Abstract
Background
The ongoing debate of whether use of cellular telephones increases the risk of developing a brain tumor was recently fueled by the launch of the fifth generation of wireless technologies. Here, we update follow-up of a large-scale prospective study on the association between cellular telephone use and brain tumors.
Methods
During 1996-2001, 1.3 million women born in 1935-1950 were recruited into the study. Questions on cellular telephone use were first asked in median year 2001 and again in median year 2011. All study participants were followed via record linkage to National Health Services databases on deaths and cancer registrations (including nonmalignant brain tumors).
Results
During 14 years follow-up of 776 156 women who completed the 2001 questionnaire, a total of 3268 incident brain tumors were registered. Adjusted relative risks for ever vs never cellular telephone use were 0.97 (95% confidence interval = 0.90 to 1.04) for all brain tumors, 0.89 (95% confidence interval = 0.80 to 0.99) for glioma, and not statistically significantly different to 1.0 for meningioma, pituitary tumors, and acoustic neuroma. Compared with never-users, no statistically significant associations were found, overall or by tumor subtype, for daily cellular telephone use or for having used cellular telephones for at least 10 years. Taking use in 2011 as baseline, there were no statistically significant associations with talking for at least 20 minutes per week or with at least 10 years use. For gliomas occurring in the temporal and parietal lobes, the parts of the brain most likely to be exposed to radiofrequency electromagnetic fields from cellular telephones, relative risks were slightly below 1.0.
Conclusion
Our findings support the accumulating evidence that cellular telephone use under usual conditions does not increase brain tumor incidence.
In just a few decades cellular telephones have become a device of everyday life, with an estimated 8.65 billion subscriptions worldwide in 2021 (1). To enable wireless communication, cellular telephones emit radiofrequency electromagnetic fields (RF-EMF). RF-EMF was ubiquitous in the environment before the first mobile technology emerged in the 1980s, because of radio and TV broadcasting. Except in some rare occupations, no RF-EMF–emitting device had been held directly to the head, until the first commercial handheld phones were released in the early to mid-1980s. Because of their close proximity to the head, RF-EMF emitted from cellular telephones penetrate several centimeters into the head and are absorbed by the tissue (exposing mainly the temporal and parietal lobes of the brain) (2). The well-established biological effect of RF-EMF on tissue is heating, and limits for human exposure to RF-EMF were developed for cellular telephones to prevent any substantial heating that could lead to adverse health effects (3). Concerns were raised, however, that there may be adverse biological effects from RF-EMF exposure below those limits, possibly caused through mechanisms other than heat (4,5).
Associations of cellular telephone use and brain tumors were investigated in many studies, as the brain was the most exposed organ (6). Recent evidence synthesized by international or national authorities generally agrees that “common” or “modest” use of cellular telephones does not appear to increase brain tumor risk [eg, European Commission (5)]. For “heavy” use of cellular telephones, there is less agreement, with some concluding that there is some evidence of an increase in risk of glioma and acoustic neuroma. As a result, the International Agency for Research on Cancer (IARC/WHO) classified RF-EMF as “possibly carcinogenic” (7). The definition of a heavy user is not generally agreed on, and largely arbitrary definitions have been applied in epidemiological studies, with definitions differing greatly across studies (8). Furthermore, the measures do not generally take into account that typical emissions from cellular telephones varied by many orders of magnitude between the wireless generations and over time (recently the fifth generation, called 5G, has been launched). With increasing popularity and calls becoming cheaper, the amount of cellular telephone use has increased over time. Yet, the more recent generations of wireless technologies emit substantially lower output power, so that on balance a very heavy user of today is unlikely to accumulate the same RF-EMF exposure as a modest user of the first 2 wireless generations (9,10).
The ongoing debate on the carcinogenicity of RF-EMF was recently fueled by 2 large animal bioassays observing an increase in heart schwannoma (11-13). However, the applied RF-EMF levels in those experiments together with the exposure duration relative to the lifetime of the animals preclude direct transferability into real-life human exposure conditions. This underscores the importance of continued epidemiological studies.
In 2013 we reported results from the large prospective UK Million Women Study on the association between self-reported cellular telephone use and the risk of various types of brain tumors (14,15). Using prospectively collected exposure data means that the participants did not know at the time of reporting cellular telephone use whether or not they would subsequently develop cancer or any other outcome of interest. Thus, information about the association between cellular telephone exposure and disease is not affected by recall bias, which can be a problem in studies where people report cellular telephone use after they have developed the disease of interest. Here, we provide an update with additional follow-up, a 60% increase in numbers of brain tumors, and new analyses by tumor laterality and location within the brain.
Methods
Study Population
Details on study design, data collection, and follow-up were described previously (14-16), and further details, including information on data access, are available through the study website (http://www.millionwomenstudy.org). In brief, during 1996-2001, 1.3 million women born in 1935-1950 were recruited through the UK National Health Service Breast Screening Programme into the study, completing a postal questionnaire about sociodemographic, medical, and lifestyle factors (16).
Women were resurveyed every 3-5 years, and questions on cellular telephone use were asked in median year 2001 (interquartile range = 2000-2003) and again in median year 2011 (interquartile range = 2010-2012). All study participants are followed via record linkage to the UK National Health Service Central Register, providing information on deaths and cancer registrations (including nonmalignant brain tumors and those of uncertain behavior) comprising the date of each such event, with tumor site and morphology (coded according to the 10th revision of the International Classification of Diseases [ICD-10] and the third edition of the International Classification of Diseases for Oncology [ICD-O]). Linkage to the Cancer Outcomes and Services Dataset from Public Health England provided data, where available, on tumor laterality for women in England. All study participants gave written consent to take part in the study, and ethical approval was provided by the Oxford and Anglia Multi-Centre Research Ethics Committee.
