Abstract
Objective
The purpose of this study was to evaluate the utility of ICD-O-3–classified local tumor behavior as a prognosticator of head and neck paraganglioma (HNP) outcomes.
Study Design
Retrospective cohort study.
Setting
National Cancer Database between 2004 and 2016.
Methods
This study included patients aged ≥18 years who were diagnosed with HNP. Clinical outcomes and clinicopathologic features were compared with regard to local tumor behavior.
Results
Our study included 525 patients, of which the majority had HNP classified as locally invasive (45.9%) or borderline (37.9%). The most common anatomic sites involved were the carotid body (33.7%), intracranial regions (29.0%), or cranial nerves (25.5%). Carotid body tumors were exclusively locally invasive, whereas intracranial and cranial nerve HNP were overwhelmingly benign or borderline (94% and 91%, respectively). One-fourth of patients underwent pathologic analysis of regional lymph nodes, of which the majority were positive for metastasis (80.6%). Metastasis to distant organs was twice as common in patients with locally invasive tumors vs benign (15% vs 7.1). For benign disease, surgery with radiotherapy (adjusted hazard ratio [aHR], 40.45; P = .006) and active surveillance (aHR, 24.23; P = .008) were associated with worse survival when compared with surgery alone. For locally invasive tumors, greater age (aHR, 1.07; P < .0001) and positive surgical margins (aHR, 4.13; P = .010) were predictors of worse survival, while combined surgery and radiotherapy were predictors of improved survival vs surgery alone (aHR, 0.31; P = .027).
Conclusion
While criteria for tumor behavior could not be defined, our results suggest that such a classification system could be used to enhance HNP risk stratification and guide clinical management decisions.
Keywords: paraganglioma, prognosis, treatment trends, invasive, survival, regional lymph nodes
Paraganglioma are rare neuroendocrine tumors arising from neural crest–derived cells associated with the autonomic nervous system of the head, neck, thorax, and abdomen. 1 The incidence of these tumors is estimated to be between 0.6 and 1.0 per 100,000 person-years, with 3% to 18% occurring within the head and neck.2-5 Head and neck paraganglioma (HNP) most often arise from the carotid body, middle ear, jugular bulb, and vagus nerve. If left untreated, they may cause significant morbidity and mortality by local invasion or metastasis.6-8
Currently there is no comprehensive risk assessment method to evaluate paraganglioma behavior in all head and neck regions. The classification systems that have been developed, such as Fisch, Glasscock-Jackson, and Shamblin, can be applied only to specific HNP sites and fail to incorporate risk of recurrence or metastatic spread.9-11 Therefore, clinicians often risk stratify cases via classification of HNP as benign or malignant. The accepted criterion for “malignant” is the occurrence of lymph node or distant metastasis 12 and is cited to be present in 10% of HNP. 13 The remaining 90% of HNP are considered benign. The median overall survival (OS) at 5 years among patients with regionally confined disease (77%) is significantly better than among those with distant metastasis (12%). 8 While it may be clinically useful to identify HNP as malignant, classification of HNP as benign may not appropriately characterize patient risk.
The literature suggests that there is some degree of heterogeneity in disease course among “benign” HNP. One study found that among patients treated for nonmetastatic HNP, recurrence rates were 8.2% and 17.1% at 4 and 10 years, respectively, with 3.2% of patients developing metastasis. 14 A separate study indicated that 17.6% of patients with nonmetastatic carotid body tumors developed metastatic recurrent disease following treatment. 15 These investigations also pinpointed mutations involving genes in the succinate dehydrogenase complex (SDHx) as risk factors for local/regional recurrence and distance metastases.15-18 Altogether, this academic work suggests that there may be a subset of HNP with more aggressive features that cannot be resolved through evaluation of distant or local metastasis upon initial presentation.
The anatomic complexity, variable behavior, and rare incidence of HNP have curtailed the development of consistent guidelines for their management. Surgical resection represents the mainstay of treatment for HNP and is associated with the highest rates of local control.19,20 However, due to the morbidity associated with surgery, recent studies have suggested that primary radiotherapy may provide similar local tumor control with reduced morbidity.21-23 Others have suggested that conservative management with serial imaging and close follow-up may be preferred for patients with advanced age, multiple comorbidities, small asymptomatic tumors, or cases in which surgical resection carries high morbidity. 24 The significant ambiguity in the literature with regard to optimal treatment paradigm stems from an incomplete understanding of disparate HNP subgroups. 12 Enhanced characterization of these tumors will provide clinicians with the tools required to pinpoint the clinical indications for each therapeutic approach.
The US National Cancer Database (NCDB) classifies all tumors with a behavior code based on histologic, cytologic, or radiologic findings. This behavior code gives insight into the local behavior of a tumor rather than information regarding regional or distant metastasis. In this study, we explore the clinical value in using local tumor behavior to develop HNP risk strata. This represents the largest analysis to date evaluating prognostic factors in HNP and the first study to develop HNP phenotypes based on local tumor behavior.
Methods
Data Source
We conducted a retrospective analysis of the NCDB, a nationwide cancer registry jointly sponsored by the American College of Surgeons and the American Cancer Society. The NCDB is a clinical oncology database sourced from >1500 Commission on Cancer–accredited facilities that accounts for about 70% of newly diagnosed cancers in the United States.25,26 The American College of Surgeons and Commission on Cancer have not reviewed and are not responsible for the analyses and conclusions drawn from these data. This study was deemed exempt from review by the University of California San Diego Institutional Review Board.
Study Cohort
Patients diagnosed with primary paraganglioma of the head and neck region from 2004 through 2016 were identified through ICD-O-3 codes (International Classification of Diseases for Oncology, Third Edition; World Health Organization). Eligible topographic codes for the head and neck region included C000-C009, C019-C069, C079-C119, C129-148, C300-C301, C310-C329, C411, C440-C444, C470, C490, C700-719, C722-C725, C739, C750-754, C758-C760, and C770. Eligible morphology codes for paraganglioma included M8680-8683, M8690-8693, M8700, and M8711. The fifth digit in the morphology code describes local tumor behavior and indicates whether the neoplasm is benign (/0), invading into surrounding tissues (/3), or of borderline or uncertain behavior (/1). The behavior code “/3” was designated as “locally invasive” because it was assigned independently of regional or distant metastasis. 27 Rather, for regional and distant metastasis status, pathologic confirmation in the NCDB of regional lymph nodes (RLNs) and distant metastasis was examined.
Demographic variables included for analysis were age, sex, and race. “Margins” refers to the surgical margins following resection of the primary tumor. The Charlson-Deyo Comorbidity Index (CCI) is an adapted comorbidity score available in the NCDB and is based on secondary diagnoses, with higher scores indicating more comorbidities.28,29 Follow-up times were reported as the number of months between the date of diagnosis and the date of patient last contact or death.
