Abstract
Aim: This systematic review examined the additional effect of taking omega-3 supplements on periodontal therapy. Methods: The focused question was “What is the possible effect of omega-3 supplementation concomitant to non-surgical periodontal therapy on clinical periodontal parameters?” Databases Cochrane, Embase, Google Scholar, PubMed, and Web of Science (January-July 2021) were searched to identify appropriate studies. Randomized clinical trials (RCT) about non-surgical therapy with omega-3 supplementation, with at least 3 months of supplementation period were included. Cochrane risk of bias tool version 2 and Grading of Recommendations Assessment, Development, and Evaluation were used. Results: A total of 1556 studies were found, of which eight studies met the inclusion criteria. All eight studies evaluated periodontal probing depth and clinical attachment loss; plaque and gingival inflammation were evaluated in seven studies. High variety of omega-3 dosage, different study lengths, questionable results from periodontal therapy (including test and control groups), high risk of bias and moderate quality of evidence prevented a satisfactory conclusion regarding the benefits of omega-3 supplementation. The studies’ high heterogeneity avoided meta-analysis. Conclusion: Notwithstanding all limitations, the promising effects of omega-3 supplementation presented in two six-month studies encourage performing RCT with better-defined treatment protocols and greater methodological rigor.
Keywords: fatty acids, omega 3, periodontal diseases, periodontitis
1. Introduction
The oral cavity harbors numerous and distinct microorganisms, collectively referred to as the oral microbiome. This microbiota is found in various oral niches, such as the tongue, saliva, mucosa, and teeth [1], and typically live in harmony and balance with the host [2]. The behavior of the biofilm on teeth is influenced by factors such as lifestyle changes, antimicrobial therapies, and the inflammatory status of the host [2]. This condition may lead to irreversible destruction of periodontal support tissues, resulting in the establishment of periodontitis—a synergistic polymicrobial and opportunistic infection [3,4,5]. According to O’Keefe et al., 2008 [6], nutritional status can directly affect inflammatory pathways and contribute to the onset and progression of chronic diseases. Nutritional issues have been identified as one contributing factor to the etiopathogenesis of periodontal diseases [7,8], as diet influences energy production, genetic modulation, and protein expression. In particular, susceptibility to periodontal diseases may be related to the consumption of lipids [9].
Lipids are essential dietary constituents that occur in a variety of forms: as fats and oils, phospholipids, steroids, and carotenoids [10]. Lipids are essential components of biological membranes, which are largely composed of phospholipids, glycolipids, and sterols. Phospholipids influence the structure and function of the cell membrane [11,12,13]. Among the components of phospholipids are fatty acids, which are classified as saturated, unsaturated, and polyunsaturated based on the number of carbon double bonds (none, one, and two or more, respectively).
Regarding polyunsaturated fatty acids (PUFAs), the human being is not able to produce linoleic acid (omega-6) and α-linolenic acid (omega-3) in the sufficient amounts. In other words, the requirement for these acids is higher than the endogenous supply [14,15] and therefore they must be included in the diet through the ingestion of specific foods or supplements. Once in the body, linoleic acid is converted by means of desaturation and elongation enzymes to arachidonic acid (AA), which is a precursor for many pro-inflammatory mediators. By means of the same enzymes, α-linolenic acid is converted to eicosapentaenoic acid (EPA) followed by conversion into resolvins (E series 1–4) with predominantly anti-inflammatory, vasodilatation and anti-aggregation properties [16,17]. Further desaturation and elongation of EPA results in docosahexaenoic acid (DHA) which is metabolized into D-series of resolvins (Resolvin1–6), protectins and maresins with anti-inflammatory and immunomodulatory properties [18]. As EPA is in competition with AA regarding COX and LOX, the AA:EPA ratio level determines the metabolic status in being more pro-inflammatory/pro-aggregatory or more anti-inflammatory/anti-aggregatory. It has been shown that EPA supplementation results in lower plasma levels of these pro-inflammatory metabolites and in a lower AA:EPA ratio being anti-inflammatory [19].
The ideal dietary intake of omega-6 and omega-3 fatty acids to obtain an optimal AA:EPA ratio is still widely debated in the literature. High dietary intake of omega-6 relative to omega-3 may result in adverse health effects and an increased prevalence of chronic diseases associated with prolonged inflammation [20]. Increased consumption of omega-3 can, therefore, improve the AA:EPA ratio and protect against inflammation. Previous studies have shown that dietary intake of omega-3 appears to reduce the risk of many diseases and disorders, including coronary artery disease [21], type II diabetes [22], rheumatoid arthritis [23], asthma [24], depression [25], and cancer [26].
As mentioned above periodontitis is a synergistic polymicrobial and opportunistic infection. This inflammatory disease is characterized by the formation of deepened periodontal pockets with a pathogenic subgingival microbial biofilm on the root surface and breakdown of the connective tissue between teeth and supporting alveolar bone. Evaluation of the periodontal condition is performed by assessment of the probing pocket depth (PPD) and clinical attachment loss (CAL). A fundamental aspect of the treatment of periodontitis is the reduction of the subgingival microbial biofilm by means of mechanical root instrumentation and removal of the supragingival biofilm by mean of oral hygiene instructions, i.e., non-surgical periodontal therapy (NSPT). This treatment results in PPD reduction and CAL gain. In respect to this treatment, it is interesting that PUFAs have demonstrated also antimicrobial properties [27]. Furthermore, it has been shown that both EPA and DHA reduced significantly bacterial strains in a multi-species subgingival biofilm model including Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans [28]. Therefore, it may be not unlikely that in addition to the anti-inflammatory effect, the antimicrobial effect of PUFAs could contribute to the effect of periodontal treatment.
Considering the importance of EPA and DHA in the anti-inflammatory process, supplementation with omega-3 may improve conditions associated with periodontal inflammation. For example, previous studies in animal models found that individuals receiving omega-3 supplements experienced less alveolar bone loss and produced fewer pro-inflammatory cytokines [29,30]. Meanwhile, a cross-sectional study by Naqvi et al. (2010) [31] used data collected between 1999 and 2004 from 9182 adults (≥20 years) as part of the NHANES (National Health and Nutrition Examination Survey) in the United States to demonstrate that dietary intake of DHA was associated with a lower prevalence of periodontitis. Dietary intake of EPA showed a similar, but more modest, relationship.
From a clinical perspective, the few studies investigating omega-3 supplementation to date exhibit many limitations—having employed different methodological designs, populations and numbers of participants, periodontal diagnoses, treatments, and supplementation protocols—that make it difficult to accurately interpret the results and draw conclusions. Consequently, the findings of systematic reviews analyzing these studies [32,33,34] should also be interpreted with caution. The null hypothesis was that supplementation of PUFAs does not have a positive effect on the results of periodontal treatment. Therefore, the objective of the current study is to examine, through a systematic review, the impact of omega-3 supplementation during NSPT on periodontal clinical parameters.
