Abstract
Exposure to particulate matter (PM) is related to various respiratory diseases, and this affects the respiratory immune system. Alveolar macrophages (AMs), which are defenders against pathogens, play a key role in respiratory inflammation through cytokine production and cellular interactions. Coconut oil demonstrates antioxidant and anti-inflammatory properties, and it is consumed worldwide for improved health. However, reports on the protective effects of coconut oil on the PM-induced respiratory immune system, especially in AMs, are limited. In this study, we generated artificial PM (APM) with a diameter approximately of 30 nm by controlling the temperature, and compared its cytotoxicity with diesel exhaust particles (DEP). We also investigated the antioxidant and anti-inflammatory effects of coconut oil in APM– and DEP–stimulated AMs, and the underlying molecular mechanisms. Our results showed that APM and DEP had high cytotoxicity in a dose-dependent manner in AMs. In particular, APM or DEP at 100 μg/mL significantly decreased cell viability (p < 0.05) and significantly increased oxidative stress markers such as reactive oxygen species (p < 0.01); the GSSH/GSH ratio (p < 0.01); and cytokine production, such as tumor necrosis factor-α (p < 0.001), interleukin (IL)-1β (p < 0.001), and IL-6 (p < 0.001). The expression of the genes for chemokine (C-X-C motif) ligand-1 (p < 0.05) and monocyte chemoattractant protein-1 (p < 0.001); and the proteins toll-like receptor (TLR) 4 (p < 0.01), mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (p < 0.001), p38 (p < 0.001); and extracellular receptor-activated kinase (p < 0.001), were also upregulated by PM. These parameters were reversed upon treatment with coconut oil in APM– or DEP–stimulated AMs. In conclusion, coconut oil can reduce APM– or DEP–induced inflammation by regulating the TLR4/MAPK pathway in AMs, and it may protect against adverse respiratory effects caused by PM exposure.
Exposure to particulate matter (PM) is related to various respiratory diseases, and this affects the respiratory immune system. Alveolar macrophages (AMs), which are defenders against pathogens, play a key role in respiratory inflammation through cytokine production and cellular interactions. Coconut oil demonstrates antioxidant and anti-inflammatory properties, and it is consumed worldwide for improved health. However, reports on the protective effects of coconut oil on the PM-induced respiratory immune system, especially in AMs, are limited. In this study, we generated artificial PM (APM) with a diameter approximately of 30 nm by controlling the temperature, and compared its cytotoxicity with diesel exhaust particles (DEP). We also investigated the antioxidant and anti-inflammatory effects of coconut oil in APM– and DEP–stimulated AMs, and the underlying molecular mechanisms. Our results showed that APM and DEP had high cytotoxicity in a dose-dependent manner in AMs. In particular, APM or DEP at 100 μg/mL significantly decreased cell viability (p < 0.05) and significantly increased oxidative stress markers such as reactive oxygen species (p < 0.01); the GSSH/GSH ratio (p < 0.01); and cytokine production, such as tumor necrosis factor-α (p < 0.001), interleukin (IL)-1β (p < 0.001), and IL-6 (p < 0.001). The expression of the genes for chemokine (C-X-C motif) ligand-1 (p < 0.05) and monocyte chemoattractant protein-1 (p < 0.001); and the proteins toll-like receptor (TLR) 4 (p < 0.01), mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (p < 0.001), p38 (p < 0.001); and extracellular receptor-activated kinase (p < 0.001), were also upregulated by PM. These parameters were reversed upon treatment with coconut oil in APM– or DEP–stimulated AMs. In conclusion, coconut oil can reduce APM– or DEP–induced inflammation by regulating the TLR4/MAPK pathway in AMs, and it may protect against adverse respiratory effects caused by PM exposure.
Keywords: artificial particulate matter, diesel exhaust particulates, coconut oil, oxidative stress, inflammation, mitogen-activated protein kinase, alveolar macrophage
1. Introduction
Particulate matter (PM), which is a mixture of solid and liquid particles suspended in the air, is an important component of air pollution [1]. Currently, the major sources of environmental PM include vehicle and industrial emissions, waste incineration, power plants, and geological materials [2,3]. PM can be divided into PM 1 (aerodynamic diameter < 1 µm), PM 2.5 (aerodynamic diameter < 2.5 µm), and PM 10 (aerodynamic diameter < 10 µm) [4,5], depending on the particle size. According to the Global Burden of Disease assessment, approximately 4.24 million premature deaths worldwide in 2015 could be attributed to exposure to PM [6]. Toxicological and epidemiological studies have shown that PM exposure can cause human diseases, such as respiratory and cardiovascular diseases [7], since these small particles can enter the respiratory tract and circulate into blood vessels or capillaries [8]. In recent years, increasing attention has been paid to the relationship between PM and public health hazards. Researchers have gradually focused on natural compounds that protect against PM, to boost or strengthen the healthy immune system after PM exposure [9,10].
Macrophages play an important role in the innate immune system [11], and they participate in host defense by engulfing and digesting pathogens [12]. In the respiratory system, alveolar macrophages (AMs) maintain clear air spaces by engulfing foreign materials and minimize the exposure of other airway cells to these substances [13]. Once inhaled and deposited into the lung, PM-pigmented AMs can induce oxidative stress and local inflammation by releasing mediators such as interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α [14] via the activation of stress kinases, such as mitogen-activated protein kinase (MAPK), which is a key signaling pathway that regulates a wide variety of cellular processes, such as proliferation, differentiation, apoptosis, and stress responses under both normal and pathological conditions [15,16,17]. Similar to these observations, our previous study showed that diesel exhaust particulates (DEP) induce endoplasmic reticulum (ER) stress-mediated neutrophil dominant lung inflammation through increasing ER stress markers such as C/EBP homologous protein, and binding immunoglobulin protein, TNF-α, and IL-1β in bronchoalveolar lavage fluids of the DEP–induced murine model and in DEP–stimulated AMs. We also found that AMs play a crucial role in the lung disease process through observing lung inflammation accompanied with the infiltration of DEP–pigment AMs in hematoxylin and eosin-stained lung tissue [18]. Additionally, PM can induce M2 macrophage polarization-enhancing lung diseases. Yan Jiang et al. have reported that PM2.5 induced airway inflammation, compromised lung function, emphysematous lesions, and deleterious airway remodeling in chronic obstructive pulmonary disease (COPD) through inducing the M2 polarization of AMs and upregulating transforming growth factor-β, matrix metallopeptidase (MMP)-9, and MMP-12 [19]. Currently, there is a growing interest in the potential roles of macrophages in the progression of lung diseases such as asthma, COPD, lung fibrosis, and cancer [11,12,19,20].
Coconut is derived from Cocos nucifera L., and the coconut oil (65–75%) obtained [21] is widely used in the food and other industries [22,23]. Traditionally, coconut oil has been used as a healthy food, and it has also been utilized for cosmeceutical purposes [24]. Previous studies have reported on the benefits of coconut oil, including several biological activities in vivo, such as antioxidant, anti-inflammatory, anticancer, analgesic, antimicrobial, and antipyretic properties [25,26,27]. Coconut oil is considered a saturated fat because it contains more than 90% saturated fatty acids; it also contains high amounts of medium-chain triacylglycerols, which are effective against the development of cardiovascular and inflammatory diseases [28,29], as well as antioxidant compounds. Luiz Henrique C. Vasconcelos [30] demonstrated that coconut oil supplementation reverses oxidative stress-induced peribronchial inflammatory infiltrate, epithelial hyperplasia, smooth muscle thickening, and hypercontractility, through its interactions with the nitric oxide (NO) pathway in guinea pigs. Although coconut oil is a potential candidate for adjuvant therapy with several biological activities, such as antioxidant and anti-inflammatory activities, to date, there are no reports on the anti-inflammatory and antioxidant activities of coconut oil in PM-stimulated AMs. Thus, we established an in vitro model of PM-stimulated AMs with inflammatory and oxidative stress responses by PM exposure and investigated the effect of coconut oil in an in vitro model.
In the present study, we generated artificial PM (APM) in the laboratory [31] and compared the toxicity of AMs with APM and DEP. In addition, we assessed the protective effects of coconut oil on PM-induced oxidative stress and inflammatory responses in AMs, and investigated its mechanism of action.