Statistical Analysis
Descriptive statistics, including proportions, means, and standard deviations, were used to report demographic and clinical features of the population. Population characteristics were compared by tumor behavior through 1-way analysis of variance with Bonferroni post hoc testing or Pearson chi-square test for continuous and categorical variables, respectively. Cox proportional hazards modeling was used to generate unadjusted and adjusted hazard ratios (aHRs) with 95% CIs evaluating OS stratified by tumor behavior. Survival analysis was performed only for patients with available vital status data. Forward stepwise selection was implemented to determine covariates to be included in the final Cox regression model with inclusion and exclusion thresholds set at P < .05 and P > .2. Prevalence ratios of treatment modalities over sequential calendar years were estimated through generalized nonparametric linear models with extension to the binomial family. All data were analyzed with Stata/SE version 17.0 for Mac.
Results
Baseline Characteristics
This study included 525 patients with HNP ( Table 1 ). Patients were on average 53 years old, mostly female (63.8%), and mostly White (69.9%). Although the sex ratio was fairly balanced among patients <40 years of age, the relative proportion of women increased with each decade of life until a female:male ratio of 4:1 was reached in patients ≥70 years old ( Figure 1 ). Most tumors were locally invasive (46%) or borderline (38%), with a minority being benign (16%) in behavior. Of the RLNs sampled (24.6%), the majority were positive for metastasis (80.6%; Table 2 ). Distant metastasis was identified in a minority of patients (7.1%).
Table 1.
Clinical Characteristics of Patients With Head and Neck Paraganglioma in NCDB From 2004 to 2016.
| Tumors, No. (%) a | P value b | ||||||
|---|---|---|---|---|---|---|---|
| Overall | Benign | Borderline | Locally invasive | Benign vs locally invasive | Borderline vs locally invasive | Benign vs borderline | |
| Patients | 525 | 85 (16.19) | 199 (37.9) | 241 (45.9) | |||
| Age at diagnosis, y, mean (SD) | 52.59 (15.9) | 56.2 (15.79) | 54.93 (14.38) | 49.38 (16.56) | .002 | .001 | .898 |
| Sex | .773 | .091 | .327 | ||||
| Male | 190 (36.19) | 32 (37.65) | 63 (31.66) | 95 (39.42) | |||
| Female | 335 (63.81) | 53 (62.35) | 136 (68.34) | 146 (60.58) | |||
| Race | .037 | .003 | .129 | ||||
| White | 367 (69.9) | 52 (61.18) | 138 (69.35) | 177 (73.44) | |||
| Black | 93 (17.71) | 21 (24.71) | 44 (22.11) | 28 (11.62) | |||
| Hispanic | 36 (6.86) | 8 (9.41) | 6 (3.02) | 22 (9.13) | |||
| Asian/Pacific Islander | 13 (2.48) | 2 (2.35) | 5 (2.51) | 6 (2.49) | |||
| Other/unknown | 16 (3.05) | 2 (2.35) | 6 (3.02) | 8 (3.32) | |||
| Primary payor | .285 | .719 | .546 | ||||
| Private insurance | 299 (56.95) | 51 (60) | 115 (57.79) | 133 (55.19) | |||
| Medicare, Medicaid, other govt | 196 (37.33) | 32 (37.65) | 74 (37.19) | 90 (37.34) | |||
| Not insured | 20 (3.81) | 1 (1.18) | 7 (3.52) | 12 (4.98) | |||
| Unknown | 10 (1.9) | 1 (1.18) | 3 (1.51) | 6 (2.49) | |||
| Facility type | .915 | .719 | .972 | ||||
| Comprehensive Community Cancer Program | 16 (3.05) | 3 (3.53) | 8 (4.02) | 5 (2.07) | |||
| Academic/research program | 89 (16.95) | 16 (18.82) | 36 (18.09) | 37 (15.35) | |||
| Integrated Network Cancer Program | 247 (47.05) | 44 (51.76) | 104 (52.26) | 99 (41.08) | |||
| Missing | 173 (32.95) | 22 (25.88) | 51 (25.63) | 100 (41.49) | |||
| Charlson-Deyo score | .145 | .16 | .967 | ||||
| 0 | 429 (81.71) | 72 (84.71) | 163 (81.91) | 194 (80.5) | |||
| 1 | 77 (14.67) | 13 (15.29) | 32 (16.08) | 32 (13.28) | |||
| 2 | 11 (2.1) | 0 (0) | 2 (1.01) | 9 (3.73) | |||
| 3 | 8 (1.52) | 0 (0) | 2 (1.01) | 6 (2.49) | |||
| Primary site, c | <.0001 | <.0001 | .007 | ||||
| Carotid body | 177 (33.71) | 0 (0) | 0 (0) | 177 (73.44) | |||
| Intracranial | 152 (28.95) | 53 (62.35) | 90 (45.23) | 9 (3.73) | |||
| Cranial nerves d | 134 (25.52) | 24 (28.24) | 98 (49.25) | 12 (4.98) | |||
| Skin, connective tissue, lymphatics | 17 (3.24) | 0 (0) | 0 (0) | 17 (7.05) | |||
| Pituitary, pineal, other endocrine structures | 15 (2.86) | 5 (5.88) | 7 (3.52) | 3 (1.24) | |||
| Acoustic nerve | 7 (1.33) | 3 (3.53) | 4 (2.01) | 0 (0) | |||
| Peripheral and autonomic nerves | 6 (1.14) | 0 (0) | 0 (0) | 6 (2.49) | |||
| Middle ear | 4 (0.76) | 0 (0) | 0 (0) | 4 (1.66) | |||
| Salivary gland | 4 (0.76) | 0 (0) | 0 (0) | 4 (1.66) | |||
| Nasal cavity, nasopharynx, sinus | 4 (0.76) | 0 (0) | 0 (0) | 4 (1.66) | |||
| Oral cavity | 3 (0.57) | 0 (0) | 0 (0) | 3 (1.24) | |||
| Oropharynx | 2 (0.38) | 0 (0) | 0 (0) | 2 (0.83) | |||
| Tumor size, cm, median (IQR) | 3.5 (2.5) | 3 (2) | 3 (2.35) | 4 (2.5) | .002 | <.0001 | .998 |
| Greatest tumor dimension, cm | <.0001 | <.0001 | .147 | ||||
| <2 | 45 (8.57) | 12 (14.12) | 24 (12.06) | 9 (3.73) | |||
| 2-4 | 149 (28.38) | 29 (34.12) | 49 (24.62) | 71 (29.46) | |||
| 4-8 | 122 (23.24) | 11 (12.94) | 39 (19.6) | 72 (29.88) | |||
| ≥8 | 21 (4) | 4 (4.71) | 3 (1.51) | 14 (5.81) | |||
| Missing/unknown | 188 (35.81) | 29 (34.12) | 84 (42.21) | 75 (31.12) | |||
| Regional lymph node status | |||||||
| Positive | 104 (19.81) | 0 (0) | 0 (0) | 104 (43.15) | |||
| Negative | 25 (4.76) | 0 (0) | 0 (0) | 25 (10.37) | |||
| No lymphadenectomy | 396 (75.43) | 85 (100) | 199 (100) | 112 (46.47) | |||
| Distant metastasis status | <.0001 | <.0001 | .492 | ||||
| Negative | 407 (77.52) | 78 (91.76) | 165 (82.91) | 164 (68.05) | |||
| Positive | 37 (7.05) | 0 (0) | 1 (0.5) | 36 (14.94) | |||
| Missing/unknown | 81 (15.43) | 7 (8.24) | 33 (16.58) | 41 (17.01) | |||
| Overall treatment | <.0001 | <.0001 | .206 | ||||
| Surgery only | 251 (47.81) | 39 (45.88) | 110 (55.28) | 102 (42.32) | |||
| Radiation only | 94 (17.9) | 22 (25.88) | 42 (21.11) | 30 (12.45) | |||
| Chemotherapy only | 5 (0.95) | 0 (0) | 0 (0) | 5 (2.07) | |||
| Surgery and radiation | 86 (16.38) | 5 (5.88) | 18 (9.05) | 63 (26.14) | |||
| Surgery and chemotherapy | 3 (0.57) | 0 (0) | 0 (0) | 3 (1.24) | |||
| Radiation and chemotherapy | 7 (1.33) | 0 (0) | 0 (0) | 7 (2.9) | |||
| Surgery, radiation, and chemotherapy | 6 (1.14) | 0 (0) | 0 (0) | 6 (2.49) | |||
| Active surveillance, no treatment | 64 (12.19) | 17 (20) | 25 (12.56) | 22 (9.13) | |||
| Unknown/missing | 9 (1.71) | 2 (2.35) | 4 (2.01) | 3 (1.24) | |||
| Margin status | .246 | .613 | .4 | ||||
| Negative | 69 (13.14) | 3 (3.53) | 1 (0.5) | 65 (26.97) | |||
| Positive | 63 (12) | 0 (0) | 2 (1.01) | 61 (25.31) | |||
| Unknown/missing | 393 (74.86) | 82 (96.47) | 196 (98.49) | 115 (47.72) | |||
| Vital status | .294 | .001 | .098 | ||||
| Dead | 80 (16.81) | 14 (17.07) | 18 (9.89) | 48 (22.64) | |||
| Alive | 396 (83.19) | 68 (82.93) | 164 (90.11) | 164 (77.36) | |||
| Follow-up, mo, median (IQR) | 53.18 (60.91) | 50.86 (71.06) | 58.63 (54.83) | 50.32 (59.3) | .59 | .187 | .763 |
Abbreviations: IQR, interquartile range; NCDB, National Cancer Database.
