Abstract
Entry of mammalian cells into DNA synthesis (S-phase) represents a key event in cell division1. According to the current cell-cycle models, the Cdc7 kinase constitutes an essential and rate-limiting trigger of DNA replication, acting together with cyclin-dependent kinase Cdk2. Here we show, using chemical-genetic systems which allow an acute shutdown of Cdc7 in in vitro cultured cells as well as in vivo in a living mouse, that Cdc7 is dispensable for cell division of many different cell types. We demonstrate that another cell-cycle kinase, Cdk1, is also active during G1/S transition both in cycling cells and in cells exiting quiescence. We show that Cdc7 and Cdk1 play functionally redundant roles during G1/S transition, and at least one of these kinases must be present to allow S-phase entry. These observations revise our understanding of cell-cycle progression by demonstrating that Cdk1 physiologically regulates two distinct transitions during cell division cycle, while Cdc7 plays a redundant function in DNA replication.
Progression of cells through G1-phase and their entry into DNA-synthesis (S-phase) represent one of the most tightly regulated steps of cell division. Mechanisms governing G1/S transition are frequently deregulated in human cancers, and they represent targets of cell-cycle-focused anti-cancer therapies1. During G1-phase, several proteins assemble on DNA replication origins and form pre-replication complexes (pre-RC), which await a signal to trigger origin firing. This signal is believed to be provided by Cdc7 kinase acting in concert with cyclin E-Cdk22,3. The major function of Cdc7 is phosphorylation of Mcm proteins within pre-RCs4–8. This, together with the action of cyclin E-Cdk2, promotes binding of Cdc45 and GINS to Mcm2–7, resulting in formation of Cdc45-Mcm-GINS (CMG) complex and activation of DNA-helicase. These events cause unwinding of double-stranded DNA, recruitment of DNA-polymerase and initiation of DNA synthesis9.
Cdc7 is a highly conserved serine-threonine kinase activated through interaction with its regulatory subunit, Dbf44–8. According to the current cell-cycle models, Cdc7 represents essential component of the cell-cycle machinery that is indispensable for S-phase entry in all organisms studied10.
We decided to revisit this notion using two independent chemical-genetic approaches, the analog-sensitive inhibition approach and generation of cells and mice that allow highly specific and acute targeted degradation of Cdc7 protein in vitro and in vivo.
Cell proliferation without Cdc7
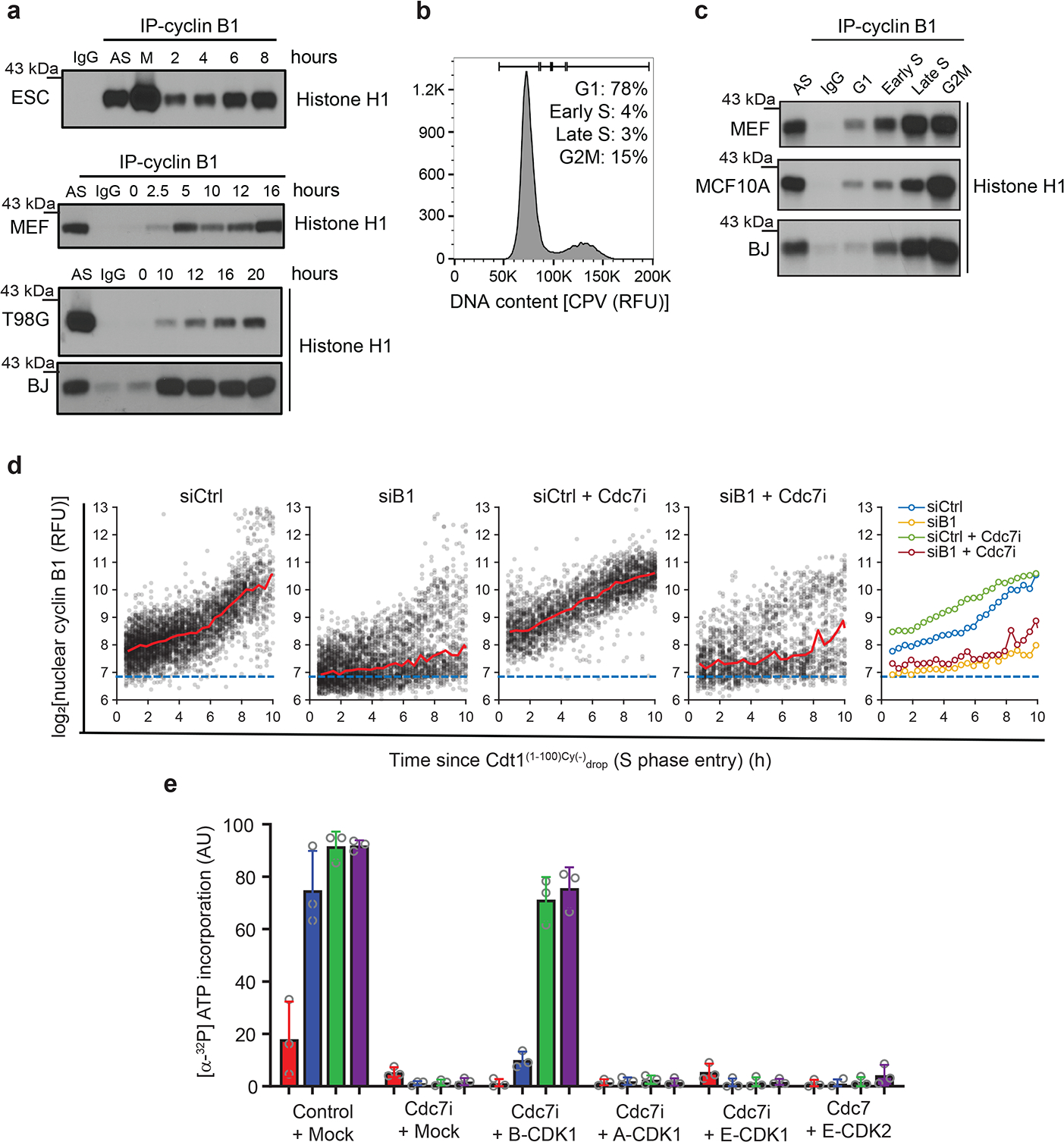
To re-assess the requirement for Cdc7 in cell proliferation, we first generated analog-sensitive (as) version of Cdc7 by mutating the bulky gatekeeper residue in the Cdc7 ATP-binding pocket from methionine-128 to glycine. Such substitution creates an enlarged ATP-binding pocket not found in wild-type kinases. While the analog-sensitive substitution does not alter kinase specificity11, the substituted kinase can be selectively inhibited by ‘bulky’ chemical compounds such as 1NM-PP1 or 3MB-PP1 that occupy the enlarged ATP-binding pocket12 (Extended Data Fig. 1a). Importantly, as inhibitors do not inhibit wild-type kinases. We knocked-in the asCdc7 mutation into the endogenous Cdc7 locus in murine embryonic stem cells (ESC) and generated homozygous Cdc7AS/AS cells (Extended Data Fig. 1b). Surprisingly, acute inhibition of Cdc7 in Cdc7AS/AS cells (by 1NM-PP1) did not arrest proliferation, although cell growth was retarded (Fig. 1a). In contrast, similar inhibition of Cdk1, an essential kinase for cell proliferation13, using Cdk1AS/AS ESC12, completely arrested cell growth (Extended Data Fig. 1c–d).
Fig. 1. Shutdown of Cdc7 in in vitro cultured cells.
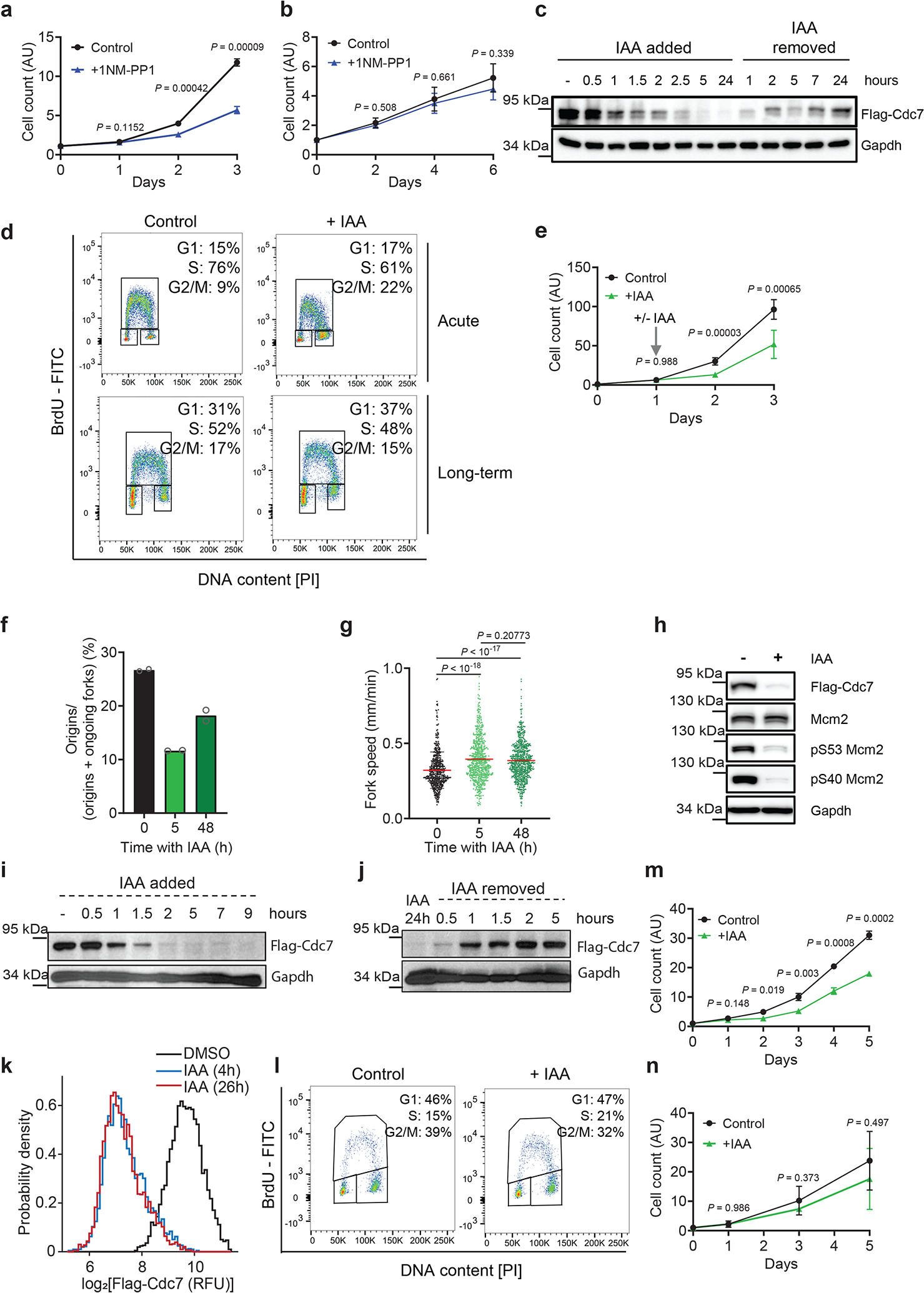
a, b, Growth curves of Cdc7AS/AS ESC (a) and MEFs (b) cultured with 1NM-PP1 (to inhibit Cdc7) or with vehicle (Control). c, Western blot analysis of Cdc7 protein levels in Cdc7AID/AID ESC treated with auxin (IAA). d, Flow cytometry of Cdc7AID/AID/Tir1 ESC treated with auxin for 12h (Acute) or 48 h (Long-term). e, Growth curves of Cdc7AID/AID/Tir1 ESC. Starting at day 1, cells were treated with auxin or vehicle. f, g, DNA fiber analysis: mean fraction of new replication origins (f), and fork speed (g) in Cdc7AID/AID/Tir1 ESC treated with auxin for the indicated times. h, Western blot analysis of phospho-Mcm2 residues in Cdc7AID/AID/Tir1 ESC treated with auxin for 2 days. i, j, Western blot analysis of Cdc7 protein levels in Cdc7AID/AID/Tir1 MEFs treated with auxin (i), or after auxin removal (j). k, Immunofluorescence analysis of Cdc7AID/AID/Tir1 MEFs treated for 4 or 26 h with auxin or vehicle (DMSO) and stained for Flag-Cdc7. l, Flow cytometry of Cdc7AID/AID/Tir1 MEFs treated with auxin or vehicle for 2 days.. m, Growth curves of Cdc7AID/AID/Tir1 MEFs cultured in the presence of auxin or vehicle n, Growth curves of Cdc7AID/AID/Tir1 MEFs immortalized by dominant-negative p53, cultured with auxin or with vehicle. a, b, e, f, g, m, n, shown are mean values; p-values were determined by two-sided t-test; error bars, SD. In a, b, e, m, n, n=3 independent replicates; f, n=2; g, n > 500 cells, c, d, h-k representative results (out of 2).
To test the requirement for Cdc7 kinase in another cell type, we injected Cdc7AS/AS ESC into mouse blastocysts and generated Cdc7AS/AS embryos, from which we derived mouse embryonic fibroblasts (MEFs). Again, Cdc7AS/AS MEFs continued to proliferate, despite an acute Cdc7 inhibition by 1NM-PP1 (Fig. 1b).
To extend these findings, we generated a system to acutely remove the Cdc7 protein. We adopted the Auxin-Inducible Degradation (AID) approach14. This plant-derived system can be ‘transplanted’ into mammalian cells by inserting the AID-domain into the protein of interest, together with simultaneous expression of plant F-box protein Tir114 (Extended Data Fig. 1e). Administration of auxin (Indole Acetic Acid, IAA) to cells engineered in this fashion triggers an acute degradation of the AID-tagged protein. This system has been used to degrade proteins in in vitro cultured cells, but not to target endogenous proteins in a living animal. We knocked-in the AID-domain into the endogenous Cdc7 locus in ESC, and generated Cdc7AID/AID cells (Extended Data Fig. 1f–g). In addition, we knocked in the Tir1 gene into the ubiquitously expressed Rosa26 locus (Extended Data Fig. 1h–k, Extended Data Fig. 2a–b).
Treatment of in vitro cultured Cdc7AID/AID/Tir1 ESC with auxin led to rapid loss of Cdc7 protein (Fig. 1c). Degradation of Cdc7 was accompanied by strong decrease in levels of Cdc7 regulatory subunit, Dbf4, suggesting that Dbf4 is unstable in the absence of its catalytic partner (Supplementary Table 1). Acute shutdown of Cdc7 resulted in a modest delay in cell-cycle progression, which lasted approximately 24h, after which cells resumed essentially normal proliferation (Fig. 1d–e, Extended Data Fig. 2c; see below). DNA fiber analysis revealed decrease in the number of new replication origins (Fig. 1f), which was compensated by increased speed of existing forks (Fig. 1g). By 48h, however, the number of new origins partly recovered, despite continued depletion of Cdc7 (Fig. 1f–g, Extended Data Fig. 2d). Analysis of Mcm2-phosphorylation revealed strongly reduced phosphorylation of Cdc7-dependent residues, which persisted as long as auxin was present (Fig. 1h). We cultured cells in the presence of auxin up to 7 weeks and observed that they proliferated normally and maintained efficient depletion of Cdc7 (Extended Data Fig. 2d).
To extend these observations to another cell type, we injected Cdc7AID/AID/Tir1 ESC into blastocysts and derived Cdc7AID/AID/Tir1 MEFs. Treatment of MEFs with auxin resulted in very efficient degradation of Cdc7 within 2h (Fig. 1i, k, Extended Data Fig. 2e); the effect was reversible upon auxin removal (Fig. 1j). Acute shutdown of Cdc7 resulted in transient and mild impairment of cell proliferation, followed by return of normal proliferation rates (Fig. 1l, m).
Inactivation of p53 is thought to render cells particularly vulnerable to Cdc7 inhibition15,16. To test this, we immortalized Cdc7AID/AID/Tir1 MEFs using dominant-negative p53. Again, these cells continued to proliferate despite Cdc7 degradation (Fig. 1n).
To better delineate the impact of Cdc7 inhibition on the first and successive cell-cycles, we performed live-cell imaging of Cdc7AID/AID/Tir1 MEFs acutely treated with auxin and human mammary epithelial MCF10A cells treated with CDC7 inhibitor. We observed lengthening of S-phase and modest prolongation of G1 and G2-phases during the first cell-cycle, resulting in increased duration of the first cell division. These parameters returned to nearly normal length in the second or third cell-cycles (Fig. 2a–c, Extended Data Fig. 3a–f). Consistent with live-cell imaging, pulse-chase analyses of ESC and MEFs treated with auxin as well as human cells treated with CDC7 inhibitor revealed lengthening of cell-cycle phases at 12–24h after Cdc7 degradation/inhibition, resulting in increased cell division time. These parameters returned to nearly normal length after 48–72h of continuous Cdc7 inhibition (Fig. 2d–g, Extended Data Fig. 3g–j). We did not observe increased cell death after Cdc7 degradation/inhibition (Fig. 2h–i, Extended Data Fig. 3k–n). The numbers of γH2AX foci were slightly increased in ESC, but not in MEFs shortly after Cdc7 depletion (Extended Data Fig. 3o, p, r); ESC cultured in the presence of auxin for 7 weeks did not display increased numbers of γH2AX foci (Extended Data Fig. 3q). We concluded that acute Cdc7 inhibition results in transient lengthening of cell-cycle, but no substantial DNA-damage or cell death.
Fig. 2. Analyses of cells following Cdc7 degradation.
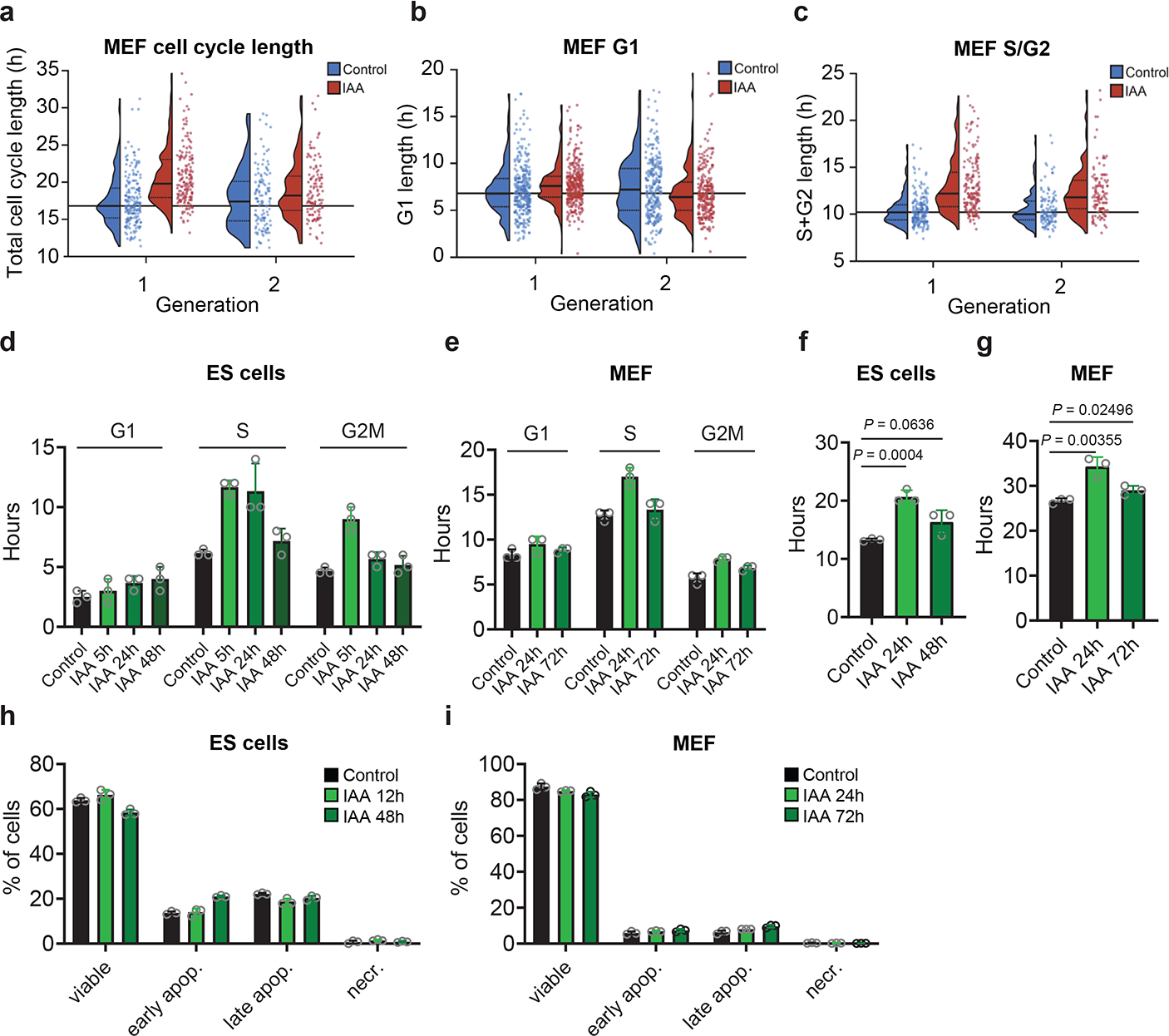
a-c, Long-term live-cell imaging. Immortalized CDC7AID/AID/Tir1 MEFs expressing Gem(1–110)-mCherry and Cdt1(1–100)Cy(−)-mVenus FUCCI(CA) cell-cycle reporters were cultured and imaged for a total of 77 h. Seven h after imaging start, cells were treated with auxin (IAA), or vehicle (Control) and imaged for additional 70 h. Shown are parameters for first two cell-cycles (generation 1 and 2) after addition of auxin (selecting cells which received IAA 0–4 h before mitosis). a, Total cell-cycle length (time between mitoses); b, G1 length (time between mitosis and Cdt1(1–100)Cy(−)drop, i.e., S-phase start). c, S/G2 length (time from Cdt1(1–100)Cy(−)drop to subsequent mitosis). Dots show values for individual cells, horizonal lines median values, dotted lines inter-quartile range, long horizonal line median value from control cells in generation 1. d-e, Length of cell-cycle phases determined by pulse-chase in Cdc7AID/AID/Tir1 ESC (d) and MEFs (e) treated for the indicated times with auxin. Control, length of cell-cycle phases in untreated cells. f, g, Doubling times of Cdc7AID/AID/Tir1 ESC (f) and MEFs (g) treated for the indicated times with auxin, determined by pulse-chase. Control, doubling time of untreated cells. h, i, Percentages of viable (Annexin V−/PI−), early apoptotic (Annexin V+/PI−), late apoptotic (Annexin V+/PI+) and necrotic (Annexin V−/PI+) Cdc7AID/AID/Tir1 ESC (h) and MEFs (i) treated with auxin for indicated times. Control, untreated cells. In d-i, bars denote mean values, p-values two-sided t-test; error bars, SD, n=3 independent replicates.
It was formally possible that vanishingly small amounts of Cdc7 activity left after auxin treatment (or after inhibition of analog-sensitive Cdc7) are sufficient to allow cell proliferation. To test this, we used CRISPR/Cas9 to knock-out Cdc7 in different mouse and human cell types. We also generated Cdc7Flox/Flox murine ESC by gene-targeting (Extended Data Fig. 1l), and acutely shut-down Cdc7 in these cells by expression of Cre-recombinase. In all cell types, we observed continued cell-cycle progression despite knocking out Cdc7 (Extended Data Fig. 4). Surprisingly, we observed that proliferating primary mouse cardiomyocytes do not express detectable Cdc7 protein (Extended Data Fig. 5a–b). Lastly, we used CRISPR/Cas9 to knock-out the activator of Cdc7, Dbf4 in immortalized murine fibroblasts. Dbf4-null cells continued to proliferate, albeit at modestly reduced rate (Extended Data Fig. 5c–g). Collectively, these observations revealed that Cdc7 is dispensable for proliferation of several cell types.
