Abstract
Introduction
Lewy body diseases are pathologically characterized by α‐synuclein pathology. Alzheimer's disease (AD) co‐pathology can influence phenotypes. In vivo AD biomarkers can suggest the presence of this co‐pathology in unusual cases, but pathological validation remains essential.
Methods
This patient originally presented with corticobasal syndrome and later developed visual hallucinations and parkinsonism consistent with a synucleinopathy. The patient underwent CSF sampling, 18F‐flortaucipir PET scanning, and brain donation with bilateral regions available for digital histological analysis.
Results
CSF Aβ42 and t‐tau were in the AD range. 18F‐flortaucipir scanning showed right‐lateralized retention in all lobes (t = 4.3‐10.0, P < .006). Neocortical stage Lewy body pathology and high levels of AD neuropathological changes were present at autopsy. There was right lateralization of α‐synuclein and tau pathology (T value = 3.1, P value = .007 and T value = 3.3, P value = .004 respectively).
Discussion
This case with overlapping tauopathy and synucleinopathy clinical features had in‐depth biomarker characterization and rare bilateral post‐mortem sampling showing lateralized tau and α‐synuclein pathology suggesting possible synergistic relationships.
Keywords: alpha‐synuclein, Alzheimer's disease, cerebrospinal fluid, corticobasal syndrome, flortaucipir, Lewy body dementia, neuropathology
1. INTRODUCTION
Lewy body disorders (LBD) are a spectrum of heterogeneous neurodegenerative diseases (i.e., Parkinson's disease [PD]; PD with dementia [PDD]; dementia with Lewy bodies [DLB]) characterized by the post mortem findings of α‐synuclein (aSyn) pathology in Lewy bodies and Lewy neurites. However, Alzheimer's disease (AD) co‐pathology is common in LBD and is associated with greater degrees of neocortical aSyn pathology, faster time to dementia, and decreased survival. 1 Tau co‐pathology in particular appears to correlate with the neocortical distribution of aSyn pathology and with specific cognitive features including episodic memory and language functioning. 2 , 3 Emerging biofluid biomarkers and molecular imaging tracers appear to be able to detect this pathology in vivo, with studies similarly suggesting a strong clinical influence of AD tau pathology in LBD. 4 , 5 The LBD clinical syndromes (PD, PDD, DLB) are fairly specific for underlying synucleinopathy; however, neocortical aSyn pathology can be found in asymptomatic individuals, in patients with clinical AD, 6 and has also been occasionally reported in association with focal lateralized cortical presentations in the frontotemporal dementia spectrum, including primary progressive aphasia 7 and corticobasal syndrome (CBS). 8 Post mortem analysis of these focal cortical syndromes can provide a unique insight into the distribution of pathology across hemispheres and suggest pathophysiological mechanisms of disease spread, 9 , 10 but detailed bilateral post mortem immunohistochemical assessment in LBD with lateralized clinical features is lacking. We are unaware of a previous assessment of combined lateralization of mixed pathology in CBS. Here, we present a novel interhemispheric assessment of pathology in an unusual case with highly lateralized clinical symptoms with overlapping features of a synucleinopathy and a tauopathy who had both ante mortem positron emission tomography (PET) imaging and rare bilateral post mortem histopathological assessments. 11
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed the literature using traditional sources (e.g., PubMed). While pathological lateralization is well described in frontotemporal lobar degeneration, less is known about lateralization of alpha‐synuclein in Lewy body disease and tau in Alzheimer's disease (AD). Tau positron emission tomography (PET) tracers can offer in vivo information about tau lateralization but detailed post mortem bi‐hemispheric analyses are rare and are needed to confirm and expand these observations.
Interpretation: This case had multimodal ante mortem biomarker assessments including cerebrospinal fluid sampling and 18F‐flortaucipir PET scanning that suggested right lateralized AD tau pathology. Bilateral brain samples were available for post mortem digital immunohistochemical analysis and showed right lateralized tau and alpha‐synuclein pathology, suggesting a synergistic relationship worthy of further study.
Future Directions: In‐depth pathological sampling with digital histological analyses provide fine‐grained assessments of pathological severity and can suggest unique patterns of disease spread and suggest mechanisms of pathophysiology. The case adds to the growing understanding of the interaction between alpha‐synuclein and tau pathology.
HIGHLIGHTS
This case had features of corticobasal syndrome and Lewy body dementia.
Cerebrospinal fluid and 18F‐flortaucipir scanning suggested right‐lateralized Alzheimer's disease (AD) tau pathology.
Right‐lateralized tau and alpha‐synuclein pathology was present at autopsy.
In vivo biomarkers can help identify AD pathology in unusual presentations.
Lateralization of tau and alpha‐synuclein suggests a synergistic relationship.
