Abstract
The binding and pore formation properties of four Bacillus thuringiensis Cry1 toxins were analyzed by using brush border membrane vesicles from Spodoptera exigua and Spodoptera frugiperda, and the results were compared to the results of toxicity bioassays. Cry1Fa was highly toxic and Cry1Ac was nontoxic to S. exigua and S. frugiperda larvae, while Cry1Ca was highly toxic to S. exigua and weakly toxic to S. frugiperda. In contrast, Cry1Bb was active against S. frugiperda but only marginally active against S. exigua. Bioassays performed with iodinated Cry1Bb, Cry1Fa, and Cry1Ca showed that the effects of iodination on toxin activity were different. The toxicities of I-labeled Cry1Bb and Cry1Fa against Spodoptera species were significantly less than the toxicities of the unlabeled toxins, while Cry1Ca retained its insecticidal activity when it was labeled with 125I. Binding assays showed that iodination prevented Cry1Fa from binding to Spodoptera brush border membrane vesicles. 125I-labeled Cry1Ac, Cry1Bb, and Cry1Ca bound with high-affinities to brush border membrane vesicles from S. exigua and S. frugiperda. Competition binding experiments performed with heterologous toxins revealed two major binding sites. Cry1Ac and Cry1Fa have a common binding site, and Cry1Bb, Cry1C, and Cry1Fa have a second common binding site. No obvious relationship between dissociation of bound toxins from brush border membrane vesicles and toxicity was detected. Cry1 toxins were also tested for the ability to alter the permeability of membrane vesicles, as measured by a light scattering assay. Cry1 proteins toxic to Spodoptera larvae permeabilized brush border membrane vesicles, but the extent of permeabilization did not necessarily correlate with in vivo toxicity.
The Cry1 δ-endotoxins of Bacillus thuringiensis are a family of 130- to 140-kDa proteins that exhibit specific toxicities to numerous lepidopteran insects (1, 19). Several Cry1 δ-endotoxins, including Cry1Bb, Cry1Ca, and Cry1Fa, are toxic to important agricultural pests belonging to the genus Spodoptera (3, 7, 46). Biopesticides based on the Cry1Ca and Cry1Fa toxins are now available for control of Spodoptera exigua and Plutella xylostella in the field (29, 37). The most recent application of Cry proteins for the control of Spodoptera larvae is the development of transgenic crops that express a synthetic cry1Ca gene (36). Evolution of insect resistance to Cry proteins will undoubtedly challenge the long-term use of these toxins. Field results obtained with P. xylostella and laboratory results obtained with S. exigua and Spodoptera littoralis have shown that pests evolve resistance to the Cry1Ca and Cry1Fa toxins (30, 31, 39).
B. thuringiensis Cry proteins intoxicate insects via the larval midgut (16, 21). Toxin binding to midgut brush border sites is a key determinant of toxicity to insects (40). For example, loss of binding sites, as measured by reduced toxin binding, is associated with acquired resistance in Plodia interpunctella and P. xylostella larvae (14, 38, 41). Cry1A-resistant P. xylostella is cross-resistant to Cry1Fa toxin apparently because of the absence of available toxin binding sites shared by the Cry1A and Cry1Fa in susceptible larvae (39). However, the extent of toxin binding does not always correlate with insecticidal activity (15, 42).
One explanation for binding that does not result in insect mortality is the fact that some Cry1 toxins bind to the brush border membrane but do not readily insert into the membrane of the insect midgut. This phenomenon is reflected by a toxin’s inability to bind to the brush border membrane irreversibly (20, 24). The relevance of “irreversible” binding was demonstrated clearly by Rajamohan et al. (34). Cry1Ab toxin domain II mutants that were 400-fold less toxic to Manduca sexta exhibited total binding to brush border membrane vesicles (BBMV) similar to the total binding of wild-type Cry1Ab toxin. However, recombinant toxin binding was reversible and thus differed from the irreversible binding of the wild-type Cry1Ab toxin.
After Cry1 toxin binds to the brush border membrane, a large portion of the molecule inserts into the membrane, forming ion channels. Carroll and Ellar (6) developed a straightforward assay for measuring Cry1 toxin-induced permeability in BBMV. When BBMV are diluted in a hyperosmotic solution, they rapidly shrink, which results in an increase in scattered light. If the solute (such as KCl) is permeable through toxin-induced pores, the vesicles rapidly reswell. Using this technique, Carroll and Ellar (6) found that Cry1Ac significantly increased the membrane permeability of M. sexta BBMV. In contrast, Cry1Ba, which is not toxic to M. sexta, had no effect on BBMV permeability. The correlation between toxicity and pore formation is now becoming better established. For example, Lorence et al. (25) observed that two insecticidal Cry toxins (Cry1Ca and Cry1D), but not inactive Cry1Ac toxin, formed cation channels in the brush border membrane of Spodoptera frugiperda. Also, mutations in Cry1Aa toxin that resulted in a loss of toxicity to Bombyx mori larvae impaired the ability of the toxin to form pores in the BBMV (43).
The purpose of this study was to compare the binding and pore formation properties of B. thuringiensis Cry1Ac, Cry1Bb, Cry1Ca, and Cry1Fa by using BBMV from S. exigua and S. frugiperda. We established that the toxicities of the four toxins examined to S. exigua and S. frugiperda larvae differ significantly. Binding experiments showed that the Cry1Ac, Cry1Ca, and Cry1Bb toxins bound with similar affinities to S. exigua and S. frugiperda BBMV, although some of these toxins recognized distinct binding sites. Our results demonstrated that toxin activities inferred from membrane-permeabilizing capacities determined by the light scattering assay are not necessarily related to 50% lethal concentrations (LC50) determined by feeding bioassays.
MATERIALS AND METHODS
B. thuringiensis strains and toxin purification.
B. thuringiensis strains that harbored the cry1Ca, cry1Fa, or cry1Bb gene were provided by Ecogen, Inc. (Langhorne, Pa.). The GenBank accession number of the cry1Ca gene cloned from B. thuringiensis subsp. entomocidus is X07518. The GenBank accession numbers of the cry1Fa gene cloned from B. thuringiensis subsp. aizawai (7) and the cry1Bb gene cloned from B. thuringiensis subsp. aizawai EG5847 are M63897 and L32020, respectively. B. thuringiensis subsp. kurstaki HD-73, which produces only one Cry protein, Cry1Ac, was obtained from the Bacillus Genetic Stock Culture Collection (Columbus, Ohio).
