Abstract
Recent attention has focused on the growing role of psychostimulants, such as methamphetamine in overdose deaths. Methamphetamine is an addictive and potent stimulant, and its use is associated with a range of physical and mental health harms, overdose, and mortality. Adding to the complexity of this resurgent methamphetamine threat is the reality that the increases in methamphetamine availability and harms are occurring in the midst of and intertwined with the ongoing opioid overdose crisis. Opioid involvement in psychostimulant-involved overdose deaths increased from 34.5% of overdose deaths in 2010 to 53.5% in 2019—an increase of more than 50%. This latest evolution of the nation’s overdose epidemic poses novel challenges for prevention, treatment, and harm reduction. This narrative review synthesizes what is known about changing patterns of methamphetamine use with and without opioids in the United States, other characteristics associated with methamphetamine use, the contributions of the changing illicit drug supply to use patterns and overdose risk, motivations for couse of methamphetamine and opioids, and awareness of exposure to opioids via the illicit methamphetamine supply. Finally, the review summarizes illustrative community and health system strategies and research opportunities to advance prevention, treatment, and harm reduction policies, programs, and practices.
Keywords: methamphetamine, opioid, overdose
Introduction
The decades-long drug overdose epidemic in the United States continues unabated. In 2019, overdose deaths reached a historical high of 70,630 deaths,1 and provisional data from the Centers for Disease Control and Prevention (CDC) indicate that this trend accelerated in 2020—with the most recent data estimating more than 93,000 overdose deaths in the 12-month period from January 2020 through December 2020.2 Although opioids, particularly synthetic opioids (e.g., fentanyl), continue to be the primary driver of increases in overdose deaths, recent attention has focused on the growing role of psychostimulants,a such as methamphetamine in overdose deaths.1,3–5 Methamphetamine is an addictive and potent synthetic central nervous system stimulant.6 Its use is associated with a range of health harms, including psychosis, depression, and other mental disorders, cognitive and neurologic deficits, cardiovascular and renal dysfunction, transmission of HIV, viral hepatitis, and sexually transmitted infections, overdose, and increased mortality.6–14
Drug overdose death rates involving psychostimulants increased more than 300%, from 1.2 per 100,000 in 2013 to 5.0 per 100,000 in 2019.3 During this same period, the percentage of overdose deaths involving psychostimulants rose from 8.2% in 2013 to 22.9% in 2019, becoming the second most numerous drug class involved in overdose deaths in 2019 (after opioids as a class).4 Importantly, increases in psychostimulant-involved overdose deaths have been seen across geographic regions and a range of demographic groups, including in areas of the country, such as the Northeast, that have not historically had extensive illicit methamphetamine markets.15 These trends were also observed among populations, such as non-Hispanic Black persons who have historically had low rates of methamphetamine use and harms as well as among those who have long been impacted by methamphetamine use, such as American Indian and Alaskan native persons.5,16,17 Similar trends have been found for psychostimulant-involved emergency department (ED) visits18,19 and methamphetamine-related calls to poison control centers in the United States.20 Moreover, the rise in psychostimulant-related harms coincides with a substantial increase in the availability of methamphetamine across the United States over the past decade. Based on drug product submissions to the National Forensic Laboratory Information System (NFLIS) of the Drug Enforcement Administration (DEA), methamphetamine submissions more than doubled from 2011 to 2019.15 Furthermore, the 2020 National Drug Threat Assessment, methamphetamine seizures and price data, and law enforcement reporting all indicate that methamphetamine is now readily available at a greater purity and potency throughout the United States.21
Data from other developed countries also show recent increases in methamphetamine availability and harms associated with its use. The U.N. World Drug Report notes that global seizures of methamphetamine continue to increase and that methamphetamine use continues to expand into new markets.22 Of particular note, Australia and Canada, which are currently experiencing their own opioid crises, have also recently reported increases in methamphetamine availability and harms.23,24 Taken together, the available evidence suggests that the resurgence of methamphetamine is widespread and not concentrated among limited populations or areas in the United States.
Adding to the complexity of the resurgent methamphetamine threat in the United States is the reality that the increases in methamphetamine availability and harms are intertwined with the ongoing opioid overdose crisis. Opioid involvement in psychostimulant-involved overdose deaths increased from 34.5% of overdose deaths in 2010 to 53.5% in 2019—an increase of more than 50% (Fig. 1).1,4 Among ED visits, the rate of visits involving both opioids and psychostimulants increased 49.9% per year from 2006 to 2011 and then increased 14.0% per year from 2011 to 2016.19 The public health implications of rising use of opioids and methamphetamine are considerable, as research indicates that people who use both substances have poorer substance use treatment outcomes, are less likely to receive medications for opioid use disorder (MOUD) treatment, have greater difficulty accessing treatment for substance use disorder, report injection drug use and injecting more frequently, have increased risk for overdose, and report engaging in high-risk behaviors that can contribute to the transmission of bloodborne infections, such as HIV and viral hepatitis.16,25–30 In addition, research suggests that some individuals using methamphetamine may unknowingly be exposed to highly potent opioids, such as illicitly manufactured fentanyl due to mixing of fentanyl into the illicit drug supply,29 further exacerbating the overdose risk among individuals who are naive to the respiratory depressant effects of opioids and may not carry naloxone or suspect they are at risk for an opioid overdose.
Figure 1.
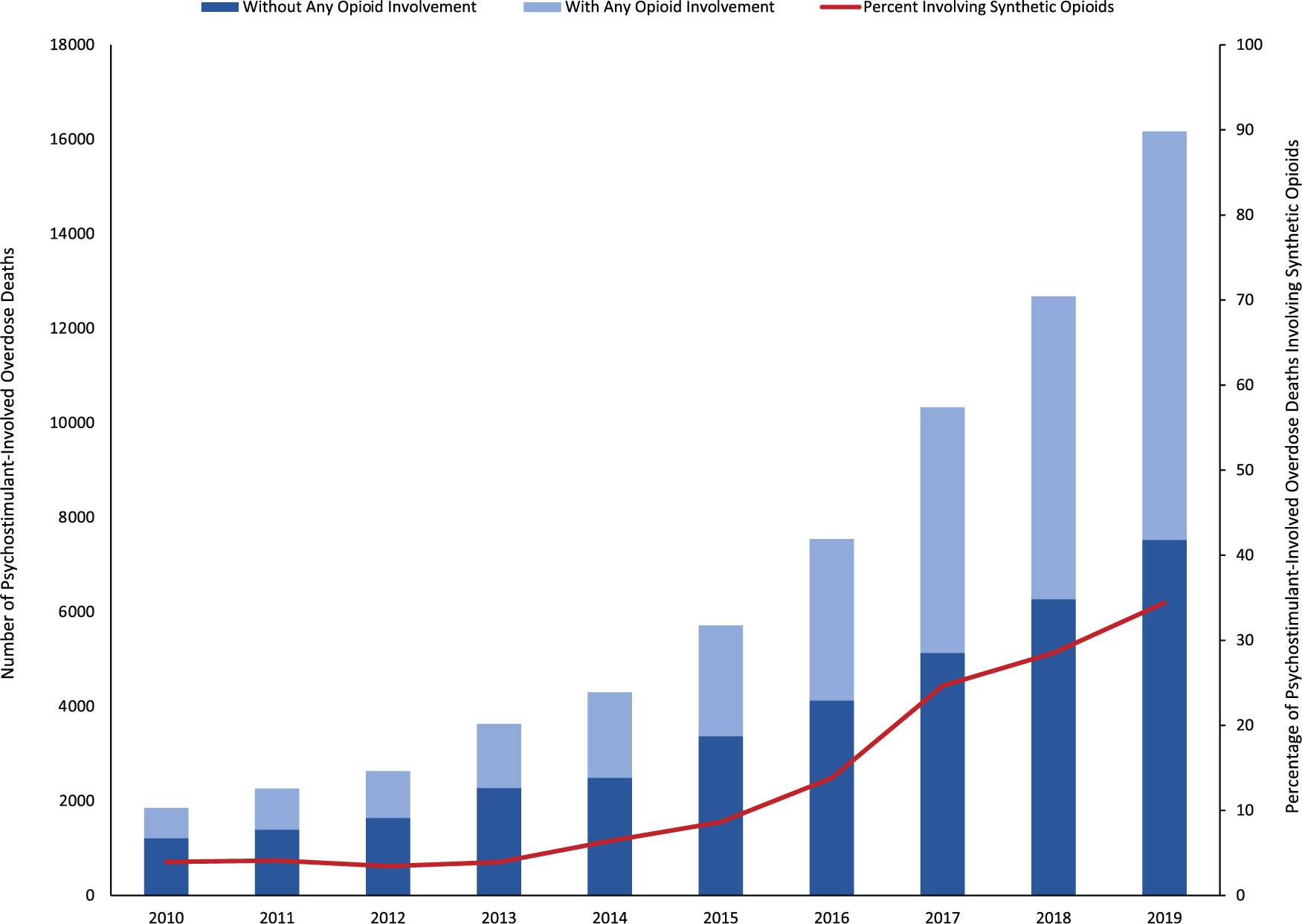
Psychostimulant-involved overdose deaths with and without opioid involvement, and the percentage of psychostimulant-involved overdose deaths involving synthetic opioids in the United States, 2010–2019. Data source: 2010–2019 National Vital Statistics System. Underlying cause of death (X40–44, X60–64, X85, Y10–14). Psychostimulant-involved overdose deaths (T43.7); any opioid (T40.0-T40.4, T40.6); synthetic opioids (T40.4).
This evolution of the nation’s overdose epidemic poses novel challenges for prevention, treatment, and harm reduction policies, programs, and practices. To inform a science-based community and health system response, this narrative review aims to: (1) assess what is known about the changing patterns of methamphetamine use with and without opioids in the United States and other clinical and social characteristics associated with methamphetamine use among nontreatment- and treatment-based populations; (2) characterize the contributions of the changing illicit drug supply to methamphetamine use patterns and overdose risk; (3) describe what is known about motivations for co-occurring use of methamphetamine and opioids, and awareness of exposure to opioids via the illicit methamphetamine supply; (4) discuss community and health system responses to resurgent methamphetamine use and harms; and (5) identify research gaps and opportunities.