2. CASE PRESENTATION
The patient initially presented with left‐sided neglect, left‐sided myoclonus, and significant visuospatial and executive impairments at age 64, consistent with a diagnosis of possible CBS. 12 The patient had several minor motor vehicle accidents and eventually was unaware of the left side of his visual field, evidenced by the history of him often leaving the left side of his plate uneaten, requiring his family to turn the plate for him to finish a meal. The minor car accidents did not result in closed head injury or loss of consciousness and did not require medical care or hospitalization. Four years later at age 68 he developed well‐formed visual hallucinations, excessive daytime sleepiness despite a full night sleep, cognitive fluctuations, along with motor Parkinsonism with left sided cogwheel rigidity and bradykinesia, fulfilling clinical criteria for DLB. 13 Alien‐limb phenomenon was also noted at that time. Tremor was not present nor was hyperreflexia or orthostatic hypotension. There was not cortical blindness on exam. The patient was enrolled into biomarker and brain donation research programs and informed consent for each study and for subsequent publication was obtained. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments with protocols being approved locally by the University of Pennsylvania's Institutional Review Board. AD cerebrospinal fluid (CSF) biomarker analysis using the Luminex platform and AlzBio3 immunoassay reagents (AlzBio3®, Innogenetics NV) and 18F‐flortaucipir PET were obtained (5 years after symptom onset and 8 months prior to death) and analyzed using previously published protocols. 4 PET images were parcellated into 116 cortical brain regions and grouped into major areas for analysis (frontal, temporal, parietal, occipital, limbic, and subcortical regions including caudate nucleus and putamen, etc.). Asymmetry indices ([Right‐Left]/[Right+Left]*100) for regional standardized uptake value ratio (SUVR) were calculated (n = 58) and compared to a hypothesized mean of 0 (i.e., symmetric disease) using one‐sided t‐tests. 9 A subset of data from this case was previously included in a series of multimodal tau biomarkers in LBD. 11 Bilateral sections were available from 17 neocortical and two subcortical regions (see supporting information), which were immunostained stained for phosphor‐tau (AT8, ThermoFisher 1:1000, no antigen retrieval), amyloid beta (Aβ; NAB228, CNDR 1:40,000, formic acid antigen retrieval), and aSyn (MJF‐R13 Abcam1:15,000, proteinase K antigen retrieval). Whole‐slide images were obtained using a digital slide scanner (Aperio AT2, Leica Biosystems) at 20x magnification, and gray matter regions were analyzed to calculate %area occupied (%AO) of reactivity for tau, Aβ, and aSyn pathology using QuPath software (version 0.2.0‐m2) as previously published. 3 , 14 Asymmetry indices were similarly calculated as above.
CSF Aβ42 was 105 pg/mL, total‐tau was 80 pg/mL, and phosphorylated tau181 was 19 pg/mL, all of which are consistent with AD co‐pathology using our previously published autopsy‐validated cutpoints in LBD. 4 Average cortical flortaucipir SUVR was 1.48 (left hemisphere SUVR = 1.40 ± 0.27 standard deviation [SD]; right hemisphere SUVR = 1.61 ± 0.36 SD) in the range consistent with AD‐related tau pathology. 11 Comparison of intrahemispheric tracer uptake showed right lateralization overall and in each major area (overall: T value = 14.4, P value < .001; regions: T value = 4.3–10.0, all P values < .006; Figure 1A). Post mortem, the brain weighed 1366 grams and neuropathological examination showed neocortical stage LBD and high‐level AD neuropathologic change by criteria (Thal phase 4: A3, Braak stage V: B3, CERAD C3). 15 Tufted astrocytes, coiled bodies, astrocytic plaques, and argyrophilic grains were not identified. There was significant right lateralization of tau and aSyn pathology in regions with bilateral sampling (Tau: right %AO = 43.3% ± 20.9 SD, left %AO = 28.3% ± 24.04 SD T value = 3.3, P value = .004; aSyn: right %AO = 32.0% ± 25.5 SD, left %AO = 18.3% ± 24.4 SD, T value = 3.1, P value = .007). Aβ pathology was not lateralized (P value = .98) (Figure 1B,C).
FIGURE 1.