Values are presented as No. (%) unless noted otherwise.
P < .05 (bold) indicates statistical significance. Missing P values indicate insufficient sample size for statistical comparison.
All sites within head and neck region.
Includes cranial nerves I-VII and IX-XII.
Figure 1.
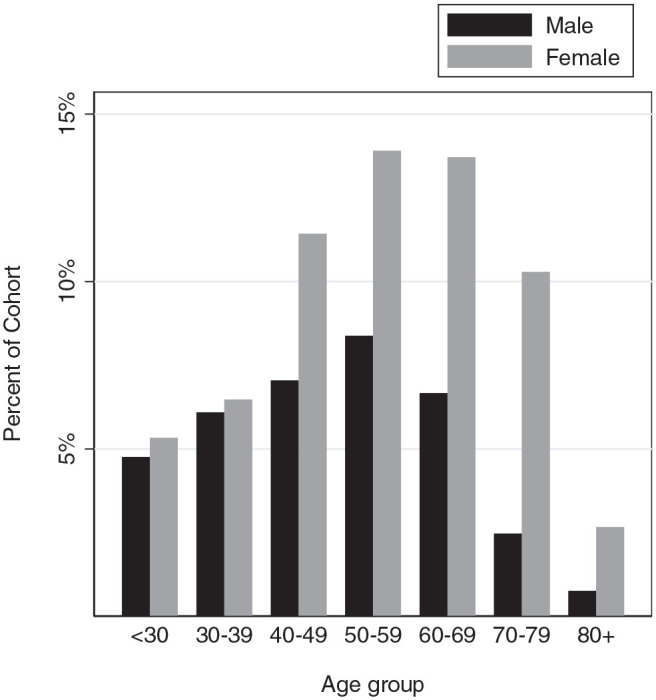
Cohort distribution by age, stratified by sex.
Table 2.
Analysis of Biopsied RLNs for Locally Invasive Head and Neck Paraganglioma.
| Primary site of tumor | Locally invasive (n = 241) | RLN biopsy (n = 129) a | Positive RLN (n = 104) b | Distant metastasis (n = 36) |
|---|---|---|---|---|
| Carotid body (n = 177) | 177 | 115 | 93 | 23 |
| Intracranial (n = 152) | 9 | 0 | 0 | 3 |
| Cranial nerves (n = 134) | 12 | 0 | 0 | 3 |
| Skin, connective tissue, and lymphatics (n = 17) | 17 | 8 | 8 | 3 |
| All other sites (n = 45) | 26 | 6 | 3 | 4 |
Abbreviation: RLN, regional lymph node.
Patients with ≥1 RLNs examined by pathologist during the first course of treatment.
Patients with ≥1 positive RLNs confirmed by pathologist during the first course of treatment.
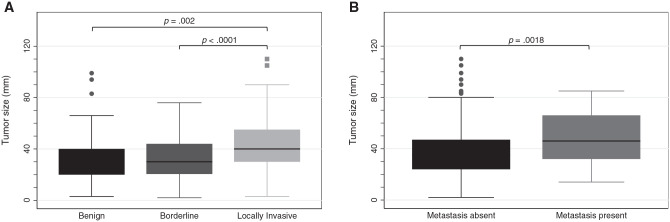
HNP most commonly involved the carotid body (33.7%), intracranial regions (29.0%), or cranial nerves (25.5%). Carotid body tumors were exclusively invasive, while intracranial and cranial nerve tumors were almost entirely benign or borderline ( Table 1 ). Benign and borderline tumors were significantly smaller than invasive tumors (median size, 3 vs 4 cm, respectively; Table 1 ). Tumors with distant metastasis were significantly larger than benign tumors ( Figure 2B ).
Figure 2.
Tumor size stratified by (A) tumor behavior and (B) presence or absence of distant metastasis. Mean comparison with t test. Line, median; box, interquartile range; error bars, 95% CI; circles/squares, outliers.
Factors Associated With OS
The median follow-up time was 53.18 months (range, 0.03-166) and did not significantly differ by tumor behavior ( Table 1 ). In total, 83%, 90%, and 77% of patients with benign, borderline, and locally invasive tumors, respectively, were alive at final follow-up. In the entire cohort, the multivariable Cox model identified age, insurance status, CCI score, and tumor behavior to be independently associated with survival. Patients with borderline HNP had a 3-fold improvement in survival (aHR, 0.36; P = .001) when compared with patients with locally invasive HNP (Supplemental Table S1, available online).
Univariate analysis of the cohort stratified by local tumor behavior revealed that, for each behavior group, unique sets of subvariables were associated with OS ( Table 3 ). Multivariate models for OS were then created with a forward stepwise procedure to select from variables evaluated in the univariate analysis. In patients with benign tumors, age, race, CCI score, and treatment modality were independently associated with survival. Single-modality surgery was independently associated with the lowest risk of death when compared with surgery with adjuvant radiotherapy (aHR, 40.45; P = .006) or active surveillance (aHR, 24.23; P = .008; Table 4 ). In patients with borderline tumors, patients with Medicare/Medicaid insurance had worse survival (aHR, 4.76; P = .005) than patients with private insurance. In patients with locally invasive tumors, age, primary site, treatment modality, and margins were independently associated with survival. As compared with single-modality surgery, treatment with surgery plus adjuvant radiotherapy was associated with a 3-fold improvement in survival (aHR, 0.31; P = .027). Positive surgical margins were also associated with worse survival (aHR, 4.13; P = .01).
