Abstract
This is the protocol for a Campbell systematic review. The objectives are as follows: to identify, describe, and summarize existing guidance and methods for multistakeholder engagement throughout the health guideline development process.
1. BACKGROUND
1.1. The problem, condition or issue
Evidence‐informed healthcare guidelines evaluate and summarize the available evidence regarding patient care, public health and health systems, weigh the benefits and risks as well as acceptability, feasibility, and potential equity considerations that accompany all care and policy options and make recommendations (IOM, 2011). There is a moral imperative to include intended end users in the development of guidelines that may affect them. Stakeholder engagement, of all those potentially affected by the recommendations included in a guideline, is critical to ensuring the right issues are addressed and the right questions are asked (Gillard, 2012; Oliver, 2014). It may improve the relevancy, transparency and usefulness of guidelines (Esmail, 2015). Stakeholder engagement helps to ensure guideline acceptability and feasibility, support for its uptake and the practices recommended, and possible effects on adherence to any treatments and practices recommended (Carroll, 2017; Schunemann, 2014). Successful guideline development and implementation requires engagement of multiple stakeholders as well as shared solutions to improve health outcomes (Dunston, 2009; Kumarasame, 2016; Suman, 2016). Stakeholder groups potentially affected by guideline recommendations include patients, caregivers and the public, providers, payers, purchasers, product makers, policy makers, program managers, peer review editors, and principal investigators (Concannon, 2012; Tugwell, 2006). Clinicians and patients/consumers are the stakeholder groups predominantly engaged in guideline development and there has been little guidance developed for including other groups of stakeholders (Armstrong, 2017; Lavis, 2008; Oxman, 2006; van de Bovenkamp, 2015).
Effective stakeholder engagement should include the equitable inclusion of different groups and those who are known to be underrepresented. We use the term ‘underrepresented’ to refer to those individuals or groups who are (1) typically excluded from guideline development and implementation and (2) those who may experience inequities. These health inequities may exist for reasons such as a lack of inclusion in research, barriers to access of services, or because of other socially stratifying factors (Shi, 2014; Wallerstein, 2010). These factors can be described using the acronym PROGRESS‐Plus (O'Neill, 2014); Place of residence, Race/ethnicity/culture/language, Occupation, Gender/sex, Religion, Education, Socioeconomic status, Social capital, or other characteristic, such as age, in the guideline development and implementation process. Engagement of those affected by the guidelines and their recommendations ensures equity, diversity, and inclusion as well as social justice issues as it democratizes the guideline development process. It allows for stakeholder to participate in decision making for recommendations, programs, and policies that affect them.
There are many existing frameworks for how to develop guidelines, however, stakeholder engagement is often included as one step of the process and guidance for stakeholder engagement throughout the individual steps of the process may be lacking. Schunemann (2014) identified 18 topics in the guideline development process. Stakeholder engagement is one topic on the checklist (item 6). Selva et al. (2017) reviewed 56 guidance documents for guideline development and found that while 72% included the engagement of patients and their views in the guideline development process, few provided details on how it may be done. In light of the importance of stakeholder engagement in guideline development, this review aims to identify and describe existing guidance for stakeholder engagement that have been or that can be employed at different stages in the guideline development process. This includes identifying who to engage, when to engage them, and how to involve them throughout the process.
1.2. Definitions
For our purposes,
Guidelines are ‘systematically developed evidence‐based statements which assist providers, recipients and other stakeholders informed decisions about appropriate health interventions’ (WHO, 2003).
Stakeholders include patients, caregivers and the public, providers, payers of health services, payers of research, purchasers, product makers, policy makers, program managers, peer review editors, principal investigators or anyone who may be affected by a guideline.
engagement entails an approach to ensure the contribution of stakeholders towards the development of the guideline, completion of any of the stages of the guideline, or dissemination of the guideline and its recommendations (Frank, 2020; Pollock, 2018). It is important to acknowledge that language in this field varies internationally and is continually evolving. Terms such as involvement, collaboration, or partnership have also been used to refer to engagement (Hoddinott, 2018). Within this review we use the term ‘engagement’.
existing guidance describe systematic approaches for stakeholder engagement (Armstrong, 2017) and we will include descriptions of process(es), checklists, concepts, models, outlines, systems, plans and or overviews on engaging stakeholders in guideline development processes.
1.3. Description of the phenomena of interest
The role and value of stakeholder engagement in guideline development is increasing and therefore there is a need to identify and describe the existing guidance as this information will be useful for creating checklists for the meaningful engagement of multiple stakeholder groups in the guideline development process.