2. Materials and Methods
2.1. APM Generation
Graphite was selected to produce APM generated from a high-voltage-based soot generator (DNP Digital 3000, Palas GmbH, Germany). It operates on the principle that a spark discharge produces nanoscale particles with consistent concentrations and a high yield. The generator maintains a high voltage of 2500 V and 700 Hz between the two graphite electrodes, using co-mixed gases (1:2) with pure argon (99.999%; 5 L/min) and air (99.999%; 10 L/min). The use of these base gases yielded pure black without volatile impurities. The jump spark rips large amounts of graphite material from the electrodes at high temperatures (Lindberg/Blue M™ 1200 °C split-hinge tube furnaces, Thermo Scientific, Waltham, MA, USA). The APM was sampled using Teflon filters with a pore size of 2 µm and a diameter of 47 mm (R2PJ047, PALL Corporation, Port Washington, NY, USA). A high concentration can result in the coagulation of these nanoparticles into agglomerates. The generated aerosol distribution was quite similar to the distribution of diesel soot particles from the furnace process [31].
2.2. Preparation of APM, DEP, and Coconut Oil
Stock suspensions with a concentration of 2 mg/mL of APM and DEP (SRM 2975; National Institute of Standards and Technology, Gaithersburg, MD, USA) were prepared in the growth medium. The suspensions were ultrasonicated using an ultrasonic cleaner (40 kHz, Branson, 1800 Cleaner, Bransonic; St. Louis, MO, USA) for 30 min and an ultrasonicate (20 kHz, VC 505, Vibra-Cell™ Ultrasonic Liquid Processors, Sonics & Materials, Inc., Newtown, CT, USA) for 30 min. Surfactant-free coconut oil in distilled water (DW) emulsion, donated by Dr. Mincheol Chu from GREENSOL Co., Ltd., was prepared in 0.5% coconut oil using high-speed agitation, high pressure, and ultrasonic dispersion methods [32]. Coconut oil has the smallest particle size (0.07~0.3 μm) and a uniform distribution, and it was the most stable during a 7 days stability test during the emulsification process [32].
2.3. Measurement of Organic Carbon/Elemental Carbon (OC/EC)
A series of sucrose solutions were used to calibrate the equipment prior to use (N = 5, R2 = 1.00). Quartz fiber filters (QMA filter 25 mm, Whatman; Little Chalfont, Buckinghamshire, UK) were pre-baked at 650 °C for 8 h to eliminate organic pollutants and stored in baked aluminum foil before sampling. A 1 cm2 punch was removed from the quartz filter after sampling, to analyze the carbonaceous components in APM/DEP using an OC-EC Aerosol Analyzer Model 5 (Sunset Laboratory, Beaverton, OR, USA) according to the revision 8.054 reference method. To avoid the premature evolution of light-absorbing carbon in the OC and EC, the IMPROVE-A temperature protocol was adopted for the OC and EC analysis [33].
2.4. Cell Culture and APM, DEP, Coconut Oil, and N-Acetylcysteine (NAC) Treatments
A murine alveolar macrophage (MH-S) cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained in a Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin solution (Gibco, Grand Island, NY, USA) at 37 °C in a humidified atmosphere of 5% CO2. To optimize the treatment concentration, cells were treated with varying concentrations of APM and DEP (50, 100, 250, 500, 1000, and 2000 μg/mL), coconut oil (0.005, 0.0125, 0.025, and 0.05%), and NAC (1, 5, 10, 15, and 20 mM). In addition, cells were treated with DW as a vehicle control for coconut oil, and cytotoxicity was evaluated. Next, to investigate the protective effect of coconut oil in PM-stimulated MH-S, cells were pretreated with coconut oil at 0.0125 or 0.025% (v/v) 6 h before adding APM or DEP (100 μg/mL); 6 h later, the cells were washed twice with Dulbecco’s phosphate-buffered saline (PBS) and harvested. The cells were treated with antioxidant NAC (10 mM) for 2 h (Sigma-Aldrich, St. Louis, MO, USA).
2.5. Cell Viability Assay
The cytotoxicity of APM, DEP, and coconut oil was assessed using the 3–(4,5–dimethylthiazol–2–yl)–2,5–diphenyltetrazolium bromide (MTT; Sigma-Aldrich) assay [34]. After the coconut oil, APM, and DEP treatments, the cells were incubated with 100 μL of 1 mg/mL MTT for 3 h at 37 °C. The medium was discarded, and 100 μL dimethyl sulfoxide (Junsei Chemical Co.; Tokyo, Japan) was added to each well to dissolve the formazan crystals. Absorbance was measured on a microplate reader (BioTek; Synergy Mx, Winooski, VT, USA) at 570 nm (with a reference wavelength of 630 nm) to obtain a sample signal (i.e., OD570-OD630 for normalization data). The cytotoxicity of NAC was measured using a lactate dehydrogenase (LDH) cytotoxicity detection kit (TaKaRa Bio, Kyoto, Japan) according to the manufacturer’s protocol. Briefly, after NAC treatment, the supernatant was harvested and centrifuged at a maximum speed for 10 min. To determine the LDH activity in the supernatant, 100 μL supernatant was transferred into the wells of an optically clear 96-well plate, and reaction mixtures containing the catalyst and dye solution (Lod tetrazolium chloride and sodium lactate) were added into each well. The cells were then incubated for 30 min at 15–25 °C in the dark. Absorbance was measured at 490 nm, and the background control was measured at 600 nm using a microplate reader (BioTek, Winooski, VT, USA). The control group was defined as 100%.
2.6. Measurement of Reactive Oxygen Species (ROS) Level
To quantify intracellular ROS production, the cells were incubated for 30 min at 37 °C in serum-free RPMI 1640 medium containing 10 μmol/L 2′,7′-dichlorofluorescein diacetate (DCF-DA) (Thermo Fisher Scientific). The cells were washed with PBS, and the DCF-DA intensity in the cells was immediately measured at 495 nm (excitation)/529 nm (emission) using a microplate reader. ROS production in the cells was represented as a percentage of DCF-DA intensity relative to cell viability in each well, which was defined as 100%.
2.7. Intracellular Glutathione (GSH) and Nicotinamide Adenine Dinucleotide Phosphate (NADP+)/Reduced Form (NADPH) Levels
Total cellular GSH and oxidized GSH (glutathione disulfide, GSSG) were measured using a GSH fluorometric assay kit (BioVision; Milpitas, CA, USA). Cells were prepared via deproteinization; subsequent reagents were added, following the manufacturer’s instructions; and fluorescence was measured at excitation/emission = 340/420 nm. Fluorescence values from the standards and the samples were subtracted from the blank control values, and the GSH and GSSG contents were calculated via interpolation from the standard curve. Results were normalized to 106 cells [35]. NADP+ and NADPH levels were analyzed using an NADP+/NADPH Quantification Colorimetric Kit (BioVision, Milpitas, CA, USA). Briefly, cells were washed with cold PBS and lysed with 800 μL NADP+/NADPH extraction buffer and were treated according to the manufacturer’s instructions. After centrifugation, the supernatant was collected for the measurement of NADP+/NADPH using a UV spectrophotometer at 450 nm.
2.8. Measurement of Cytokine Levels
At 12 h after APM and DEP stimulation, the levels of secreted IL-1β, IL-6, and TNF-α were measured in the cell culture supernatants. Quantification was performed using the respective ELISA kits (Invitrogen, Waltham, MA, USA), according to the manufacturer’s instructions.
2.9. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands), and cDNA was synthesized from total RNA using the GoScript™ Reverse Transcription System (Promega, Madison, WI, USA). The cDNA was amplified with the QuantStudio5 real-time PCR system, using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The PCR primers used are listed in Table 1. The target gene chemokine (C-S-C motif) ligand (CXCL)-1, monocyte chemoattractant protein (MCP)-1, and NADPH dehydrogenase (quinone) (NQO)-1 expression levels were normalized to that of β-actin, calculated using the 2−ΔΔCt method, and expressed as fold changes. The normalized value of the target gene expression level in the control group was set as 1.
Table 1.
The forward and reverse primer.
