Abstract
Introduction
We investigated the safety and explore potential efficacy of batoclimab administered subcutaneously in Chinese patients with generalized myasthenia gravis (gMG).
Methods
A randomized, double-blinded, placebo-controlled, parallel phase II study was conducted. First, in the double-blinded treatment period, eligible patients received batoclimab (680 mg), batoclimab (340 mg), or placebo on days 1, 8, 15, 22, 29, and 36. In the open-label treatment period, patients received batoclimab (340 mg) on days 50, 64, and 78. In the follow-up period, patients were examined on days 92, 106, and 120. The primary endpoint was Myasthenia Gravis Activities of Daily Living (MG-ADL) score change on day 43 from baseline.
Results
In total, 30 eligible patients were enrolled, with 11, 10, and 9 patients in the batoclimab 680 mg, batoclimab 340 mg, and placebo groups, respectively. MG-ADL score changes from baseline to day 43 were −2.2 ± 0.9, −4.7 ± 0.6, and −4.4 ± 1.0 in the placebo, batoclimab 340 mg, and 680 mg groups, respectively. Similar changes were observed in Quantitative Myasthenia Gravis, Myasthenia Gravis Composite, and 15-item Myasthenia Gravis Quality of Life scores in the placebo, batoclimab 340 mg, and 680 mg groups, respectively. The proportion of patients with clinically significant improvement on day 43 was higher in the batoclimab groups. On day 120, all four scales in the placebo group had more significant improvement compared with the batoclimab groups, with total serum IgG levels reaching a plateau. No death or treatment-emergent adverse events (TEAEs) led to study discontinuation.
Conclusion
Batoclimab is effective and safe in Chinese patients with gMG.
Trial Registration
This study was registered at ClinicalTrials.gov (NCT04346888) on 15 April 2020, with the first patient enrolled on 23 July 2020.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-022-00345-9.
Keywords: Batoclimab, Neonatal Fc receptor, Generalized myasthenia gravis, Safety, Efficacy
Key Summary Points
| Why carry out this study? |
| Targeting the reduction of pathogenic IgG autoantibodies is a pathophysiological strategy for myasthenia gravis treatment; however, some shortcomings, including therapeutic accessibility, treatment safety, and cost, require urgent attention to provide more effective therapies. |
| This study aimed to investigate the safety and explore potential efficacy of neonatal Fc receptor (FcRn) antagonist batoclimab administered subcutaneously in Chinese patients with generalized myasthenia gravis. |
| What was learned from the study? |
| Clinical improvement was found in batoclimab groups compared with the placebo group demonstrated by Myasthenia Gravis Activities of Daily Living score and Quantitative Myasthenia Gravis score. |
| This phase II study showed that batoclimab is clinically effective and safe in Chinese patients with generalized myasthenia gravis. The results of this study may support the next phase III study assessing batoclimab. |
Introduction
Myasthenia gravis (MG) is an autoimmune disorder characterized by antibodies against acetylcholine receptor (AChR), muscle-specific kinase (MuSK), and/or other AChR-related proteins in the postsynaptic muscle membrane [1, 2]. A large cohort study in China reported an incidence of 0.68 per million person-years for MG, with an admission mortality rate of 14.69‰ [3].
Targeting the reduction of pathogenic IgG autoantibodies is a pathophysiological strategy for MG treatment. Previous studies demonstrated exogenous immunoglobulins, e.g., intravenous immunoglobulin (IVIG), extracorporeal removal of antibodies (e.g., plasmapheresis or immunoadsorption), or depletion of antibodies producing precursor cells (e.g., CD20 monoclonal antibody) can be advantageous for patients with generalized MG (gMG) [4]. However, some shortcomings (e.g., therapeutic accessibility, as well as treatment safety and costs) require urgent attention to provide more effective therapies for patients with MG.
The neonatal Fc receptor (FcRn) plays a pivotal role in IgG recycling, and blocking IgG–FcRn interaction results in enhanced degradation of IgG [5, 6]. In recent clinical studies, the analysis of FcRn antagonists confirmed the therapeutic advantages of reducing IgG in patients with gMG [7–9].
Different from some other FcRn antagonists (humanized IgG1 Fc fragment for efgartigimod, humanized IgG4 monoclonal antibody for rozanolixizumab, etc.), batoclimab (HBM9161) is a fully humanized IgG1 monoclonal antibody targeting FcRn that accelerates the degradation of pathogenic autoantibodies. Moreover, acceptable safety, pharmacokinetics, and pharmacodynamics of batoclimab in Chinese healthy volunteers were reported [10], with a single subcutaneous dose of 680 mg, reducing total IgG levels by 41.2 ± 10.4%.
We hypothesized that reduction of total IgG, as observed in healthy volunteers in a phase I trial, leads to clinical improvement in gMG cases with batoclimab administration. Hence, this study aimed to investigate the safety and explore potential efficacy of batoclimab administered subcutaneously in Chinese patients with gMG.
Methods
Study Design
This was a randomized, double-blinded, placebo-controlled, parallel phase II study involving seven Chinese medical centers. After a 2-week screening period, study participants entered a 6-week study drug treatment period (double-blinded treatment period), followed by an open-label treatment period. A total of 30 eligible patients were planned. Study groups were: group 1, batoclimab (680 mg, double-blinded treatment period) + batoclimab (340 mg, open-label treatment period); group 2, batoclimab (340 mg, double-blinded treatment period) + batoclimab (340 mg, open-label treatment period); group 3, placebo, (double-blinded treatment period) + batoclimab (340 mg, open-label treatment period).
Ethics Approval, Registration, and Consent to Participate
This study was approved by the ethics committees of seven participating Chinese medical centers (Supplemental Table 4; with Institutional Ethics Committee for Drugs Clinical Trials of Huashan Hospital, Fudan University as the master ethics committee, approval number 2020-074), and registered at ClinicalTrials.gov (no. NCT04346888). It abided by the Declaration of Helsinki. All patients provided signed informed consent prior to enrollment.
Inclusion and Exclusion Criteria
Inclusion criteria were: (1) > 18 years old; (2) Myasthenia Gravis Foundation of America (MGFA) score of IIa-IVa; (3) AChR-Ab-positive or MuSK-Ab-positive; (4) Myasthenia Gravis Activities of Daily Living (MG-ADL) score ≥ 6, with eye muscle score accounting for < 50%; (5) stable MG treatments at baseline, including acetylcholinesterase inhibitors, corticosteroids, and/or immunosuppressants. The diagnosis of MG was supported by a history of abnormal repetitive nerve stimulation test, a positive edrophonium chloride test, or improvement with acetylcholinesterase inhibitors, and positivity of AChR-Ab or MuSK-Ab.
Main exclusion criteria were (see details in protocol): (1) a history of malignancy; (2) severe MG (e.g., type IVb or V); (3) thymectomy or radiation therapy for < 12 months; (4) intravenous administration of IgG, or plasmapheresis/plasma exchange (PLEX) for < 4 weeks before the screening; (5) immunosuppressive monoclonal antibodies (e.g., rituximab, bevacizumab, eculizumab, etc.) administered for < 6 months prior to screening; (6) other uncontrolled autoimmune diseases potentially interfering with the study course.
