Key Points
We conducted a Delphi-method survey with lymphoma experts to define modern enrollment criteria for first-line DLBCL RCTs.
We defined 31 modernized eligibility criteria to facilitate enrollment of a clinically diverse study population in first-line DLBCL RCTs.
Visual Abstract
Abstract
Recent first-line randomized controlled trials (RCTs) for patients with diffuse large B-cell lymphoma (DLBCL) have shown negative results, which may be due in part to onerous eligibility criteria limiting enrollment of poor-risk patients who require immediate treatment. We conducted a Delphi-method survey with lymphoma experts in the United States to define recommendations for essential and potentially unnecessary enrollment criteria for modern first-line DLBCL RCTs aimed at increasing clinical diversity of ensuing study groups. We first tabulated enrollment criteria from 19 DLBCL RCTs spanning the rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) era to identify common eligibility criteria from prior DLBCL RCTs for inclusion in the Delphi-method survey. We tabulated 451 total eligibility criteria comprising 51 criterion categories across 19 first-line DLBCL RCTs in the R-CHOP era. We then surveyed lymphoma clinical trial experts representing 8 academic medical centers in the United States regarding essential and unnecessary eligibility criteria for modern DLBCL RCTs. Seventeen of 29 invited clinical investigators completed the round-1 questionnaire (response rate, of 58.6%), 15 of 17 round-1 participants (88.2%) completed the round-2 survey, and all round-1 participants reviewed finalized recommendations for eligibility criteria for modern first-line DLBCL RCTs. We defined consensus recommendations for 31 modernized eligibility criteria including threshold values for 10 quantitative eligibility criteria aimed at facilitating enrollment of a clinically diverse study population in first-line DLBCL RCTs designed to improve standard-of-care therapy.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common adult non-Hodgkin lymphoma (NHL) and is clinically heterogeneous.1,2 Although 60% of patients with DLBCL who receive standard first-line treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) will experience a cure,3 the remaining 40% exhibit a median overall survival under 1 year, indicating significant unmet treatment needs for patients in poorest-risk groups.4 Recent randomized controlled clinical trials (RCTs) designed to improve first-line therapy for DLBCL have shown consistent negative results5-11 that may be due in part to time-intensive assessment of eligibility status limiting enrollment of patients who require urgent therapy.12
A key observational study showed that shorter diagnosis-to-treatment interval (DTI) is strongly associated with inferior event-free survival in newly diagnosed DLBCL.12 In the discovery cohort (n = 986 patients prospectively enrolled in the University of Iowa and Mayo Clinic Molecular Epidemiology Resource) and validation cohort (n = 1444 patients prospectively enrolled in the Lymphoma Study Association LNH-2003 clinical trials program), the median DTIs were 14 days (range, 0-155 days) and 23 days (range, 0-215 days), respectively. In both cohorts, patients with a DTI <14 days experienced reduced 24-month event-free survival when compared with patients with DTI >14 days. In the multivariable logistic regression model involving both cohorts, longer DTI predicted improvement in 24-month event-free survival, independent of clinical factors in the international prognostic index (IPI), with per-week odds ratios of 0.83 (95% confidence interval, 0.76-0.91; P < .001) and 0.93 (95% confidence interval, 0.88-0.98; P < .001) in the discovery and validation cohorts, respectively, in IPI-adjusted models.
This finding has implications for bias in DLBCL RCTs as patients who require rapid treatment are less likely to undergo the often time-consuming steps necessary to determine trial eligibility. Somewhat paradoxically, this results in reduced trial participation by the very patients who are most in need of improved treatment approaches: those with inferior outcomes on standard therapy. For instance, most recent RCTs for patients with DLBCL have described DTIs much longer than 14 days (eg, the ROBUST trial, which reported a median DTI of 31 days).11 When study populations are enriched for patients with longer DTI despite the use of poor-risk IPI eligibility criteria, we should expect improved survival in the control arms as was seen in US and European patients treated with R-CHOP,12 which may contribute to negative study results overall.5-11 Even the first-line POLARIX trial that met its primary endpoint had a mean DTI of 26 days.13
Efforts to revise trial enrollment processes and reduce DTIs among study populations have significant potential to limit bias in modern first-line DLBCL RCTs and increase the likelihood of observing true effects of novel study drugs. In the present study, we aimed to identify common and uncommon enrollment criteria from first-line DLBCL RCTs in the R-CHOP era and to develop consensus expert recommendations for modernized eligibility criteria in the interest of enrolling clinically diverse study populations that include patients with poor-risk disease in first-line DLBCL RCTs.
Methods
Categorizing eligibility criteria from prior DLBCL RCTs
We selected DLBCL RCTs representative of first-line clinical trials in the R-CHOP era. We identified studies that ranged from initial RCTs investigating R-CHOP in the first-line treatment of DLBCL through recent RCTs examining R-CHOP plus targeted therapy. We prioritized RCTs for inclusion based on access to study protocols through prior work14 or that were available as published supplemental materials. We selected this timeframe in the interest of assessing the full range of eligibility criteria in first-line clinical trials during the R-CHOP era and to capture criteria that may have been included in earlier studies and carried forward into recent trials despite lack of modern clinical indication. We tabulated inclusion and exclusion criteria from analyzed studies using study protocols, study publications, and information available on clinicaltrials.gov and calculated the average number of enrollment criteria per trial. After tabulating enrollment criteria, we further categorized enrollment criteria from selected studies into discrete criterion categories (eg, individual eligibility criteria pertaining to creatinine thresholds were categorized under the criterion category “renal function”). Common criterion categories were defined as those present in greater than or equal to two-thirds of RCTs, moderately common criterion categories were present in greater than or equal to one-third and fewer than two-thirds of RCTs, and uncommon criterion categories were present in fewer than one-third of RCTs.
Delphi-method survey
We conducted a Delphi-method survey15,16 with a panel of experts in lymphoma clinical trial design to identify consensus-essential and consensus-unnecessary eligibility criteria for first-line DLBCL RCTs. Survey participants were asked to complete a Delphi-method questionnaire regarding recommendations for modernized eligibility criteria with the specified aim of reducing DTI and increasing enrollment of patients with poorest-risk disease while maintaining patient safety in first-line DLBCL RCTs. Invited survey participants included clinical investigators and experts in biostatistics involved in the Lymphoma Epidemiology of Outcomes Cohort Study representing 8 academic medical centers in the United States. We derived eligibility criteria for inclusion in the survey based on common and moderately common eligibility criterion categories as tabulated from the prior DLBCL RCTs in order to focus expert discussion on eligibility criteria that were frequently included in first-line DLBCL RCTs in the R-CHOP era. We omitted criteria from inclusion in the survey that we believed were necessary for patient safety and would not foster discussion during the Delphi-method survey. We asked prospective survey participants to recommend additional pertinent eligibility criteria for inclusion in the survey that were not already represented after tabulation from analysis of the prior first-line DLBCL RCTs. See supplemental Figure 1 for a flowchart illustrating the criterion category selection process for inclusion in the Delphi-method survey.
