Abstract
Purpose
The purpose of this review was to explore the effects of psychosocial stress from life trauma and racial discrimination on epigenetic aging.
Design
A systematic review of the last 10 years was conducted using four databases: PubMed/MEDLINE, Web of Science, PsychInfo, and CINAHL.
Methods
Articles were identified using the following terms: ([(DNA methylation) AND (epigenetic clock)] OR [(DNA methylation) AND (epigenetic age)]) AND (discrimination OR trauma)). Original research articles published in English measuring life trauma, post-traumatic stress, experience of discrimination, and epigenetic clocks or aging were analyzed using PRISMA guidelines.
Results
Ten articles met inclusion criteria. The study sample size ranged from 96 to 1163 and most study populations had a mean age under 50 and included predominantly White male participants. One study identified accelerated epigenetic aging associated with discrimination using Hannum’s clock; 33% of studies evaluating life trauma reported epigenetic age acceleration using GrimAge or Horvath’s clock; 25% of studies evaluating childhood trauma reported epigenetic age acceleration using Horvath’s clock; and 71% of studies assessing post-traumatic stress observed epigenetic age acceleration with all clocks, while one study reported deceleration using Horvath’s clock.
Conclusions
The experiences of life trauma, post-traumatic stress, and discrimination may be associated with accelerated epigenetic aging that can be consistently detected using different epigenetic clocks. Additional studies inclusive of diverse populations and other psychosocial stressors are needed.
Relevance
Nursing scholars and other health scientists who utilize epigenetic age acceleration to assess health risks may need to consider including psychosocial stressors in their studies as covariates.
Keywords: DNA methylation age, DNA methylation age acceleration, epigenetic clock
Stress is a known risk factor for multiple disorders including cardiovascular disease, metabolic disease, cancer, psychiatric, and neurodegenerative disorders (Cohen et al., 2007; Liu et al., 2017; Steptoe & Kivimäki, 2013). Among the stressors associated with disease, traumatic life events and experiencing racial discrimination have profound effects on health (Cohen et al., 2019). According to the National Institute of Mental Health (2020), a traumatic (life) event is defined as a shocking and distressing experience such as natural disasters, acts of violence, or accidents such as car crashes, which can have emotional and physical impacts. Krieger (2014) defined racial/ethnic discrimination as socially structured unfair actions expressed in interactions among individuals and institutions. Based on racist ideology, racial/ethnic discrimination reinforces the relationship of dominance and subordination. In the United States, the dominant group has been mostly white Americans, and the subordinate group has been primarily people of color. As the examples of the impact of these stressors on health, traumatic life events have been associated with the development of metabolic syndrome among middle aged adults (Rutters et al., 2015) and perceived racial discrimination with cardiovascular disease (Lewis et al., 2014). However, our understanding of the biological pathways involved in the conversion of experiencing these psychosocial traumas to physiological disorders remains incomplete (Bailey et al., 2017).
A number of biological mechanisms have been explored to explain the physiologic relationship between stress and physical health. The most commonly described is allostasis and allostatic load, which is based on interactions across the hypothalamic–pituitary–adrenal axis (HPA) and sympatho–adrenomedullary axes. Allostatic load refers to the cumulative effects of stress on the body across the lifespan (McEwen, 2000). When stress is persistent, the response to stress overtime can become abnormal, which accelerates wear and tear on body systems and increases risk for disease (Lemelin et al., 2009). Inflammatory processes have been proposed as the mechanism responsible for this stress response (Liu et al., 2017). However, a full mechanistic explanation of how stress contributes to the development of disease has not yet been determined.
More recently, the impact of psychosocial stress on gene regulation and cellular function has been studied directly via epigenetic research (Notterman & Mitchell, 2015). It is well known that proteins, such as cytokines and stress hormones, are important in understanding mechanisms of health and disease. The production of these proteins can vary by tissue type and be influenced by multiple factors. Some of these factors include unique information tagged on the cell’s DNA and related structures, commonly referred to as epigenetic marks. These markers are known to influence gene expression, although epigenetic marks themselves can be influenced by the underlying genome sequence, environmental exposures, and other host factors, including cytokines and stress hormones (Kanherkar et al., 2014).
DNA methylation (DNAm) is one of the most commonly studied epigenetic modifications, indicating the loss or gain of methylation at the 5′ cytosine position (Allis et al., 2015). DNAm can modify gene expression by blocking (downregulating) or allowing access (upregulating) to the gene sequence by transcription factors. The amount of DNAm across the epigenome changes as people age, due to exposure to and interactions with the environment (Christensen et al., 2009). Because an individual’s DNAm signature evolves over time, research evaluating the relationship of DNAm changes related to age-related diseases is of increasing interest (Bjornsson, 2008; Fraga et al., 2005). Additionally, DNAm may be modified by social stressors, such as experiencing adverse life events (Mulligan et al., 2012), low socioeconomic status (Yehuda et al., 2015), genocidal war (McGowan et al., 2009), and combat associated post-traumatic stress disorder (PTSD). Furthermore, these epigenetic changes are heritable and reversible (Feinberg, 2018; Teschendorff et al., 2013). Therefore, understanding the effects of epigenetic changes induced by social exposures related to disease risk is of increasing importance because it may improve our ability to predict risk for age-related disease such as high blood pressure, diabetes, and cancer.
In addition to assessing genome-wide DNAm, scientists have discovered other clinically meaningful ways to analyze and interpret DNAm patterns. The concept of epigenetic aging was developed using a machine learning approach to DNA methylation analysis, and measures biological age compared to chronological calendar age. Epigenetic age, determined via variation in DNAm at specific nucleotides, is reported to be a better proxy of biological aging than telomere length or other -omic based predictors (Horvath & Raj, 2018; Jylhävä et al., 2017) and may be more useful for predicting health risks associated with age (Horvath & Raj, 2018). DNAm age is correlated with chronological age (Hannum et al., 2013; Horvath, 2013; Horvath & Raj, 2018). However, unlike chronological age that does not vary based on environmental exposures, DNAm is influenced by those exposures and these differences, accelerated or decelerated epigenetic age compared to chronological age, can be measured and serve as a quantitative measure of biological aging among individuals. Furthermore, due to the changeable and reversible nature of DNA methylation, this new method can be used to quantitatively measure interventions that accelerate or slow biological aging, improving our ability to directly measure factors that mitigate or exacerbate age-related morbidity and mortality. These intervention studies in humans are relatively new. For example, higher body mass index was associated with accelerated DNAm age among 232 women in a cross-sectional study (Li et al., 2019). A recent longitudinal study (Petersen et al., 2021) among 16 older adults, weight loss intervention showed decrease in DNAm age and improved functional ability; increased grip strength and gate speed was related with decreased DNAm age. Also, in a study with 57 participants, in women with certain genotype (MTHFR 677CC), dietary intervention such as supplementation with folic acid and Vitamin B12 was assocaited with decreased DNAm age (Sae‐Lee et al., 2018).