What is known about changing patterns and correlates of methamphetamine use with and without opioids in the United States
Methamphetamine trends and correlates among nontreatment populations
Based on nationally representative data from the National Survey on Drug Use and Health (NSDUH), the number of people 12 years or older reporting past-year use of methamphetamine in 2019 was 2.0 million (95% confidence interval (CI): 1.7–2.3 million), up from 1.4 million (95% CI: 1.2–1.6 million) in 2016; the prevalence of past-year use increased from 0.5% (95% CI: 0.4–0.6%) in 2016 to 0.7% (95% CI: 0.6–0.8%) in 2019.31 Similarly, the number of people with past-year methamphetamine use disorder increased from 684,000 (95% CI: 553,000–815,000; or 0.3%, 95% CI: 0.26–0.33%) in 2016 to 1,048,000 (95% CI: 860,000–1,236,000; or 0.4%, 95% CI: 0.32–0.48%) in 2019.31 In addition, among individuals reporting past-year methamphetamine use, the number of reporting daily or near daily use of methamphetamine increased over 92% from approximately 161,000 (95% CI: 96,000–226,000) in 2016 to 310,000 (95% CI: 204,000–416,000) in 2019.31
Past-year methamphetamine use and use disorder remained stable between 2015 and 2019 among youth aged 12–17 and adults aged 18–25 years; however, past-year use and use disorder increased significantly among adults 26 years or older between 2016 and 2019.5 Two other nationally representative surveys of U.S. high school students show declining trends in methamphetamine use over a longer time period, with lifetime use dropping from 4.7% of 12th graders in 1999 to 1.4% in 2020 in the Monitoring the Future Study,32 and lifetime use declining from 4.1% among 9th–12th graders in 2009 to 2.1% in 2019 in the Youth Risk Behavior Survey.33
Among U.S. adults aged 18–64 during 2015–2019, the prevalence of methamphetamine use disorder or methamphetamine injection surpassed the prevalence of methamphetamine use without use disorder or injection in each year during 2017–2019.34 Adults who have methamphetamine use disorder or inject methamphetamine are more likely to use methamphetamine frequently (≥100 days in the past year). Notable trends included increases in methamphetamine use disorder without injection by heterosexual men and women, gay/bisexual men, and lesbian/bisexual women; and by non-Hispanic Whites, Blacks, Asians or Other Pacific Islanders, and Hispanics. Populations at increased risk for methamphetamine use have diversified rapidly, particularly among those with socioeconomic risk factors and more comorbidities. These changes in methamphetamine use patterns increase risks for overdose and other adverse outcomes.34
A number of other demographic and geographic factors have been associated with methamphetamine use, based on NSDUH data. Among adults, past-year methamphetamine use was associated with male sex; ages 26 or older; lower educational attainment; annual household income less than $50,000; Medicaid-only or no health insurance; and residing in small metro and nonmetro counties (Table 1).35 In addition, past-year methamphetamine use was elevated among gay/lesbian and bisexual persons as well as those receiving government assistance.36 Geographically, the prevalence of past-year methamphetamine use among people 12 years of age or older in 2019 was highest in the West (1.1%, 95% CI: 0.86–1.34%), followed by the Midwest (0.8%, 95% CI: 0.66–0.94%), South (0.7%, 95% CI: 0.60–0.80%), and Northeast (0.3%, 95% CI: 0.24–0.36%).31 Co-occurring substance use and mental health problems are common among people using methamphetamine. Adults who used methamphetamine in the past year also reported past-year use of cannabis (68.7%, 95% CI: 64.8–72.4%), prescription opioid misuse (40.4%, 95% CI: 36.5–44.5%), cocaine use (30.4%, 95% CI: 27.3–33.6%), prescription sedative or tranquilizer misuse (29.1%, 95% CI: 26.0–32.4%), prescription stimulant misuse (21.6%, 95% CI: 19.0–24.4%), and heroin use (16.9%, 95% CI: 14.4–19.8%). In addition, individuals reported past-month binge drinking (46.4%, 95% CI: 42.2–50.5%) and past-month nicotine dependence (44.3%, 95% CI: 39.9–48.8%); 57.7% (95% CI: 54.0–61.3%) had any mental illness in the past year, and 25.0% (95% CI: 21.4–29.0%) had a serious mental illness. Additionally, among adults reporting past-year methamphetamine use during 2015–2018, a majority (52.9%, 95% CI: 49.6–56.3%) met diagnostic criteria for a past-year methamphetamine use disorder, and 22.3% (95% CI: 19.6–25.2%) reported injecting methamphetamine in the past year (Fig. 2).35 As with methamphetamine use, prior research has found that methamphetamine use disorder is associated with many of the same demographic factors, co-occurring substance use, and mental health comorbidities.34,37,38
Table 1.
Characteristics associated with past-year methamphetamine use among adults ≥18 years in the United States, 2015–2018
| Characteristics | Adjusted odds ratiosa (95% CI) |
|---|---|
|
| |
| Sex | |
| Female | Ref |
| Male | 1.68 (1.43–1.96) |
| Age group (years) | |
| 18–25 | Ref |
| 26–34 | 1.67 (1.36–2.05) |
| 35–49 | 2.49 (2.01–3.07) |
| ≥50 | 1.72 (1.31–2.25) |
| Race/ethnicity | |
| Non-Hispanic White | Ref |
| Non-Hispanic Black | 0.29 (0.20–0.42) |
| Non-Hispanic Other | 1.07 (0.78–1.47) |
| Hispanic | 1.08 (0.85–1.37) |
| Education level | |
| Less than high school graduation | 3.28 (2.13–5.06) |
| High school graduate | 2.65 (1.78–3.93) |
| Some college or associate’s degree | 2.04 (1.38–3.02) |
| College graduate | Ref |
| Annual household income ($) | |
| <20,000 | 2.09(1.59–2.74) |
| 20,000–49,999 | 1.42 (1.11–1.82) |
| 50,000–74,999 | 1.06 (0.77–1.46) |
| ≥75,000 | Ref |
| Insurance status | |
| Private or other insurance (including Medicare) | Ref |
| Medicaid only | 2.01 (1.55–2.61) |
| Uninsured | 1.70 (1.31–2.22) |
| County-type of residence | |
| Large metro | Ref |
| Small metro | 1.32 (1.01–1.72) |
| Nonmetro | 1.54(1.25–1.90) |
| Substance useb | |
| Past-month binge drinking | 1.06 (0.86–1.30) |
| Past-month nicotine dependence | 2.14 (1.75–2.62) |
| Past-year cannabis use | 4.61 (3.67–5.80) |
| Past-year cocaine use | 2.72 (2.12–3.50) |
| Past-year heroin use | 5.10(3.63–7.17) |
| Past-year prescription opioid misuse | 2.17(1.66–2.84) |
| Past-year prescription sedative/tranquilizer misuse | 1.85 (1.45–2.35) |
| Past-year prescription stimulant misuse | 1.91 (1.43–2.55) |
| Mental health | |
| No past-year mental illness | Ref |
| Past-year mental illness, not serious mental illnessc | 2.18 (1.82–2.60) |
| Past-year serious mental illnessd | 3.34 (2.53–4.40) |
CI, confidence interval; Ref, reference group.
Odds ratios adjusted for all other variables in the model.
Reference group is no use (misuse) in past month (past year).
Any mental illness is defined as currently or at any time in the past year having had a diagnosable mental disorder (excluding developmental and substance use disorders) of sufficient duration to meet DSM-IV diagnostic criteria. (https://www.samhsa.gov/data/report/2017-methodological-summary-and-definitions). For this analysis where the variable was defined as past-year mental illness, not serious mental illness (SMI), individuals meeting criteria for SMI were not included.
Serious mental illness is defined as currently or at any time in the past year having had a mental disorder (excluding developmental and substance use disorders) of sufficient duration to meet DSM-IV diagnostic criteria that resulted in serious functional impairment substantially interfering with or limiting one or more major life activities (https://www.samhsa.gov/data/report/2017-methodological-summary-and-definitions).
Source: National Surveys on Drug Use and Health, 2015–2018; NSDUH uses 2010 Census–based population estimates.
Adapted from Ref. 125.
Figure 2.
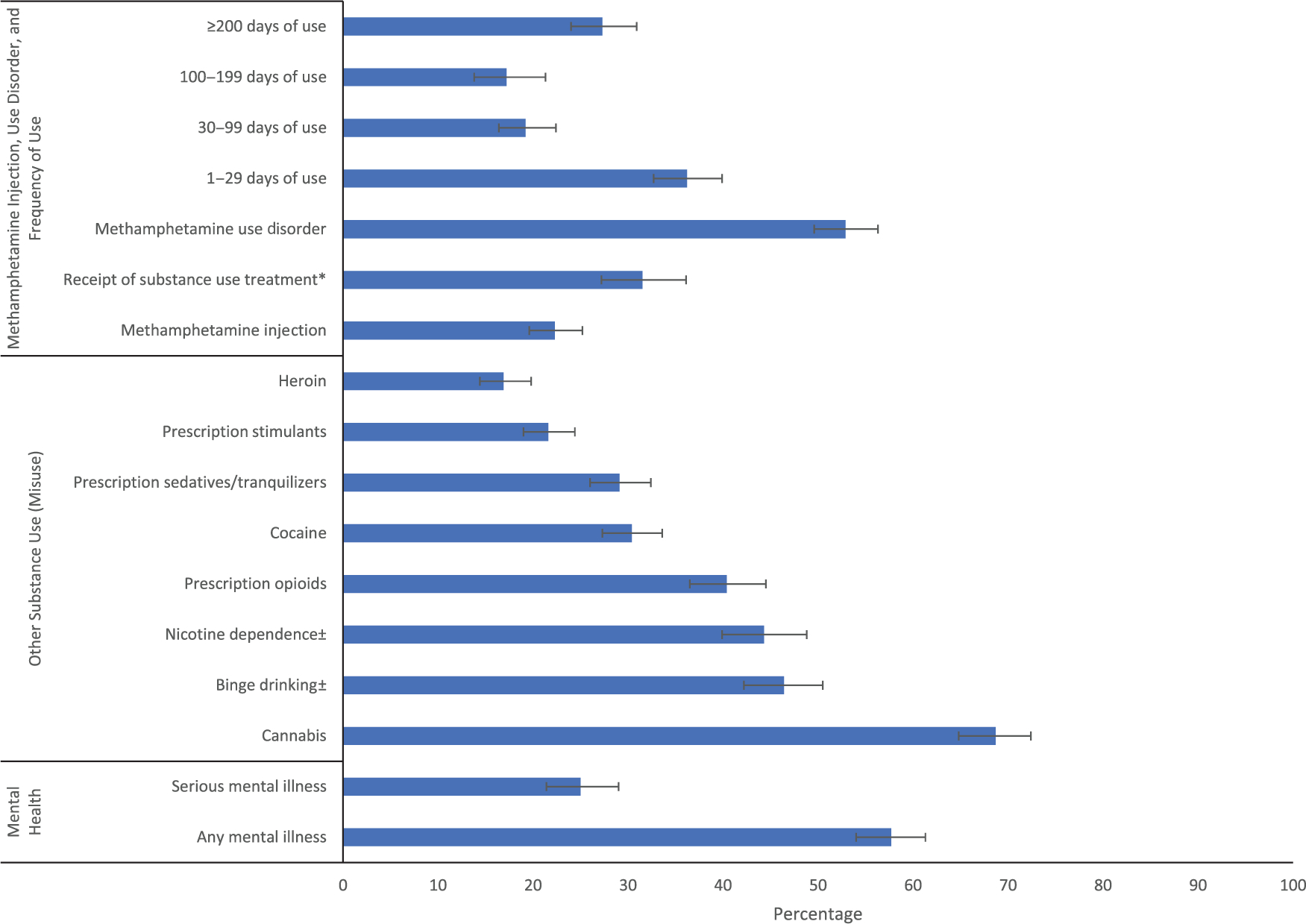
Methamphetamine injection, use disorder, receipt of substance use treatment and frequency of use, other substance use, and mental illness among adults ≥ 18 years old reporting past-year methamphetamine use in the United States, 2015–2018. Source: National Surveys on Drug Use and Health, 2015–2018; NSDUH uses 2010 Census–based population estimates. Receipt in past year among those with a methamphetamine use disorder; all other percentages are among adults reporting past-year methamphetamine use. Weighted percentages; bars represent 95% CI. *Among those with past-year methamphetamine use disorder. ±Past-month; all other variables are past-year. Adapted from Ref. 125.