OIiver et al. have described that for committees, such as those involved in guideline development, multispeciality groups are recommended over singly speciality groups to allow for a greater range of opinion. While larger groups may be more difficult to manage and require a skilled and experienced facilitator, they also offer more opportunity for diversity among members and therefore may be more reliable, may enhance credibility and lead to widespread acceptance and implementation of decisions and recommendations (Oliver, 2018).
Stakeholder engagement may occur at various steps in the process. Stakeholder intensity can vary with some stakeholder groups being involved more intensely at certain points in the process (Crowe, 2017; Oliver, 2008; Pollock, 2019). The input of different stakeholder groups at different steps of the guideline process may have different affects on the development process. For example, including peer review editors early in the process ensures that the final guidance document is written according to standards required by the journals that may publish it, including patients during question formulation and the selection of outcomes ensures that their concerns are addresses, including program managers in steps related to evaluation and use ensures that the guideline and recommendations can be evaluated. In addition, they can be involved in advisory or feedback roles, where they receive information and are invited to provide their opinions and/or experience but for which there is no commitment to act on the feedback provided, or they may be involved in decision‐making in which their views influence the overall content of the guidelines. Engagement requires certain activities to be effective (Figure 1). Stakeholder engagement is a complex process and there are many factors that may influence stakeholders' ability to engage in the guideline development process as well as barriers to guideline developer's abilities to facilitate engagement. This scoping review focuses on existing guidance for how to effectively engage stakeholders in the process as described by the activities listed in our logic model (Figure 1) and mapped to the 18 topics of the GIN‐McMaster checklist.
Figure 1.
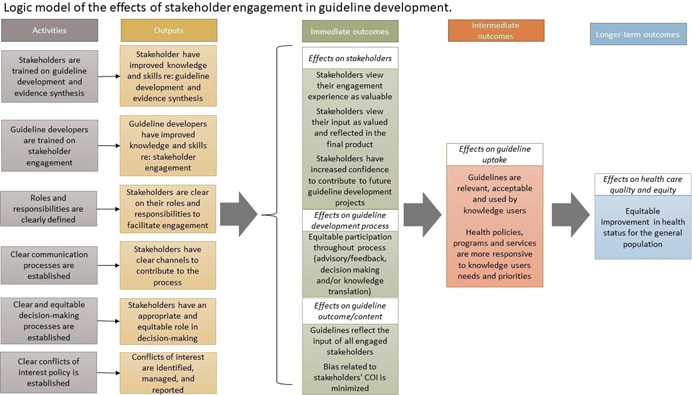
Logic model of the effects of stakeholder engagement in guideline development.
1.4. Why it is important to do the review
Guidance for guideline development, including stakeholder engagement, exists. Many guideline development organizations provide guidance for guideline development but do not provide specific information about how and when to engage multiple stakeholders. The Guidelines International Network (GIN) has proposed key elements, including stakeholder engagement, that need to be considered in developing guidelines (Qaseem, 2012). In identifying components for guidelines on guideline development, Schunemann and colleagues (Schunemann, 2006) provide practical suggestions that World Health Organization (WHO) guideline working groups can build on in their work. The GRADE Evidence to Decision (EtD) frameworks suggests 10 criteria for those making different types of guideline decisions: priority of the problem, test accuracy, benefits and harms, certainty of the evidence, outcome importance, balance (between desirable and undesirable effects), resources use, equity, acceptability, feasibility (Alonso‐Coello, 2016). Similarly, the WHO‐Integrate evidence framework includes six criteria (balance of health benefits and harms, human rights and socio‐cultural acceptability, health equity, equality, and nondiscrimination, societal implications, financial and economic considerations, feasibility and health system considerations) and suggested methods to facilitate uptake of recommendations (Rehfuess, 2018). However, guidance specific to stakeholder engagement throughout each step of guideline development is lacking. Further, given the volume of existing guidance documents, it would be useful to synthesize all existing guidance about stakeholder engagement as well as highlight items that address equitable engagement.
Other reviews have assessed existing guidance for guideline development. For example, Schunemann et al. reviewed 35 guideline manuals (from 2003 to 2012) and developed a checklist of items to be considered in guideline development and implementation (Schunemann, 2014). This overview of guidance manuals is currently being updated. However, it does not specifically focus on stakeholder engagement throughout each step of the guideline development process.
