Abstract
COVID‐19 is a dynamic disease and may affect different tissues and organs as it progresses. Therefore, the impact generated by the disease in all its stages and organs requires a functional and versatile imaging technique able to detect particularities or artifacts dynamically. Ultrasonography fulfills all these requirements and exhibit several advantages relative to other imaging modalities, including portability, lower cost and biosafety. Throughout the COVID‐19 pandemic, ultrasonography displayed a crucial role in the triage, monitoring, indicating organ damages and enabling individualized therapeutical decisions in COVID‐19 patients. This review is dedicated to highlight the main pathological effects correlated with ultrasound changes caused by COVID‐19 in the lungs, heart and liver.
Keywords: COVID‐19, echocardiography, multiorgan tropism, SARS‐CoV‐2, ultrasound
This review is dedicated to highlight the main pathological effects correlated with ultrasound changes caused by COVID‐19 in the lungs, heart and liver.
1. INTRODUCTION
The coronaviruses are zoonotic viruses that belong to the Nidovirales order and Coronaviridae family that account with three already identified species—the etiologic agents of the severe acute respiratory syndromes (SARS). 1 The first coronavirus outbreak, caused by SARS‐CoV‐1, started in China in 2002 and caused more than 8.000 cases and 774 deaths in 27 countries. 2 , 3 After 10 years, a new outbreak, caused by MERS‐CoV resulted in 1.728 confirmed cases and 624 deaths in 27 countries. 3 , 4 In 2019, the SARS‐CoV‐2 culminated in the current “Corona Virus Disease 2019” (COVID‐19) pandemic, responsible for more than 200 million cases and more than 4 million deaths worldwide. 5 , 6
The coronaviruses carry single‐stranded ribonucleic acid with positive sense (+ssRNA), which express open‐reading frames that encode for at least 27 proteins including 15 non‐structural, 8 auxiliaries and 4 structural (e.g., S “spike,” M “membrane,” E “envelope” and N “nucleocapsid” proteins). 7 To gain access to the host, the viral S protein interacts with the angiotensin II converting enzyme receptor (ACE2) and depends on auxiliary proteins located at the cell surface for successful invasion. 8 Among these peptides, the transmembrane serine protease 2 (TMPRSS2) is highlighted for its role in cleaving and activating the S protein of several classes of coronaviruses. 8 Furthermore, the furin protease mediates the binding of the S protein with ACE2 receptor. 8 , 9 , 10 After the viral particle's endocytosis process, its genetic material is released into the cytosolic environment for subsequent replication. 11
The SARS‐CoV‐2 is transmitted via respiratory droplets and by contact with infected surfaces or objects. 12 Additionally, it might be detected in several samples, including spittle, feces and blood that may also contribute with the transmission. 13 The SARS‐CoV‐2 invasion occurs primarily along the respiratory tract and may be asymptomatic or symptomatic, displaying several symptoms, such as fever, cough, dyspnea, myalgia, fatigue, confusion, headache and sore throat. 14 In some cases, the infection might progress to severe symptoms and complications that include hypoxemia, respiratory or multiple organ failure, acute cardiac injury and secondary infections. 14 , 15 , 16 In the elderly and individuals with underlying comorbidities, such as diabetes mellitus, hypertension or cardiovascular disease, the infection may result in fatal complications. 14 The SARS‐CoV‐2 infection might affect multiple organs and the lungs play a central role on the symptomatology. 17
The invasion process unbalances the renin‐angiotensin pathway caused by ACE2 function disruption and induces lung endothelial, epithelial and alveolar damage mediated by immune cells that may lead to hypoxemia and fibrosis. 18 , 19 In addition, local endothelial nitric oxide production might also cause inflammation. 18 , 20 The effect of viral invasion on other structures, such as the brain, mouth, heart, kidneys, pancreas and the gastrointestinal tract is discussed in Reference 18.
Currently, few therapies have been identified, including dexamethasone, remdesivir, lopinavir, ritonavir, recombinant ACE2, interferon 1 and convalescent plasma. 21 However, the multiple organ tropism presented by SARS‐CoV‐2 requires an individualized assessment to personalize and establish efficient therapies. 22 To monitor pathological condition, several imaging techniques are fundamental for determining the clinical management of patients with COVID‐19. 23
1.1. Ultrasonography applied to COVID‐19
Medical imaging techniques present a fundamental role in the detection, monitoring and therapeutical choices regarding the multiple damages caused by SARS‐CoV‐2. Among them, ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), X‐ray (XR) and positron emission tomography (PET) display crucial importance in COVID‐19 assessment. All are capable of revealing abnormalities in different organs as a result of the viral pathological process. In particular, chest CT allows the identification of precocious affections caused by COVID‐19 and became an important tool for determining the progress and classification of disease stages. 24 However, the wide use of CT in patients with COVID‐19 is discouraged due to the risk of cross‐contamination associated with the need for prior hygiene for each patient. 25 Likewise, XR, MRI and PET techniques present the same risk, and their use is severely limited for such patients, especially for those allocated to the intensive care unit (ICU). 25 , 26 , 27
Therefore, US has been increasingly employed as an imaging alternative to CT. US differs from other techniques for its portability, lower cost, easy transportation, sanitization and safety—a key factor to avoid viral contamination and enable imaging of patients in isolation—and, above all, US might provide images capable of indicating the nature of a wide variety of tissue damage in real‐time over relatively fast examinations, a factor that may be crucial in urgent situations. 28 , 29 , 30 , 31
Despite the several advantages of the technique, there are important diagnostic limits that prevent its wider use in cases of COVID‐19: the number of qualified professionals to interpret the test is limited during the pandemic; it is not possible to perform a “risk assessment” of the patient; mechanically ventilated patients suffer from the “curtain effect” caused by increased lung insufflation, which reduces the echocardiographic windows; and, logistically, the demand for the exam may be greater than the number of devices. 32
In the particular case of the lungs, it was recently verified that US has an accuracy similar to chest CT in pointing out elements suggestive of COVID‐19 (e.g., 100% sensitivity, 78% specificity and positive and negative predictive value of 92% and 100%, respectively). 33 A good correlation between US and CT is also observed for other organs, establishing US as an important tool in the evaluation of COVID‐19 patients. 29 , 33 Finally, US is the imaging methodology that best satisfies several requirements imposed by COVID‐19 and enable monitoring of different tissues, determine the degree of local lesion and enable the choice of therapies in an individualized manner according to the particularities of the pathological mechanism affecting different organs. 24 , 28 This review is dedicated to highlight the main US changes caused by COVID‐19 in the lungs, heart and liver.
