Abstract
Background:
Failure of humoral tolerance to red blood cell (RBC) antigens may lead to autoimmune hemolytic anemia (AIHA), a severe and sometimes fatal, disease. Previous studies have shown that although tolerance is robust in HOD mice, autoantibodies are generated upon adoptive transfer of OTII CD4+ T cells, which are specific for an epitope contained within the HOD antigen. These data imply that antigen presenting cells (APCs) are presenting RBC-derived autoantigen(s) and are capable of driving T cell activation. Given that multiple APCs participate in erythrophagocytosis, we utilized a transgenic approach to determine which cellular subsets were required for autoantigen presentation and subsequent autoreactive T cell activation.
Study Design and Methods:
HOD mice, which express an RBC-specific antigen consisting of hen egg lysozyme, ovalbumin, and human blood group molecule Duffy, were bred with IAbfl/fl and Cre-expressing transgenic animals to generate mice that lack I-Ab expression on particular cell subsets. OTII CD4+ T cell proliferation was assessed in vivo in HOD+I-Abfl/flxCre+ mice and in vitro upon co-culture with sorted APCs.
Results:
Analysis of HOD+I-Abfl/flxCre+ mice demonstrated that splenic conventional DCs, but not macrophages or monocytes, were required for autoantigen presentation to OTII CD4+ T cells. Subsequent in vitro co-culture experiments revealed that both CD8+ and CD8− dendritic cell (DC) subsets participate in erythrophagocytosis, present RBC-derived autoantigen, and stimulate autoreactive T cell proliferation.
Conclusion:
These data suggest that if erythrocyte T cell tolerance fails, DCs are capable of initiating autoimmune responses. As such, targeting DCs may be a fruitful strategy for AIHA therapies.
Keywords: Autoimmune Hemolytic Anemia, red blood cells, erythrocyte, dendritic cell, tolerance, autoimmunity
Introduction
Loss of humoral tolerance to red blood cell (RBC) antigens may lead to autoimmune hemolytic anemia (AIHA), a severe, and sometimes fatal, disease1. AIHA is characterized by shortened RBC lifespan due to detectable erythrocyte autoantibodies and patients present with pallor, fatigue, fever, hemoglobinuria, decreased hematocrit (due to hemolysis), and splenomegaly2. In most cases, AIHA treatment requires RBC transfusion support to increase hemoglobin levels. However, this approach is challenging as autoantibodies are usually specific for ubiquitous RBC antigens, thereby making compatible RBC units difficult to locate. Other treatment strategies (including corticosteroids or second-line option splenectomy) have high rates of initial response, but up to 50% of patients relapse3,4. Thus, AIHA is difficult to manage and treat successfully.
The underlying mechanisms behind the breakdown in erythrocyte autoantigens are unknown. Removal of senescent and/or damaged RBCs occurs by phagocytes in the spleen and liver5–7. And, upon erythrophagocytosis, RBC-derived antigens are presented on the surface of antigen presenting cells (APCs) in major histocompatibility complex I and II (MHC-I and MHC-II). As with many self-antigens, presentation in the absence of co-stimulation is a natural process which serves as a way to reinforce immune tolerance8. While infections or underlying disease pathology is correlated with secondary AIHA, thereby suggesting a role for inflammatory processes in the breakdown of tolerance, there are no clear correlates in primary idiopathic AIHA9. Thus, how and why autoimmunity ensues is unknown.
To elucidate which leukocytes play a role in RBC autoantigen presentation and identify which subsets can promote T cell proliferation, we utilized the HOD mouse model. HOD transgenic mice express a triple fusion protein consisting of hen egg lysozyme, a portion of ovalbumin, and human blood group molecule Duffy10. Previous studies with young HOD mice demonstrate that T cell tolerance to the HOD RBC autoantigen is profound; indeed, even immunization with HOD antigen in adjuvant does not result in autoimmunity, despite presence of autoreactive T cells11. However, circumventing T cell tolerance through adoptive transfer of OTII CD4+ T cells (which are HOD antigen-reactive) leads to autoantibody production, with specificity to the HOD antigen11. Thus, these findings show that presentation of RBC autoantigens is not tolerogenic, but instead leads to T cell activation and eventual autoantibody production. Through RBC tracking studies, multiple APCs have been identified as participating in erythrophagocytosis, making it difficult to parse out which cells are required for RBC autoantigen presentation and autoimmunity12. To address this complexity, we undertook a genetic approach to delete MHC-II from particular APCs; while the MHC locus of mice contains two functional MHC-II molecules, I-A and IE, C57BL/6 (B6) mice (and all murine strains with the b haplotype) only express I-A, as I-E is non-functional13,14. And, the HOD antigen contains the ovalbumin 323–339 epitope, which is presented in I-Ab of B6 mice and is recognized by OTII CD4+ T cells11,15. Taking advantage of this, we generated multiple murine lines whereby I-Ab (i.e. the only functional MHC-II molecule in B6 mice) expression was modulated on different APCs by breeding HOD mice (which are on a B6 background) with I-Ab floxed (referred to as I-Abfl/fl herein) and Cre-expressing transgenic mice (HOD+I-Abfl/flxCre+). With this approach, we targeted APCs previously shown to participate in RBC consumption and utilized Cre-transgenic mice with promoter-driven Cre recombinase, including: 1) Lyz2cre+ which is expressed by macrophages, monocytes and neutrophils16, 2) CD11ccre+ which is expressed by multiple dendritic cell (DC) subsets17, 3) CD19cre+ expressed by B cells18, 4) Albcre+ expressed in liver hepatocytes19, and 5) CMVcre+ for ubiquitous expression (negative control)20. Thus, upon Cre recombinase expression, I-Ab is predicted to be deleted in APC subsets that express that particular promoter. Analysis of HOD+I-Abfl/flxCre+ mice revealed that splenic CD8+ and CD8− DC subsets consume autologous RBCs, present RBC-derived autoantigens in I-Ab, and promote autoreactive T cell activation. These data suggest that if tolerization of erythrocyte-specific T cells fails, DCs can present autoantigen and initiate RBC autoimmunity.