Randomization and Blinding
The randomization was an unstratified randomization, based on block randomization method. Eligible patients were randomized in a 1:1:1 ratio to the following groups via a centralized Interactive Web Response System (IWRS): group 1, batoclimab (680 mg, double-blinded treatment period) + batoclimab (340 mg, open-label treatment period); group 2, batoclimab (340 mg, double-blinded treatment period) + batoclimab (340 mg, open-label treatment period); group 3, placebo, (double-blinded treatment period) + batoclimab (340 mg, open-label treatment period).
A screening number was assigned to each patient upon enrollment via the IWRS. The investigators, clinical staff, and patients remained blinded to the treatment process until study end.
Interventions
In the double-blinded treatment period, eligible patients received batoclimab (680 mg), batoclimab (340 mg), or placebo once weekly (QW) on days 1, 8, 15, 22, 29, and 36. In the open-label treatment period, eligible patients received batoclimab (340 mg) once every 2 weeks (Q2W) on days 50, 64, and 78. In the follow-up period, patients were followed up until day 120, including three visits on days 92, 106, and 120.
The subjects were required to keep baseline treatment stable. Adjustment of the dose of corticosteroids and/or immunosuppressants was not allowed. Rescue therapy would be provided, including IVIg and PLEX.
Efficacy was assessed by MG Activities of Daily Living (MG-ADL) [11], Quantitative Myasthenia Gravis (QMG) [12], Myasthenia Gravis Composite (MGC) [13], and revised 15-item Myasthenia Gravis Quality of Life scale (MG-QoL15r) [14] scores at prespecified visits.
Outcomes and Definitions
The primary efficacy endpoint was MG-ADL score change on day 43 from baseline. Secondary efficacy endpoints included QMG, MGC, and MG-QoL15r score changes on day 43 from baseline, the percentage of patients with clinically significant improvement based on MG-ADL (defined as 2 or more points improvement) and QMG (defined as 3 or more points improvement) scores on day 43 compared with the baseline, and MG-ADL, QMG, MGC, and MG-QoL15r score changes from baseline to day 120, and the percentage of patients with sustained improvement from baseline to day 120 (improvement in MG-ADL score ≥ 2 or in QMG score ≥ 3 for 4 consecutive weeks). Minimal symptom expression was defined as a total MG-ADL score of 0–1 [15].
Safety endpoints included the incidence of treatment-emergent AEs (TEAEs) and changes in albumin levels from baseline. Pharmacodynamic endpoints included serum total IgG level changes from baseline to day 120.
Statistical Analysis
The sample size was partly determined by the chance of observing a relatively rare safety event. With ten subjects per group, if an adverse event rate was 10% or higher, the probability of observing an event was at least 65%.
Efficacy analysis was based on the full analysis set (FAS), including all randomized patients administered at least one dose of the study drug, with MG-ADL scores between treatment initiation and day 50 recorded. MG-ADL score changes from baseline to day 43 were analyzed by mixed model for repeated measures (MMRM) analysis with data collected before treatment and on day 43. Repeated measures analysis was undertaken on the basis of the restricted maximum likelihood approach with changes from baseline as a dependent variable; treatment group, visits, and treatment–visit interaction as explanatory variables; and baseline ADL value as a covariate to establish a mixed effects model. The same MMRM model was used for the analysis of QMG/MGC/MG-QoL15r score changes from baseline to day 43, and MG-ADL/QMG/MGC/MG-QoL15r score changes from baseline to day 120. For MG-ADL, the numbers of patients with reductions of ≥ 2, ≥ 3, and ≥ 4 points from baseline, respectively, in at least one of the first two weeks after treatment were summarized. Continuous data are mean ± standard error (SE).
The safety set (SS) was used for safety analysis, including all participants administered the drug at least once after enrollment, with post-drug safety assessment data. The numbers of patients with TEAEs, treatment-emergent serious adverse events (TESAEs), and TEAEs leading to premature discontinuation of treatment were summarized.
AChR antibody was detected by enzyme-linked immunosorbent assay (ELISA) using Acetylcholine Receptor Autoantibody ELISA Kit (RSR, Cardiff, U.K), and MuSK antibody was also detected by ELISA using MuSK ELISA Kit (AESKU diagnostics, Wendelsheim, Germany).
The study protocol is available as supplemental material.
Results
Demographic and Clinical Characteristics
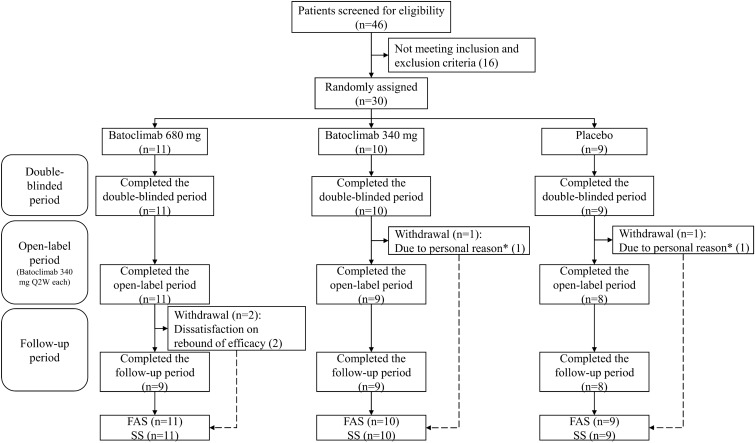
The first patient was enrolled on 8 August 2020, and the last patient completed the whole study on 11 August 2021. In total, 30 patients were involved in this phase II study. After randomization, there were 11, 10, and 9 patients in the batoclimab (680 mg), batoclimab (340 mg), and placebo groups, respectively (Fig. 1). All patients completed the double-blinded period, and 26 completed the whole study. One patient each in the batoclimab (340 mg) and placebo groups voluntarily withdrew from the study after one injection in the open-label period. Two patients in the batoclimab (680 mg) group withdrew in the follow-up period owing to dissatisfaction on rebound of efficacy.
Fig. 1.
Study flow diagram. *The two patients who withdrew in the open-label period received one injection
Table 1 presents patients’ demographic characteristics. On MGFA classification, the percentages of type III were higher in the batoclimab (340 mg, 60.0%) and batoclimab (680 mg, 54.6%) groups compared with the placebo group (33.3%). However, the percentages of type IV in the batoclimab (340 mg, 10.0%) and batoclimab (680 mg, 9.1%) groups were lower than that of the placebo group (22.2%). Besides, MG-ADL scores at baseline were comparable among the three groups. The usage proportions of corticosteroids and immunosuppressants in background therapies were higher in the batoclimab groups than in the placebo group.
Table 1.
