajat C, Stein E. The global burden of multiple chronic conditions: a narrative review. Prev Med Rep. 2018;12:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider KM, O'Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eyre H, Kahn R, Robertson RM, American Heart Association, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation 2004;109:3244–3255. [DOI] [PubMed] [Google Scholar]
- 4. Agusti A. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD Science Committee report. GOLDcopdorg. 2020. [DOI] [PubMed]
- 5. Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc. 2008;5:475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubinsztajn R, Przybyłowski T, Maskey-Warzechowska M, et al. Exacerbations of chronic obstructive pulmonary disease and quality of life of patients. Adv Exp Med Biol. 2016;884:69–74. [DOI] [PubMed] [Google Scholar]
- 8. Almagro P, Castro A. Helping COPD patients change health behavior in order to improve their quality of life. Int J Chron Obstruct Pulmon Dis. 2013;8:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet . 2007;370:765–773. [DOI] [PubMed] [Google Scholar]
- 10. van de Bool C, Mattijssen-Verdonschot C, van Melick PP, et al. Quality of dietary intake in relation to body composition in patients with chronic obstructive pulmonary disease eligible for pulmonary rehabilitation. Eur J Clin Nutr. 2014;68:159–165. [DOI] [PubMed] [Google Scholar]
- 11. Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:71–86. [DOI] [PubMed] [Google Scholar]
- 12. Stockley RA. Progression of chronic obstructive pulmonary disease: impact of inflammation, comorbidities and therapeutic intervention. Curr Med Res Opin. 2009;25:1235–1245. [DOI] [PubMed] [Google Scholar]
- 13. Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest 2013;144:266–273. [DOI] [PubMed] [Google Scholar]
- 14. de Batlle J, Barreiro E, Romieu I, et al. Dietary modulation of oxidative stress in chronic obstructive pulmonary disease patients. Free Radic Res. 2010;44:1296–1303. [DOI] [PubMed] [Google Scholar]
- 15. Zhai T, Li S, Hu W, et al. Potential micronutrients and phytochemicals against the pathogenesis of chronic obstructive pulmonary disease and lung cancer. Nutrients 2018;10: 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stijntjes F, Hassink-Franke L, Kruishoop A, et al. NHG-Standaard ADHD bij kinderen. Huisarts en Wetenschap. 2014;57:584–594. [Google Scholar]
- 18.Werkgroep JGZ-richtlijn ADHD. Signalering, begeleiding en toeleiding naar diagnostiek. 2015.. Available at: https://www.ncj.nl/richtlijnen/alle-richtlijnen/richtlijn/adhd.
- 19.GGZ-zorgstandaard autisme,. Autisme. 2017. Available at: https://www.ggzstandaarden.nl/zorgstandaarden/autisme.
- 20. van Berckelaer-Onnes I. JGZ Richtlijn Autismespectrumstoornissen; Signalering, begeleiding en toeleiding naar diagnostiek. 2015. Available at:https://www.ncj.nl/richtlijnen/alle-richtlijnen/richtlijn/autismespectrumstoornissen.
