Summary
Background
Autonomic dysfunction has been implicated in the pathophysiology of the Sudden Infant Death Syndrome (SIDS). Butyrylcholinesterase (BChE) is an enzyme of the cholinergic system, a major branch of the autonomic system, and may provide a measure of autonomic (dys)function. This study was undertaken to evaluate BChE activity in infants and young children who had died from Sudden Infant Death or Sudden Unexpected Death.
Methods
In this case-control study we measured BChE activity and total protein in the eluate of 5μL spots punched from the dried blood spots taken at birth as part of the newborn screening program. Results for each of 67 sudden unexpected deaths classified by the coroner (aged 1 week-104 weeks) = Cases, were compared to 10 date of birth - and gender-matched surviving controls (Controls), with five cases reclassified to meet criteria for SIDS, including the criterion of age 3 weeks to 1 year.
Findings
Conditional logistic regression showed that in groups where cases were reported as “SIDS death” there was strong evidence that lower BChE specific activity (BChEsa) was associated with death (OR=0·73 per U/mg, 95% CI 0·60-0·89, P=0·0014), whereas in groups with a “Non-SIDS death” as the case there was no evidence of a linear association between BChEsa and death (OR=1·001 per U/mg, 95% CI 0·89-1·13, P=0·99).
Interpretation
BChEsa, measured in dried blood spots taken 2-3 days after birth, was lower in babies who subsequently died of SIDS compared to surviving controls and other Non-SIDS deaths. We conclude that a previously unidentified cholinergic deficit, identifiable by abnormal -BChEsa, is present at birth in SIDS babies and represents a measurable, specific vulnerability prior to their death.
Funding
All funding provided by a crowd funding campaign https://www.mycause.com.au/p/184401/damiens-legacy
Keywords: Sudden Infant Death Syndrome, Sudden Unexpected Death in Infancy, Butyrylcholinesterase, Cholinergic deficit, Autonomic function, Arousal
Research in context.
Evidence before this study
Despite the effectiveness of public health campaigns in reducing the incidence of Sudden Infant Death Syndrome (SIDS), SIDS remains the major cause of infant death in western countries. The “triple risk model” hypothesises that SIDS deaths result from coincident occurrence of a vulnerable infant, a critical developmental period, and an exogenous stressor. Despite intensive research, identification of any specific vulnerability prior to the sudden death has remained elusive. And, while autonomic dysfunction has long been considered a candidate for this vulnerability, studies have been hampered by reliance on post-mortem samples.
Added value of this study
We found that Butyrylcholinesterase Activity, measured in dried blood spots taken 2-3 days after birth, was significantly lower in babies who subsequently died of SIDS compared to living controls and other Non-SIDS infant deaths. This study identifies a biochemical marker that differentiates SIDS infants from control cases and those dying from other causes, prior to their death. We postulate that this decreased activity of Butyrylcholineserase represents an autonomic cholinergic dysfunction and therefore an inherent vulnerability of the SIDS infants.
Implications of all the available evidence
This finding represents the possibility for the identification of infants at risk for SIDS infants prior to death and opens new avenues for future research into specific interventions.
Alt-text: Unlabelled box
Introduction
The term Sudden Unexpected Death in Infancy (SUDI) covers both explained and unexplained deaths. The unexplained deaths are termed Sudden Infant Death Syndrome (SIDS). Despite intensive research over the past decades, the mechanisms which lead to SIDS remain elusive. SIDS occurs in an apparently healthy infant during a period of sleep, with the cause unexplained even after a thorough post-mortem examination, death scene investigation and review of the infant's medical history.1
In recent years, the incidence of SIDS has been more than halved by public health campaigns however, despite this dramatic decline in incidence, SIDS remains the major cause of sudden and unexpected death in infants in western countries, contributing to almost 50% of all post-neonatal deaths.2
It is currently believed that SIDS is not due to a single factor, but is multi-factorial in origin. Several models have been proposed to explain the multifactorial nature of SIDS, with the most widely accepted model being the “triple risk model” for SIDS which provides a useful means for organising SIDS knowledge. According to this model, sudden death in SIDS occurs when three factors occur simultaneously: a vulnerable infant, a critical developmental period for homeostatic control and an exogenous stressor. An infant will die of SIDS only if he/she possesses all three factors; the infant's vulnerability lies latent unless subjected to an exogenous stressor during the critical period.3 Despite intensive research over the past decades, identification of any specific vulnerability has remained elusive.
Acetylcholine (ACh) is a major neurotransmitter of the autonomic nervous system and the principal neurotransmitter of the parasympathetic nervous system. It is hydrolysed at cholinergic synapses by two enzymes, Acetylcholinesterase (AChE), and Butyrlycholinesterase (BChE) (also known as pseudocholinesterase). Variable levels of AChE and BChE have been observed in parasympathetic dysfunction and inflammatory disease states and it has been suggested that levels of cholinesterases could potentially be used as biomarkers for cholinergic deficit and parasympathetic dysfunction as well as inflammation-related disease.4 As AChE and BChE (the cholinesterases) are responsible for hydrolysis of ACh they modulate cholinergic activity. The cholinesterases are present throughout almost all tissues of the body, and the activity of the different cholinesterases varies amongst those different tissues. In an exploratory study such as ours, the ideal would be to measure both AChE and BChE and our initial study plan included analysis of both cholinesterases, BChE and AChE. However, we found this was not possible in the Dried Blood Spot (DBS)samples and the methodological issues we encountered likely explain why other researchers describe the measurement only of BChE in DBSs, and not the measurement of AChE.5, 6, 7 Using the DBSs taken routinely at birth via a heel prick (Guthrie Test) we conducted a case-control study evaluating the BChE activity of SIDS infants, against Non-SIDS infants and date of birth- and gender-matched (living) controls. The present study sought to determine whether infants who succumb to SIDS have altered levels of BChE activity at time of birth, thus evaluating the activity of this enzyme as a biomarker for detecting an infant's vulnerability to SIDS.
Methods
Samples
DBSs for this study were collected on post-natal day (PND) Day 2–4 on Whatman 903 filter paper (PerkinElmer) over a 5 -year period, 2016–2020 and stored at room temperature.
