Abstract
The conclusions of the EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State, Ireland, and co‐rapporteur Member State, Portugal, for the pesticide active substance aluminium ammonium sulfate and the considerations as regards the inclusion of the substance in Annex IV of Regulation (EC) No 396/2005 are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The conclusions were reached on the basis of the evaluation of the representative uses of aluminium ammonium sulfate as a repellent on row crops (salad, brassica, carrots and other vegetable crops), combinable crops (grains, pulses and oilseed rape), grassland (primarily amenity and sports turf), orchards and forestry, amenity and ornamentals including use on hard surfaces. The reliable end points, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework and assessments not finalised are listed.
Keywords: aluminium ammonium sulfate, peer review, risk assessment, pesticide, repellent
Summary
Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659, lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012 as amended by Commission Implementing Regulation (EU) No 2016/183. Aluminium ammonium sulfate is one of the active substances listed in that Regulation.
In accordance with Article 1 of Regulation (EU) No 844/2012, the rapporteur Member State (RMS), Ireland, and co‐rapporteur Member State (co‐RMS), Portugal, received an application from Sphere Laboratories (London) Ltd, for the renewal of approval of the active substance aluminium ammonium sulfate. In addition, the applicant submitted an application for inclusion of the substance in Annex IV of Regulation (EC) No 396/2005.
An initial evaluation of the dossier on aluminium ammonium sulfate was provided by the RMS in the renewal assessment report (RAR) and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by EFSA in accordance with Article 13 of Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The following conclusions are derived.
The representative uses of aluminium ammonium sulfate using hydraulic boom sprayer, knapsack sprayer or mistblower, as a repellent against birds and mammals of various species on row crops (salad, brassica, carrots and other vegetable crops), combinable crops (grains, pulses and oilseed rape), grassland (primarily amenity and sports turf), orchards and forestry, amenity and ornamentals including use on hard surfaces, as proposed at EU level result in a sufficient repellent efficacy against the target birds and mammals.
The assessment of the data package revealed no issues that need to be included as critical areas of concern with respect to the identity, physical, chemical and technical properties of aluminium ammonium sulfate or the representative formulations.
In the area of mammalian toxicology, no issues that need to be included as critical areas of concern were identified for aluminium ammonium sulfate or the representative formulations. The use on hard surfaces (included in the representative use on amenity and ornamentals) could not be finalised in the absence of appropriate data for non‐dietary exposure estimates.
In the area of residues, the consumer dietary risk assessment could not be finalised as without the requested data, it cannot be concluded that the use of aluminium ammonium sulfate following overall spray application on row crops and combinable crops (except for the uses with barrier treatment for all crops and for the uses as trunk treatment for orchards) would not present a risk to the consumers. Toxicological reference values have been set for aluminium, and outstanding data have been identified that prevent addressing the consumer exposure to aluminium residues resulting from the representative uses of aluminium ammonium sulfate. Under these conditions and considering also the very poor information on the additional routes of exposure/natural background levels collected from the literature search, it cannot currently be demonstrated that the consumer exposure to aluminium residues resulting from the representative uses will not contribute equally or more compared to the other routes of exposure and/or to the natural background levels of aluminium. Regarding the five assessment criteria for potential inclusion of aluminium ammonium sulfate in Annex IV of Regulation (EC) No 396/2005, criteria I and II are not met. Considering criterion III, toxicological reference values were set for aluminium ion, being considered as the most toxic compound, whilst criteria IV and V cannot currently be assessed in view of the identified data gaps.
The information available regarding environmental fate and behaviour was sufficient to allow the required environmental exposure assessments to be completed with the exception that a refined groundwater exposure assessment was not available for aluminium ions when soil pH would be below 5.5 to cover the non‐agricultural intended uses of the representative formulations and environmental exposure assessments were not available for natural surface water system exposure for uses on hard surfaces. This resulted in the identification of assessments not finalised.
In the area of ecotoxicology, a high long‐term risk to birds and mammals, a high acute risk to bees and a high risk to non‐target arthropods was concluded for the representative uses of ‘Curb Powder Formulation’. The aquatic risk assessment, the chronic risk assessment to bees and the risk assessment to non‐target terrestrial plants could not be finalised.
According to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605, it can be concluded that aluminium ammonium sulfate is unlikely to be an endocrine disruptor.
Background
Commission Implementing Regulation (EU) No 844/2012 1 , as amended by Commission Implementing Regulation (EU) No 2018/1659 2 , (hereinafter referred to as ‘the Regulation’), lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/2009 3 . This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3). Furthermore, in accordance with Article 13(3a), where the information available in the dossier is not sufficient to conclude the assessment on whether the approval criteria for endocrine disruption are met, additional information can be requested to be submitted in a period of minimum 3 months, not exceeding 30 months, depending on the type of information requested.
In accordance with Article 1 of the Regulation, the RMS Ireland and co‐RMS Portugal received an application from Sphere Laboratories (London) Ltd, for the renewal of approval of the active substance aluminium ammonium sulfate. In addition, the applicant submitted an application for inclusion of the substance in Annex IV of Regulation (EC) No 396/2005 4 . Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Portugal), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on aluminium ammonium sulfate in the RAR, which was received by EFSA on 25 June 2019 (Ireland, 2019). The RAR included a proposal to include the substance into Annex IV of Regulation (EC) No 396/2005.
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, Sphere Laboratories (London) Ltd, for consultation and comments on 12 March 2020 EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 12 May 2020. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of reporting table. In addition, the applicant was invited to respond to the comments received. The comments and the applicant’s response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA, the RMS on 9 July 2020. On the basis of the comments received, the applicant’s response to the comments and the RMS’s evaluation thereof, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology and ecotoxicology.
In addition, following a consultation with Member States in the Pesticides Peer Review Experts’ Teleconference 43 in Ecotoxicology (February 2021), it was considered necessary to apply an additional clock stop of in accordance with Commission Implementing Regulation (EU) No 2018/1659, to be able to conclude whether the approval criteria for endocrine disruption in line with the scientific criteria for the determination of endocrine‐disrupting properties, as laid down in Commission Regulation (EU) 2018/605 5 , are met.
The outcome of the telephone conference, together with EFSA’s further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in March 2022.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative uses of aluminium ammonium sulfate as a repellent on row crops (salad, brassica, carrots and other vegetable crops), combinable crops (grains, pulses and oilseed rape), grassland (primarily amenity and sports turf), orchards and forestry, amenity and ornamentals including use on hard surfaces, as proposed by the applicant. In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the RAR and considered during the peer review, if any, are presented in the conclusion.
A list of the relevant end points for the active substance and the formulation is provided in Appendix B. In addition, the considerations as regards the cut‐off criteria for aluminium ammonium sulfate according to Annex II of Regulation (EC) No 1107/2009 are summarised in Appendix A.
A key supporting document to this conclusion is the peer review report (EFSA, 2022), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (7 July 2020 and 11 January 2022);
the evaluation table (25 March 2022);
the report(s) of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Ireland, 2022), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Aluminium ammonium sulfate (IUPAC) is considered by the International Organization for Standardization not to require a common name.
The representative formulated products for the evaluation were ‘Curb Liquid Formulation’, a soluble concentrate (SL), containing 42.5 g/L aluminium ammonium sulfate (anhydrous, corresponding to 81.2 g/L dodecahydrate) and ‘Curb Powder Formulation’, a water soluble powder (SP) containing 448 g/kg aluminium ammonium sulfate (anhydrous, corresponding to 858 g/kg dodecahydrate).
