Abstract
Neuroanatomical barriers with physical, chemical, and immunological properties play an essential role in preventing the spread of peripheral infections into the CNS. A failure to contain pathogens within these barriers can result in very serious CNS diseases. CNS barriers are inhabited by an elaborate conglomerate of innate and adaptive immune cells that are highly responsive to environmental challenges. The CNS and its barriers can also be protected by memory T and B cells elicited by prior infection or vaccination. Here, we discuss the different CNS barriers from a developmental, anatomical, and immunological standpoint and summarize our current understanding of how memory cells protect the CNS compartment. We then discuss a contemporary challenge to CNS-barrier system (SARS-CoV-2 infection) and highlight approaches to promote immunological protection of the CNS via vaccination.
Neuroanatomical barriers are defended by the immune system to safeguard the CNS parenchyma from pathogens. Ampie and McGavern review the anatomy and development of CNS barriers as well as their immunological composition during steady state and in response to infections. They also explore how to protect these barriers via vaccination and then discuss the immunology and neuropathogenesis of a contemporary CNS challenge (i.e., SARS-CoV-2).
Introduction
Historically, the CNS was deemed an immune-privileged tissue, but this view has been modified in recent years based on the discovery of robust immune activity along CNS borders (Fitzpatrick et al., 2020; Rustenhoven et al., 2021). The CNS comprises several physical barriers that help prevent contiguous spread of neurotropic pathogens into the parenchyma. These barriers include the meninges (Figures 1 and 2 ), nasal epithelium-olfactory bulb interface (Figure 3 ), blood-cerebrospinal fluid barrier (BCSFB) (Figure 4 ), and, lastly, the blood-brain barrier (BBB) (Figure 1). These barriers collectively form elegant infrastructures that impede neurotropic organisms from penetrating the CNS through the nasal epithelium, cerebral spinal fluid (CSF), or through hematogenous spread. In addition to these physical barriers, there are resident immune cells that vigilantly patrol these areas and add another layer of protection (Buckley and McGavern, 2022; Moseman et al., 2020).
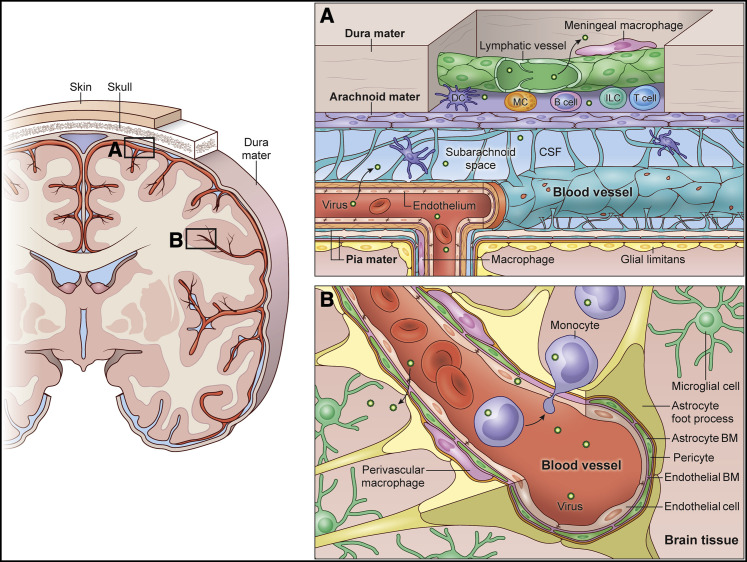
Figure 1.
The meningeal and BBBs of the CNS
A coronal brain section is depicted (left) with overlying skull, dura mater, and leptomeninges (arachnoid mater and pia mater).
(A) The meninges consist of dura mater, arachnoid mater, and pia mater, which all reside above the glia limitans superficialis, a layer of surface-associated astrocytes that creates a barrier between the meninges and brain parenchyma. The dura mater contains fenestrated blood vessels without tight junctions. There are also lymphatic vessels in the dura mater that reside primarily along large venous sinuses (Figure 2). Most blood vessels in dura mater, including the sinuses, are lined by meningeal macrophages. Dural immune cells can also be found mostly in the peri-sinus spaces, which include DCs, T cells, B cells, plasma cells, innate lymphoid cells, and mast cells (Figure 2). Under steady state, resident meningeal macrophages, DCs, and other APCs sample perivascular spaces in the dura mater and present antigens to surveying T cells. The arachnoid mater expresses tight-junction proteins and serves as a barrier between the dura mater, with its fenestrated vasculature, and the CSF-containing subarachnoid space, which contains some DCs as well. Blood vessels that run along the pial surface and enter the brain parenchyma are non-fenestrated and comprise endothelial cells that express tight-junction proteins. These vessels are also defended by perivascular macrophages. Microbes in circulation are most likely to exit fenestrated blood vessels in the dura mater, which explains the presence of lymphatic vessels and the diversity of immune cells in this meningeal layer. However, pathogens can also enter the CSF space via pial vessels, especially if components of the vessel itself become infected. Infection of the meninges usually initiates a major inflammatory response and is referred to as meningitis.
(B) The BBB is a barrier that protects the CNS parenchyma from the contents of the peripheral blood. Leptomeningeal vessels that enter the CNS parenchyma comprise endothelial cells that express tight junctions and are lined by smooth muscle cells/pericytes, basement membrane (BM), perivascular macrophages, and astrocytic foot processes. The parenchymal space adjacent to these blood vessels is surveyed by brain-resident microglia. Systemic inflammatory responses can alter the integrity of the BBB, rendering the CNS more susceptible to invasion by pathogens and immune cells. Pathogens can also enter the CNS parenchyma by infecting circulating immune cells like monocytes, which then extravasate.
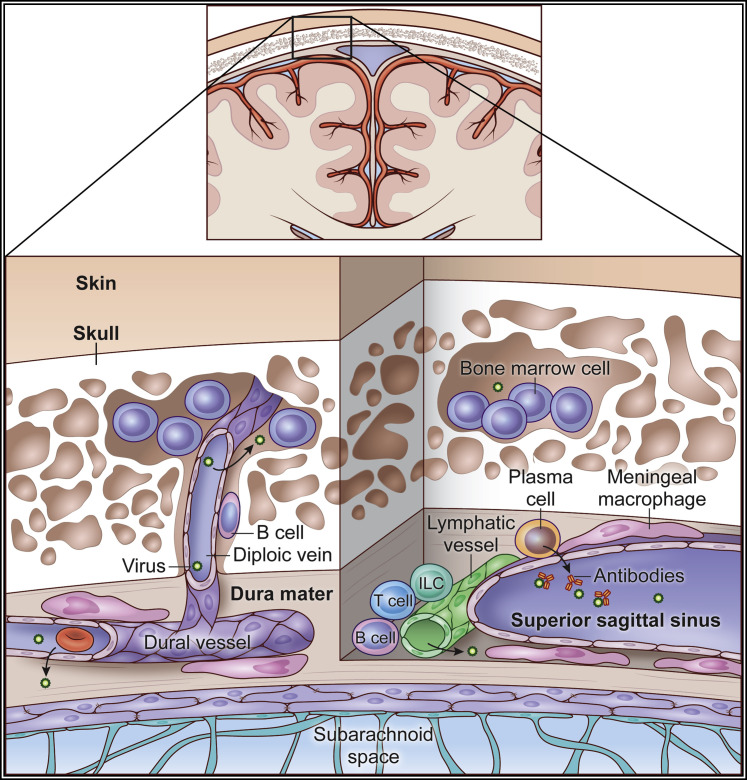
Figure 2.
Skull bone marrow niche
Pockets of bone marrow reside in porous cancellous bone within the skull. The bone marrow spaces are connected to dural vasculature via diploic veins. A chemokine gradient exists between skull bone marrow and the dura mater, which facilitates egress of bone-marrow-derived B cells into the meningeal space. It is believed that B cell education can occur within the dura mater, including clonal deletion in response to CNS-derived antigens (i.e., tolerance). During steady state, gut-derived IgA+ plasma cells inhabit the walls of the dural venous sinuses and can secrete antibodies into the lumen of these structures that prevent pathogens from entering the brain parenchyma. The meningeal space also has other immune cells such as macrophages, innate lymphoid cells, and T cells that reside along the dural venous sinuses. Lymphatic vessels run adjacent to these sinuses. Dural blood vessels are fenestrated and lined by meningeal macrophages and other APCs that present antigens to patrolling lymphocytes. The skull bone marrow and dural vasculature are especially susceptible to infection by circulating microbes.
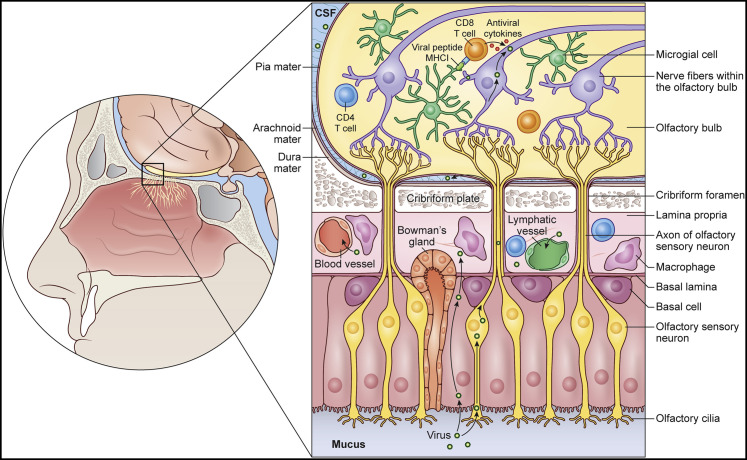
Figure 3.
Nasal epithelium and olfactory-bulb barriers
A sagittal view is shown of the olfactory mucosa and cribriform plate. The nasal mucosa is lined by both olfactory and respiratory epithelium, which is protected by macrophages, T cells, and B cells, among others. Olfactory sensory neurons, which play a role in olfaction, extend ciliary processes to the olfactory epithelium and detect odors in the airway. Certain air-borne neurotropic pathogens can infiltrate these processes and travel in a retrograde fashion, ultimately passing through the cribriform plate and into the olfactory bulbs. This route of infection allows pathogens to evade both the neurovascular and meningeal barriers. When pathogens bypass these barriers and enter the parenchyma, microglia can acquire microbes from neighboring cells including neurons and cross-present peptides to infiltrating pathogen-specific CD8+ T cells that then release antimicrobial cytokines such as IFNγ and TNFα. These cytokines can non-cytolytically purge pathogens (e.g., viruses) from adjacent neurons without harming them. After pathogen clearance, CD8+ Trms can establish residency in this space and provide protection against reinfection.
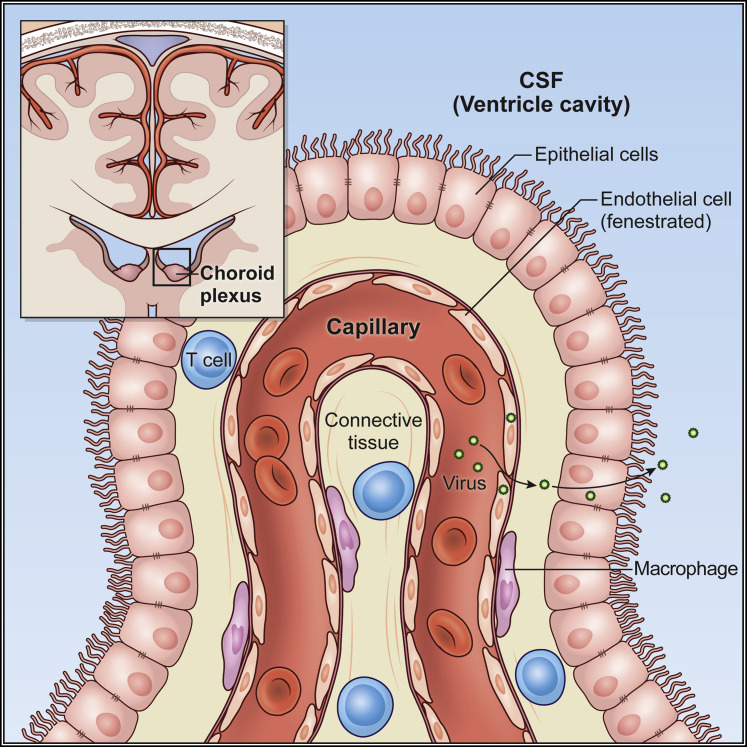
Figure 4.
Choroid plexus barrier
A schematic of the CP within the lateral ventricle is shown. The CP produces CSF and represents an important barrier interface between the blood and the CNS ventricular system. Blood vessels that enter the CP lack tight junctions and are fenestrated. However, the overlying epithelial layer has tight junctions, and this barrier limits the degree of molecular and cellular egress into the CSF-containing ventricles. The subependymal space has macrophages as well as some patrolling T cells. During states of systemic inflammation, the integrity of the epithelial cellular layer can become disrupted and lead to pathogen egress into the CSF. There are also pathogens that can directly infect the CP. Either scenario would elicit reactionary immune infiltration and movement of a pathogen into the CSF space would lead to ventriculitis.
Despite the presence of immunologically protected barriers, there are instances in which neurotropic microbes invade the CNS and elicit a neuroinflammatory response (Moseman et al., 2020; Swanson and McGavern, 2015b). In contrast to systemic infections, wherein robust immune cell infiltration is paramount in pathogen clearance, an excessive inflammatory response in the CNS can sometimes cause more harm than good. Excessive CNS immune cell infiltration or targeting of cerebrovasculature can lead to pathogenic brain swelling (Kim et al., 2009; Swanson et al., 2016), resulting in death of neurons—a cell population that for the most part cannot be replaced (Swanson and McGavern, 2015b). In situations where a pathogen is cleared from the CNS, memory immune cells are generated and retained within the CNS (Brizic et al., 2018; Wakim et al., 2010). These memory cells can protect the CNS upon reinfection and prevent a potentially injurious inflammatory response. Although vaccines promote the generation of systemic memory immune cells against certain pathogens, it is not entirely understood how and to what degree these memory cells protect CNS barriers from infection.