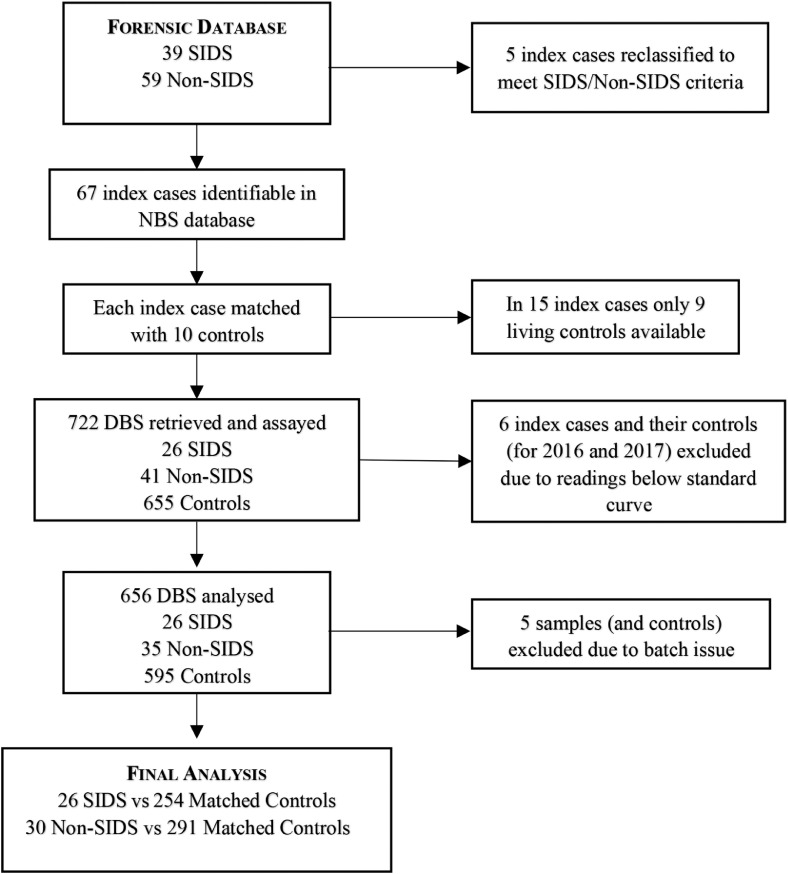
Children categorised as SUDI who died aged <24 months from unknown causes between July 2018 and July 2020 were identified from the databases of 3 Forensic Pathology sites within the state of NSW, Australia. The forensic pathologists’ classification of death was used in all but 5 cases: 2 cases classified as SIDS were reclassified as SUDI because they were aged ≤ 3 weeks, 1 case classified as SIDS was reclassified to SUDI due to being > 52 weeks, and 2 cases classified as unknown met the criteria for, and were reclassified as, SIDS. All Non-SIDS Cases had an identified cause of death.
From Forensics, for privacy reasons, we were given only the name of the deceased child while dried blood spot samples are recorded under the mother's name. Thus, only those dried blood spots recorded with the same surname for mother and child were identifiable. If these names differed those cases could not be followed. The typical example would be where the mother and father have different surnames, and the infant is identified by mother's name in the newborn period, but later identified by the paternal surname. As this was a random issue, we considered the analysed samples to be representative of the wider population.
The DBS identification number for each child was retrieved from the database of the NSW Department of Newborn screening (NBS), located at The Children's Hospital Westmead (CHW). For each case, 10 date of birth- and gender-matched samples of surviving children, born on the same day as the index case, and whose blood spot was taken on the same PND, were identified (Controls). From the 98 cases listed in the forensic medicine database 31 cases were excluded as the DBS sample could not be identified from the limited information available, or the sample was collected on day 1 which excludes their suitability for newborn screening. Certain conditions may go undetected if the blood sample is drawn before 24 h of age and this also avoids measuring maternal rather than infant-specific levels of the compounds of interest.8 Filter papers for all cases and their matched controls were retrieved from storage. Each filter paper was punched twice with a 3mm hole punch, each punch roughly equivalent to 5ul of blood. Punches were stored at room temperature, in the dark, in Eppendorf tubes until time of analysis.
During analysis experimenters were blind to group assignment and outcome assessment, for all experiments. Once the sample identification numbers were retrieved, cases and controls were de-identified and the diagnoses only re-identified at the time of statistical analysis. BChE activity as well as total protein were assayed using commercially available kits.
Study design
While we planned to measure both cholinesterases we were unable to measure AChE in the DBS samples. BChE is found in plasma, which is virtually free of AChE and the assay for BChE is specific to this enzyme as it utilises the substrate butyrylthiocholine, which shows almost zero reactivity with AChE.9 By contrast, AChE is found in erythrocytes and the assay for AChE evaluates total cholinesterase, as the substrate acetylthiocholine iodide is metabolised by both AChE and BChE. The assay recommendation is to determine total cholinesterase activity but determine AChE by subtracting BChE from that total.9 Our preliminary studies revealed that we were unable to measure AChE using that method, and we obtained only BChE, presumably because the erythrocyte is lysed during the drying process10 resulting in denatured AChE.
Sample preparation
Two punched samples from the DBS cards, approximately equivalent to 10 ul blood, were eluted with 90ul of water, giving a 1:10 initial dilution. Water was the eluate chosen as previous research has shown that this is the optimal elution medium for BChE and total protein.10 Samples were placed in a 96-deep well (Porvair Sciences, Wrexham UK) plate and placed on shaker (Ratek Instruments, Victoria Australia) at 750 rpm for 30min. Samples were then spun at 5°C for 10 min at 4500 rpm (Eppendorf, Centrifuge 5804 R, Merck Instruments, Germany). The eluate was then transferred to a new shallow 96-well plate, sealed with heat-sealing foil (Eppendorf, NSW Australia) and stored at -20°C until analysis.
Sample assays******comment*****
Butyrylcholinesterase activity: Total BChE for each sample was quantified using the DetectX® Butyrylcholinesterase Fluorescent Activity Kit (Arbor Assays, Ann Arbor, MI, USA, catalogue number K016-F1) and the assay was performed as per the manufacturer's instructions. Previous analyses of BChE in dried blood spots have used the colormetric ELISA Ellman's assay, or a modification thereof, but as the DBS samples we were analysing had been stored for some time, we decided to utilise a fluorescent ELISA assay due to the increased sensitivity of this method.
Blood samples from 2 healthy adults were used to monitor quality control (QC) of the assay. Blood from a finger prick was spotted onto a filter paper and stored in the same manner as the infant DBSs. At the same time venous blood was taken and placed in EDTA tube, spun down and the plasma frozen until required. For each assay plate, duplicate samples of the DBS QC and its matching plasma QC were assayed to ensure accuracy of the method.