Intracranial tumors are malignant neoplasms, nonmalignant neoplasms, and neoplasms of uncertain behavior at 1 of the following sites: brain (C71, D33.0-2, D43.0-2), cerebral meninges (ICD-10 C70.0, D32.0, D42.0), cranial nerves (C72.2-5, D33.3, D43.3), and cranial endocrine glands (C75.1-3, D35.2-4, D44.3-5). We hereinafter refer to this group of intracranial tumors as “brain tumors.” Where possible, brain tumors were further classed by site and morphology as glioma (ICD-O 9380-9481), glioblastoma (ICD-O 9440-9441) as a subgroup of glioma, meningioma (ICD-O 9530-9539), pituitary tumors (ICD-10 C75.1, D35.2, and D44.3), and acoustic neuromas (also known as vestibular schwannoma: ICD-10 D33.3, ICD-O 9560). Gliomas of the temporal and parietal lobes (ICD-10 C71.2 and C71.3) were also considered separately, as these areas of the brain are closest to where the phone is usually held. Cancers of the eye were also considered (ICD-10 C69).
Exposure Variables
In median year 2001, women were asked, “About how often do you use a cellular telephone [‘mobile phone’ in the original British English questionnaire]?” and given 3 options to respond:—“never,” “less than once a day,” “every day”—and “For how long have you used one (in years)?” Women who reported in 2001 that they used a cellular telephone less than once a day or every day were classified as ever-users. In median year 2011, women were asked, “How long have you used a cellular telephone (in years)?” and “How much do you talk on a cellular telephone (in minutes per week)?” Women who reported in 2011 that they talked on a cellular telephone for at least 1 minute per week were classified as ever-users. Responses to the 2001 questionnaire are used as baseline for most analyses, providing mean follow-up time of 14.2 years for cancer incidence. Responses to the 2011 questionnaire were used as baseline in some analyses, providing mean follow-up time of 6.2 years.
Statistical Analyses
The main analyses are in women who completed the 2001 questionnaire when aged 50-69 years and had no prior brain tumor or any other malignant cancer (except nonmelanoma skin cancer [ICD10 C44]). Women who completed a version of the 2001 questionnaire that did not ask about cellular telephone use were excluded (n = 14 355), as were women who left questions on cellular telephone use unanswered (n = 11 475). Women with self-reported neurofibromatosis (n = 8) were also excluded, because of their high risk of neurological tumors (Supplementary Figure 1, available online).
Cox regression models (with time in study as the underlying time variable) were used to estimate the hazard ratios (referred to as relative risks [RRs] here) for each outcome of interest in relation to cellular telephone use. Measures of phone use in 2001 (ever-use, frequency of use, and years of use vs never-use) and in 2011 (ever-talk on a cellular telephone, minutes spent talking on a cellular telephone per week, and years of use vs never-talk on a cellular telephone) were related to subsequent cancer risk. Years of cellular telephone use was treated as a time-dependent variable, by assuming that cellular telephone use continued and incrementing reported years of use annually for each additional year of follow-up. Analyses were stratified by single years of birth, single year of answering the baseline survey, and region of residence at recruitment (10 cancer registry regions) and adjusted for socioeconomic status [quintiles of Townsend deprivation index for the area of residence at recruitment (17)], smoking (never, past, current <15 cigarettes per day, current ≥15 cigarettes per day), alcohol intake (none, 1-6, 7-13, ≥14 units per week), body mass index (<25, 25-29, 30-34, ≥35 kg/m2), height (<160, 160-164, ≥165 cm), menopausal hormone therapy use (never, ever), and strenuous exercise (<1, ≥1 hour per week). Where values were missing at baseline for body mass index and alcohol intake, values reported at recruitment, on average 3 years earlier, were used instead. Missing values formed a separate category for each variable (<4% for each variable). In figures, results are shown only for the highest cellular telephone use category (eg, daily use, ≥10 years use, or ≥20 minutes talking on a cellular telephone per week vs never-use); results for all cellular telephone use categories can be found in Supplementary Tables 1 and 2 (available online). Three sensitivity analyses were carried out. First, only minimal adjustment was applied, just for year of birth, year of answering the baseline survey, and region of residence. Second, women who completed the 2001 questionnaire in 1999-2000 were excluded, as cellular telephone use was less common and a larger proportion of never-users in these years became cellular telephone users afterward. Third, the first 2 years of follow-up after the 2001 questionnaire were excluded, to minimize bias because of reverse causation (as women may alter their cellular telephone use because of early tumor symptoms, such as hearing loss).
Women contributed person-years at risk from the date they answered the relevant questions about cellular telephone use until the date of diagnosis with any brain tumor or other cancer (except nonmelanoma skin cancer), date of death, or December 31, 2017, whichever was earliest. Wald tests were used to assess statistical significance, with P values less than .05 considered to be statistically significant. All analyses were performed using Stata (version 17.0; StataCorp LLC; College Station, Texas, USA).
