Abstract
Introduction
The addition of immune checkpoint inhibitors (ICIs) to conventional chemotherapy (CT) as first-line treatment improves survival in extensive-stage small-cell lung cancer (ES-SCLC). The aim of this meta-analysis was to determine the relative efficacy of first-line ICIs compared with CT in patients with ES-SCLC.
Methods
Two independent reviewers extracted relevant data according to PRISMA guidelines and assessed the risk of bias using the Cochrane Collaboration's risk-of-bias tool. Meta-analysis was conducted using random-effects models to calculate an average effect size for overall survival (OS), progression-free survival (PFS), and safety outcomes in the overall populations and clinically relevant subgroups.
Results
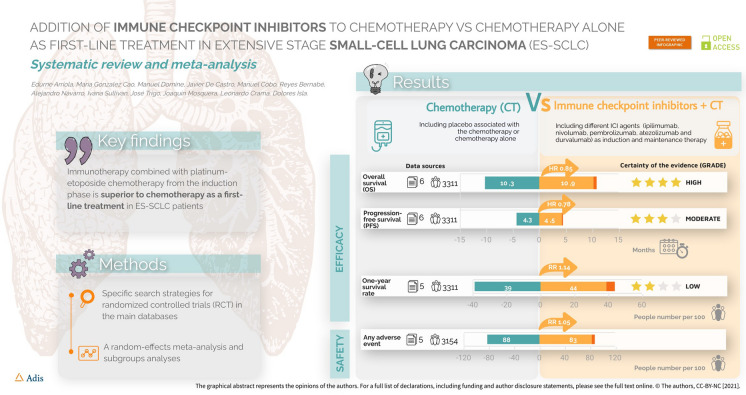
A literature search of PubMed and Embase was performed. Six randomized controlled clinical trials (IMpower133, CHECKMATE-451, CASPIAN, KEYNOTE-604, and phase II and III ipilimumab plus CT trials) with a total of 3757 patients were included. Compared with CT alone, ICIs plus CT showed a favourable effect on OS (hazard ratio [HR] 0.85; 95% confidence intervals [CI] 0.79–0.96) and PFS (HR 0.78; 95% CI 0.72–0.83) but a non-significant increase in the risk of experiencing any adverse event (relative risk, 1.05; 95% CI 0.99–1.11). The estimated HR for OS favoured ICI combinations in all planned subgroups according to age (< 65 years/≥ 65 years), sex (men/women), and ECOG performance status (0/1). Analysis by specific ICI revealed significant improvements in OS only for atezolizumab + CT (HR 1.36; 95% CI 1.09–1.69) and durvalumab + CT (HR 1.35; 95% CI 1.12–1.62) compared with CT alone.
Conclusion
Combining anti-programmed cell death ligand 1 antibodies with platinum/etoposide is a superior therapeutic approach compared to CT alone for the first-line treatment of patients with ES-SCLC.
Graphic abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40487-021-00182-0.
Keywords: Anti-PD-1/PD-L1 antibodies, Chemotherapy, Immunotherapy, Meta-analysis, Small cell lung carcinoma
Key Summary Points
| Why carry out this study? |
| Small-cell lung cancer is a very aggressive neoplasm with a dismal prognosis that accounts for 10–15% of all newly diagnosed lung cancers. |
| With the standard of care (combination of platinum agents with etoposide), most patients progress soon after initial treatment, with a median overall survival of 10 months. |
| In recent years, several studies have shown good results with the addition of checkpoint inhibitors as first-line treatment; however, more research is needed to deepen the knowledge regarding efficacy and safety, and subgroup analysis. |
| What was learned from the study? |
| The addition of immune checkpoint inhibitors to chemotherapy as first-line treatment improves the survival of patients with extensive-stage small-cell lung cancer, specifically in terms of overall survival and progression-free survival. |
| Among the analysed checkpoint inhibitors, atezolizumab and durvalumab combinations showed the best results. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.17429642.
Introduction
Lung cancer is one of the most common cancers globally, with 2.2 million new cases diagnosed in 2020 [1]. Small-cell lung cancer (SCLC) accounts for 10–15% of all newly diagnosed lung cancers, with more than 275,000 new cases diagnosed worldwide every year [2]. SCLC is a very aggressive neoplasm with few treatment options and a dismal prognosis [3]. Several factors can explain this poor outcome; the rapid growth rate and the early development of metastases mean that more than two thirds of patients present at diagnosis with extensive-stage (ES) disease [4].
Until recently, there had been no significant improvement in the effectiveness of treatments available for SCLC in the past few decades. The standard of care for ES-SCLC has been the combination of platinum agents (cisplatin or carboplatin) with etoposide as the first-line treatment [5]. Even though the objective response rate with this combination is 60–80% [6], most patients with SCLC experience disease progression soon after initial treatment, with a median overall survival of 10 months [7]. Several therapeutic approaches have been proposed in an attempt to improve the survival outcomes for SCLC patients. Among these, adding immunotherapy to conventional chemotherapy has recently been explored as a first-line strategy in ES-SCLC patients. Previous observations suggested a possible role of immunotherapy in SCLC and provided the rationale for the use of immune checkpoint inhibitors (ICIs). However, there were also reasons why immunotherapy may not be a good option for the treatment of SCLC. First, SCLC tumours have a high mutation burden due to the effect of tobacco exposure in its pathogenesis [8]. Second, immune-mediated paraneoplastic disorders are more common in SCLC than in other tumours [9]. Some paraneoplastic syndromes are associated with the production of presumed tumour neoantigen-directed humoral antibodies that cross-react with somatic antigens in other organs, such as neurological paraneoplastic syndromes. Finally, the last reason is the existence of exceptional long-term survivors after chemotherapy, accounting for fewer than 5% of cases from historical series [10]. Recent advances in immunotherapy include the development of therapeutic antibodies blocking the inhibitory checkpoint molecules programmed cell death protein-1 (PD-1)/programmed cell death ligand-1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibody that blocks the binding of CTLA-4 to the ligands CD80 and CD86. However, determining which SCLC patients derive benefit from PD-1/PD-L1-directed immunotherapy remains a key question. Unfortunately, the use of PD-L1 immunohistochemistry as a predictive biomarker may be confounded by multiple unresolved issues, such as variable detection antibodies, differing cutoffs, or tissue preparation. Indeed, preliminary data suggest that in SCLC patients, PD-L1 expression is low and does not appear to be a predictive biomarker of response to immunotherapy combined with chemotherapy [11, 12].
