Abbreviations
- AU, arbitrary units; D1
first SARS‐CoV‐2 vaccine dose
- D2
second SARS‐CoV‐2 vaccine dose
- D3
third SARS‐CoV‐2 vaccine dose
- EIA
enzyme immunoassay
- Ig
immunoglobulin
- IQR
interquartile range
- LT
liver transplantation
- MMF
mycophenolate mofetil
- mRNA
messenger RNA
- RBD
receptor‐binding domain
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
To the editor,
Liver transplantation (LT) recipients have attenuated antibody response to the two‐dose messenger RNA (mRNA) vaccine series against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2).[ 1 , 2 , 3 ] Additional vaccine doses, best studied in the kidney transplantation population, may improve response, yet many solid organ transplantation recipients appear to continue to have a poor response despite additional doses.[ 4 , 5 ] We aimed to measure antibody levels before and after a third mRNA vaccine dose (D3) in a large cohort of LT recipients to understand vaccine immunogenicity and the demographic, transplantation, and vaccine type associated with sero‐response.
PATIENTS AND METHODS
LT recipients in our longitudinal observational cohort who received three doses of homologous mRNA vaccines and had antibody levels measured both after dose 2 (D2) and after D3 were identified. Participants with multiorgan transplantation (n = 37), pre‐dose 1 (D1) SARS‐CoV‐2 infection (n = 11), or SARS‐CoV‐2 breakthrough infection (n = 3 before D3; n = 17 after D3) as antibody interpretation would not be reflective of the overall LT cohort. The pre‐D3 level was defined as the last anti‐spike antibody level collected between D2 and D3; the level was repeated 1 month after D3. Samples were tested with clinical anti‐spike assays: Roche Elecsys Anti‐SARS‐CoV‐2 enzyme immunoassay (EIA) (anti‐receptor–binding domain [RBD], positive ≥0.8 U/ml; ceiling 2500 U/ml) or EUROIMMUN EIA (anti‐S1, positive ≥1.1 AU).
RESULTS
There were 148 included participants who completed three homologous doses of BNT162b2 (49%) or mRNA‐1273 (51%) vaccines between June 5, 2021, and December 21, 2021 (Table 1). Pre‐D3 antibody levels were measured at a median (interquartile range [IQR]) of 120 days (92–178) after D2, and post‐D3 antibody levels were measured at a median (IQR) of 30 days (26–33) after D3. The median (IQR) time between D2 and D3 was 169 (149–188) days. Overall, 118/148 (80%) were seropositive before D3, increasing to 138/148 (93%) after D3. Specifically, of the 30 participants seronegative before D3, 20 (67%) seroconverted after D3. The median (IQR) time between D2 and the pre‐D3 sampling did not significantly differ between those with positive versus negative post‐D3 titers (101 [90–164] vs. 121 [92–179] days; p = 0.46; Table 1), nor versus those who seroconverted (128 [94–175] days; p = 0.91). No participant seroreverted during follow‐up. Risk factors significantly associated with seronegativity after D3 were antimetabolite use (90% vs. 41%; p = 0.005), and proximity to transplantation (3 vs. 6 years; p = 0.014). No patient made substantial decreases to their anti‐metabolite dosage before and after D3. Receipt of the BNT162b2 series (80% vs. 47%; p = 0.054) was also more common in seronegative participants, but this was not statistically significant. The median anti‐RBD level increased from before to after D3 by at least 13.6‐fold (from 184 U/ml to >2500 U/ml), and median anti‐S1 level increased by 3.6‐fold (from 2.50 AU to 8.94 AU) after D3 (Figure 1).
TABLE 1.
Baseline characteristics of LT recipients who completed three doses of homologous SARS‐CoV‐2 vaccines and categorized by positive versus negative levels at 1 month after D3
| Recipient characteristic | All participants | Antibody level after D3 | p‐value | |
|---|---|---|---|---|
| n = 148 | Positive, n = 138 (93%) | Negative, n = 10 (7%) | ||
| Age, years | 63 (51–69) | 63 (51–69) | 60 (53–66) | 0.93 |
| Female | 80 (54) | 74 (54) | 6 (60) | 0.75 |
| White | 134 (91) | 125 (91) | 9 (90) | >0.99 |
| Years between vaccination and transplantation | 6 (2–13) | 6 (3–14) | 3 (1–6) | 0.36 |
| Immunosuppression a | ||||
| Tacrolimus | 113 (76) | 104 (75) | 9 (90) | 0.45 |
| Cyclosporine | 13 (9) | 12 (9) | 1 (10) | >0.99 |
| Antimetabolite | 66 (45) | 57 (41) | 9 (90) | 0.005 |
| MMF use | 43 (29) | 37 (27) | 6 (60) | 0.064 |
| Steroid | 26 (18) | 23 (17) | 3 (30) | 0.38 |
| Sirolimus | 17 (11) | 17 (12) | 0 | 0.61 |
| Everolimus | 8 (5) | 7 (5) | 1 (10) | 0.44 |
| Triple immunosuppression b | 10 (7) | 7 (5) | 3 (30) | 0.021 |
| Vaccine series | 0.054 | |||
| BNT162b2 | 73 (49) | 65 (47) | 8 (80) | |
| mRNA‐1273 | 75 (51) | 73 (53) | 2 (20) | |
| Duration, days | ||||
| D2 to D3 | 169 (149–188) | 170 (149–189) | 164 (147–179) | 0.55 |
| D2 to pre‐D3 titer | 120 (92–178) | 121 (92–179) | 101 (90–164) | 0.46 |
| D3 to post‐D3 titer | 30 (26–33) | 30 (26–33) | 32 (23, 35) | 0.56 |
| Titers | <0.001 | |||
| Negative pre‐D3 c | 30 (20) | 20 (14) | 10 (100) | |
| Positive pre‐D3 | 118 (80) | 118 (86) | 0 | |
Note: Data are presented as median (IQR) or n (%). Fisher exact for small numbers and p‐values bolded if p < 0.05.