Several DNAm age estimators, or clocks, have been developed. The most widely used clocks and their simplified characteristics are summarized in Table 1. The most notable differences between clock types are the specific DNAm sites included in the measure and the outcomes used to develop the DNAm age estimator. Estimators developed to specifically measure “DNAm age,” such as Hannum’s clock and Horvath’s clock, evaluated patterns of DNAm at 71 or 353 methylation sites, respectively, across blood or multiple tissues and organs, and can measure the general impact of stress on biological aging. (Hannum et al., 2013; Horvath, 2013). Unlike the previous two clocks in which only chronological age was regressed on DNAm levels, DNAm PhenoAge uses 10 clinical characteristics including chronological age, such as albumin and creatinine, which result in the selection of 513 CpGs (Levine et al., 2018). In terms of DNAm GrimAge, seven plasma proteins together with a DNAm based proxy for smoking pack-years, sex, and chronological age are regressed on DNAm levels (Lu et al., 2019). Due to these differences, PhenoAge and GrimAge are strong predictors of all-cause mortality (Levine et al., 2018; Lu et al., 2019). Additionally, most clocks were derived from single-tissue analysis, whereas Horvath’s clock is the only estimator based on multiple tissues (Horvath & Raj, 2018). The single-tissue estimators, such as Hannum’s clock, Levine’s clock, and GrimAge, were created using DNAm from blood samples. This difference is meaningful when considering which DNAm age estimator should be used based on the research question. For example, Horvath’s clock can be used regardless of cell/tissue/organ types throughout lifespan and is a better measure of intrinsic cellular aging, which tends to be influenced more by genetic control. Conversely, the single-tissue, blood-based estimators more accurately measure extrinsic cellular aging, and are more likely to capture differences in DNAm age due to lifestyle and exposures. However, the cell composition in blood changes with aging and these blood-based DNAm age estimators are biased towards adults and older populations (Horvath & Raj, 2018).
Table 1.
Types of DNA Methylation (DNAm) Age Estimator.
| Tissue\DNAm Age Estimator | First Generation DNAm Age Estimators | Phenotypic Age Estimators | |
|---|---|---|---|
| Regressing on chronological age | Regressing on 10 clinical characteristics a for mortality | Regressing on seven plasma proteins, smoking pack years for mortality, chronological age, and sex | |
| Single-tissue estimator b (cell extrinsic aging) | Hannum’s DNAm age (71 CpGs) | DNAm PhenoAge (513 CpGs) | DNAm GrimAge (1030 CpGs) |
| Multi-tissue estimator c (cell intrinsic aging) | Horvath’s DNAm age (353 CpGs) | - | - |
Note. aChronological age, albumin, creatinine, glucose, C-reactive protein levels, lymphocyte percentage, mean cell volume, red blood cell distribution width, alkaline phosphatase, and white blood cell count.
bDNA of blood. As the cell-type composition of blood changes with aging, blood-based age estimators measure cell-extrinsic aging.
cDNA across tissues and cell types, such as blood, brain, liver, and saliva.
DNAm age is relatively a recent concept. In previous studies, psychosocial stressors associated with low socioeconomic status, such as low income or low levels of educational attainment, are associated with accelerated DNAm aging (Chen et al., 2016; Fiorito et al., 2017; Simons et al., 2016). However, few studies have explored the relationship between stress from traumatic life experiences, or experiences with racial discrimination, and DNAm age. Therefore, the purpose of this systematic review is to: 1) identify how many studies have explored the relationship between psychosocial stress from life trauma and perceived racial discrimination on DNAm age, 2) determine the types of DNAm age estimators that have been used in those studies, and 3) evaluate whether there are significant relationships among stress from life experiences or perceived racial discrimination and DNAm age.
Methods
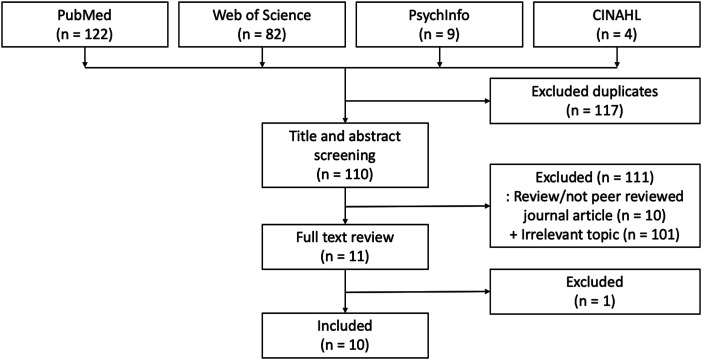
A systematic review of psychosocial factors influencing epigenetic aging in humans within 10 years was conducted using the PubMed/MEDLINE, Web of Science, PsychInfo, and CINAHL database. We focused on life trauma exposure, PTSD, and perceived racial discrimination. We used three groups of search terms: ([(DNA methylation) AND (epigenetic clock)] OR [(DNA methylation) AND (epigenetic age)]) AND (discrimination or trauma). The inclusion criteria were 1) human species, 2) original article, 3) published within 10 years, 4) published in a peer-reviewed journal, 5) full-text approachable, 6) written in English, and 7) measured DNA methylation age in relation to traumatic life events or perceived racial discrimination. Exclusion criteria included 1) review/systematic review/meta-analysis, 2) perspective/editorial/commentary, 3) abstract, and 4) book chapter. The articles from each of the four databases were combined while removing duplicates, then titles and abstracts were screened with inclusion and exclusion criteria. The full-text of articles that passed the initial screening were reviewed to ensure the accuracy of inclusion and exclusion. The articles meeting all criteria were used in this review. The data extraction process is presented in Figure 1. The initial search date was 7/21/2020, and the last updated search was 6/22/2021.
Figure 1.
Process of literature review according to Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
Initial data extraction collected the following data from all studies: author(s) and the publication date, types of psychosocial stress (life trauma exposure/PTSD/perceived racial discrimination), measurements, types of DNA methylation clock, types of collected biospecimen, study design, statistical analysis methods, whether explored causality, study results in relation to DNA methylation aging acceleration, and sample characteristics (such as sample size, race/ethnicity, sex, and age). The final data summary table included the items as follow: author(s) and the publication date, types of psychosocial stress (life trauma exposure/PTSD/perceived racial discrimination), types of DNA methylation clock, types of collected biospecimen, study design, statistical analysis methods, study results in relation to DNAm age acceleration, and sample characteristics. In this review, all authors reviewed articles separately and compared the extracted data with each other. Unanimously agreed outcomes were used. Quality assessment tool Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Analytical Cross Sectional Studies, and Cohort Studies were also used for quality control (Aromataris & Munn, 2020). Only articles agreed upon by all authors were included in this review.