Results
Splenic Dendritic Cells are Required for RBC-derived Autoantigen Presentation to T Cells
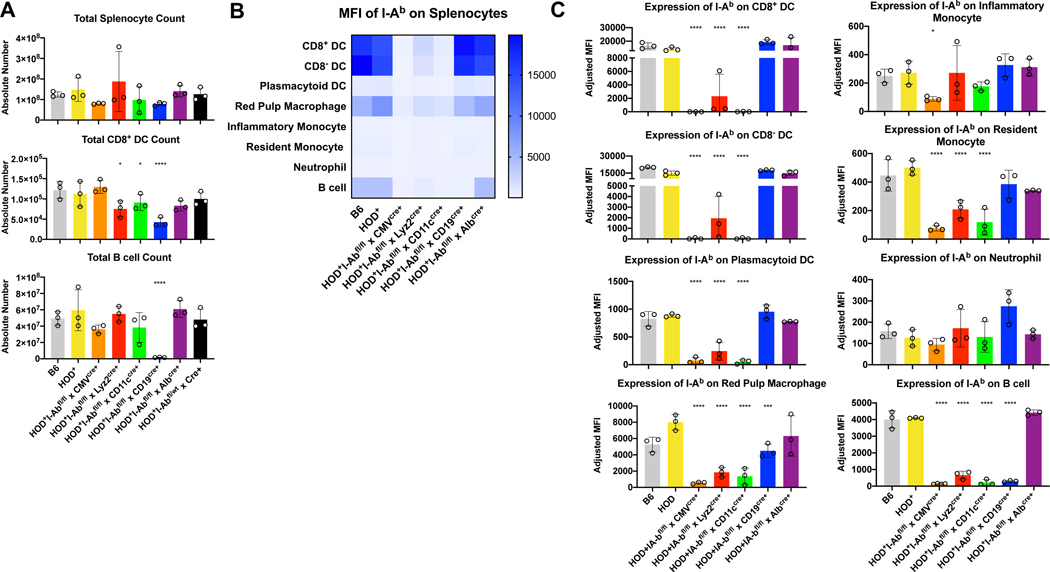
To thoroughly characterize different promoter-driven Cre recombinases affected expression of I-Ab across leukocyte subtypes, splenocytes from naïve HOD+I-Abfl/flxCre+ mice were harvested, enumerated, and stained with antibodies to delineate multiple antigen presenting cell (APC) subsets, as previously described12 (Gating strategy shown in Supplementary Figure 1A). No significant differences in total splenocyte count were detectable (Figure 1A, top). Likewise, no significant differences in the overall number of CD8− dendritic cells (DCs), plasmacytoid DCs (pDCs), red pulp macrophages (RPMs), inflammatory monocytes, resident monocytes, or neutrophils were observed (Supplementary Figure 1B). However, compared to HOD+ mice, significant reductions in CD8+ DCs were noted in HOD+I-Abfl/flxLyz2cre+, HOD+I-Abfl/flxCD11ccre+, and HOD+I-Abfl/flxCD19cre+ animals (Figure 1A, middle). Moreover, numbers of B cells were significantly reduced in HOD+I-Abfl/flxCD19cre+ mice (Figure 1A, bottom). Thus, while the overall number of many leukocyte subpopulations were not different across genotypes, significant alterations were observed within cell subsets capable of antigen presentation. Additional phenotypic analysis for I-Ab expression revealed no significant differences between splenocytes from HOD+ and B6 mice, demonstrating that expression of the HOD transgene does not affect levels of surface I-Ab (Figures 1B, 1C and representative histograms in Supplemental Figure 1C). In contrast, multiple differences were observed in mice that had active Cre recombinase. As predicted, expression of Cre downstream of the CMV promoter led to marked reductions of I-Ab across all subsets analyzed except neutrophils. Intriguingly, the I-Ab expression pattern was similar between splenocytes harvested from HOD+I-Abfl/flxLyz2cre+ and HOD+I-Abfl/flxCD11ccre+ mice; specifically, compared to control HOD+ splenocytes, significant reductions of I-Ab expression were observed in all DC subsets, RPMs, resident monocytes, and B cells. However, there were subtle differences between the two strains whereby CD8+ DCs, CD8− DCs and pDCs retained slightly higher levels of I-Ab expression in HOD+I-Abfl/flxLyz2cre+ mice, compared to HOD+I-Abfl/flxCD11ccre+ (Figure 1B and Supplemental Figure 1C). Indeed, the expression levels of I-Ab detectable on DCs from HOD+I-Abfl/flxCD11ccre+ were comparable to those from HOD+I-Abfl/flxCMVcre+ mice, which were hypothesized to represent background staining levels of the I-Ab antibody as it is predicted that CMV promoter is active in all cell types and would delete all copies of I-Ab. Within the HOD+I-Abfl/flxCD19cre+ mice, there was a significant reduction in I-Ab levels in both RPMs and B cells. Finally, analysis of HOD+I-Abfl/flxAlbcre+ mice showed no significant alterations of I-Ab expression in any spleen cell subset, when compared to control HOD+ mice.
Figure 1: Expression of Cre recombinase affects total numbers and I-Ab expression on multiple splenic subsets.
Splenocytes from naïve HOD+IAbfl/flxCre+ mice were harvested, digested, and stained with antibodies to delineate cell subsets. (A) Total splenocyte and individual cell subset counts were calculated. Each cell subset was evaluated for expression of I-Ab and data are presented in both (B) heat map and (C) individual bar graph form. For analysis, T cells and RBCs were excluded from total live leukocytes by gating out Thy1.2 and TER119 positive cells. The following phenotypes were used to delineate APC subsets: CD8+ DC: CD11chiCD8+CD11b−, CD8− DC: CD11chiCD11b+CD8−, pDC: CD11cintPDCA-1+, RPM: CD11c-/loCD11b-/loF4/80+, inflammatory monocyte: CD11b+CD11c-/loCD115+Ly6Gvar, resident monocyte: CD11b+CD11c-/loCD115−Ly6Gvar, neutrophil: CD11b+CD11c-/loLy6G+ high side scatter, B cell: CD19+. At least 3 independent experiments were performed with 3 mice per group and a representative experiment shown. Data were analyzed with a mixed model and Tukey’s post-test with multiple comparisons to HOD+ mice. Significant differences are indicated with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
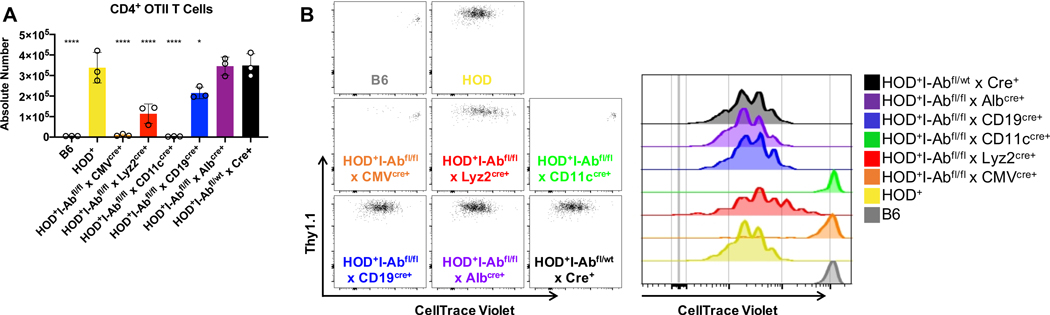
Young naïve HOD mice do not make detectable RBC-specific autoantibodies; however, adoptive transfer of OTII CD4+ T cells leads to robust anti-HOD autoantibody production11. As OTII T cells recognize an epitope within the HOD antigen presented in I-Ab, we utilized OTII T cells and HOD+I-Abfl/flxAlbcre+ to test the hypothesis that one (or several) of these APC subsets is required to present HOD RBC-derived autoantigens and promote T cell proliferation that may lead to autoantibody production. OTII CD4+ T cells, labeled with CellTrace Violet, were adoptively transferred into HOD+IAbfl/flxCre+ mice and OTII T cell numbers and proliferation were evaluated 3 days post adoptive transfer. Robust expansion and proliferation (indirectly determined by CellTrace Violet dilution) of OTII CD4+ T cells was observed in HOD+, HOD+I-Abfl/flxAlbcre+, and control HOD+I-Abfl/wtxCre+ mice (Figure 2A and 2B). Compared to HOD+ animals, significant reductions of OTII CD4+ T cell numbers were observed in HOD+I-Abfl/flxCMVcre+, HOD+I-Abfl/flxLyz2cre+, HOD+I-Abfl/flxCD11ccre+, HOD+I-Abfl/flxCD19cre+, and negative control B6 mice. Together, these data demonstrate that I-Ab expression is required for OTII CD4+ T cell proliferation, as observed in HOD+I-Abfl/flxCMVcre+ animals. Moreover, these data show that modulation of I-Ab expression affects T cell responses; for instance, decreased I-Ab expression on macrophages and B cells lead to reduced OTII T cell proliferation, compared to HOD+ controls. Further, despite similarities between I-Ab expression on APC subsets, we observed disparate T cell responses between HOD+I-Abfl/flxCD11ccre+ and HOD+I-Abfl/flxLyz2cre+ mice, as no OTII proliferation was observed in HOD+I-Abfl/flxCD11ccre+ animals. As HOD+I-Abfl/flxLyz2cre+ mice have higher expression of I-Ab on CD8+ and CD8− DCs subsets compared to DCs from HOD+I-Abfl/flxCD11ccre+ animals, these data suggest that either CD8+ DCs, CD8− DCs, or both DC subsets are required to present HOD RBC-derived autoantigens to CD4+ T cells.
