Abstract
Procyanidins, as a kind of dietary flavonoid, have excellent pharmacological properties, such as antioxidant, antibacterial, anti-inflammatory and anti-tumor properties, and so they can be used to treat various diseases, including Alzheimer’s disease, diabetes, rheumatoid arthritis, tumors, and obesity. Given the low bioavailability of procyanidins, great efforts have been made in drug delivery systems to address their limited use. Nowadays, the heavy burden of oral diseases such as dental caries, periodontitis, endodontic infections, etc., and their consequences on the patients’ quality of life indicate a strong need for developing effective therapies. Recent years, plenty of efforts are being made to develop more effective treatments. Therefore, this review summarized the latest researches on versatile effects and enhanced bioavailability of procyanidins resulting from innovative drug delivery systems, particularly focused on its potential against oral diseases.
Keywords: procyanidins, oral disease, bioavailability, drug delivery, antioxidation
1. Introduction
Procyanidins (PCs), also called proanthocyanidins and condensed tannins, are widely found in flowers, nuts, fruits, bark, and seeds of various plants [1,2]. PCs with a degree of polymerization of 2–4 or more are called oligomeric procyanidins (OPCs) and polymeric procyanidins (PPCs), respectively [3]. In most cases, the flavane-3-alcohol unit is a substituted derivative of catechin (C), epicatechin (EC), or its C4–C8 or C6 bond (type B) [4]. Recently, it has been showed PCs has predominant pharmaceutical values due to its antioxidant [5], antibacterial [6], anti-inflammatory [7], antineoplastic [8], anti-allergic [9], lipid-lowering, and anti-obesity properties [10]. As a result of these properties, they have been widely recognized and applied in the healthcare industry [11].
Oral health problems, particularly periodontal disease, dental caries, and endodontic root canal infections, are among the most damaging processes in the mouth and a costly burden on the global public [12].According to a review released by the World Health Organization (WHO), oral diseases remain a global problem despite significant progress in developing oral health in some countries [13]. The conventional oral diseases including periodontitis and dental caries are regarded as the infectious diseases, since they are initiated by plaque biofilm formation [14]. In addition, periodontitis leads to alveolar bone destruction and subsequent tooth loss, and develops due to pro-inflammatory cytokine production induced by periodontopathic bacteria [15]. Besides, recurrent aphthous ulcer is characterized by prolonged lesion with severe pain, which may induce from an overload of reactive oxygen species (ROS), occurring in up to 20% of cases [16]. Oral cancers, especially oral squamous cell carcinoma (OSCC) are the main cause of oral disease death, which are partly due to an imbalance between cell growth capacity and elimination mechanisms [17]. Thus, it is necessary to pay great attention to the treatment of oral diseases to maintain oral health and improve the quality of life.
Previous studies have shown that PCs possessed capability to reduce the excessive expression of inflammatory cytokines and inhibit production of inflammation-producing enzymes [18]. In addition, PCs can cause the disruption of bacterial membrane and lead to the leakage of intracellular substances, exerting an antibacterial activity [19]. In general, PCs exert anti-inflammatory effects by regulating nuclear factor erythroid 2-related factor 2 (Nrf2), the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, as well as inhibiting ROS production and decreasing mitochondrial membrane potential [20,21,22,23,24,25,26,27,28]. Besides, the antineoplastic effects of PCs are achieved through regulating autophagy, apoptosis, epithelial-to-mesenchymal transition (EMT) transformation, drug resistance, tumor stem cell renewal and proliferation of tumor cells [29,30,31,32,33,34,35].Moreover, PCs also have excellent pharmacological effects in the therapy of Alzheimer’s disease [36], diabetes [37], rheumatoid arthritis and obesity [38]. Thus, PCs are considered as a kind of promising candidates to use as potent pharmaceutical for disease prevention and therapeutics.
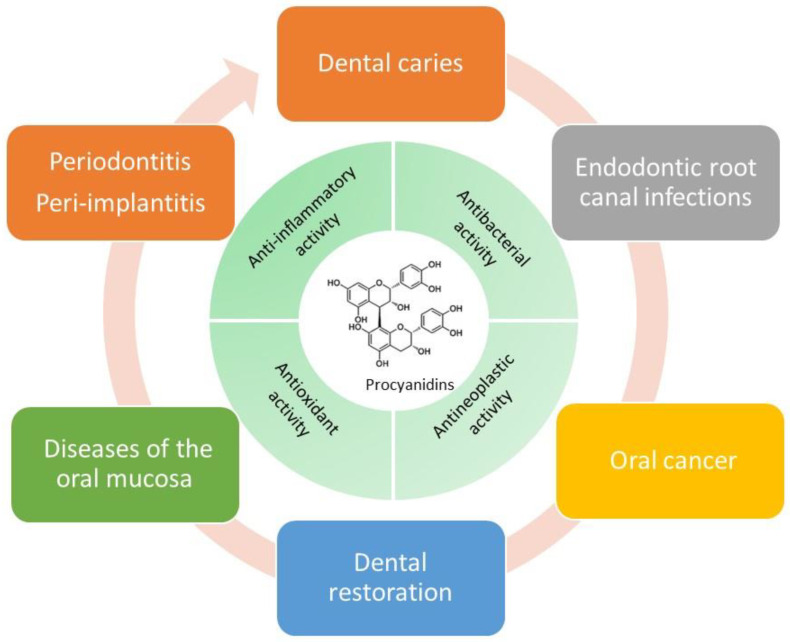
Nevertheless, with respective clinical application, the proven benefits of PCs have some stability and bioavailability issues. Therefore, extensive efforts have been made to increase the bioavailability of PCs by encapsulating it in drug delivery systems including poly(lactic-co-glycolic acid) (PLGA) and poly(D,L-lactic acid) (PLA) nanoparticles [39,40], polysaccharide-based nanoparticles [41,42,43,44,45], protein-based nanoparticles [46,47], modified hydroxyapatite inorganic nanoparticles [48], metal nanoparticles [49,50,51,52,53], ultradeformable liposomes (PUDL) and solid lipid nanoparticles (SLN) [54,55]. Significant advances in bioavailability-related fields guarantee the enhanced pharmaceutical efficacy and further clinical value of PCs as an emerging versatile drug. As shown in Figure 1, The present article aims to summarize the current state of PCs application in oral diseases treatment.
Figure 1.
Structure, properties and therapeutic applications on oral diseases of procyanidins. Notes: Procyanidins, as an emerging pharmaceutical with multifunction, have versatile properties such as antioxidant activity, antibacterial activity, anti-inflammatory activity and antineoplastic activity. It has the capacity to therapy diverse oral diseases including oral cancer, periodontitis, dental caries, diseases of the oral mucosa, endodontic root canal; peri-implantitis and dental restoration.
2. Pharmacological Mechanism of Procyanidins
2.1. Antioxidant Activity
Oxidative stress is defined as the imbalance between the production and elimination of ROS, and oxidative stress caused by excess ROS can cause oxidative damage to cells [56]. Former Studies have demonstrated that PCs can exert antioxidant effects by inhibiting enzyme activity, eliminating free radicals, and resisting oxidative stress [57,58,59].
Studies have shown that PCs have a good scavenging effect on ROS by scavenging free radicals such as O2− and NO− [52]. In addition to serve as a prominent antioxidant agent for radical scavenging, Jimenez-aspee et al. demonstrated that PCs in the pulp of Cortex pedunculata can inhibit the activities of lipoxygenase and enzymes involved in the enzyme peroxidation [57]. Moreover, Bak et al. suggest that PCs from wild grape seed effectively inhibit the production of oxidative mediators, which achieved by preventing the activation of NF-κB and p38 pathways [58].
2.2. Antibacterial Effect
Infectious diseases caused by bacteria constitute the main cause of morbidity and mortality throughout the world and mainly in developing countries [60]. So far, the mechanisms of PCs antibacterial activity mainly include: 1. Inhibition of bacterial adhesion and biofilm formation, 2. Destroy the integrity of cell membrane/wall, 3. Inhibit extracellular microbial enzymes and deprive microbial substrates needed for growth [61].
Philips et al. noted that the components of cranberry, particularly PCs, can interfere with the bacterial adhesion stage, reducing biofilm formation and/or reducing inflammation to fight pathogens [62]. Several clinical trials have shown that cranberry procyanidins have anti-adhesion properties and are important in preventing recurrent urinary tract infections [63]. Kim et al. demonstrated that PCs inhibit bacterial adhesion by restraining Streptococcus mutans derived glucose transferase (GTF) and extracellular polysaccharides (EPS) without killing the organism [64]. Lacombe et al. believed that PCs play an antibacterial role probably since they have metal ion chelation effect similar to EDTA, which can bind Ca2+ and Mg2+ on the membrane to destabilize cell membrane, release lipopolysaccharide (LPS), and increase cell membrane permeability [65]. Tamura et al. found that PCs trimers have antibacterial effects on foodborne bacteria, especially Bacillus cereus (B. cereus), the mechanism is that PCs trimer present in the peanut skin disrupts cell wall integrity of B. cereus [66]. Notably, PCs in cranberries have been shown to inhibit the activity of microbial F-ATPase, thus making bacterial survival extremely difficult [67,68].
2.3. Anti-Inflammatory Activity
Inflammation is the host’s defensive response to tissue damage or infection caused by a variety of stimulus, such as chemicals, physical trauma, and infectious agents [69].
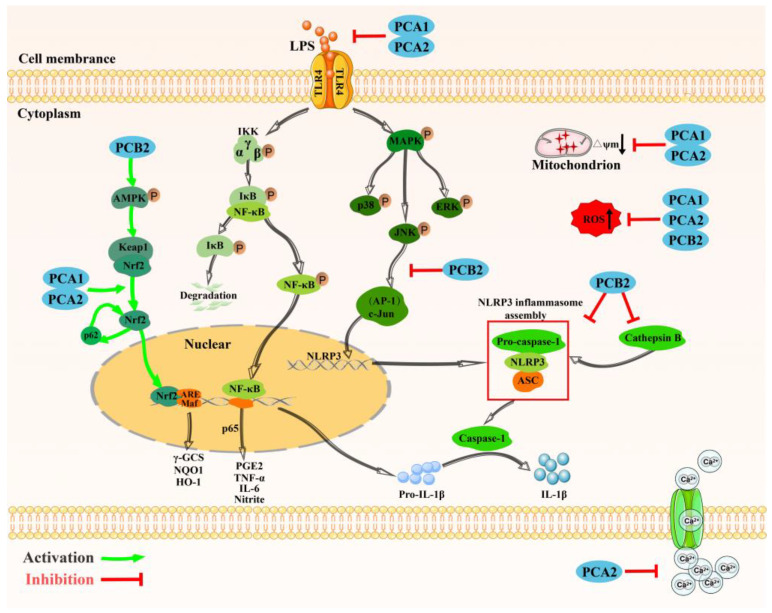
PCs exert anti-inflammatory activity by regulating MAPK, Nrf2, NF-κB signaling pathway, mitochondrial membrane potential and calcium channel. Nrf2 is an important transcription factor that benefits to cell survival during oxidative stress [20,21]. Ubiquitin binding protein p62 is important for selective autophagy and antioxidant responses [22]. It has recently been found that p62 protects cells from oxidative stress by activating Nrf2 [23]. Lu et al. demonstrated that procyanidins B2 (PCB2) enhances the system of antioxidant via adenosine monophosphate-activated protein kinase (AMPK)/Nrf2/p62 signaling axis, specifically speaking, PCB2 treatment increase p-AMPK levels, along with the letilation of Nrf2 and gather Nrf2 to nucleus, which can promote the expression of NAD(P)H quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), and γ-glutamylcysteine synthetase (γ-GCS). Besides, Nrf2 activate by PCB2 up-regulated the expression p62, and p62 stimulate the activation of Nrf2 in turn, which exert a positive loop between Nrf2 and p62 [70]. NF-κB transcription factor plays a key role in the regulation of inflammatory responses [71]. Procyanidins A2 (PCA2) inhibits the release of Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), prostaglandin E2 (PGE2), and nitric oxide (NO), through the IκB/NF-κB P65 pathway, thus exerting anti-inflammatory effect [10].