Several studies using NSDUH data have specifically examined trends and correlates of opioid and methamphetamine couse. An analysis of 2015–2018 NSDUH data found that past-year methamphetamine use among people using heroin in the past year increased from 22.5% (95% CI: 16.4–30.0%) in 2015 to 37.4% (95% CI: 26.2–50.2%) in 2018, a 66.2% increase; increases in methamphetamine use among people using prescription opioids were also found, rising from 5.4% (95% CI: 4.4–6.6%) in 2015 to 8.0% (95% CI: 6.3–10.1%) in 2018, a 49.2% increase.36 Similarly, a separate study found that past-month methamphetamine use increased from 2015 to 2017 among people reporting past-month heroin use (9.0–30.2%), among those with a past-year heroin use disorder (6.2–19.1%), and among those with a past-year prescription opioid use disorder (3.8–7.9%), whereas trends for most other substances used among these groups did not change during the study period.39 A subsequent analysis found these trends continued through 2019.40
Several small studies in the United States lend further support to the link between rising methamphetamine and opioid use. In a Denver, Colorado study of people who inject drugs (PWID), the proportion reporting methamphetamine as their most frequently injected drug steadily increased from 2.1% in 2005 to 29.6% in 2015, while the proportion reporting heroin as their most frequently injected drug fluctuated between 48.4% in 2005 and 58.4% in 2015; 50% reported injection of both heroin and methamphetamine in the past 12 months in 2015.41 Among PWID in Seattle, Washington, combined heroin and methamphetamine injection in the past 3 months increased from 18% in 2009 to 31% in 2017 among men who have sex with men (MSM) and from 10% to 53% among non-MSM.26 In a follow-up study, the proportion of PWID reporting combined heroin and methamphetamine injection as their main drug nearly doubled from 10.3% in 2017 to 20.1% in 2019.25 In a sample of adults with opioid use disorder in Ohio, 55.6% had used methamphetamine in the past 6 months. A common trajectory among this population was first misusing prescription opioids, then transitioning to heroin and illicitly manufactured fentanyl, and then initiating methamphetamine use, with 83.8% reporting their first methamphetamine use occurring after their first illicit opioid use.42 Finally, a study of 23 syringe services programs (SSPs) in 2018 found that 70% of SSPs, including programs in all U.S. Census regions and those in both rural and urban areas, reported increases in methamphetamine use among SSP participants in the past 2–3 years, often in conjunction with opioid injection.43
Particularly noteworthy are the findings from multiple studies that assessed the elevated health and social risks among individuals who use both opioids and methamphetamine. Compared with the use of opioids alone, those reporting use of both drugs have a 132% higher prevalence of injection drug use; a 55% higher prevalence of serious mental illness; a nearly two-fold higher prevalence of hepatitis B or C;44 46% more ED visits; 99% more overnight hospital stay days; 1.4 times more social service use; and 3.3 times more criminal justice involvement, potentially reflective of more severe or extensive substance use problems.45 Methamphetamine use and combined opioid and methamphetamine use is also associated with housing instability and homelessness.41,42,44,45 Injecting both heroin and methamphetamine was associated with a nearly three-fold increase in having a nonfatal overdose in the past year compared with only injecting heroin,41 as well as greater frequency of: drug use, injecting daily, sharing syringes and other injection equipment, injecting in the femoral vein or jugular vein in the past 3 months, and witnessing an overdose in the past 12 months.25
Methamphetamine trends and correlates among treatment populations
Although data are more limited, the available research on treatment populations indicates a significant rise in methamphetamine use in recent years, as was detailed above with nontreatment populations. Using data from the nationwide Treatment Episode Data Set (TEDS), prior research shows that methamphetamine use among drug-related treatment admissions increased between 2010 and 2017.16 These increases were found for both any methamphetamine reported at treatment admission, rising from 14.1% (227,372) of drug-related treatment admissions in 2010 to 23.6% (372,926) in 2017, as well as among admissions where methamphetamine was the primary drug of use, rising from 9.4% (152,351) of admissions in 2010 to 14.5% (229,336) in 2017. This increase represented an average annual growth of 8.8% per year from 2010 to 2017 for any methamphetamine use at treatment admission and 7.4% for primary methamphetamine admissions. Importantly, these increases were seen across nearly all demographic groups examined as well as all U.S. Census regions (Fig. 3).16
Figure 3.
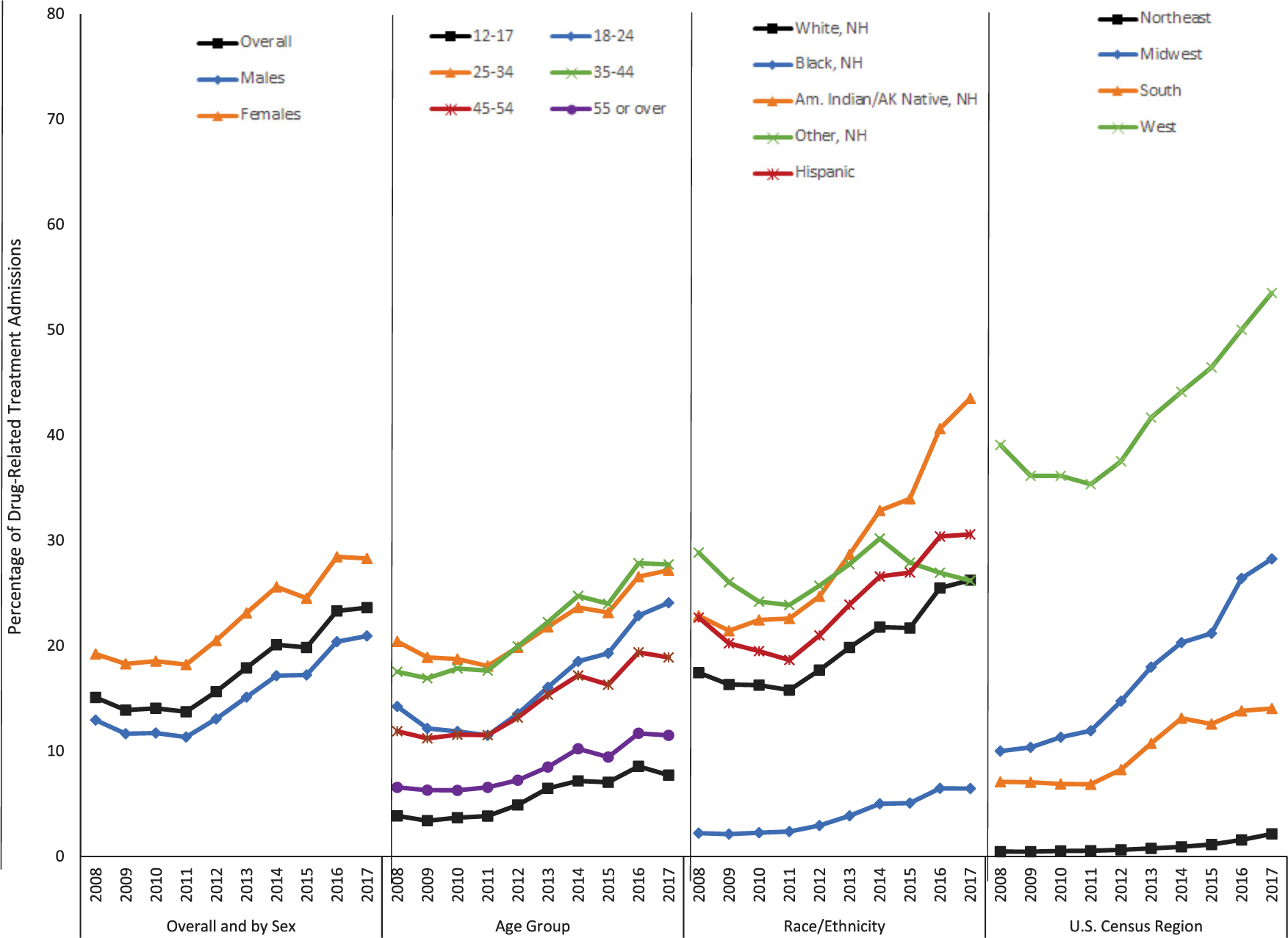
Percentage of drug-related treatment admissions reporting methamphetamine use in the United States, 2008–2017. Data source: 2008–2017 Treatment Episode Data Set. Any methamphetamine treatment admissions were defined as treatment admissions where methamphetamine was listed as a primary, secondary, or tertiary substance of use. Adapted from Ref. 126.