A recent real-world example of a virus that can affect the CNS in humans but is impeded by vaccination is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This acquired air-borne virus is responsible for the global coronavirus disease 2019 (COVID-19) pandemic. SARS-CoV-2 belongs to the coronavirus family of viruses, and other members of this family have been linked to CNS diseases. Although many symptoms experienced by COVID-19 patients are due to the impact on the respiratory and circulatory systems, there have been multiple reports of patients experiencing mental fogginess and changes in mentation (Woo et al., 2020). There are also some anecdotal reports of patients afflicted by severe neuroinflammation that are later diagnosed with the SARS-CoV-2 virus (Le Guennec et al., 2020). One question stemming from these reports is whether SARS-CoV-2 can traverse CNS barriers and cause acute and/or chronic neurological sequelae. In this review, we describe the anatomy and immunological defense of CNS barriers as well as our current understanding of how memory cells elicited by prior infection or vaccination protect these barriers. As a contemporary example, we also discuss the neuroinflammatory response to SARS-CoV-2 and how best to protect the CNS from this virus and other potentially deadly pathogens.
Development of CNS barriers
Embryonic development of hematopoietic stem cells (HSCs) is important for immune system formation and maturation. HSCs originate in the dorsal aorta around one month into embryonic development (Ivanovs et al., 2011). Later, more active hematopoiesis appears in the placenta, yolk sac, and liver. The liver ramps up the hematopoietic process until the bone marrow ultimately takes over during the second trimester of gestation (Zhang et al., 2017). The bone marrow becomes the main source of HSCs for the remainder of life. Myelopoiesis begins in the first month of the gestational period in the yolk sac (De Kleer et al., 2014). This also includes the generation of CNS-bound microglial cells that play a central role in innate and adaptive immunity. Circulating monocytes make an appearance once HSCs seed the embryonic liver. In this location, these HSC precursors develop into myeloid progenitor cells that can either become monocytes or dendritic cells, both of which have crucial roles in neuroimmunology.
The CNS has historically been considered an “immune-privileged” tissue due in part to the lack of standard lymphatic drainage and the presence of different barriers that isolate it from the periphery. Experiments conducted over a half-century ago demonstrated that implantation of foreign tissues into the intracranial compartment had a more successful survival rate than did systemic implantation (Carson et al., 2006; Medawar, 1948). More recent studies, however, rediscovered the presence of lymphatic vessels lining major venous sinuses in the dura mater and found that resident meningeal cells have a role in both innate and adaptive responses (Ahn et al., 2019; Aspelund et al., 2015; Fitzpatrick et al., 2020; Louveau et al., 2015; Rua and McGavern, 2018; Rustenhoven et al., 2021). Once a systemic immune response is elicited, the BBB, BCSFB, and meningeal barrier help regulate immune cell traffic into the CNS. These barriers impede and tightly control active leukocyte invasion of different CNS compartments and lessen the likelihood of an overt neuroinflammatory response.
In addition to regulating CNS immune cell traffic, the BBB also helps control entry of systemic substances and other cells from the periphery. Its unique anatomy is composed of pericytes, brain endothelial cells (BECs), basement membrane, and glia limitans perivascularis (Figure 1) (Haddad-Tovolli et al., 2017). Surrounding microglia, neurons, and immune cells also contribute to this anatomical structure, which is sometimes referred to as the neurovascular unit (Abbott et al., 2006; Neuwelt et al., 2011; Obermeier et al., 2013). On the abluminal surface of capillaries, a basement membrane separates BECs from pericytes and pericytes from astrocytes (Figure 1B) (Hallmann et al., 2005). Perivascular macrophages also localize to the perivascular space of postcapillary venules. These cells are bone-marrow derived with immunoregulatory roles and are activated during states of inflammation and disease (Williams et al., 2001). BECs are a key component of the BBB because they possess specific properties that peripheral endothelial cells lack (Andreone et al., 2015; Chow and Gu, 2015; Haddad-Tovolli et al., 2017). BECs are highly polarized and have tight injunctions that connect each adjacent cell. Both tight and adherens junctions in BECs facilitate cell-cell cross-talk and help maintain restrictive barrier functions (Tietz and Engelhardt, 2015). BECs also express low levels of leukocyte adhesion molecules under steady state, thereby limiting the degree of immune cell infiltration into the CNS while offering the potential to upregulate these molecules and promote immune surveillance during states of inflammation (Barkalow et al., 1996; Obermeier et al., 2013; Ransohoff and Engelhardt, 2012).
Ontogenesis of the BBB proceeds in a stepwise fashion that includes angiogenesis, differentiation, and maturation (Haddad-Tovolli et al., 2017). During the first trimester, the angiogenic phase begins during neural tube development. At this developmental stage, neuroectoderm produces vascular endothelial growth factor, which assists in developing previously established vasculature. Astrocytes and pericytes are recruited to the BBB during the differentiation phase when anti-angiogenic signals are turned on (Risau et al., 1986). The association of pericytes with endothelial cells is crucial for maintaining the BBB, because mice deficient in platelet-derived growth factor receptor β (a key pericyte growth factor) have disruptions in tight-junction distribution, leading to increased vascular permeability (Armulik et al., 2011; Bell et al., 2010; Daneman et al., 2010; Lindahl et al., 1997). Pericytes further limit permeability by enhancing tight-junction formation and decreasing the expression of leukocyte adhesion molecules through transforming growth factor β (TGF-β) signaling. A close interplay among BECs, astrocytes, and pericytes forms the functional BBB in the third trimester of life (Bauer et al., 1995; Ben-Zvi et al., 2014; Daneman et al., 2010).
Another important barrier in CNS development is the meninges. In vertebrates, the CNS is ensheathed by three protective layers collectively termed the meninges. The layers, from most superficial to deepest, are the dura mater, arachnoid mater, and pia mater (Figure 1A) (Mastorakos and McGavern, 2019). The dura mater is composed of two different layers: a periosteal layer near the skull and an endosteal layer that is adjacent to arachnoid mater. As the dura mater transcends inferiorly to cervical vertebrae that represent the start of the spinal column, it does not have a periosteal layer. Underneath the dura mater are both the arachnoid and pia mater, which together are referred to as the leptomeninges. Blood vessels and CSF are found within the subarachnoid space. The innermost layer, the pia mater, adheres to the parenchymal surface of the brain. There are astrocytes in the parenchyma beneath the pia mater that form another barrier, referred to as the glia limitans superficialis (Figure 1A) (Rua and McGavern, 2018). In the first trimester of ontogeny, mesenchymal cells are recruited and surround the hindbrain at the time of neural tube closure (Dasgupta and Jeong, 2019; O'Rahilly and Muller, 1986). This primitive mesenchymal sheath begins encasing the brain around the fifth week of human gestation (Dasgupta and Jeong, 2019; O'Rahilly and Muller, 1986). As development progresses, fibroblasts in the pial layer begin building a basement membrane through extracellular matrix (ECM) protein production that separates the brain and meningeal layers. In the middle of the first trimester, the mesenchymal layer around the CNS organizes into separate distinct layers (O'Rahilly and Muller, 1986). This sheath serves as the primordium for the scalp, skull, and meningeal layers (O'Rahilly and Muller, 1986). The meningeal layers then further differentiate into the dura mater, arachnoid mater, and pia mater in a basal to apical direction (Vivatbutsiri et al., 2008). When matured, the meningeal space contains lymphatic vasculature in the dura mater, which has been shown to continuously drain CSF into the cervical lymph nodes both in rodents and humans (Da Mesquita et al., 2018; Eide et al., 2018).
The meningeal subarachnoid space is filled with CSF that is generated by spongy material in the ventricles referred to as choroid plexus (CP) (Figure 4). Together these structures constitute the BCSFB, which is a specialized physical and biochemical barrier that helps maintain the CNS microenvironment. CP lines all ventricles except portions of the frontal horn, occipital horn, and cerebral aqueduct (Javed et al., 2021). In the human embryo, CP accounts for approximately 63% of the ventricular surface and has an impactful function in normal development (Voetmann, 1949). The BCSFB has a physical barrier consisting of tight junctions between adjacent epithelial cells that restrict molecules from entering the CNS ventricular system (Figure 4) (Liddelow, 2015). It also has a biochemical barrier that helps remove toxins and chemicals (Liddelow, 2015). During development, the anterior end of the neural tube invaginates and divides into three distinct regions (Imayoshi et al., 2008). The most medial region gives rise to CP epithelium (Imayoshi et al., 2008). The newly differentiated cells within the CP undergo maturation in four distinct stages that culminates in the epithelial cells transitioning to cuboidal cells (Imayoshi et al., 2008). Throughout the developmental process, the CP can generate CSF and restrict transfer of molecules to and from the systemic circulation.
Meningeal immune landscape
The meningeal barrier houses a diverse consortium of resident immune cells that can react to systemic perturbations and challenges (Figure 1A). Although immune cells are found throughout the meninges, they are especially concentrated and organized along vascular structures in the dura mater (Figures 1A and 2). Blood vessels within the dura mater do not have tight junctions and are often fenestrated (Mastorakos and McGavern, 2019). In addition, large fenestrated venous drains referred to as sinuses traverse the dura mater. These sinuses contain slow-moving blood (Schuchardt et al., 2015), which could render the dura mater vulnerable to circulating microbes (Fitzpatrick et al., 2020). It is likely that the immune system heavily monitors and defends vascular structures in the dura mater because a failure to do so could result in infection of underlying CNS tissue. In terms of immunology, lymphatics, and vasculature, the dura mater is unlike the rest of the CNS and resembles a peripheral tissue. The more traditional CNS-barrier system begins at the level of the arachnoid mater, which is sealed with tight-junction proteins. Blood vessels beneath the arachnoid mater (even those within the leptomeninges) also express tight-junction proteins—a defining feature of the BBB. CNS immune responses often begin in the meninges because they are easier to access and contain a more diverse immune repertoire under steady state than under the CNS parenchyma (Filiano et al., 2015; Fitzpatrick et al., 2020; Kim et al., 2009; Korin et al., 2017; Rua and McGavern, 2018; Rustenhoven et al., 2021).
Resident meningeal immune cells consist of monocytes/macrophages, neutrophils, natural killer cells, mast cells, innate lymphoid cells, B cells, T cells, and dendritic cells (DCs), among others (Brioschi et al., 2021; Cugurra et al., 2021; Fitzpatrick et al., 2020; Korin et al., 2017; Mrdjen et al., 2018; Rustenhoven et al., 2021). Meningeal T cells can access the deep cervical lymph nodes (dCLNs), and parabiotic experiments revealed that a proportion of these cells are derived hematogenously (Radjavi et al., 2014). After parabiosis, naive CD44 negative CD4+ T cells were able to equilibrate evenly between hosts, whereas CD44hi effector/memory cells did not (Radjavi et al., 2014). These data suggest the existence of a potential immunoregulatory barrier that limits the entrance of effector/memory T cells into the meninges during steady state. The influence of the dCLNs on meningeal T cell traffic was determined by surgically ablating them (Radjavi et al., 2014). dCLN removal elevated the number of meningeal CD4+ T cells two weeks after surgery. Together, these data suggest that CD4+ T cells (preferentially naive) travel through the vasculature and enter the meninges, where they survey the space, prior to egressing via dural lympahtics to secondary lymphoid tissues. Although licensing of CNS-specific T cells has been noted in other organs, such as the lungs (Odoardi et al., 2012), the dCLNs offer a more direct route for the detection of meningeal and CSF-derived antigens. In fact, recent studies have demonstrated that CSF antigens tend to accumulate around the dural venous sinuses, where they are engulfed by resident antigen-presenting cells (APCs) (Rustenhoven et al., 2021). The APCs then present these antigens to patrolling or resident T cells, which can lead to T cell activation.
The CCR7-CCL21 pathway is used by both DCs and T cells to migrate through lymphatic vasculature, assisting in both entry and egress under physiological conditions (Debes et al., 2005; Weber et al., 2013). About 40% of resident meningeal T cells express CCR7, and most were CD69− CD62L−/int—a surface phenotype suggesting that they can recirculate (Louveau et al., 2018). Meningeal T cells reside near lymphatic endothelial cells, and loss of CCR7 led to partial exclusion of T cells from the lymphatic compartment. Another study demonstrated that CCR7-deficient DCs were similarly retained in the CNS (Clarkson et al., 2017). Meningeal lymphatics appear to play a role in T cell egress from the meningeal space, because lymphatic ablation decreased their presence in both the superficial and dCLNs (Louveau et al., 2018). The functional importance of the meningeal lymphatics in local T cell immunity was established by using the experimental autoimmune encephalomyelitis (EAE) model of human multiple sclerosis. Ablation of meningeal lymphatic drainage reduced the pathogenicity of autoreactive CD4+ T cells as well as disease severity in mice with EAE (Louveau et al., 2018). However, it is important to note that disease was dampened but not aborted in these studies, which signifies that T cells directed against CNS antigens can be licensed with effector programming in other lymphoid structures besides the dCLNs.
From the standpoint of antimicrobial immunity, the meninges are home to a dense network of resident macrophages that continuously survey the environment akin to parenchymal microglia (Herz et al., 2017; Nayak et al., 2012, 2014). They are primarily situated along fenestrated blood vessels and venous sinuses in the dura mater and have the potential to influence the underlying leptomeninges and CNS parenchyma during states of inflammation and infection (Kipnis, 2016; Roth et al., 2014; Rua and McGavern, 2018). Meningeal macrophages in the dura mater are relatively long-lived cells but are replenished by monocytes during adulthood, similarly to CP macrophages (Rua et al., 2019; Utz et al., 2020). During homeostasis, hematopoietic bone-marrow niches in the skull bone marrow (Figure 2) serve as a major source of meningeal monocytes, although inflammation can promote significant recruitment from the blood as well (Cugurra et al., 2021; Herisson et al., 2018; Rua et al., 2019). These monocytes and resident macrophages can respond quickly to pathogens and provide an important barrier defense for the CNS. A blood-borne pathogen that is rapidly controlled in the dura mater will not have the ability to invade the CNS and cause a potentially life-threatening disease (Fung-Leung et al., 1991; Kang et al., 2011; Kim et al., 2009; Rua et al., 2019; Rua and McGavern, 2018). One relevant example of this scenario is the fatal meningitis caused by lymphocytic choriomeningitis virus (LCMV). LCMV is a noncytopathic arenavirus that rarely causes fatal meningitis in mice unless the CNS barrier systems are bypassed by injecting the virus intracerebrally, which triggers an uniformly fatal disease (Kang and McGavern, 2008). The defense against LCMV is mediated in part by meningeal macrophages that become infected by the virus and are then eliminated by cytotoxic T cells (CTLs) (Rua et al., 2019). Meningeal macrophages likely play a key role in limiting the degree to which LCMV enters the meninges from the blood when the virus is introduced into the periphery (e.g., intravenous inoculation). If this defense fails, then LCMV has the potential to spread throughout the meninges, causing fatal immune-mediated brain swelling (Kim et al., 2009).