Although the addition of anti-PD-L1 to first-line chemotherapy with anti-PD-L1 maintenance is emerging as a promising therapeutic option, currently, the studies analysing longer-term follow-up are not available. Data on the benefits and risks of ICIs in combination with conventional chemotherapy as a first-line therapeutic strategy in patients with ES-SCLC are heterogeneous because different immunotherapy strategies have been tried in different trials of various designs. The aim of the present systematic review and meta-analysis is to analyse the efficacy and safety of adding ICIs to conventional chemotherapy compared with conventional chemotherapy alone or associated with placebo as first-line treatment in patients with ES-SCLC.
Methods
We conducted the current systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [13]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Identifying Relevant Studies
We performed a comprehensive literature search, and specific search strategies were designed for major citation databases, including MEDLINE and Embase databases, up to 17 July 2020. Free and controlled terminology (Medical Subject Headings [MeSH] and EMTREE, respectively) were used and combined by Boolean operators to develop the search strategies. Details of the search strategies are presented in Tables S1 and S2 in the online supplementary materials. The search was restricted to human studies, with no restrictions on the language of publication. Moreover, we checked the reference lists of all eligible primary studies and reviewed articles for additional potentially eligible studies.
Study Selection
Following the removal of duplicates, the titles and abstracts of retrieved articles were screened according to the following inclusion criteria: (1) randomized controlled trials (RCTs) published in any format (full paper, conference abstracts) with sufficient data available to estimate outcomes; (2) enrolled patients who were newly diagnosed with ES-SCLC (e.g., tumour beyond ipsilateral hemithorax and regional nodes including distant metastases, malignant pericardial or pleural effusions, and contralateral supraclavicular and contralateral hilar involvement) and previously untreated; (3) compared the combination of chemotherapy plus an ICI (including atezolizumab, durvalumab, pembrolizumab, nivolumab, and/or ipilimumab) with chemotherapy alone, with or without placebo, as the first-line treatment (chemotherapy could include cisplatin, cyclophosphamide, doxorubicin, vincristine, epirubicin, carboplatin, etoposide, irinotecan, or topotecan, in any combination); and (4) the primary outcome was survival, expressed as overall survival (OS), progression-free survival (PFS) and/or survival rates at 12 months, and/or adverse events. The exclusion criteria were as follows: (1) trials enrolling participants with non-small cell lung cancer or mixed populations; (2) studies analysing ICI monotherapy or using any ICI or chemotherapy not listed above; and (3) trials with a single arm or any other design (narrative reviews, editorial comments, and letters). Full-text versions of all those articles deemed potentially relevant and those whose inclusion was doubtful were obtained to verify that they explicitly met the inclusion/exclusion criteria.
Data Extraction and Risk-of-Bias Assessment
Relevant data from each included study were extracted according to predefined criteria and recorded using a specific extraction form. The data included publication details (e.g., the name of the first author, country, and publication year), characteristics of enrolled patients (e.g., age, number, sex, functional status), intervention details (e.g. drugs, dose, route of administration, duration of treatment), information about the study design (e.g., the type of blinding, type of control, methods used for randomization and allocation), survival outcomes (expressed as hazard ratio [HR] for OS and PFS and number of patients alive at 12 months and the end of follow-up), adverse events (any grade or severe [grades 3–5]) and any other relevant information such as study funding or any notable conflict of interest according to the authors' judgement). When multiple reports from the same study were identified, the information was collated so that each study (rather than each report) was the unit of interest in the review. For each trial, the combination of ICIs with chemotherapy was considered the experimental arm, and the use of chemotherapy alone or associated with placebo as the control arm.
The potential risk of bias of each included RCT was assessed using the criteria summarized in the Cochrane Handbook for Systematic Reviews of Interventions [14]: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and staff (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); (6) selective reporting of results (reporting bias); and (7) other biases. Each study was assessed for these potential sources of bias; the risk was categorized as low, high, or unclear, and the corresponding justification recorded. We used GRADEpro GDT software to assess the quality of the evidence and to create the 'summary of findings' tables (GRADEpro GDT) based on the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias). All decisions to downgrade the certainty of the evidence were identified and explained as needed.
Data Synthesis and Statistical Analysis
For time-to-event outcomes, the HR and corresponding 95% confidence interval (CI) were estimated. For OS and PFS outcomes, we calculated the median value of the durations reported by different studies to represent the assumed risk. For dichotomous variables, the risk ratio (RR) and 95% CIs were calculated for each study. A meta-analysis was performed for each outcome with a random-effects model to account for heterogeneity, using Review Manager (version 5.4) software. Statistical heterogeneity between trials in each meta-analysis was assessed using the I2 statistic, and values of 25%, 50%, and 75% were considered to indicate low, moderate, and high heterogeneity, respectively [15]. Where heterogeneity I2 was ≥ 20%, possible sources of heterogeneity were explored by subgroup analysis. A p value of < 0.05 was regarded as statistically significant for all statistical analyses, and all tests were two-sided. Publication bias was evaluated by examining the asymmetry of the funnel plot when ≥ 10 studies were pooled. Predefined subgroup analyses were performed to investigate the sources of heterogeneity for primary outcomes (I2 ≥ 20%), according to the following characteristics: (a) different ICIs, and (b) different chemotherapy regimens. In addition, to answer specific questions about subsets of patients, subgroup analyses were performed based on the following variables: age, sex, presence of serum biomarkers (lactate dehydrogenase [LDH] levels), presence of brain or liver metastases, functional status according to Eastern Cooperative Oncology Group (ECOG)/World Health Organization (WHO) criteria, and type of ICI in long-term survivors (> 12 months). Sensitivity analyses were also conducted by (1) restricting the initial analysis to phase III trials, (2) removing from the initial analysis studies with a high risk of bias in at least one critical domain, and (3) using both fixed-effects and random-effects models for the analysis.
Public and Patient Involvement
There was no public or patient involvement in the conduct of this study.
Ethics Statement
This project was based on secondary analysis of existing data; therefore, ethics approval was not required.
Results
Eligible Studies
Our search retrieved 708 references. A single reviewer reviewed and selected the final chosen studies from the references obtained. After excluding duplicates and screening titles of the studies, 47 publications were selected based on relevance to the study topic. After abstract and full article review, 20 publications [16–35] corresponding to six studies met the inclusion criteria and were included for analysis in the present review. The PRISMA flowchart of this process is provided in Figure S1 in the online supplementary material.
