Abstract
Diet as a whole, encompassing food composition, calorie intake, and the length and frequency of fasting periods, affects the time span in which health and functional capacity are maintained. Here, we analyze aging and nutrition studies in simple organisms, rodents, monkeys and humans to link longevity to conserved growth and metabolic pathways, and outline their role in aging and age-related disease. We focus on feasible nutritional strategies shown to delay aging and/or prevent diseases through epidemiological, model organism, clinical, and centenarian studies, and underline the need to avoid malnourishment anb frailty.These findings are integrated to define a longevity diet based on a multi-pillar approach adjusted for age and health status to optimize lifespan and healthspan in humans.
Introduction
In the year 440 BCE the Greek physician Hippocrates said, “Let food be thy medicine and let thy medicine be food”. His wisdom has proven true since we now know that altering the levels, type, and timing of food consumption (i.e., fasting) is perhaps the most potent, feasible and safest intervention to improve health, extend longevity, and extend the time in which health and functional capacity are maintained (i.e., healthspan) in species ranging from bacteria to humans. In fact, the fundamental relationship between nutrients and cellular and/or organismal responses are conserved from unicellular microorganisms to humans. However, despite extensive research, the type, quantity, and combination of nutrients that optimize healthy longevity remains highly controversial. In addition, increasing evidence suggests that in humans nutrition must be adjusted to age, sex, genetics, and metabolic risk status of an individual, and that tailoring specific dietary recommendations is essential for full beneficial effects to be realized. Understanding and harnessing these evolutionary conserved mechanisms in addition to personalizing dietary interventions will be key to optimize human healthspan and longevity. Here, we explore the link between nutrients, fasting, genes, and longevity in short-lived species, and connect these links to clinical and epidemiological studies in primates and humans, including centenarians. By adopting a multi-system and multi-pillar approach based on over a century of research we can begin to define a longevity diet that represents a solid foundation for nutritional recommendation and for future research.
Nutrition and delayed aging in short-lived species
In this section we cover in broad strokes the evidence that the pace of aging can be altered by inhibiting the function of nutrient responsive genes and pathways or altering the quantity, type of nutrients and feeding patterns that regulate them. Taking examples from studies in yeast, worms, and fruit flies, we discuss the biology behind nutritional modulation of longevity and describe some of the common themes emerging from studies, which point to metabolic and growth regulatory pathways as key influences on healthspan. In particular we emphasize the conserved mechanisms and how these might play into aging regulation. Insights gleaned from studies of short-lived species provide the foundation for the fundamental biology of longevity, and for how different nutrients and their levels impact molecular processes that are vital to maintaining health with advancing age.
Yeast
Aging in yeast is assessed either by measuring survival of non-dividing cells (chronological lifespan) or the replicative capacity of individual mother cells (replicative lifespan). Here we will focus on the genes and processes that are generally involved in regulating both replicative and chronological lifespan. The quantity and type of nutrients available are at the center of the regulation of virtually every stage of the life history of simple organisms. Sugars and specific amino acids have strong effects in regulating both stress resistance and longevity pathways in yeast. In Saccharomyces cerevisiae yeast laboratory strains, nutrients are provided in the form of mixtures of carbohydrates, proteins, and lipids on which the cells grow. The presence of glucose results in the activation of the yeast Ras--adenylate cyclase (AC)--PKA pathway, whereas amino acids regulate the Pkh/PDK and Tor-Sch9/S6K pathways (Mirisola et al., 2014). Mutations that decrease the activity of either Tor- Sch9/S6K or Ras- AC -PKA pathways extend lifespan and healthspan, accompanied by the activation of stress resistance transcription factors Msn2/Msn4, increased expression of antioxidant enzymes, a reduction in DNA damage and an extension of the reproductive period (Fabrizio et al., 2001). Genetic mutations in both pathways have an additive effects on lifespan suggesting that there is more than one way to harness growth pathways as a means to extend lifespan.
Recent studies are defining the molecular impact of diet composition and fasting on aging. Yeast studies of caloric restriction (CR) usually involve lowering the availability of sugars (e.g. from 2% to 0.5% glucose), or nitrogen sources (e.g. amino acid restriction). Genetic studies of nitrogen restriction indicate that autophagy, mitochondrial function, translation, RNA processing, and the stress response are all important in conferring longevity (Campos et al., 2018). Although restriction of different carbon sources (e.g. glucose, galactose) can have different effects on lifespan extension, the shared key pathways associated with longevity involve the regulation of glycolysis and the tricarboxylic acid (TCA) cycle, oxidative phosphorylation, lipid metabolism, oxidative stress, DNA damage, apoptosis, and autophagy (Kaya et al., 2021) (Fabrizio et al., 2001). Mechanistically longevity extension is linked to increased stress resistance, altered redox metabolism, and potentially also increased engagement of lipid and peroxisomal metabolism. Thus, aging studies in this unicellular eukaryote model show interconnections among stress and nutritent signaling pathways, and indicate that signal transduction pathways activated by glucose and amino acid reduce stress resistance and accelerate metabolism and growth to shorten lifespan.
Worms
In the simple nematode Caenorhabditis elegans, the insulin signaling pathway influences longevity using key players similar to those in yeast including the insulin receptor (IR) homologue Daf-2, AKT, TOR, and the stress resistance forkhead transcription (FOXO) factor Daf-16. Genetic studies on longevity regulation in worms also implicate stress signaling, in addition to roles for mitochondrial function, metabolic adaptation, nuclear receptor signaling, translation regulation, and immune modulation (Fontana et al., 2010). Some of the first longevity genes identified in worms (age-1 and clk-1) were linked to insulin, growth signaling, and mitochondrial function. Subsequently, these mutants were associated with the mitochondrial unfolded protein response (mitoUPR), which sensitizes the innate immune response via stress response signaling (Campos et al., 2021; Wu et al., 2019). Several studies indicate that the mechanisms behind longevity conferred by dietary restriction (DR) and by reduction in insulin-like signaling are similar but not equivalent_(Greer and Brunet, 2009).
In worms, DR is often accomplished by food dilution, as the animals live on their food source, a bacterial infused layer. The term DR rather than CR is used in worm studies because actual calorie intake of individuals is not quantified. DR, which is effective in extending longevity in worm, recruits many of the same genes identified by genetic screens as modulators of longevity, including those in growth signaling, proteostasis, the stress response, and metabolic pathways. In worms, fasting induces pathways involved in proteostasis, by a mechanism involving stress response signaling factors (Uno et al., 2013), and protects against the disruption of proteostasis (Iranon et al., 2019), indicating that there is a protective aspect to the metabolic setting associated with fasting. Mitochondrial and peroxisomal function have also been implicated in mechanisms of worm longevity regulation by DR (Weir et al., 2017). Remodeling of mitochondrial architecture is required for longevity, and the peroxisomal involvement reflects the greater emphasis on lipid fuel utilization during nutrient deprivation. It is taken for granted that changes in gene activation or repression are key to implementing the longevity program, but other regulatory mechanisms are involved. The metabolic switch to lipid-based metabolism with DR involves changes to gene expression via RNA processing (Heintz et al., 2017). Regulation global protein homeostasis (proteostasis) is also important in the mechanisms of DR, and although global translation is diminished translation of subsets of transcripts is prioritized indicating a more nuanced adjustment of protein synthesis rather than a simple energy-saving reduction (Rollins et al., 2019). In terms of cellular processes, genetic strategies to augment autophagy extend longevity in worms (Kumsta et al., 2019), pointing to the importance of recycling and/or removal of damaged proteins.The protective effect of autophagy is linked to mitochondrial function (Zhou et al., 2019), indicating a metabolism-regulated proteostasis pathways. The targeted degradation of proteins through the proteasome system is also vulnerable to age (Koyuncu et al., 2021), but is rescued by genetic strategies that mimic DR or that dampen growth signaling. Aging studies in the worm model show the complexity of pathways and processes associated with longevity regulation and point to key interactions among them, where a change in growth is accompanied by a change in metabolism, and changes in metabolism influence growth and proteostasis.
Flies
There is substantial evidence that reduced insulin-like signaling also extends longevity in the fruit fly Drosophila melanogaster. Here too, factors including the fly homologues of insulin receptor substrate (Chico), AKT, and forkhead transcription factor (dFOXO) are established longevity regulatory factors (Fontana et al., 2010). Indeed, pharmacological strategies to reduce growth signaling are effective in enhancing fly lifespan (Castillo-Quan et al., 2019). One of the highly attractive features of the fly model is the amenability to studies with large numbers of organisms. That together with the increased complexity of the organism and the very well characterized genetic tools available, allows for in depth exploration of genetic and nutritient interactions in the regulation of longevity. Studies in flies reveal interactions between genetics and diet to impact longevity (McCracken et al., 2020). Metabolic hubs linked to longevity across genetic backgrounds include the glycolytic and gluconeogenic intermediate phosphoenolpyruvate, amino acids threonine and arginine, and alpha ketoglutarate, a key factor in the TCA, transamination reactions, and epigenetic regulation of gene expression (Jin et al., 2020). Interestingly, flies fed citrate or beta hydroxybutyrate (a component of ketone bodies) are healthier and live longer linking the TCA cycle and ketogenesis to longevity programs independently of other effects of fasting (Fan et al., 2021). In terms of macronutrient balance, there is a negative effect on survival when protein is either very low or very high (Savola et al., 2021) in agreement with the findings described later for mice and humans.
DR is implemented in flies by the dilution of the diet and acts in part independently of insulin-like signaling pathways, at least for the upsteam events that lead to longevity. Genetic differences among strains impact the ability of DR to increase survival (Wilson et al., 2020). Transcriptional analysis identifies phases of response to DR beginning with activation of oxidative metabolism, followed by stress signaling and lipid metabolism, and then autophagy, stress and the metabolic switch to increased expression of FAO and gluconeogenic genes (Romey-Glüsing et al., 2018). Proteomic analysis of whole flies reveals subtle differences in the response to DR depending on the age of the animals (Gao et al., 2020).
Intermittent fasting (IF) is also effective in delaying aging in adult flies, but the animals need to be switched back to ad libitum at older age, pointing to the need for age-specific dietary interventions in simple organisms (Catterson et al., 2018) as suggested by studies in humans and mice (Levine et al., 2014). IF suppresses the age-related decline in proteostasis related pathways and impacts both the stress response and inflammation. (Zhang et al., 2018). Furthermore, IF preserves the integrity of gene expression regulation and is additive with DR (Ulgherait et al., 2021). Fasting in flies induces the cAMP responsive CREB, a key transcription factor known for its role in metabolic regulation, but also influencing inflammatory and immune pathways (Shen et al., 2016). Time restricted feeding (TRF) is also beneficial and is associated with a depletion in ectopic lipid stores (Villanueva et al., 2019).