Results
We included 10 studies in the final review (Table 2). All 10 studies unanimously passed quality appraisal conducted by all authors. In these studies, a variety of instruments was used to measure life trauma experience and perceived racial discrimination (Table 3).
Table 2.
Selected Features of Studies on the Relationship between Life Trauma Experience or Perceived Racial Discrimination and DNA Methylation (DNAm) Age.
| Study | Psychosocial Stress | DNA Methylation Clock | Study Design | Statistical Analysis | DNAm Age Results | Sample Size | Population | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Life Trauma Exposure (T) | PTSD (P) | Discrimination (D) | Acceleration | Deceleration | Condition | ||||||
| Boks et al. (2015) | Tcb Tc |
P | - | Horvath | Longitudinal (two times, 1.1 years apart: before and after deployment) | Multiple linear regression | Tcb | P | PTSD measured by development of PTSD (severity) | 96 | Dutch Male soldiers - Age: mean 27.04 (SD = 9.15) |
| Brody et al. (2016) | TLs | - | D | Hannum | Longitudinal, but one-time blood collection | Multiple linear regression | D | - | Discrimination measured when adolescents, Interacted with support in the family | 616 (322 cohort 1 + 294 cohort 2) | Cohort 1 African American Male: 42.86% Mean age at blood collection: 20 Cohort 2 African American Male: 36.39% - Mean age at blood collection: 22 |
| Han et al. (2018) | Tc | - | - | Similar to Horvath | Cross-sectional | Multiple linear regression (blood), Linear mixed model (brain tissue) | Tc | - | Only in depression group | 1130 | Race/ethnicity not specified Female: 64.5% - Age: mean 41.5 (SD 13.0), range [18–64] |
| Mehta et al. (2018) | - | Pcb | - | Horvath | Cross-sectional | Generalized multiple linear regression | - | - | None of PTSD diagnosis or severity was related to DNAm age | 211 (96 discovery + 115 validation) | Discovery data Australian Male veterans Age: mean 68.67 (SD 0.45) Validation data Predominantly African American Male civilian - Age: mean 44 (SD 1.14), range: [19–64] |
| Wolf et al. (2018) | TL | P | - | Hannum | Cross-sectional | Multiple linear regression | P | - | Among PTSD severity and symptoms, only in hyperarousal symptom clusters | 339 | White, non-Hispanic veterans Male: 87% - Age: mean 52.58 (SD 10.65), range [23–72] |
| Wolf et al., 2019a | TL | P | - | Horvath, Hannum | Longitudinal (two times, 1.89 years apart) | Multiple linear regression | P | - | Only in Horvath clock, not current PTSD dx but “avoidance and numbing” PTSD symptom cluster | 179 | White (75.4%), Black (9.5%), Latino/a (11.7%), Asian American
(1.1%), American Indian (0.6%) Male: 88.3% - Age: mean 32.84 (SD 9.28), range [19–65] |
| Wolf et al. (2019b) | - | P | - | Horvath | Cross-sectional | Multiple linear regression | P | - | PTSD measured by severity Interaction significant with specific allele and older age |
532 (309 for DNAm age analysis) | Non-Hispanic White Male: 93.5% (for DNAm age analysis) - Age: mean 32.04 (SD 8.04) (for DNAm age analysis) |
| Comes et al. (2020) | TLs | - | - | Horvath | Longitudinal (two times, 1 year apart) | Multiple linear regression | - | - | Among bipolar patients | 96 | Bipolar patients German and Austrian (only European ancestries) Female: 52.1% (baseline) - Age: mean 45.2 (SD 12.4) (baseline) |
| Katrinli et al. (2020) | TL Tc | P | - | GrimAge | Cross-sectional | Multiple linear regression | TL P |
- | PTSD measured by PTSD diagnosis, only in discovery dataset | 1163 (854 discovery + 309 validation) | Discovery data Predominantly African American (93.1%) Female (70%) Age: mean around 42 (SD around 11.5) Validation data Predominantly African American (87%) Female (63%) - Age: mean around 55 (SD around 13) |
| Yang et al. (2020) | Tc | Pcb | - | GrimAge | Cross-sectional + Longitudinal (n = 26, two times, 3 years apart) | Combination of t-test, Chi-square, Pearson’s r, and ANCOVA + Logistic regression | Pcb | - | Among PTSD diagnosis and severity, only in total
severity Tc results from Pearson correlation |
215 (162 discovery + 53 validation) | Discovery data Hispanic (38%), non-Hispanic White (29%), non-Hispanic Black (25%), non-Hispanic Asian (4%) Male veterans Age: mean around 33 (SD 7.7) Validation data Hispanic (25%), non-Hispanic white (43%), non-Hispanic black (17%), non-Hispanic Asian (13%) Male veterans - Age: mean around 35 (SD 9.8) |
Note. PTSD = Post-traumatic stress disorder; Tc = childhood trauma; TL = lifetime trauma; Tcb = combat trauma; Pcb = combat PTSD; TLs = The same measurement used for TL in other articles was used for measuring current life stress; SD = standard deviation.
Table 3.
Instruments used to Measure Life Trauma Experience and Perceived Racial Discrimination.
| Childhood Trauma | Life/Combat Trauma | Post-Traumatic Stress Disorder | Current Life Stress | Perceived Racial Discrimination | |
|---|---|---|---|---|---|
| Boks et al. (2015) | 27-item Dutch version of the self-report version of the Early Trauma Inventory (ETI) | 19-item deployment experiences checklist | Self- Rating Inventory for PTSD (SRIP) | ||
| Brody et al. (2016) | A checklist of 12 events of life stress | Nine items from a version of the Schedule of Racist Events | |||
| Han et al. (2018) | Childhood trauma interview from the Netherlands Mental Health Survey and Incidence Study | ||||
| Mehta et al. (2018) | Interview-based data has been supplemented by pre-military and military data from army records a | CAPS for DSM-V | |||
| Wolf et al. (2018) | TLEQ | CAPS for DSM-IV | |||
| Wolf et al. (2019a) | TLEQ | CAPS for DSM-IV | |||
| Wolf et al. (2019b) | TLEQ a | CAPS for DSM-IV | |||
| Comes et al. (2020) | German, short version of the Childhood Trauma Questionnaire Childhood Trauma Screener (CTS) a | Life Events Questionnaire (LEQ) | |||
| Katrinli et al. (2020) | Childhood Trauma Questionnaire (CTQ) | Traumatic Events Inventory (TEI) | CAPS | ||
| Yang et al. (2020) | Early Trauma Inventory | CAPS for DSM-IV |
Note. TLEQ = Traumatic Life Events Questionnaire; CAPS = Clinician-Administered PTSD Scale.
aIndicates measurements used in the study but not for examining the relationship with DNA methylation age.