Therefore, the objective of the current study is to examine, through a systematic review, the impact of omega-3 supplementation of on periodontal clinical parameters.
2. Methodology
This systematic review was conducted based on the Cochrane Handbook for Systematic Reviews of Interventions [35] and recommendations developed to strengthen reporting of systematic reviews and meta-analyses (PRISMA) [36]. This review is registered under number CRD42021234746 in PROSPERO, the International Prospective Register of Systematic Reviews.
2.1. Search Strategy
This systematic review sought randomized clinical trials (RCTs) that evaluated the effects of omega-3 supplementation, associated with non-surgical treatment of periodontitis, on periodontal clinical parameters. The following PICO question was asked: “What is the possible effect of supplementation with omega-3, in addition to non-surgical periodontal therapy, on clinical periodontal parameters?” From January to July 2021, searches were performed in five databases: Cochrane, Embase, Google Scholar (grey literature), PubMed, and Web of Science. The search strategies used for each database are shown in Table 1. Studies meeting the following criteria were retained for further evaluation:
Population: patients >18 years old with a diagnosis of periodontitis
Intervention: non-surgical periodontal treatment with oral intake of omega-3 as the sole supplement
Comparison: non-surgical periodontal treatment with or without a control (i.e., use of a placebo)
Main outcome: periodontal probing depth (PPD)
Table 1.
Search strategies used to identify studies for inclusion in the systematic review, customized for each database.
| Database | Strategy |
|---|---|
| Cochrane | (Periodontitis OR Chronic periodontitis OR Periodontal disease OR probing pocket depth OR periodontal pocket) AND (Intervention OR Therapy OR Treatment OR Scaling and root planning OR SRP OR nonsurgical periodontal therapy OR non-surgical therapy OR Periodontal treatment OR Periodontal therapy) AND (fatty acids, omega-3 OR docosahexaenoic acids OR eicosapentaenoic acids OR fatty acids OR fish oils OR omega-3 OR ω-3 OR n-3 OR PUFA OR Long chain fatty acids) |
| Embase | #1 ‘fatty acids’ OR ‘omega-3’ OR ‘docosahexaenoic acids’ OR ‘eicosapentaenoic acids’ OR ‘fish oils’ OR ‘ω-3’ OR ‘n-3’ OR ‘pufa’ OR ‘long chain fatty acids’ #2 periodontitis OR ‘chronic periodontitis’ OR ‘periodontal disease’ OR ‘probing pocket depth’ OR ‘periodontal pocket’ #3 Intervention OR Therapy OR Treatment #4 ‘scaling and root planing’ OR ‘srp’ OR ‘nonsurgical periodontal therapy’ OR ‘non-surgical therapy’ OR ‘periodontal treatment’ OR ‘periodontal therapy’ #5 (#3 OR #4) #6 (#1 AND #2 AND #5) |
| Google Scholar | Periodontal diseases and non-surgical therapy and omega-3 |
| PubMed | #1 (fatty acids, ômega-3 [MeSH Terms]) OR (docosahexaenoic acids [Text Word]) OR (eicosapentaenoic acids [Text Word]) OR (fatty acids [Text Word]) OR (fish oils [Text Word]) OR (ômega-3 [Text Word]) OR (ω- 3 [Text Word]) OR (n-3 [Text Word]) OR (PUFA [Text Word]) OR (Long chain fatty acids [ Text Word]) #2 (periodontal diseases [ MeSH Terms]) OR (periodontitis [MeSH Terms]) OR (Chronic periodontitis [MeSH Terms]) OR (probing pocket depth [Text Word]) OR (periodontal pocket [Text Word]) #3 (Scaling and root planing [Text Word]) OR (SRP [Text Word]) OR (nonsurgical periodontal therapy [Text Word]) OR (non-surgical therapy [Text Word]) OR (Periodontal treatment [Text Word]) OR (Periodontal therapy [Text Word]) #4 (Therapy [MeSH Terms]) OR (Treatment [Text Word]) OR (Intervention [Text Word]) #5 #3 OR #4 #6 (#1) AND (#2)) AND (#5) |
| Web of Science | #1 (TS = (Fatty acids OR omega-3 OR docosahexaenoic acids OR eicosapentaenoic acids OR Fish oil OR ω-3 OR n-3 OR PUFA OR Long chain fatty acids)) #2 (TS = (Periodontitis OR Chronic periodontitis OR Periodontal disease OR probing pocket depth OR periodontal pocket)) #3 (TS = (Intervention OR Therapy OR Treatment)) #4 (TS = (Scaling and root planing OR SRP OR nonsurgical periodontal therapy OR non-surgical therapy OR Periodontal treatment OR Periodontal therapy)) #5 (#3 OR #4) #6 (#1 AND #2 AND #5) |
2.2. Screening and Selection
Titles and abstracts returned through each database search were independently evaluated by two reviewers (LMM and FBP) to select those meeting the inclusion criteria. Studies that did not provide enough information in the title or abstract to determine their eligibility for inclusion were thoroughly reviewed. Studies were categorized as eligible, ineligible, or questionable. Disagreements were resolved by consensus; in those cases where conflict persisted, the final decision was made by a third reviewer (SCG). Articles that met the inclusion criteria were read in full and processed for data extraction.
The selection of study titles and abstracts was performed according to the following inclusion criteria:
Randomized clinical trials
Studies of non-surgical treatment of periodontitis with omega-3 supplementation, with or without a control treatment
A minimum of 3 months of experimental period and supplementation length
No language restriction
Exclusion criteria were:
Study length of <3 months
Association of omega-3 with other forms of supplementation or medication
Absence of periodontal treatment
Absence of supplementation with omega-3
Pilot studies
Restricted to evaluation of gingivitis
Use of ineligible study design
Use of surgical periodontal therapy
2.3. Risk of Bias Assessment
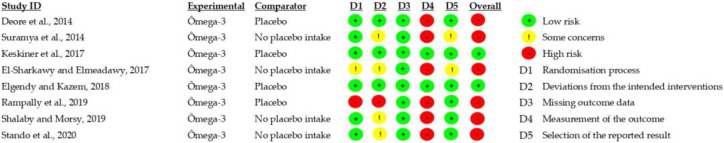
The risk of bias for each study was assessed by two independent reviewers (LMM and FBP) using the Cochrane risk of bias tool version 2 (RoB 2). The resulting score is based on five domains and classifies studies as having low risk, some concerns, or high risk of bias. The five evaluated criteria were the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported outcomes. The overall risk of bias depended on the ranking of each domain (Figure 1).