Patient basic characteristics
| Basic characteristic | Placebo (N = 9) | Batoclimab 340 mg (N = 10) | Batoclimab 680 mg (N = 11) | Batoclimab combined (N = 21) |
|---|---|---|---|---|
| Age (years), mean ± SD | 40.2 ± 9.3 | 36.4 ± 9.8 | 40.6 ± 16.8 | 38.6 ± 13.7 |
| Sex, % male/% female | 22.2%/77.8% | 20.0%/80.0% | 18.2%/81.8% | 19.0%/81.0% |
| Duration of disease (years), mean ± SD | 6.0 ± 6.8 | 9.8 ± 10.8 | 6.4 ± 5.7 | 8.0 ± 8.5 |
| MGFA classification, n (%) | ||||
| II | 4 (44.4%) | 3 (30.0%) | 4 (36.4%) | 7 (33.3%) |
| III | 3 (33.3%) | 6 (60.0%) | 6 (54.5%) | 12 (57.1%) |
| Iva | 2 (22.2%) | 1 (10.0%) | 1 (9.1%) | 2 (9.5%) |
| MG-ADL, mean ± SD | 8.2 ± 1.4 | 7.4 ± 1.6 | 9.2 ± 2.3 | 8.3 ± 2.2 |
| QMG, mean ± SD | 14.9 ± 5.0 | 17.4 ± 3.5 | 18.8 ± 6.1 | 18.1 ± 5.0 |
| MGC, mean ± SD | 17.7 ± 4.0 | 17.9 ± 3.5 | 18.2 ± 5.3 | 18.0 ± 4.5 |
| MG-QoL15r, mean ± SD | 18.8 ± 4.6 | 15.1 ± 2.8 | 19.8 ± 5.9 | 17.6 ± 5.2 |
| Background therapy, n (%) | ||||
| Acetylcholinesterase inhibitors | 7 (77.8%) | 10 (100.0%) | 10 (90.9%) | 20 (95.2%) |
| Corticosteroids | 6 (66.7%) | 6 (60.0%) | 11 (100.0%) | 17 (81.0%) |
| Immunosuppressants | 3 (33.3%) | 8 (80.0%) | 8 (72.7%) | 16 (76.2%) |
| Thymectomy | 2 (22.2%) | 3 (30.0%) | 3 (27.3%) | 6 (28.6%) |
| AChRAb positive, n (%) | 8 (88.9%) | 9 (90.0%) | 11 (100%) | 20 (95.2%) |
| MuSKAb positive, n (%) | 1 (11.1%) | 1 (10.0%) | 0 (0%) | 1 (4.8%) |
In this phase II study, there were two subjects with positive MuSK Ab, one in the 340 mg group and the other in the placebo group.
Efficacy
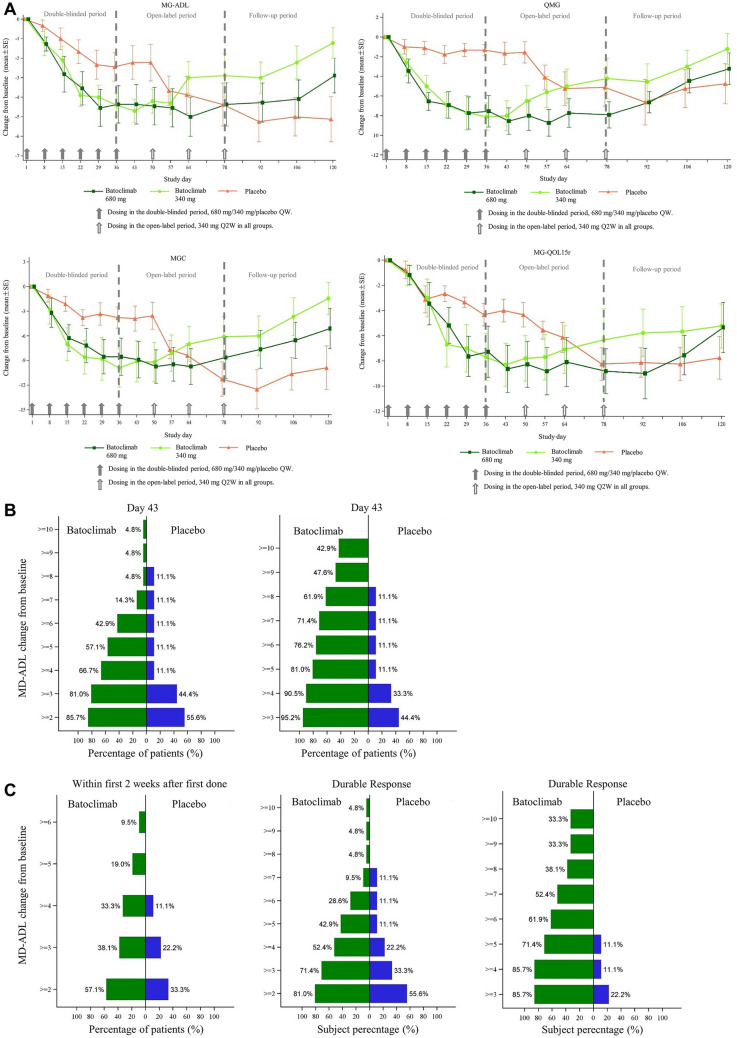
During the double-blinded treatment period, clinical improvement was found in both batoclimab groups compared with the placebo group. MG-ADL score changes from baseline to day 43 were −2.2 ± 0.9, −4.7 ± 0.6, and −4.4 ± 1.0 in the placebo, batoclimab (340 mg), and batoclimab (680 mg) groups, respectively. QMG score changes from baseline to day 43 were −1.7 ± 1.1, −8.0 ± 1.5, and −8.8 ± 1.2 in the placebo, batoclimab (340 mg), and batoclimab (680 mg) groups, respectively. MGC score changes from baseline to day 43 were −3.7 ± 1.5, −9.0 ± 2.5, and −8.8 ± 2.2 in the placebo, batoclimab (340 mg), and batoclimab (680 mg) groups, respectively. MG-QoL15r score changes from baseline to day 43 were −4.0 ± 0.9, −8.3 ± 1.7, and −8.6 ± 1.9 in the placebo, batoclimab (340 mg), and batoclimab (680 mg) groups, respectively. On day 120 from baseline, MG-ADL (−5.1 ± 1.16, −1.2 ± 0.78, and −2.9 ± 0.89), QMG (−4.8 ± 2.03, −1.2 ± 1.56, and −3.2 ± 1.61), MGC (−9.9 ± 2.64, −1.4 ± 1.94, and −5.1 ± 2.46), and MG-QoL15r (−7.8 ± 1.71, −5.2 ± 1.75, and −5.3 ± 1.99) scores were obtained in the placebo, batoclimab (340 mg), and batoclimab (680 mg) groups, respectively (Fig. 2A).
Fig. 2.
Clinical efficacy. A Changes from baseline to day 43 in Myasthenia Gravis Activities of Daily Living (MG-ADL), Quantitative Myasthenia Gravis (QMG), Myasthenia Gravis Composite (MGC), and revised 15-item Myasthenia Gravis Quality of Life (MG-QoL15r) scores. Values are mean ± standard error, expressed as point reduction from baseline. B Day 43 responder rates for Myasthenia Gravis Activities of Daily Living (MG-ADL) (reduction ≥ 2 points) and Quantitative Myasthenia Gravis (QMG) (reduction ≥ 3 points) scores. The percentage of patients with a clinical improvement of specified value is indicated next to the corresponding bar. Two batoclimab dose groups were combined. C Fast and durable clinical improvement. Patients showing fast clinical improvement by Myasthenia Gravis Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores at least 1 week within 2 weeks after the first dose. Patients showing durable response by Myasthenia Gravis Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores. MG-ADL or QMG improvement from baseline with a magnitude as indicated in the y-axis for 4 or more weeks during the double-blinded treatment period. Two batoclimab dose groups were combined
Regarding MG-ADL scores, maximal improvement was achieved on day 29 and maintained until day 57 (day 29, −4.0 ± 0.61; day 57, −4.3 ± 0.58) or day 78 (day 29, −4.5 ± 0.95; day 78, −4.4 ± 1.11) for batoclimab 340 mg and 680 mg groups, respectively. As for QMG, maximum improvement was also achieved on day 29 and maintained until day 43 (day 29, −7.7 ± 1.75; day 43, −8.0 ± 1.53) or day 78 (day 29, −7.7 ± 1.64; day 78, −7.9 ± 1.34) for batoclimab 340 mg and 680 mg groups, respectively. Similar trends were observed in MGC and MG-QOL15r scores.