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| CXCL–1 | 5′-AACCGAAGTCATAGCCACAC-3′ | 5′-GTTGGATTTGTCACTGTTCAGC-3′ |
| MCP–1 | 5′-TGATCCCAATGAGTAGGCTGGAG-3′ | 5′-ATGTCTGGACCCATTCCTTCTTG-3′ |
| NQO–1 | 5′-GGTGATATTTCAGTTCCCATTG-3′ | 5′-ACTCTCTCAAACCAGCCTTTC-3′ |
| β–Actin | 5′-GGCACCACACCT TCTACAATG-3′ | 5′-GGGGTGTTGAAGGTCTCAAAC-3′ |
2.10. Western Blotting Analyses
Cells were lysed in radioimmunoprecipitation assay buffer (Thermo Fisher Scientific) with a protease inhibitor cocktail. Protein concentrations were determined using Bradford reagent (Bio-Rad, Hercules, CA, USA). Proteins were separated using SDS-PAGE at 120 V for 90 min, then transferred to a polyvinylidene difluoride membrane (Bio-Rad) at 250 mA for 90 min via wet transfer. Nonspecific binding was blocked by incubating the membrane in 5% non-fat dry milk in Tris-buffered saline with Tween 20 (25 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, and 0.1% Tween 20) for 1 h, followed by an overnight incubation at 4 °C with antibodies against anti-phospho-c-Jun N-terminal kinase (JNK), anti-JNK, anti-phospho-extracellular signal-regulated kinase (ERK), anti-ERK, anti-phospho-p38, anti-p38 (all from Abcam, Cam-bridge, MA), toll-like receptor (TLR) 4 (Invitrogen, Carlsbad, CA, USA), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). β-actin was used as the internal reference. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Cell Signaling Technology, Beverly, MA, USA) was used to detect antibody binding, which was visualized using an iBright CL1000 imaging system (Thermo Fisher Scientific) after treatment with an enhanced chemiluminescence reagent (Thermo Fisher Scientific). The densitometric analysis of each band was performed using Bright CL1000 image software (Thermo Fisher Scientific). For the quantification of specific bands, a square of the same size was drawn around each band for density measurement, and the value was adjusted to the background density near that band. The results were expressed as the relative ratio of the target protein to the reference protein, with the relative ratio of the target protein of the control group set to 1.
2.11. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA). Data are expressed as mean ± SD. The Shapiro–Wilk test was used to assess the normality of the data. A one-way analysis of variance was performed, followed by the Tukey or Dunnett test to perform statistical comparisons. The comparisons between the two groups were performed using the t-test for paired variables, or the Mann–Whitney U test for unpaired variables. The statistical significance was set at p < 0.05.
3. Results
3.1. Characterization of DEP and APM
Table 2 presents the characterization of APM and DEP. The DEP and APM particle sizes were less than 1 μm in size (mostly <600 nm) and about 30 nm, respectively [36]. DEP was also roughly spherical and was composed of small irregular agglomerates [36]. APM exhibited typical diesel soot particle morphologies, with nearly spherical and irregular carbonaceous particles [31]. In the Raman spectra of DEP, a D peak at 1340 cm−1 and a G peak at 1600 cm−1 is visualized [37]. Similar to DEP, in the Raman spectra of APM, two typical overlapping peaks are commonly shown [31,36]. Moreover, we measured the OC/EC ratio values of DEP and APM. OC and EC in PM are widely known for their toxicity [38], and they are linked to adverse effects on human health [39]. In this study, the OC/EC ratio value of APM was similar to the level of DEP. These physical characterizations and the OC/EC ratio of DEP and APM were commonly observed, and we used DEP and APM as PM.
Table 2.
Characterization of DEP and APM.
| DEP (a) | APM | |
|---|---|---|
| Particle size (nm) | mostly <600 nm [36] | approximately 30 nm [31] |
| Pore diameter (nm) | 4−35 nm (b) | approximately 30 nm [31] |
| Morphology | small irregular agglomerates and roughly spherical [36] | spherical and irregular carbonaceous particles [31] |
| Raman spectrum | D peak: 1340, G peak: 1600 [37] | D peak: 1340, G peak: 1600 [31] |
| OC/EC ratio values (c) | 1.9 ± 0.001 | 2.2 ± 0.001 |
(a) Diesel exhaust particles, DEP (SRM2975); (b) Certified pore diameter in SRM2975; (c) Ratios of organic carbon (OC)/elemental carbon (EC).
3.2. PM Induces Oxidative Stress-Mediated Cell Damage in AMs
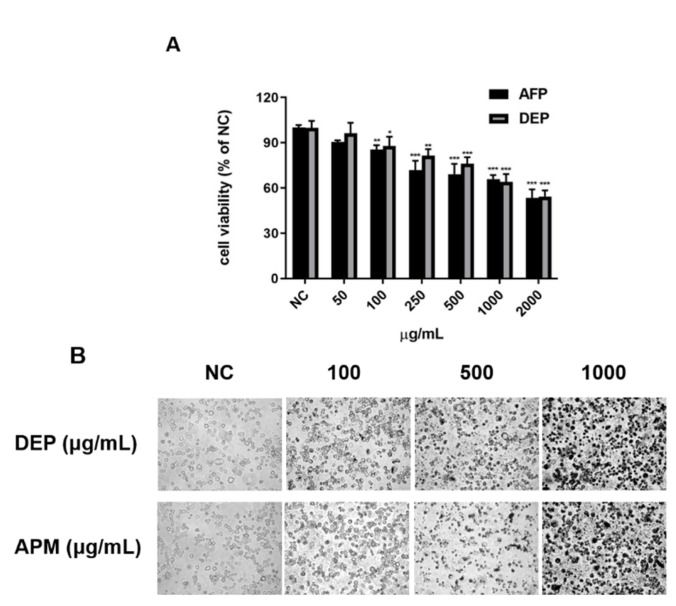
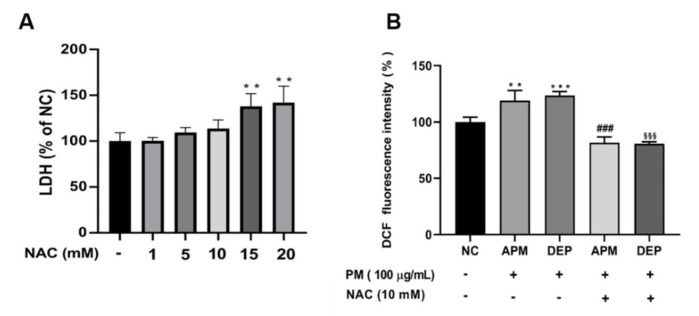
Exposure to PM induces cellular damage in the oxidative stress-mediated respiratory immune system [40,41,42,43]. First, we measured cell viability to determine the concentrations of PM, such as APM and DEP, at which cell damage occurred. Our results showed that PM, such as APM and DEP, significantly reduced cell viability from a minimum concentration of 100 μg/mL in AMs (Figure 1A); therefore, we selected 100 μg/mL APM and DEP. Morphological examinations conducted under an optical microscope indicated that several concentrations of APM/DEP induced AM death in 6 h (Figure 1B). Next, to determine whether PM-induced cell damage was mediated via oxidative stress, we evaluated cytotoxicity and ROS production via an LDH activity assay and DCF-DA fluorescence intensity measurement, respectively, after NAC treatment in PM-stimulated AMs. Compared with the untreated control, the LDH level gradually increased within AMs with increasing concentrations of NAC. We selected the non-toxic concentration of NAC at 10 mM and confirmed that PM induced cell damage by mediating ROS production in AMs, which was mitigated by NAC. Our results showed that PMs, such as APM and DEP, induce ROS-mediated cytotoxicity in AMs, and that NAC significantly inhibited the PM-induced increase of ROS production in AMs (Figure 2).
Figure 1.
Cytotoxicity of artificial particulate matter (APM) and diesel exhaust particles (DEP) in alveolar macrophages (MH-S). Negative controls (NC) are untreated samples. MH-S was treated with APM and DEP of different concentrations for 6 h. (A) Cell viability was measured using the 3–(4,5–dimethylthiazol–2–yl)–2,5–diphenyltetrazolium bromide (MTT) assay in the APM– and DEP–stimulated MH-S cells. (B) Morphological changes in MH-S by exposure to APM/DEP. Data are presented as the mean ± SD (n = 6 per group). * p < 0.05, ** p < 0.01, or *** p < 0.001 vs. NC.
Figure 2.