The survey consisted of 3 rounds and was conducted via e-mail using Google Forms. In the first round, we asked survey participants to rate the importance of each eligibility criterion using a 1 to 9 Likert-style scale (1 = unnecessary for inclusion in future RCTs; 9 = essential). Survey participants were able to see a list of prospective survey respondents but did not have access to current responses of other members of the survey group when completing questionnaires. In addition, the first-round survey requested the total number of years each participant had worked in hematology/oncology. Based on first-round results, we designated each eligibility criterion as essential, unnecessary, unresolved, or in disagreement. Essential criteria had a median Likert value ≥7. Unnecessary criteria had a median value ≤3. Criteria were in disagreement if greater than one-third of respondents selected a value ≥7 and greater than one-third selected a value ≤3. Unresolved criteria showed median values from 4 to 6 and were not in disagreement. Statistical analyses related to survey results included median, range, and interquartile range (IQR) values for number of years’ experience in hematology/oncology; frequency, median, and IQR values for Likert values by eligibility criterion; and percentage of criterion categories designated as essential, unnecessary, unresolved, or in disagreement. Respondents received a personalized summary of round-1 results including median values and IQRs for all included criteria, histograms showing the ranking value distribution for each criterion, indication of the participant’s personal Likert response by criterion, and comments from survey participants for each eligibility criterion. All survey responses including Likert rankings and comments were anonymized in personalized summaries received by survey participants.
In the second round, we invited all round-1 survey participants to reassess eligibility criteria that were unresolved or in disagreement after round 1. Round 2 employed the methods used in round 1 and requested recommendations for threshold values for quantitative criteria. Survey options for quantitative threshold values were selected from threshold values used in the analyzed DLBCL RCTs from the R-CHOP era. The second-round survey also included free-text fields for survey respondents to provide context for their recommended threshold values or to suggest other threshold values. Statistical analyses conducted after round 2 included repeating analyses conducted following the round-1 survey as well as calculating the magnitude of change between round-1 and round-2 Likert ratings by criterion (ie, round-2 Likert value minus round-1 Likert value) by respondent for each respondent who participated in both round 1 and round 2. In addition, we calculated the frequency of quantitative thresholds selected by the expert panel for quantitative eligibility criteria. Based on survey results, we developed preliminary recommendations for eligibility criteria. A final survey asked all round-1 survey participants whether they agreed or disagreed with each preliminary recommendation and to provide additional context or explanation for their response. We developed finalized recommendations for eligibility criteria based on survey results. All authors reviewed our finalized recommendations for consensus, modernized eligibility criteria in first-line DLBCL RCTs.
Statistical analysis
We performed statistical analyses using R version 3.6.2 or greater.
Results
Categorizing eligibility criteria from prior DLBCL RCTs
We selected 19 DLBCL RCTs for analysis that spanned the R-CHOP era (Table 1). Notably, this study was designed and initiated before the authors had access to eligibility criteria from the REMoDL-B17 and GOYA18 trials (see “Discussion” and supplemental data for further analysis incorporating these RCTs). Across all trials, we tabulated 451 total enrollment criteria, with an average of 23.7 criteria per study (standard deviation, 6.3; range 14 to 37 criteria). Analysis of the 451 tabulated enrollment criteria revealed 51 discrete criterion categories (Table 2) across the 19 RCTs including 18 common, 11 moderately common, and 22 uncommon criterion categories.
Table 1.
Randomized controlled trials included in analysis (n = 19)
| Study identifier | Accrual start year | n | Treatment |
|---|---|---|---|
| LNH-98.530-32 | 1998 | 399 | CHOP-21 vs R-CHOP-21 |
| ECOG 4494/CALGB 979333 | 1998 | 546; 342 | R1: CHOP-21 vs R-CHOP-21; R2: observation vs rituximab |
| LNH-98.334 | 1999 | 474; 269 | R1: ACE vs ACVBP*; R2: observation vs rituximab |
| RICOVER-6035 | 2000 | 1215 | 6 cycles CHOP-14 vs 8 cycles CHOP-14 vs 6 cycles R-CHOP-14 vs 8 cycles R-CHOP-14 |
| MInT36,37 | 2000 | 796 | CHOP-like vs R-CHOP-like |
| DSHNHL 2002-138 | 2003 | 261 | R-CHOEP-14 vs R-MegaCHOEP followed by ASCT |
| ANZINTER339 | 2003 | 224 | R-CHOP-21 vs R-miniCEOP |
| LNH03-1B40 | 2003 | 223 | ACVBP† vs R-ACVBP† |
| LNH03-2B41 | 2003 | 379 | R-CHOP-21 vs R-ACVBP† |
| LNH03-6B42 | 2003 | 600 | R-CHOP-14 vs R-CHOP-21 |
| NHL1343 | 2004 | 681 | Observation vs rituximab |
| PIX20344 | 2005 | 122 | R-CHOP-21 vs R-CPOP |
| R-CHOP-14 vs R-CHOP-2145 | 2005 | 1062 | R-CHOP-14 vs R-CHOP-21 |
| MAIN46 | 2007 | 748 | R-CHOP-14 or R-CHOP-21 vs RA-CHOP-14 or RA-CHOP-21 |
| PYRAMID7 | 2009 | 206 | R-CHOP-21 vs VR-CHOP |
| ECOG-ACRIN 141247 | 2013 | 280 | R-CHOP-21 vs R2CHOP |
| PHOENIX48 | 2013 | 844 | R-CHOP-21 vs R-CHOP + brutinib |
| ROBUST49 | 2015 | 570 | R-CHOP-21 vs R-CHOP + lenalidomide |
| POLARIX50 | 2017 | 875 | R-CHOP-21 vs R-CHP + polatuzumab vedotin |
ACE, doxorubicin, cyclophosphamide, and etoposide; ASCT, autologous stem cell transplantation; CHOP-21, cyclophosphamide, doxorubicin, vincristine, and prednisone given every 21 days; DSHNHL, Deutsche Studiengruppe Hochmaligne Non-Hodgkin-Lymphome; ECOG-ACRIN, Eastern Cooperative Oncology Group and the American College of Radiology Imaging Network; HDT, high-dose therapy; MInT, Mabthera International Trial; R1, first randomization; R2, second randomization; R2CHOP, R-CHOP plus lenalidomide; RA-CHOP, R-CHOP plus bevacizumab; R-ACVBP, ACVBP plus rituximab; R-CHOEP-14, rituximab, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone given every 14 days; R-CHOP-21, rituximab + CHOP given every 21 days; R-CHP, rituximab, cyclophosphamide, doxorubicin, and prednisone; R-CPOP, rituximab, cyclophosphamide, pixantrone, vincristine, and prednisone; R-MegaCHOEP, R-CHOEP with dose-escalated cyclophosphamide, etoposide, and doxorubicin; R-miniCEOP, rituximab, cyclophosphamide, epirubicin, vinblastine, and prednisone; VR-CHOP, R-CHOP plus bortezomib.