The number of patients with clinically significant improvement on day 43 was higher in batoclimab treatment pooled groups than in the placebo group, particularly in terms of MG-ADL (85.7% versus 55.6%) and QMG (95.2% versus 44.4%) scores (Fig. 2B).
Early response and sustained clinical symptoms were noticeable. Within the first 2 weeks after the first administration, the batoclimab treatment groups showed higher proportions of improvement in clinical status compared with the placebo group (57.1% versus 33.3%) (Fig. 2C). As for durable response, the number of patients with clinically significant improvement, particularly in MG-ADL and QMG scores, for at least 4 consecutive weeks during the double-blinded treatment period was higher in batoclimab treatment groups compared with the placebo group (MG-ADL, 81.0% versus 55.6%; QMG, 85.7% versus 22.2%) (Fig. 2C).
Minimal symptom expression on day 43 was achieved more in batoclimab-treated patients (7/21, 33.3%) versus the placebo group (1/9, 11.1%).
Pharmacodynamic Features (Serum IgG Levels)
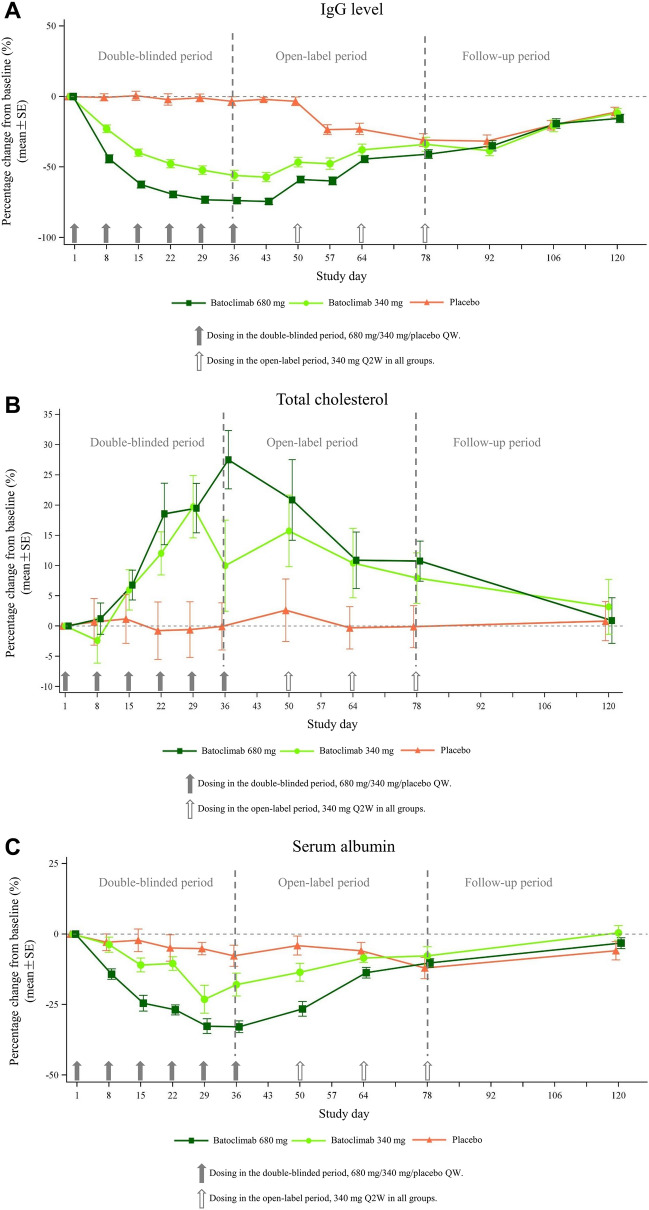
In the first week of treatment, total serum IgG level reductions approximated 23% and 44% in the batoclimab (340 mg) and batoclimab (680 mg) groups, respectively, versus 1% in the placebo group. Maximum total serum IgG level reductions were measured on day 43, i.e., 57% and 74% in the batoclimab (340 mg) and batoclimab (680 mg) groups, respectively, versus 2% in the placebo group. From day 43, serum IgG levels began to increase in both batoclimab treatment groups, with lower levels in the batoclimab (680 mg) group versus the batoclimab (340 mg) group until day 92 when equal serum IgG levels were found in both groups (Fig. 3A). On day 120, IgG level reductions decreased to about 12%, 15%, and 11% in the batoclimab (340 mg), batoclimab (680 mg), and placebo groups, respectively. In addition, both reduction and increase of serum IgG concentrations shared a mirror pattern with the clinical improvements reflected by MG-ADL and QMG scores.
Fig. 3.
Changes in IgG, total cholesterol, and albumin levels. A Serum total immunoglobulin G (IgG) levels after batoclimab and placebo treatment over the whole study period. Values are mean ± standard error, expressed as the percentage change from baseline in IgG concentration. B Serum total cholesterol levels after batoclimab and placebo treatment over the whole study period. Values are mean ± standard error, expressed as serum total cholesterol concentration. C Serum total albumin levels after batoclimab and placebo treatment over the whole study period. Values are mean ± standard error, expressed as serum albumin concentration
Safety and Tolerability
In the double-blinded period, batoclimab showed acceptable safety and tolerability profiles, with no death, SAEs, or TEAEs leading to treatment discontinuation. During follow-up, two patients in the batoclimab (680 mg) groups withdrew from the study because of unsatisfaction with rebound of efficacy, with MG-ADL scores almost back to baseline.
The reported TEAEs were well comparable among the three groups during the double-blinded period. During the double-blinded period, there were nine (100.0%), ten (100.0%), and ten (90.9%) patients who experienced TEAEs in the placebo, batoclimab (340 mg), and batoclimab (680 mg) groups, respectively, mainly of mild grade (Table 2). Only two (22.2%) and three (27.3%) patients in the placebo and batoclimab (680 mg) groups experienced moderate TEAEs. From open-label to follow-up periods, there were eight (88.9%), eight (80.0%), and ten (90.9%) patients who experienced TEAEs in the placebo, batoclimab (340 mg), and batoclimab (680 mg) groups, respectively, mainly of mild grade. Only one (11.1%) and three (27.3%) patients in the placebo and batoclimab (680 mg) groups experienced moderate TEAEs (Table 3).
Table 2.