Results
Characteristics of the study population and details of follow-up are shown in Table 1. In median year 2001, 63.1% (489 769 of 776 156) reported ever using a cellular telephone. Ever-users of cellular telephones had a higher alcohol intake, higher ever-use of menopausal hormone therapy, did more physical activity, and were slightly more likely to be ever-smokers compared with never cellular telephone users. Of those included in the main analyses, 59.0% (458 002 of 776 156) also completed a questionnaire in median year 2011, and 429 407 had no prior cancer at this time (Table 1; Supplementary Figure 1, available online). About half of the never-users in 2001 reported that they were cellular telephone users in 2011, however, those who began using a cellular telephone between the 2 dates reported substantially lower cellular telephone usage than those who were already users in 2001 (Table 1).
Table 1.
Characteristics of women by reported cellular telephone use at different times and details of follow-up
| Characteristics | Cellular telephone use reported in median year 2001 |
||
|---|---|---|---|
| Never (n = 286 387) | Ever (n = 489 769) | Daily (n = 66 362) | |
| Of those completing a questionnaire in median year 2001 | |||
| Mean age (SD), y | 59.9 (4.7) | 58.8 (4.5) | 57.6 (4.1) |
| Socioeconomic group, % in upper fifth | 18.9 | 23.7 | 20.1 |
| Mean height (SD), cm | 162.0 (6.6) | 162.5 (6.5) | 162.3 (6.7) |
| Mean body mass index (SD), kg/m2 | 26.0 (4.6) | 26.2 (4.6) | 26.7 (5.0) |
| Alcohol intake, % drinking ≥14 units/week | 7.6 | 11.2 | 13.7 |
| Smoking, % ever smoked | 44.6 | 47.1 | 54.9 |
| Hormone therapy for the menopause, % ever use | 48.2 | 57.4 | 62.0 |
| Strenuous exercise, % ≥1 hr/wk | 38.4 | 46.3 | 45.8 |
| Of those completing a questionnaire in median year 2011a | |||
| Mean age (SD), y | 68.9 (4.6) | 67.6 (4.3) | 66.2 (3.8) |
| Talk on cellular telephone, % ≥1 min/wk | 45.8 | 73.5 | 90.8 |
| Talk on cellular telephone, % ≥30 min/wk | 8.8 | 18.2 | 41.2 |
| Talk on cellular telephone, median (IQR) min/wk | 0 (0-10) | 5 (0-15) | 20 (10-45) |
| Years used a cellular telephone, mean (SD) | 4.3 (3.9) | 8.9 (4.6) | 11.0 (4.6) |
| Follow-up for cancer from the 2001 questionnaire | |||
| Women-years of follow-up (millions) | 4.1 | 6.9 | 0.9 |
| Average years of follow-up per woman | 14.4 | 14.1 | 13.8 |
| Incident brain tumors, No. | 1261 | 2007 | 271 |
Completed by 429 407 women (who also completed the questionnaire in median year 2001) with no prior cancer. In median year 2001, n = 149 024 were never users, n = 280 383 were ever users, and n = 36 338 were daily users. IQR = interquartile range.
Table 2 shows the proportions of women reporting use of cellular telephone, by age at reporting and calendar year of reporting. Answers to the questionnaire in median year 2001 were completed from 1999 to 2005, and during that time period use was greater the younger women were, and the prevalence of use at every age increased over time. Answers to the questionnaire in median year 2011 were completed from 2009 to 2013, and during that time period use was also greater the younger women were, but the prevalence showed little increase in use over time. By 2011, the prevalence of use was almost 75% at age 60-64 years and just below 50% at age 75-79 years.
Table 2.
Proportion (%) of women reporting use of a cellular telephone, by age and calendar year of reporting
| Calendar year | Age |
|||||
|---|---|---|---|---|---|---|
| 50-54 y | 55-59 y | 60-64 y | 65-69 y | 70-74 y | 75-79 y | |
| The questionnaire in median year 2001, reporting using a cellular telephone | ||||||
| 1999 | 45.7 | 36.0 | 27.9 | 20.9 | — | — |
| 2000 | 57.0 | 50.1 | 39.9 | 32.2 | — | — |
| 2001 | 70.3 | 65.4 | 57.0 | 49.6 | — | — |
| 2002 | 76.1 | 72.3 | 65.4 | 58.3 | — | — |
| 2003 | 79.6 | 76.9 | 70.7 | 62.7 | — | — |
| 2004 | 81.9 | 81.6 | 76.4 | 67.6 | — | — |
| 2005 | 87.6 | 85.4 | 80.3 | 72.2 | — | — |
| The questionnaire in median year 2011, reporting using a cellular telephone for ≥1 min per wka | ||||||
| 2009 | — | — | 74.4 | 66.6 | 55.2 | 47.3 |
| 2010 | — | — | 75.4 | 68.0 | 56.3 | 46.1 |
| 2011 | — | — | 72.0 | 64.5 | 54.8 | 45.4 |
| 2012/2013b | — | — | 73.5 | 66.6 | 57.4 | 48.3 |
Completed by 429 407 women (who also completed a questionnaire in median year 2001) with no prior cancer.
2012 and 2013 combined, as only 389 women completed the survey in 2013.
