Abstract
Schistosomiasis is an important neglected tropical disease. Interactions between the host immune system and schistosomes are complex. Neutrophils contribute to clearance of large pathogens primarily by releasing neutrophil extracellular traps (NETs). However, the functional role of NETs in clearing schistosomes remains unclear. Herein, we report that extracellular vesicles (EVs) derived from the liver of Schistosoma japonicum-infected mice (IL-EVs) induce NET release by delivering miR-142a-3p to target WASL and block the development of S. japonicum. WASL knockout accelerated the formation of NETs that blocked further development of S. japonicum. miR-142a-3p and NETs upregulated the expression of CCL2, which recruits macrophages that block S. japonicum development. However, S. japonicum inhibited NET formation in wild-type mice by upregulating host interleukin-10 (IL-10) expression. In contrast, in WASL knockout mice, IL-10 expression was downregulated, and S. japonicum-mediated inhibition of NET formation was significantly reduced. IL-EV-mediated induction of NET formation is thus an anti-schistosome response that can be counteracted by S. japonicum. These findings suggest that IL-EV-mediated induction of NET formation plays a key role in schistosome infection and that WASL is a potential therapeutic target in schistosomiasis and other infectious diseases.
Keywords: extracellular vesicles, miR-142a-3p, neutrophil extracellular traps, WASL, Schistosoma japonicum
Wu, Sun, and colleagues report that extracellular vesicles derived from the liver of Schistosoma japonicum-infected mice (IL-EVs) induce NET release by delivering miR-142a-3p to target WASL and block the development of S. japonicum. These findings suggest that IL-EV-mediated induction of NET formation plays a key role in schistosomes infection.
Introduction
Schistosomiasis is a neglected tropical disease that affects 240 million people globally and causes 250,000 deaths annually.1,2 Female Schistosoma japonicum worms live in the mesenteric veins of host organisms and produce numerous eggs, many of which are transported to the liver. Highly immunogenic substances released by these eggs elicit host immune responses, including granulomatous inflammation and fibrotic reactions. Schistosomes can flourish in the host despite the development of a pronounced immune response. Unfortunately, no schistosomiasis vaccines are available, and praziquantel is the only drug available for treatment. Therefore, a better understanding of how the immune system responds to schistosomes may facilitate the development of effective therapeutic strategies.
In schistosomiasis, schistosomes evoke responses from many immune cells, including T helper cells, eosinophils, neutrophils, and macrophages, and induce chemokine release.3 Neutrophils are the most abundant innate immune effector cells and play a pivotal role in eliminating invading pathogens.4 Neutrophils are also believed to play an important role in S. japonicum granuloma formation.5 The S. japonicum egg protein SjE16.7 recruits neutrophils and initiates inflammatory hepatic granuloma formation.6 In schistosomiasis caused by S. japonicum, interferon-γ contributes to neutrophil recruitment and the granulomatous response.7 Neutrophils are rapidly recruited to sites of infection and form the initial immunological barrier against pathogens via phagocytosis,8 degranulation,9 and the formation of neutrophil extracellular traps (NETs).10 NETs are large, extracellular, web-like structures consisting of decondensed chromatin decorated with immunostimulatory and microbicidal molecules.10, 11, 12 The “stickiness” of NETs enables efficient entrapment of pathogens to prevent further dissemination. Initial studies of NETs involved bacteria,11 fungi,13 and viruses.4 Neutrophil activation and NET release via autophagy represent one of the primary immunological defense mechanisms in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and the resulting increased NETosis can lead to sustained inflammation.14
More recent studies have described NETs in protozoan infections and infections with some helminth parasites. In particular, one study showed that the protozoan pathogen Toxoplasma gondii elicits the formation of NETs, resulting in a subsequent decrease in T. gondii viability.15 NETs drive inflammatory pathogenesis in malaria and promote Plasmodium chabaudi sequestration in the organs.16 NETs may also contribute to containment of Leishmania donovani promastigotes, thereby facilitating their uptake by mononuclear phagocytes.17 Human neutrophils co-cultured with Strongyloides stercoralis larvae induce the formation of NETs that ensnare the parasites and facilitate larval killing by immune cells.18 Hookworms, however, secrete a deoxyribonuclease that degrades NETs.19 Despite the above observations, knowledge regarding NET formation in response to parasites and the role of NETs in the resolution of parasitic diseases remains limited. The presence of infiltrating neutrophils in S. japonicum-induced granulomas was observed in a transcriptomic study of hepatic granulomas.20 However, the role of neutrophils and subsequently formed NETs in schistosome infections remains poorly understood.
Extracellular vesicles (EVs) are defined as heterogeneous plasma membrane vesicles that contain large quantities of proteins, lipids, and nucleic acids, and function in the long-distance exchange of biochemical information.21, 22, 23, 24 Emerging evidence indicates that EVs play an important role in host-parasite communication as well. MicroRNAs (miRNAs) are a class of small, non-coding RNAs that negatively regulate gene expression at the post-transcriptional or translational levels via complementary binding to the 3′ untranslated region (UTR) of target mRNAs.25, 26, 27, 28 Recent studies have shown that EVs of Schistosoma species can carry miRNAs for specific communication with the host, and Zhu et al. showed that EVs derived from S. japonicum eggs contain small RNAs.29 Meningher et al. showed that EVs derived from Schistosoma mansoni worms contain miRNAs that modulate host T helper cell differentiation.30 We previously demonstrated that Sja-miR-71a in EVs derived from Schistosoma eggs inhibits liver fibrosis in S. japonicum infection.31 However, little is known about the role of host-derived EVs in infections with Schistosoma.
In this study, we found that EVs derived from the liver of mice infected with S. japonicum (IL-EVs) induce the formation of NETs. The miRNA miR-142a-3p carried by IL-EVs plays a key role in IL-EV-mediated induction of NET formation via targeting of WASL. The resulting NETs block further development of S. japonicum in infected mice and attenuate the pathological progression of infection. However, S. japonicum is capable of inhibiting NET formation by upregulating the expression of interleukin-10 (IL-10).
Results
IL-EVs induced NET formation
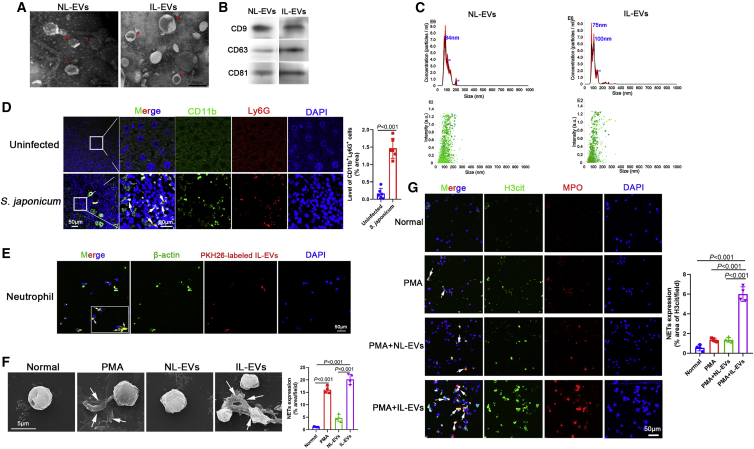
Released EVs were isolated from liver tissues of S. japonicum-infected and uninfected mice at 56 days (Table S1). EVs were confirmed by negative-staining transmission electron microscopy (TEM) as having a diameter ranging from 50 to 150 nm and a characteristic cup-shaped morphology (Figure 1A, arrows). Western blotting analysis confirmed expression of the EV surface markers CD9, CD63, and CD81 (Figure 1B). The size distribution profile of the EVs was examined by nanoparticle tracking analysis (NTA), which revealed peak sizes of 84 nm (normal liver-derived EVs [NL-EVs]) and 75 nm (IL-EVs) (Figure 1C).
Figure 1.
EVs derived from the liver of S. japonicum-infected mice (IL-EVs) induced the formation of NETs
(A) Normal liver-derived EVs (NL-EVs) and IL-EVs were purified and analyzed by negative-staining transmission electron microscopy. (B) Expression of EV surface markers (CD9, CD63, CD81) was analyzed by western blotting. (C) EV particles were investigated using nanoparticle tracking analysis (NTA). (D) Neutrophils in liver sections were examined for CD11b and Ly6G co-localization (n = 6 mice per group). (E) Neutrophils were incubated with PKH26-labeled NL-EVs, and EV internalization was examined using laser scanning confocal microscopy. (F) Neutrophils were treated with PMA (500 nM, 4 h), NL-EVs (10 μg/mL, 24 h), or IL-EVs (10 μg/mL, 24 h), and neutrophil extracellular traps (NETs) were observed using scanning electron microscopy (SEM). (G) Neutrophils were treated with PMA (500 nM, 4 h), NL-EVs (10 μg/mL, 24 h) + PMA (500 nM, 4 h), or IL-EVs (10 μg/mL, 24 h) + PMA (500 nM, 4 h), and NETs were detected based on H3cit and MPO co-localization. (D) Unpaired two-sample t test. (F and G) One-way ANOVA with Dunnett’s multiple comparison test.
A population of CD11b+Ly6G+ cells (neutrophils) surrounding the S. japonicum granuloma was observed (Figure 1D), and NL-EVs were internalized by neutrophils (Figure 1E). NETs, are released by neutrophils and neutralize and kill bacteria, fungi, viruses, and parasites.10 Interestingly, IL-EVs (but not NL-EVs) induced significant NET formation (Figure 1F). In addition, IL-EVs (but not NL-EVs) promoted phorbol-12-myristate-13-acetate (PMA)-induced NET formation (Figure 1G). Collectively, these data suggest that, during infection with S. japonicum, host liver tissue secretes EVs to induce the formation of NETs.