One study demonstrated that procyanidins A1 (PCA1) inhibit LPS-induced oxidative stress through IκB/NF-κB p65 signaling. Meanwhile, PCA1 inhibits the production of intracellular ROS in vitro and reduces the depletion of mitochondrial membrane potential [27]. NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome is a cytoplasmic protein complex, and mediates inflammatory respond by depending on the release of Cathepsin B [28,72]. Procyanidin B2 (PCB2) inhibits monosodiumurate (MSU)-induced inflammatory response in gout by inhibiting the NLRP3 inflammasome pathway, thereby reducing Interleukin-1β (Il-1β) and cathepsin B release [72].
MAPKs family members mainly include extracellular signal-regulated kinase (ERK), stress-activated protein kinase (JNK) and P38 MAPK. PCB2 inhibit LPS activated activator protein-1 (AP-1)/c-Jun pathway increasing the gene expression of NLRP3, and suppress subsequent caspase-1 activation and IL-1β secretion in endothelial cells. Besides, PCB2 also lower LPS induced the production of ROS in endothelial cells [18]. All pathways mentioned in this section are concluded in detail in Figure 2.
Figure 2.
The anti-inflammation signal pathways of procyanidins. Notes: (1). Procyanidins activated the AMPK/Nrf2 pathway; targeted its downstream gene contributing to the increased level of antioxidant genes NQO1, HO-1 and γ-GCS. (2). Procyanidins inhibits the release of inflammatory factors IL-6, TNF-α, PGE2, NO through the IκB/NF-κB p65 pathway (Note: PCA1 show no effect of PEG2). (3). Procyanidins inhibit LPS activated AP-1/c-Jun pathway decreasing the gene expression of NLRP3, and suppress subsequent caspase-1 activation and the release of IL-1β. (4). Besides, Procyanidins can also lower the production of ROS, reverse decreased mitochondrial membrane potential, inhibit Ca2+ exclusion. Abbreviation: PCA1, procyanidins A1; PCA2, procyanidins A2; PCB2, procyanidins B2; AMPK, adenosine monophosphate-activated protein kinase; Nrf2, nuclear factor erythroid 2-related factor 2; NQO1, NAD(P)H quinone oxidoreductase 1; HO-1, heme oxygenase-1; γ-GCS, γ-glutamylcysteine synthetase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; IKK, inhibitor of kappaB kinase; IκB, nuclear factor kappa-B; NF-κB, nuclear factor kappa-B; PGE2, prostaglandin E2; TNF-α, tumor necrosis factor-α; IL-6, Interleukin-6; JNK, c-Jun N-terminal kinases; ERK, extracellular signal-regulated kinase; NLRP3, NOD-like receptor family pyrin domain containing 3; IL-1β, Interleukin-1β; ROS, reactive oxygen species.
2.4. Antineoplastic Activity
According to 2020 estimates from WHO, cancer is the leading cause of death in countries around the world, which is characterized by abnormal and uncontrolled cell growth [73,74]. Tumor growth underlies all aspects of cancer development. Therefore, inhibition of tumor cell proliferation is considered as a promising cancer treatment strategy [75]. PCs have been found to have significant antitumor effects in breast cancer, lung cancer, hemangioma, colon cancer and other cancers [41,76,77,78,79,80].
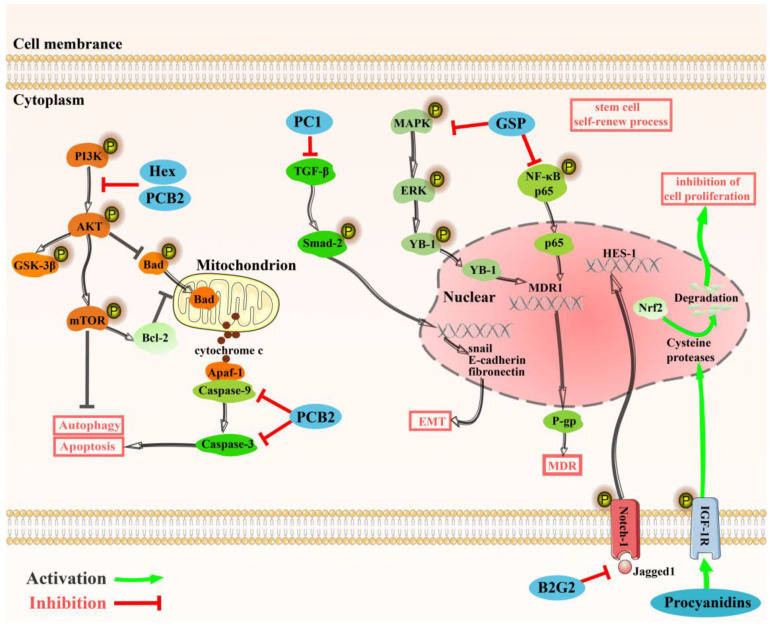
There is much evidences suggest that the activation of Nrf2 would lead to cancer cell proliferation and chemotherapy resistance and is related with poor prognosis of patients [29]. Studies have shown that PCs treatment can rapidly accelerate the degradation of activated Nrf2 expression by activating cysteine proteases in nucleus through phosphorylated insulin-like growth factor 1 receptor (IGF-1R), which can inhibit the proliferation of A549 cells [30].
Cancer stem or initiating cells (CSCs) is a rare population of undifferentiated cells, plenty of evidence shows that CSCs is closely associated with drug resistance, carcinoma recurrence and tumor metastasis [31]. A study found that both procyanidin B2 3,3″-di-O-gallate (B2G2) and grape seed procyanidins (GSP) can inhibit the self-renewal of CSCs via inhibiting Notch1 pathway activated by Jagged1 (Notch1 ligand) and restraining the transcription of Notch1 regulatory target gene HES-1, which contributes to the control of proliferating prostate cancer cells [32].
Multidrug resistance (MDR) is one of the dominant reasons of chemotherapy failure in cancer patients, and the expression of p-glycoprotein (P-gp) has close relation with MDR, which is responsible for pumping therapeutic drugs out of cancer cells [33]. Zhao et al. found GSP can significantly decrease the expression of P-gp via inhibiting the nuclear translocation of Y-box binding protein 1 (YB-1) from cytoplasm through MAPK/ERK pathway by reducing the phosphorylation of ERK1/2. Besides, GSP can also block the generation of P-gp by down-regulating NF-κB/p65 activity, eventually leading to reversal MDR. However, authors mentioned that the new mechanism is limited to human ovarian cancer cells of A2780/T and A2780 in vitro. Therefore, it is worth further exploring if GSP can reverse MDR in other cancer cells [34].
Epithelial-to-mesenchymal transition (EMT) is a process of cancer cell in which tumor cells lose their epithelial properties and turn into spindle morphology. And EMT is strongly connected with tumor metastasis. Procyanidin C1 (PC1) from the Cinnamomi Cortex extract can inhibit the process of EMT through restraining TGF-β induced phosphorylation of Smad-2, and further down-regulated the snail, E-cadherin and fibronectin in A549 cells [35].
In addition, the hexamer (Hex) which is one of trimer procyanidins, induced apoptotic cell death through the mitochondrial pathway and is involved in autophagy by upregulating genes in colorectal cancer cells (Caco-2 cells). Mechanistically, Hex inhibited both PI3K/Akt/GSK-3β and PI3K/Akt/Bad signaling pathway, increasing the translocation of Bad to the mitochondria and cytochrome c to cytoplasm, finally induced mitochondrial apoptosis pathway of cancer cell. Moreover, Hex also blocked the Caco-2 cell cycle at G2/M phase [8]. In gastric cancer cells (BGC-823 or SGC-7901 cells), PCB2 exerted the effects of anti-proliferation, more specifically, it stimulated apoptosis by enhancing the activities of executor caspase-3 and initiator caspase-9 and induced autophagy by increasing the two believable autophagic marker of Beclin1 and Atg5. More interestingly, PCB2 might boost both apoptosis and autophagy through Akt/mTOR pathway [81]. All pathways mentioned in this section are concluded in detail in Figure 3.
Figure 3.
The antineoplastic mechanism of procyanidins. Notes: (1). Procyanidins can inhibit cell proliferation by phosphorylated IGF-1R, which can activate cysteine proteases and accelerate the degradation of activated Nrf2. (2). B2G2 can inhibit stem cell self-renew process through inhibiting the activitation of Notch1 pathway. (3). GSP can reverse MDR by suppressing both MAPK/ERK/YB-1 and NF-κB p65 pathway. (4). PC1 inhibits EMT by inhibiting TGF-β-induced phosphorylation of Smad-2, and further down-regulates Snail, E-cadherin and fibronectin. (5). HEX can inhibit PI3K/Akt/GSK-3β and PI3K/Akt/Bad signaling pathways, and induce the mitochondrial apoptosis pathway of cancer cells. In addition, PCB2 also plays an anti-proliferative role and stimulates apoptosis, induces autophagy of cancer cells through the Akt/mTOR pathway. Abbreviation: IGF-1R, insulin-like growth factor 1 receptor; B2G2, B2 3;3″-di-O-gallate; MDR, multidrug resistance; GSP, grape seed procyanidins; P-gp, p-glycoprotein; YB-1, Y-box binding protein 1; EMT, Epithelial-to-mesenchymal transition; PC1, procyanidin C1; Hex, hexamer; Bcl-2, B-cell lymphoma-2.
3. Delivery Systems of Procyanidins
However, there are some stability and bioavailability issues with the proven benefits of PCs. For example, OPC is unstable under environmental conditions, while PPC has the disadvantage of low water solubility. In order to improve the stability, bioavailability and reduce adverse effects of PPC and OPC, a variety of biodegradable encapsulation materials were used to develop a sustained-release delivery system for PPC and OPC. These drug delivery systems which were described in Table 1 make quercetin easy to be absorbed and prolong drug duration.
Table 1.