2. LUNGS
2.1. Pulmonary pathophysiology
The lungs are the most affected organs by COVID‐19. 34 Recent studies indicate that the SARS‐CoV‐2 infection affects many cells along the proximal (e.g., cilia and goblet cells) and distal (e.g., type I and II pneumocytes) air tracts, especially on type 2 alveolar cells (AT2) that exhibit a high expression rate of ACE2 (~83%) 19 and TMPRSS2. 35 , 36 , 37 The infection in AT2 cells is one of the main mechanisms that triggers SARS in severe cases of COVID‐19, characterized by the accumulation of fluids in the alveolar sacs and consequent reduction in gas exchange. 38 , 39
After the viral infection, pattern recognition receptors (PRRs) detect the pathogen replication and act as “sentinels” that undergo oligomerization due to the interaction with invasive RNA structures. 40 , 41 This process generates transcription factors (e.g., NF‐κB), which induce the expression of types I and III interferons (IFN) that interfere with the viral replication. 42 The IFN activate the JAK–STAT signaling pathway and induce the expression of antiviral genes, whose products (i) restricts the viral replication within infected cells, (ii) induces an “antiviral state” in the tissue by recruiting cells of the innate immune system and (iii) prepare an adaptive immune response. 43
However, the SARS‐CoV‐2 infection is effective in delaying or preventing the production of immune responses associated with types I and III IFN, thus enabling the viral replication to proceed uncontested and delay the activation of the innate immune system. 44 This effect is particularly associated with severe cases of the disease with a high mortality risk. 45
Nonetheless, the response to the viral infection is multifaceted and a second defense strategy is mediated by the recruitment of specific leukocyte subtypes capable of releasing a wide variety of pro‐inflammatory cytokines and chemokines. 46 Therefore, the innate immune system is activated via interaction with damage‐associated molecular patterns (DAMPs) or pathogen‐associated molecular patterns (PAMPs; e.g., SARS‐CoV‐2 orf8b). 47 The exposure to PAMPs activates NLRP3 receptors which, in turn, recruit the enzyme caspase‐1, a pore‐forming protein that causes pyroptosis—cell death induced by the synthesis and activation of IL‐1β, IL‐18, TNF‐α and gasdermin‐D—a phenomenon observed in >82% of patients. 10 , 48 Different mechanisms linked to NLRP3 activation have been reported (e.g., induction of inflammasomes and consequent initiation of an inflammatory response correlated to cell death patients [ 49 , 50 ]), though only one has been so far associated with the SarS‐CoV‐2 infection. 51
The release of IL‐1β and TNF‐α activates Th17 cells, which secrete pro‐inflammatory cytokines that provoke an event defined as “cytokine storm.” 52 Among them, IL‐17, IL‐21, IL‐22 and GM‐CSF stand out, as they are responsible for the induction of neutrophils and other immune cells (e.g., CD68+ macrophages), blood‐air barrier remodeling, fever, hypoxemia and edema. 53 Recent studies suggest that due to the imbalance of the immune response caused by SARS‐CoV‐2—as it delays or prevents the preparation of an adaptive response—it triggers an exacerbated expanding response in an attempt to control the infection. 44 This response, however, results in a broad pulmonary immunopathology characterized by the elevation of neutrophils in the lung tissue, particularly in the most severe cases. 51
Although the molecular mechanisms of pulmonary pathophysiology are still unclear, multiple factors are under consideration. Among them, the functional loss and reduction of ACE2 expression upon its binding with the S protein of SARS‐CoV‐2 stands out, as it disturbs the renin‐angiotensin‐aldosterone system (RAAS) responsible for maintaining the blood volume and electrolyte concentration. 54 The consequent ACE2 blockage significantly reduces the conversion of angiotensin II—a potent vasoconstrictor and oxidizing action—into angiotensin 1–7, which interacts with the Mas receptor and stimulates vasodilation and antiproliferative effects. 55
Since the lungs are the main location of angiotensin II synthesis, its unregulated effects cause local vasoconstriction, which culminates in edema, loss of lung function, diffuse alveolar damage, AT2 cell hyperplasia, fibrin deposition in the interalveolar space and lymphocytic inflammation. 56 , 57 Another pathological mechanism of SARS‐CoV‐2 is the activation of coagulation pathways due to cellular apoptosis resulting from the inflammation of viral replication: with the loss of the air–blood barrier's integrity, the resulting exposure of thrombogenic substrates (e.g., membrane and other components of lysed cells) initiate coagulation and form microthrombi that occlude the alveoli and blood vessels. 57 This effect raises D‐dimer values up to five times and may cause disseminated intravascular coagulation. 57 Additionally, respiratory tract infection affects the vascular endothelium and smooth muscles of pulmonary arteries and arterioles, which may impact pulmonary perfusion and obstruct gas exchange by inhibiting smooth muscle contraction and reduce the concentration of nitric oxide. 58 , 59
Therefore, the SARS‐CoV‐2 infection induces a pro‐thrombotic state at the pulmonary level—not usually observed in other airway infections (Figure 1)—characterized by an increase in the levels of fibrinogen, Von Willebrand factor and D‐dimer. 61 The local hypercoagulability response is a defense mechanism aimed at preventing the entry and limit the spread of pathogens. 62
FIGURE 1.
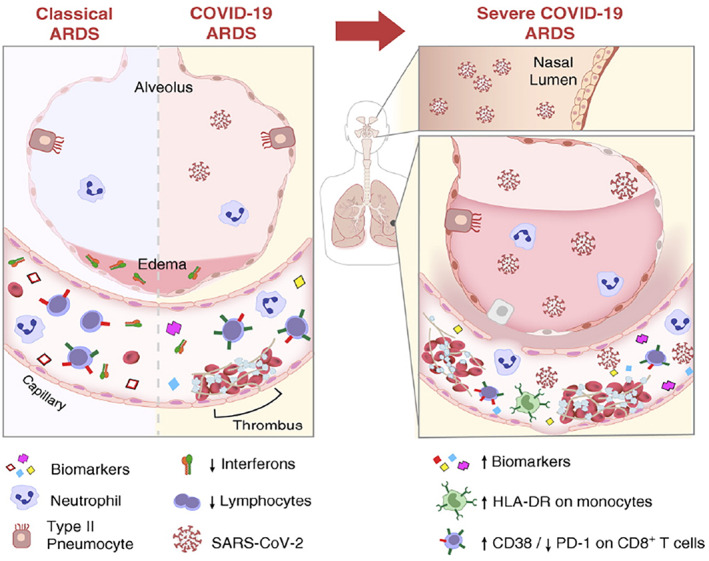
Representation of the airway and alveolar's biochemistry during classic acute respiratory distress syndrome (ARDS) and in COVID‐19‐induced ARDS. In both syndromes, the alveolar‐interstitial‐capillary structure is similarly affected. There is an increase in pro‐inflammatory biomarkers, endothelial and capillary permeability, in addition to an increase in inflammatory cells (neutrophils, monocytes and macrophages) in vessels and alveoli. However, the increased biomarkers in the two syndromes are distinct. Image adapted from 60
2.2. Pulmonary ultrasonography in patients with COVID‐19
The strategy of employing pulmonary ultrasonography (PUS) to monitor the disease evolution over the lungs revealed itself as an effective alternative for generating clinically useful images and minimize the risk of infection for the patient and/or health professional. 63 Despite the preference of CT for pulmonary diagnosis, PUS has the potential to accurately distinguish varied ultrasonic signs and patterns associated with COVID‐19, 64 , 65 as well as perform functional assessment of the tissue and the capacity for gas exchange. 66
The ultrasonographic examination of patients with COVID‐19 is characterized by the following patterns: (i) the effusion, thickening and fragmentation of the pleural and subpleural lines; (ii) the disappearance of the A lines (suggesting air content reduction); (iii) the appearance of B lines (indicating air volume reduction and local density increment), which may be separated or coalescent; (iv) consolidations in different areas and locations at different stages of the disease, which is associated with air bronchograms and whitish pulmonary pattern; (v) a “light beam signal,” which is a vertical band‐shaped artifact that moves with the pulmonary sliding during breathing—a result of the onset of interstitial involvement. 67 The observation of these patterns with bilateral pulmonary involvement was verified in >75% of COVID‐19 cases. 68
Both the A and the B lines are unique lung artifacts that are not exclusively related to COVID‐19 and may also suggest lung impairment by distinct pathologies. The A lines indicate the amount of air in the alveolar spaces and are hyperechoic horizontal arcs formed at identical intervals and parallel to the pleura and skin line, due to the repeated reflection of the transducer/skin and pleura interface (Figure 2A,B). 68 , 69 The B lines are hyperechoic and vertical to the pleura (also called “comet tails”) that move along with the pulmonary sliding. The B lines extend from the pleural line to the edge of the ultrasound image and eliminate the A lines as a result of the reverberation of the PUS bundles with edema in the alveoli (Figure 2B,C). 68 , 69 , 70
FIGURE 2.
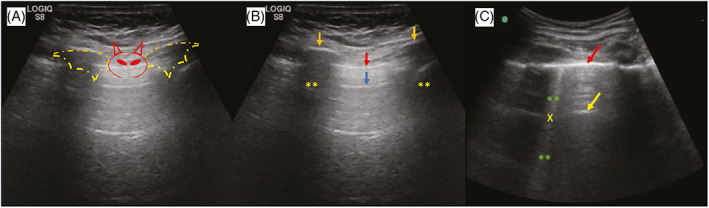
Longitudinal section of chest B‐mode ultrasound imaging. The association of ribs and pleural line generate a solid landmark called the “bat sign” (A). The A lines (blue arrow), ribs (orange arrows) and their respective acoustic shadows (asterisks) are represented in image B. The B lines (asterisks) eliminate the A lines (yellow arrow) when they cross (x) (C). Adapted from 66
The B lines indicate the degree of pulmonary impairment: the presence of up to 2 B lines per intercostal space suggests non‐severe alveolar damage; the presence of three or more lines with greater thickness point indicate compromised pulmonary aeration and a moderate reduction in compliance, as a result of interlobular septa thickening and alteration of the pulmonary interstitium 71 Another usual finding employed to indicate the evolution of the pathology are the pleural irregularities, which are associated with interstitial thickening resulting from edematous alveolar damage. 72 Subpleural consolidation indicate loss of local aeration, characterized by edema resulting from the inflammatory process. 28 , 73 In PUS, their texture is similar to the liver tissue. 73 Furthermore, large consolidations are identified by an important reduction in lung aeration. 28
As previously mentioned, PUS has excellent correlation with CT and other chest imaging techniques. 33 The evolution of the pulmonary status of patients affected by COVID‐19 monitored by PUS and CT is shown in Figure 3. 28 The following continuous monitoring shows the progressive reduction in lung capacity, revealing coalescent B lines (Figure 3B,C) compatible with ground‐glass opacification in CT. 28 The evolution of pulmonary impairment (between 9 and 13 days) leads to an increase in the area covered by the B lines and is usually followed by consolidations—initially small and restricted to the subpleural space (Figure 3D,E), but prone to evolve into other areas and locations depending on the intensification of the pathological process. 66 Thereby, by specifying the pulmonary disease of COVID‐19 through ultrasonographic images, it is possible to differentiate the disease from other pathologies, such as bacterial pneumonia, cardiogenic pulmonary edema, pulmonary fibrosis or respiratory distress syndrome. 28
FIGURE 3.