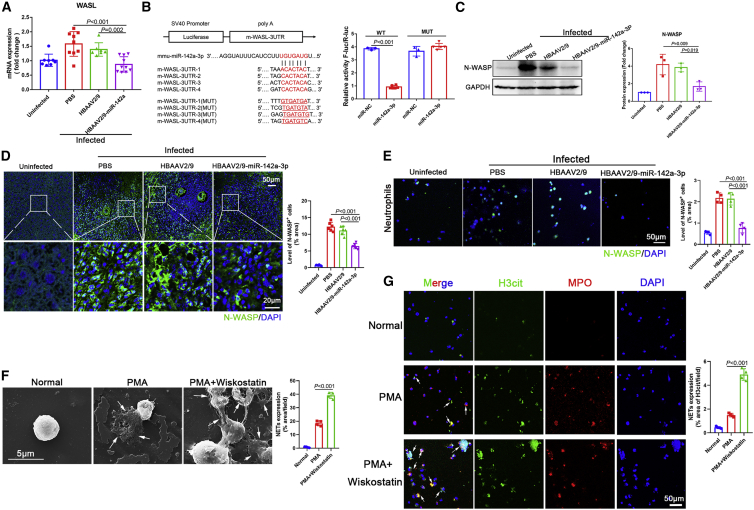
IL-EVs induced the formation of NETs via delivery of miR-142a-3p
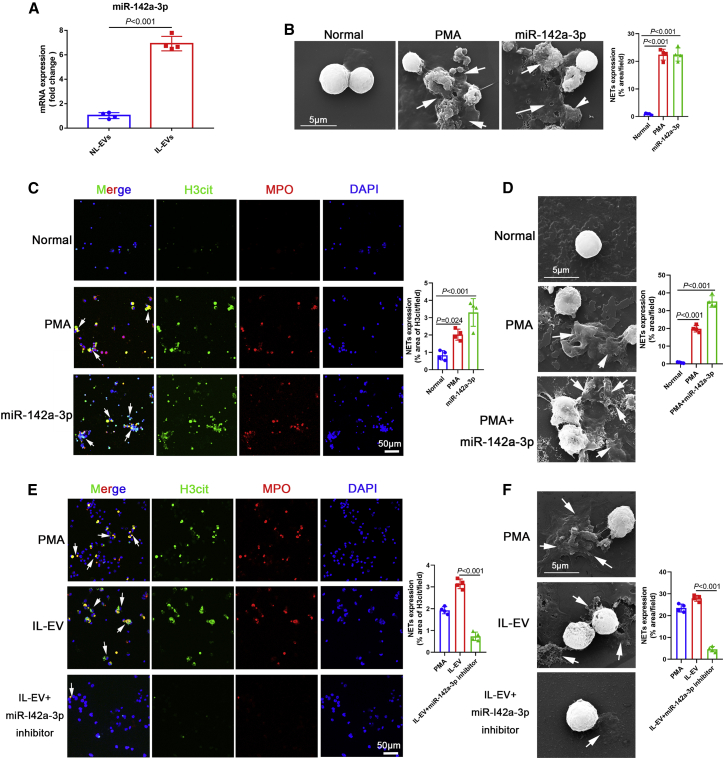
miRNAs are major components carried by EVs and play prominent roles in regulating gene expression.32 We therefore assessed the expression profiles of miRNAs in IL-EVs and NL-EVs (Figures S1A and S1B). Compared with NL-EVs, miR-142a-3p levels were significantly higher in IL-EVs (Table S2; Figure 2A). We then explored the potential role of miR-142a-3p in IL-EV-mediated induction of NET formation. Interestingly, miR-142a-3p induced significant NET formation (Figures 2B and 2C). In addition, miR-142a-3p promoted PMA-induced NET formation (Figure 2D). Furthermore, to assess the role of miR-142a-3p in IL-EV-induced NET formation, we treated cells with a miR-142a-3p inhibitor (chemically modified mature miR-142a-3p complementary single chain) to reduce miR-142a-3p levels in IL-EVs. IL-EV-mediated induction of NET formation decreased significantly after inhibition of miR-142a-3p (Figures 2E and 2F). Collectively, these results suggest that the induction of NET formation by IL-EVs is primarily mediated by the delivery of miR-142a-3p.
Figure 2.
IL-EVs induced the formation of NETs via the delivery of miR-142a-3p
(A) miR-142a-3p levels in EVs were determined using quantitative real-time polymerase chain reaction (qRT-PCR). (B) Neutrophils were treated with PMA (500 nM, 4 h) and miR-142a-3p (50 nM, 24 h), and NETs were observed using SEM. (C) Neutrophils were treated with PMA (500 nM, 4 h) and miR-142a-3p (50 nM, 24 h), and NETs were detected based on H3cit and MPO co-localization. (D) Neutrophils were treated with PMA (500 nM, 4 h) and miR-142a-3p (50 nM, 24 h) + PMA (500 nM, 4 h), and NETs were observed using SEM. (E and F) Neutrophils were treated with PMA (500 nM, 4 h), IL-EVs (10 μg/mL, 24 h), and IL-EVs (10 μg/mL, 24 h) + miR-142a-3p inhibitor (100 nM, 24 h), and NETs were detected by immunohistochemistry and observed using SEM. (A) Unpaired two-sample t test. (C–F) One-way ANOVA with Dunnett’s multiple comparison test.
miR-142a-3p induced NET formation and blocked further development of S. japonicum, thereby attenuating the pathological progression of S. japonicum infection
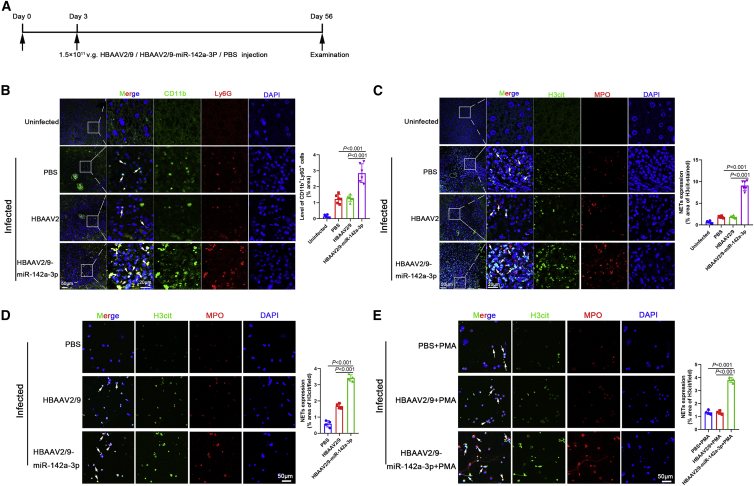
To determine whether miR-142a-3p delivery by IL-EVs is involved in the progression of schistosomiasis, HBAAV2/9-miR-142a-3p recombinant adeno-associated virus (rAAV) were constructed. Mice were infected with S. japonicum and exposed to HBAAV2/9-miR-142a-3p rAAV (Figure 3A). Liver sections reflected the highly hepatic tropism of HBAAV2/9-miR-142a-3p (Figure S2). As shown in Figure 3B, the number of CD11b+Ly6G+ neutrophils surrounding the S. japonicum granuloma significantly increased after HBAAV2/9-miR-142a-3p treatment. We then analyzed NET formation in the liver of infected mice. As expected, NETs were observed in S. japonicum granulomas (Figure S3), and a significant increase in NET formation was observed in mice treated with HBAAV2/9-miR-142a-3p (Figure 3C). Neutrophils were isolated from infected mice using Percoll density gradient centrifugation and flow cytometry (Figure S4) and then cultured in vitro for 24 h. NETs formed by these neutrophils increased markedly after HBAAV2/9-miR-142a-3p transfection (Figure 3D), and HBAAV2/9-miR-142a-3p promoted PMA-induced NET formation (Figure 3E).
Figure 3.
miR-142a-3p in IL-EVs induced NET formation
(A) Time schedule for parasite infection and intravenous injection of rAAV vectors or PBS and sample examination. (B) Neutrophils in liver sections were detected based on CD11b and Ly6G co-localization (n = 6 mice per group). (C) NETs in liver sections were detected based on H3cit and MPO co-localization (n = 6 mice per group). (D) Neutrophils were isolated from infected mice using Percoll density gradient centrifugation and flow cytometry, and cultured in vitro for 24 h. NETs were then detected based on H3cit and MPO co-localization. (E) Neutrophils were isolated from infected mice and treated with PMA (500 nM) for 4 h and then detected based on H3cit and MPO co-localization. Differences were analyzed using one-way ANOVA with Dunnett’s multiple comparison test.