Abbreviations: D2, second dose of vaccine; D3, third dose of vaccine; IQR, interquartile range; LT, liver transplantation; MMF, mycophenolate mofetil; mRNA, messenger RNA; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Listed maintenance immunosuppression is not mutually exclusive and not all dosages are reported.
Triple immunosuppression includes calcineurin inhibitors, steroids, and anti‐metabolites.
Of the 30 seronegative recipients before D3, 20 (67%) seroconverted.
FIGURE 1.
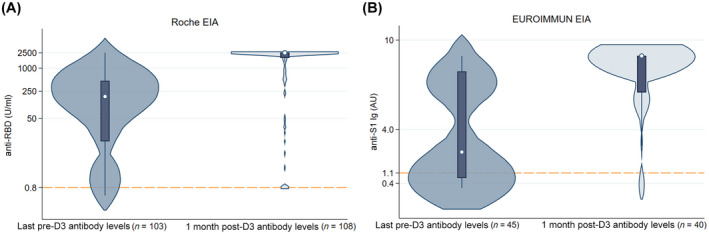
Violin plot of pre‐D3 versus 1 month post‐D3 antibody level, categorized by assay. Reference line delineates positive cut‐offs for anti‐RBD (≥0.8 U/ml) and anti‐S1 (≥1.1 AU). Anti‐RBD assay is measured along a log10 scale on the y‐axis. (A) Roche EIA; (B) EUROIMMUN EIA
As post hoc analysis, we evaluated the excluded group of participants that had breakthrough infections after D1. Among 20 participants who reported breakthrough infection after D1, 17 occurred at a median (IQR) of 125 (116–150) days after D3. Among those 17 participants, 4 (24%) were seronegative after D3. Of the 13 seropositive patients, the median (IQR) post‐D3 anti‐RBD level was 2500 (1932–2500) U/ml. The median anti‐S1 level after D3 was 8.94 AU.
DISCUSSION
Most LT recipients demonstrated excellent antibody response to mRNA vaccination, with improved seroconversion and titer levels after D3. This response is greater than that reported for other organ recipients, yet, particularly amid the changing variant landscape, this report does not provide direct evidence of seroprotection. Notably, a subgroup of recipients on anti‐metabolites and/or closer to transplantation remains seronegative after D3, and thus may warrant additional monitoring and protective interventions given the likely ongoing high risk for SARS‐CoV‐2 infection. Future directions include durability of vaccine response, including assessing for reversions after additional doses, with a link to clinical outcomes.
CONFLICT OF INTEREST
Dorry L. Segev receives honoraria from Sanofi, Novartis, Veloxis, Mallinckrodt, Jazz Pharmaceuticals, CSL Behring, Thermo Fisher Scientific, CareDx, TransMedics, Kamada, MediGO, Regeneron, AstraZeneca, Takeda, and Bridge to Life. Macey L. Levan consults for Patients Like Me and Takeda Pharmaceuticals. Robin K. Avery receives grants from AiCuris, Merck, and Takeda/Shire.
Supporting information
Figure S1
ACKNOWLEDGMENTS
The authors thank the participants of the Johns Hopkins COVID‐19 Transplant Vaccine Study, without whom this work would be possible. They also thank study team members, including Mayan Teles, BS; Jake D. Kim, BA; Carolyn Sidoti, BS; Letitia Thomas; Rivka Abedon; Chunyi Xia; Kimberly Hall; Mary Sears; Jonathan Susilo; Michael T. Ou, BS; Ross S. Greenberg, BS; Jake A. Ruddy, BS; Muhammad Asad Munir, MBBS; Michelle R. Krach, MS; Iulia Barbur, BSE. Further, they thank Andrew H. Karaba, MD, PhD, and Yolanda Eby for project support and guidance.
Alexandra T. Strauss and Amy Chang contributed equally to this article.
Funding information
This research was made possible with the generous support of the Ben‐Dov family and the Trokhan Patterson family. This work was supported by grants T32DK007732 (Amy Chang), T32DK007713 (Jennifer L. Alejo), F32DK124941 (Brian J. Boyarsky), K01DK114388–03 (Macey L. Levan), K01DK101677 (Allan B. Massie), and K23DK115908 (Jacqueline M. Garonzik‐Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases; grants K23AI157893 (William A. Werbel), K24AI144954, and U01AI138897 (Dorry L. Segev) from the National Institute of Allergy and Infectious Disease. The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
REFERENCES
- 1. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid‐19 vaccine over 6 months. N Engl J Med. 2021;385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strauss AT, Hallett AM, Boyarsky BJ, Ou MT, Werbel WA, Avery RK, et al. Antibody response to severe acute respiratory syndrome‐coronavirus‐2 messenger RNA vaccines in liver transplant recipients. Liver Transpl. 2021;27:1852–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rabinowich L, Grupper A, Baruch R, Ben‐Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS‐CoV‐2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik‐Wang JM, et al. Safety and immunogenicity of a third dose of SARS‐CoV‐2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid‐19 vaccine in solid‐organ transplant recipients. N Engl J Med. 2021;385:661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