ACVBP, doxorubicin, cyclophosphamide, vincristine, bleomycin, and prednisone.
ACVBP, doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone.
Table 2.
Criterion categories in 19 DLBCL RCTs
| Number of studies with criterion category n (%) | Common criterion categories (present in >66% of RTs; n = 18 categories) | Number of studies with criterion category n (%) | Moderately common criterion categories (present in 33% to 66% of RCTs; n = 11 categories) | Number of studies with criterion category n (%) | Uncommon criterion categories (present in <33% of RTCs; n = 22 categories) |
|---|---|---|---|---|---|
| 19 (100) | Age (y) | 11 (58) | HCV status | 6 (32) | Pulmonary function |
| 19 (100) | Histology | 11 (58) | Participation in other study | 6 (32) | Sex |
| 19 (100) | History of other malignancies | 10 (53) | Other neurologic pathology | 6 (32) | Recent surgical history |
| 19 (100) | Prior DLBCL treatment | 9 (47) | Hypersensitivity to study drugs | 5 (26) | Diabetes mellitus |
| 19 (100) | Renal function | 9 (47) | Other infectious disease status | 5 (26) | Patient compliance |
| 18 (95) | Hepatic function | 8 (42) | Imaging | 4 (21) | Adult patient under tutelage |
| 18 (95) | HIV status | 8 (42) | Minimum life expectancy | 4 (21) | Coagulopathy |
| 17 (89) | Cardiac function | 7 (37) | Contraindicated therapies | 4 (21) | Uncontrolled hypertension |
| 16 (84) | CNS involvement by lymphoma | 7 (37) | History of transformed lymphoma | 3 (16) | Hemoglobin (g/dL) |
| 16 (84) | Performance status | 7 (37) | Male reproductive concerns | 3 (16) | History of PTLD |
| 15 (79) | Contraindications to study therapy | 7 (37) | Psychiatric history | 3 (16) | Organ transplant history |
| 15 (79) | IPI score | 2 (11) | Bone marrow infiltration | ||
| 14 (74) | Female reproductive concerns | 2 (11) | Gastrointestinal function | ||
| 14 (74) | HBV status | 2 (11) | HTLV-1 status | ||
| 14 (74) | Other organ dysfunction | 1 (5) | CGA score | ||
| 14 (74) | Platelet count (platelets per µL) | 1 (5) | LDH level | ||
| 14 (74) | WBC count (cells per µL) | 1 (5) | Orthopedic history | ||
| 13 (68) | Ann Arbor stage | 1 (5) | Physical exam findings | ||
| 1 (5) | Rheumatologic disease | ||||
| 1 (5) | Substance use | ||||
| 1 (5) | Tumor invasion of major blood vessels | ||||
| 1 (5) | Vaccination history |
CGA, comprehensive geriatric assessment; CNS, central nervous system; HTLV-1, human T-lymphotropic virus 1; LDH, lactate dehydrogenase; PTLD, posttransplant lymphoproliferative disorder; WBC, white blood cell.
Delphi-method survey
We derived 30 total eligibility criterion categories for inclusion in the survey from common and moderately common criterion categories in the 19 DLBCL RCTs. From 29 common and moderately common criterion categories, 3 criterion categories were expanded for inclusion in the survey as follows: “histology” was expanded to include cell-of-origin (COO) subtype and CD20 positivity; “female reproductive concerns” was expanded to include pregnancy status, breastfeeding status, and contraception or abstinence; and “other neurologic pathology” was expanded to include peripheral neuropathy and history of stroke or intracranial hemorrhage. We omitted 3 criteria that we believed were necessary for patient safety (clinical contraindications to study therapy, known allergy or hypersensitivity reaction to study drugs, and whether the patient is receiving or has received contraindicated therapies that would preclude receiving study therapy). Prior to initiation of the first-round survey, 1 additional eligibility criterion category, “central pathology review prior to enrollment,” was proposed for inclusion in the questionnaire by a prospective survey participant for a total of 31 criterion categories included in the survey.
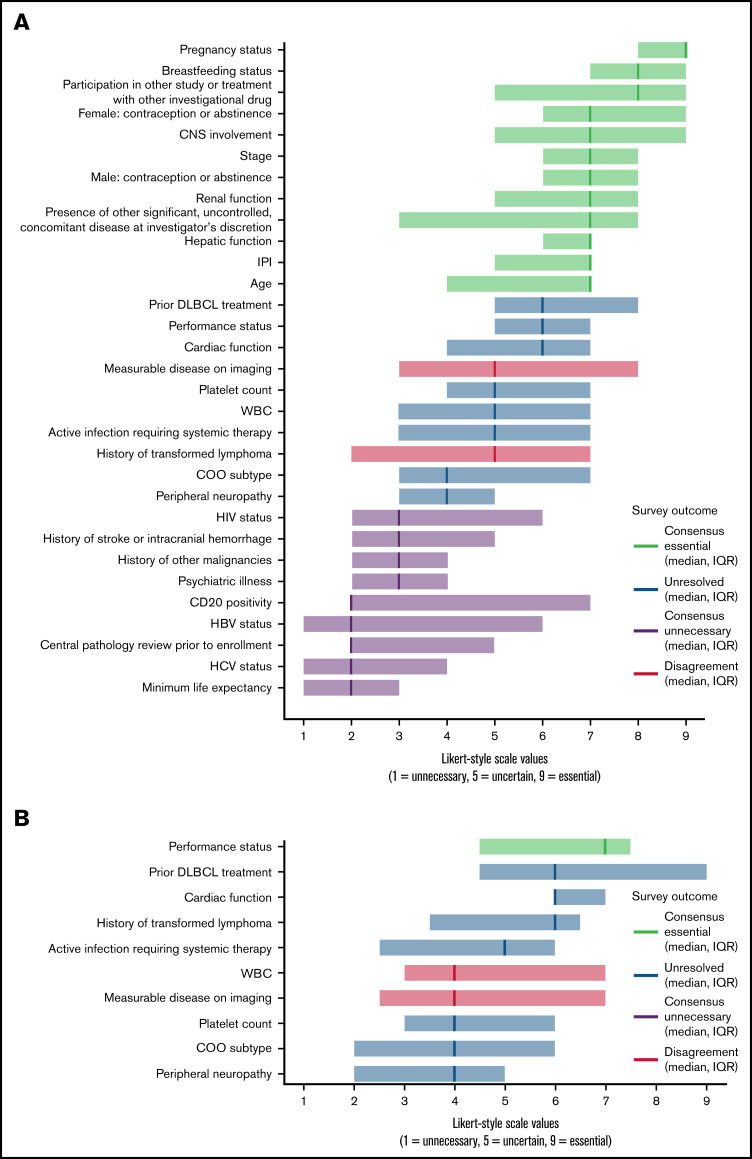
Among 29 clinical investigators invited to participate in the Delphi-method survey, 17 respondents including physicians and experts in biostatistics representing 8 academic medical centers in the United States completed the round-1 survey for a response rate of 58.6%. The median number of years’ experience in hematology/oncology among respondents was 17 years (IQR, 12 years; range, 3-30 years). The first round examined the previously derived 31 eligibility criterion categories. After the first-round survey, 12 of 31 criteria (39%) qualified as essential, 9 (29%) qualified as unnecessary, 2 (6%) showed disagreement, and 8 (26%) remained unresolved (Figure 1A). Essential criteria with the highest median Likert-style values included pregnancy status, breastfeeding status, and whether the patient was already receiving an investigational drug. Unnecessary criteria with the lowest median rankings included minimum life expectancy, HCV status, and central pathology review prior to enrollment. See supplemental data for results of all survey rounds.