Table 3.
Univariate Factors Associated With Overall Survival in Head and Neck Paraganglioma. a
| Benign (n = 82) | Borderline (n = 182) | Locally invasive (n = 212) | ||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| Age, y | 1.05 (1.01-1.09) | .022 | 1.04 (1-1.08) | .047 | 1.06 (1.04-1.08) | <.0001 |
| Sex | ||||||
| Male | 1.00 | 1.00 | 1.00 | |||
| Female | 1.95 (0.54-7.03) | .305 | 0.86 (0.3-2.42) | .772 | 1.18 (0.66-2.12) | .576 |
| Race | ||||||
| White | 1.00 | 1.00 | 1.00 | |||
| Black | 2.27 (0.72-7.18) | .162 | 0.6 (0.13-2.65) | .497 | 1.56 (0.72-3.38) | .259 |
| Hispanic | 2.96 (0.61-14.32) | .178 | 3.68 (0.83-16.37) | .087 | 0.58 (0.18-1.9) | .373 |
| Asian/Pacific Islander | 1.47 (0.19-11.35) | .709 | 1.71 (0.23-12.62) | .601 | ||
| Other/unknown | 1.34 (0.32-5.6) | .688 | ||||
| Primary payor | ||||||
| Private insurance | 1.00 | 1.00 | 1.00 | |||
| Medicare, Medicaid, other govt | 2.35 (0.81-6.77) | .115 | 3.66 (1.37-9.77) | .010 | 3.67 (1.99-6.77) | <.0001 |
| Not insured | 0.92 (0.21-4.01) | .910 | ||||
| Facility type | ||||||
| Comprehensive Community Cancer Program | 1.00 | 1.00 | 1.00 | |||
| Academic/research program | 0.42 (0.04-4.64) | .477 | 0.53 (0.1-2.89) | .462 | 0.52 (0.16-1.72) | .284 |
| Integrated Network Cancer Program | 0.52 (0.06-4.22) | .541 | 0.33 (0.07-1.58) | .163 | 0.48 (0.16-1.39) | .175 |
| Charlson-Deyo score | ||||||
| 0 | 1.00 | 1.00 | 1.00 | |||
| 1 | 2.25 (0.7-7.21) | .172 | 2.26 (0.78-6.52) | .131 | 1.44 (0.69-3.01) | .333 |
| 2 | 29.63 (6.16-142.6) | <.0001 | 1.74 (0.42-7.24) | .448 | ||
| 3 | 3.67 (1.12-12.05) | .032 | ||||
| Primary site | ||||||
| Carotid body | 1.00 | |||||
| Intracranial | 1.00 | 1.00 | 1.47 (0.45-4.83) | .525 | ||
| Cranial nerves b | 0.67 (0.19-2.41) | .539 | 0.94 (0.37-2.4) | .903 | 0.75 (0.18-3.14) | .694 |
| Skin, connective tissue, lymphatics | 1.36 (0.53-3.51) | .527 | ||||
| Pituitary, pineal, other endocrine | 6.09 (1.84-20.11) | .003 | ||||
| Peripheral and autonomic nerves | 1.74 (0.23-12.88) | .589 | ||||
| Middle ear | 1.39 (0.19-10.34) | .751 | ||||
| Nasal cavity, nasopharynx, sinus | 4.47 (1.06-18.97) | .042 | ||||
| Oral cavity | 2.03 (0.27-14.99) | .489 | ||||
| Regional lymph node status | ||||||
| Negative | 1.00 | |||||
| Positive | 0.57 (0.13-2.45) | .452 | ||||
| Distant metastasis status | ||||||
| Negative | 1.00 | |||||
| Positive | 3.55 (1.8-6.99) | <.0001 | ||||
| Overall treatment | ||||||
| Surgery only | 1.00 | 1.00 | 1.00 | |||
| XRT only | 11.11 (1.33-92.52) | .026 | 1.15 (0.31-4.25) | .838 | 1.97 (0.88-4.41) | .098 |
| Chemotherapy only | 2.6 (0.59-11.34) | .204 | ||||
| Surgery and XRT | 25.66 (2.28-288.31) | .009 | 1.45 (0.39-5.36) | .579 | 0.37 (0.15-0.92) | .032 |
| Surgery and chemotherapy | 1.65 (0.22-12.45) | .626 | ||||
| Radiation and chemotherapy | 8.83 (2.51-31.04) | .001 | ||||
| Active surveillance/no treatment | 20.46 (2.33-179.53) | .006 | 1.59 (0.43-5.87) | .490 | 2.45 (0.97-6.18) | .058 |
| Margins | ||||||
| Negative | 1.00 | |||||
| Positive | 1.8 (0.71-4.58) | .216 | ||||
Abbreviations: HR, hazard ratio; XRT, radiation.
Missing HRs indicate insufficient sample size for Cox proportional hazards model. P < .05 (bold) indicates statistical significance.
Includes cranial nerves I-VII and IX-XII.
Table 4.
Multivariate Factors Associated With Overall Survival in Head and Neck Paraganglioma. a
| Benign (n = 82) | Borderline (n = 182) | Locally invasive (n = 212) | ||||
|---|---|---|---|---|---|---|
| Variable | aHR (95% CI) | P value | aHR (95% CI) | P value | aHR (95% CI) | P value |
| Age | 1.09 (1.03-1.16) | .004 | 1.07 (1.04-1.1) | <.0001 | ||
| Race | ||||||
| White | ||||||
| Black | 6.04 (1.4-26.11) | .016 | ||||
| Hispanic | 21.23 (2.18-207.06) | .009 | ||||
| Asian/Pacific Islander | ||||||
| Other/unknown | ||||||
| Primary payor | ||||||
| Private insurance | 1.00 | |||||
| Medicare, Medicaid, other govt | 4.76 (1.61-14.07) | .005 | ||||
| Not insured | ||||||
| Charlson-Deyo score | ||||||
| 0 | 1.00 | |||||
| 1 | 4.63 (1.11-19.31) | .035 | 1.68 (0.57-4.91) | .346 | ||
| 2 | 55.17 (9.96-305.64) | <.0001 | ||||
| 3 | ||||||
| Primary site | ||||||
| Carotid body | 1.00 | |||||
| Intracranial | 0.71 (0.14-3.6) | .684 | ||||
| Cranial nerves b | 1.28 (0.24-6.65) | .772 | ||||
| Skin, connective tissue, lymphatics | 2.44 (0.83-7.14) | .105 | ||||
| Pituitary, pineal, other endocrine | 26.13 (4.66-146.42) | <.0001 | ||||
| Peripheral and autonomic nerves | 1.32 (0.16-11.04) | .798 | ||||
| Middle ear | 4.33 (0.51-36.49) | .177 | ||||
| Nasal cavity, nasopharynx, sinus | 6.34 (1.3-30.94) | .022 | ||||
| Oral cavity | 0.5 (0.06-3.88) | .506 | ||||
| Distant metastasis status | ||||||
| Negative | 1.00 | |||||
| Positive | 2.1 (0.93-4.76) | .075 | ||||
| Overall treatment | ||||||
| Surgery only | 1.00 | 1.00 | ||||
| Radiation only | 6.99 (0.67-73.43) | .105 | 1.2 (0.36-3.97) | .769 | ||
| Chemotherapy only | 0.55 (0.06-4.96) | .592 | ||||
| Surgery and XRT | 40.45 (2.94-556.66) | .006 | 0.31 (0.11-0.88) | .027 | ||
| Surgery and chemotherapy | 5.17 (0.47-57.01) | .180 | ||||
| XRT and chemotherapy | 4.08 (0.84-19.87) | .082 | ||||
| Active surveillance/no treatment | 24.23 (2.3-255.72) | .008 | 2.33 (0.63-8.6) | .206 | ||
| Margins | ||||||
| Negative | 1.00 | |||||
| Positive | 4.13 (1.41-12.05) | .010 | ||||
Abbreviations: aHR, adjusted hazard ratio; XRT, radiation.