Characteristics of Included Studies
All six studies included in this meta-analysis (IMPOWER-133 [16–24], CHECKMATE-451 [27], CASPIAN [25, 28–31], KEYNOTE-604 [35], and two studies by Reck et al. [32, 34]) were parallel-group RCTs; five were phase III studies, and one was a phase II study. These studies included 3757 patients with ES-SCLC (1628 received chemotherapy plus ICI, 1582 were treated with chemotherapy alone, and 547 were treated with other combinations). The mean age of subjects was 62.4 years (range 24–90 years), 58.6% were male patients, 56.0% were current smokers, and 26.4% were ex-smokers. The mean duration of follow-up was 13.4 months (range 9.0–21.6). None of the included studies provided data about the presence of oncogenic driver mutations in analysed populations. Patients received various ICI agents in combination with chemotherapy, including ipilimumab [33, 34], nivolumab [27], pembrolizumab [35], atezolizumab [18, 22], and durvalumab [31] as induction therapy. Only one study [27] investigated maintenance therapy with ICI plus chemotherapy in ES-SCLC patients; the ICI in this study was nivolumab. Except for the CASPIAN study, in which the comparator was chemotherapy alone [31], all trials included placebo associated with the chemotherapy comparator arm. The characteristics of the studies included in the meta-analysis are summarized in Table 1.
Table 1.
Characteristics of the trials included in the meta-analysis
| Author, year | Study acronym | Study phase | Total no. | Group | No. | Treatment arms (drug and dose) |
|---|---|---|---|---|---|---|
| Reck et al. 2013 [33] | – | II | 130 | 1 | 43 | Concurrent regimen (4 doses of ipilimumab/paclitaxel/carboplatin followed by 2 doses of placebo/paclitaxel/carboplatin) |
| 2 | 42 | Phased regimen (2 doses of placebo/paclitaxel/carboplatin followed by 4 doses of ipilimumab/paclitaxel/carboplatin) | ||||
| 3 | 45 | Control regimen (up to 6 doses of placebo/paclitaxel/carboplatin) (control group) | ||||
| Reck et al. 2016 [34] | – | III | 1132 | 1 | 566 | Etoposide 100 mg/m2 IV and cisplatin 75 mg/m2 IV or carboplatin area under the concentration–time curve 5 IV (cycles 1–4 of induction) plus ipilimumab 10 mg/kg (cycles 3–4 of induction) + ipilimumab alone (cycles 5 to 6) |
| 2 | 566 | Etoposide 100 mg/m2 IV and cisplatin 75 mg/m2 IV or carboplatin area under the concentration–time curve 5 IV (cycles 1–4 of induction) plus placebo (cycles 3–4 of induction) + placebo alone (cycles 5–6) (control group) | ||||
| Rudin et al. 2020 [35] | KEYNOTE-604 | III | 453 | 1 | 228 | Etoposide 100 mg/m2 + carboplatin/cisplatin 75 mg/m2 + pembrolizumab 200 mg (first 4 cycles) |
| 2 | 225 | Etoposide 100 mg/m2 + carboplatin/cisplatin 75 mg/m2 + placebo (first 4 cycles) (control group) | ||||
| Paz-Ares et al. 2019 [31] | CASPIAN | III | 805 | 1 | 268 | Etoposide 80–100 mg/m2 + carboplatin/cisplatin 75–80 mg/m2 + 1500 mg durvalumab (4 cycles) |
| 2 | 268 | Etoposide 80–100 mg/m2 + carboplatin/cisplatin 75–80 mg/m2 + 1500 mg durvalumab (4 cycles) + tremelimumab 75 mg | ||||
| 3 | 269 | Etoposide 80–100 mg/m2 + carboplatin/cisplatin 75–80 mg/m2 (control group) | ||||
| Horn et al. 2018 [18] | IMPOWER-133 | I/III | 403 | 1 | 201 | Etoposide 100 mg/m2 + carboplatin + atezolizumab 1200 mg (4 cycles) |
| 2 | 202 | Etoposide 100 mg/m2 + carboplatin + placebo (4 cycles) | ||||
| Owonikoko et al. 2019 [27] | CHECKMATE-451 | III | 834 | 1 | 280 | Platinum-based chemotherapy (4 cycles) + nivolumab 240 mg |
| 2 | 279 | Platinum-based chemotherapy (4 cycles) + nivolumab 1 mg/kg + ipilimumab 3 mg/kg (max 4 doses) | ||||
| 3 | 275 | Platinum-based chemotherapy (4 cycles) + placebo (control group) |
IV intravenously
Risk of Bias of the Included Studies
Overall, the studies were considered adequate for performing random sequence generation as well as having a low risk of detection and reporting bias. Five of the trials required the masking of participants and personnel, but one study [29] was open-label, and therefore the risk of selection bias for this study was considered high. Four trials [27, 31, 34, 35] reported complete outcome data, but in two studies [18, 34] there was an imbalance between the trial arms in the withdrawal rate. This is likely to have affected the comparability between the treatment arms, and the risk of attrition bias was considered high.
In four studies [18, 31, 34, 35], a significant imbalance between study groups in the number of patients undergoing prophylactic cranial irradiation may also represent a potential source of bias. Detailed information on the risk of bias of included RCTs is shown in Figure S2 in the online supplementary material.
Efficacy Meta-Analysis
Overall Survival
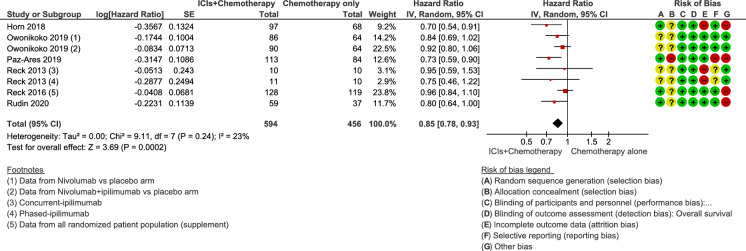
The median OS with chemotherapy plus ICIs was 10.90 months (range from 9.10 months [33] to 13.00 months [31]), while the median OS in the control group receiving chemotherapy alone or combined with placebo was 10.30 months (range 9.60 months [27] to 10.90 months [34]). The HR for OS in the individual studies ranged from 0.70 (95% CI 0.54–0.91) [18] to 0.95 (95% CI 0.59–1.54) [33]. The lowest and statistically significant HR values (0.70 and 0.73) were reported in studies with anti-PD-L1 agents (atezolizumab and durvalumab) in combination with chemotherapy. Across all the studies, the estimated HR for OS was 0.85 (95% CI 0.78–0.93; p = 0.0002), indicating a significant improvement in median OS for the combinations of chemotherapy plus ICIs versus chemotherapy alone or in combination with placebo (Fig. 1). Statistical heterogeneity among trials was low (p = 0.24, I2 = 23%), and the quality of the evidence was rated as high.
Fig. 1.