Artificial particulate matter (APM) and diesel exhaust particles (DEP) induce oxidative stress in alveolar macrophages (MH−S). (A) Cytotoxicity of N−Acetyl Cysteine (NAC). (B) Reactive oxygen species level was measured using 2′,7′−dichlorofluorescein diacetate (DCF−DA) staining. Negative controls (NC) are untreated samples. MH−S was pretreated with NAC for 3 h and was stimulated with 100 μg/mL APM or DEP for 3 h. Data are presented as the means ± SD (n = 4 per group). ** p < 0.01, or *** p < 0.001 vs. NC; ### p < 0.001 vs. APM; §§§ p < 0.001 vs. DEP group.
3.3. Effect of Coconut Oil on Cytotoxicity and Oxidative Stress in PM-Stimulated AMs
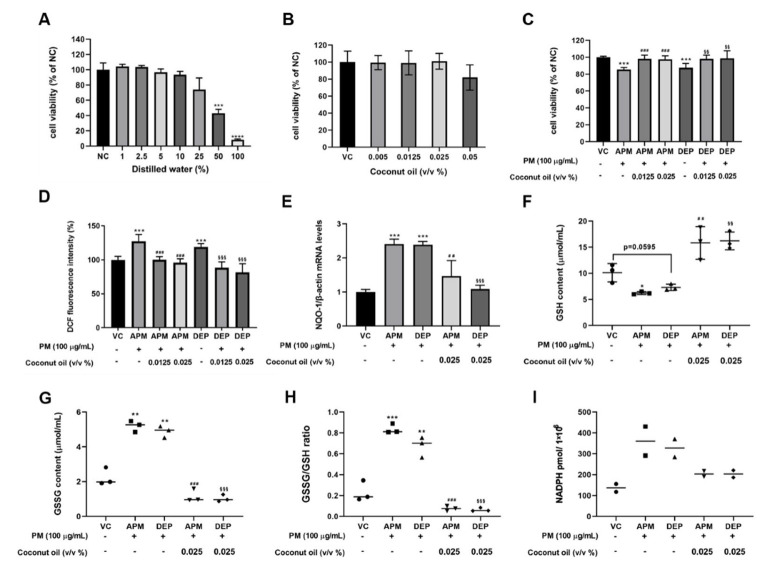
To investigate the effect of coconut oil on cytotoxicity, and its antioxidant activities in PM-stimulated AMs, we determined the concentrations of DW as a vehicle control, and of coconut oil without cytotoxicity. Our results showed that there was no change in cell viability up to 10% DW in complete medium (Figure 3A). Since there was no change in cell viability, up to 0.05% coconut oil in coconut oil-treated AMs (Figure 3B), and 0.0125% and 0.025% concentrations were chosen, for testing the efficacy of coconut oil in the in vitro study. Pretreatment with coconut oil reversed the PM-induced decrease in cell viability and the increase in oxidative stress in PM-stimulated AMs (Figure 3C,D). Furthermore, the mRNA level of NQO-1, an oxidative stress gene, was significantly increased within AMs by PM stimulation. Coconut oil pretreatment significantly decreased the PM-induced increase of NQO-1 mRNA levels (Figure 3E). Moreover, we confirmed that the PM-induced decrease of GSH levels was increased by coconut oil pretreatment in AMs (Figure 3F). The GSSG level, the ratio of GSSG-to-GSH, and the NADPH content, as indicators of cellular oxidative stress, were also reduced in PM-stimulated AMs pretreated with coconut oil (Figure 3G–I). These results indicate that PM, such as APM and DEP, induce oxidative stress-mediated cell damage in AMs, and that coconut oil can alleviate oxidative stress-mediated responses by upregulating GSH levels in PM-stimulated AMs.
Figure 3.
Effects of coconut oil on oxidative stress in artificial particulate matter (APM) and diesel exhaust particles (DEP)−stimulated alveolar macrophages (MH−S). (A,B) Cytotoxicity assessment of distilled water (DW) and coconut oil the 3–(4,5–dimethylthiazol–2–yl)–2,5–diphenyltetrazolium bromide (MTT) assay. (C) Cytotoxicity assessment of coconut oil in APM and DEP−stimulated MH−S. (D) Reactive oxygen species (ROS) production was measured using 2′,7′−dichlorofluorescein diacetate (DCF−DA) staining. Data represent the mean ± SD (n = 8 per group). (E) NADPH dehydrogenase (quinone) (NQO1) gene level was measured using real−time PCR. (F) Glutathione (GSH), (G) glutathione disulfide (GSSG), (H) GSSG/GSH ratio, and (I) Nicotinamide adenine dinucleotide phosphate (NADPH) levels in MH−S. Negative controls (NC) are untreated samples. Vehicle control (VC) was treated with cell medium including 10% DW. Data are presented as the means ± SD of three independent experiments. * p < 0.05, ** p < 0.01 or *** p < 0.001, **** p < 0.0001 vs. NC or VC; ## p < 0.01 or ### p < 0.001 vs. APM group; §§ p < 0.01 or §§§ p < 0.001 vs. DEP group.
3.4. Coconut Oil Suppresses Inflammatory Genes and Protein Expression in PM-Stimulated AMs
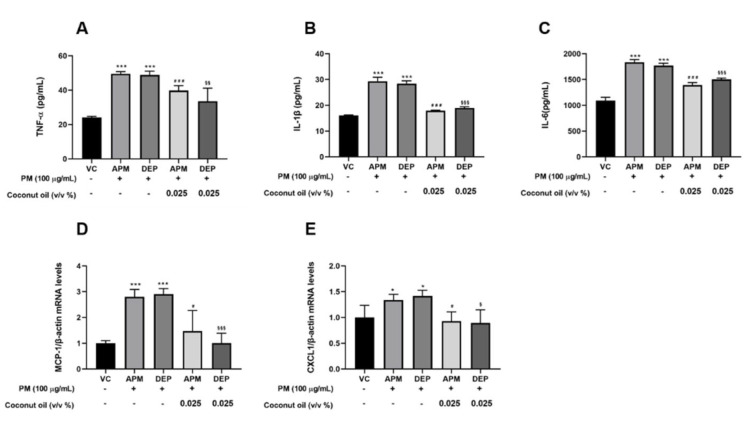
Coconut oil has anti-inflammatory activity against various diseases [25,26,27,28,29,30]. To examine whether coconut oil could regulate inflammatory responses in PM-stimulated AMs, we evaluated the levels of inflammatory genes, such as MCP-1 and CXCL-1, and inflammatory proteins, such as TNF-α, IL-1β, and IL-6, using qRT-PCR and ELISA assays, respectively. Our results showed that APM and DEP stimulation significantly increased the levels of MCP-1 and CXCL-1 genes, as well as those of the TNF-α, IL-1β, and IL-6 proteins in AMs. These levels were significantly reduced when treatment with coconut oil was used (Figure 4). These findings indicate that coconut oil potently inhibits inflammatory responses via the regulation of PM-induced inflammatory mediators in AMs.
Figure 4.
Effects of coconut oil on artificial particulate matter (APM) and diesel exhaust particles (DEP)−induced inflammation in alveolar macrophages (MH−S). Protein levels of (A) tumor necrosis factor−α (TNF−α), (B) interleukin (IL)−1β, and (C) IL−6 in MH−S were determined using enzyme−linked immunosorbent assay (ELISA). (D,E) Relative gene expression of inflammatory factors in APM/DEP–stimulated MH−S cells. Vehicle control (VC) was treated with cell medium including 10% distilled water. All experiments were independently performed in triplicate. * p < 0.5 or *** p < 0.001 vs. VC; # p < 0.05 or ### p < 0.001 vs. APM; § p < 0.05 or §§ p < 0.01, §§§ p < 0.001 vs. DEP.
