Abstract
Objective:
To compare clinical outcomes in diabetic patients with heart failure managed by insulin with those managed by non-insulin (oral hypoglycemic agents and/or lifestyle modification) based therapy.
Methods:
PubMed and Scopus databases were searched for studies conducted on diabetic patients with heart failure. Studies were to compare outcomes of patients managed by insulin versus non-insulin therapies.
Results:
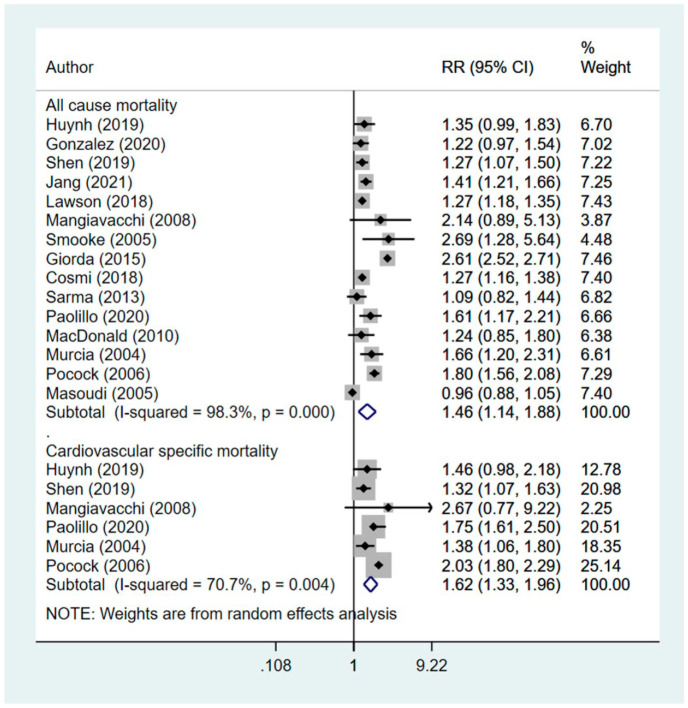
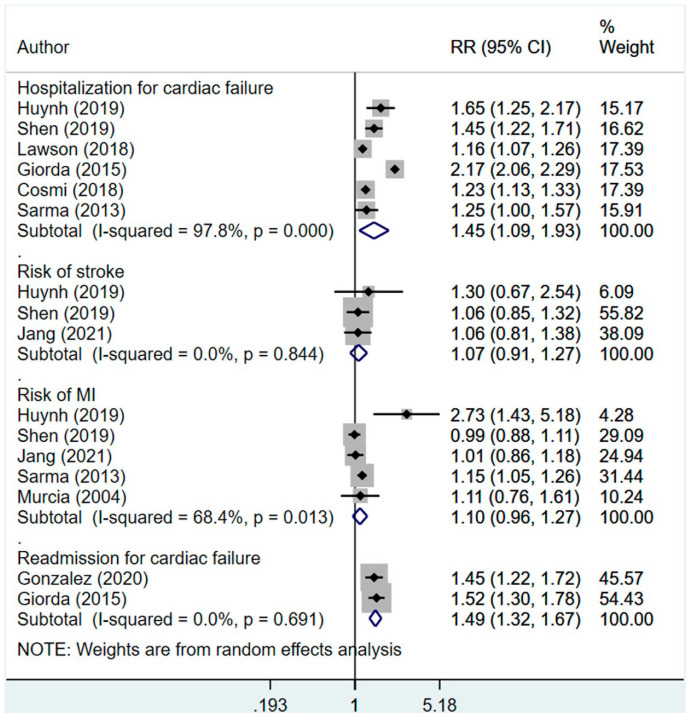
15 studies were included. Compared to those who were managed using non-insulin therapy, insulin-treated patients had increased risk of all-cause mortality (RR 1.46, 95% CI: 1.14, 1.88) and cardiovascular specific mortality (RR 1.62, 95% CI: 1.33, 1.96). Those managed using insulin also had increased risk of hospitalization (RR 1.45, 95% CI: 1.09, 1.93) and readmission (RR 1.49, 95% CI: 1.32, 1.67). There was no additional risk for stroke (RR 1.07, 95% CI: 0.91, 1.27) or myocardial infarction (MI) (RR 1.10, 95% CI: 0.96, 1.27) between the two groups of patients.
Conclusions:
Receipt of insulin among diabetic patients with heart failure was associated with an increased risk of mortality, hospitalization and readmission compared to management using oral hypoglycemic agents and/or lifestyle modification. Such patients should be closely monitored for any adverse events.
Keywords: Insulin, oral hypoglycaemic agents, non-insulin therapy, heart failure, ischemic heart disease, mortality
Introduction
There has been a global upsurge in the incidence of diabetes mellitus as well as heart failure in the recent years. According to the Global burden of disease (2017) estimates, the incidence of diabetes was ∼23 million and the disability-adjusted life-years (DALYs) associated with diabetes was ∼68 million. 1 Prevalence of type 2 diabetes mellitus has increased by ∼30% in the last decade. In 2005, the prevalence was 333 million which rose to 435 million by 2015. 2 The incidence of heart failure is also alarming with ∼26 million people affected worldwide.3,4 Both diseases share a strong inter-association. Studies indicate over 2 times higher risk of heart failure in subjects with type 2 diabetes mellitus.5,6 Additionally, presence of type 2 diabetes increases the risk of adverse cardiovascular outcomes, mortality, hospitalization, and leads to overall unfavorable prognosis among those with pre-existing cardiac disease, in comparison to those without diabetes.5,7–9
The underlying mechanisms leading to poor outcomes in diabetics have been postulated to be around disruption and dysregulation of cellular mechanisms. The increased oxidative stress, underlying inflammation, inflammation of coronary endothelium, non-regulated insulin signaling, increased levels of advanced glycated end-products, and alterations in myocardial substrate metabolism as well as signal transduction have been proposed to be some of the key factors.5,10–13 Presence of diabetes also brings some key structural and functional changes such as left ventricular systolic dysfunction, increase in left ventricular mass and wall thickness, diastolic dysfunction along with a notable increase in extracellular volume fraction .14–17 These changes also contribute to an increased risk of poor outcomes.