Forest plot of comparison of overall survival in patients receiving chemotherapy plus immune checkpoint inhibitors (ICIs) versus chemotherapy alone or combined with placebo. CI confidence interval, IV inverse variance, SE standard error
Progression-Free Survival
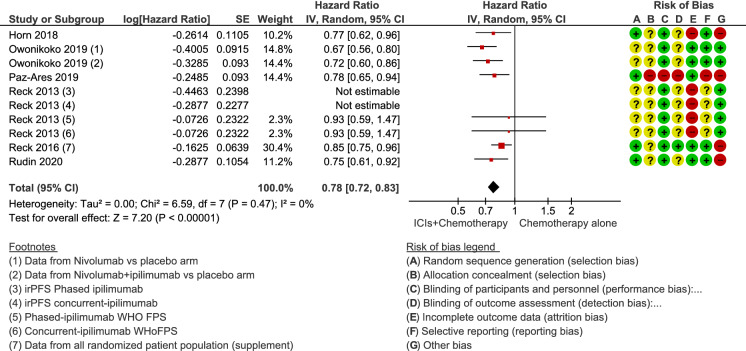
The median PFS in patients treated with chemotherapy plus ICIs was 4.55 months (range 1.70 months [27] to 5.20 months [33]), while the median PFS in those treated with chemotherapy alone or in combination with placebo was 4.35 months (range 1.40 months [27] to 5.40 months [31]). In individual studies, the HR of PFS ranged from 0.64 (95% CI 0.40–1.02) [33] to 0.85 (95% CI 0.75–0.97) [34]. In the meta-analysis, the estimated HR was 0.78 (95% CI 0.72–0.83; p < 0.00001) (Fig. 2), indicating an improvement in median PFS for the combination of ICIs and chemotherapy versus chemotherapy alone or in combination with placebo. There was no statistical heterogeneity between the studies (I2 = 0%; p = 0.47). The quality of the evidence was rated as moderate, mainly due to the inclusion of trials with a high or unclear risk of bias.
Fig. 2.
Forest plot of comparison of progression-free survival in patients receiving chemotherapy plus immune checkpoint inhibitors (ICIs) versus chemotherapy alone or combined with placebo. CI confidence interval, IV inverse variance, SE standard error
One-Year Survival Rate
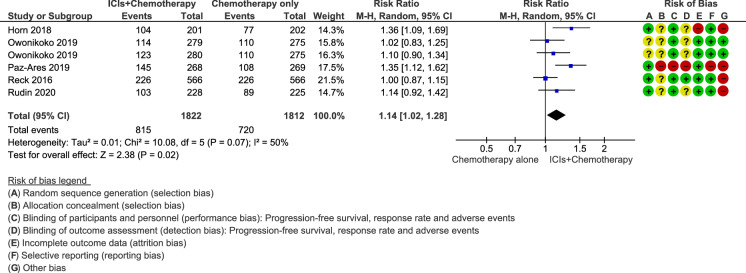
In all studies, one-year survival rates with chemotherapy plus ICIs were slightly better than those with chemotherapy alone or combined with placebo. The estimated RR was 1.14 (95% CI 1.02–1.28; p = 0.02), showing a statistically significant reduction in the risk of death in favour of the combination of ICIs and chemotherapy (Fig. 3). Moderate statistical heterogeneity was detected in this analysis (I2 = 50%; p = 0.07). The quality of the evidence was rated as low, mainly due to the inconsistency between the included studies and the inclusion of trials with a high or unclear risk of bias.
Fig. 3.
Forest plot of comparison of 12-month survival rate in patients receiving chemotherapy plus immune checkpoint inhibitors (ICIs) versus chemotherapy alone or combined with placebo. CI confidence interval, MH Mantel–Haenszel
Safety
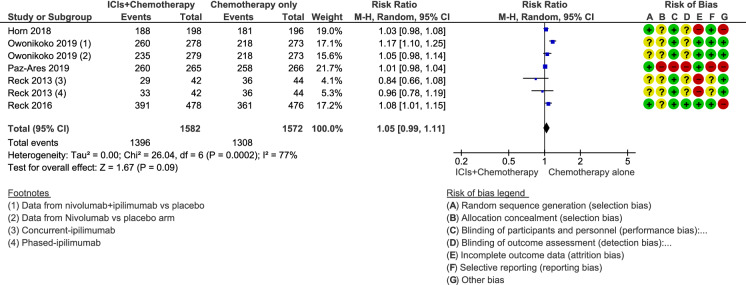
Adverse Events
The analysis showed that the addition of ICIs to chemotherapy alone or with placebo did not increase the risk of experiencing adverse events among ES-SCLC patients (RR 1.05; 95% CI 0.99–1.11; p = 0.09; Fig. 4). Statistical heterogeneity was high for this analysis (I2 = 77%; p < 0.0002). Independent meta-analysis according to the type of adverse event showed a significantly higher incidence of rash, dizziness, fatigue, loss of appetite, elevated transaminase levels, abdominal pain, colitis, thyroid function disorders, hypothermia, and pneumonitis among patients treated with ICIs and chemotherapy versus those treated with chemotherapy alone or combined with placebo.
Fig. 4.
Forest plot of comparison of adverse event rate in patients receiving chemotherapy plus immune checkpoint inhibitors (ICIs) versus chemotherapy alone or combined with placebo. CI confidence interval, MH Mantel–Haenszel
Severe Adverse Events
Adverse events of grades 3–5, according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), were reported in all included studies [18, 22, 27, 31, 33–35]. Immune-related adverse events were reported when ICIs were added to chemotherapy. However, the estimated RR showed that patients receiving ICIs in combination with chemotherapy did not have a significantly higher risk of serious adverse events than controls (RR 1.15, 95% CI 0.95–1.39).
Subgroup and Sensitivity Analyses
In terms of OS, subgroups according to age (< 65/≥ 65 years), sex (men/women), and baseline functional status (0/1) showed an estimated HR in favour of ICIs combined with chemotherapy versus chemotherapy alone or combined with placebo (Figures S3, S4 and S5, respectively, in the supplementary material). In the subgroup analysis, according to the presence of elevated levels of serum LDH (LDH ≤ upper limit of normal (ULN)/LDH > ULN), there was no significant difference in OS between ICI and chemotherapy combined treatment and chemotherapy without ICIs in either subgroup (Figure S6 in the supplementary material). The estimated HR for OS was in favour of combined therapy in the subgroup of patients without brain metastases at diagnosis, but there were few patients in the subgroup with brain metastases, so CIs were wide, and no conclusions could be drawn (Figure S7 in the supplementary material). In the subgroup analysis by liver metastases, the estimated HR for OS favoured combined treatment (Figure S8 in the supplementary material). Subgroup analysis by specific ICI agent revealed a significantly higher rate of 12-month survivors in those receiving atezolizumab (HR 1.36, 95% CI 1.09–1.69) or durvalumab (HR 1.35, 95% CI 1.12–1.62) in combination with chemotherapy versus chemotherapy alone. For the other ICIs, the HR did not reach statistical significance (pembrolizumab HR 1.14 [95% CI 0.92–1.42], ipilimumab HR 1.00 [95% CI 0.87–1.15], and nivolumab HR 1.06 [95% CI 0.92–1.22]), indicating that in these subgroups, the proportion of 12-month survivors showed little or no difference versus chemotherapy alone or combined with placebo (Figure S9 in the supplementary material).