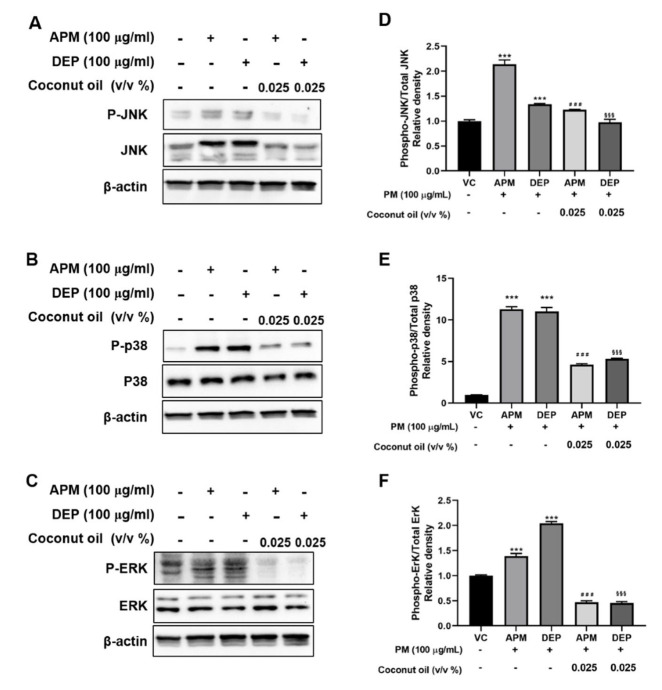
3.5. Coconut Oil Attenuates PM-Induced MAPK Signaling Activation in AMs
PM has been reported to activate intracellular MAPK signaling pathways and induce metabolic changes in human bronchial epithelial cells and mouse macrophages [17,44,45]. MAPK is an essential regulator of inflammatory mediators, including proinflammatory cytokines [45,46]. Hence, to evaluate the effect of coconut oil on MAPK signaling, we investigated the phosphorylation levels of JNK, p38, and ERK in APM– or DEP–exposed AMs and in APM– or DEP–induced AMs pretreated with coconut oil. Our results showed that phospho-JNK, total JNK, phospho-p38, and phospho-ERK expression were significantly increased within APM– and DEP–induced AMs compared with vehicle groups (Figure 5). Pretreatment with coconut oil significantly decreased APM– or DEP–induced MAPK signaling. These results indicate that coconut oil may alleviate the inflammatory response via the regulation of MAPK signaling in PM-induced AMs.
Figure 5.
Effect of coconut oil on mitogen−activated protein kinase (MAPK) pathway in artificial particulate matter (APM) and diesel exhaust particles (DEP)−stimulated alveolar macrophages (MH−S). (A–C) Phosphorylation of extracellular receptor-activated kinase (ERK), C−Jun N−terminal kinase (JNK), and p38 MAPK was detected via Western blotting. (D–F) Relative densities of p−JNK, p−p38 MAPK, and p−ERK proteins. Vehicle control (VC) was treated with cell medium including 10% distilled water. Data are presented as the means ± SD (n = 3 per group). The normalized value of target protein expression level in the control group was set to 1. *** p < 0.001 vs. VC; ### p < 0.001 vs. APM; §§§ p < 0.001 vs. DEP groups.
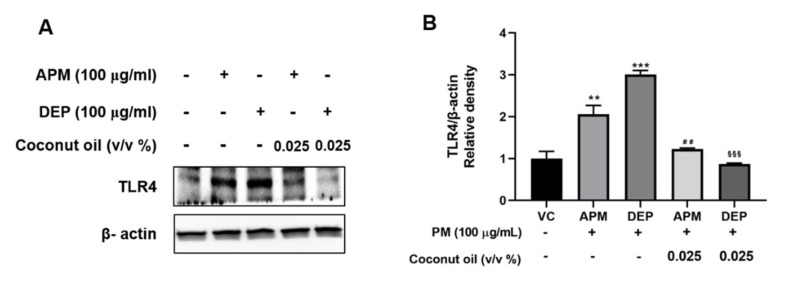
3.6. Coconut Oil Inhibits PM-Induced TLR4 Activation in AMs
TLR4 signaling plays an important role in the regulation of PM-induced inflammatory responses [47]. We investigated whether coconut oil regulated TLR4 signaling in PM-stimulated AMs, using Western blotting analysis. The results showed that PM stimulation significantly upregulated TLR4 expression in AMs, and this effect was significantly abrogated by coconut oil pretreatment (Figure 6).
Figure 6.
(A) Representative Western blots and (B) relative density of toll−like receptor (TLR) 4 expression in artificial particulate matter (APM) and diesel exhaust particles (DEP)−stimulated alveolar macrophages (MH−S). Vehicle control (VC) was treated with cell medium including 10% distilled water. Data are presented as the means ± SD (n = 3 per group). ** p < 0.01 or *** p < 0.001 vs. VC; ## p < 0.01 vs. APM group; §§§ p < 0.001 vs. DEP group.
4. Discussion
Our results, for the first time, demonstrate that coconut oil, a natural extract and functional food, attenuates oxidative stress-associated inflammatory responses via the modulation of TLR4 and MAPK activation in PM-stimulated AMs.
PM leads to health risks posed by pollutants from the ambient air or the workplace [41,48]. DEP is a mixture of various chemical components, including EC and OC, which constitute the carbon core, metals, and trace elements; organic compounds such as alkanes, branched alkanes, alkyl-cycloalkanes, alkyl-benzenes, and polycyclic aromatic hydrocarbons (PAHs); and various cyclic aromatics [49]. PAHs and related compounds, such as quinones, are metabolized to even more toxic metabolites upon ingestion [50], primarily because of their redox potential and their ability to induce oxidative stress [51]. To date, many researchers have studied in humans the toxicity of DEP as one of the natural PM components, and have reported that DEP is associated with acute irritation and the induction of respiratory symptoms. Consequently, it could exacerbate pulmonary diseases in response to known allergens and pathogens [52,53]. However, it is difficult to determine the key soot factors within a mixture of inorganic compounds, trace metals, and PAHs that play a major role in inducing toxicity [54]. Hence, we generated an APM from a single origin source as ultrafine particles. In a previous study, the APM generated in our lab maintained its original physical properties and possessed a chemically controllable surface [31]. ROS-mediated cytotoxicity is significantly increased via an increase of oxygenated functional groups on the surface of APM in epithelial cells [31]. Thus, we selected DEP and APM as PM.
AMs are responsible for the first line of defense against pathogens and pollutants, which can activate the innate immune response in the lungs. AMs participate in host defense by engulfing and digesting pathogens, and they maintain clear air spaces by engulfing foreign materials and minimizing the exposure of other airway cells to these substances [11,12]. This immune cell plays an essential role in maintaining a balance between the defense against pathogens and the tolerance towards innocuous stimuli [55]. AMs are the predominant cells in the lungs that are responsible for the processing and removal of inhaled particulate matter. When exposed to atmospheric particles, activated AM cells may release excessive amounts of mediators, such as oxygen radicals, proteases, and growth-regulating proteins, which could then be involved in the pathogenesis of acute and chronic lung diseases [56].
Coconut oil is used as a health food and has nutritional effects. In vitro and in vivo studies have reported that coconut oil exhibits various biological activities, including antioxidant, anti-inflammatory, anticancer, analgesic, antimicrobial, and antipyretic properties [23,25,26]. In fact, despite many studies, little has been reported about the effects of coconut oil on lung diseases. In an in vivo study, Luiz Henrique C reported that coconut oil prevented airway tissue from exhibiting peribronchial inflammatory infiltrate, epithelial hyperplasia, and smooth muscle thickening in guinea pigs that were submitted to ovalbumin sensitization [27]. However, there have been no reports on the biological effects of coconut oil from exposure to PM. Hence, we aimed to assess the protective effects of coconut oil in PM-stimulated AMs, focusing on its antioxidant and anti-inflammatory activities, and investigate its mechanism of action.
In this study, to investigate the protective effect of coconut oil in PM-stimulated AMs, we first optimized the concentration of APM and DEP by treating AMs with different concentrations, and examined the cell viability of PM-stimulated AMs. Researchers have shown that DEP induces the cytotoxicity of cell death over large ranges (50–3000 μg/mL) [57,58]. Therefore, we confirmed cytotoxicity by stimulating DEP or APM within those ranges in AMs, to find the optimal concentration. We found that APM and DEP induced early cytotoxicity at 100 μg/mL in AMs. We also confirmed that PM-induced ROS production and cytotoxicity in AMs are regulated by NAC treatment. Therefore, we examined the protective effect of coconut oil on oxidative activity by measuring cytotoxicity, ROS production, and GSH activity in PM-stimulated AMs. GSH plays a role in preventing damage to important cellular components caused by reactive oxygen species, such as free radicals, peroxides, and lipid peroxides, and it is associated with hypoxia, lung diseases, and aging [40]. In cells, GSH is oxidized during peroxide disposal by GSH peroxidase, and GSSG is reduced to two molecules of GSH, with reducing equivalents from the coenzyme NADPH [59]. The relative amounts of reduced (GSH) and oxidized GSH (GSSG) serve as markers of oxidative stress; thus, the GSSG/GSH ratio increases under oxidative stress [60]. In this study, our results showed that 100 μg/mL PM decreased cell viability and GSH levels, increased GSSG content, increased the GSSH/GSH ratio, increased NADPH level, and increased ROS production in AMs. The PM-induced changes were recovered by coconut oil in the AMs. Moreover, we measured via qRT-PCR the levels of NQO-1, an oxidative stress gene, in PM-stimulated AMs. PM-induced NQO-1 mRNA levels were significantly decreased by coconut oil in AMs. These results indicate that oxidative stress is a key modulator and initiator of PM-induced cell damage, and that coconut oil has potent antioxidant properties [22,24,26] in PM-stimulated AMs. Therefore, we suggest that coconut oil prevents cell damage by regulating GSH activity during PM-induced oxidative stress in AMs.