Variables eligible for inclusion in multivariate model through forward stepwise selection: age, sex, race, primary payor, Charlson-Deyo score, primary site, greatest tumor dimension, regional lymph node status, distant metastasis, overall treatment, and margins. Missing values indicate variables not included in model or insufficient sample size for Cox proportional hazards model. Overall model: P < .0001. P < .05 (bold) indicates statistical significance.
Includes cranial nerves I-VII and IX-XII.
Trends in Treatment
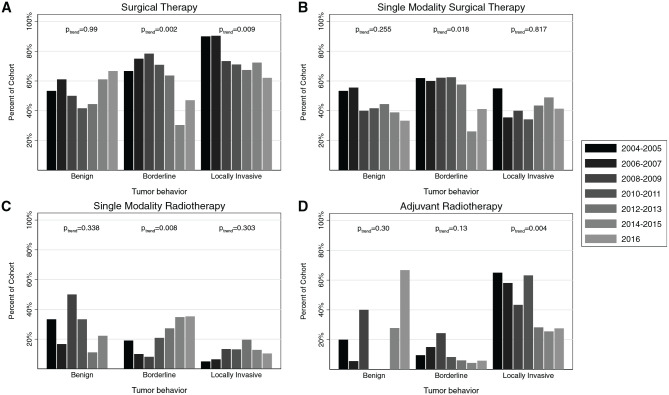
Trends in treatment strategies from 2004 to 2016 demonstrated that surgical management with or without additional therapy declined for borderline HNP (trend P = .002) and locally invasive HNP (trend P = .009; Figure 3A ). Single-modality surgical therapy also declined for borderline disease (trend P = .018; Figure 3B ), while single-modality radiotherapy increased in borderline HNP (trend P = .008; Figure 3C ). Adjuvant radiotherapy declined over time in locally invasive HNP (trend P = .004; Figure 3D ).
Figure 3.
Trends in treatment for (A) surgical therapy, (B) single-modality surgical therapy, (C) single-modality radiotherapy, and (D) adjuvant radiotherapy from 2004 to 2016.
Discussion
The purpose of this study was to explore the prognostic value of local tumor behavior in the management of HNP. In the unstratified cohort, we found that ICD-O-3 classification of tumor behavior was independently associated with survival and evenly distributed within the cohort and exhibited a plausible biological association with tumor outcomes. Further analysis revealed that locally invasive tumors pose a higher risk to patients and are optimally treated with more aggressive therapies. While the lack of a standardized tumor behavior classification system has limited the findings in the current study, the clinically meaningful differences that we identified among behavior groups highlight a promising avenue through which future investigations can develop a more robust risk-stratification system for HNP.
Given that previous studies failed to identify histopathologic and radiologic features associated with malignancy,30-34 this study sought to ask whether local tumor behavior can be correlated with survival outcomes. With malignancy rates around 10%, it is very difficult to power a study to identify differences between benign and malignant disease, especially for such a rare tumor entity. We attempted to mitigate the low statistical power by using a large national database and by focusing on a more prevalent tumor feature. Local tumor behavior can also be readily evaluated via histopathology and radiology. Although histopathology cannot be used to predict malignancy for HNP,8,35,36 it is still useful when evaluating local invasion.33,34 Similarly, HNP can be characterized on imaging, as they can be readily identified by their “salt and pepper” appearance.33,37 Several radiologic investigations have demonstrated that positron emission tomography/computed tomography, both DOTATATE and F-18 fluorodeoxyglucose, can detect HNP, identify metastatic spread, and characterize invasion into adjacent structures.38-40 While it is difficult to define strict behavior classification criteria through our current analysis, our data emphasize that there is a spectrum of risk associated with HNP that can be meaningfully stratified through a system of histopathologic and radiologically defined local tumor behavior.
We found that 43% of patients with locally invasive HNP had metastatic spread to RLNs. Interestingly, this regional metastasis was not associated with worse survival for locally invasive tumors (P = .452). Javidiparsijani et al identified a surprisingly high rate of RLN metastasis but noted that these tumors had a favorable short-term clinical course. The authors concluded that HNP metastasis to RLNs may have indolent clinical behavior with disease-free survival of up to 11 years. 41 Upon closer inspection of our cohort, we see that surgical treatment of HNP typically did not involve lymphadenectomy (75.4%; Table 1 ). When it was performed, half of patients (49.6%) had ≤5 lymph nodes examined (Supplemental Table S2, available online), which may not be sensitive enough to identify regional spread in all individuals. The inadequacy in lymph node sampling is particularly evident when we consider that among patients with distant metastatic spread, 16.7% had negative-sampled RLNs and 66.7% did not undergo lymphadenectomy (Supplemental Table S3). Therefore, there may be a high degree of unmeasured regional spread that is masking the association between regional metastasis and survival. Nonetheless, the high prevalence of regional metastasis among individuals with locally invasive tumors emphasizes the increased risk that these patients may face.
For locally invasive tumors, surgical resection with adjuvant radiation was independently associated with survival when compared with surgical resection alone, while controlling for age, primary site of tumor, distant metastasis, and positive surgical margins. Radiation is often employed if initial surgical resection results in inadequate disease control. 42 Adjuvant radiation may have provided therapeutic benefit for locally invasive HNP due to their high concordance with regional metastasis. More robust lymph node sampling may provide a clearer indication for which patients would benefit from adjuvant therapy, as it does for other head and neck malignancies.
For benign HNP, single-modality surgical excision was associated with 40- and 24-fold improved survival relative to surgery with adjuvant radiotherapy and active surveillance, respectively. This finding could be an artifact of sample size or may represent unmeasured differences in baseline disease characteristics among treatment groups. Nonetheless, we suspect that these findings represent a tradeoff between treatment and disease-related morbidity. One study indicated that while combined surgery and radiotherapy provided better local control, they led to higher rates of complications when compared with surgery alone. 43 With regard to active surveillance, a study of 43 patients revealed that 42% of tumors remained stable and 38% grew larger over 5 years. 44 A separate report noted that 30% of patients developed new cranial nerve deficits secondary to tumor progression over 3 years of conservative management. 45 Due to the limited information available in the NCDB, we were unable to determine treatment-related morbidity and its relationship with mortality. Our findings imply that the optimal management of benign HNP falls between the spectrum of active surveillance and multimodal therapy. Furthermore, in contrast to locally invasive tumors that benefit from multimodal therapy, benign HNP are associated with improved survival and therefore can tolerate some degree of therapeutic de-escalation.