Briefly, samples were diluted further as per manufacturer's instructions such that sample and QC values were within the standard curve. 100 μl of standard, diluted sample or QC was then placed in duplicate in a 96-well microtitre plate and the reaction was initiated by the addition of 50 μl reaction mix containing the butyrylthiocholine substrate and ThioStar® (proprietary, non-fluorescent molecule). After a 20-min incubation, at room temperature and in the dark, the signal was read at 510nm with excitation at 390nm. MyAssay software was used to perform a four parameter logistic curve (4PLC) to quantify total BChE activity via a calibration curve of seven standard points and activity measured in mU/ml.
Total protein in each sample was quantified using the BCA (Bicinchoninic acid) Dual Range Protein Detection Kit (Arbor assays, Ann Arbor, MI, USA, catalogue number K041-H1) and measured in duplicate. This colorimetric assay relies on the formation of a Cu2+-protein complex under alkaline conditions, followed by reduction of the Cu2+ to Cu1+. The amount of reduction is proportional to protein present. 10 µL of standard, diluted sample or QC was then placed in duplicate in a 96-well microtitre plate and the reaction initiated by the addition of 75 µL of the BCA Colour Solution. The plate was sealed and incubated at 37°C for two hours and optical density then read at 560 nm.
MyAssay software was used to perform a 4PLC to quantify total protein via a calibration curve of seven standard points, and total protein content measured in µg/ml.
Specific activity: The specific activity of BChE (BChEsa) was calculated by dividing BChE activity (mU/ml) by the total protein content (µg/ml) giving BChEsa in mU/µg. Results are reported in U/mg.
Statistical analysis
Conditional logistic regression models were used to model the outcome of case (death)/control (alive) status in each sample group using the predictors of BChE as a continuous variable, cause of death (SIDS versus other) and their interaction. If evidence of interaction was found, BChE as a predictor of case/control status was tested in conditional logistic regression models separately in SIDS and Non-SIDS sample groups.
Models with age at death as the outcome variable and BChEsa, gender and cause of death as predictors were each run individually in univariable models then all three in a multivariable model.
Ethics
Consent for use of the DBSs in future de-identified health research was obtained from parents or caregivers at the time of collection. The study was approved by the SCHN Research Ethics Committee (HREC Reference: 2019/ETH12837).
Role of funders
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report.
Results
We analysed 722 DBS including 67 DBS (58% male) from SUDI infants (26 SIDS and 41 Non-SIDS), and 655 date of birth- and gender-matched controls. SIDS cases, mean age-at death 15·7 (± 8·1) weeks, (4-35 weeks), 54% male and Non-SIDS cases, mean age at death 31·7 (± 30) weeks, (1-103 weeks), 64% male.
All DBSs were analysed for BChE activity and total protein content and the BChEsa calculated. Consistent with other studies,7 our initial experiments confirmed that BChE activity was retained despite the elution processes. There was good correlation between the BChE activity of plasma QCs and their corresponding DBS QCs (r 0·84), although the recovery of BChE activity in the DBS QC compared to the plasma QC was lower (23·4 ± 2·0%) than has been reported in other studies.6,7 BChE activity for infants born in 2016 and 2017 (a total of 6 of the DBSs from those who died during the study period) yielded several results below the standard curve that were therefore discounted from further analysis. Five Non-SIDS samples and their controls had a batch assay problem and, as there was insufficient sample to re-assay, these samples were excluded from the analysis. This left 30 Non-SIDS cases, mean age at death 23·5 (± 30) weeks, (range 1–103 weeks), 57% male, and their controls in the final analysis (Fig. 1).
Figure 1.
Flow Diagram - Sample preparation and analysis. Flow diagram of samples analysed.
To overcome variability due to differences in punch location and hematocrit we normalised BChE activity to total protein content7 and there was good recovery of the total protein in the plasma QC compared to its corresponding DBS QC (96·25 ± 11·8%).
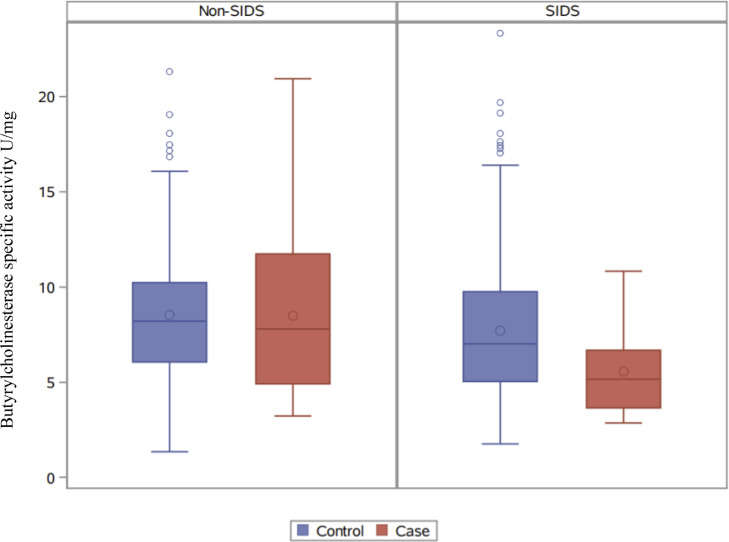
Mean values (± Standard Deviation) for BChEsa for SIDS cases (n=26) and their controls (n=254) was 5·6 (±2·1) vs 7·7 (±3·6), respectively and for Non-SIDS cases (n=30) and their controls (n=291) was 8·5 (±4·2) vs 8·5 (±3·4), respectively. Descriptive statistics are detailed in Table 1 and illustrated in Figs. 2 and 3.
Table 1.
Butyrylcholinesterase Specific Activity (U/mg) in SIDS and Non-SIDS Cases and their matched controls.
| Mean (±SD) | Range | Median | OR per U/mg BChEsa | OR 95% CI | P value | ||
|---|---|---|---|---|---|---|---|
| Non-SIDS | Control (n=291) | 8·5 ± 3·4 | 1·3-21·3 | 8·2 | |||
| Cases (n = 30) | 8·5 ± 4·2 | 3·2-20·9 | 7·8 | 1·001 | 0·89-1·13 | 0·99 | |
| SIDS | Control (n=254) | 7·7 ± 3.6 | 1·7-23·3 | 7·0 | |||
| Cases (n=26) | 5·6 ± 2.1 | 2·9-10·8 | 5·2 | 0·73 | 0·60-0·89 | 0·0014 |
Butyrylcholinesterase Specific Activity (U/mg) in infants classified as Sudden Infant Death Syndrome (SIDS) and Non-SIDS (other causes) cases and their date of birth- and gender-matched controls (SD = standard deviation). Conditional logistic regression was used to calculate Odds Ratio (OR) per U/mg Butyrylcholinesterase specific activity (BChEsa), OR 95% Confidence Intervals (95% CI) and P values.
