Abstract
Background
Both temporal changes in imaging characteristics of lymphadenopathy on US scans after COVID-19 vaccination and expected duration of radiologically evident lymphadenopathy remain uncertain.
Purpose
To longitudinally evaluate COVID-19 vaccine–associated lymphadenopathy on axillary US scans at various time intervals in both messenger (mRNA) and vector vaccine recipients.
Materials and Methods
This prospective cohort study was conducted between March 2021 and January 2022. The participants were asymptomatic women without breast cancer who had received COVID-19 vaccination. Serial follow-up US was performed in women with lymphadenopathy. The following variables were assessed: cortical thickness, number of lymph nodes, morphologic characteristics, and Doppler signal. Temporal changes in cortical thickness and number of lymph nodes during follow-up were assessed using a linear mixed model.
Results
Ninety-one women with lymphadenopathy in the vaccinated arm had undergone a total of 215 serial US examinations (mean age, 44 years ± 13 [SD]). Fifty-one participants had received a vector vaccine (ChAdOx1 nCoV-19 vaccine) and 40 had received an mRNA vaccine (BNT162b2 vaccine [n = 37] and mRNA-1273 vaccine [n = 3]). Three of the 91 women were lost to follow-up; thus, 88 women underwent serial US. Complete resolution of axillary lymphadenopathy was observed at a median of 6 weeks after vaccination (range, 4–7 weeks) in 26% of women (23 of 88). Among 49 women with follow-up US at a median of 12 weeks after vaccination (range, 8–14 weeks), persistent lymphadenopathy was observed in 25 (51%). During the follow-up period, the cortical thickness gradually decreased (P < .001) over time regardless of vaccine type; however, values were higher in recipients of the mRNA vaccine than in recipients of the vector vaccine (P = .02).
Conclusion
COVID-19 vaccine–associated axillary lymphadenopathy frequently persisted for more than 6 weeks on US scans. Lymphadenopathy should be interpreted considering vaccine type and time elapsed since vaccination. Follow-up US examination at least 12 weeks after vaccination may be reasonable, particularly for recipients of the messenger RNA vaccine.
© RSNA, 2022
Online supplemental material is available for this article.
See also the editorial by Moy and Kim in this issue.
Summary
COVID-19 vaccine–associated lymphadenopathy should be interpreted considering the vaccine type and interval from vaccination; follow-up US examination at least 12 weeks after vaccination may be reasonable, particularly for recipients of the messenger RNA vaccine.
Key Results
■ In a prospective study of 88 healthy women with COVID-19 vaccine–associated lymphadenopathy undergoing serial US, complete resolution of lymphadenopathy was observed at a median of 6 weeks (range, 4–7 weeks) after vaccination in 23 women (26%); of 49 women with follow-up US at a median of 12 weeks (range, 8–14 weeks) after vaccination, persistent lymphadenopathy was observed in 25 (51%).
■ Lymph node cortical thickness exhibited higher values in recipients of the messenger RNA vaccine than in recipients of the vector vaccine (P = .02) and gradually decreased over time (P < .001).
Introduction
Vaccination-associated reactive lymphadenopathy is considered a local adverse reaction to vaccination, more commonly observed after receiving COVID-19 messenger RNA (mRNA) vaccines (1–5). Similar to other vaccines, mRNA vaccines depend on the migration of antigen-presenting cells to the regional lymph nodes to elicit cellular (T-cell) and humoral (B-cell) immune response. Compared with protein-based vaccines, mRNA vaccines elicit more robust and rapid B-cell proliferation in the germinal center of lymph nodes, likely increasing the incidence of lymphadenopathy (6,7). Two representative mRNA COVID-19 vaccines, mRNA-1273 vaccine (Moderna) and BNT162b2 vaccine (Pfizer-BioNTech), induce host immune cells to produce antigens, whereas the attenuated viral vector ChAdOx1 nCoV-19 vaccine (AstraZeneca) induces an immune response mechanism (8). According to the Centers for Disease Control and Prevention, axillary swelling or tenderness has been reported in up to 16% of recipients of the mRNA-1273 vaccine (9).