Figure 1.
Results from a Delphi-method survey conducted with lymphoma trial experts to modernize enrollment criteria for first-line DLBCL RCTs comparing R-CHOP vs R-CHOP plus targeted therapy. Round 1 (A) included 31 total criterion categories common to first-line DLBCL RCTs spanning the R-CHOP era. Criteria with median Likert-style values ≥7 were deemed essential. Criteria with median values ≤3 were deemed unnecessary. Criteria with median values >3 and <7 were either unresolved or showed disagreement based on response distribution and were revisited in round 2 (B). CD20, cluster of differentiation 20; CNS, central nervous system; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IPI, international prognostic index; WBC, white blood cell.
Fifteen of 17 first-round respondents participated in the second round (response rate, 88.2%). Participants reassessed 10 criteria that had shown disagreement or remained unresolved. Based on round-2 results, 1 additional criterion, performance status, was deemed essential (Figure 1B). Although study participants commonly changed their Likert-style rankings between rounds 1 and 2 of the survey as illustrated in round-2 survey results available in supplemental data, the remaining 9 criteria continued to show disagreement or remain unresolved. Survey participants provided recommendations for threshold values for 10 quantitative eligibility criteria including measurements of renal, hepatic, and cardiac function, threshold levels for platelet count and white blood cell count, and recommended dimensions for measurable disease on imaging.
Twelve of 17 first-round participants responded to the final survey regarding preliminary recommendations (response rate, 70.6%). Across all criteria, the average rate of agreement among lymphoma experts in support of preliminary recommendations was 90.4% (standard deviation, 9.7%). Nine preliminary recommendations showed 100% agreement across survey respondents, and 23 of 31 criteria had ≥90% agreement. See supplemental data for data regarding agreement among experts with preliminary recommendations by criterion. Panel experts established final recommendations for all eligibility criteria assessed in prior survey rounds (Table 3).
Table 3.
Consensus recommendations for eligibility criteria in first-line DLBCL RCTs based on a Delphi-method survey of lymphoma clinical trials experts
| Criterion | Recommendation |
|---|---|
| Essential criteria | |
| Pregnancy status | Pregnant women should be excluded from enrollment. |
| Breastfeeding status | Breastfeeding should be prohibited during trial participation. |
| Female: contraception or abstinence | Effective contraception or abstinence from heterosexual intercourse is required for enrollment if of childbearing potential. |
| Male: contraception or abstinence | Effective contraception or abstinence from heterosexual intercourse is required for enrollment. |
| Participation in other study or treatment with other investigational drug | Study participants should receive no concurrent treatment with any other investigational therapy. Study participants should have received no treatment within the last 30 d with any other investigational therapy. Participation in nontherapeutic studies (eg, subject registries) is permitted. |
| IPI score | We recommend inclusion of IPI score as an eligibility criterion. No single IPI score range is recommended. IPI score range should be determined based on the target population for a given study. Alternately, consider using discrete elements of IPI as eligibility criteria rather than total IPI value. |
| Ann Arbor stage | Patients with Ann Arbor stages II-IV should be eligible for enrollment. Inclusion of patients with stage-I disease should depend on the study hypothesis and should be determined on a trial-by-trial basis. |
| Age at diagnosis | At baseline, patients aged ≥ 18 y should be eligible for trial participation. Determine final age range based on study intervention and target population, though most first-line RCTs do not require additional age cutoffs beyond age ≥ 18 y. |
| Performance status | We recommend including patients with PS of ECOG 0-2 and ECOG 3 if poor PS is due to lymphoma. |
| Renal function | Exclude patients based on a selected threshold value for renal function unless renal dysfunction is attributable to lymphoma. Selection of threshold value should take into account specific therapies in trial. Allow for use of both a Cr threshold and a CrCl threshold from the following ranges:
|
| Hepatic function | Exclude patients based on selected threshold values for hepatic function unless hepatic dysfunction is attributable to lymphoma or Gilbert’s syndrome. Selection of threshold values should take into account specific therapies in trial. Select baseline eligibility thresholds from the following ranges:
|
| CNS involvement | No known CNS involvement by lymphoma should be permitted in frontline trials evaluating strategies to improve standard of care therapy. Testing for CNS lymphoma is not required for enrollment and should be performed only when based on clinical suspicion. |
| Presence of other significant, uncontrolled, concomitant disease at investigator’s discretion | No other significant, uncontrolled, concomitant disease should be permitted at investigator’s discretion. |
| Unnecessary criteria | |
| CD20 positivity | Assessment of CD20 positivity is standard for diagnosis but should not be included as an eligibility criterion for enrollment in first-line clinical trials unless the investigational drug requires CD20 positivity to be efficacious. |
| Central pathology review prior to enrollment | Do not include central pathology review prior to enrollment as an eligibility criterion. |
| History of other malignancies | Do not enroll patients with another currently active malignancy at investigator's discretion, other malignancy requiring treatment that would preclude administration of study drugs, or other malignancy likely to be fatal during the trial evaluation window. Otherwise, do not include history of other malignancies as an eligibility criterion. |
| History of stroke or intracranial hemorrhage | If the experimental drug is known to increase risk for future CVA, do not include patients with a history of stroke or intracranial hemorrhage in the past 6 mo. |
| Psychiatric illness | Do not include psychiatric illness as an eligibility criterion. Inability to comply with study protocols, demonstrate decision-making capacity, or participate in informed consent (unless a legally authorized representative provides consent on the patient's behalf) should preclude study enrollment, regardless of the underlying reason. |
| HIV status | Although HIV testing should be performed as part of standard clinical practice, HIV infection should not preclude trial enrollment. Patients with HIV who have adequate viral suppression and disease control should be evaluated and monitored for potential drug-drug interactions with experimental therapies but otherwise should be considered eligible for trial participation. |
| HBV status | Although HBV testing should be performed as part of standard clinical practice, HBV infection should not preclude trial enrollment. Patients with HBV who have adequate viral suppression and disease control should be evaluated and monitored for potential drug-drug interactions with experimental therapies but otherwise should be considered eligible for trial participation. |
| HCV status | Although HCV testing should be performed as part of standard clinical practice, HCV infection should not preclude trial enrollment. Patients with HCV who have adequate viral suppression and disease control should be evaluated and monitored for potential drug-drug interactions with experimental therapies but otherwise should be considered eligible for trial participation. |
| Minimum life expectancy | Do not include minimum life expectancy as an eligibility criterion. |
| Unresolved criteria | |
| Prior DLBCL treatment | Generally speaking, no prior DLBCL treatment should be permitted for enrollment except treatment with corticosteroids or 1 cycle of chemotherapy at investigator’s discretion. This criterion should be tailored to the hypothesis and target population in a given study. |