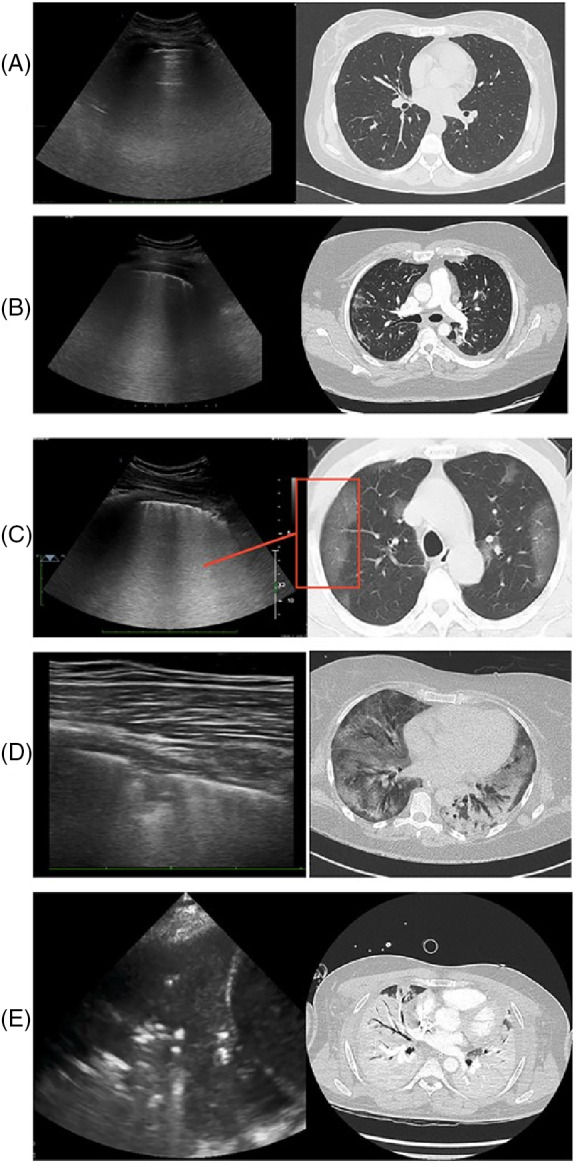
PUS and CT images of the lungs of individuals affected by COVID‐19 at different stages, from normal aeration (A), progressive deaeration states (B and C) small and peripheral consolidation (D) and large consolidation (E). Adapted from 28
To obtain effective results via PUS examination in the management of cases of infection, it is necessary to establish protocols to visualize different lung regions (Figure 4). 74 Lung division may be performed into 12 or more zones, 6 for each lung. 75 A careful analysis of the back region is suggested, as the histopathology of COVID‐19 is prominently manifested in the posterior regions. 76 It is recommended that the patient remains seated with their back to the operator. 77 However, in more severe cases, in which the patients are lied at the supine position and presents no mobility, the back areas of the lung are not visible and, therefore, the viewing should be directed close to the posterior axillary line. 74 Other ultrasound protocols include the evaluation from 10 to 14 different lung areas. 78
FIGURE 4.
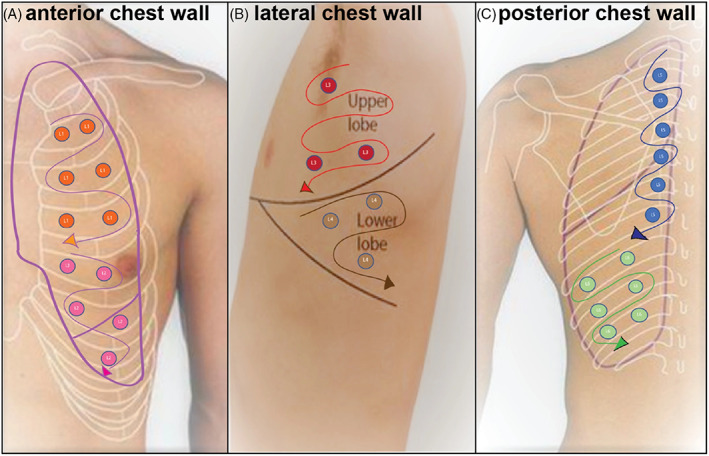
Detailing of the lung zones for the recommended PUS scan in patients with COVID‐19. Adapted from 74
In conclusion, the advantages of PUS in the examination of COVID‐19 patients are the following: portability, possibility of bedside assessments, biosafety and availability to repeat the examination during follow‐ups with no exposure to radiation. 65 , 74 In addition, this imaging method allows for the assessment of tissue damage and the degree of pulmonary impairment caused by COVID‐19. 28 , 72 , 78 Finally, PUS offers the physician a practical and safe method to obtain information during case monitoring, therapeutic response and even adaptations to mechanical ventilation. 64 , 65 , 74 , 79
3. HEART
3.1. Cardiac pathophysiology
The heart is heavily affected by COVID‐19. 75 The SARS‐CoV‐2 infection might damage the cardiac tissue in multiple ways and may cause a myriad of pathological events, such as myocardial damage, myocarditis, sudden cardiac arrest, heart failure and varying stages of shock. 80 According to epidemiological data, the following heart diseases were observed in patients with COVID‐19: myocarditis (7–17%), heart failure (24%), arrhythmias (17%) and thrombotic events (31%). 75 , 81 Such findings are associated with higher mortality rates and several complications, which include malignant arrhythmias, acute kidney injury and coagulation disorders. 80 , 82 , 83
Furthermore, the viral infection is aggravated for those patients with previous cardiovascular disease (estimated 85% more risk of fatality and a mortality rate of 10.5%, compared to 2.3% for individuals without heart disease), which is the third most prevalent comorbidity in patients hospitalized with COVID‐19. 84 As demonstrated by Zhou et al., patients with cardiac complications (pre‐existing or due to infection) exhibit higher risk of rapidly changing from a stable to an unstable condition. 85
Although still not fully understood, the pathogenesis of cardiac damage caused by SARS‐CoV‐2 is multifaceted and may be caused by direct viral infection or via indirect events. The myocardial damage caused by direct virus infection is indicated by the release of troponin T/I and detection of the viral genome in the heart. 86 Recently, Mitrani et al. revealed that almost a third of the patients exhibit some cardiac complications and the high mortality rate is associated with previous cardiovascular disease. 87 Moreover, a study by Maccio et al. showed that the endothelial cells of cardiac capillaries and epicardial nerves are the main targets of the virus due to increased ACE2 expression. 88 As a result, the infection might cause cardiac damage through several mechanisms, such as induction of cell death, inflammatory response and impairment of electromechanical functions. 89
The viral infection may provoke microvasculature impairment, particularly at the small epicardial capillaries (that display elevated ACE2 expression), which may cause vasculitis along the capillaries, arterioles and cardiac venules. 88 Therefore, patients infected by SARS‐CoV‐2 are more likely to develop thromboembolic conditions. 90 As previously discussed, the downregulation of ACE2 resulting from viral infection causes an increase in angiotensin‐II levels and a reduction in angiotensin 1–7 and 1–9 levels, which display cardioprotective effects. 75 Elevated levels of angiotensin‐II are responsible for the genesis of a cascade of cytokines via NF‐kB, IL‐6, TGF‐β and vascular endothelial growth factor (VEGF), which promote inflammation and fibrosis of the pericardial serous layer. 91 , 92 COVID‐19, as well as diabetes and atherosclerosis are ACE2 inhibitory factors, thus all these pathologies are correlated with the occurrence of myocardial ischemia and ventricular dysfunction. 91
The most frequent arrhythmia in patients with COVID‐19 is sinus tachycardia—a homeostatic response to any infection or inflammatory response whose mechanism is, however, not fully understood—though atrial fibrillation and ventricular tachycardia are also observed. 93 , 94 , 95 Among the mechanisms causing this condition, electrolyte imbalance, lung damage, drug side effects and cell signaling cascades after viral infection stands out. 75 In addition, hypoxia due to ARDS is correlated with an increased risk of arrhythmia. 96 Medicines such as azithromycin and lopinavir/ritonavir may also contribute to trigger arrhythmias by prolonging the QT interval. 97
The indirect effects of COVID‐19 might induce myocardial injury via systemic inflammatory response generated by the “cytokine storm,” characterized by high levels of IL‐1, IL‐6, IL‐1β, IFNγ, MCP‐1 and IP‐10. 98 , 99 The cytokines contribute to cardiomyopathy by facilitating the release of radical oxygen species (ROS), nitric oxide and superoxide anions. 100 Additionally, damage‐associated molecular proteins (DAMPs; e.g., heat shock proteins, oxidized lipoproteins and nuclear proteins HMGB1 proteins) are released from the damaged myocardium and induces inflammation and cardiomyopathy, establishing a continuous inflammatory cycle, resulting in septic cardiomyopathy due to COVID‐19 and hemodynamic instability. 75 , 100
The proinflammatory cytokine response mediates atherosclerosis and contribute to plaque disruption by provoking local inflammation. 101 Thereby, the exaggerated release of these inflammatory agents causes important hemodynamic changes, increasing the probability of ischemia, thrombosis, pulmonary embolism and may induce multiple organ dysfunction or disseminated intravascular coagulation. 91 , 102 Patients with more severe cases of COVID‐19 exhibit higher levels of inflammatory markers, such as C‐reactive protein, ferritin, interleukin 6, TNF‐α and thrombotic markers such as D‐dimer. 103 , 104 In addition, elevated cardiac troponin is indicative of myocardial damage, which is recurrent in COVID‐19 patients and associated with worse outcomes. 104 The increase in the level of brain natriuretic peptide (BNP) is correlated to the presence of diastolic abnormalities, right ventricular enlargement and a more severe prognosis. 104 , 105
The hypoxemia in the cardiac tissue, a condition resulting from pulmonary obstruction and consequent reduction in gas exchange is an aggravating prognosis. 106 The cellular damage caused by anaerobiosis, acidosis and ROS are also associated with an increased risk of acute myocardial infarction (AMI). 89 , 107 , 108 Moreover, patients with previous heart diseases are more susceptible to cardiac tissue damage—probably due to the inflammation caused by COVID‐19—and pre‐existing target organ damage due to cardiovascular risk factors also weakens the hearts resistance against the disease. 109 , 110 , 111
According to Capotosto et al., cardiac complications may also be related to acute pulmonary damage, causing right ventricular failure, pulmonary hypertension (with high mortality rate, up to 46%) or acute pulmonary embolism after disturbances in the coagulation and venous thromboembolism. 32 Additionally, a higher risk of death for patients with COVID‐19 who had previous coronary artery disease is observed, as the viral infection might trigger AMI. 112 , 113 , 114
3.2. Echocardiography in patients with COVID‐19
Echocardiography is widely recommended and has become part of the routine for the diagnosis and monitoring of cardiac complications caused by SARS‐CoV‐2. It is useful to indicate a wide variety of cardiac alterations caused by the infection (Figure 5) and may also suggest degeneration or necrosis of parenchymal cells as well as the formation of hyaline thrombi. 85 Thereby, the technique has a crucial role in the assessment of patients severely affected by the virus and with pre‐existing cardiovascular diseases (e.g., heart failure, cardiomegaly, and arrhythmias). 116
FIGURE 5.