The physico-chemical characters of different PCs delivery vehicles.
| Delivery System | Chemicals/Polymer Used | Preparation Methods | Size Range (nm) | Evaluations on the Encapsulated PCs | Effect | References |
|---|---|---|---|---|---|---|
| PLA nanoparticles | PLA | No data | 256 | Chemical stability | Sustained release of PCs from PLA nanoparticles. | [39] |
| PLGA nanoparticles | PLGA | Nanoprecipitation | 195.4 ± 23.8 | Chemical stability | The biodegradation resistance of demineralized dentin was improved by loading collagen crosslinking agent into biodegradable polymer nanoparticles via dentin tubules. | [40] |
| Polysaccharide-based nanoparticles | Chitosan; lauryl succinyl | Ionic gelation | 458 ± 11; 3640 ± 33 | In vitro cytotoxicity in HEK-293 cells | The encapsules of PCs reduce toxicity and improve chemical stability. | [86] |
| Polysaccharide-based nanoparticles | chitosan | Hydrogen bond | 367.3–293.2 | Chemical stability; the ExPEC invasion of gut epithelial cells in vitro. | PCs inhibited invasion of gut epithelial cells by ExPEC. | [44] |
| Polysaccharide-based nanoparticles | Gelatin; chitosan | Hydrogen bond; hydrophobic interaction and electrostatic interaction | 344.7 | Chemical stability; In vitro apoptotic; necrotic; and cytotoxic properties in THP-1 cells | The stability and biological activity of PCs were improved by nano encapsulation. | [45] |
| Protein-based nanoparticles | Poly-lactic acid | Hydrogen bond | 256 | Chemical stability | The encapsulation of PCs effectively enhanced the antioxidant activity | [46] |
| Protein-based nanoparticles | Zein | Hydrogen bond and hydrophobic interactions | 392–447 | Solubility; in vitro cytotoxicity in HL-60 cells | PCs-zein nanoparticles decreased the cytotoxicity of procyanidins in HL-60 cells | [47] |
| Modified hydroxyapatite inorganic nanoparticles | Hydroxya-patite | Metal chelation | 20–50 | Chemical stability | Enhanced the colloidal stability of nHAp particles | [48] |
| Metallic nanoparticles | Gold | Metal chelation | 20–25 | Chemical stability | PCs-gold nanoparticles can be used as biocompatible gold nanoparticles for medical applications; molecular imaging and cancer therapy. | [49] |
| Metallic nanoparticles | Gold | Metal chelation | 20–40 | Chemical stability | PCs-gold nanoparticles might serve as anticancer agents in killing cancer. | [50] |
| Metallic nanoparticles | Gold | Metal chelation | 6–24 | Stability and in-vitro methods employed in antidiabetic studies | PCs-gold nanoparticles have potential anti-diabetes and anti-oxidation effects. | [51] |
| Metallic nanoparticles | Silver; gelatin | Metal chelation | 150–230 | Chemical stability; antibacterial assessment; cytotoxicity test | GSP/Gelatin Composite Fibers Contained Silver Nanoparticles had the potential for applications in antimicrobial tissue engineering and wound dressing. | [52] |
| Metallic nanoparticles | Silver; chitosan | Metal chelation | 150 | Cytotoxicity patterns; the antipro-liferative activities; and the possible mechanisms of anticancer activity in HpG2 cells | The nanoparticles exhibited high anticancer activity against HpG2 cells and induced apoptosis by down-regulating Bcl2 gene and up-regulating p53. | [53] |
| PUDL | Ultradeformable liposomes | Thin film hydration method | 140.6 ± 19 | Chemical stability | PUDL could increase the transdermalflux; prolong the release and improve the stability of PCs; and could serve as an effective dermal delivery system for procyanidins. | [54] |
| SLN | Solid lipid | The melt-emulsion method | 243 | Chemical stability; evaluation of anti-oxidant activity | SLN loaded with GSP exhibit antioxidant effects for longer than free GSP. | [55] |
Notes: PCs, procyanidins; GSP, grape seed procyanidins; PLGA, poly(lactic-co-glycolic acid); PLA, poly(D,L-lactic acid); ExPEC, extra-intestinal pathogenic Escherichia coli; Bcl-2, B-cell lymphoma-2; PUDL, ultradeformable liposomes; SLN, solid lipid nanoparticles.
3.1. PLA and PLGA Nanoparticles
PLGA and PLA are FDA-approved biodegradable polymers that have been widely used as biomaterials for the synthesis of nanoparticles with sustained, controlled and targeted drug delivery [82]. PLGA or PLA polymers greatly improve the surface area to volume ratio of the core and the controlled release ability of nanoparticles [83]. In addition, they display a high drug loading capacity and a controlled drug release profile with improved in vivo stability for the co-delivery of various categories of anticancer agents [84].
Fernandez et al. found that PCs nanoparticles loaded with PLA have uniform spheres and smooth edges, and thermal stability increases with the improvement of PCs entrapment efficiency, suggesting that novel nanomaterials with prolonged release will enhance the potency of PCs [39]. PCs-loaded nanoparticles with the biodegradable PLGA enhance the structural stability, surface/volume mechanical and biochemical properties of demineralized dentin in collagenase-containing solutions, and resistance to biodegradation over time [40]. Although PLA/PLGA have constituted parts of a procyanidin-releasing system, most of these studies have been in vitro and need to be further expanded to complement and improve the use of PCs in vivo.
3.2. Polysaccharide Based Nanoparticles
Chitosan (CHT) is a polysaccharide obtained by the deacetylation of chitin and has been used as a nano-carrier for novel drug delivery systems due to its biodegradability and biocompatibility [85]. CHT has been reported to prevent the degradation of polyphenols and enhance their absorption in the gastrointestinal tract [86]. Previous studies have demonstrated that oligomeric procyanidins/bletilla striata polysaccharide/chitosan (OPC/BSP/CHT) microspheres have significant antioxidant activity compared with free OPC [41]. By encapsulating these OPC into biodegradable polymer bio-adhesive microspheres, the vulnerability of procyanidins to oxidation in air and its optical instability can be overcome and bioavailability can be further improved [42]. Furthermore, a cranberry procyanidins extract from the plant Vaccinium macrocarpon was encapsulated in CHT, resulting in increased stability, as well as molecular adhesion to extra-intestinal pathogenic Escherichia coli [43]. Iannone et al. synthesized chitosan microencapsulated GSP, and evaluated the pharmacological activities of GSP and CHT-containing microparticles on various cancer cells showed an increase in antitumor effect due to increased cell interaction [44]. In addition, Zou et al. prepared cacao procyanidin-gelatin-chitosan nanoparticles. Compared with PCs in solution, the stability and biological activity of PCs were significantly improved by nano encapsulation. Apoptosis of human acute monocytic leukemia THP-1 cells was observed at low concentrations [45].
3.3. Protein-Based Nanoparticles
Protein is biodegradable, metabolizable, symmetrical and easy to operate, which can be used to prepare nanoparticles. In recent years, some researchers synthesized whey protein-polyphenol aggregates, which not only increased the stability and shelf life of PCs, but also reduced the expression of inflammation-related genes [87]. Huang et al. synthesized tannic acid (TA)/PCs and gelatin (GLT) colloidal complexes due to spontaneous hydrogen bonding between PCs and gelatin. PCs is known to have strong antioxidant activity, and the antioxidant activity of GLT is greatly improved after complexing with polyphenols [46]. Zou et al. prepared cranberry procyanidins-zein nanoparticles using an improved liquid phase dispersion method. The oligomer with higher polymerization degree had higher loading efficiency than the oligomer with lower polymerization degree, indicating that it had greater binding affinity for zein. The results showed that hydrogen bonding and hydrophobicity were the main interactions between PCs-zein. Cell culture studies using human promyelocytic leukemia HL-60 cells showed that PCs encapsulated in nanoparticles reduced cytotoxicity compared to free PCs [47].
3.4. Modified Hydroxyapatite Inorganic Nanoparticles
Biomimetic hydroxyapatite (Hap, Ca10(PO4)6(OH)2) has good biocompatibility and bioactivity, which is an ideal bone replacement material and biomolecular transport matrix. Zhou et al. prepared nHAP-GSP particles with a diameter of 20–50 nm using grape polyphenols. As a biocompatibility mediated matrix, GSP can effectively regulate the nucleation and growth of hydroxyapatite nanocrystals in solution. Nucleation of nHAp crystals begins with the formation of a complex between calcium ions and phenolic hydroxyl groups in the GSP molecule. The stable aqueous dispersion of nHAP-GSP can be maintained for more than five days, which may increase its in vivo bioavailability. The strong interaction between polycarbonate and hydroxyapatite inorganic nanoparticles makes polycarbonate difficult to decompose, which is of great significance for the application of drug carrier nanocomposites with uniform organic/inorganic properties in the biomedical field [48].
3.5. Metal Nanoparticles
Metal nanoparticles, green synthetic silver nanoparticles (AgNPs) and gold nanoparticles (GNPs) are the most common [88]. It is notable for its unique physical properties related to distance, size and shape [89]. Catalytic gold (Au) with specific enzymatic activity is considered as an ideal choice for drug delivery [90]. The gold nanoparticles were synthesized using PCs-rich grape polyphenols, and catechins-the monomeric unit of PCs, were taken as the representative compound in the experiment. Catechins interact with the metal through chelation reactions (using special covalent bonding) to synthesize gold nanomaterials. In addition, PCs as reducing agents or stabilizers can also be used to control the size of gold nanomaterials [49]. Subsequently, another research group further explored GSP-gold nanoparticles using A431 cell line. The results showed that GSP-gold nanoparticles may be used as anticancer drugs to kill cancer [50]. Gold nanoparticles produced by chelation of PCs showed good stability in repeated centrifugation and re-dispersion experiments compared with PCs, indicating the formation of biostable and bioactive gold nanoparticles with potential anti-diabetes and anti-oxidation applications [51]. Due to the synergistic effects of PCs and special covalently bonded gold nanomaterials, it can conclude that the unique chemical structure of PCs also makes them excellent reductants and capping agents, with the ability to synthesize stable, safe and biologically active metal nanoparticles, and contribute to the medical effect [91].
Since they can interfere with bacterial metabolism, silver nanoparticles are commonly used as an antibacterial material, thereby extending the shelf life of drugs or nutritional medicines [92]. Due to these excellent properties, GSP/gelatin composite nanofiber films containing silver nanoparticles were successfully prepared by electrostatic spinning technology. Using GSP as reducing agent, AgNPs were synthesized in gelatin aqueous solution and then electrospun into nanofibers. The results showed that the synthesized fiber membranes had antibacterial properties and had potential application prospects in tissue engineering and wound dressing [52]. Another study reported green synthesis of CHT-PCs-Silver nanoparticles using chitosan/grape leaf water extract (GLE) nanoparticles as reductants and stabilizers. The cytotoxic pattern, antiproliferative and anticancer activity of the nanoparticles were investigated at the molecular level. The results showed that the nanoparticles exhibited high anticancer activity against HpG2 cells and induced apoptosis by down-regulating Bcl-2 gene and up-regulating p53 [53].
3.6. PUDL and SLN
Chen et al. developed a novel vesicular carrier procyanidins, namely PUDL, to expand the application range of PCs. Compared with the PCs solution, PUDL can increase the transdermal flux of PCs, prolong the release time, and improve the stability of PCs, which can be used as an effective PCs skin delivery system [54]. SLN loaded with GSP exhibit antioxidant effects for longer than free GSP, suggesting controlled release of their payload, intracellular stability and long-term persistence, and reduction of oxidative stress and inflammation [55].