To extend these studies in vivo, we generated Cdc7AID/AID/Tir1 mice using standard methods. Probing of a panel of mouse organs with anti-Flag antibody (which detects endogenous Flag-tagged Cdc7AID) revealed that Cdc7 is expressed at very low levels in most organs, including no detectable Cdc7 protein in proliferative bone marrow (Extended Data Fig. 5h–j). Administration of auxin to adult Cdc7AID/AID/Tir1 mice led to very efficient depletion of Cdc7 in their internal organs, starting at 3–6h (Extended Data Fig. 5k–l), which persisted throughout the entire study (Extended Data Fig. 5m). Prolonged shutdown of Cdc7 did not result in any visible phenotypes and did not impede proliferation of mitotically active compartments (Extended Data Fig. 5n–o). We concluded that Cdc7 is largely dispensable in in vitro cultured cells and in vivo, in the living mouse.
Synergy between Cdc7 and Cdk1
We wished to understand the molecular basis of cell proliferation in the absence of Cdc7. Since Cdk2 was postulated to play role with Cdc7 in firing of DNA replication origins2,3, we considered a possibility that in the absence of Cdc7, Cdk2 alone can drive entry of cells into S-phase. Cdk2-null mice are viable, indicating that Cdk2 is not essential for DNA synthesis17,18. We used CRISPR/Cas9 to generate Cdk2-knockout Cdc7AID/AID/Tir1 ESC or CDK2-knockout human mammary epithelial HMEC and MCF10A cells. We also engineered human cells to express analog-sensitive CDK2 in place of the endogenous CDK2. Surprisingly, combined ablation of CDK2 (or acute inhibition of CDK2 kinase) plus degradation/inhibition of CDC7 did not block asynchronous cell proliferation and had little effect on S-phase entry in cells returning from quiescence (Fig. 3a–c, Extended Data Fig. 6). We concluded that another kinase may act at G1/S to drive S-phase entry.
Fig. 3. Analyses of Cdk1 in S-phase entry.
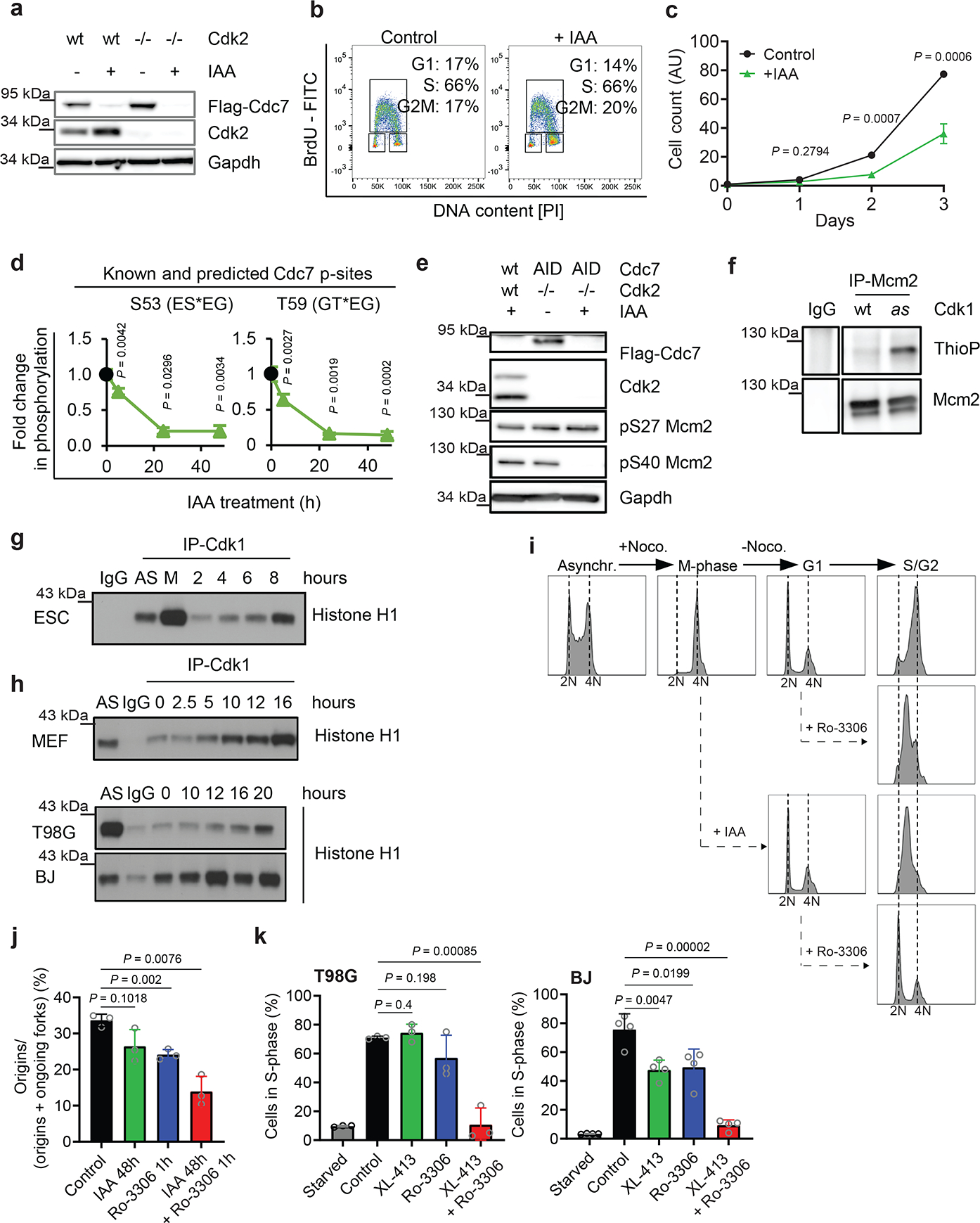
a, Immunoblotting of Cdk2+/+ (wt) and Cdk2-knockout (Cdk2−/−) Cdc7AID/AID/Tir1 ESC treated with auxin (IAA). b, Flow cytometry of Cdk2-knockout/Cdc7AID/AID/Tir1 ESC treated with auxin or vehicle (Control). c, Growth curves of Cdk2-knockout/Cdc7AID/AID/Tir1 ESC treated with auxin or vehicle. d, Quantification of Mcm2-phosphorylation in Cdc7AID/AID/Tir1 ESC treated with auxin for 5, 24, 48h. Black dots, phosphorylation levels at time 0. e, Immunoblotting of Cdk2+/+ (wt) and Cdk2−/−, Cdc7AID/AID/Tir1 (AID) ESC for Cdc7-dependent (S40) and -independent (S27) Mcm2 phosphoresidues; cells were treated with auxin for 24h. f, In-cell phosphorylation of Mcm2 by Cdk1. IgG, control immunoprecipitation from Cdk1AS/AS cells. g, h, ESC were released from mitosis (g), MEFs, glioblastoma T98G and BJ foreskin fibroblasts were released from G0 (h). Cdk1 was immunoprecipitated at the indicated time-points and used for kinase reactions with histone H1 as substrate. AS, asynchronous cells; M, mitosis-arrested cells. IgG, control immunoprecipitation. i, Asynchronously growing Cdc7AID/AID/Tir1 ESC (Asynchr.) were synchronized in mitosis by nocodazole (M-phase) and released (−Noco). Upon release, Cdc7 degradation was induced by auxin addition to account for degradation time. Two hours later, when cells reached G1, they were treated with Cdk1 inhibitor Ro-3306. Cells were cultured in the presence of inhibitor(s), collected after 12h (S/G2), stained with propidium iodide and analyzed by flow cytometry. j, Fraction of new replication origins in Cdc7AID/AID/Tir1 ESC treated with auxin for 48 h and/or Ro-3306 for the last 1h, or with vehicle. k, T98G and BJ cells were arrested in G0 and released in the presence of CDC7 inhibitor XL-413 and/or Ro-3306 or vehicle. Cells were pulsed with BrdU after 19h and analyzed by flow cytometry. c, d, j,k, mean values; p-values two-sided t-test; error bars, SD. c, d, j, k, n=3 independent replicates; a, b, e-i representative results (out of 2).
To identify this kinase, we acutely degraded Cdc7 in Cdc7AID/AID/Tir1 cells and compared phosphorylation status of Mcm proteins, well-established targets of Cdc74–8, by quantitative mass spectrometry. We found that phosphorylation of known Cdc7-specific sites and sites sharing the Cdc7-consensus sequence (acidic aminoacid-directed) within Mcm proteins was strongly decreased upon Cdc7 depletion (Fig. 3d and Supplementary Table 2). In contrast, several other Mcm sites remained phosphorylated in Cdc7-depleted cells (Supplementary Table 2); normal phosphorylation of serine-27 of Mcm2 in Cdc7-depleted and in Cdc7-depleted/Cdk2-knockout cells was confirmed by immunoblotting with phosphospecific antibodies (Fig. 3e). Several of these Cdc7-independent sites represent predicted substrates for proline-directed kinases. Therefore, we hypothesized that another proline-dependent kinase might be responsible for their phosphorylation.
Further analyses of Cdc7-depleted phosphoproteome (Supplementary Table 2) revealed changes indicative of increased Cdk1 kinase activity (increased phosphorylation of activating Thr161 and decreased phosphorylation of inhibitory Thr15 sites of Cdk1) (Extended Data Fig. 7a). Immunoprecipitation of Cdk1 followed by kinase assays confirmed mildly increased Cdk1 activity in Cdc7-deficient cells (Extended Data Fig. 4p, Extended Data Fig. 7b). These observations led us to hypothesize that Cdk1 might be responsible for phosphorylating Mcms in vivo, as suggested by previous studies19–21. Consistent with this, we found that Cdk1, but not Cdc7, can phosphorylate serine-27 of Mcm2 (Extended Data Fig. 7c).
To test whether Cdk1 phosphorylates Mcm proteins in cells under physiological conditions, we took advantage of Cdk1AS/AS knock-in ESC expressing analog-sensitive Cdk112. Analog-sensitive kinases can utilize N6-substituted bulky ATP-analogs, while wild-type kinases cannot utilize them, due to steric hindrance. Hence, by providing cells expressing analog-sensitive kinase with bulky ATP-analogs in which gamma-phosphate has been replaced with thiophosphate, one can label direct substrates of this kinase with thiophosphate tag12. We cultured Cdk1AS/AS cells in the presence of bulky ATPγS-analog, resulting in direct labelling of Cdk1 substrates. We then immunoprecipitated endogenous Mcm2 and probed immunoblots with anti-thiophosphate ester antibody. We confirmed that Cdk1 directly phosphorylates Mcm2 in cells (Fig. 3f). Moreover, inhibition of Cdk1 kinase in Cdk1AS/AS cells, by 3MB-PP1, decreased phosphorylation of endogenous Mcm2 on serine-27 (Extended Data Fig. 7d).
According to the current cell-cycle models, Cdk1 kinase operates during mitosis and in late S-phase1. However, Cdk1 has not been implicated in normal G1/S transition. To test whether Cdk1 is physiologically active during S-phase entry, we synchronized wild-type mouse ESC in M-phase (by nocodazole), released the cells and followed their synchronous exit from mitosis and progression through G1 and S-phases (Extended Data Fig. 7e). We then immunoprecipitated Cdk1 and quantified its kinase activity. Surprisingly, we detected induction of Cdk1 kinase in cells entering S-phase (Fig. 3g). We also synchronized Cdk1AS/AS cells as above, and provided cells entering S-phase with bulky ATPγS-analog, to label Cdk1 substrates. This approach confirmed that Cdk1 is active and it directly phosphorylates Mcm2 at this cell-cycle stage (Extended Data Fig. 7f).
We also rendered mouse and human cells quiescent by serum deprivation, and then stimulated them to re-enter cell-cycle (Extended Data Fig. 7g). Again, we detected upregulation of Cdk1 kinase activity as cells entered S-phase (Fig. 3h). Collectively, these observations indicated that Cdk1 might play a physiological role during G1/S transition.
To test whether Cdk1 kinase activity is required during S-phase entry, we arrested Cdc7AID/AID/Tir1 ESC in M-phase (by nocodazole), released, and monitored cell-cycle progression (Fig. 3i, upper panel). When cells reached G1-phase, we degraded Cdc7 (by auxin), and/or inhibited Cdk1 (using Cdk1 inhibitor, Ro-3306) and continued the culture in the presence of compound(s). Consistent with our earlier results, Cdc7-degraded cells entered and progressed through S-phase, although at reduced pace (Fig 3i, third panel). Cells that underwent Cdk1 inhibition also progressed through S-phase (Fig. 3i, second panel). Strikingly, combined inhibition of Cdc7 and Cdk1 essentially blocked entry of cells into S-phase (Fig. 3i, bottom panel); similar results were seen in Cdk2-knockout ESC (Extended Data Fig. 7h). Consistent with these findings, treatment of asynchronously growing ESC with auxin plus Cdk1 inhibitor inhibited firing of DNA replication origins, as revealed by DNA combing (Fig. 3j).
Virtually identical results were obtained using Cdk1AS/AS ESC, which allow highly specific Cdk1 inhibition with inhibitors of analog-sensitive kinases. Again, combined inhibition of Cdk1 (by 3MB-PP1) and Cdc7 (by Cdc7-inhibitor XL-413) in G1-phase strongly inhibited S-phase entry (Extended Data Fig. 7i, bottom panel) and impeded origin firing (Extended Data Fig. 7j), while single treatments had modest effects (Extended Data Fig. 7i–j). We also detected a similar requirement for CDC7 and CDK1 at the G1/S transition in human T98G cells (Extended Data Fig 7k–l). Importantly, we verified that inhibition of Cdk1, or Cdc7, or both, did not impede the activity of endogenous Cdk2 (Extended Data Fig. 8).
We extended these findings using four types of human non-transformed cells and T98G cells which were arrested in G0-phase and then stimulated to re-enter cell-cycle. Again, inhibition of CDC7 or CDK1 alone had only modest effects on G1/S progression, while combined inhibition blocked entry into S-phase (Fig. 3k, Extended Data Fig. 7m–o). We concluded that CDC7 and CDK1 play redundant and overlapping roles in triggering S-phase entry in different human and mouse cell types, both in cycling cells and in cells re-entering cell-cycle from quiescence, and at least one of these kinases must be present to allow DNA synthesis.
Live-cell imaging
To verify these findings at single-cell resolution, we performed combined live and fixed-cell imaging of Cdc7AID/AID/Tir1 MEFs, human mammary epithelial MCF10A cells and primary human dermal fibroblasts. We live-imaged asynchronously growing cells in the presence of auxin or CDC7 inhibitors (human cells) or vehicle. Cells were also acutely treated with CDK1 inhibitor, Ro-3306, and we analyzed cells that received CDK1 inhibitor in G1-phase (starting within the first 2h after completion of mitosis). Cdc7-inhibited or Cdk1-inhibited cells entered and progressed through S-phase (as determined by EdU-incorporation and Hoechst staining for DNA content), albeit at reduced speed. In contrast, combined inhibition of Cdc7 and Cdk1 in G1-phase essentially abrogated S-phase entry (almost no EdU-incorporation), and arrested cells with 2N content, indicating a complete G1/S block upon Cdc7/Cdk1 inhibition (Fig. 4, Extended Data Fig. 9a–c).
Fig. 4. Live-cell imaging of Cdc7-depleted cells.
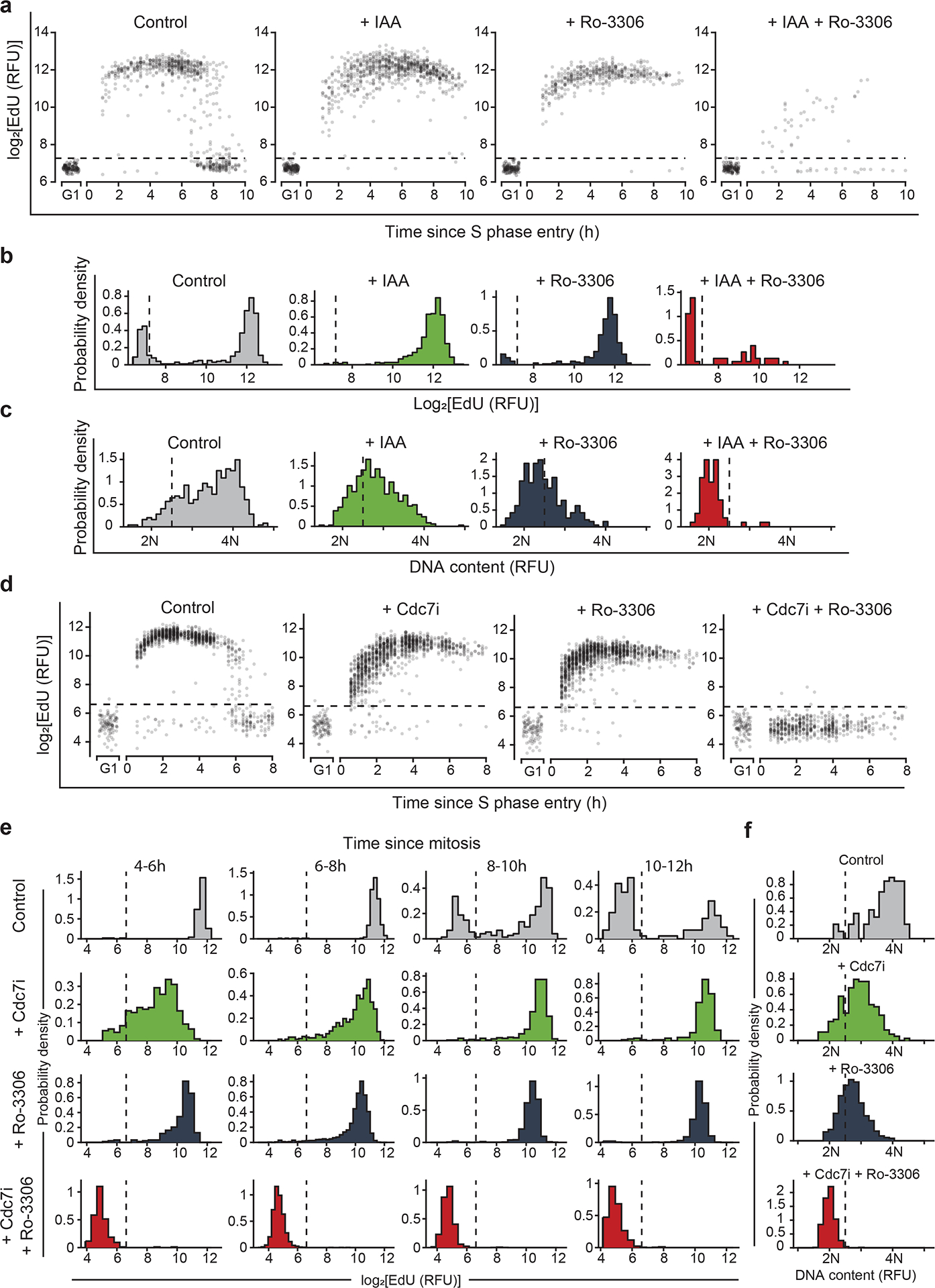
a-c, Asynchronously growing immortalized Cdc7AID/AID/Tir1 MEFs expressing FUCCI(CA)-reporters were live-imaged in the presence of auxin (IAA) or vehicle for 4 h, and then Cdk1 inhibitor Ro-3306 was added. Cells treated with Ro-3306 starting at 0–2 h after the mitotic exit (in G1-phase) were selected for analysis. a, EdU intensity at the indicated times after S-phase entry (recorded as Cdt1(1–100)Cy(−)drop), in cells treated as indicated. b, c, EdU (b) and Hoechst intensity (DNA-content) (c) in cells 16–18 h after mitosis, treated as indicated. d-f, MCF10A cells expressing FUCCI(CA)-reporters were live-imaged as in a-c. Cells were treated with vehicle, CDC7-inhibitor TAK-931 (Cdc7i), Ro-3306, or with both inhibitors. Cells treated with drug(s) starting at 0–2 h after mitotic exit were selected for analysis. d, EdU intensity at the indicated times after S-phase start (Cdt1(1–100)Cy(−)drop) in cells treated as indicated. e, Intensity of EdU incorporation at the indicated times after completion of mitosis. f, Hoechst intensity (DNA-content) in cells treated as above, 10–12h after mitotic exit. Dashed horizontal lines (a, d), cutoff values for EdU-positive signal. Dashed vertical lines: b, e, cutoff values for EdU-positive signal; c, f, signal corresponding to >2N DNA significantly above 2N noise.
We also used live-cell imaging to further dissect events at G1/S transition. We engineered Cdc7AID/AID/Tir1 MEFs and human cells to express FUCCI(CA) cell-cycle-reporters22 (Extended Data Fig. 9d). This dual fluorescent reporter system detects important events at G1/S boundary: APC/C inactivation (Gem(1–110)-mCherry reporter), which reflects commitment to S-phase entry, and the start of DNA replication (Cdt1(1–100)Cy(−)-mVenus reporter).
We live-imaged asynchronously growing Cdc7AID/AID/Tir1 MEFs in the presence of auxin or vehicle, and acutely treated cells with Cdk1 inhibitor, Ro-3306. We analyzed cells that received Cdk1 inhibitor within the first 2h after completion of mitosis (i.e., in G1). In control cells, Gem(1–110)rise (corresponding to APC/C inactivation) occurred approximately 7.5h after mitosis (Extended Data Fig. 9e). Gem(1–110)rise was followed immediately by Cdt1(1–100)Cy(−)drop (indicative of start of DNA replication) (Extended Data Fig. 9f–g), which was accompanied by the onset of EdU-incorporation (Fig. 4a, d). Inhibition of Cdc7 or Cdk1 alone or combined did not affect timing of Gem(1–110)rise (APC/C inactivation) (Extended Data Fig. 9e), consistent with Cdc7 and Cdk1 activity not being necessary for APC/C inactivation at G1/S. Cells treated with Cdc7 or Cdk1 inhibitor alone exhibited only a minor delay in Cdt1(1–100)Cy(−)drop (indicating S-phase entry, Extended Data Fig. 9h). In contrast, combined inhibition of Cdc7 and Cdk1 strongly delayed Cdt1(1–100)Cy(−)drop (S-phase entry) relative to Gem(1–110)rise (APC/C inactivation), with a large fraction of cells failing to enter S-phase (Extended Data Fig. 9f–g). Importantly, nearly all Cdc7/Cdk1-inhibited cells (including those with Cdt1(1–100)Cy(−)drop) failed to incorporate EdU and did not increase their DNA content beyond 2N (Fig. 4), indicating a complete G1/S block upon Cdc7/Cdk1 inhibition.
We recapitulated these observations using asynchronously growing human cells (Extended Data Fig. 9i–j), as well as human cells that were rendered quiescent and then stimulated to enter cell-cycle (Extended Data Fig. 9k). In these cells, combined inhibition of CDC7 and CDK1 almost completely abrogated Cdt1(1–100)Cy(−)drop (S-phase entry), while having no effect on Gem(1–110)rise (APC/C inactivation) (Extended Data Fig. 9i–k). Treatment with either CDC7 or CDK1 inhibitor alone had little effect on these parameters. Collectively, these findings demonstrated that combined inhibition of Cdc7 and Cdk1 prevents the onset of DNA synthesis in mouse and human cells.
Cyclin B-Cdk1 at G1/S
Our results indicated that in addition to its well-established role in mitosis, Cdk1 plays an important function in triggering S-phase entry. This role is performed in a functionally redundant fashion with Cdc7, and hence inhibition of Cdc7 renders Cdk1 rate-limiting for S-phase entry.
During mitosis Cdk1 is activated by cyclin B, while Cdk1 partners with cyclin A during late S-phase1. Cdk1 can also bind cyclin E in Cdk2-knockout cells23. To determine which cyclin mediates the function of Cdk1 during S-phase entry, we examined association of Cdk1 with cyclins A, B and E during G1/S-phase progression in wild-type ESC synchronized by mitotic block/release and during cell-cycle re-entry of wild-type MEFs and human cells. Surprisingly, we observed that Cdk1 associated with cyclin B, but not with detectable E or A during G1/S progression both in cells exiting mitosis and in cells returning from quiescence (Extended Data Fig. 7e, g, Extended Data Fig. 10a–d).
These findings prompted us to analyze cyclin B-associated kinase activity during G1/S progression in ESC (Fig. 5a, upper panel, Extended Data Fig. 7e), and during cell-cycle re-entry of MEFs and human cells (Fig. 5a, middle and lower panels, Extended Data Fig. 7g). We observed that Cdk1-cyclin B kinase becomes activated as cells enter S-phase, both in cycling cells and during cell-cycle re-entry (Fig. 5a).