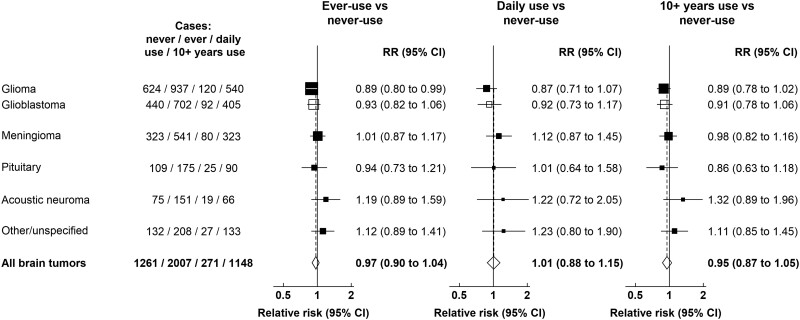
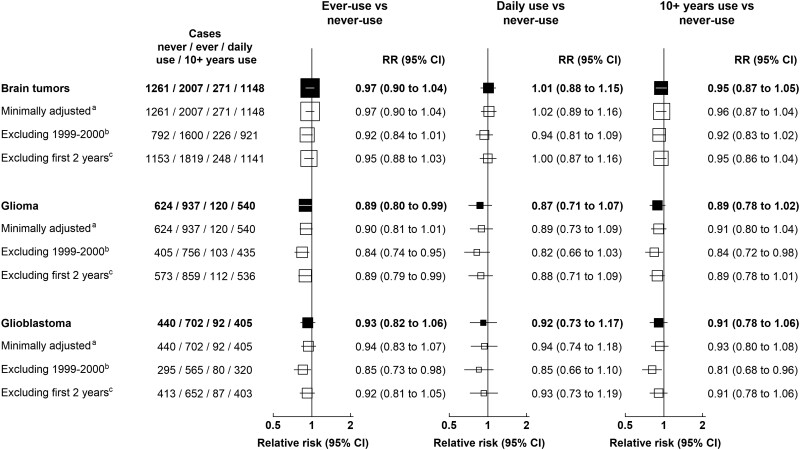
During 14 years follow-up of 776 156 women who completed the 2001 questionnaire, a total of 3268 incident brain tumors were registered. Figure 1 and Supplementary Table 1 (available online) show relative risks for cellular telephone use and brain tumor incidence, overall and by tumor subtypes, using the 2001 questionnaire as baseline. The relative risk for ever vs never cellular telephone use for all brain tumors was close to 1.0 (RR = 0.97, 95% confidence interval [CI] = 0.90 to 1.04). Relative risks for ever vs never cellular telephone use were slightly below 1.0 for glioma (RR = 0.89, 95% CI = 0.80 to 0.99) and close to 1.0 for meningioma, pituitary tumors, acoustic neuroma, and other tumors. No statistically significant increases or decreases were seen, overall or by tumor subtype, for daily use or for having used a cellular telephone for at least 10 years compared with never-use. For glioblastoma, the glioma subtype of poorest prognosis, no relative risks were statistically significantly increased. Figure 2 shows the results of sensitivity analyses. Using minimal adjustment for year of birth, year of questionnaire completion and region had little effect on the relative risks, as did excluding women who completed the questionnaire in 1999-2000 and excluding the first 2 years of follow-up (Figure 2).
Figure 1.
Relative risks for brain tumors in users vs never-users of cellular telephones in median year 2001, UK Million Women Study. Results are shown for daily use and ≥10 years use vs never-use only. Results for intermediate categories can be found in Supplementary Table 1 (available online). Relative risks are plotted as squares, with the area of each square inversely proportional to the variance of the log relative risk. Error bars represent the 95% confidence intervals. CI = confidence interval; RR = relative risk.
Figure 2.
Relative risks for brain tumors in users vs never-users of cellular telephones in median year 2001, sensitivity analysis, UK Million Women Study. aStratified by year of birth, year of answering the baseline survey, and region only. bExcluding women who completed the questionnaire in 1999-2000. cExcluding the first 2 years of follow-up. Relative risks are plotted as squares, with the area of each square inversely proportional to the variance of the log relative risk. Error bars represent the 95% confidence intervals. CI = confidence interval; RR = relative risk.
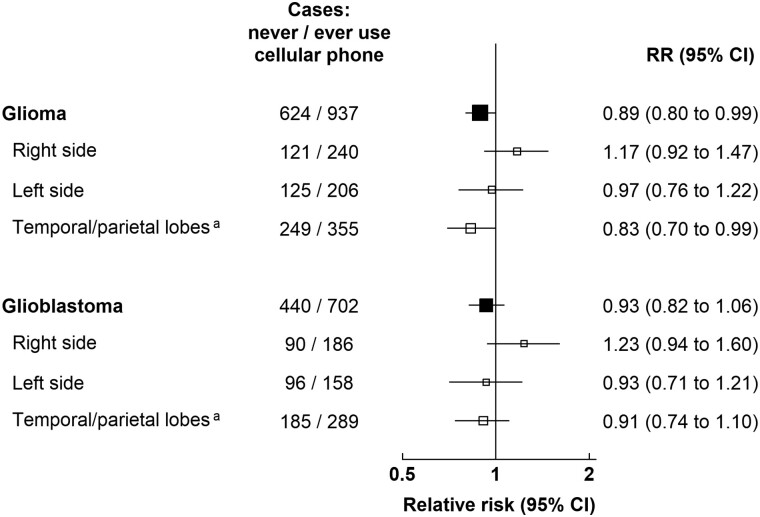
Tumor laterality (right, left) was available for 44.3% (692 of 1561) of the glioma cases and 46.4% (530 of 1142) of the glioblastoma cases (Figure 3). Almost half (47.0%; 604 of 1284) of the glioma cases with known location occurred in the temporal or parietal lobes (48.3%; 474 of 981 for glioblastoma). Relative risks did not differ statistically significantly by whether or not the tumors were right-sided or left-sided (Pheterogeneity = .3 for glioma, Pheterogeneity = .2 for glioblastoma; Figure 3). For glioma and glioblastoma in the temporal and parietal lobes, relative risks were slightly below 1.0 (Figure 3).