4. Application and Treatment for Oral Diseases
4.1. Oral Cancer
Oral cancer is a devastating disease, often disfiguring and debilitating, and in severe cases life-threatening, including cancers of the mouth, larynx, hypopharynx and oropharynx. Head and neck cancer accounts for three percent of all cancers in the United States, and about 65,000 Americans are diagnosed with it each year [93]. Furthermore, in line with incidence, the 5-year relative survival rate for oral cancer remains a dismal 18%, and the overall mortality rate for oral and pharyngeal cancer has increased by 0.5% per year from 2009 to 2018 [94]. The causes of oral cancer are complex and include lifestyle factors such as alcohol consumption and smoking, which are strongly associated with the progression and aggressiveness of most head and neck cancers [95]. Currently, surgery, chemotherapy, and radiotherapy are the main treatment methods for oral cancer [96]. Although patients’ survival time has improved with advances in surgery, chemotherapy, radiotherapy and other treatments, drug adverse effects, pain, and drug resistance remain major problems which trouble cancer patients and physicians [97]. There are many problems with current chemotherapeutic drugs. Although these drugs can delay tumor growth and prolong survival, they are controversial in cancer treatment due to their lack of desired therapeutic effect, frequent drug resistance and strong toxic adverse effects [98,99,100].
Ongoing studies of PCs have demonstrated significant chemoprophylaxis and chemotherapy potential for oral cancer [101]. Therefore, extracting highly efficient and low toxic procyanidins from natural products to replace or combine with existing chemotherapeutic drugs may become a new research trend.
Tongue squamous cell carcinoma (TSCC) is the most common oral squamous cell carcinoma. Despite significant advances in combination therapy, five-year survival in patients with TSCC has not improved significantly, which is due to local recurrence and lymph node metastasis. Yang et al. found that GSP significantly inhibited Tca8113 cell viability and induced apoptosis in a dose-dependent manner. This was associated with the significantly increased expression of pro-apoptotic regulator Bax protein and significantly decreased expression of anti-apoptotic regulator Bcl-2 protein at 100 µg/mL GSP. Additionally, GSP significantly inhibit the secretion of matrix metalloproteinase-2 (MMP-2) and MMP-9, and Tca8113 cell proliferation, migration, invasion by inhibiting the Akt/NF-κB signaling pathway. These results indicate that GSP is expected to be a novel chemoprophylaxis agent for TSCC [102]. In addition, it has been reported that PCs can induce the apoptosis of human oral squamous cell carcinoma (HSC-2) and human salivary gland tumor (HSG), and its mechanism may be through the activation of caspase-3, caspase-9 and the degradation of cytokeratin18 to promote cell apoptosis. Meanwhile, hepatocyte growth factor (HGF) of normal gingival fibroblasts was protected [103]. GSP can inhibit the spread of oral cancer by a mechanism based on the activation of key apoptotic regulatory [101].
4.2. Periodontitis/Peri-Implantitis
Periodontitis is a multifactorial, multi-microbial infection, characterized by destructive inflammatory processes affecting periodontal tissues, including supporting structures such as the gums, cementum, periodontal membrane, and alveolar bone. About 5% to 15% of the world’s population is affected by a severe form of periodontitis, which can lead to tooth loss and systemic complications [104,105]. Previous studies have demonstrated that the high antibacterial and immunomodulatory activity of procyanidins makes them an interesting class of phytonutrients for the prevention and treatment of periodontal diseases [106,107].
GSP was found to reduce periodontal inflammation and alveolar bone loss by decreasing αmatrix metalloproteinase and hypoxia-inducible factor levels and increasing osteoblast activity in diabetic rats with periodontitis [108]. For the treatment of periodontitis, PCs from blueberry and cranberry have been shown to inhibit biofilm formation and the adherence of major periodontal pathogens, such as Porphyromonas gingivalis (P. gingivalis) and Actinobacillus, exert anti-inflammatory properties, and reinforce epithelial barrier integrity [109]. The effects of PCs or flavane-3-alcohols on the growth, colony formation, and metabolic activity of potential pathogens, as well as inhibition of pathogens’ adhesion to oral mucosal cells, have been reported in a number of studies [109]. Savickiene et al. demonstrated that PCs significantly reduced the viability of P. gingivalis and the non-pathogenic commensal Streptococcus salivarius [110]. Furthermore, PCs have greater antioxidant capacity and exhibit unique antimicrobial activity, selectively targeting Gram-negative bacteria and disease-causing strains in periodontal and peri-implant conditions, such as P. gingivalis, while retaining the vitality of the beneficial oral symbiotic streptococcus salivarius [110]. Moreover, Jekabsone et al. suggested that cranberry procyanidins inhibited the attachment of P. gingivalis to periodontal tissues and reduced bacterial biofilm formation, collagenase activity and invasion by neutralizing periodontopathogen proteinases and cytotoxicity, but they did not interfere with P. gingivalis [111]. La et al., in addition to the above activities, showed dose-dependent inhibition of P. gingivalis produced by type A cranberry procyanidins on the surface of dental matrix gel-coated polystyrene and inhibition of extracellular proteases from type I collagen degradation [110].
Peri-implantitis, similar to periodontitis, is an irreversible disease involving hard and soft tissue surrounding the implant, with progressive bone resorption (biological remodeling beyond bone loss), reduced bone binding, increased pocket depth, and peri-implantitis of the functional implant [112]. Due to the versatility of procyanidins, it is speculated that procyanidin-coated implant surfaces may inhibit osteoclast activity and bacterial invasion and promote healing of surrounding tissues. La et al. used cranberry procyanidins A type to inhibit the adhesion of P. gingivalis matrix to the surface of polystyrene and found that PCs inhibited the degradation of type I collagen by the extracellular proteases produced by P. gingivalis in a dose-dependent manner [113]. This provides a promising strategy for avoiding peri-implantitis after implant surface coating.
4.3. Dental Caries
Dental caries is a chronic progressive disease occurring in the hard tissue of teeth, which is the result of many factors, among which bacteria is the most important cause [114]. Although the prevalence of dental caries in developed countries has decreased significantly and decreases with age, it remains one of the most prevalent chronic diseases in the world [115]. PCs can effectively inhibit caries through two pathways: (1) reducing caries-causing pathogens, such as Streptococcus mutans (S. mutans) and their biofilms; and (2) promoting the mineralization of hydroxyapatite. PCs have significantly reduced the incidence of caries on smooth surfaces in animal studies, and fluoride (or fluoride in combination with PCs is more effective than PCs alone [116].
EPS produced by S. mutans derived GTF is an important virulence factor related to the formation of caries-causing biofilms. PCs treatment can effectively reduce the content of insoluble EPS and prevent the growth of S. mutans in the mixed species biofilm. As a result, the 3D structure of cranberry-treated biofilms was severely impaired, suggesting that the EPS matrix was defective and unable to form microcolonies on salivary coated hydroxyapatite (sHA) surfaces. In addition, topical application of procyanidins significantly weakened the mechanical stability of biofilms [64]. In rat caries model, local application of cranberry procyanidins during biofilm formation reduced the biomass and insoluble polysaccharide of S. mutans formation in vitro, and significantly reduced the incidence of caries and light caries damage [117]. The results showed that selected water extracts of potential contained high concentrations of polyphenols, such as tannins and phenolic acids, as well as caries-preventing flavonoids, since they have shown antibacterial activity against S. mutans in vitro and inhibit plaque formation [118].
The reconstruction of inorganic matrix is an important process of dentin re-mineralization [119]. D.J. Epasinghe et al. compared the effects of pretreatment of three flavonoids (6.5% procyanidins, quercetin and naringin) on human demineralized dentin, and found that procyanidins could improve the biomechanical properties of dentin matrix while remineralizing root caries [120,121]. Cai et al. also obtained similar results, and showed that the recovery rate of microhardness, elastic modulus and creep of demineralization dentin was significantly improved after treatment with PCs combined with silver fluoride-diamine fluoride/potassium iodide (SDF/KI) for 24 h. Compared with SDF/KI alone, minerals in caries are more evenly distributed and ions are absorbed into deeper tissues [122]. PCs therapy may lead to a new alternative or adjunct to anti-biofilm/anti-caries chemotherapy agents.
4.4. Endodontic Root Canal Infections
Endodontic root canal infections, divided into primary and secondary infection, is a bacteria-caused dental disease. Enterococcus faecalis has a low prevalence in primary root canal infections (4–40%) and a high prevalence in secondary infections (24–77%) [123]. Relationships between PCs content and antioxidant capacity, antibacterial activity against Enterococcus faecalis and in vitro cytotoxicity have been documented [124]. After Enterococcus faecalis was introduced into human dentin tubules for culture for one week, the dentin specimens were pretreated with 2%, 5%, or 10% PCs. It was found that PCs could kill Enterococcus faecalis in biofilm and improve the biological stability of dehydrated dentin collagen matrix. The clinical application of PCs can assist root canal rinses to play antibacterial and anti-root fracture effects [125].
4.5. Diseases of the Oral Mucosa
Recurrent aphthous stomatitis (RAS), also known as recurrent oral ulcers and canker ulcers, is the most common oral mucosal disease, affecting about 5–25% of the general population [126]. Procyanidins therefore have the potential to be used as a treatment for RAS. A clinical trial has shown that PCs found in grape seeds, along with other flavonoids, play an important role in the healing of skin wounds [127].
In a randomized clinical trial, 24 patients with RAS were randomly divided into drug group and placebo group to be observed the occurrence of ulcer size reduction, wound healing and pain relief. Compared with placebo group, drug group met heal of ulcer in the first 10 days of treatment, the pain relief lasted for more than 4–5 h, suggesting that the propolis extract containing PCs had a strong effect on RAS [128]. This PCs-containing oral mucosal adhesive membrane provides controlled and targeted drug delivery and may serve as a novel therapeutic strategy for the treatment of recurrent oral aphthous ulcer.
Oral candidiasis is a common oral fungal disease mainly caused by Candida infection [129]. It has been shown that PCA reduce the adhesion properties of Candida albicans by reducing inflammatory responses and interfering with NF-κB P65 activation and phosphorylation of specific intracellular kinases. PCA may help alleviate oral candidiasis by affecting the adhesion properties of Candida albicans and reducing the inflammatory response caused by this pathogen [130]. Further studies have shown that PCs polymeric tannins are active on Candida albicans biofilms and, at safe doses, not only inhibit biofilm formation and reduce the metabolic activity of mature biofilm cells, but also inhibit, at least in part, the transmission of infection mediated by this cell population [131]. PCs may be a potential drug for the prevention and treatment of oral candidiasis by affecting the virulence properties of Candida.
4.6. Dental Restoration
With respect to resin-dentin bond interface, the degradation is the primary reason for the limited durability due to the existence of hybrid layer, which caused by the hydrolysis degradation of adhesive resin and the proteolysis of collagen fiber [132]. A variety of strategies have been proposed to improve the durability of resin-dentin bonding, including the use of MMP inhibitors and collagen crosslinking agents, biomimetic remineralization, and ethanol wet bonding to improve the physical and mechanical properties of the bonding matrix (i.e., dentin) [133]. PCs are considered as collagen crosslinking agents and their effectiveness in dental collagen biomodification have been demonstrated in previous studies.