Fig. 5. Analyses of Cdk1-cyclin B at G1/S.
a, ESC were synchronized in M-phase with nocodazole; MEFs, T98G and BJ cells were arrested in G0. Cells were released and collected at indicated time-points for cyclin B1 immunoprecipitation (IP) followed by kinase reactions with [γ32P]-ATP and histone H1 as substrate. AS, asynchronous cells; M, M-phase cells; IgG, control immunoprecipitation. b, Example of gating strategy for flow-sorting. BJ foreskin fibroblasts were stained with CytoPhase Violet (CPV) and sorted using gates indicated at the top. c, Cyclin B1 was immunoprecipitated from flow-sorted cells and subjected to kinase reactions as in a. d, MCF10A cells expressing Cdt1(1–100)Cy(−)-mCherry reporter were stained with anti-cyclin B1 antibody. For antibody control, cells were transfected with anti-cyclin B1 (siB1) or control siRNA (siCtrl) 24h before fixation. Shown is intensity of nuclear cyclin B1 at the indicated time-points after Cdt1(1–100)Cy(−)drop (S-phase entry). Red lines, median values within bins every two time-points (24 min, 12 min interval), blue dashed lines, mean nuclear cyclin B1 signal in cells treated with siB1 (background level). Right, median intensity of nuclear cyclin B1 at indicated time-points after S-phase entry in different treatment groups. Cdc7i, cells treated with Cdc7-inhibitor, TAK-931. e, Quantification of DNA-replication assays in Xenopus egg extracts (from Extended Data Fig. 12i). Extracts were treated with PHA-767491 (CDC7i) or DMSO (Control), cyclin-CDK complexes added, and nascent strand DNA-synthesis assessed at indicated time-points. Bars, mean values, error bars, SD, n=3 independent replicates. a, b, c, representative results (out of 2).
To evaluate the presence and activity of Cdk1-cyclin B in cells under unperturbed conditions, we stained asynchronously growing MEFs and human BJ foreskin fibroblasts and MCF10A cells with cell-permeant DNA-binding dyes CytoPhase Violet or Hoechst-33342. We then flow-sorted cells from G1, early S, late S and G2/M fractions based on DNA content (Fig. 5b), immunoprecipitated cyclin B and performed kinase and co-immunoprecipitation assays. We observed the presence of Cdk1-cyclin B complex and of cyclin B1-associated kinase activity in early S-phase in all three cell types analyzed (Fig. 5c, Extended Data Fig. 10e–g).
Cyclin B is known to be cytoplasmic during interphase and to translocate en masse to the nucleus when cells enter M-phase. We asked whether lower levels of cyclin B might be present in the nuclei at the onset of S-phase to act in DNA replication. Combined live-cell imaging and immunofluorescence staining revealed clear upregulation of nuclear cyclin B1 levels in asynchronously growing cells entering S-phase (Fig. 5d, Extended Data Fig. 10h–i). Interestingly, inhibition of CDC7 resulted in increased levels of cyclin B at S-phase start, indicating that this physiological mechanism is upregulated in the absence of CDC7 activity (Fig. 5d, Extended Data Fig. 10h–i). Moreover, combined live-cell imaging and immunofluorescence analysis revealed that CDK1 is present in cell nuclei across the cell-cycle, including G1 and early S-phase cells (Extended Data Fig. 11, Extended Data Fig. 12a–h).
Lastly, we used Xenopus egg extracts to test the ability of different cyclin-CDK combinations to drive S-phase entry in the absence of Cdc7 activity. Consistent with previous results24, treatment with Cdc7 inhibitor PHA-767491 greatly inhibited DNA replication in this cell-free system (Fig. 5e, Extended Data Fig. 12i). Addition of recombinant CDK1-cyclin B1, but not CDK1-cyclin A2, CDK1-cyclin E1, or CDK2-cyclin E1 restored DNA replication upon Cdc7 inhibition (Fig. 5e, Extended Data Fig. 12i). These results further support our findings that CDK1-cyclin B1 can promote DNA replication initiation in the absence of Cdc7 activity.
Discussion
We developed a new system that allows rapid, global and reversible degradation of a protein in vivo. This system represents a powerful tool to study essentially any protein in normal physiology and in any pathological condition. In the current work, we applied this technology to Cdc7 kinase.
According to the established models, Cdc7 represents an essential kinase that is required for origin firing10,15,24–33. However, some previous observations hinted that the requirement for Cdc7 may not be absolute. In S. cerevisiae, a recessive mutation of the MCM5/CDC46 gene bypassed the requirement for Cdc7 in cell division34–36. In fission yeast, proliferation of Hsk1(Cdc7)-null cells could be restored by deletion of Mrc1 (component of DNA replication forks) or Rif137,38. In Xenopus and in human cells, depletion of Rif1 – a protein that recruits PP1-phosphatase to replication origins to dephosphorylate Mcms – rendered phosphorylation of Mcms relatively insensitive to Cdc7 inhibition39. A very early embryonic lethality of Cdc7−/− mice could be overcome by knockout of p53; Cdc7−/−p53−/− animals survived until embryonic day 8, a stage at which several major organs have been developed33.
In the current study, we observed that acute shutdown of Cdc7 did not arrest proliferation of cells grown in vitro and in tissues of living mice, and that some murine cell types physiologically proliferate without detectable Cdc7. We conclude that Cdc7 is not uniformly required for mammalian cell proliferation.
Phosphorylation of Mcm proteins is thought to represent the essential function of Cdc74–8. Our analyses revealed that shutdown of Cdc7 strongly decreased phosphorylation of some, but not all Mcm phosphoresidues. We also found that Cdk1 can phosphorylate Mcms on ‘Cdc7-independent sites’. These observations suggest that Cdc7 and Cdk1 collaborate and independently contribute to G1/S transition by phosphorylating distinct Mcm residues, and that phosphorylation of a subset of Mcm sites (either by Cdc7 or Cdk1) is sufficient to drive S-phase entry. In contrast, Cdk2 seems to be not essential in this process.
Surprisingly, we found that during S-phase entry, Cdk1 associates with cyclin B, and these Cdk1-cyclin B complexes are catalytically active. These results are in line with some previously published observations. In Xenopus egg extracts Cdk1-cyclin B can promote DNA replication when directed to the nucleus and activated40. Also in nucleus-free Xenopus egg extracts, Cdk1-cyclin B efficiently supported DNA replication41. Cdk1-cyclin B was shown to phosphorylate Mcm2 and Mcm4 in vitro, and this augmented subsequent phosphorylation by Cdc721. Although cyclin B translocates to the nucleus at the onset of mitosis, nuclear expression of cyclin B1 during G1-phase was documented in cancer cell lines42,43, in agreement with our findings.
A recent study using human immortalized retinal pigment epithelial cells expressing analog-sensitive CDC7 revealed that inhibition of CDC7 blocked cell proliferation44. Importantly, in that study CDC7 was not required for firing of early replication origins. Firing of late origins was blocked, due to stalled replication forks and ATR-dependent S-phase checkpoint44. It is likely that under sub-optimal conditions that cause replicative stress and DNA forks stalling, CDC7 becomes rate-limiting in overcoming stalled forks. Also inactivation of Cdc7 in murine ESC was reported to cause stalled replication forks, induction of p53, activation of G2/M checkpoint, inhibition of Cdk1 kinase and ultimately p53-dependent cell death33. It is likely that this phenotype reflects stress response of cells acutely transduced with Cre-encoding adenoviruses. Also several other studies implicated CDC7 function under replicative stress45–47. Hence, Cdc7 may play mechanistically at least two distinct functions: a non-essential role in DNA replication and another major role in counteracting replicative stress.
Cancer cells display higher level of replicative stress and hence may be particularly sensitive to CDC7 inhibition. CDC7 is upregulated in cancer cells, and overexpression of CDC7 correlates with poor clinical prognosis10,16. Depletion of CDC7 or inhibition of CDC7 kinase was shown to trigger apoptosis, or senescence, predominantly in p53-mutant cancer cells10,15,16,32,48. These results – together with our findings that Cdc7 is largely dispensable in vivo – raise a possibility that Cdc7 inhibition might represent an attractive therapeutic strategy, in particular against p53-mutant tumors.
Methods
Generation of analog-sensitive Cdc7AS/AS knock-in embryonic stem cells and fibroblasts.
Cdc7 gene-targeting construct was generated by cloning three fragments of the Cdc7 gene from V6.5 mouse embryonic stem cells (mESC) cells into a pSL301 plasmid. First, a 3 kb fragment constituting the left arm of homology was inserted upstream of the puromycin resistance cassette, which was flanked by two LoxP sites. Second fragment encompassing exons 3, 4 and 5 was mutagenized to introduce the M128G (analog-sensitive) substitution. Third ~4 kb fragment served as the right arm of homology. The targeting construct was linearized and electroporated into V6.5 mESC. Cdc7puro/puro clones were identified by long-range PCR. Subsequently, cells were electroporated with plasmid encoding Cre-recombinase giving rise to Cdc7AS/AS cells. To generate homozygous mice, Cdc7AS/AS ESC were injected into mouse blastocysts. Chimeric mice were backcrossed to wild-type C57BL/6 mice to obtain Cdc7AS/+ mice. Cdc7 AS/+ animals were subsequently intercrossed. Mouse embryonic fibroblasts (MEFs) were derived from embryos at day 13.5 of gestation, as previously described49.
Generation of auxin-inducible degron Cdc7AID/AID knock-in ESC, MEFs and mice.
The targeting construct was generated by cloning two fragments of the Cdc7 gene from V6.5 mESC into a pSL301 plasmid. First, 1.3 kb fragment constituting the left arm of homology was inserted upstream of the first Cdc7 coding exon and contained an insert consisting of 3xFlag tag followed by the auxin inducible degron (AID) domain, previously described14. The second fragment was inserted in-frame with the auxin degron domain. It constituted a 1.3 kb portion of the Cdc7 locus downstream of the first translated codon and served as the right arm of homology. The targeting construct was electroporated into V6.5 mESC. To increase the efficiency of homologous recombination at the Cdc7 locus, gRNA (TGCATCCCAGTCGTGCGTAG) targeting the Cdc7 gene was designed using CRISPOR50, cloned into pX330-Cas9 vector51 (Addgene plasmid # 42230) and electroporated together with the targeting construct. In addition, cDNA encoding Oryza sativa Tir1 F-box protein, described in14 was knocked into the Rosa26 locus52 of V6.5 mESC using the approach as described53. Blastocyst injections, animal breeding and fibroblast derivation was performed as described above. Cdc7AID/AID/Tir1 mice were viable and displayed no obvious phenotypes. It was previously reported that transgenic expression of Tir1 in mice causes embryonic lethality54. We speculate that the lethality observed by these authors, but not in our study, might be related to higher level of Tir1 expression in some critical organ(s) in transgenic mice used in that report, possibly due to transgene integration site.
Generation of Cdc7Flox/Flox and Cdc7Δ/Δ embryonic stem cells.
Two targeting constructs for introducing LoxP sites into introns 2 and 6 of the Cdc7 locus were purchased from Integrated DNA Technologies, Inc. in the form of Ultramer™ DNA Oligonucleotides. Both fragments contained approximately 75 bp homology arms upstream and downstream of the LoxP sequence (ATAACTTCGTATAGCATACATTATACGAAGTTAT), corresponding to intron 2 and intron 6, respectively. In addition, the constructs contained XhoI (for intron 2) and ClaI (for intron 6) restriction sites sequences upstream of the LoxP sequence. The targeting constructs were electroporated into V6.5 mESC. To increase the efficiency of homologous recombination at the Cdc7 locus, gRNAs (GGCAGATTTTTGAGTTCGAG for intron 2 and AAGTTATGTCCGTAGCGCGA for intron 6) were designed using CRISPOR50, cloned into pX330-Cas9 vector51 (Addgene plasmid # 42230) and electroporated together with the targeting constructs. Cdc7Flox/Flox clones were identified by PCR and confirmed by DNA sequencing. To prevent p53-dependent cell death upon ablation of Cdc733, Cdc7Flox/Flox ESC were then transduced with dominant-negative p53 (DNp53), described in55, and a polyclonal population of cells stably expressing DNp53 was obtained by selection with hygromycin. To obtain Cdc7-knockout in ESC (Extended Data Fig. 4k–p), Cdc7Flox/Flox ESC expressing DNp53 were transduced with Cre-recombinase via lentiviral particle transduction in the presence of 10 μM pifithrin-α (Sigma), an inhibitor of 53. Shutdown of Cdc7 was assessed by western blotting two and three days post-transduction.
Cell culture.
All cell types were cultured at 37°C and 5% CO2. mESC were maintained on a monolayer of mitotically inactivated MEFs in SL medium consisting of Knockout DMEM medium (Gibco), 15% Fetal Bovine Serum (FBS, Sigma), 100 U/ml leukemia inhibitory factor (LIF) (Millipore), 1% MEM non-essential amino acids (Gibco), 1% penicillin/streptomycin (Gibco), 1% glutamine (Gibco) and 0.1 mM 2-mercaptoethanol (Sigma). To induce Cdc7 degradation in Cdc7AID/AID/Tir1 cells, culture media were supplemented with 500 μM Indole-3-Acetic Acid (IAA, Cayman Chemical). During long-term ESC cultures (Extended Data Fig. 2d) in presence of auxin, cells were replated every 2–3 days at ~150,000 cells/cm2 and media were changed daily. Such optimal tissue-culture conditions were required to maintain ESC viability. To inhibit Cdc7, Cdk1 or CDK2 in mouse Cdc7AS/AS, Cdk1AS/AS and in human cells expressing asCDK2, culture media were supplemented with 1 μM 1NM-PP1 (Cayman Chemical) for Cdc7AS/AS cells, or 1 μM 3MB-PP1 (Abcam) for Cdk1AS/AS and human asCDK2 cells. For 5-bromo-2’-deoxyuridine (BrdU) incorporation and DNA fiber assay experiments (described in Fig. 1d, f, g, Fig. 2d, f, Fig. 3b, j, Extended Data Fig. 2a, Extended Data Fig. 4 m, n), ESC were plated directly onto gelatin-coated 12- or 6-well plates at 50,000–250,000 cells/well. MEFs were cultured in DMEM (Gibco) with 10% FBS (Sigma), 1% penicillin/streptomycin. Immortalized MEFs were obtained by retroviral transduction of dominant-negative p53 (DNp53), described in55 and subsequent selection of a polyclonal population of cells stably expressing DNp53. Tert-immortalized BJ fibroblasts56 were kindly provided by Dr. William Hahn (Dana-Farber Cancer Institute) and cultured in DMEM (Gibco) with 15% FBS (Sigma), 1% penicillin/streptomycin. T98G glioblastoma cells were obtained from ATCC (CRL-1690) and cultured in EMEM (ATCC) with 10% FBS (Sigma). MCF10A cells were obtained from ATCC (CRL-10317), HMEC cells were kindly provided by Dr. Thomas Roberts (Dana-Farber Cancer Institute); both cell lines were cultured in MEGM™ Mammary Epithelial Cell Growth Medium BulletKit™ (Lonza). U2OS cells were obtained from ATCC (HTB-96) and cultured in DMEM (Gibco), with 10% FBS (Sigma). Primary human dermal fibroblasts (HDF) were obtained from Sigma (106–05A) and cultured in Fibroblast Growth Medium (Sigma: 116–500). For live-cell microscopy experiments, cells were seeded onto collagen-coated 96-well glass bottomed plates (Cellvis) the day before imaging. For MCF10A live-cell microscopy experiments, cells were cultured in DMEM/F12 growth media with HEPES and no phenol red (Gibco), supplemented with 5% horse serum (Gibco), 20 ng/mL EGF (PeproTech), 0.5 μg/mL hydrocortisone (Sigma), 100 ng/mL cholera toxin (Sigma) and 10 μg/mL insulin (Sigma) prior to and during experiments. Other primary cells were derived and cultured according to previously published protocols for keratinocytes57 and cardiomyocytes58.
In several experiments, inhibition of Cdk1 was achieved by addition of Ro-3306 (Sigma), final concentration: 3 μM (Extended Data Fig. 6g), 5 μM (Extended Data Fig. 6d, Extended Data Fig. 7l–o, Extended Data Fig 8d) and 10 μM (Fig. 3i–k, Figure 4a–f, Extended Data Fig. 8a–c, Extended Data Fig. 9a–c, e–k). Chemical Cdc7 inhibition was achieved by addition of XL-413 (Sigma): 10 μM (Extended Data Fig. 6b, d, g, o, t, Extended Data Fig. 7m–o), 20 μM (Fig. 3k, Extended Data Fig. 3g–n, Extended Data Fig. 6j, Extended Data Fig. 7i–l, Extended Data Fig. 8a–d), or TAK-931 (MedChem Express) 500 nM (Fig. 4d–f, Extended Data Fig. 3a–f, Extended data Fig. 9i–k, Extended Data Fig. 10h, i), or PHA-767491 (Sigma) 3.75 μM (Fig. 5e, Extended data Fig. 12i).
Cell synchronization.
For cell synchronization in M phase (Fig. 3g, i, Fig. 5a, Extended Data Fig. 7f, h, i, k, l, Extended Data Fig. 8a, Extended Data Fig. 10a), 50 ng/ml nocodazole (Sigma) was added to the culture medium for 12 h. Subsequently, cells were re-fed with a medium without nocodazole, and cell cycle progression monitored by FACS after staining with propidium iodide. Cell synchronization in G0 Fig. 3h, k, Fig. 5a, Extended Data Fig. 6c, d, g k, p, u, Extended Data Fig. 7 g, m–o, Extended Data Fig. 9k, Extended Data Fig. 10b–d) was achieved by removing FBS (MEFs, BJ and T89G) or growth factors (MCF10A, HMEC and HDF) from growth media for 48–72 h. Culture medium was then changed to the one with serum (MEFs, BJ and T89G) or growth factors (MCF10A, HMEC and HDF), in order to stimulate cell cycle re-entry.
Cdc7 degradation in mice.
All experiments involving animals were approved by the Dana-Farber Cancer Institute Institutional Animal Care and Use Committee (IACUC) and conformed to relevant regulatory standards. Animals were kept at 12 hour light/dark cycles, at 18–23°C, 40–60% humidity. Four to six week old Cdc7AID/AID/Tir1 mice were treated with auxin (or vehicle for control) by oral gavage, intraperitoneal (IP) injection, drinking water supplementation or a combination of the above treatments. In Extended Data Fig. 5k, mice received a single dose of IAA at 800 mg/kg body weight via an IP injection. In Extended Data Fig. 5m–o, mice received alternating IP injections (700 mg/kg body weight) and gavage (700 mg/kg) every 12 h in addition to a constant presence of 10 mg/ml IAA in drinking water. In some cases, such administration of IAA resulted in toxicity (irrespective of the Cdc7 genotype). In Extended Data Fig. 5l, mice were given a single IAA treatment (700 mg/kg) by IP injection or gavage and organs were harvested at the indicated times.
Immunohistochemistry staining.
For immunohistochemical analysis of BrdU incorporation presented in Extended Data Fig. 5n, Cdc7AID/AID/Tir1 mice (treated with IAA or vehicle) were injected with BrdU 1 h prior to sacrifice. Organs were fixed and stained as previously described59. In panels presented in Extended Data Fig. 5o, sections were stained with an anti-Ki-67 antibody.
Immunofluorescence.
For immunofluorescent analysis of DNA damage (Extended Data Fig. 3o–q), ESC and MEFs were treated with 500 μM IAA for the indicated times. Cells on coverslips were fixed in 4% paraformaldehyde for 10 min and subsequently washed 3 times with PBS. Cells were permeabilized with 0.25% Triton X-100 in PBS at room temperature for 5 min, followed by 3 washes in PBS and incubated in blocking buffer (1% BSA, 22.5 mg/ml glycine, 0.1% Tween 20 in PBS) for 30 min. Cells were stained with primary antibody anti-γH2AX phospho-S139 1:1000 (SCBT: sc-517348) overnight at 4°C. Next, the primary antibody was removed, cells were washed 3 times with PBS and incubated with goat anti-mouse secondary antibody, Alexa Fluor Plus 488 1:500 (Thermo Fisher Scientific: A32723) for 1 h at room temperature. Cells were washed three times with PBS, incubated in 0.5 μg/ml Hoechst in PBS to stain DNA and imaged using a Nikon Eclipse E600 microscope with Nikon Plan Fluor 40x/0.75 Ph2 DLL objective. Colocalization and quantification of γH2AX phospho-S139 foci within nuclei was performed using Fiji software. Immunofluorescence protocol for combined live and fixed-cell imaging is described in “Combined live and fixed-cell imaging” section.
Cell lysis and western blotting.
Cell lysates were prepared in RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% NP-40, 0.1% SDS, 10 mM EDTA) with Roche protease inhibitors and cleared by centrifugation at 16000 rpm, 4°C. Protein content was quantified using BCA Protein Assay Kit (Pierce). For western blotting, twenty to fifty micrograms of total proteins were mixed with reducing SDS-PAGE sample buffer, heated to 95°C for 5 min, and resolved on SDS-PAGE. Proteins were transferred to nitrocellulose membranes in Towbin buffer (1 h, 450 mA). Membranes were incubated with primary antibodies in 5% milk, TBS plus 0.1% Tween overnight at 4°C, and then with a 1:10000 dilution of Horseradish peroxidase- (HRP)-conjugated anti-mouse or anti-rabbit secondary antibodies. The signal was detected using Pierce ECL Western Blotting substrate (Thermo Fisher Scientific) on Amersham Imager 680 (GE). The following primary antibodies were used: anti-Flag 1:1000 (Sigma: F1804), anti-Cdc7 1:5000 (Abcam: ab229187), anti-Gapdh 1:10000 (CST: D16H11), anti-Mcm2 1:1000 (Abcam: ab4461), anti-Mcm2 phospho-S27 1:1000 (Abcam: ab109459), anti-Mcm2 phospho-S40 1:1000 (Abcam: ab133243), anti-Mcm2 phospho-S53 1:1000 (Epitomics: 3386–1), anti-CDC7 1:1000 (MBL: K0070–3S), anti-Cyclin A 1:1000 (SCBT: sc-596), anti-Cyclin B1 1:1000 (Abcam: ab181593), anti-Cyclin E1 1:1000 (SCBT: sc-481), anti-Cdk1 1:1000 (Abcam: ab71939), anti-Cdk2 1:1000 (SCBT: sc-163), anti-Chk1 1:500 (CST: 2360S), anti-Chk1 phospho-S317 1:500 (CST: 12302S), anti-Chk1 phospho-S345 1:500 (CST: 2348S), anti-KAP1 1:500 (Bethyl: A300–274A-M), anti-KAP1 phospho-S824 1:500 (Bethyl: A300–767A-M), anti-p53 1:500 (SCBT: sc-393031), anti-Rb 1:1000 (CST: 9313S), anti-β-actin 1:5000 (Sigma: A5441), anti-thiophosphate ester 1:2000 (Abcam: ab92570).
To identify cyclins interacting with Cdk1 (Extended Data Fig. 10a–g), Cdk1 was immunoprecipitated from whole cell lysates using an anti-Cdk1 antibody (SCBT: sc-54) and analyzed by western blotting.
Flag immunodepletion experiment (Extended Data Fig. 1j).
Four hundred micrograms of whole cell lysates from Cdc7AID/AID/Tir1 ESC were pre-cleared with 30 μl of protein A/G beads (Santa Cruz: sc-2003) at 4°C for 2 h. The cleared lysates were immunoprecipitated with 30 μl of anti-Flag antibody (Sigma: F3165) at 4°C overnight. After collecting the supernatant, the immunoprecipitate was washed 3 times with PBS plus 0.1% Tween 20. One fifth of the immunoprecipitate was mixed with reducing SDS-PAGE sample buffer, heated to 95°C for 5 min, resolved on SDS-PAGE along with 10% of the supernatant and probed with an anti-Cdc7 antibody.
DNA content-based cell sorting (Fig. 5b, c, Extended Data Fig. 10e–g and 12g–h).