Consistent with previous literature, we identified the carotid body as the most common anatomic site for HNP.24,41,46 The next-most common HNP sites were found intracranially. Although head and neck surgeons do not typically manage intracranial tumors, the majority of the intracranial HNP identified in the literature and in this study occurred along the skull base and therefore have relevance to otolaryngologists’ scope of practice. 47 All 177 carotid body tumors were exclusively coded as locally invasive in behavior, with 13% metastasizing to distant sites. In contrast, intracranial and cranial nerve HNP were more likely to be coded as benign or borderline, with just 2.5% metastasizing to distant sites. Based on the results from the current study, anatomic site may influence a clinician’s pretest probability of severe disease; however, anatomic site alone may not be sufficient to predict clinical course given that it is not associated with survival.
Similar to previous studies,8,48,49 we found that HNP were more common in women and increased with each decade above age 40 years. In addition, patients with locally invasive tumors were significantly younger than those with benign HNP (49 vs 56 years; Table 1 ). Similarly, 1 study stated that among 223 patients with pathologically confirmed paraganglioma, the age at presentation for malignant paraganglioma was significantly younger than that for benign paraganglioma (43 vs 49 years). 50 This finding may be driven by an earlier onset of clinical symptoms by more aggressive tumors or by the fact that hereditary cases tend to present earlier in life. 31
For borderline HNP, Medicare/Medicaid insurance was associated with a nearly 5-fold decrease in OS vs private insurance. This observation has been noted in several other head and neck cancer studies. 51 Patients with Medicare/Medicaid are more likely to present with more advanced tumors52,53 and seek care at nonteaching or rural medical centers.53,54 A separate study revealed that Medicare Advantage health plans, held by one-third of all Medicare beneficiaries nationally, limit utilization of specialists and access to high-volume centers. 55 The disparities presented in these studies may play an even larger role in a rare disease entity such as HNP, as reflected in our findings.
This investigation is not without limitations. In generating a broad risk-stratification model with a cohort with tremendous heterogeneity, there is a risk that the findings will not be generalizable to specific HNP sites. Inclusion of the primary tumor site in the multivariate analysis for locally invasive tumors, however, does suggest that HNP pathology related to local tumor behavior may be similar across anatomic sites. Next, our use of the NCDB relies on accurate ICD-O-3 coding by physicians, cancer registrars, and other health care staff and is susceptible to misclassification bias. Moreover, without a standardized ICD-O-3 behavior classification system, it is possible that the behavior groups analyzed in this study are not mutually exclusive. The retrospective design of the study also limited the conclusions that we could draw from this survival analysis. Without clinical data from follow-up appointments, it is difficult to determine whether mortality in patients is due to HNP or unrelated comorbidities. Finally, the treatment facility types identified in this study indicate that the patient cohort may be skewed toward representation of large academic centers as opposed to smaller community hospitals.
Conclusion
In summary, we have presented evidence that HNP local behavior can meaningfully risk-stratify cases. We found that, as compared with benign tumors, locally invasive tumors are associated with diminished survival, presumably due to higher rates of regional and distant metastasis. We suspect that this greater risk underlies the observed survival benefit of surgery with adjuvant radiotherapy for locally invasive tumors. While the current study fell short of defining criteria for tumor behavior groups, we highlighted the clinical utility of such a classification system and presented a framework through which future histopathologic and radiologic studies can standardize the measurement of this clinical feature. Ultimately, improved understanding of HNP behavioral phenotypes will aid in the development of more consistent and efficacious therapeutic guidelines.
Supplemental Material
Supplemental material, sj-docx-1-opn-10.1177_2473974X221086872 for Local Tumor Behavior Associated With Survival Among Patients With Paraganglioma of the Head and Neck by Randall J. Harley, Jason H. Lee, Benjamin T. Ostrander, Andrey Finegersh, Tammy B. Pham, Kareem O. Tawfik, Yin Ren, Farhoud Faraji and Rick A. Friedman in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation
Supplemental material, sj-docx-2-opn-10.1177_2473974X221086872 for Local Tumor Behavior Associated With Survival Among Patients With Paraganglioma of the Head and Neck by Randall J. Harley, Jason H. Lee, Benjamin T. Ostrander, Andrey Finegersh, Tammy B. Pham, Kareem O. Tawfik, Yin Ren, Farhoud Faraji and Rick A. Friedman in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation
Supplemental material, sj-docx-3-opn-10.1177_2473974X221086872 for Local Tumor Behavior Associated With Survival Among Patients With Paraganglioma of the Head and Neck by Randall J. Harley, Jason H. Lee, Benjamin T. Ostrander, Andrey Finegersh, Tammy B. Pham, Kareem O. Tawfik, Yin Ren, Farhoud Faraji and Rick A. Friedman in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation
Footnotes
Precis: This study evaluated head and neck paraganglioma in the US National Cancer Database from 2004 to 2016. ICD-O-3 tumor behavior codes can be used to stratify predictors of head and neck paraganglioma outcomes.
Author Contributions: Randall J. Harley, writing–original draft, investigation, methodology, data curation, formal analysis, visualization, writing–review and editing; Jason H. Lee, investigation, methodology, writing–review and editing; Benjamin T. Ostrander, investigation, methodology, writing–review and editing; Andrey Finegersh, writing–review and editing; Tammy B. Pham, writing–review and editing; Kareem O. Tawfik, methodology, project administration, supervision, validation, writing–review and editing; Yin Ren, methodology, project administration, supervision, validation, writing–review and editing; Farhoud Faraji, conceptualization, investigation, methodology, project administration, supervision, validation, writing–review and editing; Rick A. Friedman, conceptualization, investigation, methodology, project administration, resources, supervision, validation, writing–review and editing.
Disclosures: Competing interests: None.
Sponsorships: None.
Funding source: None.