PCs can be used as a dentine base coating due to their excellent collagenous crosslinking ability. D.J. Epasinghe et al. compared the effects of three flavonoids (6.5% procyanidins, quercetin and naringin) on the properties of human dehydrated dentin, and found that elastic modulus (MOE) and ultimate tensile strength (UTS) of dehydrated dentin increased rapidly and significantly after 4 h of PCs pretreatment. The results showed that PCs could improve the biomechanical properties of dentin matrix more effectively than quercetin and naringin, and achieve better repair effect [134]. In addition, Leme-Kraus et al. again demonstrated that PCs with a higher degree of oligomerization offer a robust bioadhesion between the hydrophilic dentin matrix and the hydrophobic adhesive [135]. In addition, PCs have some antibacterial activity and are not affected by changes in concentration. Dias et al. compared the effects of the adhesives containing 2%, 4.5%, and 6% PCs and found that the addition of 4.5% PCs was beneficial to prolong the shelf life and did not affect the bonding performance. Meanwhile, no matter how high concentration of PCs was added into the adhesive, all adhesives showed similar antibacterial activity, indicating that the antibacterial effect of the adhesive was independent of the concentration of PCs [136].
Recently, Wang’s team studied methacrylate-functionated procyanidins (MAPAs), which proved that MAPAs not only overcomes the shortcomings of PCs, but also significantly improves the biological stability and crosslinking ability of dentine collagen against enzyme degradation [137]. Then, they went on to investigate the effects of MAPAs on the polymerization, microhardness, and leaching of an experimental HEMA-based dental adhesive system, demonstrating that the novel adhesive not only stabilizes dentin collagen through its PCs components, also improves the polymerization, mechanical properties and stability of HEMA adhesives through its methacrylate composition, thus leading to long-lasting dentin bonding [138].
5. Conclusions and Prospects
As a natural polyphenol, PCs not only have no adverse effects, but also can exert antioxidant, antibacterial, anti-inflammatory, and antineoplastic activities, and are often used as dietary supplements. By encapsulating PCs in drug delivery system including PLGA, PLA nanoparticles, polysaccharide-based nanoparticles, protein nanoparticles, modified hydroxyapatite inorganic nanoparticles, metal nanoparticles, PUDL, and SLN, we are committed to improving the bioavailability, stability, and efficacy of PCs. Currently, the application of PCs in oral diseases shows its effective treatment effect in oral cancer, such as periodontitis, dental caries and other diseases. However, only in vitro studies have been carried out, and large-scale double-blind clinical studies on PCs are needed to provide more information about their clinical efficacy and safety, so that the clinical significance of PCs as therapeutic drugs can be proposed based on sufficient scientific evidence.
Author Contributions
Conceptualization, S.Z.; writing—original draft preparation, H.C., W.W., and S.Z.; writing—review and editing, S.Y., H.W., and W.W; supervision, S.Z., H.W., and Z.T.; funding acquisition, S.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Natural Science Foundation of China under the grant numbers of 82071163.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li W., He Y., Zhao H., Peng L., Li J., Rui R., Ju S. Grape Seed Proanthocyanidin Ameliorates FB(1)-Induced Meiotic Defects in Porcine Oocytes. Toxins. 2021;13:841. doi: 10.3390/toxins13120841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enomoto T., Nagasako-Akazome Y., Kanda T., Ikeda M., Dake Y. Clinical effects of apple polyphenols on persistent allergic rhinitis: A randomized double-blind placebo-controlled parallel arm study. Investig. Allergol. Clin. Immunol. 2006;16:283–289. doi: 10.1089/jir.2006.26.53. [DOI] [PubMed] [Google Scholar]
- 3.Chen S., Song J., Du L., Ma Y., Ren S., Ren J., Li S. Quantitative Analysis of Solubility Parameters and Surface Properties of Larch Bark Proanthocyanidins. Polymers. 2020;12:2800. doi: 10.3390/polym12122800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balalaie A., Rezvani M.B., Mohammadi Basir M. Dual function of proanthocyanidins as both MMP inhibitor and crosslinker in dentin biomodification: A literature review. Dent. Mater. J. 2018;37:173–182. doi: 10.4012/dmj.2017-062. [DOI] [PubMed] [Google Scholar]
- 5.Grace M.H., Xiong J., Esposito D., Ehlenfeldt M., Lila M.A. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food. Chem. 2019;277:336–346. doi: 10.1016/j.foodchem.2018.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nawrot-Hadzik I., Matkowski A., Hadzik J., Dobrowolska-Czopor B., Olchowy C., Dominiak M., Kubasiewicz-Ross P. Proanthocyanidins and Flavan-3-Ols in the Prevention and Treatment of Periodontitis-Antibacterial Effects. Nutrients. 2021;13:165. doi: 10.3390/nu13010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian Y., Yang C., Yao Q., Qian L., Liu J., Xie X., Ma W., Nie X., Lai B., Xiao L., et al. Procyanidin B2 Activates PPARγ to Induce M2 Polarization in Mouse Macrophages. Front. Immunol. 2019;10:1895. doi: 10.3389/fimmu.2019.01895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choy Y.Y., Fraga M., Mackenzie G.G., Waterhouse A.L., Cremonini E., Oteiza P.I. The PI3K/Akt pathway is involved in procyanidin-mediated suppression of human colorectal cancer cell growth. Mol. Carcinog. 2016;55:2196–2209. doi: 10.1002/mc.22461. [DOI] [PubMed] [Google Scholar]
- 9.Sazwi N.N., Nalina T., Abdul Rahim Z.H. Antioxidant and cytoprotective activities of Piper betle, Areca catechu, Uncaria gambir and betel quid with and without calcium hydroxide. BMC Complement. Altern. Med. 2013;13:351. doi: 10.1186/1472-6882-13-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q.Q., Gao H., Yuan R., Han S., Li X.X., Tang M., Dong B., Li J.X., Zhao L.C., Feng J., et al. Procyanidin A2, a polyphenolic compound, exerts anti-inflammatory and anti-oxidative activity in lipopolysaccharide-stimulated RAW264.7 cells. PLoS ONE. 2020;15:e0237017. doi: 10.1371/journal.pone.0237017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y.A., Cho E.J., Tanaka T., Yokozawa T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitaminol. 2007;53:287–292. doi: 10.3177/jnsv.53.287. [DOI] [PubMed] [Google Scholar]
- 12.Azizan N., Mohd Said S., Zainal Abidin Z., Jantan I. Composition and Antibacterial Activity of the Essential Oils of Orthosiphon stamineus Benth and Ficus deltoidea Jack against Pathogenic Oral Bacteria. Molecules. 2017;22:2135. doi: 10.3390/molecules22122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen P.E. World Health Organization global policy for improvement of oral health—World Health Assembly 2007. Community Dent. Oral Epidemiol. 2008;58:115–121. doi: 10.1111/j.1875-595X.2008.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 14.Mirhashemi A., Bahador A., Sodagar A., Pourhajibagher M., Amiri A., Gholamrezayi E. Evaluation of Antimicrobial Properties of Nano-Silver Particles Used in Orthodontics Fixed Retainer Composites: An Experimental In-Vitro Study. J. Dent. Res. Dent. Clin. Dent. Prospects. 2021;15:87–93. doi: 10.34172/joddd.2021.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giordani B., Parolin C., Vitali B. Lactobacilli as Anti-Biofilm Strategy in Oral Infectious Diseases: A Mini-Review. Front. Med. Technol. 2021;3:769172. doi: 10.3389/fmedt.2021.769172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Tao B., Wan Y., Sun Y., Wang L., Sun J., Li C. Drug delivery based pharmacological enhancement and current insights of quercetin with therapeutic potential against oral diseases. Biomed. Pharmacother. 2020;128:110372. doi: 10.1016/j.biopha.2020.110372. [DOI] [PubMed] [Google Scholar]
- 17.Shigeoka M., Koma Y.I., Kodama T., Nishio M., Akashi M., Yokozaki H. Tongue Cancer Cell-Derived CCL20 Induced by Interaction with Macrophages Promotes CD163 Expression on Macrophages. Front. Oncol. 2021;11:667174. doi: 10.3389/fonc.2021.667174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H., Xiao L., Yuan Y., Luo X., Jiang M., Ni J., Wang N. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem. Pharmacol. 2014;92:599–606. doi: 10.1016/j.bcp.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Schmuch J., Beckert S., Brandt S., Löhr G., Hermann F., Schmidt T.J., Beikler T., Hensel A. Extract from Rumex acetosa L. for Prophylaxis of Periodontitis: Inhibition of Bacterial In Vitro Adhesion and of Gingipains of Porphyromonas gingivalis by Epicatechin-3-O-(4β→8)-Epicatechin-3-O-Gallate (Procyanidin-B2-Di-Gallate) PLoS ONE. 2015;10:e0120130. doi: 10.1371/journal.pone.0120130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y., Li N., Gu J., Fu S., Peng Z., Zhao C., Zhang Y., Li X., Wang Z., Li X., et al. β-Hydroxybutyrate induces bovine hepatocyte apoptosis via an ROS-p38 signaling pathway. J. Dairy Sci. 2016;99:9184–9198. doi: 10.3168/jds.2016-11219. [DOI] [PubMed] [Google Scholar]
- 21.Du X., Shi Z., Peng Z., Zhao C., Zhang Y., Wang Z., Li X., Liu G., Li X. Acetoacetate induces hepatocytes apoptosis by the ROS-mediated MAPKs pathway in ketotic cows. J. Cell. Physiol. 2017;232:3296–3308. doi: 10.1002/jcp.25773. [DOI] [PubMed] [Google Scholar]
- 22.Katsuragi Y., Ichimura Y., Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J. 2015;282:4672–4678. doi: 10.1111/febs.13540. [DOI] [PubMed] [Google Scholar]
- 23.Su P., Zhang J., Wang S., Aschner M., Cao Z., Zhao F., Wang D., Chen J., Luo W. Genistein Alleviates Lead-Induced Neurotoxicity In Vitro and In Vivo: Involvement of Multiple Signaling Pathways. Neurotoxicology. 2016;53:153–164. doi: 10.1016/j.neuro.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Wang W., Chen R., Wang J. Procyanidin B2 ameliorates carrageenan-induced chronic nonbacterial prostatitis in rats via anti-inflammatory and activation of the Nrf2 pathway. Biochem. Biophys. Res. Commun. 2017;493:794–799. doi: 10.1016/j.bbrc.2017.08.089. [DOI] [PubMed] [Google Scholar]