For CytoPhase Violet (CPV) staining, asynchronously growing human BJ fibroblasts or human MCF10A cells were trypsinized, passed through a 70 μm strainer, counted and resuspended in culture media at 1 × 106 cells/ml. Cells were stained with CPV at 1:1000 (BioLegend: 425701) for one h at 37°C, and flow sorted based on their DNA content (Fig. 5b) on an Aria II SORP (BD) sorter using a 405 nm laser. Asynchronously growing MEFs were stained on the culture plate with 10 μg/ml Hoechst 33342 (Thermo Fisher Scientific: H3570) for one h at 37°C. After trypsinization, cells were resuspended in the media containing 10 μg/ml Hoechst, passed through a 40 μm strainer and sorted as described above. For each fraction, 1–2 × 106 cells were collected. Fifty to hundred micrograms of proteins from each fraction were used for immunoprecipitation - western blotting or immunoprecipitation - in vitro kinase assays.
For analyses shown in Extended Data Fig. 12g–h, asynchronously growing human BJ fibroblasts and mammary epithelial MCF10A and HMEC cells were stained with CPV as described above. G1/S and G2/M phase cells were flow sorted based on their DNA content as above. For each phase, 2–4 millions of cells were collected and fractionated using Cell Fractionation Kit (CST, 9038). Proteins from the cytoplasmic (10 ug) and nuclear (20 ug) fractions were resolved on SDS-PAGE. Western blots were probed with the following antibodies: anti-Cdk1 1:1000 (Abcam, ab133327), anti-Topo1 1:2000 (BD Pharmingen, BD556597), anti-SP-1 1:1000 (CST, 9389) and anti-GAPDH 1:10000 (CST 5174).
In vitro kinase activity assays.
Kinase assays were performed in a final volume of 30 μl of a kinase buffer: 50 mM HEPES pH 7.5, 10 mM MgCl2, 1 mM DTT, 1 mM EGTA, 0.1 mM NaF, containing 10 μM ATP and 0.4 μCi [32P]γATP (Perkin Elmer). To assess the kinase activity of the endogenous Cdk1, Cdk2 or cyclin B1 Fig. 3g, h, Fig. 5a, c, Extended Data Fig. 4p, Extended Data Fig. 7b, Extended Data Fig. 8a–d), Cdk1, Cdk2 or cyclin B1 were immunoprecipitated from whole cell lysates using anti-Cdk1 (SCBT: sc-54), anti-Cdk2 (SCBT: sc-6248) or anti-cyclin B1 (SCBT: sc-245 AC) antibodies. Histone H1 (EMD Millipore) was used as kinase substrate, 1 μg per reaction.
Cell cycle analyses.
For cell cycle distribution analyses (Fig. 1d, l, Fig. 3b, Extended Data Fig. 2a, Extended Data Fig. 3k, Extended Data Fig. 4b, e, j, m, n, Extended Data Fig. 5b, d, e, Extended Data Fig. 6b–d, f, g, k, j, o, p, u, t, Extended Data Fig. 7k–o), cells were pulsed with 5-bromo-2’-deoxyuridine (BrdU) for 30 min (ESC) or 1 h (MEFs, BJ fibroblasts, keratinocytes, cardiomyocytes), then stained with an anti-BrdU antibody and with propidium iodide followed by flow cytometry on a LSR Fortessa (BD) cytometer equipped with FACS Diva software. Pulse-chase experiments (Fig. 2d, e, Extended Data Fig 3g, h) were carried out as previously described53. The initial BrdU pulse was provided at indicated times after IAA addition.
Cell death.
For cell death analyses (Fig. 2h, i, Extended Data Fig. 3k–n), FITC Annexin V Apoptosis Detection Kit I (BD Biosciences) was used. Cells were washed twice with PBS and resuspended in 1x Binding Buffer at 1 × 106 cells/ml. 100 μl of cell suspension were mixed with 5 μl of FITC Annexin V and 5 μl propidium iodide, incubated for 15 min at room temperature in the dark, diluted in 400 μl of 1x Binding Buffer and analyzed by flow cytometry.
DNA fiber assays.
To analyze fork speed and the proportion of new origins (Fig. 1f, g, Fig. 3j, Extended Data Fig. 7j), nascent DNA was labeled with 50 μM 5-chloro-2’-deoxyuridine (CldU) for 20 min. CldU was then removed by washing twice with pre-warmed media and nascent DNA was further labeled with 100 μM 5-iodo-2’-deoxyuridine (IdU) for 20 min. Cells were then trypsinized, washed once with PBS and resuspended at a density of ~1,000 cells/μL in PBS. 3 μL of cell suspension was then dropped unto a glass slide and allowed to rest for 2 min. Seven μL of lysis buffer (200 mM Tris-HCl pH 7.4, 0.5% SDS, 50 mM EDTA) was carefully added to lyse cells for 3 min at room temperature. DNA was stretched along the length of slides by tilting them at a 15° angle and allowing cell lysate droplets to slide down by gravity. Slides were dried for 30 min, fixed in 3:1 methanol-acetic acid at −20°C for 5 min and dried again overnight. DNA was denatured by incubating slides in 2.5 M HCl for 45 min. Slides were then washed 5 times 1 min in PBS before blocking with PBS plus 0.05% Tween-20 (PBS-T) and 2% BSA for 30 min at 37°C. CldU and IdU were immunodetected by incubating at 37°C for 2 h in a humid chamber with rat anti-BrdU 1:100 (Abcam) and mouse anti-BrdU 1:25 (BD Biosciences) primary antibodies, respectively. Slides were washed 3 times in PBS-T and incubated for 1 h at 37°C in a humid chamber with anti-mouse Alexa-488 1:100 (Jackson ImmunoResearch) and anti-rat Cy3 1:100 (Jackson ImmunoResearch) antibodies. Slides were washed 3 times with PBS-T, dried and mounted with ProLong gold antifade reagent (Thermo Fisher Scientific). Images were acquired with a Nikon i90 fluorescence microscope equipped with NIS elements. Fibers were measured and counted using Fiji software. To obtain fork speed in μm/min, the length of IdU tracks in μm was divided by the labeling time (20 min). The fraction of new origins was calculated as the total number of origins divided by the number of origins + ongoing replication forks.
Alkaline phosphatase staining.
Alkaline Phosphatase Detection Kit (Millipore) was used. ESC were cultured for four days, fixed with 4% paraformaldehyde in PBS for 1–2 min and rinsed with TBST (20 mM Tris-HCl pH 7.4, 0.15 M NaCl, 0.05% Tween-20). Fast Red Violet/Naphthol solution (Fast Red Violet mixed with Naphthol AS-BI phosphate solution and water at 2:1:1 ratio) was added (1 ml per well). Cells were incubated in dark at room temperature for 15 min, rinsed with TBST, covered with 1x PBS and photographed (Extended Data Fig. 2c). Undifferentiated ESC colonies stain red with alkaline phosphatase.
CRISPR/Cas9 knockout.
Guide RNAs to target mouse Cdc7 (Fig. 3c–f, Extended Data Fig. 6 h–k) (ACACCTGTCACTGCCACGAA, CGAAGGGAAGAAAACGCCAT), human CDC7 (Extended Data Fig. 4i, j) (GCCACAGCACAGTTACAAGT, AGTTCAGCTGCAATTCTTAT) mouse Dbf4 (TGGTGATAGAAGGCAAGTCA), mouse Cdk2 (Fig. 3a–c, e, Extended Data Fig. 7h) (GTACTGCCATCCGAGAGATC), human CDK2 (Extended Data Fig. 6) (CAGAAACAAGTTGACGGGAG, CCGAGACCTTAAACCTCAGA) were designed using CRISPOR50 and cloned into lentivCRISPR v2 plasmids obtained from Addgene (Addgene plasmid #98291, #98293) )60. Primary MEFs (Extended Data Fig. 4a–c), DNp53-immortalized MEFs (Extended Data Fig. 4d–f, Extended Data Fig. 5c–g) or keratinocytes (Extended Data Fig. 4g, h) (all derived from Cdc7AID/AID/Tir1 embryos) were transduced with lentiviral particles carrying murine Cdc7 gRNAs, Cdc7AID/AID /Tir1 ESC (Fig. 3a–c, e, Extended Data Fig. 7h) with Cdk2 gRNAs, and BJ (Extended Data Fig. 4i, j) cells with human CDC7 gRNAs. Cells were selected with blasticidin (Extended Data Fig. 4a–h) or hygromycin (Fig. 3a–c, e, Extended Data Fig. 4i, j, Extended Data Fig. 7h) and analyzed by western blotting for knockout efficiency. In addition, in case of primary cells, DNp53-immortalized MEFs as well as BJ fibroblasts (Extended Data Fig. 4a, d, i, Extended Data Fig. 5c), genomic DNA was isolated from the selected polyclonal populations. Subsequently the genomic region flanking the Cas9-targeted site was amplified by PCR and cloned into a TOPO-TA vector (Thermo Fisher Scientific) for multiple single allele sequencing to verify the presence of frame-shift mutations within the Cdc7 or Dbf4 (Extended Data Fig. 5c) loci. Cells from the polyclonal population of DNp53-immortalized, Cdc7-knockout MEFs were plated in clonal density to purify the population of knockout cells. The resulting sub-clonal population was expanded and subjected to another round of western blotting (Extended Data Fig. 4d, ”selected”) and TOPO-TA cloning analysis. In case of Cdk2-knockout ESC, single clones were picked and expanded to obtain a cell population without detectable Cdk2 protein.
Mass spectrometry
Analysis of proteome changes upon Cdc7 degradation.
For analyses shown in Supplementary Table 1, five plates of Cdc7AID/AID/Tir1 ESC were treated with auxin or vehicle (DMSO) for 24 h. At the end of treatment, the cells were harvested by trypsinization and flash-frozen in liquid nitrogen. Protein lysates were prepared and 100 μg of proteins for each sample were digested in 100 μL 200 mM EPPS (Sigma) pH 8.5 as described before61. 30 μL acetonitrile (ACN) was added to each sample to 30% final volume. 200 μg TMT reagent (126, 127N, 127C, 128N, 128C, 129N, 129C, 130N, 130C, 131N) in 10 μL ACN was added to each sample. After 1 h of labeling, ratio check was conducted, and samples were combined, fractionated as previously described61. Twelve fractions were desalted and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS)61. Mass spectrometric data were collected on an Orbitrap Fusion Lumos (Thermo Fisher Scientific) mass spectrometer. Mass spectra were processed using a Sequest-based pipeline62; parameters were as described61. Database searching included all entries from the Mouse UniProt database (downloaded: 2011–08-10).
Time-resolved TMT-MS.
For analyses shown in Fig. 3d, Extended Data Fig. 7a, Supplementary Table 2), Cdc7AID/AID/Tir1 ESC were treated with auxin for 5 h (3 plates), 24 h (2 plates) and 48 h (3 plates) or vehicle (DMSO, 3 plates). At the end of treatment, the cells were harvested by trypsinization and flash-frozen in liquid nitrogen. Protein lysates were prepared and 1 mg of proteins for each sample was digested as described before61. Digestion was stopped by adding formic acid, and the digested peptides were subjected to C18 solid phase extraction (SPE) on Sep-Pak cartridges (100 mg bed weight). After drying down, the High-Select Fe-NTA phosphopeptide enrichment kit (Thermo Fisher Scientific) was used to enrich phosphopeptides from each sample. Phosphopeptides were desalted using Sep-Pak cartridges (10 mg bed weight), dried down and resuspended in 100 μL 200 mM EPPS pH 8.5. 30 μL ACN was added into each sample to 30% final volume. 200 μg TMT reagent (126, 127N, 127C, 128N, 128C, 129N, 129C, 130N, 130C, 131N and 131) in 10 μL ACN was added to each sample. After labeling for 1 h, 2 μL of each sample was combined, desalted, and analyzed using mass spectrometry. TMT labeling efficiency for phosphopeptides was calculated as over 99%. After quenching using 0.3% hydroxylamine, 11 samples were combined and fractionated using High pH Reversed-Phase Peptide Fractionation Kit (Pierce). Six fractions of phosphopeptides were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The unbound fraction and washes from this phosphopeptide enrichment experiment were collected and desalted using Sep-Pak cartridges (100 mg bed weight). 1/10 of the elution was dried down and resuspended in 100 μL 200 mM EPPS pH 8.5. 30 μL ACN was added into each sample to 30% final volume. 200 μg TMT reagent (126, 127N, 127C, 128N, 128C, 129N, 129C, 130N, 130C, 131N and 131) in 10 μL ACN was added to each sample.
The following steps were as described in61. Mass spectrometric data were collected on an Orbitrap Fusion mass spectrometer. Mass spectrometric data for phosphopeptides fractions were collected on an Orbitrap Lumos mass spectrometer. Data were collected in higher-energy collisional dissociation (HCD) and collision-induced dissociations (CID) method. Mass spectra were processed using a Sequest-based pipeline62; parameters were as described61. Database searching included all entries from the Mouse UniProt database (downloaded: 2014–07-02).
Mcm2 serine-27 phosphorylation analysis.
For analyses shown in Extended Data Fig. 7d, Cdk1AS/AS ESC were treated with 1 mM 3MB-PP1 for 24 h. Frozen cell pellets were processed in the same way as described above, except that TMPpro reagents (127N, 128N, 129N, 129C, 132N, 132C, 133N, 133C and 134N) were used for labeling. All data were collected on an Orbitrap Eclips mass spectrometer. Phosphopeptides were processed with FAIMS/hrMS2 using our optimized workflow for multiplexed phosphorylation analysis on an Orbitrap Eclips mass spectrometer. Mass spectra were processed using a Comet-based pipeline. Spectra were converted to mzXML using a modified version of ReAdW.exe. Database searching included all entries from the Mouse UniProt database (downloaded: 2019–04-08). Searches were performed using the same parameters as described above, except that the molecular weight of TMT tags on lysine residues and peptide N termini was 304.2071 Da and PSM filtering was performed using a linear discriminant analysis (LDA) with consideration of FAMIS.
For all the three TMT-MS experiments, the detailed parameters for MS2 and MS3 can be found in the raw data deposited to the ProteomeXchange Consortium via the PRIDE63 partner repository with the dataset identifier PXD025625. In each TMT experiment, PSMs with poor quality, MS3 spectra with all reporter ion channels missing, MS3 spectra with TMT reporter summed signal-to-noise of less than 100 or having no MS3 spectra were excluded from quantification. Each reporter ion channel was summed across all quantified proteins and normalized assuming equal protein loading of all samples.
Gel band analysis (Extended Data Fig 1k).
Cell lysates were resolved by SDS-PAGE, gels were Coomassie-stained, and five bands were excised. The gel bands were washed at room temperature by shaking for 10–20 min in 1 ml of destaining buffer (50% acetonitrile and 50% 200 mM EPPS pH 8.5, in ultrapure water) until the Coomassie dye was no longer visible in the gel. The gel pieces were dehydrated in 500 μl of acetonitrile for 10 min at room temperature. Acetonitrile was removed and gel pieces swelled in 300 μl digestion buffer (200 mM EPPS pH 8.5) with 100 ng trypsin. After digestion for 16 h at 37°C with shaking, peptides were extracted from gels as described previously and dried down in a vacuum concentrator, stage-tipped, and dried down again. Prior to liquid chromatography–tandem mass spectrometry analysis, the dried peptides were dissolved in 5% formic acid and 5% ACN and analyzed by a Orbitrap Exploris mass spectrometer (Thermo Fisher Scientific).
Mass spectra were processed using a Comet-based pipeline. Spectra were converted to mzXML using a modified version of ReAdW.EXE. Database searching included all entries from the Mouse UniProt database (downloaded: 2020–07-29). Oxidation of methionine residues (+15.995 D) was set as variable modification. Peptide-spectrum matches (PSMs) were adjusted to a 1% false discovery rate (FDR). PSM filtering was performed using a linear discriminant analysis (LDA). PSMs were identified, quantified, and collapsed to a 1% peptide false discovery rate (FDR) and then collapsed further to a final protein-level FDR of 1%, which resulted in a final peptide level FDR of <0.1%.
In vitro kinase assays – phospho-Mcm analysis (Extended Data Fig. 7c).
TCA was added to in vitro kinase assay products to a final concentration of 12.5%. After incubation on ice for 30 min, proteins were precipitated by centrifugation at 15,000 rpm for 30 min at 4 °C. Pellets were washed twice in 300 μL ice cold acetone and once in 500 μL ice cold methanol. 100 μL 200 mM EPPS pH 8.5 with 400 ng trypsin was added to the pellets. Samples were digested at 37 °C for 16 h with shaking. Formic acid was added to stop digestion and digestion products were desalted by stage-tip and analyzed by Q Exactive HF mass spectrometer.
Thiolabelling.
To thiolabel Cdk1 substrates, in vitro cultured asynchronous (Fig. 3f) or synchronized (Extended Data Fig. 7f) Cdk1AS/AS ESC were permeabilized with 0.45 μg/ml digitonin and supplemented with N6-furfuryl-ATPγS (Biolog) for 20 min. Specifically, Cdk1AS/AS ESC were incubated with the labeling buffer modified from ref.64. (20 mM HEPES pH 7.5, 100 mM KOAc, 5 mM NaOAc, 2 mM MgOAc2, 1 mM EGTA, 10 mM MgCl2, 0.5 mM DTT, 45 μg/ml digitonin, 5 mM GTP, 0.2 mM ATP, 0.1 mM N6-furfuryl ATPγS, Roche protease inhibitors cocktail) for 20 min. Subsequently, Mcm2 was immunoprecipitated from lysates using an anti-Mcm2 antibody (Proteintech: 10513–1-AP), and immunoprecipitates supplemented with 1.5 mM p-nitrobenzyl mesylate (PNBM), to alkylate proteins and generate epitopes for an anti-thiophosphate ester antibody. Proteins were then separated on SDS-PAGE gels, transferred to membranes which were probed with an anti-thiophosphate ester antibody.
Cell line and construct generation.
Cell cycle reporter cell lines were generated using third-generation lentiviral transduction65. The DNp53-immortalized, Cdc7AID/AID/Tir1 MEF cell cycle reporter cell line was generated through transduction of pLV-H2B-mTurquiose2-p2a-mCherry-Geminin(1–110)-IRES-blast (nuclear marker and mCherry-tagged Gem(1–110))66,67 and pLV-mVenus-Cdt1(1–110)Cy(−) (mVenus-tagged Cdt1(1–100)Cy(−))67 and transduced cells were selected with 5 μg/mL blasticidin. The MCF10A cell cycle reporter cell line was generated through transduction of viruses encoding pLV-H2B-miRFP670 (nuclear marker), pLV-DHB(994–1087)-mTurquoise2 (DHB not analyzed) and tFucci(CA)2/pCSII-EF (bicistronic construct for mVenus-tagged Gem(1–110) and mCherry-tagged Cdt1(1–100)Cy(−)). The U2OS cell cycle reporter cell line was generated through transduction of viruses encoding CSII-pEF-H2B-mTurquoise (nuclear marker), CSII-EF-mCherry-geminin(1–100) (mCherry-tagged Gem(1–110)), CSII-EF-DHB(994–1087)-mVenus (DHB not analyzed) and iRFP670-CRL4-Cdt2 (iRFP670-tagged Cdt1(1–100)Cy(−)). Cells expressing all 4 reporters were sorted using a BD FACSAriaII sorter (Weill Cornell Medicine CLC Flow Cytometry Core Facility). The tFucci(CA)2/pCSII-EF plasmid was obtained from RIKEN BRC (RDB15446) and described previously22. CSII-pEF-H2B-mTurquoise, CSII-EF-mCherry-geminin(1–100) and CSII-EF-DHB(994–1087)-mVenus were described previously66,68. iRFP670-CRL4-Cdt2 was a gift from the laboratory of Mary Teruel69. pLV-H2B-mTurquiose2-p2a-mCherry-Geminin(1–110)-IRES-blast, pLV-mVenus-Cdt1(1–110)Cy(−) and pLV-H2B-miRFP670 were generated through Gibson assembly of PCR amplified inserts from tFucci(CA)2/pCSII-EF, CSII-pEF-H2B-mTurquoise, and CSII-EF-mCherry-geminin(1–100).
Analog-sensitive CDK2 cell lines (Extended Data Fig. 6h–v) were generated by stable, retrovirus-mediated expression of sgRNA-resistant, Flag-tagged CDK2F80G (asCDK2) with concomitant CRISPR/Cas9-mediated knockout of the endogenous CDK2 (see CRISPR/Cas9 knockout).
siRNA transfection.
For analyses shown in Fig. 5d, Extended Data Fig. 10h–i, Extended Data Fig. 11, Extended Data Fig. 12a–d, MCF10A cells were transfected with 40 nM siRNA using DharmaFECT 1 (Dharmacon) according to the manufacturer’s protocol. Cells were incubated in transfection mixture for 6 h and then switched to growth media. Pools of 4 ON-Target-Plus siRNA from Dharmacon were used: cyclin B1 (J-003206–09, −10, −11, and −12), cyclin B2 (J-003207–09, −10, −11, and −12), CDK1 (J-003224–13, −14, −15, and −16), CDK2 (J-003236–11, −12, −13, and −14). For anti-cyclin B1 siRNA (Fig. 5d, Extended Data Fig. 10h, i), cells were transfected 24 h before fixation; cells retained sufficient cyclin B1 to progress through mitosis. When cyclin B1 was knocked down to measure cyclin B1 staining background, cyclin B2 was simultaneously knocked down to prevent cyclin B2 redistribution from impacting B1 staining levels. For anti-CDK1 siRNA (Extended Data Fig. 11b–d), cells were transfected for 6 h and then immediately imaged for 16 h. Cells depleted of CDK1 for this period of time retained sufficient amounts of CDK1 to progress through mitosis.
For western blot analyses shown in Extended Data Fig. 12e–f, cells were transfected with 40 nM siRNA using Lipofectamine RNAiMAX (Invitrogen, 13778150) according to manufacturer’s protocol. Forty eight h after transfection, cells were harvested and protein lysates extracted and resolved on SDS-PAGE. Western blots were probed with anti-CDK1 1:1000 (Abcam, ab133327), anti-CDK2 1:1000 (Abcam ab32147) and anti-HSP90 1:3000 (CST 4877) antibodies.
Single-cell microscopy
FUCCI(CA) cell cycle reporters.
Fluorescent cell cycle reporters (FUCCI(CA)22 were analyzed in time-lapse movies of live cells to determine progression through the cell cycle. This dual reporter system is made up of fluorescent protein-tagged fragments of human Cdt1 (Cdt1(1–100)Cy(−)) and human Geminin (Gem(1–110)), each of which detects distinct important events at the G1/S boundary. Cdt1(1–100)Cy(−) is a minimal fragment of human Cdt1 which is specifically degraded by E3 ubiquitin ligase CRL4Cdt2, and is not degraded by E3 ubiquitin ligase SCFSkp2 (due to the removal of a Cy motif). CRL4Cdt2-mediated degradation of Cdt1(1–100)Cy(−) depends on chromatin-bound PCNA, which is found at replication forks in S phase22. Thus, Cdt1(1–100)Cy(−) is present during G1, is rapidly degraded in response to origin firing at the start of S phase [Cdt1(1–100)Cy(−)drop] and is then stabilized at the end of S phase [Cdt1(1–100)Cy(−)rise] (Extended Data Fig. 9d). Gem(1–110) is specifically degraded by the anaphase-promoting complex/cyclosome (APC/C) from anaphase until APC/C inactivation near the end of G1. Gem(1–110) is absent from G1 cells and begins to rise at the point of APC/C inactivation [Gem(1–110)rise] (Extended Data Fig. 9d). APC/C inactivation signals the point of no-return when cells are committed to enter the S phase66. Importantly, Gem(1–110)rise and APC/C inactivation are not directly coupled to DNA synthesis, and are thus distinct readouts of cell cycle progression from Cdt1(1–100)Cy(−)drop which explicitly measures DNA synthesis and S phase entry.
General experimental scheme.
Single-cell cell cycle dynamics were determined using quantitative fluorescence microscopy in live and fixed cells. For live-cell analysis, fluorescent cell cycle reporters (FUCCI(CA)) were analyzed in time-lapse movies of live cells to determine position within the cell cycle. Combined live and fixed-cell imaging was performed in FUCCI(CA) cells to determine the dynamics of fixed-cell measurements (5-ethynyl-2′-deoxyuridine [EdU] incorporation, DNA content, immunofluorescence staining of cyclin B1 and CDK1). Cells were initially live-imaged to determine the position in the cell cycle cells were in, and then cells were immediately fixed. After fixed-cell staining, cells were reimaged at the same position in the microscope, and cells could be matched between live and fixed-cell imaging. Each fixed-cell measurement could then be assigned a position in the cell cycle (time since mitosis, time since S phase entry, etc.) and a time-course could be generated for fixed-cell measurements in asynchronous cells.