Figure 2: I-Ab expression is required to promote RBC-specific T cell proliferation.
Recipient mice were given an adoptive transfer of 1×105 enriched and CellTrace Violet-labeled OTII CD4+ T cells. (A) Absolute number and (B) CellTrace Violet dilution of OTII CD4+ T cells were evaluated 3 days post adoptive transfer. OTII T cells were identified by surface expression of CD4+Va2+Vb5+Thy1.1+. Adoptive transfer experiments were performed at least 4 times with 3–5 mice per group, with a representative experiment shown. Data were analyzed with a mixed model and Tukey’s post-test with multiple comparisons to HOD+ mice. Significant differences are indicated with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
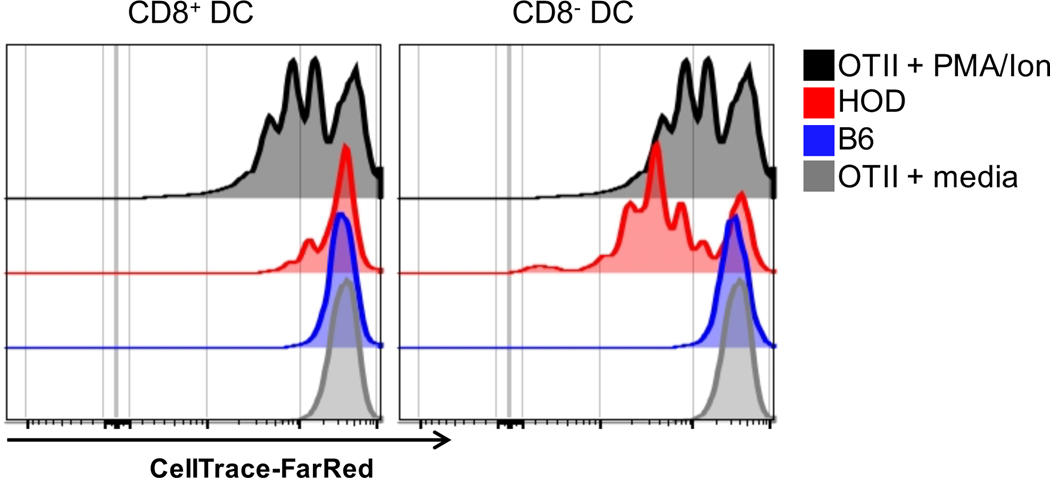
To formally test which DC subset could present HOD RBC-derived autoantigens, CD8+ and CD8− DCs were co-cultured with enriched OTII CD4+ T cells. Briefly, HOD and B6 mice were given a DiO-labeled syngeneic transfusion to identify DCs that had consumed autologous RBCs. CD8+ and CD8− DCs were sorted and co-cultured in vitro with CellTrace-FarRed labeled OTII CD4+ T cells. Diluted CellTrace-FarRed (an indirect measure of proliferation) was assessed 3 days post-stimulation. Robust proliferation was observed with both CD8+ and CD8− DC subsets sorted from HOD animals, but not with DCs derived from B6 animals (Figure 3). OTII T cells proliferated in response to PMA/Ionomycin (positive control) but not media alone (negative control). In aggregate, these data demonstrate that both conventional DC subsets participate in erythrophagocytosis, present RBC-derived autoantigen, and promote autoreactive T cell proliferation.
Figure 3: CD8+ and CD8− DCs present RBC-derived autoantigens and promote T cell proliferation.
Recipient HOD and B6 mice were transfused with 100uL of packed, leukoreduced, DiO-labeled syngeneic RBCs. Spleens were collected 18–24 hours post transfusion, processed into single cell suspensions, stained with antibodies to identify antigen presenting cells, and conventional DC subsets were sorted. Sorted DiO+ DC subsets were co-cultured at a 1:10 ratio with CD4 enriched, CellTrace-FarRed labeled OTII T cells (1 DC: 10 OTII T cells). CellTrace-FarRed dilution was assessed 3 days post-stimulation. PMA/Ion was utilized as a positive control whereas media alone served as a negative control. For sorting, T cells, B cells, and RBCs were excluded from total live leukocytes by gating out Thy1.2, CD19, and TER119 positive cells. The following phenotypes were utilized to determine DC subsets: CD8+ DC: CD11chiCD8+CD11b− and CD8− DC: CD11chiCD11b+CD8−. Experiments were performed 3 times and representative histograms are shown.