Another important function of coconut oil is its anti-inflammatory activity. We tested the levels of various inflammatory mediators in PM-stimulated AMs by qRT-PCR and Western blotting. Our results showed that PM upregulated the mRNA levels of MCP-1 and CXCL-1, as well as the protein levels of TNF-α, IL-6, and IL-1β in AMs. Coconut oil downregulates various PM-induced inflammatory mediators in AMs. In the respiratory system, AMs clearly maintain the air space between cells in the respiratory system by the phagocytosis of pathogens and debris, and foreign material-phagocytosed AMs release the prime immune responses of mediators such as TNF-α, IL-1β, IL-6, and neutrophil chemoattractants [60]. TNF-α, IL-1β, and IL-6 are widely studied pro-inflammatory cytokines that play a key role in the pathogenesis of numerous inflammatory diseases, and in the host defense mechanism [61]. In addition, the levels of these inflammatory cytokines are more sensitive to PM in COPD patients [62]. Several studies have demonstrated that tissue-resident macrophages produce chemoattractants such as CXCL-1 and MCP-1 [63,64] after engulfing particles. The chemokine CXCL-1 plays an important role in inflammation, as it is a major chemoattractant that is responsible for recruiting neutrophils. MCP-1 has been reported to promote macrophage and T-lymphocyte infiltration after tissue injury [65]. These results indicated that coconut oil can prevent PM-induced early inflammatory responses in AMs.
To elucidate the mechanism underlying the biological activities of coconut oil in APM– and DEP–stimulated AMs, we investigated the MAPK pathways. The MAPK signaling pathway is one of the most critical intracellular signal transduction systems, and it is generally activated by a broad array of extracellular or intracellular stimuli [20]. In this study, we found that the phosphorylation levels of p38, JNK, and ERK in AMs were higher than those in the control groups, using Western blotting analysis. Nevertheless, the PM-upregulated MAPK pathways were downregulated after pretreatment with coconut oil. TLR4 is an important upstream factor in the MAPK pathway, and it contributes to immune responses by regulating the levels of TNF-α, IL-1β, IL-6, inducible nitric oxide synthase, and nitric oxide via the MAPK pathway [66,67]. In this study, we showed that PM-induced TLR4 expression was significantly decreased by coconut oil in AMs. Moreover, we examined Akt signaling (Figure S1), an upstream factor of the nuclear factor kappa B (NF-κB) pathway in PM-stimulated AMs. In our previous study, we showed that NF-κB levels in nuclear protein extracts from AMs were substantially increased within DEP–stimulated AMs [9]. Consistent with these observations, we showed that PM exposure induces Akt phosphorylation in APM– and DEP–stimulated AMs. In conclusion, this study demonstrated that coconut oil can be potentially developed as an adjuvant therapy for PM-induced oxidative stress-mediated lung inflammation. This research is limited by the differences between APM, DEP, and natural atmospheric PM. We can synthesize APM with varying physical and chemical properties through temperature control. Therefore, it is necessary to evaluate the efficacy of coconut oil under various conditions. Future studies aim to investigate the potential efficacy of coconut oil in an in vivo study.
5. Conclusions
As a result of our in vitro study, we found that coconut oil had protective effects on oxidative stress and inflammatory responses that are induced by PM in AMs. Coconut oil has been shown to reduce APM/DEP–induced inflammation by modulating the TLR4/MAPK pathway in AMs, as well as by conferring protection against adverse respiratory effects caused by PM exposure. It has been demonstrated in this study that coconut oil can potentially be used as an adjuvant therapy against PM-induced oxidative stress-mediated lung inflammation.
Acknowledgments
We thank Minchul Chu for providing surfactant-free coconut oil in DW emulsion.
Abbreviations
PM: particulate matter; AMs: alveolar macrophages; APM: artificial PM; IL: interleukin; TNF: tumor necrosis factor; MAPK: mitogen-activated protein kinase; DEP: diesel exhaust particulates; COPD: chronic obstructive pulmonary disease; MMP: matrix metallopeptidase, RPMI: Roswell Park Memorial Institute, DW: distilled water; OC: organic carbon; EC: elemental carbon; NAC, N-acetylcysteine; MTT: 3–(4,5–dimethylthiazol–2–yl)–2,5–diphenyltetrazolium bromide; LDH: lactate dehydrogenase; ROS: reactive oxygen species; DCF-DA: 2′,7′-dichlorofluorescein diacetate; GSH: glutathione; GSSG: glutathione disulfide; NADP+: nicotinamide adenine dinucleotide phosphate; NADPH: reduced NADP+; PBS: phosphate-buffered saline; CXCL: chemokine (C-S-C motif) ligand; MCP: monocyte chemoattractant protein; NQO1: NADPH dehydrogenase (quinone); JNK: c-Jun N-terminal kinase; ERK: extracellular signal-regulated kinase; TLR: toll-like receptor; PAHs: polycyclic aromatic hydrocarbons; NF-κB: nuclear factor kappa B.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules27092898/s1, Figure S1: Representative Western blots and relative density of phosphorylation of AKT expression in APM– or DEP–stimulated MH-S.