ORCID iDs: Randall J. Harley  https://orcid.org/0000-0002-8519-5366
https://orcid.org/0000-0002-8519-5366
Farhoud Faraji  https://orcid.org/0000-0001-5078-813X
https://orcid.org/0000-0001-5078-813X
Supplemental Material: Additional supporting information is available at http://journals.sagepub.com/doi/suppl/10.1177/2473974X181086872
References
- 1. La Perle KMD, Dintzis SM. Endocrine system. In: Treuting PM, Dintzis SM, Montine KS, eds. Comparative Anatomy and Histology: A Mouse, Rat, and Human Atlas. 2nd ed. Academic Press; 2018:251-273. [Google Scholar]
- 2. Contrera KJ, Yong V, Reddy CA, Berber E, Lorenz RR. Second primary tumors in patients with a head and neck paraganglioma. Head Neck. 2019;41(9):3356-3361. doi: 10.1002/hed.25849 [DOI] [PubMed] [Google Scholar]
- 3. Neumann HPH, Young WF, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. doi: 10.1056/nejmra1806651 [DOI] [PubMed] [Google Scholar]
- 4. Ikram A, Rehman A. Paraganglioma. In: StatPearls. StatPearls Publishing; 2021. Updated January 6, 2021. https://www.ncbi.nlm.nih.gov/books/NBK549834/ [Google Scholar]
- 5. Woolen S, Gemmete JJ. Paragangliomas of the head and neck. Neuroimaging Clin N Am. 2016;26(2):259-278. doi: 10.1016/j.nic.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 6. Eisenhofer G, Tischler AS, de Krijger RR. Diagnostic tests and biomarkers for pheochromocytoma and extra-adrenal paraganglioma: from routine laboratory methods to disease stratification. Endocr Pathol. 2012;23(1):4-14. doi: 10.1007/s12022-011-9188-1 [DOI] [PubMed] [Google Scholar]
- 7. Bikhazi PH, Messina L, Mhatre AN, Goldstein JA, Lalwani AK. Molecular pathogenesis in sporadic head and neck paraganglioma. Laryngoscope. 2000;110(8):1346-1348. doi: 10.1097/00005537-200008000-00023 [DOI] [PubMed] [Google Scholar]
- 8. Lee JH, Barich F, Karnell LH, et al. National Cancer Data Base report on malignant paragangliomas of the head and neck. Cancer. 2002;94(3):730-737. doi: 10.1002/cncr.10252 [DOI] [PubMed] [Google Scholar]
- 9. Jansen TTG, Timmers HJLM, Marres HAM, Kaanders JHAM, Kunst HPM. Results of a systematic literature review of treatment modalities for jugulotympanic paraganglioma, stratified per Fisch class. Clin Otolaryngol. 2018;43(2):652-661. doi: 10.1111/coa.13046 [DOI] [PubMed] [Google Scholar]
- 10. Carlson ML, Sweeney AD, Pelosi S, Wanna GB, Glasscock ME, Haynes DS. Glomus tympanicum: a review of 115 cases over 4 decades. Otolaryngol Head Neck Surg. 2015;152(1):136-142. doi: 10.1177/0194599814555849 [DOI] [PubMed] [Google Scholar]
- 11. Gu G, Wu X, Ji L, et al. Proposed modification to the Shamblin’s classification of carotid body tumors: a single-center retrospective experience of 116 tumors. Eur J Surg Oncol. 2021;47(8):1953-1960. doi: 10.1016/j.ejso.2021.03.244 [DOI] [PubMed] [Google Scholar]
- 12. Offergeld C, Brase C, Yaremchuk S, et al. Head and neck paragangliomas: clinical and molecular genetic classification. Clinics (Sao Paulo). 2012;67(suppl 1):19-28. doi: 10.6061/clinics/2012(sup01)05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boedeker CC. Paragangliomas and paraganglioma syndromes. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2011;10:Doc03. doi: 10.3205/cto000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Contrera KJ, Yong V, Reddy CA, Liu SW, Lorenz RR. Recurrence and progression of head and neck paragangliomas after treatment. Otolaryngol Head Neck Surg. 2020;162(4):504-511. doi: 10.1177/0194599820902702 [DOI] [PubMed] [Google Scholar]
- 15. Ellis RJ, Patel D, Prodanov T, Nilubol N, Pacak K, Kebebew E. The presence of SDHB mutations should modify surgical indications for carotid body paragangliomas. Ann Surg. 2014;260(1):158-162. doi: 10.1097/sla.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amar L, Servais A, Gimenez-Roqueplo A-P, Zinzindohoue F, Chatellier G, Plouin P-F. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90(4):2110-2116. doi: 10.1210/jc.2004-1398 [DOI] [PubMed] [Google Scholar]
- 17. Mediouni A, Ammari S, Wassef M, et al. Malignant head/neck paragangliomas: comparative study. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(3):159-166. doi: 10.1016/j.anorl.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 18. Lee H, Jeong S, Yu Y, et al. Risk of metastatic pheochromocytoma and paraganglioma in SDHx mutation carriers: a systematic review and updated meta-analysis. J Med Genet. 2020;57(4):217-225. doi: 10.1136/jmedgenet-2019-106324 [DOI] [PubMed] [Google Scholar]
- 19. Singh S, Madan R, Singh MK, Thakar A, Sharma SC. Head-and-neck paragangliomas: an overview of 54 cases operated at a tertiary care center. South Asian J Cancer. 2019;8(4):237-240. doi: 10.4103/sajc.sajc_339_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Offergeld C, Brase C, Yaremchuk S, et al. Head and neck paragangliomas: clinical and molecular genetic classification. Clinics (Sao Paulo). 2012;67(suppl 1):19-28. doi: 10.6061/clinics/2012(sup01)05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suárez C, Rodrigo JP, Bödeker CC, et al. Jugular and vagal paragangliomas: systematic study of management with surgery and radiotherapy. Head Neck. 2013;35(8):1195-1204. doi: 10.1002/hed.22976 [DOI] [PubMed] [Google Scholar]
- 22. Kang KH, Lebow ES, Niemierko A, et al. Proton therapy for head and neck paragangliomas: a single institutional experience. Head Neck. Published online December 18, 2019. doi: 10.1002/hed.26044 [DOI] [PubMed] [Google Scholar]
- 23. Huy PT, Kania R, Duet M, Dessard-Diana B, Mazeron JJ, Benhamed R. Evolving concepts in the management of jugular paraganglioma: a comparison of radiotherapy and surgery in 88 cases. Skull Base. 2009;19(1):83-91. doi: 10.1055/s-0028-1103125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moore MG, Netterville JL, Mendenhall WM, Isaacson B, Nussenbaum B. Head and neck paragangliomas: an update on evaluation and management. Otolaryngol Head Neck Surg. 2016;154(4):597-605. doi: 10.1177/0194599815627667 [DOI] [PubMed] [Google Scholar]
- 25. American College of Surgeons Cancer Programs. National Cancer Data Base. Accessed September 9, 2019. http://www.facs.org/cancer/ncdb/index.html
- 26. Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683-690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dimakakos PB, Kotsis TE. Carotid body paraganglioma: review and surgical management. Euro J Plastic Surg. 2001;24(2):58-65. doi: 10.1007/s002380100231 [DOI] [Google Scholar]
- 28. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 29. University of Manitoba. Concept: Charlson Comorbidity Index. Accessed September 17, 2019. http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?conceptID=1098
