Abstract

Edible lotus (Nelumbo nucifera G.) is widely consumed in Asian countries and treated as a functional food and traditional medicinal herb due to its abundant bioactive compounds. Lotus rhizome peels, rhizome knots, and seed embryos are important byproducts and processing waste of edible lotus (Nelumbo nucifera G.) with commercial significance. Nevertheless, the comprehensive phenolic profiling of different parts of lotus is still scarce. Thus, this study aimed to review the phenolic contents and antioxidant potential in lotus seeds (embryo and cotyledon) and rhizomes (peel, knot, and pulp) grown in Australia. In the phenolic content and antioxidant potential estimation assays by comparing to the corresponding reference standards, the lotus seed embryo exhibited the highest total phenolic content (10.77 ± 0.66 mg GAE/gf.w.), total flavonoid content (1.61 ± 0.03 mg QE/gf.w.), 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity (9.66 ± 0.10 mg AAE/gf.w.), 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) scavenging activity (14.35 ± 0.20 mg AAE/gf.w.), and total antioxidant capacity (6.46 ± 0.30 mg AAE/g), while the highest value of ferric ion reducing antioxidant power (FRAP) activity and total tannin content was present in the lotus rhizome knot (2.30 ± 0.13 mg AAE/gf.w.). A total of 86 phenolic compounds were identified in five parts of lotus by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF-MS/MS), including phenolic acids (20), flavonoids (51), lignans (3), stilbenes (2), and other polyphenols (10). The most phenolic compounds, reaching up to 68%, were present in the lotus seed embryo (59). Furthermore, the lotus rhizome peel and lotus seed embryo exhibit significantly higher contents of selected polyphenols than other lotus parts according to high-performance liquid chromatography (HPLC) quantification analysis. The results highlighted that byproducts and processing waste of edible lotus are rich sources of phenolic compounds, which may be good candidates for further exploitation and utilization in food, animal feeding, and pharmaceutical industries.
1. Introduction
Lotus (Nelumbo nucifera G.) is an aquatic plant widely cultivated in China, Japan, India, Thailand, eastern Australia, and western Europe for more than 5000 years.1−3 At present, the output of lotus-related traditional medicine in China has exceeded 80,000 tons/year.4 Apart from its high ornamental value, almost all parts of lotus, including leaves, seeds, and rhizomes, have been used as functional foods and traditional medicine herbs due to their abundant bioactive compounds, including flavonols, procyanidins, alkaloids, and especially polyphenols.4,5 Lotus seeds consist of the seed epicarp and seed kernel (white cotyledon), in between which lies a nonedible green embryo.6 The knot and peel are nonedible parts of the lotus rhizome and are removed before consumption. Recently, many therapeutic effects of the lotus, including antiobesity, anticancer, anti-inflammatory, antioxidant, and antiaging, have been of great interest.4,7
Phenolic compounds are a group of compounds with polyhydroxy groups on the aromatic ring, which exhibit strong antioxidant properties via different mechanisms, including reactive oxygen species scavenger by donating electrons or transferring hydrogen atoms, metal chelators, oxidase inhibitors, and antioxidant enzyme cofactors.8,9 According to the number of phenol units within the molecular structure, substituent groups, and the linkage type between phenol units, phenolic compounds can be classified into monomeric polyphenols, including phenolic acids, flavonoids, stilbenes, and lignans, or polymeric polyphenols, such as tannins.10,11 Modern research has demonstrated a significant positive correlation between phenolic compounds and antioxidant capacity, suggesting that the main contributor to antioxidant capacity might be the phenolic compounds in lotus.12 The phenolic content can be estimated by various spectrometric assays, including the total phenolic content (TPC), total flavonoid content (TFC), and total tannin content (TTC), while various spectrophotometric-based in vitro antioxidant methods are used to estimate the overall antioxidant potential of plant materials, including the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay, ferric reducing antioxidant power (FRAP) assay, and total antioxidant capacity (TAC) assay.13−18 However, TPC and other colorimetric methods neither separate nor quantify individual phenolic compounds. High-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF-MS/MS) is an effective analytical tool for the characterization and quantification of individual phenolic compounds due to the high sensitivity and accuracy.19−24 Previously, lotus seeds and rhizomes were reported to be rich in flavonoids, including flavonoid C-glycosides like schaftoside, quercetin derivatives, and catechin derivatives, by LC–MS and high-performance liquid chromatography equipped with a photodiode array (HPLC-PDA).4,5
The application of the lotus and its polyphenols in the food industry has gained much attention in recent years. Although previous studies have confirmed the antioxidant activity of lotus extracts effectively against lipid oxidation of the processed meat,25,26 the comprehensive phenolic profiling of different parts of the lotus is still scarce. In this study, phenolic compounds in five parts of Australia-grown lotus including the lotus seed embryo (LSE), lotus seed cotyledon (LSC), lotus rhizome knot (LRK), lotus rhizome peel (LRP), and lotus rhizome pulp (LR) were extracted and subjected to various phenolic estimation methods (TPC, TFC, and TTC) as well as antioxidant assays (DPPH, ABTS, FRAP, and TAC). The further characterization and quantification of individual phenolic compounds in five parts of lotus were conducted by LC-ESI-QTOF-MS and HPLC-PDA, respectively. This study aimed to evaluate the antioxidant potential and provide a comprehensive phenolic profile of lotus to further explore and utilize the phenolic compounds in byproducts of lotus in the food, cosmetic, and pharmaceutical industries.
2. Results and Discussion
2.1. Polyphenol Estimation (TPC, TFC, and TTC)
Previously, lotus seeds and rhizomes were reported to contain large amounts of phenolic compounds with strong antioxidant capacity, including flavonoids and phenolic acids. Thus, TPC, TFC, and TTC were conducted to estimate the phenolic content in ethanolic extracts of lotus (Table 1). The lotus seed embryo presented a significantly higher total phenolic content (10.77 ± 0.66 mg GAE/gf.w.) at p < 0.05 than other tissues followed by the knot and peel of the lotus rhizome (3.49 ± 0.12 and 3.44 ± 0.07 mg GAE/gf.w., respectively). Our study is consistent with previous findings of Limwachiranon et al.27 and Hu and Skibsted12 that indicated that the phenolic contents of lotus knots are distinctive. The total phenolic content of our lotus rhizome peel is also comparable to that of 80% ethanolic extracts of lotus from Zhejiang, China (4.30 mg GAE/gf.w.).28 In the seeds of lotus, the pattern of the TPC results of Limwachiranon et al.27 was contradictory to our research, as they found that the cotyledon had higher phenolic contents than embryos. This variation of TPC might be explained by several factors, including different growing regions, ripening stages, and drying processes and the choice of extraction reagent.4,29−31 Yen et al.31 compared the total phenolic contents in water extracts, acetone extracts, and ethyl acetate extracts of lotus seeds and found that water extracts of lotus seeds presented the highest TPC.
Table 1. Polyphenol Estimation and Antioxidant Activities of Lotus Samples.
| antioxidant assays | LR | LRP | LRK | LSE | LSC |
|---|---|---|---|---|---|
| TPC (mg GAE/gf.w.) | 0.34 ± 0.01c | 3.44 ± 0.07b | 3.49 ± 0.12b | 10.77 ± 0.66a | 1.11 ± 0.08c |
| TFC (mg QE/gf.w.) | 0.01 ± 0.01e | 0.31 ± 0.02c | 0.24 ± 0.02d | 1.61 ± 0.03a | 0.61 ± 0.03b |
| TTC (mg CE/gf.w.) | 0.02 ± 0.01d | 0.83 ± 0.01b | 1.77 ± 0.04a | 0.27 ± 0.02c | 0.32 ± 0.01c |
| DPPH (mg AAE/gf.w.) | 0.60 ± 0.04e | 3.70 ± 0.17 c | 4.36 ± 0.36b | 9.66 ± 0.10a | 1.82 ± 0.10d |
| ABTS (mg AAE/gf.w.) | 0.58 ± 0.04e | 7.81 ± 0.15c | 8.85 ± 0.68b | 14.35 ± 0.20a | 2.09 ± 0.15d |
| FRAP (mg AAE/gf.w.) | 0.11 ± 0.01c | 2.24 ± 0.13a | 2.30 ± 0.13a | 1.72 ± 0.02b | 0.22 ± 0.01c |
| TAC (mg AAE/gf.w.) | 0.34 ± 0.01e | 1.83 ± 0.07c | 2.41 ± 0.09b | 6.46 ± 0.30a | 1.05 ± 0.04d |
The data are shown as mean ± standard deviation (n = 3); a, b indicate the means in a row with significant difference (p < 0.05) using a one-way analysis of variance (ANOVA) and Tukey’s test. LR, lotus rhizome pulp; LRP, lotus rhizome peel; LRK, lotus rhizome knot; LSC, lotus seed cotyledon; LSE, lotus seed embryo; TPC, total phenolic content; TFC, total flavonoid content; TTC, total tannin content; DPPH, 2,2-diphenyl-1-picrylhydrazyl assay; FRAP, ferric reducing antioxidant power assay; ABTS, 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid assay; TAC, total antioxidant capacity; GAE, gallic acid equivalents; QE, quercetin equivalents; CE, catechin equivalents; AAE, ascorbic acid equivalents.
Flavonoids are the predominant class of phenolic compounds, which account for over 60% of the dietary phenols and exhibit health-promoting properties.32−36 The highest flavonoid content was found in the lotus seed embryo (1.61 ± 0.03 mg QE/gf.w.) followed by the lotus seed cotyledon (0.61 ± 0.03 mg QE/gf.w.). Previously, Li et al.33 found that the TFC value in the embryo of lotus seeds was higher than that in the cotyledon, which is in agreement with our study. The high content of flavonoid C-glycosides was reported in the embryo of lotus seeds, which consists of more than 70% of the total flavonoid content.5,37
Regarding the TTC, the content of tannins varied significantly among five parts of lotus in this study. The lotus rhizome knot presented the highest tannin content (1.77 ± 0.04 mg CE/gf.w.) followed by the peel (0.83 ± 0.01 mg CE/gf.w.), while the other parts of the lotus presented a relatively low tannin content. Only limited studies have been reported on the TTC of edible lotus. Huang et al.26 found that the total tannin contents in water extracted lotus rhizome knot and lotus leaf were 13.0 ± 0.3 and 6.02 ± 0.2 (gallic acid equivalents g/100 g), respectively. Chen et al.38 indicated that the content of tannins in lotus varied with different solvent extractions with the highest tannin content in 80% methanol extraction.
2.2. Antioxidant Activities (DPPH, FRAP, ABTS, and TAC)
The phenolic contents are highly associated with their antioxidant properties.39−41 Thus, several antioxidant assays were conducted to analyze the antioxidant capacity of the lotus samples. In our study, DPPH, ABTS, FRAP, and TAC were applied to estimate the antioxidant potential of different parts of the lotus.