The representative uses evaluated comprise field spraying applications using hydraulic boom sprayer, knapsack sprayer or mistblower, as a repellent against birds and mammals of various species on row crops (salad, brassica, carrots and other vegetable crops), on grains, pulses and oilseed rape, on grassland, orchard, forestry, amenity and ornamentals, including use on hard surfaces in the Central European zone. Full details of the Good Agricultural Practice (GAP) can be found in the list of end points in Appendix B.
Data were submitted to conclude that the representative uses of aluminium ammonium sulfate proposed at CEU level result in a sufficient repellent effect following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014).
Risk managers might wish to consider if a product, which is an animal repellent with an intended use on hard surfaces (areas not intended to bear vegetation), would be in the scope of the plant protection product regulation, as these are plant‐free areas.
A data gap has been identified for a comprehensive and up‐to‐date search of peer‐reviewed scientific literature in the area of residues in accordance with the EFSA guidance on the submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009 (EFSA, 2011).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: European Commission, 2000a,b, 2010.
The minimum purity of aluminium ammonium sulfate based on the renewal data is 510 g/kg, corresponding to min. 975 g/kg aluminium ammonium sulfate dodecahydrate. The data in the evaluation refer to the variant dodecahydrate. The specification proposed is meeting the requirements of the minimum purity of the reference specification from the first approval. An FAO specification does not exist for this substance.
A data gap was identified for the determination of dilution stability and wettability after storage of the ‘SP’ formulation. The main data regarding the identity of aluminium ammonium sulfate and its physical and chemical properties are given in Appendix B.
Acceptable analytical methods for risk assessment and monitoring purposes are available for the determination of the active substance content of the technical material and the representative formulations.
The need for methods of analysis for monitoring this compound in food of plant and animal origin and in the environment has been waived due to the nature of the compound. A method for body fluids and tissues can also be waived to the nature of the compound.
2. Mammalian toxicity
The following guidance documents were followed in the production of this conclusion: EFSA, 2014, 2017.
Aluminium ammonium sulfate was discussed at the Pesticide Peer Review Experts’ Meeting TC PREV 40 in January 2021.
The analytical profile of the batches used in toxicological studies was in compliance with the technical specification provided and no impurities of concern were present in the technical material. The analytical methods used in toxicological studies were overall considered fit for purpose.
Relatively few studies were conducted with aluminium ammonium sulfate itself and only a small number of them were guideline compliant. With the exception of some bioavailability, acute toxicity, mutagenicity and reproductive toxicity studies, most of the data available were published papers/reviews on different aluminium salts. In many cases, the raw data and original studies were not part of the dossier for aluminium ammonium sulfate and therefore could not be considered for the peer review. Read across from data generated with aluminium element (the entity of concern for all forms of aluminium) and/or multiple aluminium‐containing compounds was also considered.
The absorption of aluminium is influenced by its solubility in water, its physical and chemical form. The oral bioavailability of different aluminium compounds in humans and experimental animals may vary in food and drink matrices. An overall oral absorption of 0.3% was agreed for aluminium ammonium sulfate on the basis of the evaluation of data from aluminium ammonium sulfate and other aluminium compounds and supported by EFSA ANS Panel (2018). Aluminium distributes in most organs within the body with accumulation mainly in bone, lung, muscle, liver and brain. In addition, aluminium is capable to cross the blood–brain barrier and also the placenta. Aluminium does not undergo metabolism and it is excreted unchanged mainly in the urine; since the profile of dissociation in the gastrointestinal tract, absorption, distribution and excretion are likely to be comparable within mammalian species, comparative in vitro metabolism does not need to be addressed.
Aluminium ammonium sulfate showed low acute oral and dermal toxicity and did not show skin or eye irritant properties. Acute inhalation toxicity studies were not generated for ethical reasons on the basis that the product is an animal repellent (data gap); skin sensitisation testing was considered not required since aluminium ammonium sulfate is used in veterinary and human medicine as a topical astringent to prevent bleeding. Although the experts agreed 6 that aluminium ammonium sulfate is unlikely to be acutely toxic by inhalation, particle size distribution data were not available in the dossier for the active substance (data gap relevant for the ‘Curb Powder Formulation’, see Section 10). Pending on the assessment of particle size distribution, further data on acute toxicity by inhalation might be therefore needed for aluminium ammonium sulfate; for the formulated product, it was considered not scientifically justifiable to ask for a new inhalation toxicity study and the calculation method (based on the content of aluminium ammonium sulfate) was considered acceptable instead. It is noted that no harmonised classification for inhalation toxicity is present for any of the aluminium compounds.
Phototoxicity studies were not needed since aluminium ammonium sulfate is an inorganic white substance that does not absorb any electronic radiation in the wavelength range of 290–700 nm.
No short‐term toxicity studies were available for aluminium ammonium sulfate but only published reviews on different aluminium salts administered to rats and dogs; just a few of them were considered adequate for risk assessment. The relevant no observed adverse effect level (NOAEL) for short‐term toxicity in rat was 52 mg Al/kg body weight (bw) per day on the basis of decreased body weight and mild histopathological changes in the spleen (hyperaemia in the red pulp) and liver (hyperaemia and periportal lymphomonocytic infiltrate) in two 28‐day rat studies with aluminium nitrate in drinking water. The relevant NOAEL for short‐term toxicity in dogs was 27 mg Al/kg bw per day based on decreased body and testes weight, histopathological changes in the liver (vacuolation/hypertrophy/bile stasis), kidney (tubular‐glomerular nephritis) and testes (atrophy) in a 26‐week study with sodium aluminium phosphate. Two more recent studies were summarised in JEFCA 2012 but were not considered relevant to the toxicology assessment for aluminium ammonium sulfate because of the nature of the test substances used (potassium aluminium silicate‐based pigments).
The only available genotoxicity studies for aluminium ammonium sulfate were two in vitro gene mutation assays in bacteria performed with the products ‘Curb Liquid Formulation’ and ‘Curb Powder Formulation’, respectively: They were both considered acceptable and negative. Other information available from literature, from EFSA AFC Panel (2008) and EFSA ANS Panel (2018) on different aluminium compounds confirmed the absence of mutagenicity in procaryotic or eucaryotic cells but indicated in vitro and in vivo clastogenic activity likely related to indirect mechanisms. The experts considered that this is not compromising the possibility of setting reference values as the effects were seen at relatively high exposure levels and they were considered unlikely to be relevant to human exposure to aluminium via the diet and all other possible sources of exposure to aluminium. It was, however, noted that possible genotoxic hazard by non‐dietary exposure should also be investigated. Therefore, the experts 7 considered that an in vitro micronucleus test with aluminium ammonium sulfate is needed to address also threshold‐related genotoxicity endpoints (e.g. aneugenicity) (data gap, see Section 10).
No convincing evidence for a carcinogenicity potential of aluminium ammonium sulfate was concluded 8 on the basis of limited data from available carcinogenicity studies on other aluminium salts. According to EFSA AFC Panel (2008), some evidence of carcinogenicity related to physical properties of particles/fibres was observed but not considered relevant to dietary exposure of humans; no carcinogenic risk was concluded in EFSA ANS Panel (2018). It was also noted that US EPA considered that carcinogenicity was not demonstrated (US EPA, 2006) and IARC concluded (IARC, 1984) that there is limited evidence that certain exposure in the aluminium production industry is carcinogenic to humans giving rise to cancer of the lung and bladder (IARC did not implicate aluminium itself as a human carcinogen).