Many of these underlying factors and pathophysiological mechanisms might be counteracted through use of pharmacological therapy for management of diabetes mellitus. 5 This, in turn, may reduce the risk of heart failure and its associated complications and possibly, improve prognosis. 5 Insulin is a commonly used second-line pharmacological agent for managing type 2 diabetes. 18 However, a concern with use of insulin is the associated risk of sodium and fluid retention and hypoglycemia which could further accentuate heart failure and worsen outcomes .19–21 There is a lack of systematic evidence on the safety and efficacy of use of insulin in patients with heart failure and concomitant type 2 diabetes, compared to other modes of management such as oral hypoglycemic agents and/or lifestyle modifications. Studies have attempted to analyze the impact of management using insulin on outcomes of heart failure, but the findings of these studies have not been systematically synthesized. There is a need to conduct a careful systematic review and meta-analysis to document and synthesize the findings of these studies and present conclusive and updated evidence that could guide clinical practices. The current meta-analysis was therefore undertaken with the aim to understand the effect of use of insulin therapy, compared to other non-insulin-based management strategies, on clinical outcomes in patients with concomitant diabetes and heart failure.
Methods
Search strategy
A literature search of manuscripts published, only in English, ending November 1, 2021, was executed using both PubMed and Scopus. The implemented search focused on medical subject heading (MeSH) terminology and free text words. Details of our search strategy are summarized in Supplementary Table 1. Our literature search targeted studies conducted among diabetic patients with heart failure and had presented outcomes of interest based on the mode of management of diabetes, that is, managed by insulin or non-insulin management comprising of oral hypoglycemic drugs and/or diet and lifestyle modification. The primary outcomes of interest were all-cause mortality and cardiovascular specific mortality. Secondary outcomes included hospitalization for heart failure, readmission for heart failure, risk of myocardial infarction and stroke. Study processes followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. 22 The protocol for this meta-analysis was registered in the International Prospective Registry of Systematic Reviews (PROSPERO; registration number CRD42021290253).
Selection criteria and methods
Studies, retrieved through literature searches, were both identified and reviewed by two subject experts. Initial screening focused on title and abstracts from the retrieved results. Subsequently, duplicate results were discarded. Finally, the subject experts reviewed content from the full texts of each of the remaining studies to determine inclusion or exclusion. In rare cases, disagreements between subject experts occurred. These were resolved through internal discussion and evaluation until a consensus was reached. Only studies consistent with our inclusion criteria were selected for the meta-analysis.
Inclusion criteria
Studies that were either observational in design (retrospective cohort, prospective cohort, case-control) or randomized trials were included in the meta-analysis. For inclusion studies were required to have been conducted among patients with heart failure and associated diabetes. Further, the study should have examined the outcomes of interest between the two groups of patients, that is, those managed with insulin and those managed using oral hypoglycemic agents and/or lifestyle modification.
Exclusion criteria
Studies which were reported as review articles or case-reports were excluded from the current meta-analysis. Also, studies that omitted results on outcomes of interest or excluded present comparative findings between those managed with insulin and those with non-insulin-based management were excluded.
Data extraction and quality assessment
Using a data extraction sheet, relevant data was extracted from the included studies by two separate authors. Specifically, authors focused on extracting key information such as year of publications, study author name, study settings, pertinent subject characteristics, study design including sample size and finally, results. The Newcastle-Ottawa Quality Assessment Scale was use for observational studies and consequently used for quality assessment. 23
Statistical analysis
The current study was conducted using STATA version 16.0. The effect sizes were reported as pooled relative risk (RR) with 95% CI (confidence intervals). Subgroup analysis was done based on study design and etiology of heart failure (i.e., ischemic or non-ischemic). I2 test measured heterogeneity and identify situations where I2 > 40%, random effects model was used. 24 A p-value < 0.05 was statistically significant. To assess the presence or the absence of any publication bias of the data, an Egger’s test was implemented. 25
Results
Article selection, study characteristics, and quality evaluation
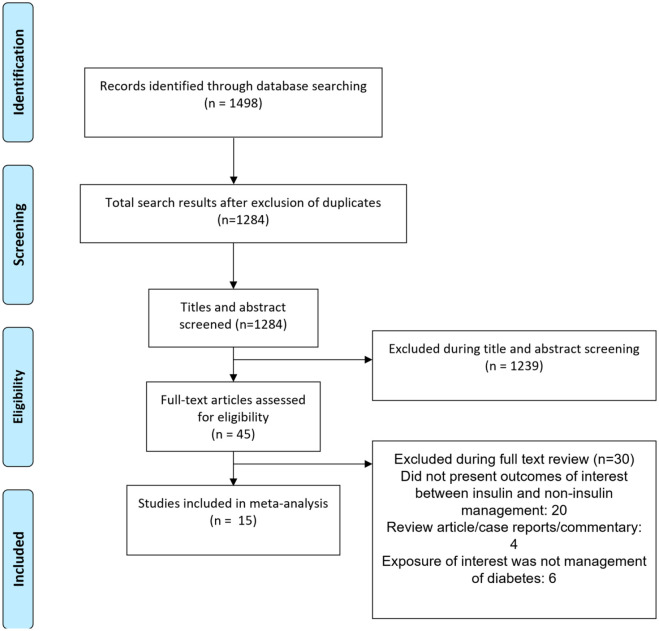
Utilizing the aforementioned literature search method, and subsequently removing duplicated results, a total of 1284 citations were obtained (Figure 1). Filtering of titles and abstracts led to removal of 1239 citations. From the remaining 45 studies, 30 were excluded after reading the full text. Finally, a total of 15 studies were selected for inclusion.26–40 Table 1 presents the details of the included studies. Six studies were based on analysis of data collected as part of randomized controlled trials (RCTs). Three each were prospective cohort and case-control studies respectively. Two studies were based on analysis of data collected as part of registry and one was a retrospective cohort in design. Five studies were multicentric and one was done in the Americas (i.e., USA, Canada, Brazil, and Argentina). Three studies were conducted in Italy, 2 in USA, 2 in United Kingdom, and one study each in Spain and South Korea. In most of the studies, majority of the patients had ischemic heart disease (n =10). In almost all the included studies, the comparison group consisted of subjects managed with oral hypoglycemic drugs. A total of 9 studies had subjects with type 2 diabetes mellitus and in remaining, data on type of diabetes was not provided. Quality evaluation of studies included in our analysis are summarized in Supplementary Tables 2 and 3. Included studies were of modest to good quality. There was a possibility that some TOPCAT data may be double counted by including both of the Huynh and Shen analyses.26,28 Similarly, some of the CHARM trial data may be duplicated by inclusion of Shen and Pocock analyses.28,39 In order to overcome, we conducted a sensitivity analysis by excluding studies by Huynh et al. and Pocock et al.26,39
Figure 1.