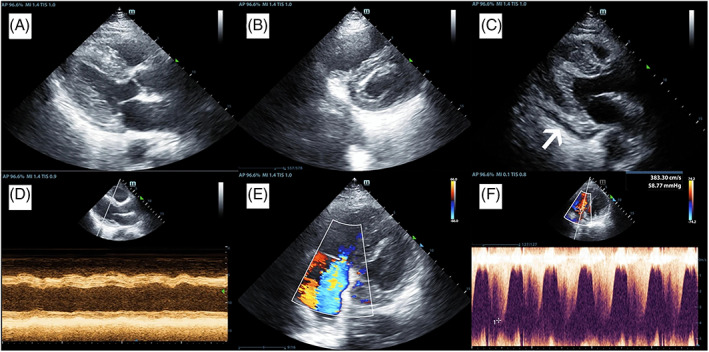
Echocardiography images of a patient with COVID‐19. The images display the thickening of the left ventricular wall (A), widening of the right ventricle and thickening of the left ventricular wall (B), pericardial effusion (indicated by the white arrow) (C), M‐mode graph of the left ventricular base with marked dyskinesia (D), Color Doppler mode showing tricuspid valve regurgitation (E) and continuous Doppler mode of the tricuspid regurgitation indicating pulmonary hypertension (E). Image adapted from 115
Echocardiography evaluation is fundamental to set individualized therapeutic interventions, such as protective ventilation to the right ventricle, therapy with anticoagulants, pulmonary vasodilation or extracorporeal oxygenation. 117 Therefore, echocardiography enables adequate management of a patient with heart failure and other complications. 118 , 119 , 120 Several findings from the clinical practice suggest this imaging modality when the patient display symptoms compatible with heart failure, high levels of BNP and pneumonia. 120 , 121 In fact, the echocardiography exam led to therapeutical modifications of 45% of COVID‐19 patients due to significant alterations. 122
As already discussed, US alterations may suggest particularities of COVID‐19, such as the extension or evolution of the pulmonary damage caused by the SARS‐CoV‐2 infection. For example, modest and severe viral infections cause local edematous foci in the interstitial pulmonary pleura, seen as “ground glass lesions” on CT and coalescent B lines on PUS. 118 In these situations, echocardiographic findings indicate ventricular interdependence, septal displacement ventricular diastolic, left ventricular hypodiastole and reduced cardiac output—caused by greater respiratory effort and higher ventilation rate per minute. 118 , 123 Moreover, the necessity of mechanical ventilation causes several functional changes that require close monitoring, such as dilation, tricuspid valve insufficiency, reduced right heart systolic capacity and left heart compression as represented in Figure 6A. 124
FIGURE 6.
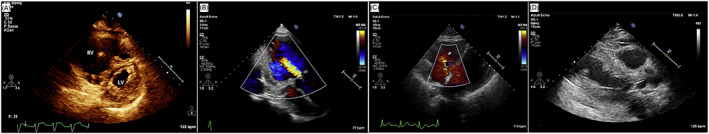
Echocardiography of a patient infected with SARS‐CoV‐2 indicating right ventricular dilatation with minor secondary left ventricular compression due to mechanical ventilation (A). Image adapted from. 118 Echocardiography images highlighting moderate aortic regurgitation (B), right ventricle and tricuspid valve regurgitation (C) and wall thickening with moderate impairment of systolic function and movement abnormality of the anterior basal wall (D). Adapted from 120
To illustrate the relevance of echocardiography, Zhang et al. reported the use of the technique in several cases throughout 2020. In all their cases, the patients presented fever, pneumonia and high levels of BNP. 120 The cardiac alterations included left ventricular enlargement, reduced ventricular ejection fraction and mitral valve regurgitation (Figures 6B,C). In severe cases, they detected abnormalities in the movement of the anterior walls, apical aneurysm with thrombosis and AMI. These assessments reinforce the discussion of Guarracino et al. that suggest a greater impairment of the right heart during COVID‐19 respiratory complications. 118
In addition, COVID‐19 patients with vein thrombosis exhibit increased risk of thromboembolism and are more susceptible to damage from this pathology. 118 , 125 Pulmonary embolism might result in pulmonary hypertension, right ventricular enlargement and tricuspid valve regurgitation. 126 According to Anile et al., around 18% of patients who manifest thromboembolic events are referred to the ICU, of which around 40% end up dying. 127
Thereby, one of the main uses of echocardiography in patients affected by COVID‐19 (especially for intubated patients) is the detailing of the right ventricular dimensions and function, usually overloaded due to pulmonary hypertension induced by (i) higher pulmonary vascular resistance, (ii) pulmonary thromboembolism or (iii) the introduction of high positive final expiratory pressures caused by mechanical ventilation. 128
This monitoring is essential to target the accurate therapy by differentiating between cases of pneumonia with or without the presence of thrombi, cases of biventricular or isolated right ventricular failure and cases with primary or secondary right ventricular dysfunction. 117 Additionally, the assessment of left ventricular function and other cardiac structures (e.g., diameter of the right heart chambers) via US is important for monitoring hemodynamic and thromboembolic phenomena, especially due to recent correlations between progressive tissue dysfunction and the worsening of the disease. 129
Therefore, the echocardiographic modality displays a paramount importance to evaluate and monitor COVID‐19 patients. This technique is relevant for the diagnosis, prognosis and treatment of any patient, as cardiac complications are not necessarily related to previous cardiovascular disease. 115 , 128 By evaluating the right ventricle, it is possible to avoid worse cardiac (e.g., regurgitation, dilation or decreased ejection fraction) and pulmonary (e.g., hypertension or lung damage) consequences. 117 , 121
4. LIVER
4.1. Hepatic pathophysiology
The SARS‐CoV‐2 infection can also affect the liver tissue directly or indirectly. The direct infection is characterized by the invasion and replication of the virus in the hepatocytes 130 via ACE2 interaction, which may progress to acute liver damage. 131 However, the hepatocyte's expression of ACE2 (~2.6%) is lower compared to cholangiocytes (~59.7%). 19 Therefore, the most probable infection process starts in the bile duct and spreads to the liver by the breakage of intratissue barriers—via reduction of tight junction proteins, an effect already demonstrated in vitro for claudin 1. 132 , 133 , 134 Furthermore, only 0.03% of hepatocytes from healthy patients co‐express ACE2 and TMPRSS, 135 reinforcing the hypothesis of a higher probability of invasion via bile duct. It is important, however, to highlight that this percentage still represents millions of cells susceptible to viral invasion. 136
The SARS‐CoV‐2 infection causes several changes in hepatic function tests (TFH; e.g., alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, gammaGT, albumin and other laboratory and imaging exams). 132 , 137 , 138 The number of patients affected by COVID‐19 that showed liver impairment ranges between 15% and 78%. 137 More severe cases exhibit liver damage with higher incidence when compared to moderate cases. 139
The associated liver damage potentializes and affect more severely individuals that are already afflicted with liver disease (e.g., non‐alcoholic fatty liver disease [NAFLD] or associated metabolic comorbidities). 132 , 134 , 140 For example, the lipogenesis that characterizes steatosis is increased by injury and dilation of the endoplasmic reticulum of hepatocytes caused by SARS‐CoV‐2. 132 According to Medeiros et al., patients with COVID‐19 are 4.7 times more likely to develop hepatic steatosis. 141 Consequently, patients with hepatic steatosis display higher risk to undergo intubation, ICU hospitalization or require dialysis. 142
The indirect impact of COVID‐19 in liver disease is identified by systemic inflammation, thromboembolism, local hypoxia, and drug use (e.g., azithromycin or remdesivir). 132 , 143 , 144 Post‐mortem histological analyses of the liver revealed the presence of vascular abnormalities, such as partial portal microthrombosis (present in 50% of cases), micro and macrovesicular steatosis (45%), portal inflammation (~65%) and portal fibrosis (60%). 145 The reason for the predisposition of thrombosis formation is the exacerbated production of pro‐inflammatory cytokines, which result in increased inflammation, platelet activation and endothelial dysfunction as previously discussed. 146
4.2. Hepatic ultrasonography in patients with COVID‐19
US can detect anatomical and/or physiological changes in the liver tissue (e.g., biliary tree obstructions, hepatic steatosis or portal fibrosis). Therefore, it is an important alternative technique for the diagnosis and monitoring of several hepatic comorbidities induced and/or potentialized by COVID‐19 since ~37% of patients are affected by viral infection at the abdominal level. 147
The main findings in the liver US in COVID‐19 patients include hepatomegaly, changes in the liver echogenicity (suggesting hepatic infarction or hepatic steatosis), heterogeneity of hepatic parenchyma, signs of necrosis, increased gallbladder thickness and drastic reduction in the Doppler signal imaging in the hepatic arteries and portal vein. 148 All such US alterations may be induced either by direct or indirect SARS‐CoV‐2 infection. 148