| Cardiac function | Determine whether to include assessment of cardiac function as an eligibility criterion based on the toxicity profile of study drugs. If the study includes an anthracycline, include assessment of cardiac function as an eligibility criterion using the following criteria:
|
| Platelet count | Patients with platelet count ≥75 000 platelets/µL are eligible for enrollment unless levels are attributable to bone marrow infiltration or spleen involvement by DLBCL. If the study drug is known to cause thrombocytopenia, consider a higher threshold value. If low platelets are due to lymphoma, patients with platelet count ≥50 000 platelets/µL are eligible for enrollment. If the study drug is known to cause thrombocytopenia, consider a higher threshold value. Alternately, if low platelets are due to lymphoma, it may be reasonable to consider no threshold value for enrollment. |
| Active infection requiring systemic therapy | Patients with a serious, active infection at investigator's discretion should not be permitted to enroll. If a patient with an active infection is enrolled, resolution of infection at investigator’s discretion is required prior to initiation of study therapy. |
| History of transformed lymphoma | Patients with a history of treated, indolent lymphoma should not be eligible for enrollment. Patients with untreated, indolent lymphoma under observation should be eligible for enrollment. Composite lymphoma does not preclude enrollment. |
| Cell-of-origin subtype | Include COO subtype as an eligibility criterion only if the study is designed to target a COO subtype using the investigational drug in question. Otherwise, do not include COO subtype as an eligibility criterion. |
| Peripheral neuropathy | If the experimental drug is known to cause peripheral neuropathy, then exclude patients with neuropathy using a severity threshold based on the experimental drug’s known toxicity profile. Otherwise, exclude only patients with severe neuropathy, and include instructions for vincristine dose adjustment for patients with mild and moderate underlying peripheral neuropathy. |
| Criteria showing disagreement | |
| Measurable disease on imaging | If the primary endpoint in the trial is treatment response, then patients with measurable disease on imaging ≥1.5 cm in ≥1 diameter should be eligible for enrollment. Otherwise, do not include measurable disease on imaging as an eligibility criterion. Any evidence of disease is sufficient for enrollment. |
| White blood cell count | Patients with ANC ≥1000 cells/µL should be eligible for enrollment. Exclude patients with ANC <1000 cells/µL unless low levels are attributable to bone marrow infiltration or spleen involvement by DLBCL. If low ANC is due to lymphoma, do not use a threshold value for enrollment. |
ANC, absolute neutrophil count; CD20, cluster of differentiation 20; CNS, central nervous system; Cr, creatinine; CrCl, creatinine clearance; CVA, cerebrovascular accident; EF, ejection fraction; PS, performance status; R-CHOP + X, R-CHOP plus targeted therapy; ULN, upper limit of normal.
Discussion
We refined eligibility criteria for frontline DLBCL RCTs utilizing a Delphi-method survey that incorporated recommendations from a multi-institution panel of lymphoma experts from academic medical centers in the United States. We defined consensus recommendations for 31 eligibility criteria including threshold values for 10 quantitative criteria, and we identified 13 essential and 9 unnecessary criteria from criteria common to DLBCL RCTs in the R-CHOP era. Notably, our survey group liberalized or identified as unnecessary multiple criteria that were frequently included in prior DLBCL RCTs, emphasizing the importance of updating preenrollment assessments that became commonplace over time but are no longer clinically indicated in modern RCTs. We believe that our recommendations for updated threshold values and for eliminating criteria deemed currently unnecessary by the survey group can streamline trial enrollment and shorten DTI. This approach aligns with recommendations from national panels of experts for enhancing enrollment in cancer clinical trials.19-21 Further, survey results address multiple issues we believe are pertinent to reduce DTI in DLBCL RCTs including eliminating central pathology review prior to enrollment and allowance of prephase corticosteroids or bridging therapy prior to administering study drugs. Final recommendations for eligibility criteria showed strong agreement among survey respondents and are readily applicable in frontline DLBCL trials. It is our hope that the consensus recommendations included in this study will streamline the enrollment process for first-line DLBCL RCTs, shorten DTI for ensuing study populations, and increase the likelihood of identifying true effects of novel drugs for first-line treatment of DLBCL.
Notably, although survey respondents designated 22 eligibility criteria as essential or unnecessary, 9 criteria remained either unresolved or showed disagreement, illustrating the challenges inherent in reaching full consensus among experts in clinical trial design. Review of survey results shows that essential criteria generally pertained to safety of study drugs (eg, pregnancy and breastfeeding status), prognostic factors (eg, IPI score, age at diagnosis, and Ann Arbor stage), and patient-specific clinical factors including renal and hepatic function. Regarding IPI score, it should be noted that the survey group advised inclusion of IPI score or elements of the IPI score in eligibility criteria at this time given that patients with low IPI score have favorable outcomes with standard therapies, allowing for selection of patients in greatest need of trials. However, survey respondents emphasized that a better understanding of DLBCL biology may someday allow us to move beyond IPI score as an eligibility criterion. Unnecessary criteria included comorbid disease states that may warrant evaluation as part of routine clinical practice but are now clinically manageable during trial participation and should not preclude eligibility (eg, HIV, HBV, and HCV status). Similarly, preenrollment steps that would slow enrollment and increase DTI (eg, central pathology review prior to trial participation) were deemed unnecessary. Regarding eligibility criteria that remained unresolved or showed disagreement, the survey group commonly concluded that assessing the importance of these criteria required additional, case-by-case information. The additional information required may depend on the purpose of a given study, with the survey group advising, for example, that assessment of COO subtype should be included as an eligibility criterion only if the study drug targets COO subtypes. Alternately, the additional needed information may relate to the investigational therapy in question; for example, the survey group recommended that inclusion of platelet count thresholds should depend on whether the study drug is known to cause thrombocytopenia. Lastly, the required case-by-case information may pertain to the medical history of the patient being evaluated for inclusion. For example, the survey group concluded that a patient’s underlying cardiac function is pertinent in any study that includes an anthracycline, but assessment of left ventricular ejection fraction for all prospective patients prior to trial participation regardless of clinical indication may unnecessarily increase DTI. Criteria that remained unresolved or showed disagreement also involved factors influencing R-CHOP dose reduction or conditions likely to be exacerbated by R-CHOP toxicity. There was not investigator consensus regarding whether these criteria should be requirements for eligibility or applied using clinical judgment as physicians determine which patients to consider for enrollment. Finalized recommendations for eligibility criteria account for the added nuance of criteria that were unresolved or showed disagreement and can be readily applied to modern first-line DLBCL RCTs.