Selection process of the studies included in the review.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Author (year of publication) | Study design | Country | Participant characteristics | Sample size; Analysis adjusted for diabetes duration/severity (Yes or No) | Key outcomes (Insulin treated vs. non-Insulin treated DM, that is, oral hypoglycemic drugs and/or diet) |
|---|---|---|---|---|---|
| Huynh et al. (2019) 26 | Analysis of data collected as part of randomized controlled trial (TOPCAT) | The Americas (United States of America, Canada, Brazil, Argentina) | Patients with ischemic heart failure and preserved left ventricular ejection fraction (HFpEF); Mean age of ∼70 years; 50% female; Mean body mass index (BMI) ⩾30 Kg/m2; mean follow-up of 3.3 years; Compared to patients with non-insulin treated, patients with insulin treated diabetes mellitus were younger, more patients belonging to ethnic minorities, with more impaired renal function, New York Heart Association (NYHA) class III/IV, and higher body mass index; duration of diabetes (in years) significantly higher in insulin treated group (median, inter-quartile range, IQR) [ 15 (10–21); 8 (3–12)]; fasting glycemia (mg/dl) significantly higher in insulin treated group (median, IQR) [ 136 (100–197); 122 (100–156)] | 796 (insulin treated, 390; non-insulin treated, 406) No adjustment done |
Effect sizes are adjusted* All-cause mortality: Relative risk (RR) 1.35 (95% Confidence interval, CI: 0.99, 1.83) Cardiovascular mortality: RR 1.46 (95% CI: 0.98, 2.18) Risk of myocardial infarction: RR 2.73 (95% CI: 1.43, 5.18) Risk of stroke: RR 1.30 (95% CI: 0.67, 2.54) Hospitalization for cardiac failure: RR 1.65 (95% CI: 1.25, 2.17) * Adjusted for randomized treatment, randomization strata, age, gender, race, body mass index, heart rate, systolic blood pressure, diastolic blood pressure, smoking status, potassium, estimated glomerular filtration, QRS duration, New York Heart Association class, atrial fibrillation, peripheral arterial disease, and left ventricular ejection fraction. |
| Gonzalez et al. (2020) 27 | Prospective cohort | Spain | Patients with acute heart failure; Mean age of ∼73.4 years; 50.8% female; 52.7% with left ventricular ejection fraction (LVEF) of at least 50%; majority with ischemic disease; follow-up period of 1 year; patients had type 2 diabetes mellitus; Patients receiving insulin had a higher proportion of prior hypertension, dyslipidemia, prior admission for acute heart failure, ischemic heart disease, worse baseline functional NYHA class, greater signs of congestion, and more Charlson co-morbidity. | 1295 (insulin treated, 527; non-insulin treated, 768) No adjustment done |
Effect sizes are adjusted* Readmission for cardiac failure: RR 1.45 (95% CI: 1.22, 1.72) All-cause mortality at 12 months: RR 1.22 (95% CI: 0.97, 1.54) * Adjusted for age, sex, prior admission for acute heart failure, last New York Heart Association class under stable condition, etiology, dyslipidaemia, Charlson co-morbidity index, peripheral edemas on admission, left bundle branch block, systolic and diastolic blood pressures, atrial fibrillation, heart rate, glomerular filtration rate, hemoglobin, sodium, N-terminal pro-brain natriuretic peptide (NT-proBNP), left ventricular ejection fraction, severe tricuspid regurgitation, left atrial diameter, and discharge treatments. |
| Shen et al. (2019) 28 | Analysis of data collected as part of randomized controlled trials (TOPCAT, CHARM-Preserved trial and I-Preserve trial) | Multicentric | Patients with heart failure (majority with hypertensive etiology, 46%); Mean age of ∼70 years; 49% male; mean BMI of ∼31 kg/m2; Duration of heart failure of ⩽5 years (57%); all patients had preserved left ventricular ejection fraction (HFpEF); majority with type 2 diabetes mellitus (∼96%); duration of diabetes (in years) significantly higher in insulin treated group (median, IQR) [ 16 (10–24); 7 (3–13)]; age at onset of diabetes significantly lower in insulin treated group (mean, SD) [48.2 (14.8); 57.8 (14.9)]; Patients with diabetes who received insulin were younger and had higher BMI, compared to patients with diabetes not on insulin. Patients with diabetes receiving insulin more often had an ischemic etiology (45%) and were more likely to have undergone coronary revascularization. The use of loop diuretics was much more frequent in insulin-treated patients than in those with diabetes not on insulin. Patients treated with insulin had worse NYHA functional status, more heart failure-related signs and symptoms, and worse health-related quality of life | 2653 (insulin treated, 979; non-insulin treated, 1674) No adjustment done |
Effect sizes are adjusted* All-cause mortality: RR 1.27 (95% CI: 1.07, 1.50) Cardiovascular mortality: RR 1.32 (95% CI: 1.07, 1.63) Risk of myocardial infarction: RR 0.99 (95% CI: 0.88, 1.11) Risk of stroke: RR 1.06 (95% CI: 0.85, 1.32) Hospitalization for cardiac failure: RR 1.45 (95% CI: 1.22, 1.71) Sudden death: RR 1.67 (95% CI: 1.20, 2.32) *adjusted for age, sex, race, heart rate, diastolic blood pressure, LVEF, NYHA class III/IV, BMI, heart failure hospitalization within the past 6months, history of myocardial infarction, hypertension, and atrial fibrillation, estimated glomerular filtration rate (eGFR), and log NT-proBNP with simple imputation of eGFR and NT-proBNP. |
| Jang et al. (2021) 29 | Analysis of data from KorAHF registry | South Korea | Patients with heart failure (mixed-ischemic as well as non-ischemic); Mean age of ∼69 years; 45% female; mean BMI of ∼24 kg/m2; majority of the patients with type 2 diabetes mellitus; Patients receiving insulin were significantly more likely to be younger and have a lower BMI. They also had significantly lower rates of hypertension, but higher rates of chronic kidney disease, and inotrope and vasodilator use during the index admission. Patients receiving insulin were significantly more likely to have severe symptoms, apparent by the higher NYHA classification | 1108 (insulin treated, 682; non-insulin treated, 426) No adjustment done |