The increase in cases of portal vein thrombosis (PVT) is an alarming complication observed in COVID‐19 cases, which may trigger intestinal infarction and ischemia. 149 Even with prophylactic measures in the ICU, the frequency of thrombotic complications in critically ill patients is around 30%. 150 The cytokine storm associated with patient immobilization and hypoxia is believed to be the cause of PVT and its primary diagnosis is performed via US and Doppler or CT due to initial complaints of abdominal pain. 151
However, the utilization of liver US usually involves the identification of consequences caused by COVID‐19, such as hepatic steatosis and fibrosis. 152 The US B‐mode is the most recommended method for diagnosis and classification of hepatic steatosis, 153 an important tool to evaluate the consequences of COVID‐19. 138 US provides useful images for the diagnosis and therapy of steatosis, especially for moderate or severely affected liver. 153 Its overall sensitivity and specificity are of 85% and 93%, respectively. 153 However, mild steatosis recognition is still poor, with 60% sensitivity. 154 More precise identification of steatosis may be performed by liver elastography that displays reduced elastic modulus due to the pathological process. 155 Inflammation and consequent elevation of transaminases is directly related to the disease's progression ( 154 , 155 ).
Furthermore, a study by Abdelmohsen et al. showed that US diagnosis in COVID‐19 patients of hepatomegaly and biliary disease was found in 56% and 41% of imaging exams, respectively. 156 US is further used for the primary analysis of hepatobiliary alterations and, therefore, may be used in cases of damage associated with cholangiocytes and cholestasis. 148
5. CONCLUSION AND PERSPECTIVES
The SARS‐CoV‐2 multiorgan tropism as well as its indirect effects might be elucidated throughout or after the infection with high sensitivity and specificity by ultrasound imaging. The ultrasonographic analysis of the lungs, heart and liver of COVID‐19 patients reveal specific artifacts that may determine the prognosis or therapy. Therefore, ultrasound, previously regarded as a complementary technique in the assessment of these patients, might be included as a key imaging modality in COVID‐19. The widespread use of ultrasound imaging, however, still requires standardization to determine specific technical settings (e.g., scanner, probes and ultrasound frequency) as well as to improve accuracy, reproducibility, scoring systems and predictive value in COVID‐19 patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors acknowledge the Pontifical Catholic University of Paraná (PUC‐PR), Federal University of Paraná (UFPR), the National council for teaching and research (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Ito GNW, Rodrigues VAC, Hümmelgen J, et al. COVID‐19 pathophysiology and ultrasound imaging: A multiorgan review. J Clin Ultrasound. 2022;50(3):326‐338. doi: 10.1002/jcu.23160
[Correction added after first online publication on February 26, 2022. Author affiliations have been amended.]
Funding information Conselho Nacional de Desenvolvimento Científico e Tecnológico; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5(4):536‐544. http://www.nature.com/articles/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan‐Yeung M, Xu R‐H. SARS: epidemiology. Respirology. 2003;8(s1):S9‐S14. doi: 10.1046/j.1440-1843.2003.00518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhong N, Zheng B, Li Y, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362(9393):1353‐1358. https://linkinghub.elsevier.com/retrieve/pii/S0140673603146302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523‐534. 10.1038/nrmicro.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burki T. Outbreak of coronavirus disease 2019. Lancet Infect Dis. 2020;20(3):292‐293. 10.1016/S1473-3099(20)30076-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coronavirus World Map: Tracking the Global Outbreak ‐ The New York Times . https://www.nytimes.com/interactive/2021/world/covid-cases.html (Accessed September 2, 2021)
- 7. Berry JD, Hay K, Rini JM, et al. Neutralizing epitopes of the SARS‐CoV S‐protein cluster independent of repertoire, antigen structure or mAb technology. MAbs. 2010;27:53‐66. doi: 10.4161/mabs.2.1.10788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marjot T, Webb GJ, Barritt AS, et al. COVID‐19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18(5):348‐364. doi: 10.1038/s41575-021-00426-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li F, Han M, Dai P, et al. Distinct mechanisms for TMPRSS2 expression explain organ‐specific inhibition of SARS‐CoV‐2 infection by enzalutamide. Nat Commun. 2021;12(1):866 http://www.nature.com/articles/s41467-021-21171-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson BA, Xie X, Bailey AL, et al. Loss of furin cleavage site attenuates SARS‐CoV‐2 pathogenesis. Nature. 2021;591(7849):293‐299. doi: 10.1038/s41586-021-03237-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kyrou I, Randeva HS, Spandidos DA, Karteris E. Not only ACE2—the quest for additional host cell mediators of SARS‐CoV‐2 infection: Neuropilin‐1 (NRP1) as a novel SARS‐CoV‐2 host cell entry mediator implicated in COVID‐19. Signal Transduct Target Therapy. 2021;6(1):2020‐2022. doi: 10.1038/s41392-020-00460-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen PZ, Koopmans M, Fisman DN, Gu FX. Understanding why superspreading drives the COVID‐19 pandemic but not the H1N1 pandemic. Lancet Infect Dis. 2021;21(9):1203‐1204. https://linkinghub.elsevier.com/retrieve/pii/S1473309921004060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of Patients infected with SARS‐CoV‐2 in Singapore. JAMA. 2020;323(15):1488‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen P, Nirula A, Heller B, et al. SARS‐CoV‐2 neutralizing antibody LY‐CoV555 in outpatients with Covid‐19. N Engl J Med. 2021;384(3):229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rendeiro AF, Ravichandran H, Bram Y, et al. The spatial landscape of lung pathology during COVID‐19 progression. Nature. 2021;593(7860):564‐569. http://www.nature.com/articles/s41586-021-03475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ashraf UM, Abokor AA, Edwards JM, et al. SARS‐CoV‐2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53(2):51‐60. doi: 10.1152/physiolgenomics.00087.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single‐cell RNA expression profiling of ACE2, the receptor of SARS‐CoV‐2. Am J Respir Crit Care Med. 2020;202(5):756‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yagil Y, Yagil C. Hypothesis: ACE2 modulates blood pressure in the mammalian organism. Hypertension. 2003;41(4):871‐873. [DOI] [PubMed] [Google Scholar]
- 21. Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID‐19: immunology and treatment options. Clin Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. dos Santos BS, dos Santos FS, Ribeiro ER. Clinical‐epidemiological relation between SARS‐COV‐2 and KAWASAKI disease: an integrative literature. Rev Paulista Pediatr. 2021;39:e2020217 http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-05822021000100503&tlng=en [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alhazzani W, Møller MH, Arabi YM, et al. Intensive Care Medicine. Springer Berlin Heidelberg; 2020:854‐887. doi: 10.1007/s00134-020-06022-5 [DOI] [Google Scholar]
- 24. Chassagnon G, Vakalopoulou M, Battistella E, et al. AI‐driven quantification, staging and outcome prediction of COVID‐19 pneumonia. Med Image Anal. 2021;67:1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsou IYY, Liew CJY, Tan BP, et al. Planning and coordination of the radiological response to the coronavirus disease 2019 (COVID‐19) pandemic: the Singapore experience. Clin Radiol. 2020;75(6):415‐422. https://linkinghub.elsevier.com/retrieve/pii/S000992602030129X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fields BKK, Demirjian NL, Dadgar H, Gholamrezanezhad A. Imaging of COVID‐19: CT, MRI, and PET. Semin Nucl Med. 2021;51(4):312‐320. 10.1053/j.semnuclmed.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meirelles G. COVID‐19: a brief update for radiologists. Radiol Bras. 2020;53(5):320‐328. doi: 10.1590/0100-3984.2020.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gargani L, Soliman‐Aboumarie H, Volpicelli G, Corradi F, Pastore MC, Cameli M. Why, when, and how to use lung ultrasound during the COVID‐19 pandemic: enthusiasm and caution. Eur Heart J Cardiovasc Imaging. 2020;21(9):941‐948. https://academic.oup.com/ehjcimaging/article/21/9/941/5855021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Piscaglia F, Stefanini F, Cantisani V, et al. Benefits, open questions and challenges of the use of Ultrasound in the COVID‐19 pandemic era. The views of a panel of worldwide international experts. Eur J Ultrasound. 2020;41(3):228‐236. doi: 10.1055/a-1149-9872 [DOI] [PubMed] [Google Scholar]