Author Contributions
X.C. conducted experiments, performed analyses, and wrote the manuscript. D.I.K. interpreted data and edited the manuscript. H.-G.M. edited the manuscript. M.C. provided coconut oil for experiment and edited the manuscript. K.L. interpreted data, edited the manuscript, and supervised the study. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Korea Institute of Toxicology (grant no. KK-2203), and the Korea Institute of Science and Technology (KIST) Institutional Program (Atmospheric Environment Research Program, grant No. 2E31700-22-P008).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li J., Liu H., Lv Z., Zhao R., Deng F., Wang C., Qin A., Yang X. Estimation of PM2.5 mortality burden in China with new exposure estimation and local concentration-response function. Environ. Pollut. 2018;243:1710–1718. doi: 10.1016/j.envpol.2018.09.089. [DOI] [PubMed] [Google Scholar]
- 2.Gilmour J.M.M.I., Duvall R.M., Dailey L., Daniels M., Boykin E., Cho S.-H., Doerfler D., Gordon T., Devlin R.B. Comparative toxicity of size-fractionated airborne particulate matter obtained from different cities in the United States. Inhal. Toxicol. 2007;19((Suppl. 1)):7–16. doi: 10.1080/08958370701490379. [DOI] [PubMed] [Google Scholar]
- 3.Chen T., Jin H., Wang H., Yao Y., Aniagu S., Tong J., Jiang Y. Aryl hydrocarbon receptor mediates the cardiac developmental toxicity of EOM from PM2.5 in P19 embryonic carcinoma cells. Chemosphere. 2018;216:372–378. doi: 10.1016/j.chemosphere.2018.10.160. [DOI] [PubMed] [Google Scholar]
- 4.Coccini T., Barni S., Mustarelli P., Locatelli C., Roda E. One-month persistence of inflammation and alteration of fibrotic marker and cytoskeletal proteins in rat kidney after Cd-doped silica nanoparticle instillation. Toxicol. Lett. 2015;232:449–457. doi: 10.1016/j.toxlet.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Dong G.-H. Perspective for Future Research Direction about Health Impact of Ambient Air Pollution in China. Adv. Exp. Med. Biol. 2017;1017:263–268. doi: 10.1007/978-981-10-5657-4_12. [DOI] [PubMed] [Google Scholar]
- 6.Bowe B., Xie Y., Li T., Yan Y., Xian H., Al-Aly Z. Particulate Matter Air Pollution and the Risk of Incident CKD and Progression to ESRD. J. Am. Soc. Nephrol. 2018;29:218–230. doi: 10.1681/ASN.2017030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone V., Miller M.R., Clift M.J.D., Elder A., Mills N.L., Moller P., Schins R.P.F., Vogel U., Kreyling W.G., Jensen K.A., et al. Nanomaterials Versus Ambient Ultrafine Particles: An Opportunity to Exchange Toxicology Knowledge. Env. Health Perspect. 2017;125:106002. doi: 10.1289/EHP424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karottki D.G., Spilak M., Frederiksen M., Andersen Z.J., Madsen A.M., Ketzel M., Massling A., Gunnarsen L., Møller P., Loft S. Indoor and Outdoor Exposure to Ultrafine, Fine and Microbiologically Derived Particulate Matter Related to Cardiovascular and Respiratory Effects in a Panel of Elderly Urban Citizens. Int. J. Environ. Res. Public Health. 2015;12:1667–1686. doi: 10.3390/ijerph120201667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D.I., Song M.K., Kim S.H., Park C.Y., Lee K. TF-343 Alleviates Diesel Exhaust Particulate-Induced Lung Inflammation via Modulation of Nuclear Factor-kappaB Signaling. J. Immunol. Res. 2019;2019:8315845. doi: 10.1155/2019/8315845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C.-P., Qin G., Shi R.-Z., Zhang M.-S., Lv J.-Y. Ginsenoside Rg1 reduces toxicity of PM2.5 on human umbilical vein endothelial cells by upregulating intracellular antioxidative state. Environ. Toxicol. Pharmacol. 2013;35:21–29. doi: 10.1016/j.etap.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Oberdorster G., Ferin J., Gelein R., Soderholm S.C., Finkelstein J. Role of the Alveolar Macrophage in Lung Injury: Studies with Ultrafine Particles. Environ. Health Perspect. 1992;97:193. doi: 10.1289/ehp.97-1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginhoux F., Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Rylance J., Fullerton D.G., Scriven J., Aljurayyan A.N., Mzinza D., Barrett S., Wright A.K.A., Wootton D.G., Glennie S.J., Baple K., et al. Household Air Pollution Causes Dose-Dependent Inflammation and Altered Phagocytosis in Human Macrophages. Am. J. Respir. Cell Mol. Biol. 2015;52:584–593. doi: 10.1165/rcmb.2014-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia J., Sun Y., Hu Z., Li Y., Ruan X. Propofol inhibits the release of interleukin-6, 8 and tumor necrosis factor-alpha correlating with high-mobility group box 1 expression in lipopolysaccharides-stimulated RAW 264.7 cells. BMC Anesthesiol. 2017;17:148. doi: 10.1186/s12871-017-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo E.Y.J., Pan W.W., Liu S.B., Shen Z.F., Xu Y., Hu L.L. RK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., Huang J., Wang L., Chen C., Yang D., Jin M., Bai C., Song Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-ĸB signaling pathway. J. Thorac. Dis. 2017;9:4398–4412. doi: 10.21037/jtd.2017.09.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Na H.G., Kim Y.D., Choi Y.S., Bae C.H., Song S.Y. Diesel exhaust particles elevate MUC5AC and MUC5B expression via the TLR4-mediated activation of ERK1/2, p38 MAPK, and NF-kappaB signaling pathways in human airway epithelial cells. Biochem. Biophys. Res. Commun. 2019;512:53–59. doi: 10.1016/j.bbrc.2019.02.146. [DOI] [PubMed] [Google Scholar]
- 18.Kim D.I., Song M.-K., Kim H.-I., Han K.M., Lee K. Diesel Exhaust Particulates Induce Neutrophilic Lung Inflammation by Modulating Endoplasmic Reticulum Stress-Mediated CXCL1/KC Expression in Alveolar Macrophages. Molecules. 2020;25:6046. doi: 10.3390/molecules25246046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y., Zhao Y., Wang Q., Chen H., Zhou X. Fine particulate matter exposure promotes M2 macrophage polarization through inhibiting histone deacetylase 2 in the pathogenesis of chronic obstructive pulmonary disease. Ann. Transl. Med. 2020;8:1303. doi: 10.21037/atm-20-6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyung S.Y., Jeong S.H. Particulate-Matter Related Respiratory Diseases. Tuberc. Respir. Dis. 2020;83:116–121. doi: 10.4046/trd.2019.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed F.R. Fruit Oils: Chemistry and Functionality. In: Osman A., editor. Oils From Fruit Nuts, Coconut (Cocos nucifera) Oil. E-Publishing Inc.; Cham, Switzerland: 2019. pp. 209–221. [Google Scholar]
- 22.Dia V.P., Garcia V.V., Mabesa R.C. Comparative physiological characteristics of virgin coconut oil produced by different methods. Philipp. Agric. Sci. Philipp. 2005;88:462–475. [Google Scholar]
- 23.DebMandal M., Mandal S. Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention. Asian Pac. J. Trop. Med. 2011;4:241–247. doi: 10.1016/S1995-7645(11)60078-3. [DOI] [PubMed] [Google Scholar]
- 24.Kamariah A.A.L., Rosmawati A., Ching M.G.W., Sivapragasam M.D.A.A., Tan C.P., Lai O.M. Physico-chemical and quality characteristics of virgin coconut oil—A Malaysian survey. J. Trop. Agric. Food Sci. 2008;36:239–248. [Google Scholar]
- 25.Winarsi H.H., Purwanto A. Virgin Coconut Oil (VCO) Enriched with Zn as Immunostimulator for Vaginal Candidiasis Patient. HAYATI J. Biosci. 2008;15:135–139. doi: 10.4308/hjb.15.4.135. [DOI] [Google Scholar]
- 26.Intahphuak S., Khonsung P., Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm. Biol. 2010;48:151–157. doi: 10.3109/13880200903062614. [DOI] [PubMed] [Google Scholar]
- 27.Nevin K.G., Rajamohan T. Effect of topical application of virgin coconut oil on skin components and antioxidant status during dermal wound healing in young rats. Ski. Pharm. Physiol. 2010;23:290–297. doi: 10.1159/000313516. [DOI] [PubMed] [Google Scholar]
- 28.Choi E.H., Kang J.I., Cho J.Y., Lee S.H., Kim T.S., Yeo I.H., Chun H.S. Supplementation of standardized lipid-soluble extract from maca (Lepidium meyenii) increases swimming endurance capacity in rats. J. Funct. Foods. 2012;4:568–573. doi: 10.1016/j.jff.2012.03.002. [DOI] [Google Scholar]
- 29.Chandrashekar P., Lokesh B.R., Krishna A.G.G. Hypolipidemic effect of blends of coconut oil with soybean oil or sunflower oil in experimental rats. Food Chem. 2010;123:728–733. doi: 10.1016/j.foodchem.2010.05.042. [DOI] [Google Scholar]
- 30.Vasconcelos L.H.C., Silva M., Costa A.C., de Oliveira G.A., de Souza I.L.L., Righetti R.F., Queiroga F.R., Cardoso G.A., Silva A.S., da Silva M.P., et al. Virgin Coconut Oil Supplementation Prevents Airway Hyperreactivity of Guinea Pigs with Chronic Allergic Lung Inflammation by Antioxidant Mechanism. Oxid. Med. Cell Longev. 2020;2020:5148503. doi: 10.1155/2020/5148503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Y.T., Youn J.S., Moon H.G., Chen X.Y., Kim D.I., Cho W.H., Lee K.-H., Joen K.-J. Relationship between Cytotoxicity and Surface Oxidation of Artificial Black Carbon. Nanomaterials. 2021;11:1455. doi: 10.3390/nano11061455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwangbo M.C.S., Moon C. Effect of oil particle size on dispersion stability in oil in water emulsion. Part. Aerosol Res. 2017;13:133–139. [Google Scholar]