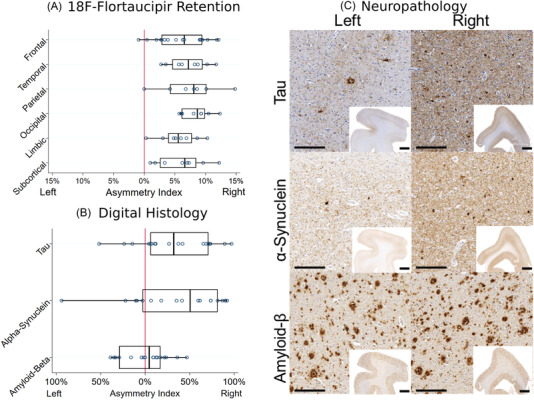
A, Asymmetry indices calculated from regional 18F‐flortaucipir positron emission tomography (PET) image standardized uptake value ratio. B, Asymmetry indices calculated from digital histological measurements of % area occupied by pathological inclusions of tau, α‐synuclein, and amyloid beta (Aβ). Right lateralization was observed for all PET region groups and for tau and α‐synuclein pathology while Aβ pathology was symmetric. C, Representative images from the bilateral sections of the dorsolateral prefrontal cortex immunostained for tau, alpha synuclein, and Aβ. High‐magnification images were taken at 10x with scale bar of 200 μm. Low‐magnification inset images were taken at 0.5x with a scale bar of 2 mm. Higher density tau and αsynuclein pathology is noted in the right hemispheric sections whereas Aβ pathology is equally distributed
3. DISCUSSION AND CONCLUSION
This is a case report of a patient with an unusual presentation originally most consistent with CBS who later developed LBD features; this patient had in‐depth biomarker analyses that suggested lateralized AD‐related tau pathology that fit with clinical symptoms of left‐sided neglect and myoclonus. This patient also participated in brain donation and rare bilateral sampling was available for modern digital histological analysis to assess pathology laterality. This analysis adds to previous work showing that AD tau pathology is influential in symptoms of CBS and LBD. 2 , 3 , 16 The features of apraxia and neglect experienced by this patient are associated with right parietal lobe dysfunction, which can be associated with both posterior cortical atrophy and CBS and AD pathology is well described to result in both phenotypes. 12 , 17 In this case, tau pathology was detectable during life using biofluid and imaging biomarkers and anatomically consistent with clinical symptoms and post mortem distribution of pathology. We and others previously reported an association of tau pathology in LBD with cortical atrophy on magnetic resonance imaging in the medial and lateral temporal lobe, and here we extend these findings to this atypical clinical presentation whose features correlate more closely to frontal and parietal regions. 18 Interestingly aSyn pathology but not Aβ plaque was also strongly lateralized to the right hemisphere potentially suggesting a synergistic interaction between tau and aSyn. Indeed, this hypothesis aligns well with prior in vitro and in vivo studies that have shown that certain strains of aSyn pathology are capable of cross seeding tau pathology. 19 It is also possible that there was early lateralization of Aβ pathology that had plateaued later in the disease course. While AD pathology is well described to result in a CBS phenotype, in the absence of current in vivo methods to detect spatial distribution of aSyn pathology, it is not possible to determine the molecular progression of proteinopathy in relationship to clinical symptoms, and thus, one cannot rule out a contribution from aSyn to the initial clinical features of this patient.
We are unaware of a previous assessment of lateralization of mixed pathology in CBS. The neuropathological substrates associated with clinical CBS include corticobasal degeneration pathology, progressive supranuclear palsy pathology, lateralized atypical AD pathology, and even rare presentations of other proteinopathies including LBD. 8 In a case series, 11/523 subjects with a clinical diagnosis of CBS had a primarily LBD pathology noted at autopsy and four had co‐occurring severe AD pathology, but this study did not include assessments of laterality of pathology across hemispheres. 8 Lateralization of frontotemporal dementia syndromes using quantitative measures have been published as have qualitative studies of laterality of pathology in AD and LBD, but the assessment of this case is a unique study of quantitative lateralization of mixed pathology in CBS. 9 , 10
A limitation of the current study was that brainstem regions were not available for comparison of laterality of pathology, which would further elucidate when some of these interactions between tau and aSyn may be occurring; however, we were able to assess the rostral end of the nigrostriatal pathway in the caudate and putamen, where tau and aSyn was lateralized similarly to the cortical regions analyzed (Tau: right %AO: 49.2% ±6.44 SD, left %AO: 1.84% ±1.67 SD; aSyn: right %AO: 59.0% ±19.8 SD, left 6.74% ±6.14 SD).
Future work combining longitudinal biomarker collection with gene expression data and detailed anatomic study may uncover insights into regional and interhemispheric neuronal vulnerabilities to neurodegeneration, which could be potentially targeted for therapeutic development in AD and LBD. Finally, the reasons for focal lateralized clinical presentations of neurodegenerative diseases are unclear but may relate in part to developmental, genetic, and environmental factors that could influence the onset and spread of pathology. 20
This case also highlights the utility of using current biomarkers in patients with unusual clinical presentation. In this case lateralized AD‐related tau pathology was suggested on PET imaging, which was confirmed along with lateralized aSyn pathology at autopsy. Future application of aSyn aggregation assays will likely aid in the detection of the presence of aSyn seeds, which can be coupled with AD‐specific biomarkers in such patients with co‐pathology.