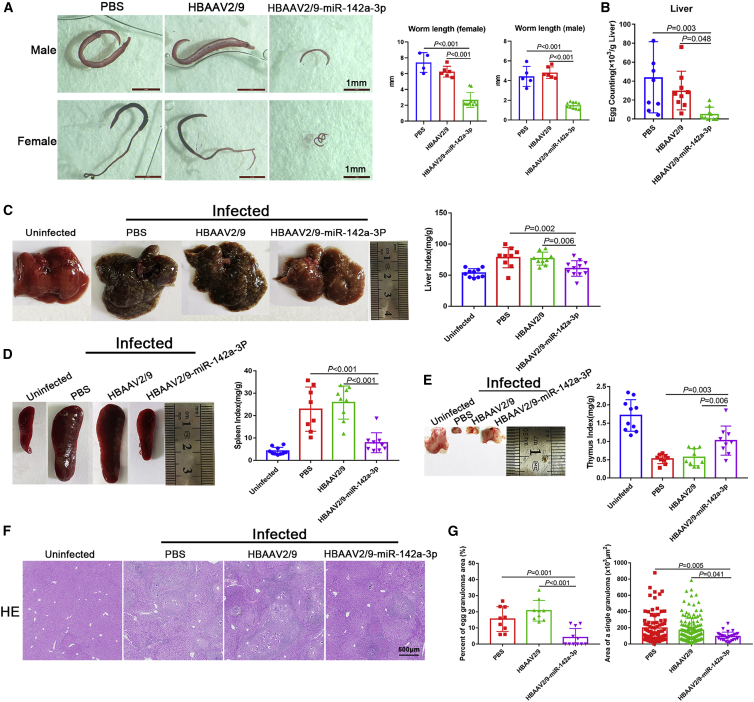
To obtain a better understanding of the effect of miR-142a-3p on IL-EV-induced NET formation in the progression of schistosomiasis, characteristics of the worms were examined. As shown in Figure 4A, development of both male and female worms was blocked by HBAAV2/9-miR-142a-3p treatment. Furthermore, worms collected from mice treated with HBAAV2/9-miR-142a-3p were significantly shorter in length than those obtained from mice treated with either PBS or HBAAV2/9 (Figure 4A). Furthermore, following exposure to HBAAV2/9-miR-142a-3p, a significant decrease in egg deposition in the liver was observed (Figure 4B). Hepatosplenomegaly was markedly attenuated after HBAAV2/9-miR-142a-3p treatment, and the liver and spleen indices declined significantly after HBAAV2/9-miR-142a-3p treatment (Figures 4C and 4D). In addition, thymus atrophy caused by S. japonicum infection diminished following HBAAV2/9-miR-142a-3p treatment (Figure 4E). Hematoxylin and eosin (H&E) staining of liver sections revealed that the percent egg granuloma area and area of a single granuloma declined significantly in the HBAAV2/9-miR-142a-3p-treated groups compared with the PBS- and HBAAV2/9-treated groups (Figures 4F and 4G). Masson’s trichrome staining of liver sections showed significantly reduced collagen deposition in HBAAV2/9-miR-142a-3p-treated mice (Figure S5A). Similarly, significantly reduced expression of α-SMA and collagen I was observed in HBAAV2/9-Sja-miR-71a-treated mice (Figures S5B and S5C). The high expression of the inflammatory cytokines IL-1β, IL-23A, and IL-33, and tumor necrosis factor alpha (TNF-α) associated with S. japonicum infection decreased significantly after HBAAV2/9-miR-142a-3p treatment (Figures S6A–S6D).
Figure 4.
miR-142a-3p induced NET formation to block further development of S. japonicum and attenuated the pathological progression of schistosomiasis
(A) Worms were stained with hydrochloric carmine red to assess development and length. (B) Liver egg burden was determined after digesting liver tissue in 4% KOH (n = 8–9 mice per group). (C–E) Macroscopic appearance of the liver, spleen, and thymus and related indices (n = 8–11 mice per group). (F) Hematoxylin and eosin (H&E) staining of liver sections. (G) Percent granulomatous area and area of a single granuloma were measured from H&E sections using Image Pro Plus 6.0 software (n = 9–11 mice per group). Differences were analyzed using one-way ANOVA with Dunnett’s multiple comparison test.
Taken together, these results suggest that IL-EVs induce NET formation by delivering miR-142a-3p, which blocks further development of S. japonicum and attenuates the pathological progression of schistosomiasis.
miR-142a-3p induced NET formation via direct targeting of WASL
To further evaluate the molecular mechanisms involved in miR-142a-3p-mediated induction of NET formation, we identified genes directly targeted and thereby controlled by miR-142a-3p. TargetScan was used to predict potential miR-142a-3p targets. We found that expression of WASL in the liver of S. japonicum-infected mice declined after HBAAV2/9-miR-142a-3p treatment (Figure 5A). Furthermore, as shown in Figure 5B, miR-142a-3p targeted four binding sites in the 3′ UTR of WASL. To confirm WASL as a direct target of miR-142a-3p, we generated a luciferase reporter plasmid containing the 3′ UTR of WASL and flanking putative miR-142a-3p-binding sites and a plasmid mutated miRNA binding sites. Dual-luciferase reporter assay results revealed that, compared with normal control mimic-transfected cells, cells transfected with miR-142a-3p mimic exhibited significantly reduced luciferase activity of the WASL construct (Figure 5B). In contrast, the miR-142a-3p mimic had no effect on the luciferase activity of the WASL construct with mutated miR-142a-3p-binding sites (Figure 5B).
Figure 5.
miR-142a-3p induced NET formation via direct targeting of WASL
(A) WASL mRNA expression was analyzed by qRT-PCR (n = 7–10 mice per group). (B) Wild-type and mutated m-WASL-3′ untranslated regions (UTRs) were cloned into psi-CHECK-2, and four predicted binding sites of miR-142a-3p were identified in the 3′ UTR of the WASL gene; dual-luciferase reporter assay performed on HEK293T cells transfected with WASL UTR reporter plasmid together with miR-142a-3p mimic or control mimic. (C and D) Expression of N-WASP (translated from WASL) in mice livers was analyzed by western blotting and immunohistochemistry (n = 6 mice per group). (E) Neutrophils of infected mice were isolated, and the expression of N-WASP was analyzed by immunohistochemistry. (F and G) Neutrophils were treated with PMA (500 nM, 4 h) or Wiskostatin (N-WASP inhibitor, 20 μM, 24 h) + PMA (500 nM, 4 h), and NET formation was evaluated by SEM and immunohistochemistry. (A, C, and D–G) One-way ANOVA with Dunnett’s multiple comparison test. (B) Unpaired two-sample t test.
We analyzed the expression of neural Wiskott-Aldrich syndrome protein (N-WASP, translated from WASL) in mouse liver. As expected, N-WASP expression was markedly downregulated in the liver of mice treated with HBAAV2/9-miR-142a-3p compared with control mice (Figures 5C and 5D). N-WASP expression was also evaluated in neutrophils of infected mice. As shown in Figure 5E, neutrophil N-WASP expression was downregulated following HBAAV2/9-miR-142a-3p treatment. In addition, to evaluate the role of N-WASP in the miR-142a-3p-induced NET formation, N-WASP expression was inhibited with Wiskostatin, which resulted a significant increase in PMA-induced NET formation (Figures 5F and 5G). Taken together, these findings indicate that miR-142a-3p induces NET formation by directly targeting WASL.
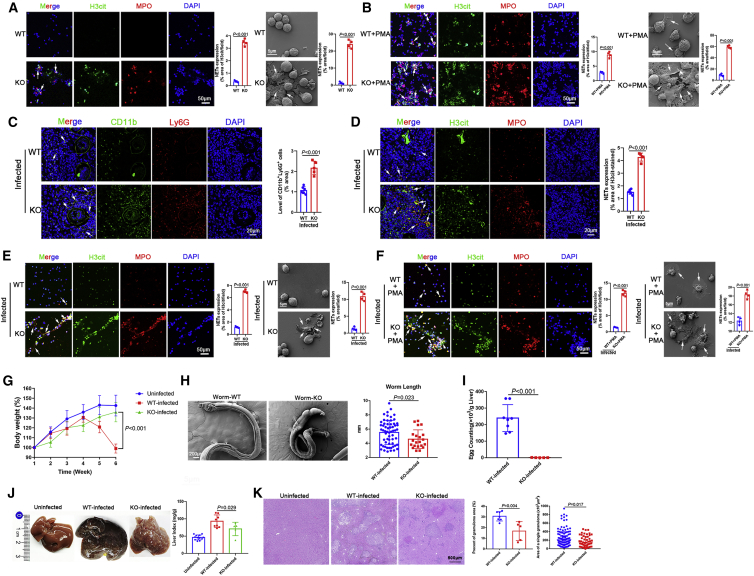
WASL deletion accelerated NET formation and blocked further development of S. japonicum
The key role of WASL in NET formation was examined using neutrophils isolated from wild-type (WT) and WASL−/− mice. Interestingly, WASL deletion significantly accelerated NET formation compared with WT mice (Figure 6A). In addition, PMA-induced NET formation was greater in WASL−/− mice than WT mice (Figure 6B). Next, we sought to gain a better understanding of miR-142a-3p-induced NET formation by directly targeting WASL. Liver sections of WASL knockout (WASL-KO) mice infected with S. japonicum exhibited a significant increase in CD11b+Ly6G+ neutrophils surrounding S. japonicum granulomas (Figure 6C). We then analyzed NET formation in the liver of infected WASL-KO mice. As expected, compared with WT mice, NET formation was significantly increased in the liver of WASL-KO mice (Figure 6D). Neutrophils were isolated from infected mice and cultured in vitro for 24 h, and we observed a marked increase in NETs after WASL deletion (Figure 6E). WASL deletion promoted PMA-induced NET formation (Figure 6F).
Figure 6.
WASL deletion accelerated NET formation and blocked further development of S. japonicum
(A) Neutrophils were cultured in vitro for 24 h, and NETs were observed by immunohistochemistry and SEM. (B) Neutrophils were treated with PMA (500 nM, 4 h), and NETs were then observed by immunohistochemistry and SEM. (C) Observation of neutrophils in liver sections (n = 6 mice per group). (D) NETs in liver sections were detected based on H3cit and MPO co-localization (n = 6 mice per group). (E) Neutrophils were isolated from WT and WASL knockout (WASL-KO) mice infected with S. japonicum (6 weeks) and cultured in vitro for 24 h. NETs were then observed by immunohistochemistry and SEM. (F) Neutrophils were isolated from WT and WASL-KO mice infected with S. japonicum (6 weeks) and treated with PMA (500 nM) for 4 h, after which NETs were observed by immunohistochemistry and SEM. (G) Body weight of mice was recorded weekly (n = 5–12 mice per group). (H) Observation and measurement of S. japonicum worm length using SEM. (I) Determination of liver egg burden (n = 5–8 mice per group). (J) Macroscopic appearance of the liver and liver index (n = 5–12 mice per group). (K) The granulomatous area was measured from H&E sections (n = 5–7 mice per group). (A–F, H, I, and K) Unpaired two-sample t test. (G and J) One-way ANOVA with Dunnett’s multiple comparison test.