DPPH is the most commonly used method to characterize the free radical scavenging capabilities of food extracts based on their hydrogen donating ability. The lotus seed embryo exhibited the highest DPPH free radical scavenging activity among five parts of lotus (9.66 ± 0.10 mg AAE/gf.w.) followed by the knot and peel of the lotus rhizome (4.36 ± 0.36 and 3.70 ± 0.17 mg AAE/gf.w., respectively). In our study, the lotus rhizome knot showed a better scavenging capacity of DPPH• compared with the pulp, which agreed with the previous study conducted by Hu and Skibsted.12
The principle of the ABTS assay is similar to the DPPH method, which is based on the fact that the antioxidants in extracts reduce the preformed ABTS•+ and form stable free radicals, resulting in decolorization.42 As shown in Table 1, the scavenging activity of ABTS radicals ranged from 14.35 ± 0.20 to 0.58 ± 0.04 mg AAE/gf.w.. The lotus seed embryo exhibited the highest scavenging activity (14.35 ± 0.20 mg AAE/gf.w.) followed by the knot and peel of the lotus rhizome (8.85 ± 0.68 and 7.81 ± 0.15 AAE/gf.w., respectively). The knot exhibited a better scavenging capacity of ABTS radicals than the lotus rhizome pulp, which is in agreement with the study conducted by Yang et al.28
The FRAP assay measures the capacity of antioxidants to reduce the ferric tripyridyltriazine complex (Fe3+-TPTZ) to the ferrous complex (Fe2+-TPTZ) at low pH. The reducing power of FRAP varied in five parts of lotus. The knot and peel of the lotus rhizome exhibited a significantly higher reducing power (2.30 ± 0.13 and 2.24 ± 0.13 mg AAE/gf.w., respectively) followed by the lotus seed embryo that presented 1.72 ± 0.02 mg AAE/gf.w. reducing power of FRAP. The FRAP value of the lotus rhizome peel was higher than that of the pulp, which agreed with the study of Yang et al.28 Based on the previous study, the reducing power values vary with different maturity in both the seed and rhizome.4,28
In the TAC assay, which was based on the capacity of reducing phosphomolybdate ions, the lotus seed embryo exhibited a significantly higher total antioxidant capacity (TAC) among five parts of lotus (6.46 ± 0.30 mg AAE/gf.w.) at p < 0.05 followed by the lotus rhizome knot obtaining a relatively high TAC value (2.41 ± 0.09 mg AAE/gf.w.).
2.3. Correlation between Phenolic Compounds and Antioxidant Potential
Pearson’s correlation between phenolic contents (TPC, TFC, and TTC) and four antioxidant assays (DPPH, ABTS, FRAP, and TAC) was performed to investigate the relationship between the phenolic contents and antioxidant capacities of lotus extracts. The correlation coefficients are summarized in Table 2.
Table 2. Pearson’s Correlation Coefficients for TPC, TFC, TTC, DPPH, FRAP, ABTS, and TAC.
| variables | TPC | TFC | TTC | DPPH | FRAP | ABTS |
|---|---|---|---|---|---|---|
| TFC | 0.879a | |||||
| TTC | 0.001 | –0.271 | ||||
| DPPH | 0.993b | 0.877a | 0.109 | |||
| FRAP | 0.523 | 0.180 | 0.746 | 0.590 | ||
| ABTS | 0.938a | 0.733 | 0.329 | 0.965b | 0.781 | |
| TAC | 0.995b | 0.911a | 0.017 | 0.993b | 0.494 | 0.929a |
The correlation between two assays is significant with p < 0.05.
Highly significant correlation with p < 0.01.
A significantly positive correlation between the content of total phenolic compounds (TPC) and all antioxidant assays except FRAP (DPPH, r = 0.993, p < 0.01; ABTS, r = 0.938, p < 0.01; TAC; r = 0.995, p < 0.01) was observed. The positive correlation between TPC and antioxidant assays (DPPH, ABTS, and TAC) was also reported by previous studies,12,28 indicating that phenolic compounds were one of the contributors to the antioxidant activity of five lotus tissues. The low correlation between FRAP with other antioxidant activity measurements may be attributed to some slowly reacting polyphenolic compounds (quercetin, caffeic, ferulic, and tannic acids) having slower reactions, requiring a longer time until the complex reduction process was completed.43
The total flavonoid content was positively correlated with the TPC (r = 0.879, p < 0.05) as well as DPPH radical scavenging activity and total antioxidant capacitive (r = 0.877, p < 0.05 and r = 0.911, p < 0.01, respectively), suggesting that flavonoids are the predominant phenolic compounds in lotus, which significantly contributed to the antioxidant activities.
In general, the phenolic compounds are one of the contributors to the antioxidant activities of lotus seeds and rhizomes. Thus, screening of these phenolic compounds is essential. In this study, LC–MS/MS and HPLC-PDA were performed to further identify, characterize, and quantify phenolic compounds present in different lotus samples.
2.4. Characterization of Phenolic Compounds by LC-ESI-QTOF-MS/MS
Table 3 shows the phenolic compounds tentatively identified in five parts of lotus based on their m/z value and MS/MS spectral data using the Agilent LC-ESI-QTOF-MS/MS Mass Hunter workstation software (Qualitative Analysis, version B.03.01, Agilent) and Personal Compound Database and Library (PCDL) with an online database of Kansas State University, USA (Supporting Information, Figures S1 and S2). Compounds with PCDL scores higher than 80 and mess error <±5 ppm were further selected for m/z verification and MS/MS analysis.
Table 3. Characterization of Phenolic Compounds in Lotus by Using LC-ESI-QTOF-MS/MSa.
| no. | proposed compounds | molecular formula | RT (min) | ionization mode | molecular weight | theoretical (m/z) | observed (m/z) | mass error (ppm) | MS/MS product ions | samples |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acid | ||||||||||
| hydroxybenzoic acids | ||||||||||
| 1 | galloyl glucose | C13H16O10 | 10.541 | [M – H]− | 332.0743 | 331.0670 | 331.0668 | –0.60 | 169, 125 | LSE |
| 2 | 2-hydroxybenzoic acid | C7H6O3 | 11.038 | c[M – H]− | 138.0317 | 137.0244 | 137.0248 | 2.92 | 93 | LRP, LR, LSC,bLSE, LRK |
| 3 | 4-hydroxybenzoic acid 4-O-glucoside | C13H16O8 | 11.054 | [M – H]− | 300.0845 | 299.0772 | 299.0770 | –0.67 | 255, 137 | LR,bLSE, LRK |
| 4 | gallic acid | C7H6O5 | 12.893 | [M – H]− | 170.0215 | 169.0142 | 169.0140 | –1.18 | 125 | LSC,bLSE |
| 5 | paeoniflorin | C23H28O11 | 34.596 | c[M – H]− | 480.1632 | 479.1559 | 479.1583 | 5.00 | 449, 357, 327 | LSC,bLSE |
| hydroxycinnamic acids | ||||||||||
| 6 | cinnamic acid | C9H8O2 | 12.479 | c[M – H]− | 148.0524 | 147.0451 | 147.0453 | 1.36 | 103 | LRP, LR, LSC,bLSE |
| 7 | 3-p-coumaroylquinic acid | C16H18O8 | 13.543 | c[M – H]− | 338.1002 | 337.0929 | 337.0921 | –2.37 | 265, 173, 162 | bLRP, LR, LSE, LRK |
| 8 | m-coumaric acid | C9H8O3 | 39.486 | c[M – H]− | 164.0473 | 163.0401 | 163.0405 | 3.03 | 119 | LRP, LR,bLSC, LSE, LRK |
| 9 | caffeoyl glucose | C15H18O9 | 19.603 | [M – H]− | 342.0951 | 341.0878 | 341.0875 | –0.88 | 179, 161 | LSE |
| 10 | caffeic acid | C9H8O4 | 19.619 | [M – H]− | 180.0423 | 179.0350 | 179.0350 | 0.00 | 143, 133 | LSE |
| 11 | 3-feruloylquinic acid | C17H20O9 | 20.847 | c[M – H]− | 368.1110 | 367.1034 | 367.1025 | –2.45 | 298, 288,192, 191 | bLSE, LRK |
| 12 | ferulic acid 4-O-glucoside | C16H20O9 | 23.330 | [M – H]− | 356.1107 | 355.1034 | 355.1031 | –0.84 | 193, 178, 149, 134 | LR, LSC,bLSE |
| 13 | isoferulic acid | C10H10O4 | 23.344 | c[M – H]− | 194.0579 | 193.0506 | 193.0513 | 3.63 | 178, 149, 134 | LRP, LR, LSC,bLSE, LRK |
| 14 | p-coumaric acid 4-O-glucoside | C15H18O8 | 23.754 | [M – H]− | 326.1002 | 325.0929 | 325.0940 | 3.38 | 163 | bLR, LSE |
| 15 | sinapic acid | C11H12O5 | 26.118 | c[M – H]− | 224.0685 | 223.0612 | 223.0618 | 2.69 | 205, 163 | LR,bLSC, LSE, LRK |
| 16 | verbascoside | C29H36O15 | 31.531 | [M – H]− | 624.2054 | 623.1981 | 623.1984 | 0.48 | 477, 461,315, 135 | LSE |
| 17 | 1-sinapoyl-2-feruloylgentiobiose | C33H40O18 | 60.158 | [M – H]− | 724.2215 | 723.2142 | 723.2122 | –2.77 | 529, 499 | LSE |
| hydroxyphenylacetic acids | ||||||||||
| 18 | 3,4-dihydroxyphenylacetic acid | C8H8O4 | 14.119 | c[M – H]− | 168.0423 | 167.0350 | 167.0346 | –2.39 | 149, 123 | LRP, LR, LSC,bLSE, LRK |
| 19 | 2-hydroxy-2-phenylacetic acid | C8H8O3 | 14.616 | c[M – H]− | 152.0473 | 151.0400 | 151.0394 | –3.97 | 136, 92 | LRP, LSC,bLSE, LRK |
| hydroxyphenylpentanoic acids | ||||||||||
| 20 | 3-hydroxy-3-(3-hydroxyphenyl)propionic acid | C9H10O4 | 18.327 | [M – H]− | 182.0579 | 181.0506 | 181.0507 | 0.55 | 163, 135, 119 | LSE |
| Flavonoids | ||||||||||
| flavanols | ||||||||||
| 21 | (+)-catechin 3-O-gallate | C22H18O10 | 10.942 | [M – H]− | 442.0900 | 441.0827 | 441.0842 | 3.40 | 289, 169, 125 | LRK |
| 22 | (+)-gallocatechin 3-O-gallate | C22H18O11 | 11.106 | c[M – H]− | 458.0849 | 457.0776 | 457.0781 | 1.10 | 305, 169 | LSE |
| 23 | (−)-epigallocatechin | C15H14O7 | 21.832 | c[M – H]− | 306.0740 | 305.0667 | 305.0675 | 2.62 | 261, 219 | LRP, LSC,bLR |