In engaging stakeholders, many guideline developers focus on a single stakeholder, particularly patients, caregivers, or service users (e.g., persons without lived experience of the medical condition related to the guideline). For example, Armstrong and colleagues have developed a 10‐step model for engaging patients in guideline development. (Armstrong, 2017). We aim to additionally consider guidance for the interactions between multiple stakeholder groups on guideline development and implementation.
The GRADE (Grading of Recommendations Assessment, Development and Evaluation) system is internationally recognized as a standard for guideline development (Guyatt, 2008). The Multi‐Stakeholder Engagement (MuSE) working group is a global consortium established in 2015 to look into the development of methods for involving multiple stakeholders in health outcomes research (Concannon, 2019; Petkovic, 2020). One goal is to develop guidance for multistakeholder engagement in guideline development. To develop this guidance, the group will conduct this systematic review to identify existing guidance for stakeholder engagement in guideline development in parallel with three other reviews focused on (a) barriers and facilitators to stake holder engagement, (b) disclosure, management and reporting of potential conflicts of interest during guideline development and c) the impact of stakeholder engagement on the guideline development process. Currently, the GRADE Handbook states that ‘the guideline panel and supporting groups … work collaboratively, informed through consumer and stakeholder involvement’ (Schunemann, 2013) but does not provide specific guidance on how this should be achieved. The results of this review, together with the other three in the series, will be used to inform the development of guidance for multistakeholder engagement in guideline development and implementation. This guidance will be official GRADE Working Group guidance.
This review will answer the questions of what guidance exists for stakeholder engagement in health guideline development and for which steps of the guideline process. The findings of this systematic review, will be used to identify and summarize existing guidance for engaging with different stakeholders in the various stages of the guideline development process. Together with the results of the other reviews in this series, the results and the GIN‐McMaster extension checklist, may be used to assist organizations who develop healthcare, public health, and health policy guidelines, such as the World Health Organization, to involve multiple stakeholders in the guideline development process to ensure the development of relevant, high quality, and transparent guidelines. This protocol and the others in this series have been developed with input from all members of the MuSE Consortium as well as stakeholders representing our identified stakeholder groups.
2. OBJECTIVES
The objective of this review is to identify, describe, and summarize existing guidance and methods for multistakeholder engagement throughout the health guideline development process.
3. METHODS
3.1. Criteria for including and excluding studies
3.1.1. Types of study designs
Our methods will follow the Cochrane Handbook for Systematic Reviews of Interventions and the Handbook for Qualitative Research, as appropriate (Higgins, 2019; Sandelowski, 2007).
To be included, papers must describe the process or methods for stakeholder engagement in guideline development using the definition of guideline described above. We will include quantitative, qualitative, and mixed‐method studies. Eligible study designs include: randomized controlled trials (RCTs), quasi‐randomized controlled trials, and nonrandomized studies (e.g., before and after studies, cohort studies, cross‐sectional studies) as well as theoretical papers, process evaluation studies, and qualitative studies. Mixed methods studies that apply a combination of the eligible quantitative and qualitative study designs and report on qualitative and quantitative outcomes separately will be eligible.
We will exclude editorials, commentaries, protocols, and conference abstracts. We will also exclude guidance provided in handbooks and other publications produced by organizations typically involved in health guideline development, such as the World Health Organization (WHO), National Health and Medical Research Council (NHMRC, Australia), National Institute for Health and Care Excellence (NICE, UK). Stakeholder engagement in the social care context will be excluded.
3.1.2. Population of interest
We have identified 13 types of stakeholders whose input can enhance the relevance and uptake of research (Concannon, 2012, 2019; Tugwell, 2006). For the purposes of this review, have grouped them as follows:
Patients, caregivers, and patient advocates
the Public
Providers of health care
Payers of health services
Payers of research
Policymakers
Program managers
Product makers
Purchasers
Principal investigators and their research teams, and
Peer review editors
Terms such as involvement, collaboration, or partnership (Hoddinott, 2018) have also been used in reference to engagement. Herein, we will use the term ‘stakeholder engagement’.
As an example, a potentially included study describes the development of a 10‐step framework for patient engagement in clinical practice guideline development (Armstrong, 2017).
3.1.3. Phenomena of interest
We will focus on studies that describe stakeholder engagement in the guideline development process. We will include studies describing the development and/or implementation of a process for stakeholder engagement in guideline development. Guidelines on healthcare‐related issues, and clinical, public, or social health will be considered.
We will include papers discussing any topic of the guideline development process as described by the GIN‐McMaster Guideline Development Checklist (Schunemann, 2014):
-
1.