Figure 2.
Butyrylcholinesterase specific activity in Non-SIDS and SIDS cases and controls. Box plot showing Butyrylcholinesterase specific activity (U/mg), from left to right, in Non-SIDS controls (n=291), Non-SIDS cases (n=30), SIDS controls (n=254) and SIDS cases (n=26), illustrating interquartile ranges, outliers, and mean (○) and median (−) values for controls and cases.
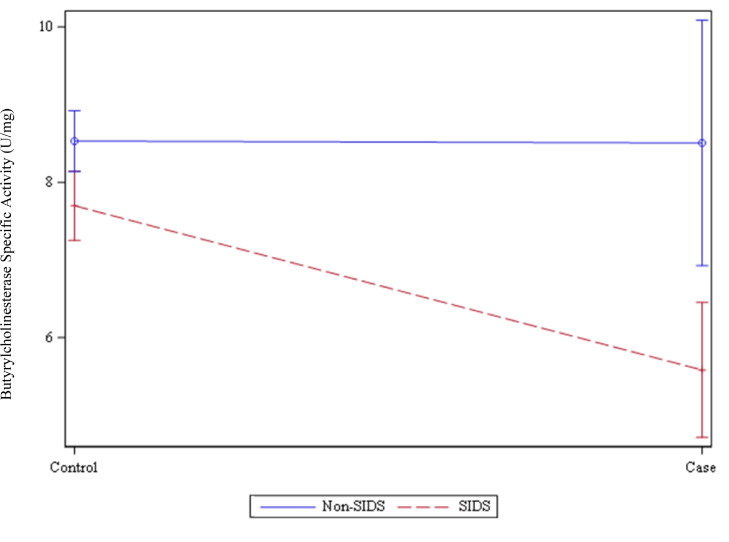
Figure 3.
Butyrylcholinesterase Specific Activity (U/mg) in Non-SIDS, SIDS and controls. Linear graph showing Mean and Standard Error of Mean (SEM) values for Butyrylcholinesterase Specific Activity (U/mg) in Sudden Infant Death Syndrome (SIDS, n=26) compared to SIDS matched controls (n=254), p=0.0014; and Non-SIDS Cases (n=30) compared to Non-SIDS matched controls (n=291), p=0.99.
The conditional logistic regression showed strong evidence of interaction between BChEsa and cause of death on the outcome of case/control status (P=0·0061), so separate models in SIDS and Non-SIDS groups were performed. In groups with a SIDS death as the case there was strong evidence that lower BChEsa was associated with death (OR=0·73 per U/mg, 95% CI 0·60-0·89, P=0·0014), whereas in groups with a Non-SIDS death as the case there was no evidence of a linear association between BChEsa and death (OR=1·001 per U/mg, 95% CI 0·89-1·13, P=0·99).
There was no evidence of an association between any of the predictors (BCHEsa, gender and cause of death) and age at death, in either univariable or multivariable models.
Discussion
SIDS infants exhibited significantly lower BChEsa, in DBSs taken on PND 2-3, compared to infants who died of other causes and compared to levels in the DBS of their date of birth- and gender-matched controls. This is the first study to identify a biochemical marker in SIDS infants, prior to their death, which differentiates them from control cases and from other causes of death. In terms of SIDS and the triple risk model we interpret this decreased activity of BChE to mean that the inherent vulnerability of a SIDS infant is autonomic cholinergic dysfunction. Thus, the finding presents the possibility of identifying infants at future risk for SIDS and it provides a specific avenue for future research into interventions prior to death.
A SIDS diagnosis is defined by characteristics surrounding the unexpected death of apparently healthy infants to encourage research into causes for these deaths. Definitions accepted throughout the literature state that ‘after thorough investigation of the death scene and autopsy, no cause of death is found' (i.e. the cause of death is unknown).11 The goal of these classifications was to “assist researchers looking for causes”,12 and ultimately reclassify some to death from specific causes or disease categories. For example, it is now accepted that up to 10% of SIDS deaths are due to arrythmias caused by channelopathies.13 Should our current finding be borne out in future studies, it may become a new disease category or ‘cause’ for those sudden deaths, in the same manner as these genetic channelopathies.
It has long been considered that the cholinergic system may be involved in SIDS deaths but there are few studies investigating the enzymes AChE and BChE. In post-mortem samples Dick et al.14 found mean levels of AChE from a group of 28 SIDS infants to be slightly lower (but not significantly) than other infant groups whereas, Livolsi et al.,15 found a significantly increased level of blood AChE. Both AChE and BChE have roles in autonomic function, although BChE has not been investigated to the same extent as AChE.