The meningeal immune landscape is not static but instead is continually shaped and reshaped by peripheral challenges. For example, a recent study showed that in a model of sub-lethal LCMV meningitis, the functional properties of meningeal macrophages in the dura mater remain altered for months after viral clearance (Rua et al., 2019). Deletion of infected meningeal macrophages by CTLs is followed by replenishment from infiltrating blood-derived monocytes. Fate-mapping studies revealed that these monocytes became meningeal macrophages that had elevated expression of major histocompatibility complex (MHC) I and MHC II but were deficient in specific bacterial and immunoregulatory sensors such as a CD209b/SIGNR-1 and the acetylcholine receptor, respectively (Rua et al., 2019). SIGNR-1, a C-type lectin receptor, participates in the recognition of microbial carbohydrates, which promotes downstream inflammatory signaling. SIGNR-1 deficiency decreases the ability of meningeal macrophages to respond to bacterial lipopolysaccharides and could render the meninges more susceptible to future infections. These data demonstrate how clearance of one type of pathogen (a virus) could open the CNS up to infection by another (bacteria).
Another functional change observed in newly engrafted meningeal macrophages was a decrease in the acetylcholine receptor (Rua et al., 2019). Acetylcholine is a neurotransmitter that can be released by CD4+ T cells and parasympathetic branches from trigeminal ganglia that innervate the dura mater (Coles et al., 2017; Rosas-Ballina et al., 2011) and is known to dampen proinflammatory responses in macrophages (Pavlov and Tracey, 2012). Release of acetylcholine is likely an important mechanism by which resident T cells and peripheral nerves prevent excessive inflammation in the meninges. However, a prior infection can decrease meningeal macrophage responsiveness to this important regulator, which has the potential to make the meninges more inflammable. Collectively, these findings demonstrate how recovery from a viral infection can imprint the dura mater with new immunological properties.
B cells represent another important immune cell population that resides in the healthy meninges. B cells represent up to 30% of resident immune cells in the dura mater (Brioschi et al., 2021; Cugurra et al., 2021; Fitzpatrick et al., 2020; Jain and Yong, 2021; Korin et al., 2017; Schafflick et al., 2021; Wang et al., 2021). Resident meningeal B cells exist in multiple stages of development, similar to those found in the bone marrow (e.g., mature naive B cells, immature B cells, pre-B cells, pro-B cells, and mitotic B cells) (Brioschi et al., 2021; Schafflick et al., 2021; Wang et al., 2021). These B cells are mostly localized extravascularly along the dural venous sinuses and are relatively immobile (Brioschi et al., 2021; Fitzpatrick et al., 2020). Recent studies have also shown that meningeal B cells are derived from bone-marrow cavities in the adjacent skull bone (Figure 2) (Brioschi et al., 2021; Wang et al., 2021). Although circulating B cells only minimally infiltrate the meningeal space during steady state, the majority migrate through specialized vascular channels (diploic veins) that traverse the inner skull bone (Brioschi et al., 2021; Wang et al., 2021). These vascular channels form a direct portal between skull bone and dura mater, allowing for immune cell egress into the meningeal space independent of periphery. It is thought that some B cells with specificity to CNS antigens undergo negative selection in the meningeal space, which helps preserve CNS tolerance (Brioschi et al., 2021; Wang et al., 2021). There is also a chemokine gradient established by dural fibroblast-like cells in the meninges to facilitate B cell recruitment to this microenvironment. Specifically, B cells express the chemokine receptor CXCR4 and migrate to fibroblast-like cells that are enriched in CXCL12 (Brioschi et al., 2021).
As mice age, they start accumulating B cells from the periphery. These aged B cells have transcriptional changes and an increased accumulation of somatic mutations, which indicates that they are antigen experienced (Brioschi et al., 2021). Aged B cells infiltrate the meningeal space from the periphery and undergo differentiation into immunoglobulin-secreting plasma cells (Brioschi et al., 2021). Under homeostasis, there is a paucity of meningeal plasma cells in young mice, and most are gut-derived IgA+ plasma cells (Fitzpatrick et al., 2020), but peripherally derived IgM+ and IgG+ plasma cells were shown to increase with age (Brioschi et al., 2021). Meningeal resident IgA+ plasma cells are located adjacent to dural venous sinuses, which are fenestrated and can potentially allow blood-borne pathogens to access the CNS (Figure 2) (Fitzpatrick et al., 2020). A recent study demonstrated that meningeal IgA+ plasma cells entrap circulating pathogens within the lumen of the dural sinuses. In mice with a blood-borne Candida albicans infection, local depletion of meningeal IgA+ plasma cells resulted in reduced fungal entrapment within the sinuses, which led to subsequent infection of the brain parenchyma (Fitzpatrick et al., 2020). These data provide a clear example of how failure to contain a pathogen in a barrier like the dural sinuses can result in parenchymal encroachment and CNS disease.
Defense of the nasal epithelium and olfactory bulbs
One method by which neurotropic viruses bypass CNS barriers is by targeting olfactory sensory neurons (OSNs), which are located within the nasal cavity (Figure 3). OSNs are in the mucosal upper airway surface and are exposed to multiple air-borne pathogens. Olfactory sensory information from the nose is conveyed via OSN axon fibers that pass through the cribriform plate and connect to the olfactory bulbs. This neural system is responsible for our sense of smell. Studies have shown that there are lymphatic vessels in the dura mater adjacent to the cribriform plate, and these vessels appear to access and sample CSF due to gaps in the arachnoid barrier (Hsu et al., 2019, 2022; Louveau et al., 2018). Studies also suggest that CSF can drain through the cribriform plate into the nasal lymphatics that reside outside of the skull (Kida et al., 1993), although this finding is more controversial (Louveau et al., 2018). Nevertheless, lymphatic ablation along the cribriform plate reduces immune cell accumulation within the superficial, but not deep, CLNs (Louveau et al., 2018). These data suggest that cribriform plate lymphatics drain primarily into the superficial cervical lymph nodes. During states of neuroinflammation, notable lymphangiogenesis was observed around the cribriform plate that did not occur with lymphatics along the dural venous sinuses (Hsu et al., 2019, 2021, 2022; Louveau et al., 2018). The vulnerability of the olfactory system to nasal pathogens could explain why lymphatic vessels along the cribriform plate expand so readily in response to inflammatory stimuli. In addition, DCs were shown to be retained in this region during an inflammatory response, which could facilitate local antigen presentation to T cells (Hsu et al., 2022). Antigen presentation along with lymphangiogenesis in cribriform-plate dura mater likely help protect the olfactory bulbs during the acute stages of an infection. Pathogens inhaled into the nose have the potential to infect OSNs (whose dendrites dangle in the airways) and travel along axon fibers through the cribriform plate into the brain parenchyma (Figure 3). This route of infection allows pathogens to evade an important olfactory barrier designed to prevent CNS invasion.
There are many viruses that use OSNs to invade the CNS, including influenza A virus, herpesviruses, vesicular stomatitis virus (VSV), and adenoviruses (van Riel et al., 2015). Pathogens can infect OSNs and be transported in a retrograde fashion to the olfactory bulb, but another mechanism is diffusion through channels formed by olfactory ensheathing cells (van Riel et al., 2015). When a pathogen does invade the CNS, a balance must be maintained between clearing the pathogen and minimizing damage to neurons, most of which lack the ability to regenerate (Swanson and McGavern, 2015b). One strategy commonly employed by the immune system to purge pathogens without causing cellular damage is release of non-cytolytic cytokines and interferons (Binder and Griffin, 2001; Burdeinick-Kerr et al., 2009; Detje et al., 2009). For example, release of interferon (IFN)γ by CD8+ T cells, CD4+ T cells, and natural killer cells is one of the primary mechanisms by which the immune system controls neurotropic viruses (Cantin et al., 1995; Liu et al., 2001; Patterson et al., 2002; Tishon et al., 2006). Cytokines like IFNγ can activate non-cytolytic antiviral machinery inside of cells that results in removal of viral nuclei acids and proteins without cellular death. Use of non-cytolytic effector mechanisms is employed when clearing viruses from neural residents to minimize CNS damage (Herz et al., 2015; Moseman et al., 2020).
VSV is a commonly studied neurotropic virus in mice that represents an excellent example of the immunological barrier defense against a nasal pathogen that infects OSNs and attempts to enter the brain. VSV preferentially infects the olfactory epithelium over respiratory epithelium when inoculated intranasally (Lundh et al., 1987). The virus replicates in OSNs and then travels via their projecting axon fibers to the olfactory bulb where it is usually halted at the glomerular layer. However, if the immune system does not stop the virus in this location, it spreads caudally throughout the brain parenchyma, eventually causing a fatal paralytic disease by infecting the brainstem and spinal cord (Detje et al., 2009). VSV that escapes the glomerular layer is believed to use retrograde axonal transport and the ventricular system as it migrates caudally (Cornish et al., 2001).
Resident microglia defend the olfactory bulb during steady state, but, in response to infection, there is a large influx of peripheral immune cells that surround infected glomeruli. These infiltrates consist of neutrophils and natural killer cells initially and then CD8+ T cells, CD4+ T cells, and Ly6chi monocytes as infection progresses. In response to VSV, virus-specific CD8+ and CD4+ T cells migrate into the olfactory bulbs after being primed in the CLNs and spleen (Moseman et al., 2020). Antiviral T cells play a crucial role in halting VSV within the olfactory bulbs, because T cell depletion results in viral spread throughout the CNS and development of fatal disease. The T cell effector molecules IFNγ, tumor necrosis factor α (TNFα), and perforin all participate in controlling the virus; however, direct T cell engagement of infected cells is not required (Moseman et al., 2020). Within the CNS parenchyma, VSV is known to primarily infect neurons, although most neurons minimize T cell engagement because they display relatively low levels of peptide MHC I on their cell surface (Joly and Oldstone, 1992; Moseman et al., 2020). In addition, genetic removal of MHC I expression from VSV-infected cells in vivo has no impact on viral control (Moseman et al., 2020). Intravital imaging studies revealed instead that virus-specific CD8+ T cells non-cytolytically clear VSV from infected neurons by engaging cross-presenting microglia (Figure 3). The microglia are not infected by the virus but instead acquire and cross-present viral antigens obtained from adjacent neurons (Moseman et al., 2020).
CD8+ T cell engagement of microglia is a mechanism that spares infected neurons from potential killing by cytolytic effector mechanisms like granzymes and perforin. T cell engagement of microglia instead of neurons allows the latter to be cleared exclusively via non-cytolytic cytokines (e.g., IFNγ and TNFα). The dependence on cytokines to purge invading viruses in a non-cytolytic manner is not unique to VSV infection but is seen with other viruses such as influenza virus, hepatitis B virus, and LCMV, among many others (Guidotti and Chisari, 2001). Protection of the olfactory system and the CNS depends on the rapid use of these effector mechanisms by innate and adaptive immune cells. The speed and efficiency of the immune response is important because the olfactory system is an especially vulnerable CNS barrier due to its continual exposure to air-borne pathogens.
Choroid plexus immunology
Relative to the BBB, the blood-CSF barrier within the CP has different properties that allow it to have a direct relationship between CSF and systemic circulation (Dani et al., 2021). CP vascular endothelial cells are fenestrated and lack tight junctions or a glia limitans, which are defining features of the BBB (Figure 4) (Ransohoff et al., 2003). Solutes can extravasate from fenestrated capillaries but cannot enter the CSF space because of a tight-junction-expressing epithelial barrier. Within the CP, there are specialized populations of macrophages as well as bone-marrow-derived DCs that can present antigens and stimulate T cells (Anandasabapathy et al., 2011; Goldmann et al., 2016; Nayak et al., 2012). CP DCs only have a half-life of 5–7 days and are replenished by migrating progenitor cells (Anandasabapathy et al., 2011). By contrast, resident macrophages have longer lifespans and are derived both from hematopoietic precursors during development as well as from blood-borne monocytes during adulthood (Cui et al., 2021; Goldmann et al., 2016).
The majority of T cells located in the CSF space under non-pathological conditions in humans are central memory cells (CD45RO+, CD27+) and CD4+ (Svenningsson et al., 1993). B cells are relatively infrequent in the CSF of healthy patients but can increase in cases of autoimmunity and infection (Cepok et al., 2001; Eggers et al., 2017). One possible mechanism by which lymphocytes enter the CSF is through the CP (Ransohoff et al., 2003). For example, blood-derived lymphocytes can extravasate through CP vasculature, interact with choroidal epithelium, and then enter the CSF (Ransohoff et al., 2003). Studies in mice have shown that lymphocytes can enter and survey the CP and meninges in a P-selectin-dependent manner (Carrithers et al., 2002). Unlike parenchymal CNS blood vessels, vasculature in the CP and meninges express P selectin during steady state, which facilitates peripheral immune surveillance of these two barrier sites (Carrithers et al., 2000, 2002). The mechanism and degree to which T cells traverse CP epithelial cells and enter the CSF space is not well understood. However, T cells found within the uninflamed CSF express the chemokine receptors CXCR3 and CCR6, suggesting that corresponding chemokines are required to recruit and retain T cells in this compartment (Kivisakk et al., 2002). It will be important in future studies to determine how frequently steady state T cells enter the CSF space by using meningeal (Figure 1A) versus CP blood vessels (Figure 4).