To further confirm that WASL deletion affects the progression of schistosomiasis by promoting NET formation, characteristics of the worms were examined. As shown in Figure 6G, after 4 weeks of S. japonicum infection, the body weight of WT mice decreased dramatically, whereas the body weight of WASL-KO mice did not change significantly compared with uninfected mice. We found that worm development was blocked by WASL deletion. Worms collected from WASL-KO mice were significantly shorter than those obtained from WT mice (Figure 6H), and a significant decrease in egg deposition in the liver was observed in WASL-KO mice (Figure 6I). Furthermore, hepatomegaly was markedly attenuated in WASL-KO mice, and the liver index was significantly reduced (Figure 6J). H&E staining of liver sections revealed that the percent egg granuloma area and the area of a single granuloma were significantly reduced in WASL-KO mice compared with WT mice (Figure 6K). Masson’s trichrome staining of liver sections showed significantly reduced collagen deposition in WASL-KO mice (Figure S7A), along with significantly reduced expression of α-SMA and collagen I (Figure S7B). Taken together, these data suggest that WASL deletion accelerates the formation of NETs, which then function to block further development of S. japonicum.
miR-142a-3p and NETs upregulated CCL2 to recruit macrophages to block further S. japonicum development
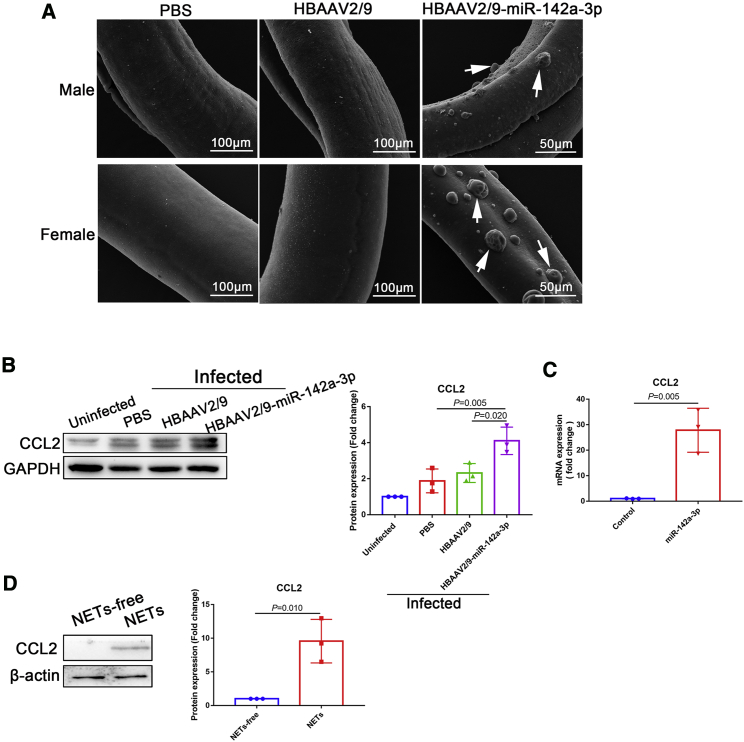
Macrophages contribute significantly to the immune response during helminth infection. Alternatively activated macrophages are induced early in the anti-helminth immune response.33 We observed numerous macrophages adhering to the surface of S. japonicum worms after HBAAV2/9-miR-142a-3p treatment (Figure 7A, arrows). Furthermore, expression of CCL2, a potent chemotactic factor for monocytes, was markedly upregulated after HBAAV2/9-miR-142a-3p treatment (Figure 7B). In vitro experiments also revealed that miR-142a-3p treatment significantly upregulated the expression of CCL2 in macrophages (Figure 7C). In addition, NETs markedly upregulated the expression of CCL2 in macrophages (Figure 7D). Collectively, these data suggest that miR-142a-3p and NETs upregulate CCL2 expression to recruit macrophages to block further development of S. japonicum.
Figure 7.
miR-142a-3p and NETs upregulated CCL2 expression to recruit macrophages to block further development of S. japonicum
(A) S. japonicum worms were collected from infected mice and analyzed using SEM. (B) Expression of CCL2 in the liver was analyzed by western blotting. (C) Macrophages were treated with miR-142a-3p (50 nM, 24 h), and CCL2 expression was analyzed by qRT-PCR. (D) Macrophages were treated with NETs (10 μg/mL, 24 h), and CCL2 expression was analyzed by western blotting. (B) One-way ANOVA with Dunnett’s multiple comparison test. (C and D) Unpaired two-sample t test.
S. japonicum inhibited NET formation by upregulating host IL-10 expression, whereas miR-142a-3p downregulated the expression of IL-10 by targeting WASL
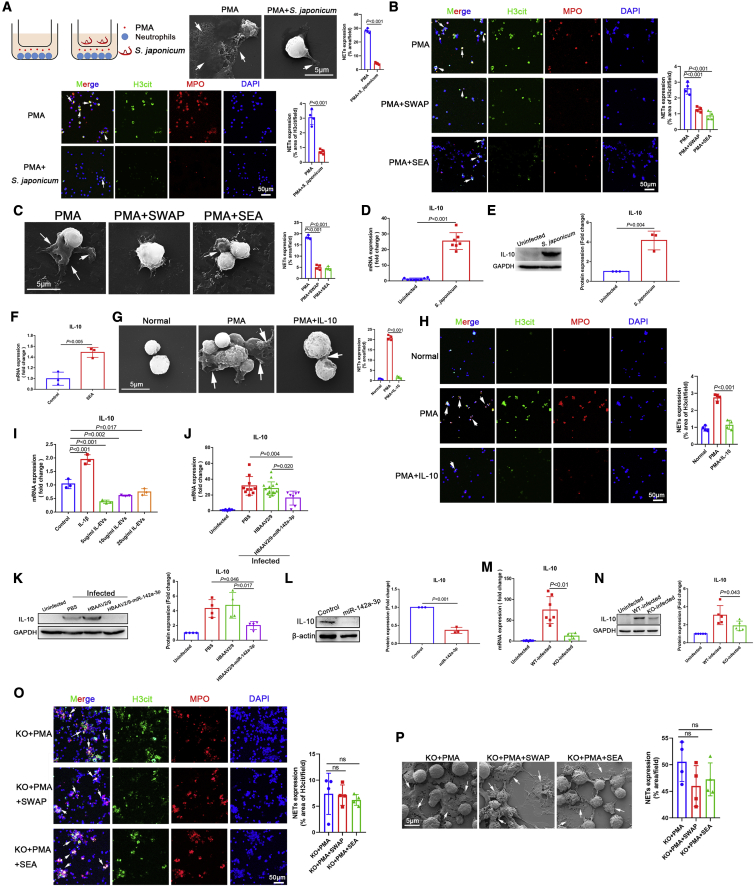
Although neutrophils are upregulated during S. japonicum infection, the worms are not killed. We therefore hypothesized that S. japonicum inhibits NET formation. Thus, we co-cultured S. japonicum with PMA-stimulated neutrophils, and the results confirmed our hypothesis that S. japonicum inhibits PMA-induced NET formation (Figure 8A). Furthermore, as shown in Figures 8B and 8C, both S. japonicum soluble worm antigenic preparation (SWAP) and S. japonicum soluble eggs antigen (SEA) inhibited PMA-induced NET formation.
Figure 8.
S. japonicum inhibited NET formation by upregulating host IL-10 expression, whereas miR-142a-3p in IL-EVs downregulated IL-10 expression
(A) S. japonicum worms were co-cultured with PMA-simulated (500 nM, 4 h) neutrophils (5 × 105 cells) using Transwells, and NETs were observed by SEM and immunohistochemistry. (B and C) Neutrophils were treated with PMA (500 nM, 4 h), SWAP (10 μg/mL, 24 h) + PMA (500 nM, 4 h), or SEA (10 μg/mL, 24 h) + PMA (500 nM, 4 h), and NETs were observed by SEM and immunohistochemistry. (D and E) Expression of IL-10 in liver tissues was analyzed by qRT-PCR (n = 5–8 mice per group) and western blotting. (F) Macrophages were treated with SEA (10 μg/mL, 24 h), and IL-10 expression was analyzed by qRT-PCR. (G and H) Neutrophils were treated with PMA (500 nM, 4 h) or IL-10 (40 ng/mL, 24 h) + PMA (500 nM, 4 h), and NETs were observed by SEM and immunohistochemistry. (I) Macrophages were treated with IL-EVs, with IL-1β (20 ng/mL, 24 h) used as a positive control, and expression of IL-10 was analyzed by qRT-PCR. (J and K) Expression of IL-10 in the liver was analyzed by qRT-PCR (n = 7–16 mice per group) and western blotting. (L) Macrophages were treated with miR-142a-3p (50 nM, 24 h), and the expression of IL-10 was analyzed by western blotting. (M and N) Expression of IL-10 in mouse liver was analyzed by qRT-PCR (n = 5–8 mice per group) and western blotting. (O and P) Neutrophils from WASL-KO mice were treated with PMA (500 nM, 4 h), SWAP (10 μg/mL, 24 h) + PMA (500 nM, 4 h), or SEA (10 μg/mL, 24 h) + PMA (500 nM, 4 h), and NETs were observed by immunohistochemistry and SEM. (A, D, E, F, and L) Unpaired two-sample t test. (B, C, G–K, and M–P) One-way ANOVA with Dunnett’s multiple comparison test.
S. japonicum also significantly upregulated the expression of IL-10 in the host (Figures 8D and 8E). In in vitro experiments, SEA significantly upregulated the expression of IL-10 in macrophages (Figure 8F), and PMA-induced NET formation was subsequently inhibited by IL-10 (Figures 8G and 8H). Interestingly, IL-EVs downregulated the expression of IL-10 in macrophages (Figure 8I). Furthermore, in vivo experiments showed that IL-10 expression was upregulated in the liver by S. japonicum but downregulated after HBAAV2/9-miR-142a-3p treatment (Figures 8J and 8K). In addition, miR-142a-3p significantly downregulated the expression of IL-10 in macrophages in vitro (Figure 8L). To investigate whether miR-142a-3p downregulates the expression of IL-10 by targeting WASL, we determined the expression level of IL-10 in WASL-KO mice infected with S. japonicum. IL-10 expression was significantly reduced in WASL-KO mice compared with WT mice (Figures 8M and 8N). Similarly, compared with the WT mice shown in Figures 8B and 8C, the inhibitory effects of SWAP and SEA on PMA-induced NET formation in WASL-KO mice were significantly attenuated (Figures 8O and 8P).