Recent research on the cholinergic neurotransmitter system has focused on the dementias and on neurotoxins (such as organophosphate poisoning), but a growing body of research has led to targeted studies of BChE (rather than AChE), for example as a potential specific target for treatments in Alzheimer's disease.16 A role for BChE regulating ACh at the neuromuscular junction has also been suggested, where BChE is involved in the modulation of ACh at the pre-synapse.17 Reviews have also been devoted to the growing body of literature regarding BChE as a measure of autonomic function, having a role coregulating the levels of ACh in the brain in neurodegenerative disorders such as Alzheimer's Disease (AD),18 and its usefulness as a biomarker in Alzheimer's Disease19,20 and other disease states21 together providing sufficient theoretical underpinning for a study restricted to the measurement of BChE. The function of BChE is not well understood and has been considered by some to be vestigial and physiologically irrelevant since, apart from a heightened sensitivity to the muscle relaxant suxamethonium, individuals deficient in BChE activity appear asymptomatic.22 However, evolutionary biology suggests otherwise as an adult human has ten times more BChE than AChE and it has survived as a functional protein for over 500 million years.23 Currently, 75 genetic variants of BChE have been described.24 This suggests that it is more than likely to have some physiological relevance especially given that while AChE is expressed after the last mitosis coincident with the start of differentiation, BChE is transiently expressed in neural crest cells at an earlier stage both before and during mitosis, which hints at a participatory role in the expression of AChE as well as its possible involvement in cell differentiation and development.25
Low BChE activity is thought to reflect decreased availability of ACh and thus an altered cholinergic homeostasis and several studies have found that low BChE activity is associated with severe systemic inflammation26, 27, 28 and a significant higher mortality after sepsis and cardiac events.4,29,30 Interestingly, in terms of our present findings, inflammation has long been thought to play a role in SIDS and as far back as 1889 mild inflammatory changes in the walls of the bronchioles were observed in SIDS infants.31
The abnormality we identified ties into several previously identified abnormalities in SIDS. Respiratory and cardiovascular control mechanisms undergo significant maturation during the first year of life. Although prematurity also carries a risk for SIDS, a study from 1957 evaluating BChE in infancy32 found that there was no difference between the BChE levels of premature and mature newborn infants. A characteristic of a SIDS death is the occurrence during sleep and the failure to arouse in response to an exogenous stressor has long been considered a key component of an infant's vulnerability.33
There are two distinct arousal types defined in infants, sub-cortical activation and full cortical arousal, reflecting the hierarchical activation from the brainstem (including heart rate, blood pressure and ventilation changes) to the cortex.34 Any impairment of these protective responses may render an infant vulnerable to the respiratory and cardiovascular instabilities and it has been suggested that decreased cholinergic activity could impair arousal responses.35
It will be important to identify whether the decreased BChEsa we observed in the peripheral blood of our SIDS cases is also evident in the brain because of the possible role BChE plays in maintaining ACh levels. In the brain, while BChE is largely associated with glia, it is also associated with neurons particularly in the hippocampus and amygdala36 and thalamus.18 These neurons express Choline Acetyl Transferase (ChAT) and therefore ACh, and appear to operate normally except they are under the control of BChE, rather than AChE.23 Particularly, BChE-positive neuropils have been observed in close relationship with ChAT - positive neurons within the pedunculopontine and laterodorsal tegemental nuclei in the pons.37 This caudal cholinergic cell column innervates numerous areas of the brain, including the thalamus,38 pontomedullary reticular formation and mesolimbic regions and is involved in, amongst other things, the regulation of the sleep-wake cycle and arousal.39 Given this close association it has been suggested that BChE may be involved in maintaining ACh levels in the arousal systems of the Central Nervous System (CNS).40 This is highly relevant as numerous studies have observed an arousal deficit in infants who eventually succumbed to SIDS.40,41 It may be that a decrease in BChEsa implies a decrease in available ACh and thus a resultant impaired arousal response to a given environmental challenge, whether it be infection,42 apnoea,43 or CO2 rebreathing as a result of prone sleeping.44 In support of this hypothesis decreased ChAT positive neuron immunoreactivity has been observed in the brain of SIDS babies,45 as well as decreased muscarinic acetyl choline receptors (mAChRs).35
Within the CNS, decreased BChE may also be related to the consistent finding of decreased serotonergic activity in SIDS infants.46 The caudal serotonergic network in the medulla is intimately involved in autonomic parasympathetic outflow and activation of protective respiratory and cardiac autonomic reactions, via bronchovagal preganglionic neurons and cardiac vagal preganglionic neurons,47 leading to the suggestion that decreased serotonergic receptor binding in this area leads to failure of the autoresuscitation and arousal in sleep that ultimately causes SIDS.48 Bidirectional interactions exist between cholinergic and serotonergic brain systems and recent studies highlight the interactions between these two systems.49,50 Cholinergic receptors control serotonin release in hypothalamic slices of rat brain, and striatal cholinergic interneurons are modulated via serotoninergic projections from the raphe.51 Arousal and maintenance of wakefulness involves serotonergic neurones in the dorsal raphe and cholinergic neurones in the pons.52 A study in 2019 by Davis et al.50 describes “a previously unknown interaction between the serotonergic and cholinergic systems in the control of breathing in early life in rat pups” with the authors concluding that the findings are relevant to SIDS. Another study investigated the effects of prenatal dexamethasone and a cholinesterase inhibitor and found suppression of serotoninergic synaptic function.53 While this possibly related to effects of both compounds on synaptic function it also demonstrated simultaneously reduced function in both systems.53
In this study we found decreased BChE activity at birth which indicates that the vulnerability probably originates in the gestational period. Smoking during pregnancy is associated with more than a threefold increase in SIDS events,54,55 along with impairment of autonomic system,56,57 and cardiovascular and arousal responses.58 One of the few studies evaluating effects of cigarette smoke exposure on BChE levels evaluated chronic exposure to cigarette smoke on pregnant rats and demonstrated increased BChE activity in the rat pups.59 While the direction of change opposes our finding, the result supports an impact of gestational exposure to cigarette smoke on the development of the autonomic system. Interestingly, the increased level in rat pups was detected on post-natal day 7, a time equating approximately to about 8–12 weeks in human terms, and the period of highest risk for SIDS. At this stage we do not know how BChE levels change beyond day 2, 3 when the heel prick samples were collected. Studies evaluating the impact of cigarette smoke have also found that both cholinergic and serotonergic systems are impacted: in the brain tissue of a high-risk group of SIDS infants with pre-natal exposure to alcohol and cigarette smoke, reduced cholinergic receptor binding in the brainstem was found and was reported as being linked to the adverse impact on the serotonergic nuclei in the same region.60 And, in a mouse model, the combination of serotonin deficiency and cigarette smoke exposure results in failure of autoresuscitation.61
Limitations to this study include difficulties comparing our findings of BChEsa with known BChE reference intervals (0-1 month: 1·5kU/l-8·2 kU/l; 1 month-6 years: 4·2kU/l-13·6 kU/l), firstly because the reference intervals refer to serum, not DBSs and secondly, they refer to activity per litre and not to activity per gram (of protein). Additionally, the samples were over 2 years old and so would not accurately reflect BChEsa in fresh DBSs. Previous studies have shown that DBS samples retain > 90% of their initial BChE activity after 40 days of storage at room temperature7 and that proteins in DBSs are stable over a 22 week period.62 In this study we sought to minimise the effect of the long storage period by including 10 date of birth- and gender-matched, surviving controls, stored under the same conditions and whose sample was collected on the same PND as that of the case.