Sample Characteristics
Six studies were predominantly male (Boks et al., 2015; Mehta et al., 2018; Wolf et al., 2018, 2019a, 2019b; Yang et al., 2020), and remaining studies included 33–70% females (Brody et al., 2016; Comes et al., 2020; Han et al., 2018; Katrinli et al., 2020). Six studies included predominantly participants of European ancestry (Boks et al., 2015; Comes et al., 2020; Wolf et al., 2018, 2019a, 2019b; Yang et al., 2020), and two were completed with primarily Black or African American participants (Brody et al., 2016; Katrinli et al., 2020). Four studies evaluated the experiences of male veterans (Boks et al., 2015; Mehta et al., 2018; Wolf et al., 2018; Yang et al., 2020). Due to the limited number of the studies in the topic area, we tried to include as many studies as possible from the screening. This resulted in including studies about participants with certain conditions such as depression (Han et al., 2018) or bipolar disorder (Comes et al., 2020). The mean sample size was 458 and the sample sizes ranged from 96 to 1163. Among 10 studies, 60% had sample size less than 500. All studies used peripheral blood.
Study Design and Statistical Analysis
Six studies used a cross-sectional design (Han et al., 2018; Katrinli et al., 2020; Mehta et al., 2018; Wolf et al., 2018, 2019b; Yang et al., 2020), and one of these studies completed longitudinal analysis among a subset of individuals (n = 26; Yang et al., 2020). Four studies used longitudinal study design analyzing two samples collected 1–3 years apart (Boks et al., 2015; Comes et al., 2020; Wolf et al., 2019a; Yang et al., 2020). They examined the changes of various stress from traumatic life events, life stress, childhood trauma, and PTSD, with the change of DNAm age. One study evaluated DNAm that was collected after a previous exposure of life stress and perceived racial discrimination (Brody et al., 2016). Four studies evaluated discovery and validation groups (Brody et al., 2016; Katrinli et al., 2020; Mehta et al., 2018; Yang et al., 2020). All studies utilized regression modeling to evaluate relationships between psychological variables and DNAm age.
DNA Methylation Clocks
Three types of epigenetic clock were used: Horvath, Hannum, and GrimAge. Horvath’s clock was most frequently used (60% of studies) (Boks et al., 2015; Comes et al., 2020; Han et al., 2018; Mehta et al., 2018; Wolf et al., 2019a, 2019b). One study modified the clock calculation, but indicated their approach was similar to Horvath’s method (Han et al., 2018). Three studies used Hannum’s clock (Brody et al., 2016; Wolf et al., 2018, 2019a) and the two most recent articles used GrimAge (Katrinli et al., 2020; Yang et al., 2020).
Study Findings
As shown in Table 2, six studies explored the relationship between life trauma or life stress and DNAm age (Boks et al., 2015; Brody et al., 2016; Comes et al., 2020; Katrinli et al., 2020; Wolf et al., 2018, 2019a). One study focused on combat trauma (Boks et al., 2015) and two evaluated life stress (Brody et al., 2016; Comes et al., 2020). Among the six studies, two (33.3%) reported positive relationships (Boks et al., 2015; Katrinli et al., 2020), one of which assessed the effects of combat trauma. These two studies examined life trauma, not non-traumatic life stress. The remaining four studies did not show significant relationships including the one that included participants with bipolar disorder. When it comes to the participants’ demographic characteristics, four of the six studies enrolled participants of European ancestry and two included predominantly African Americans. Only one study included Hispanic individuals (12% of the participants). In these studies, the mean age ranged from 27 to 55.
Among the four studies that explored the relationship between childhood trauma and DNAm age (Boks et al., 2015; Han et al., 2018; Katrinli et al., 2020; Yang et al., 2020), one (25%) reported a positive relationship (Han et al., 2018). This study included participants with depression and a control group, and the significant relationship was detected only in those with depression. In the remaining three studies, there were no significant relationships. One study did not specify participants’ race and/or ethnicity, while the remaining three studies included individuals of European, African, and mixed ancestry with mostly Hispanic or non-Hispanic white participants. The mean age ranged from 22 to 55.
Seven studies explored the relationship between post-traumatic stress disorder and DNAm age (Boks et al., 2015; Katrinli et al., 2020; Mehta et al., 2018; Wolf et al., 2018, 2019a, 2019b; Yang et al., 2020), two of which focused on combat PTSD (Mehta et al., 2018; Yang et al., 2020). In these seven studies, PTSD was measured and evaluated by various concepts. For example, five studies evaluated the severity of PTSD (Boks et al., 2015; Mehta et al., 2018; Wolf et al., 2018, 2019b; Yang et al., 2020), four used PTSD diagnosis (Katrinli et al., 2020; Mehta et al., 2018; 2019a; Yang et al., 2020), and two assessed PTSD symptoms related to DNAm aging (Wolf et al., 2018, 2019a). In this review, we aggregated these results and considered them all measures of PTSD. As a result, five studies (71.4%) reported positive relationships (Katrinli et al., 2020; Wolf et al., 2018, 2019a, 2019b; Yang et al., 2020), one of which focused on combat PTSD (Yang et al., 2020). On the other hand, one (14.3%) reported a negative relationship (Boks et al., 2015). The remaining study found no significant relationship. As participants’ demographic characteristics, seven of the studies enrolled individuals of European ancestry and two studies included African American participants. Two studies included Hispanic participants. Mean ages ranged from 27 to 69.
Only one study explored the relationship between perceived racial discrimination and DNAm age among African Americans and reported that perceived racial discrimination was associated with accelerated epigenetic aging (Brody et al., 2016). The participants’ mean age at the time of blood draw was 21.
Some studies explored additional factors than may influence the impact of traumatic life experience or perceived racial discrimination on DNAm aging. For example, one study also evaluated resilience and reported that higher resilience was associated with accelerated DNAm aging (Mehta et al., 2018). A separate study examined the interaction effect of a specific allele on the relationship between PTSD and DNAm age. Specific details of additional study variables are listed in Table 2, under the “DNAm Age Results” column.
Discussion
In this review, we explored the influence of psychosocial stress from traumatic life events and perceived racial discrimination on epigenetic aging reported in previous studies. From the 10 studies published within the past 10 years identified from four databases, we found that the experiences of life trauma, post-traumatic stress, and perceived racial discrimination may be associated with accelerated epigenetic aging.