- 25.Knatko E.V., Higgins M., Fahey J.W., Dinkova-Kostova A.T. Loss of Nrf2 abrogates the protective effect of Keap1 downregulation in a preclinical model of cutaneous squamous cell carcinoma. Sci. Rep. 2016;6:25804. doi: 10.1038/srep25804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sydykov A., Mamazhakypov A., Petrovic A., Kosanovic D., Sarybaev A.S., Weissmann N., Ghofrani H.A., Schermuly R.T. Inflammatory Mediators Drive Adverse Right Ventricular Remodeling and Dysfunction and Serve as Potential Biomarkers. Front. Physiol. 2018;9:609. doi: 10.3389/fphys.2018.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han S., Gao H., Chen S., Wang Q., Li X., Du L.J., Li J., Luo Y.Y., Li J.X., Zhao L.C., et al. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci. Rep. 2019;9:15087. doi: 10.1038/s41598-019-51614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molyvdas A., Georgopoulou U., Lazaridis N., Hytiroglou P., Dimitriadis A., Foka P., Vassiliadis T., Loli G., Phillipidis A., Zebekakis P., et al. The role of the NLRP3 inflammasome and the activation of IL-1β in the pathogenesis of chronic viral hepatic inflammation. Cytokine. 2018;110:389–396. doi: 10.1016/j.cyto.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T., Sonobe M., Menju T., Nakayama E., Mino N., Iwakiri S., Nagai S., Sato K., Miyahara R., Okubo K., et al. Mutations in Keap1 are a potential prognostic factor in resected non-small cell lung cancer. J. Surg. Oncol. 2010;101:500–506. doi: 10.1002/jso.21520. [DOI] [PubMed] [Google Scholar]
- 30.Ohnuma T., Sakamoto K., Shinoda A., Takagi C., Ohno S., Nishiyama T., Ogura K., Hiratsuka A. Procyanidins from Cinnamomi Cortex promote proteasome-independent degradation of nuclear Nrf2 through phosphorylation of insulin-like growth factor-1 receptor in A549 cells. Arch. Biochem. Biophys. 2017;635:66–73. doi: 10.1016/j.abb.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed N., Escalona R., Leung D., Chan E., Kannourakis G. Tumour microenvironment and metabolic plasticity in cancer and cancer stem cells: Perspectives on metabolic and immune regulatory signatures in chemoresistant ovarian cancer stem cells. Semin. Cancer Biol. 2018;53:265–281. doi: 10.1016/j.semcancer.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Tyagi A., Kumar S., Raina K., Wempe M.F., Maroni P.D., Agarwal R., Agarwal C. Differential effect of grape seed extract and its active constituent procyanidin B2 3,3″-di-O-gallate against prostate cancer stem cells. Mol. Carcinog. 2019;58:1105–1117. doi: 10.1002/mc.22995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding Y., Du C., Qian J., Dong C.M. NIR-Responsive Polypeptide Nanocomposite Generates NO Gas, Mild Photothermia, and Chemotherapy to Reverse Multidrug-Resistant Cancer. Nano. Lett. 2019;19:4362–4370. doi: 10.1021/acs.nanolett.9b00975. [DOI] [PubMed] [Google Scholar]
- 34.Zhao B.X., Sun Y.B., Wang S.Q., Duan L., Huo Q.L., Ren F., Li G.F. Grape seed procyanidin reversal of p-glycoprotein associated multi-drug resistance via down-regulation of NF-κB and MAPK/ERK mediated YB-1 activity in A2780/T cells. PLoS ONE. 2013;8:e71071. doi: 10.1371/journal.pone.0071071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kin R., Kato S., Kaneto N., Sakurai H., Hayakawa Y., Li F., Tanaka K., Saiki I., Yokoyama S. Procyanidin C1 from Cinnamomi Cortex inhibits TGF-β-induced epithelial-to-mesenchymal transition in the A549 lung cancer cell line. Int. J. Oncol. 2013;43:1901–1906. doi: 10.3892/ijo.2013.2139. [DOI] [PubMed] [Google Scholar]
- 36.Zhao S., Zhang L., Yang C., Li Z., Rong S. Procyanidins and Alzheimer’s Disease. Mol. Neurobiol. 2019;56:5556–5567. doi: 10.1007/s12035-019-1469-6. [DOI] [PubMed] [Google Scholar]
- 37.Nie X., Tang W., Zhang Z., Yang C., Qian L., Xie X., Qiang E., Zhao J., Zhao W., Xiao L., et al. Procyanidin B2 mitigates endothelial endoplasmic reticulum stress through a PPARδ-Dependent mechanism. Redox Biol. 2020;37:101728. doi: 10.1016/j.redox.2020.101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pauwels R.A., Kips J.C., Peleman R.A., Van Der Straeten M.E. The effect of endotoxin inhalation on airway responsiveness and cellular influx in rats. Am. Rev. Respir. Dis. 1990;141:540–545. doi: 10.1164/ajrccm/141.3.540. [DOI] [PubMed] [Google Scholar]
- 39.Fernández K., Aburto J., von Plessing C., Rockel M., Aspé E. Factorial design optimization and characterization of poly-lactic acid (PLA) nanoparticle formation for the delivery of grape extracts. Food Chem. 2016;207:75–85. doi: 10.1016/j.foodchem.2016.03.083. [DOI] [PubMed] [Google Scholar]
- 40.Fawzy A.S., Priyadarshini B.M., Selvan S.T., Lu T.B., Neo J. Proanthocyanidins-Loaded Nanoparticles Enhance Dentin Degradation Resistance. J. Dent. Res. 2017;96:780–789. doi: 10.1177/0022034517691757. [DOI] [PubMed] [Google Scholar]
- 41.Liu K., Feng Z., Shan L., Yang T., Zhang W.J.I.C. Preparation, Characterization, and Antioxidative Activity of Bletilla striata Polysaccharide/Chitosan Microspheres for Oligomeric Proanthocyanidins. Inorg. Chem. 2017;35:3889–3896. doi: 10.1080/07373937.2016.1269123. [DOI] [Google Scholar]
- 42.Sharma S.D., Katiyar S.K. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 43.Alfaro-Viquez E., Esquivel-Alvarado D., Madrigal-Carballo S., Krueger C.G., Reed J.D. Cranberry proanthocyanidin-chitosan hybrid nanoparticles as a potential inhibitor of extra-intestinal pathogenic Escherichia coli invasion of gut epithelial cells. Int. J. Biol. Macromol. 2018;111:415–420. doi: 10.1016/j.ijbiomac.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 44.Iannone M., Mare R., Paolino D., Gagliardi A., Froiio F., Cosco D., Fresta M. Characterization and In Vitro Anticancer Properties of Chitosan-Microencapsulated Flavan-3-Ols-Rich Grape Seed Extracts. Int. J. Biol. Macromol. 2017;104:1039–1045. doi: 10.1016/j.ijbiomac.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Zou T., Percival S.S., Cheng Q., Li Z., Rowe C.A., Gu L. Preparation, characterization, and induction of cell apoptosis of cocoa procyanidins-gelatin-chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2012;82:36–42. doi: 10.1016/j.ejpb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y., Li A., Qiu C., Teng Y., Wang Y. Self-assembled colloidal complexes of polyphenol-gelatin and their stabilizing effects on emulsions. Food Funct. 2017;8:3145–3154. doi: 10.1039/C7FO00705A. [DOI] [PubMed] [Google Scholar]
- 47.Zou T., Li Z., Percival S.S., Bonard S., Gu L. Fabrication, characterization, and cytotoxicity evaluation of cranberry procyanidins-zein nanoparticles. Food Hydrocoll. 2012;27:293–300. doi: 10.1016/j.foodhyd.2011.10.002. [DOI] [Google Scholar]
- 48.Zhou R., Si S., Zhang Q.J.A.S.S. Water-dispersible hydroxyapatite nanoparticles synthesized in aqueous solution containing grape seed extract. Appl. Surf. Sci. 2012;258:3578–3583. doi: 10.1016/j.apsusc.2011.11.119. [DOI] [Google Scholar]
- 49.Krishnaswamy K., Vali H., Orsat V. Value-adding to grape waste: Green synthesis of gold nanoparticles. J. Food Eng. 2014;142:210–220. doi: 10.1016/j.jfoodeng.2014.06.014. [DOI] [Google Scholar]
- 50.Nirmala J.G., Akila S., Narendhirakannan R.T., Chatterjee S. Vitis vinifera peel polyphenols stabilized gold nanoparticles induce cytotoxicity and apoptotic cell death in A431 skin cancer cell lines. Adv. Powder Technol. 2017;28:1170–1184. doi: 10.1016/j.apt.2017.02.003. [DOI] [Google Scholar]
- 51.Badeggi U.M., Ismail E., Adeloye A.O., Botha S., Badmus J.A., Marnewick J.L., Cupido C.N., Hussein A.A. Green Synthesis of Gold Nanoparticles Capped with Procyanidins from Leucosidea sericea as Potential Antidiabetic and Antioxidant Agents. Biomolecules. 2020;10:452. doi: 10.3390/biom10030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han X., Xing Z., Si S., Yao Y., Zhang Q.J.F. Electrospun grape seed polyphenols/gelatin composite fibers contained silver nanoparticles as biomaterials. Fiber. Polym. 2014;15:2572–2580. doi: 10.1007/s12221-014-2572-y. [DOI] [Google Scholar]
- 53.El-Sherbiny I.M., Salih E., Yassin A.M., Hafez E.E. Newly developed chitosan-silver hybrid nanoparticles: Biosafety and apoptosis induction in HepG2 cells. J. Nanopart. Res. 2016;18:172. doi: 10.1007/s11051-016-3477-z. [DOI] [Google Scholar]
- 54.Chen R., Li R., Liu Q., Bai C., Qin B., Ma Y., Han J. Ultradeformable Liposomes: A Novel Vesicular Carrier for Enhanced Transdermal Delivery of Procyanidins: Effect of Surfactants on the Formation, Stability, and Transdermal Delivery. AAPS PharmSciTech. 2017;18:1823–1832. doi: 10.1208/s12249-016-0661-5. [DOI] [PubMed] [Google Scholar]
- 55.Castellani S., Trapani A., Spagnoletta A., di Toma L., Magrone T., Di Gioia S., Mandracchia D., Trapani G., Jirillo E., Conese M. Nanoparticle delivery of grape seed-derived proanthocyanidins to airway epithelial cells dampens oxidative stress and inflammation. J. Transl. Med. 2018;16:140. doi: 10.1186/s12967-018-1509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snijders K.E., Fehér A., Táncos Z., Bock I., Téglási A., van den Berk L., Niemeijer M., Bouwman P., Le Dévédec S.E., Moné M.J., et al. Fluorescent tagging of endogenous Heme oxygenase-1 in human induced pluripotent stem cells for high content imaging of oxidative stress in various differentiated lineages. Arch. Toxicol. 2021;95:3285–3302. doi: 10.1007/s00204-021-03127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiménez-Aspee F., Theoduloz C., Soriano M., Ugalde-Arbizu M., Alberto M.R., Zampini I.C., Isla M.I., Simirigiotis M.J., Schmeda-Hirschmann G. The Native Fruit Geoffroea decorticans from Arid Northern Chile: Phenolic Composition, Antioxidant Activities and In Vitro Inhibition of Pro-Inflammatory and Metabolic Syndrome-Associated Enzymes. Molecules. 2017;22:1565. doi: 10.3390/molecules22091565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ali K., Maltese F., Choi Y.H., Verpoorte R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010;9:357–378. doi: 10.1007/s11101-009-9158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mendoza-Wilson A.M., Castro-Arredondo S.I., Balandrán-Quintana R.R. Computational study of the structure-free radical scavenging relationship of procyanidins. Food Chem. 2014;161:155–161. doi: 10.1016/j.foodchem.2014.03.111. [DOI] [PubMed] [Google Scholar]