The results of the sensitivity analyses were consistent with the main analysis for primary outcomes after limiting the analysis to phase III trials or removing studies with a high risk of bias, or using different statistical models for the analysis.
Discussion
This meta-analysis included six phase-II/III RCTs in a total of 3757 patients, evaluating the benefits and risks of adding ICIs to conventional chemotherapy compared with conventional chemotherapy alone or associated with placebo as first-line treatment for patients with ES-SCLC. High, moderate, and low quality of evidence, respectively, showed that the OS and PFS were longer and 12-month survival rates higher in patients receiving chemotherapy plus ICIs than in those receiving chemotherapy alone or combined with placebo, without a statistically significant increase in the risk of experiencing adverse events of any severity. The addition of an ICI agent to chemotherapy reduced the risk of death by 15% (HR 0.85, 95% CI 0.78–0.93) and improved the relative 1-year survival rate by 14% (RR 1.14, 95% CI 1.02–1.28), with absolute improvement in 1-year survival of 5%. The subgroup analyses for OS showed that the benefit from the addition of ICIs did not differ significantly across subgroups defined by age, sex, or baseline functional status. However, in the subgroup analysis by individual ICI agent, the combination of chemotherapy with either atezolizumab or durvalumab (PD-L1 inhibitors) significantly increased the proportion of 12-month survivors compared with chemotherapy only, whereas the combinations with ipilimumab (CTLA-4 inhibitor), pembrolizumab, or nivolumab (PD-L1 inhibitors) did not significantly increase the proportion of 12-month survivors compared with chemotherapy alone ± placebo. Although all studies included in the present meta-analysis were RCTs, some factors such as study limitations or inconsistency of results downgraded the quality of evidence for some outcomes.
Our findings suggest several implications for clinical practice. Notably, the debate regarding the superiority of ICI as induction or maintenance therapy in combination with chemotherapy persists. In our meta-analysis, only one study [27] investigated nivolumab plus chemotherapy as maintenance therapy in ES-SCLC patients. The results demonstrated that maintenance therapy with nivolumab did not prolong OS vs placebo for ES-SCLC patients. Indeed, our subgroup analysis looking at the relative efficacy for each type of ICI showed that only atezolizumab and durvalumab combined with chemotherapy exhibited improved OS compared with chemotherapy alone ± placebo, but no difference in the proportion of 12-month survivors was seen with nivolumab maintenance therapy compared with chemotherapy ± placebo. In addition, in the nivolumab study, the analysis of PFS took into account data starting from the randomization phase; this may explain why the PFS results with nivolumab are clearly different from those with the other ICIs. Therefore, ICI as maintenance therapy in combination with a chemotherapy regimen deserves further exploration, including the study of the mechanisms involved.
SCLC often causes brain or liver metastases, which are historically associated with a poor prognosis and for which no specific treatment is available. However, few data are available about the efficacy of immunotherapy in SCLC patients with brain or liver metastases, since most RCTs assessing immunotherapy in first-line settings excluded patients with active or untreated metastases. In our subgroup analysis, an OS benefit of the combination of ICI with chemotherapy was observed only in the subgroup without brain metastases at diagnosis. However, the ICI and chemotherapy combination did confer a benefit irrespective of the presence of liver metastases, despite these patients having a worse prognosis. It should be remembered, however, that these observations are based on subgroup analyses from larger trials that did not assess the specific characteristics of the central nervous system (CNS) disease or the role of prophylactic cranial irradiation (PCI). In this sense, the role of PCI in ES-SCLC patients and its potential benefit in combination with immunotherapy have been poorly explored, and efficacy and safety data regarding the combination of cranial radiation plus ICIs remain sparse. In our meta-analysis, only two trials [19, 33] reported the number of participants who underwent PCI, and neither study included more than 12% of PCI patients in any treatment arm, whereas up to 23% of patients with ES-SCLC undergo PCI in clinical practice [36]. Data from an exploratory analysis of the IMpower133 study showed a delay in intra-cranial progression with atezolizumab combined with chemotherapy vs placebo combined with chemotherapy [35], suggesting potential CNS efficacy in a disease in which brain metastases are common. However, to date, studies assessing the role of ICIs in patients with brain metastases are limited, and the generalizability of ICI data to this group is still challenging.
Biomarkers may play a key role in the treatment of ES-SCLC, which is characterized by multiple genetic defects and cellular heterogeneity. In our subgroup analysis, there was little or no difference in the effect of ICI plus chemotherapy on OS between subgroups with elevated or low levels of serum LDH. The two trials included in this analysis were consistent in showing that LDH level is not a biomarker for lack of benefit [34, 35]. These results demonstrate that there is still a lack of biomarkers to select treatment for SCLC patients and highlight the need to identify biomarkers that are able to distinguish between patients likely or unlikely to benefit from ICI plus chemotherapy combined therapy.
Our meta-analysis suggests that combination treatment with ICI plus chemotherapy is an effective and tolerable option for first-line treatment for previously untreated ES-SCLC patients. Our results are consistent with those of previous meta-analyses that have also evaluated the efficacy and safety of ICIs in combination with chemotherapy [37–42] and reported survival results in favour of the combination of immunotherapy with chemotherapy. The HR for survival varied slightly between these meta-analyses, from 0.76 [39] to 0.84 [40] for OS and from 0.75 [39] to 0.82 [37] for PFS. Moreover, as in our analysis, two of the previous meta-analyses reported responses at 12 months that also favoured combined therapy [38, 39]. The earlier meta-analyses were based on four or five trials (in some cases including only completed phase II–III RCTs [42]), whereas our analysis included all these studies in addition to one new trial [27], as well as data from longer-term follow-up (at 18 and 24 months) in two of the included studies [20, 31]. Previous meta-analyses also included populations with limited-stage SCLC (not only ES-SCLC) [41] or other therapeutic strategies in addition to immunotherapy-based combination (e.g., standard-of-care or ICI monotherapy) [40, 41]. In contrast, we excluded all trials enrolling patients with limited-stage SCLC or exploring ICI monotherapy or other immunotherapeutic agents, in order to maintain uniformity across the two arms in our meta-analysis. These differences may explain the slight discrepancies between our results and those of the previous meta-analyses.