All cells were imaged on a Ti2-E inverted microscope (Nikon). Multichannel fluorescent images were taken with triple-band (Chroma) or penta-band (Chroma) filter sets using an LED light source (Lumencor Spectra X) and Hamamatsu ORCA-Flash4.0 V3 camera. Live cells expressing cell cycle reporters were imaged using appropriate fluorescent channels within an enclosed 37°C, 5% CO2 environmental chamber. Nine images per well were taken every 12 min (10 min for human dermal fibroblasts) for the duration of imaging, while minimizing light exposure to cells. A 0.45 NA 10x objective (live-cell imaging in MEF, MCF10A, U2OS) and 0.75 NA 20x objective (fixed-cell imaging and human dermal fibroblasts live-cell imaging) were used to acquire images. When imaging fixed cells which were to be matched back to previously live-imaged cells, the stage position was aligned to approximately the same location, and further aligned computationally after image acquisition.
Live-cell microscopy experiments.
For cell cycle analyses by live-cell imaging alone (Fig. 2a–c, Extended Data Fig. 3a–f, Extended Data Fig. 9e–k), drug treatments were started acutely during imaging in asynchronously cycling cells to determine the cell cycle phase at the time of drug treatment (e.g. to avoid cells which would be arrested in G2/M due to treatment with Ro-3306). For drug treatment during live-cell imaging, cells were quickly taken off the microscope, treated with drugs and then immediately put back onto the microscope for continued imaging. All drug additions were performed through media replacement and media was refreshed daily for multi-day imaging.
When treating asynchronously cycling Cdc7AID/AID/Tir1 MEF cells with IAA and Ro-3306 (Extended Data Fig. 9e–h), 500 μM IAA was initially added to degrade Cdc7, and cells were immediately live-imaged for 4 h and then treated with Ro-3306. During image analysis, cells which received Ro-3306 0–2 h after mitosis (cells in G1) were selected on the basis of the automatically tracked mitotic time of each cell (see Image analysis section). For multi-day imaging of asynchronously cycling Cdc7AID/AID/Tir1 MEFs, cells were initially imaged for 7 h and then IAA was added to cells and imaged for an additional 70 h (Fig. 2a–c). Cells which had IAA added 0–4 h before mitosis were selected.
For MCF10A and U2OS asynchronous cycling experiments (Extended Data Fig. 9i, j), cells were live-imaged for 2 h and then 10 μM Ro-3306 and 500 nM TAK-931 were added to cells and analyzed as described for MEFs. For MCF10A serum release experiments, drugs were added at the time of serum release and all tracked cells were analyzed (Extended Data Fig. 9k). For multi-day imaging of asynchronously MCF10A cells, cells were initially imaged for 8 h and then 500 nM TAK-931 was added to cells and imaged for an additional 69 h (Extended Data Fig. 3a–f).
Combined live and fixed-cell imaging.
For combined-live and fixed-cell imaging with drug treatments, cells were treated with drugs in the same way as in live-cell imaging only experiments unless otherwise stated. Analysis in Fig. 4b, c shows EdU intensity and log2 of Hoechst intensity (DNA content) in cells 16–18 h after mitosis, selecting cells with which underwent Gem(1–110)rise (inactivated APC/C) to focus on cells which committed to S phase entry. In Fig. 4d–f, MCF10A cells expressing FUCCI(CA) were live imaged for a total of 12 h and then pulsed with EdU for 8 min and fixed. At 4, 6, 8 and 10 h before fixation, cells were treated with vehicle (control), CDC7 inhibitor TAK-931 (Cdc7i), CDK1 inhibitor Ro-3306, or with both inhibitors. Cells which were treated with drug(s) starting at 0–2 h after exit from mitosis were selected for analysis (corresponding to cells fixed 4–6, 6–8, 8–10 and 10–12 h after mitosis) of EdU incorporation and DNA content from Hoechst stain. Panels 4e, f, show cells which underwent Gem(1–110)rise (corresponding to APC/C inactivation).
For human dermal fibroblasts asynchronous cycling experiments (Extended Data Fig. 9a–c, Extended Data Fig. 11h,i), primary human dermal fibroblasts with no fluorescent proteins expressed were stained with 50 nM SiR-Hoechst (Cytoskeleton, Inc.) in growth media for nuclear visualization. In Extended Data Fig. 9a–c, cells were pre-treated with for 3 h to stain the nuclei and were then live-imaged for an additional 3.5 h. 10 μM Ro-3306 and 500 nM TAK-931 were then added to cells in growth media with 50 nM SiR-Hoechst and imaged for an additional 12 h before fixation and staining (Hoechst, EdU incorporation, endogenous Geminin). Cells which were treated 0–2 h after the exit of mitosis and which were positive for endogenous Geminin staining (APC/C inactivation allows for accumulation of Geminin, similarly to Gem(1–110)) were selected for analysis (corresponding to cells fixed 12–14 h after mitosis). Human dermal fibroblasts were only annotated based on mitotic time due to the lack of fluorescent reporters.
For fixed-cell staining after live-cell imaging (Fig. 1k, Fig. 4, Fig. 5d, Extended Data Fig. 2e, Extended Data Fig. 9a–c, Extended Data Fig. 10h, i, Extended Data Fig. 11b–i), cells were immediately fixed after the end of imaging in 4% paraformaldehyde in PBS for 10 min at room temperature followed by PBS wash 3 times. To measure EdU incorporation, cells were pulsed with 50 μM EdU (Cayman Chemical) in growth media together with any drugs for 8 min prior to fixation. Fluorescent proteins were chemically bleached70 for 1 h after fixation to free fluorescent channels for use in fixed imaging. Fixed cells were permeabilized in 0.2% Triton X-100 in PBS for 10 min and then blocked in 10% FBS, 1% BSA, 0.1% Triton X-100, 0.01% NaN3 in PBS for 1 h. To stain incorporated EdU, a click reaction (incubation in 2 mM CuSO4, 20 mg/mL sodium ascorbate in Tris 50 mM, 150 mM NaCl pH 8.3) was performed with 3 μM AFDye 488, 568 or 647 picolyl azide (Click Chemistry Tools) for 30 min. Cells were then incubated in primary antibodies overnight in blocking buffer at 4°C, followed by secondary antibodies (goat anti-rabbit or goat anti-mouse Alexa Fluor 488 or 647 1:1000; Thermo Fisher Scientific) in blocking buffer for 1 h at room temperature, and 1 μg/mL Hoechst 33342 in PBS for 10 min. Cells were washed 3 times in PBS after each step. Rabbit anti-cyclin B1 mAb 1:400 (CST: 12231), rabbit anti-CDK1 mAb 1:200 (Abcam: ab133327; antibody #1 in Extended Data Fig. 11 and 12), rabbit anti-CDK1 pAb 1:100 (Atlas Antibodies: HPA003387; antibody #2 in Extended Data Fig. 11 and 12), mouse anti-cdc2 (CDK1) mAb 1:100 (CST: 9116; antibody #3 in Extended Data Fig. 11 and 12), rabbit anti-CDK2 mAb 1:800 (CST: 18048), rabbit anti-GMNN (Geminin) pAb 1:500 (Atlas Antibodies: HPA049977), and mouse anti-Flag mAb 1:2000 (Sigma: F3165) were used. Note that in Fig. 1k, cells were not live-imaged and the stained cells represent a population gated for Gem(1–110) -positive cells (S and G2-phase) in fixed cells. In Extended Data Fig. 12 a–d, cells were not live-imaged and quantification included nuclear signal in all imaged cells.
Image analysis.
Automated image analysis was performed using custom MATLAB scripts as described previously66. (tracking pipeline available at Zenodo, https://zenodo.org/record/6347643, DOI: 10.5281/zenodo.6347643) In short, raw images were flat-field corrected using an empirically determined illumination profile. Nuclei were automatically segmented from H2B or Hoechst channels, and tracked using a nearest-neighbor algorithm. Mitoses were annotated by tracking the splitting of cell-nuclei with the condition that the intensity of both daughter cell nuclei approximately equals the mother cell intensity. For live-cell imaging, the mean signal within the nucleus after background subtraction was used to quantify the Cdt1(1–100)Cy(−) and Gem(1–110) fluorescence levels, which were automatically annotated using a fall- and rise-time detection algorithm respectively. For matching fixed-cell images to live cells, fixed-cell images were computationally aligned to live-cell images and cells were assigned to its nearest neighbor. EdU incorporation and nuclear Cdc7 (anti-Flag-tag), CDK1, cyclin B1, CDK2 and geminin were quantified by the intensity within the nucleus. To quantify nuclear cyclin B1 levels, nuclear masks were eroded by 3 pixels and median value was taken to avoid cytoplasmic signal near the edge of the nucleus from confounding results. DNA content was quantified as the integrated Hoechst stain intensity within the nucleus. 2N DNA content was estimated from cells from G1. Thresholds for >2N DNA and EdU positive cells were calculated by taking the 99th percentile of G1 cells. Annotated mitosis or Cdt1(1–100)Cy(−)drop times from live-cell imaging were used to determine the cell cycle timing of each fixed-cell measurement.
Xenopus egg extracts and DNA replication assays (Fig. 5e, Extended Data Fig. 12i).
All experiments involving animals were approved by the Harvard Medical School Institutional Animal Care and Use Committee (IACUC) and conformed to relevant regulatory standards. Xenopus egg extracts including High Speed Supernatant (HSS) and Nucleoplasmic egg extract (NPE) were prepared as described71,72. Replication of plasmid DNA was performed as previously described73. Briefly, a 4.8 kb plasmid DNA was first incubated in HSS at a concentration of 7.5 ng DNA/μL HSS for 30 min at room temperature to license the DNA for replication (“licensing”), followed by addition of 2 volumes of NPE to initiate replication. NPE was supplemented with trace amounts of [α-32P]-dATP to label the nascent strands. NPE was treated either with 3.75 μM of Cdc7 inhibitor PHA-767491 or DMSO (control). To assess the effects of cyclin-CDK complexes on DNA replication initiation, the NPE was supplemented with 25 ng/μl of recombinant cyclin-CDK complexes (ProQinase) and incubated for 5 min prior to addition to the licensing mixture. At the indicated timepoints, 2 μL of the replication reaction were sampled into 5 μL of stop solution A (5% SDS, 80 mM Tris pH 8.0, 0.13% phosphoric acid, 10% Ficoll). The replicated DNA was then treated with 1 μL 20 mg/ml Proteinase K (Roche, Nutley, NJ) for 1 h at 37°C prior to resolution by 0.9% native agarose gel electrophoresis in TBE buffer. Gels were dried and imaged using phosphoimaging on a Typhoon FLA 7000 (GE Healthcare).
Statistical analyses.
Statistical analyses were conducted using two-tailed unpaired t-tests. Statistical significance was defined as a p-value of less than 0.05 using the appropriate statistical test method. Error bars are represented as either mean ± SD or mean ± SEM, and this is indicated in the figure legends.
Data Availability.
Source data for mass spectrometry analyses (Fig. 3d; Extended Data Fig. 7a, d; Supplementary Table 1; Supplementary Table 2) has been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD025625.
ProteomeXchange title:
The role of Cdk1-cyclin B in G1/S transition revealed by chemical genetic inhibition of Cdc7. ProteomeXchange accession: PXD025625. Project Webpage: http://www.ebi.ac.uk/pride/archive/projects/PXD025625. Raw imagining datasets are available upon request. Source data for western blots and flow cytometry gating strategies are provided with this paper.
Extended Data
Extended Data Fig. 1. Gene-targeting to generate mutant Cdc7 alleles.
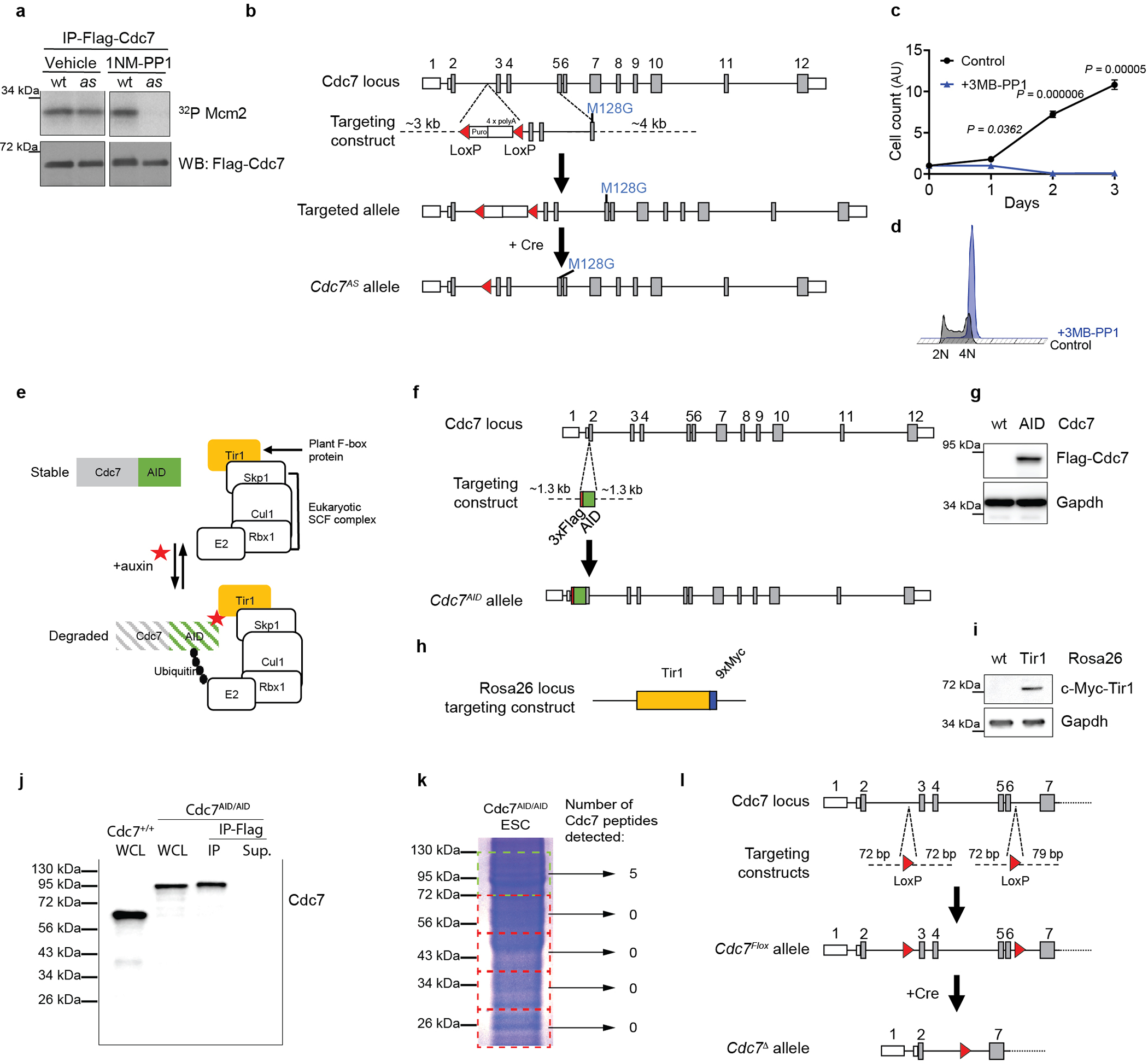
a, Verification that analog-sensitive (as) Cdc7, but not wild-type Cdc7 can be potently inhibited by 1NM-PP1. 293T cells were transfected with constructs encoding Flag-tagged wild-type (wt) or as Cdc7. Cells were treated with DMSO (Vehicle) or 1NM-PP1, Cdc7 immunoprecipitated using an anti-Flag antibody and subjected to kinase reactions with [γ32P]-ATP and Mcm2 as a substrate. Immunoprecipitates were also immunoblotted with an anti-Flag antibody. b, Gene-targeting strategy to generate the Cdc7AS allele. Cdc7 exons are numbered, coding exons are in grey. M128G, as substitution; Puro, puromycin resistance cassette; 4x polyA, polyadenylation signal; red triangles, LoxP sites. c, Growth curves of Cdk1AS/AS ESC cultured in the presence of vehicle (Control) or 3MB-PP1. d, Cdk1AS/AS ESC treated as in c for 24h were stained with propidium iodide and analyzed by flow cytometry. Note that Cdk1-inhibited cells arrested in G2-phase, as expected. e, The principle of auxin-inducible degradation system. A plant hormone auxin recruits proteins containing the auxin-inducible degron (AID) domain to E3 ubiquitin ligase Cul1–Rbx1–Skp1–Tir1 (SCFTir1) for polyubiquitination and subsequent degradation by the proteasome. f, Gene-targeting strategy to knock-in the AID domain and 3xFlag-tag into the Cdc7 gene in ESC. g, Immunoblot analysis of wild-type (wt) and Cdc7AID/AID/Tir1 (AID) ESC for expression of Flag-tagged Cdc7. h, Gene-targeting strategy to knock-in the Myc-tagged Tir1 gene into Rosa 26 locus. i, Immunoblot analysis of wild-type (wt) and Tir1 knock-in ESC for expression of Myc-tagged Tir1. Gapdh was used as loading control. j, To verify the absence of truncated, untagged Cdc7 species in Cdc7AID/AID/Tir1 ESC, we prepared whole cell lysates (WCL) from these cells (lane 2), and probed immunoblots with an anti-Cdc7 antibody. In addition, we immunodepleted tagged Cdc7 from WCL using an anti-Flag antibody (IP-Flag) and probed immunoprecipitates (IP, lane 3) and supernatants (Sup, lane 4) with an anti-Cdc7 antibody. WCL from wild-type ESC were also immunoblotted (lane 1). We did not detect the presence of truncated Cdc7. k, To further exclude the presence of truncated Cdc7, we resolved WCL from Cdc7AID/AID /Tir1 ESC on an SDS-PAGE gel, cut the gel into smaller fragments corresponding to different protein sizes and evaluated the presence of Cdc7 peptides in these fractions by mass spectrometry. We detected the presence of Cdc7 only in the fraction corresponding to the full-length protein. l, Gene-targeting strategy to generate Cdc7Flox allele in ESC. Red triangles, LoxP sites. c, n=3 independent replicates; a, d, g, i, j show representative results (out of 2); c, p-values determined by two-sided t-test; error bars, SD.
Extended Data Fig. 2. Analyses of Cdc7AID/AID cells.
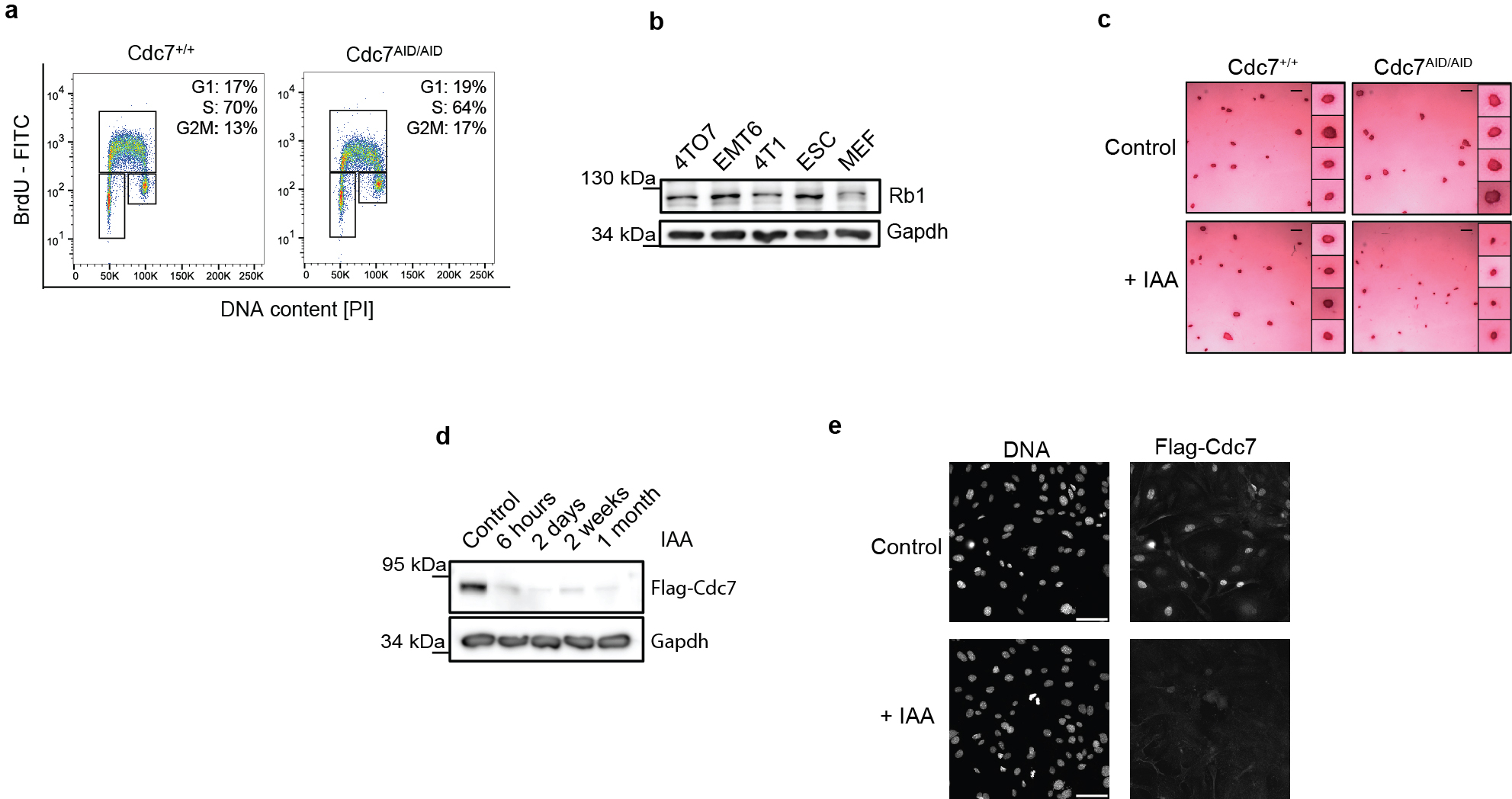
a, wild-type (Cdc7+/+) and Cdc7AID/AID/Tir1 ESC were cultured without auxin. Cells were pulsed with BrdU, stained with an anti-BrdU antibody and propidium iodide and analyzed by flow cytometry. Note normal cell cycle profile of Cdc7AID/AID/Tir1 ESC in the absence of auxin, as expected. b, Immunoblot analysis to demonstrate expression of the retinoblastoma protein, Rb1, in wild-type ESC. Whole cell lysates from MEFs and mouse breast cancer cell lines (4TD8, EMT6, 4T1) were immunoblotted for control. c, Cdc7+/+ and Cdc7AID/AID/Tir1 ESC were cultured on feeders with auxin (IAA) or vehicle (Control) for 72 h and stained for alkaline phosphatase, a marker of undifferentiated pluripotent stem cells. Scale bar, 500 μm. Inserts, higher magnification of colonies. Inhibition of Cdc7 did not decrease alkaline phosphatase staining, suggesting that Cdc7 is not required to maintain pluripotency. d, Cdc7 protein levels in Cdc7AID/AID/Tir1 ESC cultured with auxin for the indicated times. Immunoblots of whole cell lysates were probed with an anti-Flag antibody to detect Flag-tagged endogenous Cdc7. Efficient degradation of Cdc7 was maintained after 1 month of continuous culture with auxin. e, Immunostaining of Cdc7AID/AID/Tir1 MEFs for expression of Cdc7. Cells were cultured with vehicle or auxin for 4 h, and stained with an anti-Flag antibody. Nuclei were counterstained with Hoechst. Note the loss Cdc7 staining after addition of auxin. See Fig. 1k for quantification. a-e, representative results (out of 2).
Extended Data Fig. 3. Analyses of CDC7-inhibited cells.