In early reviews, both EFSA AFC Panel (2008) and JECFA (2007) concluded that a potential for effects on reproduction (more specifically spermatogenesis) was identified with soluble aluminium compounds. Two guideline multigeneration studies were subsequently conducted with aluminium sulfate and aluminium ammonium sulfate administered in rats via drinking water, and have been reviewed by JEFCA (2012) and by EFSA ANS Panel (2018) who concluded that the studies did not provide any evidence of reproductive toxicity. In the multigeneration study performed with aluminium ammonium sulfate, the experts agreed to set the parental NOAEL at 33.5 mg Al/kg bw per day (based on effects on food consumption and body weight/body weight gains and on changes in pituitary and kidney weights). The offspring NOAEL was set at 33.5 mg/kg bw per day based on retardation of sexual development in the F1 females, decreased body weight gain and liver and spleen weights in the F1 and F2 offspring, and the reproductive NOAEL was set at > 305 mg Al/kg bw per day.
With regard to adverse effects on spermatogenesis and testes, it was noted that rat is a poor model for effect on spermatogenesis and male fertility; therefore, potential effects (as reported in previous reviews) might not have been detected in these last two studies. In the review by Yokel (2020), adverse effects on spermatogenesis and testes have also been indicated. However, the experts agreed that there is no concern for a potential reproductive effect following exposure to aluminium ammonium sulfate applied as plant protection product (PPP) because it is not expected to contribute significantly to the general exposure to aluminium via other sources (e.g. packaging and vaccines).
An overview of all developmental toxicity studies (for different aluminium compounds) has been provided and no consistent evidence of a potential for developmental toxicity was observed. Some evidence of developmental effects was available for aluminium compounds other than aluminium ammonium sulfate in the published literature, but these effects were observed at doses far above the proposed toxicological reference values for aluminium ammonium sulfate. The experts agreed 9 that, although some uncertainties are present, no further developmental toxicity study should be required for aluminium ammonium sulfate.
Some evidence of neurotoxicity for aluminium were available in the published literature. However, the studies presented several limitations and many inconsistencies. Given the extent of the database, there is no consistent evidence for a potential neurotoxicity and the experts agreed 10 that, although no clear conclusion on neurotoxicity could be reached, a new guideline compliant study should not be required. However, further robust considerations of all the available evidence are still missing (data gap, see Section 10). Regarding developmental neurotoxicity, some review studies were available in addition to the two multigeneration studies performed with aluminium sulfate and aluminium ammonium sulfate, and a DNT study was available with aluminium citrate (NOAEL set at 30 mg Al/kg bw per day). No indication of specific developmental neurotoxicity relevant for aluminium ammonium sulfate was concluded in these studies.
Reference values were set for aluminium ion, being considered as the most toxic compound: an acceptable daily intake (ADI) of 0.3 mg/kg bw per day was agreed on the basis of the NOAEL of 33.5 mg Al/kg bw per day in the two‐generation study in rats with aluminium ammonium sulfate and supported by the DNT study performed with aluminium citrate, applying an uncertainty factor of 100. The agreed acceptable operator exposure level (AOEL) is 0.001 mg/kg bw per day based on the NOAEL of 33.5 mg Al/kg bw per day from the same study used to derive the ADI (two‐generation study), applying an uncertainty factor of 100 and a correction for an oral absorption of 0.3%. In the absence of acute toxicity, acute reference dose (ARfD) and acute AOEL (AAOEL) were not considered needed. In the previous assessment, the ADI and ARfD for aluminium were 0.14 mg/kg bw (per day), and the AOEL was 0.002 mg/kg bw per day (EFSA, 2012).
In the absence of specific data and in agreement with the EFSA Guidance 2017, dermal absorption was agreed to have the same value as the oral absorption (0.3%), being oral absorption considered as surrogate for dermal absorption.
Non‐dietary exposure estimates were provided according to the EFSA, 2014 model.
For ‘Curb Powder formulation’, the operator exposure estimates are exceeding the AOEL for tractor‐mounted applications on row crops, combinable crops, grassland/amenity; and are below the AOEL for orchards and ornamentals (with the use of personal protective equipment (PPE) and RPE). For knapsack application on all representative crops, the estimates are below the AOEL with use of gloves and RPE. The residential exposure estimates for children are below the AOEL for row crops, combinable crops and grassland/amenity when excluding vapour (due to the nature and volatility of aluminium); below the AOEL for ornamentals when drift reduction and 10 m buffer zone are applied (and vapour excluded); and above the AOEL for orchards (even considering the available risk mitigation measures and exclusion of vapour). The worker exposure estimates are above the AOEL for the uses on high crops and ornamentals, even with the use of gloves, and below the AOEL for the other uses (wearing gloves where appropriate).
For ‘Curb Liquid Formulation’, the operator exposure estimates are below the AOEL for all representative uses (excluding hard surfaces) with the use of PPE or not (for tractor‐mounted application on ornamentals). The residential exposure (for children and adults) is also predicted to be below the AOEL in all scenarios (excluding hard surfaces) when excluding vapour exposure. The worker exposure estimates are below the AOEL for all uses (excluding hard surfaces), wearing gloves where appropriate.
It is noted that no exposure estimates were provided for the use on hard surfaces (included in the representative use on amenity and ornamentals) (data gap leading to an issue not finalised, see Section 9.1).
3. Residues
The assessment in the residue section is based on the following guidance documents: OECD (2009, 2000a, 2000b, 2011, 2019), European Commission (2011) and JMPR (2004, 2007).
Studies to assess the nature of the residues in plants upon foliar treatment of aluminium ammonium sulfate were not submitted and are not required. When used to prepare a spray solution, aluminium ammonium sulfate readily dissolves whereupon it dissociates into aluminium ions, ammonium ions and sulfate ions forming an acidic solution. The nitrogenous and sulfate moieties of the parent molecule are further metabolised into the known processes of living matter whilst transportation and distribution of aluminium ion in plants are expected to be limited and residues are likely to occur only on treated plant surfaces without any further metabolisation and formation of degradation products. The plant residue definition for risk assessment is therefore set as aluminium ion only.
As for the toxic potential of aluminium, the peer review concluded on an ADI of 0.3 mg/kg bw per day whilst an ARfD was not allocated (see Section 2).
It is noted that residue trials addressing the magnitude of aluminium residues according to the representative uses being assessed were not provided, and therefore, a consumer exposure assessment to aluminium residues from the representative uses cannot be conducted. As already identified in the previous EFSA assessment (EFSA, 2012), a data gap is identified for sufficient residue trials addressing the residue levels of aluminium and compliant with the relevant representative uses respectively for row crops (salad, brassica, carrots and other vegetable crops) and for combinable crops (grains, pulses and oilseed rape) where an overall spray treatment of the crops is intended. These residue trials should also determine the natural background levels of aluminium in the untreated crops grown under identical conditions (data gap, see Section 9.1). As for the uses with barrier treatment for all crops and for the uses as trunk treatment for orchards, a data gap for residue trials is not relevant as it can reasonably be assumed that residues of aluminium resulting from the treatment with aluminium ammonium sulfate will be insignificant if the application to the crops/orchards trees is conducted in a way that precludes any contamination of the edible parts of the crops and of the fruits. Pending upon the results of the requested residue trials, the need to conduct a livestock exposure assessment might need to be reconsidered accordingly.
Aluminium is not expected to be translocated to plant tissues as it is not systemic. It is therefore unlikely that aluminium residues may be found in pollen and bee products.
With regard to the five assessment criteria according to the Commission guidance SANCO/11188/2013 Rev. 2 (European Commission, 2015) for potential inclusion in Annex IV of Regulation (EC) No 396/2005, i.e. approval as basic substance (criterion I), listed in Annex I of Regulation (EC) No 396/2005 (criterion II), having no identified hazardous properties (criterion III), natural exposure is higher than the one linked to the use as plant protection product (criterion IV) and consumer exposure is not expected considering the representative uses (criterion V), none of these criteria were considered to be met for aluminium ammonium sulfate for the following reasons:
Criteria I and II are not met.