Adoptively Transferred RBC-specific Autoreactive CD4+ T cells are not Detectable in the Liver
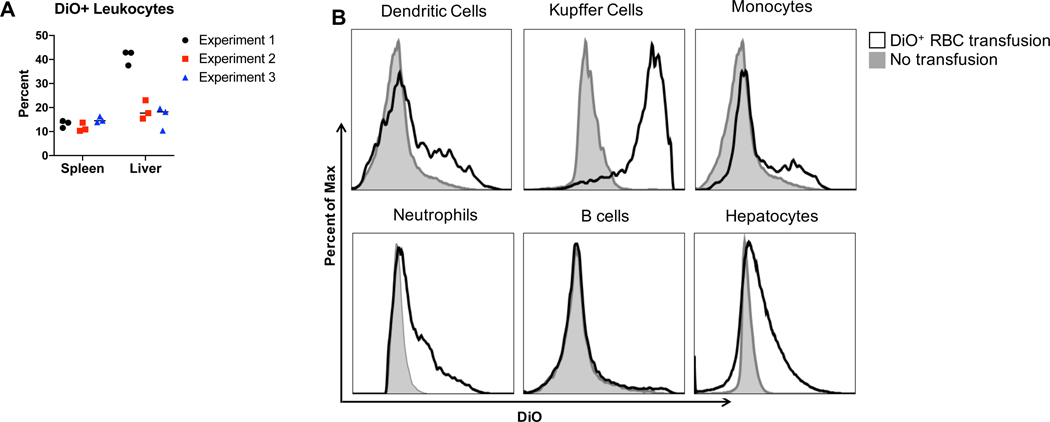
Erythrophagocytosis occurs not only in the spleen, but also in the liver. Kupffer cells play a large role in clearing damaged and/or stressed RBCs from circulation21 and RBC consumption by liver CD11c+ DCs and F4/80+ macrophages has been observed6. To build on those studies and evaluate erythrophagocytosis of additional leukocyte subsets, syngeneic, leukoreduced, DiO-labeled RBCs were transfused into recipient B6 mice. Spleens and livers were harvested and leukocytes were assessed for DiO+ signal, an indirect measure of RBC consumption. In all 3 experiments, RBCs were consumed by leukocytes in both the spleen and liver (Figure 4A). Consistent with previous reports, Kupffer cells had the highest frequency of DiO+ leukocytes, suggesting they play a major role in erythrophagocytosis (Figure 4B, gating strategy shown in Supplemental Figure 2). DCs, monocytes, neutrophils and hepatocytes also participated in RBC consumption. No significant RBC consumption was observed in B cells. Thus, these data show that syngeneic RBCs are consumed in the both the spleen and liver, and of the cells participating in erythrophagocytosis, Kupffer cells play a key role.
Figure 4: RBCs are phagocytosed by Kupffer cells in the liver.
Recipient B6 mice were transfused with 100uL of packed leukoreduced DiO-labeled, syngeneic B6 RBCs. Spleens and livers were collected from recipient mice 18–24 hours post transfusion, processed into single cells suspensions, and stained with antibodies to identify leukocytes. (A) The percentage of DiO+ cells from CD45+Thy1.2−TER119− leukocytes was determined. (B) DiO+ RBC consumption was determined for each liver leukocyte subset. Gray lines: untransfused B6 mice, black lines: DiO+ RBC transfused B6 mice. Experiments were performed 3 times with 3 mice per group. Data from all 3 experiments are shown in (A) whereas representative histograms are shown in (B).
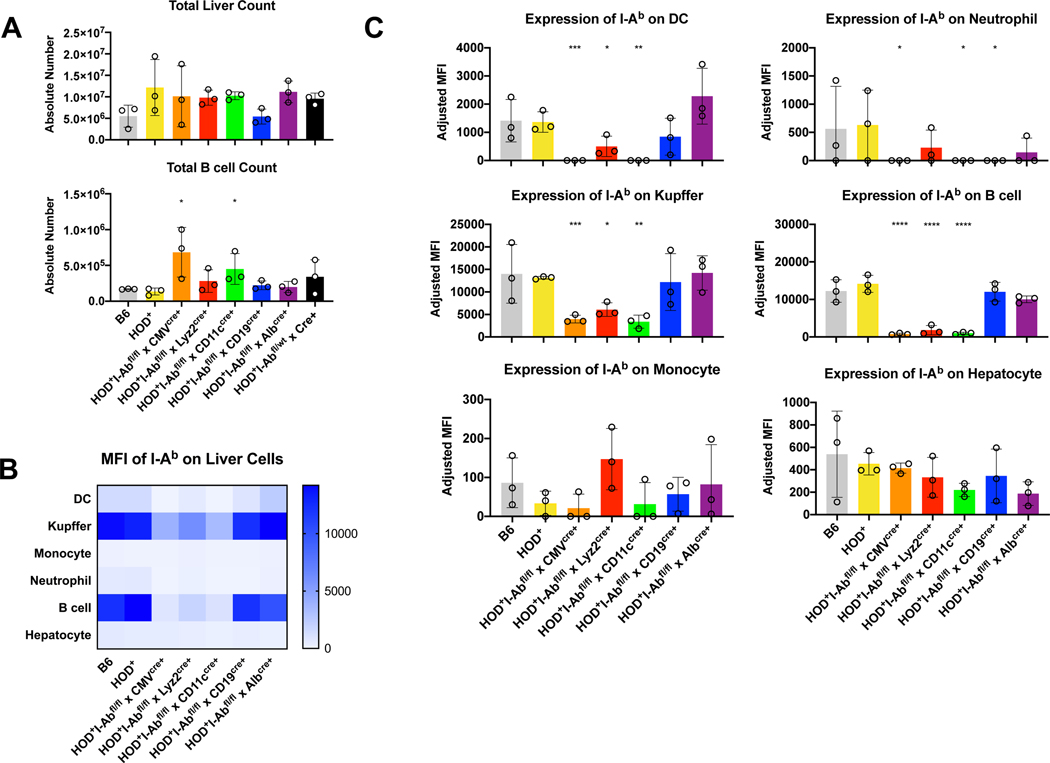
While multiple studies have shown that stored and/or stressed RBCs are preferentially removed from circulation in the liver, it is unclear whether the liver can initiate an immune response in the absence of underlying pathology or damaged erythrocytes. As such, to test whether autoreactive T cells could be primed by APC subsets in the liver, in the presence of a normal functional spleen, we also analyzed the OTII proliferation profile in the liver of control and HOD+I-Abfl/flxCre+ mice. Intriguingly, of the recipient mice given an adoptive transfer of OTII CD4+ T cells, no OTIIs were detectable in the liver after 3 days (data not shown). Additional analysis was performed to determine leukocyte numbers and I-Ab expression. No significant differences in overall liver counts were observed between control and HOD+I-Abfl/flxCre+ mice (Figure 5A, top). Similarly, no significant modulations in cell numbers were observed within populations of DCs, Kupffer macrophages, monocytes, neutrophils or hepatocytes (Supplemental Figure 3A). In contrast, there was a significant increase in the number of B cells in the livers from HOD+I-Abfl/flxCMVcre+ and HOD+I-Abfl/flxCD11ccre+ mice (Figure 5A, bottom). Expression of I-Ab was significantly reduced in DCs, Kupffer cells, neutrophils, and B cells from HOD+I-Abfl/flxCMVcre+ and HOD+I-Abfl/flxCD11ccre+ mice (Figures 5B, 5C, and representative histograms in Supplemental Figure 3B). Similarly, reduced expression of I-Ab was observed on DCs, Kupffer cells, and B cells from HOD+I-Abfl/flxLyz2cre+ animals. Intriguingly, I-Ab was significantly reduced in neutrophils, but not B cells, from HOD+I-Abfl/flxCD19cre+ mice. No significant alterations of I-Ab was observed in monocytes or hepatocytes. In aggregate, these data demonstrate that in this model, expression of Cre recombinase leads to alterations of I-Ab expression on multiple cellular subsets within the liver. However, these data show that adoptively transferred T cells do not appear in the liver at early time points. Taken together, these data suggest that the liver may not play a large role in the initiation of an immune response to RBCs when the spleen is present.