Treatment-emergent safety outcomes in the double-blinded periods
| TEAEs during double-blinded period, n (%) | Batoclimab 680 mg (N = 11) | Batoclimab 340 mg (N = 10) | Placebo (N = 9) |
|---|---|---|---|
| Total | 10 (90.9) | 10 (100) | 9 (100) |
| Metabolism and nutrition disorders | 7 (63.6) | 7 (70.0) | 5 (55.6) |
| Hypoalbuminemia | 4 (36.4) | 0 | 0 |
| Hypercholesterolemia | 3 (27.3) | 2 (20.0) | 2 (22.2) |
| Hyponatremia | 1 (9.1) | 5 (50.0) | 4 (44.4) |
| Hypomagnesemia | 1 (9.1) | 2 (20.0) | 3 (33.3) |
| Hypoproteinemia | 1 (9.1) | 1 (10.0) | 0 |
| Decreased appetite | 1 (9.1) | 0 | 0 |
| Hypertriglyceridemia | 1 (9.1) | 0 | 0 |
| Hyperuricemia | 0 | 2 (20.0) | 0 |
| Hypocalcemia | 0 | 1 (10.0) | 0 |
| Hypokalemia | 0 | 0 | 1 (11.1) |
| General disorders and administration site conditions | 5 (45.5) | 5 (50.0) | 1 (11.1) |
| Injection site hemorrhage | 3 (27.3) | 2 (20.0) | 1 (11.1) |
| Edema peripheral | 2 (18.2) | 2 (20.0) | 0 |
| Injection site pain | 1 (9.1) | 0 | 1 (11.1) |
| Injection site reactions | 0 | 1 (10.0) | 0 |
| Injection site pruritus | 0 | 1 (10.0) | 0 |
| Injection site nodule | 0 | 1 (10.0) | 0 |
| Facial edema | 0 | 1 (10.0) | 0 |
| Injection site erythema | 0 | 0 | 1 (11.1) |
| Infections and infestations | 3 (27.3) | 4 (40.0) | 6 (66.7) |
| Urinary tract infection | 2 (18.2) | 3 (30.0) | 2 (22.2) |
| Upper respiratory infection | 1 (9.1) | 1 (10.0) | 2 (22.2) |
| Herpes simplex | 1 (9.1) | 0 | 0 |
| Folliculitis | 1 (9.1) | 0 | 0 |
| Urethritis | 0 | 0 | 1 (11.1) |
| Blepharitis | 0 | 0 | 1 (11.1) |
| Gastrointestinal disorders | 3 (27.3) | 3 (30.0) | 3 (33.3) |
| Abdominal pain upper | 1 (9.1) | 1 (10.0) | 0 |
| Abdominal pain | 1 (9.1) | 1 (10.0) | 1 (11.1) |
| Nausea | 1 (9.1) | 0 | 0 |
| Toothache | 1 (9.1) | 0 | 1 (11.1) |
| Diarrhea | 1 (9.1) | 0 | 2 (22.2) |
| Gingival pain | 0 | 1 (10.0) | 0 |
| Lower abdominal pain | 0 | 0 | 1 (11.1) |
| Investigations | 2 (18.2) | 5 (50.0) | 3 (33.3) |
| Blood albumin decreased | 2 (18.2) | 2 (20.0) | 1 (11.1) |
| Blood cholesterol increased | 1 (9.1) | 1 (10.0) | 1 (11.1) |
| Blood in urine | 0 | 3 (30.0) | 1 (11.1) |
| Weight gain | 0 | 1 (10.0) | 0 |
| Urine ketone body present | 0 | 1 (10.0) | 0 |
| Blood calcium decreased | 0 | 1 (10.0) | 1 (11.1) |
| White blood cell urine positive | 0 | 0 | 1 (11.1) |
| Blood bicarbonate decreased | 0 | 0 | 1 (11.1) |
| Musculoskeletal and connective tissue disorders | 2 (18.2) | 0 | 0 |
| Muscle twitching | 2 (18.2) | 0 | 0 |
| Skin and subcutaneous tissue disorders | 1 (9.1) | 1 (10.0) | 0 |
| Pruritus | 1 (9.1) | 0 | 0 |
| Rash | 1 (9.1) | 0 | 0 |
| Night sweats | 0 | 1 (10.0) | 0 |
| Respiratory, thoracic, and mediastinal disorders | 1 (9.1) | 0 | 0 |
| Cough | 1 (9.1) | 0 | 0 |
| Psychiatric disorders | 1 (9.1) | 0 | 1 (11.1) |
| Trouble sleeping | 1 (9.1) | 0 | 0 |
| Anxious | 0 | 0 | 1 (11.1) |
| Hepatobiliary disorders | 1 (9.1) | 0 | 0 |
| Hepatic function abnormal | 1 (9.1) | 0 | 0 |
| Vascular disorders | 1 (9.1) | 0 | 0 |
| Flushing | 1 (9.1) | 0 | 0 |
| Nervous system disorders | 0 | 4 (40.0) | 1 (11.1) |
| Dizziness | 0 | 4 (40.0) | 0 |
| Headache | 0 | 0 | 1 (11.1) |
| Cardiac disorders | 0 | 1 (10.0) | 0 |
| Palpitations | 0 | 1 (10.0) | 0 |
| Eye disorders | 0 | 1 (10.0) | 0 |
| Blurred vision | 0 | 1 (10.0) | 0 |
| Renal and urinary disorders | 0 | 0 | 1 (11.1) |
| Urinary frequency | 0 | 0 | 1 (11.1) |
Table 3.
Treatment-emergent safety outcomes in the open-label and follow-up periods
| TEAEs during open-label and follow-up periods, n (%) | Batoclimab 680 mg (N = 11) | Batoclimab 340 mg (N = 10) | Placebo (N = 9) | Total (N = 30) |
|---|---|---|---|---|
| Total | 10 (90.9) | 8 (80.0) | 8 (88.9) | 26 (86.7) |
| Infections and infestations | 3 (27.3) | 2 (20.0) | 4 (44.4) | 9 (30.0) |
| Upper respiratory infection | 2 (18.2) | 1 (10.0) | 2 (22.2) | 5 (16.7) |
| Urinary tract infection | 2 (18.2) | 1 (10.0) | 2 (22.2) | 5 (16.7) |
| Nervous system disorders | 3 (27.3) | 1 (10.0) | 0 | 4 (13.3) |
| Myasthenia gravis | 2 (18.2) | 0 | 0 | 2 (6.7) |
| Headache | 1 (9.1) | 0 | 0 | 1 (3.3) |
| Dizziness | 0 | 1 (10.0) | 0 | 1 (3.3) |
| Metabolism and nutrition disorders | 2 (18.2) | 5 (50.0) | 4 (44.4) | 11 (36.7) |
| Hyponatremia | 2 (18.2) | 4 (40.0) | 2 (22.2) | 8 (26.7) |
| Hypomagnesemia | 2 (18.2) | 1 (10.0) | 0 | 3 (10.0) |
| Hypercholesterolemia | 1 (9.1) | 3 (30.0) | 1 (11.1) | 5 (16.7) |
| Hypertriglyceridemia | 1 (9.1) | 1 (10.0) | 1 (11.1) | 3 (10.0) |
| Hypochloremia | 1 (9.1) | 0 | 0 | 1 (3.3) |
| Hyperuricemia | 0 | 2 (20.0) | 0 | 2 (6.7) |
| Hypokalemia | 0 | 0 | 1 (11.1) | 1 (3.3) |
| General disorders and administration site conditions | 2 (18.2) | 1 (10.0) | 1 (11.1) | 4 (13.3) |
| Edema peripheral | 2 (18.2) | 1 (10.0) | 0 | 3 (10.0) |
| Facial edema | 1 (9.1) | 0 | 0 | 1 (3.3) |
| Chest pain | 0 | 0 | 1 (11.1) | 1 (3.3) |
| Chest discomfort | 0 | 0 | 1 (11.1) | 1 (3.3) |
| Musculoskeletal and connective tissue disorders | 2 (18.2) | 1 (10.0) | 1 (11.1) | 4 (13.3) |
| Muscle twitching | 2 (18.2) | 1 (10.0) | 0 | 3 (10.0) |
| Limb discomfort | 0 | 0 | 1 (11.1) | 1 (3.3) |
| Pain in extremity | 0 | 0 | 1 (11.1) | 1 (3.3) |
| Musculoskeletal chest pain | 0 | 0 | 1 (11.1) | 1 (3.3) |
| Gastrointestinal disorders | 2 (18.2) | 0 | 1 (11.1) | 3 (10.0) |
| Diarrhea | 1 (9.1) | 0 | 1 (11.1) | 2 (6.7) |
| Tongue edema | 1 (9.1) | 0 | 0 | 1 (3.3) |
| Investigations | 1 (9.1) | 5 (50.0) | 1 (11.1) | 7 (23.3) |
| Blood in urine | 1 (9.1) | 1 (10.0) | 0 | 2 (6.7) |
| Blood chloride increased | 1 (9.1) | 0 | 0 | 1 (3.3) |
| Blood cholesterol increased | 1 (9.1) | 0 | 0 | 1 (3.3) |
| Blood glucose increased | 1 (9.1) | 0 | 1 (11.1) | 2 (6.7) |
| Blood sodium increased | 1 (9.1) | 0 | 0 | 1 (3.3) |
| Blood calcium decreased | 0 | 2 (20.0) | 0 | 2 (6.7) |