- 60.Ebelle Etame R., Mouokeu R.S., Cidjeu Pouaha C.L., Voukeng Kenfack I., Tchientcheu R., Assam Assam J.P., Monthe Poundeu F.S., Tchinda Tiabou A., Etoa F.X., Kuiate J.R., et al. Effect of Fractioning on Antibacterial Activity of Enantia chlorantha Oliver (Annonaceae) Methanol Extract and Mode of Action. Evid.-Based Complement. Alternat. Med. 2018;2018:4831593. doi: 10.1155/2018/4831593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smeriglio A., Barreca D., Bellocco E., Trombetta D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017;174:1244–1262. doi: 10.1111/bph.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sánchez M.C., Ribeiro-Vidal H., Bartolomé B., Figuero E., Moreno-Arribas M.V., Sanz M., Herrera D. New Evidences of Antibacterial Effects of Cranberry Against Periodontal Pathogens. Foods. 2020;9:246. doi: 10.3390/foods9020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Micali S., Isgro G., Bianchi G., Miceli N., Calapai G., Navarra M. Cranberry and recurrent cystitis: More than marketing? Crit. Rev. Food Sci. Nutr. 2014;54:1063–1075. doi: 10.1080/10408398.2011.625574. [DOI] [PubMed] [Google Scholar]
- 64.Kim D., Hwang G., Liu Y., Wang Y., Singh A.P., Vorsa N., Koo H. Cranberry Flavonoids Modulate Cariogenic Properties of Mixed-Species Biofilm through Exopolysaccharides-Matrix Disruption. PLoS ONE. 2015;10:e0145844. doi: 10.1371/journal.pone.0145844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lacombe A., Wu V.C., Tyler S., Edwards K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. Int. J. Food Microbiol. 2010;139:102–107. doi: 10.1016/j.ijfoodmicro.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 66.Tamura T., Ozawa M., Tanaka N., Arai S., Mura K. Bacillus cereus Response to a Proanthocyanidin Trimer, a Transcriptional and Functional Analysis. Curr. Microbiol. 2016;73:115–123. doi: 10.1007/s00284-016-1032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson-White B., Buquo L., Zeinali M., Ligler F.S. Prevention of nonspecific bacterial cell adhesion in immunoassays by use of cranberry juice. Anal. Chem. 2006;78:853–857. doi: 10.1021/ac051700v. [DOI] [PubMed] [Google Scholar]
- 68.Abachi S., Lee S., Rupasinghe H.P. Molecular Mechanisms of Inhibition of Streptococcus Species by Phytochemicals. Molecules. 2016;21:215. doi: 10.3390/molecules21020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mbaoji F.N., Onwuka A.M., Onu S., Peter I.E., Nweze J.A., Okonta L.E. Evaluation of Methanol-Dichloromethane Extract of Stemonocoleus micranthus Harms (Fabaceae) Stem Bark for Anti-Inflammatory and Immunomodulatory Activities. Evid.-Based Complement. Alternat. Med. 2020;2020:1738163. doi: 10.1155/2020/1738163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu J., Jiang H., Liu B., Baiyun R., Li S., Lv Y., Li D., Qiao S., Tan X., Zhang Z. Grape seed procyanidin extract protects against Pb-induced lung toxicity by activating the AMPK/Nrf2/p62 signaling axis. Food Chem. Toxicol. 2018;116:59–69. doi: 10.1016/j.fct.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 71.Ma X., Li X., Ma L., Chen Y., He S. Soy isoflavones alleviate polycystic ovary syndrome in rats by regulating NF-κB signaling pathway. Bioengineered. 2021;12:7215–7223. doi: 10.1080/21655979.2021.1979864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiao C.Y., Li Y., Shang Y., Jiang M., Liu J., Zhan Z.Y., Ye H., Lin Y.C., Jiao J.Y., Sun R.H., et al. Management of Gout-associated MSU crystals-induced NLRP3 inflammasome activation by procyanidin B2: Targeting IL-1β and Cathepsin B in macrophages. Inflammopharmacology. 2020;28:1481–1493. doi: 10.1007/s10787-020-00758-8. [DOI] [PubMed] [Google Scholar]
- 73.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer. J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 74.Mei F., Xie M., Huang X., Long Y., Lu X., Wang X., Chen L. Porphyromonas gingivalis and Its Systemic Impact: Current Status. Pathogens. 2020;9:944. doi: 10.3390/pathogens9110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan Z., Wang X., Li P., Mei C., Zhang M., Zhao C. Overexpression of lncRNA GATA6-AS inhibits cancer cell proliferation in mantle cell lymphoma by downregulating GLUT1. Oncol. Lett. 2019;18:2443–2447. doi: 10.3892/ol.2019.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sochorova L., Prusova B., Cebova M., Jurikova T., Mlcek J., Adamkova A., Nedomova S., Baron M., Sochor J. Health Effects of Grape Seed and Skin Extracts and Their Influence on Biochemical Markers. Molecules. 2020;25:5311. doi: 10.3390/molecules25225311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou Y., Zheng J., Li Y., Xu D.P., Li S., Chen Y.M., Li H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients. 2016;8:515. doi: 10.3390/nu8080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang R., Xian D., Xu J., Peng H., Pan S., Zhong J. Proanthocyanidins as a Potential Novel Way for the Treatment of Hemangioma. Biomed. Res. Int. 2021;2021:5695378. doi: 10.1155/2021/5695378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li A.N., Li S., Zhang Y.J., Xu X.R., Chen Y.M., Li H.B. Resources and biological activities of natural polyphenols. Nutrients. 2014;6:6020–6047. doi: 10.3390/nu6126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu C., Li M., Yang J., Xiong L., Sun Q.J.F.H. Fabrication and characterization of biocompatible hybrid nanoparticles from spontaneous co-assembly of casein/gliadin and proanthocyanidin. Food Hydrocoll. 2017;73:74–89. doi: 10.1016/j.foodhyd.2017.06.036. [DOI] [Google Scholar]
- 81.Li Y., Lu X., Tian P., Wang K., Shi J. Procyanidin B2 induces apoptosis and autophagy in gastric cancer cells by inhibiting Akt/mTOR signaling pathway. BMC Complement. Med. Ther. 2021;21:76. doi: 10.1186/s12906-021-03225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei W., Rosenkrans Z.T., Luo Q.Y., Lan X., Cai W. Exploiting Nanomaterial-Mediated Autophagy for Cancer Therapy. Small Methods. 2019;3:1800365. doi: 10.1002/smtd.201800365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang C., Merlin D. Lipid-Based Drug Delivery Nanoplatforms for Colorectal Cancer Therapy. Nanomaterials. 2020;10:1424. doi: 10.3390/nano10071424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alven S., Aderibigbe B.A. Efficacy of Polymer-Based Nanocarriers for Co-Delivery of Curcumin and Selected Anticancer Drugs. Nanomaterials. 2020;10:1556. doi: 10.3390/nano10081556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang J., Yan H., Puligundla P., Gao X., Zhou Y., Wan X.J.F.H. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017;69:286–292. doi: 10.1016/j.foodhyd.2017.01.041. [DOI] [Google Scholar]
- 86.Muñoz V., Kappes T., Roeckel M., Vera J.C., Fernández K. Modification of chitosan to deliver grapes proanthocyanidins: Physicochemical and biological evaluation. LWT Food Sci. Technol. 2016;73:640–648. doi: 10.1016/j.lwt.2016.07.006. [DOI] [Google Scholar]
- 87.Schneider M., Esposito D., Lila M.A., Foegeding E.A. Formation of whey protein-polyphenol meso-structures as a natural means of creating functional particles. Food Funct. 2016;7:1306–1318. doi: 10.1039/C5FO01499A. [DOI] [PubMed] [Google Scholar]
- 88.Kumar P., Shivam P., Mandal S., Prasanna P., Kumar S., Prasad S.R., Kumar A., Das P., Ali V., Singh S.K., et al. Synthesis, characterization, and mechanistic studies of a gold nanoparticle-amphotericin B covalent conjugate with enhanced antileishmanial efficacy and reduced cytotoxicity. Int. J. Nanomed. 2019;14:6073–6101. doi: 10.2147/IJN.S196421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma Y., Zhu Y., Liu B., Quan G., Cui L. Colorimetric Determination of Hypochlorite Based on the Oxidative Leaching of Gold Nanorods. Materials. 2018;11:1629. doi: 10.3390/ma11091629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jadczak P., Kulpa D., Drozd R., Przewodowski W., Przewodowska A. Effect of AuNPs and AgNPs on the Antioxidant System and Antioxidant Activity of Lavender (Lavandula angustifolia Mill.) from In Vitro Cultures. Molecules. 2020;25:5511. doi: 10.3390/molecules25235511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Badeggi U.M., Badmus J.A., Botha S.S., Ismail E., Marnewick J.L., Africa C.W.J., Hussein A.A. Biosynthesis, Characterization, and Biological Activities of Procyanidin Capped Silver Nanoparticles. J. Funct. Biomater. 2020;11:66. doi: 10.3390/jfb11030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh M., Mallick A.K., Banerjee M., Kumar R. Loss of outer membrane integrity in Gram-negative bacteria by silver nanoparticles loaded with Camellia sinensis leaf phytochemicals: Plausible mechanism of bacterial cell disintegration. Bull. Mater. Sci. 2016;39:1871–1878. doi: 10.1007/s12034-016-1317-5. [DOI] [Google Scholar]
- 93.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 94.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 95.Jethwa A.R., Khariwala S.S. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017;36:411–423. doi: 10.1007/s10555-017-9689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L., Song H., Yang S. MicroRNA-206 has a bright application prospect in the diagnosis of cases with oral cancer. J. Cell. Mol. Med. 2021;25:8169–8173. doi: 10.1111/jcmm.16598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng X., Wang W., Wang G., Liu S. Could Jinfukang alleviate the chemotherapy-related adverse effects in non-small cell lung cancer patients? A protocol for a double-blind, randomized controlled trial. Medicine. 2021;100:e25002. doi: 10.1097/MD.0000000000025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi Y., Moon M., Dawood S., Mcmanus B., Liu P.P. Mechanisms and management of doxorubicin cardiotoxicity. Herz. 2011;36:296–305. doi: 10.1007/s00059-011-3470-3. [DOI] [PubMed] [Google Scholar]
- 99.Parambil J.G., Myers J.L., Ryu J.H. Diffuse Alveolar Damage: Uncommon Manifestation of Pulmonary Involvement in Patients with Connective Tissue Diseases. Chest. 2006;130:553–558. doi: 10.1378/chest.130.2.553. [DOI] [PubMed] [Google Scholar]
- 100.Naranjo T.W., Lopera D.E., Diaz-Granados L.R., Duque J.J., Restrepo A.M., Cano L.E. Combined itraconazole-pentoxifylline treatment promptly reduces lung fibrosis induced by chronic pulmonary paracoccidioidomycosis in mice. Pulm. Pharmacol. Ther. 2011;24:81–91. doi: 10.1016/j.pupt.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Chatelain K., Phippen S., McCabe J., Teeters C.A., O’Malley S., Kingsley K. Cranberry and grape seed extracts inhibit the proliferative phenotype of oral squamous cell carcinomas. Evid.-Based Complement. Alternat. Med. 2011;2011:467691. doi: 10.1093/ecam/nen047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang N., Gao J., Cheng X., Hou C., Yang Y., Qiu Y., Xu M., Zhang Y., Huang S. Grape seed proanthocyanidins inhibit the proliferation, migration and invasion of tongue squamous cell carcinoma cells through suppressing the protein kinase B/nuclear factor-κB signaling pathway. Int. J. Mol. Med. 2017;40:1881–1888. doi: 10.3892/ijmm.2017.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ito H., Kobayashi E., Takamatsu Y., Li S.H., Hatano T., Sakagami H., Kusama K., Satoh K., Sugita D., Shimura S., et al. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem. Pharm. Bull. 2000;48:687–693. doi: 10.1248/cpb.48.687. [DOI] [PubMed] [Google Scholar]