Take home message from short-lived species
Studies in short lived species are invaluable for advancing the field in nutrition and aging research. It is clear that genetics regulates the health and longevity of these organisms and that many of the key aging genes are regulated by nutrients levels and composition. Studies in simple organisms also indicate that genes play a role in how an individual organism responds to nutritional cues to promote health and longevity. It is also clear that there are complex interactions between nutrient composition and the engagement of longevity pathways. Furthermore, the age of onset influences diet efficacy, a feature that is clear also in mammalian studies. In short-lived species, aging appears to be regulated though inhibition of growth and alteration of metabolic pathways. Mechanisms that are associated with fasting, including greater stress resistance, reliance on lipid fuel use and activation of proteostatic mechanisms, are shared features of delayed aging (Figure 1). A substantial body of evidence indicates that cellular processes including mitochondrial energy metabolism, autophagy, and the stress response are likely to be causal in implementing longevity induced by diet manipulation. Importantly, these signatures are at least partially conserved in mammals.
Figure 1.
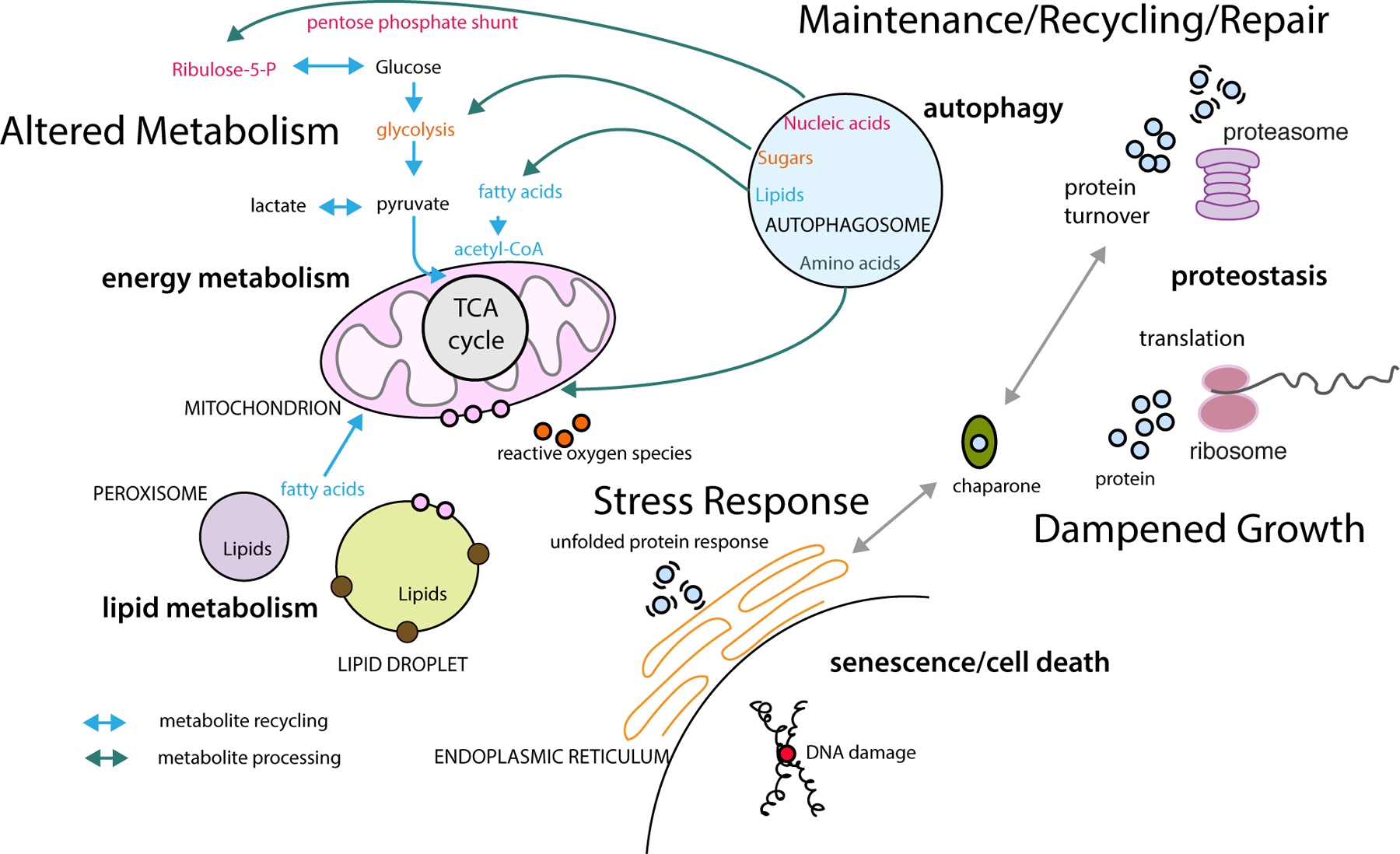
Conserved pathways associated with longer lifespan identified in yeast, worms, flies, and mice. Altered metabolism involves signature changes related to energy saving, activation of lipid fuel use, and dampened growth and synthetic pathways. At the cellular level the delayed aging phenotype is associated with increased metabolite recycling, autophagy, reduced translation, protein turnover, and enhanced maintenance and repair linked to antioxidant and other stress response pathways. Interactions among organelles are influenced by energy status and associations shift to accommodate the metabolic state linked to lower nutrient availability and low growth signaling conditions. The overall outcome is a reprogrammed metabolism, enhanced repair and recycling mechanisms, and reduced growth and macromolecular synthesis.
Nutrient Response Pathways in Mammals
In this section we explore the effects of specific nutrients on genetic pathways that regulate aging and diseases in mammals. We focus on those identified in the prior section that point to conserved mechanisms in longevity regulation across species.
The protein-endocrine axis
Within non-restrictived feeding strategies, diets with increased levels of proteins and certain amino acids including methionine are the most effective in increasing GH signaling and IGF-1 levels, and, not surprisingly, in shortening the lifespan of rodents by activating a pro-aging axis including higher levels of circulating IGF-1 (Figure 2) (Bartke et al., 2013). For example, the switch from 18% to 7% of calorie intake obtained from proteins whether derived from casein or soy, caused an over 30% decrease in IGF-1 levels and a doubling in the levels of IGFBP1, an inhibitor of IGF-1 signaling, in mice (Levine et al., 2014). Similarly, blood IGF-1 levels are significantly higher in human subjects in the US reporting a high protein diet compared to those on a low protein diet. Genetics of aging studies also revolutionized our understanding of the mechanisms responsible for the effect of dietary restrictions on aging and lifespan in mammals. As observed for yeast and flies, mutations that cause severe deficiencies in growth genes (for mice, growth hormone (GH) and growth hormone receptor (GHR)) extend lifespan by 35–50% (Bartke et al., 2013). The ability of the deficiency in growth hormone releasing hormone receptor (GHRHD) upstream of GH and GHR to also extend the mouse lifespan by 20–25% point to a role for the GHRH-GH-GHR axis as a master regulator of aging and lifespan (Fig. 2). Both growth hormone deficiency (GHD) and growth hormone receptor deficiency (GHRD) cause a severe reduction in the levels of circulating insulin-like growth factor 1 (IGF-1), which is the central factor promoting the growth of mammals (Bartke et al., 2013). This circulating IGF-1 reduction and the lowering of insulin levels caused by GHRD but potentially also a reduction in the cell autonomous GHR signaling, appear to be important for longevity extension (Bartke et al., 2013). The relative contribution of the lowering of insulin, versus IGF-1, versus the down-regulation of GHR signaling in various cell types on lifespan extension of GH or GHR deficient mice remains poorly understood and in need of further investigation.
Figure 2. Dietary modulation of longevity.
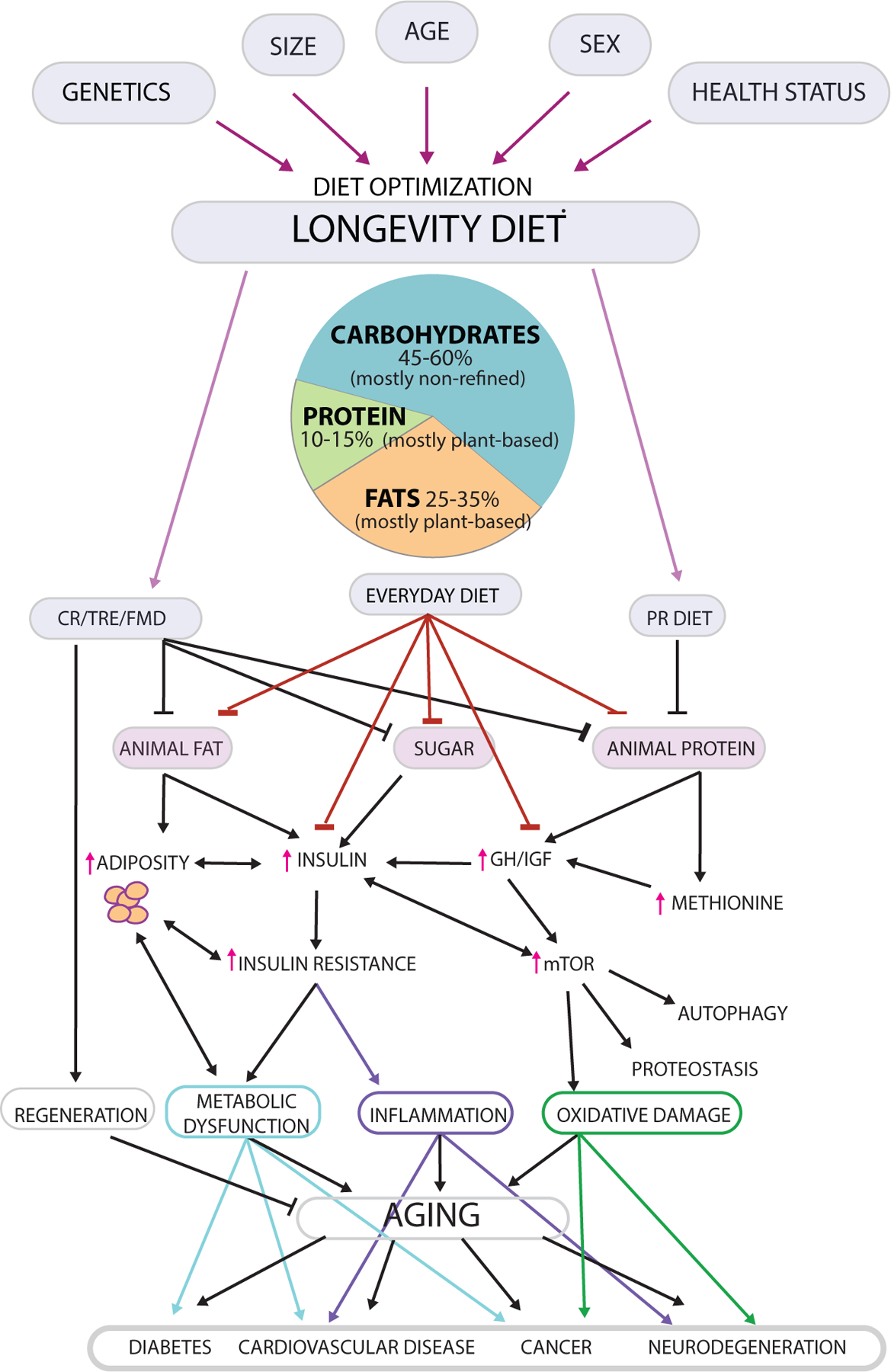
Longevity can be promoted in different ways; however, diet composition and levels must be optimized to avoid malnourishment and frailty and should be personalized based on characteristics including the genome, size and adiposity, biological age range, sex and health status (Top). The Longevity Diet includes limiting calorie intake (left: calorie restriction (CR), adopting 11–12 hour time restricted eating (TRE) as well as a few yearly cycles of 5 day fasting/FMDs) or selective age range-sepcific reduction in specific dietary components (right: protein restriction (PR), methionine restriction (MR)), through a high legume, high whole grain pesco-vegan diet or mostly plant-based everyday diet including nut consumption which provides 45–60% of calories from non-refined complex carbohydrates, 10–15% from mostly plant-based proteins, and 25–35% from mostly plant-based fats. Together, these nutritional patterns promote low insulin and insulin resistance, low adiposity, moderate levels/activity of GH/IGF-1, and reduced mTOR signaling and potentially increased autophagy in different cell types. Prolonged and periodic FMDs and possibly other fasting methods may activate autophagy during the late portion of the fasting period and increase the levels of stem cells and regeneration in various tissues, especially during the re-feeding period. Downstream of these changes are improved metabolic function, reduced inflammation with delayed immunosenescence, reduced oxidative damage and improved proteostasis. The regulation of this pro-longevity network can delay aging, and reduce the risk factors and/or incidence of age-related diseases including diabetes, cancer, cardiovascular and neurodegenerative diseases. The effects of the Longevity Diet on diseases appears to be both dependent and independent of its effect on aging and biological age.