In our study, we combined non-traumatic life stress together with life trauma as there were scarce sources of articles assessing the effects of lifetime trauma. Of the studies we identified that evaluated types of stress and traumatic life events, there was no significant relationship between non-traumatic life stress and DNAm age. However, we did find more heterogenous results across studies that evaluated the relationship between other types of stress and DNAm age. Among the included studies, one identified a positive relationship between childhood trauma and DNAm age. However, a separate study that analyzed childhood trauma, life trauma, and PTSD concurrently, there was no significant relationship between childhood trauma and DNAm age. In two different studies, when life trauma was measured with childhood trauma and PTSD, accelerated DNAm aging was significantly associated with life trauma. In many cases in which PTSD was measured, there was a significant relationship with DNAm age acceleration. The heterogeneity of results may be partly due to the fact multiple different types of life trauma were assessed and different types of life trauma may differentially impact DNAm age. This multivariable approach has potential to identify effects of multiple types of life trauma on DNAm age. However, many of the studies that evaluated multiple kinds of traumatic life events did not look at the types of life trauma independent of each other or combined them into a single variable, which made it difficult to estimate the effects of specific types of life trauma on DNAm age.
Traumatic life events can have a critical impact on mental and physical health with different manifestations in affected individuals, such as PTSD and maladaptive health behaviors. Life trauma can even have a bigger impact on individual health when traumatic life events occur during childhood (Dunn et al., 2018). A recent study reported that the toxicity of stress can changes the brain structure, and affect the coping style of a child (Oral et al., 2016). Childhood trauma has also been linked to increased risk for psychiatric illness in adulthood (Trotta et al., 2015). Not only can experiencing life trauma influence mental health by altering psychological pathways, but also through biological pathways such as chronic inflammation or altered corticosteroids stress response (Danese & Baldwin, 2017; Nemeroff, 2004). In many of the studies that evaluated PTSD in our study, life trauma was measured, but it was not included in analysis related to DNAm age. Due to the variety of symptoms and disorders that are associated with experiencing life trauma, more research is needed to examine the effects of different types of life trauma on DNAm age. Further, more nuanced exploration of traumatic life events and DNAm aging may illuminate biological imprints of life trauma associated with different symptom presentations.
Similar to the studies evaluating traumatic life events, studies assessing the impact of PTSD on DNAm age evaluated different features of PTSD. For example, some studies used PTSD diagnosis as their predictor variable, whereas other used PTSD symptom severity or PTSD symptoms which may have contributed to mixed results across all of the PTSD studies. Additionally, there were interesting findings in two PTSD studies (Wolf et al., 2018, 2019a). Although neither PTSD diagnosis nor PTSD symptom severity had a significant relationship with DNAm age; PTSD symptoms, such as hyperarousal or avoidance and numbing symptoms showed a positive relationship with DNAm age acceleration. As DNAm age is reversible, these findings suggest that people with specific symptom profiles may benefit from more personalized therapy to help mitigate this acceleration by developing therapeutic interventions based on DNAm age (Horvath & Raj, 2018). Similarly in Mehta and colleagues’ (2018) study, neither PTSD diagnosis nor PTSD symptom severity had significant relationship with DNAm age, although higher resilience among individuals with PTSD had accelerated DNAm aging. These findings suggest that how an individual experiences PTSD may result in a differential impact on DNAm age, and further highlights the importance of precision in biobehavioral intervention to mitigate the impact of PTSD on health.
The limitations of the psychometric instruments used to measure life trauma may have also contributed to heterogeneity in the results. When measuring life trauma, we need to not only consider its type/situation and the frequency, but also the age that event occurred and how severe/impactful the event was to the individual. Many of the studies only evaluated frequency of traumatic life events and scarcely evaluated the severity of life trauma. When severity was evaluated, some studies reduced or dichotomized life trauma severity for the analysis, which could have led to a loss of information and inability to detect variation based on severity.
Future studies in this area can be strengthened by using Common Data Elements (CDEs) (Sheehan et al., 2016). CDEs are precisely defined measurements by research communities that can be commonly used across studies. Broad use of CDEs can improve cross-study comparisons and data aggregation and therefore overall efficiency (https://cde.nlm.nih.gov/). In our review, most of the studies that evaluated PTSD used Clinician-Administered PTSD Scale for DSM-IV (CAPS) (or DSM-V), which is a measure recommend for use to generate CDEs (Blake et al., 1995). However, measures used to capture experiences of life trauma related to DNAm age were diverse, and only 40% of the studies included CDEs. Currently, there are a number of CDEs for life trauma assessment including the Traumatic Life Events Questionnaire (TLEQ) (for adults), Adverse Life Events Scale (for children), and Childhood Trauma Questionnaire (CTQ) (https://www.phenxtoolkit.org/) (Bernstein et al., 1997; Dohrenwend, 2006; Tiet et al., 2001). Although some limitations of CDEs remain, such as validity among different population and differences in interpretation, using CDEs should be considered when designing future studies to facilitate research efficiency through seamless data sharing and easier subsequent meta-analysis.
In our review, one study identified accelerated epigenetic aging associated with perceived racial discrimination. The results remained significant after controlling for stressful life events, suggesting that experiencing racial discrimination has an independent impact on DNAm aging acceleration from that of other stressful life events. The effect of perceived racial discrimination on health outcomes has long been studied (Krieger et al., 1993; Lewis et al., 2014; Panza et al., 2019). Earlier reports have linked experiencing racial discrimination with adverse birth outcomes, hypertension, and increased morbidity and mortality (Matoba & Collins, 2017; Panza et al., 2019; Stewart et al., 2020). In order to try to determine how these experiences increase a variety of health risks, research has been done to examine multiple potential biological pathways influenced or altered when experiencing racism that increase health risks (de Mendoza et al., 2018; Taylor et al., 2017). Other biological markers for age acceleration, such as telomere length, also suggest there is accelerated biological aging associated with experiencing racial discrimination (Chae et al., 2016; Lee et al., 2017). More studies are needed to investigate the effects of experiencing racial discrimination and biological aging and the impact on health comes associated with aging.
The fact that there are different epigenetic clocks developed using different components will contribute to heterogeneity in outcomes regardless of the predictors (i.e., traumatic life events, PTSD, or perceived racial discrimination). For example, only GrimAge identified accelerated DNAm aging related to PTSD diagnosis and symptom severity. This may be due to the fact that GrimAge uses the most up-to-date extrinsic cell estimator, and therefore more sensitive for detecting stress related cellular aging than other DNAm age estimators. In studies using Horvath’s clock, results were less consistent. For example, PTSD symptom severity was only related with DNAm age when considering the interaction of PTSD with genetic variants of a longevity related gene (rs9563121 & rs9315202) (Wolf et al., 2019b). Conversely, in Boks and colleagues’ (2015) study, which also used Horvath’s clock, PTSD symptom severity was inversely related with DNAm age acceleration. This may indicate stress related biological aging is a complex process and compensatory mechanism in cellular aging toward stress may influence the effects on epigenetic aging.