Collectively, these data suggest that S. japonicum inhibits NET formation by upregulating host IL-10 expression to escape the host immune response, whereas miR-142a-3p contained in IL-EVs downregulates the expression of IL-10 by targeting WASL.
Discussion
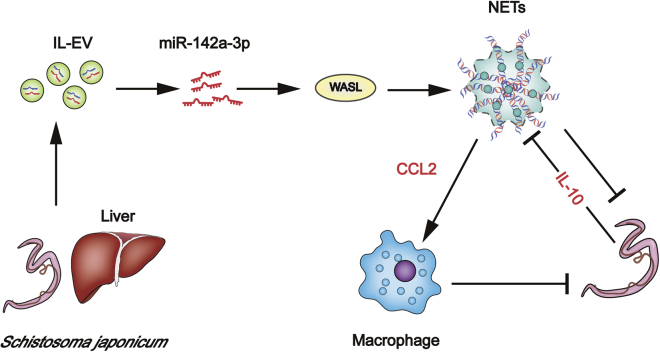
In this study, we demonstrate that host EVs and EVs carrying miR-142a-3p contribute to block further development of S. japonicum in infected mice and attenuate the pathological progression of S. japonicum infection by inducing NET formation. Our data showed that IL-EVs contain high levels of miR-142a-3p, which induces NET formation by directly targeting WASL. WASL KO accelerates the formation of NETs that block further development of S. japonicum. In addition, miR-142a-3p and NETs upregulate CCL2 expression, leading to recruitment of macrophages that also block development of S. japonicum. We also demonstrated that S. japonicum inhibits NET formation by upregulating host IL-10 expression, whereas miR-142a-3p in IL-EVs downregulates the expression of IL-10 by targeting WASL (Figure 9).
Figure 9.
Host liver-derived EVs deliver miR-142a-3p, which induces NET formation by targeting WASL to block further development of S. japonicum
NETs additionally upregulate CCL2 expression to recruit macrophages that also block development of S. japonicum. Nevertheless, S. japonicum can inhibit NET formation in wild-type mice by upregulating host IL-10 expression.
The interactions between the host immune system and schistosomes is complex. The host immune system likely plays an important role in suppressing the enormous reproductive potential of schistosomes.34 Immune responses against schistosomes are important for the development of resistance to reinfection.35 Granulomas are a collection of inflammatory cells that include lymphocytes, eosinophils, macrophages, and neutrophils.35 Granulomas induced by S. japonicum are primarily composed of neutrophils, whereas the core of hepatic granulomas induced by S. mansoni is composed primarily of eosinophils.3 Hsü et al. first reported that S. japonicum egg lesions induce a stronger neutrophilic chemotaxis response than S. mansoni egg lesions.36 Neutrophils are recruited to the areas surrounding S. japonicum eggs as early as 8 days post-deposition.37 In this study, we identified a group of CD11b+Ly6G+ cells (neutrophils) surrounding granulomas of S. japonicum. However, the role of neutrophils in schistosome infection is not fully understood. NETs, which are released by neutrophils, are highly “sticky” and can capture bacteria,11,38 fungi,13 and viruses4 to prevent their dissemination. Studies of patients with coronavirus disease 2019 (COVID-19) have shown that elevated levels of NET-specific markers (e.g., free DNA, MPO, and H3cit) are strongly associated with total neutrophil count.39 SARS-CoV-2-triggered NETs mediate the pathology of COVID-19.40 NETs contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome.41 Targeting NETs may therefore reduce the clinical severity of COVID-19.42
A number of recent studies have reported the presence of NETs in infections involving protozoans and a few helminth parasites, including T. gondii,15 L. donovani,17 Plasmodium,43 S. stercoralis,18 and Haemonchus contortus.44 Chuah et al. reported observing NET-like structures surrounding S. japonicum eggs.20 Nevertheless, the role of NETs in schistosome infection remains largely unknown.
Recent studies have also shown that EVs secreted from S. japonicum play important roles in host-parasite communication.29, 30, 31 However, little is known about the role of host-derived EVs in schistosome infection. We hypothesized that host-derived EVs also play important roles in host-schistosome communication. Interestingly, we isolated EVs from the liver of S. japonicum-infected mice (i.e., IL-EVs) by ultracentrifugation and found that they induce NET formation. Among the different active components harbored by EVs, miRNAs are regarded as essential to EVs function.45,46 To investigate the role of the key components in IL-EVs that induce NET formation, we sequenced and analyzed IL-EV miRNAs. Compared with NL-EVs, we found that the expression of miR-142a-3p was significantly increased in IL-EVs.
miR-142-3p was originally described as a hematopoietic-specific miRNA.47 Studies have indicated that miR-142-3p is required for hematopoietic development. In Xenopus, knockdown of miR-142-3p impairs the specification of definitive hemangioblasts.48 Furthermore, miR-142-3p plays a prominent role in CD25+CD4+ T cell proliferation by targeting the expression of glycoprotein A repetitions.49 In zebrafish and mice, miR-142-3p is specifically expressed in hematopoietic stem cells, and knockdown of miR-142a-3p in zebrafish leads to a reduced population of hematopoietic stem cells.50 In zebrafish, miR-142-3p also acts as an essential modulator of neutrophil development.51 However, no studies have demonstrated an association between miR-142-3p and NET formation. Interestingly, we found that miR-142a-3p plays a key role in IL-EV-induced NET formation, whereas IL-EV-induced NET formation is significantly decreased following inhibition of miR-142a-3p.
Several studies have shown that N-WASP (encoded by the Wasl gene) plays an important role in B cell. The combined activity of N-WASP and WASP is required for peripheral B cell development and function.52 For example, N-WASP is required for B cell-mediated autoimmunity in Wiskott-Aldrich syndrome.53 Deletion of N-WASP and WASP in B cells results in the production of IgM autoantibodies.54 However, the role of N-WASP in the development and function of neutrophils remains unknown. We demonstrated that miR-142a-3p induces NET formation by directly targeting WASL, which blocks further development of S. japonicum and attenuates the pathological progression of S. japonicum infection. Consistent with these observations, we found that WASL deletion accelerates NET formation and blocks the development of S. japonicum.
Macrophages contribute significantly to the immune response during helminth infection. Indeed, alternatively activated macrophages are induced early in the anti-helminth response.33 We observed numerous macrophages adhering to the surface of worms after HBAAV2/9-miR-142a-3p treatment. Expression of CCL2, a potent chemotactic factor for monocytes,55 was markedly upregulated after miR-142a-3p treatment, suggesting that miR-142a-3p and NETs upregulate CCL2 expression to recruit macrophages to block further development of S. japonicum.
Hepatic fibrosis is one of the primary pathological features of schistosomiasis. Female S. japonicum worms produce eggs that are transported to the liver, and these eggs elicit host immune responses and fibrotic reactions.56 The function of neutrophils varies in schistosome-induced fibrosis depending on when they are recruited and their location in the granuloma.3 In this study, we found that IL-EVs deliver miR-142a-3p to induce NET formation by targeting WASL, thereby blocking further development of S. japonicum. This causes egg deposition in the liver to decrease significantly, thereby alleviating fibrosis. In schistosome infection, macrophages in the periphery of granulomas exhibit matrix-degrading properties, and they may promote the recruitment of neutrophils.3 We found that miR-142a-3p and NETs upregulate CCL2 expression to recruit macrophages and thereby block further development of S. japonicum and alleviate host fibrosis.
The host immune system works toward killing schistosomes; accordingly, schistosomes have developed immunoregulatory mechanisms to counteract the host immune response and enable the schistosomes to persist and complete their life cycle.57 Interestingly, we observed that S. japonicum inhibited NET formation. Furthermore, both SWAP and SEA also inhibited NET formation, which may explain why neutrophils are upregulated during schistosome infection, but the worms are not killed. However, the development of S. japonicum was blocked after overexpression of miR-142a-3p, resulting in the recruitment of large numbers of neutrophils and inducing NET formation. These findings suggest that miR-142a-3p may be an important target for preventing schistosome infection.
Helminths produce numerous specialized molecules that alter the host microenvironment and affect specific types of immune cells. Helminth infection also induces elevated expression of immunosuppressive cytokines, such as IL-10.58 A previous study showed that expression of IL-4 induced primarily by schistosome eggs in turn induces IL-10 production by B cells.57 Our previous study found that Sj16, a 16-kDa secreted protein of S. japonicum, significantly stimulates IL-10 production in dendritic cells.59 The upregulation of IL-10 promotes Treg cell development, shifting the host immune response to a downregulated phenotype.57 We found that S. japonicum significantly upregulates the expression of IL-10 in the host, which subsequently inhibits NET formation. The level of IL-10 was downregulated after treatment with miR-142a-3p. IL-10 expression was significantly decreased in WASL-KO mice, and the inhibitory effects of SWAP and SEA on PMA-induced NET formation were significantly diminished in WASL-KO mice. These data suggest that S. japonicum inhibits NET formation by upregulating host IL-10 expression to escape the host immune response, whereas miR-142a-3p downregulates the expression of IL-10 by directly targeting WASL.
Conclusion
In conclusion, we demonstrated that IL-EVs deliver miR-142a-3p to induce the formation of NETs, which then block further development of S. japonicum. However, S. japonicum counteracts this response by upregulating host IL-10 expression. Our study enhances current understanding of the molecular mechanisms underlying Schistosoma-host interactions. Our data indicate that miR-142a-3p and WASL may be potential therapeutic targets for treating infectious diseases such as schistosomiasis and COVID-19.