Figure 1.
Risk of bias assessment of included studies.
2.4. Data Extraction
Data extraction was performed independently by two reviewers. In cases of discordance, a third reviewer would be decisive (SCG). The following data were extracted from each study: main authorship, study title, country, type of study, study period, supplementation period length, number of participants, age, systemic conditions, periodontal diagnosis, placebo use, dosage of omega-3 supplementation, capsules intake control, oral hygiene instructions, adverse effects, author’s conflict of interest statement, and initial and final values for each periodontal clinical parameter.
2.5. Data Analysis
Relevant data and comparisons of the included studies were presented descriptively. When applicable, delta values (i.e., the difference between the baseline and final values) were calculated for the clinical parameters. Due to the high methodological heterogeneity of the studies, it was decided to do not perform meta-analysis.
2.6. Classification of Evidence
The GRADE system (Grading of Recommendations Assessment, Development, and Evaluation) was used to classify the evidence produced through this review [37]. Two reviewers assessed the quality of evidence and the strength of the recommendations (LMM and SCG) according to the following factors: risk of bias, consistency of results, objectivity of evidence, accuracy of data, publication bias, and magnitude of effect.
3. Results
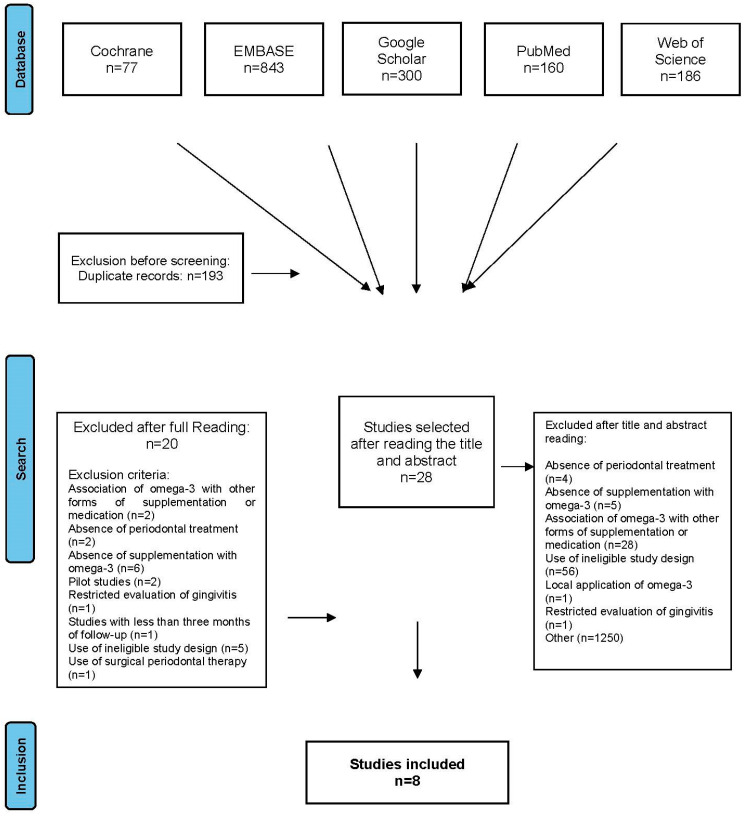
The initial database search resulted in 1556 articles, of which 193 were excluded due to duplication and 1345 were excluded after reading the titles and abstracts. The following criteria led to exclusion:
Absence of periodontal treatment (n = 4)
Absence of supplementation with omega-3 (n = 5)
Association of omega-3 with other forms of supplementation or medication (n = 28)
Use of ineligible study design (n = 56)
Local application of omega-3 (n = 1)
Restricted evaluation of gingivitis (n = 1)
Lack of connection with periodontal evaluation (n = 1250)
Screening of titles and abstracts by both independent reviewers (LMM and FBP) resulted in retention of the same 28 articles (Figure 2). After reading all the articles thoroughly, additional exclusions were made due to the following criteria:
Study length of <3 months (n = 1)
Association of omega-3 with other forms of supplementation or medication (n = 2)
Absence of periodontal treatment (n = 2)
Absence of supplementation with omega-3 (n = 6)
Pilot studies (n = 2)
Restricted to evaluation of gingivitis (n = 1)
Use of ineligible study design (n = 5)
Use of surgical periodontal therapy (n = 1)
Figure 2.
Flowchart of the process used to select studies for inclusion in the systematic review.
Ultimately, eight studies were included in this systematic review. The general characteristics of each study are presented in detail in Table 2. The number of participants in the studies ranged from 30 to 60, and the study length from 3 to 6 months. All studies included adult participants, of at least 22 years. In the same table, it can be noted that one study included people with diabetes (type 2 diabetes mellitus) [38], other obese individuals [39], and a third menopausal participants [40].
Table 2.
Study characteristics of selected studies.