This is the first review that explored the relationship among psychosocial stress from life trauma and perceived racial discrimination and DNA methylation age acceleration. Considering the long-term effect of psychosocial stress on health (Lewis et al., 2014; Rutters et al., 2015), utilizing this new biomarker may provide additional insight into the effects of stress at the cellular level, which may be detectable before alterations in health outcomes develop.
Limitations
Due to the various aforementioned factors that could affect the results, such as 1) multiple kinds of traumatic life experiences while sometimes combining them into a single variable, 2) different features of predictor variables, such as diagnosis or severity, 3) imitations of the psychometric measurements (mostly measuring frequency, not intensity), and 4) different types of epigenetic clocks, it was not possible to definitively discern racial effects across studies. Moreover, many studies only included white males or have few racial/ethnic minority populations being included. This makes it difficult to generalize our findings from these studies to the overall population. Future research including more women and racial/ethnic minorities are needed. Future studies would also benefit by obtaining larger samples sizes when possible, as sample sizes analyzed here were relatively small. We also recommend use of CDEs to improve our ability to compare results across studies and allow for meta-analysis of results. Approximately half of studies we evaluated had longitudinal design, which is a stronger design to evaluate causality of psychosocial stress on epigenetic age. However, the time intervals between assessments were short (1–3 year apart) which could impact the results if the timing of the experience related to DNAm change is important.
Conclusions
Although there was heterogeneity in measures and results across studies, our analysis suggests that life trauma and perceived racial discrimination are likely associated with epigenetic aging acceleration. Utilizing DNAm age as a tool to explore the mechanisms between stressful life events, traumatic life events, PTSD, and perceived racial discrimination will help our understanding of how psychosocial stress can developed into disease and provide intervention targets to minimize their impact on health. Therefore, additional studies that are inclusive of more diverse populations and psychosocial stressors are needed to validate previous findings and determine the effect and magnitude of these associations. Nursing scholars and other health scientists who utilize epigenetic age acceleration to assess health risks may need to consider including psychosocial stressors in their studies as covariates.
Acknowledgments
Michelle L Wright is supported by the National Institute of Nursing Research (K01NR017903). The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH. The authors wish to acknowledge the support of Ye Ryn Jeong with data collection and analysis.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Sungju Lim https://orcid.org/0000-0002-0150-5188
Michelle L Wright https://orcid.org/0000-0002-9348-8740
References
- Allis D., Caparros M. L., Jenuwein T., Reinberg D. (2015). Epigenetics (2nd ed.). Cold Spring Harbor Laboratory Press. [Google Scholar]
- Aromataris E., Munn Z. (2020). Chapter 1: JBI Systematic Reviews. In Aromataris E., Munn Z. (Eds.), JBI manual for evidence synthesis. JBI. 10.46658/JBIMES-20-02 [DOI] [Google Scholar]
- Bailey Z. D., Krieger N., Agénor M., Graves J., Linos N., Bassett M. T. (2017). Structural racism and health inequities in the USA: Evidence and interventions. The Lancet, 389(10077), 1453–1463. 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- Bernstein D. P., Ahluvalia T., Pogge D., Handelsman L. (1997). Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry, 36(3), 340–348. 10.1097/00004583-199703000-00012 [DOI] [PubMed] [Google Scholar]
- Bjornsson H. T. (2008). Intra-individual change over time in DNA methylation with familial clustering. JAMA, 299(24), 2877. 10.1001/jama.299.24.2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. D., Weathers F. W., Nagy L. M., Kaloupek D. G., Gusman F. D., Charney D. S., Keane T. M. (1995). The development of a Clinician-Administered PTSD Scale. Journal of Trauma Stress, 8, 75–90. 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- Boks M. P., van Mierlo H. C., Rutten B. P. F., Radstake T. R. D. J., De Witte L., Geuze E., Horvath S., Schalkwyk L. C., Vinkers C. H., Broen J. C. A., Vermetten E. (2015). Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology, 51, 506–512. 10.1016/j.psyneuen.2014.07.011 [DOI] [PubMed] [Google Scholar]
- Brody G. H., Miller G. E., Yu T., Beach S. R. H., Chen E. (2016). Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: A replication across two longitudinal cohorts. Psychological Science, 27(4), 530–541. 10.1177/0956797615626703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae D. H., Epel E. S., Nuru-Jeter A. M., Lincoln K. D., Taylor R. J., Lin J., Blackburn E. H., Thomas S. B. (2016). Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology, 63, 10–16. 10.1016/j.psyneuen.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E., Miller G. E., Yu T., Brody G. H. (2016). The Great Recession and health risks in African American youth. Brain, Behavior, and Immunity, 53, 234–241. 10.1016/j.bbi.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. C., Houseman E. A., Marsit C. J., Zheng S., Wrensch M. R., Wiemels J. L., Nelson H. H., Karagas M. R., Padbury J. F., Bueno R., Sugarbaker D. J., Yeh R.-F., Wiencke J. K., Kelsey K. T. (2009). Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genetics, 5(8), e1000602. 10.1371/journal.pgen.1000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Janicki-Deverts D., Miller G. E. (2007). Psychological stress and disease. JAMA, 298(14), 1685. 10.1001/jama.298.14.1685 [DOI] [PubMed] [Google Scholar]
- Cohen S., Murphy M. L. M., Prather A. A. (2019). Ten surprising facts about stressful life events and disease risk. Annual Review of Psychology, 70(1), 577–597. 10.1146/annurev-psych-010418-102857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comes A. L., Czamara D., Adorjan K., Anderson-Schmidt H., Andlauer T. F. M., Budde M., Gade K., Hake M., Kalman J. L., Papiol S., Reich-Erkelenz D., Klöhn-Saghatolislam F., Schaupp S. K., Schulte E. C., Senner F., Juckel G., Schmauß M., Zimmermann J., Reimer J., Heilbronner U. ( 2020). The role of environmental stress and DNA methylation in the longitudinal course of bipolar disorder. International Journal of Bipolar Disorders, 8(1), 9. 10.1186/s40345-019-0176-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Baldwin J. R. (2017). Hidden wounds? Inflammatory links between childhood trauma and psychopathology. Annual Review of Psychology, 68(1), 517–544. 10.1146/annurev-psych-010416-044208 [DOI] [PubMed] [Google Scholar]