Organization, budget, planning and training
-
2.
Priority‐setting
-
3.
Guideline group membership
-
4.
Establishing guideline group processes
-
5.
Identifying target audience and topic selection
-
6.
Consumer and stakeholder involvement
-
7.
Conflict of interest considerations
-
8.
Question generation
-
9.
Considering importance of outcomes and interventions, values, preferences, and utilities
-
10.
Deciding what evidence to include and searching for evidence
-
11.
Summarizing evidence and considering additional information
-
12.
Judging quality, strength or certainty of body of evidence
-
13.
Developing recommendations and determining their strength
-
14.
Wording of recommendations and of considerations about implementation, feasibility and equity
-
15.
Reporting and peer review
-
16.
Dissemination and implementation
-
17.
Evaluation and use
-
18.
Updating
3.1.4. Types of outcome measures
Outcomes for this review include
Methods for identifying stakeholders at each step of the guideline development process
Methods for engaging stakeholders at each step of the guideline development process
Methods for training stakeholder to facilitate participation
Frequency and level of engagement of stakeholders at each step of the guideline development process
Level of engagement (Crowe, 2017; Oliver, 2008; Pollock, 2019) in each step of the guideline development process (advisory/feedback, decision‐making)
How stakeholders contributed at each step of the guideline development process
Methods for resolving disagreements
Evaluation of engagement processes
3.1.5. Types of settings
We will place no restrictions on setting.
3.2. Search strategy
We will develop one comprehensive search strategy for all four systematic reviews in consultation with a medical librarian which will be reviewed by a second medical librarian. We will search the following databases: MEDLINE (OVID), CINAHL (EBSCO), EMBASE (OVID), PsycInfo (OVID) and SCOPUS. Limits will not be placed on date, study design or language.
We will conduct an extensive grey literature search. We will search the websites of agencies who actively engage stakeholder groups in their work such as the Agency for Healthcare Research and Quality (AHRQ), CIHR Strategy for Patient‐Oriented Research (SPOR), INVOLVE, Guidelines Internation Network (G‐I‐N), the National Institute for Health and Care Excellence, and the Patient‐Centred Outcomes Research Institute (PCORI). We will also search the websites of guideline‐producing agencies, such as the American Academy of Paediatrics, Australia's National Health Medical Research Council (NHMRC), and the World Health Organization (WHO) including Latin American and Caribbean Health Sciences Literature (LILACS).
We will solicit suggestions for additional grey literature sources from the MuSE working group members and via social media, such as Twitter.
3.3. Description of methods used in primary research
It is expected that included papers will describe frameworks or guidance for who, when and how to engage stakeholders in guideline development and implementation.
3.4. Details of study coding categories
Two reviewers will independently screen titles and abstracts to identify relevant studies meeting the pre‐specified inclusion criteria. The full text of potentially included studies will be screened independently by two authors. We will use Covidence software (https://www.covidence.org/) for screening of studies. All completed studies will be included if they meet the inclusion criteria listed above.
The data extraction form will be pre‐tested and extracted independently in duplicate by two reviewers using Eppi‐Reviewer to facilitate qualitative synthesis (described below) (Thomas, 2010).
Disagreements will be resolved by discussion and with a third member of the research team when necessary.
We will extract details related to guidance for stakeholder engagement for each of our identified stakeholder groups for each of the 18 topics (146 steps) of the GIN‐McMaster checklist (Table 1). We will also extract data on:
General study characteristics such as study design and year of publication
Stakeholder groups and definition of stakeholder
Definition of engagement
Methods for identifying stakeholders
Characteristics of stakeholder panel
Whether stakeholders were part of the guideline development panel or external
Methods for recruitment
Methods for balancing stakeholder group representation
Methods for engaging/method of communication
Frequency of engagement
Level of engagement (advisory/feedback or decision‐making)
How stakeholders contributed
Methods for resolving disagreement
Table 1.