Effect sizes are adjusted* Overall All-cause mortality: RR 1.41 (95% CI: 1.21, 1.66) Risk of myocardial infarction: RR 1.01 (95% CI: 0.86, 1.18) Risk of stroke: RR 1.06 (95% CI: 0.81, 1.38) In those with ischemic etiology All-cause mortality: RR 1.32 (95% CI: 1.02, 1.68) In those with non-ischemic etiology All-cause mortality: RR 1.46 (95% CI: 1.18, 1.80) *adjusted for age, hypertension, inotropes/vasodilators management at admission and angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin II receptor blockers (ARBs) management at discharge |
| Lawson et al. (2018) 30 | Nested case-control | UK | Patients with heart failure (majority with ischemic etiology); Mean age of ∼80 years; 45% female; mean BMI of ∼26 kg/m2; median follow up of 2.6 years for mortality and 99 days for hospitalization; all patients with type 2 diabetes mellitus; | 17358 (insulin treated, 3287; non-insulin treated, 14071) No adjustment done |
Effect sizes are adjusted* All-cause mortality: RR 1.27 (95% CI: 1.18, 1.35) Hospitalization for cardiac failure: RR 1.16 (95% CI: 1.07, 1.26) *Adjusted for current age, gender, hemoglobin, BMI, beta-blocker, ACE inhibitor (ACEIs), ARBs, diuretic, aspirin, ischemic heart disease (IHD), previous myocardial infarction, atrial fibrillation (AF), chronic obstructive pulmonary disease (COPD), eGFR, cholesterol, systolic blood pressure, smoking and alcohol use |
| Mangiavacchi et al. (2008) 31 | Prospective cohort | Italy | Patients with heart failure (majority with ischemic cardiomyopathy, 54.1%) undergoing cardiac resynchronization therapy (CRT); Mean age of ∼66 years; 81% male; Ischemic cardiomyopathy (54%); median follow up of 23.8 months after CRT; all patients with type 2 diabetes mellitus; data on diabetes duration and metabolic control at baseline not collected; similar baseline characteristics (mean age, NYHA class, proportion with coronary artery disease, proportion with renal insufficiency and % left ventricular ejection fraction) in diabetic patients treated with insulin and non-insulin based management | 91 (insulin treated, 29; non-insulin treated, 62) No adjustment done |
Effect sizes are adjusted* All-cause mortality: RR 2.14 (95% CI: 0.89, 5.13) Cardiovascular mortality: RR 2.67 (95% CI: 0.77, 9.22) * Adjusted for age, coronary artery disease, renal insufficiency, use of beta-blockers and of diuretics, and presence of ischemic cardiac disease |
| Smooke et al. (2005) 32 | Prospective cohort | USA | Patients with advanced systolic heart failure; Mean age of ∼52 years; >70% male; Ischemic etiology in majority; Mean BMI of 29 kg/m2; median follow up of 11.7 months; around 96% had type 2 diabetes; duration of diabetes (in years) significantly higher in insulin treated group (mean, SD) [10.7 (10.1); 5.7 (5.7)]; HbA1c (mean, SD) statistically similar in both groups (insulin treated; non-insulin treated) [6.93 (1.33); 7.85 (2.06)]; There were no statistically significant differences between the groups for ejective fraction, smoking history, mitral regurgitation, cardiac index, serum sodium, creatinine, and total cholesterol levels. Diabetic patients treated with insulin were found to have a higher percentage of coronary artery disease, history of hypertension, and higher blood urea nitrogen (BUN) levels. Hemoglobin levels were also significantly lower in patients with insulin-treated diabetes. | 132 (insulin treated, 43; non-insulin treated, 89) No adjustment done |
Effect sizes are adjusted* All-cause mortality: RR 2.69 (95% CI: 1.28, 5.64) * Adjustment for age, sex, history of coronary artery disease, hypertension, BMI, serum creatinine, and ejection fraction |
| Giorda et al. (2015) 33 | Matched case–control study | Italy | Patients with heart failure (ischemic heart disease in majority); Mean age of ∼78 years; 53% male; all patients with type 2 diabetes mellitus | 137359 (insulin treated, 78636; non-insulin treated, 58723) Adjusted for severity of diabetes (Treatment with insulin in the 6 months prior to the date of hospital admission regarded as proxy of severity of disease) |
Effect sizes are adjusted* All-cause mortality: RR 2.61 (95% CI: 2.52, 2.71) Hospitalization for cardiac failure: RR 2.17 (95% CI: 2.06, 2.29) Readmission for cardiac failure: RR 1.52 (95% CI: 1.30, 1.78) * adjusted for past history of ischemic heart disease and glimepiride or glibenclamide use |
| Cosmi et al. (2018) 34 | Analysis of data from 4 large clinical trials | Multicentric | Patients with heart failure; Mean age of ∼65 years; >65% male; mean BMI >25 kg/m2; >50% with ischemic etiology; majority patients with type 2 diabetes mellitus | 6671 (insulin treated, 1860; non-insulin treated, 4811) Adjusted for diabetes duration |
Effect sizes are adjusted* All-cause mortality: RR 1.27 (95% CI: 1.16, 1.38) Hospitalization for cardiac failure: RR 1.23 (95% CI: 1.13, 1.33) * Adjusted for age, sex, BMI, heart rate, QRS duration, systolic and diastolic blood pressure, NYHA class, ischemic etiology, duration of diabetes, LVEF, flutter, peripheral artery disease, COPD, peripheral edema, beta-blockers, digoxin, concentrations of creatinine and bilirubin, third heart sound, calcium antagonists, diuretics, concentrations of bilirubin and triglycerides. |
| Sarma et al. (2013) 35 | Analysis of data from EVEREST trial | Multicentric | Patients with heart failure (ischemic etiology); Mean age of ∼66 years; >70% male; >60% with associated coronary artery disease; follow up until 2.5 years; did not distinguish between type 1 and type 2 DM, baseline serum glucose (random; mg/dl) significantly higher in insulin treated group (median, IQR) [171 (124–225); 161 (122–205)]; Patients treated with insulin had higher proportion of subjects with co-morbidities, such as previous myocardial infarction (62.3% vs. 54.3%), hypertension (82.1% vs. 79.4%), hypercholesterolemia (66.5% vs. 56.4%), chronic kidney disease (43.5% vs. 30.8%) | 1338 (insulin treated, 766; non-insulin treated, 572) No adjustment done |
