Abstract
Nucleotide-binding oligomeric domain (NOD)-like receptor protein 3 (NLRP3) is a recently discovered cytoplasmic multiprotein complex involved in inflammation. The NLRP3 inflammasome contains NLRP3, apoptosis-related specific protein (ASC) and precursor caspase-1. The NLRP3 inflammasome is involved in many diseases, including diabetes. H2S is a harmful gas with a rotten egg smell. Recently, it has been identified as the third gas signal molecule after nitric oxide and carbon monoxide. It has many biological functions and plays an important role in many diseases, including diabetes. In recent years, it has been reported that H2S regulation of the NLRP3 inflammasome contributes to a variety of diseases. However, the mechanism has not been fully understood. In this review, we summarized the recent role and mechanism of H2S in regulating the NLRP3 inflammasome in diabetes, in order to provide a theoretical basis for future research.
Keywords: hydrogen sulfide, NLRP3 inflammasome, diabetic cardiomyopathy, diabetes-accelerated atherosclerosis, diabetic retinopathy
1. Introduction
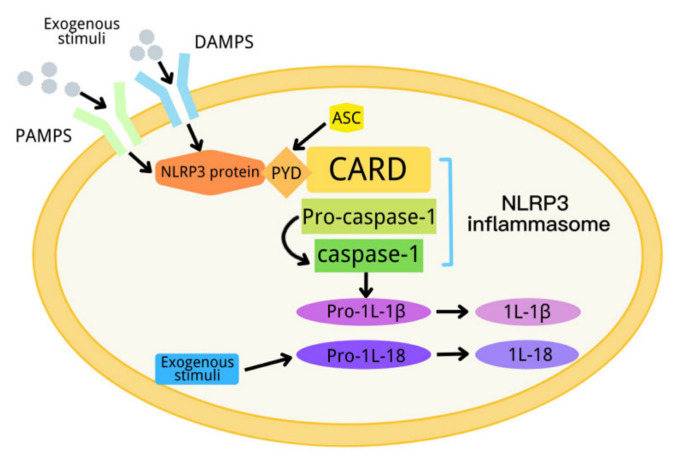
In 2002, Tschopp et al., first proposed a definition of inflammasome [1]. An inflammasome is a complex composed of a variety of proteins. It is an important part of the innate immune system, and can detect metabolic alarms, pathogens and infections in cells [2,3]. At present, there are five kinds of inflammasomes: NLRP1, NLRP3, NLRC4, AIM2 and RIG-I [4,5,6]. The NLRP3 inflammasome, consisting of NLRP3, apoptosis-associated spot-like protein (ASC) and a precursor of caspase-1, is the most deeply studied inflammasome [7,8,9,10,11,12]. NLRP3 has a molecular weight of 115 kDa, and mainly exists in monocytes, lymphocytes, epithelial cells, dendritic cells, osteoblasts and neutrophils. It contains three domains: one domain is the N-terminal pyran domain (PYD), which can recruit ASC; another domain is the central nucleotide-binding oligomeric domain NACHT, which possesses ATPase activity; and the third domain is the C-terminal leucine-rich repeat (LRR) [13,14]. ASC contains the carboxyl terminal CARD and the amino terminal PYD. When the NLRP3 inflammasome is activated, ASC interacts with NLRP3 through the PYD domain, on the one hand, and recruits pro-caspase-1 through a CARD–CARD domain interaction, on the other hand [5,15,16]. When the body is stimulated by exogenous or endogenous stimuli, NLRP3 is activated. The activated NLRP3 interacts with ASC and pre-caspase-1 to form a large protein complex, so as to activate pre-caspase-1. The activated caspase-1 converts pre-IL-1β and pre-IL-18 into their active form to induce inflammation [17]. The activation process of the NLRP3 inflammasome can be divided into two steps, which are mediated by the first and second signals, respectively. The first signal (signal 1) represents tissue injury or infection, including Toll-like receptor 4 (a pattern recognition receptor), which can recognize lipopolysaccharides (LPS), and a series of endogenous risk signals to activate NF-ĸB to increase the expression of the NLRP3 inflammasome, pro-IL-1β and pro-IL-18 [18,19]. Then, the second activation signal (signal 2) represents cell damage, including urate and cholesterol crystals and extracellular adenosine triphosphate (ATP), and induces the assembly of the inflammasome and the autolysis of pro-caspase-1. The activated caspase-1 converts the precursors IL-1β and IL-18 into their active form (Figure 1) [20]. NLRP3 inflammasome dysfunction can contribute to many diseases, including diabetes [21].
Figure 1.
Schematic diagram of the NLRP3 inflammasome activation process.
Hydrogen sulfide (H2S) has long been regarded as a toxic pollutant gas, but, recently, it has been considered as the third gas signal molecule after nitric oxide (NO) and carbon monoxide (CO) [22,23,24]. There are three enzymes that catalyze the synthesis of endogenous H2S: cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS) and 3-mercaptopyruvate thiotransferase (3-MST) [25,26]. During the generation of endogenous H2S, L-cystathionine is produced by the β-substitution reaction of homocysteine with serine, catalyzed by CBS. The elimination of α- and γ-cysteine in L-cystathionine catalyzed by CSE produces L-cystenine. L-cystenine is then catalyzed by CSE/CBS to produce H2S via a β-elimination reaction. L-cystenine is also catalyzed by cysteine aminotransferase (CAT) to produce 3-mercaptopyruvate (3-MP), by transferring its amines to α-ketoglutarate. 3-MP is catalyzed by 3-MST to be converted into H2S (Figure 2) [27,28]. H2S contributes to many physiological processes, including anti-apoptosis [29], antioxidant stress [30], anti-inflammation [31], vasodilation, decreased blood pressure [32,33], cell survival/death, cell differentiation, cell proliferation/hypertrophy and mitochondrial bioenergy/biogenesis [34]. In recent years, more and more evidence has shown that exogenous H2S regulates the NLRP3 inflammasome and plays a vital role in a variety of pathological processes [24]. In this review, we summarized the recent research on H2S regulation of the NLRP3 inflammasome in diabetes to provide a theoretical reference for future related research.
Figure 2.