Framework for data extraction.
| Stakeholder groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Guideline development topics | Patients, caregivers, patient organizations | Public | Providers | Principal investigators + team | Policy makers | Program managers | Payers/Purchasers of health services | Payers of health research | Peer Review editors | Product makers |
| 1. Organization, budget, planning and training | ||||||||||
| 2. Priority setting | ||||||||||
| 3. Guideline group membership | ||||||||||
| 4. Establishing guideline group processes | ||||||||||
| 5. Identifying target audience and topic selection | ||||||||||
| 6. Consumer and stakeholder involvement | ||||||||||
| 7. Conflict of interest (COI) considerations | ||||||||||
| 8. (PICO) question generation | ||||||||||
| 9. Considering importance of outcomes and interventions, values, preferences, and utilities | ||||||||||
| 10. Deciding what evidence to include and searching for evidence | ||||||||||
| 11. Summarizing evidence and considering additional information | ||||||||||
| 12. Judging quality, strength or certainty of a body of evidence | ||||||||||
| 13. Developing recommendations and determining their strength | ||||||||||
| 14. Wording of recommendations and of considerations of implementation, feasibility and equity | ||||||||||
| 15. Reporting and peer review | ||||||||||
| 16. Dissemination and implementation | ||||||||||
| 17. Evaluation and use | ||||||||||
| 18. Updating | ||||||||||
3.5. Quality assessment
We will assess the quality of our included papers using the criteria: practicality, relevance, and legitimacy as described in the tool developed by Movsisyan et al. (2019). This tool assesses, for example, whether the paper in describes key concepts clearly, whether the guidance can be easily applied without additional information, whether there is information related to its adaptation to other settings, and whether the underlying theory and principles have been described (see Table 2). We will also assess whether the development of the guidance has included input from relevant stakeholders.
Table 2.
Quality appraisal criteria.
| Quality Appraisal (adapted from Movsisyan, 2019) | Rating | Comments |
|---|---|---|
| Practicality | ||
| Key concepts/nomenclature and their definitions related to guidance development | −/+/++ | |
| Definition and description—understandability and clarity of key constructs | ||
| Guiding question: | ||
| Are the key items/guidance clearly specified? | ||
| Ease of use and operationalisability | −/+/++ | |
| Guiding questions: | ||
| Can the guidance be applied easily without the need to search for additional information? | ||
| Can the guidance be adequately operationalised? | ||
| Comprehensiveness | −/+/++ | |
| Guiding questions: | ||
| Does the guidance thoroughly describe information related to adaptation in a new setting? | ||
| Does the guidance thoroughly describe information related to evaluation in a new setting? | ||
| Relevance | ||
| Relevance for use in different settings and with different stakeholders | −/+/++ | |
| Guiding question: | ||
| Can the guidance be applied to different types of guidelines? | ||
| Can the guidance be used in different settings? | ||
| Can the guidance be adapted to additional stakeholders? | ||
| Legitimacy | ||
| Legitimacy—Scientific basis and development process | −/+/++ | |
| Guiding questions: | ||
| Does the guidance describe its underlying theory and principles? | ||
| Does the guidance describe a rigorous development process (such as a comprehensive literature review and/or a consensus‐based methodology) |
Abbreviations: −, not addressed; +, partially addressed; ++, fully addressed.
Quality will be assessed independently, in duplicate, by two authors and any discrepancies will be resolved by consensus and consultation with a third author, when necessary.
3.6. Statistical procedures and conventions
We will not have data for statistical analysis.
3.7. Treatment of qualitative research
We will narratively summarize the existing guidance identified in our included studies by mapping the descriptions of stakeholder engagement in the included papers to the 18 topics (146 steps) of the guideline development process outlined by the GIN‐McMaster checklist (Schunemann, 2014). We will present this information as a matrix indicating the guidance that exists for each stakeholder group and for each step of guideline development.
We will use thematic synthesis to combine guidance for stakeholder involvement in each step.
We will follow the guidance for qualitative synthesis in systematic reviews as outlined by Thomas (2008) which includes coding the text and developing descriptive themes (Thomas, 2008). This involves translating concepts from one study to another and looking for similarities and differences between codes to allow for grouping concepts into themes for analysis (Thomas, 2008).
SOURCES OF SUPPORT
Internal sources
No sources of support provided
External sources
Canadian Institutes of Health Research, Canada
CIHR Project Grant
CONTRIBUTIONS OF AUTHORS
Conceiving the review: PT, VW, TC, JP
Designing the review: JP, AR, VW, PT, TC
Coordinating the review: JP, AR, PA
Writing the protocol: JP, PA, AR, JK, LL
Providing general advice on the review: JK, LL, EA, VW, OM, IDG, SG, DJ, SVK, AS, HS, TC, PT
Securing funding for the review: PT, VW, JP
DECLARATIONS OF INTEREST
SG's spouse is a salaried employee of Eli Lilly and Company and owns stock. SG has accompanied his spouse on company‐sponsored travel. SVK acknowledges research funding from the UK Medical Research Council, Scottish Government Chief Scientist Office and a NRS Senior Clinical Fellowship. SVK is an honorary Consultant in Public Health at Public Health Scotland and has provided unpaid advice to Obesity Action Scotland, Scottish Government and UK Government. VW is editor in chief of the Campbell Collaboration. This review will be handled by an independent editor, and the cochairs of the relevant group will act in lieu of editor in chief. TC has developed and published several peer‐reviewed publications that could potentially be included in the review. TC currently holds one research contract with the Patient‐Centred Outcomes Research Institute and another with the Pharmaceutical Research and Manufacturers of America Foundation that address a similar topic.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
The MuSE project has been funded by the Canadian Institutes of Health Research. The authors acknowledge the valuable contributions and advice provided by the entire MuSE Consortium membership throughout the many stages of this project. We thank Ms. Layal Hneiny of the American University of Beirut who helped refine and translate the search strategies.