Figure 5: Expression of Cre recombinase affects total numbers and I-Ab expression on multiple liver subsets.
Livers from naïve HOD+IAbfl/flxCre+ mice were harvested, digested and stained with antibodies to delineate cell subsets. (A) Total liver and individual cell subset counts were calculated. Each cell subset was evaluated for expression of I-Ab and data are presented in both (B) heat map and (C) individual bar graph form. For analysis, T cells and RBCs were excluded from total live leukocytes by gating out Thy1.2 and TER119 positive cells. The following phenotypes were used to identify APC subsets: hepatocytes: ASGR-1+CD45var, neutrophils: CD45+Ly6G+ high side scatter, B cells: CD45+I-Ab+CD24+, T cells: CD45+I-Ab−CD24−, monocytes: CD45+Ly6G−I-Ab−FcyRI+CD11b+, Kupffer cells: CD45+Ly6G−I-Ab+FcyRI+, dendritic cells: CD45+CD11c+I-Ab+FcyRI−. At least 3 independent experiments were performed with 3 mice per group and a representative experiment shown. Data were analyzed with a mixed model and Tukey’s post-test with multiple comparisons. Significant differences, when compared to HOD+ mice, are indicated with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Discussion
Herein, we utilized a transgenic approach to elucidate which leukocyte populations are required for the generation of an RBC-specific autoreactive immune response. Using HOD mice, a well-described RBC model, previous studies have shown that adoptive transfer of OTII CD4+ T cells (which recognize an ovalbumin epitope within the HOD antigen) leads to RBC-specific autoantibodies, suggesting that erythrocyte-derived autoantigens are being actively presented to lymphocytes. To test which leukocytes were required for RBC-specific autoantigen presentation, OTII CD4+ T cells were adoptively transferred into newly-generated transgenic mice whereby I-Ab expression was selectively deleted on particular cellular subsets. With this model, we show that OTII CD4+ T cells do not proliferate in HOD+I-Abfl/flxCD11ccre+ animals, which lack I-Ab expression on splenic CD8+ and CD8− DCs, but retain expression on macrophages and monocytes. In contrast, significant reductions of I-Ab on macrophages and monocytes in HOD+I-Abfl/flxLyz2cre+ mice led to OTII proliferation, albeit at reduced numbers compared to controls. As such, these data demonstrate that I-Ab expression on DCs, but not macrophages, is required for autoreactive T cell activation. Additional studies show that while erythrophagocytosis is performed by phagocytes in both the spleen and liver, the spleen is essential for initiation of the immune response. In aggregate, these data provide evidence that splenic conventional DCs are capable of priming RBC-specific autoreactive T cells and suggest that these DCs may play a key role in the breakdown of tolerance to erythrocyte autoantigens.
Multiple studies have shown that macrophages (from the spleen and liver) consume the majority of RBCs in circulation5–7. However, despite significant erythrophagocytosis, RBC-containing macrophages do not stimulate an immune response; indeed, even in response to allogeneic RBCs, macrophages fail to form productive synapses with T cells and do not elicit activation nor proliferation15. As such, it is not surprising that macrophages do not play a significant role in priming OTII CD4+ T cells to HOD RBC-derived autoantigens. In addition to macrophages, many other subsets are involved in erythrophagocytosis (i.e. monocytes) and patterns of RBC consumption can be altered due to environmental factors (e.g. inflammatory stimuli) or the fitness of RBCs (i.e. damage due to storage)5,12. In unmanipulated mice, syngeneic RBCs are consumed by many cell subsets, including DC and monocyte subsets. However, data presented herein show that only I-Ab expression on conventional DC subsets is required for autoreactive T cell activation and proliferation. And, there is a correlation between lower frequencies of I-Ab-expressing DCs and reduced OTII T cell proliferation, as observed in HOD+I-Abfl/flxLyz2cre+ mice. One limitation of utilizing the in vivo transgenic murine approach is the inability to discern whether CD8+ or CD8− DCs were required as CD11ccre+ affects both populations, including pDCs. As an attempt to narrow the scope, we also bred HOD+I-Abfl/flxZbtbcre+ mice22; the Zbtb promoter has expression in both CD8+ and CD8− DCs, but not pDCs. However, upon phenotypic analysis, HOD+I-Abfl/flxZbtbcre+ mice exhibited widespread deletion of I-Ab and were undiscernible from HOD+I-Abfl/flxCMVcre+ animals (data not shown). As such, they were not included in this manuscript. To circumvent this limitation and test which DC subset was required for T cell proliferation, sorted CD8+ and CD8− DCs from HOD+ mice were co-cultured with OTII CD4+ T cells. Both DC subsets prompted T cell proliferation, although more divisions were evident with CD8− DCs. These data are consistent with prior findings as CD8− DCs have been shown to stimulate CD4+ T cells in multiple settings, including stored RBCs23.
The use of a transgenic mice to evaluate which APC subsets are required for antigen presentation has advantages over in vitro analysis. Results obtained by co-culture of APCs with T cells ex vivo should be verified in vivo (if possible) as it is plausible that those particular cells would be segregated within the splenic architecture and never come into contact. Moreover, processing cells (e.g. purification or sorting) for in-vitro co-culture experiments may inadvertently cause activation. The use of an in vivo mouse model circumvents these limitations; however, there are challenges with this transgenic approach. First, the utilization of the Cre-expressing transgenic mice did not lead to expected patterns of I-Ab expression, an observation that has been reported previously24. Indeed, in the HOD+I-Abfl/flxCD11ccre+ animals, multiple cell subsets beyond DCs were affected. Similarly, in HOD+I-Abfl/flxLyz2cre+ animals, I-Ab expression on DCs was affected, in addition to the predicted macrophages and monocytes. One unexpected complication was that all Cre-expressing mouse lines, except HOD+I-Abfl/flxCD19cre+, led to significant reductions of I-Ab on B cells, which limited the ability to test whether OTII T cell proliferation in this setting led directly to autoantibody production. With that observation, OTII T cells numbers used for adoptive transfer were titrated down to 1×105, a number that was reliably detectable in total splenocytes from naïve B6 mice 3 days after transfer. Utilization of a lower precursor frequency of autoreactive OTII T cells allowed for the observation of fine differences between HOD+I-Abfl/flxCD11ccre+ and HOD+I-Abfl/flxLyz2cre+ animals that might not have been possible otherwise. Taken together, however, despite the overlapping patterns of I-Ab expression on APC subsets in HOD+I-Abfl/flxCre+ mice and in combination with the in vitro co-culture experiments, we were still able to determine that absence of I-Ab on DCs prevented autoreactive immune responses.