| Urine ketone body present | 0 | 1 (10.0) | 0 | 1 (3.3) |
| Blood chloride decreased | 0 | 1 (10.0) | 0 | 1 (3.3) |
| Blood creatinine increased | 0 | 1 (10.0) | 0 | 1 (3.3) |
| Blood magnesium decreased | 0 | 1 (10.0) | 0 | 1 (3.3) |
| Blood glucose abnormal | 0 | 0 | 1 (11.1) | 1 (3.3) |
| Eye disorders | 1 (9.1) | 1 (10.0) | 0 | 2 (6.7) |
| Paraesthesia eye | 1 (9.1) | 0 | 0 | 1 (3.3) |
| Eye pruritus | 1 (9.1) | 0 | 0 | 1 (3.3) |
| Visual fatigue | 0 | 1 (10.0) | 0 | 1 (3.3) |
| Skin and subcutaneous tissue disorders | 0 | 1 (10.0) | 0 | 1 (3.3) |
| Pruritus | 0 | 1 (10.0) | 0 | 1 (3.3) |
| Renal and urinary disorders | 0 | 0 | 1 (11.1) | 1 (3.3) |
| Urinary frequency | 0 | 0 | 1 (11.1) | 1 (3.3) |
The most frequently reported TEAEs were hypercholesterolemia, hyponatremia, urinary tract infection, injection site reaction, and peripheral edema (Table 4). Serum cholesterol levels were increased in batoclimab treatment groups. During the double-blinded period, maximum increases of serum cholesterol were 19.7 ± 5.1% (day 29), 27.5 ± 4.8% (day 36), and 2.6 ± 5.2% (day 50) in the batoclimab (340 mg), batoclimab (680 mg), and placebo groups, respectively (Fig. 3B). There was no hypercholesterolemia of CTCAE grade ≥ 3, and cases of hypercholesterolemia were of grade 1 at baseline, recovering soon after discontinuation of the study drug. No clinical abnormality or serious cardiovascular events related to hypercholesterolemia were identified.
Table 4.
Treatment-emergent safety outcomes in all treated patients during the double-blinded period (overall reported in five or more patients)
| TEAEs during the double-blinded treatment period (reported in five or more patients), n (%) | Placebo (N = 9) | Batoclimab 340 mg (N = 10) | Batoclimab 680 mg (N = 11) |
|---|---|---|---|
| Hypercholesterolemiaa | 3 (33.3) | 3 (30.0) | 4 (36.4) |
| Hyponatremia | 4 (44.4) | 4 (40.0) | 1 (9.1) |
| Urinary tract infection | 3 (33.3) | 3 (30.0) | 2 (18.2) |
| Injection site reactionb | 1 (11.1) | 3 (30.0) | 3 (27.3) |
| Peripheral edema | 1 (11.1) | 2 (20.0) | 4 (36.4) |
| Hypomagnesemia | 3 (33.3) | 2 (20.0) | 1 (9.1) |
| Abdominal painc | 2 (22.2) | 2 (20.0) | 2 (18.2) |
aIncludes hypercholesterolemia and blood cholesterol increase
bIncludes injection site hemorrhage, injection site pruritus, injection site hematoma, injection site pain, and injection site nodule
c Includes abdominal pain, upper abdominal pain, and lower abdominal pain
During the study, serum albumin was dose-dependently decreased in both batoclimab (340 mg) and batoclimab (680 mg) groups, returning to the normal range 6 weeks after study drug discontinuation. Maximum decreases of serum albumin were 23.1 ± 4.9% (day 29) and 32.9 ± 2.1% (day 36) in the batoclimab (340 mg) and batoclimab (680 mg) groups, respectively, versus 7.7 ± 3.8% (day 36) in the placebo group (Fig. 3C). Mild peripheral edema was reported in one (11.1%), two (20.0%), and four (36.4%) patients in the placebo, batoclimab (340 mg), and batoclimab (680 mg) groups, respectively, accounting for one-third of patients with hypoproteinemia. One 74-year-old female in the batoclimab (680 mg) group showed sustained edema when serum albumin returned to the normal range after study drug discontinuation, indicating this adverse event (AE) was attributed to the underlying disease rather than the study drug.
Besides, ADA was detected in none of the patients throughout the study. IgM, IgA, and IgE were unchanged (Supplementary Fig. 1).
Discussion
Compared with traditional therapy of MG such as corticosteroid and immunosuppressants (azathioprine, mycophenolate mofetil, and calcineurin inhibitor) that suppress a wide range of lymphocytes and cytokine production, batoclimab selectively targets IgG, which is central to MG pathology. Batoclimab has the advantage of rapid onset of effect, as well as avoiding side effects of traditional therapy, including glucose intolerance, osteoporosis, and renal dysfunction.
In this phase II study, batoclimab showed a noticeable efficacy on gMG, including MG-ADL, QMG, MGC, and MG-QoL15r score improvements on day 43, and the maximum efficacy was observed 4 weeks after the first intervention, and maintained for about 3 weeks. Both doses of batoclimab showed a favorable safety profile and were well tolerated. Besides, TEAEs were balanced among the placebo and batoclimab treatment groups. No death, SAEs, or TEAEs leading to study discontinuation were reported, and AEs were mainly mild and moderate. Rescue therapy (IVIg) was used in one subject whose MG condition worsened during the follow-up period.
In double-blinded, open-label and follow-up periods, the incidence of TEAEs associated with infections was similar among the two batoclimab groups and the placebo group, and the incidence is even slightly higher in the placebo group; therefore, the treatment with batoclimab does not increase the risk of infections. Urinary tract infection was the most common infection (asymptomatic urinary leukocyte elevation), followed by upper respiratory infection. However, all infectious AEs were of mild grade, and a higher incidence was noted in the placebo group. Serum IgG level decreases by FcRn antagonist appeared to be transient and reversible [16, 17]. In the current study, at least 25.6% of serum IgG levels at baseline were retained after treatment. In addition, evidence suggests inhibition of FcRn selectively reduces serum IgG levels, leaving IgM, IgA, and IgE levels unaffected [17]. Moreover, inhibition of FcRn is not expected to affect other cells or components of the innate and adaptive immune systems [16, 18]. Therefore, batoclimab is less likely to be associated with increased risk of infection compared with other immune modulators.