As expected, based on the role of aging as a major risk factor for many diseases, the portion of GHRDs mice developing neoplasms was reduced from 83.3% in wild type mice to 42.1 %, with adenocarcinomas affecting 20% of wild type but none of the GHRDs (Ikeno et al., 2009). GHRD mice are also protected from insulin resistance and age-dependent cognitive decline (Bartke, 2005). Insulin and IGF-1 can activate the insulin and IGF-1 receptors and the downstream IRS, PI3K-AKT and TOR-S6K pathways in many different cell types (Bartke et al., 2013). In fact, compared to wild type mice, mice lacking one copy of the IGF-1R gene live 16–33% longer, with females displaying a stronger extension, and mice with mutations in the IRS-1 gene, which encodes proteins functioning downstream of both the IGF-1 and insulin receptors, also live 16–30% longer (Bartke et al., 2013). Furthermore, knockout mutations of the S6K gene result in lifespan extension in mice (Selman et al., 2009) and administration of the TOR-S6K inhibitor rapamycin starting after middle age extends longevity in genetically heterogeneous mice (Harrison et al., 2009). The role of reduced IGF-1 and insulin signaling in mouse lifespan extension is in agreement with the role for reduced insulin-like signaling in the lifespan extension of worms and flies, discussed earlier.
In agreement with studies in yeast, flies, and mice showing that mutation in either TOR-S6K or GH-IGF-1/Insulin signaling cause dwarfisms and record longevity extension, studies of humans with mutations in the GHR gene that causes severe GHR and circulating IGF-1 deficiency show protection against age related diseases. In fact in the Ecudaorian GHRD subjects cancer incidence was extremely low (Guevara-Aguirre et al., 2011) in agreement with the observation of Zvi Laron for GHRD subjects in other parts of the world. Ecuadorian GHRDs also display a very low incidence of diabetes despite high obesity prevalence which is explained by the increased insulin sensitivity in these subjects (Guevara-Aguirre et al., 2011). Furthermore, GHRDs display a cognitive performance similar to that of younger individuals (Nashiro et al., 2017). Notably, the effects of GHRD on obesity, insulin sensitization, diabetes, cancer, and cognitive decline are consistent with those described above for mice (Bartke et al., 2013)
The sugar-endocrine axis
In addition to the protein-endocrine axis, sugars can also play a central role in signaling leading to the acceleration of the aging process. As well established for S. cerevisiae, glucose may also contribute to mammalian aging by both increasing the release of insulin and possibly by directly activating certain pro-aging pathways. In rat cardiomyocytes glucose restriction induces the activation of early growth response protein 1 (Egr1) transcription factor, the ortholog of yeast Msn2/Msn4 through the regulation of protein kinase A (PKA)- and AMP-activated protein kinase (AMPK) (Longo et al., 2021). Glucose can also activate mTORC1 which senses dihydroxyacetone phosphate (DHAP), a glycolytic metabolite (Orozco et al., 2020). Not surprisingly, both mutations in AC and in subunits of PKA extend longevity and reduce morbidity in mice. Disruption of adenylyl cyclase type 5, predominantly expressed in the heart and brain, increased median lifespan by 30% and protected mice from cardiomyopathy, possibly through protection against oxidative stress, analogous to the findings related to AC and longevity in yeast (Yan et al., 2015). The disruption of the RIIb subunit of PKA is also associated with extended longevity in male mice, reduced fasting glucose and insulin levels, and protection against left ventricular hypertrophy (Enns et al., 2009).
In summary, the increased levels/activity of hormones, factors and genetic pathways caused by either proteins, certain amino acids, or sugars have been consistently associated with accelerated aging and/or age-related disease in organisms ranging from yeast to humans. Among them, the GH-IGF-1 axis, the Tor-S6K pathway, and glucose-dependent responses either through elevated insulin or direct cellular signaling appear to be the most conserved and most likely to serve as targets of effective pro-longevity drugs. However, either continuous, intermittent or periodic dietary intervention may, at least initially, be preferable choices to regulate these pathways because they can generate coordinated responses which are effective against aging and diseases but also safe both after short- and long-term use. These responses may have evolved to allow organisms to enter a high protection and slow aging maintenance mode when food is not sufficient for growth or reproduction
Caloric restriction
Caloric restriction in rodents
There is a large literature on the beneficial impact of CR on indices of health including the onset and progression of multiple age-related diseases and conditions in rodents (Richardson, 2021). The mechanisms responsible for the effects of CR on longevity and disease involve the nutrient responsive signaling genes discussed above, although additional mechanisms, including the prevention of insulin resistance and metabolic diseases, are also involved. In fact, decreased adiposity is a hallmark of CR in rodents and is associated with changes in fat function, including the secretion of protein and lipid factors associated with metabolic homeostasis (Miller et al., 2017). Not all strains respond equivalently to the same imposed degree of restriction, and more recently sex dimorphism has been a focal point in nutritional studies of aging (Mitchell et al., 2016). Despite a well-established dampening of growth response in rodents on CR, there is evidence that the immune response is actually improved (Palma et al., 2021), although it will be important to determine whether CR renders organisms sensitive to specific infectious diseases. In rodent studies the pressing question is whether the documented benefits of CR can be harnessed through manipulation of diet composition or timing of feeding, and it seems there may be interactions between the two (see below). Since the literature on CR in rodents has been reviewed extensively over the years, in this review we will focus on the effects of CR in monkeys and humans.
Caloric Restriction in monkeys
Nonhuman primates are a highly translational model for biomedical research and the rhesus monkey Macaca mullata is one of the best characterized for aging studies (Balasubramanian et al., 2017). Two prominent studies of the impact of long-term CR on aging and health were conducted over 30 years, one at the Wisconsin National Primate Research Center, and the other within the National Institute on Aging intramural program at the National Institutes of Health (Colman et al., 2009, 2014; Mattison et al., 2012). Although initial reports seemed contradictory, subsequent comparisons between the studies resolved that the monkeys that weighed less and ate less lived longer and were healthier until later in life (Mattison et al., 2017).
The hallmarks of CR in monkeys mirror those identified in mouse studies and include lower adiposity, lower fasting glucose and insulin, greater insulin sensitivity, and more favorable lipid profiles (Kemnitz, 2011). Importantly, these same features are also detected in a short-term clinical trial of CR in humans (Most et al., 2017). CR in nonhuman primates is associated with several indices of healthy aging. MRI studies of monkeys on CR indicate delayed brain aging, based on the preservation of age-related loss of gray matter volume (Colman et al., 2009), which was subsequently linked to improved insulin sensitivity (Willette et al., 2012), and preservation of white matter (Bendlin et al., 2011). CR was also effective in delaying sarcopenia, preserving muscle mass, preventing an age-related decline in physical activity, and lowering the metabolic cost of movement that becomes elevated with age (Yamada et al., 2013). There is also evidence that CR preserves the T cell repertoire (Messaoudi et al., 2006), opposing a phenotype of age that is thought to be directly linked to disease vulnerability. In skeletal muscle, CR increases expression of genes involved in energy metabolism and proteostasis, decreases the expression of genes involved of immune and inflammatory pathways, preserves fiber metabolism and cross-sectional area, and delays fibrosis and fat infiltration (Rhoads et al., 2020). At the system level, delayed muscle aging is linked to insulin sensitivity. In hepatic tissue, CR induces gene expression related to oxidative phosphorylation, lipid metabolism and peroxisomal pathways, proteostasis, and RNA processing, while down-regulating immune and inflammatory pathways (Rhoads et al., 2018). Serum metabolomics reveal similarities between the rodent and monkey responses to CR (Aon et al., 2020), including enrichment of ketone bodies, fatty acids, and factors associated with fasting including succinate, glutamine and lactate. These studies suggest that the biology of CR is at least partially conserved from mice to nonhuman primates.
Caloric restriction in humans
The NIH/NIA-sponsored 2 year CALERIE study demonstrated that the systemic hallmarks of CR observed in rodents and monkeys are largely recapitulated in humans (Most et al., 2017). CR induced a loss of total body weight and a reduction in adiposity resulting in fat free mass being higher as a percent of total body mass in individuals on the CR regimen. CR was associated with greater insulin sensitivity (Ravussin et al., 2015), lower risk scores for cardiovascular disease (Kraus et al., 2019), and improved biomarkers of liver health (Dorling et al., 2021). Analysis of clinical and plasma biomarkers from CALERIE subjects indicates that the pace of aging is delayed (Belsky et al., 2017), which was later corroborated using the methylation clock (Belsky et al., 2020). The similarities between the human, nonhuman primate, and mouse responses to CR argue for strong conservation in the underlying mechanisms by which CR impacts health in mammals, with links to improved longevity consistent between mice and monkeys.