With increasing COVID-19 vaccine availability and more widely vaccinated patient populations, we anticipate that more patients will demonstrate subclinical lymphadenopathy on images. Several reports have identified COVID-19 vaccine–associated lymphadenopathy with mammography (3% of recipients of COVID-19 vaccine) (4) or PET/CT (1%–45% of recipients of the mRNA vaccine) (10). Imaging findings of axillary lymphadenopathy following COVID-19 vaccination have been reported in up to 15% and 57% of recipients of the BNT162b2 vaccine and mRNA-1273 vaccine, respectively (11). A recent study reported that 44% of patients who had received COVID-19 vaccination and underwent breast imaging had lymphadenopathy with at least one imaging modality—9% of lymphadenopathy was identified with mammography alone, 61% with US alone, and 30% with both examinations (12). However, there is still no information on the temporal changes of these imaging characteristics and the duration for which the enlarged nodes remain asymmetrically detectable, as well as the time to lymphadenopathy resolution on US scans following COVID-19 vaccination (1). There is also a lack of consensus regarding the management recommendations for COVID-19 vaccine–associated lymphadenopathy observed on images, ranging from screening at least 6 weeks after (1,2) or at least 12 weeks after (5,12,13) the second vaccination dose. This uncertainty causes diagnostic confusion for clinicians and radiologists, resulting in unwarranted biopsies and follow-up examinations. Because US is the primary method for axillary lymph node evaluation that can be repeatedly performed without concerns of radiation or contrast material, we hypothesized that serial follow-up axillary US examinations after COVID-19 vaccination would provide more information on the temporal imaging changes and the optimal timeline for resolution.
Therefore, the purpose of our study was to prospectively and longitudinally evaluate COVID-19 vaccine–associated lymphadenopathy at axillary US performed at various time intervals, in both mRNA and vector vaccine recipients.
Materials and Methods
Participants
The institutional review board of the Seoul National University Hospital approved this prospective, observational, longitudinal single-center study, and written informed consent was obtained from all participants. The first COVID-19 vaccination was administered in the Republic of Korea on February 26, 2021, for high-risk groups, which included people aged 65 years or older, persons with disability, and health care workers, and the large-scale vaccination for the general population began in May 2021 (14). Thus, between March 2021 and January 2022, we prospectively recruited healthy recipients of the COVID-19 vaccine aged 18 years or older who were willing to undergo screening of bilateral breasts and axillae after COVID-19 vaccination or who presented with axillary lymphadenopathy detected at screening or follow-up US examinations. All patients were required to have negative or benign findings within 6 months on the breast and axillary US examination. Recruitment was consecutive and was censored when participants showed near or complete resolution of axillary lymphadenopathy at follow-up US examinations. Women were included in this study if they (a) had received COVID-19 vaccination, (b) had no symptoms in both breasts, (c) had no recent diagnosis of breast cancer, and (d) provided voluntary, written informed consent for follow-up examinations. We excluded women with known current malignancy and those who were pregnant or lactating.
Imaging Protocol
One of the two attending breast radiologists (J.M.C. and S.M.H., with 15 and 9 years of experience in breast imaging, respectively) performed axillary US using a 13–5-MHz linear transducer (Arietta 850; Fujifilm Health Care). Details on standard axillary US, including B-mode and color Doppler images, are described in Appendix E1 (online).
Image Analysis
The following criteria were used to identify suspicious lymph nodes: cortical abnormalities, including focal or diffuse thickening greater than 3 mm with partial or complete fatty hilum loss or rounded hypoechoic lymph node, and nonhilar or diffuse flow (15). At the first US examination after vaccination, the radiologists counted the number of lymph nodes and identified the most suspicious lymph node among them. The maximal cortical thickness, morphologic characteristics, and Doppler findings of the most suspicious lymph node were assessed and recorded. We recommended follow-up US at 4–6 weeks from vaccination after the detection of suspicious lymph nodes. An additional US examination at 10–12 weeks from vaccination was recommended for unresolved lymph nodes at the previous follow-up. When the participant requested an earlier or later visit for follow-up examination, we allowed a minimum of 1–2 weeks between the specified follow-up examinations. Thus, despite recommended follow-up examinations at 4–6 weeks and an additional 10–12-week interval for unresolved lymph nodes, variability in time from vaccination persisted. Hence, we calculated the time between follow-up US and vaccination and categorized the scheduled visits accordingly (≤2, >2 and ≤4, >4 and ≤6, >6 and ≤10, >10 and ≤12, and >12 weeks). During the follow-up US examination, the radiologists reviewed the recent preceding US findings to identify the most suspicious lymph node that was detected previously and repeatedly assessed maximal cortical thickness, morphologic characteristics, and Doppler findings of the predefined lymph node and the number of visible lymph nodes to evaluate temporal changes. The same imaging assessment was performed for lymph nodes in the contralateral axilla. The complete resolution of axillary lymphadenopathy was defined as normalization of cortical thickness showing a difference less than 0.5 mm compared with the contralateral side or prevaccination state for previously available US images. Among variable imaging parameters we obtained during different time intervals, the maximal cortical thickness was selected as the main imaging feature for assessment of normalization of lymphadenopathy because it is a quantitative feature that is less subject to observer variability, directly shows the temporal changes of its values, and is easily applicable in clinical practice.