Petkovic, J. , Riddle, A. , Lytvyn, L. , Khabsa, J. , Akl, E. A. , Welch, V. , Magwood, O. , Atwere, P. , Graham, I. D. , Grant, S. , John, D. , Katikireddi, S. V. , Langlois, E. , Mustafa, R. A. , Todhunter‐Brown, A. , Schünemann, H. , Smith, M. , Stein, A. T. , Concannon, T. , & Tugwell, P. (2022). PROTOCOL: Guidance for stakeholder engagement in guideline development: A systematic review. Campbell Systematic Reviews, 18, e1242. 10.1002/cl2.1242
REFERENCES
- OTHER REFERENCES
- ADDITIONAL REFERENCES
- Alonso‐Coello, A. , Schunemann, H. J. , Moberg, J. , Brignardello‐Petersen, R. , Akl, E. A. , Davoli, M. , Treweek, S. , Mustafa, R. A. , Rada, G. , Rosenbaum, S. , Morelli, A. , Guyatt, G. H. , Oxman, A. D. , & the GRADE Working Group . (2016). GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ, 353, i2016. [DOI] [PubMed] [Google Scholar]
- Armstrong, M. J. , & Bloom, J. A. (2017). Patient involvement in guidelines is poor five years after institute of medicine standards: Review of guideline methodologies. Research Involvement and Engagement, 3(1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, M. J. , Rueda, J. D. , Gronseth, G. S. , & Mullins, C. D. (2017). Framework for enhancing clinical practice guidelines through continuous patient engagement. Health Expectations, 20(1), 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, C. (2017). Qualitative evidence synthesis to improve implementation of clinical guidelines. BMJ, 356, j80. [DOI] [PubMed] [Google Scholar]
- Concannon, T. W. , Meissner, P. , Grunbaum, J. A. , McElwee, N. , Guise, J. M. , Santa, J. , Conway, P. H. , Daudelin, D. , Morrato, E. H. , & Leslie, L. K. (2012). A new taxonomy for stakeholder engagement in patient‐centered outcomes research. Journal of General Internal Medicine, 27(8), 985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon, T. W. , Grant, S. , Welch, V. , Petkovic, J. , Selby, J. , Crowe, S. , Synnot, A. , Greer‐Smith, R. , Mayo‐Wilson, E. , Tambor, E. , Tugwell, P. , & Multi Stakeholder Engagement (MuSE), C. (2019). Practical guidance for involving stakeholders in health research. Journal of General Internal Medicine, 34(3), 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe, S. (2017). Who inspired my thinking?—Sherry Arnstein. Research for All, 1(1), 143–146. [Google Scholar]
- Dunston, R. , Lee, A. , Boud, D. , Brodie, P. , & Chiarella, M. (2009). Co‐production and health system reform—From re‐imagining to re‐making. Australian Journal of Public Administration, 68(1), 39–52. [Google Scholar]
- Esmail, L. , Moore, E. , & Rein, A. (2015). Evaluating patient and stakeholder engagement in research: Moving from theory to practice. Journal of Comparative Effectiveness Research, 4(2), 133–145. [DOI] [PubMed] [Google Scholar]
- Frank, L. , Morton, S. C. , Guise, J. M. , Jull, J. , Concannon, T. , & Tugwell, P. (2020). Engaging patients and other non‐researchers in health research: Defining research engagement. Journal of General Internal Medicine, 35(1), 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard, S. , Simons, L. , Turner, K. , Lucock, M. , & Edwards, C. (2012). Patient and public involvement in the coproduction of knowledge: Reflection on the analysis of qualitative data in a mental health study. Qualitative Health Research, 22(8), 1126–1137. [DOI] [PubMed] [Google Scholar]
- Guyatt, G. H. , Oxman, A. D. , Vist, G. E. , Kunz, R. , Falck‐Ytter, Y. , Alonso‐Coello, P. , & Schunemann, H. J. (2008). GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ, 336, 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T. , Thomas, J. , Chandler, J. , Cumpston, M. , Li, T. , Page, M. J. , & Welch, V. A. (Eds.). (2019). Cochrane handbook for systematic reviews of interventions. Vol. version 6.0 (updated July 2019). Cochrane. [Google Scholar]
- Hoddinott, P. , Pollock, A. , O'Cathain, A. , Boyer, I. , Taylor, J. , MacDonald, C. , Oliver, S. , & Donovan, J. L. (2018). How to incorporate patient and public perspectives into the design and conduct of research. F1000 Research, 7, 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines . (2011). Clinical practice guidelines we can trust. National Academies Press. [PubMed]