Figure 3.
Relative risks for glioma by location in the brain, in users vs never-users of cellular telephones in median year 2001, UK Million Women Study. Results are shown for ever-use vs never-use only. Results by frequency of use and by years of use can be found in Supplementary Table 1 (available online). aTemporal lobe (C71.2) and parietal lobe (C71.3) only. Relative risks are plotted as squares, with the area of each square inversely proportional to the variance of the log relative risk. Error bars represent the 95% confidence intervals. CI = confidence interval; RR = relative risk.
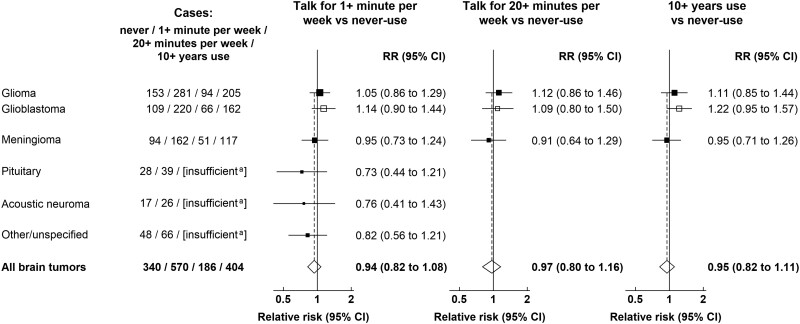
Figure 4 and Supplementary Table 2 (available online) show the associations between brain tumors and different subtypes related to cellular telephone use using the 2011 questionnaire as baseline. Although numbers are small, compared with women who did not talk on their cellular telephone, relative risks for at least 1 minute talking per week, 20 or more minutes talking per week, and at least 10 years of cellular telephone use were close to 1.0 for all groups.
Figure 4.
Relative risks for brain tumors in users vs never-users of cellular telephones in median year 2011, UK Million Women Study. Results are shown for ≥20 minutes talking per week and ≥10 years use vs never-use only. Results for intermediate categories can be found in Supplementary Table 2 (available online). aMarked as insufficient if there were fewer than 50 cases in ever-users of cellular phones. Relative risks are plotted as squares, with the area of each square inversely proportional to the variance of the log relative risk. Error bars represent the 95% confidence intervals. CI = confidence interval; RR = relative risk.
No increase in relative risks were seen for eye tumors: compared with never cellular telephone users in 2001, relative risks were 0.99 (95% CI = 0.76 to 1.30; 161 cases) for ever cellular telephone use, 0.78 (95% CI = 0.45 to 1.32; 17 cases) for daily cellular telephone use, and 0.99 (95% CI = 0.71 to 1.44; 92 cases) for at least 10 years of cellular telephone use.
Discussion
In this large prospective study of about 1 in every 4 UK women born in 1935-1950, use of cellular telephones was not associated with an increased risk of brain tumors overall or by brain tumor subtype or its location. For the main malignant subtypes, glioma and glioblastoma, there was no indication of an increase, based on 937 and 702 cases, respectively, in cellular telephone users. There was also no statistically significantly increased risk of brain tumors or of any brain tumor subtype in cellular telephone users of at least 10 years or for temporal and parietal lobe tumors, which are the most exposed parts of the brain. The incidence of right-sided and left-sided tumors were similar in cellular telephone users, even though cellular telephone use tends to be considerably greater on the right than the left side (18).
Our results are in line with the only other published prospective study, which subdivided the entire Danish adult population into those who had subscribed for a cellular telephone in 1995 or earlier and those who did not; no association with any type of brain tumor was observed even after at least 13 years of subscription (19–21). Information on subscription rather than cellular telephone use may, however, introduce exposure misclassification (22), although any effect was estimated to be modest (20). The 2 prospective studies have the strong advantage of recording exposure before the diagnosis of brain tumors. Retrospective studies, which record information on cellular telephone use after the brain tumors are diagnosed, are prone to differential reporting of use between those who know that they have a brain tumor and those who know that they do not have a brain tumor.
In the largest retrospective study to date, the 13-country INTERPHONE study, odds ratios for ever-use and time since first use consistently showed inverse associations, which are implausible as suggesting causality, because there are no mechanisms suggesting that RF-EMF should provide protection against the development of brain tumors (23,24). Selection bias has been identified as a possible major bias in its study population (25). Findings in smaller retrospective studies are, likewise, difficult to interpret (26). In INTERPHONE, a modest positive association was seen between glioma risk and the heaviest (top decile of) cellular telephone use (odds ratio = 1.40, 95% CI = 1.03 to 1.89). This specific group of cellular telephone users is estimated to represent not more than 3% of the women in our study, so that overall, the results of the 2 studies are not in contradiction (23). In a series of case-control studies in Sweden, consistently strong positive associations for ever cellular telephone use were observed, even within a short time after first use (27,28). If true, this would by now have led to a massive epidemic of brain tumors that—fortunately—has not happened (29-31). Hence, some major underlying bias in either the recruitment of study participants or in assessing exposure is the likely explanation for their findings.