Data Collection
We recorded the age, vaccine type (vector vaccine = ChAdOx1 nCoV-19 vaccine, mRNA vaccine = BNT162b2 vaccine and mRNA-1273 vaccine), time from vaccination (interval between US and vaccination), dose (first or second), injection site (left or right arm), and associated symptoms.
Statistical Analysis
The temporal changes of axillary lymphadenopathy on US images obtained from recipients of vector vaccine and recipients of mRNA vaccine were analyzed. Due to variability in time intervals from vaccination and inconsistency in the number of follow-up examinations from participants, we used a linear mixed model of our repeated measured data acquired from scheduled visits to estimate the interaction between vaccine and time effect, vaccine effect, and time effect on cortical thickness and number of lymph nodes. We used the least squares mean with SE for comparison. Differences between categorical and continuous variables of recipients of vector and mRNA vaccines were compared using the χ2 or Fisher exact test and Student t test, respectively. Statistical analyses were performed using R statistical software (version 4.1.2; The R Foundation). Statistical significance was set at P < .05.
Results
Participant Characteristics
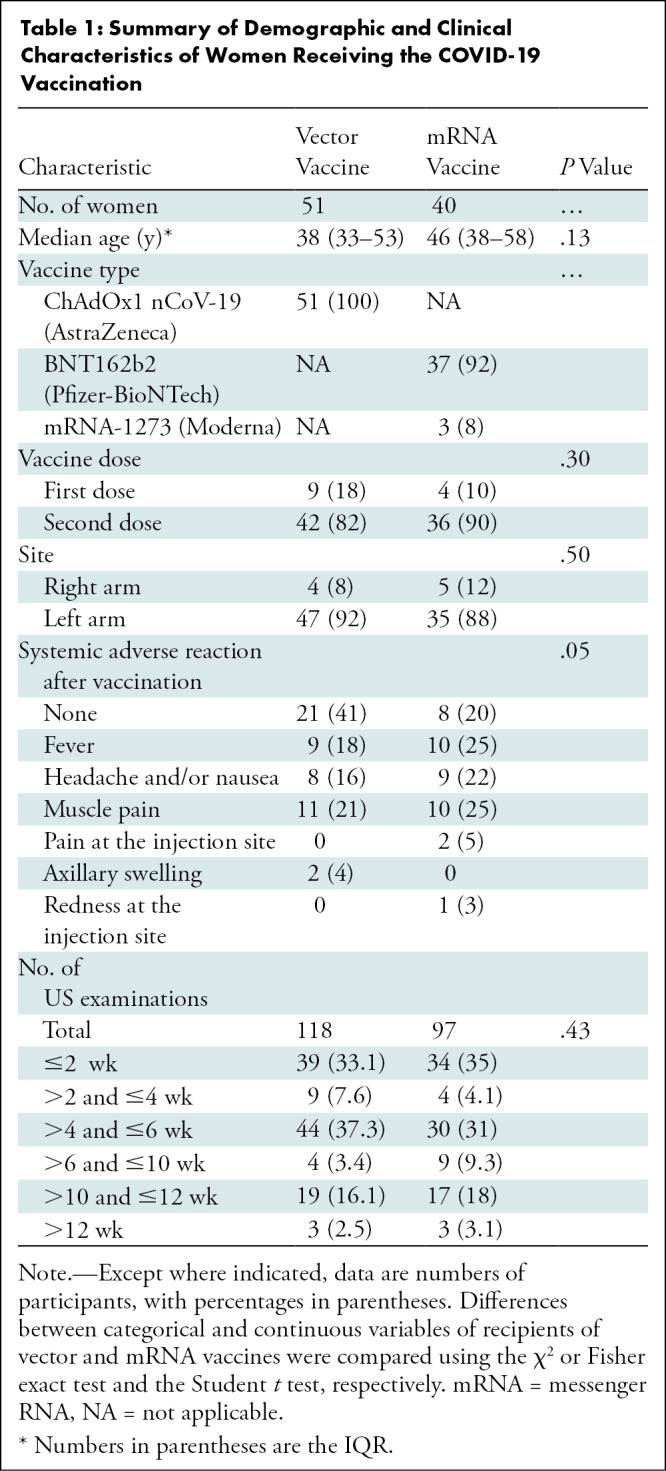
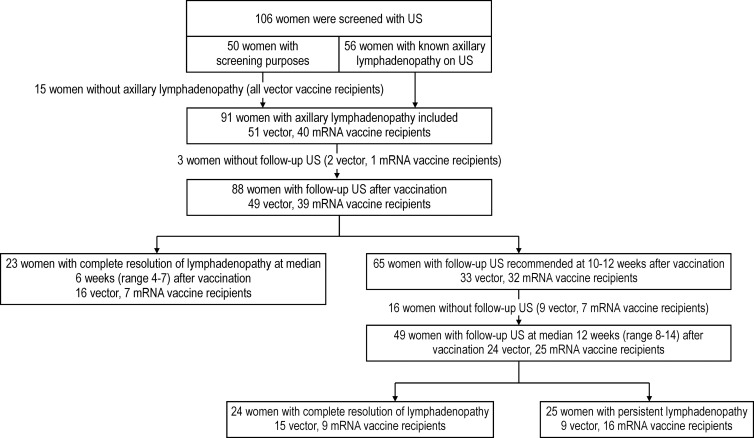
A total of 106 eligible women were screened using US to identify axillary lymphadenopathy at the first visit. Of 106 women, 50 (47%) underwent US for screening purposes after COVID-19 vaccination and 56 (53%) underwent US because axillary lymphadenopathy was detected at screening or follow-up US examinations. Of the 50 women who volunteered for screening purposes, 35 (70%) showed axillary lymphadenopathy within 1 week of vaccination. All 15 women who did not develop lymphadenopathy had received the vector vaccine; thus, they did not undergo further US examination. Ninety-one women exhibiting lymphadenopathy in the vaccinated arm were included and underwent 215 serial axillary US examinations (median number of examinations, three; IQR, two to three). The mean interval ± SD between vaccination and the last follow-up image acquisition was 8.9 weeks ± 3.2 (range, 4–16 weeks) (Fig 1). Fifty-one women had received a vector vaccine (ChAdOx1 nCoV-19 vaccine), and 40 had received an mRNA vaccine (BNT162b2 vaccine [n = 37] and mRNA-1273 vaccine [n = 3]). Table 1 lists the baseline and clinical characteristics of participants. Further details are presented in Appendix E1 (online).