CP vasculature and epithelial cells is a common entry point for pathogens to access the ventricular system and meninges (Figure 4) (Swanson and McGavern, 2015a). Viruses such as LCMV, coxsackievirus B3, chikungunya virus, cytomegalovirus, HIV, and influenza virus, among others have all been shown to infect the CP, which provides a potential gateway into the CNS (Swanson and McGavern, 2015a). Other pathogens such as bacteria and fungi can also access this barrier site causing CNS diseases. For example, there are multiple bacteria (e.g., Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis) capable of causing meningitis, which is a potentially fatal CNS disease driven by microbial invasion of the meninges and CSF spaces. When bacterial loads elevate in the blood, CNS barriers like the CP and meninges become susceptible to infection. To fend off bacteria, rapid recognition of the pathogen must occur via toll-like receptors (TLRs) and other pattern recognition receptors (PRRs). This is typically followed by a massive recruitment of myelomonocytic cells and lymphocytes. The S. pneumoniae model provides a good example of how CNS barriers respond immunologically to a bacterial infection (Prager et al., 2017). S. pneumoniae typically colonize nasopharynx mucosa in humans but can move from there into the blood supply. From the blood, S. pneumoniae has developed strategies to traverse the BBB and CP epithelium. This triggers a massive CSF inflammatory response that is dependent in part of TLR-2 and TLR-4 signaling (Echchannaoui et al., 2002; Klein et al., 2008). In fact, mice deficient in TLR2 signaling showed decreased survival even after administration of antibiotics (Echchannaoui et al., 2002)—a standard treatment for bacterial meningitis. The key defenses against a microbe like S. pneumoniae include free radical release, engulfment, complement, and antibodies. The challenge for the host, however, is to elicit these responses before bacteria or other pathogens traverse CNS barriers and begin damaging the parenchyma.
Cerebrovasculature and the BBB
The BBB is a physical and immunological vascular barrier that coordinates the exchange of substances between the CNS parenchyma and systemic circulation. This vascular barrier consists of tight-junction-expressing endothelial cells, basement membrane, pericytes/smooth-muscle cells, and perivascular macrophages. Within the parenchyma, this structure interacts with adjacent astrocytes, neurons, and microglia (Abbott et al., 2006; Mastorakos and McGavern, 2019; Nedergaard et al., 2003). Two important features of the BBB are its endothelial tight junctions and secondary wrapping by astrocytic endfeet (referred to as the glia limitans perivascularis) that regulate the movement of molecules and immune cells into the CNS parenchyma (Kacem et al., 1998; Mastorakos and McGavern, 2019). From an immunological perspective, cerebrovascular endothelial cells are the first line of defense in the CNS parenchyma, and these cells express TLRs and other PRRs responsible for detecting pathogen-associated molecular patterns (Medzhitov and Janeway, 2002). These PRRs alert cerebrovasculature to the presence of pathogens in the blood. For example, cerebral endothelial cells express TLR2, TLR3, TLR4, and TLR6 (Nagyoszi et al., 2010) and, by using TLR4, can respond to lipopolysaccharide (LPS), an outer membrane component Gram-negative bacteria. LPS can in fact disrupt the function of cerebrovascular endothelial cells by reducing the activity of P-glycoprotein (Salkeni et al., 2009), which is an ATP-dependent brain-to-blood efflux pump that limits entry of drugs, toxins, and other blood-derived substances into the CNS. Reduction of P-glycoprotein expression provides one mechanism by which a pathogen in circulation can initiate an inflammatory response that disrupts BBB function and promotes subsequent CNS dysregulation. P-glycoprotein was also shown to facilitate the release of chemoattractants that promote extravasation of CD8+ T cells across brain vasculature. By using the EAE model of autoimmune disease, it was shown that P-glycoprotein regulates the secretion of CCL2 (Kooij et al., 2014)—a chemokine known to recruit monocytes and T cells to sites of inflammation. Reduction of P-glycoprotein or CCL2 in endothelial cells decreased accumulation of CNS CD8+ T cells (Kooij et al., 2014). Together these data suggest that activation of TLRs in cerebrovascular endothelial cells can help reduce immune cell traffic to the CNS by down-regulating P-glycoprotein, but this could also expose the CNS to disruptive inflammatory mediators once the function of this important efflux pump becomes suboptimal. Regulation of BBB integrity and CNS function depends on maintenance of a careful balance that can become completely disrupted during states of infection.
Even though immune cells and inflammatory mediators can disrupt the BBB, it is important to remember that the system is designed to help protect the barrier and prevent the entry of pathogens into the CNS. Cytokines and peptides produced during an acute inflammatory response can, in fact, reduce pathogen access to the CNS parenchyma. For example, it was shown in a model of West Nile virus (WNV) infection that IFN-λ (a type III interferon) increases transendothelial electrical resistance in the BBB and reduces its permeability by modulating the localization of tight-junction proteins (Lazear et al., 2015). BBB fortification by IFN-λ helped protect the CNS from a lethal WNV infection without affecting the amount of virus in circulation. These data demonstrate that when a pathogen enters circulation, one way for a host to protect the CNS is to strengthen the BBB. Another is to rapidly to defend the barrier. Local production of TNFα after viral infection can lead to production of the CCR7 ligands, CCL19 and CCL21, by cerebrovascular endothelial cells and fibroblastic reticular-like cells residing in perivascular spaces. This response promotes the recruitment and local reactivation of antiviral CD8+ T cells that help protect the brain from developing a fatal encephalitis like the one induced by mouse hepatitis virus (MHV) (Cupovic et al., 2016).
Despite the existence of BBB defense mechanisms to protect the CNS parenchyma from irreparable damage after peripheral infection, the immune system is not always perfect and can generate unfavorable outcomes. In response to Japanese encephalitis virus infection, microglia produce a plethora of different proinflammatory mediators, such as inducible interleukin (IL)-6, IL-1β, TNFα, nitric oxide synthase, and cyclooxygenase-2, that contribute to inadvertent neuronal cell death (Ghoshal et al., 2007). Microglia can also strip neurons of their synapses in a complement-dependent manner after CNS viral infection, leading to long-term memory loss and cognitive impairment (Vasek et al., 2016). Even immune cell extravasation across the BBB can cause serious CNS damage. Orderly peripheral immune cell extravasation does not disrupt endothelial tight junctions (Wolburg et al., 2005), which allows the barrier to quickly reseal. However, during states of heavy CNS damage or infection, intravital imaging studies have shown that myelomonocytic cells can synchronously extravasation from CNS vasculature, causing life-threatening brain swelling (Kim et al., 2009; Mastorakos et al., 2021). An uncontrolled buildup of water in the brain due to BBB breakdown can lead rapid brainstem herniation and death. This can also occur when the BBB itself is directly attacked by the immune system. During cerebral malaria in rodents and humans, infection of red blood cells by the parasite Plasmodium falciparum (humans) or berghei (rodents) results in cross-presentation of parasite antigen (acquired from the blood) by cerebrovascular endothelial cells. This causes circulating parasite-specific CD8+ T cells to engage cerebrovascular endothelial cells, resulting in BBB leakage and fatal brain swelling due to altered tight-junction protein expression (Howland et al., 2013; Riggle et al., 2020; Seydel et al., 2015; Swanson et al., 2016). This fatal disease represents a misdirected immune attack, because cerebrovascular endothelial cells are never actually infected by the parasite. These studies combined demonstrate that disruption of vascular integrity by the immune system is a common mechanism by which the CNS becomes injured in response to infection.
Impact of age on CNS barriers
With age there are many immunological changes in CNS barriers, including an increase in class-switched plasma cells; shifts in T cell number, location, and activation status; decay in lymphatic drainage; alterations cytokine production and signaling; etc. (Baruch et al., 2013, 2014; Brioschi et al., 2021; Da Mesquita et al., 2018, 2021; Fitzpatrick et al., 2020). For example, T cells in the meninges of young mice localize primarily along the dural venous sinuses, where they are in proximity to lymphatic vasculature and interact with local antigen-presenting cells (Rustenhoven et al., 2021). In older mice, CD8+ and CD4+ T cells increase in number and broaden their meningeal distribution to include non-sinus locations (Rustenhoven et al., 2021), suggesting enhanced surveillance. Lymphocytic CCR7 expression also decreases with age in the meninges, which leads to accumulation of both effector and regulatory T cells (Da Mesquita et al., 2021). This accumulation is associated with a decline in cognition, which is likely due to alterations in meningeal homeostasis. In addition to the meninges, an increased number of CD8+ T cells is also found in the choroid plexus and CNS parenchyma with age (Ritzel et al., 2016). Within the choroid plexus, enhanced T cell surveillance is explained in part by increased type-I IFN signaling (Baruch et al., 2014), an innate immune response that is typically associated with antiviral immunity. Importantly, inhibition of type-I IFN signaling in aged mice partially restores hippocampal neurogenesis and improves cognition (Baruch et al., 2013, 2014). These data demonstrate that CNS barriers become more inflamed with age, which could help ward off invading pathogens. However, disruptions in barrier homeostasis and the associated cognitive decline might predispose aged mice and humans to unfavorable outcomes and poor recovery trajectories when pathogens do invade the CNS.
Protection of CNS barriers by immunological memory and vaccination
Although the CNS is relatively sealed off compared to most peripheral tissues, it must still be protected from rechallenge by commonly encountered pathogens. When the CNS or its barriers become infected, some of the infiltrating lymphocytes with specificity to the pathogen establish long-term residency. Systemically, after pathogen clearance, expanded effector T cells diminish in numbers, but some become memory T cells (Gourley et al., 2004; Harty and Badovinac, 2008; Masopust and Soerens, 2019; Sallusto et al., 2004). There are three subtypes of memory T cells: tissue resident memory T cells (Trms), central memory T cells (Tcms), and effector memory T cells (Tems). Trms are non-circulating memory T cells that reside in previously infected tissues and can elicit a local protective response upon reinfection (Farber et al., 2014; Masopust and Soerens, 2019; Steinert et al., 2015). Similar to other peripheral organs, once a pathogen is cleared from the CNS, a specialized subset of brain-resident memory T cells are generated and reside in situ, often in close proximity to vasculature or CNS barriers (Wakim et al., 2010). By contrast, Tcms express both CD62L and CCR7, allowing them to enter and reside within secondary lymphoid tissues (like CCR7+ T cells draining into CLNs from the meningeal space) (Cupovic et al., 2016; Louveau et al., 2018; Prasad and Lokensgard, 2019; Sallusto et al., 2004). Conversely, Tems lack CD62L and CCR7, which allows them to remain in the bloodstream and recirculate, thus representing a memory cell population that can be recruited to a tissue upon reinfection (Sallusto et al., 2004).
These three different memory T cell subsets play a crucial role in protecting against tissue reinfection throughout the body (Gebhardt et al., 2009; Hogan et al., 2001; Liang et al., 1994). This protection is especially important for pathogens that are encountered routinely in the environment or that establish latency within host cells and reactivate over time. Surveillance of tissues by circulating microbe-specific memory T cells varies. Parabiosis studies have revealed that circulating memory CD8+ T cells rapidly equilibrate in lymphoid tissues, lung, and liver, but not the brain, peritoneal cavity, and intestinal lamina propria (Klonowski et al., 2004). These data suggest memory T cell entry is restricted and/or tightly regulated in certain tissues. Within the CNS, memory T cells are more likely to access and survey barrier sites like the meninges, CP, olfactory borders, and peri-sinus regions than the parenchyma, which is sealed by the BBB and glia limitans. Study of T cell surveillance during the early stages of an autoimmune disease (EAE) has shed light on how effector/memory T cells scan the CNS borders. By using intravital microscopy, it was revealed that autoreactive CD4+ T cells extravasated from leptomeningeal vessels in a very late antigen 4 (VLA-4)-dependent manner and then interacted with APCs (Bartholomaus et al., 2009). Expression of the adhesion molecule, vascular cell adhesion molecule 1 (VCAM-1), is commonly used by vascular endothelial cells to promote surveillance of CNS barriers by VLA-4+ effector/memory T cells (Yednock et al., 1992). T cell scanning of the dural venous sinuses is also dependent in part on VLA-4/VCAM-1 interactions (Rustenhoven et al., 2021). These barrier surveillance programs used by effector/memory T cells are crucial for detecting and rapidly responding to pathogens but can sometimes give rise to CNS autoimmune diseases.
In situations where a pathogen invades and is controlled within the CNS, studies have shown that Trms can establish residency in the parenchyma and surrounding barriers. After intranasal inoculation of immunocompetent adult mice, VSV minimally invades the brain parenchyma but then is cleared. This results in the deposition of CD8+ Trms in the brain parenchyma that appear to cluster in areas of prior infection but do not require antigen for their maintenance (Wakim et al., 2010). Injection of DCs displaying viral peptides into the parenchyma (a method of promoting transient antigen presentation) also generates CD8+ Trms with a similar phenotype to those observed after VSV infection (Wakim et al., 2010). These data demonstrate that local antigen presentation, but not direct infection of the CNS, is required for the generation of brain-resident Trm. In fact, CNS infection by several different pathogens, including mouse cytomegalovirus (Prasad et al., 2017), VSV (Wakim et al., 2010), LCMV (Steinbach et al., 2016), Toxoplasma gondii (Landrith et al., 2017), polyomavirus (Mockus et al., 2018), and replication-deficient adenovirus (Scholler et al., 2019), all promote inhabitance by CD8+ Trms. A recent study also suggested that CNS Trms could be generated by peripheral (intravenous) immunization, an approach that almost certainly challenges CNS barriers like the dural venous sinuses, choroid plexus, etc. (Urban et al., 2020). Similar to Trms in other tissues, a high proportion of those in the brain have elevated expression of CD103, an E-cadherin ligand (Brizic et al., 2018; Wakim et al., 2010). CD103 knockdown in virus-specific CD8+ T cells substantially decreased Trms in the brain after VSV clearance (Wakim et al., 2010), and it is postulated that this ligand contributes to Trm retention in tissues. However, CD103 is not the only molecule responsible for Trm retention, as it is not found on all Trms, and its expression varies depending on the model of infection/immunization (Brizic et al., 2018; Steinert et al., 2015; Urban et al., 2020; Wakim et al., 2010). Trms have been shown to protect the CNS after reinfection, even in the absence of circulating memory T cells. They accomplish this by rapidly reacquiring effector functions, utilizing both perforin and IFN-γ to limit pathogen spread (Steinbach et al., 2016). Collectively, these data demonstrate that the presence of Trms in the CNS parenchyma and its borders provide these compartments with crucial protection against reinfection.