Phase I study indicated that subjects administered a single dose of batoclimab experienced a transient, dose-dependent reduction of serum albumin level, which fully resolved spontaneously after drug withdrawal [10]. In the current study, the batoclimab groups also experienced serum albumin level reductions, in which maximum decreases from baseline were 23% and 32% in the 340 mg and 680 mg groups, respectively. The mean decrease of serum albumin was about 20% for nipocalimab (15 or 30 mg/kg QW) in a phase I study [17], and 14% for nipocalimab (60 mg/kg Q2W) in the corresponding phase II study [19]. FcRn binds to albumin and IgG at two different sites, respectively, prolonging the molecular half-life [20]. Thus, smaller specific FcRn inhibitors, such as the IgG1 Fc fragment efgartigimod, should not hamper the binding of albumin, leading to no change in serum albumin concentration [8, 16]. However, the albumin binding could be affected by monoclonal antibodies such as batoclimab and nipocalimab, possibly owing to bigger molecular size. Accumulating evidence reveals reduction of serum albumin during treatment with FcRn inhibitors is recoverable and often asymptomatic.
Mild-to-moderate elevation of total cholesterol levels was noted in the current study, in which the highest increases from baseline were 27% and 19% in the 680 mg and 340 mg groups, respectively, all returned to baseline during follow-up. No elevation was observed in the placebo group. It was postulated that a negative correlation exists between cholesterol and albumin, indicating that cholesterol levels might increase as a result of decreased albumin levels, which deserves further investigation [21]. The correlation between albumin and cholesterol levels was not assessed in the current phase II study, but an increase in low-density lipoprotein cholesterol (LDL-C) levels has been reported in patients with thyroid-associated ophthalmopathy administered batoclimab (680 mg, 65%; 340 mg, 40%) after 12 weeks, which recovered after discontinuation [22]. Collectively, the levels of cholesterol require further attention during treatment with batoclimab in patients with gMG.
In the double-blinded period, there was only one (11%) TEAE of headache in the placebo group, and in the open-label and follow-up periods, there was only one (9%) TEAE of headache in the batoclimab 680 mg group;therefore, the incidence of headache was much lower than in the studies on other FcRn antagonists. After administration of efgartigimod, 33.3% (phase II) and 29% (phase III) of patients had headache [7, 8]. The most common TEAE in patients administered rozanolixizumab is headache, as reported previously [9, 23]. Similarly, the most common TEAE among subjects administered nipocalimab is headache [17]. In contrast, batoclimab may not lead to headache, while further research needs to be conducted to confirm this hypothesis.
A rapid and sharp fall in total IgG levels was observed after batoclimab treatment. Total serum IgG level reduction from baseline at 1 week after the first administration of batoclimab (680 mg) was 44.2%, which was similar to a previously reported rate [10]; the decrease from baseline to the second week in the batoclimab (680 mg) group was 62.4%, reaching 74.4% at 1 week after the sixth injection. In a phase II study of efgartigimod, serum IgG levels were reduced by nearly 40% after the first week of treatment, and was further reduced by 70% of pretreatment levels [7]. The total serum IgG level after rozanolixizumab treatment was reduced by 68% of the highest level in a phase II study [9]. As extracorporeal removal procedures, PLEX could maximally reduce 73.4% of total serum IgG [24] and immunoadsorption (IA) could reduce 26.5% after one session with 2.0 L processing plasma volume (PPV) [25].
The results of the double-blinded period of present study preliminarily confirmed the close relationship between total serum IgG level decrease and the improvement of gMG symptoms, corroborating previous findings [24] and trials of efgartigimod [7, 8]. Treatment with batoclimab provided a rapid effect, as well as a durable and meaningful improvement of the clinical condition. On day 7 after the first administration, MG-ADL scores were significantly decreased from baseline in both batoclimab groups, In the ADAPT study, maximal clinical improvement was detected at 1 week after the fourth administration and was maintained for another week [8]. In a phase II study of rozanolixizumab, the maximal clinical improvement was maintained 1 week after the last dose, but dramatically decreased thereafter, revealing QW intervention for 3 weeks is not sufficient to maintain the maximal clinical improvement [9]. Similarly, in this study, the maximal clinical improvement was achieved at 1 week after the fourth injection, and maintained until 4–6 weeks after the last injection of the double-blinded period. In addition, minimal symptom expression was achieved in 33.3% of batoclimab-treated patients in this study, which was close to the rate of 40% reported previously in ADAPT study [8].
Current pharmacodynamic evidence regarding FcRn inhibitors indicates that serum IgG level reduction mirrors MG-ADL, QMG, MGC, and MG-QOL15r score improvements [7, 9, 17]. In the double-blinded period, this correlation was maintained very well. However, in the open-label and follow-up periods, the IgG reduction amplitude was distinctly mismatched to score improvement. For example, on day 92, the placebo-340 mg Q2W group only reduced serum IgG by 28%, while the MG-ADL improvement exceeded the best outcome of the active drug group in the double-blinded period. Further investigation of such discrepancy is required.
During the open-label period, all three groups received batoclimab of 340 mg Q2W, to explore whether this dose could maintain the clinical improvement from the double-blinded period. At the same time, the placebo group was exposed to batoclimab. The IgG level of the 340 mg group begin to increase in the open-label period, from day 43, and ADL and QMG score started to increase accordingly from day 50, showing that 340 mg Q2W regimen could not maintain the reduction of total IgG level and the improvement of clinical symptoms. Similar trend was observed in 680 mg group; however, on day 50, the IgG level of the 680 mg group remained lower than that of the 340 mg group until day 92. Since the 680 mg group got more IgG reduction and clinical improvement from the double-blinded period, the ADL and QMG score did not rebound as fast as in the 340 mg group.
We noted similar efficacy for both batoclimab groups in the double-blinded period; however, the 680 mg group had higher MGFA score, higher efficacy scale score, and a larger proportion of patients using concomitant steroids. Owing to the small sample size and the imbalanced baseline characteristics, it is premature to conclude that 340 mg was close to or reached the efficacy plateau dose. In the open-label period, symptom recurrence and IgG levels rose again in both 340 mg and 680 mg groups, suggesting that the 340 mg Q2W regimen could not fully maintain the reduction of total IgG levels and clinical improvement while 680 mg better maintained symptom and IgG improvement. Therefore, 680 mg was recommended and selected for the phase III study.
In line with MG-ADL and QMG score, the MG QoL15r improved significantly in the batoclimab group, which reflected the increased life quality of patients with MG undergoing batoclimab treatment. A higher ratio of batoclimab group subjects achieved minimal symptom expression with which the disease affects patients’ normal life and work very little. For most patients with MG, corticosteroid and other immunosuppressants are options for chronic treatment, with severe burden of adverse event; despite their minimal effective dose, IVIg and PLEX are mostly used as rescue therapies owing to their invasiveness, limited access, and high cost. Batoclimab, with similar mechanism and effects of PLEX, may provide a new therapy that could reduce the disease financial burden both for the patients and society.
The limitations of the present research should be pointed out. First, this was an exploratory study, and only descriptive statistics were performed. Secondly, the small sample size of this study restricts the generalization of the current results. Thus, the above findings should be further verified in a larger phase III study.
Conclusion
In summary, this phase II study showed that batoclimab is clinically effective and safe in Chinese patients with gMG. The results of this study may support the next phase III study assessing batoclimab.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients who took part and their families as well as the principal investigators and their teams who contributed to this trial.
Funding
This study was funded by Harbour BioMed. The journal’s Rapid Service Fee was funded by Harbour BioMed.