Figure 1:
Flowchart displays participant inclusion. mRNA = messenger RNA.
Table 1:
Summary of Demographic and Clinical Characteristics of Women Receiving the COVID-19 Vaccination
Effect of Vaccine Type and Time on COVID-19 Lymphadenopathy
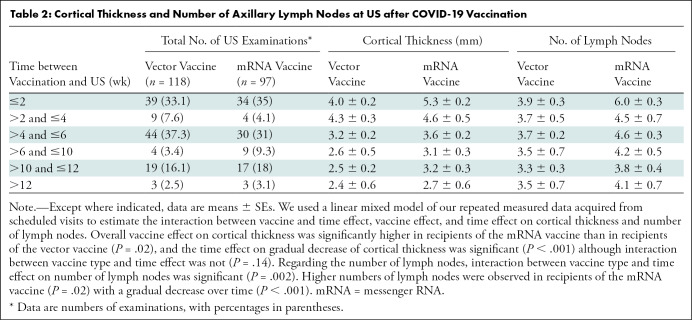
Of the 91 women, 88 underwent serial follow-up US examinations after vaccination; temporal changes could not be evaluated in three women because they were lost to follow-up. Of these 88 women, complete resolution of axillary lymphadenopathy was observed at a median of 6 weeks (range, 4–7 weeks) in 23 women (26%; 16 of 49 vector vaccine recipients [33%] and seven of 39 mRNA vaccine recipients [18%]) (Fig 1). Lymph nodes were not resolved in 65 women (33 vector and 32 mRNA vaccine recipients), warranting an additional follow-up at 10–12 weeks. Follow-up US was performed at a median of 12 weeks (range, 8–14 weeks) for 49 women (24 vector and 25 mRNA vaccine recipients), and complete resolution was observed in 24 of the 49 women (49%; 63% [15 of 24] of vector vaccine recipients and 36% [nine of 25] of mRNA vaccine recipients). In contrast, persistent lymphadenopathy was observed in 25 of the 49 women (51%; 38% [nine of 24] of vector vaccine recipients and 64% [16 of 25] of mRNA vaccine recipients). Three women underwent further clinical follow-up after 12 weeks, and complete resolution was observed in two vector vaccine recipients and one mRNA vaccine recipient at 13 and 16 weeks, respectively. There were no women in whom suspicious lymphadenopathy was aggravated or biopsy was performed. In our linear mixed model analysis, overall vaccine effect on cortical thickness was significantly higher in recipients of the mRNA vaccine than in recipients of the vector vaccine (P = .02) and the time effect on gradual decrease of cortical thickness was significant (P < .001), although interaction between vaccine type and time effect was not (P = .14). With regard to the number of lymph nodes, interaction between vaccine type and time effect on number of lymph nodes was significant (P = .002) along with higher numbers observed in recipients of the mRNA vaccine (P = .02), with a gradual decrease over time (P < .001) (Table 2; Figs 2, 3).