All of the B. thuringiensis strains were grown for 3 days at 28°C in 1 liter of half-strength L broth until sporulation and cell lysis. The crystals, spores, and debris were collected by centrifugation at 7,500 × g for 30 min, and the pellet was washed with 1 M NaCl containing 0.1% Triton X-100 and then with distilled water. The crystals were dissolved in 50 ml of 50 mM Na2CO3 (pH 9.6) containing 0.1% 2-mercaptoethanol by incubating the preparation for 2 h at room temperature and then centrifuged at 27,000 × g for 30 min to remove the insoluble debris. The protoxin was treated with 10 mg of l-1-tosylamide-2-phenylethylchloromethyketone-treated trypsin (Sigma) at room temperature for 15 min (Cry1Ca) or 30 min (Cry1Fa, Cry1Bb, and Cry1Ac). Toxin samples were filtered through a 0.2-μm-pore-size filter (Nalge Co.) and then were loaded onto an Econo-Pac Q anion-exchange column (Bio-Rad) that previously had been equilibrated with Na2CO3 buffer (pH 9.6) and was connected to a fast-performance liquid chromatograph (Pharmacia). The toxin was eluted from the column with a 0 to 0.6 M NaCl gradient in 20 mM Na2CO3 (pH 9.6). Fractions containing purified toxin were pooled and stored at 4°C. For the Cry1Ca toxin, an additional purification step was performed to remove contaminating small peptides. The Cry1Ca toxin that eluted from the Econo-Pac Q column was dialyzed against 20 mM Na2CO3 buffer (pH 9.6) overnight and then loaded onto a Mono Q HR 5/5 column (Pharmacia). The bound toxin was eluted by using the same NaCl gradient. Fractions containing purified Cry1Ca toxin were pooled and stored at −20°C until they were used. We found that this additional purification step was important for labeling the Cry1Ca toxin to a high specific activity.
Insect bioassays.
Toxin preparations were diluted with phosphate-buffered saline (10 mM Na2HPO4, 1.7 mM KH2PO4, 2.7 mM KCl, 136.9 mM NaCl [pH 7.4]) containing 0.1% bovine serum albumin (BSA). Seven toxin concentrations were tested by using 20 neonate S. exigua or S. frugiperda larvae per concentration. S. exigua was obtained from the USDA/ARS Southern Field Crop Insect Management Laboratory, Stoneville, Miss., and S. frugiperda was obtained from the USDA/ARS Turf Grass Laboratory Tifton, Ga. Cry1 toxin samples (50 μl) were applied uniformly to the surface (area, 2 cm2) of an artificial diet preparation (Southland Products, Lake Village, Ark.) and then allowed to dry. Each larva was placed onto the diet surface and reared at 26°C and 70% relative humidity with a photoperiod consisting of 12 h of light and 12 h of darkness. Insect mortality was scored after 7 days, and the data were analyzed with the POLO-PC computer program (9).
Iodination.
Cry1Ac was iodinated by the chloramine T method described by Garczynski et al. (15). Cry1Ca, Cry1Fa, and Cry1Bb were iodinated by the Iodobead (Pierce) method as described previously (27), except that each reaction vial was gently shaken during iodination. The specific radioactivities were 20, 6.1, 10.1, and 8.6 μCi/μg of input toxin for Cry1Ac, Cry1Ca, Cry1Fa, and Cry1Bb, respectively. Cry1Bb, Cry1Ca, and Cry1Fa were labeled with Nal by the Iodobead (Pierce) method (27) in order to examine the effects of iodination on toxin activity. Briefly, 50 μl of 50 mM Nal (pH 9.0) was added to a reaction mixture containing 300 to 400 μg of toxin. After incubation for 10 min at room temperature, the free Nal molecules were removed by three 1-ml washes with 20 mM Na2CO3 (pH 9.6) by using a Centriprep-30 ultrafiltration device (Amicon) at 4°C, and then the toxins were concentrated to concentrations of 300 to 400 μg/ml. The protein concentrations were determined after the preparations were washed with a Bio-Rad protein assay kit; BSA was used as the standard as described by Bradford (5).
Gel electrophoresis.
Cry1 toxin preparations (approximately 10 μg for gels that were stained with Coomassie brilliant blue R-250 or 105 cpm for 125I-labeled proteins) were analyzed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS-PAGE). The gels were stained with Coomassie brilliant blue R-250 or were exposed to a Kodak XAR-5 film with an intensifying screen at −80°C for 3 h.
Preparation of BBMV.
Midguts were excised from fifth-instar S. exigua and S. frugiperda larvae and frozen on dry ice. BBMV were prepared by the MgCl2 precipitation method (44), as modified by Ferre et al. (14). Each final BBMV pellet was suspended in 0.3 M mannitol–5 mM EGTA–17 mM Tris-Cl (pH 7.5) and stored at −80°C until it was used.
Membrane vesicle binding assays.
Binding assays were performed as described by Garczynski et al. (15). Qualitative binding experiments were done to determine if each labeled Cry1 toxin bound to S. exigua and S. frugiperda BBMV and to identify a BBMV concentration suitable for competition binding experiments. Duplicate samples of various concentrations of BBMV from S. exigua and S. frugiperda were incubated with 0.1 nM 125I-labeled toxin in 100 μl of phosphate-buffered saline containing 0.1% BSA at room temperature for 30 min (Cry1Ac, Cry1Fa, and Cry1Bb) or 60 min (Cry1Ca). The samples were centrifuged at 15,000 × g for 5 min, and then the pellets were washed twice with binding buffer. The radioactivity was measured with a Beckman model Gamma 4000 detector.
To evaluate binding at a quantitative level, we performed homologous competition assays (in which competition between a labeled ligand and an unlabeled ligand was examined) with different amounts of unlabeled Cry1Ac, Cry1Ca, and Cry1Bb as described above by using BBMV from S. exigua and S. frugiperda. Using the results of these binding experiments, we calculated the dissociation constants (Kd) and the binding site concentrations (Bmax) with the LIGAND computer program (Biosoft). We performed heterologous competition binding assays (in which competition between a labeled toxin and a different unlabeled ligand was examined) with different amounts of unlabeled Cry1Ac, Cry1Bb, Cry1Ca, and Cry1Fa toxins to investigate whether these toxins recognized the same binding site.
To measure toxin dissociation, BBMV (10 μg of protein) were incubated with 125I-labeled toxins (concentration, 0.1 nM) at room temperature for 30 min (Cry1Ac) or 60 min (Cry1Ca and Cry1Bb) in order to achieve equilibrium binding, and then unlabeled toxins (final concentration, 100 nM) were added into the reaction mixtures. The amounts of the bound 125I-labeled toxins were then determined at different times after the excess unlabeled toxins were added as described by Van Rie et al. (40).
Permeability assays.