The biology of mammalian CR
Despite species specificity in how aging manifests and in the final determinants of mortality, the underlying cellular biology of CR is conserved. Key processes involved in making the transition to healthier status include autophagy, proteostasis, energy metabolism and the switch to lipid fuel usage, changes in growth signaling including translation and synthetic pathways, and engagement of gene regulatory mechanisms such as RNA processing, all of which have been linked to longevity regulation in short-lived species (Figure 1). The overall picture indicates that longevity is associated with a reduction in the activity of growth pathways and a switch to metabolic patterns associated with fasting responses. These changes are coincident with reduced inflammation without a general impairment of immune function, which could contribute to protection against diseases ranging from cancer, to cardiovascular disease, to Alzheimer’s and autoimmune diseases. Although these outcomes are shared in effective models of longevity enhancement there are important caveats. Timing of onset of the diet, sex, and existing metabolic and genetic status all influence the efficacy of these diets in producing beneficial effects. The striking influence of genetics on the health and longevity response to CR observed in shorter-lived laboratory animals, many of which are substantialy in-bred, is unlikely to be so dramatic in outbred populations or in primates and humans, but genetics must be considered as a factor in optimizing dietary interventions. The take home message from aging and nutrition research is that one size does not fit all, but it is almost certain that specific nutrition patterns can optimize health and longevity.
Fasting
In this section we bring together evidence from mammalian studies on the beneficial effects of fasting, describing the different models of fasting implementation, and their effects on disease risk factors, health, and longevity.
Intermittent fasting
Although there are many different types of intermittent fasting regimens, ranging from restricting eating to a limited number of hours (time restricted feeding or TRF in model organism and time restricted eating TRE in humans) to alternate day fasting, to fasting for 2 days a week, here we will focus on the most common form of IF, which in most cases entails 12–23 hours of fasting per day (Longo et al., 2021).
TRF in rodents
Time restricted feeding (TRF) has gained a lot of public attention due to ease of implementation and the promise of health benefit. In this regimen, the time period in which there is access to food is reduced but composition of the food is not changed. Much of the early work was not focused on aging but on correcting or avoiding metabolic dysfunction associated with obesogenic diets (Chaix et al., 2014). Compared to a high fat diet (60% fat) fed ad libitum without timing limitations, the same diet limited to 9hr of feeding followed by 15hr of fasting (TRF) activated pathways in liver including those involved in metabolism, proteostasis, RNA processing, and repair and defense pathways (Chaix et al., 2019). On a standard diet, benefits of TRF are also observed, and although there are similarities with CR even at the molecular level, TRF is not as effective as CR in delaying aging (Aon et al., 2020; Mitchell et al., 2019; Velingkaar et al., 2020). The beneficial metabolic effects of TRF (9hr feeding/15hr fasting) in mice fed a “western” diet (45% fat) ad libitum compared to those fed the same ad libitum diet without imposed fasting periods are observed in young and mature adult mice, and also include improved survival in response to LPS (Chaix et al., 2021). There are interesting sex-specific effects of TRF benefits, reflecting differences in the innate response to high fat diet feeding, and adding to the growing evidence across aging studies that females are not the same as males. Thus, the impact of nutrient limitation by CR and the effect of TRF on health indices is observed whether animals are on standard diets or on high fat diets although the specifics of the metabolic reprograming are not identical (Diaz-Ruiz et al., 2021). It seems that the context matters, perhaps reflecting the importance of metabolic status and the best strategy to harness health and longevity associated pathways that may be different when starting from a metabolically compromised position.
TRE in Humans
There is growing evidence of the beneficial effects of time restricted eating (TRE) in humans (Duregon et al., 2021). Most of the clinical trials to date have focused on weight loss or correcting existing metabolic impairment, with studies involving subjects with obesity, metabolic syndrome, or Type 2 Diabetes (T2D). The regimen used is usually a 8–10 hour daily eating window, with duration varying from 4 to 12 weeks, and with some studies imposing TRE only 5 out of 7 days per week. Almost all studies of TRE report weight loss, and a reduction in adiposity (Wilkinson et al., 2020) or waist circumference (Schroder et al., 2021) when measured. Several studies report improvements in circulating factors linked to cardiovascular disease (Che et al., 2021; Schroder et al., 2021; Wilkinson et al., 2020), but this is not always the case. Few studies report improvements in glucoregulatory parameters. A notable exception is a study involving healthy weight individuals, where cardiovascular disease indices were unaltered but circulating glucose levels were lowered (Martens et al., 2020). More stringent TREs (6 hours) are effective in improving insulin sensitivity but are more challenging to undertake (Sutton et al., 2018). Epidemiology studies are less clear about TRE. Longer daily fasting periods which involve breakfast skipping have been consistently associated with increased mortality, which is particularly high for cardiovascular disease (Rong et al., 2019). Four weeks of another form of intermittent fasting in which subjects fast every other day (alternate day fasting) was also effective In improving cardiovascular markers, reducing trunk fat, improving the fat-to- lean ratio, and increasing b-hydroxybutyrate, even on non-fasting days (Stekovic et al., 2020)
In summary, TRE appears to have beneficial effects in both rodents and humans, but both compliance issues and side effects point to a 11–12 hours daily eating period as ideal at least until additional studies identify TRE lengths that are safe, feasible and effective.
Periodic fasting and fasting mimicking diets
In humans, markers or risk factors for aging and age-related disease, including IGF-1, insulin, glucose, insulin resistance, HbA1c, C reactive protein (CRP), hypertension, high cholesterol, can be affected by dietary composition and by fasting periods. As described in the earlier section, Intermittent fasting (IF) regimens--ranging from time restricted eating, to alternate day fasting, to 2 days of fasting per week--require frequent and long-term restrictions, and only specific types are effective and not associated with side effects. Periodic fasting (PF) cycles, which do not require frequent fasting, are emerging as an alternative to IF. The disadvantage is that they require longer periods of fasting lasting 2 days and in most cases 4 sequential days, but the advantage is that they are in the great majority of cases adopted twice a month or less and may be effective if applied only a few times a year, since they have been shown to provide long term protective effects even months after PF cycles end (Wei et al., 2017). Thus, PF can be adopted at regular intervals such as once a month and can be used analogously to drugs based on the need to treat a condition or disease such as cancer (Longo et al., 2021). Although periods of water-only fasting (i.e., only water is consumed) lasting 3 or more days are feasible, the extreme nature of this intervention underlines both safety and compliance concerns, particularly when involving relatively healthy subjects who are not motivated by the need to treat a disease or condition. In fact, the initial trials focused on the use of water-only fasting in cancer patients proceeded very slowly, since the intervention was difficult for patients and was met with skepticism by oncologists (Longo et al., 2021). For this reason, but also in search for nutritional compositions able to enhance the effects of water only fasting, Fasting mimicking diets (FMDs) were developed and tested both in animal and clinical studies.
Periodic Fasting/FMD in rodents
FMDs are plant-based low calories, low protein, low sugar, and high fat nutritional compositions normally provided to animals or human subjects in a pre-packaged form developed and studied for the purpose of replacing water-only fasting while maintaining and possibly exceeding its effect on key markers of the fasting response including changes in IGF-1, IGFBP1, glucose and ketone bodies (Longo et al., 2021). They are part of an emerging nutri-technology field focused on applying both specific ingredients and complex food compositions as medicine to accompany or replace pharmacological or biological therapies.
In mice, FMD cycles are protective in both type 1 and type 2 diabetes models, prevent the premature death caused by a high fat/calorie diet, reduce the symptoms and pathology associated with multiple autoimmune diseases, reduce the incidence and progression of a range of tumors, and extend lifespan (Longo et al., 2021). Notably, in humans the beneficial effects of FMD cycles on disease markers/risk factors including IGF-1 and leptin continue for weeks after the return to a normal diet, consistent with what has been observed in mice (Caffa et al., 2020). Although the specific mechanisms responsible for the protective and rejuvenating effects of FMD cycles are only beginning to be understood many of the beneficial outcomes induced by CR including reduced adiposity, improved insulin sensitivity, and lowered inflammation are also detected in response to periodic FMD treatments. Specific mechanisms observed during the FMD cycles in mice include the activation of stem cells and developmental-like programs in multiple systems, but the re-feeding phase in which mice are switched from the FMD to a high protein and calorie nutrition appears to be central for the regenerative effects. Consistent with both the anti-inflammatory and regenerative effects, FMDs cause a reduction in autoimmune cells leading to reduced inflammation, re-myelination, and reduced pathology in mouse models of multiple sclerosis (Longo et al., 2021). In leptin receptor deficient a type 2 diabetes db/db mouse model, FMD lowers insulin resistance pointing to a fundamental role of fasting periods in recalibrating metabolic integrity. FMD also promotes a gene expression profile in the pancreas similar to that observed during embryonic development, leading to the reversal of the depletion in functional beta cells and reduced insulin production in a type 1 diabetes model (Cheng et al., 2017). FMD cycles applied for 5 days once a month to mice on a high fat/calorie diet, lower body fat, improve cardiac function, lower cholesterol and restore lifespan to the levels observed in mice on a standard diet (Mishra et al., 2021). Additional health benefits of FMD cycles lasting 4 days include extended longevity, reduced tumor incidence and delayed cognitive decline, even when started at middle age (Brandhorst et al., 2015). Furthermore, either CR or FMD cycles protect against cancer in an implanted xenograft model (Pomatto-Watson et al., 2021). Metabolite signaling is integral to fasting biology and recent studies demonstrated enhanced longevity in mice fed the TCA intermediate alpha ketoglutarate (Asadi Shahmirzadi et al., 2020). Perhaps the stimulation of mitochondrial activity by ketone bodies or TCA cycles intermediates, in addition to increased stress resistance, anti-inflammatory and regenerative effects, could mediate part of the effects of periodic fasting/FMDs.
Periodic Fasting/FMD in Humans
In humans, PF and FMDs have been studied in both normal subjects, and in disease treatment. A randomized crossover study of 100 patients of which 71 received 3 monthly 5 day FMD cycles showed reduced body weight, trunk, and total body fat; lowered blood pressure and decreased insulin-like growth factor 1 (IGF-1). A post hoc analysis also indicated a reduction in fasting glucose, triglycerides, total and low-density lipoprotein cholesterol, and C-reactive protein in participants with high levels of these risk factors at baseline (Wei et al., 2017). A number of studies have now also investigated the role of FMD in cancer treatment including a 125 patient randomized study indicating that FMD increases the efficacy of chemotherapy on clinical and pathological responses in women with breast cancer, even if the majority of patients complete only 2 cycles of the dietary intervention (de Groot et al., 2020). In addition, a 36-patient feasibility study in which FMD combined with hormone therapy to treat breast cancer was found to be safe and reduce markers and risk factors associated with cancer progression without reducing muscle function or mass (Caffa et al., 2020).
Therefore, FMD cycles have been associated with potent anti-inflammatory, metabolic and regenerative effects in mice and with improvemens in disease risk factors or clinical response in multiple clinical studies. Because the beneficial changes caused by FMD cycles can last for months, this dietary intervention has the potential to be effective and should be tested in clinical trials for the prevention and treatment of many diseases when applied for only 3–4 times per year without requiring but while preferring improvements in the daily eating habits.