| 24 | procyanidin trimer C1 | C45H38O18 | 22.246 | c[M – H]− | 866.2058 | 865.1985 | 865.1989 | 0.46 | 739, 713, 695 | LRP,b LR, LSC |
| 25 | cinnamtannin A2 | C60H50O24 | 24.081 | c[M – H]− | 1154.2692 | 1153.2619 | 1153.2656 | 3.21 | 739 | LR,bLSC |
| 26 | (−)-epicatechin | C15H14O6 | 24.208 | c[M – H]− | 290.0790 | 289.0717 | 289.0714 | –1.04 | 245, 205, 179 | LRP, LR, LRK, LSC,bLSE |
| 27 | 4″-O-methylepigallocatechin 3-O-gallate | C23H20O11 | 32.575 | c[M – H]− | 472.1006 | 471.0933 | 471.0927 | –1.27 | 169, 319 | LRP, LSC,bLSE |
| 28 | procyanidin dimer B1 | C30H26O12 | 78.369 | c[M – H]− | 578.1424 | 577.1351 | 577.1340 | –1.90 | 451 | bLRP, LR, LSC, LRK |
| flavanones | ||||||||||
| 29 | eriocitrin | C27H32O15 | 34.931 | c[M – H]− | 596.1741 | 595.1668 | 595.1650 | –3.00 | 431, 287 | bLSC, LSE |
| 30 | naringin | C27H32O14 | 41.624 | c[M – H]− | 580.1792 | 579.1719 | 579.1696 | –4.00 | 271 | bLRP, LSE |
| 31 | 8-prenylnaringenin | C20H20O5 | 45.721 | [M + H]+ | 340.1311 | 341.1384 | 341.1389 | 1.47 | 323, 137 | bLRP, LR, LRK |
| 32 | hesperidin | C28H34O15 | 52.573 | [M + H]+ | 610.1898 | 611.1971 | 611.1962 | –1.47 | 593, 465, 449, 303 | LSE |
| 33 | hesperetin 3′-O-glucuronide | C22H22O12 | 52.779 | c[M – H]− | 478.1111 | 477.1038 | 477.1048 | 2.10 | 301, 175, 113, 85 | LRP,bLR, LSE, LRK |
| flavones | ||||||||||
| 34 | apigenin 7-O-apiosyl-glucoside | C26H28O14 | 14.031 | c[M + H]+ | 564.1479 | 565.1552 | 565.1552 | 0.00 | 296 | LR,bLSE |
| 35 | apigenin 7-O-glucuronide | C21H18O11 | 22.201 | [M + H]+ | 446.0849 | 447.0922 | 447.0933 | 2.46 | 271, 253 | bLRP, LR |
| 36 | apigenin 6,8-di-C-glucoside | C27H30O15 | 32.309 | c[M – H]− | 594.1585 | 593.1512 | 593.1532 | 3.37 | 503, 473 | LSC,bLSE |
| 37 | chrysoeriol 7-O-glucoside | C22H22O11 | 40.657 | c[M + H]+ | 462.1162 | 463.1235 | 463.1221 | –3.00 | 445, 427, 409, 381 | LSE |
| 38 | apigenin 6-C-glucoside | C21H20O10 | 41.736 | c[M – H]− | 432.1056 | 431.0983 | 431.0984 | 0.23 | 413, 341, 311 | LR, LRP, LSC,bLSE |
| 39 | neodiosmin | C28H32O15 | 52.580 | c[M + H]+ | 608.1741 | 609.1814 | 609.1826 | 1.97 | 301, 286 | bLSC, LSE |
| flavonols | ||||||||||
| 40 | patuletin 3-O-glucosyl-(1->6)-[apiosyl(1->2)]-glucoside | C33H40O22 | 21.872 | [M – H]− | 788.2011 | 787.1938 | 787.1907 | –3.94 | 625, 463, 301, 271 | LSE |
| 41 | quercetin 3-O-xylosyl-rutinoside | C32H38O20 | 26.493 | [M + H]+ | 742.1956 | 743.2029 | 743.2058 | 3.90 | 479, 317 | LSC |
| 42 | myricetin 3-O-rutinoside | C27H30O17 | 27.025 | c[M – H]− | 626.1483 | 625.1410 | 625.1393 | –2.72 | 301 | LSC,bLSE |
| 43 | quercetin 3-O-glucosyl-xyloside | C26H28O16 | 27.754 | c[M – H]− | 596.1377 | 595.1304 | 595.1306 | 0.34 | 265, 138, 116 | LSC,bLSE |
| 44 | kaempferol 3,7-O-diglucoside | C27H30O16 | 28.897 | c[M – H]− | 610.1534 | 609.1461 | 609.1479 | 2.95 | 447, 285 | LSC,bLSE |
| 45 | myricetin 3-O-glucoside | C21H20O13 | 38.995 | c[M – H]− | 480.0904 | 479.0831 | 479.0834 | 0.63 | 317 | LRP,bLR, LSC, LRK |
| 46 | kaempferol 3-O-glucosyl-rhamnosyl-galactoside | C33H40O20 | 40.180 | c[M – H]− | 756.2113 | 755.2040 | 755.2025 | –2.00 | 285 | LSC |
| 47 | kaempferol 3-O-(2″-rhamnosyl-galactoside) 7-O-rhamnoside | C33H40O19 | 41.143 | [M – H]− | 740.2164 | 739.2091 | 739.2106 | 2.03 | 593, 447, 285 | bLRP, LSE |
| 48 | quercetin 3′-O-glucuronide | C21H18O13 | 45.016 | [M – H]− | 478.0747 | 477.0674 | 477.0653 | –4.40 | 301 | LSC,bLSE |
| 49 | myricetin 3-O-rhamnoside | C21H20O12 | 45.314 | c[M – H]− | 464.0955 | 463.0882 | 463.0871 | –2.38 | 317 | LRP, LR, LSC,bLSE, LRK |
| 50 | quercetin 3-O-arabinoside | C20H18O11 | 45.598 | c[M – H]− | 434.0849 | 433.0776 | 433.0780 | 0.90 | 301 | LRP,bLR, LSE |
| 51 | isorhamnetin | C16H12O7 | 85.555 | c[M – H]− | 316.0583 | 315.0510 | 315.0510 | 0.00 | 300, 271 | LSC,bLSE |
| dihydrochalcones | ||||||||||
| 52 | 3-hydroxyphloretin 2′-O-xylosyl-glucoside | C26H32O15 | 12.115 | [M – H]− | 584.1741 | 583.1668 | 583.1688 | 3.43 | 289 | LSE |
| 53 | 3-hydroxyphloretin 2′-O-glucoside | C21H24O11 | 38.973 | c[M – H]− | 452.1319 | 451.1246 | 451.1247 | 0.22 | 289, 273 | LRP, LSC,bLRK |
| 54 | phloridzin | C21H24O10 | 47.041 | c[M – H]− | 436.1369 | 435.1296 | 435.1303 | 1.61 | 273 | LRP, LR,bLRK |
| anthocyanins | ||||||||||
| 55 | peonidin 3-O-diglucoside-5-O-glucoside | C34H43O21 | 10.988 | c[M + H]+ | 787.2297 | 788.2370 | 788.2399 | 3.68 | 625, 478, 317 | bLRP, LR, LSE |
| 56 | cyanidin 3-O-(6″-p-coumaroyl-glucoside) | C30H27O13 | 16.009 | c[M + H]+ | 595.1452 | 596.1525 | 596.1515 | –1.68 | 287 | bLR, LRP |
| 57 | delphinidin 3-O-glucoside | C21H21O12 | 22.187 | c[M + H]+ | 465.1033 | 466.1106 | 466.1095 | –2.36 | 303 | LRP,bLR, LSC, LSK |
| 58 | delphinidin 3-O-glucosyl-glucoside | C27H31O17 | 26.933 | [M + H]+ | 627.1561 | 628.1634 | 628.1664 | 4.78 | 465, 303 | LSE |
| 59 | isopeonidin 3-O-arabinoside | C21H21O10 | 41.565 | [M + H]+ | 433.1135 | 434.1208 | 434.1200 | –1.84 | 271, 253, 243 | bLSC, LSE |
| 60 | cyanidin 3,5-O-diglucoside | C27H31O16 | 42.675 | c[M + H]+ | 611.1612 | 612.1685 | 612.1672 | –2.12 | 449, 287 | bLSC, LSE |
| 61 | pelargonidin 3-O-rutinoside | C27H31O14 | 50.950 | [M + H]+ | 579.1714 | 580.1787 | 580.1814 | 4.65 | 271, 433 | LSE |
| isoflavonoids | ||||||||||
| 62 | 6″-O-malonylglycitin | C25H24O13 | 37.252 | [M + H]+ | 532.1217 | 533.1290 | 533.1274 | –3.00 | 285, 270, 253 | LSE |
| 63 | 5,6,7,3′,4′-pentahydroxyisoflavone | C15H10O7 | 37.837 | c[M + H]+ | 302.0427 | 303.0500 | 303.0503 | 0.99 | 285, 257 | bLRP, LR, LSC, LSE, LRK |
| 64 | 6″-O-acetyldaidzin | C23H22O10 | 41.868 | c[M – H]− | 458.1213 | 457.1140 | 457.1129 | –2.41 | 221 | LRP, LR, LSC,bLSE |
| 65 | violanone | C17H16O6 | 47.057 | cM – H]− | 316.0947 | 315.0874 | 315.0881 | 2.22 | 300, 285, 135 | LRP, LR, LSE,bLRK |
| 66 | 3′-hydroxydaidzein | C15H10O5 | 50.933 | [M + H]+ | 270.0528 | 271.0601 | 271.0613 | 4.43 | 253, 241, 225 | LRP, LR, LSC,bLSE |
| 67 | 6″-O-acetylglycitin | C24H24O11 | 50.950 | c[M + H]+ | 488.1319 | 489.1392 | 489.1410 | 3.68 | 285, 270 | bLSE, LRP |
| 68 | 3′-hydroxygenistein | C15H10O6 | 51.305 | c[M + H]+ | 286.0477 | 287.0550 | 287.0546 | –1.39 | 269, 259 | LSC, LSE,bLRK |
| 69 | dihydrobiochanin A | C16H14O5 | 54.847 | [M + H]+ | 286.0841 | 287.0914 | 287.0922 | 2.79 | 269, 203,201, 175 | LRP |
| 70 | 2-dehydro-O-desmethylangolensin | C15H12O4 | 75.685 | c[M – H]− | 256.0736 | 255.0663 | 255.0655 | –3.14 | 135, 119 | LRP,bLRK |
| 71 | 3′,4′,7-trihydroxyisoflavanone | C15H12O5 | 83.053 | c[M – H]− | 272.0685 | 271.0612 | 271.0608 | –1.48 | 177, 151, 119, 107 | LRP, LR, LSC,bLSE, LRK |
| Other polyphenols | ||||||||||
| hydroxycoumarins | ||||||||||
| 72 | coumarin | C9H6O2 | 8.486 | [M + H]+ | 146.0368 | 147.0441 | 147.0442 | 0.68 | 103, 91 | LRP |
| 73 | esculin | C15H16O9 | 13.406 | [M + H]+ | 340.0794 | 341.0867 | 341.0862 | –1.47 | 179, 151 | bLRP, LR, LSC |
| 74 | salvianolic acid B | C36H30O16 | 27.074 | [M – H]− | 718.1534 | 717.1461 | 717.1485 | 3.35 | 519, 339, 321, 295 | LSE |
| 75 | scopoletin | C10H8O4 | 41.480 | c[M – H]− | 192.0423 | 191.0350 | 191.0357 | 3.66 | 176 | LRP,bLR, LRK |
| alkylmethoxyphenols | ||||||||||
| 76 | 4-vinylsyringol | C15H14O3 | 21.803 | c[M + H]+ | 242.0943 | 243.1016 | 243.1019 | 1.23 | 225, 211, 197 | bLRP, LSE |
| hydroxybenzoketones | ||||||||||
| 77 | 2,3-dihydroxy-1-guaiacylpropanone | C10H12O5 | 9.879 | c[M – H]− | 212.0685 | 211.0612 | 211.0602 | –4.70 | 167, 123, 105, 93 | LRP |
| 78 | 2-hydroxy-4-methoxyacetophenone 5-sulfate | C9H10O7S | 12.844 | [M – H]− | 262.0147 | 261.0074 | 261.0069 | –1.92 | 181, 97 | LSE |
| tyrosols | ||||||||||
| 79 | hydroxytyrosol 4-O-glucoside | C14H20O8 | 9.777 | c[M – H]− | 316.1158 | 315.1085 | 315.1076 | –2.90 | 153, 123 | bLSC, LR |
| 80 | demethyloleuropein | C24H30O13 | 12.181 | [M – H]− | 526.1686 | 525.1613 | 525.1633 | 3.81 | 495 | LSE |
| 81 | 3,4-DHPEA-AC | C10H12O4 | 37.614 | c[M – H]− | 196.0736 | 195.0663 | 195.0659 | –2.05 | 135 | LRP, LR,bLRK |
| Lignans | ||||||||||
| 82 | todolactol A | C20H24O7 | 39.718 | c[M – H]− | 376.1522 | 375.1449 | 375.1439 | –2.67 | 313, 137 | LRP,bLRK |
| 83 | 7-hydroxymatairesinol | C20H22O7 | 41.309 | c[M – H]− | 374.1366 | 373.1293 | 373.1298 | 1.30 | 343, 313, 298, 285 | LR |
| 84 | matairesinol | C20H22O6 | 45.898 | [M – H]− | 358.1416 | 357.1343 | 357.1338 | –1.40 | 342, 327, 313, 221 | LRP, LR,bLRK |
| Stilbenes | ||||||||||
| 85 | resveratrol | C14H12O3 | 31.317 | c[M – H]− | 228.0786 | 227.0713 | 227.0709 | –1.80 | 212, 185, 157, 143 | bLRP, LR, LSC, LRK |
| 86 | resveratrol 3-O-glucoside | C20H22O8 | 42.667 | [M – H]− | 390.1315 | 389.1242 | 389.1240 | –0.51 | 227 | LRK |
Lotus samples mentioned in abbreviations are lotus rhizome pulp (LR), lotus rhizome peel (LRP), lotus rhizome knot (LRK), lotus seed cotyledon (LSC), and lotus seed embryo (LSE).