Light scattering assays were performed with a stop flow spectrofluorimeter (model RSM 1000; On-line Instrument Systems, Bogart, Ga.). BBMV from S. exigua and S. frugiperda were diluted to a concentration of 0.5 mg/ml with 10 mM HEPES–Tris (pH 8.0) containing 0.1% BSA. Aliquots (1.0 ml) of BBMV were incubated with Cry1Ac, Cry1Bb, Cry1Ca, or Cry1Fa toxin (final concentration, 2.5 μg/ml) at room temperature for 30 min. The toxin-BBMV mixture and 0.5 M KCl in 10 mM HEPES–Tris (pH 8.0) were manually transferred into separate reservoirs by using syringes. Assays were initiated by simultaneously injecting 35 μl of a toxin-BBMV mixture and 35 μl of KCl into the cuvette in the spectrofluorimeter sample compartment. Incident 450-nm light was scattered at 90° from incidence and was monitored for 120 s by obtaining five measurements per s. The model RSM-1000 spectrofluorimeter has a time lag of about 3 ms between the injection time and the onset of data collection by the photodetector. To determine the amount of light scattered by control BBMV before osmotically induced shrinkage (i.e., in buffer alone), measurements were obtained after simultaneous injection of 35 μl of 10 mM HEPES–Tris (pH 8.0) and 35 μl of BBMV in 10 mM HEPES–Tris (pH 8.0). To determine the maximum amount of light scattered after BBMV shrinkage, the scattered light was measured after BBMV and 0.5 M KCl were injected simultaneously. The cuvette was washed three times with 50 mM Tris (pH 8.0) between measurements involving different insect BBMV. Assays were conducted in triplicate for both species for each toxin.
RESULTS
Toxicity of four B. thuringiensis toxins to S. exigua and S. frugiperda.
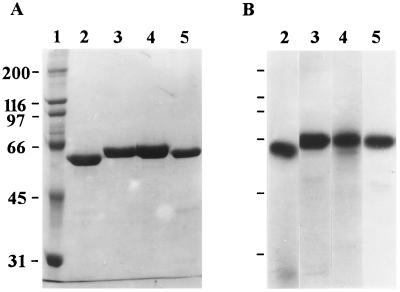
Figure 1 shows the purity of the Cry1 toxins used in this study. Each toxin appeared as a single band on an SDS-PAGE gel after staining for unlabeled toxins and after autoradiography for 125I-labeled toxins. In bioassays, Cry1Ac did not kill S. exigua or S. frugiperda (Table 1). Cry1Ca was highly toxic to S. exigua (LC50, 127 ng/cm2) and weakly toxic to S. frugiperda (LC50, 1,144 ng/cm2). In contrast, Cry1Bb was active against S. frugiperda (LC50, 308 ng/cm2) but only marginally active against S. exigua (LC50, 1,468 ng/cm2). Only Cry1Fa exhibited high levels of activity against both S. exigua and S. frugiperda (LC50, 177 and 109 ng/cm2, respectively). Bioassays performed with iodinated Cry1Bb, Cry1Fa, and Cry1Ca showed that the effects of iodination on toxin activity differed between toxins. I-labeled Cry1Bb was not toxic to S. frugiperda, I-labeled Cry1Fa exhibited greatly reduced toxicity against both Spodoptera species compared with unlabeled Cry1Fa, and I-labeled Cry1Ca retained insecticidal activity (Table 1).
FIG. 1.
SDS-PAGE and autoradiography analyses of purified Cry1 toxins. (A) Coomassie blue-stained SDS-PAGE gel. Lane 1, molecular size markers; lane 2, Cry1Ac; lane 3, Cry1Ca; lane 4, Cry1Fa; lane 5, Cry1Ba. (B) Autoradiograph of 125I-labeled toxins. Lane 2, Cry1Ac; lane 3, Cry1Ca; lane 4, Cry1Fa; lane 5, Cry1Ba. The numbers on the left are molecular masses (in kilodaltons).
TABLE 1.
Toxicities of different B. thuringiensis toxins toward S. exigua and S. frugiperdaa
| Toxin | Insect | LC50 (ng/cm2)b | Slope |
|---|---|---|---|
| Cry1Ac | S. exigua | >2,000 | |
| S. frugiperda | >2,000 | ||
| Cry1Bb | S. exigua | 1,468 (1,035–3,750) | 3.3 |
| S. frugiperda | 308 (233–393) | 3.9 | |
| Cry1Ca | S. exigua | 127 (49–187) | 4.6 |
| S. frugiperda | 1,144 (813–3,227) | 3.5 | |
| Cry1Fa | S. exigua | 177 (96–247) | 4.4 |
| S. frugiperda | 109 (31–168) | 4.6 | |
| I-labeled Cry1Bb | S. frugiperda | >1,500 | |
| I-labeled Cry1Ca | S. exigua | 242 (165–373) | 4.2 |
| I-labeled Cry1Fa | S. exigua | 923 (676–1,830) | 3.3 |
| S. frugiperda | 608 (488–844) | 3.5 |
LC50, 95% confidence intervals, and slopes were calculated by using data from two bioassay experiments.
Nanograms of protein per square centimeter of artificial diet. The values in parentheses are 95% confidence intervals.
Maximal binding of labeled toxins to BBMV from S. exigua and S. frugiperda.
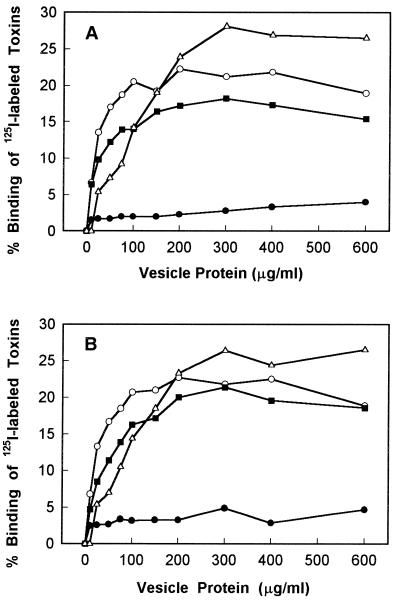
Qualitative binding experiments were done to determine if each labeled Cry1 toxin bound to S. exigua and S. frugiperda BBMV and to identify a BBMV concentration suitable for competition binding experiments. 125I-labeled Cry1Ac, Cry1Bb, Cry1Ca, and Cry1Fa were incubated with various concentrations of BBMV from S. exigua and S. frugiperda. Maximal binding of Cry1Ac, Cry1Ca, and Cry1Bb was observed at concentrations between 200 and 300 μg of vesicle protein per ml (Fig. 2). Cry1Ca and Cry1Bb bound similarly to S. exigua and S. frugiperda BBMV (Fig. 2), although their toxicities against the two species were different (Table 1). Cry1Ac, which is not toxic to S. exigua and S. frugiperda, bound strongly to BBMV from both species (27 and 28% of the input 125I-Cry1Ac bound to S. exigua and S. frugiperda BBMV, respectively) (Fig. 2).
FIG. 2.