Our findings are consistent with time trends of glioma incidence rates from high-quality population-based cancer registries in the Nordic countries, the United States, and Australia (29-31). Overall age-adjusted incidence rates have increased since the 1970s, mainly at older ages, which are thought to be an effect of improved imaging diagnostics and registration. Age- and sex-specific incidence rates do not suggest any adverse effects due to cellular telephone use, as cellular telephone use increased steeply first among middle-aged men.
We found no statistically significant excess of acoustic neuroma in ever cellular telephone users, overall or with at least 10 years of cellular telephone use. In INTERPHONE and other retrospective studies, there was no association with at least 10 years of cellular telephone use (0.76, 95% CI = 0.52 to 1.11), but there was a non-statistically significant positive association among the heaviest (top decile of) cellular telephone users (1.32, 95% CI = 0.88 to 1.97) (24). One possible reason for not finding an association with acoustic neuroma as observed in our first follow-up could be that more exposure misclassification with longer follow-up may have masked any association (14,15). However, a more likely explanation for the previous positive results is that for a very slow growing tumor, there may be detection bias if cellular telephone users seek medical advice because of awareness of typical symptoms of acoustic neuroma, such as unilateral hearing problems, earlier than non-users (19). We also did not see an association with eye tumors, consistent with the evidence from previous studies (20,32).
Some evidence for carcinogenicity was recently reported in 2 independent animal bioassays, although findings were based on small numbers and were inconsistent across species (11–13). The whole-body exposure of the animals at very high RF-EMF levels for 9 (12,13) to 19 (11) hours every day of their life was orders of magnitude higher than the typical localized brain exposure in humans under real-life conditions of cellular telephone use.
The totality of human evidence, from observational studies, time trends, and bioassays, suggests little or no increase in the risk of cellular telephone users developing a brain tumor. Heavy cellular telephone users are now advised to reduce exposure by using headphones or loudspeakers for calls of long durations.
Strengths of our study include its large size and that it is population-based and has information on tumor subtype, tumor location, and tumor laterality, but most of all, the prospective nature of the research. The main limitation is the relatively simple exposure assessment (with a lack of detailed cellular telephone use history) and the lack of information on the type of cellular telephone technology used. Exposure misclassification may have occurred especially in the earlier years because of the rapidly growing use of cellular telephones, as shown in studies comparing self-reported cellular telephone use and traffic records from cellular telephone operators (33). Tumor laterality was only available for about half of the glioma cases, which leaves some uncertainty related to the location-specific results. Because of small numbers, results for acoustic neuroma have wide confidence intervals, reflecting the reality that this is a very rare tumor. In addition, the cohort consists only of women of middle to older ages, who generally have lower cellular telephone use than younger women or men (9,24). Our study did not include children, but such studies have been done (34,35).
In conclusion, in this large-scale prospective study of UK women, there was little to suggest that the use of cellular telephones increases the risk of brain tumors, overall or by subtype or by tumor location. This finding supports the accumulating evidence that cellular telephone use under usual conditions does not increase brain tumor risk. Future research should target specifically the very heavy cellular telephone users, with attention to new features of a continuously evolving technology; hence, advising cellular telephone users on how to reduce unnecessary exposures remains a good precautionary approach. An ongoing international prospective cohort designed to investigate adverse health effects of cellular telephone use should eventually provide further evidence (36).
Funding
UK Medical Research Council (grant no. MR/K02700X/1), Cancer Research UK (grant no. C570/A16491 and A29186).
Notes
Role of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors have no conflict of interest to declare.
Author contributions: Conceptualization- JS, KP, VB. Methodology- JS, KP, VB. Formal analysis- KP. Investigation- JS, KP, VB. Visualization- KP. Project administration- JS, KP, VB. Writing- original draft- JS, KP, VB. Writing- review & editing- JS, KP, GKR, SF, VB. Funding acquisition- GKR, SF, VB.
Acknowledgements: The authors thank the women who have participated in the Million Women Study and National Health Service (NHS) breast screening centre staff. We also thank NHS Digital in England and the Information Services Division, NHS Scotland for data on cancers and deaths, and Public Health England for data based on information collected and quality assured by the Public Health England National Cancer Registration and Analysis Service, for which access was facilitated by the Office for Data Release. Data for this study include information collected and provided by the Office for National Statistics. Those who carried out the original collection and analysis of the data bear no responsibility for their further analysis or interpretation.