Table 2:
Cortical Thickness and Number of Axillary Lymph Nodes at US after COVID-19 Vaccination
Figure 2:
Graphs show changes in (A) temporal cortical thickness and (B) number of lymph nodes over time between the recipients of a messenger RNA (mRNA) vaccine (BNT162b2 [n = 37] and mRNA-1273 [n = 3]) and a vector vaccine (ChAdOx1 nCoV-19 [n = 51]). (A) Lymphadenopathy was most commonly observed in the first 2 weeks of mRNA vaccine administration, with larger cortical thickness (mean, 5.26 mm ± 0.2). Lymphadenopathy was most commonly observed 2–4 weeks after vector vaccination, with lower cortical thickness (mean, 4.35 mm ± 0.3). The cortical thickness of lymph nodes gradually decreased over time regardless of vaccine type; nonetheless, its thickness was higher in recipients of the mRNA vaccine than in recipients of the vector vaccine. (B) The number of lymph nodes was higher in recipients of the mRNA vaccine than in recipients of the vector vaccine (mean, 6.03 nodes ± 0.3 vs 3.9 nodes ± 0.3) and gradually decreased regardless of vaccine type. A linear mixed model was established to estimate the time effect on cortical thickness and number of lymph nodes.
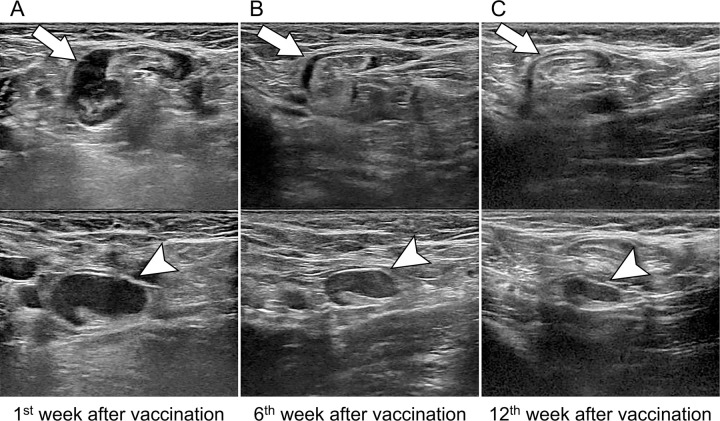
Figure 3:
Images in a 43-year-old asymptomatic woman without breast cancer and unilateral left axillary adenopathy after messenger RNA COVID-19 vaccination. Axial US images of the left axilla show two enlarged lymph nodes (arrow, top; arrowhead, bottom). (A) US scan obtained within the 1st week of BNT162b2 vaccination shows the cortex measuring up to 7.7 mm (arrowhead). (B, C) Follow-up US images obtained within 6 weeks (B) and 12 weeks (C) show decreased axillary lymphadenopathy of variable degrees, with the cortex measuring up to 5.7 and 3.4 mm (arrowhead), respectively.