- 30. Gogna A, Yogendra P, Lee SHE, et al. Diagnostic Ultrasound services during the coronavirus disease (COVID‐19) pandemic. Am J Roentgenol. 2020;215(5):1130‐1135. doi: 10.2214/AJR.20.23167 [DOI] [PubMed] [Google Scholar]
- 31. Antúnez‐Montes OY, Buonsenso D. Routine use of point‐of‐care lung ultrasound during the COVID‐19 pandemic. Med Int. 2020;46(1):42‐45. https://linkinghub.elsevier.com/retrieve/pii/S0210569120301170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Capotosto L, Nguyen BL, Ciardi MR, Mastroianni C, Vitarelli A. Heart, COVID‐19, and echocardiography. Echocardiography. 2020;37(9):1454‐1464. doi: 10.1111/echo.14834 [DOI] [PubMed] [Google Scholar]
- 33. Tung‐Chen Y, Martí de Gracia M, Díez‐Tascón A, et al. Correlation between chest computed tomography and lung ultrasonography in patients with coronavirus disease 2019 (COVID‐19). Ultrasound Med Biol. 2020;46(11):2918‐2926. https://linkinghub.elsevier.com/retrieve/pii/S030156292030301X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yi Y, Lagniton PNP, Ye S, Li E, Xu R‐H. COVID‐19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16(10):1753‐1766. http://www.ijbs.com/v16p1753.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sungnak W, Huang N, Bécavin C, et al. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lukassen S, Chua RL, Trefzer T, et al. SARS ‐CoV‐2 receptor ACE 2 and TMPRSS 2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39(10):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mulay A, Konda B, Garcia G, et al. SARS‐CoV‐2 infection of primary human lung epithelium for COVID‐19 modeling and drug discovery. Cell Rep. 2021;35(5):109055. doi: 10.1016/j.celrep.2021.109055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Katsura H, Sontake V, Tata A, et al. Human lung stem cell‐based Alveolospheres provide insights into SARS‐CoV‐2‐mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell. 2020;27(6):890‐904. 10.1016/j.stem.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post‐mortem findings in a series of COVID‐19 cases from northern Italy: a two‐centre descriptive study. Lancet Infect Dis. 2020;20(10):1135‐1140. doi: 10.1016/S1473-3099(20)30434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blanco‐Melo D, Nilsson‐Payant BE, Liu W‐C, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell. 2020;181(5):1036‐1045. doi: 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20(1):197‐216. doi: 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- 42. Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond Ser B Biol Sci. 1957;147(927):258‐267.13465720 [Google Scholar]
- 43. Mesev E, RA LD, Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol. 2019;4(6):914‐924. doi: 10.1038/s41564-019-0421-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sette A, Crotty S. Adaptive immunity to SARS‐CoV‐2 and COVID‐19. Cell. 2021. Feb;184(4):861‐880. doi: 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Galani IE, Rovina N, Lampropoulou V, et al. Untuned antiviral immunity in COVID‐19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol. 2021;22(1):32‐40. doi: 10.1038/s41590-020-00840-x [DOI] [PubMed] [Google Scholar]
- 46. Bonaventura A, Vecchié A, Dagna L, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID‐19. Nat Rev Immunol. 2021;21(5):319‐329. doi: 10.1038/s41577-021-00536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shi CS, Nabar NR, Huang NN, Kehrl JH. SARS‐Coronavirus Open Reading Frame‐8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Dis. 2019;5(1):1‐12. doi: 10.1038/s41420-019-0181-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Zoete MR, Palm NW, Zhu S, Flavell RA. Inflammasomes. Cold Spring Harb Perspect Biol. 2014;6(12):a016287‐a016287. doi: 10.1101/cshperspect.a016287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zahid A, Li B, AJK K, Jin T, Tao J. Pharmacological inhibitors of the NLRP3 inflammasome. Front Immunol. 2019;25(10):1‐10. doi: 10.3389/fimmu.2019.02538/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li S, Jiang L, Li X, et al. Clinical and pathological investigation of patients with severe COVID‐19. JCI Insight. 2020;5(12):1‐13. https://insight.jci.org/articles/view/138070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ramos‐Casals M, Brito‐Zerón P, Mariette X. Systemic and organ‐specific immune‐related manifestations of COVID‐19. Nat Rev Rheumatol. 2021;17(6):315‐332. doi: 10.1038/s41584-021-00608-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Asrani P, Hassan MI. SARS‐CoV‐2 mediated lung inflammatory responses in host: targeting the cytokine storm for therapeutic interventions. Mol Cell Biochem. 2021;476(2):675‐687. doi: 10.1007/s11010-020-03935-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beyerstedt S, Casaro EB, Rangel ÉB. COVID‐19: angiotensin‐converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS‐CoV‐2 infection. Eur J Clin Microbiol Infect Dis. 2021;40(5):905‐919. doi: 10.1007/s10096-020-04138-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tikellis C, Bernardi S, Burns WC. Angiotensin‐converting enzyme 2 is a key modulator of the renin–angiotensin system in cardiovascular and renal disease. Curr Opin Nephrol Hypertens. 2011;20(1):62‐68. http://journals.lww.com/00041552-201101000-00011 [DOI] [PubMed] [Google Scholar]
- 56. Sarzani R, Giulietti F, di Pentima C, Giordano P, Spannella F. Disequilibrium between the classic renin‐angiotensin system and its opposing arm in SARS‐CoV‐2‐related lung injury. Am J Physiol Lung Cell Mol Physiol. 2020;319(2):L325‐L336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120‐128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McFadyen JD, Stevens H, Peter K. The emerging threat of (micro)thrombosis in COVID‐19 and its therapeutic implications. Circ Res. 2020;127(4):571‐587. doi: 10.1161/CIRCRESAHA.120.317447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID‐19: vascular and perfusion abnormalities on dual‐energy CT. Lancet Infect Dis. 2020;20(12):1365‐1366. doi: 10.1016/S1473-3099(20)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Matthay MA, Leligdowicz A, Liu KD. Biological Mechanisms of COVID‐19 Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;202(11):1489‐1491. doi: 10.1164/rccm.202009-3629ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48(9):1358‐1364. doi: 10.1097/CCM.0000000000004458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013. Jan 7;13(1):34‐45. doi: 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]
- 63. Denina M, Scolfaro C, Silvestro E, et al. Lung ultrasound in children with COVID‐19. Pediatrics. 2020. Jul;146(1):e20201157. doi: 10.1542/peds.2020-1157 [DOI] [PubMed] [Google Scholar]
- 64. Volpicelli G, Gargani L, Perlini S, et al. Lung ultrasound for the early diagnosis of COVID‐19 pneumonia: an international multicenter study. Intensive Care Med. 2021;47(4):444‐454. doi: 10.1007/s00134-021-06373-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Volpicelli G, Lamorte A, Villén T. What's new in lung ultrasound during the COVID‐19 pandemic. Intensive Care Med. 2020;46(7):1445‐1448. doi: 10.1007/s00134-020-06048-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. de Oliveira RR, Rodrigues TP, da PSD S, Gomes AC, Chammas MC. Lung ultrasound: an additional tool in COVID‐19. Radiol Bras. 2020;53(4):241‐251. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-39842020000400241&tlng=en [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peixoto AO, Costa RM, Uzun R, AMA F, Ribeiro JD, FAL M. Applicability of lung ultrasound in COVID‐19 diagnosis and evaluation of the disease progression: a systematic review. Pulmonology. 2021;27(6):529‐562. doi: 10.1016/j.pulmoe.2021.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Secco G, Delorenzo M, Salinaro F, et al. Lung ultrasound presentation of COVID‐19 patients: phenotypes and correlations. Intern Emerg Med. 2021;16(5):1317‐1327. doi: 10.1007/s11739-020-02620-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lichtenstein DA, Mezière GA, Lagoueyte J‐F, Biderman P, Goldstein I, Gepner A. A‐Lines and B‐Lines. Chest. 2009;136(4):1014‐1020. https://linkinghub.elsevier.com/retrieve/pii/S0012369209605997 [DOI] [PubMed] [Google Scholar]