Macronutrient composition and levels
The role of nutrition on lifespan and age-related disease is widely accepted yet we are far from a consensus on what type of nutrition affects healthspan. Fortunately, the nutrition response mechanisms affecting health and longevity are quite well conserved in species ranging from simple organisms to rodents to humans, making it possible to take advantage of both basic science and human studies to identify the type and levels of macronutrients and nutrition patterns that will be effective in regulating adiposity and aging in most individuals, although the diet will also need be tailored to account for not only age, sex, and genetics, but also lifestyle and the health status of an individual. It is now well established that diets that augment central adiposity can cause major increases in insulin resistance and the risk for diabetes, cancer and neurodegenerative disease in mice and humans (Saltiel and Olefsky, 2017). In the sections below we focus on how macronutrient composition, levels, and source affect biomarkers and risk factors for aging and age-related diseases in rodents and humans.
High calorie diets
In rodents and humans, increasing calorie intake above the level required for the required energy expenditure increases lipogenesis, fat storage and obesity, contributing to major age-related disease (Janssen, 2021). Excess glucose is directed to the synthesis of triglycerides in the liver, which are transported to adipose tissue and muscle by VLDL. Genetics influence the response to dietary interventions, but in general a diet providing high levels of saturated fats and sugars appears to be effective in generating obesity, insulin resistance, high cholesterol and a shortened lifespan (Mishra et al., 2021; Wali et al., 2020). In mice and rats, the calories from fat intake that promote obesity and insulin resistance is typically in the 40–60% range, but these high fat diets in general also contain high levels of sugars (Wali et al., 2020).
Since 1970, daily calorie intake in the US has increased by 20% or by about 425 Kcal/day (Janssen, 2021) but the increase in total calorie intake is not the only qualitative difference of the “western diet.” With high calories comes also elevated sugar, starch, saturated fat and protein content. Collectively western diets result in elevated insulin, hyperglycemia, high IGF-1 and high cholesterol and triglyceride levels and on one hand activate pro-aging pathways and on the other hand promote insulin resistance and obesity, outcomes linked to a host of age-related diseases (Figure 2). Thus, the combination of these factors appears to contribute to disease and mortality both by accelerating the aging process and by promoting morbidities independently of aging.
Low carbohydrate and ketogenic diets
In humans, most low carbohydrate diets limit daily carbohydrate consumption to 50–60 grams, with the rest of the calories coming from high levels of fat and moderate to high levels of proteins. 100 years ago Dr. Wilder at Mayo Clinic described how the previously known benefits of fasting in children with epilepsy could be also achieved by a diet able to produce higher levels of ketone bodies and called it “ketogenic diet” (KD) (Wilder, 1921). This was later described as a diet providing 1 g of protein per Kg of body weight, less than 15 g of carbohydrates/day, and the rest of it coming from fat. In the 1970’s, the ketogenic diet was modified and made popular by Robert Atkins to achieve a higher compliance and weight loss in adults by allowing a much higher level of proteins but maintaining carbohydrate intake low to very low (Weber et al., 2020). However, this popularization of the KD allowed the adoption of a low carbohydrate “western diet” that allows more than 15 g a day of carbohydrates but also promotes the consumption of ingredients typical of western diets.
The ability of very high fat content diets to confer health benefits has prompted widespread interest in the KD. In mice, a KD applied in fully mature adult animals modestly increases lifespan, and improves indices of metabolic, physical, and cognitive function (Roberts et al., 2017). A cyclical application of the diet also affords metabolic and cognitive benefits (Newman et al., 2017). In mice KD improves cerebrovascular function (Ma et al., 2018), and in rats it improves cognitive scores together with changes in metabolite transport systems in the prefrontal cortex (Hernandez et al., 2018). Cognitive performance also improves in Alzheimer’s disease models treated with KD (Pawlosky et al., 2020), and treatment with ketone bodies, beta hydroxybutyrate, in Alzheimer’s disease mice fed a standard diet improves cognition and lowers plaque burden in a manner that was linked to hippocampal neuronal mitochondrial function (Wu et al., 2020). Within hours, the KD induces autophagy in liver, a step critical for the synthesis of ketone bodies. Mechanistically, the induction of the ketogenic program is linked to key lipid metabolism regulator PPARa and the autophagy dependent removal of an inhibitor complex (Saito et al., 2019). The links between lipid metabolism and autophagy is likely to contribute to the health benefits of the KD as both are linked to longevity regulation in shorter-lived species. Notably, in many studies the KD involves a low protein intake, so it is possible that some of the reported benefits of KD on longevity and disease may be linked at least in part to lower protein/amino acid intake. It will also be important to know how different animal and plant-derived fats affect the effect of KD on aging and disease.
Ketogenic and other low carbohydrates diets have also been studied extensively in humans. In obese humans, a recent meta-analysis suggested that ketogenic/low carbohydrate consumption was no more effective than a balanced diet including a low calorie, or low fat/high carb, or low protein/high carb diets, with equivalent effects on body mass index (BMI), circulating levels of total cholesterol, lipoprotein profiles, and triglycerides (López-Espinoza et al., 2021). Some large epidemiological studies have specifically focused on carbohydrate intake and mortality. One of these studies followed 85,168 women (aged 34 to 59 years at baseline) and 44,548 men (aged 40 to 75 years at baseline) without heart disease, cancer, or diabetes, for 26 years and 20 years, respectively. This study showed that a low-carbohydrate diet based on animal sources was associated with higher all-cause mortality in both men and women, whereas a low-carbohydrate diet with a higher content of plant-based food was associated with lower all-cause and cardiovascular disease mortality rates. Men on an animal products-based low carb diet also displayed a 66% increased risk of cancer mortality, whereas women on the same diet displayed a 26% increased risk of dying of cancer (Fung et al., 2010). In a meta-analysis of multiple cohorts involving 432,179 participants, both a low carbohydrate consumption (<40% of energy) and high carbohydrate consumption (>70% of energy) increased mortality risk compared to moderate carbohydrate intake. The risk of overall mortality increased by over 50% in the group consuming less than 20% of energy from carbohydrates compared to that for the group consuming 50–55% of energy from carbohydrates (Seidelmann et al., 2018). However, the low carbohydrate intake necessitates increases in protein and fat intake, rasising the possibility that the higher protein and/or fat intake may be more important for mortality than the low carbohydrate consumption. In addition to macronutrient balance the source of macronutrients was also found to be key. Mortality risk was about 18% higher when animal-derived proteins or fats replaced carbohydrates but 18% lower when plant-based proteins or fats replaced carbohydrates. These epidemiological studies considered groups that were consuming low carbohydrate levels but far from the very low levels (<50 grams) allowed in the strict ketogenic diets. Although it is well established that long term consumption of the very restrictive KD is not feasible for the great majority of the population, these studies are important to understand whether certain plant based diets providing moderately low carbohydrate levels could represent a more realistic option for the public. They also underline the importance of analyzing the relative macronutrient content instead of focusing on a specific one, but also point to very different effects of animal versus plant-based sources of fats and proteins on health, mortality and longevity. These results also clarify the importance of combining basic and human studies to begin to identify the age-specific nutrition that can extend healthspan.
Low protein and amino acids diets
A groundbreaking study in mice compared 25 different diets varying in fat, protein, and carbohydrate (Solon-Biet et al., 2014). Quantitation of survival and health outcomes indicated that the low protein and high carbohydrate diets were beneficial, although there was sex dimorphism in how diet interacts with mortality risk. In a follow-up study, low protein diets could recapitulate to some extent the beneficial effects of CR on cognition with evidence of nutrient signaling pathway activation and preservation of neuronal architecture related to connectivity in the hippocampus (Wahl et al., 2018). In contrast, a very low protein diet causes mice to eat less, due in part to altered signaling in the hypothalamus (Wu et al., 2021). These studies indicate that the composition of the diet influences feeding behaviors and may illicit distinct feeding signaling patterns in hypothalamic centers.
The relative importance of specific amino acids in the diet is an active area of investigation. Methionine restriction (MR) increases longevity in mice, and recent studies have identified the potential use of this restriction in combination with standard treatments for cancer (Gao et al., 2019). Notably, methionine levels are very low in legumes and other plant based protein sources compared to those in animals. A key signaling molecule in the mechanisms of both protein restriction (PR) and MR is the liver derived signaling peptide FGF21 (Hill et al., 2020). The remodeling of adipose tissue by MR requires activation of FGF21 receptors in the brain, indicating a cross-talk among tissues (Forney et al., 2020). In a high fat diet background, MR dampens inflammation locally in tissues and systemically, although the effect on inflammation is independent of FGF21 (Sharma et al., 2019). MR has beneficial effects on cognition even when applied at advanced age, and in this case the metabolic and structural changes induced in the hippocampus are FGF21 dependent (Ren et al., 2021). The increase in circulating levels of branched chain amino acids (BCAA) in models of metabolic dysfunction has prompted considerable research interest. Glucoregulatory improvement and the anti-inflammatory effects of PR are dependent on low levels of BCAA, and increasing the levels of dietary BCAA under standard diet feeding conditions is sufficient to drive over eating and increased adiposity (Solon-Biet et al., 2019). Lifelong restriction of BCAAs improves health and extends lifespan in males but not females in mice (Richardson et al., 2021). Molecular analysis of skeletal muscle from these animals shows the enrichment of pathways involved in peroxisomes, lipid metabolism, and growth signaling in males but not females.
The ability of protein/amino acid restriction to extend rodent longevity is linked to a reduction in the levels of IGF-1, in agreement with the role of pro-growth signaling in blunting longevity in organisms ranging from yeast to mice (Longo et al., 2021). In humans, CR results in beneficial changes in cardiometabolic risk factors but is not associated with reduced IGF-1 levels unless participants are also protein restricted (Fontana et al., 2008). In both mice and humans, a low protein diet imposes a reduction in growth factors/signaling both upstream of IGF-1 (GHRH, GH) and downstream of it (mTOR, S6K). With PR diets lower growth signaling goes hand in hand with lower insulin and improved insulin sensitivity, and although clinical studies more often focus on insulin it is clear that there is a connection between these pathways.
The role of protein intake in increasing mortality and reducing longevity appears to be also conserved in humans, although this relationship is complex. There is evidence that diet should be tailored to age. Whereas consumption of more than 20% of calories in the form of proteins is associated with a 75% increase in overall mortality and 400% increase in the risk of cancer mortality in subjects 65 years old or younger compared to consumption of less than 10% of calories from proteins, these associations are not observed in those 66 and older (Levine et al., 2014).These results are in agreement with those in mice in which prior to 85 weeks of age, mortality is minimized by a low protein consumption but as animals aged beyond 85 weeks, a major increase in the protein to carbohydrate ratio is necessary to minimize mortality (Senior et al., 2019).