In the same study, characteristics associated with increased odds of reporting methamphetamine use at treatment admission included the following: female sex; admissions aged 35–44 years compared with those aged 25–34 years; treatment admission in the Midwest, South, or West compared with the Northeast; being unemployed or not in the labor force; dependent living or being homeless; and having a referral from a healthcare provider, other community referral, or criminal justice referral compared with individual/self-referral.16 Consistent with increases in methamphetamine injection seen in nontreatment populations, using TEDS data from 2008 to 2017, admissions reporting injection as the primary route of methamphetamine use increased from 17.5% of methamphetamine-related admissions in 2008 to 28.4% in 2017, a 62.3% increase. By contrast, smoking as the primary route of use declined from 67.3% in 2008 to 58.8% in 2017, a 12.6% decline; snorting declined from 10.8% in 2008 to 9.4% in 2017; and oral/other routes declined from 4.3% in 2008 to 3.4% in 2017. Similar results were found for primary methamphetamine admissions, where injection increased from 16.8% in 2008 to 25.9% in 2017; smoking dropped from 70.3% to 62.0%; snorting remained stable at about 9.0%; and oral/other routes dropped slightly to approximately 3.0% in 2017.16
Co-occurring substance use was also common among methamphetamine treatment admissions. In 2017, the most common substances reported at treatment admission among any methamphetamine treatment admissions were marijuana (33.7%), alcohol (25.9%), and heroin (23.6%). However, there were significant shifts in the percentage of methamphetamine treatment admissions reporting use of other substances during 2008–2017, with declines in alcohol (28.4% decline, from 36.1% of admissions to 25.9%), marijuana (10.8%, from 37.8% to 33.7%), and cocaine (43.5%, from 10.1% to 5.7%); however, benzodiazepine use increased 196.7%, from 0.9% to 2.7% of any methamphetamine treatment admissions.16 Of particular relevance to the link between rising methamphetamine and opioid use during 2008–2017 was the substantial increase in heroin use among people reporting methamphetamine use at treatment admission, rising 345.8%, from 5.3% of admissions to 23.6%, with prescription opioid use increasing 115.1%, from 3.8% to 8.3%; among primary methamphetamine admissions, heroin use increased 281.1%, from 2.1% of admissions to 8.0%; prescription opioid use increased 147.1%, from 2.2% to 5.6%.16
Examining trends in methamphetamine use among people entering substance use treatment for primary heroin use disorder in the national TEDS data from 2008 to 2017, the percentage of heroin admissions also reporting methamphetamine use at treatment admission increased nearly five-fold, from 2.1% of admission in 2008 to 12.4% in 2017—an average annual growth rate of 23.4%.27 As with methamphetamine treatment admissions, the rise in methamphetamine use among primary heroin treatment admissions was seen among both males and females, among all age and race/ethnicity groups examined, and in all U.S. Census regions. Characteristics associated with increased odds of reporting methamphetamine use among heroin treatment admissions included the following: female sex; age 25–34 years compared with those aged 35–44 years; non-Hispanic American Indian/Alaska Native persons compared with non-Hispanic White persons; treatment admissions in the Midwest, South, or West compared with the Northeast; part-time employment, unemployment, or not in the labor force; dependent living or being homeless; treatment referral by a healthcare provider, other community referral, or criminal justice referral compared with individual/self-referral; and reporting heroin injection. Concerningly, treatment admissions reporting both heroin and methamphetamine use were 35% less likely to receive MOUD as part of their treatment plan compared with those reporting heroin alone.27
A small number of other recent studies have examined methamphetamine and opioid use among treatment populations. Among people seeking treatment for opioid use disorder at approximately 170 treatment facilities in the United States, past-month use of methamphetamine increased from 18.8% of individuals in the second quarter of 2011 to 34.2% in the first quarter of 2017.46 Increases were seen among males and females, in urban and rural areas, in White and non-White persons, and in all U.S. Census regions.46 In a subsequent study, among people entering treatment for opioid use disorder, past-month use of methamphetamine increased from 19.6% in the second half of 2011 to 36.4% in the first half of 2018, whereas past-month use of alcohol, nicotine, and other prescription and illicit drugs either remained stable or declined during the study period.47
How illicit drug supply changes are contributing to methamphetamine use patterns and overdose risk
In 2006, the Combat Methamphetamine Epidemic Act (CMEA) was signed into law, which regulated over-the-counter sales of methamphetamine precursor chemicals (i.e., ephedrine, pseudoephedrine, and phenylpropanolamine). Provisions of CMEA implemented daily sales limits and 30-day purchase limits; placed products out of direct customer access; and required a logbook and valid identification for purchase, among other requirements.48 As a result, domestic production of methamphetamine declined substantially; however, the drop in domestic production has now been replaced by the production of high purity methamphetamine from Mexico that is primarily transited into the United States via the Southwest border.21
The overall availability of methamphetamine in the United States has increased substantially in recent years. After declining from 2005 through 2010, methamphetamine submissions to DEA’s NFLIS more than doubled from 2011 to 2019. In fact, in 2019, methamphetamine was the most frequently identified drug in products tested by state and local laboratories participating in NFLIS, with 417,867 reports out of 1,521,360 submissions (27.4%).15 Regional variation exists in the availability of methamphetamine, and several important regional changes have occurred over time as the availability of methamphetamine has increased. In general, methamphetamine has been predominately concentrated in the Western region of the United States, largely due to its proximity to the southwest border with Mexico, where trafficking of methamphetamine has thrived.21 However, in recent years, increases in methamphetamine in other regions of the United States have created cause for concern. For example, though almost half of methamphetamine drug submissions in 2019 occurred in the West (47.0%), steep increases were seen in the South and Midwest, accounting for 29.5% and 28.4% of submissions in 2019.15 Furthermore, although the Northeast accounted for the lowest percentage of methamphetamine submissions in 2019, 4.6%, this region has experienced significant increases in methamphetamine submissions in recent years.15 This is particularly concerning because the Northeast has historically not been a major market for methamphetamine—opening up new avenues of access for people who use drugs.21
In addition to increased availability of methamphetamine throughout the United States, there has also been concern in recent years about mixing of highly potent synthetic opioids like illicitly manufactured fentanyl into the illicit methamphetamine supply, and thus unknowingly exposing opioid-naive individuals to potentially lethal amounts of opioids. Between 2015 and 2017, the number of drug submissions with mixtures of fentanyl and methamphetamine increased 744%; yet, methamphetamine–fentanyl mixtures accounted for less than 2% of all methamphetamine reports in 2017.49,50 More recently, the percentage of methamphetamine reports in NFLIS that also contained fentanyl (either as combinations mixed together or separately but part of the same law enforcement report) increased from 3% in 2018 to 7% in 2020, suggesting that the mixing of fentanyl with methamphetamine continues to increase, underscoring the potential for individuals who are using methamphetamine to unknowingly be exposed to highly potent synthetic opioids, such as fentanyl.51 Geographic variability is also present in these drug combinations, with methamphetamine–fentanyl drug submissions most often found in the Northeast, specifically New Hampshire, Massachusetts, Vermont, and Maine.52
With these changes in availability, there has also been a proliferation of new product forms. Traditionally, methamphetamine is sold in powder or crystal form,53,54 but increasingly, new forms, including precursor chemicals, are being seized. For example, liquid methamphetamine can easily be dissolved and transported in other fluids, making it more difficult for drug detection and easier to traffic and distribute.21 In addition, pressed methamphetamine powder has been identified in counterfeit 3,4-methylenedioxymethamphetamine (i.e., MDMA or ecstasy) and amphetamine-type prescription stimulant tablets (e.g., Adderall®) in several states.21,50 Equally concerning to diversification in the types of methamphetamine available is that the purity and potency of methamphetamine has increased substantially in recent years, while the price in the illicit market has remained low. From 2007 to 2017, the purity and potency of methamphetamine increased nearly 100%; in the first half of 2019, DEA data show that the purity of methamphetamine was 97.2% and the potency was 97.5%.21,50 These changes are further expanding the reach of methamphetamine markets and products in the United States, and likely contributing to the increasing number of fatal and nonfatal overdoses as well as other rising health harms associated with methamphetamine use.
What is known about motivations for co-occurring use of methamphetamine and opioids, and awareness of exposure to opioids via the illicit methamphetamine
Typically, people who report use of methamphetamine have used other substances prior to initiating methamphetamine. Thus, understanding motivations for methamphetamine use is important to inform prevention, treatment, and harm reduction strategies. A survey of people with methamphetamine dependence who did not meet criteria for dependence on other substances found that the majority used methamphetamine for its positive reinforcement or “pleasure seeking,” with smaller but generally nonoverlapping groups reporting “pain avoidance” or negative reinforcement as important motivations for use.55 Among methamphetamine-related treatment admissions in Texas, motivations for use among females centered around activities, such as increased energy, staying awake, housework, childcare, weight loss, and mood, whereas increased energy and enhanced sexual pleasure were cited by male respondents as a benefit to using methamphetamine.56 The term chemsex has been coined recently to describe the use of drugs in conjunction with planned sexual experiences. Methamphetamine, in particular, is linked with chemsex, especially among MSM due to its association with enhanced sexual desire, arousal, and sexual pleasure.57–61
Given the rising couse of methamphetamine and opioids, it is particularly important to understand motivations contributing to these trends. Drawing from a sample of individuals with opioid use disorder receiving treatment in approximately 170 treatment facilities in the United States, the primary motivations for co-occurring use included high seeking and synergistic effects (51% of respondents), to balance the effect between the two drugs (38.6%), and using methamphetamine as a substitute when opioids were not available (15.2%). Individuals also reported that methamphetamine had increased in their local area because of increased accessibility (38.9%), increased popularity (37.5%), low cost (30.6%), restrictions on opioids (13.9%), and available dealer supply (11.1%).46
A small qualitative study conducted among rural residents at SSPs in Oregon who coused methamphetamine and opioids reported heroin (56%) was their primary drug for getting high and methamphetamine use was driven by the increased availability and lower costs compared with opioids in their communities. Participants also reported using methamphetamine to help with opioid withdrawal symptoms or to decrease their opioid use, which they viewed as more harmful.62 An additional study among treatment staff and patients receiving treatment at two treatment facilities in Oregon identified several individual-level factors contributing to the rise in methamphetamine and opioid use: methamphetamine as a strategy to detoxify or titrate impacts of heroin; financial benefits of methamphetamine to maintain a high; easy access to purchasing heroin and methamphetamine in combination; and methamphetamine being perceived as a safer alternative to heroin.63
Three additional studies have identified similar motivations for methamphetamine use in the context of heroin or illicit fentanyl. A study exploring overdose risk factors in a sample of people who use drugs in Ohio noted that “a preliminary finding from our current research focusing on methamphetamine suggests the existence of lay beliefs among people who use drugs in Dayton that use of methamphetamine in conjunction with heroin/non-prescribed fentanyl may reduce overdose risk. This incorrect lay belief may contribute to increased methamphetamine use in conjunction with non-prescribed fentanyl, and may, in part, explain the significant role of methamphetamine use as a correlate of overdose.”64 A subsequent study found that 78% of individuals participating in an in-depth qualitative study of methamphetamine and opioid use endorsed using methamphetamine to manage opioid withdrawal and 54.6% reported ever using methamphetamine to help quit opioid use; other motivations for methamphetamine use included to enjoy the high (93.9%), help with emotional problems (42.4%), get more energy (93.9%), balance out the effects of heroin/fentanyl (72.7%), and to enhance sexual experiences (60.6%).65 A study of 23 SSPs across the United States found that not only were programs seeing an increase in combined injection of opioids and methamphetamine, but that some individuals were switching from opioids to methamphetamine due to concerns about increased risk for overdose from fentanyl and the contaminated heroin supply.43
In addition to motivations for use, there has been interest in understanding the patterns of couse of opioids and methamphetamine, for example, whether individuals are using them together at the same time or at different times and days, as this is undoubtedly linked to the motivations for use and risk for harm. The available data are limited; however, several small studies have examined the patterns of couse. In a sample of adults seeking treatment for opioid use disorder, 79.9% of respondents used both opioids and methamphetamine on the same day, with 38.9% reporting use at the same time, 9.4% reporting use immediately before or immediately after one another, and 31.5% reporting use on the same day but at different times. The mean number of days of co-occurring use was 14.6 days per month in an average month.46 Among people who were injecting both heroin and methamphetamine in Denver, Colorado, 43.9% reported injecting the drugs separately at different times, 28.0% reported mixing the drugs together and injecting, and 24.0% reported doing both.41 Among SSP participants in Seattle, 55.3% of participants reported using the combination in the past 3 months.25
Finally, while there are clear trends in people knowingly using opioids and methamphetamine together in both treatment and nontreatment samples, there remains very real concern about unknowing exposure to opioids leading to overdose due to mixing of fentanyl and other synthetic opioids in illicit drug supplies of methamphetamine. The literature is very limited on this topic. Among people who use drugs and engage with harm reduction programs in British Columbia in 2015, 73% of individuals testing positive for fentanyl reported that they had not knowingly used fentanyl in the past 3 days.29 Among this sample, most reported recent methamphetamine use (59% of the sample, with 38% of these testing positive for fentanyl).25 However, as awareness of fentanyl in the illicit drug supply has increased in recent years, awareness and motivations may be changing. For example, a subsequent study of individuals engaging with harm reduction programs in British Columbia in 2018 reported that 60.3% of participants tested positive for fentanyl, and among those testing positive, nearly two-thirds (64%) reported knowingly having used fentanyl within the past 3 days. People reporting the use of methamphetamine were significantly more likely to report recent known fentanyl use compared with unknown fentanyl use in recent years.66
How do community and health system responses to resurgent methamphetamine use and harms need to evolve?