- 21. Zheng PF, Shu L, Si CJ, et al. Dietary patterns and chronic obstructive pulmonary disease: a meta-analysis. COPD 2016;13:515–522. [DOI] [PubMed] [Google Scholar]
- 22. Guenther PM, Reedy J, Krebs-Smith SM. Development of the healthy eating index-2005. J Am Diet Assoc. 2008;108:1896–1901. [DOI] [PubMed] [Google Scholar]
- 23. Sorli-Aguilar M, Martin-Lujan F, Flores-Mateo G; for the RESET Study Group investigators. Dietary patterns are associated with lung function among Spanish smokers without respiratory disease. BMC Pulm Med. 2016;16:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cho Y, Chung HK, Kim SS, Shin MJ. Dietary patterns and pulmonary function in Korean women: findings from the Korea National Health and Nutrition Examination Survey 2007-2011. Food Chem Toxicol. 2014;74:177–183. [DOI] [PubMed] [Google Scholar]
- 25. Fischer A, Johansson I, Blomberg A, et al. Adherence to a Mediterranean-like diet as a protective factor against COPD: a nested case-control study. COPD 2019;16:272–277. [DOI] [PubMed] [Google Scholar]
- 26. Root MM, Houser SM, Anderson JJ, et al. Healthy Eating Index 2005 and selected macronutrients are correlated with improved lung function in humans. Nutr Res (New York, NY). 2014;34:277–284. [DOI] [PubMed] [Google Scholar]
- 27. Hanson C, Sayles H, Rutten E, et al. The association between dietary intake and phenotypical characteristics of COPD in the ECLIPSE cohort. Chronic Obstr Pulm Dis. 2014;1:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yazdanpanah L, Paknahad Z, Moosavi AJ, et al. The relationship between different diet quality indices and severity of airflow obstruction among COPD patients. Med J Islam Repub Iran 2016;30:380. [PMC free article] [PubMed] [Google Scholar]
- 29. Salari-Moghaddam A, Milajerdi A, Larijani B, et al. Processed red meat intake and risk of COPD: a systematic review and dose-response meta-analysis of prospective cohort studies. Clin Nutr (Edinburgh, Scotland). 2019;38:1109–1116. [DOI] [PubMed] [Google Scholar]
- 30. Varraso R, Dumas O, Boggs KM, et al. Processed meat intake and risk of chronic obstructive pulmonary disease among middle-aged women. EClinicalMedicine 2019;14:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okubo H, Shaheen SO, Ntani G, Hertfordshire Cohort Study Group, et al. Processed meat consumption and lung function: modification by antioxidants and smoking. Eur Respir J. 2014;43:972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garshick E, Segal MR, Worobec TG, et al. Alcohol consumption and chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;140:373–378. [DOI] [PubMed] [Google Scholar]
- 33. Frantz S, Wollmer P, Dencker M, et al. Associations between lung function and alcohol consumption–assessed by both a questionnaire and a blood marker. Respir Med. 2014;108:114–121. [DOI] [PubMed] [Google Scholar]
- 34. Schünemann HJ, Grant BJ, Freudenheim JL, et al. Beverage specific alcohol intake in a population-based study: evidence for a positive association between pulmonary function and wine intake. BMC Pulm Med. 2002;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sparrow D, Rosner B, Cohen M, et al. Alcohol consumption and pulmonary function. A cross-sectional and longitudinal study. Am Rev Respir Dis. 1983;127:735–738. [DOI] [PubMed] [Google Scholar]
- 36. Sisson JH, Stoner JA, Romberger DJ, et al. Alcohol intake is associated with altered pulmonary function. Alcohol 2005;36:19–30. [DOI] [PubMed] [Google Scholar]
- 37. Tabak C, Smit HA, Räsänen L, et al. Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology 2001;12:239–245. [DOI] [PubMed] [Google Scholar]
- 38. Lange P, Groth S, Mortensen J, et al. Pulmonary function is influenced by heavy alcohol consumption. Am Rev Respir Dis. 1988;137:1119–1123. [DOI] [PubMed] [Google Scholar]
- 39. Kaluza J, Harris HR, Linden A, et al. Alcohol consumption and risk of chronic obstructive pulmonary disease: a prospective cohort study of men. Am J Epidemiol. 2019;188:907–916. [DOI] [PubMed] [Google Scholar]
- 40. Zhai H, Wang Y, Jiang W. Fruit and vegetable intake and the risk of chronic obstructive pulmonary disease: a dose-response meta-analysis of observational studies. Biomed Res Int. 2020;2020:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tabak C, Smit HA, Heederik D, et al. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin Exp Allergy. 2001;31:747–755. [DOI] [PubMed] [Google Scholar]