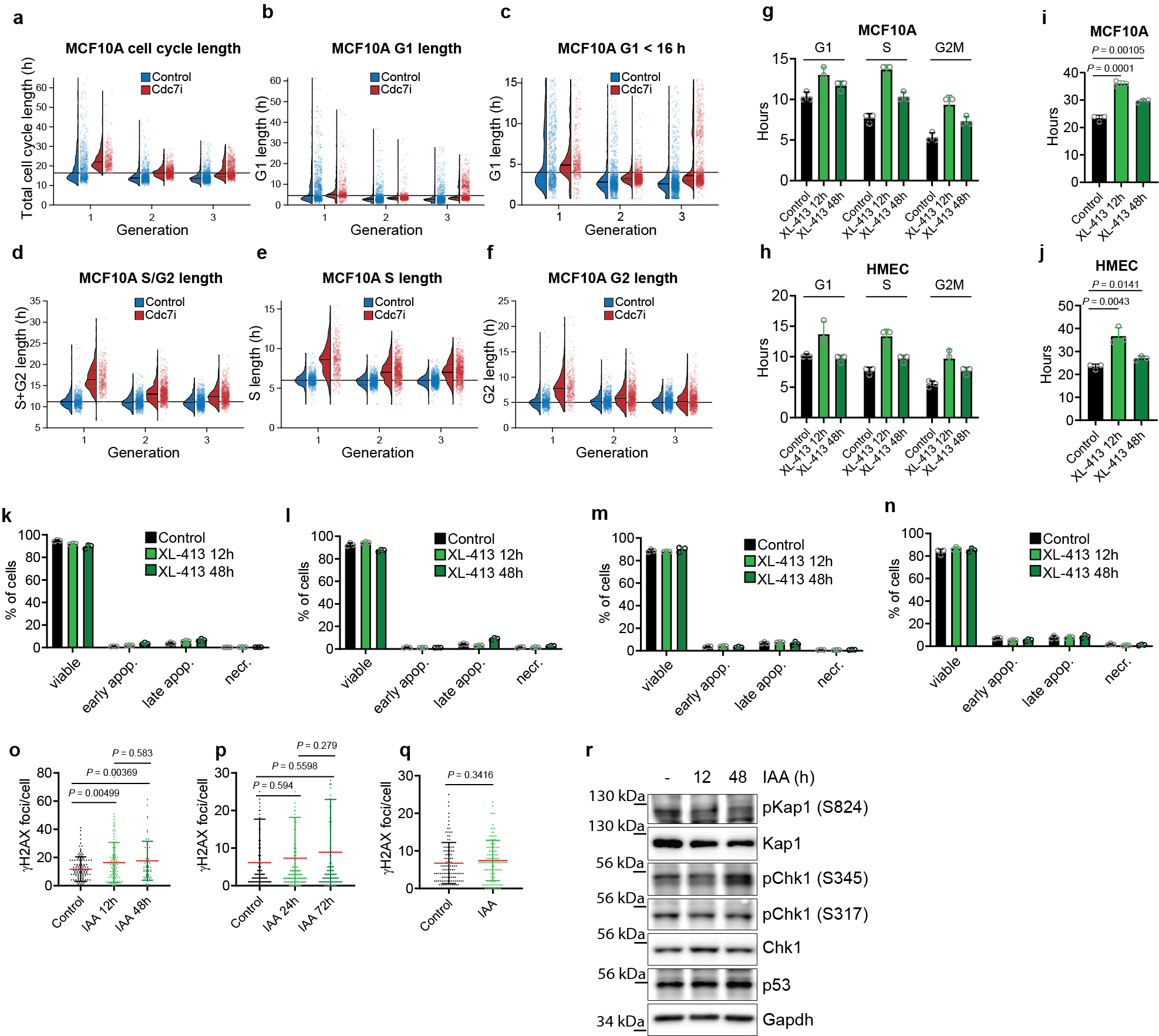
a-f, Long-term live-cell imaging. MCF10A cells expressing Gem(1–110)-mVenus and Cdt1(1–100)Cy(−)-mCherry FUCCI(CA) reporters were cultured and imaged for a total of 77 h. 8 h after imaging start, CDC7 inhibitor, TAK-931, was added to the culture medium, and cells were imaged for an additional 69 h. Shown are parameters for the first three cell cycles after addition of TAK-931 (generations 1–3, selecting cells which received TAK-931 0–2 h after mitosis). a, Total cell cycle length (time between two mitoses). b, G1 length (time between mitosis and Cdt1(1–100)Cy(−)drop [S phase start]). c, Shown is the subset of G1 cells from b, with G1 phase lasting less than 16 h. d, S/G2 length (time from Cdt1(1–100)Cy(−)drop [S phase start]) to subsequent mitosis. e, S phase length (time from Cdt1(1–100)Cy(−)drop [S phase start] to Cdt1(1–100)Cy(−)rise [S phase end]). f, G2 length (time from Cdt1(1–100)Cy(−)rise [S phase end]) to subsequent mitosis). In a-f, dots show values for individual cells; thin horizonal lines, median values; dotted lines above and below median, inter-quartile range; long horizonal lines, median values from control cells in generation 1. g, h, The length of G1, S and G2/M as determined by pulse-chase analysis in MCF10A (g) and HMEC human mammary epithelial cells (h) treated for the indicated times with CDC7 inhibitor, XL-413. Control, the length of cell cycle phases in untreated cells. i, j, Doubling times of MCF10A (i) and HMEC cells (j) treated for the indicated times with XL-413, determined by pulse-chase analysis. Control, untreated cells. k-n, human BJ foreskin fibroblasts (k), HMEC (l), human primary dermal fibroblasts (m), and MCF10A cells (n) were treated for the indicated times with XL-413, stained with Annexin V and propidium iodide (PI) and analyzed by flow cytometry. Shown are percentages of viable (Annexin V−/PI−), early apoptotic (Annexin V+/PI−), late apoptotic (Annexin V+/PI+) and necrotic (Annexin V−/PI+) cells. Control, untreated cells. o, p, mean numbers of γH2AX foci/cell in Cdc7AID/AID/Tir1 ESC (o) and MEFs (p) cultured for the indicates times with auxin. Control, untreated cells. q, similar analysis for Cdc7AID/AID/Tir1 ESC cultured with auxin for 7 weeks. r, Cdc7AID/AID/Tir1 MEFs were treated with vehicle (−) or auxin for the indicated times. Lysates were immunoblotted with antibodies recognizing Chk1, p53, Kap1 (Trim28), phospho-Kap1 (serine 824), phospho-Chk1 (serine 317) and phospho-Chk1 (serine 345). Gapdh was used as loading control. g-q, show mean values; i, j, o-q, p-values determined by two-sided t-test; error bars, SD. g-n, n=3 independent replicates; a-f, n=2 independent replicates, o-q, data of n > 60 cells from at least 5 independent fields per experimental condition, r, representative results (out of 2).
Extended Data Fig. 4. Knockout of Cdc7.
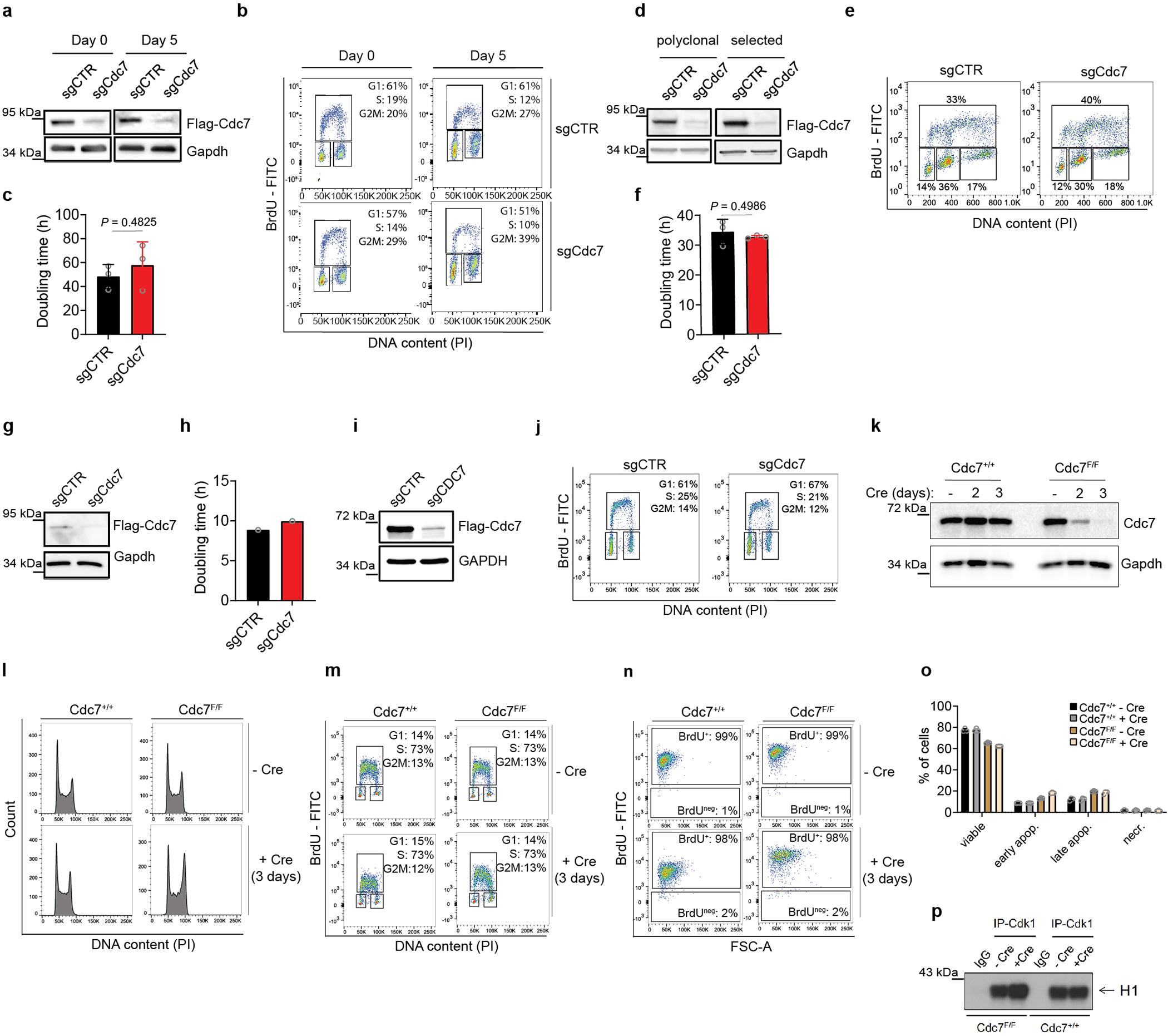
a-c, CRISPR/Cas9-mediated knockout of Cdc7 in primary mouse fibroblasts (MEFs). Cdc7AID/AID/Tir1 MEFs were transduced with viruses encoding control sgRNA (sgCTR) or anti-Cdc7 sgRNA (sgCdc7). a, Whole cell lysates were immunoblotted and probed with an anti-Flag antibody to detect Flag-tagged endogenous Cdc7. Day 0, cells at the end of selection; Day 5, cells after 5 days of culture. b, Cell cycle analysis of cells from a. Cells were pulsed with BrdU, stained with an anti-BrdU antibody and propidium iodide and analyzed by flow cytometry. c, Doubling times. d-f, CRISPR/Cas9-mediated knockout of Cdc7 in MEFs immortalized by dominant-negative p53. d, Immunoblotting as in a. Polyclonal, total polyclonal cell population after transduction with sgRNAs; selected, further purified population containing over 90% of Cdc7 frame-shift alleles. e, cell cycle analysis (polyclonal population), f, doubling times (polyclonal population). g, h CRISPR/Cas9-mediated knockout of Cdc7 in keratinocytes; g, immunoblotting, h, doubling time. i-j, CRISPR/Cas9-mediated knockout of CDC7 in human BJ foreskin fibroblasts. i, Immunoblotting with anti-CDC7 antibody, j, Cell cycle analysis. k-p, Acute ablation of Cdc7 in ESC. Cdc7Flox/Flox (Cdc7F/F) and Cdc7+/+ ESC were engineered to stably express dominant-negative p53 (to prevent p53-dependent cell death upon shutdown of Cdc733). Cells were transduced with lentiviruses encoding Cre-recombinase, or mock transduced. k, Cells were harvested at days 2 and 3, and extracts immunoblotted with an anti-Cdc7 antibody. l, m, at day 3, cells were stained with propidium iodide (l), or pulsed with BrdU and stained with propidium iodide and an anti-BrdU antibody (m) and analyzed by flow cytometry. n, Cells were cultured in the presence of BrdU for 15 h, stained with an anti-BrdU antibody and the percentage of BrdU+ cells was evaluated by flow cytometry. Note that 98% of Cdc7-deleted cells (and the same fraction of Cdc7+/+ cells) incorporated BrdU, indicating active DNA synthesis. FSC, forward scatter. o, Cells were stained with Annexin V and propidium iodide (PI) and analyzed by flow cytometry. Shown are percentages of viable (Annexin V−/PI−), early apoptotic (Annexin V+/PI−), late apoptotic (Annexin V+/PI+) and necrotic (Annexin V−/PI+) cells. p, Cdk1 was immunoprecipitated (IP-Cdk1) from cells and used for kinase reactions with [γ32P]-ATP and histone H1 as a substrate. IgG, control immunoprecipitation with IgG. Note that ablation of Cdc7 resulted in modestly elevated levels of Cdk1 kinase - like an acute degradation of Cdc7 in Cdc7AID/AID/Tir1 ESC (Extended Data Fig. 7b). a, g, i, k, Gapdh was used as loading control. c, f, o, show means; p-values by two-sided t-test; error bars, SD; n=3 independent replicates; other panels show representative results (out of 2).
Extended Data Fig. 5. Studies of Cdc7-Dbf4 in cells, and in vivo analyses using Cdc7AID/AID/Tir1 mice.
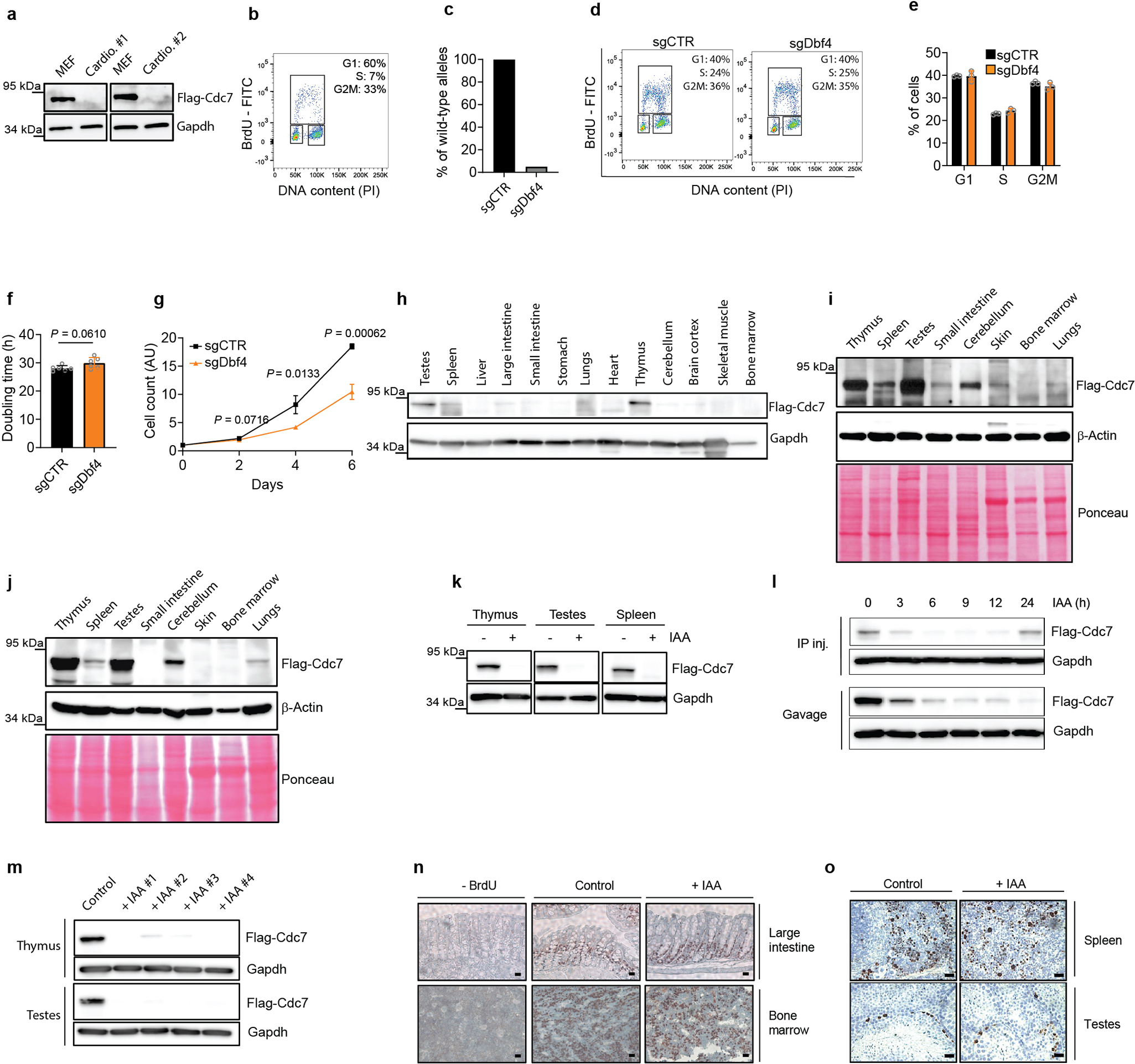
a, Analysis of cultured cardiomyocytes isolated from two independent litters of Cdc7AID/AID/Tir1 embryos (Cardio. #1 and #2) for expression of Cdc7. Whole cell lysates were immunoblotted and probed with an anti-Flag antibody to detect Flag-tagged endogenous Cdc7. MEFs served as a positive control. Note that cardiomyocytes do not express appreciable levels of Cdc7, despite active proliferation. b, Cell cycle analysis. Cells were pulsed with BrdU, stained with an anti-BrdU antibody and propidium iodide and analyzed by flow cytometry. c-g, CRISPR/Cas9-mediated knockout of Dbf4 in MEFs immortalized by dominant-negative p53. c, Results of DNA sequencing of the Dbf4 alleles in cells transduced with viruses encoding control sgRNA (sgCTR) or anti-Dbf4 sgRNA (sgDbf4). Shown is percentage of wild-type Dbf4 alleles in cell populations. d, Cell cycle analysis as in b. e, Distribution of cell cycle phases (from d). f, Doubling times; g, growth curves. h-o, In vivo analyses using Cdc7AID/AID/Tir1 mice. h, i, Analysis of Cdc7 protein levels in the indicated organs of Cdc7AID/AID/Tir1 mice. Lysates were immunoblotted and probed with an anti-Flag antibody to detect Flag-tagged endogenous Cdc7. In i, the immunoblot was overexposed to illustrate undetectable levels of Cdc7 in bone marrow. Note that except for testes and thymi, Cdc7 is expressed at very low levels. j, A similar analysis as in h, using organs from Cdc7AID/AID Tir1-negative mice. k, The levels of Cdc7 protein in the indicated mouse organs after treatment of the animals with auxin for 12 h. l, This panel illustrates the efficiency of two routes of auxin administration: a single intraperitoneal injection (IP inj.) or a single oral gavage (Gavage). Mice received auxin via the indicated routes and thymi collected at the indicated time-points for immunoblot analysis. Each of these routes resulted in an efficient Cdc7 degradation that lasted approximately 12 h. m, The levels of Cdc7 protein in thymi and testes of four Cdc7AID/AID/Tir1 mice (#1 to #4) treated with auxin for 8 days. Control, a mouse treated with vehicle for 8 days. n, After 8 days of auxin treatment, Cdc7AID/AID/Tir1 mice were injected with BrdU and sections stained with an anti-BrdU antibody. Control, a mouse treated for 8 days with vehicle, –BrdU, staining of sections from a mouse not injected with BrdU. Scale bars, 100 μm. o, Sections of organs from Cdc7AID/AID/Tir1 mice treated with auxin for 4 days were stained with an anti-Ki67 antibody (to detect proliferating cells). Scale bars, 100 μm. Gapdh and β-actin were used as loading controls; i, j, show Ponceau S-stained membrane. e-g show means; p-values by two-sided t-test; error bars, SD. e, n=3; f, g, n=6, a-d, h-l representative results (out of 2); m, n=4 n, o, representative images (of at least 5 independent fields).
Extended Data Fig. 6. Analyses of human CDK2-deficient cells.
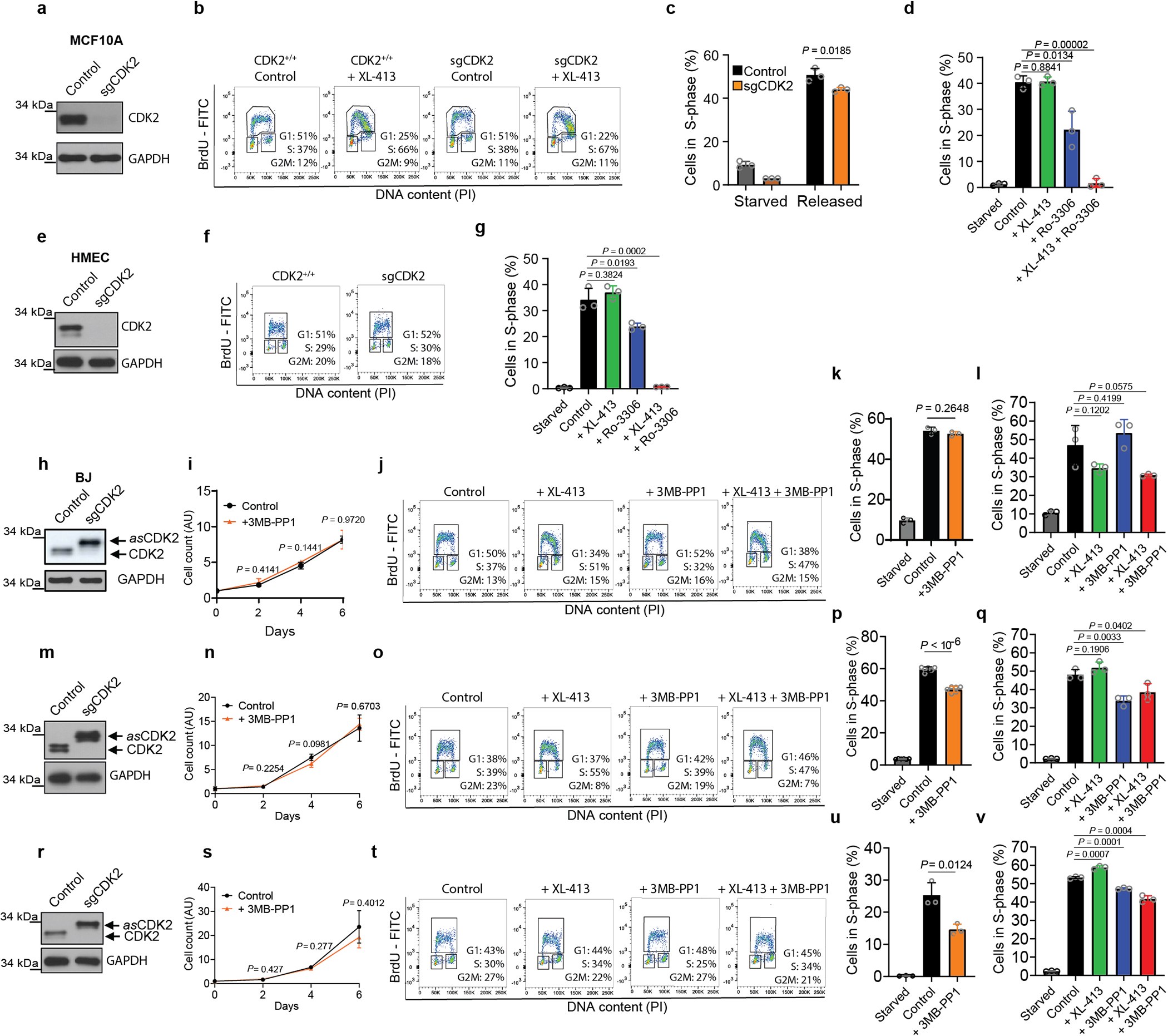
a-d, Analyses of CDK2-knockout human mammary epithelial MCF10A cells. a, Whole cell lysates from parental (Control) and CDK2-knockout (sgCDK2) MCF10A cells were immunoblotted and probed with an anti-CDK2 antibody. b, CDK2+/+ and CDK2-knockout (sgCDK2) MCF10A cells were cultured in the absence (Control) or presence of CDC7 inhibitor (XL-413) for 12 h. Cells were pulsed with BrdU, stained with an anti-BrdU antibody and propidium iodide and analyzed by flow cytometry. c, Parental and CDK2-knockout (sgCDK2) MCF10A cells were arrested in G0 by growth factor deprivation (Starved) and stimulated to re-enter the cell cycle by addition of growth factors (Released). Cells were pulsed with BrdU after 19 h, stained with propidium iodide and anti-BrdU antibody and analyzed by flow cytometry. d, CDK2-knockout MCF10A cells were arrested in G0 (Starved) and stimulated (as in c), in the presence of vehicle (Control), XL-413, CDK1 inhibitor (Ro-3306), or both inhibitors and analyzed as in c. e-g, CDK2-knockout human mammary epithelial HMEC cells. e, Whole cell lysates from parental and CDK2-knockout (sgCDK2) HMEC cells were immunoblotted as in a. f, Asynchronously growing CDK2+/+ and CDK2-knockout HMEC cells were analyzed as in b. g, CDK2-knockout HMEC cells were arrested in G0 (Starved), stimulated to re-enter cell cycle in the presence of inhibitors and analyzed as in d. h-l, Analyses of human BJ foreskin fibroblasts expressing analog-sensitive (as) CDK2 in place of the endogenous CDK2. h, Immunoblots of whole cell lysates of parental and CDK2-knockout cells expressing asCDK2 (asCDK2) probed with an anti-CDK2 antibody. asCDK2 migrates slower due to the presence of 3xFlag-tag. i, Growth curves of asCDK2 cells treated with vehicle (Control) or 3MB-PP1 (to inhibit asCDK2). j, asCDK2 BJ fibroblasts were cultured with XL-413, 3MB-PP1, or both and analyzed as in b. k, l, asCDK2 cells were arrested in G0 (Starved) and stimulated with serum in the presence of vehicle, 3MB-PP1, XL-413, or both inhibitors. Cells were analyzed as in d. m-q, Analyses of human mammary epithelial MCF10A cells expressing analog-sensitive CDK2 (asCDK2). m, Immunoblotting as in h. n, Growth curves as in i. o, Analyses of asynchronously growing cells, as in j. p, q, analyses of cell cycle re-entry as in k, l. r-v, Analyses of human mammary epithelial HMEC cells expressing analog-sensitive CDK2 (asCDK2). r, Immunoblotting; s, growth curves (as in i); t, analyses of asynchronous cells (as in j); u, v, analyses of cell cycle re-entry (as in k, l). GAPDH was used as loading control. c, d, g, i, k, l, n, p, q, s, u, v show mean values; p-values by two-sided t-test; error bars, SD. c, d, g, i, k, l, n, p, q, s, u, v, n=3 independent replicates; panels a, b, e, f, h, j, m, o, p, r, t, show representative results (out of 2).