- 33.Birch M.E., Cary R.A. Elemental Carbon-Based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust. Aerosol Sci. Technol. 1996;25:221–241. doi: 10.1080/02786829608965393. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Peterson D.A., Kimura H., Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 1997;69:581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- 35.Afzal M., Afzal A., Jones A., Armstrong D., Afzal M., Afzal A., Jones A., Armstrong D. A Rapid Method for the Quantification of GSH and GSSG in Biological Samples. In: Armstrong D., editor. Oxidative Stress Biomarkers and Antioxidant Protocols. Humana Press; Totova, NJ, USA: 2002. [Google Scholar]
- 36.Robinson R.K., Birrell M.A., Adcock J.J., Wortley M.A., Dubuis E.D., Chen S., McGilvery C.M., Hu S., Shaffer M.S.P., Bonvini S.J., et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J. Allergy. Clin. Immunol. 2018;141:1074–1084. doi: 10.1016/j.jaci.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le K.C., Lefumeux C., Pino T. Differential Raman backscattering cross sections of black carbon nanoparticles. Sci. Rep. 2017;7:17124. doi: 10.1038/s41598-017-17300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi Z.H., Zhang Y.H., Chen Z.F., Yang C., Song Y.Y., Liao X.L., Li W.Q., Tsang S.Y., Liu G.G., Cai W.Z. Chemical identity and cardiovascular toxicity of hydrophobic organic components in PM2.5 Ecotoxicol. Environ. Saf. 2020;201:110827. doi: 10.1016/j.ecoenv.2020.110827. [DOI] [PubMed] [Google Scholar]
- 39.Janssen N.A.H., Gerlofs-Nijland M.E., Lanki T., Salonen R.O., Cassee F., Brunekreef B., Hoek G., Fischer P., Krzyzanowski M. Health Effects of Black Carbon. WHO Regional Office for Europe; Copenhagen, Denmark: 2012. [Google Scholar]
- 40.Kitz K., Windischhofer W., Leis H.J., Huber E., Kollroser M., Malle E. 15-Deoxy-Delta12,14-prostaglandin J2 induces Cox-2 expression in human osteosarcoma cells through MAPK and EGFR activation involving reactive oxygen species. Free Radic. Biol. Med. 2011;50:854–865. doi: 10.1016/j.freeradbiomed.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 41.Totlandsdal A.I., Cassee F.R., Schwarze P., Refsnes M., Lag M. Diesel exhaust particles induce CYP1A1 and pro-inflammatory responses via differential pathways in human bronchial epithelial cells. Part Fibre. Toxicol. 2010;7:41. doi: 10.1186/1743-8977-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunes C., Pereira A.M., Morais-Almeida M. Asthma costs and social impact. Asthma. Res. Pract. 2017;3:1. doi: 10.1186/s40733-016-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang G., Huang L., Gao S., Gao S., Wang L. Measurements of PM10 and PM2.5 in urban area of Nanjing, China and the assessment of pulmonary deposition of particle mass. Chemosphere. 2002;48:689–695. doi: 10.1016/S0045-6535(02)00197-2. [DOI] [PubMed] [Google Scholar]
- 44.Cavanagh J.A., Trought K., Brown L., Duggan S. Exploratory investigation of the chemical characteristics and relative toxicity of ambient air particulates from two New Zealand cities. Sci. Total Environ. 2009;407:5007–5018. doi: 10.1016/j.scitotenv.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Huang X., Shi X., Zhou J., Li S., Zhang L., Zhao H. The activation of antioxidant and apoptosis pathways involved in damage of human proximal tubule epithelial cells by PM2.5 exposure. Environ. Sci. Europe. 2020;32:2. doi: 10.1186/s12302-019-0284-z. [DOI] [Google Scholar]
- 46.Seriani R., Mde S.J., de Toledo A.C., Martins M.A., Seckler M., Alencar A.M., Negri E.M., Silva L.F.F., Mauad T., Saldiva P.H.N., et al. Diesel exhaust particulates affect cell signaling, mucin profiles, and apoptosis in trachea explants of Balb/C mice. Environ. Toxicol. 2015;30:1297–1308. doi: 10.1002/tox.22000. [DOI] [PubMed] [Google Scholar]
- 47.Hashimoto S., Gon Y., Takeshita I., Matsumoto K., Jibiki I., Takizawa H., Kudoh S., Horie T. Diesel exhaust particles activate p38 MAP kinase to produce interleukin 8 and RANTES by human bronchial epithelial cells and N-acetylcysteine attenuates p38 MAP kinase activation. Am. J. Respir. Crit. Care. Med. 2000;161:280–285. doi: 10.1164/ajrccm.161.1.9904110. [DOI] [PubMed] [Google Scholar]
- 48.Nemmar A., Al-Salam S., Zia S., Dhanasekaran S., Shudadevi M., Ali B.H. Time-course effects of systemically administered diesel exhaust particles in rats. Toxicol. Lett. 2010;194:58–65. doi: 10.1016/j.toxlet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 49.De Filippo K., Henderson R.B., Laschinger M., Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J. Immunol. 2008;180:4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- 50.Hussein M.S.M.M., Abdel-Shafy I. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016;25:107–123. [Google Scholar]
- 51.Gurbani S.K.B.D., Kumar A., Pandey A.K., Ana G.R.E.E., Verma A., Khan A.H., Patel D.K., Mudiam M.K.R., Jain S.K., Roy R., et al. Polycyclic aromatic hydrocarbons and their quinones modulate the metabolic profile and induce DNA damage in human alveolar and bronchiolar cells. Int. J. Hyg. Environ. Health. 2013;216:553–565. doi: 10.1016/j.ijheh.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Guo C., Xia Y., Niu P., Jiang L., Duan J., Yu Y., Zhou X., Li Y., Sun Z. Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-kappaB signaling. Int. J. Nanomed. 2015;10:1463–1477. doi: 10.2147/IJN.S76114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saito H. Toxico-pharmacological perspective of the Nrf2-Keap1 defense system against oxidative stress in kidney diseases. Biochem. Pharmacol. 2013;85:865–872. doi: 10.1016/j.bcp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Varma S.R., Sivaprakasam T.O., Arumugam I., Dilip N., Raghuraman M., Pavan K.B., Rafiq M., Paramesh R. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement. Med. 2019;9:5–14. doi: 10.1016/j.jtcme.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allard B., Panariti A., Martin J.M. Alveolar macrophages in the resolution of inflammation, tissue repair, and tolerance to infection. Front. Immunol. 2018;9:1777. doi: 10.3389/fimmu.2018.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hiraiwa K., Stephan F., Eeden v. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediat. Inflamm. 2013;2013:619523. doi: 10.1155/2013/619523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mauderly J., Hoyer M. Health Assessment Document for Diesel Engine Exhaust. National Center for Environmental Assessment, Environmental Protection Agency; Washington, DC, USA: 2002. p. 669. [Google Scholar]
- 58.Turner J., Hernandez M., Snawder J.E., Handorean A., McCabe K.M. A toxicology suite adapted for comparing parallel toxicity responses of model human lung cells to diesel exhaust particles and their extracts. Aerosol. Sci. Technol. 2015;49:599–610. doi: 10.1080/02786826.2015.1053559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roh J.L., Jang H., Kim E.H., Shin D. Targeting of the Glutathione, Thioredoxin, and Nrf2 Antioxidant Systems in Head and Neck Cancer. Antioxid Redox Signal. 2017;27:106–114. doi: 10.1089/ars.2016.6841. [DOI] [PubMed] [Google Scholar]
- 60.Katharine B., Stefania S., Miller F., Jr., Krause K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012;142:13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 61.Kany S., Vollrath J.T., Relja B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ling S.H., Eeden S.F.v. Particulate matter air pollution exposure: Role in the development and exacerbation of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2009;4:233–243. doi: 10.2147/COPD.S5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sanchez-Perez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 65.Schwarze P.E., Totlandsdal A.I., Lag M., Refsnes M., Holme J.A., Ovrevik J. Inflammation-related effects of diesel engine exhaust particles: Studies on lung cells in vitro. Biomed Res. Int. 2013;2013:685142. doi: 10.1155/2013/685142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pushpakumar S., Ren L., Kundu S., Gamon A., Tyagi S.C., Sen U. Toll-like Receptor 4 Deficiency Reduces Oxidative Stress and Macrophage Mediated Inflammation in Hypertensive Kidney. Sci. Rep. 2017;7:6349. doi: 10.1038/s41598-017-06484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jasek-Gajda E., Jurkowska H., Jasinska M., Lis G.J. Targeting the MAPK/ERK and PI3K/AKT Signaling Pathways Affects NRF2, Trx and GSH Antioxidant Systems in Leukemia Cells. Antioxidants. 2020;9:633. doi: 10.3390/antiox9070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.