- 42. Garcia-Larsen V, Potts JF, Omenaas E, . et al. Dietary antioxidants and 10-year lung function decline in adults from the ECRHS survey. Eur Respir J. 2017;50:1602286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Celik F, Topcu F. Nutritional risk factors for the development of chronic obstructive pulmonary disease (COPD) in male smokers. Clin Nutr (Edinburgh, Scotland). 2006;25:955–961. [DOI] [PubMed] [Google Scholar]
- 44. Lin YC, Wu TC, Chen PY, et al. Comparison of plasma and intake levels of antioxidant nutrients in patients with chronic obstructive pulmonary disease and healthy people in Taiwan: a case-control study. Asia Pacific J Clin Nutr. 2010;19:393–401. [PubMed] [Google Scholar]
- 45. Rodriguez-Rodriguez E, Ortega RM, Andres P, et al. Antioxidant status in a group of institutionalised elderly people with chronic obstructive pulmonary disease. Br J Nutr. 2016;115:1740–1747. [DOI] [PubMed] [Google Scholar]
- 46. Keranis E, Makris D, Rodopoulou P, et al. Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. Eur Respir J. 2010;36:774–780. [DOI] [PubMed] [Google Scholar]
- 47. Pounis G, Arcari A, Costanzo S, et al. Favorable association of polyphenol-rich diets with lung function: cross-sectional findings from the Moli-sani study. Respir Med. 2018;136:48–57. [DOI] [PubMed] [Google Scholar]
- 48. Tabak C, Arts IC, Smit HA, et al. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: the MORGEN Study. Am J Respir Crit Care Med. 2001;164:61–64. [DOI] [PubMed] [Google Scholar]
- 49. Mehta AJ, Cassidy A, Litonjua AA, et al. Dietary anthocyanin intake and age-related decline in lung function: longitudinal findings from the VA Normative Aging Study. Am J Clin Nutr. 2016;103:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oh CM, Oh IH, Choe BK, et al. Consuming green tea at least twice each day is associated with reduced odds of chronic obstructive lung disease in middle-aged and older Korean adults. J Nutr. 2018;148:70–76. [DOI] [PubMed] [Google Scholar]
- 51. Ng TP, Gao Q, Gwee X, et al. Tea consumption and risk of chronic obstructive pulmonary disease in middle-aged and older Singaporean adults. Copd. 2021;16:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Siedlinski M, Boer JM, Smit HA, et al. Dietary factors and lung function in the general population: wine and resveratrol intake. Eur Respir J. 2012;39:385–391. [DOI] [PubMed] [Google Scholar]
- 53. di Giuseppe R, Arcari A, Serafini M, et al. ; Moli-sani Project Investigators. Total dietary antioxidant capacity and lung function in an Italian population: a favorable role in premenopausal/never smoker women. Eur J Clin Nutr. 2012;66:61–68. [DOI] [PubMed] [Google Scholar]
- 54. Cerdá B, Soto C, Albaladejo MD, et al. Pomegranate juice supplementation in chronic obstructive pulmonary disease: a 5-week randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2006;60:245–253. [DOI] [PubMed] [Google Scholar]
- 55. Shaheen SO, Jameson KA, Robinson SM, et al. Relationship of vitamin D status to adult lung function and COPD. Thorax. 2011;66:692–698. [DOI] [PubMed] [Google Scholar]
- 56. Larose TL, Brumpton BM, Langhammer A, et al. Serum 25-hydroxyvitamin D level, smoking and lung function in adults: the HUNT Study. Eur Respir J. 2015;46:355–363. [DOI] [PubMed] [Google Scholar]
- 57. Shim SY, Chae WJ, Kim HC, et al. Association between serum 25-hydroxyvitamin D levels and pulmonary function, among Korean adults, during 2010-2014, by sex, age, and body mass index. Respir Med. 2018;140:32–38. [DOI] [PubMed] [Google Scholar]
- 58. Lee J, Park HK, Kwon MJ, et al. Decreased lung function is associated with vitamin D deficiency in apparently health, middle aged Koreans: the Kangbuk Samsung Health Study. Eur J Clin Nutr. 2021;75:501–512. [DOI] [PubMed] [Google Scholar]
- 59. Choi CJ, Seo M, Choi WS, et al. Relationship between serum 25-hydroxyvitamin D and lung function among Korean adults in Korea National Health and Nutrition Examination Survey (KNHANES), 2008-2010. J Clin Endocrinol Metabol. 2013;98:1703–1710. [DOI] [PubMed] [Google Scholar]
- 60. Moghaddassi M, Pazoki M, Salimzadeh A, et al. Association of serum level of 25-hydroxy vitamin D deficiency and pulmonary function in healthy individuals. Scientificworldjournal. 2018;2018:3860921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Berry DJ, Hesketh K, Power C, et al. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106:1433–1440. [DOI] [PubMed] [Google Scholar]