Effect sizes are adjusted* All-cause mortality: RR 1.09 (95% CI: 0.82, 1.44) Hospitalization for cardiac failure: RR 1.25 (95% CI: 1.00, 1.57) Risk of myocardial infarction: RR 1.15 (95% CI: 1.05, 1.26) * adjusted for sex, age, body mass index, ejection fraction, sodium, blood urea nitrogen (BUN), baseline systolic blood pressure, QRS duration, brain natriuretic peptide (BNP)/N-terminal pro BNP (NT-proBNP), presence of atrial fibrillation or flutter on baseline electrocardiogram (ECG), baseline medication use [angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, beta-blockers, aldosterone blockers, digoxin, and intravenous inotropes), and comorbidities (history of hypertension, CKD, stroke, and smoking history). |
| Paolillo et al. (2020) 36 | Analysis of database (MECKI score database) | Italy | Patients with heart failure and reduced ejection fraction; Mean age of ∼65 years; ∼85% male; mean follow up of 3.36 years; majority with ischemic etiology (57%); did not distinguish between type 1 and type 2 DM; diabetes duration at study enrollment was not considered | 783 (insulin treated, 304; non-insulin treated, 479) No adjustment done |
Unadjusted estimates All-cause mortality: RR 1.61 (95% CI: 1.17, 2.21) Cardiovascular mortality: RR 1.75 (95% CI: 1.61, 2.50) |
| MacDonald et al. (2010) 37 | Case-control | UK | Patients with heart failure; mean age of around 78 yrs; males (53%); etiology (whether ischemic or non-ischemic) unclear; all patients with type 2 diabetes mellitus | 1536 (insulin treated, 230; non-insulin treated, 1306) No adjustment done |
Effect sizes are adjusted* All-cause mortality: RR 1.24 (95% CI: 0.85, 1.80) * Adjusted for age, systolic blood pressure, diastolic blood pressure, body mass index (BMI), hemoglobin, glycosylated hemoglobin (HbA1c), eGFR, previous myocardial infarction, medication use within previous 90 days (ACEIs, ARBs, beta-blocker, aspirin, digoxin, statin, spironolactone) |
| Murcia et al. (2004) 38 | Analysis of data from SAVE trial | Multicentric | Patients with heart failure; mean age of around 60 yrs; males (70%); mean BMI of 28 kg/m2; around 25% were obese; patients with non-ischemic heart disease; there were no differences in the baseline characteristics between the two group of subjects (BMI, prior myocardial infarction, hypertension, smoking status, % LVEF); type of diabetes not specified | 496 (insulin treated, 168; non-insulin treated, 328) No adjustment done |
Effect sizes are adjusted* All-cause mortality: RR 1.66 (95% CI: 1.20, 2.31) Cardiovascular mortality: RR 1.38 (95% CI: 1.06, 1.80) Risk of myocardial infarction: RR 1.11 (95% CI: 0.76, 1.61) *Adjusted for age, sex, history of myocardial infarction, LVEF, Killip class II or greater, thrombolytic therapy, beta-blocker use, and captopril assignment. |
| Pocock et al. (2006) 39 | Analysis of data from CHARM trial | Multicentric | Patients with heart failure; mean age of around 65 yrs; males (70%); mean BMI of 27 kg/m2; majority with ischemic heart disease (70%); mean follow up of 38 months; type of diabetes not specified | 2160 (insulin treated, 706; non-insulin treated, 1454) No adjustment done |
Effect sizes are adjusted* All-cause mortality: RR 1.80 (95% CI: 1.56, 2.08) Cardiovascular mortality: RR 2.03 (95% CI: 1.80, 2.29) *Adjusted for age, ejection fraction, BMI, sex, NYHA class III/IV, current smoker, bundle branch block, cardiomegaly, Prior heart failure hospitalization within 6 months, diastolic blood pressure, previous myocardial infarction, dependent edema, pulmonary crackles, pulmonary edema, mitral regurgitation, atrial fibrillation, rest dyspnea |
| Masoudi et al. (2005) 40 | Retrospective cohort | USA | Patients with heart failure; mean age of around 77 yrs; females (58%); 70% with associated hypertension; associated CAD (66%); Outcomes reported at 1 year follow up; etiology (whether ischemic or non-ischemic) unclear; did not distinguish between type 1 and type 2 DM | 16417 (insulin treated, 8187; non-insulin treated, 8230) No adjustment done |
Effect sizes are adjusted* All-cause mortality: RR 0.96 (95% CI: 0.88, 1.05) *Adjustment done for patient, physician, and hospital variables and accounting for the clustering of patients within hospitals. |
Effect on mortality outcomes
Compared to those who were managed using non-insulin therapy (i.e., oral hypoglycemic agents and/or diet), those managed using insulin had increased risk of all-cause mortality (RR 1.46, 95% CI: 1.14, 1.88; N = 15; I2 = 98.3%) and cardiovascular specific mortality (RR 1.62, 95% CI: 1.33, 1.96; N=6; I2 = 70.7%) (Figure 2). Results from the Egger’s test indicate no publication bias (p = 0.27 for all-cause mortality; p = 0.18 for cardiovascular specific mortality). The findings on sensitivity analysis after exclusion of studies by Huynh et al. and Pocock et al. were statistically similar to the overall pooled findings (supplementary figure 1).
Figure 2.
Effect of management of diabetes using insulin, compared to non-insulin management in patients with heart disease on mortality related outcomes.
Subgroup analysis indicated that in both ischemic and non-ischemic heart disease, the pooled effect size for all-cause mortality as well as cardiovascular specific mortality was higher in diabetics managed with insulin, compared to those undergoing non-insulin management Table 2). However, the number of studies with non-ischemic heart failure were few. Further, the risk of mortality was higher when only studies with observational design were pooled, compared to when only RCTs were pooled (Table 2).
Table 2.