Despite evaluating over 600 control samples we do not know how common this abnormality is in the wider population, although this will become clearer with future testing of greater numbers. Further, we do not know the status of BChEsa at time of death and many of the post-natal changes that occur in the first 6 months of life are also likely to affect the cholinesterases and the autonomic nervous system.63
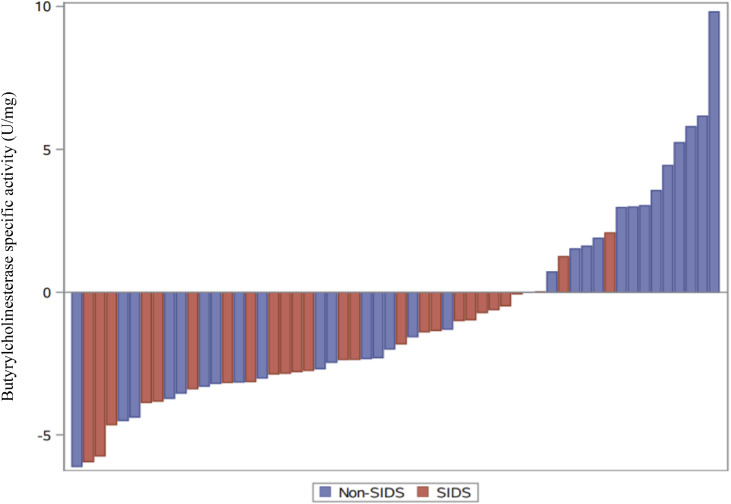
Finally, we did not access the autopsy details but used the Coroner's diagnosis where possible, only reclassifying 5 cases at the time of the original diagnostic groupings to meet currently accepted SIDS definitions: age (3 weeks to 12 months) and autopsy (unknown) definitions.64 While the biochemical abnormality we identified fits well with children diagnosed as SIDS, Fig. 4 illustrates that the abnormality crosses current SIDS and Non-SIDS groups and future research will be needed to further identify common phenotypic and pathological characteristics of the affected children. While those children aged 12–24 months would be considered sudden unexplained deaths in childhood (SUDC), in designing the study we noted growing interest in those babies that die suddenly, and from no determined cause, aged 12 months–24 months. As it has been suggested that there is a developmental continuum,65 we decided to include this age group in our investigation and chose the term ‘Non-SIDS’ to refer to this group.
Figure 4.
Difference in Butyrylcholinesterase specific activity (Case-mean of matched controls). Waterfall plot showing the difference between the Butyrylcholinesterase specific activity (U/mg) for each case of Sudden Infant Death Syndrome (SIDS) (n=26) or Non-SIDS (n=30) and the mean of their matched controls.
In conclusion, decreased BChEsa was a biochemical marker that distinguished infants who succumbed to SIDS from date of birth- and gender-matched (surviving) controls and from infants with known causes of death. We hypothesise that this is evidence of an altered cholinergic homeostasis. This is the first study to identify a measurable biochemical marker in infants who succumb to SIDS, during their newborn period while they are still alive, and one that could plausibly produce functional alterations to an infant's autonomic and arousal responses to an exogenous stressor leaving them vulnerable to sudden death. Further work investigating this area needs to be undertaken with urgency, to determine if specific activity of BChE could potentially be used as a biomarker to identify and prevent future SIDS deaths.
Contributors
CH conceptualised the study, devised the study protocol, acquired funding and is the principal investigator. NA and KW participated in the study design. NA developed the methodology and assayed all samples. Data and statistical analysis was done by CH. CH, NA, and KW contributed to data interpretation. CH drafted the initial manuscript, with further contributions from KW and NA. CH, NA, and KW reviewed successive drafts, and all authors approved the final version of the manuscript. CH, KW, and NA have full access to, and verify, the underlying data and accept responsibility to submit for publication.
Data sharing statement
Due to the sensitivity of the data, individual participant data will not be made available. De-identified Case and Control BChEsa values along with all statistical analysis will be made available.
Data will be available to be shared upon publication by correspondence with either Carmel Harrington (carmel.harrington@health.nsw.gov.au) or Karen Waters (karen.waters@health.nsw.gov.au), after approval of a proposal, with a signed access agreement, and relevant ethics consent.
Declaration of interests
CH, NA, and KW declare no competing interests.
Acknowledgments
This research was supported by NSW Health Pathology (http://www.pathology.nsw.gov.au). The authors would like to acknowledge the NSW Newborn Screening Programme for having provided access to the DBS samples as well as to their laboratory facilities in order to undertake this study. We thank Ms Liz Barnes, Sydney University, for her input to data analysis and Dr Rita Machaalani, Faculty of Medicine and Health, University of Sydney, for her valuable theoretic and technical discussions. This study was funded by the Damien's Legacy crowd-funding campaign (https://www.mycause.com.au/p/184401/damiens-legacy) and we wish to thank the hundreds of donors who contributed to this campaign, without whose support this study could not have been done.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104041.
Appendix. Supplementary materials
References
- 1.Krous H.F., Beckwith J.B., Byard R.W., et al. Sudden Infant Death Syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114:234–238. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter R.G., Irgens L.M., Blair P.S., et al. Sudden unexplained infant death in 20 regions in Europe: case control study. Lancet. 2004;363:185–191. doi: 10.1016/s0140-6736(03)15323-8. [DOI] [PubMed] [Google Scholar]
- 3.Filiano J.J., Kinney H.C. A perspective on neuropathologic findings in victims of the Sudden Infant Death Syndrome: the triple-risk model. Biol Neonate. 1994;65:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- 4.Das U.N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med Sci Monit. 2007;13:RA214–RA221. [PubMed] [Google Scholar]
- 5.Augustinsson K.B., Holmstedt B. Determination of cholinesterase in blood samples dried on filter-paper and its practical application. Scand J Clin Lab Investig. 1965;17:573–583. doi: 10.1080/00365516509083366. [DOI] [PubMed] [Google Scholar]
- 6.Hilborn E.D., Padilla S. A dried blood spot method to evaluate cholinesterase activity in young children. Arch Environ Health. 2004;59:467–470. doi: 10.1080/00039890409603427. [DOI] [PubMed] [Google Scholar]
- 7.Perez J.W., Pantazides B.G., Watson C.M., Thomas J.D., Blake T.A., Johnson R.C. Enhanced stability of blood matrices using a dried sample spot assay to measure human butyrylcholinesterase activity and nerve agent adducts. Anal Chem. 2015;87:5723–5729. doi: 10.1021/acs.analchem.5b00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilcken B., Wiley V. Newborn screening. Pathology. 2008;40:104–115. doi: 10.1080/00313020701813743. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y.C., Chou W.H., Fang C.P., et al. Serum Level and activity of butylcholinesterase: a biomarker for post-stroke dementia. J Clin Med. 2019;8:1778. doi: 10.3390/jcm8111778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ait Belkacem I., Mossadegh-Keller N., Bourgoin P., et al. Cell analysis from dried blood spots: new opportunities in immunology, hematology, and infectious diseases. Adv Sci (Weinh) 2021 doi: 10.1002/advs.202100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon R.Y., Horne R.S., Hauck F.R. Sudden Infant Death Syndrome. Lancet. 2007;370:1578–1587. doi: 10.1016/S0140-6736(07)61662-6. [DOI] [PubMed] [Google Scholar]