| Author/Year/ (Reference Number) | Sample Size | Age Years | Systemic Conditions | Periodontitis Diagnosis |
Number of Teeth Present (and Mean Number of Teeth Examined) | Study Duration | Omega-3 | Capsules Intake Control | Side Effects Reported | Conflict of Interest | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | (n/Group) | Number of Sites Examined | EPA Dose per Day | DHA Dose per Day | |||||||
| Study Period | Periodontal Parameters Evaluated | ||||||||||
| Deore et al., 2014 [41] | 60 | T: 45.40 ± 40.90 | Healthy | Moderate/severe chronic periodontitis | NR (NR) |
180 mg | None | Not declared | |||
| India | (T:30/C:30) | 4 sites | 3 months | 120 mg | Yes | ||||||
| 3 months | C: 44.47 ± 5.20 | Plaque, GI, SBI, PPD, CAL | |||||||||
| Suramya et al., 2014 [39] | 40 | T + C > 30 | Obese | Generalized chronic periodontitis |
At least 20 (NR) |
550 mg | None | Not declared | |||
| India | (T:20/C:20) | 6 sites | 3 months | 450 mg | Yes | ||||||
| 3 months | Plaque, GI, SBI, PPD, BOP, CAL | ||||||||||
| Keskiner et al., 2017 [42] | 30 | T: 40.87 ± 9.7 | Healthy | Chronic periodontitis | NR (T: 25.87 ± 1.19 C: 26.33 ± 1.23) |
12.5 mg | None | Not declared | |||
| Turkey | (T:15/C:15) | C: 42.54 ± 5.82 | 6 sites | 6 months | 38.38 mg | Yes | |||||
| 3 months | Plaque, GI, PPD, BOP, CAL | ||||||||||
| El-Sharkawy and Elmeadawy, 2017 [43] | 34 | T: 45.75 ± 2.05 | Healthy | Untreated advanced chronic periodontitis |
At least 18 (T: 24.1 ± 3.2 C: 25.1 ± 2.1) |
2000 mg Omega-3 |
None | Not declared | |||
| Egypt | (T:17/C:17) | C: 47.82 ± 2.21 | NI | 3 months | Yes | ||||||
| 3 months | Plaque, MGI, PPD, BOP, CAL | ||||||||||
| Elgendy and Kazem, 2018 [40] | 50 | T: 50.24 ± 3.04 | Post-menopause | Generalized chronic periodontitis | At least 6 (NR) |
600 mg | None | Not declared | |||
| Egypt | (T:25/C:25) | C: 51.44 ± 3.36 | 6 sites | 6 months | 400 mg | Yes | |||||
| 6 months | Plaque, GI, PPD, CAL | ||||||||||
| Rampally et al., 2019 [38] | 42 | T + C 30–65 |
Diabetes II | Chronic periodontitis | At least 15 (NR) |
1000 mg | None | Not declared | |||
| India | (T:14) | NR | 3 months | No | |||||||
| 3 months | (C:14) | GI, PPD, CAL | |||||||||
| Shalaby and Morsy, 2019 [44] | 45 | T + C 35–55 |
Healthy | Stage II and III, grade B periodontitis |
NR (NR) |
3000 mg Omega-3 |
None | Not declared | |||
| Egypt | (T1:15) (T2:15) |
6 sites | 6 months | Yes | |||||||
| 6 months | (C:15) | Plaque, GI II, PPD, CAL | |||||||||
| Stando et al., 2020 [45] | 40 | T: 45 ± 8 | Healthy | Stage III and IV periodontitis |
At least 18 (NR) |
Nausea and irritating fish- scented halitosis (6 subjects) |
Not declared | ||||
| Poland | (T:16/C:14) | C: 54 ± 11 | 6 sites | 3 months | 2600 mg | 1800 mg | Yes | ||||
| 3 months | Plaque, PPD, BOP, CAL | ||||||||||
GI: gingival index; MGI: modified gingival index; GI II: GI inflammation index; SBI: sulcus bleeding index; PPD: probing pocket depth; BOP: bleeding on probing; CAL: clinical attachment loss; NR: not reported.
The diagnosis of the patients in the included studies varied from moderate/severe chronic [41], untreated advanced chronic [43], chronic [38,42], generalized chronic [39,40] to stage II/III periodontitis [44] and stage III/IV periodontitis [45] according to the most recent classification [46]. Six out of eight studies presented detailed criteria for patient selection. Four studies used presence of sites with PPD (probing depth) ≥ 5 mm [38,40,41,42] and two studies presence of sites with PPD ≥ 6 mm [43,45]. Of the remaining two studies one study included patients with stage II and III grade B periodontitis [44] and the other mentioned only chronic periodontitis, both without any further information [39].
The number of teeth, as far as presented, varied greatly among studies. Two studies reported the number of teeth examined [42,43]. Four authors did not report the number of teeth examined but defined the inclusion criteria based on the minimum number of teeth present: at least 6 [40], 15 [38], ≥18 [45] and 20 [39]. In contrast, Deore et al. (2014) [41] and Shalaby and Morsy (2019) [44] did not provide any information about number of teeth. Besides, information about the number of sites in which the clinical parameters were assessed varied as well. For example, four studies evaluated clinical parameters at six sites per tooth [39,40,42,44,45], one at four sites [41] and the remaining two studies did not provide any information about the number of sites examined [38,43].
PPD and CAL were evaluated in all studies, and presence of plaque in seven of them [39,40,41,42,43,44,45] (Supplementary Material Table S1). Gingival inflammation was assessed in seven studies by means of the Gingival Index (GI) [47] of which in one study [43] the modified GI (MGI) [48] was used and in another one [44] the GI inflammation index (GI II) [49]. In two studies both the Sulcus Bleeding Index and the GI were applied [39,41] and in two other studies [42,43] a combination of the GI and BOP (bleeding assessment after PPD) was used. In terms of results, there were no differences which method was performed. One study [45] used BOP assessment as sole parameter of inflammation.
The length of the omega-3-supplementation period coincided with the experimental period in all studies, starting at baseline. However, the dose of omega-3 administered per day in the studies varied widely, from 50.88 mg to 3000 mg. Five studies reported the exact amount of EPA and DHA used [39,40,41,42,45]. Capsule intake control was reported in seven studies. [39,40,41,42,43,44,45]. Mild adverse effects (nausea or fish breath) were reported in one study in 6 out of 30 participants [45], while all other studies reported no side effects (Table 2). No authors declared conflict of interest regarding their participation.
Randomization of participants was in all studies but one [42] performed before treatment. The provided periodontal therapy included in six studies scaling and root planning (SRP) in conjunction with oral hygiene instructions (OHI) and in two studies SRP solely [38,39]. Repetition of OHI during the experiment was carried out in the sixth and twelfth weeks of the study by Deore et al. (2014) [41], in the third and sixth month by Elgendy and Kazem (2018) [40] and Shalaby and Morsy (2019) [44], and in the third week after baseline by Stando et al. (2020) [45]. At baseline no differences were observed between test and control groups regarding PPD, CAL and gingival inflammation assessments. This was found for plaque as well in seven studies. Only in one study baseline plaque scores were in the control group higher compared to that of the test group (Table 3) [45]. With regard to plaque reduction during the study, results of all studies showed no differences between test and control groups (Supplementary Material Table S1).
Table 3.
Mean values (standard deviation) of clinical periodontal parameters of test and control groups at baseline and final examination.