Findings of the subgroup analysis.
| All-cause mortality |
Cardiovascular specific mortality |
Hospitalization for cardiac failure (CF) |
Readmission for CF | |
|---|---|---|---|---|
| Pooled effect size (95% CI); (N = total number of studies; I2) | ||||
| Analysis of RCT data | RR 1.39 (1.19, 1.62); (N=6; I2= 76.6%) * | RR 1.55 (1.19, 2.02); (N = 4; I2 = 81.9%) * | RR 1.34 (1.19, 1.52); (N = 4; I2 = 52.3%) * | — |
| Observational data (prospective cohort; case-control; analysis of registry data) | RR 1.53 (1.06, 2.21); (N = 9; I2 = 98.8%) * | RR 1.77 (1.43, 2.20); (N = 2; I2 = 0.0%) * | RR 1.59 (0.86, 2.93); (N = 2; I2 = 99.4%) | RR 1.49 (1.32, 1.67); (N = 2; I2 = 0.0%) * |
| Ischemic heart disease | RR 1.55 (1.17, 2.05); (N = 11; I2 = 98.2%) * | RR 1.89 (1.66, 2.16); (N = 4; I2= 16.0%) * | RR 1.45 (1.04, 2.01); (N = 5; I2= 98.3%) * | RR 1.49 (1.32, 1.67); (N = 2; I2= 0.0%) * |
| Non-ischemic heart disease | RR 1.38 (1.22, 1.56); (N = 3; I2 = 17.5%) * | RR 1.34 (1.14, 1.58); (N = 2; I2 = 0.0%) * | RR 1.45 (1.22, 1.72); (N = 1) * | — |
Effect on secondary outcomes
Compared to those who were managed using non-insulin therapy, those managed using insulin had increased risk of hospitalization (RR 1.45, 95% CI: 1.09, 1.93; N = 6; I2 = 97.8%) and readmission (RR 1.49, 95% CI: 1.32, 1.67; N = 2; I2 = 0.0%) (Figure 3). There was no additional risk for stroke (RR 1.07, 95% CI: 0.91, 1.27; N = 3; I2 = 0.0%) or myocardial infarction (MI) (RR 1.10, 95% CI: 0.96, 1.27; N = 5; I2 = 68.4%) between the two groups. Egger’s test did not indicate the presence of publication bias (p = 0.42 for hospitalization; p = 0.13 for readmission; p = 0.65 for stroke and p = 0.43 for MI). The findings on sensitivity analysis after exclusion of studies by Huynh et al. and Pocock et al. were statistically similar to the overall pooled findings (supplementary figure 2).
Figure 3.
Effect of management of diabetes using insulin, compared to non-insulin management in patients with heart disease on hospitalization, readmission, stroke, and myocardial infarction.
In subgroup analysis, the risk of hospitalization was higher when only studies with observational design were pooled (RR 1.59, 95% CI: 0.86, 2.93; N = 2; I2 = 99.4%), compared to when only RCTs were pooled (RR 1.34, 95% CI: 1.19, 1.52; N = 4; I2 = 52.3%) (Table 2). In both ischemic and non-ischemic heart disease, the pooled effect size for hospitalization was higher in diabetics managed with insulin, compared to those undergoing non-insulin management; however, the number of studies with patients having non-ischemic heart failure were few (Table 2).
Discussion
It is well established that presence of diabetes adversely affects the outcomes and prognosis of heart failure.5,8,9,41 In addition, available literature does provide support that pharmacological management of diabetes offers some improvement in the clinical outcomes of heart failure. 5 However, the nature of pharmacological management also has an impact on outcomes. Our review showed that use of insulin was associated with an increased the risk of adverse outcomes, compared to non-insulin-based management. One of the potential explanations for an increased risk of adverse outcome in patients managed with insulin is related to its sodium and water retention properties.20,42 The anti-natriuretic effect of insulin is due to reduction in glycosuria and a consequent decrease in sodium excretion. 42 Further, insulin increases sodium and water absorption in the nephrons. This results in increase in vascular volume and leads to increased cardiac work overload. 42 Another plausible explanation is related to the increased risk of hypoglycemia which is common in patients managed with insulin. 43 Hypoglycemia has adverse cardiovascular effects through sympathetic activation culminating into increased heart rate (i.e., tachycardia), myocardial infarction, and a substantial lowering of blood potassium level (i.e., hypokalemia).44,45 All these predispose to a pro-thrombotic state and increase the risk of arrhythmias. There has been recent emerging evidence that insulin might lead to reduced contractility of heart through induction of Gi-biased beta 2-adrenergic signaling in hearts. 46
One of the known adverse effects of exogenous insulin is the increase in insulin levels above normal physiological threshold in systemic circulation leading to hyperinsulinemia. 47 A state of hyperinsulinemia has a wide variety of negative effects such as increased insulin resistance, excessive weight gain, derangement in lipid profile, inflammation, and exhaustion of beta-cells.48–51 Cardiac muscles derive their energy from oxidation of free fatty acids and not from glucose. This is the reason why cardiac myocardium is relatively resistant to insulin action. 50 Exogenous insulin administration led hyperinsulinemia counteracts the natural insulin resistance that myocardium has, and this leads to increased glucose entry and consequent glucolipotoxicity. 50 Studies have shown that hyperinsulinemia can lead to cardiomyopathy, induce endothelial dysfunction and increase the risk of atherosclerosis.52–54 Such adverse effects could be attributed to underlying pathophysiological derangements such as suppression of important pathways like those involved in production of nitric oxide and phosphatidylinositol-3-kinase signaling.53,54 Exogenous insulin predisposes to weight gain and increased adiposity that can accentuate underlying inflammatory environment and increase the levels of circulating inflammatory cytokines. 55 This could also increase the risk of poor cardiovascular outcomes.