- 104.Caton J.G., Armitage G., Berglundh T., Chapple I.L.C., Jepsen S., Kornman K.S., Mealey B.L., Papapanou P.N., Sanz M., Tonetti M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018;45((Suppl. 20)):S1–S8. doi: 10.1111/jcpe.12935. [DOI] [PubMed] [Google Scholar]
- 105.Isola G., Polizzi A., Muraglie S., Leonardi R., Lo Giudice A. Assessment of Vitamin C and Antioxidant Profiles in Saliva and Serum in Patients with Periodontitis and Ischemic Heart Disease. Nutrients. 2019;11:2956. doi: 10.3390/nu11122956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rauf A., Imran M., Abu-Izneid T., Iahtisham Ul H., Patel S., Pan X., Naz S., Sanches Silva A., Saeed F., Rasul Suleria H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019;116:108999. doi: 10.1016/j.biopha.2019.108999. [DOI] [PubMed] [Google Scholar]
- 107.Odai T., Terauchi M., Kato K., Hirose A., Miyasaka N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2019;11:2844. doi: 10.3390/nu11122844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toker H., Balci Yuce H., Lektemur Alpan A., Gevrek F., Elmastas M. Morphometric and histopathological evaluation of the effect of grape seed proanthocyanidin on alveolar bone loss in experimental diabetes and periodontitis. J. Periodontal. Res. 2018;53:478–486. doi: 10.1111/jre.12536. [DOI] [PubMed] [Google Scholar]
- 109.Ben Lagha A., Pellerin G., Vaillancourt K., Grenier D. Effects of a tart cherry (Prunus cerasus L.) phenolic extract on Porphyromonas gingivalis and its ability to impair the oral epithelial barrier. PLoS ONE. 2021;16:e0246194. doi: 10.1371/journal.pone.0246194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Savickiene N., Jekabsone A., Raudone L., Abdelgeliel A.S., Cochis A., Rimondini L., Makarova E., Grinberga S., Pugovics O., Dambrova M., et al. Efficacy of Proanthocyanidins from Pelargonium sidoides Root Extract in Reducing P. gingivalis Viability While Preserving Oral Commensal S. salivarius. Materials. 2018;11:1499. doi: 10.3390/ma11091499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jekabsone A., Sile I., Cochis A., Makrecka-Kuka M., Laucaityte G., Makarova E., Rimondini L., Bernotiene R., Raudone L., Vedlugaite E., et al. Investigation of Antibacterial and Antiinflammatory Activities of Proanthocyanidins from Pelargonium sidoides DC Root Extract. Nutrients. 2019;11:2829. doi: 10.3390/nu11112829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chala M., Anagnostaki E., Mylona V., Chalas A., Parker S., Lynch E. Adjunctive Use of Lasers in Peri-Implant Mucositis and Peri-Implantitis Treatment: A Systematic Review. Dent. J. 2020;8:68. doi: 10.3390/dj8030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.La V.D., Howell A.B., Grenier D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrob. Agents Chemother. 2010;54:1778–1784. doi: 10.1128/AAC.01432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang W., Deng X., Zhou X., Hao Y., Li Y. Influence of Helicobacter pylori culture supernatant on the ecological balance of a dual-species oral biofilm. J. Appl. Oral Sci. 2018;26:e20170113. doi: 10.1590/1678-7757-2017-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mejàre I., Stenlund H., Zelezny-Holmlund C. Caries incidence and lesion progression from adolescence to young adulthood: A prospective 15-year cohort study in Sweden. Caries Res. 2004;38:130–141. doi: 10.1159/000075937. [DOI] [PubMed] [Google Scholar]
- 116.Yoo S., Murata R.M., Duarte S. Antimicrobial traits of tea- and cranberry-derived polyphenols against Streptococcus mutans. Caries Res. 2011;45:327–335. doi: 10.1159/000329181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Slobodníková L., Fialová S., Rendeková K., Kováč J., Mučaji P. Antibiofilm Activity of Plant Polyphenols. Molecules. 2016;21:1717. doi: 10.3390/molecules21121717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tomczyk M., Pleszczyńska M., Wiater A. Variation in total polyphenolics contents of aerial parts of Potentilla species and their anticariogenic activity. Molecules. 2010;15:4639–4651. doi: 10.3390/molecules15074639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu Z., Neoh K.G., Lin C.C., Kishen A. Biomimetic deposition of calcium phosphate minerals on the surface of partially demineralized dentine modified with phosphorylated chitosan. J. Biomed. Mater. Res. B Appl. Biomater. 2011;98:150–159. doi: 10.1002/jbm.b.31844. [DOI] [PubMed] [Google Scholar]
- 120.Dávila-Sánchez A., Gutierrez M.F., Bermudez J.P., Méndez-Bauer M.L., Hilgemberg B., Sauro S., Loguercio A.D., Arrais C.A.G. Influence of flavonoids on long-term bonding stability on caries-affected dentin. Dent. Mater. 2020;36:1151–1160. doi: 10.1016/j.dental.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 121.Epasinghe D.J., Yiu C., Burrow M.F. Effect of flavonoids on remineralization of artificial root caries. Aust. Dent. J. 2016;61:196–202. doi: 10.1111/adj.12367. [DOI] [PubMed] [Google Scholar]
- 122.Cai J., Burrow M.F., Manton D.J., Tsuda Y., Sobh E.G., Palamara J.E.A. Effects of silver diamine fluoride/potassium iodide on artificial root caries lesions with adjunctive application of proanthocyanidin. Acta Biomater. 2019;88:491–502. doi: 10.1016/j.actbio.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 123.Al-Madi E.M., Al-Jamie M.A., Al-Owaid N.M., Almohaimede A.A., Al-Owid A.M. Antibacterial efficacy of silver diamine fluoride as a root canal irrigant. Clin. Exp. Dent. Res. 2019;5:551–556. doi: 10.1002/cre2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Navarro-Hoyos M., Alvarado-Corella D., Moreira-Gonzalez I., Arnaez-Serrano E., Monagas-Juan M. Polyphenolic Composition and Antioxidant Activity of Aqueous and Ethanolic Extracts from Uncaria tomentosa Bark and Leaves. Antioxidants. 2018;7:65. doi: 10.3390/antiox7050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang S.Y., Liu Y., Mao J., Wu Y.B., Deng Y.L., Qi S.C., Zhou Y.C., Gong S.Q. The antibiofilm and collagen-stabilizing effects of proanthocyanidin as an auxiliary endodontic irrigant. Int. Endod. J. 2020;53:824–833. doi: 10.1111/iej.13280. [DOI] [PubMed] [Google Scholar]
- 126.Al-Maweri S.A., Halboub E., Ashraf S., Alqutaibi A.Y., Qaid N.M., Yahya K., Alhajj M.N. Single application of topical doxycycline in management of recurrent aphthous stomatitis: A systematic review and meta-analysis of the available evidence. BMC Oral Health. 2020;20:231. doi: 10.1186/s12903-020-01220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hemmati A.A., Foroozan M., Houshmand G., Moosavi Z.B., Bahadoram M., Maram N.S. The topical effect of grape seed extract 2% cream on surgery wound healing. Glob. J. Health Sci. 2014;7:52–58. doi: 10.5539/gjhs.v7n3p52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arafa M.G., Ghalwash D., El-Kersh D.M., Elmazar M.M. Propolis-based niosomes as oromuco-adhesive films: A randomized clinical trial of a therapeutic drug delivery platform for the treatment of oral recurrent aphthous ulcers. Sci. Rep. 2018;8:18056. doi: 10.1038/s41598-018-37157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liang J., Peng X., Zhou X., Zou J., Cheng L. Emerging Applications of Drug Delivery Systems in Oral Infectious Diseases Prevention and Treatment. Molecules. 2020;25:516. doi: 10.3390/molecules25030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feldman M., Tanabe S., Howell A., Grenier D. Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement. Altern. Med. 2012;12:6. doi: 10.1186/1472-6882-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luiz R.L., Vila T.V., de Mello J.C., Nakamura C.V., Rozental S., Ishida K. Proanthocyanidins polymeric tannin from Stryphnodendron adstringens are active against Candida albicans biofilms. BMC Complement. Altern. Med. 2015;15:68. doi: 10.1186/s12906-015-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li F., Majd H., Weir M.D., Arola D.D., Xu H.H. Inhibition of matrix metalloproteinase activity in human dentin via novel antibacterial monomer. Dent. Mater. 2015;31:284–292. doi: 10.1016/j.dental.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bedran-Russo A.K., Vidal C.M., Dos Santos P.H., Castellan C.S. Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. J. Biomed. Mater. Res. B Appl. Biomater. 2010;94:250–255. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Epasinghe D.J., Yiu C.K., Burrow M.F., Tsoi J.K., Tay F.R. Effect of flavonoids on the mechanical properties of demineralised dentine. J. Dent. 2014;42:1178–1184. doi: 10.1016/j.jdent.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 135.Leme-Kraus A.A., Phansalkar R.S., Dos Reis M.C., Aydin B., Sousa A.B.S., Alania Y., McAlpine J., Chen S.N., Pauli G.F., Bedran-Russo A.K. Dimeric Proanthocyanidins on the Stability of Dentin and Adhesive Biointerfaces. J. Dent. Res. 2020;99:175–181. doi: 10.1177/0022034519892959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dias P.G., da Silva E.M., Carvalho C.M., Miranda M., Portela M.B., Amaral C.M. Characterization and Antibacterial Effect of an Experimental Adhesive Containing Different Concentrations of Proanthocyanidin. J. Adhes. Dent. 2020;22:139–147. doi: 10.3290/j.jad.a44280. [DOI] [PubMed] [Google Scholar]
- 137.Hass V., Li Y., Wang R., Nguyen D., Peng Z., Wang Y. Methacrylate-functionalized proanthocyanidins as novel polymerizable collagen cross-linkers—Part 1: Efficacy in dentin collagen bio-stabilization and cross-linking. Dent. Mater. 2021;37:1183–1192. doi: 10.1016/j.dental.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang R., Li Y., Hass V., Peng Z., Wang Y. Methacrylate-functionalized proanthocyanidins as novel polymerizable collagen cross-linkers—Part 2: Effects on polymerization, microhardness and leaching of adhesives. Dent. Mater. 2021;37:1193–1201. doi: 10.1016/j.dental.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.