In comparison to what we know about memory T cells, our knowledge about CNS memory B cells is sparse. Humoral immunity contributes an important part to immunological memory that is maintained by memory B cells and long-lived plasma cells, which are responsible for secreting antimicrobial antibodies (Akkaya et al., 2020; Phan and Tangye, 2017; Weisel and Shlomchik, 2017). CNS infection induces B cell recruitment to the brain and spinal cord that changes with time, indicating turnover and differentiation (Atkinson et al., 2019; DiSano et al., 2017; Metcalf et al., 2013; Phares et al., 2014). For example, naive B cells are recruited in the early stages of a MHV infection that localize primarily to the CNS meninges and perivascular spaces (Atkinson et al., 2019; Phares et al., 2014). These cells are replaced over time by class-switched B cells and antibody-secreting plasma cells that enter the CNS parenchyma as the virus establishes persistence (Atkinson et al., 2019; Phares et al., 2014). Antiviral B cells against MHV appear to be generated within germinal centers (GCs) formed in secondary lymphoid tissues, because no evidence of local GC formation was discovered in the CNS of infected mice (DiSano et al., 2017). Infection with Sindbis virus (SINV), an encephalitic alphavirus, also induces recruitment of class-switched B cells and antibody secreting cells (ASCs) into the CNS (Metcalf et al., 2013). Recruited B cells expressed CXCR4, CXCR5, and CCR7 that were drawn into the CNS via local production of CCL1, CCL2, CCL5, CXCL9, and CXCL10. Importantly, SINV-specific ASCs were found in the CNS for up to six months after viral clearance (Metcalf et al., 2013), suggesting that humoral immune memory can be maintained in this compartment. The location of the long-lived ASCs is unclear. It will therefore be important in future studies to determine how and where different memory B cell subsets are maintained in the CNS after infection. It will also be interesting to explore any CNS niches supportive of local B cell class switching and affinity maturation.
Vaccines are used to induce an immune response and equip the body with immunological memory. They can be live or inactivated, although the former is potentially more dangerous in immunocompromised individuals. Most vaccines confer their benefit by generating long-lived memory ASCs capable of producing pathogen-specific antibodies. The BBB limits the entry of most molecules, including antibodies, into the CNS (Yu et al., 2011), which means that ASCs elicited by peripheral vaccination are unlikely to exert their protection locally. It is also unlikely that a peripheral vaccine would promote the establishment of Trms in the CNS unless the delivered antigen traverses its barriers, which most are not designed to do. However, it might be possible for a peripheral vaccine to enter circulation and generate Trms in the dura mater by traversing its fenestrated vasculature. This could in turn provide some level of protection for the underlying CNS. Nevertheless, because vaccines are not designed to elicit Trms that reside behind CNS barriers, they often protect this compartment by eliciting peripheral memory cells that prevent the spread of pathogen. For example, having ASCs, Tcms, and Tems distributed throughout the periphery limits the early expansion of a pathogen and decreases the probability that it will enter the CNS. If a pathogen does enter the CNS parenchyma, then circulating Tems and antibodies should help facilitate rapid local control. However, pathogens that circumvent CNS barriers by traveling in nerve fibers (Figure 3) or circulating immune cells (Figure 1) could still pose some challenges to peripheral memory cells (Swanson and McGavern, 2015a). The nasal barrier in particular is regularly exposed to inhaled pathogens, which must require a heightened degree of immunological vigilance to prevent CNS infections.
Many modern vaccine strategies aim to induce immunological responses by introducing genes that encode for microbial antigens as opposed to directly inoculating patients with the antigen or the pathogen itself (Sasso et al., 2020). This approach termed “genetic vaccines” includes introduction of viral, bacterial, DNA, and RNA vaccine vectors (Sasso et al., 2020). Some excellent examples of genetic vaccines are the mRNA or viral vectors used to prime the human immune system against SARS-CoV-2 infection (Kyriakidis et al., 2021). A benefit of the former vector is that it can be administered several times, because the immune reaction against the RNA scaffold itself is minimal, whereas viral vectors (e.g., adenoviruses) could be targeted by preexisting host immunity against the vector (Sasso et al., 2020). In clinical trials that studied a failed vaccine against HIV, it was revealed that individuals with high antibody titers against the adenovirus vaccine vector actually had an increased risk of being infected by HIV after vaccination (Sekaly, 2008). Therefore, selection of the vaccine scaffold is incredibly important when attempting to generate immunological memory against a microbe. Immune recognition of the scaffold can lessen the magnitude of the response or even enhance infection like in the case of HIV. It is also important to consider the tropism of the pathogen when designing a vaccine. A nasal vaccine, for example, might be more effective in preventing an upper respiratory pathogen from invading the brain than a vaccine injected into the arm.
An important question is whether systemic vaccination with conventional or genetic vectors has any impact on CNS immunity against a neurotropic pathogen. LCMV is a very well-studied neurotropic pathogen in rodents. Upon intracerebral inoculation, LCMV causes a uniformly fatal meningitis in mice that results from immune-mediated brain swelling (Gledhill, 1967; Kang and McGavern, 2008; Kim et al., 2009; Shedlock et al., 2011). Peripheral vaccination approaches have therefore been used in this model to determine if it is possible to prevent fatal meningitis. Systemic vaccination of mice with a recombinant vaccinia virus vector expressing the LCMV nucleoprotein completely prevented fatal meningitis when mice were challenged six weeks later with intracranial LCMV (Klavinskis et al., 1989). This protection was mediated primarily by virus-specific CD8+ T cells. Similarly, a DNA vaccine encoding the same LCMV protein was able to prevent fatal meningitis in mice when injected intramuscularly, which was linked to the antiviral T cell response rather than humoral immunity (Shedlock et al., 2011). Mice that received one, two, or three injections of the DNA vaccine had 67%, 84%, and 100% survival rates, respectively. Although these studies demonstrate that systemic vaccination can elicit T cell memory that protects against fatal viral meningitis, future studies will need to determine the degree to which other CNS barriers are protected against a broad range of pathogens when vaccines are administered systemically versus locally. More specifically, it will be important to determine if Trms generated by local barrier immunization is required to protect against specific types of pathogens (e.g., a microbe that infects that olfactory epithelium). Given the speed of viral replication, it could be beneficial to have Trms defending CNS barriers before circulating Tems have a chance to arrive. The benefit of memory B cells in protecting CNS barriers after vaccination must also be explored in greater detail.
Neuroimmunology of SARS-CoV-2 infection
Coronaviruses are RNA viruses with zoonotic origins that can cause symptoms ranging from those experienced during the common cold to pneumonia and respiratory failure (Gandhi et al., 2020; Holmes et al., 2021; V'Kovski et al., 2021). In 2019, a new coronavirus named SARS-CoV-2 was reported, initially in Wuhan, China (Holmes et al., 2021). This virus subsequently spread rapidly, leading to a global pandemic and a disease in humans referred to as coronavirus disease 2019 (COVID-19). The COVID-19 pandemic became one of the leading causes of death in the United States, with up to 2,856 deaths per day in the spring of 2020 (Woolf et al., 2021). The acute and chronic effects of COVID-19 have been of great interest, because surviving patients sometimes experience subjective mental fogginess and anosmia (loss of smell) (Woo et al., 2020). The cause of these neurological issues has led to a great deal of speculation (Spudich and Nath, 2022) but might be linked in part to gray-matter damage in the brains of patients (Douaud et al., 2022). Other neurological complications include strokes, meningitis, and acute demyelinating encephalopathy (Helms et al., 2020; Moriguchi et al., 2020; Reichard et al., 2020).
SARS-CoV-2 is primarily a respiratory pathogen transmitted through air-borne particles and droplets. However, there are case reports that describe encephalitis and neuroinflammation in COVID-19 patients as well as patients infected by other coronaviruses (Gu et al., 2005; Moriguchi et al., 2020; Novi et al., 2020). In fact, there is one interesting case where a patient with COVID-19 developed status epilepticus (Le Guennec et al., 2020). A brain MRI image from this patient revealed hyperintensity (indicative of an abnormality) in the right orbital prefrontal cortex, which lies adjacent to the olfactory bulb (Le Guennec et al., 2020). Interestingly, the CSF of this patient was negative for SARS-CoV-2, but alterations in this frontal brain region suggest that SARS-CoV-2 can access and/or inflame the CNS via the olfactory barrier (Figure 3). The loss of smell commonly observed in COVID-19 patients (Shelton et al., 2022) and the corresponding loss of olfactory brain tissue also support a direct impact of SARS-CoV-2 on the olfactory system (Douaud et al., 2022).
Studies have revealed that angiotensin-converting enzyme 2 (ACE2) is the main receptor for SARS-CoV infection (Li et al., 2003; Ziegler et al., 2020). Upon binding of viral spike protein to ACE2, it activates proteases, such as transmembrane serine protease 2 (TMPRSS2), that help facilitate cellular entry of the virus (Glowacka et al., 2011; Hoffmann et al., 2020; Ziegler et al., 2020). By using a single-cell RNA-sequencing (RNA-seq) approach, it was discovered that absorptive enterocytes in the gut, goblet secretory cells of the nasal mucosa, and type II pneumocytes in the lung were the main cell types expressing both ACE2 and TMPRSS2 (Ziegler et al., 2020); however, this approach might not accurately reflect the actual in vivo expression pattern during homeostasis and states of inflammation. In addition, type I and type II IFNs, which are produced in response to SARS-CoV-2 and most viruses, were shown to upregulate ACE2 and likely alter its expression pattern after infection (Ziegler et al., 2020). Expression of ACE2 and TMPRSS2 was further explored in a separate study that focused specifically on the olfactory epithelium and bulbs (Brann et al., 2020), which are both affected in COVID-19 patients. Within the olfactory epithelium, ACE2 protein was found on sustentacular cells (a type of support cell) and basal cells (an olfactory stem cell), but not on OSNs (Brann et al., 2020). Examination of the olfactory bulbs demonstrated ACE2 protein expression on vascular support cells like pericytes/smooth muscle cells but not olfactory-bulb neurons or OSN axon fibers projecting from the nasal epithelium. Vascular expression of ACE2 was confirmed in olfactory-bulb sections from COVID-19 patients, which co-localized with staining for viral spike protein, vascular breakdown, and significant demyelination/axonal damage in surrounding brain tissue (Schwabenland et al., 2021). Comparable vascular pathology was observed in the brainstem of these patients. In separate studies, SARS-CoV-2 RNA was found in the olfactory mucosa and bulbs of COVID-19 patients (Matschke et al., 2020; Meinhardt et al., 2021). Although SARS-CoV-2 does not appear to infect neurons or invade the CNS parenchyma directly, it can disrupt the olfactory system by infecting support cells in the nasal epithelium and vascular cells in the olfactory bulb—two important barrier systems. This would explain the olfactory pathology and loss of smell commonly observed in COVID-19 patients.
Given that SARS-CoV-2 can enter circulation and establish viremia in COVID-19 patients (Jacobs et al., 2021), this affords the virus an opportunity to potentially traverse CNS barriers with fenestrated vasculature such as the dura mater (Figures 1 and 2), CP (Figure 4), and circumventricular organs (CVO; not shown). CVOs, which were not discussed previously in this review, are specialized midline brain regions bordering the third and fourth ventricles that are responsible for maintaining body homeostasis (Ganong, 2000). In postmortem studies of COVID-19 patients, SARS-CoV-2 viral RNA was detected in the brainstem and cerebellum (Al-Dalahmah et al., 2020; Meinhardt et al., 2021). These structures are near the area postrema, a CVO that detects hormones and can induce vomiting if it senses certain toxins (Boldrini et al., 2021; Meinhardt et al., 2021). Although it is unknown whether SARS-CoV-2 infects CVOs, it will be important in future studies to determine whether these vulnerable structures serve as portals of viral entry into other brain regions like brainstem and cerebellum.
Similarly, the dura mater and CP possess fenestrated vessels and are potentially susceptible to blood-borne pathogens (Mastorakos and McGavern, 2019). The degree to which SARS-CoV-2 enters the meninges is not known; however, meningitis and meningoencephalitis have been observed in COVID-19 patients (Lv et al., 2021; Moriguchi et al., 2020), suggesting that the virus does enter this compartment in at least some individuals. SARS-CoV-2 is not typically found in the CSF of COVID-19 patients (Spudich and Nath, 2022), and, consistent with this observation, a recent single-nucleus RNA-seq study found no evidence of virus in the CP (Yang et al., 2021), the ventricular structure responsible for producing CSF. This study did, however, uncover evidence of a substantial inflammatory response within the CP, including upregulation of chemokine, complement, IFN, and antiviral genes. The SARS-CoV-2 receptor, ACE2, was also found to be expressed in this CNS barrier (Yang et al., 2021). Although the failure to detect SARS-CoV-2 in the CP does not exclude the possibility of direct infection, this structure is clearly inflamed in COVID-19 patients, which has the potential to disrupt CNS homeostasis.