Considering criterion III, toxicological reference values were set for aluminium ion, being considered as the most toxic compound (see Section 2).
Criterion IV cannot be assessed in view of the very poor information on the additional routes of exposure/natural background levels collected from the literature search (see data gap).
Criterion V cannot also be evaluated considering the data gap identified to address the consumer exposure to aluminium residues resulting from the use of aluminium ammonium sulfate on row crops and combinable crops following overall spray treatment.
Considering both criteria IV and V, it cannot currently be demonstrated that the consumer exposure to aluminium residues resulting from the representative uses of aluminium ammonium sulfate will not contribute equally or more compared to the other routes of exposure and/or to the natural background levels of aluminium.
It is acknowledged that the total consumer exposure towards aluminium residues includes besides contributions from foodstuffs from the representative uses being assessed, other sources of exposure such as its natural food occurrence where it is a normal constituent, as food additives, food contact materials, personal care products (cosmetic and pharmaceutical products) and an aggregate exposure estimation to aluminium from all these uses should therefore be conducted considering these different sources of exposure. For that purpose and as very limited published literature data have been included in the dossier and summarised in the RAR, EFSA is of the opinion that a more comprehensive and up‐to‐date search of peer‐reviewed scientific literature should be carried out by the applicant as there are indications that more recent publications compared to those reported in the RAR are available; e.g. ‘Aggregated aluminium exposure: risk assessment for the general population (Tietz et al., 2019)’ (data gap, see Section 9.1).
It is noted that EFSA has prepared a statement to waive the review of maximum residue levels (MRLs) for aluminium ammonium sulfate as no codex maximum residue limits (CXLs) are currently in place and all the MRLs are set at the default value of 0.01 mg/kg according to Art.18(1)(b) of Regulation (EC) No 396/2005 (EFSA, 2020).
In the framework of this assessment, a plant residue definition for monitoring is currently not proposed but might be reconsidered upon the outcome of the required residue trials.
4. Environmental fate and behaviour
Aluminium ammonium sulfate when used to prepare a spray solution readily dissolves whereupon it dissociates into aluminium ions, ammonium ions and sulfate ions forming an acidic solution.
When aluminium ions following spray application are present in soil pore water or surface water, the pH of the water present along with the presence of complexing ligands such as fluoride ions and dissolved organic material such as humic acid determine the speciation and water solubility of aluminium. At pH levels below pH 5.5, the more water soluble, positively charged aluminium species (Al3+, AlOH2+ and Al(OH)2+) predominate. At around neutral pH the less soluble, neutral hydroxylated Al(OH)3 species predominates and the total amount of dissolved aluminium is very low with aluminium solubility being at a minimum near pH 6.5 at 20°C. Solubility then increases again as Al(OH)− 4 begins to form at higher pH. At pH over 4.5, dissolved aluminium species can also polymerise. At pH above 5.5, the aluminium is insoluble and becomes immobilised in soils and sediments. Many clay minerals present in rocks, soil and sediment are aluminium rich. The amount of aluminium ions being added to soil, water or sediment by the uses of aluminium ammonium sulfate being assessed (Predicted environmental concentrations (PEC) for soil, water and sediment for aluminium ions covering the representative uses assessed, can be found in Appendix B, 11 ) can be concluded as insignificant considering the amounts that can already be present in these matrices. However, in situations where soil or water system pH is above pH 5.5 (which is the usual situation), natural surface water concentrations of aluminium ions will be lower than those estimated by the available PEC for the representative uses.
When ammonium following spray application is present in soil pore water or surface water, like the other ammonium sources (such as those resulting from organic matter breakdown or the use of ammonium and urea‐based fertilisers), they will be further mineralised via microbial activity to nitrate under aerobic conditions and nitrite under anaerobic reducing conditions. In this context, negligible amounts of ammonium would be added to soil, water or sediment through the application of aluminium ammonium sulfate from the representative uses being assessed (PEC for soil and water for ammonium, nitrate and nitrite covering the representative uses assessed can be found in Appendix B) in comparison to these other ammonium/nitrate/nitrite sources.
Organic matter is the principal source of sulfur and sulfate in soil, sediment and natural surface waters. Wet and dry deposition from the atmosphere as well as the application to soil of organic manures and inorganic sulfate fertilisers are other sources. In this context, negligible amounts of sulfate would be added to soil, surface water and sediment through the application of aluminium ammonium sulfate from the representative uses being assessed (PEC for soil, and water for sulfate covering the representative uses assessed can be found in Appendix B) in comparison to these other sulfate sources.
Published literature data included in the dossier summarised in the RAR were sufficient to identify the levels of aluminium ions, ammonium, nitrate, nitrite and sulfate typically present in soil, surface water and sediment used to make the comparisons discussed above. Information was also available that demonstrated that the environmental matrices would have sufficient buffering capacity such that their pH would not be adversely lowered as a result of the representative uses assessed.
PEC in groundwater were calculated for aluminium ions, ammonium, nitrate, nitrite and sulfate covering the representative uses assessed, assuming that the annual total dose would be leached to groundwater using the worst‐case annual average recharge volume from the FOCUS groundwater scenarios (i.e. 493 mm from the Sevilla scenario) and complete transformation to each moiety in the calculations. The resulting PEC in groundwater are presented in Section 7, Table 2 and Appendix B. These PEC in groundwater are less than the parametric drinking water limits set in the drinking water directive 12 for ammonium (0.5 mg/L), nitrate (50 mg/L) and sulfate (250 mg/L). Though the calculated nitrite concentration exceeded its drinking water limit (0.5 mg/L), as topsoil would be expected to usually remain aerobic, the majority of the ammonium would be expected to transform to nitrate and not nitrite. Therefore, in practice, it is concluded that nitrite from the plant protection uses assessed would be below 0.5 mg/L.
Table 2.
Groundwater (a)
|
Compound (name and/or code) |
> 0.1 μg/L at 1 m depth for the representative uses(b), (c) Step 2 |
Biological (pesticidal) activity/relevance Step 3a. |
Hazard identified Steps 3b. and 3c. |
Consumer RA triggered Steps 4 and 5 |
Human health relevance |
|---|---|---|---|---|---|
| Aluminium ions |
0.638 mg/L when soil pH < 5.5 Essentially 0 when soil pH > 5.5 |
Not applicable for inorganic compounds (c) |
– |
– |
Yes |
| Ammonium | 0.427 mg/L | Not applicable for inorganic compounds (c) | – | – | – |
| Nitrate | 1.47 mg/L | Not applicable for inorganic compounds (c) | – | – | – |
| Nitrite |
Calculated maximum 1.09 mg/L, but as topsoil would be expected to remain aerobic, the majority of the ammonium will transform to nitrate and not nitrite |
Not applicable for inorganic compounds (c) | – | – | – |
| Sulfate | 4.54 mg/L | Not applicable for inorganic compounds (c) | – | – | – |
Assessment according to European Commission guidance of the relevance of groundwater metabolites (2003).
FOCUS scenarios or relevant lysimeter.
Repellents and inorganic compounds are not defined as pesticides in Council Directive 98/83/EC 17 The drinking water standards applicable from Council Directive 98/83/EC are: aluminium ions 0.2 mg/L. ammonium 0.5 mg/L, nitrate 50 mg/L, nitrite 0.5 mg/L and sulfate 250 mg/L.