Materials and methods
Animals and ethics
Male BALB/c mice (6 weeks old) were purchased from the Guangdong Medical Laboratory Animal Center, China. WASL gene KO mice (male, 6 weeks old, C57BL/6NCrl, Gene: WASL ENSMUSG00000031165) were provided by Professor Yinmin Liang of Xinxiang University. Mice were infected by percutaneous exposure to S. japonicum cercariae shed from lab-infected snails (Oncomelania hupensis), which were purchased from the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention. All procedures were approved by the Animal Care and Use Committee of Sun-Yat-Sen University (SYSU-IACUC-2020-B0063) and conformed to the Guidelines for the Care and Use of Laboratory Animals of the National Institute of Health in China.
EV purification and identification
To prepare liver-derived EVs, the livers were collected from normal mice (n = 10) and infected mice (n = 10) at 56 days post-infection, and the gallbladder, bile ducts, and large vessels were removed. Single-cell (parenchymal cells and nonparenchymal cells) suspensions were obtained by gentle grinding. Single cells were resuspended in 90% Percoll and centrifuged at 1,000 × g for 5 min to remove dead-cell debris. The cells were maintained in RPMI-1640 culture medium (1 × 106 cells/mL, total of 60 mL/mouse). After 24 h, the culture supernatant was collected for further experiments. EVs were purified by differential centrifugation and identified by electron microscopy, western blotting, and NTA. In brief, cell culture supernatants were centrifuged at low speed (300 × g for 10 min at 4°C) in 15-mL polypropylene tubes using a swinging-bucket rotor centrifuge (model A-4-44 5804R Refrigerated Centrifuge, Eppendorf, Germany), and the resulting supernatants were centrifuged at 2,000 × g for 10 min at 4°C. These supernatants were transferred into 1.5-mL polypropylene tubes (Eppendorf) using a micropipette and then centrifuged at 10,000 × g for 60 min at 4°C (fixed-angle rotor, angle of 45°, model no. 3331, D-37520 Refrigerated Centrifuge, Thermo Electron Corporation, USA). The resulting supernatants were transferred to Quick-Seal centrifuge tubes (Beckman Coulter, USA) and centrifuged at 120,000 × g for 90 min at 4°C in an Optima L-100xp ultracentrifuge (swinging-bucket rotor, model SW40 Ti, Optima L-100xp, Beckman Coulter). The resultant pellets (EVs) were diluted with PBS and centrifuged again at 120,000 × g for 90 min at 4°C in an Optima L-100xp ultracentrifuge (swinging-bucket rotor, model SW40 Ti, Optima L-100xp, Beckman Coulter). Negative-staining TEM was used to analyze the EVs. EVs were loaded on a copper grid and negatively stained with 3% (w/v) aqueous phosphotungstic acid for 1 min. The grid was then examined using a FEI Tecnai G2 Sprit Twin TEM (FEI, USA). Expression of the EV markers CD9, CD63, and CD81 was analyzed by western blotting. EV particles were also analyzed by NTA (NanoSight NS300, Malvern Instruments, UK) using a NanoSight NS300 instrument equipped with a sCMOS camera, 488-nm laser (blue), and NTA 3.3 Dev Build 3.3.30 software (number of frames: 749).
Immunofluorescence analysis
Liver tissues were fixed in 4% neutral buffered formalin and embedded in paraffin. The paraffin sections were deparaffinized by baking and then dehydrated using xylene and ethanol. After blocking with 1% bovine serum albumin (BSA) for 1 h, the sections were incubated with anti-CD11b (1:100, BD Biosciences, USA), anti-Ly6G (1:100; BD Biosciences), anti-H3cit (1:400; CST, USA), anti-MPO (1:100; Abcam, UK), and anti-N-WASP (1:100; Abcam) antibodies overnight at 4°C. Cells were fixed in 4% paraformaldehyde for 25 min at room temperature and then permeabilized with 0.2% Triton X-100 in PBS for 10 min. The cells were blocked in PBS with 2% BSA for 30 min at room temperature and then incubated with anti-H3Cit (1:400; CST), anti-N-WASP (1:100; Abcam), or anti-MPO (1:100; Abcam) antibodies overnight at 4°C. The sections and cells were then incubated with the indicated Alexa Fluor-conjugated secondary antibodies. DAPI was used to detect nuclei. Between all steps, samples were washed three times in PBS for 5 min each. The sections were visualized using an LSM 800 laser scanning confocal microscope (Zeiss, Germany).
Neutrophil and macrophage isolation
The femurs and tibia of mice were excised, and cells within the bone marrow were prepared as a single-cell suspension. Neutrophils were isolated from the single-cell suspension using Percoll density gradient centrifugation and identified and separated by positive selection for CD11b+Ly6G+ cells by flow cytometry (BD Influx, USA). For macrophage isolation, cells within the bone marrow were cultured in DMEM (Gibco, Germany) with 10% (v/v) fetal bovine serum and recombinant murine macrophage colony-stimulating factor (20 ng/mL, PeproTech, USA). On the seventh day, bone marrow-derived macrophages were harvested. Neutrophils and macrophages were cultured in a humidified, 5% CO2 incubator at 37°C.
Analysis of EVs uptake
EVs were incubated with PKH26 (Sigma-Aldrich, USA) for 5 min, and staining was terminated by adding an equal volume of 1% BSA. The liquid was then transferred to a Quick-Seal centrifuge tube (Beckman Coulter) and centrifuged at 120,000 × g for 90 min at 4°C in an Optima L-100xp ultracentrifuge (swinging-bucket rotor, model SW60 Ti, Optima L-100xp, Beckman Coulter). The resultant pellet (PKH26-labeled EVs) was resuspended with PBS and centrifuged again at 120,000 × g for 90 min at 4°C in an Optima L-100xp ultracentrifuge (swinging-bucket rotor, model SW60 Ti, Optima L-100xp, Beckman Coulter). Neutrophils were incubated with PKH26-labeled EVs for 1 h, and the cells were analyzed using confocal microscopy to evaluate EV internalization. Alexa Fluor phalloidin-FITC (CST) was used to label actin, and DAPI was used to detect nuclei.
Scanning electron microscopy
Neutrophils were cultured on coverslips and treated as follows: PMA (500 nM; NET-inducer) for 4 h, NL-EVs (10 μg/mL) for 24 h; IL-EVs (10 μg/mL) for 24 h; S. japonicum SWAP (10 μg/mL) for 24 h; S. japonicum SEA (10 μg/mL) for 24 h; miR-142a-3p mimic (50 nM) for 24 h; Wiskostatin (N-WASP inhibitor, Abcam) (20 μM) for 24 h; or IL-10 (40 ng/mL) for 24 h. Neutrophils and S. japonicum worms were fixed in 2.5% glutaraldehyde overnight. After fixation, the samples were washed with PBS before dehydration using an ethanol gradient, followed by exchange of ethanol with acetone and isoamyl acetate. The samples were critical point-dried and coated with gold using an ion coater (E−102; Hitachi) and observed and photographed using an FEI Quanta 200 scanning electron microscope.
Small RNA sequencing and analysis
Small RNAs were sequenced and analyzed as described previously.31 In brief, total RNA was isolated from EVs and used as the input material to generate the small RNA library. Following cluster generation, the library preparations were sequenced on an Illumina HiSeq 2500 platform (Illumina, USA). After sequencing, the data were subjected to preliminary analyses, including quality control analysis, comparative analysis, target gene functional annotation, quantification of miRNA expression levels, mRNA differential gene expression analysis, and Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analyses.
RNA extraction and qRT-PCR
Expression of mRNAs was quantified using qRT-PCR. In brief, RNA was extracted from cells by lysing 100 mg of mouse liver tissue and cells (106 cells/sample) in TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. The extracted RNA was then quantified using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific, USA). Complementary DNA (cDNA) was synthesized from 1.0 μg of total RNA using oligo (dT) primers and a Thermo Scientific RevertAid First Strand cDNA synthesis kit (Thermo Scientific) according to the manufacturer's protocol. Expression of miR-142a-3p, WASL, IL-10, CCL2, α-SMA, collagen I, IL-1β, IL-23A, and IL-33 was analyzed using a SYBR Green Master Mix kit (Takara, Japan) with the primers listed in Table 1. GAPDH or U6 snRNA were used as internal control, and the fold-change in expression was calculated according to the 2−ΔΔCT method.
Table 1.
Primers used for qRT-PCR
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| WASL | TCCTTCTAGGCCTGGTGTTG | CTGTGGACCCTGCCATAGAT |
| IL-10 | AGTACAGCCGGGAAGACAAT | TCTAGGAGCATGTGGCTCTG |
| CCL2 | AACTGCATCTGCCCTAAGGT | CTGTCACACTGGTCACTCCT |
| α-SMA | CACAGCCCTGGTGTGCGACAAT | TTGCTCTGGGCTTCATCCCCCA |
| Collagen I | TCCTGCGCCTAATGTCCACCGA | AAGCGACTGTTGCCTTCGCCTC |
| IL-1β | CTCACAAGCAGAGCACAAGC | TCCAGCCCATACTTTAGGAAGA |
| IL-23A | ATGCTGGATTGCAGAGCAGTA | ACGGGGCACATTATTTTTAGTCT |
| IL-33 | ACACAGTCTCCTGCCTCCCT | CCACACCGTCGCCTGATTGA |
| GAPDH (mouse) | ACTCCACTCACGGCAAATTC | TCTCCATGGTGGTGAAGACA |
| miR-142a-3p | CTGTAGTGTTTCCTACTTTATG | mRQ 3′ Primer (Takara, Kyoto, Japan) |
| U6 | Takara, Kyoto, Japan | Takara, Kyoto, Japan |
rAAV vectors and transduction
The rAAVs HBAAV2/9-miR-142a-3p-green fluorescent protein (GFP) and HBAAV2/9-GFP were purchased from Hanbio Biotechnology. Mice were injected via the tail vein with viral particles (1.5 × 1011 v.g./mouse) 3 days after infection with S. japonicum. GFP expression associated with HBAAV2/9 in the liver was observed using fluorescence microscopy.