Unlike other previous studies comparing OS and PFS across different therapeutic regimens plus chemotherapy, our work provides unique and valuable information regarding specific subgroup analysis (including levels of serum LDH or presence of metastases, for example) and longer-term safety analysis for previously untreated ES-SCLC, all supported by robust methods. Subgroup analyses are often performed as part of the analysis of intervention studies to assess whether treatment effects vary across subpopulations. Such analyses can provide valuable information for hypothesis generation, but they are only conducted in an exploratory manner. Consequently, the results identified through these analyses require further studies correctly designed and powered as the primary objective for drawing firm conclusions. Furthermore, other strengths of our study include the application of a strict set of eligibility criteria, especially regarding population and intervention characteristics and specific analysis of the effects of the combination of ICI with chemotherapy in several different subsets of patients. Another strength is the use of the random-effects model to minimize the influence of heterogeneity and performing subgroup analyses to explore the detected heterogeneity and identify possible modifiers, which are often not taken into account by researchers and which are important when evaluating the results of immunotherapy.
However, the present meta-analysis has certain limitations. First, we explored a limited number of databases and therefore may have missed studies published in journals not covered by those databases. Second, arguments have been made that the single screening of the titles and abstracts of studies retrieved in bibliographic searches is not equivalent to screening by two reviewers, as substantially more studies may be missed. However, in our work, the study selection was conducted by an experienced reviewer and the results were validated independently by another investigator, minimizing the likelihood of errors and reducing the possibility that the selection process was influenced by a single reviewer's interpretation and the missing studies. Third, few studies were included in our review, and most of them had small sample sizes, although we did not detect publication bias in the main analyses. Fourth, the trials analysed in our review did not take into account gene mutation status or potential predictive factors of benefit to ICI (e.g., tumour mutational burden and PD-L1 expression) in deciding the initial approach in ES-SCLC patients. Lastly, although our meta-analysis was undertaken using frequentist methods, in general terms, a Bayesian approach is increasingly used to assess the variation between studies in a random-effects analysis. This Bayesian approach is particularly advantageous when the number of studies in the meta-analysis is small (fewer than 5 or 10). In our review, Tau2 (a measure of the between-study variability in treatment effect) is mostly zero, likely not because there is no variability in the treatment effect but because there are too few studies to estimate it. These limitations may have affected our final results analysis, although it is difficult to quantify their impact. Therefore, more randomized, multicentre, and large-scale studies are needed to further explore these unaddressed questions and novel combinations of ICI and chemotherapy as the first-line treatment in untreated ES-SCLC patients.
Conclusion
The addition of ICIs to chemotherapy as first-line treatment improves the survival of patients with ES-SCLC compared with chemotherapy alone. Benefits were observed in terms of OS and PFS, while there was no significant increase in the rate of adverse events. Atezolizumab and durvalumab combinations showed the best results. These data confirm that adding immunotherapy, specifically anti-PD-L1 agents, to platinum-etoposide chemotherapy from the induction phase is currently a superior therapeutic approach for patients with ES-SCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This work was supported by Roche, including the Journal’s Rapid Service Fee.
Medical writing/Editorial assistance
The authors thank Esther Martin, who wrote this manuscript on behalf of Springer Healthcare Communications, and to Catherine Rees from Springer Healthcare Communications, who edited the English language. This medical writing assistance was funded by Roche.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
Conceptualization: all authors; methodology: EA, MGC, DI, LC; formal analysis: all authors; data curation: EA, MGC, DI, LC; writing (original draft preparation): MGC, EA, LC; writing (reviewing and editing): all authors; supervision: LC, MGC, EA. All authors have read, approved and agreed to submit this version of the manuscript for publication.
Disclosures
Dr Arriola has received honoraria (self) from Roche, Merck, BMS, AstraZeneca, Lilly, Pfizer, Boehringer, Takeda; advisory/consultancy fees from Roche, Merck, BMS, AstraZeneca, Lilly, Pfizer, Boehringer, Takeda; speaker fees from Roche, Merck, BMS, AstraZeneca, Lilly, Pfizer, Boehringer and Takeda; research grant/funding (institution) from Roche, BMS, Pfizer, Boehringer; travel/accommodation/expenses from Roche, Merck, BMS, AstraZeneca, Lilly, Pfizer and Boehringer. Dr Domine has received advisory/consultancy fees from Roche, Merck, BMS, AstraZeneca, Pfizer, Abbvie, Boehringer; speaker fees from Roche, Merck, BMS, AstraZeneca, Pfizer; travel/accommodation/expenses from Roche, BMS, AstraZeneca, Lilly, Pfizer and Boehringer. Dr Castro has received advisory/consultancy fees from Roche, Merck, BMS, AstraZeneca, Pfizer, PharmaMar, Boehringer, Takeda, Tesauro; speaker fees from Roche, Merck, AstraZeneca, Boehringer, Takeda; travel/accommodation/expenses from Roche, Merck, BMS and AstraZeneca. Dr Cobo has received advisory/consultancy fees from Roche, BMS, AstraZeneca, Pfizer, Boehringer; travel/accommodation/expenses from Roche, BMS, AstraZeneca. Dr Navarro has received advisory/consultancy from Roche, Pfizer, Boehringer; speaker fees from Roche and BMS; travel/accommodation/expenses from Pfizer and Boehringer; and has had a leadership role (medical monitor) for Oryzon Genomics. Dr Sullivan has participated in advisory boards for Roche, Novartis and Boehringer Ingelheim; acted as a speaker for Roche, Merck Sharp & Dohme, Pfizer, Bristol-Myers Squibb, AstraZeneca; received travel grants from Roche, Novartis, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim. Dr Trigo has received advisory/consultancy fees from Merck, BMS, GSK, Boehringer, Takeda; speaker fees from Roche, Merck, BMS, AstraZeneca, Bayer, Boehringer; travel/accommodation/expenses from Roche, Merck, BMS and AstraZeneca. Dr Mosquera has acted as a speaker for Roche, BMS, AstraZeneca and received travel/accommodation/expenses from Roche, Merck and Lilly. Dr Isla has received advisory/consultancy fees from Roche, BMS, AstraZeneca, Lilly, Pfizer, Bayer, Abbvie, Boehringer, Takeda, Sanofi; speaker fees from Roche, BMS, AstraZeneca, Lilly, Pfizer, Bayer, Abbvie, Boehringer and Takeda; research grant/funding (self) from Roche, BMS and AstraZeneca. Dr Crama is an employee of Roche Farma, Spain. The other authors (Dr González Cao and Dr Bernabé) have declared no potential conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analysed during the current study are available in the vivli repository (https://vivli.org/). Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Contributor Information
Edurne Arriola, Email: earriola@parcdesalutmar.cat.