- 70. Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound. 2011;9(1):6. doi: 10.1186/1476-7120-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH. Essentials for radiologists on COVID‐19: an update‐radiology scientific expert panel. Radiology. 2020;296(2):E113‐E114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Almeida Monteiro RA, de Oliveira EP, Nascimento Saldiva PH, et al. Histological–ultrasonographical correlation of pulmonary involvement in severe COVID‐19. Intensive Care Med. 2020;46(9):1766‐1768. doi: 10.1007/s00134-020-06125-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang G, Ji X, Xu Y, Xiang X. Lung ultrasound: a promising tool to monitor ventilator‐associated pneumonia in critically ill patients. Crit Care. 2016;20(1):1‐10. doi: 10.1186/s13054-016-1487-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Manivel V, Lesnewski A, Shamim S, Carbonatto G, Govindan T. CLUE: COVID‐19 lung ultrasound in emergency department. Emerg Med Australas. 2020;32(4):694‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sattar Y, Ullah W, Rauf H, et al. COVID‐19 cardiovascular epidemiology, cellular pathogenesis, clinical manifestations and management. IJC Heart Vasculture. 2020;29:100589. doi: 10.1016/j.ijcha.2020.100589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Millington SJ, Koenig S, Mayo P, Volpicelli G. Lung ultrasound for patients with coronavirus disease 2019 pulmonary disease. Chest. 2021. Jan;159(1):205‐211. doi: 10.1016/j.chest.2020.08.2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yan J‐H, Pan L, Gao Y‐B, Cui G‐H, Wang Y‐H. Utility of lung ultrasound to identify interstitial lung disease. Medicine. 2021;100(12):e25217. doi: 10.1097/MD.0000000000025217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mento F, Perrone T, Fiengo A, et al. Limiting the areas inspected by lung ultrasound leads to an underestimation of COVID‐19 patients' condition. Intensive Care Med. 2021;47(7):811‐812. doi: 10.1007/s00134-021-06407-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pietersen PI, Madsen KR, Graumann O, Konge L, Nielsen BU, Laursen CB. Lung ultrasound training: a systematic review of published literature in clinical lung ultrasound training. Crit Ultrasound J. 2018;10(1):23. doi: 10.1186/s13089-018-0103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Palevsky PM. COVID‐19 and AKI: where do we stand? J Am Soc Nephrol. 2021;32(5):1029‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Capone V, Cuomo V, Esposito R, et al. Epidemiology, prognosis and clinical manifestation of cardiovascular disease in COVID‐19. Expert Rev Cardiovasc Ther. 2020;18(8):531‐540. doi: 10.1080/14779072.2020.1797491 [DOI] [PubMed] [Google Scholar]
- 82. Babapoor‐Farrokhran S, Rasekhi RT, Gill D, Babapoor S, Amanullah A. Arrhythmia in COVID‐19. SN Compr Clin Med. 2020;2(9):1430‐1435. doi: 10.1007/s42399-020-00454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(9):2103‐2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cameli M, Pastore MC, Soliman Aboumarie H, et al. Usefulness of echocardiography to detect cardiac involvement in COVID‐19 patients. Echocardiography. 2020;37(8):1278‐1286. doi: 10.1111/echo.14779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lippi G, Lavie CJ, Sanchis‐Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID‐19): evidence from a meta‐analysis. Prog Cardiovasc Dis. 2020;63(3):390‐391. https://linkinghub.elsevier.com/retrieve/pii/S0033062020300554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mitrani RD, Dabas N, Goldberger JJ. COVID‐19 cardiac injury: implications for long‐term surveillance and outcomes in survivors. Heart Rhythm. 2020;17(11):1984‐1990. doi: 10.1016/j.hrthm.2020.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Maccio U, Zinkernagel AS, Shambat SM, et al. SARS‐CoV‐2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021;63:103182. doi: 10.1016/j.ebiom.2020.103182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Babapoor‐Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID‐19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Khan IH, Savarimuthu S, Leung MST, Harky A. The need to manage the risk of thromboembolism in COVID‐19 patients. J Vasc Surg. 2020;72(3):799‐804. doi: 10.1016/j.jvs.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hirano T, Murakami M. COVID‐19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731‐733. doi: 10.1016/j.immuni.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cizgici AY, Zencirkiran Agus H, Yildiz M. COVID‐19 myopericarditis: it should be kept in mind in today's conditions. Am J Emerg Med. 2020;38(7):1547. doi: 10.1016/j.ajem.2020.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Long B, Brady WJ, Bridwell RE, et al. Electrocardiographic manifestations of COVID‐19. Am J Emerg Med. 2021;41:96‐103. https://linkinghub.elsevier.com/retrieve/pii/S0735675720311803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. He J, Wu B, Chen Y, et al. Characteristic Electrocardiographic Manifestations in Patients With COVID‐19. Can J Cardiol. 2020;36(6):966.e1‐966.e4. https://linkinghub.elsevier.com/retrieve/pii/S0828282X20303019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wasim D, Alme B, Jordal S, et al. Characteristics of 24‐hour ambulatory blood pressure monitoring in a COVID‐19 survivor. Futur Cardiol. 2021;17(8):1321‐1326. doi: 10.2217/fca-2020-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526‐2533. [DOI] [PubMed] [Google Scholar]
- 97. Bansal M. Cardiovascular disease and COVID‐19. Diabetes Metab Syndr Clin Res Rev. 2020;14(3):247‐250. doi: 10.1016/j.dsx.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lu Q, Zhu Z, Tan C, et al. Changes of serum IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 in COVID‐19 patients. Int J Clin Pract. 2020;2021:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lu Q, Zhu Z, Zhou H, et al. Discussion about clinical value of detection of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 for the diagnosis of COVID‐19. Authorea preprints. 2020;1‐11. [Google Scholar]
- 100. Hendren NS, Drazner MH, Bozkurt B, Cooper LT. Description and proposed management of the acute COVID‐19 cardiovascular syndrome. Circulation. 2020;141(23):1903‐1914. doi: 10.1161/CIRCULATIONAHA.120.047349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Grzegorowska O, Lorkowski J. Possible correlations between atherosclerosis, acute coronary syndromes and COVID‐19. J Clin Med. 2020;9(11):3746 https://www.mdpi.com/2077-0383/9/11/3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mountantonakis SE, Saleh M, Fishbein J, et al. Atrial fibrillation is an independent predictor for in‐hospital mortality in patients admitted with SARS‐CoV‐2 infection. Heart Rhythm. 2021;18(4):501‐507. doi: 10.1016/j.hrthm.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sandoval Y, Januzzi JL, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID‐19: JACC review topic of the week. J Am Coll Cardiol. 2020;76(10):1244‐1258. doi: 10.1016/j.jacc.2020.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Januzzi JL Jr. Troponin and BNP use in COVID‐19. Am Coll Cardiol. 2020;1:1‐2. doi: 10.1101/2020.03.25.20043133v1 [DOI] [Google Scholar]
- 106. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID‐19. Mayo Clin Proc. 2020;95(6):1138‐1147. doi: 10.1016/j.mayocp.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Stefanini GG, Montorfano M, Trabattoni D, et al. ST‐elevation myocardial infarction in patients with COVID‐19. Circulation. 2020;141(25):2113‐2116. doi: 10.1161/CIRCULATIONAHA.120.047525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911‐915. doi: 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen Y, Gong X, Wang L, Guo J. Effects of hypertension, diabetes and coronary heart disease on COVID‐19 diseases severity: a systematic review and meta‐analysis 2020;(280). [Google Scholar]
- 110. Alsaied T, Aboulhosn JA, Cotts TB, et al. Coronavirus disease 2019 (COVID‐19) pandemic implications in pediatric and adult congenital heart disease. J Am Heart Assoc. 2020;9(12):1‐29. doi: 10.1161/JAHA.120.017224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Saeed S, Tadic M, Larsen TH, Grassi G, Mancia G. Coronavirus disease 2019 and cardiovascular complications: focused clinical review. J Hypertens. 2021;39(7):1282‐1292. doi: 10.1097/HJH.0000000000002819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schiavone M, Gasperetti A, Mancone M, et al. Redefining the prognostic value of high‐sensitivity troponin in COVID‐19 patients: the importance of concomitant coronary artery disease. J Clin Med. 2020;9(10):3263 https://www.mdpi.com/2077-0383/9/10/3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Barman HA, Atici A, Sahin I, et al. Prognostic significance of cardiac injury in COVID‐19 patients with and without coronary artery disease. Coron Artery Dis. 2021;32(5):359‐366. doi: 10.1097/MCA.0000000000000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ganjali S, Bianconi V, Penson PE, et al. Commentary: statins, COVID‐19, and coronary artery disease: killing two birds with one stone. Metabolism. 2020;113:1‐5. doi: 10.1016/j.metabol.2020.154375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zeng J‐H, Wu W‐B, Qu J‐X, et al. Cardiac manifestations of COVID‐19 in Shenzhen, China. Infection. 2020;48(6):861‐870. doi: 10.1007/s15010-020-01473-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hung J, Abraham TP, Cohen MS, et al. ASE statement on the reintroduction of echocardiographic services during the COVID‐19 pandemic. J Am Soc Echocardiogr. 2020;33(8):1034‐1039. https://linkinghub.elsevier.com/retrieve/pii/S0894731720303138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dandel M. Cardiac manifestations of COVID‐19 infection: the role of echocardiography in patient management. Infection. 2021;49(1):187‐189. doi: 10.1007/s15010-020-01507-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Guarracino F, Vetrugno L, Forfori F, et al. Lung, heart, vascular, and diaphragm ultrasound examination of COVID‐19 patients: a comprehensive approach. j Cardiothorac Vasc Anesth. 2021;35(6):1866‐1874. doi: 10.1053/j.jvca.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Li L, Yong RJ, Kaye AD, Urman RD. Perioperative point of care ultrasound (POCUS) for anesthesiologists: an overview. Curr Pain Headache Rep. 2020;24(5):20. doi: 10.1007/s11916-020-0847-0 [DOI] [PubMed] [Google Scholar]