The available data from both nontreatment populations and populations engaging with the substance use treatment system demonstrate that methamphetamine use is increasing in the United States, and that these increases are occurring among a range of demographic groups and across geographic areas. Of particular importance, the rise in methamphetamine use appears to be intimately linked to the ongoing opioid overdose crisis, with substantial increases in methamphetamine use among people using opioids and those with opioid use disorder. In addition, motivations for use vary, with some individuals reporting that methamphetamine serves as a substitute when opioids are not available, as a means to modulate or moderate their opioid use, or as a purported risk reduction strategy due to the perceived improved safety profile of methamphetamine compared with heroin or fentanyl. The changing methamphetamine use trends noted in this review are consistent with the well-documented rise in overdose deaths and ED visits involving psychostimulants, both with and without opioid involvement.1–5,18,19 Underlying these changing patterns of substance use and overdose are substantial expansions in the availability of methamphetamine that is of high purity and potency, increasingly available in various forms, and being sold at historically relatively low cost.21 The mixing of fentanyl and other synthetic opioids into illicit supplies of methamphetamine may also be contributing to overdose risk and other harms among people using methamphetamine.
As the nation faces an accelerating overdose epidemic, these evolving substance use patterns present novel challenges for prevention, treatment, and harm reduction efforts that demand expanded, innovative community and health system responses. This response must engage multiple sectors in the community to comprehensively address the immediate challenge of rising overdose deaths and other health harms from substance use while simultaneously working to prevent future substance use, addiction, and overdose.
Effective community response begins by maintaining near real-time situational awareness about local use patterns and overdose trends. CDC’s Overdose Data to Action (OD2A) cooperative agreement program supports state, territorial, county, and city health departments collecting high-quality, comprehensive, and timely data on overdoses and in using those data to inform prevention and response efforts.67 The two surveillance components of OD2A leverage data from EDs (Drug Overdose Surveillance and Epidemiology or DOSE) and medical examiner/coroner reports, including death scene investigation findings and postmortem toxicology (State Unintentional Drug Overdose Reporting System or SUDORS), to better track and understand the complex and changing nature of the drug overdose epidemic in local communities.68,69 Law enforcement drug seizure data and mixed-methods monitoring of drug use patterns by groups, such as the National Drug Early Warning System (NDEWS), have also provided clues about changes and variation in drug availability contributing to changing substance use and overdose patterns.70 Such programs as DOSE, SUDORS, and NDEWS and other monitoring systems may be a model that communities, states, and regions can utilize.
Of note, some monitoring systems track overdose spikes and activate resources on demand in collaboration with community partners.71 One tool, known as OD-MAP (Overdose Detection – Mapping Application Program), uses smart phone technology or existing record management systems to track the geospatial location of fatal and nonfatal overdoses.72 OD-MAP alerts allow communities to rapidly implement a response protocol and disseminate messaging to mitigate harms as part of a comprehensive emergency management plan.72 Broad stakeholder engagement is essential to facilitate secure and HIPAA-compliant data sharing and coordination of activities between community partners to maximize integration in service delivery.72 Larger community awareness about overdose incidents can be maintained by leveraging dashboards to track progress and areas for opportunity over time.73
To complement this, communities can conduct overdose fatality reviews (OFRs).74,75 This involves interdisciplinary teams coming together to critically review case-level information about overdose deaths in an area, identify opportunities to intervene across sectors, and address the gaps pinpointed via programmatic and policy changes.74,75 For example, OFRs might identify unmet needs, such as treating co-occurring mental illness, or highlight avenues to better target resources to reach at-risk populations, such as persons experiencing homelessness, those transitioning from institutional settings, such as the criminal justice system, those with a previous nonfatal overdose, and those with a history of suicide attempts or suicidal ideation.74,75 OFRs can also lead to service enhancement through novel service delivery strategies, such as the use of telehealth, which was proven effective during the COVID-19 pandemic.76,77
These activities fit into broader frameworks that communities can leverage like Public Health and Safety Teams (PHAST).78 Importantly, PHAST focuses on engaging community partners to increase their understanding of the local overdose crisis, optimize jurisdictional capacity, and establish shared accountability for continuous improvement.78 PHAST teams analyze data on overdose burden, treatment and harm reduction service availability and use, changes in the local drug supply from drug-related arrests and seizure information, and input from a wide array of local agencies and community organizations to inform strategic priorities.78 A centerpiece of the PHAST approach is performance management where progress is monitored, and setbacks are learning opportunities to refocus activities to improve long-term outcomes.78
The Overdose Response Strategy, an ongoing initiative in 21 high-intensity drug trafficking areas in 34 states, widens public health and public safety data sharing to the regional level and is examining cutting-edge evidence-based community-level interventions.79,80 This includes capitalizing on unique opportunities to link persons to treatment from public safety venues, such as prearrest diversion programs, fire or police stations, or drug courts.80–83 Programs like Law Enforcement Assisted Diversion (LEAD) and Police Assisted Addiction & Recovery Initiative (PAARI) are noteworthy examples that use nonarrest, low barrier, and flexible pathways for treatment and recovery.84–87
Community partners are well positioned to help link and retain people who use drugs to comprehensive treatment and recovery support services.88–90 Linkage to care is an intentional strategy that works by connecting persons with a substance use disorder who are not currently accessing care with intensive support to initiate and sustain engagement with a treatment provider. A holistic approach involves complementary services, such as behavioral health supports, treatment for infectious disease complications of injection drug use, such as HIV and viral hepatitis, and access to housing, transportation, and employment assistance.90 Use of persons with lived experience like peer recovery coaches or patient navigators can be expanded to fit into broader efforts to adapt the “cascade of care” framework that has proven effective at retaining persons in HIV treatment.91–93 Community partners are central to this linkage and retention given their access to high-risk populations.
Given the increase in potency, affordability, availability, and geographic dispersion of methamphetamine as well as the mixing or couse of it with illicitly manufactured fentanyl and fentanyl analogs, communities can also increase the number of venues offering overdose prevention education and providing take-home naloxone through primary care settings, retail pharmacies, inpatient and outpatient substance use treatment programs, SSPs, support groups, mobile outreach, and other community-based settings.94 This may be especially important in rural areas where these services are often absent or not widely available.95,96 Harm reduction approaches and efforts to link and retain persons in treatment and care are critical, given the lack of Food and Drug Administration (FDA)–approved pharmacologic treatment for methamphetamine use disorder and in an illicit drug market rampant with synthetic opioids.94,97 Recently, SAMHSA and CDC amended the State Opioid Response Grant and the OD2A cooperative agreement programs, respectively, to allow funds to address stimulant misuse and use disorders, as well as to allow the purchase of fentanyl test strips to help drug users identify fentanyl contamination in the drug supply.98–100
It is critical for the U.S. healthcare system to provide treatment to individuals with methamphetamine use disorder because it is paramount to supporting long-term recovery and reducing the health harms associated with methamphetamine use. Currently, there are no FDA-approved medications for the treatment of methamphetamine use disorder. Several evidence-based nonpharmacological treatments have been identified for management of methamphetamine use disorder and other stimulant use disorders, including contingency management (CM), cognitive behavioral therapy, community reinforcement approach, and the Matrix Model (a framework to engage patients in treatment and to help them achieve abstinence, with treatment materials, including elements of relapse prevention, family and group therapies, drug education, and self-help participation). A review of systematic reviews of these management strategies found that CM demonstrated a significant benefit in treatment of stimulant use disorder, and suggested an additive effect of CM when combined with other nonpharmacological interventions.101 De Crescenzo et al. reviewed 50 randomized controlled trials of structured psychosocial interventions against an active control (or treatment as usual) for the treatment of cocaine and/or amphetamine addiction in adults. They reported that CM plus community reinforcement approach, a comprehensive behavioral treatment approach that focuses on the management of substance-related behaviors and other disrupted life areas, was the only intervention that increased the number of abstinent patients at the end of treatment compared with other interventions with noncontingent rewards and 12-step programs.102 These findings are supported by other systematic reviews subsequently published.103,104
Given the need to expand access to treatment services, such as CM, health systems, treatment facilities, and clinicians can institute CM as part of their substance use disorder or other outpatient clinics.105 For example, the Department of Veterans Affairs (VA) instituted a nationwide CM initiative in 94 substance use disorder treatment locations and enrolled 2060 patients. The 12-week CM intervention consisted of rapid, onsite testing for stimulants twice weekly, with participants receiving a prize slip for a drawing with each stimulant-negative sample. Prize slip draws started at one for the first stimulant-negative sample and increased for each consecutive negative sample up to a maximum of eight draws. A refused, stimulant-positive, or missed sample (i.e., an unexcused absence on a testing day) reset the draws for the next negative sample down to one with escalation resuming for sustained abstinence. After 55 months, evaluation results found that the average proportion of CM sessions attended was 55.6%, with 61 programs (64.9%) having an average proportion of sessions attended above 50%. In addition, of the 27,850 submitted samples, 25,593 (91.9%) tested negative for the targeted substance(s).105
While addressing overdose prevention and increasing use of effective treatments for methamphetamine use disorder are essential responses, other issues related to methamphetamine require attention as well. Major psychiatric and medical harms related to methamphetamine use include psychosis, agitation, severe anxiety, depression, cardiovascular issues (e.g., arrhythmias, myocardial infarction, and cerebrovascular accidents), infectious diseases (e.g., HIV and hepatitis), and dental problems, all of which require interventions within various health settings.6–14 Primary care, EDs, and other health services settings all need to maximize their ability to respond to these complex sequelae.106 Even health services for pregnant women may need to increase their attention to the use of methamphetamine.