Multiple recent studies describe the importance of modernizing eligibility criteria for clinical trials in oncology including analyses highlighting criteria pertaining to minimum age threshold,21 comorbid organ dysfunction,22 and HIV status.23 In addition, recent examinations focused on DLBCL in particular indicate that organ-function based eligibility criteria in DLBCL RCTs limit inclusion of patients with poorest-risk disease24,25 and that DLBCL trial eligibility criteria have become increasingly restrictive over time, likely limiting recruitment.26 Our work addresses this urgent research gap by updating and streamlining enrollment criteria with important ramifications for inclusion of poor-risk patients in first-line DLBCL RCTs. It is our hope that by focusing on first-line DLBCL RCTs, our study will build on these prior analyses to streamline enrollment specific to first-line DLBCL clinical trials.
Limitations of our study include a round-1 response rate of 58.6% with 17 out of 29 invited experts responding to the initial questionnaire. A systematic review of Delphi-method studies showed that the median number of individuals invited to participate across 80 analyzed Delphi-method studies was 17 prospective participants,15 indicating that the total number of respondents in our survey was greater than the median number of respondents for studies of this kind. Nonetheless, the round-1 response rate and sample size may limit generalizability of study results. Response rates for subsequent rounds of the survey showed sustained participation among panel experts, with 88.2% and 70.6% of e-mailed survey participants replying to the round-2 and preliminary-recommendations questionnaires, respectively. Additionally, it should be noted that invited respondents in the present study were limited to physicians and experts in biostatistics representing major academic medical centers in the United States. The selection of experts for the survey panel has an important impact on survey results, and a similar study conducted with different stakeholders (eg, representatives of community health centers, ethics committees, or pharmaceutical companies) or conducted in a different region (eg, outside the catchment area of major academic medical centers or outside the United States) may reach different consensus recommendations for eligibility criteria in first-line DLBCL RCTs. However, our survey respondents represent a diverse group of lymphoma clinical trials research experts with considerable depth and breadth of clinical research experience, which achieves a key objective for enhancing enrollment in clinical trials for diverse populations based on prior recommendations.19,27 The scope of the present study was limited to identifying consensus recommendations for eligibility criteria from expert clinical investigators in the United States, and survey results may not be representative of opinions, perspectives, or local issues relevant to trial design in other areas. Recommendations from additional stakeholders could enrich the discussion regarding modern eligibility criteria in first-line DLBCL RCTs.
Strengths of the present study include the incorporation of expert opinion from clinical investigators with significant experience in first-line DLBCL RCTs to define streamlined essential and unnecessary eligibility criteria. The expert panel included senior clinical investigators; Lymphoma Committee chairs from SWOG, ECOG-ACRIN, and the Alliance; and members and chairs from the National Cancer Institute Lymphoma Steering Committee. Additionally, survey results exhibited high agreement rates for recommendations among lymphoma experts across proposed eligibility criteria (see supplemental data for concordance rates for preliminary criteria prior to development of finalized recommendations). Lastly, incorporation of inclusion and exclusion criteria from 19 DLBCL clinical trials from the R-CHOP era places our methods in context with pivotal trials spanning the relevant history of DLBCL RCTs. We considered whether these methods overemphasized eligibility criteria that were included early in the R-CHOP era but were excluded from more recent studies. When restricting analyzed trials to recent DLBCL RCTs that examined targeted therapies and included R-CHOP in the control arm, categorization of common and moderately common criteria yielded largely similar results when compared with criterion categories incorporating all 19 analyzed studies (supplemental Table 1). We also considered whether inclusion of additional first-line DLBCL RCTs from the R-CHOP era would have altered our results and yielded different survey questions in the Delphi-method survey. We examined inclusion and exclusion criteria from 2 DLBCL RCTs not included in analysis, REMoDL-B17 and GOYA,18 and reassessed common, moderately common, and uncommon eligibility criterion categories with the addition of these RCTs (supplemental Table 2). Eligibility criteria from both studies showed considerable overlap with common criteria from the previously analyzed 19 DLBCL RCTs, indicating that our survey was representative of eligibility criteria from first-line DLBCL RCTs in the R-CHOP era. With the REMoDL-B and GOYA studies included, 2 criterion categories, pulmonary function and recent surgical history, changed from uncommon to moderately common criterion categories and thereby would have been included in the Delphi-method survey if these additional studies had been incorporated in analysis. All other common and moderately common criterion categories remained the same with inclusion of the REMoDL-B and GOYA studies, yielding no further changes to the Delphi-method survey. It should be noted that the majority of RCTs included in analysis that incorporated assessment of pulmonary function in enrollment criteria stipulated exclusion based on substantial pulmonary impairment or uncontrolled chronic obstructive pulmonary disease, which were arguably captured in our Delphi-method survey under the criterion category “presence of other significant, uncontrolled, concomitant disease at investigator’s discretion.”
We believe that the recommended eligibility criteria included in this study are ready for application to novel first-line DLBCL RCTs. S1918 (NCT 04799275), a phase 2/3 RCT focused on treatment of newly diagnosed DLBCL and other lymphomas in patients ages 75 or older, is currently recruiting patients and adheres to many recommendations included in the present study, illustrating the potential of finalized recommendations to help guide development of future DLBCL clinical trials. Next steps for this work beyond direct application in modern DLBCL RCTs include combining recommended eligibility criteria with large DLBCL patient data sets to analyze demographic and outcomes data of prospective patient cohorts defined using proposed criteria. A recent study examining the impact of trial eligibility criteria on outcomes in a nationwide cohort of patients with newly diagnosed DLBCL suggests that real-world data can be used to evaluate the impact of eligibility criteria on prospective first-line DLBCL study populations.25 This would enable modification of recommended criteria as needed prior to development of future studies and could further increase the likelihood of enrolling a more clinically diverse patient population that includes poor-risk groups. Additionally, application of recommended eligibility criteria to DLBCL data sets that incorporate patient-level genetic characteristics would allow for anticipation of ensuing genetic cohorts when using finalized eligibility criteria and would facilitate development of future clinical trials incorporating novel targeted therapies. Although we believe that modernizing eligibility criteria is an important step toward increasing enrollment of poor-risk groups, additional research is needed in other avenues that may address DTI and barriers to trial participation including efforts to increase access, engagement of community sites, timely drug delivery, streamlined molecular testing when applicable, selection of time windows for meeting screening eligibility criteria, baseline imaging modalities, and other factors beyond the scope of the present study.