- 12.Byard R.W., Becker L.E., Berry P.J., et al. The pathological approach to sudden infant death–consensus or confusion? Recommendations from the Second SIDS Global Strategy Meeting, Stavangar, Norway, August 1994, and the Third Australasian SIDS Global Strategy Meeting, Gold Coast, Australia, May 1995. Am J Forensic Med Pathol. 1996;17:103–105. doi: 10.1097/00000433-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Cannon S.C. Skeletal muscle channelopathy: a new risk for Sudden Infant Death Syndrome. Lancet. 2018;391:1457–1458. doi: 10.1016/S0140-6736(18)30477-X. [DOI] [PubMed] [Google Scholar]
- 14.Dick A., Ford R. Cholinergic and oxidative stress mechanisms in Sudden Infant Death Syndrome. Acta Paediatr. 2009;98:1768–1775. doi: 10.1111/j.1651-2227.2009.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livolsi A., Niederhoffer N., Dali-Youcef N., et al. Cardiac muscarinic receptor overexpression in Sudden Infant Death Syndrome. PLoS One. 2010;5:e9464. doi: 10.1371/journal.pone.0009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darvesh S. Butyrylcholinesterase as a diagnostic and therapeutic target for Alzheimer's Disease. Curr Alzheimer Res. 2016;13:1173–1177. doi: 10.2174/1567205013666160404120542. [DOI] [PubMed] [Google Scholar]
- 17.Minic J., Chatonnet A., Krejci E., Molgo J. Butyrylcholinesterase and acetylcholinesterase activity and quantal transmitter release at normal and acetylcholinesterase knockout mouse neuromuscular junctions. Br J Pharmacol. 2003;138:177–187. doi: 10.1038/sj.bjp.0705010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darvesh S., Hopkins D.A. Differential distribution of butyrylcholinesterase and acetylcholinesterase in the human thalamus. J Comp Neurol. 2003;463:25–43. doi: 10.1002/cne.10751. [DOI] [PubMed] [Google Scholar]
- 19.Ha Z.Y., Mathew S., Yeong K.Y. Butyrylcholinesterase: a multifaceted pharmacological target and tool. Curr Protein Pept Sci. 2020;21:99–109. doi: 10.2174/1389203720666191107094949. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald I.R., Maxwell S.P., Reid G.A., Cash M.K., Debay D.R., Darvesh S. Quantification of butyrylcholinesterase activity as a sensitive and specific biomarker of Alzheimer's disease. J Alzheimers Dis. 2017;58:491–505. doi: 10.3233/JAD-170164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santarpia L., Grandone I., Contaldo F., Pasanisi F. Butyrylcholinesterase as a prognostic marker: a review of the literature. J Cachexia Sarcopenia Muscle. 2013;4:31–39. doi: 10.1007/s13539-012-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Primo-Parmo S.L., Bartels C.F., Wiersema B., Van Der Spek A.F., Innis J.W., La Du B.N. Characterization of 12 silent alleles of the human butyrylcholinesterase (BCHE) gene. Am J Hum Genet. 1996;58:52–64. [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson G., Moore S.W. Why has butyrylcholinesterase been retained? Structural and functional diversification in a duplicated gene. Neurochem Int. 2012;61:783–797. doi: 10.1016/j.neuint.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Lockridge O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther. 2015;148:34–46. doi: 10.1016/j.pharmthera.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Massoulie J. The origin of the molecular diversity and functional anchoring of cholinesterases. Neurosignals. 2002;11:130–143. doi: 10.1159/000065054. [DOI] [PubMed] [Google Scholar]
- 26.Zivkovic A.R., Tourelle K.M., Brenner T., Weigand M.A., Hofer S., Schmidt K. Reduced serum cholinesterase activity indicates splenic modulation of the sterile inflammation. J Surg Res. 2017;220:275–283. doi: 10.1016/j.jss.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Arbel Y., Shenhar-Tsarfaty S., Waiskopf N., et al. Decline in serum cholinesterase activities predicts 2-year major adverse cardiac events. Mol Med. 2014;20:38–45. doi: 10.2119/molmed.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampon N., Hermida-Cadahia E.F., Riveiro A., Tutor J.C. Association between butyrylcholinesterase activity and low-grade systemic inflammation. Ann Hepatol. 2012;11:356–363. [PubMed] [Google Scholar]
- 29.Zivkovic A.R., Decker S.O., Zirnstein A.C., et al. A sustained reduction in serum cholinesterase enzyme activity predicts patient outcome following sepsis. Mediat Inflamm. 2018;2018 doi: 10.1155/2018/1942193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L., Qi X., Tan Q., Yang H., Qi X. Low serum-butyrylcholinesterase activity as a prognostic marker of mortality associates with poor cardiac function in acute myocardial infarction. Clin Lab. 2016;62:1093–1099. doi: 10.7754/clin.lab.2015.151013. [DOI] [PubMed] [Google Scholar]
- 31.Wright J.R. A fresh look at the history of SIDS. Acad Forensic Pathol. 2017;7:146–162. doi: 10.23907/2017.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann H., Cook J., Ryan E. Pseudocholinesterase in early infancy. Proc R Soc Med. 1957;50:147–150. doi: 10.1177/003591575705000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillipson E.A., Sullivan C.E. Arousal: the forgotten response to respiratory stimuli. Am Rev Respir Dis. 1978;118:807–809. doi: 10.1164/arrd.1978.118.5.807. [DOI] [PubMed] [Google Scholar]
- 34.Harrington C., Kirjavainen T., Teng A., Sullivan C.E. Altered autonomic function and reduced arousability in apparent life-threatening event infants with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1048–1054. doi: 10.1164/ajrccm.165.8.2102059. [DOI] [PubMed] [Google Scholar]
- 35.Kinney H.C., Filiano J.J., Sleeper L.A., Mandell F., Valdes-Dapena M., White W.F. Decreased muscarinic receptor binding in the arcuate nucleus in Sudden Infant Death Syndrome. Science. 1995;269:1446–1450. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- 36.Darvesh S., Grantham D.L., Hopkins D.A. Distribution of butyrylcholinesterase in the human amygdala and hippocampal formation. J Comp Neurol. 1998;393:374–390. [PubMed] [Google Scholar]
- 37.Reid G.A., Chilukuri N., Darvesh S. Butyrylcholinesterase and the cholinergic system. Neuroscience. 2013;234:53–68. doi: 10.1016/j.neuroscience.2012.12.054. [DOI] [PubMed] [Google Scholar]