- 62. Flexeder C, Thiering E, Koletzko S, et al. Higher serum 25(OH)D concentrations are associated with improved FEV(1) and FVC in adolescence. Eur Respir J 2017;49: 1601804. [DOI] [PubMed] [Google Scholar]
- 63. Hansen JG, Gao W, Dupuis J, SUNLIGHT Consortium, et al. Association of 25-hydroxyvitamin D status and genetic variation in the vitamin D metabolic pathway with FEV1 in the Framingham Heart Study. Respir Res. 2015;16:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Afzal S, Lange P, Bojesen SE, et al. Plasma 25-hydroxyvitamin D, lung function and risk of chronic obstructive pulmonary disease. Thorax. 2014;69:24–31. [DOI] [PubMed] [Google Scholar]
- 65. Skaaby T, Husemoen LL, Thuesen BH, et al. Vitamin D status and chronic obstructive pulmonary disease: a prospective general population study. PloS One. 2014;9:e90654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rafiq R, Thijs W, Prein R, et al. Associations of serum 25(OH)D concentrations with lung function, airway inflammation and common cold in the general population. Nutrients. 2018;10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Craveiro V, Cabral M, Araújo J, et al. Association of serum 25-hydroxyvitamin D concentration with pulmonary function in young adults. Nutrients 2018;10:1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ng TP, Niti M, Yap KB, et al. Dietary and supplemental antioxidant and anti-inflammatory nutrient intakes and pulmonary function. Public Health Nutr. 2014;17:2081–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ghosh AJ, Moll M, Hayden LP, COPDGene Investigators, et al. Vitamin D deficiency is associated with respiratory symptoms and airway wall thickening in smokers with and without COPD: a prospective cohort study. BMC Pulm Med. 2020;20:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. de Batlle J, Romieu I, Anto JM, et al. Dietary habits of firstly admitted Spanish COPD patients. Respir Med. 2009;103:1904–1910. [DOI] [PubMed] [Google Scholar]
- 71. Zhu M, Wang T, Wang C, et al. The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Copd. 2016;11:2597–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Al-Azzawi MA, Ghoneim AH, Elmadbouh I. Evaluation of vitamin D, vitamin D binding protein gene polymorphism with oxidant - antioxidant profiles in chronic obstructive pulmonary disease. J Med Biochem. 2017;36:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sanket S, Madireddi J, Stanley W, et al. Relation between vitamin D deficiency and severity of chronic obstructive pulmonary disease-a case control study. JCDR. 2016;10:oc1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lokesh KS, Chaya SK, Jayaraj BS, et al. Vitamin D deficiency is associated with chronic obstructive pulmonary disease and exacerbation of COPD. Clin Respir J. 2020;15:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jolliffe DA, James WY, Hooper RL, et al. Prevalence, determinants and clinical correlates of vitamin D deficiency in patients with chronic obstructive pulmonary disease in London, UK. J Steroid Biochem Mol Biol. 2018;175:138–145. [DOI] [PubMed] [Google Scholar]
- 76. Baneen U, Naseem S. Correlation of severity of chronic obstructive pulmonary disease with serum vitamin-D level. J Family Med Prim Care. 2019;8:2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Burkes RM, Ceppe AS, Doerschuk CM, et al. Associations among 25-hydroxyvitamin D levels, lung function, and exacerbation outcomes in COPD: an analysis of the SPIROMICS cohort. Chest 2020;157:856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mishra NK, Mishra JK, Srivastava GN, et al. Should vitamin D be routinely checked for all chronic obstructive pulmonary disease patients? Lung India. 2019;36:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hashim Ali Hussein S, Nielsen LP, Konow Bogebjerg Dolberg M, et al. Serum magnesium and not vitamin D is associated with better QoL in COPD: a cross-sectional study. Respir Med. 2015;109:727–733. [DOI] [PubMed] [Google Scholar]
- 80. Monadi M, Heidari B, Asgharpour M, et al. Relationship between serum vitamin D and forced expiratory volume in patients with chronic obstructive pulmonary disease (COPD). Caspian J Intern Med 2012;3:451–455. [PMC free article] [PubMed] [Google Scholar]
- 81. Chen FY, Xiao M, Ling B, et al. Vitamin D does not improve lung function decline in COPD: a meta-analysis. Eur Rev Med Pharmacol Sci 2019;23:8637–8644. [DOI] [PubMed] [Google Scholar]