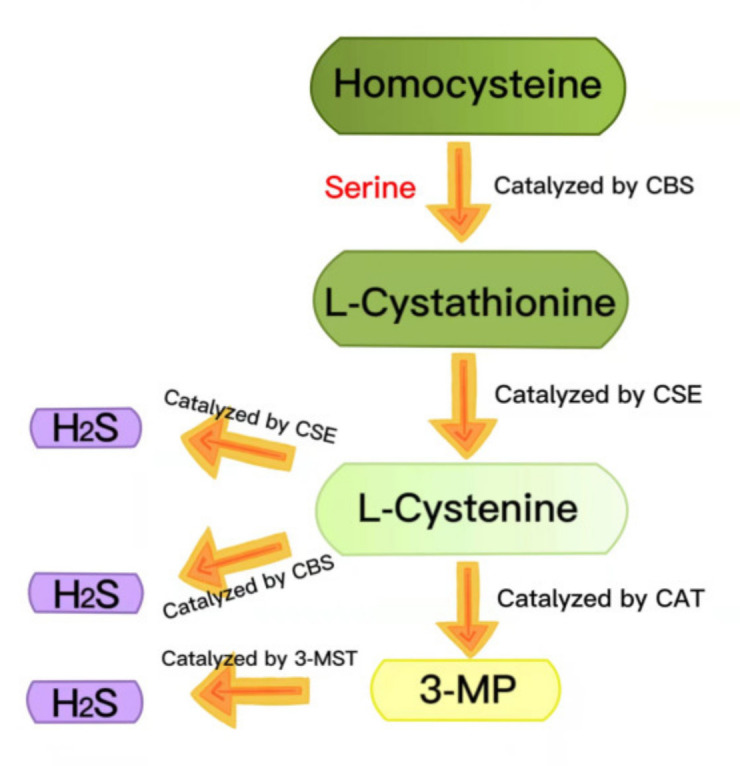
Summary of the production of endogenous H2S. CBS: cystathionine-beta-synthase; CSE: cystathionine-gamma-lyase; 3-MST: 3-mercaptopyruvate thiotransferase; 3-MP: 3-mercaptopyruvate; CAT: cysteine aminotransferase.
2. The Role of H2S in Regulating the NLRP3 Inflammasome in Diabetic Cardiomyopathy
Diabetic cardiomyopathy (DC) is characterized by early diastolic dysfunction. After that, heart failure occurs without coronary artery disease, hypertension and dyslipidemia. The pathogenesis of diabetic cardiomyopathy is closely related to insulin resistance, hyperinsulinemia and hyperglycemia. The pathophysiological factors of diabetes include systemic metabolic disorder, oxidative stress, inflammation and dysfunctional immune regulation, which promote cardiac interstitial fibrosis, cardiac stiffness/diastolic dysfunction and subsequent systolic dysfunction, thus inducing clinical heart failure syndrome and causing cardiomyopathy [35,36,37]. Diabetic cardiomyopathy is one of the main causes of death in diabetic patients. At present, specific strategies for its prevention and treatment have not yet been elucidated [38,39]. Apoptosis, inflammation and oxidative stress play an important role in diabetic cardiomyopathy [40,41,42]. The results of the study conducted by Zena Huang et al., showed that high glucose (HG) promoted apoptosis by increasing the level of caspase-3 protein and induced inflammation by upregulating the level of IL-1β and IL-18 in H9c2 cells, which was abolished by exogenous H2S, suggesting that exogenous H2S could improve HG-induced cardiotoxicity. HG also induced mitochondrial membrane potential (MMP) loss and ROS expression, and downregulated the expression of the NLRP3 inflammasome, IL-1β and IL-18, which was abolished by exogenous H2S. The in-depth research showed that H2S suppressed the activation of TLR4 and NF-κB, induced by HG. NLRP3 gene silencing with siRNA significantly reduced HG-induced cell apoptosis, inflammation, the ROS level and MMP loss, indicating that the NLRP3 inflammasome mediated the H2S improvement in cardiotoxicity induced by HG. In HG-treated H9c2 cells, pretreatment with TAK-242 (TLR4 inhibitor) reduced the HG-induced phosphorylation of NF-κB; furthermore, treatment with BAY11-7082 (NF-κB inhibitor) downregulated the protein level of the NLRP3 inflammasome, prompting that HG promoted NLRP3 inflammasome activation through the TLR4/NF-κB pathway. Collectively, exogenous H2S improved HG-induced cardiotoxicity by suppressing NLRP3 inflammasome activation, by inhibiting the TLR4/NF-κB pathway in H9c2 cells [43]. It can be observed from the above study that H2S inhibits apoptosis by inhibiting the NLRP3 inflammasome in cardiomyocytes, which is involved in only a few studies. Further research is needed to clarify the relationship and mechanism between H2S, NLRP3 and apoptosis.
Low chronic inflammation in the myocardium is one of the main causes of diabetic cardiomyopathy [44,45]; hence, the inhibition of NLRP3 inflammasome-mediated inflammation can effectively improve diabetic cardiomyopathy [46,47]. Qiang Jia et al., used streptozotocin to establish a type 1 diabetic rat model, and found that H2S improved DC by ameliorating cardiac hypertrophy, enhancing the systolic and diastolic capacity of the heart, and alleviating histopathological and ultrastructural lesions in diabetic rats. Further research revealed that exogenous H2S also notably reduced the serum level of cardiac enzymes and pro-inflammatory factors in the DC rat model, indicating that H2S could dampen cardiac damage and inflammation in DC. Hyperglycemia induced redox perturbation by upregulating TXNIP protein expression and downregulating thioredoxin protein expression in DC, which was mitigated by H2S. H2S inhibited NLRP3 inflammasome activation in DC, whereas PAG (an inhibitor of CSE) counteracted the above protective effects of exogenous H2S. Overall, exogenous H2S alleviated myocardial inflammation by suppressing NLRP3 inflammasome activation in diabetic rats, which needs to be further demonstrated using NLRP3 inhibitors [48]. Thioredoxin is an important antioxidant in the body [49,50]. TXNIP has been reported to promote oxidative stress by inhibiting thioredoxin [51,52]. Moreover, TXNIP is an important regulator of the NLRP3 inflammasome [53,54]. Therefore, it can be deduced that H2S inhibits NLRP3 inflammasome activation in diabetic rats through the suppression of TXNIP, although this requires further verification [48].