Binding of 125I-labeled Cry1Ac (▵), Cry1Bb (■), Cry1Ca (○), and Cry1Fa (•) to S. exigua BBMV (A) and S. frugiperda BBMV (B). Vesicles at the concentrations indicated were incubated with 125I-labeled toxins at a concentration of 0.1 nM. Each data point is a mean based on the results for duplicate samples.
Much less 125I-Cry1Fa than other Cry1 toxins bound to BBMV from Spodoptera species. The maximal levels of binding to both species were only 4 to 5% of the input 125I-labeled toxin (Fig. 2). Perhaps attachment of 125I to the Cry1Fa toxin reduces its toxicity to larvae and its binding to BBMV. Even when the lower toxicity of labeled Cry1Fa was taken into account, toxicity and maximal binding were not well correlated for the toxins. In particular, labeled Cry1Fa was more toxic to both species than Cry1Ac was, yet Cry1Fa exhibited much lower maximal binding than Cry1Ac exhibited.
Homologous and heterologous competition binding.
As shown in Table 2, three toxins bound to BBMV from S. exigua and S. frugiperda with similar Kd and Bmax values. We could not calculate Kd and Bmax values for Cry1Fa due to the low level of binding of 125I-labeled Cry1Fa to BBMV from the two insects.
TABLE 2.
Binding characteristics of different B. thuringiensis toxinsa
| Toxin |
S. exigua
|
S. frugiperda
|
||
|---|---|---|---|---|
| Kd (nM) | Bmax (pmol/mg)b | Kd (nM) | Bmax (pmol/mg)b | |
| Cry1Ac | 7.4 ± 3.1c | 6.3 ± 0.4 | 3.0 ± 1.9 | 5.4 ± 0.3 |
| Cry1Ca | 4.7 ± 0.2 | 2.1 ± 0.1 | 5.5 ± 0.4 | 2.1 ± 0.1 |
| Cry1Bb | 6.6 ± 0.4 | 2.4 ± 0.2 | 8.5 ± 0.3 | 3.5 ± 0.3 |
Kd and Bmax were calculated by using the LIGAND program.
Picomoles per milligram of vesicle protein.
Mean ± standard error.
In both S. exigua and S. frugiperda, unlabeled Cry1Bb and Cry1Ca did not compete with bound 125I-Cry1Ac (Fig. 3A and B). Similarly, unlabeled Cry1Ac did not compete with 125I-Cry1Ca and 125I-Cry1Bb (Fig. 3C to F). Cry1Fa exhibited high-affinity competition for the binding sites of 125I-Cry1Ac (Fig. 3A and B), 125I-Cry1Bb (Fig. 3C and D), and 125I-Cry1Ca (Fig. 3E and F). In both S. exigua and S. frugiperda, unlabeled Cry1Ca exhibited significant competition for bound 125I-Cry1Bb (Fig. 3E and F). Similar competition was also observed with labeled Cry1Ca and unlabeled Cry1Bb (Fig. 3C and D). These binding data revealed that there are two high-affinity Cry1 binding sites in S. exigua and S. frugiperda. Cry1Ac and Cry1Fa recognize one site, and Cry1Bb, Cry1Ca, and Cry1Fa recognize a second site.
FIG. 3.
Competition between 125I-labeled Cry1Ac (A and B), Cry1Bb (C and D), and Cry1Ca (E and F) toxins and unlabeled Cry1Ac (▵), Cry1Bb (■), Cry1Ca (○), and Cry1Fa (•) toxins. S. exigua BBMV (A, C, and E) and S. frugiperda BBMV (B, D, and F) were incubated with 125I-labeled toxins at a concentration of 0.1 nM plus different concentrations of unlabeled toxins. Binding was expressed as a percentage of the maximum amount of toxin bound during incubation with labeled toxin. Each data point is a mean based on the results for duplicate samples.
Dissociation binding assays.
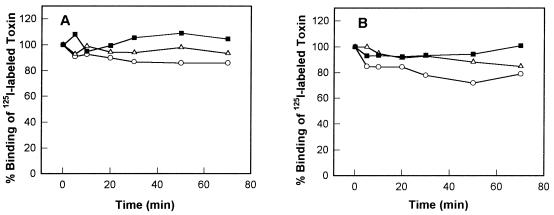
Since Cry1Ac, Cry1Ca, and Cry1Bb had different toxicities for S. exigua and S. frugiperda larvae but bound with similar Kd and Bmax values to BBMV from the two insects, we investigated whether the extents of dissociation of membrane-bound toxins are major factors in their toxicities. As shown in Fig. 4, the extents of Cry1Ac, Cry1Ca, and Cry1Bb toxin dissociation from S. exigua and S. frugiperda BBMV were not different (Fig. 4A and B). For example, while the LC50 of Cry1Ca for S. exigua was 127 ng/cm2 and the LC50 of Cry1Ca for S. frugiperda was 1,144 ng/cm2, the amount of 125I-Cry1Ca displaced by excess unlabeled Cry1Ca was the same for both species. Also, Cry1Ac was the least toxic of the three toxins tested, yet its dissociation was intermediate between the dissociation of Cry1Bb and the dissociation of Cry1Ca. Furthermore, binding of the three toxins to BBMV was for the most part irreversible after incubation for 70 min with excess unlabeled toxins (Fig. 4A and B). The difference between the insecticidal and noninsecticidal Cry1 toxins could not be accounted for by dissociation of inactive toxins from the membrane surface.
FIG. 4.
Dissociation of Cry1Ac (▵), Cry1Bb (■), and Cry1Ca (○) from S. exigua BBMV (A) and S. frugiperda BBMV (B). BBMV (10 μg of protein) were incubated with 125I-labeled toxin at a concentration of 0.1 nM for 30 min (Cry1Ac) or 60 min (Cry1Bb and Cry1Ca) in order to obtain equilibrium binding, and then unlabeled toxins (concentration, 100 nM) were added to the reaction mixtures. The bound toxins were measured at different times. Each data point is a mean based on the results of two independent experiments.
Permeability assays.