Compound was detected in more than one lotus sample; data presented in this table are from the asterisk sample.
Compounds were detected in both negative [M – H]− and positive [M + H]+ modes of ionization, while only single mode data were presented.
Previously, more than 90 flavonoids and 12 phenolic acids have been reported in various parts of lotus, including leaves, seeds, rhizomes, and flowers.27,38 In this study, a total of 86 phenolic compounds were characterized in lotus, including 20 phenolic acids (23%), 51 flavonoids (59%), 3 lignans (4%), 2 stilbenes (2%), and 10 other polyphenols (12%). Lignans, stilbene, other polyphenols, and some phenolic acids were first characterized in lotus.
2.4.1. Phenolic Acid
In our study, phenolic acids in five parts of lotus were tentatively characterized into four subclasses, including hydroxybenzoic acids, hydroxycinnamic acids, hydroxyphenylpentanoic acids, and hydroxyphenylacetic acids.
2.4.1.1. Hydroxybenzoic Acid Derivatives
Seventeen hydroxybenzoic acid derivatives were detected in five parts of lotus. Compound 1 with [M – H]− at m/z 331.0668 was tentatively identified as galloyl glucose in LSE. Upon fragmentation, it produced the product ions at m/z 169 and 125 due to the loss of the hexosyl moiety (162 Da) and a further loss of carbon dioxide (44 Da) from the precursor ion, respectively. The further confirmation of galloyl glucose was achieved by comparing the MS/MS spectra with a previous study, in which a similar MS/MS fragmentation behavior of galloyl glucose standard was observed.44 Compound 2 detected in both modes with observed [M – H]−m/z at 137.0248 was discovered in all five parts of lotus and characterized as 2-hydroxybenzoic acid based on the product ion at m/z 93, corresponding to the loss of CO2 (44 Da) from the precursor ion.45 Previously, this compound was also characterized in lotus leaves and lotus seeds with the help of HPLC.31,46 Compound 4 with [M – H]−m/z at 169.0140 was only detected from LSC and LSE and was characterized as gallic acid based on the product ion at m/z 125, corresponding to the loss of CO2 from the precursor ion, which has already been reported in lotus leaves,47 lotus seeds,31 and lotus rhizomes.7 Compound 5 with observed [M – H]−m/z at 479.1583 was tentatively characterized as paeoniflorin. The characteristic fragment ions at m/z 449 [M – H – CH2O]−, m/z 357 [M – H – C7H6O2]−, m/z 327 [M – H – CH2O – C7H6O2]−, and m/z 121 [M – H – C16H22O9]− confirmed the presence of paeoniflorin in the lotus seed. Previously, Tu et al.48 isolated paeoniflorin from Paeonia lactiflora, a well-known traditional Chinese herb, and also reported the potent anti-inflammatory and immune regulatory effects of paeoniflorin.
2.4.1.2. Hydroxycinnamic Acids and Other Phenolic Acid Derivatives
In our study, 12 hydroxycinnamic acid derivatives, 2 hydroxyphenylacetic acids, and 1 hydroxyphenylpentanoic acid were identified in five parts of lotus. Compound 6 was tentatively characterized as cinnamic acid found in LRP, LR, LSC, and LSE based on m/z at 147.0453 in the negative mode. The identification was further supported by the MS2 spectrum, which exhibited a typical product ion at m/z 103 formed by the neutral loss of a carboxylic acid moiety (45 Da).49 Previously, cinnamic acid was found in bilberry fruit (Vaccinium) and reported to be a precursor for the synthesis of a vast number of plant substances, including lignin, tannins, flavonoids, and various alkaloids.50
m-Coumaric acid (compound 8) were detected in both positive (ESI+) and negative (ESI–) modes in all five parts of lotus, with an observed [M – H]−m/z at 163.0405 and the primary product ion at m/z 119, corresponding to the loss of CO2 (44 Da).51 Compound 9 having a precursor ion [M – H]−m/z at 341.0875 was tentatively characterized as caffeoyl glucose and was present in LSE. The MS2 fragmentation showed the product ions at m/z 179 [M – H – 162]− and m/z 161 [M – H – 180]−, consistent with losses of the hexosyl moiety and further loss of H2O.52 Previously, derivatives of coumaric acids and caffeic acids have already been reported in different solvent extracts of lotus leaves and seeds by HPLC.31,46
Three other phenolic acid derivatives were also detected, including two hydroxyphenylacetic acid derivatives (compounds 18 and 19) and one hydroxyphenylpentanoic acid derivative (compound 20). To the best of our knowledge, this is the first time that hydroxyphenylacetic acids and hydroxyphenylpentanoic acids were identified in lotus.
2.4.2. Flavonoids
The study of flavonoids has always been the priority of research related to phenolic compounds in N. nucifera. In addition, flavonoids might be the predominant contributors to the antioxidant activity of lotus, as shown in the correlation section. In our study, a total of 51 flavonoids classified into 8 subclasses were characterized in 5 parts of lotus, including 8 flavanols, 5 flavanones, 6 flavones, 12 flavonols, 3 dihydrochalcones, 7 anthocyanins, and 10 isoflavonoids, as shown in Table 3.
2.4.2.1. Flavanol Derivatives
Compound 21 was characterized as (+)-catechin 3-O-gallate in LRK based on the precursor ion [M – H]− at m/z 441.0842, with product ions at m/z 289 ([C15H13O6]−), m/z 169 ([C7H5O5]−), and m/z 125 ([C6H5O3]−).51 Previously, catechins and its derivatives have been detected in many tissues of lotus, including rhizomes,53 leaves,54 and seed epicarp,4 which have been proved to be able to regulate the insulin secretion and blood glucose level in both in vitro and in vivo models.55
(−)-Epicatechin was proposed as compound 26, detected from all five parts of lotus in both modes, with a precursor ion [M – H]−m/z of 289.0714. The MS2 spectrum showed the product ions at m/z 245, 205, and 179, indicating the loss of CO2 (44 Da), flavonid A ring (84 Da), and flavonid B ring (110 Da) from the precursor ion, respectively.45 The procyanidin trimer C1 and procyanidin dimer B1 (compounds 24 and 28) were also identified in LRP, LR, LSC, and LRK.56,57 The presence of epicatechin and procyanidin in lotus has already been reported by Chen et al.38 In addition, Xu et al.58 extracted procyanidins in the seedpod of lotus and found that procyanidins improved age-related antioxidant deficits in an animal model and had antiaging effects.
2.4.2.2. Flavanone and Flavone Derivatives
Four apigenin derivatives (compounds 34, 35, 36, and 38) were tentatively identified from both seeds and rhizomes of lotus in our study. Compounds 36 and 38 were tentatively assigned as apigenin 6,8-di-C-glucoside and apigenin 6-C-glucoside, respectively, based on the [M – H]− ions at m/z 593.1532 and 431.0984, respectively.
The characteristic loss of 90 and 120 Da caused by the loss of cross-ring cleavages of the glycoside moiety was observed in these two compounds in the MS/MS fragmentation, confirming the identification of these two compounds.59 Different from other apigenin derivatives, compounds 36 and 38 were only present in seeds of lotus, which are in agreement with the finding that the flavonoid C-glycosides were only present in lotus seeds33 [Li, 2014 #40]. Previously, apigenin 6,8-di-C-glucoside (compound 36) has been found in a 70% ethanolic extract of lotus seed embryo by HPLC–MS in the study of Zhu et al.5 In contrast to the O-glucosyl bond, the C-glucosyl bond between the flavonoid carbon skeleton and the glycosyl group is more stable under acidity and enzymatic hydrolysis, resulting in significant differences in the bioactivity and pharmacokinetics.60 Currently, the research of flavonoid C-glycosides is rare. Thus, the flavonoid C-glycosides need further study, which may have an application potential in the food industry.
2.4.2.3. Flavonol Derivatives
Based on the composition of the aglycone, a total of 12 flavonols identified in five parts of lotus were mainly classified into 4 different groups, including 3 kaempferol derivatives, 4 quercetin derivatives, 3 myricetin derivatives, and 1 isorhamnetin derivative.