The absence of detectable OTII CD4+ T cells in the liver was unexpected. A prior study by Hendrickson et al.25 showed that adoptively transferred T cells could be detected in the liver, and upon transfusion, the T cells proliferated; however, these experiments were completed in an allogeneic transfusion setting and utilized higher numbers of adoptively transferred T cells. Thus, one explanation for the absence of OTII T cells detectable in livers from HOD+I-Abfl/flxCre+ mice is that 1×105 adoptively transferred T cells are too few for dissemination throughout all of the lymphoid and non-lymphoid compartments. Indeed, studies that include liver analysis routinely utilize at least 1×106 T cells for adoptive transfer26. Another hypothesis is that, upon adoptive transfer, the OTII T cells remained in the spleen upon recognition of their cognate antigen presented in I-Ab on APCs. However, this seems unlikely as no OTII T cells were observed in livers from B6 mice, which do not contain the HOD antigen or ovalbumin. Additional studies in splenectomized mice will provide additional insight into the capacity of the liver to promote RBC-specific autoimmunity.
In summary, we report that I-Ab expression on splenic conventional DCs is required for autoreactive CD4+ T cell proliferation in response to RBC-derived antigens. Intriguingly, these data suggest that DCs are constantly consuming autologous RBCs and presenting autoantigens in I-Ab. Further, these DCs are receptive to CD4+ T cell help and prompt T cell activation, even in the absence of danger signals (e.g. inflammation). As such, in the event that T cell tolerance fails (i.e. due to infection, immunodeficiency, age, etc.), autoimmune responses can be initiated. For instance, in observational studies, RBC autoantibodies are detectable in 0.1% of asymptomatic blood donors; however, the prevalence is higher in hospitalized patients (i.e. up to 8%) and in individuals with defects in T cell tolerance mechanisms, these rates can be as high as 30%27–31. In aggregate, these data demonstrate that in the event that RBC-specific T cell tolerance fails, DCs can promote autoreactive immune responses. And, as such, DCs may be a potential target for future therapies for AIHA patients.
Materials and Methods
Mice.
C57BL/6 (B6) mice were purchased from Charles River Laboratories (Wilmington, MA). CD11cCre+ (B6.Cg-Tg(ItgaxCre+)1–1Reiz/J; stock #008068), Lyz2Cre+ (B6.129P2-Lyz2tm1(cre)Ifo/J; stock #004781), zDCCre+ (B6.Cg-Zbtb46tm3.1(cre)Mnz/J, stock #028538), CMVCre+ (B6.C-Tg(CMVCre+)1Cgn/J; stock #006054), AlbCre+ (B6.Cg-Speer6-ps1Tg(Alb-cre)21Mgn/J, stock #003574), CD19Cre+ (B6.129P2(C)-Cd19tm1(cre)Cgn/J, stock #006785), IA-bfl/fl (B6.129X1-H2-Ab1tm1Koni/J, stock #013181), OTII (B6.Cg-Tg(TcraTcrb)425CBn/J; stock #004194), and B6-Thy1.1 (B6.PL-Thy1a/CyJ; stock #000406) mice were purchased from the Jackson Laboratory. HOD mice, expressing a triple fusion protein consisting of hen egg lysozyme, ovalbumin and human Duffy, were developed previously and bred in the Bloodworks Northwest and Columbia University vivarium facility. All transgenic mice used were genotyped with PCR and phenotyped with flow cytometry. All mice were maintained in a pathogen-free environment on standard rodent chow and water in a light and temperature-controlled environment. Unless otherwise stated, mice were 6–24 weeks old and both male and female mice were used. All protocols and experiments were carried out in accordance with relevant guidelines and regulations as approved by both Bloodworks Northwest and Columbia University Institutional Animal Care and Use Committees (IACUC).
CD4+ T cell enrichment, labeling, and adoptive transfer.
Total splenocytes were harvested from OTIIxB6.Thy1.1 mice and CD4+ T cells were purified by negative selection (ThermoFisher). Purity was assessed by staining with antibodies against CD3, CD19 and CD4 (ThermoFisher). CD4+ T cells were labeled with 5μM CellTrace Violet (ThermoFisher) and resuspended in PBS; labeling efficiency was evaluated on a flow cytometer. A total of 1×105 OTII CD4+ CellTrace Violet-labeled T cells were adoptively transferred into recipient mice via tail vein injection. Recipient mice were euthanized 3 days post OTII T cell adoptive transfer. For in vitro co-culture experiments with sorted DCs, enriched OTII CD4+ T cells were labeled with 1μM of CellTrace-FarRed (ThermoFisher).
DiO labeling of RBCs and transfusion.
RBCs from donor B6 and HOD mice were obtained through cardiac puncture and collected in CPDA-1. RBCs were leukoreduced (Acrodisc WBC filter, Pall Life Sciences), washed with sterile PBS and labeled with a lipophilic dye, DiO, as previously described12. Labeling was confirmed by flow cytometry. Recipient mice were given an i.v. transfusion of 100uL of packed DiO-labeled RBCs at 20% hematocrit in PBS.
Tissue processing.
Recipient mice were euthanized with isoflurane 3 days post OTII adoptive transfer. After euthanasia, mice were perfused through the left ventricle with 1mg/mL collagenase A in PBS, and the liver and spleen were collected. Liver. The liver was cut into small pieces and incubated with 5mL of digestion solution (Hanks Balanced Salt Solution supplemented with 1.5mg/mL collagenase A, 0.4mg/mL DNase I, 5% FBS and 10mM HEPES) in a 37C shaking incubator (250rpm) for 45 minutes, with intermittent vortexing every 8–10 minutes as described32. Upon completion of digestion, 10mL of PBS was added and tissue was vortexed at maximum speed for 30 seconds. The cell suspension was then strained through a 70um cell strainer, pelleted by centrifugation at 1,200 rpm for 10 minutes, RBC lysed, and resuspended in FACS buffer (PBS supplemented with 0.4mL/L 0.5M EDTA and 0.2mg/mL BSA). Spleen. The spleen was prepared as previously described12. Briefly, spleens were collagenase digested, cut into small pieces, and then incubated at 37C for 20 minutes. Afterwards, the spleen was smashed through a 70um cell strainer, washed with complete RPMI, RBC lysed, and resuspended in FACS buffer. Single cell suspensions of leukocytes from the liver and spleen were than stained with antibodies to delineate individual cell subsets.
In vitro co-culture of DCs and OTII T cells.
HOD and B6 mice were transfused with syngeneic leukoreduced DiO-labeled RBCs. Splenocytes were harvested and processed 18–24hr post transfusion, stained with antibodies to delineate antigen presenting cells, and CD8+ and CD8− DCs were sorted. Sorted DiO+ DC subsets were co-cultured at a 10:1 ratio with enriched OTII CD4+ T cells, as described previously15. OTII CD4+ T cells cultured in media alone served as a negative control whereas stimulation with PMA (10ng/mL) plus ionomycin (1ug/mL) was a positive control for proliferation. Co-cultured cells were harvested 3 days post-stimulation and CellTrace-FarRed dilution was assessed in CD3+CD4+Thy1.2+Va2+Vb5+ OTII T cells.