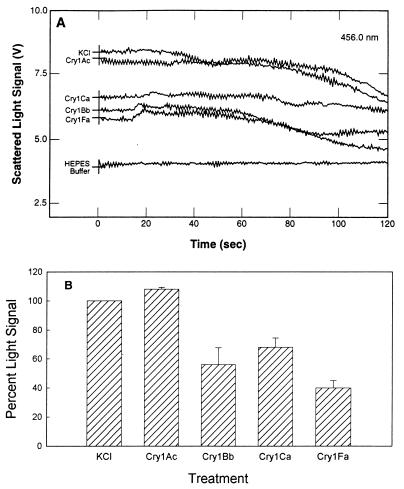
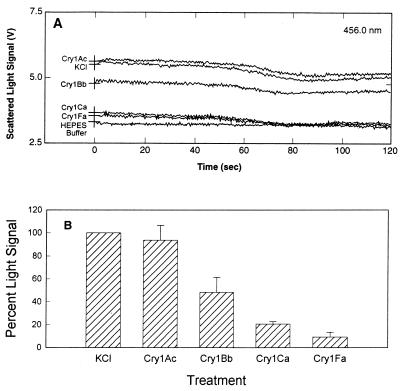
Since reversible and irreversible binding of Cry1 toxins to BBMV from S. exigua and S. frugiperda did not account for the differences in toxicity, we analyzed the next step in toxin action, pore formation. When external KCl was added to BBMV, the amount of scattered light rapidly increased due to vesicle shrinkage. The shrinkage response (measured by determining the increase in the scattered light signal, expressed in volts) was determined by comparing the light scattered from BBMV that were coinjected with buffer having the same osmotic strength (HEPES buffer) and the light scattered from BBMV that were coinjected with 0.5 M KCl (Fig. 5A). The decrease in the signal after 30 s may have been due to endogenous ion channels in the BBMV. When S. frugiperda BBMV were made more permeable to KCl (for example, with insecticidal toxins), the size of the vesicles changed rapidly and less light was scattered compared to the untreated control membranes (Fig. 5A). The BBMV response to toxin shown in Fig. 5A was deduced by comparing the results for BBMV mixed with HEPES buffer, BBMV mixed with KCl, and toxin-treated BBMV mixed with KCl. The light scattering value (in volts) for the no-salt treatment (buffer alone) (NS) was subtracted from the value for the salt treatment (S): S − NS = X. This was done to correct for the salt effect. The value for the no-salt treatment (buffer alone) was subtracted from the values for toxin treatments (T) (in volts): T − NS = Y. This was done to correct for the buffer effect. (Y/X) × 100 was calculated in order to determine the percent change in light scattering corrected for both salt and buffer effects. Figure 5B shows the proportion of scattered light change for toxin-treated BBMV compared to untreated BBMV from S. frugiperda for three separate experiments. The results of the light scattering assays indicated that membrane permeability induced by Cry1 toxins in S. frugiperda increased in the following increasing order: Cry1Ca, Cry1Bb, Cry1Fa. Cry1Ca and Cry1Bb are not significantly different from each other. Cry1Fa is significantly different from Cry1Ca and Cry1Bb. Cry1Ca, Cry1Bb, and Cry1Fa are all significantly different from Cry1Ac. Cry1Fa, the most insecticidal toxin, changed membrane permeability the most, and the nontoxic compound Cry1Ac had no overall effect on membrane permeability.
FIG. 5.
Scattered light signal of toxin-treated S. frugiperda BBMV after an increase in medium osmolarity. (A) Direct traces of scattered light from toxin-treated BBMV. BBMV were resuspended at a concentration of 0.5 mg/ml in 10 mM HEPES–Tris (pH 8.0) containing 0.1% BSA. Aliquots (1 ml) of BBMV were incubated with toxins (2.5 μg/ml) at room temperature for 30 min. Assays were initiated by simultaneously injecting 35 μl of a toxin-BBMV mixture and 35 μl of 0.5 M KCl into a cuvette in the spectrofluorimeter sample compartment. Light scattered at 90° from incidence was monitored for 120 s by using five measurements per s. In the HEPES sample, untreated BBMV were coinjected with 10 mM HEPES–Tris (pH 8.0) containing 0.1% BSA. The KCl sample contained untreated BBMV coinjected with 0.5 M KCl. (B) Proportion of scattered light signal change for toxin-treated S. frugiperda BBMV compared to KCl-treated BBMV, calculated from three independent assays. Five scattered light signal values (in volts) were extracted from raw data obtained at 1, 10, 20, 30, and 40 s in each assay, and then an average was calculated from these values. The standard deviations were calculated from the means of the three independent assays and are indicated as error bars.
Since 125I-Cry1Bb was able to bind specifically to S. frugiperda BBMV but was not insecticidal in feeding bioassays, pore formation assays were performed with iodinated Cry1Bb to determine if iodination of this toxin affected the membrane-permeabilizing capacities in vitro. We expected that iodinated Cry1Bb toxin would not increase membrane permeability in the light scattering assay, as iodinated Cry1Bb toxin activity is dramatically reduced in vivo compared with Cry1Bb toxin activity. However, there was no difference between the ability of native Cry1Bb and the ability of iodinated Cry1Bb (data not shown) to increase membrane permeability in S. frugiperda BBMV. The membrane-permeabilizing effects of iodinated Cry1Bb were not examined with S. exigua BBMV since the LC50 of this toxin is relatively high for this insect compared to S. frugiperda (Table 1). In addition, the membrane-permeabilizing effects of iodinated Cry1Ca were not examined with S. exigua and S. frugiperda BBMV since the activity of iodinated Cry1Ca was not affected by labeling, as determined by feeding bioassays (Table 1).
The tracings obtained in the S. exigua light scattering experiments are shown in Fig. 6A, and the relative changes are shown in Fig. 6B. The light scattering assays showed that Cry1Ac had no effect on S. exigua membrane permeability. The three other toxins increased the membrane permeability of S. exigua BBMV in the following increasing order: Cry1Bb, Cry1Ca, Cry1Fa. The membrane permeabilities induced by Cry1Bb, Cry1Ca, and Cry1Fa were significantly different from each other, and the effects of these toxins were significantly different from the effect of Cry1Ac.
FIG. 6.
Change in scattered light signal of toxin-treated S. exigua BBMV. (A) Direct traces of scattered light from toxin-treated BBMV. Data were collected as described in the legend to Fig. 4. (B) Proportion of scattered light signal change for toxin-treated BBMV compared to KCl-treated BBMV, calculated as described in the legend to Fig. 4.
The relationship between toxin concentration and the magnitude of light scattering was tested by using the most insecticidal toxin (Cry1Fa) and S. exigua BBMV. The data in Fig. 7 confirm that an increase in the concentration of Cry1Fa resulted in a decrease in scattered light. This dose-dependent response is an indirect measure of pore formation in S. exigua BBMV.
FIG. 7.
Relationship between Cry1Fa concentration and light scattering signal. S. frugiperda BBMV were preincubated with different concentrations of Cry1Fa. Experiments were conducted as described in the legend to Fig. 4.