| Authors, Year, [Reference Number] | Groups | PPD (mm) | p-Value | Delta | CAL | p-Value | Delta | GI | p-Value | Delta | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Inter-Group | PPD | Baseline | Final | Inter- Group |
CAL | Baseline | Final | Inter-Group | GI | ||
| Deore et al., 2014 [41] | T | 4.26 ± 1.10 | 2.15 ± 0.53 | p < 0.05 | 2.11 | 5.53 ± 0.95 | 2.73 ± 0.98 | p < 0.05 | 2.80 | 1.93 ± 0.29 | 1.12 ± 0.14 | p < 0.01 | 0.81 |
| C | 4.05 ± 1.03 | 2.77 ± 0.47 | 1.28 | 5.20 ± 0.90 | 3.72 ± 0.62 | 1.48 | 2.04 ± 0.34 | 1.43 ± 0.33 | 0.61 | ||||
| Suramya et al., 2014 [39] | T | 5.45 ± 0.42 | 4.30 ± 0.79 | p > 0.05 | 1.15 | 5.55 ± 0.52 | 4.56 ± 0.80 | p > 0.05 | 0.99 | 2.38 ± 0.31 | 1.08 ± 0.0.16 | p > 0.05 | 1.30 |
| C | 5.50 ± 0.53 | 4.56 ± 0.80 | 0.94 | 5.42 ± 0.37 | 4.56 ± 0.80 | 0.86 | 2.31 ± 0.31 | 1.05 ± 0.12 | 1.26 | ||||
| Keskiner et al., 2017 [42] | T | 3.72 (2.23–4.75) 4 | 2.46 (1.83–3.32) | p > 0.05 | 1.26 | 4.59 (3.04–5.31) 4 | 3.53 (2.42–4.08) | p > 0.05 | 1.06 | 1.82(1.49–2.12) 4 | 1.23(0.67–1.44) | p > 0.05 | 0.59 |
| C | 3.73 (2.43–4.25) | 2.38 (2.04–3.23) | 1.35 | 4.20 (2.73–5.33) | 3.10 (2.68–4.16) | 1.10 | 1.68(1.45–1.92) | 1.18(1.06–1.34) | 0.50 | ||||
| El-Sharkawy and Elmeadawy, 2017 [43] | T | 4.76 ± 0.84 | 2.24 ± 0.27 | p < 0.001 | 2.52 | 5.16 ± 0.52 | 2.78 ± 0.38 | p < 0.001 | 2.38 | 2.32 ± 0.19 1 | 0.66 ± 0.16 | p > 0.05 | 1.66 |
| C | 4.46 ± 0.57 | 3.37 ± 0.64 | 1.09 | 5.08 ± 0.46 | 3.84 ± 0.49 | 1.24 | 2.27 ± 0.13 | 0.72 ± 0.14 | 1.55 | ||||
| Elgendy and Kazem, 2018 [40] | T | 6.00 ± 0.59 | 3.46 ± 0.49 | p < 0.05 | 2.54 | 5.96 ± 0.61 | 3.40 ± 0.50 | p < 0.05 | 2.56 | 1.98 ± 0.30 | 0.30 ± 0.23 | p < 0.05 | 1.68 |
| C | 5.84 ± 0.61 | 4.29 ± 0.75 | 1.55 | 5.79 ± 0.72 | 4.06 ± 0.59 | 1.73 | 2.06 ± 0.39 | 0.55 ± 0.32 | 1.51 | ||||
| Rampally et al., 2019 [38] | T | 6.71 ± 0.47 | 4.71 ± 0.47 | p > 0.05 | 2.00 | 5.71 ± 0.47 | 3.71 ± 0.47 | p > 0.05 | 2.00 | 2.03 ± 0.30 | 1.26 ± 0.44 | p > 0.05 | 0.77 |
| C | 6.43 ± 0.51 | 4.43 ± 0.51 | 2.00 | 5.43 ± 0.51 | 3.43 ± 0.51 | 2.00 | 1.96 ± 0.44 | 1.14 ± 0.57 | 0.82 | ||||
| Shalaby and Morsy, 2019 [44] | T | 5.47 ± 0.94 | 3.14 ± 0.64 | p < 0.01 | 2.33 | 4.08 ± 0.96 | 2.60 ± 0.52 | p < 0.01 | 1.48 | 1.95 ± 0.53 2 | 0.47 ± 0.25 | p > 0.05 | 1.48 |
| C | 5.82 ± 0.61 | 4.31 ± 0.84 | 1.51 | 4.28 ± 0.57 | 3.49 ± 0.85 | 0.79 | 2.03 ± 0.49 | 0.57 ± 0.30 | 1.46 | ||||
| Stando et al., 2020 [45] | T | 5.0 ± 0.5 | 3.7 ± 0.7 | p > 0.05 | 1.3 | 5.8 ± 0.8 | 4.4 ± 1.1 | p < 0.05 | 1.4 | 28 ± 16 3 | 14 ± 6 | p > 0.05 | 14 |
| C | 5.1 ± 0.8 | 4.0 ± 0.7 | 1.1 | 6.1 ± 1.1 | 5.3 ± 1.0 | 0.8 | 36 ± 19 | 21 ± 7 | 15 | ||||
T: test group; C: control group; PPD: probing pocket depth; CAL: clinical attachment loss; GI: gingival index; 1 modified gingival index; 2 GI modification; 3 bleeding on probing (%); 4 median and percentiles (25–75).
Comparison of the treatment results of the included studies is presented in Table 3. In all studies it was found that the provided treatment resulted both in test- and control groups in significant PPD reduction, CAL gain and less inflammation. Omega-3 supplementation resulted in four of eight studies in significant greater PPD reduction and CAL gain compared to the control group [40,41,43,44]. In one study [45] a significant CAL gain in favor of the test group did not coincide with greater PPD reduction. A significant reduction of gingival inflammation in favor of the test group was found in two studies [40,41]. There was no indication that the amount omega-3 supplementation was related to a possible treatment effect.
The estimated risk of bias of the included studies is shown in detail in Figure 2. Selection bias was observed in two studies [38,43] where insufficient information was provided regarding the confidentiality of the allocation of experimental groups. The blinding of participants was not possible in those studies that did not use a placebo in their interventions [39,43,44,45]. The lack of information about which teeth were examined, led to the classification of one study, in domain two, as high risk [38]. The absence of a placebo in four studies [39,43,44,45] and led to their classification, in domain two, as moderate risk. The overall assessment of the potential risk of bias resulted in six studies with a high risk of bias and only two with low risk. Due to the serious risk of bias and inconsistency found here, the studies included have moderate certainty regarding PPD, CAL and gingival inflammation results. Table 4 summarizes the factors used to assess the quality of evidence according to the GRADE system.
Table 4.
Summary of findings based on quality and body of evidence. GRADE.
| Participants (Studies) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Overall Certainty of Evidence | Impact |
|---|---|---|---|---|---|---|---|
| Probing depth (PPD) | |||||||
| 302 (8 RCTs) |
very serious | serious | not serious | not serious | All plausible residual confounding would reduce the demonstrated effect dose response gradient | +++○ | Six studies have a high risk of bias. In general, studies report different results for PPD |
| Clinical attachment loss (CAL) | |||||||
| 302 (8 RCTs) |
very serious | serious | not serious | not serious | All plausible residual confounding would reduce the demonstrated effect dose response gradient | +++○ | Six studies have a high risk of bias. In general, studies report different results for CAL |
| Gingival inflammation | |||||||
| 302 (8 RCTs) |
very serious | serious | not serious | not serious | All plausible residual confounding would reduce the demonstrated effect dose response gradient | +++○ | Six studies have a high risk of bias. In general, studies report different results for gingival inflammation |
+++○, Moderate.