In subjects younger than 65, IGF-1 levels correlate with level of protein intake but not in subjects 66 and older (Levine et al., 2014). These findings suggest that protein restriction in the elderly may no longer provide protection against overall and cancer mortality in part because it no longer inhibits pro-aging pathways. In light of these results, the low carbohydrate and high mortality correlation described earlier may also be re-interpreted by focusing on the protein content of the different diets. The group on the lowest carbohydrate diet consumed 37.2% energy from carbohydrate versus 60.5% in the highest intake group, but the lowest carb group also displayed a 22.3% of the energy from proteins versus 15% in the high carb group (Fung et al., 2010) raising the possibility that the increased all-cause, cardiovascular, and cancer mortality observed for the low carbohydrate group may be also due to the high protein intake. Instead, for the group consuming a vegetable-based low carbohydrate diet, which was associated with a reduced all-cause and cardiovascular mortality, the protein intake was similar to that of the high carbohydrate group (18.7% versus 17.5%)(Fung et al., 2010). These studies suggest that animal-derived proteins play an important role in age-related mortality and diseases, underline the importance of the balance of all macronutrients within the diet, and demonstrate that diet efficacy can be age range specific.
Low fat and high fat diets
For decades low fat diets have been adopted by the public but have been a recommended intervention on the part of the medical community to combat obesity. Although the consumption of fat has decreased in the US, obesity has continued to increase pointing to increased total calorie intake and to modern diet composition as culprits rather than simply the intake of fat. In fact, when 7447 participants at high risk for cardiovascular disease were randomized to a Mediterranean diet supplemented with extra-virgin olive oil, or mixed nuts, or to a control diet with the advice to reduce dietary fat, the risk of major cardiovascular events was about 30% lower in the Mediterranean diet groups supplemented with fats from olive oil or nuts compared to the group recommended a low fat diet (Estruch et al., 2018). These results are also in agreement with the epidemiological data discussed earlier and showing that a diet high in animal fat and animal protein increases mortality compared to a high carbohydrate diet, but that a low carbohydrate diet is beneficial when high in vegetable-based food sources (Fung et al., 2010). The consensus from these studies is that a relatively high carbohydrate diet is ideal but that the balance of macronutrients is important, and the source of nutrients can determine whether the diet is more or less healthy.
Vegan diets
Several studies indicate that pesco-vegetarians but not vegans display reduced risk for overall mortality compared to meat eaters, although a vegan dietary pattern is also associated with reduced risk of cancer, hypertension and diabetes compared to that for regular meat eaters (Segovia-Siapco and Sabaté, 2019). Notably, the vegan diet has been associated with a 43% increased risk in all fractures and 2.3 fold increase in hip fractures compared to non-vegan diets (Tong et al., 2020). This frailty may be explained in part by deficiencies of certain amino acids. In fact in the EPIC-Oxford study, 16.5% of vegan men and 8.1% of vegan women had a protein intake lower than their requirement, which could be made worse by the reliance on amino acids solely from legumes, which provide very low levels of methionine and other amino acids (Mariotti and Gardner, 2019). In summary, the data are consistent with remarkable benefits of a vegan diet against aging and diseases but also an association of vegan diets with less benefits compared to vegetarian or pesco-vegetarian diets, possibly because these diets prevent the frailty associated with vegan diets in the general population.
A multi-pillar approach for nutrition and healthspan
The evidence from the literature to date underscores the need for hypothesis-driven and multi-disciplinary assessment of nutrition and healthspan to identify the complex dietary patterns that promote healthy longevity. Alone, an “epidemiological” comparison of how a low versus a high consumption of an isolated macronutrient and its association with health and mortality may not only fail to identify protective or detrimental nutrition patterns but may lead to misleading interpretations. For example, many epidemiological studies have pointed to the increased mortality risk in subjects with low IGF-1 leading to the conclusion that IGF-1 should be maintained higher, but several studies have pointed to both the lowest and highest IGF-1 levels being associated with higher mortality pointing to mid-range IGF-1 as consistently linked to low mortality (Burgers et al., 2011). Thus, epidemiology, which is clearly a central pillar in determining the ideal ranges of a nutrient or factor for health and longevity, should be complemented by at least 3 additional pillars that account for age, sex, and underlying metabolic status and that assess risk factors in addition to biological age: 1) basic research focused on lifespan and healthspan, 2) carefully controlled clinical trials, 3) studies of individuals and populations with record longevity.
The Longevity Diet
Based on all of the studies discussed in this review and representing all the pillars of longevity listed above, we can begin to point to a common denominator for healthy longevity. These pillars indicate that the everyday normocaloric Longevity Diet associated with low or very low side effects and extended lifespan and healthspan is characterized by a mid to high carbohydrate and low but sufficient protein intake which is mostly plant-based but includes regular consumption of pesco-vegetarian-derived proteins (Longo, Valter, 2019). For example, animal products represented about 1 % of the traditional diet of the record longevity Okinawans (Willcox et al., 2007), and occasional meat or animal product consumption also characterized the populations of the Sardinian and Loma Linda areas with high prevalence of centenarians or high average lifespan (Levine et al., 2014). The benefits of such a diet is supported by evidence from the calorie and protein restriction studies in short-lived species, is in agreement with the epidemiological data described in earlier sections, and consistent with the evidence from large clinical trials. Thus, the low but sufficient protein diet or a normal protein intake with high legume consumption and therefore relatively low content of methionine and other amino acids contributes to the reduction in the levels/activity of the pro-aging GHR, IGF-1 and TOR-S6K signaling (Fig. 2). However, in over 65 individuals the low protein diet does not appear to reduce further the circulating IGF-1 already lowered during the aging process, and may instead contribute to lean body mass loss and frailty. In the absence of obesity and insulin resistance, the relatively high carbohydrate consumption may also contribute to avoiding frailty at all ages but particulary in the elderly, thus providing energy without increasing insulin and activating glucose signaling pathways.
A fat consumption providing about the 30% of energy mostly from plant-based sources is also part of the longevity diet and is again consistent with the basic research, and epidemiological and clinical data, although the traditional Okinawan diet provided a much lower level of fats, confirming that thre are variations of the optimal longevity diet that could be equally effective. The high circulating fat content, does not appear to have the pro-aging effects of the protein- and sugar-endocrine axes, possibly because fat catabolism, fatty acids and ketone bodies are at the center of fasting responses. A recent study based on meta-analyses and data form the Global Burden of Disease 2019 study including studies from the US, China and Europe, provides evidence in support of the Longevity Diet. A sustained change from the typical western diet to an optimal diet rich in legumes, whole grains and nuts with reduced red and processed meats is associated with an increase in life expectancy of 10.7 years in females and 13 years in males if started at age 20, and over 8 years of increased life expectancy when started at age 60 (Fadnes et al., 2022),
An important caveat is that the longevity diet should be designed to avoid malnourishment, particularly in the over 65 population to prevent frailty and diseases that may result from reduced bone or muscle mass or low blood cell counts. Ideally, the longevity diet would also include a 12–13 hours daily fasting period that has been shown to be safe, feasible, and effective in many studies. The periodic use of a periodic FMD in those age 18 to 70 may be key in reversing the insulin resistance generated by a high calorie diet. In fact, maintaining a Body Mass Index lower than 25 and an ideal sex- and age-specific fat composition, lean body mass and abdominal circumference and not a set calorie level should be used as guidelines to establish daily food intake. FMD cycles may also lower IGF-1, blood pressure, total cholesterol and inflammation, particularly in at risk subjects.
In summary, we propose that the Longevity Diet would be a valuable complement to standard healthcare, and that taken as a preventative measure it could aid in avoiding morbidity, sustaining health into advanced age.
Acknowledgements
This work was supported in part by awards to VDL including the Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG#17605 and IG#21820.), the BC161452 grant of the Breast Cancer Research Program (US Department of Defense) and the US National Institute on Aging-National Institutes of Health (NIA–NIH) grants P01 AG055369). RMA is supported by NIH-NIA RF1AG057408, R01AG067330, R01AG074503, Veterans Adminstration Merit Award BX003846, and by Impetus Grants and the Simons Foundation. This work was made possible by support from the William S. Middleton Memorial Veterans Hospial Madison Wisconsin. VDL has equity interest in L-Nutra, a company that commercializes medical food.