María González-Cao, Email: mgonzalezcao@oncorosell.com.
Manuel Domine, Email: mdomine@fjd.es.
Javier De Castro, Email: javier.decastro@salud.madrid.org.
Manuel Cobo, Email: manuelcobodols@yahoo.es.
Reyes Bernabé, Email: mariar.bernabe.sspa@juntadeandalucia.es.
Alejandro Navarro, Email: anavarro@vhio.net.
Ivana Sullivan, Email: ISullivan@santpau.cat.
José Manuel Trigo, Email: jmtrigo@seom.org.
Joaquín Mosquera, Email: Joaquin.mosquera.martinez@sergas.es.
Leonardo Crama, Email: leonardo.crama@roche.com.
Dolores Isla, Email: disla@salud.aragon.es.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.van Meerbeeck JP, Fennell DA, De Ruysscher DKM (2011) Small-cell lung cancer. Lancet378:1741–1755. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673611601657 [DOI] [PubMed]
- 3.Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol. 2020;17:300–312. doi: 10.1038/s41571-019-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM (2008) Bronchopulmonary neuroendocrine tumors. Cancer [Internet]. Cancer 113: 5–21. Cited 14 Dec 2020. Available from: https://pubmed.ncbi.nlm.nih.gov/18473355/ [DOI] [PubMed]
- 5.Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E (2013) Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24:vi99–vi105. Cited 17 Aug 2020. Available from: https://pubmed.ncbi.nlm.nih.gov/23813929/ [DOI] [PubMed]
- 6.Rossi A, Di Maio M, Chiodini P, Rudd RM, Okamoto H, Skarlos DV, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. JCO. 2012;30:1692–1698. doi: 10.1200/JCO.2011.40.4905. [DOI] [PubMed] [Google Scholar]
- 7.Konala VM, Madhira BR, Ashraf S, Graziano S (2020) Use of immunotherapy in extensive-stage small cell lung cancer. Oncology 98: 749–754. Cited 9 Dec 2020. Available from: https://pubmed.ncbi.nlm.nih.gov/32663833/ [DOI] [PubMed]
- 8.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al (2012) Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482: 400–404. Available from: http://www.nature.com/articles/nature10755 [DOI] [PMC free article] [PubMed]
- 9.Pelosof LC, Gerber DE (2010) Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc 85: 838–854. Cited 15 Dec 2020. Available from: https://pubmed.ncbi.nlm.nih.gov/20810794/ [DOI] [PMC free article] [PubMed]
- 10.Lassen U, Osterlind K, Hansen M, Dombernowsky P, Bergman B, Hansen HH (1995) Long-term survival in small-cell lung cancer: posttreatment characteristics in patients surviving 5 to 18+ years--an analysis of 1,714 consecutive patients. J Clin Oncol 13:1215–1220. Cited 10 Dec 2020. Available from: https://pubmed.ncbi.nlm.nih.gov/7738624/ [DOI] [PubMed]
- 11.Paz-Ares L, Goldman JW, Garassino MC, Dvorkin M, Trukhin D, Statsenko G, et al. PD-L1 expression, patterns of progression and patient-reported outcomes (PROs) with durvalumab plus platinum-etoposide in ES-SCLC: results from CASPIAN. Ann Oncol. 2019;30:v928–v929. doi: 10.1093/annonc/mdz394.089. [DOI] [Google Scholar]
- 12.Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133) JCO. 2021;39:619–630. doi: 10.1200/JCO.20.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. Cited 6 Aug 2020. Available from: https://pubmed.ncbi.nlm.nih.gov/19621072/ [DOI] [PMC free article] [PubMed]
- 14.Higgins JPT GS (2019) (eds.) Higgins. Cochrane Handbook for Systematic Reviews of Interventions. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (eds.). Cochrane Handb. Syst. Rev. Interv. Wiley. 10.1002/9781119536604 [DOI] [PMC free article] [PubMed]
- 15.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Califano R, Każarnowicz A, Karaseva N, Sánchez A, Liu SV, Horn L, et al (2018) IMpower133: Patient-reported outcomes (PROs) in a ph1/3 study of first-line (1L) atezolizumab (atezo) + carboplatin + etoposide (CP/ET) in extensive-stage SCLC (ES-SCLC). Ann Oncol [Internet]. R. Califano, Department of Medical Oncology, Christie NHS Foundation Trust, Manchester, United Kingdom 29: x17. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L628089636%0Ahttp://dx.doi.org/10.1093/annonc/mdy486
- 17.Horn L, Reck M, Mok TSK, Johnson M, Waterkamp D, Lam S, et al (2016) A Phase III study of atezolizumab with carboplatin plus etoposide in patients with extensive-stage small cell lung cancer (IMpower133). Ann Oncol. L. Horn, Department of Medicine, Vanderbilt Ingram Cancer Center, Nashville, TN, United States 27: vi496. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L613912449
- 18.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al (2018) First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. L. Horn, Vanderbilt University Medical Center, 2220 Pierce Ave., 777 PRB, Nashville, TN, United States, United States 379: 2220–2229. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L625385974 [DOI] [PubMed]
- 19.Horn L, Mansfield AS, Reck M, Mok T, Spira AI, Tang X, et al (2017) Phase I/III trial of atezolizumab with carboplatin and etoposide in ES-SCLC in first-line setting (IMpower133). J Clin Oncol 35: TPS8584–TPS8584. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L617435133
- 20.Kawashima Y, Sugawara S, Atagi S, Akamatsu H, Sakai H, Okamoto I et al (2019) Subgroup Analysis of Japanese Patients in a Phase I/III Study of Atezolizumab in ES-SCLC (IMpower133). Ann Oncol [Internet]. Y. Kawashima, Department of Pulmonary Medicine, Sendai Kousei Hospital 30: vi114–vi115. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2004528213
- 21.Liu S, Mansfield A, Szczesna A, Havel L, Krzakowski M, Hochmair M, et al (2018) PL02.07 IMpower 133: primary PFS, OS and safety in a PH1/3 study of 1L Atezolizumab + Carboplatin + Etoposide in extensive-stage SCLC. J Thorac Oncol 13: S185–S186. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2001208380
- 22.Mansfield AS, Każarnowicz A, Karaseva N, Sánchez A, De Boer R, Andric Z, et al (2020) Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol [Internet]. A.S. Mansfield, Division of Medical Oncology, Mayo Clinic, 200 1st St NW, Rochester, MN, United States, England 31: 310–317. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2004165090 [DOI] [PubMed]
- 23.Mansfield AS, Liu S V., Szczęsna A, Havel L, Kzrakowski M, Hochmair MJ, et al (2019) Abstract CT199: IMpower133: Primary efficacy and safety + CNS-related adverse events in a Ph1/3 study of first-line (1L) atezolizumab (atezo) + carboplatin + etoposide in extensive-stage SCLC (ES-SCLC). Clin Trials. A.S. Mansfield, Mayo Clinic, Rochester, MN, United States: American Association for Cancer Research CT199–CT199. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L628858708