- 120. Zhang L, Wang B, Zhou J, Kirkpatrick J, Xie M, Johri AM. Bedside focused cardiac ultrasound in COVID‐19 from the Wuhan epicenter: the role of cardiac point‐of‐care ultrasound, limited transthoracic echocardiography, and critical care echocardiography. J Am Soc Echocardiogr. 2020;33(6):676‐682. https://linkinghub.elsevier.com/retrieve/pii/S0894731720302157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Agricola E, Beneduce A, Esposito A, et al. Heart and lung multimodality imaging in COVID‐19. J Am Coll Cardiol Img. 2020;13(8):1792‐1808. doi: 10.1016/j.jcmg.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID‐19. Eur Heart J Cardiovasc Imaging. 2020;21(9):949‐958. https://academic.oup.com/ehjcimaging/article/21/9/949/5859292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gattinoni L, Chiumello D, Caironi P, et al. COVID‐19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099‐1102. doi: 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dandel M. Heart–lung interactions in COVID‐19: prognostic impact and usefulness of bedside echocardiography for monitoring of the right ventricle involvement. Heart Fail Rev. 2021:1‐15. doi: 10.1007/s10741-021-10108-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Liu RB, Tayal VS, Panebianco NL, et al. Ultrasound on the frontlines of COVID‐19: report from an international webinar. Acad Emerg Med. 2020;27(6):523‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med. 2002;136(9):691‐700. [DOI] [PubMed] [Google Scholar]
- 127. Anile A, Castiglione G, Zangara C, Calabrò C, Vaccaro M, Sorbello M. COVID‐19: the new ultrasound alphabet in SARS‐CoV‐2 era. Anesth Analg. 2020;131(5):e232‐e234. doi: 10.1213/ANE.0000000000005142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;26(7):1017‐1032. doi: 10.1038/s41591-020-0968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sattarzadeh Badkoubeh R, Khoshavi M, Lalehfar V, et al. Imaging data in COVID‐19 patients: focused on echocardiographic findings. Int J Cardiovasc Imaging. 2021;37(5):1629‐1636. doi: 10.1007/s10554-020-02148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wang Y, Liu S, Liu H, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol. 2020;73(4):807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Porta G, SMG V, de Albuquerque AT, et al. Doenças hepáticas crônicas e transplante hepático em tempos de COVID‐19. Nota de Alerta SBP. 2020;5:1‐9. [Google Scholar]
- 132. Nardo AD, Schneeweiss‐Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID‐19. Liver Int. 2021;41(1):20‐32. doi: 10.1111/liv.14730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. bioRxiv. 2020. [Google Scholar]
- 134. Moon AM, Webb GJ, Aloman C, et al. High mortality rates for SARS‐CoV‐2 infection in patients with pre‐existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73(3):705‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. MacParland SA, Liu JC, Ma X‐Z, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9(1):4383 http://www.nature.com/articles/s41467-018-06318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. de Smet V, Verhulst S, van Grunsven LA. Single cell RNA sequencing analysis did not predict hepatocyte infection by SARS‐CoV‐2. J Hepatol. 2020;73(4):993‐995. https://linkinghub.elsevier.com/retrieve/pii/S0168827820303494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zarifian A, Zamiri Bidary M, Arekhi S, et al. Gastrointestinal and hepatic abnormalities in patients with confirmed COVID‐19: a systematic review and meta‐analysis. J Med Virol. 2021;93(1):336‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID‐19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613‐628. doi: 10.1007/s10735-020-09915-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428‐430. doi: 10.1016/S2468-1253(20)30057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Campos MR, de A Schramm JM, Emmerick ICM, Rodrigues JM, de Avelar FG, Pimentel TG. Carga de doença da COVID‐19 e de suas complicações agudas e crônicas: reflexões sobre a mensuração (DALY) e perspectivas no Sistema Único de Saúde. Cad Saude Publica. 2020;36(11): http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-311X2020001103001&tlng=pt [DOI] [PubMed] [Google Scholar]
- 141. Medeiros AK, Barbisan CC, Cruz IR, et al. Higher frequency of hepatic steatosis at CT among COVID‐19‐positive patients. Abdominal Radiology. 2020;45(9):2748‐2754. doi: 10.1007/s00261-020-02648-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chen VL, Hawa F, Berinstein JA, et al. Hepatic steatosis is associated with increased disease severity and liver injury in coronavirus disease‐19. Dig Dis Sci. 2020. doi: 10.1007/s10620-020-06618-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Brito CA, Barros FM, Lopes EP. Mechanisms and consequences of COVID‐19 associated liver injury: what can we affirm? World J Hepatol. 2020;12(8):413‐422. https://www.wjgnet.com/1948-5182/full/v12/i8/413.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zampino R, Mele F, Florio LL, et al. Liver injury in remdesivir‐treated COVID‐19 patients. Hepatol Int. 2020;14(5):881‐883. doi: 10.1007/s12072-020-10077-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sonzogni A, Previtali G, Seghezzi M, et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40(9):2110‐2116. doi: 10.1111/liv.14601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Brandão SCS, Godoi ETAM, de OX RJ, de LMMP M, ESC S. COVID‐19 grave: entenda o papel da imunidade, do endotélio e da coagulação na prática clínica. J Vasc Brasil. 2020;19:1‐11. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1677-54492020000100414&tlng=pt [Google Scholar]
- 147. Bhayana R, Som A, Li MD, et al. Abdominal imaging findings in COVID‐19: preliminary observations. Radiology. 2020;297(1):E207‐E215. doi: 10.1148/radiol.2020201908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Spogis J, Hagen F, Thaiss WM, et al. Sonographic findings in coronavirus disease‐19 associated liver damage. di Gennaro F, editor. PLoS One. 2021;16(2):e0244781. doi: 10.1371/journal.pone.0244781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Buso G, Becchetti C, Berzigotti A. Acute splanchnic vein thrombosis in patients with COVID‐19: a systematic review. Dig Liver Dis. 2021;53(8):937‐949. https://linkinghub.elsevier.com/retrieve/pii/S1590865821002723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Barco S, Konstantinides SV. Thrombosis and thromboembolism related to COVID‐19: a clarion call for obtaining solid estimates from large‐scale multicenter data. Res Pract Thrombosis Haemostasis. 2020;4(5):741‐743. doi: 10.1002/rth2.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lopez‐Mendez I, Aquino‐Matus J, Gall SM‐B, et al. Association of liver steatosis and fibrosis with clinical outcomes in patients with SARS‐CoV‐2 infection (COVID‐19). Ann Hepatol. 2021;20:100271 https://linkinghub.elsevier.com/retrieve/pii/S1665268120301861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ferraioli G, Monteiro LBS. Ultrasound‐based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25(40):6053‐6062. https://www.wjgnet.com/1007-9327/full/v25/i40/6053.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta‐analysis. Hepatology. 2011;54(3):1082‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Effenberger M, Grander C, Fritsche G, et al. Liver stiffness by transient elastography accompanies illness severity in COVID‐19. BMJ Open Gastroenterol. 2020;7(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Abdelmohsen MA, Alkandari BM, Gupta VK, ElBeheiry AA. Diagnostic value of abdominal sonography in confirmed COVID‐19 intensive care patients. Egypt J Radiol Nucl Med. 2020;51(1). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