4. Discussion
This systematic review investigated the effect of omega-3 supplementation on NSPT in randomized clinical trials. In this respect, the omega-3 dose administered and the duration time of the administration are important variables. It has been shown that incorporation rates of EPA and DHA vary according to the sample evaluated; incorporation may take days (for triglycerides and phosphatidylcholine in plasma fractions), months (6–9 months for mononuclear cells), or years (for adipose tissue) [50]. The benefits of supplementation also manifest over varying time intervals for different diseases. In addition, the greatest benefits with omega-3 are believed to come from the daily intake of fatty fish (salmon, tuna, mackerel, herring and sardines) and from some seeds and vegetables [51]. However, none of the studies included here evaluated the current dietary habits of the participants, which could lead to significant bias. Besides, the most current international recommendations for intake of DHA and EPA are based mainly on epidemiological and clinical studies that aimed to assess the benefits of omega-3 intake for the treatment of cardiovascular diseases, suggesting a dose ranging from 0.4 to 1.0 g/day [52,53,54].
In the studies included here, the highest omega-3 dosage used was 4400 mg per day consisting of 2600 mg of EPA and 1800 mg of DHA [45]; compared to the control group this resulted in more CAL gain but not more PPD and BOP reduction. These results might be partially explained by the short supplementation time (three months), which contradicts data suggesting a minimum period of six months for EPA and DHA to be incorporated into mononuclear cells [50]. The three-months supplementation period was used in five of the eight included studies. In two studies a significant effect on PPD reduction in favor of the test group was found and in three not. The two positive studies used a daily dosage of 300 mg omega-3 [41] and 2000 mg [43], respectively. The daily omega-3 dosage of the three negative studies was 1000 mg in two studies [38,39] and 4400 mg in the study as mentioned above [45]. With regard to CAL, the three months supplementation resulted in three studies with a significant CAL gain [41,43,45] and in two not [38,39]. These findings may suggest that three months omega-3 supplementation has a greater effect on CAL gain than PPD reduction. However, to put it in perspective, the PPD reduction in control groups of the above five studies varied from 0.94 mm to 2.0 mm (mean 1.3 mm) which is within the expected range of the effect of non-surgical periodontal therapy (NSPT) after three months [55]. CAL gain in the control group varied from 0.8 mm to 2.0 with a mean value of 1.3 mm, comparable to the PPD reduction. This is in contrast to the results as presented by Cobb (2002) [55] showing CAL gain after NSPT of about 0.5 mm. This may indicate bias in the supplementation studies.
Regarding the performed periodontal therapy, two three-month 1000 mg omega-3 supplementation studies without significant differences between test and control [38,39] did not carry out oral hygiene instructions, in contrast to the other studies. The lack of oral hygiene instructions is an important issue because it has been shown that supragingival plaque control reduces PPD values, improves CAL gain [56,57], as well as maintenance the results obtained after NSPT [58]. On the other hand, these two studies were the only studies that included participants with systemic conditions that interfere with the individual’s inflammatory state and are considered a risk factor (diabetes mellitus) or risk indicator (obesity) for periodontal diseases. Including participants with systemic conditions that alter the host response to periodontal treatment may be premature, as existing evidence is insufficient to identify the patient profile and severity of periodontal disease that will benefit from omega-3 supplementation in individuals with such conditions.
A longer period of daily omega-3 supplementation for 6 months was used in three studies. In two studies a significant effect was found for PPD reduction and CAL gain in favor of the test group [40,44] and in one study not [42]. This discrepancy may be linked to the omega-3 dosage used. Elgendy and Kazem (2018) [40] used 1000 mg omega-3 and Shalaby and Morsy (2019) [44] 3000 mg omega-3. In contrast, Keskiner et al. (2017) [42] used 50.88 mg omega-3 which may be too low to be effective. These two positive 6-month studies would suggest that supplementation of NSPT with daily omega-3 ≥ 1000 mg over a longer period of time is effective, confirming the time period needed for substantial incorporation of EPA and DHA in mononuclear cells [50]. Nevertheless, as in the three-month studies, the amount of PPD reduction in the control groups is within the expected range of the effect of non-surgical periodontal therapy (NSPT) after six months [55]. However, regarding CAL gain this was not the case. Both Elgendy and Kazem (2018) [40] and Shalaby and Morsy (2019) [44] show CAL gain of 1.5 mm, which is according to the data provided by Cobb (2002) [55] highly exceptional.
The above observations underscore that new RCTs on periodontal treatment and omega-3 supplementation should include, not only six months of omega-3 supplementation, but also evaluation of the gingival condition in combination with the level of supragingival plaque control on a regular basis as well as evaluation of the regular consumption of omega-3 from the daily diet. In addition, the medical condition of study participants should be checked in order to selects patients suffering from no other disease or condition than periodontal disease.
The studies included in this systematic review present several limitations. The few RCTs available to date exhibit a high degree of methodological heterogeneity, which precluded the performance of a meta-analysis. Based on the RoB tool, six studies included RCTs were also characterized by a high risk of bias. Meanwhile, the GRADE system classified the quality of evidence for the results of this review as ‘moderate.’ These limitations prevent us from reaching a satisfactory conclusion on the benefits of omega-3 supplementation.
5. Conclusions
Notwithstanding all limitations, a promising effect of omega-3 supplementation on the effect of NSPT was present in two six months studies with daily omega-3 supplementation ≥ 1000 mg. Both showing significant more PPD reduction and CAL gain after NSPT in the test group compared to the control. These findings encourage performing RCTs with better-defined treatment protocols and greater methodological rigor.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14091838/s1, Table S1: Data about dental plaque and oral hygiene instructions (OHI) and reinforcement (OHR) along over the study period.