- 24.Mok TSK, Reck M, Horn L, Lam S, Shames DS, Liu J, et al (2018) IMpower133: Primary efficacy and safety + CNS-related adverse events in a phase I/III study of first-line (1L) atezolizumab + carboplatin + etoposide in extensive-stage SCLC (ES-SCLC). Ann Oncol 29: ix173. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L627304826
- 25.Nishio M, Ji JH, Hotta K, Chiu C-H, Lee J-S, Azuma K, et al (2019) Overall survival with first-line durvalumab plus platinum-etoposide in patients with extensive-stage (ES)-SCLC in CASPIAN: subgroup findings from Asia. Ann Oncol. M. Nishio, Department of Thoracic Medical Oncology, Cancer Institute Hospital OfJFCR, Tokyo, Japan 30: ix197–ix198. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L630552831
- 26.Owonikoko T.K., Kim H.R., Govindan R. et al (2019) LBA1_PR—Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer. Ann Oncol 30: ii77–ii80. Cited 17 Jul 2020. Available from: https://oncologypro.esmo.org/meeting-resources/european-lung-cancer-congress-2019/Nivolumab-nivo-plus-ipilimumab-ipi-nivo-or-placebo-pbo-as-maintenance-therapy-in-patients-pts-with-extensive-disease-small-cell-lung-cancer-ED-SCLC-after-first-line-1L-plati
- 27.Owonikoko TK, Kim HR, Govindan R, Ready N, Reck M, Peters S, et al (2019) Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): Results from the double-blind, rando. Ann Oncol. T.K. Owonikoko, Winship Cancer Institute, Emory University, Atlanta, GA, United States 30: ii77. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2005032071%0A. http://dx.doi.org/10.1093/annonc/mdz094%0A. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L628119084
- 28.Özgüroğlu M, Goldman JW, Reinmuth N, Chen Y, Dvorkin M, Trukhin D, et al (2019) LBA2 First-line durvalumab plus platinum-etoposide in extensive-stage (ES)-SCLC: Safety, pharmacokinetics (PK) and immunogenicity in CASPIAN. Ann Oncol. M. Özgüroǧlu, Cerrahpaşa School of Medicine, Istanbul University-Cerrahpaşa, Istanbul, Turkey 30: xi66. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L630741289
- 29.Paz-Ares LG, Jiang H, Huang Y, Dennis PA (2018) A phase 3, randomized study of first-line durvalumab (D) +/- tremelimumab (T) + platinum-based chemotherapy (CT) vs CT alone in extensive disease small-cell lung cancer (ED-SCLC): CASPIAN. Oncol Res Treat 41: 103. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L621265499
- 30.Paz-Ares L, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al (2019) PL02.11 overall survival with durvalumab plus etoposide-platinum in first-line extensive-stage SCLC: results from the CASPIAN Study. J Thorac Oncol 14:S7–S8. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2003407744
- 31.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al (2019) Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet [Internet]. L. Paz-Ares, Department of Medical Oncology, Hospital Universitario 12 de Octubre, Madrid, Spain, England 394: 1929–1939. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2003873320 [DOI] [PubMed]
- 32.Reck M, Liu SV, Mansfield AS et al (2019) Impower133: updated overall survival (OS) analysis of first-line (1L) atezolizumab (Atezo) + carboplatin + etoposide in extensive-stage SCLC (ES-SCLC). Ann Oncol 30: v710–v711. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L631302251
- 33.Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al (2013) Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 24: 75–83. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2004528213 [DOI] [PubMed]
- 34.Reck M, Luft A, Szczesna A, Havel L, Kim S-W, Akerley W, et al (2016) Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 34:3740–3748. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L612965007 [DOI] [PubMed]
- 35.Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al (2020) Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 38: 2369–2379. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L631926467 [DOI] [PMC free article] [PubMed]
- 36.Seute T, Leffers P, ten Velde GPM, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer. 2004;100:801–806. doi: 10.1002/cncr.20043. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Bi M (2020) The efficiency and safety of immune checkpoint inhibitors in the treatment of small cell lung cancer: a meta-analysis. Ann Palliat Med 9: 4081–4088. Available from: http://apm.amegroups.com/article/view/56721/html [DOI] [PubMed]
- 38.Landre T, Chouahnia K, Des Guetz G, Duchemann B, Assié JB, Chouaïd C. First-line immune-checkpoint inhibitor plus chemotherapy versus chemotherapy alone for extensive-stage small-cell lung cancer: a meta-analysis. Ther Adv Med Oncol. 2020 doi: 10.1177/1758835920977137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Facchinetti F, Di Maio M, Tiseo M (2020) Adding PD-1/PD-L1 inhibitors to chemotherapy for the first-line treatment of extensive stage small cell lung cancer (SCLC): a meta-analysis of randomized trials. Cancers 12: 2645. Available from: https://www.mdpi.com/2072-6694/12/9/2645 [DOI] [PMC free article] [PubMed]
- 40.Zhou T, Zhang Z, Luo F, Zhao Y, Hou X, Liu T, et al (2020) Comparison of first-line treatments for patients with extensive-stage small cell lung cancer. JAMA Netw Open 3: e2015748. Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2771854 [DOI] [PMC free article] [PubMed]
- 41.Niu Z, Guo S, Cao J, Zhang Y, Guo X, Grossi F, et al (2021) Immune checkpoint inhibitors for treatment of small-cell lung cancer: a systematic review and meta-analysis. Ann Transl Med 9: 705–705. Cited 25 Oct 2021. Available from: https://pubmed.ncbi.nlm.nih.gov/33987403/ [DOI] [PMC free article] [PubMed]
- 42.Chen HL, Tu YK, Chang HM, Lee TH, Wu KL, Tsai YC, et al (2020) Systematic review and network meta-analysis of immune checkpoint inhibitors in combination with chemotherapy as a first-line therapy for extensive-stage small cell carcinoma. Cancers 12: 1–17. Cited 25 Oct 2021. Available from: https://pubmed.ncbi.nlm.nih.gov/33287455/ [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the vivli repository (https://vivli.org/). Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).