Antibody staining and analysis.
Directly conjugated antibodies against IAb, Ly6G, F4/80, Thy1.2, Thy1.1, TER119, CD45, CD4, CD3, Vb5.1/5.2, PDCA1, CD115, CD11c, and CD11b were purchased from ThermoFisher Scientific. Purified ASGR1 and secondary rabbit anti-goat IgG Alexa-Fluor 647 were also purchased from ThermoFisher Scientific. Antibodies against FcyRI, CD24, CD11b, CD19, Va2, and CD8a were purchased from BioLegend. Splenocytes were treated with Fc Block (anti-mouse CD16/32; BD Biosciences) prior to surface staining. Leukocytes from the liver and spleen were incubated with cell surface antibodies for 20 minutes at 4C, washed with FACS buffer, and then fixed with 4% paraformaldehyde. Cells were collected with an LSRII (BD Biosciences) and data analysis was performed with FlowJo software (Treestar); cell sorting was performed with a BD Influx.
Statistics
All data from each individual experiment was compiled and SAS studio was used to perform a mixed model analysis with a Tukey-Kramer correction; experimental groups were compared against positive control HOD animals. A p-value of ≤ 0.05 was considered significant and *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Supplementary Material
Figure 1: Total cell numbers and I-Ab expression in spleens from HOD+IAbfl/flxCre+ mice.
Splenocytes from naïve HOD+IAbfl/flxCre+ mice were harvested, digested, and stained with antibodies to delineate cell subset. (A) Gating strategy used to identify specific cell subsets. For analysis, T cells and RBCs were excluded from total live leukocytes by gating out Thy1.2 and TER119 positive cells. The following phenotypes were used to delineate APC subsets: CD8+ DCs: CD11chiCD8+CD11b−, CD8− DCs: CD11chiCD11b+CD8−, pDC: CD11cintPDCA-1+, RPMs: CD11c-/loCD11b-/loF4/80+, inflammatory monocytes: CD11b+CD11c-/loCD115+Ly6Gvar, resident monocytes: CD11b+CD11c-/loCD115−Ly6Gvar, neutrophils: CD11b+CD11c-/loLy6G+ high side scatter, B cells: CD19+. (B) Total counts for individual APC subsets and (C) representative histograms for I-Ab expression on individual cell subsets are shown. At least 3 independent experiments were performed with 3 mice per group, with a representative experiment shown. Data were analyzed with a mixed model and Tukey’s post-test with multiple comparisons. Significant differences, when compared to HOD+ mice, are indicated with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Figure 2: Gating strategy for liver leukocytes.
Single cell suspensions were generated from livers from experimental mice and stained with antibodies to delineate leukocyte subsets. For analysis, TER119+ RBCs were excluded from total live single cells. The following phenotypes were used to delineate leukocyte subsets: hepatocytes: ASGR-1+CD45var, neutrophils: CD45+Ly6G+ high side scatter, B cells: CD45+I-Ab+CD24+, T cells: CD45+I-Ab−CD24−, monocytes: CD45+Ly6G−I-Ab−FcyRI+CD11b+, Kupffer cells: CD45+Ly6G−I-Ab+FcyRI+, dendritic cells: CD45+CD11c+I-Ab+FcyRI−.
Figure 3: Total cell numbers and I-Ab expression on liver cell subsets in HOD+IAbfl/flxCre+ mice.
Livers from naïve HOD+IAbfl/flxCre+ mice were harvested, digested and stained with antibodies to delineate cell subsets. For analysis, T cells and RBCs were excluded from total live leukocytes by gating out Thy1.2 and TER119 positive cells. The following phenotypes were used to identify APC subsets: hepatocytes: ASGR-1+CD45var, neutrophils: CD45+Ly6G+ high side scatter, B cells: CD45+I-Ab+CD24+, T cells: CD45+I-Ab−CD24−, monocytes: CD45+Ly6G−I-Ab−FcyRI+CD11b+, Kupffer cells: CD45+Ly6G−I-Ab+FcyRI+, dendritic cells: CD45+CD11c+I-Ab+FcyRI−. (A) Total counts for individual APC subsets and (B) representative histograms of I-Ab expression on individual cell subsets are shown. At least 3 independent experiments were performed with 3 mice per group and a representative experiment shown. Data were analyzed with a mixed model and Tukey’s post-test with multiple comparisons. Significant differences, when compared to HOD+ mice, are indicated with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Acknowledgements
The authors thank Dr. Eldad Hod for guidance on statistical methods, Dr. James C. Zimring for valuable discussions, Dr. Xiaoping Wu in the Bloodworks NW flow core, and the vivarium staff at Bloodworks NW Research Institute and Columbia University Irving Medical Center.
Funding:
These studies were supported by grants to KEH from the National Institutes of Health (NHLBI R01HL133325) and the National Blood Foundation. Cell sorting experiments were performed in the CCTI Flow Cytometry Core, which is supported in part by the National Institutes of Health (S10OD020056).
Footnotes
Additional Information
COI: The authors have no relevant conflicts of interest.