The authors also thank the Million Women Study Collaborators. Million Women Study Co-ordinating Centre staff: Simon Abbott, Rupert Alison, Sarah Atkinson, Krys Baker, Angela Balkwill, Isobel Barnes, Valerie Beral, Judith Black, Roger Blanks, Anna Brown, Andrew Chadwick, Dave Ewart, Sarah Floud, Kezia Gaitskell, Toral Gathani, Laura Gerrard, Adrian Goodill, Jane Green, Jane Henderson, Carol Hermon, Darren Hogg, Isobel Lingard, Sau Wan Kan, Nicky Langston, Kirstin Pirie, Alison Price, Gillian Reeves, Keith Shaw, Emma Sherman, Helena Strange, Sian Sweetland, Ruth Travis, Lyndsey Trickett, Clare Wotton, Owen Yang, Heather Young. Million Women Study Advisory Committee: Emily Banks, Valerie Beral, Lucy Carpenter, Carol Dezateux, Sarah Floud, Jane Green, Julietta Patnick, Richard Peto, Gillian Reeves, Cathie Sudlow. The NHS Breast Screening Centres that took part in the recruitment of participants were Avon, Aylesbury, Barnsley, Basingstoke, Bedfordshire and Hertfordshire, Cambridge and Huntingdon, Chelmsford and Colchester, Chester, Cornwall, Crewe, Cumbria, Doncaster, Dorset, East Berkshire, East Cheshire, East Devon, East of Scotland, East Suffolk, East Sussex, Gateshead, Gloucestershire, Great Yarmouth, Hereford and Worcester, Kent, Kings Lynn, Leicestershire, Liverpool, Manchester, Milton Keynes, Newcastle, North Birmingham, North East Scotland, North Lancashire, North Middlesex, North Nottingham, North of Scotland, North Tees, North Yorkshire, Nottingham, Oxford, Portsmouth, Rotherham, Sheffield, Shropshire, Somerset, South Birmingham, South East Scotland, South East Staffordshire, South Derbyshire, South Essex, South Lancashire, South West Scotland, Surrey, Warrington Halton St Helens and Knowsley, Warwickshire Solihull and Coventry, West Berkshire, West Devon, West London, West Suffolk, West Sussex, Wiltshire, Winchester, Wirral, Wycombe.
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Data Availability
Information on data access for the Million Women Study is available at www.millionwomenstudy.org/data_access/.
Supplementary Material
Contributor Information
Joachim Schüz, International Agency for Research on Cancer (IARC/WHO), Environment and Lifestyle Epidemiology Branch, Lyon, France.
Kirstin Pirie, Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Gillian K Reeves, Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Sarah Floud, Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Valerie Beral, Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
References
- 1.International Telecommunication Union (ITU). ICT statistics homepage. https://www.itu.int/en/ITU-D/Statistics/Pages/stat/default.aspx. Accessed January 20, 2022.
- 2. Cardis E, Deltour I, Mann S, et al. Distribution of RF energy emitted by mobile phones in anatomical structures of the brain. Phys Med Biol. 2008;53(11):2771–2783. doi: 10.1088/0031-9155/53/11/001. [DOI] [PubMed] [Google Scholar]
- 3.International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020;118(5):483–524. doi:10.1097/HP. 0000000000001210. [DOI] [PubMed] [Google Scholar]
- 4. VijayalaxmiScarfi MR. International and national expert group evaluations: biological/health effects of radiofrequency fields. Int J Environ Res Public Health. 2014;11(9):9376–9408. doi: 10.3390/ijerph110909376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Opinion on Potential Health Effects of Exposure to Electromagnetic Fields (EMF). European Commission; 2015. https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_041.pdf. Accessed January 20, 2022.
- 6. Blettner M, Michaelis J, Wahrendorf J. Workshop on research into the health effects of cellular telephones. Epidemiology. 2000;11(5):609–611. [DOI] [PubMed] [Google Scholar]
- 7. Baan R, Grosse Y, Lauby-Secretan B, et al. ; for the WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12(7):624–626. [DOI] [PubMed] [Google Scholar]
- 8. Röösli M, Lagorio S, Schoemaker MJ, Schüz J, Feychting M. Brain and salivary gland tumors and mobile phone use: evaluating the evidence from various epidemiological study designs. Annu Rev Public Health. 2019;40:221–238. [DOI] [PubMed] [Google Scholar]
- 9. Kühn S, Kuster N. Field evaluation of the human exposure from multiband, multisystem mobile phones. IEEE Trans Electromagn Compat. 2012;55(2):275–287. [Google Scholar]
- 10. Mazloum T, Aerts S, Joseph W, Wiart J. RF-EMF exposure induced by mobile phones operating in LTE small cells in two different urban cities. Ann Telecommun. 2019;74(1-2):35–42. doi: 10.1007/s12243-018-0680-1. [DOI] [Google Scholar]
- 11. Falcioni L, Bua L, Tibaldi E, et al. Report of final results regarding brain and heart tumors in Sprague-Dawley rats exposed from prenatal life until natural death to mobile phone radiofrequency field representative of a 1.8 GHz GSM base station environmental emission. Environ Res. 2018;165:496–503. doi: 10.1016/j.envres.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 12.National Toxicology Program (NTP). NTP Technical Report on the Toxicology and Carcinogenesis Studies in Hsd:Sprague Dawley SD Rats exposed to Whole-Body Radio Frequency Radiation at a Frequency (900 MHz) and Modulations (GSM and CDMA) Used by Cell Phones. National Institutes of Health, Public Health Service, US Department of Health and Human Services; November 2018. https://ntp.niehs.nih.gov/publications/reports/tr/500s/tr595/. Accessed March 8, 2022. [DOI] [PMC free article] [PubMed]
- 13.National Toxicology Program (NTP). NTP Technical Report on the Toxicology and Carcinogenesis Studies in B6C3F1/N Mice Exposed to Whole-Body Radio Frequency Radiation at a Frequency (1900 MHz) and Modulations (GSM and CDMA) Used by Cell Phones. National Institutes of Health, Public Health Service, US Department of Health and Human Services; November 2018. https://ntp.niehs.nih.gov/publications/reports/tr/500s/tr596/. Accessed March 8, 2022.