There is increasing evidence that necroptosis contributes to DC [46,55,56]. In order to further study the mechanism of necroptosis in the pathogenesis of diabetic cardiomyopathy, Weiwei Gong et al., carried out a series of experiments and found that myocardial H2S production, CSE mRNA expression and plasma H2S levels were significantly downregulated in STZ-induced diabetic mice. CSE deficiency aggravated DC and promoted oxidative stress, necroptosis and the NLRP3 inflammasome. Similarly, the CSE inhibitor (PAG) promoted cell injury and oxidative stress, and promoted cardiomyocyte necroticptosis and NLRP3 inflammasome activation in cardiomyocytes induced by HG. However, in vivo and in vitro, exogenous H2S significantly improved diabetic cardiomyopathy, alleviating oxidative stress, necroptosis and the NLRP3 inflammasome. In conclusion, exogenous H2S could improve DC by inhibiting necroticptosis, the NLRP3 inflammasome and oxidative stress [57]. It has been reported that excessive oxidative stress is correlated with necroptosis [58,59,60]. Necroptosis also mediates the activation of the NLRP3 inflammasome [61]. Therefore, in the above study, it can be deduced that exogenous H2S inhibits the NLRP3 inflammasome by suppressing necroptosis [57], although this requires further study.
3. The Role of H2S in Regulating the NLRP3 Inflammasome in Diabetes-Accelerated Atherosclerosis
Diabetes-accelerated atherosclerosis is one of the most common cardiovascular complications of diabetes. Compared with non-diabetic patients, it has a high incidence rate, early onset time, fast course and high mortality [62,63,64]. When examining atherosclerosis in a diabetic mouse model, H2S inhibited the formation of aortic root plaques, and downregulated the levels of vascular cell adhesion molecule 1 (VCAM1) and intercellular adhesion molecule 1 (ICAM1). Meanwhile, the inflammatory factors IL-1β, IL-6, TNF-α and MCP1 were notably downregulated by H2S. The mechanism research revealed that exogenous H2S inhibited NLRP3 inflammasome activation in diabetes-accelerated atherosclerosis conditions. Silencing NLRP3 with siRNA dampened the increase in ICAM1 and VCAM1, induced by HG and oxLDL, suggesting that H2S protected the endothelium by suppressing NLRP3 inflammasome activation. In summary, exogenous H2S significantly ameliorated diabetes-accelerated atherosclerosis by inhibiting NLRP3 inflammasome activation and oxidative stress, thus prompting that H2S inhibited NLRP3 inflammasome activation through the inhibition of oxidative stress in diabetes-accelerated atherosclerosis [65].
4. The Role of H2S in Regulating the NLRP3 Inflammasome in Gestational Diabetes Mellitus
Gestational diabetes mellitus (GDM) is the most common complication during pregnancy. It is characterized by insulin resistance and glucose intolerance, and is associated with adverse consequences for parturient women and newborns [66,67,68]. It has been reported that excessive activation of the NLRP3 inflammasome contributes to GDM [69,70,71]. The results of the study conducted by Wei Wu et al., revealed that the protein expression levels of NLRP3 and cleaved caspase-1 were upregulated, whereas the protein expression levels of CBS and CSE were downregulated in GDM placenta. Moreover, the CBS and CSE levels were negatively correlated with the levels of NLRP3 and cleaved caspase-1. The in-depth research showed that exogenous H2S and H2S precursor L-cysteine notably reduced the protein expression of NLRP3 and cleaved caspase-1, and reduced the levels of IL-1β and IL-18 in human placental cells, while the NLRP3 inflammasome inhibitor Ac-YVAD-CMK reduced the levels of IL-1β and IL-18. Overall, exogenous H2S inhibited NLRP3 inflammasome activation in GDM [72]. In the above studies, the mechanism of exogenous H2S regulating the NLRP3 inflammasome needs to be clarified.
5. The Role of H2S in Regulating the NLRP3 Inflammasome in Diabetic Adipose Tissue Inflammation
Adipose tissue is an important endocrine organ, which is involved in regulating inflammation [73,74,75]. The NLRP3 inflammasome plays an important role in inflammation in adipose tissue [76,77]. H2S has an anti-inflammatory function in many tissues and cells [78,79,80]; however, the anti-inflammatory properties of H2S in adipose tissue have not been reported. Tian-Xiao Hu et al., found that HG upregulated the expression of NLRP3, ASC, cleaved caspase-1, and the levels of IL-1β and IL-18 in adipocytes. The inhibition of caspase-1 with inhibitors could eliminate the increase in IL-1β and IL-18, induced by HG, in adipocytes. Exogenous H2S inhibited the induction of NLRP3, ASC, cleaved caspase-1, IL-1β and IL-18, induced by HG, in adipocytes. Overall, exogenous H2S inhibited NLRP3 inflammasome-mediated inflammation, induced by HG, in adipocytes [81]. At present, there are few studies on the role of H2S in regulating the NLRP3 inflammasome in adipose tissue, which is worthy of in-depth study; in particular, the relevant mechanism needs to be clarified.
6. The Role of H2S in Regulating the NLRP3 Inflammasome in Human Diabetic Retinopathy
Diabetic retinopathy (DR), one of the most common complications of diabetes, is the main cause of blindness [82,83,84]. It has been reported that oxidative stress and inflammation are closely related with DR [85,86,87,88]. Peng Wang et al., found that in ARPE-19 (human retinal pigment epithelial) cells, HG promoted cell death and upregulated the expression levels of IL-18 and IL-1β. Furthermore, HG also upregulated NLRP3 inflammasome expression and promoted ROS production, whereas the ROS scavenger NAC inhibited HG-induced ROS production, downregulated the expression levels of IL-18 and IL-1β, and abolished NLRP3 inflammasome activation, induced by FG. Moreover, silencing NLRP3 gene expression with siRNA had similar results to those with NAC. The above indicated that HG induced NLRP3 inflammasome-mediated inflammation by promoting ROS production. The in-depth study showed that exogenous H2S was able to completely abolish the increase in ROS production and NLRP3 inflammasome-mediated inflammation induced by HG in ARPE-19 cells. Collectively, exogenous H2S protected human retinal pigment epithelial cells against NLRP3 inflammasome-mediated inflammation, induced by HG, by inhibiting ROS production [89]. Contrary to the above conclusion, H2S can promote inflammation and elevate oxidative stress [90,91,92], which may be related to the concentration of H2S. A low concentration of H2S inhibits inflammation and oxidative stress and has a protective effect on the body, while a high concentration of H2S has the opposite effect.
7. The Role of H2S in Regulating the NLRP3 Inflammasome in Diabetic Fibrosis of the Diaphragm
The contractile function of the diaphragm in diabetic rats, induced by streptozotocin (STZ), decreased significantly, which was related to the excessive inflammation and oxidative stress in the diaphragm tissue [93,94]. The available evidence suggests that hyperglycemia can promote the deposition of collagen in muscle, which leads to fibrosis [95], and the excessive activation of the NLRP3 inflammasome contributes to tissue fibrosis [96,97,98]. Rui Yang et al., found that the biomechanical parameters of the diaphragm in diabetic rats were reduced, while the levels of inflammatory cytokines, NLRP3 inflammasome and collagen were upregulated, suggesting that diabetes induced diaphragm muscle fibrosis by activating the NLRP3 inflammasome. Exogenous H2S improved the histological and ultrastructural lesions, diaphragmatic biomechanical alterations and fibrosis of the diaphragm, and inhibited NLRP3 inflammasome activation in streptozotocin-induced diabetic rats. Collectively, exogenous H2S partly ameliorated the diaphragm muscle fibrosis of streptozotocin-induced diabetic rats by suppressing NLRP3 inflammasome activation, which needed to be further confirmed [99]. TGF-β1 is a contributor to tissue fibrosis [29]. Overactivation of the NLRP3 inflammasome can lead to the excessive production of TGF-β1, which can lead to fibrosis [100]. In the above study, how H2S regulates the NLRP3 inflammasome remains to be clarified.
8. Conclusions
Much evidence indicates that H2S plays an important role in diabetes. In this review, we summarized the role of H2S regulation in the NLRP3 inflammasome and its mechanism in many type of diabetes-related diseases, reaching the following conclusions: (1) exogenous H2S improves HG-induced cardiotoxicity through the inhibition of NLRP3 inflammasome activation, by suppressing the TLR4/NF-κB pathway in H9c2 cells; (2) H2S suppresses NLRP3 inflammasome activation in diabetic rats through the inhibition of TXNIP, which needs to be further verified; (3) exogenous H2S could improve diabetic cardiomyopathy through inhibition of the NLRP3 inflammasome, by inhibiting necroticptosis; (4) H2S mitigates diabetes-accelerated atherosclerosis through the inhibition of NLRP3 inflammasome activation, by suppressing oxidative stress; (5) exogenous H2S suppresses NLRP3 inflammasome activation in gestational diabetes mellitus; (6) exogenous H2S inhibits NLRP3 inflammasome activation induced by HG in adipocytes; (7) exogenous H2S inhibits the NLRP3 inflammasome, induced by HG, through the inhibition of ROS production in human retinal pigment epithelial cells; (8) exogenous H2S partly improves diaphragm muscle fibrosis through the inhibition of NLRP3 inflammasome activation in streptozotocin-induced diabetic rats, which needed to be further confirmed (Table 1).
Table 1.
Summary of the role of H2S in regulating the NLRP3 inflammasome in diabetes.
| Type of Diabetes-Related Diseases | Role of Hydrogen Sulfide Regulation of NLRP3 Inflammasome | Experimental Materials | Reference |
|---|---|---|---|
| diabetic cardiomyopathy | exogenous H2S improves HG-induced cardiotoxicity through the inhibition of NLRP3 inflammasome activation by suppressing the TLR4/NF-κB pathway | high glucose (HG)-induced H9c2 cells | [43] |
| diabetic cardiomyopathy | H2S suppresses NLRP3 inflammasome activation in diabetic cardiomyopathy through the inhibition of TXNIP, which requires further verification | diabetic rats | [48] |
| diabetic cardiomyopathy | exogenous H2S improves diabetic cardiomyopathy through suppression of the NLRP3 inflammasome by inhibiting necroticptosis | diabetic mice/HG-induced cardiomyocyte | [57] |
| diabetes-accelerated atherosclerosis | H2S ameliorates diabetes-accelerated atherosclerosis through the inhibition of NLRP3 inflammasome activation by suppressing oxidative stress | HG-induced human umbilical vein endothelial cells/diabetic mice | [65] |
| gestational diabetes mellitus | exogenous H2S inhibits NLRP3 inflammasome activation in gestational diabetes mellitus | HG-induced human diabetic placenta tissues and placental cell | [72] |
| diabetic adipose tissue inflammation | exogenous H2S inhibits HG-induced NLRP3 inflammasome activation | HG-induced rat adipocytes | [81] |
| diabetic retinopathy | exogenous H2S inhibits the NLRP3 inflammasome induced by HG through the inhibition of ROS production | HG-induced human retinal pigment epithelium cell line ARPE-19 |
[89] |
| diabetic fibrosis of the diaphragm | exogenous H2S improves diaphragm muscle fibrosis partly through the inhibition of NLRP3 inflammasome activation | diabetic rats | [99] |
It can be observed from the above that H2S may inhibit NLRP3 inflammasome activation through suppression of the TLR4/NF-κB pathway, TXNIP, necroticptosis and oxidative stress in diabetes, some of which need to be further confirmed. Whether there are other mechanisms of H2S inhibiting the NLRP3 inflammasome in diabetes requires further study. In addition, in the above studies, H2S played a role in diabetes by inhibiting the NLRP3 inflammasome. It has been reported that H2S has both anti-inflammatory and pro-inflammatory effects, so whether H2S can promote the NLRP3 inflammasome in diabetes also remains to be studied. The H2S/NLRP3 inflammasome has been involved in diabetes in many studies, respectively, but the roles and the mechanisms of the two in the occurrence and development of diabetes need to be further elucidated. Furthermore, the NLRP3 inflammasome is closely related to endoplasmic reticulum stress (ERS)/autophagy, and the latter two are the targets of H2S regulation. Therefore, the role of H2S in regulating the NLRP3 inflammasome/ERS or NLRP3 inflammasome/autophagy in diabetes is worth studying. Finally, the current donor of exogenous H2S is not ideal; therefore, the development of better H2S donors will be more conducive to the application of H2S in the regulation of the NLRP3 inflammasome in diabetes.
In conclusion, with the further development of related research, H2S regulation of the NLRP3 inflammasome will become a new strategy for the treatment of diabetes.
Author Contributions
H.W. devised and wrote the manuscript; H.Z. wrote and fund the manuscript; H.L. wrote the manuscript; Y.Y. drew the figures. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics Approval and Consent to Participate
Not applicable.
Funding Statement
This work was supported by grants from key scientific and technological projects in Henan Province, China (Grant No. 202102310153), the construction of first-class medical disciplines at the Medical College of Henan University from 2020 to 2021 for H.W. (Grant No: 20211021002) and the Innovation and Entrepreneurship Training Program for Henan University Students in 2021 (Grant No: 20217003002).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martinon F., Burns K., Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–4126. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 2.Elliott E.I., Sutterwala F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015;265:35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jo E.K., Kim J.K., Shin D.M., Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol. Immunol. 2016;13:148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbe F., Douglas T., Saleh M. Advances in Nod-like receptors (NLR) biology. Cytokine Growth Factor Rev. 2014;25:681–697. doi: 10.1016/j.cytogfr.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Minkiewicz J., de Rivero Vaccari J.P., Keane R.W. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013;61:1113–1121. doi: 10.1002/glia.22499. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor W., Jr., Harton J.A., Zhu X., Linhoff M.W., Ting J.P. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. J. Immunol. 2003;171:6329–6333. doi: 10.4049/jimmunol.171.12.6329. [DOI] [PubMed] [Google Scholar]
- 8.Volt H., Garcia J.A., Doerrier C., Diaz-Casado M.E., Guerra-Librero A., Lopez L.C., Escames G., Tresguerres J.A., Acuna-Castroviejo D. Same molecule but different expression: Aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 2016;60:193–205. doi: 10.1111/jpi.12303. [DOI] [PubMed] [Google Scholar]
- 9.Sharif H., Wang L., Wang W.L., Magupalli V.G., Andreeva L., Qiao Q., Hauenstein A.V., Wu Z., Nunez G., Mao Y., et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–343. doi: 10.1038/s41586-019-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agostini L., Martinon F., Burns K., McDermott M.F., Hawkins P.N., Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/S1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 11.Zahid A., Li B., Kombe A.J.K., Jin T., Tao J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front. Immunol. 2019;10:2538. doi: 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao Y., Wang N., Qiu T., Sun X. The role of autophagy and NLRP3 inflammasome in liver fibrosis. Biomed. Res. Int. 2020;2020:7269150. doi: 10.1155/2020/7269150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T., Fitzgerald K.A., Latz E., Moore K.J., Golenbock D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong Y., Kinio A., Saleh M. Functions of NOD-Like Receptors in Human Diseases. Front. Immunol. 2013;4:333. doi: 10.3389/fimmu.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai X., Chen J., Xu H., Liu S., Jiang Q.X., Halfmann R., Chen Z.J. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu A., Magupalli V.G., Ruan J., Yin Q., Atianand M.K., Vos M.R., Schroder G.F., Fitzgerald K.A., Wu H., Egelman E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prochnicki T., Mangan M.S., Latz E. Recent insights into the molecular mechanisms of the NLRP3 inflammasome activation. F1000Research. 2016;5:1469. doi: 10.12688/f1000research.8614.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanson K.V., Junkins R.D., Kurkjian C.J., Holley-Guthrie E., Pendse A.A., El Morabiti R., Petrucelli A., Barber G.N., Benedict C.A., Ting J.P. A noncanonical function of cGAMP in inflammasome priming and activation. J. Exp. Med. 2017;214:3611–3626. doi: 10.1084/jem.20171749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokolova M., Ranheim T., Louwe M.C., Halvorsen B., Yndestad A., Aukrust P. NLRP3 Inflammasome: A Novel Player in Metabolically Induced Inflammation-Potential Influence on the Myocardium. J. Cardiovasc. Pharmacol. 2019;74:276–284. doi: 10.1097/FJC.0000000000000704. [DOI] [PubMed] [Google Scholar]
- 21.Meyers A.K., Zhu X. The NLRP3 Inflammasome: Metabolic Regulation and Contribution to Inflammaging. Cells. 2020;9:1808. doi: 10.3390/cells9081808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 23.Powell C.R., Dillon K.M., Matson J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018;149:110–123. doi: 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H., Shi X., Qiu M., Lv S., Zheng H., Niu B., Liu H. Hydrogen sulfide plays an important role by influencing NLRP3 inflammasome. Int. J. Biol. Sci. 2020;16:2752–2760. doi: 10.7150/ijbs.47595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose P., Moore P.K., Zhu Y.Z. H2S biosynthesis and catabolism: New insights from molecular studies. Cell Mol. Life Sci. 2017;74:1391–1412. doi: 10.1007/s00018-016-2406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polhemus D.J., Lefer D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H., Shi X., Qiu M., Lv S., Liu H. Hydrogen sulfide plays an important protective role through influencing endoplasmic reticulum stress in diseases. Int. J. Biol. Sci. 2020;16:264–271. doi: 10.7150/ijbs.38143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behera J., Tyagi S.C., Tyagi N. Role of hydrogen sulfide in the musculoskeletal system. Bone. 2019;124:33–39. doi: 10.1016/j.bone.2019.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J., Yuan Y.Q., Zhang L., Zhang H., Zhang S.W., Zhang Y., Xuan X.X., Wang M.J., Zhang J.Y. Exogenous hydrogen sulfide protects against high glucose-induced apoptosis and oxidative stress by inhibiting the STAT3/HIF-1alpha pathway in H9c2 cardiomyocytes. Exp. Ther. Med. 2019;18:3948–3958. doi: 10.3892/etm.2019.8036. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Tocmo R., Parkin K. S-1-propenylmercaptocysteine protects murine hepatocytes against oxidative stress via persulfidation of Keap1 and activation of Nrf2. Free Radic. Biol. Med. 2019;143:164–175. doi: 10.1016/j.freeradbiomed.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Zhao A.S., Zou D., Wang H.H., Han X., Yang P., Huang N. Hydrogen sulphide-releasing aspirin enhances cell capabilities of anti-oxidative lesions and anti-inflammation. Med. Gas Res. 2019;9:145–152. doi: 10.4103/2045-9912.266990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greaney J.L., Kutz J.L., Shank S.W., Jandu S., Santhanam L., Alexander L.M. Impaired hydrogen sulfide-mediated vasodilation contributes to microvascular endothelial dysfunction in hypertensive adults. Hypertension. 2017;69:902–909. doi: 10.1161/HYPERTENSIONAHA.116.08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin S., Teng X., Xiao L., Xue H., Guo Q., Duan X., Chen Y., Wu Y. Hydrogen sulfide ameliorated L-NAME-induced hypertensive heart disease by the Akt/eNOS/NO pathway. Exp. Biol. Med. 2017;242:1831–1841. doi: 10.1177/1535370217732325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D., Du J., Tang C., Huang Y., Jin H. H2S-Induced sulfhydration: Biological function and detection methodology. Front. Pharmacol. 2017;8:608. doi: 10.3389/fphar.2017.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia G., Whaley-Connell A., Sowers J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61:21–28. doi: 10.1007/s00125-017-4390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knapp M., Tu X., Wu R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol. Sin. 2019;40:1–8. doi: 10.1038/s41401-018-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L., Cai Y., Jian L., Cheung C.W., Zhang L., Xia Z. Impact of peroxisome proliferator-activated receptor-alpha on diabetic cardiomyopathy. Cardiovasc. Diabetol. 2021;20:2. doi: 10.1186/s12933-020-01188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji L., Liu F., Jing Z., Huang Q., Zhao Y., Cao H., Li J., Yin C., Xing J., Li F. MICU1 alleviates diabetic cardiomyopathy through mitochondrial Ca2+-dependent antioxidant response. Diabetes. 2017;66:1586–1600. doi: 10.2337/db16-1237. [DOI] [PubMed] [Google Scholar]
- 39.Palomer X., Aguilar-Recarte D., Garcia R., Nistal J.F., Vazquez-Carrera M. Sirtuins: To be or not to be in diabetic cardiomyopathy. Trends Mol. Med. 2021;27:554–571. doi: 10.1016/j.molmed.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Ge Q., Zhao L., Ren X.M., Ye P., Hu Z.Y. LCZ696, an angiotensin receptor-neprilysin inhibitor, ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis. Exp. Biol. Med. 2019;244:1028–1039. doi: 10.1177/1535370219861283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jubaidi F.F., Zainalabidin S., Taib I.S., Hamid Z.A., Budin S.B. The potential role of flavonoids in ameliorating diabetic cardiomyopathy via alleviation of cardiac oxidative stress, inflammation and apoptosis. Int. J. Mol. Sci. 2021;22:5094. doi: 10.3390/ijms22105094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abukhalil M.H., Althunibat O.Y., Aladaileh S.H., Al-Amarat W., Obeidat H.M., Al-Khawalde A.A.A., Hussein O.E., Alfwuaires M.A., Algefare A.I., Alanazi K.M., et al. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2021;138:111410. doi: 10.1016/j.biopha.2021.111410. [DOI] [PubMed] [Google Scholar]
- 43.Huang Z., Zhuang X., Xie C., Hu X., Dong X., Guo Y., Li S., Liao X. Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation by suppressing TLR4/NF-kappaB pathway in H9c2 cells. Cell Physiol. Biochem. 2016;40:1578–1590. doi: 10.1159/000453208. [DOI] [PubMed] [Google Scholar]
- 44.Liang R.K., Zhao Y.Y., Shi M.L., Zhang G., Zhao Y.J., Zhang B.G., Liang R.J. Skimmin protects diabetic cardiomyopathy in streptozotocin-induced diabetic rats. Kaohsiung J. Med. Sci. 2021;37:136–144. doi: 10.1002/kjm2.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia G., Hill M.A., Sowers J.R. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song S., Ding Y., Dai G.L., Zhang Y., Xu M.T., Shen J.R., Chen T.T., Chen Y., Meng G.L. Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation. Acta Pharmacol. Sin. 2021;42:230–241. doi: 10.1038/s41401-020-0490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang F., Qin Y., Wang Y., Meng S., Xian H., Che H., Lv J., Li Y., Yu Y., Bai Y., et al. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int. J. Biol. Sci. 2019;15:1010–1019. doi: 10.7150/ijbs.29680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia Q., Mehmood S., Liu X., Ma S., Yang R. Hydrogen sulfide mitigates myocardial inflammation by inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation in diabetic rats. Exp. Biol. Med. 2020;245:221–230. doi: 10.1177/1535370219899899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan X.L., Ju G.Y., Xu L., Yang H.M., Wang Z.Y. Dietary selenomethionine increases antioxidant capacity of geese by improving glutathione and thioredoxin systems. Poult. Sci. 2019;98:3763–3769. doi: 10.3382/ps/pez066. [DOI] [PubMed] [Google Scholar]
- 50.Canesi F., Mateo V., Couchie D., Karabina S., Negre-Salvayre A., Rouis M., El Hadri K. A thioredoxin-mimetic peptide exerts potent anti-inflammatory, antioxidant, and atheroprotective effects in ApoE2.Ki mice fed high fat diet. Cardiovasc. Res. 2019;115:292–301. doi: 10.1093/cvr/cvy183. [DOI] [PubMed] [Google Scholar]
- 51.Muri J., Thut H., Kopf M. The thioredoxin-1 inhibitor Txnip restrains effector T-cell and germinal center B-cell expansion. Eur. J. Immunol. 2021;51:115–124. doi: 10.1002/eji.202048851. [DOI] [PubMed] [Google Scholar]
- 52.Zuo H., Yuan J., Yang L., Liang Z., Weng S., He J., Xu X. Characterization and immune function of the thioredoxin-interacting protein (TXNIP) from Litopenaeus vannamei. Fish. Shellfish Immunol. 2019;84:20–27. doi: 10.1016/j.fsi.2018.09.064. [DOI] [PubMed] [Google Scholar]
- 53.Wang C.Y., Xu Y., Wang X., Guo C., Wang T., Wang Z.Y. Dl-3-n-Butylphthalide inhibits NLRP3 inflammasome and mitigates Alzheimer’s-like pathology via Nrf2-TXNIP-TrX axis. Antioxid. Redox Signal. 2019;30:1411–1431. doi: 10.1089/ars.2017.7440. [DOI] [PubMed] [Google Scholar]
- 54.Dai X., Liao R., Liu C., Liu S., Huang H., Liu J., Jin T., Guo H., Zheng Z., Xia M., et al. Epigenetic regulation of TXNIP-mediated oxidative stress and NLRP3 inflammasome activation contributes to SAHH inhibition-aggravated diabetic nephropathy. Redox Biol. 2021;45:102033. doi: 10.1016/j.redox.2021.102033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y., Li X., Hua Y., Ding Y., Meng G., Zhang W. RIPK3-mediated necroptosis in diabetic cardiomyopathy requires CaMKII activation. Oxid. Med. Cell Longev. 2021;2021:6617816. doi: 10.1155/2021/6617816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Q., Tan X., Xian W., Geng J., Li H., Tang B., Zhang H., Wang H., Gao Q., Kang P. Changes of necroptosis in irbesartan medicated cardioprotection in diabetic rats. Diabetes Metab. Syndr. Obes. 2021;14:3851–3863. doi: 10.2147/DMSO.S300388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong W., Zhang S., Chen Y., Shen J., Zheng Y., Liu X., Zhu M., Meng G. Protective role of hydrogen sulfide against diabetic cardiomyopathy via alleviating necroptosis. Free Radic. Biol. Med. 2022;181:29–42. doi: 10.1016/j.freeradbiomed.2022.01.028. [DOI] [PubMed] [Google Scholar]
- 58.Mohammed S., Nicklas E.H., Thadathil N., Selvarani R., Royce G.H., Kinter M., Richardson A., Deepa S.S. Role of necroptosis in chronic hepatic inflammation and fibrosis in a mouse model of increased oxidative stress. Free Radic. Biol. Med. 2021;164:315–328. doi: 10.1016/j.freeradbiomed.2020.12.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen S., Lv X., Hu B., Zhao L., Li S., Li Z., Qing X., Liu H., Xu J., Shao Z. Critical contribution of RIPK1 mediated mitochondrial dysfunction and oxidative stress to compression-induced rat nucleus pulposus cells necroptosis and apoptosis. Apoptosis. 2018;23:299–313. doi: 10.1007/s10495-018-1455-x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y., Yu D., Zhang J., Bao J., Tang C., Zhang Z. The role of necroptosis and apoptosis through the oxidative stress pathway in the liver of selenium-deficient swine. Metallomics. 2020;12:607–616. doi: 10.1039/c9mt00295b. [DOI] [PubMed] [Google Scholar]
- 61.Wang B., Cui Y., Zhang Q., Wang S., Xu S. Selenomethionine alleviates LPS-induced JNK/NLRP3 inflammasome-dependent necroptosis by modulating miR-15a and oxidative stress in chicken lungs. Metallomics. 2021;13:mfab048. doi: 10.1093/mtomcs/mfab048. [DOI] [PubMed] [Google Scholar]
- 62.Chao M.L., Luo S., Zhang C., Zhou X., Zhou M., Wang J., Kong C., Chen J., Lin Z., Tang X., et al. S-nitrosylation-mediated coupling of G-protein alpha-2 with CXCR5 induces Hippo/YAP-dependent diabetes-accelerated atherosclerosis. Nat. Commun. 2021;12:4452. doi: 10.1038/s41467-021-24736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 64.Eckel R.H., Wassef M., Chait A., Sobel B., Barrett E., King G., Lopes-Virella M., Reusch J., Ruderman N., Steiner G., et al. Prevention conference VI: Diabetes and cardiovascular disease: Writing group II: Pathogenesis of atherosclerosis in diabetes. Circulation. 2002;105:e138–e143. doi: 10.1161/01.CIR.0000013954.65303.C5. [DOI] [PubMed] [Google Scholar]
- 65.Zheng Q., Pan L., Ji Y. H 2S protects against diabetes-accelerated atherosclerosis by preventing the activation of NLRP3 inflammasome. J. Biomed. Res. 2019;34:94–102. doi: 10.7555/JBR.33.20190071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alfadhli E.M. Gestational diabetes mellitus. Saudi Med. J. 2015;36:399–406. doi: 10.15537/smj.2015.4.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.ACOG ACOG Practice bulletin No. 190: Gestational diabetes mellitus. Obstet. Gynecol. 2018;131:e49–e64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 68.Catalano P.M. Trying to understand gestational diabetes. Diabet. Med. 2014;31:273–281. doi: 10.1111/dme.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang R., Zhang X., Xing B., Zhao J., Zhang P., Shi D., Yang F. Astragaloside IV attenuates gestational diabetes mellitus via targeting NLRP3 inflammasome in genetic mice. Reprod. Biol. Endocrinol. 2019;17:77. doi: 10.1186/s12958-019-0522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao J., Peng Q. NLRP3 inhibitor tranilast attenuates gestational diabetes mellitus in a genetic mouse model. Drugs R D. 2022;22:105–112. doi: 10.1007/s40268-022-00382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H., Luan S., Xiao X., Lin L., Zhao X., Liu X. Silenced microRNA-222 suppresses inflammatory response in gestational diabetes mellitus mice by promoting CXCR4. Life Sci. 2021;266:118850. doi: 10.1016/j.lfs.2020.118850. [DOI] [PubMed] [Google Scholar]
- 72.Wu W., Tan Q.Y., Xi F.F., Ruan Y., Wang J., Luo Q., Dou X.B., Hu T.X. NLRP3 inflammasome activation in gestational diabetes mellitus placentas is associated with hydrogen sulfide synthetase deficiency. Exp. Ther. Med. 2022;23:94. doi: 10.3892/etm.2021.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Unamuno X., Gomez-Ambrosi J., Rodriguez A., Becerril S., Fruhbeck G., Catalan V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018;48:e12997. doi: 10.1111/eci.12997. [DOI] [PubMed] [Google Scholar]
- 74.Stolarczyk E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr. Opin. Pharmacol. 2017;37:35–40. doi: 10.1016/j.coph.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Anthony S.R., Guarnieri A.R., Gozdiff A., Helsley R.N., Phillip Owens A., Tranter M. Mechanisms linking adipose tissue inflammation to cardiac hypertrophy and fibrosis. Clin. Sci. 2019;133:2329–2344. doi: 10.1042/CS20190578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Unamuno X., Gomez-Ambrosi J., Ramirez B., Rodriguez A., Becerril S., Valenti V., Moncada R., Silva C., Salvador J., Fruhbeck G., et al. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol. Immunol. 2021;18:1045–1057. doi: 10.1038/s41423-019-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen X., Huang Q., Feng J., Xiao Z., Zhang X., Zhao L. GLP-1 alleviates NLRP3 inflammasome-dependent inflammation in perivascular adipose tissue by inhibiting the NF-kappaB signalling pathway. J. Int. Med. Res. 2021;49:300060521992981. doi: 10.1177/0300060521992981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H., Zhong P., Sun L. Exogenous hydrogen sulfide mitigates NLRP3 inflammasome-mediated inflammation through promoting autophagy via the AMPK-mTOR pathway. Biol. Open. 2019;8:bio043653. doi: 10.1242/bio.043653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo C., Liang F., Shah Masood W., Yan X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-kappaB dependent anti-inflammation pathway. Eur. J. Pharmacol. 2014;725:70–78. doi: 10.1016/j.ejphar.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 80.Chen Y.H., Wang P.P., Wang X.M., He Y.J., Yao W.Z., Qi Y.F., Tang C.S. Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine. 2011;53:334–341. doi: 10.1016/j.cyto.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 81.Hu T.X., Zhang N.N., Ruan Y., Tan Q.Y., Wang J. Hydrogen sulfide modulates high glucose-induced NLRP3 inflammasome activation in 3T3-L1 adipocytes. Exp. Ther. Med. 2020;19:771–776. doi: 10.3892/etm.2019.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang W., Lo A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018;19:1816. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simo-Servat O., Hernandez C., Simo R. Diabetic retinopathy in the context of patients with diabetes. Ophthalmic Res. 2019;62:211–217. doi: 10.1159/000499541. [DOI] [PubMed] [Google Scholar]
- 84.Sabanayagam C., Banu R., Chee M.L., Lee R., Wang Y.X., Tan G., Jonas J.B., Lamoureux E.L., Cheng C.Y., Klein B.E.K., et al. Incidence and progression of diabetic retinopathy: A systematic review. Lancet Diabetes Endocrinol. 2019;7:140–149. doi: 10.1016/S2213-8587(18)30128-1. [DOI] [PubMed] [Google Scholar]
- 85.Wang H., Zhang M., Zhou H., Cao L., Zhou J., Chen Q., Zhang X. Salusin-beta mediates high glucose-induced inflammation and apoptosis in retinal capillary endothelial cells via a ROS-dependent pathway in diabetic retinopathy. Diabetes Metab. Syndr. Obes. 2021;14:2291–2308. doi: 10.2147/DMSO.S301157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tu Y., Li L., Zhu L., Guo Y., Du S., Zhang Y., Wang Z., Zhang Y., Zhu M. Geniposide attenuates hyperglycemia-induced oxidative stress and inflammation by activating the Nrf2 signaling pathway in experimental diabetic retinopathy. Oxid. Med. Cell Longev. 2021;2021:9247947. doi: 10.1155/2021/9247947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu S., Zheng Y., Sun Y., Lai M., Qiu J., Gui F., Zeng Q., Liu F. Suppressing long noncoding RNA OGRU ameliorates diabetic retinopathy by inhibition of oxidative stress and inflammation via miR-320/USP14 axis. Free Radic. Biol. Med. 2021;169:361–381. doi: 10.1016/j.freeradbiomed.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 88.Zeng Q., Luo Y., Fang J., Xu S., Hu Y.H., Yin M. Circ_0000615 promotes high glucose-induced human retinal pigment epithelium cell apoptosis, inflammation and oxidative stress via miR-646/YAP1 axis in diabetic retinopathy. Eur. J. Ophthalmol. 2021:11206721211020200. doi: 10.1177/11206721211020200. [DOI] [PubMed] [Google Scholar]
- 89.Wang P., Chen F., Wang W., Zhang X.D. Hydrogen sulfide attenuates high glucose-induced human retinal pigment epithelial cell inflammation by inhibiting ROS formation and NLRP3 inflammasome activation. Mediators Inflamm. 2019;2019:8908960. doi: 10.1155/2019/8908960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ni K., Hua Y. Hydrogen sulfide exacerbated periodontal inflammation and induced autophagy in experimental periodontitis. Int. Immunopharmacol. 2021;93:107399. doi: 10.1016/j.intimp.2021.107399. [DOI] [PubMed] [Google Scholar]
- 91.Kabil O., Motl N., Banerjee R. H2S and its role in redox signaling. Biochim. Biophys. Acta. 2014;1844:1355–1366. doi: 10.1016/j.bbapap.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chi Q., Wang D., Hu X., Li S., Li S. Hydrogen sulfide gas exposure induces necroptosis and promotes inflammation through the MAPK/NF-kappaB pathway in broiler spleen. Oxid. Med. Cell Longev. 2019;2019:8061823. doi: 10.1155/2019/8061823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trevisan C.S.C., Garcia-Araujo A.S., Duarte A., Furino V.O., Russo T.L., Fujimoto A., Souza H.C.D., Jaenisch R.B., Arena R., Borghi-Silva A. Effects of respiratory muscle training on parasympathetic activity in diabetes mellitus. Braz. J. Med. Biol. Res. 2021;54:e10865. doi: 10.1590/1414-431x2020e10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jia Q., Yang R., Ma S., Liu X. Protective effect of genistein on diaphragm injury in diabetic rats. Lat. Am. J. Pharm. 2018;37:2145–2153. [Google Scholar]
- 95.Fanning K.M., Pfisterer B., Davis A.T., Presley T.D., Williams I.M., Wasserman D.H., Cline J.M., Kavanagh K. Changes in microvascular density differentiate metabolic health outcomes in monkeys with prior radiation exposure and subsequent skeletal muscle ECM remodeling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;313:R290–R297. doi: 10.1152/ajpregu.00108.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wree A., McGeough M.D., Inzaugarat M.E., Eguchi A., Schuster S., Johnson C.D., Pena C.A., Geisler L.J., Papouchado B.G., Hoffman H.M., et al. NLRP3 inflammasome driven liver injury and fibrosis: Roles of IL-17 and TNF in mice. Hepatology. 2018;67:736–749. doi: 10.1002/hep.29523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y., Li H., Liu S., Pan P., Su X., Tan H., Wu D., Zhang L., Song C., Dai M., et al. Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol. Immunol. 2018;99:134–144. doi: 10.1016/j.molimm.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 98.Wu M., Han W., Song S., Du Y., Liu C., Chen N., Wu H., Shi Y., Duan H. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol. Cell Endocrinol. 2018;478:115–125. doi: 10.1016/j.mce.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Yang R., Jia Q., Li Y., Mehmood S. Protective effect of exogenous hydrogen sulfide on diaphragm muscle fibrosis in streptozotocin-induced diabetic rats. Exp. Biol. Med. 2020;245:1280–1289. doi: 10.1177/1535370220931038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li R., Lu K., Wang Y., Chen M., Zhang F., Shen H., Yao D., Gong K., Zhang Z. Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochem. Biophys. Res. Commun. 2017;485:69–75. doi: 10.1016/j.bbrc.2017.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.