There are certain limitations of the current study. Insulin therapy is never the first line for the treatment of type 2 diabetes and reflects more severe or longer disease duration compared to patients managed with oral hypoglycemics or lifestyle advice. In the included studies, not all provided a comparison of the duration and severity of diabetes among the two groups. Further, a total of 9 studies only had subjects with type 2 diabetes and in the remaining 6 studies, no clear distinction was made based on type of diabetes. In studies that provided data on diabetes duration, the age at onset was earlier and the duration of diabetes was more in those that were managed with insulin, compared to non-insulin treated group. Also, the data provided by the included studies clearly demonstrates that those receiving insulin therapy had comparatively worse baseline clinical parameters and a higher prevalence of comorbidities, compared to those receiving non-insulin based management. It is not surprising therefore, that patients treated with insulin could have comparatively worse outcomes. Further, in most of the studies, the statistical model was not adjusted for diabetes duration and/or severity and therefore, it may not be able to conclude conclusively that receipt of insulin worsens outcomes. This argument is further supported by the observations from some of the trials, such as ORIGIN, UKPDS and BARI-2D,56–58 that investigated the effect of insulin monotherapy on cardiovascular safety in apparently healthy individuals, including incident heart failure. These trials found no difference in any cardiovascular outcomes, including hospitalization for heart failure, when compared to standard care. On the contrary, observational studies among apparently healthy subjects with diabetes suggested an increase in risk of incident heart failure with insulin therapy. However, the possibility that the effect is modified by residual confounding cannot be ruled out. These are important considerations while interpreting the findings of this meta-analysis. This analysis includes a diverse range of participants (stable outpatients, hospitalized patients, reduced and preserved ejection fraction) and study designs. This could be a potential methodological weakness and limits the generalizability of the findings.
Another important point is that there could be a switch in treatment provided to the study subjects, that is, there would be subjects that were initially started on oral hypoglycemic drugs and then shifted to insulin treatment or vice-versa. While this shift in treatment may impact the outcome, most of the included studies did not account for this in the analysis. It would have been interesting to know whether there were differences, if any, between the ambulatory/hospitalized patients or those with reduced and preserved ejection fraction. These subgroup analyses could not be done as the included studies did not present findings stratified by these subgroups. Majority of the studies included in this meta-analysis were observational in design and some of them analyzed data from a randomized controlled trial that was not designed to test the hypothesis under consideration. Therefore, the possibility that some important confounders are not adjusted for, cannot be ruled out. The present meta-analysis could not conclusively ascertain the differential risk of insulin therapy, if any, in individuals suffering from either ischemic or non-ischemic heart failure mainly because only few studies included patients with non-ischemic heart failure. An additional limitation was the use of only PubMed and Scopus databases, with the possibility that other studies might have been identified using wider search criteria.
Conclusion
Based on pooling of findings from 15 studies, the meta-analysis noted a significant association between insulin-based management and all-cause mortality, cardiovascular specific mortality, hospitalization due to cardiac failure and readmission among subjects with heart failure and concomitant type 2 diabetes. However, there are certain methodological limitations and therefore careful interpretation of the findings of this meta-analysis is warranted. Nonetheless, necessary caution should be exercised in patients with type 2 diabetes mellitus that are started on insulin therapy. For those who are undergoing insulin-based management, regular follow up and careful supervision is necessitated.
Key messages.
We compared clinical outcomes in diabetic patients with heart failure managed by insulin.
Management of diabetes among patients with heart failure using insulin might be associated with an increased risk of mortality, hospitalization, and readmission.
This increased risk of adverse outcomes should be carefully interpreted as the findings may be influenced by methodological limitations of the studies
Supplemental Material
Supplemental material, sj-docx-1-dvr-10.1177_14791641221093175 for Impact of insulin therapy on outcomes of diabetic patients with heart failure: A systematic review and meta-analysis by Jingxing Liu and Xinhua Hu in Diabetes & Vascular Disease Research
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data availability: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ORCID iD: Xinhua Hu  https://orcid.org/0000-0002-6586-2715
https://orcid.org/0000-0002-6586-2715
Supplemental material: Supplemental material for this article is available online.
References
- 1. Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep 2020; 10: 14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 2016; 388: 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Savarese G, Lund LH. Global Public Health Burden of Heart Failure. Card Fail Rev 2017; 03: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Failure 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 5. Kenny HC, Abel ED. Heart Failure in Type 2 Diabetes Mellitus. Circ Res 2019; 124: 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacDonald MR, Petrie MC, Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J 2008; 29: 1377–1385. [DOI] [PubMed] [Google Scholar]
- 7. Kong MG, Jang SY, Jang J, et al. Impact of diabetes mellitus on mortality in patients with acute heart failure: a prospective cohort study. Cardiovascular Diabetology 2020; 19: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilkinson MJ, Zadourian A, Taub PR. Heart Failure and Diabetes Mellitus: Defining the Problem and Exploring the Interrelationship. Am J Cardiol 2019; 124(Suppl 1): S3–S11. [DOI] [PubMed] [Google Scholar]
- 9. Bauters C, Lamblin N, Mc Fadden EP, et al. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc Diabetol 2003; 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marwick TH, Ritchie R, Shaw JE, et al. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol 2018; 71: 339–351. [DOI] [PubMed] [Google Scholar]
- 11. Montaigne D, Marechal X, Coisne A, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 2014; 130: 554–564. [DOI] [PubMed] [Google Scholar]
- 12. Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 Diabetes mellitus and heart failure: a scientific statement from the american heart association and the heart failure society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 2019; 140: e294–e324. [DOI] [PubMed] [Google Scholar]
- 13. Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med 2008; 5: 715–724. [DOI] [PubMed] [Google Scholar]
- 14. Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 2000; 101: 2271–2276. [DOI] [PubMed] [Google Scholar]
- 15. Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol 2006; 48: 1548–1551. [DOI] [PubMed] [Google Scholar]