It might be expected that for the representative uses on grassland (in particular in upland areas, but rarely for amenity and sports turf), orchards and forestry (in particular coniferous forestry) and amenity and ornamental uses (in particular ericaceous ornamental plant growing), the pH of the soil matrix will be below pH 5.5. These are the situations where aluminium ion concentrations in groundwater might be expected to exceed its parametric drinking water limit of 0.2 mg/L. However, for other use situations where agricultural soil pH would usually be above pH 5.5, aluminium ions will be insoluble and immobilised, and therefore would not leach through the soil column to groundwater above 0.2 mg/L. Conversely, for surface water, it is the use situations with matrix pH above 5.5 where the uses would be expected to result in aluminium ion concentrations above the levels that might be found naturally. A data gap and assessment not finalised have been identified for information to produce a refined groundwater exposure assessment for aluminium ions in situations where the soil pH is less than 5.5 to cover the non‐agricultural intended uses of the representative formulations (e.g. of pertinent situations are grassland, coniferous forestry, ericaceous ornamental plants in parks) (see Section 9.1).
An environmental exposure assessment was not provided consequent to the representative amenity and ornamental use on hard surfaces. This resulted in the identification of a data gap and assessment not finalised (see Section 9.1).
Though the representative uses can result in aluminium ion concentrations in surface water and groundwater above its drinking water limit of 0.2 mg/L, at abstraction points for the production of drinking water, the applicant clarified that aluminium sulfate is used in water treatment plants as a flocculation agent so the water finishing process is set up to ensure that aluminium ion concentrations in finished drinking water are below 0.2 mg/L. Therefore, an immediate or delayed harmful effect on human or animal health from the consumption of drinking water can be excluded.
5. Ecotoxicology
The risk assessment was based on the following documents: European Commission (2002a, 2002b), SETAC (2001), EFSA (2009, 2013) and EFSA PPR Panel (2013).
Several aspects of the risk assessment for aluminium ammonium sulfate were discussed at the Peer Review Experts’ Meeting PREV 43 which took place in February 2021.
The analytical profile of the batches used in the ecotoxicological studies was in compliance with the technical specification provided.
All ecotoxicity studies were performed with material prepared from aluminium ammonium sulfate; thus, toxicity to non‐target organisms was not evaluated with regard to effects from either of the two representative formulations (i.e. ‘Curb Liquid Formulation’ and ‘Curb Powder Formulation’). However, the co‐formulants present in both ‘Curb’ formulations are not expected to enhance the toxicity of aluminium ammonium sulfate.
Acute and long‐term oral toxicity data were available for mammals. No toxicity data for birds were available. Instead, a qualitative risk assessment was performed by considering (i) the risk assessment for mammals using the acute and chronic endpoints as surrogate values; (ii) a supporting study with juvenile ringed turtle‐doves; and (iii) the lack of mortality reported in the available vertebrate studies. 13 Based on the available evidence and the large margin of safety obtained in the risk assessment for mammals, a low acute risk to both birds and mammals was concluded for all representative uses. Low long‐term risk to birds and mammals was only concluded for the uses of ‘Curb Liquid Formulation’. For the uses of ‘Curb Powder Formulation’, no higher tier refinements were provided and a high long‐term risk to both groups of non‐target organisms could not be excluded. Due to the characteristics of aluminium ammonium sulfate, no bioaccumulation is expected; therefore, a quantitative risk assessment for exposure via secondary poisoning was not considered necessary. Relevant plant metabolites were not identified; therefore, exposure to plant metabolites has not been considered further.
Acute data for assessing the oral toxicity of aluminium ammonium sulfate were available for fish (the rainbow trout, Oncorhynchus mykiss), aquatic invertebrates (the water flea, Daphnia magna) and green algae (Pseudokirchneriella subcapitata). No toxicity studies were submitted to assess the chronic risk to aquatic organisms. The experts concluded that the endpoints for fish and D. magna are not valid due to the lack of analytical measurement of the test item concentrations at the end of the study and that the endpoint for algae has limited reliability. However, the majority of the experts agreed to use the endpoints in a quantitative risk assessment but only as supporting information. The outcome of the risk assessment at the FOCUS Step 2 scenario indicated a low risk only for the uses of ‘Curb Liquid Formulation’ in row crops, combinable crops and amenity and ornamental use (excluding use on hard surfaces as the environmental exposure in surface water could not be finalised; see Sections 4 and 9.1). Considering the available information, the shortcomings identified in all acute studies and the lack of chronic toxicity data, the risk assessment could not be finalised. Acute and chronic toxicity studies with aluminium ion, which is the driving ion for toxicity, are needed to finalise the risk assessment for aquatic organisms (data gap and issue not finalised, see Section 9.1). 14
The applicant submitted valid acute (contact and oral) toxicity studies with honey bees. The experts agreed that, with the available information, environmental exposure to bees cannot be excluded for any of the representative uses of aluminium ammonium sulfate. 15 An acute risk assessment following the EFSA (2013) bee guidance document was not conducted. Based on the SANCO guidance on terrestrial ecotoxicology (European Commission, 2002a, 2002b), a low acute contact and oral risk was identified for the representative uses of ‘Curb Liquid Formulation’. However, a high acute risk was concluded for the uses of ‘Curb Powder Formulation’. Studies assessing the chronic toxicity to adult and honey bee larvae were not submitted (data gap and issue not finalised, see Section 9.1). The risk from exposure to contaminated water was not assessed (data gap, see Section 10). An assessment of accumulative effects was not available. Also, no data were submitted on sublethal effects, e.g. hypopharyngeal glands (data gap, see Section 10). No toxicity tests were available on bumblebees and solitary bees. The exposure to plant metabolites has not been considered (see Section 3 for further details).
Tier‐1 (glass plate) toxicity tests on the indicator non‐target arthropods Aphidius rhopalosiphi (parasitic wasp) and Typhlodromus pyri (predatory mite) were available. In addition, an extended laboratory test using a natural substrate on Aphidius rhopalosiphi was submitted. Based on the available toxicity data and risk assessment, a high in‐field and off‐field risk was concluded for all the representative uses of ‘Curb Powder Formulation’ with the exception of the uses on grassland for which a low off‐field risk was indicated. At the meeting, the experts agreed that a tier‐1 study with the formulated product on A. rhopalosiphi or higher tier studies with additional species should be provided to refine the risk. 16 A low risk was concluded for all the representative uses of ‘Curb Liquid Formulation’ at higher tier risk assessment.
Suitable toxicity studies were not available on earthworms, other soil macroorganisms and soil microorganisms. However, since the amounts of aluminium ions, ammonium and sulfate being added to soil under the proposed conditions of use can all be considered insignificant when compared to the amounts already present in the soil compartment (see Section 4 for further details), a low risk to soil organisms was concluded for all representative uses. This conclusion is also applicable to the ammonium transformation products nitrate and nitrite (see Section 4).
The applicant did not submit seedling emergence or vegetative vigour studies on non‐target terrestrial plants (data gap). Considering the available information, the exposure to non‐target plants in the field margins could not be excluded for any of the representative uses of aluminium ammonium sulfate, and thus, the risk assessment could not be finalised (see Section 9.1).
The risk to organisms involved in organisms involved in sewage treatment processes was considered to be low.
6. Endocrine disruption properties
With regard to the assessment of the endocrine disruption (ED) potential of aluminium ammonium sulfate for humans, an ED assessment was carried out according to the EFSA/ECHA (2018) ED Guidance. All available evidence and an uncertainty analysis were contextualised to conclude that, although the available data set was not complete for the assessment of the oestrogen, androgen, steroidogenesis and thyroid (EATS) modalities, there was enough evidence to conclude that the sparse effects observed on potential endocrine‐related targets (e.g. effects observed on spermatogenesis) were likely consequent to a non‐endocrine mode of action. In particular, a cytotoxic mediated mode of action was considered as plausible mechanisms for the observed effects. No additional data were therefore considered necessary based on: the common use of the substance as a food additive, use as a cosmetic ingredient and ubiquitous distribution in the environment. In addition, the cytotoxic properties of the active substance will be a likely limiting factor for any further testing. The ED criteria for aluminium ammonium sulfate were not met for humans and a waiver for additional data was considered acceptable despite the data set was not complete.