For example, overdoses due to methamphetamine have clinical presentations that are quite distinct from overdoses due to opioids. Emergency medical services (including law enforcement as first responders) who have become particularly familiar with opioid overdose may require education and practice support regarding methamphetamine overdose, while recognizing that overdose with multiple substances, including opioids, is very common. Furthermore, serious disinhibited and agitated behavior due to methamphetamine intoxication can be extremely challenging and approaches to managing such clinical presentations may need to be enhanced. Entry into treatment and retention in care may be more difficult in persons with methamphetamine use disorder compared with other persons with other substance use disorders.107 Persons who use methamphetamine may not recognize their own use patterns as problematic and may drop out of treatment more readily than people who use other drugs.108 Such issues suggest that health services other than substance treatment and harm reduction activities may be particularly important for persons who use methamphetamine.
Finally, community resources can be utilized to prevent problematic substance use before it begins by addressing adverse childhood experiences and other environmental and contextual factors that create and sustain health disparities.109,110 Universal preventive interventions, such as Promoting School–Community–University Partnerships to Enhance Resilience (PROSPER), should be scaled up further, given the short- and long-term protective effects they have shown on youth substance use—including on methamphetamine and opioid use specifically.111–114 Together, these strategies mitigate harms and integrate, coordinate, and strengthen community prevention and response efforts.
What are the research gaps and opportunities?
As methamphetamine harms escalate, the need for improved surveillance, prevention, and treatment options becomes particularly salient. Research spanning epidemiology, basic neuroscience, prevention intervention development, treatment development (including both medication and nonmedication approaches), and health services (including policy) research are all needed.
The studies detailed in this review document national rates of methamphetamine use and use disorders; however, research to understand the trajectories of methamphetamine use from initiation to desistance is needed. Understanding the interplay of methamphetamine and co-occurring opioid use is of particular importance given the evidence of dynamic relationships of these substances in multiple areas and in their overlap in overdose mortality in recent years. While much has been written about the use of methamphetamine by MSM,61,115 documentation of the ongoing prevalence and patterns of use by this subpopulation, along with the overlap with HIV incidence, is an important area for study. Building on descriptive, epidemiological research, primary prevention interventions are sorely needed to reduce the initial onset of methamphetamine use,113 as well as secondary prevention interventions to minimize the progression to methamphetamine addiction and reduce the risks of overdose. Tertiary prevention of the serious harms associated with methamphetamine use is also warranted. To be successful, such work may need to account for the overlap of methamphetamine with other substances, in particular opioids, and with multiple risk and protective factors.
While significant research has been conducted on the impacts of methamphetamine on the central reward circuitry (and related pathways),116,117 basic neuroscience research is essential to developing a full understanding of how methamphetamine use affects the brain. Such research can be helpful in targeting both prevention and treatment targets. For example, new targets for brain circuitry through brain stimulation interventions (both transcranial magnetic stimulation and deep brain stimulation) show some promise for development.118 In addition, targets for medication development have been uncovered by basic science research and are sorely needed given the lack of FDA-approved medications for treatment of methamphetamine use disorder. While multiple promising pharmacological targets have been identified for development,119 it is also noteworthy that vaccines and monoclonal antibodies targeting methamphetamine are under study for both application in supporting methamphetamine use disorder treatment and as treatment for acute overdose.120–122
Although research on new prevention and treatment interventions is sorely needed, making sure that proven approaches to avoiding or treating methamphetamine use disorder are widely available is a key research goal. For example, while CM is effective for methamphetamine use disorder treatment,103 it is not widely available in the United States, in part, because of challenges to using monetary (or similar) incentives for behavior change. While groups such as the VA have overcome these challenges and have begun to offer CM as part of care of persons with methamphetamine use disorder in the VA,123 the large number of patients insured through Medicaid (and Medicare) are not able to access this as a reimbursed service,124 yet those with Medicaid are more likely to have methamphetamine use disorder compared with those with private health insurance only.35 Finding novel ways to address this gap in services is a key goal for research.
Finally, given the range of demographic and geographic groups experiencing increases in methamphetamine-related use and harms, addressing the acute needs of subpopulations, including racial and ethnic minority groups and sexual minorities, for prevention, treatment, and harm reduction interventions as well as underlying factors contributing to health inequities is particularly needed.
Conclusion
Methamphetamine use and harms are increasing in the United States among a broad spectrum of demographic groups and geographic areas. Furthermore, the available data demonstrate that the rise in methamphetamine use and harms is intimately linked to the ongoing opioid crisis, with substantial increases in methamphetamine use among people using opioids and those with opioid use disorder. Motivations for use vary, including methamphetamine serving as a substitute when opioids are not available, as a means to modulate or moderate opioid use, for synergistic euphoric effects, or as a purported risk reduction strategy due to the perceived improved safety profile of methamphetamine compared with heroin or illicitly manufactured fentanyl. Underlying these changing patterns of use are substantial expansions in the availability of methamphetamine throughout the United States. As the nation faces an accelerating overdose epidemic, these evolving use patterns demand an expanded, innovative community and health system responses. This response must engage multiple sectors in the community to comprehensively address the immediate challenge of rising overdose deaths and other health harms, better address the complex medical needs of persons using methamphetamine, and work to prevent future substance use, addiction, and overdose.
Footnotes
Competing interests
Unrelated to the submitted work, W.M.C. reports the ownership of stock in General Electric Co., 3M Co., and Pfizer Inc. All other authors declare no competing interests.
Disclaimer
The findings and conclusions of this study are those of the authors and do not necessarily reflect the views of the National Institute on Drug Abuse of the National Institutes of Health, the Centers for Disease Control and Prevention, or the U.S. Department of Health and Human Services.
Psychostimulants refers to the class of drugs included in the ICD-10 code T43.6—psychostimulants with abuse potential. This class of drugs does not include cocaine, which is coded under a separate ICD-10 code, T40.5 (cocaine). Some deaths identified as involving psychostimulants included in T43.6 may also involve cocaine.
References
- 1.Hedegaard H, Minino AM & Warner M. 2020. Drug overdose deaths in the United States, 1999–2019. NCHS Data Brief, no 394. Accessed July 14, 2021. https://www.cdc.gov/nchs/data/databriefs/db394-H.pdf. [PubMed]
- 2.Centers for Disease Control and Prevention. 2021. Accessed July 14, 2021. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm.
- 3.Mattson CL, Tanz LJ, Quinn K, et al. 2021. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013–2019. Morb. Mortal. Wkly. Rep 70: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedegaard H, Minino AM & Warner M. 2021. Co-involvement of opioids in drug overdose deaths involving cocaine and psychostimulants. NCHS Data Brief, no 406. Accessed July 14, 2021. https://www.cdc.gov/nchs/products/databriefs/db406.htm. [PubMed]
- 5.Han B, Cotto J, Etz K, et al. 2021. Methamphetamine overdose deaths in the US by sex and race and ethnicity. JAMA Psychiatry 78: 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr AM, Panenka WJ, MacEwan GW, et al. 2006. The need for speed: an update on methamphetamine addiction. J. Psychiatry Neurosci 31: 301–313. [PMC free article] [PubMed] [Google Scholar]
- 7.Voce A, Calabria B, Burns R, et al. 2019. A systematic review of the symptom profile and course of methamphetamine-associated psychosis. Subst. Use Misuse 54: 549–559. [DOI] [PubMed] [Google Scholar]
- 8.Prakash MD, Tangalakis K, Antonipillai J, et al. 2017. Methamphetamine: effects on the brain, gut and immune system. Pharmacol. Res 120: L60–L67. [DOI] [PubMed] [Google Scholar]
- 9.Wang TY, Fan TT, Bao YP, et al. 2017. Pattern and related factors of cognitive impairment among chronic methamphetamine users. Am. J. Addict 26: 145–151. [DOI] [PubMed] [Google Scholar]
- 10.Darke S, Duflou J & Kaye S. 2017. Prevalence and nature of cardiovascular disease in methamphetamine-related death: a national study. Drug Alcohol Depend. 179: 174–179. [DOI] [PubMed] [Google Scholar]
- 11.Cheng WS, Garfein RS, Semple SJ, et al. 2010. Increased drug use and STI risk with injection drug use among HIV-seronegative heterosexual methamphetamine users. J. Psychoactive Drugs 42: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strathdee SA & Stockman JK. 2010. Epidemiology of HIV among injecting and non-injecting drug users: current trends and implications for interventions. Curr. HIV/AIDS Rep 7: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham EB, Jacka B, DeBeck K, et al. 2015. Methamphetamine injecting is associated with phylogenetic clustering of hepatitis C virus infection among street-involved youth in Vancouver, Canada. Drug Alcohol Depend. 152: 272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darke S, Kaye S & Duflou J. 2017. Rates, characteristics and circumstances of methamphetamine-related death in Australia: a national 7-year study. Addiction 112: 2191–2201. [DOI] [PubMed] [Google Scholar]
- 15.Drug Enforcement Administration, Diversion Control Division. 2020. National Forensic Laboratory Information System: NFLIS-drug annual report 2019. Accessed July 23, 2021. https://www.nflis.deadiversion.usdoj.gov/nflisdata/docs/NFLIS-DRUG_2019_Annual_Report.pdf.
- 16.Jones CM, Olsen EO, O’Donnell J & Mustaquim D. 2020. Resurgent methamphetamine use at treatment admission in the United States, 2008–2017. Am. J. Public Health 110: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han B, Cotto J, Etz K, et al. 2021. Methamphetamine overdose deaths in the U.S. by sex and race and ethnicity. JAMA Psychiatry 7895: 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivolo-Kantor AM, Hoots BE, Seth P & Jones CM. 2020. Recent trends and associated factors of amphetamine-type stimulant overdoses in emergency departments. Drug Alcohol Depend. 216: 108323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoots B, Vivolo-Kantor A & Seth P. 2020. The rise in non-fatal and fatal overdoses involving stimulants with and without opioids in the United States. Addiction 115: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T, Spiller HA, Badeti J, et al. 2021. Methamphetamine exposures reported to United States poison control centers, 2000–2019. Clin. Toxicol 59: 705–714. [DOI] [PubMed] [Google Scholar]
- 21.Drug Enforcement Administration. 2020. National Drug Threat Assessment. Accessed July 20, 2021. https://www.dea.gov/sites/default/files/2021-02/DIR-008-21%202020%20National%20Drug%20Threat%20Assessment_WEB.pdf.