Extended Data Fig. 7. Analyses of Cdc7-deficient cells.

a, Quantification of Cdk1 phosphorylation on threonine-161 (T161) and threonine-15 (T15), using mass spectrometry. Cdc7AID/AID/Tir1 ESC were treated with auxin and analyzed after 5, 24 and 48 h. Black dots, phosphorylation levels at time 0. b, Cdk1 was immunoprecipitated (IP-Cdk1) from Cdc7AID/AID/Tir1 ESC treated with auxin or vehicle (−) for 24 h, and used for kinase reactions with [γ32P]-ATP and histone H1 as a substrate. IgG, control immunoprecipitation. c, Recombinant Mcm2 was phosphorylated in vitro with recombinant CDK1-cyclin B1 or CDC7-DBF4 and incorporation of phosphate into serine-27 quantified by mass spectrometry. d, Cdk1AS/AS ESC were treated with 3MB-PP1 for 24 h and phosphorylation of the endogenous Mcm2 on serine-27 quantified by mass spectrometry. e, Wild-type ESC were arrested in mitosis by nocodazole, and released. At the indicated time-points after the release, cells were stained with propidium iodide and analyzed by flow cytometry. Asynchronous, asynchronously growing cells; M-phase, cells arrested in mitosis. f, In-cell phosphorylation of Mcm2 by Cdk1 during early S phase. Cdk1AS/AS (as) and wild-type (wt) ESC were synchronized in mitosis with nocodazole and released. 4 h after the release, cells were provided for 20 min with bulky ATPγS-analog, N6-furfuryl-ATPγS, to label Cdk1 substrates. Mcm2 was immunoprecipitated (IP-Mcm2) and immunoblots probed with an anti-thiophosphate ester antibody (ThioP) to detect thio-phosphorylation of Mcm2, and with anti-Mcm2 antibody. IgG, control, immunoprecipitation from Cdk1AS/AS cells. Note the absence of signal for thio-phosphorylated Mcm2 in wild-type ESC cells, as expected. Shown is analysis of two independent cultures. g, MEFs, human glioblastoma T98G cells and BJ foreskin fibroblasts were arrested in G0 by serum starvation, and stimulated to enter the cell cycle by serum addition. At the indicated time-points after stimulation, cells were stained with propidium iodide and analyzed by flow cytometry. SS, serum starved cells. h, Asynchronously growing Cdk2-knockout/Cdc7AID/AID/Tir1 ESC (Asynchr.) were synchronized in mitosis by nocodazole (M-phase) and released (−Noco). Upon release, Cdc7 degradation was induced by auxin (IAA) addition to account for degradation time. Two h later, when cells reached G1 phase (G1), they were treated with Cdk1 inhibitor (Ro-3306). Cells were cultured in the presence of inhibitor(s), collected after 15 h (S/G2), stained with propidium iodide and analyzed by flow cytometry along with asynchronous, M phase and G1 phase cells. Upper row, vehicle-treated cells; second, cells treated with Ro-3306; third, auxin treatment; fourth row, treatment with auxin plus Ro-3306. i, Asynchronously growing Cdk1AS/AS ESC (Asynchr.) were synchronized in mitosis by nocodazole (M-phase) and released (−Noco). Two h later, when cells reached G1 phase (G1), they were treated with 3MB-PP1 (to inhibit Cdk1) and/or with XL-413 (Cdc7 inhibitor). Cells were cultured in the presence of inhibitor(s), collected after 12 h (S/G2) and analyzed as in h. Upper row, cells treated with vehicle; second, cells treated with 3MB-PP1; third, XL-413 treatment; fourth, treatment with 3MB-PP1 plus XL-413. j, Asynchronously growing Cdk1AS/AS ESC were treated with XL413 for 48 h and/or with 3MB-PP1 for the last 1 h, or with vehicle (Control), and subjected to DNA fiber analysis. Shown is the percentage of new replication origins, over the total number of forks analyzed. k, l, T98G cells were synchronized in M phase by nocodazole, released and treated in G1 phase with vehicle, XL-413, Ro-3306, or with both inhibitors, as in panels h, i. After 9 h of culture with inhibitor(s), cells were pulsed with BrdU, stained with an anti-BrdU antibody and propidium iodide and analyzed by flow cytometry (k). l, Mean percentage of S phase cells (from k). m-o, Human mammary epithelial MCF10A cells (m), mammary epithelial HMEC cells (n), and primary dermal fibroblasts (o) were arrested in G0 by growth factor deprivation and stimulated to re-enter the cell cycle by addition of growth factors in the presence of vehicle, XL-413, Ro-3306, or both inhibitors. Cells were pulsed with BrdU after 19 h, stained with propidium iodide and an anti-BrdU antibody and analyzed by flow cytometry along with serum-starved, G0 cells. a, d, j, l-o show mean values; p-values using two-sided t-test; Error bars, SD. a, d, j, l-o, n=3 independent replicates, b, e, g, h, i, k, representative results (out of 2).
Extended Data Fig. 8.

a, Cdc7AID/AID/Tir1 ESC (upper panel) or Cdk1AS/AS ESC (lower panel) were synchronized in mitosis by nocodazole and released as in Fig. 3i and Extended Data Fig. 7h, i. Two hours after the release, Cdc7AID/AID/Tir1 ESC were treated for 2 h with vehicle (Control), auxin (IAA), Cdk1 inhibitor Ro-3306, or with both compounds. Cdk1AS/AS ESC were treated for 2 h with vehicle, Cdc7 inhibitor XL-413, 3MB-PP1 (to inhibit Cdk1), or with both compounds. b, Upper panel: asynchronously growing Cdc7AID/AID/Tir1 ESC were treated for 2 h with vehicle, auxin, Ro-3306 or with both compounds. Lower panel: Cdk1AS/AS ESC were treated for 2 h with vehicle, XL-413, 3MB-PP1, or with both compounds. c, Cdc7AID/AID/Tir1 MEFs were treated for 2 h with vehicle, auxin, Ro-3306 or with both compounds. d, MCF10A, HMEC, BJ fibroblasts and primary human dermal fibroblasts (HDF) were treated for 2 h with vehicle, XL-413, Ro-3306 or with both compounds. a-d, The endogenous Cdk2 was immunoprecipitated (IP-Cdk2) from treated cells and used for kinase reactions with [γ32P]-ATP and histone H1 as a substrate. IgG, control immunoprecipitation with IgG. Note that treatment of cells with any of these inhibitors (either singly, or in combination) did not decrease the activity of the endogenous Cdk2. a-d, representative results (out of 2).
Extended Data Fig. 9. Live-cell imaging.
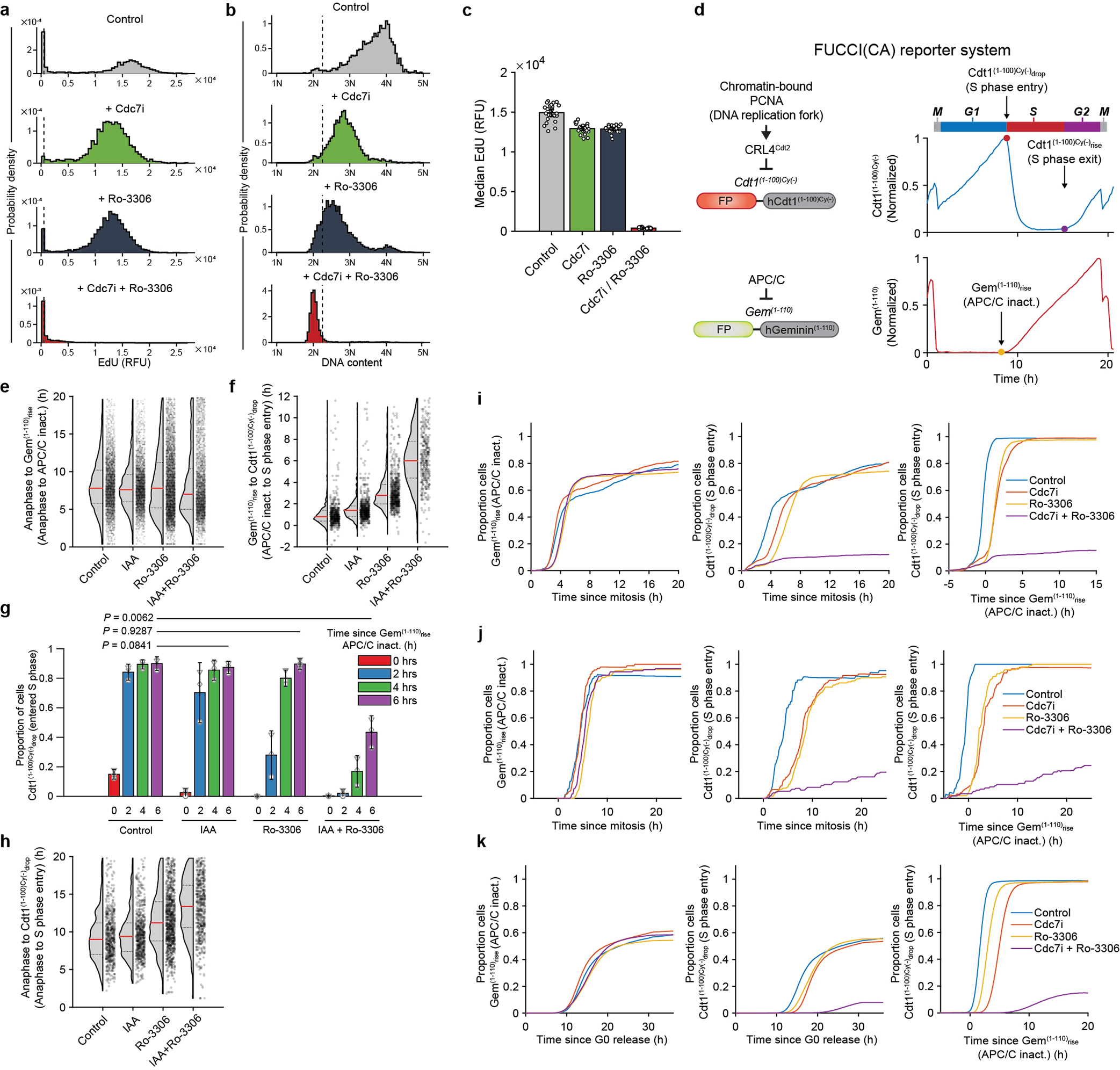
a-c, Primary human dermal fibroblasts were live-imaged and treated with vehicle (Control), CDC7 inhibitor TAK-931 (Cdc7i), CDK1 inhibitor Ro-3306, or with both inhibitors. Cells which received the inhibitor(s) starting 0–2 h after the exit of mitosis were selected for analysis. Cells were pulsed with EdU and analyzed for EdU incorporation intensity (a), and DNA content from Hoechst staining (b) 12–14 h after mitosis. Dashed vertical lines in a represent cutoff values for EdU-positive signal, in b for signal corresponding to >2N DNA significantly above 2N noise. c, Median EdU incorporation (from a). Each circle corresponds to a replicate well. d, FUCCI(CA) cell cycle reporter system used in the study. The system consists of fluorescent protein (FP)-tagged fragments of human Cdt1 (Cdt1(1–100)Cy(−)) and human Geminin (Gem(1–110)). Cdt1(1–100)Cy(−) is present during G1, and is rapidly degraded in response to origin firing at the start of S phase (Cdt1(1–100)Cy(−)drop). After the S phase ends, the reporter reaccumulates (Cdt1(1–100)Cy(−)rise). Gem(1–110) is degraded by the anaphase-promoting complex/cyclosome (APC/C) starting at anaphase and throughout G1 phase, until APC/C inactivation takes place at the end of G1 (Gem(1–110)rise). e-h, Asynchronously growing Cdc7AID/AID/Tir1 MEFs expressing FUCCI(CA) reporters were live-imaged in the presence of auxin or vehicle for 4 h and then Ro-3306 was added to cells. Cells which received Ro-3306 0–2 h after the exit from mitosis were selected for analysis. e, Time from the end of mitosis to Gem(1–110)rise (APC/C inactivation), in cells treated as indicated. Shown are cells with Gem(1–110)rise by 20 h after mitosis. f, g, Timing of Cdt1(1–100)Cy(−)drop (S phase entry) relative to Gem(1–110)rise (APC/C inactivation), in cells treated as above. f, Values for cells with Cdt1(1–100)Cy(−)drop within 12 h of Gem(1–110)rise. g, Fraction of cells which had Cdt1(1–100)Cy(−)drop (S phase start) at given times following Gem(1–110)rise (APC/C inactivation). h, Time from the end of mitosis to Cdt1(1–100)Cy(−)drop (S phase entry), in cells treated as indicated. In e, f, h, dots represent values for individual cells, pooled from independent experiments. Red horizontal lines, median values; dotted lines above and below the median, inter-quartile range. Control, vehicle-treated cells. i, j, Analysis of human mammary epithelial MCF10A (i) and osteosarcoma U2OS cells (j) expressing FUCCI(CA) system. Cells were live-imaged and treated with TAK-931 (CDC7i) and/or Ro-3306, or with vehicle (Control). Cells which were treated 0–2 h after the exit from mitosis were selected for analysis. Left panel: proportion of cells which underwent Gem(1–110)rise (APC/C inactivation) over time following the end of mitosis; middle: proportion of cells which underwent Cdt1(1–100)Cy(−)drop (S phase entry) over time following the end of mitosis; right: proportion of cells which underwent S phase entry (Cdt1(1–100)Cy(−)drop) over time following APC/C inactivation (Gem(1–110)rise). k, Analyses of MCF10A cells engineered as in i. Cells were arrested in G0, then induced to re-enter the cell cycle by re-addition of growth factors in the presence of TAK-931 and/or Ro-3306, or with vehicle, and analyzed as in i. c, g, Show mean values; error bars 2×SEM; p-values using two-sided t-test. e-h, n=3 independent replicates.
Extended Data Fig. 10. Analyses of Cdk1-cyclin B in mouse and human cells.
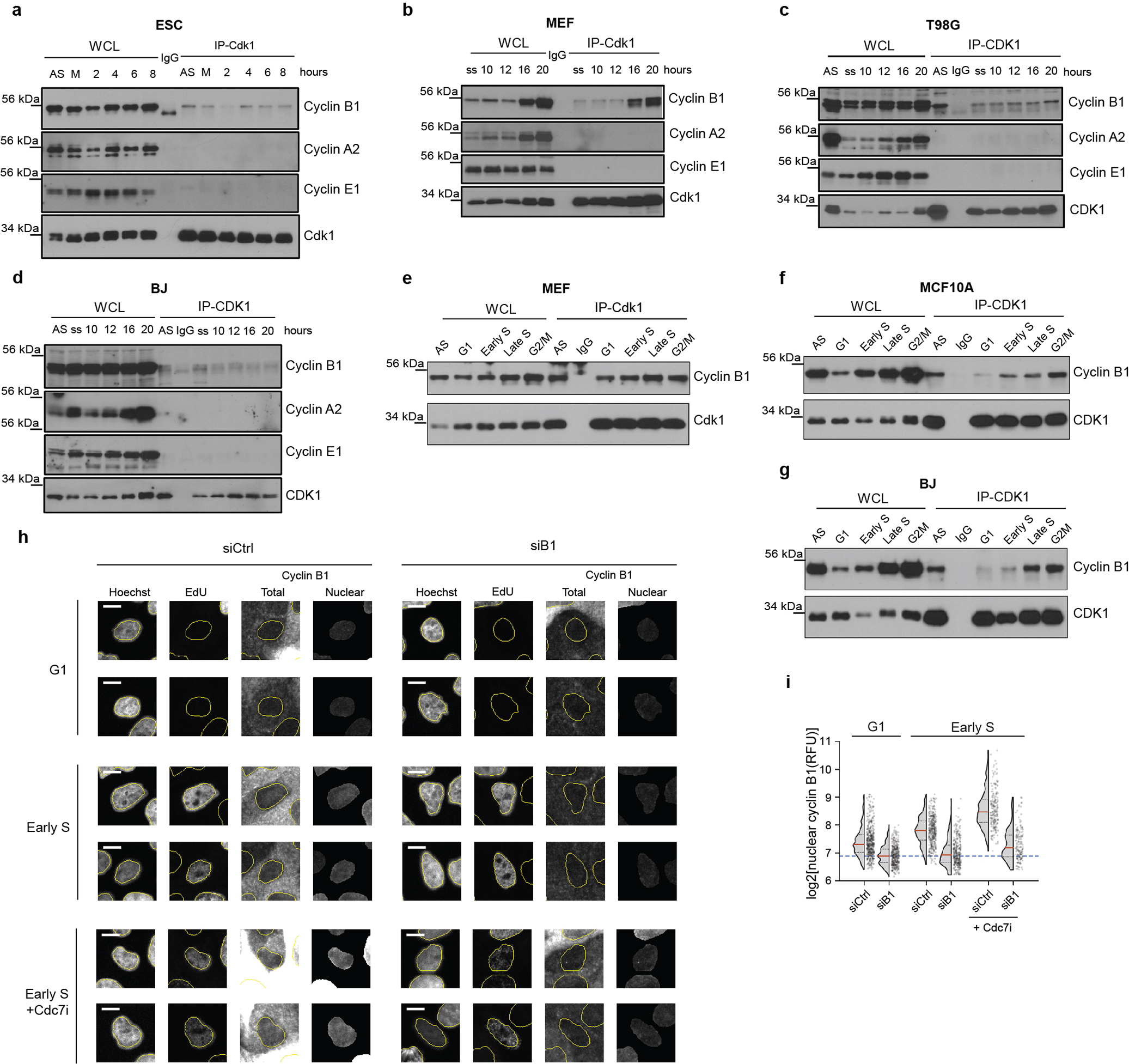
a-d, ESC (a) were synchronized in M phase with nocodazole, released, and cell cycle progression monitored (Extended Data Fig. 7e). MEFs (b), human glioblastoma T98G cells (c), and BJ foreskin fibroblasts (d) were serum-starved, stimulated to re-enter the cell cycle by serum addition, and progression through the cell cycle was monitored (Extended Data Fig. 7g). Cdk1 was immunoprecipitated (IP-Cdk1) from cells at the indicated times after the release/stimulation and immunoblots probed for cyclins B1, A2 and E1, and for Cdk1. Whole cell lysates (WCL) were also immunoblotted. AS, asynchronously growing cells; M, M phase-arrested cells; ss, serum-starved cells; IgG, control immunoprecipitation. e-g, Asynchronously growing MEFs (e), human mammary epithelial MCF10A cells (f), and BJ fibroblasts (g) were stained with cell-permeant DNA-binding dyes Hoechst 33342 (MEFs) or CytoPhase Violet (MCF10A and BJ cells). Cells were sorted using FACS based on DNA content into G1, early S, late S and G2/M fractions as in Fig. 5b. Cdk1 was immunoprecipitated from flow-sorted cells and immunoblots probed with antibodies against cyclin B1 or Cdk1. Whole cell lysates (WCL) prepared from sorted cells were also immunoblotted. AS, asynchronously growing cells; IgG, control immunoprecipitation. h, MCF10A cells expressing Cdt1(1–100)Cy(−)-mCherry reporter were used for combined live and fixed-cell imaging. Cells were stained with an anti-cyclin B1 antibody, and nuclear cyclin B1 staining assessed 1–2 h after mitosis in cells which had not entered S phase (G1 cells) or in cells 0.5 to 1 h after Cdt1(1–100)Cy(−)drop (early S phase). For control of antibody specificity, cells were transfected with anti-cyclin B1 siRNA (siB1), or control siRNA (siCtrl) 24 h before fixation (cells retained sufficient cyclin B1 to progress through mitosis). Cdc7i, cells treated with a Cdc7 inhibitor, TAK-931. Total, images of cells stained for cyclin B1; nuclear, cyclin B1 nuclear signal. Cells were also stained for EdU incorporation (to confirm DNA synthesis in S not in G1 phase nuclei) and with Hoechst (DNA stain). Scale bar, 10 μm. i, log2 intensity of nuclear cyclin B1 staining in different treatment groups (data from Fig. 5d; G1 and early S phase cells were defined as above in h). Dots represent values for individual cells; red horizontal lines, median values; dotted lines above and below the median, inter-quartile range. Long dashed line denotes median nuclear cyclin B1 signal in cells treated with anti-cyclin B1 siRNA, which we consider background level. Note that anti-cyclin B1 siRNA decreased nuclear cyclin B1 staining in G1 and in early S phase cells (h, i), confirming the specificity of the antibody used. a-g, representative results (out of 2).
Extended Data Fig. 11. Analyses of nuclear CDK1 during cell cycle progression.