Medical Writing Assistance
The authors acknowledge MedSci (funded by Harbour) and Stephen Chen (Harbour) for providing medical writing assistance in the development of this manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Chong Yan, Michael Lee, Yu Chen, Xiaoxiang Chen and Chongbo Zhao contributed to the study’s conception and design. Chong Yan, Rui-Sheng Duan, Huan Yang, Haifeng Li, Zhangyu Zou, Hua Zhang, Zhongyu Zhou, Xiao-Li Li, Hao Zhou, Lidong Jiao, Jialin Chen, Jian Yin, Qin Du, and Chongbo Zhao contributed to data collection. Chong Yan, Michael Lee, Yu Chen, Xiaoxiang Chen and Chongbo Zhao analyzed and interpreted the data. Michael Lee and Yu Chen drafted the manuscript, and Yan Chong, Xiaoxiang Chen and Chongbo Zhao revised the manuscript. All authors read and approved the final manuscript.
Disclosures
Chong Yan, Rui-Sheng Duan, Huan Yang, Haifeng Li, Zhangyu Zou, Hua Zhang, Zhongyu Zhou, Xiao-Li Li, Hao Zhou, Lidong Jiao, Jialin Chen, Jian Yin, Qin Du, and Chongbo Zhao do not have conflict of interests; Michael Lee, Yu Chen, and Xiaoxiang Chen are employees of Harbour BioMed.
Compliance with Ethics Guidelines
The study protocol was reviewed and approved by the ethics committee of seven participating Chinese medical centers (Supplemental Table 4; with Institutional Ethics Committee for Drugs Clinical Trials of Huashan Hospital, Fudan University as the master ethics committee, approval number 2020–074). Written informed consent was obtained from each subject before participating in the study. All authors confirm that our study was performed following Good Clinical Practice (GCP) as well as the Helsinki Declaration of 1964 and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Chong Yan, Rui-Sheng Duan, and Huan Yang contributed equally to the present study.
References
- 1.Dresser L, Wlodarski R, Rezania K, Soliven B. Myasthenia gravis: epidemiology, pathophysiology and clinical manifestations. J Clin Med. 2021;2021:10. doi: 10.3390/jcm10112235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazaridis K, Tzartos SJ. Myasthenia gravis: autoantibody specificities and their role in MG management. Front Neurol. 2020;11:596981. doi: 10.3389/fneur.2020.596981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Tian DC, Zhang C, et al. Incidence, mortality, and economic burden of myasthenia gravis in China: a nationwide population-based study. Lancet Reg Health West Pac. 2020;5:100063. doi: 10.1016/j.lanwpc.2020.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren J. Myasthenia gravis. Nat Rev Dis Primers. 2019;5:30. doi: 10.1038/s41572-019-0079-y. [DOI] [PubMed] [Google Scholar]
- 5.Zuercher AW, Spirig R, Baz Morelli A, Rowe T, Kasermann F. Next-generation Fc receptor-targeting biologics for autoimmune diseases. Autoimmun Rev. 2019;18:102366. doi: 10.1016/j.autrev.2019.102366. [DOI] [PubMed] [Google Scholar]
- 6.Lascano AM, Lalive PH. Update in immunosuppressive therapy of myasthenia gravis. Autoimmun Rev. 2021;20:102712. doi: 10.1016/j.autrev.2020.102712. [DOI] [PubMed] [Google Scholar]
- 7.Howard JF, Jr, Bril V, Burns TM, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92:e2661–e2673. doi: 10.1212/WNL.0000000000007600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard JF, Jr, Bril V, Vu T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20:526–536. doi: 10.1016/S1474-4422(21)00159-9. [DOI] [PubMed] [Google Scholar]
- 9.Bril V, Benatar M, Andersen H, et al. Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: a phase 2 randomized control trial. Neurology. 2021;96:e853–e865. doi: 10.1212/WNL.0000000000011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yap DYH, Hai J, Lee PCH, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of HBM9161, a novel FcRn inhibitor, in a phase I study for healthy Chinese volunteers. Clin Transl Sci. 2021;20:21. doi: 10.1111/cts.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52:1487–1489. doi: 10.1212/WNL.52.7.1487. [DOI] [PubMed] [Google Scholar]
- 12.Barohn RJ, McIntire D, Herbelin L, Wolfe GI, Nations S, Bryan WW. Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci. 1998;841:769–772. doi: 10.1111/j.1749-6632.1998.tb11015.x. [DOI] [PubMed] [Google Scholar]
- 13.Burns TM, Conaway M, Sanders DB, Composite MG, Group M-QS. The MG composite: a valid and reliable outcome measure for myasthenia gravis. Neurology. 2010;74:1434–1440. doi: 10.1212/WNL.0b013e3181dc1b1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns TM, Sadjadi R, Utsugisawa K, et al. International clinimetric evaluation of the MG-QOL15, resulting in slight revision and subsequent validation of the MG-QOL15r. Muscle Nerve. 2016;54:1015–1022. doi: 10.1002/mus.25198. [DOI] [PubMed] [Google Scholar]
- 15.Vissing J, Jacob S, Fujita KP, O'Brien F, Howard JF, Group RS. 'Minimal symptom expression' in patients with acetylcholine receptor antibody-positive refractory generalized myasthenia gravis treated with eculizumab. J Neurol. 2020;267:1991–2001. doi: 10.1007/s00415-020-09770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peter HH, Ochs HD, Cunningham-Rundles C, et al. Targeting FcRn for immunomodulation: benefits, risks, and practical considerations. J Allergy Clin Immunol. 2020;146:479–491e475. doi: 10.1016/j.jaci.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling LE, Hillson JL, Tiessen RG, et al. M281, an anti-FcRn antibody: pharmacodynamics, pharmacokinetics, and safety across the full range of IgG reduction in a first-in-human study. Clin Pharmacol Ther. 2019;105:1031–1039. doi: 10.1002/cpt.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumberg LJ, Humphries JE, Jones SD, et al. Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses. Sci Adv. 2019;5:eaax9586. doi: 10.1126/sciadv.aax9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Incorporation MP. Vivacity-MG phase 2 interim analysis topline results. In: Incorporation MP (ed) Investor and analyst conference call. Online: Momenta Pharmaceuticals Incorporation, 2020.
- 20.Sand KM, Bern M, Nilsen J, Noordzij HT, Sandlie I, Andersen JT. Unraveling the interaction between FcRn and albumin: opportunities for design of albumin-based therapeutics. Front Immunol. 2014;5:682. doi: 10.3389/fimmu.2014.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Md AH, Deb KP, Hossain MA, Mannan KA, Mostafa G, Hossain MM. Correlation between serum cholesterol and serum albumin level in childhood nephrotic syndrome. Urol Nephrol Open Access J. 2016;3:00086. [Google Scholar]
- 22.Urquhart L. Cholesterol fears rock Immunovant. Evaluate Vantage, 2021.
- 23.Kiessling P, Lledo-Garcia R, Watanabe S, et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci Transl Med. 2017;9:25. doi: 10.1126/scitranslmed.aan1208. [DOI] [PubMed] [Google Scholar]
- 24.Guptill JT, Juel VC, Massey JM, et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity. 2016;49:472–479. doi: 10.1080/08916934.2016.1214823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkubo A, Okado T, Sakurasawa T, et al. Removal characteristics of immunoadsorption with the tryptophan-immobilized column using conventional and selective plasma separators in the treatment of myasthenia gravis. Ther Apher Dial. 2019;23:271–278. doi: 10.1111/1744-9987.12820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.