The systemic immune response in COVID-19 patients, accessible in the peripheral blood, is complex and heterogeneous. Some patients mount a robust response consisting of activated cytotoxic and helper T cells as well as plasma blasts, whereas other patients show minimal immune activation (Lucas et al., 2020; Mathew et al., 2020). The type of immune response mounted has been associated with different clinical outcomes, although there is still much to learn about engagement of the immune system against SARS-CoV-2 in specific tissues throughout the body like the CNS. It has been postulated that because SARS-CoV-2 is minimally neuroinvasive, CNS-related symptoms must be linked to the systemic immune reaction (i.e., inflammatory mediators released into circulation) (Spudich and Nath, 2022). Indeed, cytokines released into circulation during viral infection have the potential to increase or strengthen BBB permeability (Daniels et al., 2014). Thus, it is conceivable that generalized states of neural dysfunction observed in COVID-19 patients could be due to the peripheral immune reaction against SARS-CoV-2 or lack thereof. For example, type-I IFNs are known as antiviral cytokines, but they can also contribute to tightening the BBB after viral infection (Daniels et al., 2014). A hallmark of severe disease in COVID-19 patients is an impaired type-I IFN response, which would certainly explain elevated viral titers in the blood but could also contribute to BBB dysfunction, especially given the exacerbated inflammation observed in these patients (Hadjadj et al., 2020).
Although general CNS complications are certainly possible after SARS-CoV-2 infection, it is also important to consider that specific patterns of vascular pathology have been observed in olfactory-bulb, basal ganglia, brainstem, and cerebellum tissue from COVID-19 patients (Lee et al., 2021; Matschke et al., 2020; Meinhardt et al., 2021; Schwabenland et al., 2021). By using imaging mass cytometry, a recent study of COVID-19 patients provided a detailed high-dimensional spatial map of virus, immune cells, and associated pathology in olfactory-bulb and brainstem tissue (Schwabenland et al., 2021). This study revealed the presence of SARS-CoV-2 in ACE2-positive cells comprising brain vasculature. These brain regions also contained CD8+ T cells (consistent with viral infection) that were found interacting with clusters of activated microglia. As demonstrated in rodents, microglia, without being infected, are capable of cross-presenting viral antigen to CD8+ T cells that contribute to the antiviral defense against a nasal VSV infection (Figure 3) (Moseman et al., 2020). The pattern of immune engagement observed in the brains of COVID-19 patients is consistent with this mode of defense against SARS-CoV-2. It is also important to mention that SARS-CoV-2 does not need to be neuroinvasive (i.e., infect the CNS parenchyma) to induce vascular pathology. As discussed, cerebral malaria is a potentially fatal disease in humans that occurs when antigen from parasite-infected red blood cells is cross-presented by cerebrovascular endothelial cells, which are then engaged by parasite-specific CD8+ T cells (Howland et al., 2013; Riggle et al., 2020; Swanson et al., 2016). Endothelial cells are not infected by the parasite but nevertheless acquire parasite antigen from the blood. This promotes CD8+ T cell-mediated cerebrovascular breakdown and fatal brain swelling (Seydel et al., 2015). Expression of ACE2 on cells associated with vasculature (e.g., pericytes and endothelial cells) gives SARS-CoV-2 the opportunity to infect CNS blood vessels and promote CD8+ T cell engagement (Schwabenland et al., 2021). However, cerebrovascular endothelial cells and microglia also can cross-present viral antigen, which could promote CD8+ T cell interactions and release of effector molecules even in the absence of direct infection. Either way, it is clear that vascular pathology and surrounding brain tissue damage does occur in the olfactory bulbs and brainstem of COVID-19 patients, which would explain some of the observed neurological symptoms.
It is not entirely clear how SARS-CoV-2 accesses vasculature in specific neuroanatomic regions within the CNS. The presence of virus in the blood would certainly provide one mode of entry. Another possibility is viral entry via the nasal epithelium or mucosa (Meinhardt et al., 2021). This mode of entry would explain that pattern of pathology and gray-matter reduction observed in the olfactory bulbs of COVID-19 patients (Douaud et al., 2022; Meinhardt et al., 2021; Schwabenland et al., 2021). The nasal mucosa of COVID-19 patients is heavily invaded by immune cells such as monocytes, NK cells, granulocytes, and T cells during the acute phase, and this is followed by the establishment of CD8+ Trms after pathogen clearance. The degree to which the immune system can contain SARS-CoV-2 in the nasal mucosa could determine whether the olfactory bulbs become infected or not. If this is true, then the presence of CD8+ Trms in the nasal mucosa will be an important immunological variable to consider when evaluating the type of protection afforded by the different SARS-CoV-2 vaccines.
Concluding remarks
The CNS is protected by sophisticated barriers, resident immune cells, and sometimes memory cells that all help prevent invasion by neurotropic pathogens. CNS barriers develop at crucial time periods during ontogenesis to ensure some degree of protection once a neonate is no longer defended by maternal immunity. Despite the presence of these barriers, there are situations where pathogens do infiltrate the CNS and trigger neuroinflammatory reactions that must be regulated. An uncontrolled CNS infection and subsequent inflammatory reaction can quickly cause irreparable damage or even fatal brain swelling, especially when vasculature is involved. A strong immunological defense of the different barriers discussed in this review is a necessary component of CNS homeostasis. It is likely that barriers such as the olfactory epithelium/mucosa are constantly challenged by air-borne pathogens inhaled into the nose. Immune cells inhabiting this barrier must therefore maintain a high degree of responsiveness to prevent pathogen entry into the olfactory bulbs. Similarly, immune cells along the olfactory bulbs must be able to rapidly stop pathogens in their tracks that traverse the cribriform plate. Other CNS barriers containing specialized vasculature (e.g., dura mater, choroid plexus, and circumventricular organs) are also challenged but in a different way. Microbes in circulation have the potential to extravasate from fenestrated vasculature in these barriers, which could result in invasion of the CNS meninges and/or parenchyma if not contained by the immune system. Evolutionary pressures have likely equipped CNS barriers with strong immune defenses because these structures interface with the periphery and are exposed to regular environmental challenges.
Once a pathogen is cleared from the CNS, the immune landscape can become imprinted with new features. As an example, newly engrafted innate immune cells like monocyte-derived meningeal macrophages do not always possess the same functional properties as the ones that existed before infection. In addition, both memory T and B cells can establish CNS residency after infection. We postulate that having these memory cells inhabit different CNS barriers is the best way to reduce the possibility of reinfection. However, we still have much to learn about how to optimally elicit barrier protective T and B cell responses. Although it is clear that CD8+ Trms and long-lived ASCs can establish residency in the CNS after infection, it is less clear where these cells localize and how they are maintained. Vaccines have the capacity to promote the generation of Trms, Tems, Tcms as well as B cell memory and ASCs, but in humans they are typically administered into a peripheral site like the arm. Defending the CNS olfactory bulbs against a respiratory pathogen like SARS-CoV-2 might instead require a nasal vaccine that results in the deposition of memory cells along the olfactory epithelial barrier. It is also worth considering how best to protect internal CNS barriers such as the dural venous sinuses, choroid plexus vasculature, etc. Introduction of a vaccine into the blood supply could help promote resident T and B cell memory in these locations if surveillance by circulating memory cells (e.g., Tems) alone is not capable of providing rapid protection. SARS-CoV-2 represents one contemporary challenge among many that taxes the CNS barrier defense systems and requires our full attention. A more thorough understanding of how naive and memory immune cells defend the different CNS barriers will help us design the most efficacious vaccines against a wide range of pathogens, including SARS-CoV-2.
Acknowledgments
This work was supported by the intramural program at the National Institute of Neurological Disorders & Stroke (NINDS), National Institutes of Health (NIH). We thank Alan Hoofring and Ethan Tyler in the NIH Medical Arts Design Section for their help with the illustrations shown in Figures 1, 2, 3, and 4.
Declaration of interests
The authors declare no competing interests.
References
- Abbott N.J., Ronnback L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Ahn J.H., Cho H., Kim J.H., Kim S.H., Ham J.S., Park I., Suh S.H., Hong S.P., Song J.H., Hong Y.K., et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572:62–66. doi: 10.1038/s41586-019-1419-5. [DOI] [PubMed] [Google Scholar]
- Akkaya M., Kwak K., Pierce S.K. B cell memory: building two walls of protection against pathogens. Nat. Rev. Immunol. 2020;20:229–238. doi: 10.1038/s41577-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dalahmah O., Thakur K.T., Nordvig A.S., Prust M.L., Roth W., Lignelli A., Uhlemann A.C., Miller E.H., Kunnath-Velayudhan S., Del Portillo A., et al. Neuronophagia and microglial nodules in a SARS-CoV-2 patient with cerebellar hemorrhage. Acta Neuropathol. Commun. 2020;8:147. doi: 10.1186/s40478-020-01024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandasabapathy N., Victora G.D., Meredith M., Feder R., Dong B., Kluger C., Yao K., Dustin M.L., Nussenzweig M.C., Steinman R.M., Liu K. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J. Exp. Med. 2011;208:1695–1705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreone B.J., Lacoste B., Gu C. Neuronal and vascular interactions. Annu. Rev. Neurosci. 2015;38:25–46. doi: 10.1146/annurev-neuro-071714-033835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A., Genove G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Aspelund A., Antila S., Proulx S.T., Karlsen T.V., Karaman S., Detmar M., Wiig H., Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J.R., Hwang M., Reyes-Rodriguez A., Bergmann C.C. Dynamics of virus-specific memory B cells and plasmablasts following viral infection of the central nervous system. J. Virol. 2019;93 doi: 10.1128/JVI.00875-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkalow F.J., Goodman M.J., Gerritsen M.E., Mayadas T.N. Brain endothelium lack one of two pathways of P-selectin-mediated neutrophil adhesion. Blood. 1996;88:4585–4593. doi: 10.1182/blood.v88.12.4585.bloodjournal88124585. [DOI] [PubMed] [Google Scholar]
- Bartholomaus I., Kawakami N., Odoardi F., Schlager C., Miljkovic D., Ellwart J.W., Klinkert W.E., Flugel-Koch C., Issekutz T.B., Wekerle H., Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- Baruch K., Deczkowska A., David E., Castellano J.M., Miller O., Kertser A., Berkutzki T., Barnett-Itzhaki Z., Bezalel D., Wyss-Coray T., et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014;346:89–93. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K., Ron-Harel N., Gal H., Deczkowska A., Shifrut E., Ndifon W., Mirlas-Neisberg N., Cardon M., Vaknin I., Cahalon L., et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc. Natl. Acad. Sci. U S A. 2013;110:2264–2269. doi: 10.1073/pnas.1211270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H., Sonnleitner U., Lametschwandtner A., Steiner M., Adam H., Bauer H.C. Ontogenic expression of the erythroid-type glucose transporter (Glut 1) in the telencephalon of the mouse: correlation to the tightening of the blood-brain barrier. Brain Res. Dev. Brain Res. 1995;86:317–325. doi: 10.1016/0165-3806(95)00044-e. [DOI] [PubMed] [Google Scholar]
- Bell R.D., Winkler E.A., Sagare A.P., Singh I., LaRue B., Deane R., Zlokovic B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A., Lacoste B., Kur E., Andreone B.J., Mayshar Y., Yan H., Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder G.K., Griffin D.E. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science. 2001;293:303–306. doi: 10.1126/science.1059742. [DOI] [PubMed] [Google Scholar]
- Boldrini M., Canoll P.D., Klein R.S. How COVID-19 affects the brain. JAMA Psychiatry. 2021;78:682–683. doi: 10.1001/jamapsychiatry.2021.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I.C., Chou H.J., Fletcher R.B., et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioschi S., Wang W.L., Peng V., Wang M., Shchukina I., Greenberg Z.J., Bando J.K., Jaeger N., Czepielewski R.S., Swain A., et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science. 2021;373 doi: 10.1126/science.abf9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizic I., Susak B., Arapovic M., Huszthy P.C., Hirsl L., Kvestak D., Juranic Lisnic V., Golemac M., Pernjak Pugel E., Tomac J., et al. Brain-resident memory CD8(+) T cells induced by congenital CMV infection prevent brain pathology and virus reactivation. Eur. J. Immunol. 2018;48:950–964. doi: 10.1002/eji.201847526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley M.W., McGavern D.B. Immune dynamics in the CNS and its barriers during homeostasis and disease. Immunol. Rev. 2022;306:58–75. doi: 10.1111/imr.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdeinick-Kerr R., Govindarajan D., Griffin D.E. Noncytolytic clearance of sindbis virus infection from neurons by gamma interferon is dependent on Jak/STAT signaling. J. Virol. 2009;83:3429–3435. doi: 10.1128/JVI.02381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin E.M., Hinton D.R., Chen J., Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 1995;69:4898–4905. doi: 10.1128/JVI.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers M.D., Visintin I., Kang S.J., Janeway C.A., Jr. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 2000;123(Pt 6):1092–1101. doi: 10.1093/brain/123.6.1092. [DOI] [PubMed] [Google Scholar]
- Carrithers M.D., Visintin I., Viret C., Janeway C.S., Jr. Role of genetic background in P selectin-dependent immune surveillance of the central nervous system. J. Neuroimmunol. 2002;129:51–57. doi: 10.1016/s0165-5728(02)00172-8. [DOI] [PubMed] [Google Scholar]
- Carson M.J., Doose J.M., Melchior B., Schmid C.D., Ploix C.C. CNS immune privilege: hiding in plain sight. Immunol. Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepok S., Jacobsen M., Schock S., Omer B., Jaekel S., Boddeker I., Oertel W.H., Sommer N., Hemmer B. Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain. 2001;124:2169–2176. doi: 10.1093/brain/124.11.2169. [DOI] [PubMed] [Google Scholar]