- de Mendoza V. B., Huang Y., Crusto C. A., Sun Y. V., Taylor J. Y. (2018). Perceived racial discrimination and DNA methylation among African American women in the InterGEN Study. Biological Research for Nursing, 20(2), 145–152. 10.1177/1099800417748759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend B. P. (2006). Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychological Bulletin, 132(3), 477–495. 10.1037/0033-2909.132.3.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn E. C., Nishimi K., Gomez S. H., Powers A., Bradley B. (2018). Developmental timing of trauma exposure and emotion dysregulation in adulthood: Are there sensitive periods when trauma is most harmful? Journal of Affective Disorders, 227, 869–877. 10.1016/j.jad.2017.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P. (2018). The key role of epigenetics in human disease prevention and mitigation. New England Journal of Medicine, 378(14), 1323–1334. 10.1056/NEJMra1402513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito G., Polidoro S., Dugué P. A., Kivimaki M., Ponzi E., Matullo G., Guarrera S., Assumma M. B., Georgiadis P., Kyrtopoulos S. A., Krogh V., Palli D., Panico S., Sacerdote C., Tumino R., Chadeau-Hyam M., Stringhini S., Severi G., Hodge A. M., Vineis P. (2017). Social adversity and epigenetic aging: A multi-cohort study on socioeconomic differences in peripheral blood DNA methylation. Scientific Reports, 7(1), 16266. 10.1038/s41598-017-16391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga M. F., Ballestar E., Paz M. F., Ropero S., Setien F., Ballestar M. L., Heine-Suner D., Cigudosa J. C., Urioste M., Benitez J., Boix-Chornet M., Sanchez-Aguilera A., Ling C., Carlsson E., Poulsen P., Vaag A., Stephan Z., Spector T. D., Wu Y.-Z., Esteller M. (2005). Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America, 102(30), 10604–10609. 10.1073/pnas.0500398102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L. K. M., Aghajani M., Clark S. L., Chan R. F., Hattab M. W., Shabalin A. A., Zhao M., Kumar G., Xie L. Y., Jansen R., Milaneschi Y., Dean B., Aberg K. A., Van Den Oord E. J. C. G., Penninx B. W. J. H. (2018). Epigenetic aging in major depressive disorder. American Journal of Psychiatry, 175(8), 774–782. 10.1176/appi.ajp.2018.17060595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum G., Guinney J., Zhao L., Zhang L., Hughes G., Sadda S., Klotzle B., Bibikova M., Fan J.-B., Gao Y., Deconde R., Chen M., Rajapakse I., Friend S., Ideker T., Zhang K. (2013). Genome-wide methylation profiles reveal quantitative views of human aging rates. Molecular Cell, 49(2), 359–367. 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. (2013). DNA methylation age of human tissues and cell types. Genome Biology, 14(10), R115. 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Raj K. (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics, 19(6), 371–384. 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- Jylhävä J., Pedersen N. L., Hägg S. (2017). Biological age predictors. EBioMedicine, 21, 29–36. 10.1016/j.ebiom.2017.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanherkar R. R., Bhatia-Dey N., Csoka A. B. (2014). Epigenetics across the human lifespan. Frontiers in Cell and Developmental Biology, 2, 49. 10.3389/fcell.2014.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katrinli S., Stevens J., Wani A. H., Lori A., Kilaru V., van Rooij S. J. H., Hinrichs R., Powers A., Gillespie C. F., Michopoulos V., Gautam A., Jett M., Hammamieh R., Yang R., Wildman D., Qu A., Koenen K., Aiello A. E., Jovanovic T., Smith A. K. (2020). Evaluating the impact of trauma and PTSD on epigenetic prediction of lifespan and neural integrity. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 45(10), 1609–1616. 10.1038/s41386-020-0700-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. (2014). Discrimination and health inequities. International Journal of Health Services, 44(4), 643–710. 10.2190/HS.44.4.b [DOI] [PubMed] [Google Scholar]
- Krieger N., Rowley D. L., Herman A. A., Avery B., Phillips M. T. (1993). Racism, sexism, and social class: Implications for studies of health, disease, and well-being. American Journal of Preventive Medicine, 9(6), 82–122. 10.1016/S0749-3797(18)30666-4 [DOI] [PubMed] [Google Scholar]
- Lee D. B., Kim E. S., Neblett E. W. (2017). The link between discrimination and telomere length in African American adults. Health Psychology, 36(5), 458–467. 10.1037/hea0000450 [DOI] [PubMed] [Google Scholar]
- Lemelin E. T., Diez Roux A. V., Franklin T. G., Carnethon M., Lutsey P. L., Ni H., O’Meara E., Shrager S. (2009). Life-course socioeconomic positions and subclinical atherosclerosis in the multi-ethnic study of atherosclerosis. Social Science & Medicine, 68(3), 444–451. 10.1016/j.socscimed.2008.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. E., Lu A. T., Quach A., Chen B. H., Assimes T. L., Bandinelli S., Hou L., Baccarelli A. A., Stewart J. D., Li Y., Whitsel E. A., Wilson J. G., Reiner A. P., Aviv A., Lohman K., Liu Y., Ferrucci L., Horvath S. (2018). An epigenetic biomarker of aging for lifespan and healthspan. Aging, 10(4), 573–591. 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T. T., Williams D. R., Tamene M., Clark C. R. (2014). Self-reported experiences of discrimination and cardiovascular disease. Current Cardiovascular Risk Reports, 8(1), 365. 10.1007/s12170-013-0365-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang Z., Hardy T., Huang Y., Hui Q., Crusto C. A., Wright M. L., Taylor J. Y., Sun Y. V. (2019). Association of obesity with DNA methylation age acceleration in African American mothers from the InterGEN study. International Journal of Molecular Sciences, 20(17), 4273. 10.3390/ijms20174273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-Z., Wang Y.-X., Jiang C.-L. (2017). Inflammation: The common pathway of stress-related diseases. Frontiers in Human Neuroscience, 11, 316. 10.3389/fnhum.2017.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. T., Quach A., Wilson J. G., Reiner A. P., Aviv A., Raj K., Hou L., Baccarelli A. A., Li Y., Stewart J. D., Whitsel E. A., Assimes T. L., Ferrucci L., Horvath S. (2019). DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging, 11(2), 303–327. 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba N., Collins J. W. (2017). Racial disparity in infant mortality. Seminars in Perinatology, 41(6), 354–359. 10.1053/j.semperi.2017.07.003 [DOI] [PubMed] [Google Scholar]
- McEwen B. (2000). Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 22(2), 108–124. 10.1016/S0893-133X(99)00129-3 [DOI] [PubMed] [Google Scholar]