- Kumarasame, M. A. , & Sanfilippo, F. P. (2016). Breaking down silos: Engaging students to help fix the US health care system. Journal of Multidisciplinary Healthcare, 8, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavis, J. N. , Paulsen, E. J. , Oxman, A. D. , & Moynihan, R. (2008). Evidence‐informed health policy 2—Survey of organizations that support the use of research evidence. Implementation Science, 3(1), 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsisyan, A. , Evans, L. A. , Hallingberg, B. , Moore, G. , O'Cathain, A. , Pfadenhauer, L. M. , Segrott, J. , & Rehfuess, E. (2019). Adapting evidence‐informed complex population health interventions for new contexts: A systematic review of guidance. Implementation Science, 14, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, J. , Tabish, H. , Welch, V. , Petticrew, M. , Pottie, K. , Clarke, M. , Evans, T. , Pardo Pardo, J. , Waters, E. , White, H. , & Tugwell, P. (2014). Applying an equity lens to interventions: Using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. Journal of Clinical Epidemiology, 67(1), 56–64. [DOI] [PubMed] [Google Scholar]
- Oliver, S. R. , Rees, R. W. , Clarke‐Jones, L. , Milne, R. , Oakley, A. R. , Gabbay, J. , Stein, K. , Buchanan, P. , & Gyte, G. (2008). A multidimensional conceptual framework for analysing public involvement in health services research. Health Expectations, 11(1), 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, K. , Lorenc, T. , & Innvaer, S. (2014). New directions in evidence‐based policy research: A critical analysis of the literature. Health Research Policy and Systems/BioMed Central, 12, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, S. , Hollingworth, K. , Briner, R. , Swann, C. , Hinds, K. , & Roche, C. (2018). Effective and efficient committee work: A systematic overview of multidisciplinary literatures. Evidence Base, 2, 1–21. [Google Scholar]
- Oxman, A. D. , Schunemann, H. J. , & Fretheim, A. (2006). Improving the use of research evidence in guideline development: 8. Synthesis and presentation of evidence. Health Research Policy and Systems, 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic, J. , RIddle, A. , Akl, E. A. , Khabsa, J. , Lytvyn, L. , Atwere, P. , Campbell, P. , Chalkidou, K. , Chang, S. M. , Crowe, S. , Dans, L. , Jardali, F. E. , Ghersi, D. , Graham, I. D. , Grant, S. , Greer‐Smith, R. , Guise, J. M. , Hazlewood, G. , Jull, J. , … Tugwell, P. (2020). Protocol for the development of guidance for stakeholder engagement in health and healthcare guideline development and implementation. Systematic Reviews, 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, A. , Campbell, P. , Struthers, C. , Synnot, A. , Nunn, J. , Hill, S. , Goodare, H. , Morris, J. , Watts, C. , & Morley, R. (2018). Stakeholder involvement in systematic reviews: a scoping review. Systematic Reviews, 7, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, A. , Campbell, P. , Struthers, C. , Synnot, A. , Nunn, J. , Hill, S. , Goodare, H. , Morris, J. , Watts, C. , & Morley, R. (2019). Development of the ACTIVE framework to describe stakeholder involvement in systematic reviews. Journal of Health Services Research & Policy, 24(4), 245–255. [DOI] [PubMed] [Google Scholar]
- Qaseem, A. , Forland, F. , Macbeth, F. , Ollenschlager, G. , Phillips, S. , & van der Wees, P. (2012). Guidelines International Network: Toward international standards for clinical practice guidelines. Annals of Internal Medicine, 156(7), 525–531. [DOI] [PubMed] [Google Scholar]
- Rehfuess, E. A. , Stratil, J. M. , Scheel, I. B. , Portela, A. , Norris, S. L. , & Baltussen, R. (2018). The WHO‐INTEGRATE evidence to decision framework version 1.0: Integrating WHO norms and values and a complexity perspective. BMJ Global Health, 4, e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelowski, M. , & Barroso, J. (2007). Handbook for synthesizing qualitative research. Springer Publishing Company. [Google Scholar]