The outcome of the assessment reported above for humans also applies to wild mammals as non‐target organisms.
For non‐target organisms other than mammals, neither the endocrine activity nor the endocrine adversity was sufficiently investigated. In accordance with the ECHA/EFSA (2018) Guidance, for aluminium ammonium sulfate, an ED assessment was not considered scientifically necessary due to the knowledge on the physico‐chemical and (eco)toxicological properties. In particular, a waiver was considered sufficiently justified based on the ubiquitous nature of aluminium being the third most abundant element; the cytotoxic properties of the active substance which may limit the possibility of further testing and the lack of literature reporting any positive mechanistic or in vivo evidence.
According to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605, it can be concluded that aluminium ammonium sulfate is unlikely to be an endocrine disruptor.
7. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1, 2, 3, 4)
Table 1.
Soil
|
Compound (name and/or code) |
Ecotoxicology |
|---|---|
| None | Low risk to soil organisms |
Table 3.
Surface water and sediment
|
Compound (name and/or code) |
Ecotoxicology |
|---|---|
| Aluminium ions | Data gap |
Table 4.
Air
|
Compound (name and/or code) |
Toxicology |
|---|---|
| Aluminium ions | Data gap |
| Ammonium | No data, not required |
| Sulfate | No data, not required |
8. Particular conditions proposed to be taken into account by risk managers
Risk mitigation measures (RMMs) identified following consideration of Member State (MS) and/or applicant’s proposal(s) during the peer review, if any, are presented in this section. These measures applicable for human health and/or the environment leading to a reduction of exposure levels of operators, workers, bystanders/residents, environmental compartments and/or non‐target organisms for the representative uses are listed below. The list may also cover any RMMs as appropriate, leading to an acceptable level of risks for the respective non‐target organisms.
It is noted that final decisions on the need of RMMs to ensure the safe use of the plant protection product containing the concerned active substance will be taken by risk managers during the decision‐making phase. Consideration of the validity and appropriateness of the RMMs remains the responsibility of MSs at product authorisation, taking into account their specific agricultural, plant health and environmental conditions at national level.
Table 5 Risk mitigation measures proposed for the representative uses assessed
| Representative use |
Row crops (salad, brassica, carrots and other vegetable crops) |
Combinable crops (grains, pulses and oilseed rape) |
Grassland (primarily amenity and sports turf) |
Orchards and forestry | Amenity and ornamental use including use on hard surfaces | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soluble powder (SP) | Soluble concentrate (SL) | Soluble powder (SP) | Soluble concentrate (SL) | Soluble powder (SP) | Soluble concentrate (SL) | Soluble powder (SP) | Soluble concentrate (SL) | Soluble powder (SP) | Soluble concentrate (SL) | |
| Operator risk | Use of PPE is required(a) | Use of PPE is required(e) | Use of PPE is required(a) |
Use of PPE is required(e) |
Use of PPE is required(a) | Use of PPE is required(e) | Use of PPE is required(b) | Use of PPE is required(f) | Use of PPE is required(c) (excluding hard surfaces) |
Use of PPE is required(g) (excluding hard surfaces) |
| Worker exposure | Use of protective gloves is required |
RMM not required |
RMM not required |
RMM not required |
RMM not required |
RMM not required |
Available RMM are insufficient |
Use of protective gloves is required |
Available RMM are insufficient for ornamentals |
Use of protective gloves is required |
|
Bystander/ resident exposure(h) |
RMM not required |
RMM not required |
RMM not required |
RMM not required |
RMM not required |
RMM not required |
Available RMM are insufficient |
RMM not required |
Drift reducing nozzles and 10 m buffer zone(d) |
RMM not required for ornamentals |
For tractor‐mounted applications: available RMM are insufficient. For knapsack applications: gloves during mixing/loading (ML) and application (A) and RPE during ML (EFSA, 2014).
For tractor‐mounted applications: gloves and RPE during ML and A, water‐soluble bags (ML) and closed cab (A). For knapsack applications: gloves and RPE (ML and A) (EFSA, 2014).
For tractor‐mounted applications: gloves and RPE (ML and A), water‐soluble bags. For knapsack applications: gloves (ML and A) and RPE (ML) (EFSA, 2014).
Please note that drift reducing nozzles are not applicable to knapsack applications (EFSA, 2014).
For tractor‐mounted applications: gloves (ML). For knapsack applications: gloves (ML and A) and RPE (ML) (EFSA, 2014).
For tractor‐mounted applications: gloves (ML and A), RPE (ML) and closed cab (A). For knapsack applications: gloves (ML) (EFSA, 2014).
For tractor‐mounted applications: no PPE/RPE required. For knapsack application: gloves (ML and A), RPE (ML) (EFSA, 2014).
For bystander/resident, the default buffer zone of 2–3 m for low crop application and 5 m for high crop application is not indicated as RMM.
9. Concerns and related data gaps
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for one or more of the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011 18 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following issues or assessments that could not be finalised have been identified, together with the reasons including the associated data gaps where relevant, which are reported directly under the specific issue to which they are related:
-
The consumer dietary risk assessment could not be finalised as without the requested data, it cannot be concluded that the use of aluminium ammonium sulfate following overall spray application on row crops and combinable crops would not present a risk to the consumers. The consumer dietary risk assessment is considered as finalised with regard to the uses with barrier treatment for all crops and for the uses as trunk treatment for orchards, as it can reasonably be assumed that residues of aluminium resulting from the treatment with aluminium ammonium sulfate will be insignificant if application to the crops/orchards trees is conducted in a way that precludes any contamination of the edible parts of the crops and of the fruits (see Section 3).
Sufficient residue trials addressing the residue levels of aluminium and compliant with the relevant representative uses, respectively, for row crops (salad, brassica, carrots and other vegetable crops) and for combinable crops (grains, pulses and oilseed rape) where an overall foliar spray treatment of the crops is intended. The natural background levels of aluminium in the untreated crops grown under identical conditions should also be determined (relevant for the uses in row crops and combinable crops following overall spray treatment – except for the uses with barrier treatment for all crops and for the uses as trunk treatment for orchards, see Section 3).
A comprehensive and up‐to‐date search of peer‐reviewed scientific literature should be carried out in accordance with the EFSA guidance on the submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009 (EFSA, 2011), as there are indications that more recent publications compared to those reported in the RAR are available; e.g. ‘Aggregated aluminium exposure: risk assessment for the general population (Tietz et al., 2000a, 2000b, 2011, 2019) (relevant for the uses in row crops and combinable crops following overall spray treatment ‐ except for the uses with barrier treatment for all crops and for the uses as trunk treatment for orchards, see Section 3)’.
-
A groundwater exposure assessment for aluminium ions demonstrating the parametric drinking water limit for aluminium ions would not be breached, could not be finalised for situations with soil pH below 5.5, that are relevant for some of the grassland and non‐agricultural uses requested for the representative products (see Section 4).
Information regarding a refined groundwater exposure assessment for aluminium ions for situations with soil pH less than 5.5 which can include grassland, coniferous forestry and ericaceous ornamental plant growing was not available (relevant for some use situations that fall within the requested uses on grassland, in orchards and forestry and the amenity and ornamental uses, see Section 4).