- McGowan P. O., Sasaki A., D’Alessio A. C., Dymov S., Labonté B., Szyf M., Turecki G., Meaney M. J. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience, 12(3), 342–348. 10.1038/nn.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D., Bruenig D., Lawford B., Harvey W., Carrillo-Roa T., Morris C. P., Jovanovic T., Young R. M., Binder E. B., Voisey J. (2018). Accelerated DNA methylation aging and increased resilience in veterans: The biological cost for soldiering on. Neurobiology of Stress, 8, 112–119. 10.1016/j.ynstr.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan C., D’Errico N., Stees J., Hughes D. (2012). Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics: Official Journal of the DNA Methylation Society, 7(8), 853–857. 10.4161/epi.21180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health (2020). Coping with traumatic events. Retrieved October 20, 2021, from https://www.nimh.nih.gov/health/topics/coping-with-traumatic-events [Google Scholar]
- Nemeroff C. B. (2004). Neurobiological consequences of childhood trauma. Journal of Clinical Psychiatry, 65(Suppl 1), 18–28. [PubMed] [Google Scholar]
- Notterman D. A., Mitchell C. (2015). Epigenetics and understanding the impact of social determinants of health. Pediatric Clinics of North America, 62(5), 1227–1240. 10.1016/j.pcl.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral R., Ramirez M., Coohey C., Nakada S., Walz A., Kuntz A., Benoit J., Peek-Asa C. (2016). Adverse childhood experiences and trauma informed care: The future of health care. Pediatric Research, 79(1–2), 227–233. 10.1038/pr.2015.197 [DOI] [PubMed] [Google Scholar]
- Panza G. A., Puhl R. M., Taylor B. A., Zaleski A. L., Livingston J., Pescatello L. S. (2019). Links between discrimination and cardiovascular health among socially stigmatized groups: A systematic review. PLoS One, 14(6), e0217623. 10.1371/journal.pone.0217623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. L., Christensen B. C., Batsis J. A. (2021). Weight management intervention identifies association of decreased DNA methylation age with improved functional age measures in older adults with obesity. Clinical Epigenetics, 13(1), 1–8. 10.1186/s13148-021-01031-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutters F., Pilz S., Koopman A. D. M., Rauh S. P., Pouwer F., Stehouwer C. D. A., Elders P. J., Nijpels G., Dekker J. M. (2015). Stressful life events and incident metabolic syndrome: The Hoorn study. Stress: The International Journal on the Biology of Stress, 18(5), 507–513. 10.3109/10253890.2015.1064891 [DOI] [PubMed] [Google Scholar]
- Sae‐Lee C., Corsi S., Barrow T. M., Kuhnle G. G., Bollati V., Mathers J. C., Byun H. M. (2018). Dietary intervention modifies DNA methylation age assessed by the epigenetic clock. Molecular Nutrition & Food Research, 62(23), e1800092. 10.1002/mnfr.201800092 [DOI] [PubMed] [Google Scholar]
- Sheehan J., Hirschfeld S., Foster E., Ghitza U., Goetz K., Karpinski J., Lang L., Moser R. P., Odenkirchen J., Reeves D., Rubinstein Y., Werner E., Huerta M. (2016). Improving the value of clinical research through the use of Common Data Elements. Clinical Trials, 13(6), 671–676. 10.1177/1740774516653238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. L., Lei M. K., Beach S. R. H., Philibert R. A., Cutrona C. E., Gibbons F. X., Barr A. (2016). Economic hardship and biological weathering: The epigenetics of aging in a U.S. sample of black women. Social Science & Medicine, 150, 192–200. 10.1016/j.socscimed.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A., Kivimäki M. (2013). Stress and cardiovascular disease: An update on current knowledge. Annual Review of Public Health, 34(1), 337–354. 10.1146/annurev-publhealth-031912-114452 [DOI] [PubMed] [Google Scholar]
- Stewart Q. T., Cobb R. J., Keith V. M. (2020). The color of death: Race, observed skin tone, and all-cause mortality in the United States. Ethnicity & Health, 25(7), 1018–1040. 10.1080/13557858.2018.1469735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. Y., Sun Y. V., Barcelona de Mendoza V., Ifatunji M., Rafferty J., Fox E. R., Musani S. K., Sims M., Jackson J. S. (2017). The combined effects of genetic risk and perceived discrimination on blood pressure among African Americans in the Jackson Heart Study. Medicine, 96(43), e8369. 10.1097/MD.0000000000008369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff A. E., West J., Beck S. (2013). Age-associated epigenetic drift: Implications, and a case of epigenetic thrift? Human Molecular Genetics, 22(R1), R7–R15. 10.1093/hmg/ddt375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiet Q. Q., Bird H. R., Hoven C. W., Moore R., Wu P., Wicks J., Jensen P. S., Goodman S., Cohen P. (2001). Relationship between specific adverse life events and psychiatric disorders. Journal of Abnormal Child Psychology, 29(2), 153–164. 10.1023/a:1005288130494 [DOI] [PubMed] [Google Scholar]
- Trotta A., Murray R. M., Fisher H. L. (2015). The impact of childhood adversity on the persistence of psychotic symptoms: A systematic review and meta-analysis. Psychological Medicine, 45(12), 2481–2498. 10.1017/S0033291715000574 [DOI] [PubMed] [Google Scholar]
- Wolf E. J., Logue M. W., Morrison F. G., Wilcox E. S., Stone A., Schichman S. A., McGlinchey R. E., Milberg W. P., Miller M. W. (2019. a). Posttraumatic psychopathology and the pace of the epigenetic clock: A longitudinal investigation. Psychological Medicine, 49(5), 791–800. 10.1017/S0033291718001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E. J., Logue M. W., Stoop T. B., Schichman S. A., Stone A., Sadeh N., Hayes J. P., Miller M. W. (2018). Accelerated DNA methylation age: Associations with posttraumatic stress disorder and mortality. Psychosomatic Medicine, 80(1), 42–48. 10.1097/PSY.0000000000000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E. J., Morrison F. G., Sullivan D. R., Logue M. W., Guetta R. E., Stone A., Schichman S. A., McGlinchey R. E., Milberg W. P., Miller M. W. (2019. b). The goddess who spins the thread of life: Klotho, psychiatric stress, and accelerated aging. Brain, Behavior, and Immunity, 80, 193–203. 10.1016/j.bbi.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Wu G. W. Y., Verhoeven J. E., Gautam A., Reus V. I., Kang J. I., Flory J. D., Abu-Amara D., Hood L., Doyle F. J., Yehuda R., Marmar C. R., Jett M., Hammamieh R., Mellon S. H., Wolkowitz O. M., PTSD Systems Biology Consortium (2020). A DNA methylation clock associated with age-related illnesses and mortality is accelerated in men with combat PTSD. Molecular Psychiatry. 10.1038/s41380-020-0755-z [DOI] [PubMed] [Google Scholar]
- Yehuda R., Flory J. D., Bierer L. M., Henn-Haase C., Lehrner A., Desarnaud F., Makotkine I., Daskalakis N. P., Marmar C. R., Meaney M. J. (2015). Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans with posttraumatic stress disorder. Biological Psychiatry, 77(4), 356–364. 10.1016/j.biopsych.2014.02.006 [DOI] [PubMed] [Google Scholar]