Compound 47 with [M – H]−m/z at 739.2106 exhibiting characteristic fragment ions at m/z 593 [M – H – C6H10O4]−, m/z 447 [M – H – 2C6H10O4]−, and m/z 285 [M – H – 2C6H10O4 – C6H10O5]− was identified as kaempferol 3-O-(2″-rhamnosyl-galactoside) 7-O-rhamnoside.61 Kaempferol derivatives were previously reported to spread in almost all lotus tissues.62−67 Liao et al.68 found that the kaempferol derivatives extracted from lotus leaves could prevent diabetes type 2 through the inhibition of α-amylase.
2.4.2.4. Dihydrochalcone, Anthocyanin, and Isoflavonoid Derivatives
Compound 54 was only detected in lotus rhizomes in both positive (ESI+) and negative (ESI–) modes with an observed molecular ion peak [M – H]−m/z at 435.1303. This compound was assigned to phloridzin, a characteristic flavonoid found in apples based on its fragment at m/z 273 for the phloretin aglycon.69 Compound 57 having a precursor ion [M + H]+m/z at 466.1095 was tentatively characterized as delphinidin 3-O-glucoside and was present in LRP, LR, LSC, and LRK. The MS2 analysis showed the product ion at m/z 303 [M + H – 162]+, consistent with losses of the hexosyl moiety.70 Previously, delphinidin 3-O-glucoside has been identified in a methanolic extract of the lotus flower petal by HPLC–electrospray ionization–mass spectrometry in the study of Li et al.33
Compound 65 with [M – H]−m/z at 315.0881 exhibiting characteristic fragment ions at m/z 300 [M – H – CH3]−, m/z 285 [M – H – 2CH3]−, m/z 135 [M – H – C10H12O3]−, and m/z 91 [M – H – C10H12O3 – CO2]− was identified as violanone, which was previously found in the essential oil of traditional Chinese medicine Dalbergia odorifera by LC–MS.71
2.4.3. Other Polyphenols
Four hydroxycoumarins, one alkylmethoxyphenol, two hydroxybenzoketones, and three tyrosols were tentatively identified in our study. All of these compounds were first reported in lotus. Compound 81 from lotus rhizomes with [M – H]− ion at m/z 195.0659 was identified as 3,4-DHPEA-AC and showed the main fragment ion at m/z 135 was depicted on the cause of the C2H4O2 deletion, which fit the fragment generated by the hydrolysis of the bound ester group from 3,4-DHPEA-AC. The hydroxytyrosol acetate was a critical antioxidant present in olive oil.72
2.4.4. Lignans and Stilbenes
Lignans were minor components present in the lotus. In the present study, a total of three lignans were shown to be only present in lotus rhizomes. Compound 84 was tentatively characterized as matairesinol and only found in lotus rhizomes based on [M – H]−m/z at 357.1338. The identification was further supported by the MS2 spectrum that exhibited the product ions at m/z 342 [M – H – 15]−, 327 [M – H – 30]−, 313 [M – H – 44]−, 221 [M – H – 136]−, and 161 [M – H – 196]−, corresponding to the loss of CH3, two CH3, CO2, C8H8O2, and C10H12O4, respectively.52 Matairesinol was previously found in the stem of traditional Chinese medicine Acanthopanax senticosus.52
Compounds 85 and 86 were aligned as resveratrol and resveratrol 3-O-glucoside by MS2 spectrum73 [Stella, 2008 #30]. According to previous research, resveratrol was identified primarily in fruit samples such as grape and was reported to be a new cancer chemopreventive agent that inhibits cellular events related to the initiation, promotion, and progression of tumors.74 The lotus seed and rhizome are rich resources of phenolic compounds that might have a wide application prospect in pharmacy, feed, cosmetics, and food industries.
2.5. HPLC and Heat Map
Based on the characterization of phenolic compounds by LC-ESI-QTOF-MS (Table 3) and previous investigations involved in the phenolic composition of different lotus tissues,5,31,33,46 a total of 10 polyphenols were selected for quantitative analysis in lotus by HPLC-PDA, including five phenolic acids (gallic acid, p-hydroxybenzoic acid, chlorogenic acid, syringic acid, and coumaric acid) and five flavonoids (catechin, epicatechin, quercetin-3-galactoside, quercetin, and kaempferol).
A heat map (Figure 1) shows the hierarchical clustering of targeted phenolic compounds in five parts of the lotus. The axis of the map has samples and phenolic compounds, whereas the branching exhibits the similarity of the samples. The darker color (brown) represents a higher concentration, while the blue color has a lower content.
Figure 1.
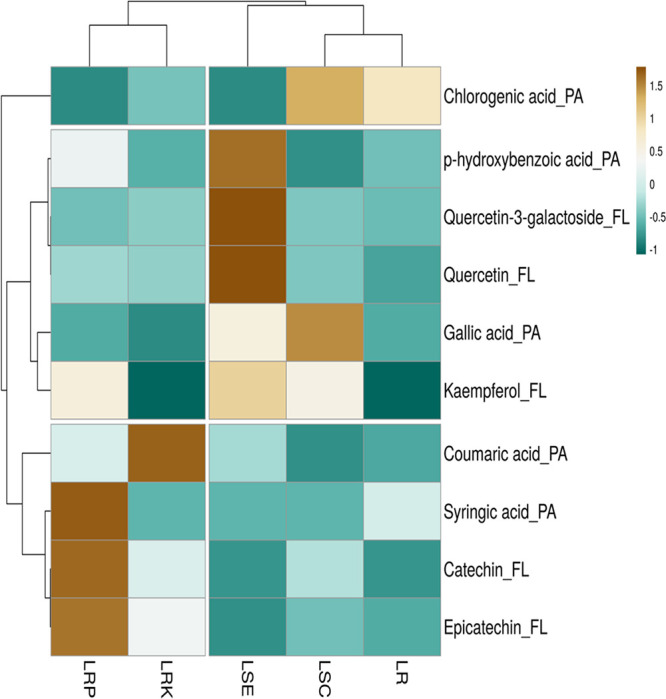
Heat map showing the distribution and concentration of phenolic compounds in five parts of lotus. Brown boxes show that constructions are higher among five samples. Blue boxes indicate lower concentrations. FL: flavonoids and PA: phenolic acids. Fruit peel samples are mentioned in abbreviations. LR: lotus rhizome pulp; LRP, lotus rhizome peel; LRK, lotus rhizome knot; LSC, lotus seed cotyledon; and LSE, lotus seed embryo.
In general, quercetin-3-galactoside and quercetin were found in high concentrations in LSE (marked with dark brown color). Previously, Chen et al.67 reported the concentration of quercetin in different tissues of lotus leaves ranging from 11.0 to 15.1 μg/g. In addition, gallic acid, chlorogenic acid, and kaempferol were also quantified in LSC. Previously, Yen et al.31 already reported the concentration of caffeic acid, chlorogenic acid, p-hydroxybenzoic acid, and gallic acid in an aqueous extract of lotus seeds by HPLC and suggested that those phenolic acids may make contributions to the antioxidant activities of lotus seeds. Catechin and epicatechin were the most abundant flavanols quantified in the lotus rhizome. The LRP presents the highest syringic acid content, and the LRK exhibits the highest content of coumaric acid. Chlorogenic acid was the most abundant phenolic acid quantified in the LR.
3. Materials and Methods
3.1. Chemicals and Reagents
The bioassay for the determination of phenolic compounds and antioxidant potential and the standards used including vanillin, catechin, gallic acid, and ascorbic acid were obtained from Sigma-Aldrich (Castle Hill, NSW, Australia), and the chemicals including aluminum chloride hexahydrate, Folin–Ciocalteu reagent, ferric(III) chloride anhydrous, quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), sodium phosphate, 2,4,6-tripyridyl-s-triazine (TPTZ), potassium persulfate, ammonium molybdate, 2,2-azino-bis(3-ethylbenz-thiazoline-6-sulfonate) (ABTS), and ammonium molybdate were obtained from Sigma-Aldrich Chemical Co. Ltd. (St. Louis, MO, USA). Hydrated sodium acetate, anhydrous sodium acetate, hydrochloric acid, methanol, acetic acid, sodium carbonate (anhydrous), and sulfuric acid (98%) were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA) and Chem-Supply Pty Ltd. (Adelaide, SA, Australia). Acetonitrile and acetic acid used in HPLC and LC–MS were analytical grade and purchased from Fisher Chemical Company (San Jose, CA, USA). Water used in this study was deionized by Millipore Milli-Q Gradient Water Purification System (Darmstadt, Germany). All standards used in HPLC analysis, including gallic acid, protocatechuic acid, caftaric acid, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid, syringic acid, coumaric acid, ferulic acid, sinapic acid, catechin, epicatechin, epicatechin gallate, quercetin-3-glucuronide, quercetin-3-galactoside, quercetin-3-glucoside, quercetin-3-rhamnoside, kaempferol-3-glucoside, diosmin, quercetin, kaempferol, polydatin, and resveratrol, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Sample Preparation
Seeds and rhizomes of ripened edible lotus were purchased from the Preston market in Melbourne, Victoria, Australia, in August 2019. Lotus rhizomes were cleaned before peeling and cutting into pulp (0.5 × 1 cm), peel (0.1 cm thick), and knot (0.5 × 1 cm). The peel, knot, and pulp were then blended into slurries using a 1.5 L blender (Russell Hobbs Classic, model DZ-1613, Melbourne, VIC, Australia). Lotus seeds were separated in half by a hammer to remove embryos from cotyledons followed by grinding in a grinder (Sunbeam Multi Grinder, EM0405, Melbourne, VIC, Australia). All materials were stored at −20 °C for further analysis.
3.3. Extraction of Phenolic Compounds
The polyphenols were extracted by modifying the method of Liu et al.4 Each sample (5 g) was mixed with 15 mL of ethanol (80%, v/v) and homogenized by the Ultra-Turrax T25 Homogenizer (IKA, Staufen, Germany) at 10,000 rpm for 30 s followed by incubation in a ZWYR-240 incubator shaker (Labwit, Ashwood, VIC, Australia) at 120 rpm at 4 °C overnight. The extracts were centrifuged by a centrifugation incubator (Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany) at 10, 000 rpm for 10 min. The supernatants were transferred and filtered through a 0.45 μm syringe filter (Thermo Fisher Scientific Inc., Waltham, MA, USA) before being stored at −20 °C for further analysis.
3.4. Estimation of Polyphenols and Antioxidant Activities
All phytochemical and antioxidant assays were performed in triplicate and measured by a Multiskan Go microplate photometer (Thermo Fisher Scientific, Waltham, MA, USA). The standard curves were plotted with R2 > 0.995. All results were based on fresh weight (f.w.).