Histopathology and fibrosis measurement
The indexes of the liver, spleen, and thymus were calculated based on the following formula: total weight of mouse liver or spleen or thymus (mg)/total weight of mouse (g). The area of egg granulomas was measured on H&E-stained tissue sections using Image Pro Plus 6.0 software. The area of each granuloma in H&E-stained sections was measured. Liver tissues were weighed and digested overnight in 4% potassium hydroxide, and released eggs were then counted. Worms were fixed with 95% ethanol, 3% formalin, and 2% glacial acetic acid and then stained with 2.5% hydrochloric carmine red (Merck) for 1 h and preserved on glass slides. The worms were observed under a stereomicroscope (Leica M205FA, Germany), and worm length was calculated using the stereomicroscope (Leica M205FA). Fibrosis was detected by staining liver sections with Masson’s trichrome.
Prediction of potential miR-142a-3p target genes and dual-luciferase reporter assay
TargetScan was used to predict potential miR-142a-3p target genes. Target genes were selected for further study by combining scores and conserved binding sites in TargetScan. HEK293T cells were transfected with reporter plasmids encoding the un-mutated or mutated 3′ UTR of WASL and with miR-142a-3p mimic or control mimic for 48 h using RNAiMAX (Invitrogen). Luciferase assay reagent (Promega, USA) was added to the cells, and luciferase activity was detected using an Infinite F500 Multimarker Analyzer (Tecan, Austria). Luciferase activity was normalized to Renilla luciferase activity.
Western blotting
Liver tissues, neutrophils, and macrophages were homogenized using RIPA lysis buffer in the presence of freshly added phosphatase and protease inhibitors (Thermo Fisher Scientific, USA). Lysates were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the resolved proteins were transferred onto polyvinylidene fluoride blotting membranes (GE Healthcare Life Sciences, UK) and blocked using 5% skim milk. The membranes were incubated with the following primary antibodies (overnight at 4°C): anti-mouse N-WASP (1:1,000; Abcam), IL-10 (1:500; Proteintech, China), CCL2 (1:500; Boster, China), α-SMA (1:1,000; Abcam), collagen I (1:2,000; Proteintech), and TNF-α (1:1,000; Abcam). GAPDH (1:5,000; Sigma-Aldrich, USA) and β-actin (1:5,000; Sigma-Aldrich) were used as internal standards. Blots were incubated with anti-IgG conjugated to horseradish peroxidase as the secondary antibody for 2 h at room temperature. The membranes were visualized using an ECL western blotting detection system (Amersham, USA).
Purification of NETs
Neutrophils were treated with 500 nM PMA for 4 h. The culture supernatant containing NETs was centrifuged at 1,000 × g for 10 min at 4°C, and isolated NETs were used for subsequent experiments.
Statistical analysis
Results are expressed as mean ± SD. Differences between two groups were analyzed using unpaired two-sample t tests. Multiple comparisons between more than two groups were analyzed by one-way ANOVA. p values < 0.05 were considered statistically significant. Immunofluorescence and scanning electron microscopy data for NETs were quantified using ImageJ software. Statistical analyses were performed using GraphPad Prism 8.0.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (nos. 2021YFC2300800, 2021YFC2300801), the National Natural Science Foundation of China (grant nos. 81871682 and 81902081), the Natural Science Foundation of Guangdong Province (grant nos. 2020A1515011573, 2019A1515012068, and 2021A1515010976), and the Guangzhou Science and Technology Program (grant no. 202102100003). Generation of WASL gene knockout mice in the C57BL/6 background was supported by the 111 program (D20036).
Author contributions
L.W., X.S., and Z.W. conceived and designed the experiments. L.W., Z.Y., and Y.L. acquired the data. Z.Y. performed and analyzed the data for most of the experiments. L.Z., R.Y., and J.L. assisted with performing experiments and analyzing data. L.W., X.S., and Z.W. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.03.016.
Contributor Information
Zhongdao Wu, Email: wuzhd@mail.sysu.edu.cn.
Xi Sun, Email: sunxi2@mail.sysu.edu.cn.
Supplemental information
References
- 1.Wendt G., Zhao L., Chen R., Liu C., O'Donoghue A.J., Caffrey C.R., Reese M.L., Collins J.J. A single-cell RNA-seq atlas of Schistosoma mansoni identifies a key regulator of blood feeding. Science. 2020;369:1644–1649. doi: 10.1126/science.abb7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S., Zhang X., Liu Y., Yuan X., Yang L., Zhang R., Wang X., Xu F., Zhu C. Early application of caffeine improves white matter development in very preterm infants. Respir. Physiol. Neurobiol. 2020;281:103495. doi: 10.1016/j.resp.2020.103495. [DOI] [PubMed] [Google Scholar]
- 3.Chuah C., Jones M.K., Burke M.L., McManus D.P., Gobert G.N. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol. 2014;30:141–150. doi: 10.1016/j.pt.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., Uehata T., Iwasaki H., Omori H., Yamaoka S., et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Burke M.L., McManus D.P., Ramm G.A., Duke M., Li Y., Jones M.K., Gobert G.N. Temporal expression of chemokines dictates the hepatic inflammatory infiltrate in a murine model of schistosomiasis. PLoS Negl. Trop. Dis. 2010;4:e598. doi: 10.1371/journal.pntd.0000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Chen Q., Fang Y., Wu J., Han Y., Wang Y., Yang Y., Chu M., Feng Y., Tan L., et al. Schistosoma japonicum egg specific protein SjE16.7 recruits neutrophils and induces inflammatory hepatic granuloma initiation. PLos Negl. Trop. Dis. 2014;8:e2703. doi: 10.1371/journal.pntd.0002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirata M., Kage M., Hara T., Yoneda Y., Zhang M., Fukuma T. Schistosoma japonicum egg granuloma formation in the interleukin-4 or interferon-gamma deficient host. Parasite Immunol. 2001;23:271–280. doi: 10.1046/j.1365-3024.2001.00382.x. [DOI] [PubMed] [Google Scholar]
- 8.van Kessel K.P.M., Bestebroer J., van Strijp J.A.G. Neutrophil-mediated phagocytosis of Staphylococcus aureus. Front. Immunol. 2014;5:467. doi: 10.3389/fimmu.2014.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swamydas M., Gao J.-L., Break T.J., Johnson M.D., Jaeger M., Rodriguez C.A., Lim J.K., Green N.M., Collar A.L., Fischer B.G., et al. CXCR1-mediated neutrophil degranulation and fungal killing promote Candida clearance and host survival. Sci. Transl. Med. 2016;8:322ra310. doi: 10.1126/scitranslmed.aac7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 11.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science (New York, N.Y.) 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 12.Sollberger G., Choidas A., Burn G.L., Habenberger P., Di Lucrezia R., Kordes S., Menninger S., Eickhoff J., Nussbaumer P., Klebl B., et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018;3:eaar6689. doi: 10.1126/sciimmunol.aar6689. [DOI] [PubMed] [Google Scholar]
- 13.Urban C.F., Reichard U., Brinkmann V., Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 14.Cicco S., Cicco G., Racanelli V., Vacca A. Neutrophil extracellular traps (NETs) and damage-associated molecular patterns (DAMPs): two potential targets for COVID-19 treatment. Mediators Inflamm. 2020;2020:1–25. doi: 10.1155/2020/7527953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abi Abdallah D.S., Lin C., Ball C.J., King M.R., Duhamel G.E., Denkers E.Y. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 2012;80:768–777. doi: 10.1128/iai.05730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knackstedt S.L., Georgiadou A., Apel F., Abu-Abed U., Moxon C.A., Cunnington A.J., Raupach B., Cunningham D., Langhorne J., Kruger R., et al. Neutrophil extracellular traps drive inflammatory pathogenesis in malaria. Sci. Immunol. 2019;4:eaaw0336. doi: 10.1126/sciimmunol.aaw0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabriel C., McMaster W.R., Girard D., Descoteaux A. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J. Immunol. 2010;185:4319–4327. doi: 10.4049/jimmunol.1000893. Baltimore, Md, 1950. [DOI] [PubMed] [Google Scholar]
- 18.Bonne-Année S., Kerepesi L.A., Hess J.A., Wesolowski J., Paumet F., Lok J.B., Nolan T.J., Abraham D. Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes Infect. 2014;16:502–511. doi: 10.1016/j.micinf.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchery T., Moyat M., Sotillo J., Silverstein S., Volpe B., Coakley G., Tsourouktsoglou T.D., Becker L., Shah K., Kulagin M., et al. Hookworms evade host immunity by secreting a deoxyribonuclease to degrade neutrophil extracellular traps. Cell Host Microbe. 2020;27:277–289.e6. doi: 10.1016/j.chom.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Chuah C., Jones M.K., Burke M.L., Owen H.C., Anthony B.J., McManus D.P., Ramm G.A., Gobert G.N. Spatial and temporal transcriptomics of Schistosoma japonicum-induced hepatic granuloma formation reveals novel roles for neutrophils. J. Leukoc. Biol. 2013;94:353–365. doi: 10.1189/jlb.1212653. [DOI] [PubMed] [Google Scholar]
- 21.Lőrincz Á.M., Bartos B., Szombath D., Szeifert V., Timár C.I., Turiák L., Drahos L., Kittel A., Veres D.S., Kolonics F., et al. Role of Mac-1 integrin in generation of extracellular vesicles with antibacterial capacity from neutrophilic granulocytes. J. Extracell. Vesicles. 2020;9:1698889. doi: 10.1080/20013078.2019.1698889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angioni R., Liboni C., Herkenne S., Sánchez-Rodríguez R., Borile G., Marcuzzi E., Cali B., Muraca M., Viola A. CD73+extracellular vesicles inhibit angiogenesis through adenosine A2Breceptor signalling. J. Extracell. Vesicles. 2020;9:1757900. doi: 10.1080/20013078.2020.1757900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavallaro S., Pevere F., Stridfeldt F., Görgens A., Paba C., Sahu S.S., Mamand D.R., Gupta D., El Andaloussi S., Linnros J., et al. Multiparametric profiling of single nanoscale extracellular vesicles by combined atomic force and fluorescence microscopy: correlation and heterogeneity in their molecular and biophysical features. Small. 2021;17:e2008155. doi: 10.1002/smll.202008155. [DOI] [PubMed] [Google Scholar]