Author Contributions
S.C.G. and U.v.d.V. contributed to conception and design of the study; L.M.M., F.B.P. and S.C.G. conducted the database search; S.C.G., U.v.d.V., L.M.M. and F.B.P. made substantial contribution to interpretation of data and drafted the manuscript. S.C.G., U.v.d.V., L.M.M. and F.B.P. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be obtained from the referred publications.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Faust K., Sathirapongsasuti F., Izard J., Segata N., Gevers D., Raes J., Huttenhower C. Microbial Co-occurrence Relationships in the Human Microbiome. PLoS Comput. Biol. 2012;8:e1002606. doi: 10.1371/journal.pcbi.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh P.D., Head D.A., Devine D.A. Ecological Approaches to Oral Biofilms: Control without Killing. Caries Res. 2015;49:46–54. doi: 10.1159/000377732. [DOI] [PubMed] [Google Scholar]
- 3.Curtis M.A., Diaz P.I., Van Dyke T.E. The role of the microbiota in periodontal disease. Periodontol. 2000. 2020;83:14–25. doi: 10.1111/prd.12296. [DOI] [PubMed] [Google Scholar]
- 4.Hajishengallis G., Lamont R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamont R.J., Koo H., Hajishengallis G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Keefe J.H., Gheewala N.M., O’Keefe J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2008;51:249–255. doi: 10.1016/j.jacc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Saito T., Shimazaki Y. Metabolic disorders related to obesity and periodontal disease. Periodontol. 2000. 2007;43:254–266. doi: 10.1111/j.1600-0757.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 8.Saito T., Yamaguchi N., Shimazaki Y., Hayashida H., Yonemoto K., Doi Y., Kiyohara Y., Iida M., Yamashita Y. Serum Levels of Resistin and Adiponectin in Women with Periodontitis: The Hisayama Study. J. Dent. Res. 2008;87:319–322. doi: 10.1177/154405910808700416. [DOI] [PubMed] [Google Scholar]
- 9.Dawson D.R., 3rd, Branch-Mays G., Gonzalez O.A., Ebersole J.L. Dietary modulation of the inflammatory cascade. Periodontol. 2000. 2014;64:161–197. doi: 10.1111/j.1600-0757.2012.00458.x. [DOI] [PubMed] [Google Scholar]
- 10.Ball D.W., Hill J.W., Scott R.J. The Basics of General, Organic, and Biological Chemistry. 1st ed. Flatword; Boston, MA, USA: 2011. pp. 957–1003. [Google Scholar]
- 11.Fahy E., Subramaniam S., Brown H.A., Glass C.K., Merrill A.H., Murphy R.C., Raetz C.R.H., Russell D.W., Seyama Y., Shaw W., et al. A comprehensive classification system for lipids. J. Lipid Res. 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Alikhani M., MacLellan C.M., Raptis M., Vora S., Trackman P.C., Graves D.T. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. American journal of physiology. Cell Physiol. 2007;292:2. doi: 10.1152/ajpcell.00356.2006. [DOI] [PubMed] [Google Scholar]
- 13.Innes J.K., Calder P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Plourde M., Cunnane S.C. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. 2007;32:619–634. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- 15.Wiktorowska-Owczarek A., Berezinska M., Nowak J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015;24:931–941. doi: 10.17219/acem/31243. [DOI] [PubMed] [Google Scholar]
- 16.Poorani R., Bhatt A.N., Dwarakanath B., Das U.N. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur. J. Pharmacol. 2016;785:116–132. doi: 10.1016/j.ejphar.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 17.Nelson J.R., Budoff M.J., Wani O.R., Le V., Patel D.K., Nelson A., Nemiroff R.L. EPA’s pleiotropic mechanisms of action: A narrative review. Postgrad. Med. 2021;133:651–664. doi: 10.1080/00325481.2021.1921491. [DOI] [PubMed] [Google Scholar]
- 18.Saini R.K., Keum Y. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018;203:255–267. doi: 10.1016/j.lfs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 19.Nelson J.R., Raskin S. The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019;131:268–277. doi: 10.1080/00325481.2019.1607414. [DOI] [PubMed] [Google Scholar]
- 20.Simopoulos A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 21.GISSI Prevezione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin e after myocardial infarction: Results of the gissi-prevenzione trial. Lancet. 1999;354:447–455. doi: 10.1016/S0140-6736(99)07072-5. [DOI] [PubMed] [Google Scholar]
- 22.Raheja B.S., Sadikot S.M., Phatak R.B., Rao M.B. Significance of the N-6/N-3 ratio for insulin action in diabetes. Ann. N. Y. Acad. Sci. 1993;683:258–271. doi: 10.1111/j.1749-6632.1993.tb35715.x. [DOI] [PubMed] [Google Scholar]
- 23.James M.J., Cleland L.G. Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin. Arthritis Rheum. 1997;27:85–97. doi: 10.1016/S0049-0172(97)80009-1. [DOI] [PubMed] [Google Scholar]
- 24.Broughton K.S., Johnson C.S., Pace B.K., Liebman M., Kleppinger K.M. Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5-series leukotriene production. Am. J. Clin. Nutr. 1997;65:1011–1017. doi: 10.1093/ajcn/65.4.1011. [DOI] [PubMed] [Google Scholar]
- 25.Kiecolt-Glaser J.K., Belury M.A., Porter K., Beversdorf D., Lemeshow S., Glaser R. Depressive Symptoms, omega-6:omega-3 Fatty Acids, and Inflammation in Older Adults. Psychosom. Med. 2007;69:217–224. doi: 10.1097/PSY.0b013e3180313a45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maillard V., Bougnoux P., Ferrari P., Jourdan M.-L., Pinault M., Lavillonnière F., Body G., Le Floch O., Chajès V. N-3 and N-6 fatty acids in breast adipose tissue and relative risk of breast cancer in a case-control study in Tours, France. Int. J. Cancer. 2002;98:78–83. doi: 10.1002/ijc.10130. [DOI] [PubMed] [Google Scholar]
- 27.Huang C., Ebersole J. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral Microbiol. 2010;25:75–80. doi: 10.1111/j.2041-1014.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro-Vidal H., Sánchez M.C., Alonso-Español A., Figuero E., Ciudad M.J., Collado L., Herrera D., Sanz M. Antimicrobial Activity of EPA and DHA against Oral Pathogenic Bacteria Using an In Vitro Multi-Species Subgingival Biofilm Model. Nutrients. 2020;12:2812. doi: 10.3390/nu12092812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesavalu L., Vasudevan B., Raghu B., Browning E., Dawson D., Novak J.M., Correll M., Steffen M., Bhattacharya A., Fernandes G., et al. Omega-3 Fatty Acid Effect on Alveolar Bone Loss in Rats. J. Dent. Res. 2006;85:648–652. doi: 10.1177/154405910608500713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kesavalu L., Sathishkumar S., Bakthavatchalu V., Matthews C., Dawson D., Steffen M., Ebersole J.L. Rat Model of Polymicrobial Infection, Immunity, and Alveolar Bone Resorption in Periodontal Disease. Infect. Immun. 2007;75:1704–1712. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naqvi A.Z., Buettner C., Phillips R.S., Davis R.B., Mukamal K.J. n-3 Fatty Acids and Periodontitis in US Adults. J. Am. Diet. Assoc. 2010;110:1669–1675. doi: 10.1016/j.jada.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azzi D.V., Viafara J.A.S., Zangeronimo M.G., Lima R.R., Marques L.S., Pereira L.J. n-3 Ingestion may modulate the severity of periodontal disease? Systematic review. Crit. Rev. Food Sci. Nutr. 2018;58:1937–1942. doi: 10.1080/10408398.2017.1278677. [DOI] [PubMed] [Google Scholar]
- 33.Chatterjee D., Chatterjee A., Kalra D., Kapoor A., Vijay S., Jain S. Role of adjunct use of omega 3 fatty acids in periodontal therapy of periodontitis. A systematic review and meta-analysis. J. Oral Biol. Craniofacial Res. 2022;12:55–62. doi: 10.1016/j.jobcr.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruse A.B., Kowalski C.D., Leuthold S., Vach K., Ratka-Krüger P., Woelber J.P. What is the impact of the adjunctive use of omega-3 fatty acids in the treatment of periodontitis? A systematic review and meta-analysis. Lipids Health Dis. 2020;19:100. doi: 10.1186/s12944-020-01267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins J.P., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Volume 5 John Wiley & Sons; Hoboken, NJ, USA: 2011. [Google Scholar]
- 36.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:7. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group. 2019. [(accessed on 20 February 2022)]. Available online: http://www.gradeworkinggroup.org.