3.4.1. Determination of Total Phenolic Content (TPC)
The total polyphenol content was measured using a modified Folin–Ciocalteu method of Gu et al.13 A total of 25 μL of samples, 25 μL of 25% (v/v) Folin–Ciocalteu reagent, and 200 μL of deionized water were mixed in 96-well plates (Costar, Corning, NY, USA) followed by incubation for 5 min at 25 °C. After that, 25 μL of 10% (w/w) sodium carbonate was added followed by further incubation in the darkroom for 60 min at 25 °C. The absorbance was measured at 764 nm in a plate reader. The results were converted to total phenolic content expressed as mg of gallic acid equivalents per gram of the sample based on fresh weight (mg GAE/gf.w.). Gallic acid ranging from 0 to 200 μg/mL was used to plot the calibration curve.
3.4.2. Determination of Total Flavonoid Content (TFC)
A modified aluminum chloride coloration method was applied to evaluate the TFC values of lotus seeds and rhizomes.13 Eighty milliliters of sample extracts, 80 μL of 2% (w/v) aluminum chloride ethanolic solution (analytical grade), and 120 μL 50 g/L sodium acetate were sequentially added into the 96-well plate. The mixture was incubated in the darkroom for 1 h at 25 °C before measuring the absorbance at 440 nm. The quercetin standard ranging from 0 to 50 μg/mL was used to plot the standard curve, and the result was expressed in quercetin equivalents (mg QE/gf.w.).
3.4.3. Determination of Total Tannin Content (TTC)
The TTC was determined by the colorimetric method of Gu et al.13 with some modifications. The sample extract (25 μL) was mixed with 150 μL of 4% (w/v) methanolic vanillin solution and 25 μL of 32% (v/v) sulfuric acid (diluted with methanol) in the 96-well plates followed by incubation for 15 min at 25 °C. The absorbance was measured at 500 nm. Catechin (0–1000 μg/mL) was used as a calibration standard. The results were expressed as mg of catechin equivalents per gram of sample (mg CE/gf.w.).
3.4.4. Determination of DPPH Free Radical Scavenging Activity
The ability to scavenge the DPPH radical was evaluated based on the method of Braca et al.75 [Braca, 2001 #33] with some modifications. Forty microliters of lotus extracts was mixed with 260 μL of the DPPH radical methanol solution (0.1 mM). Absorbance was measured at 517 nm after 30 min incubation in the darkroom at 25 °C, and ascorbic acid (0–50 μg/mL) was used as the standard. The DPPH free radical scavenging activity was expressed as units of ascorbic acid equivalent (mg AAE/gf.w.).
3.4.5. Determination of Ferric Reducing Antioxidant Power (FRAP)
For the ferric reducing antioxidant power of lotus in this study, the method of Kim and Shin76 was engaged with some modifications. In the reaction, the Fe3+-TPTZ complex (ferric-2,4,6-tripyridyl-s-triazine) was reduced to a colored product (Fe2+-TPTZ). The FRAP reagent was prepared freshly by mixing 10 mL of 20 mM FeCl3, 10 mL of TPTZ solution (10 mM TPTZ and 40 mM HCl), and 100 mL of 300 mM sodium acetate solution. Then, 20 μL of extracts was mixed with 280 μL of the FRAP reagent in the 96-well plates. The absorbance was measured at 593 nm after incubation at 37 °C for 10 min, and ascorbic acid (0–50 μg/mL) was used as the control. The results were expressed as mg ascorbic acid equivalents per gram of sample weight (mg AAE/gf.w.).
3.4.6. Determination of ABTS Free Radical Scavenging Activity
The determination of ABTS radical scavenging activity was based on a modified method of Sogi et al.77 First, ABTS was dissolved in 140 mM potassium persulfate solution to a 7 mM concentration to produce the ABTS radical cation (ABTS•+) followed by incubation in the darkroom overnight before use. The stock solution was further diluted with ethanol (analytical grade) to give an absorbance of 0.70 ± 0.02 at 734 nm. After that, 10 μL of lotus extracts was added to 290 μL of the ABTS working solution in the 96-well plates. The absorbance was measured at 734 nm immediately after 6 min incubation at 25 °C, and ascorbic acid was used as control (0–2000 μg/mL). The results were expressed as mg ascorbic acid equivalent per gram of sample (mg AAE/gf.w.).
3.4.7. Determination of Total Antioxidant Capacity (TAC)
The total antioxidant capacity assay was carried out by modifying the method of Jan et al.,78 which is based on reducing phosphomolybdate ions. Antioxidants in extracts reduce phosphomolybdate ion and form a green phosphate/MoV complex, which can be measured spectrophotometrically78 [Jan, 2013 #13]. The sample (25 μL) was mixed with 250 μL of the prepared dye solution (0.6 M H2SO4 sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate, with the volume ratio of 1:1:1). The mixtures were incubated in the darkroom at 95 °C for 90 min. The absorbance was measured at 695 nm after cooling at room temperature for 10 min. Ascorbic acid (0–300 μg/mL) was used as standard, and the results were expressed as mg ascorbic acid equivalent per gram of sample (mg AAE/gf.w.).
3.5. LC-ESI-QTOF-MS/MS Characterization
The characterization of phenolic compounds by LC-ESI-QTOF-MS/MS was carried out by the modified method of Ma et al.79 An Agilent 1200 series HPLC (Agilent Technologies, CA, USA) equipped with an Agilent 6520 Accurate-Mass Q-TOF LC/MS (Agilent Technologies, CA, USA) was used in this work. The separation was achieved by a Synergi Hydro-RP (250 × 4.6 mm i.d.) reversed-phase column with a particle size of 4 μm (Phenomenex, Lane Cove, NSW, Australia) coupled with a Phenomenex 4.0 × 2.0 mm i.d. C18 ODS guard column. The column temperature was set at 25 °C, and the injection volume was 5 μL. The mobile phase consisted of two eluents. Eluent A is 0.5% acetic acid in water (0.5:99.5, v/v), and eluent B consists of acetonitrile/water/acetic acid (50:49.5:0.5, v/v/v). The gradient profile was 10–25% eluent B from 0 to 20 min, 25–35% eluent B from 20 to 30 min, 35–40% eluent B from 30 to 40 min, 40–55% eluent B from 40 to 70 min, 55–80% eluent B from 70 to 75 min, 80–90% B from 75 to 77 min, 90–100% B from 77 to 79 min, 100–10% B from 79 to 82 min, and isocratic 10% B from 82 to 85 min. The flow rate was 0.8 mL/min. The nitrogen gas pressure was set at 45 psi with a flow rate of 5 L/min at 300 °C, while the sheath gas was set at 11 L/min at 250 °C. The capillary was set at 3.5 kV. The nozzle voltage was set at 500 V. A complete mass scan ranging from m/z 50 to 1300 was used. MS/MS analyses were carried out in automatic mode with collision energy (10, 15, and 30 eV) for fragmentation. Peak identification was performed in both positive and negative modes, while the instrument control, data acquisition, and processing were performed using the MassHunter workstation software (Qualitative Analysis, version B.03.01) (Agilent Technologies, Santa Clara, CA, USA).
3.6. HPLC-PDA Quantification Analysis
The quantification of 10 targeted phenolic compounds present in five parts of the lotus was carried out by the modified method of Ma et al.79 HPLC (chromatography separation module, Waters Alliance 2690) was equipped along with a photodiode array and a detector. The mention column and condition as stated in the LC-ESI-QTOF-MS/MS section were practical, excluding for a sample injection volume of 20 μL. The PDA detector noticed the phenolics of extracts under λ 280, 320, and 370 nm. The individual phenolic compound was quantified based on linear regression of the external standards’ plotting peak area against concentration.
3.7. Statistical Analysis
Results from seven independent experiments (Sections 3.4.1 to 3.4.7: TPC, TFC, TTC, ABTS, DPPH, FRAP, and TAC) in spectrophotometric assays were expressed as mean ± standard deviation (SD). The analysis of variance was conducted using one-way analysis of variance (ANOVA), and the differences between the means of samples were carried out by Tukey’s test using Minitab 18 Statistical Software (Minitab Inc., State College, PA, USA) at a significance level of p < 0.05. Pearson’s correlation coefficient was used to analyze the correlation between antioxidant activities and total phenolic and flavonoid content in the extracts of lotus seeds and rhizomes.
4. Conclusions
Remarkable phenolic contents and antioxidant potentials were observed in all lotus samples, while among the five parts of lotus, the lotus seed embryo exhibits the highest total phenolic content, total flavonoid content, and total antioxidant capacity (DPPH, ABTS, and TAC). A total of 86 phenolic compounds were successfully separated and characterized in five parts of lotus seeds and rhizomes by the application of the LC-ESI-QTOF-MS/MS technique. Most compounds were discovered in the lotus seed embryo followed by the lotus seed cotyledon and lotus rhizome peel. In addition, the flavonoid C-glycosides identified in lotus seeds are not commonly found in most plants, which are valuable for further investigations on their effects on human health. Thus, the results of the present study revealed that byproducts of lotus seeds and rhizomes (lotus seed embryo, lotus rhizome knot, and lotus rhizome peel) have a prominent antioxidant effect and could be good sources of natural antioxidants. However, further investigations involving more detailed activity studies in vitro and in vivo are required to support further utilization in the food and pharmaceutical industries.
Funding
This research was funded by the University of Melbourne under the ″McKenzie Fellowship Scheme″ (Grant UoM-18/21); the ″Richard WS Nicholas Agricultural Science Scholarship″ and the ″Faculty Research Initiative Funds″ funded by the Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Australia; and ″The Alfred Deakin Research Fellowship″ funded by Deakin University, Australia.
Acknowledgments
We would like to thank Nicholas Williamson, Shuai Nie, and Michael Leeming from the Mass Spectrometry and Proteomics Facility, Bio21 Molecular Science and Biotechnology Institute, the University of Melbourne, VIC, Australia, for providing access and support for the use of HPLC-PDA and LC-ESI-QTOF-MS/MS and data analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c07018.