Last, typical neuropathological assessments incorporate immunohistochemistry from a single hemisphere. While laterality is well described in CBS, posterior cortical atrophy, and frontotemporal dementia syndromes, less is known about laterality in LBD. 7 , 17 Bilateral sampling and the use of digital histology methods can aid in improving understanding of unique patterns and suggest mechanisms of disease spread.
CONFLICTS OF INTEREST
The authors have no competing interests or conflicts of interest to declare that are relevant to the content of this article.
AUTHOR CONTRIBUTIONS
David G. Coughlin and David J. Irwin: conception, organization and execution of the project, writing the first draft and revisions. Claire Peterson, H. Branch Coslett, Jeffrey S. Phillips, Corey McMillan, Edward B. Lee, John Q. Trojanowski, Murray Grossman: execution of the project and review of the manuscript.
AUTHOR INFORMATION
The work was completed while David Coughlin was affiliated at the University of Pennsylvania and now at the University of California San Diego.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Avid Radiopharmaceuticals provided 18F‐flortaucipir tracer used in this study and were involved in the decision to publish the paper. They were not involved in study design, collection, analysis or interpretation of data, or the writing of this report. This work was funded by the AAN (CRTS 2059), the National Institutes of Health (P30AG072979, AG043503, AG062429, AG062418, NS053488, NS109260).
Coughlin DG, Coslett HB, Peterson C, et al. Lateralized ante mortem and post mortem pathology in a case of Lewy body disease with corticobasal syndrome. Alzheimer's Dement. 2022;8:e12294. 10.1002/trc2.12294
Contributor Information
David G. Coughlin, Email: dirwin@pennmedicine.upenn.edu.
David J. Irwin, Email: dirwin@pennmedicine.upenn.edu.
REFERENCES
- 1. Coughlin DG, Hurtig HI, Irwin DJ. Pathological influences on clinical heterogeneity in lewy body diseases. Mov Disord 2019. 10.1002/mds.27867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peavy GM, Edland SD, Toole BM, et al. Phenotypic differences based on staging of Alzheimer's neuropathology in autopsy‐confirmed dementia with Lewy bodies. Parkinsonism Relat Disord 2016;31:72‐78. 10.1016/j.parkreldis.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coughlin D, Xie SX, Liang M, et al. Cognitive and pathological influences of tau pathology in lewy body disorders. Ann Neurol 2019;85:259‐271. 10.1002/ana.25392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Irwin DJ, Xie SX, Coughlin D, et al. CSF tau and amyloid‐beta predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology 2018. 10.1212/wnl.0000000000005166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kantarci K, Lowe VJ, Boeve BF, et al. AV‐1451 tau and β‐amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol 2017;81:58‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parkkinen L, Pirttila T, Alafuzoff I. Applicability of current staging/categorization of alpha‐synuclein pathology and their clinical relevance. Acta Neuropathol 2008;115:399‐407. 10.1007/s00401-008-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of Frontotemporal Lobar Degeneration‐Tau (FTLD‐Tau). J Mol Neurosci 2011;45:384‐389. 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasanuki K, Josephs KA, Ferman TJ, et al. Diffuse Lewy body disease manifesting as corticobasal syndrome A rare form of Lewy body disease. Neurology 2018;91:E268‐79. 10.1212/WNL.0000000000005828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giannini LAA, Xie SX, McMillan CT, et al. Divergent patterns of TDP‐43 and tau pathologies in primary progressive aphasia. Ann Neurol 2019;85:630‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stefanits H, Budka H, Kovacs GG. Asymmetry of neurodegenerative disease‐related pathologies: a cautionary note. Acta Neuropathol 2012;123:449‐52. 10.1007/S00401-011-0936-6. [DOI] [PubMed] [Google Scholar]
- 11. Coughlin DG, Phillips JS, Roll E, et al. Multimodal in vivo and post‐mortem assessments of tau in lewy body disorders. Neurobiol Aging 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496‐503. 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep 2017;7:16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging‐Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1‐11. 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 2017;16:55‐65. 10.1016/s1474-4422(16)30291‐30295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Renner JA, Burns JM, Hou CE, McKeel DW, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology 2004;63:1175‐80. 10.1212/01.WNL.0000140290.80962.BF. [DOI] [PubMed] [Google Scholar]
- 18. Spotorno N, Coughlin DG, Olm CA, et al. Tau pathology associates with in vivo cortical thinning in Lewy body disorders. Ann Clin Transl Neurol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bassil F, Meymand ES, Brown HJ, et al. α‐Synuclein modulates tau spreading in mouse brains. J Exp Med 2021;218. 10.1084/jem.20192193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rogalski E, Weintraub S, Mesulam MM. Are there susceptibility factors for primary progressive aphasia? Brain Lang 2013;127:135‐8. 10.1016/j.bandl.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