- 38.Koduganti R.R., Rampally P., Ganapathi S.N., Panthula V.R., Surya P.J. Comparison of effectiveness of low-dose aspirin versus omega-3 fatty acids as adjuvants to nonsurgical periodontal therapy in Type II diabetic patients with chronic periodontitis. J. Indian Soc. Periodontol. 2019;23:249–256. doi: 10.4103/jisp.jisp_528_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suramya S., Gujjari S.K., Ali N. Evaluation of Efficacy of Omega 3 Fatty Acid Supplementation in Obese Patients: A Pilot Study. J. Dent. Med. Sci. 2014;13:49–60. [Google Scholar]
- 40.Elgendy E.A., Kazem H.H. Effect of Omega-3 Fatty Acids on Chronic Periodontitis Patients in Postmenopausal Women: A Randomised Controlled Clinical Study. Oral Heal. Prev. Dent. 2018;16:327–332. doi: 10.3290/j.ohpd.a40957. [DOI] [PubMed] [Google Scholar]
- 41.Deore G.D., Gurav A.N., Patil R., Shete A.R., NaikTari R.S., Inamdar S.P. Omega 3 fatty acids as a host modulator in chronic periodontitis patients: A randomised, double-blind, palcebo-controlled, clinical trial. J. Periodontal Implant. Sci. 2014;44:25–32. doi: 10.5051/jpis.2014.44.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keskiner I., Saygun I., Bal V., Serdar M., Kantarci A. Dietary supplementation with low-dose omega-3 fatty acids reduces salivary tumor necrosis factor-α levels in patients with chronic periodontitis: A randomized controlled clinical study. J. Periodontal Res. 2017;52:695–703. doi: 10.1111/jre.12434. [DOI] [PubMed] [Google Scholar]
- 43.El-Sharkawy H.M., Elmeadawy S.H. The impact of ômega-3 fatty acids combined with initial periodontal therapy on salivar visfatin and tnf-a levels in chronic periodontitis patients. Egypt. Dent. J. 2017;63:1437–1447. doi: 10.21608/edj.2017.74541. [DOI] [Google Scholar]
- 44.Shalaby H.K., Morsy S.M. Evaluation of adjunctive effect of daily dietary supplements with omega 3 and propolis to non-surgical periodontal therapy: Randomized Clinical Trial. Egypt. Dent. J. 2019;65:3543–3553. doi: 10.21608/edj.2019.74811. [DOI] [Google Scholar]
- 45.Stańdo M., Piatek P., Namiecinska M., Lewkowicz P., Lewkowicz N. Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial. Nutrients. 2020;12:2614. doi: 10.3390/nu12092614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tonetti M.S., Greenwell H., Kornman K.S. Staging and gradin g of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018;89:S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 47.Löe H., Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 48.Gordon J.M., Lamster I.B., Seiger M.C. Efficacy of Listerine antiseptic in inhibiting the development of plaque and gingivitis. J. Clin. Periodontol. 1985;12:697–704. doi: 10.1111/j.1600-051X.1985.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 49.Saxton C.A., Van Der Ouderaa F.J. The effect of a dentifrice containing zinc citrate and Triclosan on developing gingivitis. J. Periodontal Res. 1989;24:75–80. doi: 10.1111/j.1600-0765.1989.tb00860.x. [DOI] [PubMed] [Google Scholar]
- 50.Browning L.M., Walker C.G., Mander A.P., West A.L., Madden J., Gambell J.M., Young S., Wang L., Jebb S.A.A., Calder P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012;96:748–758. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calder P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017;45:1105–1115. doi: 10.1042/BST20160474. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization . Diet, Nutrition and the Prevention of Chronic Diseases. WHO; Geneva, Switzerland: 2003. (WHO Technical Report Series). no. 916. [PubMed] [Google Scholar]
- 53.UK Scientific Advisory Committee on Nutrition . Advice on Fish Consumption: Benefits and Risks. UK Scientific Advisory Committee on Nutrition; London, UK: 2004. [Google Scholar]
- 54.Cunnane S.C. Recommendations for Intake of Polyunsaturated Fatty Acids in Healthy Adults. Int. Soc. Study Fat. Acids Lipids. 2004;11:12–25. [Google Scholar]
- 55.Cobb C.M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002;29((Suppl. 2)):22–32. doi: 10.1034/j.1600-051X.29.s2.4.x. [DOI] [PubMed] [Google Scholar]
- 56.Gomes S.C., Piccinin F.B., Susin C., Oppermann R.V., Marcantonio R.A. Effect of Supragingival Plaque Control in Smokers and Never-Smokers: 6-Month Evaluation of Patients With Periodontitis. J. Periodontol. 2007;78:1515–1521. doi: 10.1902/jop.2007.060462. [DOI] [PubMed] [Google Scholar]
- 57.Gomes S.C., Romagna R., Rossi V., Corvello P.C., Angst P.D.M. Supragingival treatment as an aid to reduce subgingival needs: A 450-day investigation. Braz. Oral Res. 2014;28:1–7. doi: 10.1590/S1806-83242014.50000004. [DOI] [PubMed] [Google Scholar]
- 58.Angst P.D.M., Stadler A.F., Mendez M., Oppermann R.V., Van Der Velden U., Gomes S.C. Supportive periodontal therapy in moderate-to-severe periodontitis patients: A two-year randomized clinical trial. J. Clin. Periodontol. 2019;46:1083–1093. doi: 10.1111/jcpe.13178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be obtained from the referred publications.