Applying modernized eligibility criteria has important implications for reducing the number and nature of preenrollment evaluations for all patients, enabling participation of patients who require urgent therapy in particular, and increasing generalizability of study results for first-line DLBCL RCTs. Given the identified impact of DTI on enrollment of poor-risk patients in first-line DLBCL trials,11 it is our hope that application of our expert consensus recommendations for eligibility criteria in first-line DLBCL RCTs will facilitate enrollment of a more clinically diverse patient population and increase generalizability of study results while maintaining patient safety.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) (grants TL1TR002382 and UL1TR002378) (R.A.H.). C.R.F. is a Cancer Prevention and Research Institute of Texas (CPRIT) Scholar of Cancer Research. The project described was supported in part by the National Cancer Institute (NCI) (K24 CA208132 award for mentored patient-oriented research in lymphoma) and CPRIT Award RR190079 (C.R.F.). Research reported in this publication was supported in part by the Biostatistics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292.
Authorship
Contribution: R.A.H. analyzed results and made tables and figures; S.P.P. and M.J.L. assisted in data collection; J.M.S. assisted in statistical analysis; S.M.A., N.L.B., K.A.B., A.F.C., C.C., J.W.F., P.B.J., B.S.K., J.P.L., B.K.L., I.S.L., P.M., M.J.M., N.M.-S., P.M.R., J.R.W., and C.R.F. developed consensus recommendations and edited the manuscript; J.L.K. wrote and edited the manuscript; and R.A.H. and C.R.F. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: C.C. receives research funding from Gilead, BMS, Genentech, and Verastem. J.W.F. discloses data safety monitoring committee participation for Acerta and Novartis. B.S.K. receives research funding from Genentech, ADC Therapeutics, Abbvie, AstraZeneca, and Beigene and provides consulting for Genentech, ADC Therapeutics, Abbvie, AstraZeneca, BeiGene, Pharmacyclics, Celgene/BMS, TG Therapeutics, Teva, Janssen, and MEI. J.P.L. provided consulting advice to Sutro, Epizyme, BMS/Celgene, Bayer, Gilead/Kite, GenMab, Genentech/Roche, Abbvie, Incyte, Janssen, Eisai, Mustang Bio, and Second Genome, and received research support from Genentech. I.S.L. has served on the advisory boards of Seattle Genetics, Janssen Scientific, and Verastem. P.M. is a consultant for ADCT, AstraZeneca, Bayer, Beigene, BMS, Cellectar, Epizyme, Gilead, Incyte, Janssen, Karyopharm, Morphosys, Regeneron, and Verastem. M.J.M. discloses consulting or advisory roles with MorphoSys, Kite Pharma, and Pfizer and research funding from Celgene, NanoString Technologies, Genentech, and Morphosys. P.M.R. has consultancy for Kite Pharma and research funding from SeaGen and Genentech. J.R.W. discloses consulting work with Novartis, Kite/Gilead, BMS, Morphosys, AstraZeneca, Janssen, Genentech, Iksuda, Umoja, and ADC Therapeutics and research funding from Novartis, Kite/Gilead, BMS, Morphosys, AstraZeneca, Janssen, Genentech, Curus, Unum, and ADC Therapeutics. J.L.K. has participated on advisory boards for Janssen, MorphoSys, TG Therapeutics, and Gamida Cell and received research funding from Oncternal Therapeutics, Viracta Therapeutics, and Atara Biotherapeutics. C.R.F. is a consultant for Abbvie, Bayer, BeiGene, Celgene, Denovo Biopharma, Genentech/Roche, Genmab, Gilead, Karyopharm, Pharmacyclics/Janssen, SeaGen, and Spectrum and has received research funding from 4D, Abbvie, Acerta, Adaptimmune, Allogene, Amgen, Bayer, Celgene, Cellectis, EMD, Gilead, Genentech/Roche, Guardant, Iovance, Janssen Pharmaceutical, Kite, Morphosys, Nektar, Novartis, Pfizer, Pharmacyclics, Sanofi, Takeda, TG Therapeutics, Xencor, Ziopharm, Burroughs Wellcome Fund, Eastern Cooperative Oncology Group, National Cancer Institute, V Foundation, and Cancer Prevention and Research Institute of Texas as the CPRIT Scholar in Cancer Research. N.M.-S. has institutional research support from Genentech/Roche, Bristol Myers Squibb/Celgene, AstraZeneca, Innate Pharmaceuticals, Corvus Pharmaceuticals, C4 Therapeutics, Secura Bio, Verastem, and Daiichi Sankyo and has served as a consultant for Daiichi Sankyo, Secura Bio, Kiowa Hakka Kirin, Ono Pharmaceuticals, C4 Therapeutics, and Karyopharm.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. R.A.H. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Correspondence: R. Andrew Harkins, Department of Internal Medicine, Emory University School of Medicine, 49 Jesse Hill Dr. SE, Suite 491, Atlanta, GA 30303; e-mail: andrew.harkins@emory.edu.