Glossary:
- Dietary restriction (DR)
A broad term describing the reduction in specific dietary components or in amounts of food provided
- Caloric restriction (CR)
Reduction in total calorie intake
- Protein restriction (PR)
Reduction in protein content of the diet
- Methionine restriction (MR)
Reduction in levels of the amino acid methionine in the diet
- Time-restricted feeding (TRF)
Reduction in the daily period of food intake (animal studies)
- Time-restricted eating (TRE)
Reduction in the daily period of food intake (clinical studies)
- Intermittant fasting (IF)
Short term daily or weekly fasting periods of 12 or more hours
- Periodic Fasting (PF)
prolonged fasting periods lasting 48 or more hours and normally occurring twice a month or less
- Fasting-mimicking diet (FMD)
A nutritional program containing ingredients at quantities that do not interfere with the fasting response
- Ketogenic diet (KD)
Diet very high in fat, and very low in carbohydrates
- Healthspan
the period of life during which health and functional capacity are maintained
- Longevity Diet (LD)
Diet composition or feeding regimen designed to enhance longevity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aon MA, Bernier M, Mitchell SJ, Di Germanio C, Mattison JA, Ehrlich MR, Colman RJ, Anderson RM, and de Cabo R (2020). Untangling Determinants of Enhanced Health and Lifespan through a Multi-omics Approach in Mice [DOI] [PMC free article] [PubMed]
- Asadi Shahmirzadi A, Edgar D, Liao C-Y, Hsu Y-M, Lucanic M, Asadi Shahmirzadi A, Wiley CD, Gan G, Kim DE, Kasler HG, et al. (2020). Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab 32, 447–456.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian P, Mattison JA, and Anderson RM (2017). Nutrition, metabolism, and targeting aging in nonhuman primates. Ageing Research Reviews [DOI] [PMC free article] [PubMed]
- Bartke A (2005). Insulin resistance and cognitive aging in long-lived and short-lived mice. J Gerontol A Biol Sci Med Sci 60, 133–134. [DOI] [PubMed] [Google Scholar]
- Bartke A, Sun LY, and Longo V (2013). Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev 93, 571–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Huffman KM, Pieper CF, Shalev I, and Kraus WE (2017). Change in the Rate of Biological Aging in Response to Caloric Restriction: CALERIE Biobank Analysis. J Gerontol A Biol Sci Med Sci 73, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Arseneault L, Baccarelli A, Corcoran DL, Gao X, Hannon E, Harrington HL, Rasmussen LJ, Houts R, et al. (2020). Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife 9, e54870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin BB, Canu E, Willette A, Kastman EK, McLaren DG, Kosmatka KJ, Xu G, Field AS, Colman RJ, Coe CL, et al. (2011). Effects of aging and calorie restriction on white matter in rhesus macaques. Neurobiol Aging 32, 2319 e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, et al. (2015). A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab 22, 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers AMG, Biermasz NR, Schoones JW, Pereira AM, Renehan AG, Zwahlen M, Egger M, and Dekkers OM (2011). Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab 96, 2912–2920. [DOI] [PubMed] [Google Scholar]
- Caffa I, Spagnolo V, Vernieri C, Valdemarin F, Becherini P, Wei M, Brandhorst S, Zucal C, Driehuis E, Ferrando L, et al. (2020). Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 583, 620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos JC, Wu Z, Rudich PD, Soo SK, Mistry M, Ferreira JC, Blackwell TK, and Van Raamsdonk JM (2021). Mild mitochondrial impairment enhances innate immunity and longevity through ATFS-1 and p38 signaling. EMBO Rep e52964. [DOI] [PMC free article] [PubMed]
- Campos SE, Avelar-Rivas JA, Garay E, Juárez-Reyes A, and DeLuna A (2018). Genomewide mechanisms of chronological longevity by dietary restriction in budding yeast. Aging Cell 17, e12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Quan JI, Tain LS, Kinghorn KJ, Li L, Grönke S, Hinze Y, Blackwell TK, Bjedov I, and Partridge L (2019). A triple drug combination targeting components of the nutrient-sensing network maximizes longevity. Proc Natl Acad Sci U S A 116, 20817–20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterson JH, Khericha M, Dyson MC, Vincent AJ, Callard R, Haveron SM, Rajasingam A, Ahmad M, and Partridge L (2018). Short-Term, Intermittent Fasting Induces Long-Lasting Gut Health and TOR-Independent Lifespan Extension. Curr Biol 28, 1714–1724.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, and Panda S (2014). Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Lin T, Le HD, Chang MW, and Panda S (2019). Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab 29, 303–319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Deota S, Bhardwaj R, Lin T, and Panda S (2021). Sex- and age-dependent outcomes of 9-hour time-restricted feeding of a Western high-fat high-sucrose diet in C57BL/6J mice. Cell Rep 36, 109543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che T, Yan C, Tian D, Zhang X, Liu X, and Wu Z (2021). Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr Metab (Lond) 18, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C-W, Villani V, Buono R, Wei M, Kumar S, Yilmaz OH, Cohen P, Sneddon JB, Perin L, and Longo VD (2017). Fasting-Mimicking Diet Promotes Ngn3-Driven β-Cell Regeneration to Reverse Diabetes. Cell 168, 775–788.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, and Anderson RM (2014). Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 5, 3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ruiz A, Rhinesmith T, Pomatto-Watson LCD, Price NL, Eshaghi F, Ehrlich MR, Moats JM, Carpenter M, Rudderow A, Brandhorst S, et al. (2021). Diet composition influences the metabolic benefits of short cycles of very low caloric intake. Nat Commun 12, 6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorling JL, Ravussin E, Redman LM, Bhapkar M, Huffman KM, Racette SB, Das SK, Apolzan JW, Kraus WE, Höchsmann C, et al. (2021). Effect of 2 years of calorie restriction on liver biomarkers: results from the CALERIE phase 2 randomized controlled trial. Eur J Nutr 60, 1633–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duregon E, Pomatto-Watson LCDD, Bernier M, Price NL, and de Cabo R (2021). Intermittent fasting: from calories to time restriction. Geroscience 43, 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns LC, Morton JF, Treuting PR, Emond MJ, Wolf NS, Dai D-F, McKnight GS, Rabinovitch PS, and Ladiges WC (2009). Disruption of protein kinase A in mice enhances healthy aging. PLoS One 4, e5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, et al. (2018). Retraction and Republication: Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N Engl J Med 2013;368:1279–90. N Engl J Med 378, 2441–2442. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, and Longo VD (2001). Regulation of longevity and stress resistance by Sch9 in yeast. Science 292, 288–90. [DOI] [PubMed] [Google Scholar]
- Fadnes LT, Økland J-M, Haaland ØA, and Johansson KA (2022). Estimating impact of food choices on life expectancy: A modeling study. PLoS Med 19, e1003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S-Z, Lin C-S, Wei Y-W, Yeh S-R, Tsai Y-H, Lee AC, Lin W-S, and Wang P-Y (2021). Dietary citrate supplementation enhances longevity, metabolic health, and memory performance through promoting ketogenesis. Aging Cell e13510. [DOI] [PMC free article] [PubMed]
- Fontana L, Weiss EP, Villareal DT, Klein S, and Holloszy JO (2008). Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, and Longo VD (2010). Extending healthy life span--from yeast to humans. Science 328, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney LA, Fang H, Sims LC, Stone KP, Vincik LY, Vick AM, Gibson AN, Burk DH, and Gettys TW (2020). Dietary Methionine Restriction Signals to the Brain Through Fibroblast Growth Factor 21 to Regulate Energy Balance and Remodeling of Adipose Tissue. Obesity (Silver Spring) 28, 1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, and Hu FB (2010). Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med 153, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, Richie JP, Ciccarella A, Calcagnotto A, Mikhael PG, et al. (2019). Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhu C, Li K, Cheng X, Du Y, Yang D, Fan X, Gaur U, and Yang M (2020). Comparative proteomics analysis of dietary restriction in Drosophila. PLoS One 15, e0240596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, and Brunet A (2009). Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell 8, 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot S, Lugtenberg RT, Cohen D, Welters MJP, Ehsan I, Vreeswijk MPG, Smit VTHBM, de Graaf H, Heijns JB, Portielje JEA, et al. (2020). Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat Commun 11, 3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng C-W, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, et al. (2011). Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3, 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz C, Doktor TK, Lanjuin A, Escoubas CC, Zhang Y, Weir HJ, Dutta S, Silva-Garcia CG, Bruun GH, Morantte I, et al. (2017). Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature 541, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Hernandez CM, Campos K, Truckenbrod L, Federico Q, Moon B, McQuail JA, Maurer AP, Bizon JL, and Burke SN (2018). A Ketogenic Diet Improves Cognition and Has Biochemical Effects in Prefrontal Cortex That Are Dissociable From Hippocampus. Front Aging Neurosci 10, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Qualls-Creekmore E, Berthoud H-R, Soto P, Yu S, McDougal DH, Münzberg H, and Morrison CD (2020). FGF21 and the Physiological Regulation of Macronutrient Preference. Endocrinology 161, bqaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, and Bartke A (2009). Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci 64, 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranon NN, Jochim BE, and Miller DL (2019). Fasting prevents hypoxia-induced defects of proteostasis in C. elegans. PLoS Genet 15, e1008242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen JAMJL (2021). Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int J Mol Sci 22, 7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wilson KA, Beck JN, Nelson CS, Brownridge GW, Harrison BR, Djukovic D, Raftery D, Brem RB, Yu S, et al. (2020). Genetic and metabolomic architecture of variation in diet restriction-mediated lifespan extension in Drosophila. PLoS Genet 16, e1008835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya A, Phua CZJ, Lee M, Wang L, Tyshkovskiy A, Ma S, Barre B, Liu W, Harrison BR, Zhao X, et al. (2021). Evolution of natural lifespan variation and molecular strategies of extended lifespan. Elife 10, e64860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz JW (2011). Calorie restriction and aging in nonhuman primates. ILAR J 52, 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu S, Loureiro R, Lee HJ, Wagle P, Krueger M, and Vilchez D (2021). Rewiring of the ubiquitinated proteome determines ageing in C. elegans. Nature 596, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WE, Bhapkar M, Huffman KM, Pieper CF, Krupa Das S, Redman LM, Villareal DT, Rochon J, Roberts SB, Ravussin E, et al. (2019). 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol 7, 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta C, Chang JT, Lee R, Tan EP, Yang Y, Loureiro R, Choy EH, Lim SHY, Saez I, Springhorn A, et al. (2019). The autophagy receptor p62/SQST-1 promotes proteostasis and longevity in C. elegans by inducing autophagy. Nat Commun 10, 5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, et al. (2014). Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Tano MD, Mattson MP, and Guidi N (2021). Intermittent and periodic fasting, longevity and disease. Nature Aging 1, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo Valter (2019). The Longevity Diet (Avery Hill)