LC-ESI-QTOF-MS/MS basic peak chromatograph (BPC) for the characterization of phenolic compounds of Australia-grown lotus (Figure S1) and LC-ESI-QTOF-MS/MS characterization of 2-hydroxybenzoic acid (Figure S2) (PDF)
Author Contributions
# Z.Z. and B.Z. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Guo X.; Wang D.; Duan W.; Du J.; Wang X. Preparative isolation and purification of four flavonoids from the petals of Nelumbo nucifera by high-speed counter-current chromatography. Phytochem. Anal. 2010, 21, 268–272. 10.1002/pca.1196. [DOI] [PubMed] [Google Scholar]
- Paudel K. R.; Panth N. Phytochemical Profile and Biological Activity of Nelumbo nucifera. J. Evidence-Based Complementary Altern. Med. 2015, 2015, 1. 10.1155/2015/789124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungmunnithum D.; Pinthong D.; Hano C. Flavonoids from Nelumbo nucifera Gaertn., a Medicinal Plant: Uses in Traditional Medicine, Phytochemistry and Pharmacological Activities. Medicines 2018, 5, 127. 10.3390/medicines5040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Ma S. S.; Ibrahim S. A.; Li E. H.; Yang H.; Huang W. Identification and antioxidant properties of polyphenols in lotus seed epicarp at different ripening stages. Food Chem. 2015, 185, 159–164. 10.1016/j.foodchem.2015.03.117. [DOI] [PubMed] [Google Scholar]
- Zhu M.; Liu T.; Zhang C.; Guo M. Flavonoids of Lotus (Nelumbo nucifera) Seed Embryos and Their Antioxidant Potential. J. Food Sci. 2017, 82, 1834–1841. 10.1111/1750-3841.13784. [DOI] [PubMed] [Google Scholar]
- Arooj M.; Imran S.; Inam-Ur-Raheem M.; Rajoka M. S. R.; Sameen A.; Siddique R.; Sahar A.; Tariq S.; Riaz A.; Hussain A.; Siddeeg A.; Aadil R. M. Lotus seeds (Nelumbinis semen) as an emerging therapeutic seed: A comprehensive review. Food. Sci. Nutr. 2021, 9, 3971–3987. 10.1002/fsn3.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J.; Chen S.; Yin X.; Wang K.; Liu Y.; Li S.; Yang P. Systematic qualitative and quantitative assessment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars. Food Chem. 2013, 139, 307–312. 10.1016/j.foodchem.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Yi Y.; Sun J.; Xie J.; Min T.; Wang L. M.; Wang H. X. Phenolic Profiles and Antioxidant Activity of Lotus Root Varieties. Molecules 2016, 21, 863. 10.3390/molecules21070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas M.; Saeed F.; Anjum F. M.; Afzaal M.; Tufail T.; Bashir M. S.; Ishtiaq A.; Hussain S.; Suleria H. A. R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. 10.1080/10942912.2016.1220393. [DOI] [Google Scholar]
- Etxeberria U.; Fernandez-Quintela A.; Milagro F. I.; Aguirre L.; Martinez J. A.; Portillo M. P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. 10.1021/jf402506c. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Zhong B.; Barrow C. J.; Dunshea F. R.; Suleria H. A. Identification of phenolic compounds in Australian grown dragon fruits by LC-ESI-QTOF-MS/MS and determination of their antioxidant potential. Arab. J. Chem. 2021, 14, 103151. 10.1016/j.arabjc.2021.103151. [DOI] [Google Scholar]
- Hu M.; Skibsted L. H. Antioxidative capacity of rhizome extract and rhizome knot extract of edible lotus (Nelumbo nuficera). Food Chem. 2002, 76, 327–333. 10.1016/S0308-8146(01)00280-1. [DOI] [Google Scholar]
- Gu C.; Howell K.; Dunshea F. R.; Suleria H. A. R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. 10.3390/antiox8090405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou O.; Ali A.; Subbiah V.; Barrow C. J.; Dunshea F. R.; Suleria H. A. LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential. Fermentation 2021, 7, 73. 10.3390/fermentation7020073. [DOI] [Google Scholar]
- Du J.; Zhong B.; Subbiah V.; Barrow C. J.; Dunshea F. R.; Suleria H. A. LC-ESI-QTOF-MS/MS Profiling and Antioxidant Activity of Phenolics from Custard Apple Fruit and By-Products. Separations 2021, 8, 62. 10.3390/separations8050062. [DOI] [Google Scholar]
- Feng Y.; Dunshea F. R.; Suleria H. A. Lc-esi-qtof/ms characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Sci. Technol. 2020, 57, 4671–4687. 10.1007/s13197-020-04504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y.; Wang Z.; Barrow C. J.; Dunshea F. R.; Suleria H. A. High-Throughput Screening and Characterization of Phenolic Compounds in Stone Fruits Waste by LC-ESI-QTOF-MS/MS and Their Potential Antioxidant Activities. Antioxidants 2021, 10, 234. 10.3390/antiox10020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah V.; Zhong B.; Nawaz M. A.; Barrow C. J.; Dunshea F. R.; Suleria H. A. Screening of phenolic compounds in australian grown berries by lc-esi-qtof-ms/ms and determination of their antioxidant potential. Antioxidants 2021, 10, 26. 10.3390/antiox10010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herchi W.; Arraez-Roman D.; Trabelsi H.; Bouali I.; Boukhchina S.; Kallel H.; Segura-Carretero A.; Fernandez-Gutierrez A. Phenolic compounds in flaxseed: a review of their properties and analytical methods. An overview of the last decade. J. Oleo Sci. 2014, 63, 7–14. 10.5650/jos.ess13135. [DOI] [PubMed] [Google Scholar]
- Lee F. Y.; Vo G. T.; Barrow C. J.; Dunshea F. R.; Suleria H. A. Mango rejects and mango waste; Characterization and quantification of phenolic compounds and their antioxidant potential. J. Food Process. Preserv. 2021, e15618 10.1111/jfpp.15618. [DOI] [Google Scholar]
- Li H.; Subbiah V.; Barrow C. J.; Dunshea F. R.; Suleria H. A. Phenolic Profiling of Five Different Australian Grown Apples. Appl. Sci. 2021, 11, 2421. 10.3390/app11052421. [DOI] [Google Scholar]
- Peng D.; Zahid H. F.; Ajlouni S.; Dunshea F. R.; Suleria H. A. Lc-esi-qtof/ms profiling of australian mango peel by-product polyphenols and their potential antioxidant activities. Processes 2019, 7, 764. 10.3390/pr7100764. [DOI] [Google Scholar]
- Sharifi-Rad J.; Zhong J.; Ayatollahi S. A.; Kobarfard F.; Faizi M.; Khosravi-Dehaghi N.; Suleria H. A. LC-ESI-QTOF-MS/MS characterization of phenolic compounds from Prosopis farcta (Banks & Sol.) JF Macbr. and their potential antioxidant activities. Cell. Mol. Biol. 2021, 67, 189–200. 10.14715/cmb/2021.67.1.28. [DOI] [PubMed] [Google Scholar]
- Suleria H. A.; Barrow C. J.; Dunshea F. R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. 10.3390/foods9091206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D.-J.; Choe J.; Hwang K.-E.; Kim C.-J.; Jo C. Antioxidant effects of lotus (Nelumbo nucifera) root and leaf extracts and their application on pork patties as inhibitors of lipid oxidation, alone and in combination. Int. J. Food. Prop. 2019, 22, 383–394. 10.1080/10942912.2019.1588295. [DOI] [Google Scholar]
- Huang B.; He J.; Ban X.; Zeng H.; Yao X.; Wang Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat. Sci. 2011, 87, 46–53. 10.1016/j.meatsci.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Limwachiranon J.; Huang H.; Shi Z.; Li L.; Luo Z. Lotus Flavonoids and Phenolic Acids: Health Promotion and Safe Consumption Dosages. Compr. Rev. Food Sci. Food Saf. 2018, 17, 458–471. 10.1111/1541-4337.12333. [DOI] [PubMed] [Google Scholar]
- Yang D.; Zhang Q.; Ren G.; Ying T. A comparative study on antioxidant activity of different parts of lotus (Nelumbo nuficera Gaertn) rhizome. Food Sci. Technol. 2017, 37, 135–138. 10.1590/1678-457X.10816. [DOI] [Google Scholar]
- Huang B.; Ban X.; He J.; Tong J.; Tian J.; Wang Y. Comparative analysis of essential oil components and antioxidant activity of extracts of Nelumbo nucifera from various areas of China. J. Agric. Food Chem. 2010, 58, 441–448. 10.1021/jf902643e. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Shen J.; Chang K. J.; Kim S. H. Comparative analysis of antioxidant activity and functional components of the ethanol extract of lotus (Nelumbo nucifera) from various growing regions. J. Agric. Food Chem. 2014, 62, 6227–6235. 10.1021/jf501644t. [DOI] [PubMed] [Google Scholar]
- Yen G.-C.; Duh P.-D.; Su H.-J. Antioxidant properties of lotus seed and its effect on DNA damage in human lymphocytes. Food Chem. 2005, 89, 379–385. 10.1016/j.foodchem.2004.02.045. [DOI] [Google Scholar]
- Robbins R. J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- Li S. S.; Wu J.; Chen L. G.; Du H.; Xu Y. J.; Wang L. J.; Zhang H. J.; Zheng X. C.; Wang L. S. Biogenesis of C-glycosyl flavones and profiling of flavonoid glycosides in lotus (Nelumbo nucifera). PLoS One 2014, 9, e108860 10.1371/journal.pone.0108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.; Dunshea F. R.; Suleria H. A. LC-ESI-QTOF/MS characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 2020, 9, 7. 10.3390/foods9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Barrow C. J.; Dunshea F. R.; Suleria H. A. A comparative investigation on phenolic composition, characterization and antioxidant potentials of five different australian grown pear varieties. Antioxidants 2021, 10, 151. 10.3390/antiox10020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.; Dunshea F. R.; Suleria H. A. LC-ESI-QTOF/MS characterization of Australian herb and spices (garlic, ginger, and onion) and potential antioxidant activity. J. Food Process. Preserv. 2020, 44, e14497 10.1111/jfpp.14497. [DOI] [Google Scholar]
- Plaza M.; Pozzo T.; Liu J.; Gulshan Ara K. Z.; Turner C.; Nordberg Karlsson E. Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J. Agric. Food Chem. 2014, 62, 3321–3333. 10.1021/jf405570u. [DOI] [PubMed] [Google Scholar]
- Chen H.; Sun K.; Yang Z.; Guo X.; Wei S. Identification of Antioxidant and Anti-alpha-amylase Components in Lotus (Nelumbo nucifera, Gaertn.) Seed Epicarp. Appl. Biochem. Biotechnol. 2019, 187, 677–690. 10.1007/s12010-018-2844-x. [DOI] [PubMed] [Google Scholar]
- Zhong B.; Robinson N. A.; Warner R. D.; Barrow C. J.; Dunshea F. R.; Suleria H. A. Lc-esi-qtof-ms/ms characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. 10.3390/md18060331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.; Chou O.; Lee F. Y.; Wang Z.; Barrow C. J.; Dunshea F. R.; Suleria H. A. Characterization of phenolics in rejected kiwifruit and their antioxidant potential. Processes 2021, 9, 781. 10.3390/pr9050781. [DOI] [Google Scholar]