- 38.Mesulam M.M., Mufson E.J., Wainer B.H., Levey A.I. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 39.Vanderhorst V.G., Ulfhake B. The organization of the brainstem and spinal cord of the mouse: relationships between monoaminergic, cholinergic, and spinal projection systems. J Chem Neuroanat. 2006;31:2–36. doi: 10.1016/j.jchemneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Kahn A., Groswasser J., Rebuffat E., et al. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep. 1992;15:287–292. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- 41.Kato I., Franco P., Groswasser J., et al. Incomplete arousal processes in infants who were victims of sudden death. Am J Respir Crit Care Med. 2003;168:1298–1303. doi: 10.1164/rccm.200301-134OC. [DOI] [PubMed] [Google Scholar]
- 42.Duncan JR, Byard RW. In: OSH. Cytokines, Infection, and Immunity, SIDS Sudden Infant and Early Childhood Death: the Past, the Present and the Future. Adelaide (AU) 2018, (eds.). [PubMed]
- 43.Thach BT. The role of the upper airway in SIDS and sudden unexpected infant deaths and the importance of external airway-protective behaviors. In: Duncan JR, Byard RW, (Eds.), SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future. Adelaide (AU) 2018. [PubMed]
- 44.Sperhake J., Jorch G., Bajanowski T. The prone sleeping position and SIDS. Historical aspects and possible pathomechanisms. Int J Leg Med. 2018;132:181–185. doi: 10.1007/s00414-017-1749-5. [DOI] [PubMed] [Google Scholar]
- 45.Mallard C., Tolcos M., Leditschke J., Campbell P., Rees S. Reduction in choline acetyltransferase immunoreactivity but not muscarinic-m2 receptor immunoreactivity in the brainstem of SIDS infants. J Neuropathol Exp Neurol. 1999;58:255–264. doi: 10.1097/00005072-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Duncan J.R., Paterson D.S., Hoffman J.M., et al. Brainstem serotonergic deficiency in Sudden Infant Death Syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Ramage A.G. The role of central 5-HT(1A) receptors in the control of B-fibre cardiac and bronchoconstrictor vagal preganglionic neurones in anaesthetized cats. J Physiol. 2001;536:753–767. doi: 10.1111/j.1469-7793.2001.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panigrahy A., Filiano J., Sleeper L.A., et al. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the Sudden Infant Death Syndrome. J Neuropathol Exp Neurol. 2000;59:377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- 49.Padua-Reis M., Aquino N.S., Oliveira V.E.M., et al. Reduced Vesicular Acetylcholine Transporter favors antidepressant behaviors and modulates serotonin and dopamine in female mouse brain. Behav Brain Res. 2017;330:127–132. doi: 10.1016/j.bbr.2017.04.049. [DOI] [PubMed] [Google Scholar]
- 50.Davis M.R., Magnusson J.L., Cummings K.J. Increased central cholinergic drive contributes to the apneas of serotonin-deficient rat pups during active sleep. J Appl Physiol. 2019;126:1175–1183. doi: 10.1152/japplphysiol.00909.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumari A., Sreetama S., Mohanakumar K.P. Atropine, a muscarinic cholinergic receptor antagonist increases serotonin, but not dopamine levels in discrete brain regions of mice. Neurosci Lett. 2007;423:100–103. doi: 10.1016/j.neulet.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 52.Luppi P.H., Fort P. Sleep-wake physiology. Handbook of Clinical Neurology. 2019;160:359–370. doi: 10.1016/B978-0-444-64032-1.00023-0. [DOI] [PubMed] [Google Scholar]
- 53.Slotkin T.A., Card J., Seidler F.J. Prenatal dexamethasone, as used in preterm labor, worsens the impact of postnatal chlorpyrifos exposure on serotonergic pathways. Brain Res Bull. 2014;100:44–54. doi: 10.1016/j.brainresbull.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson T.M., Lavista Ferres J.M., Ren S.Y., et al. Maternal smoking before and during pregnancy and the risk of Sudden Unexpected Infant Death. Pediatrics. 2019:143. doi: 10.1542/peds.2018-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dietz P.M., England L.J., Shapiro-Mendoza C.K., Tong V.T., Farr S.L., Callaghan W.M. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med. 2010;39:45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Fifer W.P., Fingers S.T., Youngman M., Gomez-Gribben E., Myers M.M. Effects of alcohol and smoking during pregnancy on infant autonomic control. Dev Psychobiol. 2009;51:234–242. doi: 10.1002/dev.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duncan J.R., Garland M., Myers M.M., et al. Prenatal nicotine-exposure alters fetal autonomic activity and medullary neurotransmitter receptors: implications for Sudden Infant Death Syndrome. J Appl Physiol. 2009;107:1579–1590. doi: 10.1152/japplphysiol.91629.2008. (1985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richardson H.L., Walker A.M., Horne R.S. Maternal smoking impairs arousal patterns in sleeping infants. Sleep. 2009;32:515–521. doi: 10.1093/sleep/32.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zugno A.I., Fraga D.B., De Luca R.D., et al. Chronic exposure to cigarette smoke during gestation results in altered cholinesterase enzyme activity and behavioral deficits in adult rat offspring: potential relevance to schizophrenia. J Psychiatr Res. 2013;47:740–746. doi: 10.1016/j.jpsychires.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Vivekanandarajah A., Nelson M.E., Kinney H.C., et al. Nicotinic receptors in the brainstem ascending arousal system in SIDS with analysis of pre-natal exposures to maternal smoking and alcohol in high-risk populations of the safe passage study. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.636668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee S.Y., Sirieix C.M., Nattie E., Li A. Pre- and early postnatal nicotine exposure exacerbates autoresuscitation failure in serotonin-deficient rat neonates. J Physiol. 2018;596:5977–5991. doi: 10.1113/JP275885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chambers A.G., Percy A.J., Yang J., Borchers C.H. Multiple reaction monitoring enables precise quantification of 97 proteins in dried blood spots. Mol Cell Proteomics. 2015;14:3094–3104. doi: 10.1074/mcp.O115.049957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shideler K.K., Yan J. M1 muscarinic receptor for the development of auditory cortical function. Mol Brain. 2010;3:29. doi: 10.1186/1756-6606-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ambrose N., Waters K.A., Rodriguez M.L., Bailey K., Machaalani R. Neuronal apoptosis in the brainstem medulla of Sudden Unexpected Death In Infancy (SUDI), and the importance of standardized SUDI classification. Forensic Sci Med Pathol. 2018;14:42–56. doi: 10.1007/s12024-018-9954-1. [DOI] [PubMed] [Google Scholar]
- 65.Mcguone D., Crandall L.G., Devinsky O. Sudden unexplained death in childhood: a neuropathology review. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.582051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.