- Schunemann, H. J. , Fretheim, A. , & Oxman, A. (2006). Improving the use of research evidence in guideline development: 1. Guidelines for guidelines. Health Research Policy and Systems, 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunemann, H. , Brozek, J. , Guyatt, G. , & Oxman, A. (2013). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. https://gdt.gradepro.org/app/handbook/handbook.html#h.svwngs6pm0f2
- Schunemann, H. J. , Wiercioch, W. , Etxeandia, I. , Falavigna, M. , Santesso, N. , Mustafa, R. , Ventresca, M. , Brignardello‐Petersen, R. , Laisaar, K. T. , Kowalski, S. , Baldeh, T. , Zhang, Y. , Raid, U. , Neumann, I. , Norris, S. L. , Thornton, J. , Harbour, R. , Treweek, S. , Guyatt, G. , … Akl, E. A. (2014). Guidelines 2.0: Systematic development of a comprehensive checklist for a successful guideline enterprise. Canadian Medical Association Journal/Journal de l'Association Medicale Canadienne, 186(3), E123–E142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva, A. , Sanabria, A. J. , Pequeño, S. , Zhang, Y. , Solà, I. , Pardo‐Hernandez, H. , Selva, C. , Schünemann, H. , & Alonso‐Coello, P. (2017). Incorporating patients' views in guideline development: A systematic review of guidance documents. Journal of Clinical Epidemiology, 88, 102–112. [DOI] [PubMed] [Google Scholar]
- Shi, C. , Tian, J. , Wang, Q. , Petkovic, J. , Ren, D. , Yang, K. , & Yang, Y. (2014). How equity is addressed in clinical practice guidelines: A content analysis. BMJ Open, 4(12), e005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniszewska, S. , Brett, J. , Simera, I. , Seers, K. , Mockford, C. , Goodlad, S. , Altman, D. G. , Moher, D. , Barber, R. , Denegri, S. , Entwistle, A. , Littlejohns, P. , Morris, C. , Suleman, R. , Thomas, V. , & Tysall, C. (2017). GRIPP2 reporting checklists: Tools to improve reporting of patient and public involvement in research. BMJ, 358, j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne, J. A. , Hernan, M. A. , Reeves, B. C. , Savovic, J. , Berkman, N. D. , Viswanathan, M. , Henry, D. , Altman, D. G. , Ansari, M. T. , Bourton, I. , Carpenter, J. R. , Phelan, A. , Churchill, R. , Deeks, J. J. , Hrobjartsson, A. , Kirkham, J. , Juni, P. , Loke, Y. K. , Pigott, T. D. , … Higgins, J. P. T. (2016). ROBINS‐I: A tool for assessing risk of bias in non‐randomised studies of interventions. BMJ, 355, i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman, A. , Dikkers, M. F. , Schaafsma, F. G. , van Tulder, M. W. , & Anema, J. R. (2016). Effectiveness of multifaceted implementation strategies for the implementation of back and neck pain guidelines in health care: A systematic review. Implementation Science, 11, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. , & Harden, A. (2008). Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Medical Research Methodology, 8, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. , Brunton, J. , & Graziosi, S. (2010). Eppi‐Reviewer 4.0: Software for research synthesis. EPPI‐Centre Software. Social Science Research Unit, Institute of Education, University of London. [Google Scholar]
- Tugwell, P. , Robinson, V. , Grimshaw, J. , & Santesso, N. (2006). Systematic reviews and knowledge translation. Bulletin of the World Health Organization, 84(8), 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Bovenkamp, H. M. , & Zuiderent‐Jerak, T. (2015). An empirical study of patient participation in guideline development: Exploring the potential for articulating patient knowledge in evidence‐based epistemic settings. Health Expectations, 15(5), 942–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerstein, N. , & Duran, B. (2010). Community‐based participatory research contributions to intervention research: The intersection of science and practice to improve health equity. American Journal of Public Health, 100(s1), s40–s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2003). Guidelines for WHO guidelines. Global programme on evidence for health policy. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