- 16. Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation 2003; 107: 448–454. [DOI] [PubMed] [Google Scholar]
- 17. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol 2010; 55: 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swinnen SG, Hoekstra JB, DeVries JH. Insulin therapy for type 2 diabetes. Diabetes Care 2009; 32(Suppl 2): S253–S259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeFronzo RA. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia 1981; 21: 165–171. [DOI] [PubMed] [Google Scholar]
- 20. Brands MW, Manhiani MM. Sodium-retaining effect of insulin in diabetes. Am J Physiol Regul Integr Comp Physiol 2012; 303: R1101–R1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCall AL. Insulin therapy and hypoglycemia. Endocrinol Metab Clin North Am 2012; 41: 57–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. PRISMA. Transparent Reporting of systematic Reviews and meta-analyses. Columbia, SC: PRISMA. http://www.prisma-statement.org/ [Google Scholar]
- 23. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. 21. Ottawa, Canada: Ottawa Hospital Research Institute. [Google Scholar]
- 24. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. 674. London, UK: The Cochrane Collaboration. [Google Scholar]
- 25. Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huynh T, Harty BJ, Claggett B, et al. Comparison of outcomes in patients with diabetes mellitus treated with versus without insulin + heart failure with preserved left ventricular ejection fraction (from the TOPCAT Study). Am J Cardiol 2019; 123: 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bertomeu-Gonzalez V, Fácila L, Palau P, et al. Effect of insulin on readmission for heart failure following a hospitalization for acute heart failure. ESC Heart Failure 2020, 7, 3320–3328. Epub ahead of print 13 August 2020. DOI: 10.1002/ehf2.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen L, Rørth R, Cosmi D, et al. Insulin treatment and clinical outcomes in patients with diabetes and heart failure with preserved ejection fraction. Eur J Heart Fail 2019; 21: 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jang SY, Jang J, Yang DH, et al. Impact of insulin therapy on the mortality of acute heart failure patients with diabetes mellitus. Cardiovascular Diabetology 2021; 20: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lawson CA, Jones PW, Teece L, et al. Association Between Type 2 Diabetes and All-Cause Hospitalization and Mortality in the UK general heart failure population: stratification by diabetic glycemic control and medication intensification. JACC: Heart Failure 2018; 6: 18–26. [DOI] [PubMed] [Google Scholar]
- 31. Mangiavacchi M, Gasparini M, Genovese S, et al. Insulin-treated type 2 diabetes is associated with a decreased survival in heart failure patients after cardiac resynchronization therapy. Pacing Clin Electrophysiol 2008; 31: 1425–1432. [DOI] [PubMed] [Google Scholar]
- 32. Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149: 168–174. [DOI] [PubMed] [Google Scholar]
- 33. Giorda CB, Picariello R, Tartaglino B, et al. Hospitalisation for heart failure and mortality associated with dipeptidyl peptidase 4 (DPP-4) inhibitor use in an unselected population of subjects with type 2 diabetes: a nested case-control study. BMJ Open 2015; 5: e007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cosmi F, Shen L, Magnoli M, et al. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail 2018; 20: 888–895. [DOI] [PubMed] [Google Scholar]
- 35. Sarma S, Mentz RJ, Kwasny MJ, et al. Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. Eur J Heart Fail 2013; 15: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paolillo S, Salvioni E, Perrone Filardi P, et al. Long-term prognostic role of diabetes mellitus and glycemic control in heart failure patients with reduced ejection fraction: Insights from the MECKI Score database. Int J Cardiol 2020; 317: 103–110. [DOI] [PubMed] [Google Scholar]
- 37. MacDonald MR, Eurich DT, Majumdar SR, et al. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the U.K. General Practice Research Database. Diabetes Care 2010; 33: 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murcia AM, Hennekens CH, Lamas GA, et al. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch Intern Med 2004; 164: 2273–2279. [DOI] [PubMed] [Google Scholar]
- 39. Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 2006; 27: 65–75. [DOI] [PubMed] [Google Scholar]
- 40. Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111: 583–590. [DOI] [PubMed] [Google Scholar]
- 41. Dauriz M, Mantovani A, Bonapace S, et al. Prognostic impact of diabetes on long-term survival outcomes in patients with heart failure: a meta-analysis. Diabetes Care 2017; 40: 1597–1605. [DOI] [PubMed] [Google Scholar]
- 42. Brands MW. Role of Insulin-Mediated Antinatriuresis in Sodium Homeostasis and Hypertension. Hypertension 2018; 72: 1255–1262. [DOI] [PubMed] [Google Scholar]
- 43. Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 Diabetes: a meta-analysis. JAMA 2016; 316: 313–324. [DOI] [PubMed] [Google Scholar]
- 44. Hanefeld M, Frier BM, Pistrosch F. Hypoglycemia and cardiovascular risk: is there a major link? Diabetes Care 2016; 39(Suppl 2): S205–S209. [DOI] [PubMed] [Google Scholar]
- 45. Eshaghian S, Horwich TB, Fonarow GC. An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure. Am Heart J 2006; 151: 91. [DOI] [PubMed] [Google Scholar]
- 46. Wang Q, Liu Y, Fu Q, et al. Inhibiting Insulin-Mediated β2-Adrenergic receptor activation prevents diabetes-associated cardiac dysfunction. Circulation 2017; 135: 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Herman ME, O’Keefe JH, Bell DSH, et al. Insulin therapy increases cardiovascular risk in Type 2 Diabetes. Prog Cardiovasc Dis 2017; 60: 422–434. [DOI] [PubMed] [Google Scholar]
- 48. Bittencourt MS, Hajjar LA. Insulin therapy in insulin resistance: could it be part of a lethal pathway? Atherosclerosis 2015; 240: 400–401. [DOI] [PubMed] [Google Scholar]
- 49. Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128: 609–619. [DOI] [PubMed] [Google Scholar]
- 50. Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1: 9–10. [DOI] [PubMed] [Google Scholar]
- 51. Draznin B. Mechanism of the mitogenic influence of hyperinsulinemia. Diabetol Metab Syndr 2011; 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang X, Yu C, Zhang B, et al. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69: 213–218. [DOI] [PubMed] [Google Scholar]
- 53. Madonna R, De Caterina R. Prolonged exposure to high insulin impairs the endothelial PI3-kinase/Akt/nitric oxide signalling. Thromb Haemost 2009; 101: 345–350. [PubMed] [Google Scholar]
- 54. Nandish S, Bailon O, Wyatt J, et al. Vasculotoxic effects of insulin and its role in atherosclerosis: what is the evidence? Curr Atheroscler Rep 2011; 13: 123–128. [DOI] [PubMed] [Google Scholar]
- 55. Gin H, Rigalleau V, Perlemoine C. Insulin therapy and body weight, body composition and muscular strength in patients with type 2 diabetes mellitus. J Nutr Metab 2010; 2010: 340570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia. New Engl J Med 2012; 367: 319–328. [DOI] [PubMed] [Google Scholar]
- 57.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 58.A Randomized Trial of Therapies for Type 2 Diabetes and Coronary Artery Disease. New Engl J Med 2009; 360: 2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dvr-10.1177_14791641221093175 for Impact of insulin therapy on outcomes of diabetic patients with heart failure: A systematic review and meta-analysis by Jingxing Liu and Xinhua Hu in Diabetes & Vascular Disease Research