References
- 1.Shulman IA et al. Autoimmune Hemolytic Anemia With Both Cold and Warm Autoantibodies. JAMA 253, 1746–1748, doi: 10.1001/jama.1985.03350360072021 (1985). [DOI] [PubMed] [Google Scholar]
- 2.Brodsky RA Warm Autoimmune Hemolytic Anemia. New England Journal of Medicine 381, 647–654, doi: 10.1056/NEJMcp1900554 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Go RS, Winters JL & Kay NE How I treat autoimmune hemolytic anemia. Blood 129, 2971, doi: 10.1182/blood-2016-11-693689 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Lechner K. & Jäger U. How I treat autoimmune hemolytic anemias in adults. Blood 116, 1831, doi: 10.1182/blood-2010-03-259325 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD & Zimring JC Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood 110, 2736, doi: 10.1182/blood-2007-03-083105 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Theurl I. et al. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nature Medicine 22, 945, doi: 10.1038/nm.4146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Back DZ, Kostova EB, van Kraaij M, van den Berg TK & van Bruggen R. Of macrophages and red blood cells; a complex love story. Front Physiol 5, 9–9, doi: 10.3389/fphys.2014.00009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugliese A. Central and peripheral autoantigen presentation in immune tolerance. Immunology 111, 138–146, doi: 10.1111/j.0019-2805.2003.01804.x (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packman CH The Clinical Pictures of Autoimmune Hemolytic Anemia. Transfus Med Hemother 42, 317–324, doi: 10.1159/000440656 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desmarets M, Cadwell CM, Peterson KR, Neades R. & Zimring JC Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood 114, 2315, doi: 10.1182/blood-2009-04-214387 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudson KE, Hendrickson JE, Cadwell CM, Iwakoshi NN & Zimring JC Partial tolerance of autoreactive B and T cells to erythrocyte-specific self-antigens in mice. Haematologica 97, 1836–1844, doi: 10.3324/haematol.2012.065144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards AL, Hendrickson JE, Zimring JC & Hudson KE Erythrophagocytosis by plasmacytoid dendritic cells and monocytes is enhanced during inflammation. Transfusion 56, 905–916, doi: 10.1111/trf.13497 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Stuart PM Major Histocompatibility Complex (MHC): Mouse. eLS, 1–7, doi: 10.1002/9780470015902.a0000921.pub4 (2015). [DOI] [Google Scholar]
- 14.Mathis DJ, Benoist C, Williams VE, Kanter M. & McDevitt HO Several mechanisms can account for defective E alpha gene expression in different mouse haplotypes. Proceedings of the National Academy of Sciences 80, 273, doi: 10.1073/pnas.80.1.273 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards AL, Sheldon K, Wu X, Gruber DR & Hudson KE The Role of the Immunological Synapse in Differential Effects of APC Subsets in Alloimmunization to Fresh, Non-stored RBCs. Front Immunol 9, 2200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clausen B, Kurkhardt C, Reith W, Renkawitz R. & Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Research 8, 265–277 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Caton ML, Smith-Raska MR & Reizis B. Notch–RBP-J signaling controls the homeostasis of CD8<sup>−</sup> dendritic cells in the spleen. The Journal of Experimental Medicine 204, 1653, doi: 10.1084/jem.20062648 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rickert RC, Roes J. & Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res 25, 1317–1318, doi: 10.1093/nar/25.6.1317 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postic C. et al. Dual Roles for Glucokinase in Glucose Homeostasis as Determined by Liver and Pancreatic β Cell-specific Gene Knock-outs Using Cre Recombinase. Journal of Biological Chemistry 274, 305–315 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Schwenk F, Baron U. & Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23, 5080–5081, doi: 10.1093/nar/23.24.5080 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klei TRL, Meinderts SM, van den Berg TK & van Bruggen R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front Immunol 8, 73–73, doi: 10.3389/fimmu.2017.00073 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loschko J. et al. Absence of MHC class II on cDCs results in microbial-dependent intestinal inflammation. The Journal of Experimental Medicine 213, 517, doi: 10.1084/jem.20160062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabro S. et al. Bridging channel dendritic cells induce immunity to transfused red blood cells. The Journal of Experimental Medicine 213, 887, doi: 10.1084/jem.20151720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abram CL, Roberge GL, Hu Y. & Lowell CA Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods 408, 89–100, doi: 10.1016/j.jim.2014.05.009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendrickson JE et al. The spleen plays a central role in primary humoral alloimmunization to transfused mHEL red blood cells. Transfusion 49, 1678–1684, doi: 10.1111/j.1537-2995.2009.02200.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochel A. et al. Effective intrahepatic CD8+ T-cell immune responses are induced by low but not high numbers of antigen-expressing hepatocytes. Cellular And Molecular Immunology 13, 805, doi: 10.1038/cmi.2015.80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garratty G. The significance of IgG on the red cell surface. Transfusion Med (1987). [DOI] [PubMed] [Google Scholar]
- 28.Zantek ND, Koepsell SA, Tharp DR Jr & Cohn CS The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol 87, 707–709, doi: 10.1002/ajh.23218 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Rao VK & Oliveira JB How I treat autoimmune lymphoproliferative syndrome. Blood 118, 5741–5751, doi: 10.1182/blood-2011-07-325217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalfa TA Warm antibody autoimmune hemolytic anemia. Hematology Am Soc Hematol Edu Program 2016, 690–697 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westerberg LS, Klein C. & Snapper SB Breakdown of T cell tolerance and autoimmunity in primary immunodeficiency--lessons learned from monogenic disorders in mice and men. Curr Opin Immunol 20, 646–654, doi: 10.1016/j.coi.2008.10.004 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Y-RA et al. A Protocol for the Comprehensive Flow Cytometric Analysis of Immune Cells in Normal and Inflamed Murine Non-Lymphoid Tissues. PLOS ONE 11, e0150606, doi: 10.1371/journal.pone.0150606 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1: Total cell numbers and I-Ab expression in spleens from HOD+IAbfl/flxCre+ mice.
Splenocytes from naïve HOD+IAbfl/flxCre+ mice were harvested, digested, and stained with antibodies to delineate cell subset. (A) Gating strategy used to identify specific cell subsets. For analysis, T cells and RBCs were excluded from total live leukocytes by gating out Thy1.2 and TER119 positive cells. The following phenotypes were used to delineate APC subsets: CD8+ DCs: CD11chiCD8+CD11b−, CD8− DCs: CD11chiCD11b+CD8−, pDC: CD11cintPDCA-1+, RPMs: CD11c-/loCD11b-/loF4/80+, inflammatory monocytes: CD11b+CD11c-/loCD115+Ly6Gvar, resident monocytes: CD11b+CD11c-/loCD115−Ly6Gvar, neutrophils: CD11b+CD11c-/loLy6G+ high side scatter, B cells: CD19+. (B) Total counts for individual APC subsets and (C) representative histograms for I-Ab expression on individual cell subsets are shown. At least 3 independent experiments were performed with 3 mice per group, with a representative experiment shown. Data were analyzed with a mixed model and Tukey’s post-test with multiple comparisons. Significant differences, when compared to HOD+ mice, are indicated with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Figure 2: Gating strategy for liver leukocytes.
Single cell suspensions were generated from livers from experimental mice and stained with antibodies to delineate leukocyte subsets. For analysis, TER119+ RBCs were excluded from total live single cells. The following phenotypes were used to delineate leukocyte subsets: hepatocytes: ASGR-1+CD45var, neutrophils: CD45+Ly6G+ high side scatter, B cells: CD45+I-Ab+CD24+, T cells: CD45+I-Ab−CD24−, monocytes: CD45+Ly6G−I-Ab−FcyRI+CD11b+, Kupffer cells: CD45+Ly6G−I-Ab+FcyRI+, dendritic cells: CD45+CD11c+I-Ab+FcyRI−.
Figure 3: Total cell numbers and I-Ab expression on liver cell subsets in HOD+IAbfl/flxCre+ mice.
Livers from naïve HOD+IAbfl/flxCre+ mice were harvested, digested and stained with antibodies to delineate cell subsets. For analysis, T cells and RBCs were excluded from total live leukocytes by gating out Thy1.2 and TER119 positive cells. The following phenotypes were used to identify APC subsets: hepatocytes: ASGR-1+CD45var, neutrophils: CD45+Ly6G+ high side scatter, B cells: CD45+I-Ab+CD24+, T cells: CD45+I-Ab−CD24−, monocytes: CD45+Ly6G−I-Ab−FcyRI+CD11b+, Kupffer cells: CD45+Ly6G−I-Ab+FcyRI+, dendritic cells: CD45+CD11c+I-Ab+FcyRI−. (A) Total counts for individual APC subsets and (B) representative histograms of I-Ab expression on individual cell subsets are shown. At least 3 independent experiments were performed with 3 mice per group and a representative experiment shown. Data were analyzed with a mixed model and Tukey’s post-test with multiple comparisons. Significant differences, when compared to HOD+ mice, are indicated with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.