Temporal Changes to US Findings of COVID-19 Lymphadenopathy
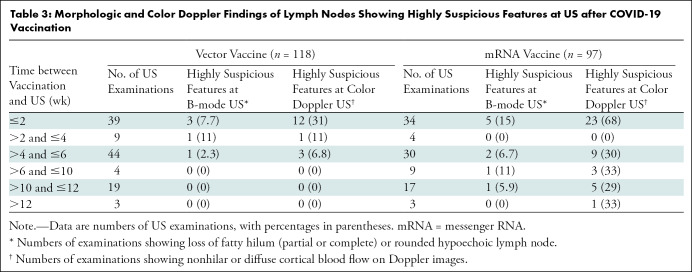
All 91 women showed ipsilateral axillary lymph node with focal or diffuse cortical thickening greater than 3 mm, and the cortical thickness was largest within 2 and 2–4 weeks following mRNA (mean, 5.3 mm ± 0.2) and vector (mean, 4.3 mm ± 0.3) vaccine administration, respectively (Table 2). At 2 weeks after vaccination in recipients of mRNA and vector vaccines, the mean numbers of lymph nodes (±SE) were 6.0 ± 0.3 and 3.9 ± 0.3, respectively. In most cases, fatty hilum of ipsilateral axillary lymph node was preserved. Suspicious morphologic characteristics including fatty hilum loss (partial or complete) or rounded hypoechoic features were noted in 4.2% of US examinations (five of 118) in five recipients of the vector vaccine and 9.3% of US examinations (nine of 97) in six recipients of an mRNA vaccine. Nonhilar or diffuse increased blood flow of lymph nodes was noted in 13.6% of US examinations (16 of 118) in 14 recipients of the vector vaccine and 42% of US examinations (41 of 97) in 25 recipients of mRNA vaccine. These findings were predominantly observed within 2 weeks in both vaccine types (Table 3). Among 118 US examinations in recipients of the vector vaccine, 7.7% (three of 39 examinations) showed morphologic abnormalities and 31% (12 of 39 examinations) showed abnormal Doppler signals within 2 weeks. Among 97 US examinations in recipients of the mRNA vaccine, 15% (five of 34 examinations) showed morphologic abnormalities within 2 weeks and 68% (23 of 34 examinations) showed abnormal Doppler signals within 2 weeks.
Table 3:
Morphologic and Color Doppler Findings of Lymph Nodes Showing Highly Suspicious Features at US after COVID-19 Vaccination
Discussion
With increasing COVID-19 vaccine availability and more widely vaccinated patient populations, we anticipate that more patients will demonstrate subclinical lymphadenopathy at imaging. However, the information on the temporal changes of lymphadenopathy following COVID-19 vaccination according to the vaccine types is still limited. We therefore prospectively and longitudinally evaluated COVID-19 vaccine–associated ipsilateral lymphadenopathy. Among 88 women who underwent serial US, complete resolution of axillary lymphadenopathy occurred at a median of 6 weeks in only 23 women (26%). Among 49 women with follow-up US at a median of 12 weeks, persistent lymphadenopathy was observed in 25 (51%) and lasted up to 16 weeks after vaccination. The cortical thickness of the ipsilateral axillary lymph node was largest within 2 and 2–4 weeks following messenger RNA (mRNA) vaccine (mean, 5.3 mm ± 0.2) and vector vaccine (mean, 4.3 mm ± 0.3) administration, respectively, with higher values in recipients of the mRNA vaccine than in recipients of the vector vaccine. Furthermore, suspicious morphologic characteristics and Doppler signal, as well as increased numbers of lymph nodes, were predominantly observed within 2 weeks with both vaccine types, with higher values and incidence in recipients of mRNA vaccine.
Our study elucidated the temporal changes of COVID-19 vaccine–associated lymphadenopathy at axillary US and optimal follow-up timing. Although imaging follow-up is not recommended for axillary adenopathy after vaccination in healthy women, cautious interpretation may be needed in those with recently diagnosed breast cancer (3) or in asymptomatic patients with a history of cancer undergoing monitoring for recurrence (16). In patients with a new or active cancer diagnosis, reactive lymphadenopathy cannot be easily distinguished from metastatic disease by using their morphologic characteristics or location (3,17). Different time effects on complete resolution attributed to vaccine administration provide clues for deciding the time for follow-up examination or directly proceeding to biopsy in women with higher risk of metastatic lymphadenopathy; thus, our findings can be especially helpful for determining optimal management for women who have recently diagnosed breast cancer or personal history of breast cancer. Indeed, the timing of the recommended follow-up imaging is debatable. The National Comprehensive Cancer Network recommends scheduling screening examinations before the first COVID-19 vaccination or 4–6 weeks after the second dose, postponing the short-interval follow-up for 4–6 weeks after the final dose (18). A previous study reported that the incidence of COVID-19 vaccine–associated lymphadenopathy at mammography decreased over time, disappearing more than 28 days after vaccination (4), thereby supporting the recommendations from the Society of Breast Imaging (2). However, the European Society of Breast Imaging recommends imaging in cases of axillary lymphadenopathy at least 12 weeks from the second vaccine dose in patients without a history of breast cancer (5), which was consistent with our study results. In a recent study, the time to resolution of reactive lymphadenopathy varied, with persistent axillary lymphadenopathy observed up to 43 weeks after vaccination (12), which led to the suggestion of no need for short-term follow-up imaging. Considering the extended time to resolution, short-term follow-up imaging examination within 6 weeks is not helpful in making a differential diagnosis of benign reactive lymphadenopathy regardless of vaccine type. Instead, follow-up imaging examination at least 12 weeks after vaccination may be reasonable, particularly for recipients of the mRNA vaccine.