DISCUSSION
The effects of iodination on Cry1Aa, Cry1Ab, Cry1Ac, Cry1Ba, Cry1Ca, Cry1D, and Cry1E have been reported previously (11, 15, 17, 18, 40). Only Cry1Ba was found to lose its toxicity and its ability to bind to Ostrinia nubilalis and Pieris brassicae when it was labeled with 125I (17). In this study, we found that the toxicities of Cry1Bb and Cry1Fa decreased significantly when they were labeled with 125I, while Cry1Ca retained its insecticidal activity when it was labeled with 125I (Table 1). Binding assays showed that labeling Cry1Fa with Na125I resulted in a significant decrease in binding to BBMV from both Spodoptera species (Fig. 2). However, the ability of Cry1Bb to bind to BBMV from either Spodoptera species was not altered by 125I labeling (Fig. 2). Cummings and Ellar (8) reported that chemical modification of 7 of 21 tyrosine residues in the Cry1Ac toxin reduced its binding and toxicity to M. sexta. Therefore, some tyrosine residues are critical to toxin conformation and receptor recognition. If Cry1Fa tyrosine residues that are involved in receptor binding are modified by attachment of 125I, the toxin binding and membrane-permeabilizing abilities of this toxin could be inhibited. Since 125I-Cry1Bb exhibited high-affinity binding and 126I-Cry1Bb was an effective pore former (data not shown), iodination of Cry1Bb tyrosine residues seems to alter a step in the intoxication process prior to interaction with the brush border membrane. Perhaps iodinated Cry1Bb is more susceptible to proteinases than noniodinated Cry1Bb is and is inactivated in the midgut lumen. These data also demonstrate that the effects of iodination on binding and toxicity of Cry1 toxins may be different.
Multiple binding sites for the B. thuringiensis Cry1 toxins are present in many insects (2, 13–15, 22, 32, 40). For example, Aranda et al. (2) reported that Cry1Ab and Cry1Ca recognized different binding sites on the brush border membrane of S. frugiperda. In P. xylostella, Cry1Ab and Cry1Fa recognized the same binding site, while Cry1Ca bound to a distinct site (14, 45). The results of the present study show that there are at least two high-affinity Cry1 binding sites in S. exigua and S. frugiperda; one site is for Cry1Ac, and the other site is for Cry1Bb and Cry1Ca. Cry1Fa competed not only with Cry1Ac but also with Cry1Bb and Cry1Ca in both Spodoptera species (Fig. 3). This suggests that Cry1Fa binds to both sites. One possible explanation for this duality is that the Cry1Fa toxin molecule has two binding determinants; one determinant might recognize a Cry1Ac receptor, while the other might bind to a Cry1Bb-Cry1Ca receptor. Indeed, de Maagd et al. (10) reported that domain II of Cry1Ab and Cry1Ac recognized the same binding molecule, while domain III of these toxins bound to a distinct molecule in S. exigua and M. sexta. Alternatively, Cry1Fa might have a binding determinant that recognizes only one binding site. Although our data show that Cry1Fa binding competes with binding of Cry1Ac, Cry1Bb, and Cry1Ca, they do not indicate whether these toxins bind to the same epitope or bind to different sites on the same molecule. Masson et al. (28) reported that purified Cry1Ac-binding aminopeptidase N from M. sexta has multiple binding sites for three Cry1A toxins. If both Cry1Ac and Cry1Ca receptor molecules had a separate binding site for Cry1Fa toxin, Cry1Fa might compete with Cry1Ac, Cry1Bb, and Cry1Ca binding by steric hindrance.
B. thuringiensis toxin binding to BBMV is a two-step process which involves (i) recognition of a receptor and (ii) irreversible association with the membrane (20, 24). The irreversible binding of toxins to BBMV has been reported to correlate with insecticidal potencies of B. thuringiensis toxins (20, 24). However, this positive relationship was not observed in our experiments. We found that Cry1Ac, Cry1Ca, and Cry1Bb irreversibly bound to BBMV from S. exigua and S. frugiperda (Fig. 4), although the toxicities of these toxins against both Spodoptera insects were different (Table 1). This phenomenon has been described for other insects. Lee et al. (23) reported that Cry1Ac irreversibly bound to susceptible and resistant strains of Heliothis virescens. Therefore, the relationship between irreversible binding and insecticidal activity requires further investigation.
Several workers have identified pore formation as a key step in B. thuringiensis Cry1 toxin action (12, 33). The results of our light scattering assays indicate that Cry1Bb, Cry1Ca, and Cry1Fa significantly alter the membrane permeability of S. exigua and S. frugiperda BBMV, whereas Cry1Ac does not (Fig. 5 and 6). These results are consistent with previous reports that BBMV permeability is related to Cry1 toxin insecticidal potencies (6, 25, 43). However, our results also indicate that the ability of a Cry1 toxin to increase membrane permeability in vitro does not always correspond to toxin activity measured in vivo. For example, Fig. 5 shows that there was no significant difference in the abilities of Cry1Ca and Cry1Bb to increase membrane permeability in S. frugiperda, but the LC50 of these two toxins differed fourfold for this insect (Table 1). In addition, we observed that while the activity of iodinated (cold-labeled) Cry1Bb decreases in vivo, this toxin retains the ability to bind to BBMV and increase membrane permeability in vitro (Table 1 and Fig. 2; data not shown). The data obtained with iodinated Cry1Bb suggest that iodination caused a structural modification of the toxin molecule that was detected by the insect bioassay but not by binding or light scattering assays. Wolfersberger et al. (43), in their analyses of mutant Cry1Aa toxins by a light scattering assay, noted a similar phenomenon. These authors commented that while the results of light scattering assays might reflect the true membrane-permeabilizing potencies of the toxins, feeding bioassay results not only reflect membrane-permeabilizing potencies but also include other factors, such as toxin resistance to midgut proteases.
In our binding experiments, Cry1Ac irreversibly bound to S. exigua and S. frugiperda BBMV (Fig. 2 to 4). However, Cry1Ac did not permeabilize BBMV from either Spodoptera species (Fig. 5 and 6). These results suggest that some membrane components are required for Cry1Ac-induced pore formation. We do not know what the Spodoptera membrane components are and why they do not facilitate Cry1Ac channel formation in Spodoptera BBMV. In M. sexta, components associated with the 120-kDa aminopeptidase modify Cry1Ac-induced pore formation. The 120-kDa Cry1A-binding aminopeptidase is isolated as a complex with other membrane proteins and lipids (26). When incorporated into planar lipid bilayers, this complex catalyzed channel formation by Cry1Ac with physical properties that were similar to those of whole BBMV proteins but distinct from those of the purified 115-kDa aminopeptidase (35; unpublished data). Other as-yet-unidentified membrane components seem to be critical factors that determine the ability of toxins to permeabilize the brush border membrane.
In conclusion, our results confirm that Cry1 toxin binding is necessary but not sufficient for toxicity (15, 42). The similarity of the data obtained from the Cry1 toxin binding experiments (as determined by saturation, homologous competition, and heterologous competition assays) performed with Spodoptera BBMV is striking. Our light scattering results support the conclusion that this assay could be a useful tool for identifying active toxins, with the caveat that in vivo toxicity is determined not just by membrane-permeabilizing capacity but also by other factors in the intoxication process.