References
- 1.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459. [DOI] [PubMed] [Google Scholar]
- 2.Pasqualucci L, Dalla-Favera R. Genetics of diffuse large B-cell lymphoma. Blood. 2018;131(21):2307-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:498-505. [DOI] [PubMed] [Google Scholar]
- 4.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study [published correction appears in Blood. 2018;131(5):587–588]. Blood. 2017;130(16):1800-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Offner F, Samoilova O, Osmanov E, et al. Frontline rituximab, cyclophosphamide, doxorubicin, and prednisone with bortezomib (VR-CAP) or vincristine (R-CHOP) for non-GCB DLBCL. Blood. 2015;126(16):1893-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitolo U, Trněný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3529-3537. [DOI] [PubMed] [Google Scholar]
- 7.Leonard JP, Kolibaba KS, Reeves JA, et al. Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3538-3546. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett NL, Wilson WH, Jung SH, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: clinical outcomes of the phase III intergroup trial alliance/CALGB 50303. J Clin Oncol. 2019;37(21):1790-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies AJ, Barrans S, Maishman T, et al. Differential efficacy of bortezomib in subtypes of diffuse large b-cell lymphoma (DLBL): a prospective randomised study stratified by transcriptome profiling: REMoDL-B. Hematol Oncol. 2017;35(9):130-131.26228379 [Google Scholar]
- 10.Younes A, Sehn LH, Johnson P, et al. ; PHOENIX investigators . Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-Cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowakowski GS, Chiappella A, Gascoyne RD, et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol. 2021;39(12):1317-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurer MJ, Ghesquières H, Link BK, et al. Diagnosis-to-treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials. J Clin Oncol. 2018;36(16):1603-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022;386(4):351-363. [DOI] [PubMed] [Google Scholar]
- 14.Shi Q, Schmitz N, Ou FS, et al. Progression-free survival as a surrogate end point for overall survival in first-line diffuse large B-cell lymphoma: an individual patient-level analysis of multiple randomized trials (SEAL). J Clin Oncol. 2018;36(25):2593-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6(6):e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38(3):655-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies A, Cummin TE, Barrans S, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(5):649-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sehn LH, Martelli M, Trněný M, et al. A randomized, open-label, Phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-Cell lymphoma: final analysis of GOYA. J Hematol Oncol. 2020;13(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polite BN, Adams-Campbell LL, Brawley OW, et al. Charting the future of cancer health disparities research: a position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. J Clin Oncol. 2017;35(26):3075-3082. [DOI] [PubMed] [Google Scholar]
- 20.Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol. 2017;35(33):3737-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gore L, Ivy SP, Balis FM, et al. Modernizing clinical trial eligibility: recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Minimum Age Working Group. J Clin Oncol. 2017;35(33):3781-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtman SM, Harvey RD, Damiette Smit MA, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol. 2017;35(33):3753-3759. [DOI] [PubMed] [Google Scholar]
- 23.Uldrick TS, Ison G, Rudek MA, et al. Modernizing clinical trial eligibility criteria: recommendations of the american society of clinical oncology-friends of Cancer Research HIV Working Group. J Clin Oncol. 2017;35(33):3774-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khurana A, Mwangi R, Nowakowski GS, et al. Impact of organ function-based clinical trial eligibility criteria in patients with diffuse large B-cell lymphoma: who gets left behind? J Clin Oncol. 2021;39(15):1641-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Severinsen FT, Haunstrup LM, Jensen RK, et al. The impact of trial eligibility criteria on outcomes in a nationwide cohort of newly diagnosed DLBCL patients treated with R-CHOP. Blood. 2021;138(supplement 1):53. [Google Scholar]
- 26.Loh Z, Salvaris R, Chong G, et al. Evolution of eligibility criteria for diffuse large B-cell lymphoma randomised controlled trials over 30 years. Br J Haematol. 2021;193(4):741-749. [DOI] [PubMed] [Google Scholar]
- 27.Durant RW, Wenzel JA, Scarinci IC, et al. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT). Cancer. 2014;120(suppl 7):1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harkins RA, Patel SP, Lee MJ, Switchenko JM, Flowers CR. A data-driven approach to define parsimonious eligibility criteria in first-line clinical trials for diffuse large B-cell lymphoma. Blood. 2019;134(supplement_1):3416. [Google Scholar]
- 29.Harkins RA, Flowers CR. Modernizing Eligibility Criteria in First-Line Clinical Trials for Diffuse Large B-Cell Lymphoma: Consensus Recommendations Using a Modified Delphi-Method Survey. Poster presented at: the 62nd ASH Annual Meeting and Exposition; 5-8 December 2020; virtual conference.
- 30.Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. [DOI] [PubMed] [Google Scholar]
- 31.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23(18):4117-4126. [DOI] [PubMed] [Google Scholar]
- 32.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121-3127. [DOI] [PubMed] [Google Scholar]
- 34.Haioun C, Mounier N, Emile JF, et al. Rituximab versus observation after high-dose consolidative first-line chemotherapy with autologous stem-cell transplantation in patients with poor-risk diffuse large B-cell lymphoma. Ann Oncol. 2009;20(12):1985-1992. [DOI] [PubMed] [Google Scholar]
- 35.Pfreundschuh M, Schubert J, Ziepert M, et al. ; German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) . Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105-116. [DOI] [PubMed] [Google Scholar]
- 36.Pfreundschuh M, Trümper L, Osterborg A, et al. ; MabThera International Trial Group . CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379-391. [DOI] [PubMed] [Google Scholar]
- 37.Pfreundschuh M, Kuhnt E, Trümper L, et al. ; MabThera International Trial (MInT) Group . CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013-1022. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz N, Nickelsen M, Ziepert M, et al. ; German High-Grade Lymphoma Study Group (DSHNHL) . Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol. 2012;13(12):1250-1259. [DOI] [PubMed] [Google Scholar]
- 39.Merli F, Luminari S, Rossi G, et al. Cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab versus epirubicin, cyclophosphamide, vinblastine, prednisone and rituximab for the initial treatment of elderly “fit” patients with diffuse large B-cell lymphoma: results from the ANZINTER3 trial of the Intergruppo Italiano Linfomi. Leuk Lymphoma. 2012;53(4):581-588. [DOI] [PubMed] [Google Scholar]
- 40.Ketterer N, Coiffier B, Thieblemont C, et al. Phase III study of ACVBP versus ACVBP plus rituximab for patients with localized low-risk diffuse large B-cell lymphoma (LNH03-1B). Ann Oncol. 2013;24(4):1032-1037. [DOI] [PubMed] [Google Scholar]
- 41.Récher C, Coiffier B, Haioun C, et al. ; Groupe d’Etude des Lymphomes de l’Adulte . Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378(9806):1858-1867. [DOI] [PubMed] [Google Scholar]
- 42.Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013;14(6):525-533. [DOI] [PubMed] [Google Scholar]
- 43.Jaeger U, Trneny M, Melzer H, et al. ; AGMT-NHL13 Investigators . Rituximab maintenance for patients with aggressive B-cell lymphoma in first remission: results of the randomized NHL13 trial. Haematologica. 2015;100(7):955-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herbrecht R, Cernohous P, Engert A, et al. Comparison of pixantrone-based regimen (CPOP-R) with doxorubicin-based therapy (CHOP-R) for treatment of diffuse large B-cell lymphoma. Ann Oncol. 2013;24(10):2618-2623. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817-1826. [DOI] [PubMed] [Google Scholar]
- 46.Seymour JF, Pfreundschuh M, Trnĕný M, et al. ; MAIN Study Investigators . R-CHOP with or without bevacizumab in patients with previously untreated diffuse large B-cell lymphoma: final MAIN study outcomes. Haematologica. 2014;99(8):1343-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowakowski GS, Hong F, Scott DW, et al. Addition of lenalidomide to R-CHOP improves outcomes in newly diagnosed diffuse large B-cell lymphoma in a randomized phase II US intergroup study ECOG-ACRIN E1412. Hematol Oncol. 2021;39(12):1329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Younes A, Zinzani PL, Sehn LH, et al. A randomized, double-blind, placebo-controlled phase 3 study of ibrutinib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in subjects with newly diagnosed nongerminal center B-cell subtype of diffuse large B-cell lymphoma (DLBCL). J Clin Oncol. 2014;32(15 suppl). [Google Scholar]
- 49.Vitolo U, Witzig TE, Gascoyne RD, et al. ROBUST: First report of phase III randomized study of lenalidomide/R-CHOP (R2-CHOP) vs placebo/R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma. Hematol Oncol. 2019;37(S2):36-37. [Google Scholar]
- 50.Tilly H, Flowers C, Friedberg JW, et al. A phase 3 study comparing polatuzumab vedotin plus R-CHP versus R-CHOP in patients with DLBCL (POLARIX). J Clin Oncol. 2018;36(15 suppl). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.