-
The environmental exposure and risk assessments and human non‐dietary exposure and risk assessments could not be finalised consequent to amenity and ornamental use on hard surfaces (see Sections 2, 4 and 5).
-
The risk assessment for aquatic organisms could not be finalised considering the shortcomings identified in all acute studies and the lack of chronic toxicity data (relevant for all representative uses; see Section 5).
Acute and chronic toxicity data were not available (see Section 5).
-
The chronic risk assessment for bees could not be finalised as there were no chronic studies with adults and the bee brood study did not cover the full developmental period (relevant for all representative uses, see Section 5).
Chronic toxicity data with adult and bee larvae were not available (see Section 5).
-
The risk assessment for non‐target terrestrial plants could not be finalised (relevant for all representative uses, see Section 5).
Toxicity studies (seedling emergence or vegetative vigour) were not available (see Section 5).
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following critical areas of concern are identified, together with any associated data gaps, where relevant, which are reported directly under the specific critical area of concern to which they are related:
Critical areas of concern were not identified
9.3. Overview of the concerns identified for each representative use considered (Table 6)
Table 6.
Overview of concerns reflecting the issues not finalised, critical areas of concerns and the risks identified that may be applicable for some but not for all uses or risk assessment scenarios
| Representative use |
Row crops (salad, brassica, carrots and other vegetable crops) |
Combinable crops (grains, pulses and oilseed rape) |
Grassland (primarily amenity and sports turf) |
Orchards and forestry | Amenity and ornamental use including use on hard surfaces | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soluble powder (SP) | Soluble concentrate (SL) | Soluble powder (SP) | Soluble concentrate (SL) | Soluble powder (SP) | Soluble concentrate (SL) | Soluble powder (SP) | Soluble concentrate (SL) | Soluble powder (SP) | Soluble concentrate (SL) | ||
| Operator risk | Risk identified | X(e) | X(e) | X(e) | |||||||
| Assessment not finalised | X3 | ||||||||||
| Worker risk | Risk identified | X | X | ||||||||
| Assessment not finalised | X3 | ||||||||||
| Resident/bystander risk | Risk identified | X | |||||||||
| Assessment not finalised | X3 | ||||||||||
| Consumer risk | Risk identified | ||||||||||
| Assessment not finalised | X1;(d) | X1;(d) | |||||||||
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X(a) | X(a) | X(a) | X(a) | X(a) | |||||
| Assessment not finalised | |||||||||||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | X(b) | X(b) | X(b) | X(b) | X(b) | |||||
| Assessment not finalised | X5,6 | X5,6 | X5,6 | X5,6 | X5,6 | ||||||
| Risk to aquatic organisms | Risk identified | ||||||||||
| Assessment not finalised | X4 | X4 | X4 | X4 | X3,4 | ||||||
| Groundwater exposure to active substance | Legal parametric value breached | ||||||||||
| Assessment not finalised | |||||||||||
| Groundwater exposure to metabolites | Legal parametric value breached | X(c) | X(c) | X(c) | |||||||
| Assessment not finalised | X2 | X2 | X2 | ||||||||
The superscript numbers relate to the numbered points indicated in Sections 9.1 and 9.2. Where there is no superscript number, see Sections 2, 3, 4, 5, 6–7 for further information.
Long‐term risk to birds and mammals.
Acute risk to bees and risk to non‐target arthropods other than bees.
With the available assessment, concentrations have been calculated above the parametric value of 0.2 mg/L for aluminium ions in use situations when soil pH < 5.5, e.g. grassland in upland areas, coniferous forestry and ericaceous ornamental plant growing.
Assessment not finalised except for the uses with barrier treatment for all crops and for the uses as trunk treatment for orchards.
For tractor‐mounted application, not for knapsack application.
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 6.)
10. List of other outstanding issues
Remaining data gaps not leading to critical areas of concern or issues not finalised but considered necessary to comply with the data requirements, and which are relevant for some or all of the representative uses assessed at EU level. Although not critical, these data gaps may lead to uncertainties in the assessment and are considered relevant.
These data gaps refer only to the representative uses assessed and are listed in the order of the sections:
Determination of dilution stability and wettability after storage of the ‘SP’ formulation (relevant for the representative uses evaluated for the ‘SP’ formulation; see Section 1).
Data on particle size distribution of the active substance to assess acute toxicity by inhalation were not available (relevant for the representative uses evaluated for the ’SP’ formulation; see Section 2).
Data (in vitro micronucleus test) to address also threshold‐related genotoxicity endpoints (e.g. aneugenicity) (relevant for all representative uses; see Section 2).
Further robust considerations of the available evidence to address the neurotoxic potential of aluminium ammonium sulfate (relevant for all representative uses; see Section 2).
Further data were not available to address the risk to honeybees from sublethal effects (e.g. effects on hypopharyngeal glands) and the risk from exposure to contaminated water (relevant for all representative uses, see Section 5).
Abbreviations
- 1/n
slope of Freundlich isotherm
- λ
wavelength
- ε
decadic molar extinction coefficient
- ADI
acceptable daily intake
- AAOEL
acute acceptable operator exposure level
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- AR
androgen receptor
- ARfD
acute reference dose
- AUC
area under the blood concentration/time curve
- Bw
body weight
- EC50
effective concentration
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- Iv
intravenous
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- Mm
millimetre (also used for mean measured concentrations)
- MRL
maximum residue level
- MS
mass spectrometry
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- Pa
pascal
- PEC
predicted environmental concentration
- PPE
personal protective equipment
- Ppm
parts per million (10–6)
- r2
coefficient of determination
- RAC
regulatory acceptable concentration
- RAR
Renewal Assessment Report
- RPE
respiratory protective equipment
- SC
suspension concentrate
- SMILES
simplified molecular‐input line‐entry system
- WHO
World Health Organization
Appendix A – Consideration of cut‐off criteria for aluminium ammonium sulfate according to Annex II of Regulation (EC) No 1107/2009 of the European Parliament and of the Council
| Properties | Conclusion (a) | |
|---|---|---|
| CMR | Carcinogenicity (C) | Classification criteria not met. Aluminium ammonium sulfate is not considered to be carcinogenic, mutagenic or toxic for reproduction according to points 3.6.2, 3.6.3 and 3.6.4 of Annex II of Regulation No 1107/2009. |
| Mutagenicity (M) | ||
| Toxic for Reproduction (R) | ||
| Endocrine‐disrupting properties | Aluminium ammonium sulfate is not considered to meet the criteria for endocrine disruption for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II of Regulation No 1107/2009, as amended by Commission Regulation (EU) 2018/605. | |
| POP |
Persistence |
Aluminium ammonium sulfate is not considered to be a persistent organic pollutant (POP) according to point 3.7.1 of Annex II of Regulation (EC) 1107/2009. |
|
Bioaccumulation | ||
|
Long‐range transport | ||
| PBT |
Persistence |
Aluminium ammonium sulfate not considered to be a persistent, bioaccumulative and toxic (PBT) substance according to point 3.7.2 of Annex II of Regulation (EC) 1107/2009. |
|
Bioaccumulation | ||
|
Toxicity | ||
| vPvB |
Persistence |
Aluminium ammonium sulfate not considered to be a very persistent, very bioaccumulative substance according to point 3.7.3 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
Origin of data to be included where applicable (e.g. EFSA, ECHA RAC, Regulation).
Appendix B – List of end points for the active substance and the representative formulation
Appendix B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7319
Appendix C – Used compound codes
| Code/trivial name (a) | IUPAC name/SMILES notation/InChiKey (b) | Structural formula (c) |
|---|---|---|
| aluminium ammonium sulfate |
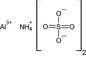
aluminium ammonium sulfate [Al+3].[NH4+].[O‐]S(=O)(=O)[O‐].[O‐]S([O‐])(=O)=O LCQXXBOSCBRNNT‐UHFFFAOYSA‐K |
|
The compound name in bold is the name used in the conclusion.