- 14. Benson VS, Pirie K, Schüz J, Reeves GK, Beral V, Green J; for the Million Women Study Collaborators. Mobile phone use and risk of brain neoplasms and other cancers: prospective study. Int J Epidemiol. 2013;42(3):792–802. [DOI] [PubMed] [Google Scholar]
- 15. Benson VS, Pirie K, Schüz J, Reeves GK, Beral V, Green J. Authors’ response to: the case of acoustic neuroma: comment on mobile phone use and risk of brain neoplasms and other cancers. Int J Epidemiol. 2014;43(1):275. [DOI] [PubMed] [Google Scholar]
- 16. Green J, Reeves GK, Floud S, et al. ; for the Million Women Study Collaborators. Cohort profile: the Million Women Study. Int J Epidemiol. 2019;48(1):28–29e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. London: Croon Helm; 1988. [Google Scholar]
- 18. Seidman MD, Siegel B, Shah P, Bowyer SM. Hemispheric dominance and cell phone use. JAMA Otolaryngol Head Neck Surg. 2013;139(5):466–470. [DOI] [PubMed] [Google Scholar]
- 19. Schüz J, Steding-Jessen M, Hansen S, et al. Long-term mobile phone use and the risk of vestibular Schwannoma: a Danish nationwide cohort study. Am J Epidemiol. 2011;174(4):416–422. [DOI] [PubMed] [Google Scholar]
- 20. Schüz J, Jacobsen R, Olsen JH, Boice JD Jr, McLaughlin JK, Johansen C. Cellular telephone use and cancer risk: an update of a nationwide Danish cohort. J Natl Cancer Inst. 2006;98(23):1707–1713. [DOI] [PubMed] [Google Scholar]
- 21. Frei P, Poulsen AH, Johansen C, Olsen JH, Steding-Jessen M, Schüz J. Use of mobile phones and risk of brain tumours: update of Danish cohort study. BMJ. 2011;343:d6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schüz J, Johansen C. A comparison of self-reported cellular telephone use with subscriber data: agreement between the two methods and implications for risk estimation. Bioelectromagnetics. 2007;28(2):130–136. [DOI] [PubMed] [Google Scholar]
- 23.INTERPHONE Study Group. Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J Epidemiol. 2010;39(3):675–694. [DOI] [PubMed] [Google Scholar]
- 24.INTERPHONE Study Group. Acoustic neuroma risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Cancer Epidemiol. 2011;35(5):453–464. [DOI] [PubMed] [Google Scholar]
- 25. Vrijheid M, Richardson L, Armstrong BK, et al. Quantifying the impact of selection bias caused by nonparticipation in a case-control study of mobile phone use. Ann Epidemiol. 2009;19(1):33–41. [DOI] [PubMed] [Google Scholar]
- 26. Coureau G, Bouvier G, Lebailly P, et al. Mobile phone use and brain tumours in the CERENAT case-control study. Occup Environ Med. 2014;71(7):514–522. [DOI] [PubMed] [Google Scholar]
- 27. Hardell L, Carlberg M. Mobile phone and cordless phone use and the risk for glioma – analysis of pooled case-control studies in Sweden, 1997-2003 and 2007-2009. Pathophysiology. 2015;22(1):1–13. [DOI] [PubMed] [Google Scholar]
- 28. Carlberg M, Hardell L. Pooled analysis of Swedish case-control studies during 1997-2003 and 2007-2009 on meningioma risk associated with the use of mobile and cordless phones. Oncol Rep. 2015;33(6):3093–3098. [DOI] [PubMed] [Google Scholar]
- 29. Deltour I, Auvinen A, Feychting M, et al. Mobile phone use and incidence of glioma in the Nordic countries 1979-2008: consistency check. Epidemiology. 2012;23(2):301–307. [DOI] [PubMed] [Google Scholar]
- 30. Little MP, Rajaraman P, Curtis RE, et al. Mobile phone use and glioma risk: comparison of epidemiological study results with incidence trends in the United States. BMJ. 2012;344:e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chapman S, Azizi L, Luo Q, Sitas F. Has the incidence of brain cancer risen in Australia since the introduction of mobile phones 29 years ago? Cancer Epidemiol. 2016;42:199–205. [DOI] [PubMed] [Google Scholar]
- 32. Stang A, Schmidt-Pokrzywniak A, Lash TL, et al. Mobile phone use and risk of uveal melanoma: results of the risk factors for uveal melanoma case-control study. J Natl Cancer Inst. 2009;101(2):120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toledano MB, Auvinen A, Tettamanti G, et al. An international prospective cohort study of mobile phone users and health (COSMOS): factors affecting validity of self-reported mobile phone use. Int J Hyg Environ Health. 2018;221(1):1–8. [DOI] [PubMed] [Google Scholar]
- 34. Aydin D, Feychting M, Schüz J, et al. Mobile phone use and brain tumors in children and adolescents: a multicenter case-control study. J Natl Cancer Inst. 2011;103(16):1264–1276. doi: 10.1093/jnci/djr244. [DOI] [PubMed] [Google Scholar]
- 35. Castaño-Vinyals G, Sadetzki S, Vermeulen R, et al. Wireless phone use in childhood and adolescence and neuroepithelial brain tumours: results from the international MOBI-Kids study. Environ Int. 2022;160:107069. doi: 10.1016/j.envint.2021.107069. [DOI] [PubMed] [Google Scholar]
- 36. Schüz J, Elliott P, Auvinen A, et al. An international prospective cohort study of mobile phone users and health (Cosmos): design considerations and enrolment. Cancer Epidemiol. 2011;35(1):37–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Information on data access for the Million Women Study is available at www.millionwomenstudy.org/data_access/.