- 24.Fan Z., Xiao K., Lin J., Liao Y., Huang X. Functionalized DNA enables programming exosomes/vesicles for tumor imaging and therapy. Small. 2019;15:e1903761. doi: 10.1002/smll.201903761. [DOI] [PubMed] [Google Scholar]
- 25.Benmoussa A., Ly S., Shan S.T., Laugier J., Boilard E., Gilbert C., Provost P. A subset of extracellular vesicles carries the bulk of microRNAs in commercial dairy cow’s milk. J. Extracell. Vesicles. 2017;6:1401897. doi: 10.1080/20013078.2017.1401897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min L., Zhu S., Chen L., Liu X., Wei R., Zhao L., Yang Y., Zhang Z., Kong G., Li P., Zhang S. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: a comparison with plasma total miRNAs. J. Extracell. Vesicles. 2019;8:1643670. doi: 10.1080/20013078.2019.1643670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y., Chen L., Yan C., Zhou W., Endo Y., Liu J., Hu L., Hu Y., Mi B., Liu G. Circulating exosomal miR-20b-5p inhibition restores Wnt9b signaling and reverses diabetes-associated impaired wound healing. Small. 2020;16:e1904044. doi: 10.1002/smll.201904044. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L., Hao C., Yao S., Tang R., Guo W., Cong H., Li J., Bao L., Wang D., Li Y., et al. Exosomal miRNA profiling to identify nanoparticle phagocytic mechanisms. Small. 2018;14:e1704008. doi: 10.1002/smll.201704008. [DOI] [PubMed] [Google Scholar]
- 29.Zhu S., Wang S., Lin Y., Jiang P., Cui X., Wang X., Zhang Y., Pan W. Release of extracellular vesicles containing small RNAs from the eggs of Schistosoma japonicum. Parasites Vectors. 2016;9:574. doi: 10.1186/s13071-016-1845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meningher T., Barsheshet Y., Ofir-Birin Y., Gold D., Brant B., Dekel E., Sidi Y., Schwartz E., Regev-Rudzki N., Avni O., Avni D. Schistosomal extracellular vesicle-enclosed miRNAs modulate host T helper cell differentiation. EMBO Rep. 2020;21:e47882. doi: 10.15252/embr.201947882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L., Liao Y., Yang R., Yu Z., Zhang L., Zhu Z., Wu X., Shen J., Liu J., Xu L., et al. Sja-miR-71a in Schistosome egg-derived extracellular vesicles suppresses liver fibrosis caused by schistosomiasis via targeting semaphorin 4D. J. Extracell. Vesicles. 2020;9:1785738. doi: 10.1080/20013078.2020.1785738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori M.A., Ludwig R.G., Garcia-Martin R., Brandão B.B., Kahn C.R. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019;30:656–673. doi: 10.1016/j.cmet.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coakley G., Harris N.L. Interactions between macrophages and helminths. Parasite Immunol. 2020;42:e12717. doi: 10.1111/pim.12717. [DOI] [PubMed] [Google Scholar]
- 34.Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet (London, England) 2006;368:1106–1118. doi: 10.1016/s0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 35.McManus D.P., Dunne D.W., Sacko M., Utzinger J., Vennervald B.J., Zhou X.-N. Schistosomiasis. Nat. Rev. Dis. Primers. 2018;4:13. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 36.Li Hsü S.Y., Hsü H.F., Davis J.R., Lust G.L. Comparative studies on the lesions caused by eggs of Schistosoma japonicum and Schistosoma mansoni in livers of albino mice and rhesus monkeys. Ann. Trop. Med. Parasitol. 1972;66:89–97. doi: 10.1080/00034983.1972.11686801. [DOI] [PubMed] [Google Scholar]
- 37.Hirata M., Fukuma T. Cytokine regulation in experimentally-induced Schistosoma japonicum egg granuloma formation. Parasitol. Int. 2003;52:341–349. doi: 10.1016/s1383-5769(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 38.Chen K.W., Monteleone M., Boucher D., Sollberger G., Ramnath D., Condon N.D., von Pein J.B., Broz P., Sweet M.J., Schroder K. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 2018;3:eaar6676. doi: 10.1126/sciimmunol.aar6676. [DOI] [PubMed] [Google Scholar]
- 39.Bautista-Becerril B., Campi-Caballero R., Sevilla-Fuentes S., Hernández-Regino L.M., Hanono A., Flores-Bustamante A., Gonzalez-Flores J., Garcia-Avila C.A., Aquino-Galvez A., Castillejos-Lopez M., et al. Immunothrombosis in COVID-19: implications of neutrophil extracellular traps. Biomolecules. 2021;11:694. doi: 10.3390/biom11050694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetite D., Tavares L.A., Paiva I.M., et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Middleton E.A., He X.-Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., DaBler-Plenker J., Guerci P., Huynh C., Knight J.S., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217:e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kho S., Minigo G., Andries B., Leonardo L., Prayoga P., Poespoprodjo J.R., Kenangalem E., Price R.N., Woodberry T., Anstey N.M., Yeo T.W. Circulating neutrophil extracellular traps and neutrophil activation are increased in proportion to disease severity in human malaria. J. Infect. Dis. 2019;219:1994–2004. doi: 10.1093/infdis/jiy661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muñoz-Caro T., Rubio R.M.C., Silva L.M.R., Magdowski G., Gärtner U., McNeilly T.N., Taubert A., Hermosilla C. Leucocyte-derived extracellular trap formation significantly contributes to Haemonchus contortus larval entrapment. Parasites Vectors. 2015;8:607. doi: 10.1186/s13071-015-1219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cel. Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 46.Ren X.-S., Tong Y., Qiu Y., Ye C., Wu N., Xiong X.-Q., Wang J., Han Y., Zhou Y., Zhang F., et al. MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression. J. Extracell. Vesicles. 2020;9:1698795. doi: 10.1080/20013078.2019.1698795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C.-Z., Li L., Lodish H.F., Bartel D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science (New York, N.Y.) 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 48.Nimmo R., Ciau-Uitz A., Ruiz-Herguido C., Soneji S., Bigas A., Patient R., Enver T. MiR-142-3p controls the specification of definitive hemangioblasts during ontogeny. Dev. Cell. 2013;26:237–249. doi: 10.1016/j.devcel.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Q., Haupt S., Prots I., Thümmler K., Kremmer E., Lipsky P.E., Schulze-Koops H., Skapenko A. miR-142-3p is involved in CD25+ CD4 T cell proliferation by targeting the expression of glycoprotein A repetitions predominant. J. Immunol. 2013;190:6579–6588. doi: 10.4049/jimmunol.1202993. Baltimore, Md, 1950. [DOI] [PubMed] [Google Scholar]
- 50.Lu X., Li X., He Q., Gao J., Gao Y., Liu B., Liu F. miR-142-3p regulates the formation and differentiation of hematopoietic stem cells in vertebrates. Cell Res. 2013;23:1356–1368. doi: 10.1038/cr.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan H.-B., Liu Y.-J., Wang L., Du T.-T., Dong M., Gao L., Meng Z.Z., Jin Y., Chen Y., Deng M., et al. miR-142-3p acts as an essential modulator of neutrophil development in zebrafish. Blood. 2014;124:1320–1330. doi: 10.1182/blood-2013-12-545012. [DOI] [PubMed] [Google Scholar]
- 52.Westerberg L.S., Dahlberg C., Baptista M., Moran C.J., Detre C., Keszei M., Eston M.A., Alt F.W., Terhorst C., Notarangelo L.D., Snapper S.B. Wiskott-Aldrich syndrome protein (WASP) and N-WASP are critical for peripheral B-cell development and function. Blood. 2012;119:3966–3974. doi: 10.1182/blood-2010-09-308197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volpi S., Santori E., Abernethy K., Mizui M., Dahlberg C.I.M., Recher M., Capuder K., Csizmadia E., Ryan D., Mathew D., et al. N-WASP is required for B-cell-mediated autoimmunity in Wiskott-Aldrich syndrome. Blood. 2016;127:216–220. doi: 10.1182/blood-2015-05-643817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dahlberg C.I.M., Torres M.-L., Petersen S.H., Baptista M.A.P., Keszei M., Volpi S., Grasset E.K., Karlsson M.C., Walter J.E., Snapper S.B., et al. Deletion of WASp and N-WASp in B cells cripples the germinal center response and results in production of IgM autoantibodies. J. Autoimmun. 2015;62:81–92. doi: 10.1016/j.jaut.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian B.-Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R., Kaiser E.A., Snyder L.A., Pollard J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He X., Sun Y., Lei N., Fan X., Zhang C., Wang Y., Zheng K., Zhang D., Pan W. MicroRNA-351 promotes schistosomiasis-induced hepatic fibrosis by targeting the vitamin D receptor. Proc. Natl. Acad. Sci. U S A. 2018;115:180–185. doi: 10.1073/pnas.1715965115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiedemann M., Voehringer D. Immunomodulation and immune escape strategies of gastrointestinal helminths and schistosomes. Front. Immunol. 2020;11:572865. doi: 10.3389/fimmu.2020.572865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen J.E., Maizels R.M. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 59.Sun X., Yang F., Shen J., Liu Z., Liang J., Zheng H., Fung M., Wu Z. Recombinant Sj16 from Schistosoma japonicum contains a functional N-terminal nuclear localization signal necessary for nuclear translocation in dendritic cells and interleukin-10 production. Parasitol. Res. 2016;115:4559–4571. doi: 10.1007/s00436-016-5247-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.