ACKNOWLEDGMENTS
We thank Jim Baum, Bruce Tabashnik, and Michael Wolfersberger for critically reading a draft of the manuscript.
The toxin binding experiments were partially funded by Ecogen Inc. This research was also supported by the NRI Competitive Grants Program of the U.S. Department of Agriculture.
REFERENCES
- 1.Adang M J. Bacillus thuringiensis insecticidal crystal proteins: gene structure, action and utilization. In: Maramorosch K, editor. Biotechnology for biological control of pests and vectors. Boca Raton, Fla: CRC Press; 1991. pp. 3–24. [Google Scholar]
- 2.Aranda E, Sanchez J, Peferoen M, Guereca L, Bravo A. Interactions of Bacillus thuringiensis crystal proteins with the midgut epithelial cells of Spodoptera frugiperda (Lepidoptera: Noctuidae) J Invertebr Pathol. 1996;68:202–212. doi: 10.1006/jipa.1996.0087. [DOI] [PubMed] [Google Scholar]
- 3.Bai C, Degheele D, Jansens S, Lambert B. Activity of insecticidal crystal proteins and strains of Bacillus thuringiensis against Spodoptera exempta. J Invertebr Pathol. 1993;62:211–215. [Google Scholar]
- 4.Bohrova N, Cabrera M, Abarca C, Quintero R, Maciel A M, Brito R M, Hoisington D, Bravo A. Susceptibility of four lepidopteran maize pests to Bacillus thuringiensis CryI-type insecticidal toxins. J Econ Entomol. 1997;90:412–415. [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Carroll J, Ellar D J. An analysis of Bacillus thuringiensis δ-endotoxin action on insect-midgut-membrane permeability using a light-scattering assay. Eur J Biochem. 1993;214:771–778. doi: 10.1111/j.1432-1033.1993.tb17979.x. [DOI] [PubMed] [Google Scholar]
- 7.Chambers J A, Jelen A, Gilbert M P, Jany C S, Johnson T B, Gawron-Burke C. Isolation and characterization of a novel insecticidal crystal protein gene from Bacillus thuringiensis subsp. aizawai. J Bacteriol. 1991;173:3966–3976. doi: 10.1128/jb.173.13.3966-3976.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings C E, Ellar D J. Chemical modification of Bacillus thuringiensis activated δ-endotoxin and its effect on toxicity and binding to Manduca sexta midgut membranes. Microbiology. 1994;140:2737–2747. [Google Scholar]
- 9.Daum R. Revision of two computer programs for probit analysis. Bull Entomol Soc Am. 1970;16:10–15. [Google Scholar]
- 10.de Maagd R A, Kwa M S G, ven der Klei H, Yamamoto T, Schipper B, Vlak J, Stiekema W J, Bosch D. Domain III substitution in Bacillus thuringiensis delta-endotoxin CryIA(b) results in superior toxicity for Spodoptera exigua and altered membrane protein recognition. Appl Environ Microbiol. 1996;62:1537–1543. doi: 10.1128/aem.62.5.1537-1543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denolf P, Jansens S, Peferoen M, Degheele D, Van Rie J. Two different Bacillus thuringiensis delta-endotoxin receptors in the midgut brush border membrane of the European corn borer, Ostrinia nubilalis (Hubner) (Lepidoptera: Pyrallidae) Appl Environ Microbiol. 1993;59:1828–1837. doi: 10.1128/aem.59.6.1828-1837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.English L, Readdy T L, Bastian A E. Delta endotoxin-induced leakage of 86Rb+-K+ and H2O from phospholipid vesicles is catalyzed by reconstituted midgut membrane. Insect Biochem. 1991;21:177–184. [Google Scholar]
- 13.Escriche B, Martinez-Ramirez A C, Real M D, Silva F J, Ferre J. Occurrence of three different binding sites for Bacillus thuringiensis δ-endotoxins in the midgut brush border membrane of potato tuber moth, Pthorimaea operculella (Zeller) Arch Insect Biochem Physiol. 1994;26:315–327. [Google Scholar]
- 14.Ferre J, Real M D, Van Rie J, Jansens S, Peferoen M. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella is due to a change in a midgut membrane receptor. Proc Natl Acad Sci USA. 1991;88:5119–5123. doi: 10.1073/pnas.88.12.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garczynski S F, Crim J W, Adang M J. Identification of putative brush border membrane binding proteins specific to Bacillus thuringiensis delta-endotoxin by protein blot analysis. Appl Environ Microbiol. 1991;57:2816–2820. doi: 10.1128/aem.57.10.2816-2820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill S, Cowles E A, Pietrantonio P V. The mode of action of Bacillus thuringiensis delta-endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann C, Luthy P, Hutter R, Pliska V. Binding of the delta-endotoxin from Bacillus thuringiensis to brush-border membrane vesicles of the cabbage butterfly (Pieris brassicae) Eur J Biochem. 1988;173:85–91. doi: 10.1111/j.1432-1033.1988.tb13970.x. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann C, Vanderbruggen H, Höfte H, Van Rie J, Jansens S, Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins is correlated with the presence of high-affinity binding sites in the brush border membrane of target insect midguts. Proc Natl Acad Sci USA. 1988;85:7844–7848. doi: 10.1073/pnas.85.21.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Höfte H, Whiteley H R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989;53:242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ihara H, Kuroda E, Wadano A, Himeno M. Specific toxicity of δ-endotoxins from Bacillus thuringiensis to Bombyx mori. Biosci Biotechnol Biochem. 1993;57:200–204. doi: 10.1271/bbb.57.200. [DOI] [PubMed] [Google Scholar]
- 21.Knowles B H, Dow J A T. The crystal δ-endotoxins of Bacillus thuringiensis: models for their mechanisms of action on the insect gut. Bioessays. 1993;15:469–476. [Google Scholar]
- 22.Lee M K, Aguda R M, Cohen M B, Gould F L, Dean D H. Determination of binding of Bacillus thuringiensis delta-endotoxin receptors to rice stem borer midguts. Appl Environ Microbiol. 1997;63:1453–1459. doi: 10.1128/aem.63.4.1453-1459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee M K, Rajamohan F, Gould F, Dean D H. Resistance to Bacillus thuringiensis CryIA δ-endotoxins in a laboratory-selected Heliothis virescens strain is related to receptor alteration. Appl Environ Microbiol. 1995;61:3836–3842. doi: 10.1128/aem.61.11.3836-3842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang Y, Patel S S, Dean D H. Irreversible binding kinetics of Bacillus thuringiensis CryIA δ-endotoxins to gypsy moth brush border membrane vesicles is directly correlated to toxicity. J Biol Chem. 1995;270:24719–24724. doi: 10.1074/jbc.270.42.24719. [DOI] [PubMed] [Google Scholar]