ACD/Name 2018.2.2 ACD/Labs 2018 Release (File version N50E41, Build 103230, 21 July 2018).
ACD/ChemSketch 2018.2.2 ACD/Labs 2018 Release (File version C60H41, Build 106041, 7 December 2018).
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, Binaglia M, Castoldi AF, Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Ferilli F, Gouliarmou V, Nogareda LH, Ippolito A, Istace F, Jarrah S, Kardassi D, Kienzler A, Lanzoni A, Lava R, Leuschner R, Linguadoca A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Serafimova R, Sharp R, Szentes C, Terron A, Theobald A, Tiramani M and Villamar‐Bouza L, 2022. Conclusion on the peer review of the pesticide risk assessment of the active substance aluminium ammonium sulfate. EFSA Journal 2022;20(5):7319, 27 pp. 10.2903/j.efsa.2022.7319
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00694
Note: This scientific output, approved on 12 April 2022, supersedes the previous output published on 15 March 2012 (EFSA, 2012).
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to thank the rapporteur Member State, Ireland, for the preparatory work on this scientific output.
Approved: 12 April 2022
Note
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Commission Implementing Regulation (EU) No 2018/1659 of 7 November 2018 amending Implementing Regulation (EU) No 844/2012 in view of the scientific criteria for the determination of endocrine disrupting properties introduced by Regulation (EU) 2018/605
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the determination of endocrine disrupting properties. OJ L 101, 20.4.2018, p. 33–36.
For full details on the experts’ discussion, please refer to experts’ consultation point 2.2 of the Pesticides Peer Review Experts’ Meeting in January 2021 (EFSA 2022).
For full details on the experts’ discussion, please refer to experts’ consultation point 2.3 of the Pesticides Peer Review Experts’ Meeting in January 2021 (EFSA, 2022).
For full details on the experts’ discussion, please refer to experts’ consultation point 2.4 of the Pesticides Peer Review Experts’ Meeting in January 2021 (EFSA, 2022).
For full details on the experts’ discussion, please refer to experts’ consultation point 2.5 of the Pesticides Peer Review Experts’ Meeting in January 2021 (EFSA, 2022).
For full details on the experts’ discussion, please refer to experts’ consultation point 2.6 of the Pesticides Peer Review Experts’ Meeting in January 2021 (EFSA, 2022).
PEC in surface water and sediment have been calculated at Step 2 (following FOCUS, 2001 guidance) using v3.2 of the FOCUS Step 1 and 2 calculator.
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.1998, p. 32–54.
See expert consultation point 5.1 in the report of the Peer Review Experts’ meeting PREV 43, February 2021 (EFSA, 2022).
See expert consultation point 5.2 in the report of the Peer Review Experts’ meeting PREV 43, February 2021 (EFSA, 2022).
See expert consultation point 5.3 in the report of the Peer Review Experts’ meeting PREV 43, February 2021 (EFSA, 2022).
See expert consultation point 5.4 in the report of the Peer Review Experts’ meeting PREV 43, February 2021 (EFSA, 2022).
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.1988, p. 32–54
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- ECHA (European Chemicals Agency) and EFSA (European Food Safety Authority) with the technical support of the Joint Research Centre (JRC) , Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311 [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority) , 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011. Submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009. EFSA Journal, 2011;9(2):2092, 49 pp. 10.2903/j.efsa.2011.2092 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2012. Conclusion on the peer review of the pesticide risk assessment of the active substance aluminium ammonium sulfate. EFSA Journal 2012;10(3):2491, 46 pp. 10.2903/j.efsa.2012.2491 [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority) , 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal 2014;12(10):3874, 55. pp. 10.2903/j.efsa.2014.3874 Available online: www.efsa.europa.eu/efsajournal [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2017. Guidance on dermal absorption. EFSA Journal 2017;15(6):4873, 45 pp.. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2020. Pesticide active substances that do not require a review of the existing maximum residue levels under Article 12 of Regulation (EC)No 396/2005. EFSA Journal, 2020;18(12):6318, 13 pp. 10.2903/j.efsa.2020.6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2022. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance aluminium ammonium sulfate. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA AFC Panel , 2008. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials on a request from European Commission on Safety of aluminium from dietary intake. EFSA Journal 2008;754, 34 pp. 10.2903/j.efsa.2008.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipic M, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Kuhnle GG, Lambre C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Wright M, Di Domenico A, van Loveren H, Giarola A, Zs H, Lodi F, Tard A and Woutersen RA, 2018. Scientific Opinion on the re‐evaluation of aluminium sulphates (E 520–523) and sodium aluminium phosphate (E 541) as food additives. EFSA Journal 2018;16(7):5372, 40 pp. 10.2903/j.efsa.2018.5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002.
- European Commission , 2002b. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council. Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9. March 2011. p.1–46.
- European Commission , 2014. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- European Commission , 2015. Guidance document on criteria for the inclusion of active substances into Annex IV of Regulation (EC) No 396/2005. SANCO/11188/2013, Rev. 2, 14 September 2015.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.4, May 2015.
- IARC , 1984. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 34.
- Ireland , 2019. Renewal Assessment Report (RAR) on the active substance aluminium ammonium sulfate prepared by the rapporteur Member State Ireland, in the framework of Commission Implementing Regulation (EU). No 844/2012, June 2019. Available online: www.efsa.europa.eu
- Ireland , 2022. Revised Renewal Assessment Report (RAR) on aluminium ammonium sulfate prepared by the rapporteur Member State Ireland in the framework of Commission Implementing Regulation (EU). No 844/2012, January 2022. Available online: www.efsa.europa.eu
- JEFCA (Joint FAO, WHO Expert Committee on Food Additives) , 2012. Safety evaluation of certain food additives and contaminants. WHO Food Additives Series, 65. [Google Scholar]
- JEFCA (Joint FAO/WHO Expert Committee on Food Additives) , 2007. Safety evaluation of certain food additives and contaminants. WHO Food Additives Series:58.
- JMPR (Joint Meeting on Pesticide Residues) , 2004. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20–29 September 2004, 383 pp. [Google Scholar]
- JMPR (Joint Meeting on Pesticide Residues) , 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 18–27 September 2007, 164 pp. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2009. Guidance document on overview of residue chemistry studies. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: www.oecd.org [Google Scholar]
- SETAC (Society of Environmental Toxicology and Chemistry) , Candolfi MP, Barrett KL, Campbell PJ, Forster R, Grandy N, Huet MC, Lewis G, Oomen PA, Schmuck R and Vogt H (eds.), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2 workshop.
- Tietz T, Lenzner A, Kolbaum AE, Zellmer S, Riebeling C, Gürtler R, Jung C, Kappenstein O, Tentschert J, Giulbudagian M, Merkel S, Pirow R, Lindtner O, Tralau T, Schäfer B, Laux P, Greiner M, Lampen A, Luch A, Wittkowski R and Hensel A, 2019. Aggregated aluminium exposure: risk assessment for the general population. Archives of Toxicology, 93, 3503–3521. [DOI] [PubMed] [Google Scholar]
- US EPA , 2006. Provisional Peer‐Reviewed Toxicity Values for Aluminum. U.S. Environmental Protection Agency, Washington, DC, EPA/690/R‐06/001F, 2006.
- Yokel , 2020. Aluminium reproductive toxicity: a summary and interpretation of scientific reports. Critical Reviews in Toxicology, 50, 551–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