- 30. Wang B, Qiu J. Progress in the diagnosis and treatment of paraganglioma. Transl Cancer Res. 2019;8(7):2624-2635. doi: 10.21037/tcr.2019.10.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williams MD, Tischler AS. Update from the 4th edition of the World Health Organization classification of head and neck tumours: paragangliomas. Head Neck Pathol. 2017;11(1):88-95. doi: 10.1007/s12105-017-0786-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ilona Linnoila R, Keiser HR, Steinberg SM, Lack EE. Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Hum Pathol. 1990;21(11):1168-1180. doi: 10.1016/0046-8177(90)90155-x [DOI] [PubMed] [Google Scholar]
- 33. Chapman DB, Lippert D, Geer CP, et al. Clinical, histopathologic, and radiographic indicators of malignancy in head and neck paragangliomas. Otolaryngol Head Neck Surg. 2010;143(4):531-537. doi: 10.1016/j.otohns.2010.05.031 [DOI] [PubMed] [Google Scholar]
- 34. Thompson LDR, Gill AJ, Asa SL, et al. Data set for the reporting of pheochromocytoma and paraganglioma: explanations and recommendations of the guidelines from the International Collaboration on Cancer Reporting. Hum Pathol. Published online May 11, 2020. doi: 10.1016/j.humpath.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier; 2020. [Google Scholar]
- 36. Antúnez Plaza P, Santos-Briz Terrón A, Sáncho de Salas M, Flores Corral T. Anatomía patológica de los paragangliomas cervicocefálicos. Histological characteristics of head and neck paragangliomas. Acta Otorrinolaringol Esp. 2009;60(suppl 1):18-23. [PubMed] [Google Scholar]
- 37. Som P, Curtin H. Head and neck imaging. AJNR Am J Neuroradiol. 2003;24(8):1969-1972. [Google Scholar]
- 38. Chang CA, Pattison DA, Tothill RW, et al. (68)Ga-DOTATATE and (18)F-FDG PET/CT in paraganglioma and pheochromocytoma: utility, patterns and heterogeneity. Cancer Imaging. 2016;16(1):22. doi: 10.1186/s40644-016-0084-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boughdad S, O’Connor A, Cook GJ, et al. FDG PET-CT imaging in head and neck paragangliomas: a centre experience. Clin Endocrinol (Oxf). 2021;95(2):315-322. doi: 10.1111/cen.14446 [DOI] [PubMed] [Google Scholar]
- 40. Kim SH, Roytman M, Kamen E, et al. [68Ga]-DOTATATE PET/MRI in the diagnosis and management of recurrent head and neck paraganglioma with spinal metastasis. Clin Imaging. 2021;79:314-318. doi: 10.1016/j.clinimag.2021.07.028 [DOI] [PubMed] [Google Scholar]
- 41. Javidiparsijani S, Brickman A, Lin DM, et al. Is regional lymph node metastasis of head and neck paraganglioma a sign of aggressive clinical behavior: a clinical/pathologic review. Ear Nose Throat J. Published online September 29, 2019. doi: 10.1177/0145561319863373 [DOI] [PubMed] [Google Scholar]
- 42. Hu K, Persky MS. Treatment of head and neck paragangliomas. Cancer Control. 2016;23(3):228-241. doi: 10.1177/107327481602300306 [DOI] [PubMed] [Google Scholar]
- 43. Jansen TTG, Kaanders JHAM, Beute GN, Timmers HJLM, Marres HAM, Kunst HPM. Surgery, radiotherapy or a combined modality for jugulotympanic paraganglioma of Fisch class C and D. Clin Otolaryngol. 2018;43(6):1566-1572. doi: 10.1111/coa.13216 [DOI] [PubMed] [Google Scholar]
- 44. Langerman A, Athavale SM, Rangarajan SV, Sinard RJ, Netterville JL. Natural history of cervical paragangliomas: outcomes of observation of 43 patients. Arch Otolaryngol Head Neck Surg. 2012;138(4):341-345. doi: 10.1001/archoto.2012.37 [DOI] [PubMed] [Google Scholar]
- 45. Prasad SC, Mimoune HA, D’Orazio F, et al. The role of wait-and-scan and the efficacy of radiotherapy in the treatment of temporal bone paragangliomas. Otol Neurotol. 2014;35(5):922-931. doi: 10.1097/MAO.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 46. Jennings AW, Preskitt JT, Vallera RD. Extraadrenal pheochromocytoma and vagal paraganglioma. Proc (Bayl Univ Med Cent). 2012;25(2):152-154. doi: 10.1080/08998280.2012.11928813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Al Jishi A, Cenic A, Elgheriani A, Kachur E, Lach B. Primary supratentorial intracerebral malignant paraganglioma. Neuroimmunology and Neuroinflammation. 2015;2(2):121. doi: 10.4103/2347-8659.154431 [DOI] [Google Scholar]
- 48. Smith JD, Harvey RN, Darr OA, et al. Head and neck paragangliomas: a two-decade institutional experience and algorithm for management. Laryngoscope Investig Otolaryngol. 2017;2(6):380-389. doi: 10.1002/lio2.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Letouzé E, Martinelli C, Loriot C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23(6):739-752. doi: 10.1016/j.ccr.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 50. Kim KY, Kim JH, Hong AR, et al. Disentangling of malignancy from benign pheochromocytomas/paragangliomas. PLoS One. 2016;11(12):e0168413. doi: 10.1371/journal.pone.0168413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abt NB, Miller LE, Parikh A, Bhattacharyya N. Insurance status effect on laryngeal cancer survival: a population based study. Ann Otol Rhinol Laryngol. Published online September 4, 2021. doi: 10.1177/00034894211044231 [DOI] [PubMed] [Google Scholar]
- 52. Naghavi AO, Echevarria MI, Grass GD, et al. Having Medicaid insurance negatively impacts outcomes in patients with head and neck malignancies. Cancer. 2016;122(22):3529-3537. doi: 10.1002/cncr.30212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gupta A, Sonis ST, Schneider EB, Villa A. Impact of the insurance type of head and neck cancer patients on their hospitalization utilization patterns. Cancer. 2018;124(4):760-768. doi: 10.1002/cncr.31095 [DOI] [PubMed] [Google Scholar]
- 54. Gourin CG, Stewart CM, Frick KD, et al. Association of hospital volume with laryngectomy outcomes in patients with larynx cancer. JAMA Otolaryngol Head Neck Surg. 2019;145(1):62. doi: 10.1001/jamaoto.2018.2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Raoof M, Jacobson G, Fong Y. Medicare Advantage networks and access to high-volume cancer surgery hospitals. Ann Surg. 2021;274(4):e315-e319. doi: 10.1097/sla.0000000000005098 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-opn-10.1177_2473974X221086872 for Local Tumor Behavior Associated With Survival Among Patients With Paraganglioma of the Head and Neck by Randall J. Harley, Jason H. Lee, Benjamin T. Ostrander, Andrey Finegersh, Tammy B. Pham, Kareem O. Tawfik, Yin Ren, Farhoud Faraji and Rick A. Friedman in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation
Supplemental material, sj-docx-2-opn-10.1177_2473974X221086872 for Local Tumor Behavior Associated With Survival Among Patients With Paraganglioma of the Head and Neck by Randall J. Harley, Jason H. Lee, Benjamin T. Ostrander, Andrey Finegersh, Tammy B. Pham, Kareem O. Tawfik, Yin Ren, Farhoud Faraji and Rick A. Friedman in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation
Supplemental material, sj-docx-3-opn-10.1177_2473974X221086872 for Local Tumor Behavior Associated With Survival Among Patients With Paraganglioma of the Head and Neck by Randall J. Harley, Jason H. Lee, Benjamin T. Ostrander, Andrey Finegersh, Tammy B. Pham, Kareem O. Tawfik, Yin Ren, Farhoud Faraji and Rick A. Friedman in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation