Abstract
Antimicrobial compounds from the commensal gut microbiota have gained much attention due to their multifunctionality in maintaining good health in the host and killing multidrug-resistant bacteria. Our previous study showed that Paenibacillus jilinensis YPG26 isolated from chicken intestine can antagonize multiple pathogens. Herein, we characterized a bacteriocin-like inhibitory substance, jileicin, purified from P. jilinensis YPG26. Mass spectrometry analysis revealed that jileicin was a protein consisting of 211 amino acids, which showed 88.98% identity to the SIMPL domain-containing protein. The jileicin showed a relatively broad-spectrum antibacterial ability, especially against enterococci. Additionally, the jileicin exhibited good stability after various treatments, no detectable resistance, no significant cytotoxicity, and very low levels of hemolytic activity. The mode of action against Enterococcus faecium demonstrated that jileicin could destroy cell membrane integrity, increase cell membrane permeability, and eventually lead to cell death. Furthermore, jileicin was efficient in controlling the growth of E. faecium in milk. In conclusion, jileicin, as a newly identified antibacterial agent, is expected to be a promising candidate for application in the food, pharmaceutical, and biomedical industries.
Keywords: Paenibacillus jilinensis, jileicin, purification, mode of action, Enterococcus faecium
Introduction
The intestinal tracts of humans and animals are colonized by an enormous number of microbes, including bacteria, viruses, fungi, and archaea, collectively known as the gut microbiota. Bacteria account for the greatest proportion, with an estimated abundance of 1011 bacteria/mL in the human colon.1 The gut microbiota plays a crucial role in the host physiology, metabolism, immunity, digestion, and nutrition uptake. Furthermore, the gut microbiota, which has not yet been fully explored, is gradually emerging as a promising source of novel antimicrobials.2 In recent years, several gut microbiota-derived antimicrobial substances have been developed to protect the host from multidrug-resistant pathogens.3−5 These effective antimicrobials help to maintain the health of the host and overcome the growing problem of antibiotic-resistant bacteria.
Bacteriocin, or bacteriocin-like inhibitory substance (BLIS), is a class of peptide or protein synthesized by ribosomes, which are classified into three main classes based on their structural and physicochemical properties.6 Most bacteriocins kill bacteria by destroying the cell structure and leading to leakage of intracellular molecules. Thus, it is for this reason that bacteriocin may be more effective in inhibiting or killing multidrug-resistant bacteria.6 In addition, bacteriocins unusually display excellent stability, low cell cytotoxicity, and low hemolytic activity and are not easy to develop resistance. These advantages allow bacteriocin to have a wide range of applications, for example, applied in food preservation as natural bio-preservatives and in human and animal health as alternatives to conventional antibiotics, and other related applications.7 Nonetheless, it is still necessary to explore novel antimicrobials with broad-spectrum antibacterial activity.
In our previous study, we isolated Paenibacillus jilinensis YPG26 from the chicken intestine, which can antagonize multiple pathogens. The study aims to purify and identify a bacteriocin-like inhibitory substance, jileicin, obtained from P. jilinensis YPG26. The stability and safety of jileicin and the mode of action against Enterococcus faecium will also be determined. Furthermore, the application of jilieicin in milk will be evaluated. The findings will be helpful in providing a promising candidate for application in the food, pharmaceutical, and biomedical industries.
Materials and Methods
Bacterial Strains and Culture Conditions
Paenibacillus jilinensis YPG26 (P. jilinensis YPG26, the GenBank accession of 16S rRNA gene sequence is OK324374) reserved in our lab was isolated from chicken intestine and was cultivated aerobically in tryptic soy broth (TSB, Qingdao Hope Biotechnology Co., Ltd., China) at 37 °C. All indicator organisms preserved in our laboratory and culture conditions are listed in Table 1. E. faecium M8-3 was selected as the indicator strain for all subsequent experiments, including antimicrobial assays at purification steps and determining the mode of action, etc.
Table 1. Antimicrobial Spectrum of Jileicin Produced from P. jilinensis YPG26.
| species | strain | medium | MIC (mg/mL) | MIC (μM) |
|---|---|---|---|---|
| Enterococcus faecium | M8-3 | TSB | 0.256 | 11.3 |
| Enterococcus faecium | 4P-SA | TSB | 0.256 | 11.3 |
| Enterococcus faecalis | ATCC 51299 | TSB | 0.32 | 14.2 |
| Enterococcus faecalis | BL-4 | TSB | 0.32 | 14.2 |
| Enterococcus gallinarum | SOT-5 | TSB | 0.64 | 28.4 |
| Enterococcus hirae | GY-6 | TSB | 0.64 | 28.4 |
| Enterococcus villorum | LB-2 | TSB | 0.512 | 22.7 |
| Bacillus subtilis | LG-1 | TSB | 0.32 | 14.2 |
| Listeria monocytogenes | 10403S | TSB | 0.64 | 28.4 |
| Staphylococcus aureus | USA300 | TSB | 0.64 | 28.4 |
| Salmonella pullorum | ATCC 19945 | LB | 0.64 | 28.4 |
| Pseudomonas aeruginosa | ATCC 27853 | LB | 0.64 | 28.4 |
| Salmonella typhimurium | SL1344 | LB | >0.64 | >28.4 |
| Klebsiella pneumoniae | K7 | LB | >0.64 | >28.4 |
| Escherichia coli | O157:H7 | LB | >0.64 | >28.4 |
Production and Purification of Jileicin
According to the previously described methods8,9 with some modifications, P. jilinensis YPG26 was activated in 3 mL of TSB at 37 °C for 12 h with shaking at 200 rpm, then 1% (v/v) of the above culture was inoculated into 1000 mL of TSB and incubated for 10 h in the same way. After incubation, bacterial suspension was centrifuged at 8000g for 10 min. Supernatants were collected after filter sterilization with a 0.22 μm filter (Millipore). Next, the saturated ammonium sulfate was gently added to cell-free supernatants (CFS) to reach 70% (v/v) saturation. After stirring overnight at 4 °C, the precipitate was collected after centrifugation at 10,000g for 20 min, then redissolved in sterile double-distilled water, and dialyzed extensively to remove ammonium sulfate using a 1000 Da cutoff dialysis bag (Yibo biological, China). The active sample was loaded onto a Sephadex G-75 (Solarbio, China) gel filtration column (40 × 2.5 cm2) after equilibrating with double-distilled water and then eluted at a flow rate of 1 mL/min using double-distilled water. The separated fractions were collected, and the antibacterial activity was determined after freeze-drying. Next, the absorption spectra of antibacterial fraction were obtained using a UV–visible spectrophotometer and were further purified by reversed-phase high-performance liquid chromatography (PuriMaster-2000, Shanghai Kezhe Bio-Tech Co., Ltd., Shanghai, China) equipped with a preparative reversed-phase chromatography C18 column by a 5–100% linear gradient of acetonitrile (Tedia) containing 0.1% (v/v) trifluoroacetic acid (Aladdin, China) elution at 280 nm. The purified substance with antibacterial activity was freeze-dried to determine its primary structure.
Molecular Mass Determination and MALDI-TOF/TOF Analysis of Jileicin
After purification of jileicin using a C18 column, the SDS-PAGE was applied to determine the molecular size according to the previously described methods.10 Briefly, the active samples were applied to a 12% (m/v) separating gel for SDS-PAGE at 60 V for 30 min and then at 120 V for 1 h. Then, the gel was stained using the Coomassie brilliant blue staining method to determine the molecular mass. Subsequently, a single band was cut and sent to Sangon Biotech (Shanghai, China) to determine the protein sequence using mass spectrometry MALDI-TOF/TOF (Shimadzu, Japan).
Antimicrobial Spectrum of Jileicin
The minimum inhibitory concentration (MIC) was determined by broth microplate dilution using the Clinical and Laboratory Standards Institute (CLSI) interpretive criteria according to the methods described previously.11 Briefly, all indicator organisms were cultured overnight in Mueller-Hinton broth (MHB, Qingdao Hope Biotechnology Co., Ltd., China), and cell concentration was adjusted to 5 × 105 CFU/mL before being deposited in a 96-well microplate. Concurrently, different concentrations of 10× dilutions of jileicin were added to each well. After incubation at 37 °C for 16–20 h, the MIC was defined as the lowest concentration of jileicin with no visible growth of bacteria. The experiment was performed three times.
Stability Assays of Jileicin
The purified jileicin was used to measure the effects of enzymes, pH, temperature, ultraviolet rays, sodium chloride, metal ions, organic solvents, and detergents on antimicrobial activity. The effects of enzyme on antimicrobial activity were determined by incubating with trypsin, amylase, and catalase (1 mg/mL, all from Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China) according to the method described by Zhao et al.12 After incubation at 37 °C for 2 h, the enzymes were inactivated by heating for 5 min at 100 °C, and the residual antimicrobial activity against the indicator strain was determined by the agar well diffusion method.13 The effects of pH value on antimicrobial activity were determined by adjusting the pH value of the jileicin with 1 M NaOH or 1 M HCl in a range of 3.0–11.0 according to the method previously described.14 After incubation at 37 °C for 3 h, the residual antimicrobial activity was determined. The thermal stability of jileicin was determined by exposure to temperatures of 60, 80, and 100 °C for 30 min in a thermostatic water bath, respectively, as well as to 121 °C for 20 min in an autoclave,12 and then, the residual antimicrobial activity was determined. The effect of ultraviolet rays on antimicrobial activity was determined by measuring residual antimicrobial activity after different times (20, 40, 60 min) of ultraviolet radiation according to the previously described methods with appropriate modifications.15 The effect of sodium chloride on antimicrobial activity was determined by supplementing different concentrations of sodium chloride (3, 4, and 5%) to jileicin and incubating at 37 °C for 2 h according to the previously described with appropriately modifications,16 and then the residual antimicrobial activity was determined. The effect of metal ions on antimicrobial activity was determined by assaying the relative activity with the addition of 5 mM metal ions (Mg2+, Fe2+, Ca2+, and Ba2+) according to the previously described methods with appropriate modifications.15 After incubation at 37 °C for 3 h, the residual antimicrobial activity was determined. The effect of organic solvents and detergents on antimicrobial activity was determined by adding different organic solvents and detergents at their working concentrations: methanol, ethanol, butanol, acetone (10%, v/v), Tween 20, Triton X-100 (5%, v/v), SDS (5%, m/v), and EDTA (5 mM) to jileicin and incubated at 37 °C for 3 h according to the previously described with appropriate modifications,10 and then the residual antimicrobial activity was determined. All experiments were performed with two biological replicates.
Resistance Development Study
The resistance development was determined as previously described with appropriate modifications.5 First, 1× MICs of jileicin, nisin, and oxacillin against E. faecium were determined as described above. Subsequently, the E. faecium cells at the logarithmic growth phase in the TSB medium were adjusted to approximately 5 × 105 CFU/mL and dispensed into 96-well microtiter plates with 100 μL/well and added jileicin, nisin, and oxacillin at concentrations of 0.25×, 0.5×, 1×, 2×, and 4× MIC. After incubation at 37 °C for 16–20 h, wells with subinhibitory concentration were passaged, the MIC was determined as described above. Experiments were performed with two biological replicates.
Time-Killing Kinetics
The time-kill kinetics of E. faecium were determined as previously described.9 In brief, the E. faecium cells at the logarithmic growth phase in TSB medium were adjusted to an OD600 of 0.0005 (approximately 105 CFU/mL). Then, bacteria were challenged with jileicin at a final concentration of 2× MIC in culture tubes at 37 °C, and the culture without jileicin was used as a control. During the period of incubation, 100 μL aliquots were taken at intervals of 0, 1, 2, 4, 6, and 8 h for 10-fold serial dilutions, then 100 μL of the dilutions were plated on the TSB agar plates and incubated overnight at 37 °C. Colonies were counted and CFU/mL was calculated. Experiments were performed with two biological replicates.
Nucleic Acid and Protein Leakage Measurements
Nucleic acid and protein leakage were measured as detailed in Yang et al., with some modifications.17 The bacteria cells were treated as described in the time-killing kinetics assay method (jileicin at a final concentration of 1× MIC), and the culture without jileicin was used as a control. During the period of incubation, 500 μL of bacterial suspension was taken at intervals (0, 0.5, 1, 2, 4, and 8 h) and centrifuged at 8000g for 5 min. The supernatants were collected after filter sterilization with a 0.22 μm filter and measured for nucleic acid and protein at UV absorption wavelengths of 260 and 280 nm, respectively. The assay was performed in duplicate.
Cell Membrane Permeability Assay
The effect of jileicin on the membrane permeability of E. faecium was assessed as previously described with appropriate modifications.17 The bacteria cells were treated as described in the time-killing kinetics assay method (jileicin at a final concentration of 1× MIC). After treatment, the suspension was washed with PBS and divided into two equal portions. Portion 1 was treated with sodium dodecyl sulfate (SDS) solution at a final concentration of 0.001% (g/v, it was determined that SDS at the concentration of 0.001% did not impair the growth of bacteria), and portion 2 was treated with PBS. SDS acts as a permeabilizing probe that causes cell death when a sudden influx occurred. The OD600 value of cultures was measured at intervals of 0, 20, 40, and 60 min. The assay was performed in duplicate.
In addition, the effect of jileicin on the membrane permeability of E. faecium was also assessed by measuring lactate dehydrogenase (LDH) as previously described with appropriate modifications.18 The bacteria cells were treated as described in the time-killing kinetics assay method (jileicin at a final concentration of 1× MIC), and the culture without jileicin was used as a control. After treatment, 500 μL of bacterial suspension was taken at 0, 3, and 5 h, and the supernatants were collected after centrifuging at 8000g for 5 min and filter-sterilizing with a 0.22 μm filter. The LDH concentration of the supernatant was determined with an LDH Kit (Solarbio, Beijing, China) according to the manufacturer’s instructions. The assay was performed in duplicate.
Scanning Electron Microscopy
The effect of jileicin on the membrane integrity of E. faecium was visualized using scanning electron microscopy as previously described with appropriate modifications.19 Briefly, the E. faecium cells at the logarithmic growth phase in TSB medium were adjusted to approximately 108 CFU/mL. Then, the bacteria were challenged with jileicin at a final concentration of 1× MIC for 2 and 4 h at 37 °C, and the culture without jileicin was used as a control. The bacterial cells were collected after centrifuging at 8000g for 5 min and then fixed with 0.25% glutaraldehyde. Subsequently, the fixed cells were dried by the vacuum freeze-drying method after dehydrating through a grade series of ethanol (30, 50, 70, 90, and 100%). Finally, the samples were sputter-coated with gold and observed via a Hitachi S3400 scanning electron microscope (Hitachi, Tokyo, Japan) at 5 kV.
Safety Assays of Jileicin
The effect of jileicin on mammalian cells and red blood cells was used to evaluate the safety of jileicin as previously described with appropriate modifications.20,21
For mammalian cytotoxicity of jileicin, HepG2 cells, L929 cells, and Raw 264.7 cells (1 × 104/well) are seeded into a 96-well microtiter plate for 24 h at 37 °C with 5% CO2. The cells were then treated with different concentrations of jileicin for 48 h at 37 °C. After incubation, the CCK8 solution (Beyotime, Shanghai, China) was added to each well and incubated for 1 h at 37 °C. The OD490 value was measured using a microplate reader (BioTek), and cell viability was calculated according to the manufacturer’s instructions. The experiment was performed in triplicate.
For the hemolytic activity of jileicin, fresh mouse, chicken, and bovine red blood cells were washed with PBS until the upper phase was clear after centrifugation. The supernatant fraction was discarded and suspended at 2% (v/v) in PBS, and then added to the wells of a 96-well microtiter plate. Subsequently, jileicin of serial twofold dilution was added to the wells, resulting in a final concentration ranging from 0.03125 to 2.0 mg/mL, and the samples were incubated for 1 h at 37 °C. After incubation, 100 μL of supernatant was transferred to a 96-well plate after centrifuging at 1000g for 5 min. The 100% hemolysis was determined in 1.0% Triton X-100. The OD450 value was measured using a microplate reader (BioTek), and the percentage of hemolysis was calculated. The experiments were performed in triplicate.
Antimicrobial Application of Jileicin in Milk
The effect of the jileicin on the growth of E. faecium inoculated in milk was evaluated as previously described with appropriate modifications.22 In brief, the milk (pure milk, Yili, China) purchased from a local market was inoculated with E. faecium at the logarithmic growth phase at a concentration of 106 CFU/mL, and then jileicin was added to the milk, resulting in a final concentration 25, 50, and 100 mg/kg. All groups were incubated for 1 week at 4 °C, and during the period of incubation, 100 μL aliquots were taken every 24 h for 10-fold serial dilutions. The dilutions (100 μL) were plated on the TSB agar plates and incubated overnight at 37 °C. Colonies were counted, and CFU/mL was calculated. Experiments were performed with two biological replicates.
Statistical Analysis
GraphPad Prism 7.0 was used to analyze the data, which were expressed as mean ± standard error from two or three independent replicates. The significance of differences (P < 0.05) was calculated by one-way ANOVA or Student’s t-test.
Results
Purification and Identification of Jileicin
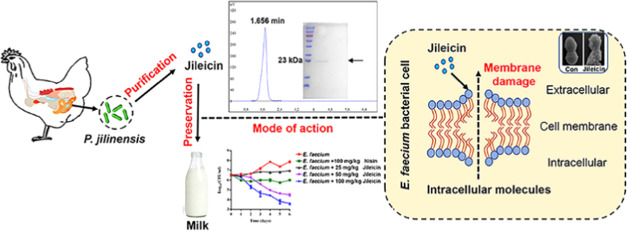
Obtaining an adequate antibacterial is crucial for the subsequent steps. Thus, partial fermentation parameters of P. jilinensis YPG26 are optimized, including growth medium, time, and with or without oxygen. The antibacterial activity of the cell-free supernatants against E. faecium was shown to reach its maximum when cultivating aerobically in TSB for 10 h at 37 °C with shaking at 200 rpm (Figure S1). Supernatants (5.0 L) as material for purification were collected after centrifuging at 10 000g for 10 min and filter-sterilizing with a 0.22 μm filter. The supernatants were initially purified by precipitation with ammonium sulfate at 70% saturations, followed by extensive dialysis (Figure S2A,B). The material was then fractionated using Sephadex G-75 gel filtration chromatography, and the antibacterial test revealed that peak 1 possessed antibacterial activity (Figure S2C). The absorption spectrum of peak 1 showed prominent absorption at 280 nm (Figure S2Dinset). The active samples were loaded onto the C18 column using reversed-phase HPLC at 280 nm, and peak 1 (Figure S2D), demonstrating antibacterial activity, was detected at a retention time of 1.656 min (Figure S2E, the purity >95%). The peak was collected, and molecular mass was determined by SDS-PAGE. The results revealed a single protein band with a molecular mass of approximately 23 kDa (Figure S2F). The target protein band was cut and identified by MALDI-TOF/TOF mass spectrometry.
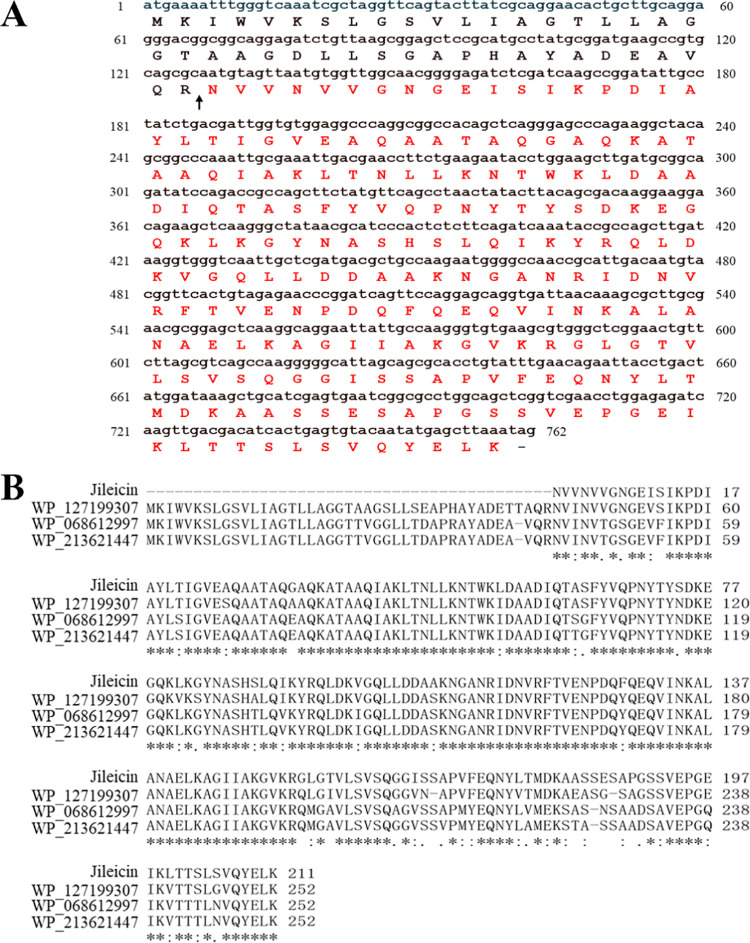
By mass spectrometry analysis, a total of 31 peptide fragments were identified (Table S1) and spliced into a protein that consisted of 211 amino acids. The comparison with the genome of P. jilinensis YPG26 (GenBank accession: CP084530) determined the deduced coding DNA sequences of the target protein (Figure 1A, locus = Chr1: 221935–222696). In addition, protein sequence alignment and analysis by the BLASTp algorithm showed 88.98% identity to the SIMPL domain-containing protein (GenBank accession no. WP_127199307) compared with the GenBank database (Figure 1B). The target protein showed an isoelectric point of 8.00 and a theoretical molecular mass of 22.561 kDa using the ProtParam tool (https://web.expasy.org/protparam/). The molecular mass of the target protein was almost consistent between the SDS-PAGE and MALDI-TOF/TOF. The antibacterial protein purified in this study was designated jileicin. It could tentatively be assigned to a bacteriocin-like inhibitory substance (BLIS). However, further studies are needed to verify whether it can be assigned to class III bacteriocin.
Figure 1.
Amino acid sequence analysis of jileicin. (A) Amino acid sequence of jileicin (marked by red text) spliced with peptide fragments from MALDI-TOF/TOF and deduced coding DNA sequences from genome of P. jilinensis YPG26 (GenBank accession no. CP084530), The black arrow indicates the possible cleavage site. (B) Spliced amino acid sequence of jileicin comparison with the SIMPL domain-containing protein (GenBank accession no. WP_127199307, WP_068612997, WP_213621447) from the GenBank database.
Antimicrobial Spectrum of Jileicin
The antimicrobial activity of jileicin was assessed against a range of pathogens. Jileicin showed higher antibacterial activity against Gram-positive bacteria than against Gram-negative bacteria. Among these, the best antibacterial activity was shown against E. faecium (vancomycin-resistance), and the lowest MIC value was 11.3 μM. For antibacterial activity against Gram-negative bacteria, the MIC value against Salmonella pullorum and Pseudomonas aeruginosa was 28.4 μM but showed no antibacterial activity against other Gram-negative bacteria tested at the concentration of 28.4 μM (Table 1).
Stability Assays of Jileicin
The effects of some factors of treatment on antibacterial activity were evaluated. Jileicin lost antibacterial activity in the presence of trypsin but had antibacterial activity in the presence of catalase and amylase (Figure S3A). The result revealed the proteinaceous nature of jileicin and excluded the effect of hydrogen peroxide. Different pH value treatment results showed that only pH 11 led to the activity loss, while there was antibacterial activity at pH 3–10 (Figure S3B). The antibacterial activity of jileicin gradually decreased with increasing temperature and lost antibacterial activity after incubation at 120 °C (Figure S3C). Moreover, the effect of ultraviolet rays, sodium chloride, metal ions, organic solvents, and detergents on the antibacterial activity of jileicin has also been tested, and the results showed that the antibacterial activity was not affected by any treatment, with the exception of N-butanol, which partly inhibited antibacterial activity (Figure S3D–H).
Resistance Development
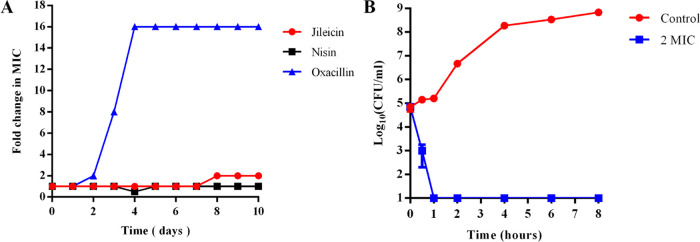
Resistant mutants in the serial passage of E. faecium were not generated in the presence of subinhibitory concentrations of jileicin and nisin over a period of 7 days. In contrast, E. faecium developed resistance to oxacillin rapidly only after 3 days of exposure (Figure 2A).
Figure 2.
Determination of resistance development and time-kill kinetics. (A) Resistance development during serial passaging in the presence of antimicrobials at the concentration of sub-MIC; (B) Time-kill kinetics of jileicin on E. faecium.
Time-Killing Kinetics
To reveal the concentration and time of the antibacterial activity of jileicin, the time-kill curve was measured. The results showed that the number of bacteria treated with jileicin at the concentrations of 2× MIC significantly decreased compared with the control group, and after 1 h of incubation, the number of bacteria decreased by 4.20 log10 units based on the colony counts. In addition, no bacteria were detected (detection limit: 1.0 log10 CFU/mL) during the 8 h period (Figure 2B). These results indicated that the jileicin could kill the bacteria over a short time period.
Cell Membrane Integrity Assay
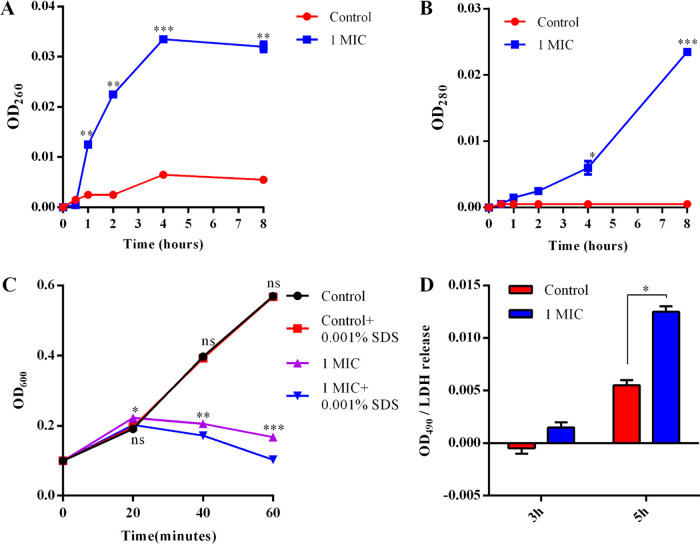
When the bacterial cell membrane integrity is disrupted, some intracellular molecules such as nucleic acid and protein will flow out. Thus, the influence of jileicin on bacterial cell membrane integrity can be examined by measuring intracellular nucleic acid and protein leakage. The leakage assay results showed that no nucleic acid or protein was detected at the initial treatment, but compared with the control group, the OD260 value increased significantly after treatment with jileicin at 1× MIC for 1 h (Figure 3A). Similarly, the OD280 value increased significantly after treatment with jileicin at 1× MIC for 4 h (Figure 3B). These results indicate that jileicin can effectively disrupt the cell membrane integrity, which results in the leakage of intracellular molecules.
Figure 3.
Effect of the jileicin on cell membrane integrity and permeability of E. faecium. (A) Intracellular nucleic acid leakage after treatment with 1× MIC jileicin; (B) intracellular protein leakage after treatment with 1× MIC jileicin; (C) membrane permeability of E. faecium cells exposed to 0.001% SDS after treatment with 1× MIC jileicin; and (D) LDH concentrations in the E. faecium culture supernatant after treatment with 1× MIC jileicin. The culture without jileicin was used as a control.
Cell Membrane Permeability Assay
The effect of jileicin on the membrane permeability was assessed by measuring the growth of E. faecium cells after jileicin treatment with supplemental 0.001% (g/v) SDS. The results showed that the control group grew normally in the absence or presence of SDS. However, the OD600 value decreased significantly after treatment with jileicin compared with the nontreatment group. And upon the addition of SDS, the OD600 value further decreased significantly compared with the control and jileicin treatment groups without SDS (Figure 3C). The result indicates that jileicin can cause an influx of SDS into the cell and accelerate cell death.
In addition, the influence of jileicin on bacterial cell membrane permeability was also determined by measuring secreted LDH levels in culture supernatants. The results showed that bacteria treated with jileicin for 5 h showed a significant increase in LDH release in the supernatant compared to untreated bacterial cells (Figure 3D). These results indicate that jileicin can improve cell membrane permeability.
Morphological Changes in E. faecium Cells after Treatment with Jileicin
The scanning electron micrograph was used to reveal morphological changes of E. faecium cells and further assess the effect of jileicin on the membrane integrity. The results showed that untreated E. faecium retained smooth and regular membrane morphology (Figure 4A), while E. faecium treated with jileicin at a concentration of 1× MIC showed membrane damage, distorting, breakage, and leakage of intracellular materials (Figure 4B,C). The results further demonstrated that the jileicin elicited bacterial death by disrupting the bacterial cell membrane.
Figure 4.
Effect of jileicin on cell morphology of E. faecium by SEM. (A) Control; (B) treated with 1× MIC jileicin for 2 h; and (C) treated with 1× MIC jileicin for 4 h.
Safety Assays of Jileicin
The cytotoxic effect of jileicin was determined against HepG2, L929, and RAW 264.7 cells. The results revealed no significant cytotoxicity to RAW 264.7 cells below a 1.0 mg/mL concentration of jileicin (Figure S4A). HepG2 cells treated with 1.0 mg/mL jileicin maintained a viability of 77.08%, while no significant cytotoxicity to HepG2 cells below 0.5 mg/mL was observed (Figure S4B). Similarly, L929 cells still showed a cell viability of more than 70% when the concentration of jileicin was up to 1.0 mg/mL (Figure S4C).
The hemolytic activity of jileicin was determined as the percentage of lysis of mouse, chicken, and bovine red blood cells. The result showed little hemolytic activity after exposure to jileicin at the concentrations of 0.03125–2.0 mg/mL compared with controls of PBS (0% hemolysis) and 1% Triton X-100 (v/v, 100% hemolysis), and at the highest concentrations of 2.0 mg/mL tested, the relative hemolysis for mouse and chicken red blood cells was less than 5% (Figure S4D–F).
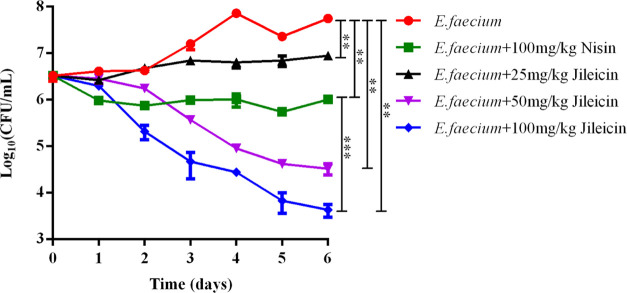
Antimicrobial Application of Jileicin in Milk
The effect of jileicin on the cell viability of E. faecium inoculated in milk was evaluated. The result showed that at 48 h treated with jileicin at the concentrations of 50 and 100 mg/kg (below the Chinese limit for milk of 500 mg/kg, GB2760-2014), a growth inhibition of E. faecium was observed compared with the control. And after 6 days treated with jileicin, they showed a decrease of E. faecium of 3.23 log10 in the 50 mg/kg jileicin treatment group and a decrease of 4.12 log10 in the 100 mg/kg jileicin treatment group compared with the control (Figure 5), which was much lower than the Chinese detection limit for milk of 2 × 106 CFU/g (mL) (GB19301-2010). Thus, it could be considered a successful result for jileicin in food application.
Figure 5.
Effect of jileicin on the cell viability of E. faecium inoculated in milk.
Discussion
The gut microbiota plays a crucial role in the host physiology, metabolism, immunity, digestion, and nutrition uptake. In addition, the gut microbiota is also an important source of antimicrobial compounds that could be developed to combat certain antibiotic-resistant bacterial infections.23 A rich and taxonomically diverse microbial community inhabiting the host intestinal tract has been reported to produce a variety of antimicrobial substances, such as gassericin A, reurerin, plantaricin, pediocin, amylopeptins, cytolysin, ruminococcin A and C, microcin, thuricin CD, and so on.24 In this study, we reported an antimicrobial protein designated jileicin from P. jilinensis YPG26, isolated from chicken intestine. The purified antimicrobial protein showed 88.98% identity to the SIMPL domain-containing protein (GenBank accession no. WP_127199307). However, there is no report on the antibacterial activity of SIMPL domain-containing protein. We further determined the proteinaceous nature of the jileicin by trypsin treatment, primary structural characterization, and stability analysis. Thus, it is tentatively attributed to a bacteriocin-like inhibitory substance (BLIS). As to whether it can be assigned to class III bacteriocin, further studies are still needed.25 As a bacteriocin-like inhibitory substance from Paenibacillus ehimensis NPUST1, peocin is assigned to class III bacteriocin.26
The antibacterial spectrum results showed that jileicin had inhibitory activity against both Gram-negative and Gram-positive bacteria but showed better antibacterial activity against Gram-positive bacteria, especially against the genus Enterococcus. Bacteriocins, or BLIS, show a narrow antibacterial activity spectrum generally and have weak activity against Gram-negative bacteria due to the protection of an inner and outer membrane.23 Antimicrobial agents usually fail preclinical studies because of their low stability.27 Stability assays of jileicin treated with enzymes, pH, temperature, ultraviolet rays, sodium chloride, metal ions, organic solvents, and detergents showed trypsin, pH 11, and 120 °C treatment abolished completely the antimicrobial activity of jileicin, while the antibacterial activity was little affected by other treatments. A vast majority of class III bacteriocins or BLIS are heat-labile, such as enterolysin A and helveticin J, which are both inactivated after treatment at 100 °C temperature,28,29 whereas peocin is heat-stable and its the antimicrobial activity does not decrease even at temperatures ranging from 100 to 121 °C.26,30 The thermal stability of jileicin is between that of enterolysin A, helveticin J, and peocin. These evaluations suggest a decent stability of jileicin.
In contrast to antibiotics, ribosomally synthesized antimicrobial peptides rarely develop resistance because of the unique mechanism of action.31 Similarly, resistant mutants in the serial passage of E. faecium were also not generated in the presence of subinhibitory concentrations of jileicin over a period of 7 days.
The jileicin at the concentrations of 2× MIC could kill the bacteria over a short time period by time-killing kinetics analysis, and scanning electron microscopy observation showed that jileicin could induce membrane damage, distorting, breakage, and leakage of intracellular materials. Moreover, the increased intracellular nucleic acid and protein leakage also further indicates disruption of cell membrane integrity, and the alteration of cell membrane permeability has been demonstrated again by the influx of SDS into the cell to accelerate cell death and LDH release in the supernatant. In short, jileicin exerts antibacterial activity by causing membrane damage. Different bacteriocins have different modes of action. Class I and class II bacteriocins carry out antibacterial activity through causing permeabilization and forming pores, while most class III bacteriocins kill cells by hydrolysis of peptidoglycan (cell-wall degradation) because of their function directly on the cell wall.6 Furthermore, some bacteriocins can also target bacteria by interfering with DNA, RNA, and protein metabolism.6 Therefore, to completely determine the mode of action of jileicin, more extensive studies should be performed in the future.
An antimicrobial drug candidate needs to generally show low or no toxicity toward mammalian cells and no hemolysis to avoid the limitation of its clinical applications.32 Our results revealed that jileicin at concentrations below 0.5 mg/mL exhibited no significant cytotoxicity to HepG2, L929, and RAW 264.7 cells and showed very low levels of hemolytic activity, which indicates that a certain dose of jileicin was safe for animal application.
Apart from its use in antagonizing clinical pathogens, bacteriocins, or BLIS, have been widely used in food preservation, particularly in dairy products and meat products.6 The numbers of Enterococci may reach 108 CFU/mL in dairy products, and one of the most common bacteria in raw milk, such as E. faecalis, E. faecium, E. durans, E. hirae, and so on, have been isolated from the raw milk of cows, goats, and sheep.33 These enterococci harbor a few genes of virulence and antibiotic resistance. In addition, they can also obtain and disseminate virulence and antibiotic virulence genes in the environment and in their hosts.34 And E. faecium (vancomycin-resistant) has been listed as a bacterium for which new antibiotics are urgently needed by the World Health Organization. Our results showed that 50 mg/kg jileicin reduced E. faecium (vancomycin-resistant) by 3.23 log10 in milk products after 6 days of incubation, and a decrease of 4.12 log10 was achieved with 100 mg/kg jileicin treatment, which showed promising potential to be used in food preservation.
In summary, we purified and identified a bacteriocin-like inhibitory substance, jileicin, obtained from P. jilinensis YPG26 using a series of purification methods. The jileicin showed a relatively broad-spectrum antibacterial ability, especially against enterococci. Additionally, the jileicin had good stability after different treatments, no detectable resistance, no significant cytotoxicity, and very low levels of hemolytic activity. The mode of action of jileicin against E. faecium demonstrated that jileicin could destroy cell membrane integrity, increase cell membrane permeability, and eventually lead to cell death. Furthermore, jileicin controlled the growth of E. faecium in milk and thus possessed desirable advantages for future application in dairy products. In conclusion, these results indicate that jileicin is a promising and attractive candidate for application in the food, pharmaceutical, and biomedical industries.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [nos. 32070119, 31972682, and 31872457].
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.2c01458.
Optimization of partial fermentation conditions (Figure S1); purification of jileicin (Figure S2); stability assays of jileicin (Figure S3); safety assays of jileicin (Figure S4); and peptide fragments of jileicin identified by MALDI-TOF/TOF (Table S1) (PDF)
Author Contributions
† K.M. and W.C. contributed equally to this work. Y.-J.Y., K.M., and W.C. designed experiments. K.M., S.-Q.Y., Z.-Z.L., X.-Q.L., J.-B.Z., Y.G., T.W., and J.-G.Z. performed the experiments. K.M., Y.-J.Y., and W.C. analyzed data, K.M. wrote the manuscript, and Y.-J.Y. revised the manuscript. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Sender R.; Fuchs S.; Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez E.; Mayer M. J.; Cotter P. D.; Narbad A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2019, 10, 1–21. 10.1080/19490976.2018.1455790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiumento S.; R C.; Kieffer-Jaquinod S.; et al. Ruminococcin C, a promising antibiotic produced by a human gut symbiont. Sci. Adv. 2019, 5, eaaw9969 10.1126/sciadv.aaw9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. G.; Becattini S.; Moody T. U.; Shliaha P. V.; Littmann E. R.; Seok R.; Gjonbalaj M.; Eaton V.; Fontana E.; Amoretti L.; Wright R.; Caballero S.; Wang Z. X.; Jung H. J.; Morjaria S. M.; Leiner I. M.; Qin W.; Ramos R.; Cross J. R.; Narushima S.; Honda K.; Peled J. U.; Hendrickson R. C.; Taur Y.; van den Brink M. R. M.; Pamer E. G. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nature 2019, 572, 665–669. 10.1038/s41586-019-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipperer A.; Konnerth M. C.; Laux C.; Berscheid A.; Janek D.; Weidenmaier C.; Burian M.; Schilling N. A.; Slavetinsky C.; Marschal M.; Willmann M.; Kalbacher H.; Schittek B.; Brotz-Oesterhelt H.; Grond S.; Peschel A.; Krismer B. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]
- Negash A. W.; Tsehai B. A. Current Applications of Bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891 10.1155/2020/4374891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abriouel H.; Franz C. M.; Ben Omar N.; Galvez A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. 10.1111/j.1574-6976.2010.00244.x. [DOI] [PubMed] [Google Scholar]
- Hu M.; Zhao H.; Zhang C.; Yu J.; Lu Z. Purification and characterization of plantaricin 163, a novel bacteriocin produced by Lactobacillus plantarum 163 isolated from traditional Chinese fermented vegetables. J. Agric. Food Chem. 2013, 61, 11676–11682. 10.1021/jf403370y. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Chen J.; Liu Y.; Xu Q.; Inam M.; He C.; Jiang X.; Jia Y.; Ma H.; Kong L. Discovery of a novel antibacterial protein CB6-C to target methicillin-resistant Staphylococcus aureus. Microb. Cell Fact. 2022, 21, 4 10.1186/s12934-021-01726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X.; Du R.; Wang Y.; Han Y.; Zhou Z. Purification, characterization and mode of action of enterocin, a novel bacteriocin produced by Enterococcus faecium TJUQ1. Int. J. Biol. Macromol. 2020, 144, 151–159. 10.1016/j.ijbiomac.2019.12.090. [DOI] [PubMed] [Google Scholar]
- Karczewski J.; Krasucki S. P.; Asare-Okai P. N.; Diehl C.; Friedman A.; Brown C. M.; Maezato Y.; Streatfield S. J. Isolation, Characterization and Structure Elucidation of a Novel Lantibiotic From Paenibacillus sp. Front. Microbiol. 2020, 11, 598789 10.3389/fmicb.2020.598789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S.; Han J.; Bie X.; Lu Z.; Zhang C.; Lv F. Purification and Characterization of Plantaricin JLA-9: A Novel Bacteriocin against Bacillus spp. Produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a Traditional Chinese Fermented Cabbage. J. Agric. Food Chem. 2016, 64, 2754–2764. 10.1021/acs.jafc.5b05717. [DOI] [PubMed] [Google Scholar]
- Okella H.; Ikiriza H.; Ochwo S.; Ajayi C. O.; Ndekezi C.; Nkamwesiga J.; Kaggwa B.; Aber J.; Mtewa A. G.; Koffi T. K.; Odongo S.; Vertommen D.; Kato C. D.; Ogwang P. E. Identification of Antimicrobial Peptides Isolated From the Skin Mucus of African Catfish, Clarias gariepinus (Burchell, 1822). Front. Microbiol. 2021, 12, 794631 10.3389/fmicb.2021.794631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarski Y.; Deseva I.; Mihaylova D.; Stoyanova M.; Krastev L.; Nikolova R.; Yanakieva V.; Ivanov I. Isolation, Characterization and Amino Acid Composition of a Bacteriocin Produced by Bacillus methylotrophicus Strain BM47. Food Technol. Biotechnol. 2018, 56, 546–552. 10.17113/ftb.56.04.18.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhi Sudha S.; Aranganathan V. Experimental elucidation of an antimycobacterial bacteriocin produced by ethnomedicinal plant-derived Bacillus subtilis (MK733983). Arch. Microbiol. 2021, 203, 1995–2006. 10.1007/s00203-020-02173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Yang Y.; Yang H.; Bu Y.; Yi H.; Zhang L.; Han X.; Ai L. Purification and Partial Characterization of Bacteriocin Lac-B23, a Novel Bacteriocin Production by Lactobacillus plantarum J23, Isolated From Chinese Traditional Fermented Milk. Front. Microbiol. 2018, 9, 2165 10.3389/fmicb.2018.02165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. K.; Yusoff K.; Ajat M.; Wee C. Y.; Yap P. S.; Lim S. H.; Lai K. S. Combinatorial Antimicrobial Efficacy and Mechanism of Linalool Against Clinically Relevant Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 635016 10.3389/fmicb.2021.635016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamida R. S.; Ali M. A.; Goda D. A.; Al-Zaban M. I. Lethal Mechanisms of Nostoc-Synthesized Silver Nanoparticles Against Different Pathogenic Bacteria. Int. J. Nanomed. 2020, 15, 10499–10517. 10.2147/IJN.S289243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.-h.; Zhou T.-t.; Wei C.-h.; Lan W.-q.; Zhao Y.; Pan Y.-j.; Wu V. C. H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. 10.1016/j.foodcont.2018.07.012. [DOI] [Google Scholar]
- Ling L. L.; Schneider T.; Peoples A. J.; Spoering A. L.; Engels I.; Conlon B. P.; Mueller A.; Schaberle T. F.; Hughes D. E.; Epstein S.; Jones M.; Lazarides L.; Steadman V. A.; Cohen D. R.; Felix C. R.; Fetterman K. A.; Millett W. P.; Nitti A. G.; Zullo A. M.; Chen C.; Lewis K. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y. Z.; Wu G.; Yang L. Y.; Yang X. J.; Zhang Y. M.; Lin L. B.; Deng X. Y.; Zhang Q. L. Antibacterial effect of bacteriocin XJS01 and its application as antibiofilm agents to treat multidrug-resistant Staphylococcus aureus infection. Int. J. Biol. Macromol. 2022, 196, 13–22. 10.1016/j.ijbiomac.2021.11.136. [DOI] [PubMed] [Google Scholar]
- Rahmeh R.; Akbar A.; Alonaizi T.; Kishk M.; Shajan A.; Akbar B. Characterization and application of antimicrobials produced by Enterococcus faecium S6 isolated from raw camel milk. J. Dairy Sci. 2020, 103, 11106–11115. 10.3168/jds.2020-18871. [DOI] [PubMed] [Google Scholar]
- Drider D. Gut Microbiota is an Important Source of Bacteriocins and Their In Situ Expression Can Be Explored for Treatment of Bacterial Infections. Probiotics Antimicrob. Proteins 2021, 13, 1759–1765. 10.1007/s12602-021-09843-y. [DOI] [PubMed] [Google Scholar]
- Mousa W. K.; Athar B.; Merwin N. J.; Magarvey N. A. Antibiotics and specialized metabolites from the human microbiota. Nat. Prod. Rep. 2017, 34, 1302–1331. 10.1039/C7NP00021A. [DOI] [PubMed] [Google Scholar]
- Rani A.; Saini K. C.; Bast F.; Varjani S.; Mehariya S.; Bhatia S. K.; Sharma N.; Funk C. A Review on Microbial Products and Their Perspective Application as Antimicrobial Agents. Biomolecules 2021, 11, 1860 10.3390/biom11121860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C. C.; Murni L.; Han T. W.; Arfiati D.; Shih H. T.; Hu S. Y. Molecular Characterization and Heterologous Production of the Bacteriocin Peocin, a DNA Starvation/Stationary Phase Protection Protein, from Paenibacillus ehimensis NPUST1. Molecules 2019, 24, 2516 10.3390/molecules24132516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila J.; Moreno-Morales J.; Balleste-Delpierre C. Current landscape in the discovery of novel antibacterial agents. Clin. Microbiol. Infect. 2020, 26, 596–603. 10.1016/j.cmi.2019.09.015. [DOI] [PubMed] [Google Scholar]
- Nilsen T.; Nes I. F.; Holo H. Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl. Environ. Microbiol. 2003, 69, 2975–2984. 10.1128/AEM.69.5.2975-2984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger M. C.; K T. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J. Bacteriol. 1986, 167, 439–446. 10.1128/jb.167.2.439-446.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. W.; Liu C. H.; Hu S. Y. Dietary administration of probiotic Paenibacillus ehimensis NPUST1 with bacteriocin-like activity improves growth performance and immunity against Aeromonas hydrophila and Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 84, 695–703. 10.1016/j.fsi.2018.10.059. [DOI] [PubMed] [Google Scholar]
- Huang F.; Teng K.; Liu Y.; Cao Y.; Wang T.; Ma C.; Zhang J.; Zhong J. Bacteriocins: Potential for Human Health. Oxid. Med. Cell. Longevity 2021, 2021, 5518825 10.1155/2021/5518825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco I.; Molchanova N.; Holmedal E.; Jenssen H.; Hummel B. D.; Watts J. L.; Hakansson J.; Hansen P. R.; Svenson J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206 10.1038/s41598-020-69995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapkevicius M. d. L. E.; Sgardioli B.; Camara S. P. A.; Poeta P.; Malcata F. X. Current Trends of Enterococci in Dairy Products: A Comprehensive Review of Their Multiple Roles. Foods 2021, 10, 821 10.3390/foods10040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore E.; Van Tyne D.; Gilmore M. S. Pathogenicity of Enterococci. Microbiol. Spectrum 2019, 7, 189–211. 10.1128/microbiolspec.GPP3-0053-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.