a, Immunostaining of human mammary epithelial MCF10A cells treated with control siRNA (siCtrl) or anti-CDK1 siRNA (siCDK1) for 26 h, stained with anti-CDK1 antibody #1 (Abcam). b-d, MCF10A cells expressing Gem(1–110)-mVenus and Cdt1(1–100)Cy(−)-mCherry FUCCI(CA) reporters were live-imaged and then fixed. Cells were stained with three different anti-CDK1 antibodies: (b) #1 from Abcam, (c) #2 from Atlas Antibodies, (d) #3 from Cell Signaling Technology (CST), and nuclear CDK1 staining was assessed. Upper rows, log2 intensity of nuclear CDK1 staining at the indicated times after Cdt1(1–100)Cy(−)drop (S phase start); lower panels, CDK1 nuclear intensity after the end of last mitosis. For control of antibody specificity, cells were transfected with anti-CDK1 siRNA (siCDK1), or control siRNA (siCtrl) for 6 h and then immediately imaged for 16 h. Cells depleted of CDK1 for this time retained sufficient amounts of CDK1 to progress through mitosis. Red lines represent median values within bins every 3 time-points (36 min, 12 min interval). Third panels from left show median log2 intensity of nuclear CDK1 staining in control cells (blue) and cells treated with anti-CDK1 siRNA (red). Note decreased nuclear CDK1 staining (using all three antibodies) in cells transfected with anti-CDK1 siRNA, which confirms the specificity of the antibodies. Right panels (images of cells) depict examples of nuclear CDK1 staining used for quantification in the previous panels. Upper panels (set of 4 pictures labelled ‘early S phase’), Hoechst (DNA) and CDK1 staining for cells fixed 48 min (4 time-points) after Cdt1(1–100)Cy(−)drop (S phase start); lower panels labelled ‘G1phase’, cells fixed 3 h after the end of last mitosis in G1. Cells were transfected with control siRNA or anti-CDK1 siRNA. CDK1 depletion (siCDK1) decreased nuclear CDK1 staining in G1 and early S phase cells. e-g, Another representation of values from b-d, for cells in G1 (3–4 h after mitosis) and early S phase (0–1 h after Cdt1(1–100)Cy(−)drop), transfected with control siRNA (siCDK1 −) or with anti-CDK1 siRNA (siCDK1 +) and stained with anti-CDK1 antibody #1 (e), #2 (f) or #3 (g). Dots show values for individual cells, horizonal lines median values, dotted lines above and below the median represent inter-quartile range. h-i, Live and fixed-cell analysis of primary human dermal fibroblasts, similar to b-d. h, Since cells did not express FUCCI reporters, we analyzed nuclear CDK1 staining relative to the end of last mitosis (left panel). Right panels (cell images) show nuclear CDK1 staining in cells stained 3 h after the end of last mitosis (labelled G1 phase). Nuclei were stained with Hoechst. i, log2 of EdU incorporation in cells from the left panel (to illustrate their cell cycle progression), pulsed with EdU for 8 min prior to fixation; EdU-high cells correspond to S phase cells, EdU-low to G1 or G2 cells. h-i, Red lines represent median values within bins every 6 time-points (60 min, 10 min interval). Scale bars, 100 μm (a); 10 μm (b-d, h).
Extended Data Fig. 12. Analyses of anti-CDK1 antibody specificity and subcellular fractionation.
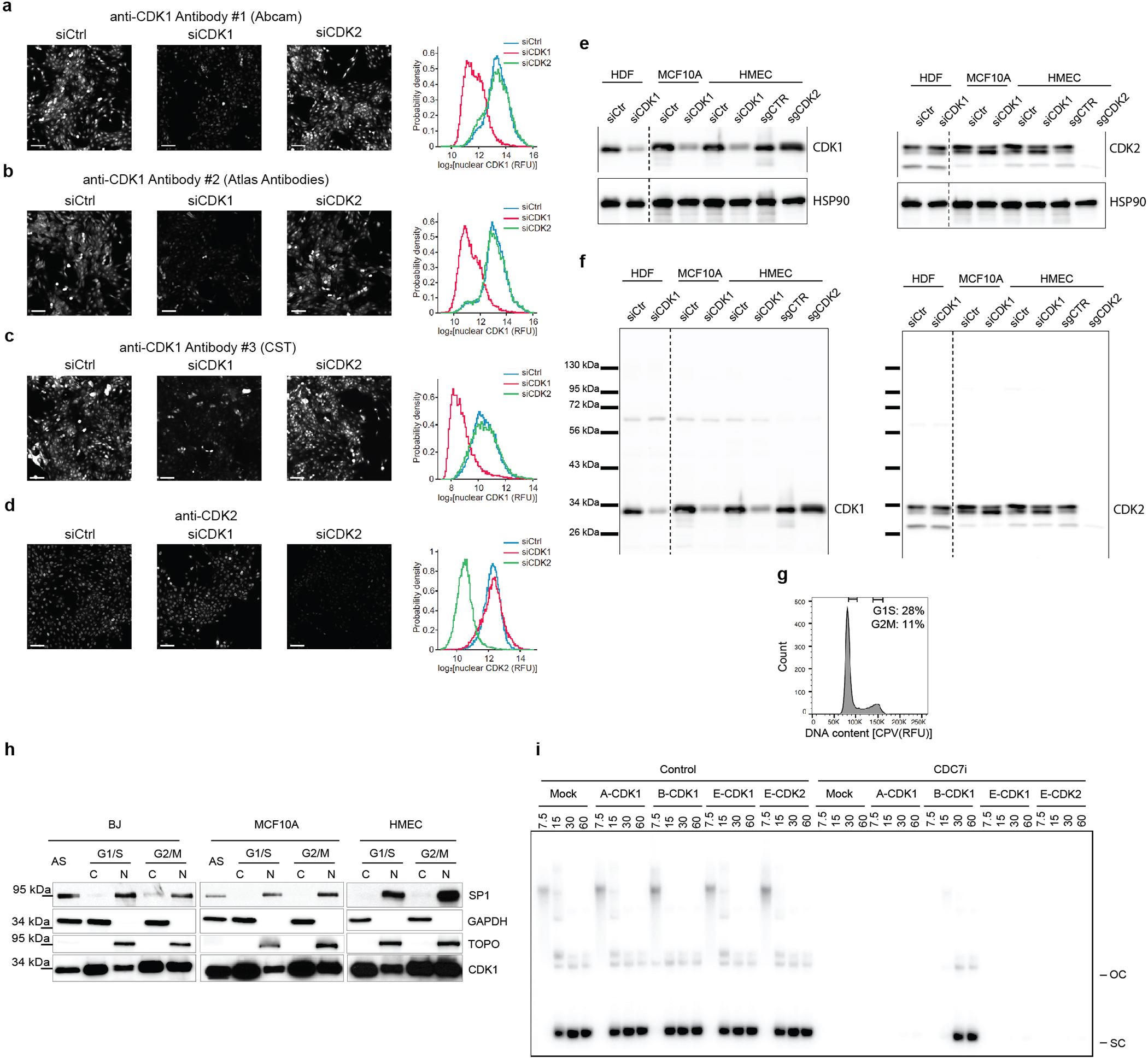
a-d, Immunostaining of MCF10A cells transfected with control siRNA (siCtrl), anti-CDK1 siRNA (siCDK1), or anti-CDK2 siRNA (siCDK2) for 24 h, stained with (a) anti-CDK1 antibody #1 (Abcam), (b) anti-CDK1 antibody #2 (Atlas Antibodies), (c) anti-CDK1 antibody #3 (Cell Signaling Technology, CST) or (d) anti-CDK2 antibody. Scale bars, 100 μm. Quantification of fluorescence intensity within the nuclei is shown on the right of each panel. Note that the anti-CDK1 immunofluorescence signal decreases upon depletion of CDK1 (siCDK1), but not following depletion of CDK2 (siCDK2). The anti-CDK2 signal decreases after depletion of CDK2 (siCDK2) but not of CDK1 (siCDK1). e, f, primary human dermal fibroblasts (HDF), MCF10A and HMEC cells and were transfected with control siRNA (siCtrl) or anti-CDK1 siRNA (siCDK1) for 48 h. Protein lysates were immunoblotted with anti-CDK1 antibody #1 (Abcam, left panel) or anti-CDK2 antibody (right panel). Lysates from CDK2-knockout (sgCDK2) and control (sgCTR) HMEC cells were immunoblotted in parallel. HSP90 was used as a loading control. The CDK1 signal decreased following depletion of CDK1, while the CDK2 signal remained unaffected. Knockout of CDK2 abrogated the CDK2 signal, but not the CDK1 signal. Panel e shows bands corresponding to CDK1, CDK2 and HSP90, panel f full-length blots probed with anti-CDK1 and anti-CDK2 antibodies. Dashed vertical lines indicate that the middle portions of the blots were spliced out; first two left lanes (HDF cells) were exposed for a longer time than the rest of the blot. g, h, Asynchronously growing human BJ foreskin fibroblasts and human mammary epithelial MCF10A and HMEC cells were stained with cell-permeant DNA-binding dye CytoPhase Violet. Cells were sorted using FACS, based on DNA content, into G1/S and G/M fractions. Cytoplasmic and nuclear fractions were prepared from cells, resolved on gels (10 μg of cytoplasmic, 20 μg of nuclear) and immunoblots probed for CDK1 (antibody #1, Abcam), GAPDH (a cytoplasmic marker), SP1 and topoisomerase I (TOPO) (both markers of the nuclear fraction; in BJ cells TOPO band migrated at ~75 kD). g, example of gating strategy for flow-sorting (BJ cells), h, immunoblot analysis; C, cytoplasmic fraction; N, nuclear fraction; AS, whole cell lysates from asynchronously growing non-sorted cells. In agreement with immunostaining (Extended Data Fig. 11), we detected nuclear Cdk1 in G1/S cells. i, DNA replication assays in nucleus-free Xenopus egg extracts (see Fig. 5e for quantification). Extracts were treated with Cdc7 inhibitor PHA-767491 (CDC7i) or with DMSO (Control). The indicated recombinant cyclin-CDK complexes were added, and nascent strand DNA synthesis assessed by incorporation of [α-32P]-dATP at 7.5, 15, 30 and 60 min following replication initiation. OC, open circular plasmid; SC, supercoiled plasmid. a-h Show representative results (out of 2), i out of 3.
Supplementary Material
Acknowledgements.
We thank Drs. Stephen P. Bell, Lorraine De Jesús-Kim, John F. X. Diffley and Lucy S. Drury for discussions and help, and Prasad V. Jallepalli for sharing unpublished results. Supported by grants R01 CA247375, CA202634 and P01 CA250959 from NIH (to PS) and GM097645 (to SPG). JMS was supported by the Mobilność Plus postdoctoral fellowship from the Ministry of Science and Higher Education of Poland (1085/MOB/2013/0), MB by the Polish National Agency for Academic Exchange (PPN/WAL/2019/1/00023) and by the Foundation for Polish Science (START Programme), YG is partially supported by R50 CA243769 from NIH.
Footnotes
Competing interests statement. PS has been a consultant at Novartis, Genovis, Guidepoint, The Planning Shop, ORIC Pharmaceuticals, Cedilla Therapeutics, Syros Pharmaceuticals and Exo Therapeutics; his laboratory receives research funding from Novartis. JMS is currently an employee of AstraZeneca, WM of Cedilla Therapeutics.
References
- 1.Asghar U, Witkiewicz AK, Turner NC & Knudsen ES The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 14, 130–146, doi: 10.1038/nrd4504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuang LC et al. Phosphorylation of Mcm2 by Cdc7 promotes pre-replication complex assembly during cell-cycle re-entry. Mol Cell 35, 206–216, doi: 10.1016/j.molcel.2009.06.014 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heller RC et al. Eukaryotic origin-dependent DNA replication in vitro reveals sequential action of DDK and S-CDK kinases. Cell 146, 80–91, doi: 10.1016/j.cell.2011.06.012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei M et al. Mcm2 is a target of regulation by Cdc7-Dbf4 during the initiation of DNA synthesis. Genes Dev 11, 3365–3374, doi: 10.1101/gad.11.24.3365 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheu YJ & Stillman B Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression. Mol Cell 24, 101–113, doi: 10.1016/j.molcel.2006.07.033 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheu YJ & Stillman B The Dbf4-Cdc7 kinase promotes S phase by alleviating an inhibitory activity in Mcm4. Nature 463, 113–117, doi: 10.1038/nature08647 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji T, Ficarro SB & Jiang W Essential role of phosphorylation of MCM2 by Cdc7/Dbf4 in the initiation of DNA replication in mammalian cells. Mol Biol Cell 17, 4459–4472, doi: 10.1091/mbc.e06-03-0241 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeeles JT, Deegan TD, Janska A, Early A & Diffley JF Regulated eukaryotic DNA replication origin firing with purified proteins. Nature 519, 431–435, doi: 10.1038/nature14285 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas ME, Ali FA, Costa A & Diffley JFX The mechanism of eukaryotic CMG helicase activation. Nature 555, 265–268, doi: 10.1038/nature25787 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonte D et al. Cdc7-Dbf4 kinase overexpression in multiple cancers and tumor cell lines is correlated with p53 inactivation. Neoplasia 10, 920–931, doi: 10.1593/neo.08216 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witucki LA et al. Mutant tyrosine kinases with unnatural nucleotide specificity retain the structure and phospho-acceptor specificity of the wild-type enzyme. Chem Biol 9, 25–33, doi: 10.1016/s1074-5521(02)00091-1 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Michowski W et al. Cdk1 Controls Global Epigenetic Landscape in Embryonic Stem Cells. Mol Cell 78, 459–476 e413, doi: 10.1016/j.molcel.2020.03.010 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santamaria D et al. Cdk1 is sufficient to drive the mammalian cell-cycle. Nature 448, 811–815, doi: 10.1038/nature06046 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Nishimura K, Fukagawa T, Takisawa H, Kakimoto T & Kanemaki M An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods 6, 917–922, doi: 10.1038/nmeth.1401 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Montagnoli A et al. Cdc7 inhibition reveals a p53-dependent replication checkpoint that is defective in cancer cells. Cancer Res 64, 7110–7116, doi: 10.1158/0008-5472.CAN-04-1547 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Acebes S et al. Targeting DNA replication before it starts: Cdc7 as a therapeutic target in p53-mutant breast cancers. Am J Pathol 177, 2034–2045, doi: 10.2353/ajpath.2010.100421 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthet C, Aleem E, Coppola V, Tessarollo L & Kaldis P Cdk2 knockout mice are viable. Curr Biol 13, 1775–1785, doi: 10.1016/j.cub.2003.09.024 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Ortega S et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet 35, 25–31, doi: 10.1038/ng1232 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Komamura-Kohno Y et al. Site-specific phosphorylation of MCM4 during the cell-cycle in mammalian cells. FEBS J 273, 1224–1239, doi: 10.1111/j.1742-4658.2006.05146.x (2006). [DOI] [PubMed] [Google Scholar]
- 20.Lin DI, Aggarwal P & Diehl JA Phosphorylation of MCM3 on Ser-112 regulates its incorporation into the MCM2–7 complex. Proc Natl Acad Sci U S A 105, 8079–8084, doi: 10.1073/pnas.0800077105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masai H et al. Human Cdc7-related kinase complex. In vitro phosphorylation of MCM by concerted actions of Cdks and Cdc7 and that of a criticial threonine residue of Cdc7 bY Cdks. J Biol Chem 275, 29042–29052, doi: 10.1074/jbc.M002713200 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Sakaue-Sawano A et al. Genetically Encoded Tools for Optical Dissection of the Mammalian Cell-cycle. Mol Cell 68, 626–640 e625, doi: 10.1016/j.molcel.2017.10.001 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Aleem E, Kiyokawa H & Kaldis P Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol 7, 831–836, doi: 10.1038/ncb1284 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Takahashi TS & Walter JC Cdc7-Drf1 is a developmentally regulated protein kinase required for the initiation of vertebrate DNA replication. Genes Dev 19, 2295–2300, doi: 10.1101/gad.1339805 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bahman M, Buck V, White A & Rosamond J Characterisation of the CDC7 gene product of Saccharomyces cerevisiae as a protein kinase needed for the initiation of mitotic DNA synthesis. Biochim Biophys Acta 951, 335–343, doi: 10.1016/0167-4781(88)90104-2 (1988). [DOI] [PubMed] [Google Scholar]
- 26.Bousset K & Diffley JF The Cdc7 protein kinase is required for origin firing during S phase. Genes Dev 12, 480–490, doi: 10.1101/gad.12.4.480 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donaldson AD, Fangman WL & Brewer BJ Cdc7 is required throughout the yeast S phase to activate replication origins. Genes Dev 12, 491–501, doi: 10.1101/gad.12.4.491 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masai H, Miyake T & Arai K hsk1+, a Schizosaccharomyces pombe gene related to Saccharomyces cerevisiae CDC7, is required for chromosomal replication. EMBO J 14, 3094–3104 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts BT, Ying CY, Gautier J & Maller JL DNA replication in vertebrates requires a homolog of the Cdc7 protein kinase. Proc Natl Acad Sci U S A 96, 2800–2804, doi: 10.1073/pnas.96.6.2800 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva T, Bradley RH, Gao Y & Coue M Xenopus CDC7/DRF1 complex is required for the initiation of DNA replication. J Biol Chem 281, 11569–11576, doi: 10.1074/jbc.M510278200 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, McDonald D, Hope TJ & Hunter T Mammalian Cdc7-Dbf4 protein kinase complex is essential for initiation of DNA replication. EMBO J 18, 5703–5713, doi: 10.1093/emboj/18.20.5703 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montagnoli A et al. A Cdc7 kinase inhibitor restricts initiation of DNA replication and has antitumor activity. Nat Chem Biol 4, 357–365, doi: 10.1038/nchembio.90 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Kim JM et al. Inactivation of Cdc7 kinase in mouse ES cells results in S-phase arrest and p53-dependent cell death. EMBO J 21, 2168–2179, doi: 10.1093/emboj/21.9.2168 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy CF, Dryga O, Seematter S, Pahl PM & Sclafani RA mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p. Proc Natl Acad Sci U S A 94, 3151–3155, doi: 10.1073/pnas.94.7.3151 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoang ML et al. Structural changes in Mcm5 protein bypass Cdc7-Dbf4 function and reduce replication origin efficiency in Saccharomyces cerevisiae. Mol Cell Biol 27, 7594–7602, doi: 10.1128/MCB.00997-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson AL, Pahl PM, Harrison K, Rosamond J & Sclafani RA Cell-cycle regulation of the yeast Cdc7 protein kinase by association with the Dbf4 protein. Mol Cell Biol 13, 2899–2908, doi: 10.1128/mcb.13.5.2899 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayano M et al. Rif1 is a global regulator of timing of replication origin firing in fission yeast. Genes Dev 26, 137–150, doi: 10.1101/gad.178491.111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumoto S, Hayano M, Kanoh Y & Masai H Multiple pathways can bypass the essential role of fission yeast Hsk1 kinase in DNA replication initiation. J Cell Biol 195, 387–401, doi: 10.1083/jcb.201107025 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alver RC, Chadha GS, Gillespie PJ & Blow JJ Reversal of DDK-Mediated MCM Phosphorylation by Rif1-PP1 Regulates Replication Initiation and Replisome Stability Independently of ATR/Chk1. Cell Rep 18, 2508–2520, doi: 10.1016/j.celrep.2017.02.042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore JD, Kirk JA & Hunt T Unmasking the S-phase-promoting potential of cyclin B1. Science 300, 987–990, doi: 10.1126/science.1081418 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Prokhorova TA, Mowrer K, Gilbert CH & Walter JC DNA replication of mitotic chromatin in Xenopus egg extracts. Proc Natl Acad Sci U S A 100, 13241–13246, doi: 10.1073/pnas.2336104100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.David-Pfeuty T & Nouvian-Dooghe Y Human cyclin B1 is targeted to the nucleus in G1 phase prior to its accumulation in the cytoplasm. Oncogene 13, 1447–1460 (1996). [PubMed] [Google Scholar]
- 43.Shen M et al. Detection of cyclin b1 expression in g(1)-phase cancer cell lines and cancer tissues by postsorting Western blot analysis. Cancer Res 64, 1607–1610, doi: 10.1158/0008-5472.can-03-3321 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Jones MJK et al. Human DDK rescues stalled forks and counteracts checkpoint inhibition at unfired origins to complete DNA replication. Mol Cell 81, 426–441 e428, doi: 10.1016/j.molcel.2021.01.004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang CC, Kato H, Shindo M & Masai H Cdc7 activates replication checkpoint by phosphorylating the Chk1-binding domain of Claspin in human cells. Elife 8, doi: 10.7554/eLife.50796 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada M et al. ATR-Chk1-APC/CCdh1-dependent stabilization of Cdc7-ASK (Dbf4) kinase is required for DNA lesion bypass under replication stress. Genes Dev 27, 2459–2472, doi: 10.1101/gad.224568.113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasi NK et al. DDK Has a Primary Role in Processing Stalled Replication Forks to Initiate Downstream Checkpoint Signaling. Neoplasia 20, 985–995, doi: 10.1016/j.neo.2018.08.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 574, 268–272, doi: 10.1038/s41586-019-1607-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geng Y et al. Cyclin E ablation in the mouse. Cell 114, 431–443, doi: 10.1016/s0092-8674(03)00645-7 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Haeussler M et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol 17, 148, doi: 10.1186/s13059-016-1012-2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cong L et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823, doi: 10.1126/science.1231143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zambrowicz BP et al. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A 94, 3789–3794, doi: 10.1073/pnas.94.8.3789 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L et al. G1 cyclins link proliferation, pluripotency and differentiation of embryonic stem cells. Nat Cell Biol 19, 177–188, doi: 10.1038/ncb3474 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yesbolatova A et al. The auxin-inducible degron 2 technology provides sharp degradation control in yeast, mammalian cells, and mice. Nat Commun 11, 5701, doi: 10.1038/s41467-020-19532-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaulian E, Zauberman A, Ginsberg D & Oren M Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol Cell Biol 12, 5581–5592, doi: 10.1128/mcb.12.12.5581-5592.1992 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masutomi K et al. Telomerase maintains telomere structure in normal human cells. Cell 114, 241–253, doi: 10.1016/s0092-8674(03)00550-6 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Li F, Adase CA & Zhang LJ Isolation and Culture of Primary Mouse Keratinocytes from Neonatal and Adult Mouse Skin. J Vis Exp, doi: 10.3791/56027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehler E, Moore-Morris T & Lange S Isolation and culture of neonatal mouse cardiomyocytes. J Vis Exp, doi: 10.3791/50154 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma S et al. Targeting the cyclin-dependent kinase 5 in metastatic melanoma. Proc Natl Acad Sci U S A 117, 8001–8012, doi: 10.1073/pnas.1912617117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanjana NE, Shalem O & Zhang F Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 11, 783–784, doi: 10.1038/nmeth.3047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Navarrete-Perea J, Yu Q, Gygi SP & Paulo JA Streamlined Tandem Mass Tag (SL-TMT) Protocol: An Efficient Strategy for Quantitative (Phospho)proteome Profiling Using Tandem Mass Tag-Synchronous Precursor Selection-MS3. J Proteome Res 17, 2226–2236, doi: 10.1021/acs.jproteome.8b00217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huttlin EL et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189, doi: 10.1016/j.cell.2010.12.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez-Riverol Y et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 47, D442–D450, doi: 10.1093/nar/gky1106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banko MR et al. Chemical genetic screen for AMPKalpha2 substrates uncovers a network of proteins involved in mitosis. Mol Cell 44, 878–892, doi: 10.1016/j.molcel.2011.11.005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dull T et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 72, 8463–8471, doi: 10.1128/JVI.72.11.8463-8471.1998 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cappell SD, Chung M, Jaimovich A, Spencer SL & Meyer T Irreversible APC(Cdh1) Inactivation Underlies the Point of No Return for Cell-Cycle Entry. Cell 166, 167–180, doi: 10.1016/j.cell.2016.05.077 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakaue-Sawano A et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487–498, doi: 10.1016/j.cell.2007.12.033 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Spencer SL et al. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155, 369–383, doi: 10.1016/j.cell.2013.08.062 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao ML et al. Molecular Competition in G1 Controls When Cells Simultaneously Commit to Terminally Differentiate and Exit the Cell Cycle. Cell Rep 31, 107769, doi: 10.1016/j.celrep.2020.107769 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin JR, Fallahi-Sichani M & Sorger PK Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat Commun 6, 8390, doi: 10.1038/ncomms9390 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blow JJ & Laskey RA Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell 47, 577–587, doi: 10.1016/0092-8674(86)90622-7 (1986). [DOI] [PubMed] [Google Scholar]
- 72.Lebofsky R, Takahashi T & Walter JC DNA replication in nucleus-free Xenopus egg extracts. Methods Mol Biol 521, 229–252, doi: 10.1007/978-1-60327-815-7_13 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Deng L et al. Mitotic CDK Promotes Replisome Disassembly, Fork Breakage, and Complex DNA Rearrangements. Mol Cell 73, 915–929 e916, doi: 10.1016/j.molcel.2018.12.021 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data for mass spectrometry analyses (Fig. 3d; Extended Data Fig. 7a, d; Supplementary Table 1; Supplementary Table 2) has been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD025625.