- López-Espinoza MÁ, Chacón-Moscoso S, Sanduvete-Chaves S, Ortega-Maureira MJ, and Barrientos-Bravo T (2021). Effect of a Ketogenic Diet on the Nutritional Parameters of Obese Patients: A Systematic Review and Meta-Analysis. Nutrients 13, 2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Wang AC, Parikh I, Green SJ, Hoffman JD, Chlipala G, Murphy MP, Sokola BS, Bauer B, Hartz AMS, et al. (2018). Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci Rep 8, 6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti F, and Gardner CD (2019). Dietary Protein and Amino Acids in Vegetarian Diets-A Review. Nutrients 11, E2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens CR, Rossman MJ, Mazzo MR, Jankowski LR, Nagy EE, Denman BA, Richey JJ, Johnson SA, Ziemba BP, Wang Y, et al. (2020). Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience 42, 667–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. (2012). Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, and Anderson RM (2017). Caloric restriction improves health and survival of rhesus monkeys. Nature Communications 8, 14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken AW, Buckle E, and Simons MJP (2020). The relationship between longevity and diet is genotype dependent and sensitive to desiccation in Drosophila melanogaster. J Exp Biol 223, jeb230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ, et al. (2006). Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A 103, 19448–19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KN, Burhans MS, Clark JP, Howell PR, Polewski MA, DeMuth TM, Eliceiri KW, Lindstrom MJ, Ntambi JM, and Anderson RM (2017). Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell 16, 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirisola MG, Taormina G, Fabrizio P, Wei M, Hu J, and Longo VD (2014). Serine- and threonine/valine-dependent activation of PDK and Tor orthologs converge on Sch9 to promote aging. PLoS Genet 10, e1004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Mirzaei H, Guidi N, Vinciguerra M, Mouton A, Linardic M, Rappa F, Barone R, Navarrete G, Wei M, et al. (2021). Fasting-mimicking diet prevents high-fat diet effect on cardiometabolic risk and lifespan. Nat Metab 3, 1342–1356. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, Gonzalez-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, et al. (2016). Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metabolism 23, 1093–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Bernier M, Mattison JA, Aon MA, Kaiser TA, Anson RM, Ikeno Y, Anderson RM, Ingram DK, and de Cabo R (2019). Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metab 29, 221–228.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Tosti V, Redman LM, and Fontana L (2017). Calorie restriction in humans: An update. Ageing Res Rev 39, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Guevara-Aguirre J, Braskie MN, Hafzalla GW, Velasco R, Balasubramanian P, Wei M, Thompson PM, Mather M, Nelson MD, et al. (2017). Brain Structure and Function Associated with Younger Adults in Growth Hormone Receptor-Deficient Humans. J Neurosci 37, 1696–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, and Verdin E (2017). Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metabolism 26, 547–557.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco JM, Krawczyk PA, Scaria SM, Cangelosi AL, Chan SH, Kunchok T, Lewis CA, and Sabatini DM (2020). Dihydroxyacetone phosphate signals glucose availability to mTORC1. Nat Metab 2, 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma C, La Rocca C, Gigantino V, Aquino G, Piccaro G, Di Silvestre D, Brambilla F, Rossi R, Bonacina F, Lepore MT, et al. (2021). Caloric Restriction Promotes Immunometabolic Reprogramming Leading to Protection from Tuberculosis. Cell Metab 33, 300–318.e12. [DOI] [PubMed] [Google Scholar]
- Pawlosky RJ, Kashiwaya Y, King MT, and Veech RL (2020). A Dietary Ketone Ester Normalizes Abnormal Behavior in a Mouse Model of Alzheimer’s Disease. Int J Mol Sci 21, E1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomatto-Watson LCD, Bodogai M, Bosompra O, Kato J, Wong S, Carpenter M, Duregon E, Chowdhury D, Krishna P, Ng S, et al. (2021). Daily caloric restriction limits tumor growth more effectively than caloric cycling regardless of dietary composition. Nat Commun 12, 6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, et al. (2015). A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci 70, 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Wang L, Shi L, Jin X, Liu Y, Liu RH, Yin F, Cadenas E, Dai X, Liu Z, et al. (2021). Methionine restriction alleviates age-associated cognitive decline via fibroblast growth factor 21. Redox Biol 41, 101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads TW, Burhans MS, Chen VB, Hutchins PD, Rush MJP, Clark JP, Stark JL, McIlwain SJ, Eghbalnia HR, Pavelec DM, et al. (2018). Caloric Restriction Engages Hepatic RNA Processing Mechanisms in Rhesus Monkeys. Cell Metabolism 27, 677–688.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads TW, Clark JP, Gustafson GE, Miller KN, Conklin MW, DeMuth TM, Berres ME, Eliceiri KW, Vaughan LK, Lary CW, et al. (2020). Molecular and Functional Networks Linked to Sarcopenia Prevention by Caloric Restriction in Rhesus Monkeys. Cell Syst 10, 156–168.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A (2021). You Have Come A Long Way Baby: Five Decades of Research on the Biology of Aging From the Perspective of a Researcher Studying Aging. J Gerontol A Biol Sci Med Sci 76, 57–63. [DOI] [PubMed] [Google Scholar]
- Richardson NE, Konon EN, Schuster HS, Mitchell AT, Boyle C, Rodgers AC, Finke M, Haider LR, Yu D, Flores V, et al. (2021). Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat Aging 1, 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, et al. (2017). A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab 26, 539–546.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins JA, Shaffer D, Snow SS, Kapahi P, and Rogers AN (2019). Dietary restriction induces posttranscriptional regulation of longevity genes. Life Sci Alliance 2, e201800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romey-Glüsing R, Li Y, Hoffmann J, von Frieling J, Knop M, Pfefferkorn R, Bruchhaus I, Fink C, and Roeder T (2018). Nutritional regimens with periodically recurring phases of dietary restriction extend lifespan in Drosophila. FASEB J 32, 1993–2003. [DOI] [PubMed] [Google Scholar]
- Rong S, Snetselaar LG, Xu G, Sun Y, Liu B, Wallace RB, and Bao W (2019). Association of Skipping Breakfast With Cardiovascular and All-Cause Mortality. J Am Coll Cardiol 73, 2025–2032. [DOI] [PubMed] [Google Scholar]
- Saito T, Kuma A, Sugiura Y, Ichimura Y, Obata M, Kitamura H, Okuda S, Lee H-C, Ikeda K, Kanegae Y, et al. (2019). Autophagy regulates lipid metabolism through selective turnover of NCoR1. Nat Commun 10, 1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, and Olefsky JM (2017). Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 127, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savola E, Montgomery C, Waldron FM, Monteith KM, Vale P, and Walling C (2021). Testing evolutionary explanations for the lifespan benefit of dietary restriction in fruit flies (Drosophila melanogaster). Evolution 75, 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder JD, Falqueto H, Mânica A, Zanini D, de Oliveira T, de Sá CA, Cardoso AM, and Manfredi LH (2021). Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J Transl Med 19, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia-Siapco G, and Sabaté J (2019). Health and sustainability outcomes of vegetarian dietary patterns: a revisit of the EPIC-Oxford and the Adventist Health Study-2 cohorts. Eur J Clin Nutr 72, 60– 70. [DOI] [PubMed] [Google Scholar]
- Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, Folsom AR, Rimm EB, Willett WC, and Solomon SD (2018). Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 3, e419–e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. (2009). Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior AM, Solon-Biet SM, Cogger VC, Le Couteur DG, Nakagawa S, Raubenheimer D, and Simpson SJ (2019). Dietary macronutrient content, age-specific mortality and lifespan. Proc Biol Sci 286, 20190393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Dixon T, Jung S, Graff EC, Forney LA, Gettys TW, and Wanders D (2019). Dietary Methionine Restriction Reduces Inflammation Independent of FGF21 Action. Obesity (Silver Spring) 27, 1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Wang B, Giribaldi MG, Ayres J, Thomas JB, and Montminy M (2016). Neuronal energy-sensing pathway promotes energy balance by modulating disease tolerance. Proc Natl Acad Sci U S A 113, E3307–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Cogger VC, Pulpitel T, Wahl D, Clark X, Bagley E, Gregoriou GC, Senior AM, Wang Q-P, Brandon AE, et al. (2019). Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat Metab 1, 532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J, et al. (2020). Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab 31, 878–881. [DOI] [PubMed] [Google Scholar]
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, and Peterson CM (2018). Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 27, 1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong TYN, Appleby PN, Armstrong MEG, Fensom GK, Knuppel A, Papier K, Perez-Cornago A, Travis RC, and Key TJ (2020). Vegetarian and vegan diets and risks of total and site-specific fractures: results from the prospective EPIC-Oxford study. BMC Med 18, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulgherait M, Midoun AM, Park SJ, Gatto JA, Tener SJ, Siewert J, Klickstein N, Canman JC, Ja WW, and Shirasu-Hiza M (2021). Circadian autophagy drives iTRF-mediated longevity. Nature 598, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno M, Honjoh S, Matsuda M, Hoshikawa H, Kishimoto S, Yamamoto T, Ebisuya M, Yamamoto T, Matsumoto K, and Nishida E (2013). A fasting-responsive signaling pathway that extends life span in C. elegans. Cell Rep 3, 79–91. [DOI] [PubMed] [Google Scholar]
- Velingkaar N, Mezhnina V, Poe A, Makwana K, Tulsian R, and Kondratov RV (2020). Reduced caloric intake and periodic fasting independently contribute to metabolic effects of caloric restriction. Aging Cell 19, e13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva JE, Livelo C, Trujillo AS, Chandran S, Woodworth B, Andrade L, Le HD, Manor U, Panda S, and Melkani GC (2019). Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat Commun 10, 2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl D, Solon-Biet SM, Wang Q-P, Wali JA, Pulpitel T, Clark X, Raubenheimer D, Senior AM, Sinclair DA, Cooney GJ, et al. (2018). Comparing the Effects of Low-Protein and High-Carbohydrate Diets and Caloric Restriction on Brain Aging in Mice. Cell Rep 25, 2234–2243.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali JA, Jarzebska N, Raubenheimer D, Simpson SJ, Rodionov RN, and O’Sullivan JF (2020). Cardio-Metabolic Effects of High-Fat Diets and Their Underlying Mechanisms-A Narrative Review. Nutrients 12, E1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, and Kofler B (2020). Ketogenic diet in the treatment of cancer - Where do we stand? Mol Metab 33, 102–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, Groshen S, Mack WJ, Guen E, Di Biase S, et al. (2017). Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med 9, eaai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir HJ, Yao P, Huynh FK, Escoubas CC, Goncalves RL, Burkewitz K, Laboy R, Hirschey MD, and Mair WB (2017). Dietary Restriction and AMPK Increase Lifespan via Mitochondrial Network and Peroxisome Remodeling. Cell Metab 26, 884–896.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder RM (1921). The effect on ketonemia on the course of epilepsy. Mayo Clin Proc 2, 307. [Google Scholar]
- Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, et al. (2020). Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab 31, 92–104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb JD, and Suzuki M (2007). Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci 1114, 434–455. [DOI] [PubMed] [Google Scholar]
- Willette AA, Bendlin BB, Colman RJ, Kastman EK, Field AS, Alexander AL, Sridharan A, Allison DB, Anderson R, Voytko ML, et al. (2012). Calorie restriction reduces the influence of glucoregulatory dysfunction on regional brain volume in aged rhesus monkeys. Diabetes 61, 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KA, Beck JN, Nelson CS, Hilsabeck TA, Promislow D, Brem RB, and Kapahi P (2020). GWAS for Lifespan and Decline in Climbing Ability in Flies upon Dietary Restriction Reveal decima as a Mediator of Insulin-like Peptide Production. Curr Biol 30, 2749–2760.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Gong Y, Luan Y, Li Y, Liu J, Yue Z, Yuan B, Sun J, Xie C, Li L, et al. (2020). BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer’s disease. FASEB J 34, 1412–1429. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li B, Li L, Mitchell SE, Green CL, D’Agostino G, Wang G, Wang L, Li M, Li J, et al. (2021). Very-low-protein diets lead to reduced food intake and weight loss, linked to inhibition of hypothalamic mTOR signaling, in mice. Cell Metab 33, 888–904.e6. [DOI] [PubMed] [Google Scholar]
- Wu Z, Isik M, Moroz N, Steinbaugh MJ, Zhang P, and Blackwell TK (2019). Dietary Restriction Extends Lifespan through Metabolic Regulation of Innate Immunity. Cell Metab 29, 1192–1205.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Colman RJ, Kemnitz JW, Baum ST, Anderson RM, Weindruch R, and Schoeller DA (2013). Long-term calorie restriction decreases metabolic cost of movement and prevents decrease of physical activity during aging in rhesus monkeys. Exp Gerontol 48, 1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan WW, Chen GH, Wang F, Tong JJ, and Tao F (2015). Long-term acarbose administration alleviating the impairment of spatial learning and memory in the SAMP8 mice was associated with alleviated reduction of insulin system and acetylated H4K8. Brain Research 1603, 22–31. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ratliff EP, Molina B, El-Mecharrafie N, Mastroianni J, Kotzebue RW, Achal M, Mauntz RE, Gonzalez A, Barekat A, et al. (2018). Aging and Intermittent Fasting Impact on Transcriptional Regulation and Physiological Responses of Adult Drosophila Neuronal and Muscle Tissues. Int J Mol Sci 19, E1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Kreuzer J, Kumsta C, Wu L, Kamer KJ, Cedillo L, Zhang Y, Li S, Kacergis MC, Webster CM, et al. (2019). Mitochondrial Permeability Uncouples Elevated Autophagy and Lifespan Extension. Cell 177, 299–314.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]