- 22.United Nations Office on Drugs and Crime. 2018. World drug report. Accessed July 21, 2021. https://www.unodc.org/wdr2018/.
- 23.Canadian Centre on Substance Use and Addiction. 2019. Canadian Community Epidemiology Network on Drug Use Bulletin: changes in stimulant use and related harms: focus on methamphetamine and cocaine. Accessed April 16, 2021. https://www.ccsa.ca/sites/default/files/2019-05/CCSA-CCENDU-Stimulant-Use-Related-Harms-Bulletin-2019-en.pdf.
- 24.Degenhardt L, Grant S, McKetin R, et al. 2017. Crystalline methamphetamine use and methamphetamine-related harms in Australia. Drug Alcohol Rev. 36: 160–170. [DOI] [PubMed] [Google Scholar]
- 25.Glick SN, Klein KS, Tinsley J & Golden MR. 2021. Increasing heroin-methamphetamine (Goofball) use and related morbidity among Seattle area people who inject drugs. Am. J. Addict 30: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glick SN, Burt R, Kummer K, et al. 2018. Increasing methamphetamine injection among non-MSM who inject drugs in King County, Washington. Drug Alcohol Depend. 182: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones CM, Underwood N & Compton WM. 2020. Increases in methamphetamine use among heroin treatment admissions in the United States, 2008–2017. Addiction 115: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsui JL, Mayfield J, Speaker EC, et al. 2020. Association between methamphetamine use and retention among patients with opioid use disorders treated with buprenorphine. J. Subst. Abuse Treat 109: 80–85. [DOI] [PubMed] [Google Scholar]
- 29.Amlani A, McKee G, Khamis N, et al. 2015. Why the FUSS (Fentanyl Urine Screen Study)? A cross-sectional survey to characterize an emerging threat to people who use drugs in British Columbia, Canada. Harm Reduct. J 12: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mellis AM, Potenza MN & Hulsey JN. 2021. COVID-19 related treatment service disruptions among people with single-and polysubstance use concerns. J. Subs. Abuse Treat 121: 108180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Center for Behavioral Health Statistics and Quality. 2020. Results from the 2019 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- 32.Miech RA, Johnston LD, O’Malley PM, et al. 2021. Monitoring the Future National Survey Results on drug use, 1975–2020: volume I, secondary school students. Ann Arbor, MI: Institute for Social Research, The University of Michigan. [Google Scholar]
- 33.Jones CM, Clayton HB, Deputy NP, et al. 2020. Prescription opioid misuse and use of alcohol and other substances among high school students – Youth Risk Behavior Survey, United States, 2019. MMWR Suppl. 69: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han B, Compton WM, Jones CM, et al. 2021. Trends in methamphetamine use, use disorder, and injection among adults in the United States. JAMA Psychiatry. Published online on September 22, 2021. http://Jamanetwork/2021/psy/09_22_2021/yoi210059pap. [Google Scholar]
- 35.Jones CM, Compton WM & Mustaquim D. 2020. Patterns and characteristics of methamphetamine use among adults – United States, 2015–2018. Morb. Mortal. Wkly. Rep 69: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palamar JJ, Han BH & Keyes KM. 2020. Trends in characteristics of individuals who use methamphetamine in the United States, 2015–2018. Drug Alcohol Depend. 213: 108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute on Drug Abuse, National Institutes of Health. 2021. Special internal analysis.
- 38.Fletcher JB, Swendeman D & Reback CJ. 2018. Mental health and substance use disorder comorbidity among methamphetamine—using men who have sex with men. J. Psychoactive Drugs 50: 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strickland JC, Havens JR & Stoops WW. 2019. A nationally representative analysis of “twin epidemics”: rising rates of methamphetamine use among persons who use opioids. Drug Alcohol Depend. 204: 107592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strickland JC, Stoops WW, Dunn KE, et al. 2021. The continued rise of methamphetamine use among people who use heroin in the United States. Drug Alcohol Depend. 225: 108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Tayyib A, Koester S, Langegger S & Raville L. 2017. Heroin and methamphetamine injection: an emerging drug use pattern. Subst. Use Misuse 52: 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniulaityte R, Silverstein SM, Crawford TN, et al. 2020. Methamphetamine use and its correlates among individuals with opioid use disorder in a midwestern U.S. city. Subst. Use Misuse 55: 1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones CM 2019. Syringe services programs: an examination of legal, policy, and funding barriers in the midst of the evolving opioid overdose crisis in the U.S. Int. J. Drug Policy 70: 22–32. [DOI] [PubMed] [Google Scholar]
- 44.Shearer RD, Howell BA, Bart G & Winkelman TNA. 2020. Substance use patterns and health profiles among US adults who use opioids, methamphetamine, or both, 2015–2018. Drug Alcohol Depend. 214: 108162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howell BA, Bart G, Wang EA & Winkelman TNA. 2021. Service involvement across multiple sectors among people who use opioids, methamphetamine, or both, United States—2015–2018. Med. Care 59: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellis MS, Kasper ZA & Cicero TJ. 2018. Twin epidemics: the surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend. 193: 14–20. [DOI] [PubMed] [Google Scholar]
- 47.Cicero TJ, Ellis MS & Kasper ZA. 2020. Polysubstance use: a broader understanding of substance use during the opioid crisis. Am. J. Public Health 110: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Public Law 109–177. Accessed July 14, 2021. https://www.deadiversion.usdoj.gov/meth/pl109_177.pdf.
- 49.U.S. Department of Justice, Drug Enforcement Administration. 2019. National Drug Threat Assessment. Washington, DC: U.S. Department of Justice, Drug Enforcement Administration. Accessed July 14, 2021. [Google Scholar]
- 50.U.S. Department of Justice, Drug Enforcement Administration. 2019. NFLIS-drug special report: methamphetamine reported in NFLIS, 2001–2017. Accessed July 14, 2021. https://www.nflis.deadiversion.usdoj.gov/publicationsRedesign.xhtml.
- 51.U.S. Department of Justice, Drug Enforcement Administration. 2021. NFLIS Drug snapshot. Accessed August 18, 2021. https://www.nflis.deadiversion.usdoj.gov/nflisdata/docs/NFLIS_Snapshot_032021.pdf.
- 52.Park JN, Rashidi E, Foti K, et al. 2021. Fentanyl and fentanyl analogs in the illicit stimulant supply: results from US drug seizure data, 2011–2016. Drug Alcohol Depend. 218: 108416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shukla RK, Crump JL & Chrisco ES. 2012. An evolving problem: methamphetamine production and trafficking in the United States. Int. J. Drug Policy 23: 426–435. [DOI] [PubMed] [Google Scholar]
- 54.National Institute on Drug Abuse. 2019. How is methamphetamine manufactured. Methamphetamine Research Report. North Bethesda, MD: National Institute on Drug Abuse. [Google Scholar]
- 55.Newton TF, De La Garza R, Kalechstein AD, et al. 2009. Theories of addiction: methamphetamine users’ explanations for continuing drug use and relapse. Am. J. Addict 18: 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maxwell JC 2014. A new survey of methamphetamine users in treatment: who they are, why they like “meth”, and why they need additional services. Subst. Use Misuse 49: 639–644. [DOI] [PubMed] [Google Scholar]
- 57.Diaz RM, Heckert AL & Sanchez J. 2005. Reasons for stimulant use among Latino gay men in San Francisco: a comparison between methamphetamine and cocaine users. J. Urban Health 82(1 Suppl. 1): i71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edmundson C, Heinsbroek E, Glass R, et al. 2018. Sexualized drug use in the United Kingdom (UK): a review of the literature. Int. J. Drug Policy 55: 131–148. [DOI] [PubMed] [Google Scholar]
- 59.McCall H, Adams N, Mason D & Willis J. 2015. What is chemsex and why does it matter? BMJ 351: h5790. [DOI] [PubMed] [Google Scholar]
- 60.Gilhart VL, Simms I, Jenkins C, et al. 2015. Sex, drugs, and smart phone applications: findings from semistructured interviews with men who have sex with men diagnosed Shigella flexneri 3a in England and Wales. Sex. Transm. Infect 91: 598–602. [DOI] [PubMed] [Google Scholar]
- 61.Compton WM & Jones CM. 2021. Substance use among men who have sex with men. N. Engl. J. Med 385: 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker R, Leichtling G, Hildebran C, et al. 2020. “Like Yin and Yang”: perceptions of methamphetamine benefits and consequences among people who use opioids in rural communities. J. Addict. Med 9: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopez AM, Dhatt Z, Howe M, et al. 2021. Co-use of methamphetamine and opioids among people in treatment in Oregon: a qualitative examination of interrelated structural, community, and individual-level factors. Int. J. Drug Policy 91: 103098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carlson RG, Daniulaityte R, Silverstein SM, et al. 2020. Unintentional drug overdose: is more frequent use of non-prescribed buprenorphine associated with lower risk of overdose? Int. J. Drug Policy 79: 102722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silverstein SM, Daniulaityte R, Getz K & Zule W. 2021. “It’s crazy what meth can help you do”: lay beliefs, practices, and experiences of using methamphetamine to selftreat symptoms of opioid withdrawal. Subst. Use Misuse 19: 1–10. [DOI] [PubMed] [Google Scholar]
- 66.Karamouzian M, Papamihali K, Graham B, et al. 2020. Known fentanyl use among clients of harm reduction sites in British Columbia, Canada. Int. J. Drug Policy 77: 103665. [DOI] [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2021. Overdose data to action. Accessed July 10, 2021. https://www.cdc.gov/drugoverdose/od2a/index.html.
- 68.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2021. Drug overdose surveillance and epidemiology. Accessed July 10, 2021. https://www.cdc.gov/drugoverdose/nonfatal/case.html.
- 69.O’Donnell J, Gladden RM, Mattson CL, et al. 2020. Vital signs: characteristics of drug overdose deaths involving opioid and stimulants – 24 states and the District of Columbia, January–June 2019. MMWR Morb. Mortal. Wkly. Rep 69: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palamar JJ, Lea A, Carr TH & Cottler LB. 2021. Shifts in drug seizures in the United States during the COVID-19 pandemic. Drug Alcohol Depend. 221: 108580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Community response plan template: guidance for coordinated response to rapid increases in drug overdose. Ohio Department of Health. [Google Scholar]
- 72.Washington/Baltimore high intensity drug trafficking area. ODMAP – Overdose Detection and Mapping Application. Accessed February 7, 2021. http://www.odmap.org/.