A recent study (19) reported a high incidence (49%) of COVID-19 vaccine–induced axillary lymphadenopathy at US, demonstrating that mRNA vaccine (odds ratio [OR] = 6.7), intervals of 1–14 days (OR = 4.3) and 15–28 days (OR = 4.3), younger age (≤40 years, OR = 2.2), and first vaccination (OR = 1.6) independently influenced the occurrence of lymphadenopathy on US scans. Similarly, our results showed a larger number of mRNA vaccine recipients with suspected axillary lymphadenopathy and longer duration of lymphadenopathy, with only 18% of recipients (seven of 39) displaying complete resolution at a median of 6 weeks (range, 4–7 weeks) after mRNA vaccination. In addition to cortical thickening, suspicious morphologic features of loss in fatty hilum, rounded shape, increased cortical flow, and increase in overall number of lymph nodes were more frequently observed in women receiving mRNA vaccines. Although no women needed biopsy in our study, these imaging findings may raise more concern for malignant lymphadenopathy (3,12,17,20); thus, cautious interpretation is needed.
Our study had some limitations. First, this is a single-center design with a small sample size. In this prospective observational study, we could only enroll a limited number of women owing to time, cost, and compliance issues related to the serial US examinations planned for each participant. However, we believe our study is distinct from other retrospective studies because we only included women with a clear antecedent relationship between COVID-19 vaccination and lymphadenopathy. Second, in our linear mixed model, the analysis was conducted on the premise of “missing at random,” and cortical thickness and number of lymph nodes were not evaluated at 10–12 weeks in participants who showed complete resolution within 6 weeks. This may influence our results; however, comparison between two groups was still possible with no significant difference in the number of objects measured at each time point. Third, fewer women received the mRNA-1273 vaccine in our country because the ChAdOx1 nCoV-19 vaccine had been initiated earlier. Last, we did not have information on the incidence of lymphadenopathy, which was beyond the scope of this study as we intended to evaluate the temporal changes of lymphadenopathy resolution on US scans. However, we can indirectly infer the incidence in our study population because 47% of the participants (50 of 106 eligible women) were part of the screening population and 70% (35 of 50) of them showed axillary lymphadenopathy, which is higher than in other studies (4,10–12). This high incidence should be interpreted with caution because our study population was enriched with women in whom lymphadenopathy had been detected. The use of various types of vaccine and US examination performed within a short interval from vaccination could explain this high incidence; however, further larger studies are warranted.
In conclusion, COVID-19 vaccine–associated axillary lymphadenopathy as identified at US developed within the first 2 weeks of vaccination and frequently persisted for 6 weeks. Clinicians and radiologists should understand the different timelines for lymphadenopathy resolution according to the vaccine type and cautiously interpret lymphadenopathy considering the timeframe from vaccination and overall nodal metastatic risk of individual patients. Even in situations that require follow-up US examinations, short-term follow-up within 6 weeks is not helpful for identifying the resolution of lymphadenopathy; thus, follow-up US examination at least 12 weeks after vaccination may be reasonable, particularly for recipients of the messenger RNA vaccine.
Supported by a research grant from JW Medical and Seoul National University Hospital (grant 06-2021-1410).
Disclosures of conflicts of interest: S.M.H. No relevant relationships. A.J.C. No relevant relationships. J.B.L. No relevant relationships. S.Y.K. No relevant relationships. S.H.L. No relevant relationships. H.Y. Trainee editorial board. N.C. No relevant relationships. W.K.M. No relevant relationships. J.M.C. No relevant relationships.
Abbreviations:
- mRNA
- messenger RNA
References
- 1. Becker AS , Perez-Johnston R , Chikarmane SA , et al . Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel . Radiology 2021. ; 300 ( 2 ): E323 – E327 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grimm L , Destounis S , Dogan B , et al . SBI Recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination . Society of Breast Imaging Web site . https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf. Accessed February 6, 2022.