- 25.Lorence A, Darszon A, Diaz C, Lievano A, Quintero R, Bravo A. δ-Endotoxins induce cation channels in Spodoptera frugiperda brush border membranes in suspension and in planar lipid bilayers. FEBS Lett. 1995;360:217–222. doi: 10.1016/0014-5793(95)00092-n. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Adang M J. Conversion of Bacillus thuringiensis CryIAc-binding aminopeptidase to a soluble form by endogenous phosphatidylinositol phospholipase C. Insect Biochem Mol Biol. 1996;226:33–40. [Google Scholar]
- 27.Luo K, Lu Y, Adang M J. A 106-kDa form of aminopeptidase is a receptor for Bacillus thuringiensis CryIC δ-endotoxin in the brush border membrane of Manduca sexta. Insect Biochem Mol Biol. 1996;26:783–791. [Google Scholar]
- 28.Masson L, Lu Y, Mazza A, Brosseau R, Adang M J. The CryIA(c) receptor purified from Manduca sexta displays multiple specificities. J Biol Chem. 1995;270:20309–20315. doi: 10.1074/jbc.270.35.20309. [DOI] [PubMed] [Google Scholar]
- 29.Moar W J, McCollum R C. Microbial control of tobacco budworm and beet armyworm in Alabama cotton. Beltwide Cotton Conf Proc. 1996;2:1048–1050. [Google Scholar]
- 30.Moar W J, Pusztai-Carey M, Van Fassen H, Bosch D, Frutos R, Rang C, Luo K, Adang M J. Development of Bacillus thuringiensis CryIC resistance by Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) Appl Environ Microbiol. 1995;61:2086–2092. doi: 10.1128/aem.61.6.2086-2092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller-Cohn J, Chaufaux J, Buisson C, Gilois N, Sanchis V, Lereclus D. Spodoptera littoralis (Lepidoptera: Noctuidae) resistance to CryIC and cross-resistance to other Bacillus thuringiensis toxins. J Econ Entomol. 1996;89:791–797. [Google Scholar]
- 32.Oddou P, Hartmann H, Geiser M. Immunologically unrelated Heliothis sp. and Spodoptera sp. midgut membrane-proteins bind Bacillus thuringiensis CryIA(b) delta-endotoxin. Eur J Biochem. 1993;212:145–150. doi: 10.1111/j.1432-1033.1993.tb17644.x. [DOI] [PubMed] [Google Scholar]
- 33.Peyronnet O, Vachon V, Brousseau R, Baines D, Schwartz J-L, Laprade R. Effect of Bacillus thuringiensis toxins on the membrane potential of lepidopteran insect midgut cells. Appl Environ Microbiol. 1997;63:1679–1684. doi: 10.1128/aem.63.5.1679-1684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajamohan F, Cotrill J A, Gould F, Dean D H. Role of domain II, loop 2 residues of Bacillus thuringiensis CryIAb δ-endotoxin in reversible and irreversible binding to Manduca sexta and Heliothis virescens. J Biol Chem. 1996;271:2390–2396. doi: 10.1074/jbc.271.5.2390. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz J-L, Lu Y J, Soehnlein P, Brousseau R, Masson L, Laprade R, Adang M J. Ion channels formed in planar lipid bilayers by Bacillus thuringiensis toxins in the presence of Manduca sexta midgut receptors. FEBS Lett. 1997;412:270–276. doi: 10.1016/s0014-5793(97)00801-6. [DOI] [PubMed] [Google Scholar]
- 36.Strizhov N, Keller M, Mathur J, Koncz-Kalman Z, Bosch D, Prudovsky E, Schell J, Sneh B K C, Zilberstein A. A synthetic cryIC gene, encoding a Bacillus thuringiensis δ-endotoxin, confers Spodoptera resistance in alfalfa and tobacco. Proc Natl Acad Sci USA. 1996;93:15012–15017. doi: 10.1073/pnas.93.26.15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabashnik B E. Evolution of resistance to Bacillus thuringiensis. Annu Rev Entomol. 1994;39:47–79. doi: 10.1146/annurev.ento.54.110807.090518. [DOI] [PubMed] [Google Scholar]
- 38.Tabashnik B E, Finson N, Groeters F R, Moar W J, Johnson M W, Luo K, Adang M J. Reversal of resistance to Bacillus thuringiensis in Plutella xylostella. Proc Natl Acad Sci USA. 1994;91:4120–4124. doi: 10.1073/pnas.91.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabashnik B E, Malvar T, Liu Y-B, Finson N, Boethakur D, Shin B S, Park S-H, Masson L, DeMaagd R, Bosch D. Cross-resistance of diamondback moth implies altered interactions with domain II of Bacillus thuringiensis toxins. Appl Environ Microbiol. 1996;62:2839–2844. doi: 10.1128/aem.62.8.2839-2844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Receptors on the brush border membrane of the insect midgut as determinants of the specificity of Bacillus thuringiensis delta-endotoxins. Appl Environ Microbiol. 1990;56:1378–1385. doi: 10.1128/aem.56.5.1378-1385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Rie J, McGaughey W H, Johnson D E, Barnett B D, Van Mellaert H. Mechanism of insect resistance to the microbial insecticide Bacillus thuringiensis. Science. 1990;247:72–74. doi: 10.1126/science.2294593. [DOI] [PubMed] [Google Scholar]
- 42.Wolfersberger M G. The toxicity of two Bacillus thuringiensis δ-endotoxins to gypsy moth larvae is inversely related to the affinity of binding sites on midgut brush border membranes for the toxins. Experientia. 1990;46:475–477. doi: 10.1007/BF01954236. [DOI] [PubMed] [Google Scholar]
- 43.Wolfersberger M G, Chen X J, Dean D H. Site-directed mutations in the third domain of Bacillus thuringiensis δ-endotoxin Cry1Aa affect its ability to increase the permeability of Bombyx mori midgut brush border membrane vesicles. Appl Environ Microbiol. 1996;62:279–283. doi: 10.1128/aem.62.1.279-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfersberger M G, Luthy P, Maurer A, Parenti P, Sacchi V F, Giordana B, Hanozet G M. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae) Comp Biochem Physiol A Comp Physiol. 1987;86:301–308. [Google Scholar]
- 45.Wright D J, Iqbal M, Granero F, Ferre J. A change in a single midgut receptor in the diamondback moth (Plutella xylostella) is only in part responsible for field resistance to Bacillus thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai. Appl Environ Microbiol. 1997;63:1814–1819. doi: 10.1128/aem.63.5.1814-1819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto T, Powell G K. Bacillus thuringiensis crystal proteins: recent advances in understanding its insecticidal activity. In: Kim L, editor. Advanced engineered pesticides. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 3–42. [Google Scholar]