- 73.Marshall BD, Yedinak JL, Goyer J, et al. Development of a statewide, publicly accessible drug overdose surveillance and information system. Am. J. Public Health 107: 1760–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haas E, Truong C, Bartolomei-Hill L, et al. 2019. Rebbert-Franklin K. Local overdose fatality review team recommendations for overdose death prevention. Health Prom. Pract 20: 553–564. [DOI] [PubMed] [Google Scholar]
- 75.Bureau of Justice Assistance. Overdose fatality review data system guidance version 1.0. 2020. Accessed May 7, 2021. https://www.cossapresources.org/Content/Documents/Articles/OFR_Data_System_Guidance_Oct_2020.pdf.
- 76.Samuels EA, Clark SA, Wunsch C, et al. 2020. Innovation during COVID-19: improving addiction treatment access. J. Addict. Med 14: e8–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin LA, Fernandez AC & Bonar EE. 2020. Telehealth for substance-using populations in the age of coronavirus disease 2019: recommendations to enhance adoption. JAMA Psychiatry 77: 1209–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.CDC Foundation. 2020. Public Health and Safety Team (PHAST) Toolkit: guidance for data-driven overdose response coordination among public health, criminal justice, law enforcement, and first responders - draft version 2.0 for piloting. Accessed May 7, 2021. https://www.cdcfoundation.org/sites/default/files/files/PHAST_Web_Toolkit_Pilot_Version_2.0_For_Dissemination.pdf.
- 79.Carroll JJ, Noonan RK & Wolff J. 2020. Building effective public health and public safety collaborations to prevent opioid overdose at the local, state, and federal levels. In A Public Health Guide to Ending the Opioid Epidemic. Butler JC & Fraser MR. 241–252. Oxford University Press. [Google Scholar]
- 80.Office of National Drug Control Policy (ONDCP) and the Centers for Disease Control and Prevention. Overdose response strategy. Accessed July 14, 2021. https://www.hidta.org/ors/.
- 81.Yatsco AJ, Champagne-Langabeer T, Holder TF, et al. 2020. Developing interagency collaboration to address the opioid epidemic: a scoping review of joint criminal justice and healthcare initiatives. Int. J. Drug Policy 83: 102849. [DOI] [PubMed] [Google Scholar]
- 82.Brinkley-Rubinstein L, Zaller N, Martino S, et al. 2018. Criminal justice continuum for opioid users at risk of overdose. Addict. Behav 86: 104–110. [DOI] [PubMed] [Google Scholar]
- 83.Mitchell O, Wilson DB, Eggers A & MacKenzie DL. 2012. Assessing the effectiveness of drug courts on recidivism: a meta-analytic review of traditional and nontraditional drug courts. J. Crim. Justice 40: 60–71. [Google Scholar]
- 84.Collins SE, Lonczak HS & Clifasefi SL. 2017. Seattle’s Law Enforcement Assisted Diversion (LEAD): program effects on recidivism outcomes. Eval. Progr. Plann 64: 49–56. [DOI] [PubMed] [Google Scholar]
- 85.Schiff DM, Drainoni ML, Weinstein ZM, et al. 2017. A police-led addiction treatment referral program in Gloucester, MA: implementation and participants’ experiences. J. Subst. Abuse Treat 82: 41–47. [DOI] [PubMed] [Google Scholar]
- 86.LEAD National Support Bureau. Accessed May 10, 2021. https://www.leadbureau.org/.
- 87.The Police Assisted Addiction and Recovery Initiative. Accessed May 10, 2021. https://paariusa.org/.
- 88.Langabeer J, Champagne-Langabeer T, Luber SD, et al. 2020. Outreach to people who survive opioid overdose: linkage and retention in treatment. J. Subst. Abuse Treat 111: 11–15. [DOI] [PubMed] [Google Scholar]
- 89.Formica SW, Apsler R, Wilkins L, et al. 2018. Post opioid overdose outreach by public health and public safety agencies: exploration of emerging programs in Massachusetts. Int. J. Drug Policy 54: 43–50. [DOI] [PubMed] [Google Scholar]
- 90.Bagley SM, Schoenberger SF, Waye KM & Walley AY. 2019. A scoping review of post opioid-overdose interventions. Prev. Med 128: 105813. [DOI] [PubMed] [Google Scholar]
- 91.Bassuk EL, Hanson J, Greene RN, et al. 2016. Peer-delivered recovery support services for addictions in the United States: a systematic review. J. Subst. Abuse Treat 63: 1–9. [DOI] [PubMed] [Google Scholar]
- 92.Tracy K & Wallace SP. 2016. Benefits of peer support groups in the treatment of addiction. Subst. Abuse Rehabil 7: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams A, Nunes E & Olfson M. 2016. To battle the opioid overdose epidemic, deploy the ‘cascade of care’ model.
- 94.Centers for Disease Control and Prevention. Health Alert Network (HAN) No. 438 – increase in fatal drug overdoses across the United States driven by synthetic opioids before and during the COVID-19 pandemic. Accessed May 20, 2021. https://emergency.cdc.gov/han/2020/han00438.asp.
- 95.Kishore S, Hayden M & Rich J. 2019. Lessons from Scott County—progress or paralysis on harm reduction? N. Engl. J. Med 380: 1988–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lister JJ, Weaver A, Ellis JD, et al. 2020. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am. J. Drug Alcohol Abuse 46: 273–288. [DOI] [PubMed] [Google Scholar]
- 97.McMahan VM, Kingston S, Newman A, et al. 2020. Interest in reducing methamphetamine and opioid use among syringe services program participants in Washington State. Drug Alcohol Depend. 216: 108243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Substance Abuse and Mental Health Services Administration. State opioid response grants – funding opportunity announcement information – March 2020. Accessed May 20, 2021. https://www.samhsa.gov/grants/grant-announcements/ti-20-012.
- 99.Centers for Disease Control and Prevention. 2021. OD2A guidance.
- 100.Centers for Disease Control and Prevention. 2021. News Release. Accessed May 20, 2021. https://www.cdc.gov/media/releases/2021/p0407-Fentanyl-Test-Strips.html.
- 101.Ronsely C, Nolan S, Knight R, et al. 2020. Treatment of stimulant use disorder: a systematic review of review. PLoS One 15: e0234809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Crescenzo F, Ciabattini M, D’Alo GL, et al. 2018. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: a systematic review and network meta-analysis. PLoS Med. 15: e1002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown HD & DeFulio A. 2020. Contingency management for the treatment of methamphetamine use disorder: a systematic review. Drug Alcohol Depend. 216: 108307. [DOI] [PubMed] [Google Scholar]
- 104.Ginley MK, Pfund RA, Rash CJ & Zajac K. 2021. Long-term efficacy of contingency management treatment based on objective indicators of abstinence from illicit substance use up to 1 year following treatment: a meta-analysis. J. Consult. Clin. Psychol 89: 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.DePhillipis D, Petry NM, Bonn-Miller MO, et al. 2018. The national implementation of contingency management (CM) in the Department of Veterans Affairs: attendance at CM sessions and substance use outcomes. Drug Alcohol Depend. 185: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paulus MP & Stewart JL. 2020. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry 77: 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lappan SN, Brown AW & Hendricks PS. 2020. Dropout rates of in-person psychosocial substance use disorder treatments: a systematic review and meta-analysis. Addiction 115: 201–2017. [DOI] [PubMed] [Google Scholar]
- 108.McMahan WM, Kingston S, Newman A, Stekler JD, et al. 2020. Interest in reducing methamphetamine and opioid use among syringe services program participants in Washington State. Drug Alcohol Depend. 216: 108243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swedo EA, Sumner SA, de Fijter S, et al. 2020. Adolescent opioid misuse attributable to adverse childhood experiences. J. Pediatr 224: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Galea S & Vlahov D. 2002. Social determinants and the health of drug users: socioeconomic status, homelessness, and incarceration. Public Health Rep. 117(Suppl 1): S135. [PMC free article] [PubMed] [Google Scholar]
- 111.Compton WM, Jones CM, Baldwin GT, et al. 2019. Targeting youth to prevent later substance use disorder: an underutilized response to the US opioid crisis. Am. J. Public Health 109: S185–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spoth R, Redmond C, Shin C, et al. 2017. PROSPER delivery of universal preventive interventions with young adolescents: long-term effects on emerging adult substance misuse and associated risk behaviors. Psychol. Med 47: 2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spoth RL, Clair S, Shin C & Redmond C. 2006. Long-term effects of universal preventive interventions on methamphetamine use among adolescents. Arch. Pediatr. Adolesc. Med 160: 876–882. [DOI] [PubMed] [Google Scholar]
- 114.Spoth R, Redmond C, Shin C, Greenberg M, et al. 2007. Substance-use outcomes at 18 months past baseline: the PROSPER community-university partnership trial. Am. J. Prev. Med 32: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rivera AV, Harriman G, Carrillo SA & Braunstein SL. 2020. Trends in methamphetamine use among men who have sex with men in New York City, 2004–2017. AIDS Behav. 25: 1210–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ashok AH, Mizuno Y, Volkow ND & Howes OD. 2017. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry 74: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abbruscato TJ & Trippier PC. 2018. DARK classics in chemical neuroscience: methamphetamine. ACS Chem. Neurosci 9: 2373–2378. [DOI] [PubMed] [Google Scholar]
- 118.Mahoney JJ 3rd, Hanlon CA, Marshalek PJ, et al. 2020. Transcranial magnetic stimulation, deep brain stimulation, and other forms of neuromodulation for substance use disorders: review of modalities and implications for treatment. J. Neurol. Sci 418: 117149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ballester J, Valentine G & Sofuoglu M. 2017. Pharmacological treatments for methamphetamine addiction: current status and future directions, Expert Rev. Clin. Pharmacol 10: 305–314. [DOI] [PubMed] [Google Scholar]
- 120.Hossain MK, Hassanzadeganroudsari M, Nurgali K & Apostolopoulos V. 2020. Vaccine development against methamphetamine drug addiction. Expert Rev. Vaccines 19: 1105–1114. [DOI] [PubMed] [Google Scholar]
- 121.Xiaoshan T, Junjie Y, Wenqing W, et al. 2020. Immunotherapy for treating methamphetamine, heroin and cocaine use disorders. Drug Discov. Today 25: 610–619. [DOI] [PubMed] [Google Scholar]
- 122.Stevens MW, Tawney RL, West CM, et al. 2014. Preclinical characterization of an anti-methamphetamine monoclonal antibody for human use. mAbs 6: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Petry NM, DePhilippis D, Rash CJ, et al. 2014. Nationwide dissemination of contingency management: the Veterans Administration initiative. Am. J. Addict 23: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Petry NM 2010. Contingency management treatments: controversies and challenges. Addiction 105: 1507–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jones CM, Compton WM & Mustaquim D. 2020. Patterns and characteristics of methamphetamine use among adults – United States, 2015–2018. Morb. Mortal. Wkly. Rep 69: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jones CM, Olsen EO, O’Donnell J, et al. 2020. Resurgent methamphetamine use at treatment admission in the United States, 2008–2017. Am. J. Public Health 110: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]