- Zou X. S.; Al-Duais M. A.; Bahattab O.; Hamayoon Khan M.; Suleria H. A. Screening of Polyphenols in Tobacco (Nicotiana tabacum) and Determination of Their Antioxidant Activity in Different Tobacco Varieties. ACS Omega 2021, 6, 25361–25371. 10.1021/acsomega.1c03275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proteggente A. R.; Pannala A. S.; Paganga G.; Van Buren L.; Wagner E.; Wiseman S.; Van De Put F.; Dacombe C.; Rice-Evans C. A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radical Res. 2002, 36, 217–233. 10.1080/10715760290006484. [DOI] [PubMed] [Google Scholar]
- Sunitha D. A Review on Antioxidant Methods. J. Pharm. Clin. Res. 2016, 9, 14–32. [Google Scholar]
- Rajauria G.; Foley B.; Abu-Ghannam N. Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innovative Food Sci. Emerging Technol. 2016, 37, 261–268. 10.1016/j.ifset.2016.02.005. [DOI] [Google Scholar]
- Saez V.; Riquelme S.; Baer D. V.; Vallverdu-Queralt A. Phenolic Profile of Grape Canes: Novel Compounds Identified by LC-ESI-LTQ-Orbitrap-MS. Molecules 2019, 24, 20. 10.3390/molecules24203763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. B.; Kim D. H.; Je J. Y. Antioxidant and Cytoprotective Effects of Lotus (Nelumbo nucifera) Leaves Phenolic Fraction. Prev. Nutr. Food. Sci. 2015, 20, 22–28. 10.3746/pnf.2015.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H. H.; Hsu L. S.; Chan K. C.; Chen H. M.; Wu C. H.; Wang C. J. Extract from the leaf of nucifera reduced the development of atherosclerosis via inhibition of vascular smooth muscle cell proliferation and migration. Food Chem. Toxicol. 2010, 48, 159–168. 10.1016/j.fct.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Tu J.; Guo Y.; Hong W.; Fang Y.; Han D.; Zhang P.; Wang X.; Korner H.; Wei W. The Regulatory Effects of Paeoniflorin and Its Derivative Paeoniflorin-6’-O-Benzene Sulfonate CP-25 on Inflammation and Immune Diseases. Front. Pharmacol. 2019, 10, 57. 10.3389/fphar.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K. M.; Cheng Y. Y.; Tsai T. H. Integrated LC-MS/MS Analytical Systems and Physical Inspection for the Analysis of a Botanical Herbal Preparation. Molecules 2015, 20, 10641–10656. 10.3390/molecules200610641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson B. G.TANNINS AND POLYPHENOLS. In Encyclopedia of Food Sciences and Nutrition (SecondEdition), Caballero B., Ed. Academic Press: Oxford, 2003; pp. 5729–5733. [Google Scholar]
- Wang J.; Jia Z.; Zhang Z.; Wang Y.; Liu X.; Wang L.; Lin R. Analysis of Chemical Constituents of Melastoma dodecandrum Lour. by UPLC-ESI-Q-Exactive Focus-MS/MS. Molecules 2017, 22, 3. 10.3390/molecules22030476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Liu J.; Zhang A.; Sun H.; Zhang Y.. Chapter 23 - Systematic Characterization of the Absorbed Components of Acanthopanax senticosus Stem. In Serum Pharmacochemistry of Traditional Chinese Medicine, Wang X., Ed. Academic Press: 2017, 2, 313–336. [Google Scholar]
- Jiang Y.; Ng Tzi B.; Liu Z.; Wang C.; Li N.; Qiao W.; Liua F. Immunoregulatory and anti-HIV-1 enzyme activities of antioxidant components from lotus (Nelumbo nucifera Gaertn.) rhizome. Biosci. Rep. 2011, 31, 381–390. 10.1042/BSR20100062. [DOI] [PubMed] [Google Scholar]
- Chang C. H.; Ou T. T.; Yang M. Y.; Huang C. C.; Wang C. J. Nelumbo nucifera Gaertn leaves extract inhibits the angiogenesis and metastasis of breast cancer cells by downregulation connective tissue growth factor (CTGF) mediated PI3K/AKT/ERK signaling. J. Ethnopharmacol. 2016, 188, 111–122. 10.1016/j.jep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Yang M.-Y.; Chang Y.-C.; Chan K.-C.; Lee Y.-J.; Wang C.-J. Flavonoid-enriched extracts from Nelumbo nucifera leaves inhibits proliferation of breast cancer in vitro and in vivo. Eur. J. Integr. Med. 2011, 3, e153–e163. 10.1016/j.eujim.2011.08.008. [DOI] [Google Scholar]
- Enomoto H.; Takahashi S.; Takeda S.; Hatta H. Distribution of Flavan-3-ol Species in Ripe Strawberry Fruit Revealed by Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry Imaging. Molecules 2020, 25, 11. 10.3390/molecules25010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q.; Luo F.; Zhao X.; Liu Y.; Hu G.; Sun C.; Li X.; Chen K. Identification of proanthocyanidins from litchi (Litchi chinensis Sonn.) pulp by LC-ESI-Q-TOF-MS and their antioxidant activity. PLoS One 2015, 10, e0120480 10.1371/journal.pone.0120480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Rong S.; Xie B.; Sun Z.; Zhang L.; Wu H.; Yao P.; Hao L.; Liu L. Procyanidins extracted from the lotus seedpod ameliorate age-related antioxidant deficit in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 236–241. [DOI] [PubMed] [Google Scholar]
- Singh A.; Kumar S.; Bajpai V.; Reddy T. J.; Rameshkumar K. B.; Kumar B. Structural characterization of flavonoid C- and O-glycosides in an extract of Adhatoda vasica leaves by liquid chromatography with quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 1095–1106. 10.1002/rcm.7202. [DOI] [PubMed] [Google Scholar]
- Courts F. L.; Williamson G. The Occurrence, Fate and Biological Activities of C-glycosyl Flavonoids in the Human Diet. Crit. Rev. Food. Sci. Nutr. 2015, 55, 1352–1367. 10.1080/10408398.2012.694497. [DOI] [PubMed] [Google Scholar]
- Gong L.; Haiyu X.; Wang L.; Xiaojie Y.; Huijun Y.; Songsong W.; Cheng L.; Ma X.; Gao S.; Liang R.; Yang H. Identification and evaluation of the chemical similarity of Yindan xinnaotong samples by ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry fingerprinting. J. Sep. Sci. 2016, 39, 611–622. 10.1002/jssc.201500836. [DOI] [PubMed] [Google Scholar]
- Ahn J. H.; Kim E. S.; Lee C.; Kim S.; Cho S. H.; Hwang B. Y.; Lee M. K. Chemical constituents from Nelumbo nucifera leaves and their anti-obesity effects. Bioorg. Med. Chem. Lett. 2013, 23, 3604–3608. 10.1016/j.bmcl.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Liu H.; Liu J.; Zhang J.; Qi Y.; Jia X.; Zhang B.; Xiao P. Simultaneous Quantitative and Chemical Fingerprint Analysis of Receptaculum Nelumbinis Based on HPLC-DAD-MS Combined with Chemometrics. J. Chromatogr. Sci. 2016, 54, 618–624. 10.1093/chromsci/bmv229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredy H. M.; Huang D.; Xie B.; He H.; Yang E.; Tian B.; Xiao D. Flavonols of lotus (Nelumbo nucifera, Gaertn.) seed epicarp and their antioxidant potential. Eur. Food Res. Technol. 2010, 231, 387–394. 10.1007/s00217-010-1287-6. [DOI] [Google Scholar]
- Lim S. S.; Jung Y. J.; Hyun S. K.; Lee Y. S.; Choi J. S. Rat lens aldose reductase inhibitory constituents of Nelumbo nucifera stamens. Phytother. Res. 2006, 20, 825–830. 10.1002/ptr.1847. [DOI] [PubMed] [Google Scholar]
- Zhu M. Z.; Wu W.; Jiao L. L.; Yang P. F.; Guo M. Q. Analysis of Flavonoids in Lotus (Nelumbo nucifera) Leaves and Their Antioxidant Activity Using Macroporous Resin Chromatography Coupled with LC-MS/MS and Antioxidant Biochemical Assays. Molecules 2015, 20, 10553–10565. 10.3390/molecules200610553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Fang L.; Xi H.; Guan L.; Fang J.; Liu Y.; Wu B.; Li S. Simultaneous qualitative assessment and quantitative analysis of flavonoids in various tissues of lotus (Nelumbo nucifera) using high performance liquid chromatography coupled with triple quad mass spectrometry. Anal. Chim. Acta 2012, 724, 127–135. 10.1016/j.aca.2012.02.051. [DOI] [PubMed] [Google Scholar]
- Liao L.; Chen J.; Liu L.; Xiao A. Screening and Binding Analysis of Flavonoids with Alpha-Amylase Inhibitory Activity from Lotus Leaf. J. Braz. Chem. Soc 2017, 29, 33. 10.21577/0103-5053.20170171. [DOI] [Google Scholar]
- Kelebek H.; Kadiroğlu P.; Demircan N. B.; Selli S. Screening of bioactive components in grape and apple vinegars: Antioxidant and antimicrobial potential. J. Inst. Brew. 2017, 123, 407–416. 10.1002/jib.432. [DOI] [Google Scholar]
- Tourino S.; Fuguet E.; Jauregui O.; Saura-Calixto F.; Cascante M.; Torres J. L. High-resolution liquid chromatography/electrospray ionization time-of-flight mass spectrometry combined with liquid chromatography/electrospray ionization tandem mass spectrometry to identify polyphenols from grape antioxidant dietary fiber. Rapid Commun. Mass Spectrom. 2008, 22, 3489–3500. 10.1002/rcm.3756. [DOI] [PubMed] [Google Scholar]
- Li Z.; Zhang X.; Liao J.; Fan X.; Cheng Y. An ultra-robust fingerprinting method for quality assessment of traditional Chinese medicine using multiple reaction monitoring mass spectrometry. J. Pharm. Anal. 2021, 11, 88–95. 10.1016/j.jpha.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drira M.; Kelebek H.; Guclu G.; Jabeur H.; Selli S.; Bouaziz M. Targeted analysis for detection the adulteration in extra virgin olive oil’s using LC-DAD/ESI–MS/MS and combined with chemometrics tools. Eur. Food Res. Technol. 2020, 246, 1661–1677. 10.1007/s00217-020-03522-y. [DOI] [Google Scholar]
- Stella L.; De Rosso M.; Panighel A.; Vedova A. D.; Flamini R.; Traldi P. Collisionally induced fragmentation of [M-H](−) species of resveratrol and piceatannol investigated by deuterium labelling and accurate mass measurements. Rapid Commun. Mass Spectrom. 2008, 22, 3867–3872. 10.1002/rcm.3811. [DOI] [PubMed] [Google Scholar]
- Smoliga J. M.; Baur J. A.; Hausenblas H. A. Resveratrol and health--a comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- Braca A.; De Tommasi N.; Di Bari L.; Pizza C.; Politi M.; Morelli I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64. 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- Kim M.-J.; Shin H.-S. Antioxidative effect of lotus seed and seedpod extracts. Food. Sci. Biotechnol. 2012, 21, 1761–1766. 10.1007/s10068-012-0234-7. [DOI] [Google Scholar]
- Sogi D. S.; Siddiq M.; Greiby I.; Dolan K. D. Total phenolics, antioxidant activity, and functional properties of ’Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. 10.1016/j.foodchem.2013.05.053. [DOI] [PubMed] [Google Scholar]
- Jan S.; Khan M. R.; Rashid U.; Bokhari J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of monotheca buxifolia fruit. Osong Public Health Res. Perspect. 2013, 4, 246–254. 10.1016/j.phrp.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.; Dunshea F. R.; Suleria H. A. R. LC-ESI-QTOF/MS Characterization of Phenolic Compounds in Palm Fruits (Jelly and Fishtail Palm) and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 483. 10.3390/antiox8100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