- Chow B.W., Gu C. The molecular constituents of the blood-brain barrier. Trends Neurosci. 2015;38:598–608. doi: 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson B.D., Walker A., Harris M.G., Rayasam A., Hsu M., Sandor M., Fabry Z. CCR7 deficient inflammatory dendritic cells are retained in the central nervous system. Sci. Rep. 2017;7:42856. doi: 10.1038/srep42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J.A., Myburgh E., Brewer J.M., McMenamin P.G. Where are we? The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Prog. Neurobiol. 2017;156:107–148. doi: 10.1016/j.pneurobio.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Cornish T.E., Stallknecht D.E., Brown C.C., Seal B.S., Howerth E.W. Pathogenesis of experimental vesicular stomatitis virus (New Jersey serotype) infection in the deer mouse (Peromyscus maniculatus) Vet. Pathol. 2001;38:396–406. doi: 10.1354/vp.38-4-396. [DOI] [PubMed] [Google Scholar]
- Cugurra A., Mamuladze T., Rustenhoven J., Dykstra T., Beroshvili G., Greenberg Z.J., Baker W., Papadopoulos Z., Drieu A., Blackburn S., et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021;373 doi: 10.1126/science.abf7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Xu H., Lehtinen M.K. Macrophages on the margin: choroid plexus immune responses. Trends Neurosci. 2021;44:864–875. doi: 10.1016/j.tins.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupovic J., Onder L., Gil-Cruz C., Weiler E., Caviezel-Firner S., Perez-Shibayama C., Rulicke T., Bechmann I., Ludewig B. Central nervous system stromal cells control local CD8(+) T cell responses during virus-induced neuroinflammation. Immunity. 2016;44:622–633. doi: 10.1016/j.immuni.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S., Herz J., Wall M., Dykstra T., de Lima K.A., Norris G.T., Dabhi N., Kennedy T., Baker W., Kipnis J. Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and beta-amyloid pathology. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abe4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S., Louveau A., Vaccari A., Smirnov I., Cornelison R.C., Kingsmore K.M., Contarino C., Onengut-Gumuscu S., Farber E., Raper D., et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018;560:185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani N., Herbst R.H., McCabe C., Green G.S., Kaiser K., Head J.P., Cui J., Shipley F.B., Jang A., Dionne D., et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184:3056–3074.e21. doi: 10.1016/j.cell.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B.P., Holman D.W., Cruz-Orengo L., Jujjavarapu H., Durrant D.M., Klein R.S. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. mBio. 2014;5 doi: 10.1128/mBio.01476-14. e01476–01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta K., Jeong J. Developmental biology of the meninges. Genesis. 2019;57:e23288. doi: 10.1002/dvg.23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kleer I., Willems F., Lambrecht B., Goriely S. Ontogeny of myeloid cells. Front Immunol. 2014;5:423. doi: 10.3389/fimmu.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes G.F., Arnold C.N., Young A.J., Krautwald S., Lipp M., Hay J.B., Butcher E.C. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detje C.N., Meyer T., Schmidt H., Kreuz D., Rose J.K., Bechmann I., Prinz M., Kalinke U. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J. Immunol. 2009;182:2297–2304. doi: 10.4049/jimmunol.0800596. [DOI] [PubMed] [Google Scholar]
- DiSano K.D., Stohlman S.A., Bergmann C.C. Activated GL7(+) B cells are maintained within the inflamed CNS in the absence of follicle formation during viral encephalomyelitis. Brain Behav. Immun. 2017;60:71–83. doi: 10.1016/j.bbi.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echchannaoui H., Frei K., Schnell C., Leib S.L., Zimmerli W., Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- Eggers E.L., Michel B.A., Wu H., Wang S.Z., Bevan C.J., Abounasr A., Pierson N.S., Bischof A., Kazer M., Leitner E., et al. Clonal relationships of CSF B cells in treatment-naive multiple sclerosis patients. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide P.K., Vatnehol S.A.S., Emblem K.E., Ringstad G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci. Rep. 2018;8:7194. doi: 10.1038/s41598-018-25666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber D.L., Yudanin N.A., Restifo N.P. Human memory T cells: generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano A.J., Gadani S.P., Kipnis J. Interactions of innate and adaptive immunity in brain development and function. Brain Res. 2015;1617:18–27. doi: 10.1016/j.brainres.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick Z., Frazer G., Ferro A., Clare S., Bouladoux N., Ferdinand J., Tuong Z.K., Negro-Demontel M.L., Kumar N., Suchanek O., et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. 2020;587:472–476. doi: 10.1038/s41586-020-2886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung W.P., Kundig T.M., Zinkernagel R.M., Mak T.W. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J. Exp. Med. 1991;174:1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate Covid-19. N. Engl. J. Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- Ganong W.F. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 2000;27:422–427. doi: 10.1046/j.1440-1681.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- Gebhardt T., Wakim L.M., Eidsmo L., Reading P.C., Heath W.R., Carbone F.R. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Ghoshal A., Das S., Ghosh S., Mishra M.K., Sharma V., Koli P., Sen E., Basu A. Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia. 2007;55:483–496. doi: 10.1002/glia.20474. [DOI] [PubMed] [Google Scholar]
- Gledhill A.W. Protective effect of anti-lymphocytic serum on murine lymphocytic choriomeningitis. Nature. 1967;214:178–179. doi: 10.1038/214178c0. [DOI] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T., Wieghofer P., Jordao M.J., Prutek F., Hagemeyer N., Frenzel K., Amann L., Staszewski O., Kierdorf K., Krueger M., et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016;17:797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley T.S., Wherry E.J., Masopust D., Ahmed R. Generation and maintenance of immunological memory. Semin. Immunol. 2004;16:323–333. doi: 10.1016/j.smim.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L.G., Chisari F.V. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- Haddad-Tovolli R., Dragano N.R.V., Ramalho A.F.S., Velloso L.A. Development and function of the blood-brain barrier in the Context of Metabolic control. Front Neurosci. 2017;11:224. doi: 10.3389/fnins.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Pere H., Charbit B., Bondet V., Chenevier-Gobeaux C., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmann R., Horn N., Selg M., Wendler O., Pausch F., Sorokin L.M. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- Harty J.T., Badovinac V.P. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herisson F., Frodermann V., Courties G., Rohde D., Sun Y., Vandoorne K., Wojtkiewicz G.R., Masson G.S., Vinegoni C., Kim J., et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 2018;21:1209–1217. doi: 10.1038/s41593-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Filiano A.J., Smith A., Yogev N., Kipnis J. Myeloid cells in the central nervous system. Immunity. 2017;46:943–956. doi: 10.1016/j.immuni.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Johnson K.R., McGavern D.B. Therapeutic antiviral T cells noncytopathically clear persistently infected microglia after conversion into antigen-presenting cells. J. Exp. Med. 2015;212:1153–1169. doi: 10.1084/jem.20142047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan R.J., Zhong W., Usherwood E.J., Cookenham T., Roberts A.D., Woodland D.L. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J. Exp. Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E.C., Goldstein S.A., Rasmussen A.L., Robertson D.L., Crits-Christoph A., Wertheim J.O., Anthony S.J., Barclay W.S., Boni M.F., Doherty P.C., et al. The origins of SARS-CoV-2: a critical review. Cell. 2021;184:4848–4856. doi: 10.1016/j.cell.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland S.W., Poh C.M., Gun S.Y., Claser C., Malleret B., Shastri N., Ginhoux F., Grotenbreg G.M., Renia L. Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria. EMBO Mol. Med. 2013;5:984–999. doi: 10.1002/emmm.201202273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Laaker C., Madrid A., Herbath M., Choi Y.H., Sandor M., Fabry Z. Neuroinflammation creates an immune regulatory niche at the meningeal lymphatic vasculature near the cribriform plate. Nat. Immunol. 2022;23:581–593. doi: 10.1038/s41590-022-01158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Laaker C., Sandor M., Fabry Z. Neuroinflammation-driven lymphangiogenesis in CNS diseases. Front Cell Neurosci. 2021;15:683676. doi: 10.3389/fncel.2021.683676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Rayasam A., Kijak J.A., Choi Y.H., Harding J.S., Marcus S.A., Karpus W.J., Sandor M., Fabry Z. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 2019;10:229. doi: 10.1038/s41467-018-08163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I., Shimogori T., Ohtsuka T., Kageyama R. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development. 2008;135:2531–2541. doi: 10.1242/dev.021535. [DOI] [PubMed] [Google Scholar]
- Ivanovs A., Rybtsov S., Welch L., Anderson R.A., Turner M.L., Medvinsky A. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J. Exp. Med. 2011;208:2417–2427. doi: 10.1084/jem.20111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J.L., Bain W., Naqvi A., Staines B., Castanha P.M.S., Yang H., Boltz V.F., Barratt-Boyes S., Marques E.T.A., Mitchell S.L., et al. SARS-CoV-2 viremia is associated with COVID-19 severity and predicts clinical outcomes. Clin. Infect Dis. 2021 doi: 10.1093/cid/ciab686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R.W., Yong V.W. B cells in central nervous system disease: diversity, locations and pathophysiology. Nat. Rev. Immunol. 2021 doi: 10.1038/s41577-021-00652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed K., Reddy V., Lui F. StatPearls. 2021. Neuroanatomy, choroid plexus. [Google Scholar]
- Joly E., Oldstone M.B. Neuronal cells are deficient in loading peptides onto MHC class I molecules. Neuron. 1992;8:1185–1190. doi: 10.1016/0896-6273(92)90138-4. [DOI] [PubMed] [Google Scholar]
- Kacem K., Lacombe P., Seylaz J., Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. doi: 10.1002/(sici)1098-1136(199805)23:1<1::aid-glia1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kang S.S., Herz J., Kim J.V., Nayak D., Stewart-Hutchinson P., Dustin M.L., McGavern D.B. Migration of cytotoxic lymphocytes in cell cycle permits local MHC I-dependent control of division at sites of viral infection. J. Exp. Med. 2011;208:747–759. doi: 10.1084/jem.20101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.S., McGavern D.B. Lymphocytic choriomeningitis infection of the central nervous system. Front Biosci. 2008;13:4529–4543. doi: 10.2741/3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S., Pantazis A., Weller R.O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Kim J.V., Kang S.S., Dustin M.L., McGavern D.B. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisakk P., Trebst C., Liu Z., Tucky B.H., Sorensen T.L., Rudick R.A., Mack M., Ransohoff R.M. T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin. Exp. Immunol. 2002;129:510–518. doi: 10.1046/j.1365-2249.2002.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavinskis L.S., Whitton J.L., Oldstone M.B. Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection. J. Virol. 1989;63:4311–4316. doi: 10.1128/JVI.63.10.4311-4316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Obermaier B., Angele B., Pfister H.W., Wagner H., Koedel U., Kirschning C.J. Innate immunity to pneumococcal infection of the central nervous system depends on toll-like receptor (TLR) 2 and TLR4. J. Infect Dis. 2008;198:1028–1036. doi: 10.1086/591626. [DOI] [PubMed] [Google Scholar]
- Klonowski K.D., Williams K.J., Marzo A.L., Blair D.A., Lingenheld E.G., Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- Kooij G., Kroon J., Paul D., Reijerkerk A., Geerts D., van der Pol S.M., van Het Hof B., Drexhage J.A., van Vliet S.J., Hekking L.H., et al. P-glycoprotein regulates trafficking of CD8(+) T cells to the brain parenchyma. Acta Neuropathol. 2014;127:699–711. doi: 10.1007/s00401-014-1244-8. [DOI] [PubMed] [Google Scholar]
- Korin B., Ben-Shaanan T.L., Schiller M., Dubovik T., Azulay-Debby H., Boshnak N.T., Koren T., Rolls A. High-dimensional, single-cell characterization of the brain's immune compartment. Nat. Neurosci. 2017;20:1300–1309. doi: 10.1038/nn.4610. [DOI] [PubMed] [Google Scholar]
- Kyriakidis N.C., Lopez-Cortes A., Gonzalez E.V., Grimaldos A.B., Prado E.O. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccin. 2021;6:28. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrith T.A., Sureshchandra S., Rivera A., Jang J.C., Rais M., Nair M.G., Messaoudi I., Wilson E.H. CD103(+) CD8 T cells in the Toxoplasma-infected brain exhibit a tissue-resident memory transcriptional Profile. Front Immunol. 2017;8:335. doi: 10.3389/fimmu.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Daniels B.P., Pinto A.K., Huang A.C., Vick S.C., Doyle S.E., Gale M., Jr., Klein R.S., Diamond M.S. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci. Transl Med. 2015;7:284ra259. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guennec L., Devianne J., Jalin L., Cao A., Galanaud D., Navarro V., Boutolleau D., Rohaut B., Weiss N., Demeret S. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020;61:e90–e94. doi: 10.1111/epi.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.H., Perl D.P., Nair G., Li W., Maric D., Murray H., Dodd S.J., Koretsky A.P., Watts J.A., Cheung V., et al. Microvascular injury in the brains of patients with Covid-19. N. Engl. J. Med. 2021;384:481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Mozdzanowska K., Palladino G., Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J. Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- Liddelow S.A. Development of the choroid plexus and blood-CSF barrier. Front Neurosci. 2015;9:32. doi: 10.3389/fnins.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P., Johansson B.R., Leveen P., Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Liu T., Khanna K.M., Carriere B.N., Hendricks R.L. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A., Herz J., Alme M.N., Salvador A.F., Dong M.Q., Viar K.E., Herod S.G., Knopp J., Setliff J.C., Lupi A.L., et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018;21:1380–1391. doi: 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., Derecki N.C., Castle D., Mandell J.W., Lee K.S., et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh B., Kristensson K., Norrby E. Selective infections of olfactory and respiratory epithelium by vesicular stomatitis and Sendai viruses. Neuropathol. Appl. Neurobiol. 1987;13:111–122. doi: 10.1111/j.1365-2990.1987.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Lv P., Peng F., Zhang Y., Zhang L., Li N., Sun L., Wang Y., Hou P., Huang T., Wang X. COVID-19-associated meningoencephalitis: a care report and literature review. Exp. Ther. Med. 2021;21:362. doi: 10.3892/etm.2021.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D., Soerens A.G. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 2019;37:521–546. doi: 10.1146/annurev-immunol-042617-053214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos P., McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aav0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos P., Mihelson N., Luby M., Burks S.R., Johnson K., Hsia A.W., Witko J., Frank J.A., Latour L., McGavern D.B. Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat. Neurosci. 2021;24:245–258. doi: 10.1038/s41593-020-00773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., Alanio C., Kuri-Cervantes L., Pampena M.B., D'Andrea K., et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369 doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lutgehetmann M., Hagel C., Sperhake J.P., Schroder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar P.B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brunink S., Greuel S., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Metcalf T.U., Baxter V.K., Nilaratanakul V., Griffin D.E. Recruitment and retention of B cells in the central nervous system in response to alphavirus encephalomyelitis. J. Virol. 2013;87:2420–2429. doi: 10.1128/JVI.01769-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockus T.E., Shwetank, Lauver M.D., Ren H.M., Netherby C.S., Salameh T., Kawasawa Y.I., Yue F., Broach J.R., Lukacher A.E. CD4 T cells control development and maintenance of brain-resident CD8 T cells during polyomavirus infection. Plos Pathog. 2018;14:e1007365. doi: 10.1371/journal.ppat.1007365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseman E.A., Blanchard A.C., Nayak D., McGavern D.B. T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abb1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrdjen D., Pavlovic A., Hartmann F.J., Schreiner B., Utz S.G., Leung B.P., Lelios I., Heppner F.L., Kipnis J., Merkler D., et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48:380–395.e6. doi: 10.1016/j.immuni.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Nagyoszi P., Wilhelm I., Farkas A.E., Fazakas C., Dung N.T.K., Hasko J., Krizbai I.A. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem. Int. 2010;57:556–564. doi: 10.1016/j.neuint.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Nayak D., Roth T.L., McGavern D.B. Microglia development and function. Annu. Rev. Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D., Zinselmeyer B.H., Corps K.N., McGavern D.B. In vivo dynamics of innate immune sentinels in the CNS. Intravital. 2012;1:95–106. doi: 10.4161/intv.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M., Ransom B., Goldman S.A. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Neuwelt E.A., Bauer B., Fahlke C., Fricker G., Iadecola C., Janigro D., Leybaert L., Molnar Z., O'Donnell M.E., Povlishock J.T., et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novi G., Rossi T., Pedemonte E., Saitta L., Rolla C., Roccatagliata L., Inglese M., Farinini D. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol. Neuroimmunol Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R., Muller F. The meninges in human development. J. Neuropathol. Exp. Neurol. 1986;45:588–608. [PubMed] [Google Scholar]
- Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odoardi F., Sie C., Streyl K., Ulaganathan V.K., Schlager C., Lodygin D., Heckelsmiller K., Nietfeld W., Ellwart J., Klinkert W.E., et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- Patterson C.E., Lawrence D.M., Echols L.A., Rall G.F. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 2002;76:4497–4506. doi: 10.1128/jvi.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A., Tracey K.J. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T.G., Tangye S.G. Memory B cells: total recall. Curr. Opin. Immunol. 2017;45:132–140. doi: 10.1016/j.coi.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Phares T.W., DiSano K.D., Stohlman S.A., Bergmann C.C. Progression from IgD+ IgM+ to isotype-switched B cells is site specific during coronavirus-induced encephalomyelitis. J. Virol. 2014;88:8853–8867. doi: 10.1128/JVI.00861-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager O., Friedman A., Nebenzahl Y.M. Role of neural barriers in the pathogenesis and outcome of Streptococcus pneumoniae meningitis. Exp. Ther. Med. 2017;13:799–809. doi: 10.3892/etm.2017.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., Hu S., Sheng W.S., Chauhan P., Singh A., Lokensgard J.R. The PD-1: PD-L1 pathway promotes development of brain-resident memory T cells following acute viral encephalitis. J. Neuroinflammation. 2017;14:82. doi: 10.1186/s12974-017-0860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., Lokensgard J.R. Brain-resident T cells following viral infection. Viral Immunol. 2019;32:48–54. doi: 10.1089/vim.2018.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjavi A., Smirnov I., Derecki N., Kipnis J. Dynamics of the meningeal CD4(+) T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol. Psychiatry. 2014;19:531–532. doi: 10.1038/mp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R.M., Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M., Kivisakk P., Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggle B.A., Manglani M., Maric D., Johnson K.R., Lee M.H., Neto O.L.A., Taylor T.E., Seydel K.B., Nath A., Miller L.H., et al. CD8+ T cells target cerebrovasculature in children with cerebral malaria. J. Clin. Invest. 2020;130:1128–1138. doi: 10.1172/JCI133474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W., Hallmann R., Albrecht U. Differentiation-dependent expression of proteins in brain endothelium during development of the blood-brain barrier. Dev. Biol. 1986;117:537–545. doi: 10.1016/0012-1606(86)90321-0. [DOI] [PubMed] [Google Scholar]
- Ritzel R.M., Crapser J., Patel A.R., Verma R., Grenier J.M., Chauhan A., Jellison E.R., McCullough L.D. Age-associated resident memory CD8 T cells in the central nervous system are primed to potentiate inflammation after ischemic brain injury. J. Immunol. 2016;196:3318–3330. doi: 10.4049/jimmunol.1502021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M., Olofsson P.S., Ochani M., Valdes-Ferrer S.I., Levine Y.A., Reardon C., Tusche M.W., Pavlov V.A., Andersson U., Chavan S., et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T.L., Nayak D., Atanasijevic T., Koretsky A.P., Latour L.L., McGavern D.B. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rua R., Lee J.Y., Silva A.B., Swafford I.S., Maric D., Johnson K.R., McGavern D.B. Infection drives meningeal engraftment by inflammatory monocytes that impairs CNS immunity. Nat. Immunol. 2019;20:407–419. doi: 10.1038/s41590-019-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rua R., McGavern D.B. Advances in meningeal immunity. Trends Mol. Med. 2018;24:542–559. doi: 10.1016/j.molmed.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustenhoven J., Drieu A., Mamuladze T., de Lima K.A., Dykstra T., Wall M., Papadopoulos Z., Kanamori M., Salvador A.F., Baker W., et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184:1000–1016.e27. doi: 10.1016/j.cell.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkeni M.A., Lynch J.L., Otamis-Price T., Banks W.A. Lipopolysaccharide impairs blood-brain barrier P-glycoprotein function in mice through prostaglandin- and nitric oxide-independent pathways. J. Neuroimmune Pharmacol. 2009;4:276–282. doi: 10.1007/s11481-008-9138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Geginat J., Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sasso E., D’Alise A.M., Zambrano N., Scarselli E., Folgori A., Nicosia A. New viral vectors for infectious diseases and cancer. Semin. Immunol. 2020;50:101430. doi: 10.1016/j.smim.2020.101430. [DOI] [PubMed] [Google Scholar]
- Schafflick D., Wolbert J., Heming M., Thomas C., Hartlehnert M., Borsch A.L., Ricci A., Martin-Salamanca S., Li X., Lu I.N., et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat. Neurosci. 2021;24:1225–1234. doi: 10.1038/s41593-021-00880-y. [DOI] [PubMed] [Google Scholar]
- Scholler A.S., Fonnes M., Nazerai L., Christensen J.P., Thomsen A.R. Local antigen encounter is essential for establishing persistent CD8(+) T-cell memory in the CNS. Front Immunol. 2019;10:351. doi: 10.3389/fimmu.2019.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt F., Schroeder L., Anastasopoulos C., Markl M., Bauerle J., Hennemuth A., Drexl J., Valdueza J.M., Mader I., Harloff A. In vivo analysis of physiological 3D blood flow of cerebral veins. Eur. Radiol. 2015;25:2371–2380. doi: 10.1007/s00330-014-3587-x. [DOI] [PubMed] [Google Scholar]
- Schwabenland M., Salie H., Tanevski J., Killmer S., Lago M.S., Schlaak A.E., Mayer L., Matschke J., Puschel K., Fitzek A., et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. 2021;54:1594–1610.e11. doi: 10.1016/j.immuni.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaly R.P. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydel K.B., Kampondeni S.D., Valim C., Potchen M.J., Milner D.A., Muwalo F.W., Birbeck G.L., Bradley W.G., Fox L.L., Glover S.J., et al. Brain swelling and death in children with cerebral malaria. N. Engl. J. Med. 2015;372:1126–1137. doi: 10.1056/NEJMoa1400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock D.J., Talbott K.T., Cress C., Ferraro B., Tuyishme S., Mallilankaraman K., Cisper N.J., Morrow M.P., Wu S.J., Kawalekar O.U., et al. A highly optimized DNA vaccine confers complete protective immunity against high-dose lethal lymphocytic choriomeningitis virus challenge. Vaccine. 2011;29:6755–6762. doi: 10.1016/j.vaccine.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton J.F., Shastri A.J., Fletez-Brant K., andMe C.-T., Kung A., Altman A., Kill A., Symons A., Weldon C., Coker D., Tan J., Pollard J., McCreight J., Bielenberg J., Matthews J., Lee J., Tran L., Lowe M., Royce M., Tang N., Gandhi P., d’Amore R., Tennen R., Dvorak S., Hadly S., Park S., Morrow T., Sonmez T.F., Le T., Zheng Y., The 23andMe COVID-19 Team. Chubb A., Aslibekyan S., Fitch A., Auton A. The UGT2A1/UGT2A2 locus is associated with COVID-19-related loss of smell or taste. Nat. Genet. 2022;54:121–124. doi: 10.1038/s41588-021-00986-w. [DOI] [PubMed] [Google Scholar]
- Spudich S., Nath A. Nervous system consequences of COVID-19. Science. 2022;375:267–269. doi: 10.1126/science.abm2052. [DOI] [PubMed] [Google Scholar]
- Steinbach K., Vincenti I., Kreutzfeldt M., Page N., Muschaweckh A., Wagner I., Drexler I., Pinschewer D., Korn T., Merkler D. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J. Exp. Med. 2016;213:1571–1587. doi: 10.1084/jem.20151916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert E.M., Schenkel J.M., Fraser K.A., Beura L.K., Manlove L.S., Igyarto B.Z., Southern P.J., Masopust D. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson A., Hansson G.K., Andersen O., Andersson R., Patarroyo M., Stemme S. Adhesion molecule expression on cerebrospinal fluid T lymphocytes: evidence for common recruitment mechanisms in multiple sclerosis, aseptic meningitis, and normal controls. Ann. Neurol. 1993;34:155–161. doi: 10.1002/ana.410340210. [DOI] [PubMed] [Google Scholar]
- Swanson P.A., 2nd, Hart G.T., Russo M.V., Nayak D., Yazew T., Pena M., Khan S.M., Janse C.J., Pierce S.K., McGavern D.B. CD8+ T cells induce fatal brainstem pathology during cerebral malaria via luminal antigen-specific engagement of brain vasculature. PLoS Pathog. 2016;12:e1006022. doi: 10.1371/journal.ppat.1006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson P.A., 2nd, McGavern D.B. In: The Blood-Brain Barrier in Health and Disease, Volume Two: Pathophysiology and Pathology. Dorovini-Zis K., editor. CRC Press; 2015. Portals of viral entry into the central nervous system; p. 23. [Google Scholar]
- Swanson P.A., 2nd, McGavern D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015;11:44–54. doi: 10.1016/j.coviro.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz S., Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015;209:493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishon A., Lewicki H., Andaya A., McGavern D., Martin L., Oldstone M.B. CD4 T cell control primary measles virus infection of the CNS: regulation is dependent on combined activity with either CD8 T cells or with B cells: CD4, CD8 or B cells alone are ineffective. Virology. 2006;347:234–245. doi: 10.1016/j.virol.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Urban S.L., Jensen I.J., Shan Q., Pewe L.L., Xue H.H., Badovinac V.P., Harty J.T. Peripherally induced brain tissue-resident memory CD8(+) T cells mediate protection against CNS infection. Nat. Immunol. 2020;21:938–949. doi: 10.1038/s41590-020-0711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz S.G., See P., Mildenberger W., Thion M.S., Silvin A., Lutz M., Ingelfinger F., Rayan N.A., Lelios I., Buttgereit A., et al. Early fate defines microglia and non-parenchymal brain macrophage development. Cell. 2020;181:557–573.e18. doi: 10.1016/j.cell.2020.03.021. [DOI] [PubMed] [Google Scholar]
- V'Kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D., Verdijk R., Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 2015;235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- Vasek M.J., Garber C., Dorsey D., Durrant D.M., Bollman B., Soung A., Yu J., Perez-Torres C., Frouin A., Wilton D.K., et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivatbutsiri P., Ichinose S., Hytonen M., Sainio K., Eto K., Iseki S. Impaired meningeal development in association with apical expansion of calvarial bone osteogenesis in the Foxc1 mutant. J. Anat. 2008;212:603–611. doi: 10.1111/j.1469-7580.2008.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voetmann E. On the structure and surface area of the human choroid plexuses. Ugeskr Laeger. 1949;111:1051. [PubMed] [Google Scholar]
- Wakim L.M., Woodward-Davis A., Bevan M.J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. U S A. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen D., Xu D., Huang C., Xing R., He D., Xu H. Early developing B cells undergo negative selection by central nervous system-specific antigens in the meninges. Immunity. 2021;54:2784–2794.e6. doi: 10.1016/j.immuni.2021.09.016. [DOI] [PubMed] [Google Scholar]
- Weber M., Hauschild R., Schwarz J., Moussion C., de Vries I., Legler D.F., Luther S.A., Bollenbach T., Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- Weisel F., Shlomchik M. Memory B cells of mice and humans. Annu. Rev. Immunol. 2017;35:255–284. doi: 10.1146/annurev-immunol-041015-055531. [DOI] [PubMed] [Google Scholar]
- Williams K., Alvarez X., Lackner A.A. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- Wolburg H., Wolburg-Buchholz K., Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109:181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- Woo M.S., Malsy J., Pottgen J., Seddiq Zai S., Ufer F., Hadjilaou A., Schmiedel S., Addo M.M., Gerloff C., Heesen C., et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2:fcaa205. doi: 10.1093/braincomms/fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf S.H., Chapman D.A., Lee J.H. COVID-19 as the leading cause of death in the United States. JAMA. 2021;325:123–124. doi: 10.1001/jama.2020.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A.C., Kern F., Losada P.M., Agam M.R., Maat C.A., Schmartz G.P., Fehlmann T., Stein J.A., Schaum N., Lee D.P., et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock T.A., Cannon C., Fritz L.C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4βl integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Yu Y.J., Zhang Y., Kenrick M., Hoyte K., Luk W., Lu Y., Atwal J., Elliott J.M., Prabhu S., Watts R.J., Dennis M.S. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhivaki D., Lo-Man R. Unique aspects of the perinatal immune system. Nat. Rev. Immunol. 2017;17:495–507. doi: 10.1038/nri.2017.54. [DOI] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035.e9. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]