- 3. Lam DL , Flanagan MR . Axillary lymphadenopathy after COVID-19 vaccination in a woman with breast cancer . JAMA 2022. ; 327 ( 2 ): 175 – 176 . [DOI] [PubMed] [Google Scholar]
- 4. Robinson KA , Maimone S , Gococo-Benore DA , Li Z , Advani PP , Chumsri S . Incidence of axillary adenopathy in breast imaging after COVID-19 vaccination . JAMA Oncol 2021. ; 7 ( 9 ): 1395 – 1397 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schiaffino S , Pinker K , Magni V , et al . Axillary lymphadenopathy at the time of COVID-19 vaccination: ten recommendations from the European Society of Breast Imaging (EUSOBI) . Insights Imaging 2021. ; 12 ( 1 ): 119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bettini E , Locci M . SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond . Vaccines (Basel) 2021. ; 9 ( 2 ): 147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lederer K , Castaño D , Gómez Atria D , et al . SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation . Immunity 2020. ; 53 ( 6 ): 1281 – 1295.e5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rijkers GT , Weterings N , Obregon-Henao A , et al . Antigen presentation of mRNA-based and virus-vectored SARS-CoV-2 vaccines . Vaccines (Basel) 2021. ; 9 ( 8 ): 848 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Moderna COVID-19 Vaccine’s Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events. Centers for Disease Control and Prevention . http://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html. Accessed February 22, 2022
- 10. Cohen D , Krauthammer SH , Wolf I , Even-Sapir E . Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation . Eur J Nucl Med Mol Imaging 2021. ; 48 ( 6 ): 1854 – 1863 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adin ME , Isufi E , Kulon M , Pucar D . Association of COVID-19 mRNA vaccine with ipsilateral axillary lymph node reactivity on imaging . JAMA Oncol 2021. ; 7 ( 8 ): 1241 – 1242 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolfson S , Kim E , Plaunova A , et al . Axillary adenopathy after COVID-19 vaccine: No reason to delay screening mammogram . Radiology 2022. . 10.1148/radiol.213227. Published online February 8, 2022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eshet Y , Tau N , Alhoubani Y , Kanana N , Domachevsky L , Eifer M . Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 weeks after mRNA COVID-19 vaccination . Radiology 2021. ; 300 ( 3 ): E345 – E347 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ritchie H , Mathieu E , Rodés-Guirao L , et al . Coronavirus (COVID-19) Vaccinations . Our World in Data Web site . http://ourworldindata.org/covid-vaccinations. Accessed March 22, 2022 . [Google Scholar]
- 15. Abe H , Schacht D , Sennett CA , Newstead GM , Schmidt RA . Utility of preoperative ultrasound for predicting pN2 or higher stage axillary lymph node involvement in patients with newly diagnosed breast cancer . AJR Am J Roentgenol 2013. ; 200 ( 3 ): 696 – 702 . [DOI] [PubMed] [Google Scholar]
- 16. Moon HJ , Kim MJ , Kim EK , et al . US surveillance of regional lymph node recurrence after breast cancer surgery . Radiology 2009. ; 252 ( 3 ): 673 – 681 . [DOI] [PubMed] [Google Scholar]
- 17. Ha SM , Cheun J-H , Lee SH , et al . Ipsilateral lymphadenopathy after COVID-19 vaccination in patients with newly diagnosed breast cancer . J Breast Cancer 2022. ; 25 : e10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Comprehensive Cancer Network (NCCN). Recommendations of the NCCN COVID-19 vaccination advisory committee . https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v5-0.pdf. Accessed February 22, 2022 .
- 19. Park JY , Lee JY , Yi SY . Axillary Lymphadenopathy on Ultrasound after COVID-19 vaccination and its influencing factors: a single-center study . J Clin Med 2022. ; 11 ( 1 ): 238 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Özütemiz C , Krystosek LA , Church AL , et al . Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients . Radiology 2021. ; 300 ( 1 ): E296 – E300 . [DOI] [PMC free article] [PubMed] [Google Scholar]



![Graphs show changes in (A) temporal cortical thickness and (B) number of lymph nodes over time between the recipients of a messenger RNA (mRNA) vaccine (BNT162b2 [n = 37] and mRNA-1273 [n = 3]) and a vector vaccine (ChAdOx1 nCoV-19 [n = 51]). (A) Lymphadenopathy was most commonly observed in the first 2 weeks of mRNA vaccine administration, with larger cortical thickness (mean, 5.26 mm ± 0.2). Lymphadenopathy was most commonly observed 2–4 weeks after vector vaccination, with lower cortical thickness (mean, 4.35 mm ± 0.3). The cortical thickness of lymph nodes gradually decreased over time regardless of vaccine type; nonetheless, its thickness was higher in recipients of the mRNA vaccine than in recipients of the vector vaccine. (B) The number of lymph nodes was higher in recipients of the mRNA vaccine than in recipients of the vector vaccine (mean, 6.03 nodes ± 0.3 vs 3.9 nodes ± 0.3) and gradually decreased regardless of vaccine type. A linear mixed model was established to estimate the time effect on cortical thickness and number of lymph nodes.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/d984/9527338/3a44fd549efe/radiol.220543.fig2.jpg)