- 82. Li X, He J, Yu M, et al. The efficacy of vitamin D therapy for patients with COPD: a meta-analysis of randomized controlled trials. Ann Palliat Med. 2020;9:286–297. [DOI] [PubMed] [Google Scholar]
- 83. Sluyter JD, Camargo CA, Waayer D, et al. Effect of monthly, high-dose, long-term vitamin D on lung function: a randomized controlled trial. Nutrients. 2017;9: 1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hu G, Cassano PA. Antioxidant nutrients and pulmonary function: the Third National Health and Nutrition Examination Survey (NHANES III). Am J Epidemiol. 2000;151:975–981. [DOI] [PubMed] [Google Scholar]
- 85. Schünemann HJ, Grant BJ, Freudenheim JL, et al. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med. 2001;163:1246–1255. [DOI] [PubMed] [Google Scholar]
- 86. Park HJ, Byun MK, Kim HJ, et al. Dietary vitamin C intake protects against COPD: the Korea National Health and Nutrition Examination Survey in 2012. Int J Chron Obstruct Pulmon Dis. 2016;11:2721–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bentley AR, Kritchevsky SB, Harris TB, et al. Dietary antioxidants and forced expiratory volume in 1 s decline: the Health, Aging and Body Composition study. Eur Respir J. 2012;39:979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guénégou A, Leynaert B, Pin I, et al. Serum carotenoids, vitamins A and E, and 8 year lung function decline in a general population. Thorax. 2006;61:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hanson C, Lyden E, Furtado J, et al. Serum tocopherol levels and vitamin E intake are associated with lung function in the normative aging study. Clin Nutr. 2016;35:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schunemann HJ, McCann S, Grant BJ, et al. Lung function in relation to intake of carotenoids and other antioxidant vitamins in a population-based study. Am J Epidemiol. 2002;155:463–471. [DOI] [PubMed] [Google Scholar]
- 91. Joshi P, Kim WJ, Lee SA. The effect of dietary antioxidant on the COPD risk: the community-based KoGES (Ansan-Anseong) cohort. Int J Chron Obstruct Pulmon Dis. 2015;10:2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tsiligianni IG, van der Molen T. A systematic review of the role of vitamin insufficiencies and supplementation in COPD. Respir Res. 2010;11:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hirayama F, Lee AH, Terasawa K, et al. Folate intake associated with lung function, breathlessness and the prevalence of chronic obstructive pulmonary disease. Asia Pac J Clin Nutr. 2010;19:103–109. [PubMed] [Google Scholar]
- 94. Paiva SA, Godoy I, Vannucchi H, et al. Assessment of vitamin A status in chronic obstructive pulmonary disease patients and healthy smokers. Am J Clin Nutr. 1996;64:928–934. [DOI] [PubMed] [Google Scholar]
- 95. Piscaer I, van den Ouweland JMW, Vermeersch K, et al. Low vitamin K status is associated with increased elastin degradation in chronic obstructive pulmonary disease. J Clin Med 2019;81116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Daga MK, Chhabra R, Sharma B, et al. Effects of exogenous vitamin E supplementation on the levels of oxidants and antioxidants in chronic obstructive pulmonary disease. J Biosci. 2003;28:7–11. [DOI] [PubMed] [Google Scholar]
- 97. Cassano PA, Guertin KA, Kristal AR, et al. A randomized controlled trial of vitamin E and selenium on rate of decline in lung function. Respir Res. 2015;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fonseca Wald ELA, van den Borst B, Gosker HR, et al. Dietary fibre and fatty acids in chronic obstructive pulmonary disease risk and progression: a systematic review. Respirology. 2014;19:176–184. [DOI] [PubMed] [Google Scholar]
- 99. Varraso R, Barr RG, Willett WC, et al. Fish intake and risk of chronic obstructive pulmonary disease in 2 large US cohorts. Am J Clin Nutr. 2015;101:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shahar E, Folsom AR, Melnick SL, et al. ; for the Atherosclerosis Risk in Communities Study Investigators. Dietary n-3 polyunsaturated acids and smoking-related chronic obstructive pulmonary disease. Am J Epidemiol. 2008;168:796–801. [DOI] [PubMed] [Google Scholar]
- 101. Shahar E, Boland LL, Folsom AR, et al. Docosahexaenoic acid and smoking-related chronic obstructive pulmonary disease. The Atherosclerosis Risk in Communities Study Investigators. Am J Respir Crit Care Med. 1999;159:1780–1785. [DOI] [PubMed] [Google Scholar]
- 102. Schwartz J, Weiss ST. The relationship of dietary fish intake to level of pulmonary function in the first National Health and Nutrition Survey (NHANES I). Eur Respir J. 1994;7:1821–1824. [DOI] [PubMed] [Google Scholar]
- 103. Kompauer I, Demmelmair H, Koletzko B, et al. Association of fatty acids in serum phospholipids with lung function and bronchial hyperresponsiveness in adults. Eur J Epidemiol. 2008;23:175–190. [DOI] [PubMed] [Google Scholar]
- 104. Cornell K, Alam M, Lyden E, et al. Saturated fat intake is associated with lung function in individuals with airflow obstruction: results from NHANES 2007-2012. Nutrients. 2019;11:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jimenez-Cepeda A, Davila-Said G, Orea-Tejeda A, et al. Dietary intake of fatty acids and its relationship with FEV1/FVC in patients with chronic obstructive pulmonary disease. Clin Nutr Espen. 2019;29:92–96. [DOI] [PubMed] [Google Scholar]
- 106. Choi H, Kim T. Polyunsaturated fatty acids, lung function, and health-related quality of life in patients with chronic obstructive pulmonary disease. Yeungnam Univ J Med. 2020;37:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hanson C, Lyden E, Rennard S, et al. The relationship between dietary fiber intake and lung function in the National Health and Nutrition Examination surveys. Ann Am Thorac Soc. 2016;13:643–650. [DOI] [PubMed] [Google Scholar]
- 108. Kaluza J, Harris H, Wallin A, et al. Dietary fiber intake and risk of chronic obstructive pulmonary disease: a prospective cohort study of men. Epidemiology (Cambridge, Mass.). 2018;29:254–260. [DOI] [PubMed] [Google Scholar]
- 109. Szmidt MK, Kaluza J, Harris HR, et al. Long-term dietary fiber intake and risk of chronic obstructive pulmonary disease: a prospective cohort study of women. Eur J Nutr. 2019;59:1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ben Moussa S, Sfaxi I, Tabka Z, et al. Oxidative stress and lung function profiles of male smokers free from COPD compared to those with COPD: a case-control study. Libyan J Med. 2014;9:23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med. 1996;21:669–681. [DOI] [PubMed] [Google Scholar]
- 112. MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429:195–207. [DOI] [PubMed] [Google Scholar]
- 113. Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Young RP, Hopkins RJ, Marsland B. The gut-liver-lung axis. Modulation of the innate immune response and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2016;54:161–169. [DOI] [PubMed] [Google Scholar]
- 115. Walter RE, Wilk JB, Larson MG, et al. Systemic inflammation and COPD: the Framingham Heart Study. Chest. 2008;133:19–25. [DOI] [PubMed] [Google Scholar]
- 116. Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. [DOI] [PubMed] [Google Scholar]
- 117. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ma Y, Griffith JA, Chasan-Taber L, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ma Y, Hébert JR, Li W, et al. Association between dietary fiber and markers of systemic inflammation in the Women's Health Initiative Observational Study. Nutrition. 2008;24:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pandey R, Singh M, Singhal U, et al. Oxidative/nitrosative stress and the pathobiology of chronic obstructive pulmonary disease. J Clin Diagn Res. 2013;7:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ricciardolo FL, Di Stefano A, Sabatini F, et al. Reactive nitrogen species in the respiratory tract. Eur J Pharmacol. 2006;533:240–252. [DOI] [PubMed] [Google Scholar]
- 122. Uribarri J, Cai W, Peppa M, et al. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–916.e912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hirayama F, Lee AH, Oura A, et al. Dietary intake of six minerals in relation to the risk of chronic obstructive pulmonary disease. Asia Pacific J Clin Nutr. 2010;19:572–577. [PubMed] [Google Scholar]
- 125. Rathi V, Ish P, Singh G, et al. Iron deficiency in non-anemic chronic obstructive pulmonary disease in a predominantly male population: an ignored entity. Monaldi Arch Chest Dis. 2020;90: 39–42. [DOI] [PubMed] [Google Scholar]
- 126. Pizzini A, Aichner M, Sonnweber T, et al. The significance of iron deficiency and anemia in a real-life COPD cohort. Int J Med Sci. 2020;17:2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.